Abstract
Purpose
A reduction in cancer services during the coronavirus disease of 2019 pandemic has affected cancer diagnoses. The purpose of this study is to quantitatively determine the impact on cancer diagnostic service in public facilities across Hong Kong. Quantifying the temporal changes in the number of cancer diagnoses before, during, and after the outbreak is useful to establish the scale of the problem and assess if there has been an adequate level of response.
Methods and Materials
This is a retrospective cohort study using a territory-wide database in Hong Kong from 2017 to 2020, and consecutive specimens received for pathologic diagnosis in public laboratories in 41 hospitals were retrieved.
Results
In 2020, a total of 455,453 pathologic specimens were received, which amounted to a 15.5% reduction compared with the prior 3-year average (P < .001). An analysis of confirmed malignant pathologic diagnoses revealed a statistically significant reduction in colorectal (–10.0%; P < .001) and prostate (–19.7%; P < .001), nonsignificant reduction in lung (–3.0%; P = .0526), and a marginal but nonsignificant increase for breast (0.7%; P = .7592) regions. Based on time series projection data, the estimated missed cancers for the 3 regions with reduced investigations were colorectal (10.0%), lung (3.0%), and prostate (19.7%).
Conclusions
Variable impact on actual malignant pathologic diagnoses based on 4 body regions was observed, with a statistically significant reduction in colorectal, lung, and prostate regions, and marginal but insignificant increase in breast regions. The findings could help public health policy with future planning and intervention.
Introduction
The coronavirus disease of 2019 (COVID-19) pandemic has brought disruptions to all aspects of health care, including cancer diagnostic services.1 The pandemic lockdown has reduced patients’ willingness to seek care2 and prevented patients’ usual care.3 In addition, hospital services were repurposed to address COVID-19–related services, contributing to a reduction in normal cancer diagnostic services.4 In the short term, the disruption to cancer treatment, alteration of treatment plans and intervals, as well as delays in cancer treatment, have been previously documented.5, 6, 7 The long-term effect of delay in diagnosis is more difficult to measure. A potential early indicator for delayed diagnosis may be gleaned from the number of cancer diagnostic investigations that have been performed during this period.
We reasoned that by comparing data from previous years, we can extrapolate quantitatively the potential shortfall in cancer diagnoses during the current pandemic period. We can do this by examining diagnostic services, such as pathologic investigations, during this period. In this study, we systematically examined territory-wide data in Hong Kong to explore the temporal relationship before, during, and after different waves of COVID-19 infections, and examine its impact on cancer-related diagnostic services. Furthermore, we extrapolated the impact on missed cancer diagnoses in 4 of the most common cancer body regions in Hong Kong.
Methods and Materials
A retrospective search of patients’ electronic records using a nationwide database was performed using the Hong Kong Hospital Authority Clinical Data Analysis and Reporting System, which has been utilized in several prior studies.8 , 9 This study method adhered to the Strengthening the Reporting of Observational Studies in Epidemiology reporting guideline, and was approved by an institutional review board. Patient consent was waived due to the study's retrospective nature. The study period was from January 2017 to December 2020. A search for the total number of pathologic specimens received by public laboratories in Hong Kong was performed with associated body regions and pathologic diagnoses. A subanalysis of the 4 most common cancer regions in Hong Kong (ie, colorectal, lung, breast, and prostate) were performed. The number and percentages of the year 2020 were compared with the prior 3-year average. To predict the change in the number of malignant lesions in 2020 more accurately, we performed a time-series model based on data in the prior years of 2017 to 2019 to derive the predicted number of malignant lesions in 2020. The best forecasting model was chosen based on the best fitting univariate autoregressive integrated moving average (ARIMA) model:
where P is the order of the autoregressive part; d the degree of the first differencing involved, q the order of the moving average part, m the number of periods in each season, and uppercase P, D, and Q the autoregressive, differencing, and moving average terms, respectively, for the seasonal part of the ARIMA model. Winter's additive is similar to an ARIMA model with 0 orders of autoregression, 1 order of differencing, 1 order of seasonal differencing, and 13 orders of moving average. Simple seasonal is similar to an ARIMA model with 0 orders of autoregression, 1 order of differencing, 1 order of seasonal differencing, and orders 1, 12, and 13 of moving average.
Differences in counts between the years were compared for statistical significance using Poisson regression (P < .05 is considered statistical significance). The comparators were prior 3-year average or the predicted number based on the time-series analysis. The positivity rate for each of the 4 regions was calculated based on the total number of malignant lesions, divided by the total number of specimens received for that region. The number of potentially missed malignant lesions was also calculated (Table 1; Appendix E1).
Table 1.
Total number and percentage change of pathologic specimens received, malignant lesions, positivity rate, and predicted missed cases
| Total | Colorectal | Lung | Breast | Prostate | |
|---|---|---|---|---|---|
| Pathologic specimens received | |||||
| 2017 | 528,916 | 57,481 | 91,885 | 19,031 | 8747 |
| 2018 | 535,896 | 59,507 | 95,405 | 19,322 | 8516 |
| 2019 | 552,820 | 62,754 | 106,001 | 19,139 | 9229 |
| 2020 | 455,453 | 48,955 | 87,497 | 18,756 | 7149 |
| % change | –15.53 | –18.29 | –10.5 | –2.13 | –19.04 |
| Malignant lesions | |||||
| 2017 | 41,736 | 6136 | 7046 | 4101 | 1953 |
| 2018 | 42,002 | 6050 | 7120 | 3980 | 1959 |
| 2019 | 43,653 | 5987 | 7736 | 4035 | 2202 |
| 2020 | 41,550 | 5410 | 7802 | 4057 | 1939 |
| 2020 predicted | 44,071.51 | 6011.58 | 8045.98 | 4029.43 | 2416 |
| % change | –5.7 | –10.0 | –3.0 | 0.7 | –19.7 |
| 2020 missed cancer diagnosis | 2521.51 | 601.58 | 243.98 | N/A | 477 |
| Positivity rate | |||||
| 2020 | 9.12 | 11.05 | 8.92 | 21.63 | 27.12 |
| 2020 predicted | 7.84 | 9.23 | 7.34 | 21.04 | 25.44 |
| % change | 16.3 | 19.7 | 21.5 | 2.8 | 6.6 |
Abbreviation: N/A = not applicable.
The percentage change of pathologic specimens received and positivity rate was based on the prior 3-year average. The percentage change of malignant lesions and predicted missed cases was based on the predicted number using a time-series model.
Results
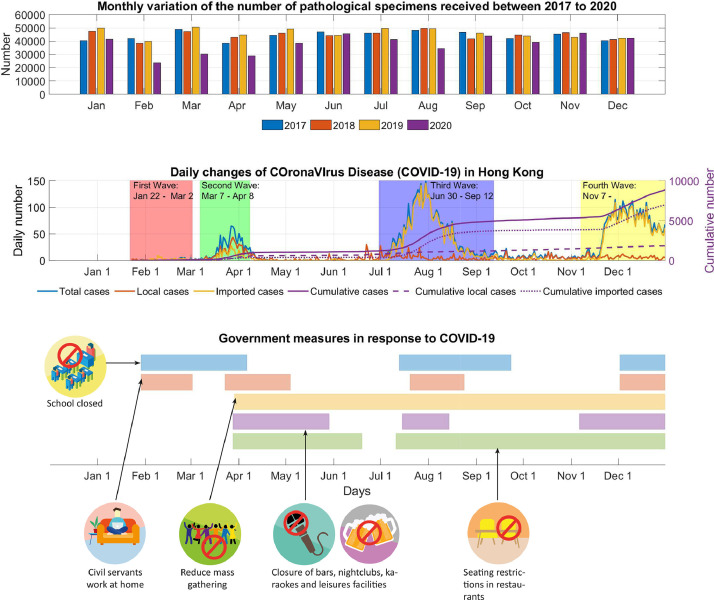
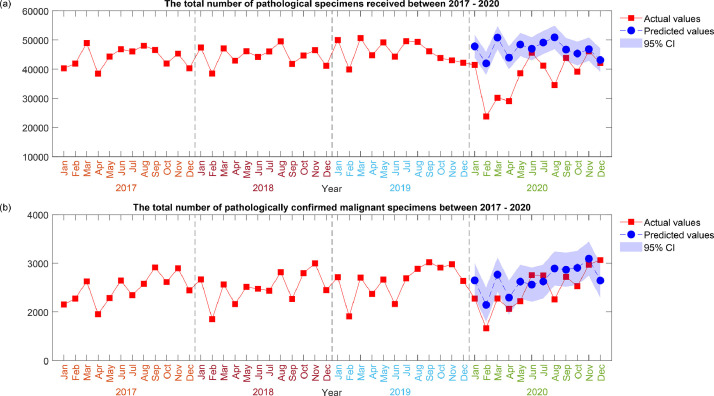
In 2020, there was a total number of 455,453 pathologic specimens received from 41 hospitals. A 15.5% reduction in the total number of specimens was observed in 2020 compared with the prior 3-year average (Table 1 ). A monthly analysis revealed a sharp drop in February during the start of the COVID-19 outbreak in Hong Kong, followed by a more modest reduction in August, which coincided with the third wave of infections (Table 2 ; Fig. 1, Fig. 2 ; Figs. E1 and E3). Using Poisson regression for count, a statistically significant reduction based on body regions was observed for the colorectal (18.3%; P < .001), lung (10.5%; P < .001), breast (2.1%; P < .05), and prostate (19.0%; P < .001) regions (Fig. E6).
Table 2.
Actual predicted number using time-series model and percentage change of pathologic specimens received and malignant lesions in 2020
| Pathologic specimens received |
Malignant lesions |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Colorectal | Lung | Breast | Prostate | Total | Colorectal | Lung | Breast | Prostate | ||
| Model type | Winters' additive | Winters' additive | Winters' additive | Simple seasonal | Simple seasonal | Winters' additive | Simple seasonal | Winters' additive | Simple seasonal | Winters' additive | |
| Jan | Actual | 41,445 | 4928 | 8407 | 1589 | 761 | 3275 | 460 | 626 | 315 | 176 |
| predicted* | 47,809 | 5517 | 9432 | 1649 | 840 | 3646 | 530 | 648 | 338 | 211 | |
| % change | –13.31 | –10.67 | –10.87 | –3.63 | –9.46 | –10.19 | –13.23 | –3.46 | –6.68 | –16.52 | |
| Feb | Actual | 23,752 | 2013 | 5676 | 1145 | 294 | 2664 | 383 | 527 | 260 | 88 |
| predicted | 41,987 | 4917 | 8731 | 1417 | 759 | 3146 | 443 | 605 | 260 | 183 | |
| % change | –43.43 | –59.06 | –34.99 | –19.21 | –61.29 | –15.31 | –13.58 | –12.86 | 0.04 | –51.87 | |
| Mar | Actual | 30,195 | 2413 | 6973 | 1389 | 364 | 3277 | 452 | 643 | 293 | 131 |
| predicted | 50,780 | 5839 | 10,195 | 1669 | 862 | 3767 | 551 | 691 | 330 | 216 | |
| % change | –40.54 | –58.67 | –31.6 | –16.77 | –57.76 | –13.01 | –18.04 | –6.96 | –11.19 | –39.30 | |
| Apr | Actual | 29,019 | 2631 | 6348 | 1479 | 479 | 3062 | 388 | 571 | 317 | 150 |
| predicted | 43,922 | 5060 | 8991 | 1470 | 702 | 3296 | 457 | 608 | 313 | 184 | |
| % change | –33.93 | –48.01 | –29.4 | 0.62 | –31.75 | –7.09 | –15.19 | –6.10 | 1.31 | –18.26 | |
| May | Actual | 38,555 | 3932 | 7275 | 1610 | 525 | 3219 | 394 | 616 | 331 | 147 |
| predicted | 48,415 | 5612 | 9764 | 1621 | 792 | 3622 | 512 | 674 | 317 | 192 | |
| % change | –20.36 | –29.93 | –25.49 | –0.69 | –33.75 | –11.14 | –23.07 | –8.66 | 4.34 | –23.24 | |
| Jun | Actual | 45,586 | 5103 | 8265 | 1846 | 713 | 3756 | 469 | 726 | 383 | 172 |
| predicted | 46,994 | 5530 | 9238 | 1603 | 795 | 3562 | 494 | 629 | 321 | 200 | |
| % change | –3 | –7.71 | –10.53 | 15.17 | –10.37 | 5.46 | –5.15 | 15.46 | 19.23 | –14.07 | |
| Jul | Actual | 41,172 | 4771 | 7440 | 1842 | 702 | 3752 | 517 | 678 | 357 | 160 |
| predicted | 49,117 | 5727 | 9485 | 1677 | 786 | 3624 | 495 | 648 | 340 | 205 | |
| % change | –16.18 | –16.69 | –21.56 | 9.83 | –10.74 | 3.53 | 4.34 | 4.56 | 5.14 | –21.76 | |
| Aug | Actual | 34,541 | 3461 | 7175 | 1508 | 535 | 3258 | 428 | 693 | 315 | 149 |
| predicted | 50,865 | 6118 | 9792 | 1783 | 832 | 3895 | 542 | 742 | 350 | 199 | |
| % change | –32.09 | –43.43 | –26.73 | –15.42 | –35.71 | –16.35 | –21.06 | –6.66 | –9.89 | –24.94 | |
| Sep | Actual | 43,827 | 5233 | 8325 | 1738 | 683 | 3722 | 473 | 659 | 368 | 163 |
| predicted | 46,707 | 5507 | 8973 | 1654 | 810 | 3869 | 491 | 720 | 347 | 207 | |
| % change | –6.17 | –4.97 | –7.22 | 5.09 | –15.66 | –3.80 | –3.63 | –8.53 | 6.08 | –21.07 | |
| Oct | Actual | 39,128 | 4423 | 6896 | 1434 | 603 | 3529 | 461 | 643 | 320 | 171 |
| predicted | 45,360 | 5156 | 8184 | 1549 | 782 | 3907 | 486 | 712 | 371 | 203 | |
| % change | –13.74 | –14.22 | –15.74 | –7.42 | –22.9 | –9.68 | –5.18 | –9.66 | –13.8 | –15.83 | |
| Nov | Actual | 46,093 | 5286 | 7313 | 1618 | 758 | 3970 | 482 | 674 | 373 | 209 |
| predicted | 46,837 | 5357 | 8445 | 1609 | 824 | 4093 | 534 | 716 | 407 | 224 | |
| % change | –1.59 | –1.32 | –13.41 | 0.57 | –8.02 | –3.01 | –9.76 | –5.92 | –8.25 | –6.49 | |
| Dec | Actual | 42,140 | 4761 | 7404 | 1558 | 732 | 4066 | 503 | 746 | 425 | 223 |
| predicted | 43,106 | 4812 | 8386 | 1454 | 710 | 3644 | 474 | 651 | 337 | 195 | |
| % change | –2.24 | –1.07 | –11.71 | 7.16 | 3.08 | 11.57 | 6.16 | 14.63 | 26.15 | 14.26 | |
| Model Fit statistics | Stationary R2 | 0.8571 | 0.8818 | 0.7396 | 0.8777 | 0.7648 | 0.8785 | 0.8687 | 0.8758 | 0.8829 | 0.7981 |
| RMSE | 1913.30 | 243.53 | 410.08 | 92.59 | 47.32 | 171.83 | 37.1 | 46.56 | 22.75 | 15.41 | |
| MAE | 1468.69 | 194.86 | 299.53 | 72.83 | 36.45 | 126.06 | 29.49 | 34.99 | 18.26 | 12.42 | |
| MaxAPE | 10.72 | 10.71 | 11.97 | 11.84 | 11.96 | 13.72 | 21.46 | 17.43 | 17.7 | 21.93 | |
| MaxAE | 4319.55 | 534.00 | 966.20 | 191.51 | 87.22 | 448.33 | 88.86 | 109.73 | 52.93 | 28.72 | |
| Normalized BIC | 15.41 | 11.29 | 12.33 | 9.26 | 7.91 | 10.59 | 7.43 | 7.98 | 6.45 | 5.77 | |
Abbreviations: BIC = Bayesian information criterion; MAE = mean absolute error; MaxAE = maximum absolute error; MaxAPE = maximum absolute percentage error; RMSE = root mean square error.
Predicted values were rounded to nearest integer, and percentage changes were based on the full digits of the predicted value.
Fig. 1.
Monthly variation in numbers of pathologic specimens received from January to December 2020, matched with graphical illustrations of coronavirus disease of 2019 cases and governmental measures during and after each outbreak in Hong Kong.
Fig. 2.
Time-series analysis of pathologic specimens received and malignant lesions.
The impact on malignant pathologic diagnoses was variable depending on the body region (Table 2; Figs. E2, E4 and E5). For colorectal and prostate cancer, there was a statistically significant reduction of 10.0% (P < .001) and 19.7% (P < .001), respectively, in 2020 compared with the predicted number. For lung cancer, there was a nonsignificant reduction of 3.0% (P = .0526). For breast cancer, there was a marginal but nonsignificant increase of 0.7% (P = .7592) in 2020 compared with the predicted number. The highest total number and largest increase from the 2020 average were observed in December 2020 for confirmed malignant specimens.
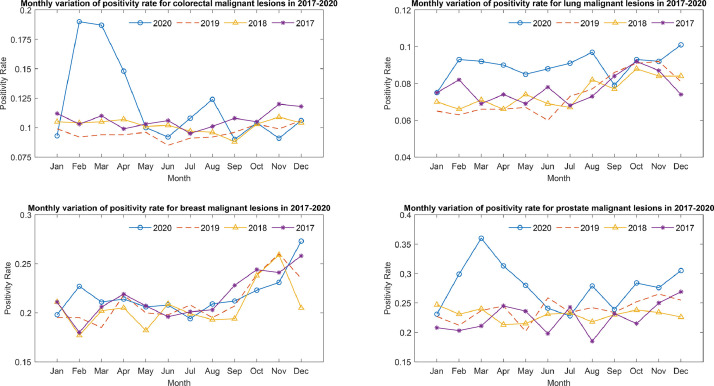
The positivity rate for malignant lesion detection increased during 2020 for breast, colorectal, lung, and prostate regions despite a reduction in the number of investigations (Fig. 3 ; Tables E1 and E2). A significant increase in positivity rate was observed in colorectal (19.7%; P < .05), lung (21.5%; P < .001), and prostate (6.6%; P < .005) sections compared with 2019. Despite this, the estimated numbers of missed cancer cases were 602 (10.0%), 244 (3.0%), and 477 (19.7%) for colorectal, lung, and prostate regions, respectively (Table 2).
Fig. 3.
Positivity rate of 4 body regions between January 2017 and December 2020.
Discussion
In Hong Kong, a differential impact was observed in 2020 depending on body region, with colorectal and prostate more affected compared with breast and lung regions in terms of actual malignant pathologic diagnosis. The reduction in the number of malignant pathologic diagnoses in colorectal (10.0%) and prostate (19.7%) regions was mainly driven by the decrease in the number of specimens received from the laboratories. Cancellation of elective colonoscopy and prostate biopsy lists during the pandemic have been previous documented.10, 11, 12 Despite a modest reduction of 10.5% in the number of lung specimens received, there was a negligible effect on malignant pathologic diagnoses. The high positivity rate for lung malignant lesions suggests that, despite a decrease in the number of investigations, the malignant cases were still being diagnosed. Conceivably, more respiratory-related investigations were performed during the COVID-19 outbreak, which may have led to more targeted confirmatory investigations. For the breast region, the reduction in specimens received was small (2.1%), and there was indeed a marginal but nonsignificant increase (0.7%) in malignant breast lesions diagnoses compared with the predicted number in 2020. Breast-related investigations, such as mammogram, ultrasound and U.S.-guided biopsies can be performed relatively noninvasively, and services were scaled up more quickly after an initial reduction, as observed in June and July 2020 (Fig. E3). Based on our projection data, the percentage of missed cancer diagnoses were seen in the colorectal (10.0%), lung (3.0%), and prostate (19.7%) regions, although the numbers were perhaps not as high as previously anticipated. This may be explained partly by the robust response during the trough period of infections, but also less impact was observed during the subsequent phases of infections despite high COVID-19 infection rates in the community, likely due to enhanced precautions in hospital services with the maintenance of diagnostic services, as well as a significant increase in services in December 2020 and a consequent increase in malignant lesions detection and positivity rate. Due to the robustness of the response in Hong Kong, the impact of previously hypothesized delayed diagnoses potentially leading to patients presenting later with more advanced-stage disease and potentially affecting their treatment options and survival may not be as severe as previously anticipated.13 The numbers of breast- and lung-region malignant pathologic diagnoses were not significantly different from those of prior years. However, a 10.0% reduction in colorectal malignant pathologic diagnoses was observed. The impact on prostate cancer based on the numbers appeared high, but prostate cancers are known to be mostly indolent, and a proportion of patients with low-grade malignant disease were likely put on active surveillance without active intervention. Therefore, although the actual impact may be minimal, nevertheless, from a public health perspective, increasing diagnostic services related to colorectal and prostate regions may be prudent if such provisions are possible. However, this must be balanced against the backdrop of overdiagnosis and overtreatment regarding which there are ongoing debates, particularly for prostate cancer.
There are several limitations to this study. First, malignant diagnosis was based on specimens received, but we did not have the actual breakdown numbers of newly diagnosed cancers, recurrence or metastatic lesions, nor staging or grading of malignant lesions (eg, prostate cancers Gleason score). However, the general trend in reduction is likely to be true because the same criteria were applied also to the previous years. Second, the search system only covered public hospitals in Hong Kong, and did not include private hospitals. Approximately 90% of inpatient care is estimated to be in public hospital systems, but differential usage during the pandemic could not be accounted for.
Third, there was a lack of specific oncologic-related outcomes (eg, disease stage at diagnosis, survival). The study is broad, encompassing 41 centers in Hong Kong; thus, establishing staging information was not possible and survival data would take longer to manifest. Determining whether pathologic diagnoses appropriately or inappropriately lag based on patient outcome data would be useful, and should be the focus of future studies. However, while establishing that there was a significant delay in diagnosis with real impact on morbidity and mortality, we may have missed the window to intervene.
Finally, we could not account for the idiosyncrasies of local practices (eg, loss or new services during the study period), which may affect the actual numbers. However, this is unlikely to have a major impact on the overall conclusion, which takes a more global perspective.
Conclusions
Cancer diagnoses were expected to be significantly impacted during the COVID-19 outbreak due to the reduction and cancelation of diagnostic services. In Hong Kong, a differential impact was observed in 2020 depending on body region, with colorectal and prostate regions more affected compared with breast and lung regions in terms of actual malignant pathologic diagnoses. This was the result of a robust response after the initial impact with maintenance and increasing services later in the year despite increasing case rates due to the subsequent waves of active infections. Nevertheless, a significant reduction in colorectal and prostate malignant pathologic diagnoses was observed, and increasing diagnostic services may be a more optimal way to reallocate additional resources in the post-COVID-19 era.
Footnotes
Disclosures: none.
Data Sharing Statement: Research data are not available at this time.
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ijrobp.2021.05.010.
Appendix. Supplementary materials
References
- 1.Disis ML. Oncology and COVID-19. JAMA. 2020;324:1141. doi: 10.1001/jama.2020.16945. [DOI] [PubMed] [Google Scholar]
- 2.LoGiudice SH, Liebhaber A, Schöder H. Overcoming the COVID-19 crisis and planning for the future. J Nucl Med. 2020;61:1096–1101. doi: 10.2967/jnumed.120.250522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xie C, Wang X, Liu H, et al. Outcomes in radiotherapy-treated patients with cancer during the COVID-19 outbreak in Wuhan, China. JAMA Oncol. 2020;6:1457. doi: 10.1001/jamaoncol.2020.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaufman HW, Chen Z, Niles J, Fesko Y. Changes in the number of U.S. patients with newly identified cancer before and during the coronavirus disease 2019 (COVID-19) pandemic. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.17267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elkrief A, Kazandjian S, Bouganim N. Changes in lung cancer treatment as a result of the coronavirus disease 2019 pandemic. JAMA Oncol. 2020;6:1805–1806. doi: 10.1001/jamaoncol.2020.4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldstein DA, Ratain MJ, Saltz LB. Weight-based dosing of pembrolizumab every 6 weeks in the time of COVID-19. JAMA Oncol. 2020;6:1694–1695. doi: 10.1001/jamaoncol.2020.2493. [DOI] [PubMed] [Google Scholar]
- 7.Papautsky EL, Hamlish T. Patient-reported treatment delays in breast cancer care during the COVID-19 pandemic. Breast Cancer Res Treat. 2020;184:249–254. doi: 10.1007/s10549-020-05828-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lui TKL, Leung K, Guo CG, Tsui VWM, Wu JT, Leung WK. Impacts of the coronavirus 2019 pandemic on gastrointestinal endoscopy volume and diagnosis of gastric and colorectal cancers: A population-based study. Gastroenterology. 2020;159:1164–1166. doi: 10.1053/j.gastro.2020.05.037. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yip TCF, Lui GCY, Wong VWS, et al. Liver injury is independently associated with adverse clinical outcomes in patients with COVID-19. Gut. 2021;70:733–742. doi: 10.1136/gutjnl-2020-321726. [DOI] [PubMed] [Google Scholar]
- 10.Maida M. Screening of gastrointestinal cancers during COVID-19: A new emergency. Lancet Oncol. 2020;21:e338. doi: 10.1016/S1470-2045(20)30341-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Forbes N, Smith ZL, Spitzer RL, et al. Changes in gastroenterology and endoscopy practices in response to the coronavirus disease 2019 pandemic: Results from a North American survey. Gastroenterology. 2020;159:772–774. doi: 10.1053/j.gastro.2020.04.071. e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roscigno M, Naspro R, Piccichè A, et al. A snapshot from the department of urology in Bergamo evaluating the timeline of the SARS-CoV-2 outbreak: Which patients are we missing? Eur Urol Focus. 2020;6:1120–1123. doi: 10.1016/j.euf.2020.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamilton W. Cancer diagnostic delay in the COVID-19 era: What happens next? Lancet Oncol. 2020;21:1000–1002. doi: 10.1016/S1470-2045(20)30391-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.