Abstract
Background
Delirium is a deleterious condition affecting up to 60% of patients in the surgical ICU (SICU). Few SICU-focused delirium interventions have been implemented, including those addressing sleep-wake disruption, a modifiable delirium risk factor common in critically ill patients.
Research Question
What is the effect on delirium and sleep quality of a multicomponent nonpharmacologic intervention aimed at improving sleep-wake disruption in patients in the SICU setting?
Study Design and Methods
Using a staggered pre-post design, we implemented a quality improvement intervention in two SICUs (general surgery or trauma and cardiovascular) in an academic medical center. After a preintervention (baseline) period, a multicomponent unit-wide nighttime (ie, efforts to minimize unnecessary sound and light, provision of earplugs and eye masks) and daytime (ie, raising blinds, promotion of physical activity) intervention bundle was implemented. A daily checklist was used to prompt staff to complete intervention bundle elements. Delirium was evaluated twice daily using the Confusion Assessment Method for the Intensive Care Unit. Patient sleep quality ratings were evaluated daily using the Richards-Campbell Sleep Questionnaire (RCSQ).
Results
Six hundred forty-six SICU admissions (332 baseline, 314 intervention) were analyzed. Median age was 61 years (interquartile range, 49-70 years); 35% of the cohort were women and 83% were White. During the intervention period, patients experienced fewer days of delirium (proportion ± SD of ICU days, 15 ± 27%) as compared with the preintervention period (20 ± 31%; P = .022), with an adjusted pre-post decrease of 4.9% (95% CI, 0.5%-9.2%; P = .03). Overall RCSQ-perceived sleep quality ratings did not change, but the RCSQ noise subscore increased (9.5% [95% CI, 1.1%-17.5%; P = .02).
Interpretation
Our multicomponent intervention was associated with a significant reduction in the proportion of days patients experienced delirium, reinforcing the feasibility and effectiveness of a nonpharmacologic sleep-wake bundle to reduce delirium in critically ill patients in the SICU.
Trial Registry
ClinicalTrials.gov; No.: NCT03313115; URL: www.clinicaltrials.gov
Key Words: critical care, delirium, sleep, sleep hygiene, wakefulness
Abbreviations: CAM-ICU, Confusion Assessment Method for the Intensive Care Unit; CCI, Charlson Comorbidity Index; CVICU, cardiovascular ICU; IQR, interquartile range; LOS, length of stay; QI, quality improvement; RCSQ, Richards-Campbell Sleep Questionnaire; SAS, Sedation-Agitation Scale; SICU, surgical ICU
Critically ill patients in the ICU often experience delirium, a deleterious problem associated with short-term and long-term cognitive, physical, and mental health impairments, prolonged length of stay (LOS), and early death.1, 2, 3, 4, 5, 6 As a preventable and often hospital-acquired problem, ICU delirium is expensive, costing the US health care system more than $148 billion annually.2,4,7,8
Rising awareness about delirium has brought attention to the surgical ICU (SICU) setting, where delirium affects up to 60% of patients after surgery.9 Although SICU and nonsurgical ICU patients share many delirium risk factors,10,11 several perioperative factors are specific to surgical patients, including anesthesia medications, pain, and operative time.12, 13, 14, 15 These and other modifiable factors10,11 make delirium an attractive target for quality improvement (QI) interventions, including those focused on sleep-wake disruption, a common problem during critical illness believed to be associated with delirium.16, 17, 18 Sleep in the ICU is characterized by fragmentation, a lack of restorative stages, and predominance during the daytime hours.19,20 Prior ICU sleep QI interventions led to reductions in delirium, but involved fewer than 350 patients in predominantly medical or medical-surgical units.21, 22, 23 Evaluating the effect of sleep interventions on delirium is needed,23,24 and because prior efforts, to our knowledge, did not focus on SICU patients, we performed a multicomponent QI intervention to improve delirium and sleep-wake disruption in two ICUs in an academic medical center.
Methods
Intervention Setting and Design
This QI intervention was conducted in two ICUs in a tertiary care academic medical center, a 12-bed SICU and a 20-bed cardiovascular ICU (CVICU). The SICU comprised mostly trauma and general surgical patients, whereas the CVICU comprised cardiothoracic surgery and cardiac patients undergoing procedures, including those requiring mechanical circulatory support. Patients primarily were in a postoperative period, except for nonsurgical cardiovascular patients requiring ICU admission (ie, after Impella [Abiomed] or intra-aortic balloon pump placement). ICU care teams included a 24-h in-house intensivist, fellows, residents, a nurse practitioner or physician assistant, and a respiratory therapist. Daytime teams also included a critical care pharmacist; physical, occupational, and speech therapists; and a dietician. Both ICUs were staffed at a 1:2 or 1:1 nurse to patient ratio.
Following an established QI framework25 and building on prior successful ICU-based sleep interventions, we assembled a multidisciplinary team and designed and implemented a multicomponent sleep-wake intervention comprising staff-led patient-centered and unit-wide actions to improve the ICU environment (e-Appendix 1).21,22 Because the 2018 Pain, Agitation/Sedation, Delirium, Immobility, and Sleep guidelines had not been published at the time of the intervention,26 our team leveraged experiences of nurses and aids and used a previously published sleep QI checklist21 and a Plan-Do-Study-Act process to identify candidate sleep improvement interventions, which then were reviewed by an ICU sleep expert (B. B. K.).27 In the two involved ICUs, the intervention was implemented in stages, starting with an 8- or 12-week baseline usual care period (SICU, October-December 2017; CVICU, October 2017-January 2018) and followed by an 8- or 12-week intervention period (SICU, December 2017-March 2018; CVICU, January-March 2018). Environmental interventions to promote daytime wakefulness included: turning on lights, opening windows and doors, provision of eyeglasses and hearing aids, encouragement of physical and cognitive activities to prevent napping, daytime supply restocking, and avoiding caffeine after 3 pm. At 10 pm each day, nighttime interventions to promote sleep were encouraged, including: turning televisions off, closing doors and curtains, minimizing unnecessary alarms, dimming lights, encouraging family to facilitate bedtime (by leaving or not disturbing the patient), offering eye masks and earplugs, and clustering patient care activities (e-Appendix 1). Because our effort was ICU-wide, all patients in both ICUs were considered for all intervention components.
Before intervention implementation, designated ICU sleep champions (J. E. T., A. D., K. L., S. H., and J. B.) educated staff regarding sleep-wake disruption, delirium, and daytime and nighttime interventions. A daily sleep-wake bundle checklist prompted intervention completion (e-Appendix 1) and was reinforced by champions using verbal prompts, e-mails, and flyers, along with weekly education and interdisciplinary meetings and daily sleep champion rounds to obtain feedback from the overnight team regarding the previous night’s interventions. During this effort, no other delirium interventions were carried out in the involved ICUs. Because this intervention aimed to promote standard bedside practices, the local institutional review board deemed it QI. All findings were reported according to the Standards for Quality Improvement Reporting Excellence guidelines (e-Appendix 1).28
Outcome Measures
Data were collected until ICU discharge or death for all patients ≥ 18 years of age admitted to the SICU or CVICU during the preintervention (baseline) and intervention periods, with mortality data collected until hospital discharge. The primary outcome was ICU delirium, evaluated by bedside staff using the Confusion Assessment Method for the ICU (CAM-ICU).29,30 As part of this assessment, level of consciousness was evaluated using the Riker Sedation-Agitation Scale (SAS),31 a 1-to-7 scale (1 = unarousable; 2 = very sedated, responsive only to physical stimuli; 3 = sedated, responsive to verbal stimuli or gentle touch; 4 = calm and cooperative; 5 = agitated; 6 = very agitated; and 7 = dangerously agitated). Per unit standard, the CAM-ICU and Riker SAS were completed by nurses twice daily, including on ICU admission. In patients with coma, defined as a Riker SAS score of 1 or 2, sedative agents were reduced or stopped and were re-evaluated for delirium when Riker SAS score was ≥ 3.
Perceived nighttime sleep quality was evaluated using the Richards-Campbell Sleep Questionnaire (RCSQ), a five-item validated questionnaire evaluating the following characteristics of nighttime sleep: (1) depth, (2) latency (time to fall asleep), (3) number of awakenings, (4) efficiency (percent of time awake), and (5) quality.21 RCSQ responses were recorded on 100-mm visual analog scales, with higher scores representing better sleep and the mean of all five items representing overall sleep quality (primary sleep outcome). Each morning during the preintervention and intervention periods, patients were asked to complete the RCSQ; if not comatose (ie, Riker SAS score ≥ 3) but delirious, the RCSQ was completed by the patient’s nighttime nurse, as described previously.21 Consistent with prior studies, our questionnaire included a separate sixth item evaluating perceived nighttime noise.21 All forms were entered into the Research Electronic Data Capture application.32
Demographic and Clinical Variables
Demographic and clinical data were obtained from the institutional electronic data warehouse and included age, race, and ethnicity; clinical variables included admission diagnosis category, Charlson Comorbidity Index (CCI),33 Medicare Severity-Diagnosis Related Group score, and daily presence of life-supporting therapies, including mechanical ventilation, continuous renal replacement therapy, and mechanical circulatory support devices (ie, extracorporeal membrane oxygenation, Impella, and intra-aortic balloon pump). Additionally, patients (or proxies if unable) completed a brief home sleep questionnaire, adapted from the Pittsburgh Sleep Quality Index,34 inquiring about home sleep quality, presence of pre-existing sleep disorders, and using television and medications to sleep at home.21
Statistical Analysis
Demographic and clinical characteristics were stratified across the preintervention and intervention periods. Continuous variables were compared using Student t test or the Wilcoxon rank-sum test, and categorical variables were compared using the χ 2 or Fisher exact test. The analysis was conducted at the patient level and involved only each patient’s first admission.
Our primary analysis involved comparison of delirium, as measured using the CAM-ICU, across the preintervention and intervention periods. Our analysis required consideration of death, discharge, and comatose status, factors that could preclude delirium evaluation. For example, the presence of death or coma could suggest lower delirium prevalence, whereas a patient with a shorter LOS could experience a high daily delirium rate. To address these issues, we considered a statistical method involving joint modeling of daily delirium status and death or discharge35; however, existing software packages misclassified death and discharge as identical events, and therefore, we used a simpler model involving the percentage of days that noncomatose ICU patients experienced delirium, with ≥ 1 positive CAM-ICU assessment constituting a delirious day. Compared with using a daily binary variable or days of delirium, the proportion of ICU days with delirium weighs each admission equally because delirium potentially was associated with a patient’s LOS. Although this approach loses power to detect a pre-post intervention effect on delirium, it avoids misclassification of death and discharge as identical events.
The effect of our intervention was modeled using multivariate linear regression for the outcome of the average proportion of ICU days a patient experienced delirium comparing the postintervention and preintervention periods. To account for possible nonconstant variance, we used a bootstrap procedure resampling patients 2,000 times and computing 95% bias-corrected and accelerated CIs.36 Further methodologic details are reported in e-Appendix 1.
The main secondary outcome included patient-reported sleep quality and noise ratings, as measured using the RCSQ. The effect of our intervention on RCSQ outcomes was analyzed using an interrupted time-series framework using a generalized estimating equation linear regression model with project time, a main effect of postintervention vs preintervention, and time after the intervention. Under this approach, both the immediate intervention effect and a change in RCSQ slope across the preintervention and intervention periods was estimated.37 CIs were estimated using percentile method bootstrapping, and P values came from a generalized estimating equation model with robust SEs. To account for possible interrater differences in RCSQ scores, our regression model also included a covariate for rater (nurse vs patient).21
Covariates for the primary analysis were selected a priori based on variables previously shown to be associated with delirium and included type of ICU (SICU or CVICU),8,11,38 age,38 sex,8 race, admission category8,11,38 (ie, cardiothoracic surgery, trauma or general surgery, cardiology), sleep medication use at home,21,38 CCI,38,39 and coma on first ICU assessment.11 Covariates for the secondary analysis were selected a priori based on variables previously associated with sleep quality in critically ill patients and included age,40, 41, 42, 43 sex,41,42 CCI,44 history of sleep problems,41,44 respondent (nurse vs patient),21 using television to sleep at home,41 admission category,41 coma at first ICU assessment,45 and mechanical ventilation.41,46,47 During the project period, nearly all patients received continuous nasojejunal tube feedings; hence, nutrition status was not included as a covariate.
To test the durability of our findings, we performed several sensitivity analyses. First, we examined the efficacy of our intervention on delirium status using a joint modeling approach (e-Appendix 1).35 We also conducted sensitivity analyses for our main model using different ICU exposure times (7 days, 21 days, and all ICU days), combining coma and delirium as a composite outcome, analyzing postoperative patients only (excluding nonoperative cardiac patients), and restricting the analyses to patients with coma on first ICU assessment who subsequently became more alert (e-Appendix 1). All statistical analyses were conducted in R version 3.4 software (R Foundation for Statistical Computing). Statistical significance was defined as a two-sided P < .05.
Results
Baseline and ICU Variables
During the project, 646 surgical ICU patients (332 preintervention, 314 intervention) underwent at least one CAM-ICU assessment during their first admission and were included in our analysis. These 646 patients had a median age of 61 years (interquartile range [IQR], 49-70 years), 35% were women, and 83% were White (Table 1). Patients present during the preintervention vs intervention period demonstrated fewer pre-existing comorbidities (median CCI, 2 [IQR, 1-5] vs 3 [IQR, 1-5]; P < .04) and were less likely to report a history of sleep problems (28% vs 37%; P = .01), to report bad home sleep quality (15% vs 23%; P < .001), and to report use of a television to sleep at home (16% vs 24%; P < .001). No significant difference in patient distribution between ICUs was noted (P = .06 for group). Similarly, the preintervention vs intervention periods included more general or trauma surgery patients (45% vs 36%) and fewer cardiovascular patients (47% vs 58%; P = .03 for group). No significant preintervention vs intervention differences were observed in ICU or hospital LOS or in the proportion of days patients received mechanical ventilation, mechanical circulatory support, or continuous renal replacement therapy (Table 1).
Table 1.
Patient Characteristics
| Variable | All Patients (N = 646) | Preintervention (n = 332) | Intervention (n = 314) | P Valuea |
|---|---|---|---|---|
| Baseline variables | ||||
| Age, y | 58 ± 17 | 58 ± 16 | 58 ± 17 | .94 |
| Female sex | 226 (35) | 118 (36) | 108 (34) | .76 |
| White race | 535 (83) | 274 (83) | 261 (83) | .84 |
| Hispanic/Latino ethnicityb | 49 (8) | 24 (7) | 25 (8) | .47 |
| Charlson Comorbidity Indexc | 2 (1-5) | 2 (1-5) | 3 (1-5) | .04 |
| History of sleep problems | 208 (32) | 92 (28) | 116 (37) | .01 |
| Home sleep quality | ||||
| Very good | 113 (18) | 50 (15) | 63 (20) | |
| Somewhat good | 161 (25) | 82 (25) | 79 (25) | .001 |
| Bad | 123 (19) | 50 (15) | 73 (23) | |
| Unknown | 249 (39) | 150 (45) | 99 (32) | |
| Use television to sleep at home | 130 (20) | 54 (16) | 76 (24) | < .001 |
| Home sleep medication use | 152 (24) | 73 (22) | 79 (25) | < .001 |
| ICU variables | ||||
| Admitting ICUd | ||||
| Surgical | 295 (46) | 165 (50) | 130 (41) | .06 |
| Cardiovascular | 340 (53) | 160 (48) | 180 (57) | |
| Admission categorye | ||||
| General or trauma surgery | 256 (40) | 146 (45) | 110 (36) | |
| Cardiothoracic surgery | 207 (32) | 100 (31) | 107 (35) | .03 |
| Cardiovascular | 127 (20) | 54 (17) | 73 (24) | |
| MS-DRG weight | 5 (2-7) | 4 (2-7) | 5 (2-7) | .89 |
| Days with mechanical ventilation, %f | 31 ± 40 | 33 ± 41 | 29 ± 39 | .33 |
| Days with mechanical circulatory support, %f | 3 ± 13 | 2 ± 13 | 3 ± 14 | .50 |
| Days with continuous renal replacement therapy, %f | 4 ± 17 | 4 ± 18 | 4 ± 16 | .75 |
| ICU LOS, d | 3 (2-6) | 3 (2-6) | 4 (2-7) | .28 |
| Hospital LOS, d | 9 (5-14) | 9 (5-14) | 8 (5-15) | .76 |
| Died in ICU | 40 (6) | 20 (6) | 20 (6) | .86 |
| Delirium outcomes | ||||
| Absolute days with a recorded CAM-ICU assessment | 3 (2-6) | 3 (2-5) | 3 (2-6) | .22 |
| Absolute CAM-ICU assessments/d | 1.7 (1-2) | 1.7 (1-2) | 1.7 (1-2) | .29 |
| Days with ≥ 1 CAM-ICU assessment with positive results, %g | 15 ± 27 | 17 ± 28 | 13 ± 25 | .02 |
| Days with CAM-ICU assessment with positive results, %g | 18 ± 30 | 20 ± 31 | 15 ± 28 | .02 |
Data are presented as No. (%), mean ± SD, or median (interquartile range), unless otherwise indicated. CAM-ICU = Confusion Assessment Method for the Intensive Care Unit; CCI = Charlson Comorbidity Index; LOS = length of stay; MS-DRG = Medicare Severity-Diagnostic Related Group.
Calculated using the Student t test or Wilcoxon rank-sum test for continuous variables and the χ 2 test or Fisher exact test for categorical variables.
Unknown/no response: all, n = 22 (3%); preintervention, n = 8 (2%); and intervention, n = 14 (5%).
Data missing for 2 (1%) preintervention and 1 (0%) intervention patient.
Both ICUs response: all, n = 11 (2%); preintervention, n = 7 (2%); and intervention, n = 4 (1%).
Other response: all, n = 48 (8%); preintervention, n = 28 (9%); and intervention, n = 20 (7%). Data missing for 4 (1%) preintervention and 4 (1%) intervention patients.
Expressed as a proportion of hospital days; data missing for 19 (6%) preintervention and 52 (17%) intervention patients.
Calculated over the first 14 days.
Sleep-Promoting Interventions
The environmental intervention completion rate during the intervention stage is reported in e-Table 1. Briefly, some sleep-wake-promoting interventions were highly completed (eg, day: blinds raised, 93% of patient days; mobility or chair position daily, 58%; night: lights dimmed, 93% of patient nights; stop sign on door, 93%; minimizing nurse interruptions after 10 pm, 86%), whereas some were carried out infrequently (use of eye masks, 2%).
Delirium Outcome
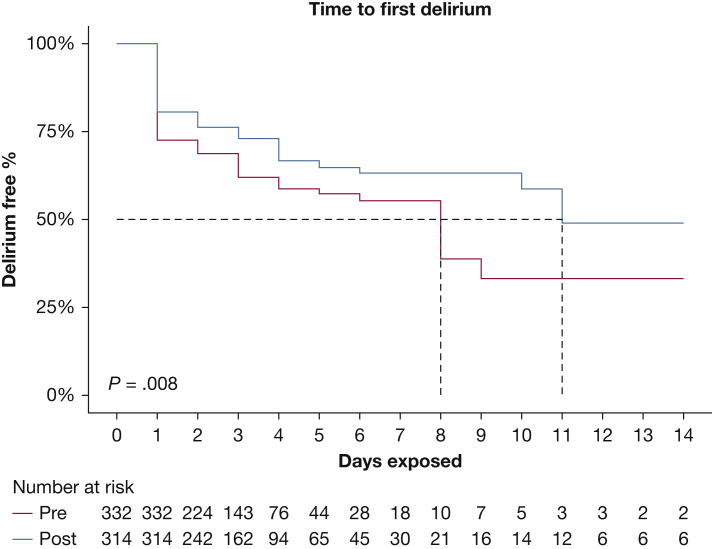
Comparing the preintervention with the intervention period, SICU patients saw a similar number of days with a recorded CAM-ICU (median, 3 days [IQR, 2-5 days] before intervention vs 3 days [IQR, 2-6 days] during intervention; P = .22) and number of daily CAM-ICU assessments (median, 1.7 days [IQR, 1-2 days] vs 1.7 days [IQR, 1-2 days]; P = .29) (Table 1). Across the first 14 ICU days, a significant reduction was found in proportion of days with ≥ 1 CAM-ICU assessments with positive results per day per patient (mean ± SD, 17 ± 28% before intervention vs 13 ± 25% during intervention; P = .02 [Table 1]; unadjusted difference, –5.3% [95% CI, –9.8% to –0.7%]; P = .02 [Table 2]) and proportion of positive CAM-ICU assessments (mean ± SD, 20 ± 31% vs 15 ± 28%; P = .02 [Table 1]; adjusted difference, –4.9% [95% CI, –9.2% to –0.5%]; P = .03 [Table 2]). Unadjusted time to first delirium is shown in Figure 1.
Table 2.
Factors Associated With Proportion of Days That Patients Experienced Deliriuma
| Variable | Unadjusted |
Adjusted |
||||
|---|---|---|---|---|---|---|
| Difference | 95% CI | P Value | Difference | 95% CI | P Value | |
| Intervention vs preintervention | –5.3% | –9.8 to –0.7 | .02 | –4.9% | –9.2% to –0.5% | .03 |
| Age per y | 0.1% | 0.0% to 0.3% | .06 | 0.1% | 0.0% to 0.3% | .07 |
| Female vs male sex | 3.1% | –1.6% to 8.2% | .21 | 3.5% | –1.1% to 8.4% | .16 |
| Other vs White race | 7.6% | 0.7% to 14.5% | .03 | 8.4% | 2.2% to 15.7% | .01 |
| Charlson Comorbidity Index, per point | 0.4% | –0.4% to 1.3% | .40 | 0.6% | –0.2% to 1.6% | .16 |
| Home sleep medication, yes vs no | –1.1% | –6.2% to 5.0% | .71 | –1.5% | –6.7% to 3.9% | .58 |
| SICU vs CVICU | 5.6% | 0.9% to 10.5% | .02 | 5.8% | –7.9% to 16.2% | .30 |
| Admission category, vs general or trauma surgery | ||||||
| Cardiology | –9.7% | –14.9% to –3.5% | < .001 | –3.6% | –16.6% to 4.2% | .24 |
| Cardiothoracic surgery | –1.7% | –7.0% to 3.6% | .52 | 0.2% | –13.4% to 10.6% | .97 |
| Coma at first ICU assessment vs none | 21.1% | 14.3% to 28.6% | < .001 | 21.4% | 14.4% to 29.3% | < .001 |
CCI = Charlson Comorbidity Index; CVICU = cardiovascular ICU; SICU = surgical ICU.
Values represent proportion of days with a positive delirium assessment during an admission among the first 14 d of the ICU stay that delirium was assessed. P values calculated using linear regression with generalized estimating equations under an independence correlation structure and with robust Huber-White sandwich SEs to account for variance across patients (heteroscedasticity). 95% CIs estimated using the bias-corrected and accelerated bootstrap method with 2,000 samples. Delirium proportion data missing for 7 (2%) and 8 (2%) preintervention and intervention patients, respectively.
Figure 1.
Line graph showing time to first delirium during the intervention phase compared with the preintervention phase.
RCSQ Ratings
Overall, 1,821 RCSQs were collected, of which 564 (31%) were completed by patients. Across the preintervention and intervention periods, no significant difference was found in RCSQ overall sleep quality rating (median, 52 [IQR, 37-69] vs 55 [IQR, 41-69]; adjusted difference, 3.4 [95% CI, –5.2 to 11.5]; P = .43) (Table 3). In the intervention period, patients reported higher perceived noise scores in the unadjusted (median, 65 [IQR, 50-79] vs 71 [IQR, 59-85]) and adjusted (adjusted difference, 9.5 [95% CI, 1.1-17.3]; P = .02) analyses (Table 3).
Table 3.
Adjusted Pre-Post Association of Intervention on RCSQ Overall and Noise Ratings
| Variable | Overall Sleep Qualitya | P Value | Noiseb | P Value |
|---|---|---|---|---|
| Intervention vs preintervention | 3.4 (–5.2 to 11.5)b | .43 | 9.51 (1.1-17.5) | .02 |
| Project dayc | 0.1 (–0.1 to 0.3) | .39 | 0.02 (–0.2 to 0.2) | .81 |
| Days afterd | –0.2 (–0.4 to 0.1) | .20 | –0.07 (–0.3 to 0.2) | .58 |
| Age, per y | –0.1 (–0.3 to 0.0) | .09 | –0.03 (–0.2 to 0.1) | .72 |
| Female vs male | 0.5 (–4.1 to 5.2) | .82 | 0.6 (–4.0 to 5.3) | .79 |
| CCI, per point | –0.0 (–0.9 to 0.8) | .93 | –0.2 (–1.2 to 0.6) | .60 |
| History of sleep problem, yes vs no | –4.4 (–10.1 to 1.0) | .12 | –1.9 (–7.3 to 3.4) | .49 |
| Use television to sleep at home, vs Yes | ||||
| No | 0.2 (–5.8 to 6.2) | .96 | 1.2 (–4.5 to 7.2) | .70 |
| Unknown | –6.6 (–22.8 to 9.4) | .42 | 10.3 (–5.5 to 29.3) | .22 |
| Admission category | ||||
| Cardiology | 5.5 (–1.4 to 11.8) | .11 | 2.6 (–4.2 to 9.6) | .45 |
| Cardiothoracic surgery | –5.9 (–11.7 to –0.1) | .046 | –3.6 (–9.7 to 2.2) | .22 |
| Mechanical ventilation, yes | 6.8 (1.8-12.1) | .01 | –5.3 (–10.5 to 0.1) | .05 |
| Coma on first ICU assessment | 1.2 (–4.8 to 7.2) | .70 | –1 (–6.9 to 4.6) | .74 |
| Nurse vs patient completing the questionnaire | 1.1 (–8.5 to 10.6) | .82 | –5.9 (–17.0 to 4.6) | .27 |
| Other vs patient completing the questionnaire | 6.2 (–8.3 to 20.9) | .82 | –10 (–27.1 to 5.0) | .17 |
Data are presented as difference (95% CI). CCI = Charlson Comorbidity Index; RCSQ = Richards-Campbell Sleep Questionnaire.
Values represent adjusted difference in RCSQ ratings, on a 0 to 100 visual analog scale, with positive differences representing improved sleep quality ratings across preintervention and intervention periods. The overall sleep quality rating represents the average of five RCSQ items. 95% CIs were estimated using bootstrap percentile method with 2,000 samples.
Unadjusted P > .1 for each of the five RCSQ items: (1) depth, (2) latency (time to fall asleep), (3) number of awakenings, (4) efficiency (percent of time awake), and (5) quality.
A time measure inherent in segmented regression analysis. In this analysis, it represents the number of days from intervention (day 0). It is negative for days before intervention and positive for days after intervention. Its coefficient measures a time trend assuming no intervention at all.
A time measure inherent in segmented regression analysis. In our analyses, it is the number of days after intervention. For days after intervention, the value equals project days. For days before the intervention, it is 0 (so it has no effect). Its coefficient measures additional time trend after the intervention not accounted for by the effect of project days alone.
Sensitivity Analyses
In sensitivity analyses, when delirium was modeled jointly as a recurrent event with death or discharge, the intervention was associated with a reduced hazard of delirium (e-Table 2). Significant reductions in delirium also were observed in sensitivity analyses involving varying lengths of ICU stay (e-Tables 3, 4, and 5), a composite outcome of days with either delirium or coma (e-Tables 6 and 7), excluding nonoperative cardiovascular patients (e-Table 8) and involving only patients who had coma on first ICU assessment, but subsequently were more alert (e-Table 9).
Discussion
We demonstrated that a prospective, sequentially implemented QI intervention to ameliorate sleep-wake disruption was associated with reduced prevalence of delirium among patients in surgical ICU settings. In addition, we observed that the time to first delirium was delayed with the intervention. Our findings were robust to different modeling approaches, including exclusion of nonoperative patients, and with a composite outcome of delirium or coma. Notably, we found that coma on first ICU assessment was associated strongly with development of ICU delirium, suggesting that residual anesthetic or oversedation may be a risk factor for ICU delirium in postoperative and surgical patients. Finally, across the preintervention and intervention periods, we did not observe improvements in sleep quality ratings.
Our findings are consistent with two prior efforts in medical or mixed medical and surgical patients that demonstrated an association between multicomponent staff-based interventions to improve sleep-wake disruption and reductions in ICU delirium.21,22 Building on these efforts, our intervention involved a larger postoperative and surgical population comprising general, trauma, and subspecialty surgical, cardiothoracic, and periprocedural cardiovascular patients. Surgical patients are at risk of delirium, having been exposed to operative time, anesthetic medications, and painful procedures.3,12, 13, 14, 15,48, 49, 50 Additionally, surgical patients may receive nerve blocks or have open wounds, which may limit mobility, and may need to return to the operating room. Conversely, these patients often have shorter stays, thus lowering their exposure to common delirium risk factors and opportunities to lose sleep. For this reason, surgical patients may not have been the focus of ICU-based interventions to improve sleep. Regarding a potential mechanism, it is widely believed that sleep-wake disruption, which is common in critically ill patients, increases delirium risk.51 Notably, we were not able to corroborate our delirium finding with an improvement in subjective sleep quality ratings, perhaps speaking to the lack of sensitivity of the measurement tool used. Future studies can evaluate the influence of sleep-wake disruption in the surgical ICU setting while considering other sleep evaluation approaches.
Because delirium is a multifactorial, expensive, and often hospital-acquired syndrome,10 major cost reduction could be achieved through targeted, efficacious interventions.4 Given inconsistent evidence regarding the effect of medications for delirium reduction in postoperative patients,12,52, 53, 54, 55 we chose to focus on environmental improvements, despite the inherent challenges of environmental vs pharmacologic intervention implementation (ie, inconsistent compliance).56 A trial of 160 carefully selected elderly patients with oral cancer demonstrated reduced secondary outcomes of postoperative delirium from a nonpharmacologic behavioral intervention.23 To our knowledge, our intervention is the first to include an unrestricted surgical ICU population and the largest to demonstrate a significant relationship between an environmental sleep-wake improvements and delirium reduction. Moreover, our findings were robust in sensitivity analyses examining alternative modeling approaches, durations of measurement, and focusing only on postoperative patients.
Despite our project’s strengths, we acknowledge several limitations. First, given the observational pre-post design, potential confounding variables and temporal factors may explain some of our findings. To minimize these effects, we implemented our intervention in a staggered fashion across two ICUs. Second, because this was a QI effort, our team regularly educated staff and solicited feedback on intervention implementation, so the observed effect on delirium could not be traced to a single event or action. Third, we did not use polysomnography, the gold standard to assess sleep, and instead relied on the RCSQ based on its use in prior intervention studies.21,27 Polysomnography is complicated in the ICU because it is physically cumbersome and the data are not scorable using established criteria.18,57 No perfect metric currently exists to measure sleep in the ICU; however, future studies can consider more objective tools like actigraphy, an accelerometry-based technology gaining attention for its ability to measure rest-activity cycles on a large scale.58, 59, 60, 61 Fourth, because our results consisted of predominantly surgical ICU patients from a single center, one could question the generalizability of our findings. We believe our findings are broadly applicable; however, further studies are required. Fifth, because no other delirium efforts were ongoing in the involved ICUs, we believed our sleep-based intervention was responsible for the observed improvements, but also acknowledge that the intervention itself may have motivated changes in delirium assessment. We would have expected the intervention to increase delirium recognition and diagnosis, but instead observed decreased delirium prevalence, suggesting that our intervention was beneficial while also highlighting the notion that negative CAM-ICU scores may have been confounded by unblinded administration. Sixth, factors not included in our analysis, such as seasonal light changes, may have influenced the outcome; however, we believe this influence was negligible given evidence that natural light tends to degrade substantially from the window to the ICU bed.62 Finally, we were not able to adjust for the administration of medications on the delirium outcome or the RCSQ, because this variable from the sleep checklist was not collected before the intervention phase. Although medications for sleep were administered on < 5% of the nights, melatonin was administered 16% of nights and may have influenced the delirium or RCSQ outcome. Despite these limitations, we view our findings as robust and encourage further research to help guide clinical practice.
Interpretation
In critically ill surgical ICU patients, we demonstrated that a nonpharmacologic environmental intervention to improve sleep-wake disruption was associated with a significant reduction in delirium. Acknowledging intense interest in this topic, we believe our data provide an important foundation for future delirium efforts in surgical ICU populations.24,26 Given the adverse outcomes and major health care costs associated with delirium, our findings suggest that simple environmental and staff behavioral interventions are feasible and should be a target of future multicenter trials.
Take-home Points.
Study Question: In two academic surgical ICUs, what is the effect of a multicomponent nonpharmacologic intervention aimed at improving sleep-wake disruption on delirium and sleep quality ratings?
Results: Compared with an 8- to 12-week preintervention baseline period, the 8- to 12-week intervention led to significant reductions in the proportion of days patients in the surgical ICU experienced delirium (15% vs 20%) with no significant changes in overall sleep quality ratings.
Interpretation: Implementation of a multicomponent nonpharmacologic sleep-wake intervention in surgical ICU settings is feasible and is associated with a reduced prevalence of delirium.
Acknowledgments
Author contributions: J. E. T. is the guarantor of the content of the manuscript, having had full access to the project data, and takes responsibility for the data integrity and accuracy and the integrity of the submission as a whole. J. E. T., A. D., K. L., S. H., J. B., A. P. P., E. C., C. Z., and B. B. K. conceived the work. J. E. T., A. D., K. L., and S. H. conducted the project. J. E. T., A. D., K. L., S. H., E. C., A. P. P., C. Z., and B. B. K. conducted the data acquisition and analysis. J. E. T., C. Z., E. C., A. P. P., and B. B. K. drafted the manuscript. All authors revised the article for important intellectual content and approved the final manuscript for publication.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: J. E. T. received speaker fees and travel compensation from LivaNova and Philips Healthcare unrelated to this work. None declared (A. D., A. P. P., C. Z., E. C., K. L., S. H., J. B., B. B. K.)
Role of sponsors: None of the funding sources were involved in the design or conduct of the project; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript.
Other contributions: The authors thank the nurses and staff of the SICU and CVICU of University of Utah Health, without whom this intervention would not have been possible, and Mary Mone, RN, who assisted in data analysis.
Additional information: The e-Appendixes and e-Tables can be found in the Supplemental Materials section of the online article.
Footnotes
Drs Tonna and Dalton contributed equally to this manuscript.
FUNDING/SUPPORT: J. E. T. is supported by a Career Development Award from the National Heart, Lung, and Blood Institute, National Institutes of Health [Grant K23 HL141596]. B. B. K. is supported by a Paul B. Beeson Career Development Award through the National Institute on Aging, National Institutes of Health [Grant K76 AG059936]. This project also was supported by the National Center for Advancing Translational Sciences, National Institutes of Health [Grant UL1TR002538; formerly Grants 5UL1TR001067-05, 8UL1TR000105, and UL1RR025764].
Supplementary Data
References
- 1.Francis J., Martin D., Kapoor W.N. A prospective study of delirium in hospitalized elderly. JAMA. 1990;263(8):1097–1101. [PubMed] [Google Scholar]
- 2.Ely E., Gautam S., Margolin R. The impact of delirium in the intensive care unit on hospital length of stay. Intensive Care Med. 2001;27(12):1892–1900. doi: 10.1007/s00134-001-1132-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pandharipande P., Shintani A., Peterson J. Lorazepam is an independent risk factor for transitioning to delirium in intensive care unit patients. Anesthesiology. 2006;104(1):21–26. doi: 10.1097/00000542-200601000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Inouye S.K., Bogardus S.T., Jr., Charpentier P.A. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340(9):669–676. doi: 10.1056/NEJM199903043400901. [DOI] [PubMed] [Google Scholar]
- 5.Pandharipande P.P., Girard T.D., Jackson J.C. Long-term cognitive impairment after critical illness. N Engl J Med. 2013;369(14):1306–1316. doi: 10.1056/NEJMoa1301372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Austin C.A., O’Gorman T., Stern E. Association between postoperative delirium and long-term cognitive function after major nonemergent surgery. JAMA Surg. 2019;154(4):328–334. doi: 10.1001/jamasurg.2018.5093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leslie D.L., Inouye S.K. The importance of delirium: economic and societal costs. J Am Geriatr Soc. 2011;59:S241–S243. doi: 10.1111/j.1532-5415.2011.03671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCusker J., Cole M.G., Dendukuri N., Belzile E. Does delirium increase hospital stay? J Am Geriatr Soc. 2003;51(11):1539–1546. doi: 10.1046/j.1532-5415.2003.51509.x. [DOI] [PubMed] [Google Scholar]
- 9.Lat I., McMillian W., Taylor S. The impact of delirium on clinical outcomes in mechanically ventilated surgical and trauma patients. Crit Care Med. 2009;37(6):1898–1905. doi: 10.1097/CCM.0b013e31819ffe38. [DOI] [PubMed] [Google Scholar]
- 10.Patel S.B., Poston J.T., Pohlman A. Rapidly reversible, sedation-related delirium versus persistent delirium in the intensive care unit. Am J Respir Crit Care Med. 2014;189(6):658–665. doi: 10.1164/rccm.201310-1815OC. [DOI] [PubMed] [Google Scholar]
- 11.Van Rompaey B., Elseviers M.M., Van Drom W., Fromont V., Jorens P.G. The effect of earplugs during the night on the onset of delirium and sleep perception: a randomized controlled trial in intensive care patients. Crit Care. 2012;16(3):R73. doi: 10.1186/cc11330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pandharipande P.P., Pun B.T., Herr D.L. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA. 2007;298(22):2644–2653. doi: 10.1001/jama.298.22.2644. [DOI] [PubMed] [Google Scholar]
- 13.Hollinger A., Siegemund M., Goettel N., Steiner L.A. Postoperative delirium in cardiac surgery: an unavoidable menace? J Cardiothorac Vasc Anesth. 2015;29(6):1677–1687. doi: 10.1053/j.jvca.2014.08.021. [DOI] [PubMed] [Google Scholar]
- 14.Kang T., Park S.Y., Lee J.H. Incidence & risk factors of postoperative delirium after spinal surgery in older patients. Sci Rep. 2020;10(1):9232. doi: 10.1038/s41598-020-66276-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yildizeli B., Ozyurtkan M.O., Batirel H.F., Kuscu K., Bekiroglu N., Yuksel M. Factors associated with postoperative delirium after thoracic surgery. Ann Thorac Surg. 2005;79(3):1004. doi: 10.1016/j.athoracsur.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 16.Elliott R., McKinley S., Cistulli P., Fien M. Characterisation of sleep in intensive care using 24-hour polysomnography: an observational study. Crit Care. 2013;17(2):R46. doi: 10.1186/cc12565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friese R.S., Diaz-Arrastia R., McBride D., Frankel H., Gentilello L.M. Quantity and quality of sleep in the surgical intensive care unit: are our patients sleeping? J Trauma. 2007;63(6):1210–1214. doi: 10.1097/TA.0b013e31815b83d7. [DOI] [PubMed] [Google Scholar]
- 18.Watson P.L., Pandharipande P., Gehlbach B.K. Atypical sleep in ventilated patients: empirical electroencephalography findings and the path toward revised ICU sleep scoring criteria. Crit Care Med. 2013;41(8):1958–1967. doi: 10.1097/CCM.0b013e31828a3f75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dorsch J.J., Martin J.L., Malhotra A., Owens R.L., Kamdar B.B. Sleep in the intensive care unit: strategies for improvement. Semin Respir Crit Care Med. 2019;40(5):614–628. doi: 10.1055/s-0039-1698378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Honarmand K., Rafay H., Le J. A Systematic review of risk factors for sleep disruption in critically ill adults. Crit.Care Med. 2020;48(7):1066–1074. doi: 10.1097/CCM.0000000000004405. [DOI] [PubMed] [Google Scholar]
- 21.Kamdar B.B., King L.M., Collop N.A. The effect of a quality improvement intervention on perceived sleep quality and cognition in a medical ICU∗. Critical Care Med. 2013;41(3):800–809. doi: 10.1097/CCM.0b013e3182746442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patel J., Baldwin J., Bunting P., Laha S. The effect of a multicomponent multidisciplinary bundle of interventions on sleep and delirium in medical and surgical intensive care patients. Anaesthesia. 2014;69(6):540–549. doi: 10.1111/anae.12638. [DOI] [PubMed] [Google Scholar]
- 23.Flannery A.H., Oyler D.R., Weinhouse G.L. The impact of interventions to improve sleep on delirium in the ICU: a systematic review and research framework. Crit Care Med. 2016;44(12):2231–2240. doi: 10.1097/CCM.0000000000001952. [DOI] [PubMed] [Google Scholar]
- 24.Pandharipande P.P., Ely E.W., Arora R.C. The intensive care delirium research agenda: a multinational, interprofessional perspective. Intensive Care Med. 2017;43(9):1329–1339. doi: 10.1007/s00134-017-4860-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pronovost P.J., Berenholtz S.M., Needham D.M. Translating evidence into practice: a model for large scale knowledge translation. BMJ. 2008;337:a1714. doi: 10.1136/bmj.a1714. [DOI] [PubMed] [Google Scholar]
- 26.Devlin J.W., Skrobik Y., Gelinas C. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46(9):e825–e873. doi: 10.1097/CCM.0000000000003299. [DOI] [PubMed] [Google Scholar]
- 27.Kamdar B.B., Yang J., King L.M. Developing, implementing, and evaluating a multifaceted quality improvement intervention to promote sleep in an ICU. Am J Med Qual. 2013;29(6):546–554. doi: 10.1177/1062860613509684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ogrinc G., Davies L., Goodman D., Batalden P., Davidoff F., Stevens D. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf. 2016;25(12):986–992. doi: 10.1136/bmjqs-2015-004411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ely E.W., Margolin R., Francis J. Evaluation of delirium in critically ill patients: validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) Crit Care Med. 2001;29(7):1370–1379. doi: 10.1097/00003246-200107000-00012. [DOI] [PubMed] [Google Scholar]
- 30.Tullmann D.F. Assessment of delirium: another step forward. Crit Care Med. 2001;29(7):1481–1482. doi: 10.1097/00003246-200107000-00034. [DOI] [PubMed] [Google Scholar]
- 31.Riker R.R., Picard J.T., Fraser G.L. Prospective evaluation of the sedation-agitation scale for adult critically ill patients. Crit Care Med. 1999;27(7):1325–1329. doi: 10.1097/00003246-199907000-00022. [DOI] [PubMed] [Google Scholar]
- 32.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Charlson M.E., Pompei P., Ales K.L., MacKenzie C.R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 34.Buysse D.J., Reynolds C.F., III, Monk T.H., Berman S.R., Kupfer D.J. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 35.Colantuoni E., Dinglas V.D., Ely E.W., Hopkins R.O., Needham D.M. Statistical methods for evaluating delirium in the ICU. Lancet Respir Med. 2016;4(7):534–536. doi: 10.1016/S2213-2600(16)30138-2. [DOI] [PubMed] [Google Scholar]
- 36.Efron B. Better bootstrap confidence intervals. J Am Stat Assoc. 1987;82(397):171–185. [Google Scholar]
- 37.Wagner A.K., Soumerai S.B., Zhang F., Ross-Degnan D. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther. 2002;27(4):299–309. doi: 10.1046/j.1365-2710.2002.00430.x. [DOI] [PubMed] [Google Scholar]
- 38.Chaiwat O., Chanidnuan M., Pancharoen W. Postoperative delirium in critically ill surgical patients: incidence, risk factors, and predictive scores. BMC Anesthesiol. 2019;19(1):39. doi: 10.1186/s12871-019-0694-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McCusker J., Cole M., Abrahamowicz M., Primeau F., Belzile E. Delirium predicts 12-month mortality. Arch Intern Med. 2002;162(4):457–463. doi: 10.1001/archinte.162.4.457. [DOI] [PubMed] [Google Scholar]
- 40.Blackman M.R. Age-related alterations in sleep quality and neuroendocrine function: interrelationships and implications. JAMA. 2000;284(7):879–881. doi: 10.1001/jama.284.7.879. [DOI] [PubMed] [Google Scholar]
- 41.Kamdar B.B., Niessen T., Colantuoni E. Delirium transitions in the medical ICU: exploring the role of sleep quality and other factors. Crit Care Med. 2015;43(1):135–141. doi: 10.1097/CCM.0000000000000610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Young T., Palta M., Dempsey J., Skatrud J., Weber S., Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328(17):1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 43.Van Cauter E., Leproult R., Plat L. Age-related changes in slow wave sleep and REM sleep and relationship with growth hormone and cortisol levels in healthy men. JAMA. 2000;284(7):861–868. doi: 10.1001/jama.284.7.861. [DOI] [PubMed] [Google Scholar]
- 44.Altman M.T., Knauert M.P., Pisani M.A. Sleep disturbance after hospitalization and critical illness: a systematic review. Ann Am Thorac Soc. 2017;14(9):1457–1468. doi: 10.1513/AnnalsATS.201702-148SR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jean R., Shah P., Yudelevich E. Effects of deep sedation on sleep in critically ill medical patients on mechanical ventilation. J Sleep Res. 2019 doi: 10.1111/jsr.12894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Freedman N.S., Kotzer N., Schwab R.J. Patient perception of sleep quality and etiology of sleep disruption in the intensive care unit. Am J Respir Crit Care Med. 1999;159(4 Pt 1):1155–1162. doi: 10.1164/ajrccm.159.4.9806141. [DOI] [PubMed] [Google Scholar]
- 47.Parthasarathy S., Tobin M.J. Effect of ventilator mode on sleep quality in critically ill patients. Am J Respir Crit Care Med. 2002;166(11):1423–1429. doi: 10.1164/rccm.200209-999OC. [DOI] [PubMed] [Google Scholar]
- 48.Ravi B., Pincus D., Choi S., Jenkinson R., Wasserstein D.N., Redelmeier D.A. Association of duration of surgery with postoperative delirium among patients receiving hip fracture repair. JAMA Netw Open. 2019;2(2) doi: 10.1001/jamanetworkopen.2019.0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sampson E.L., West E., Fischer T. Pain and delirium: mechanisms, assessment, and management. Eur Geriatr Med. 2020;11(1):45–52. doi: 10.1007/s41999-019-00281-2. [DOI] [PubMed] [Google Scholar]
- 50.Meier D.E. Pain as a cause of agitated delirium. Arch Intern Med. 2012;172(15) doi: 10.1001/archinternmed.2012.2088. 1130-1130. [DOI] [PubMed] [Google Scholar]
- 51.Kamdar B.B., Martin J.L., Needham D.M., Ong M.K. Promoting sleep to improve delirium in the ICU. Crit Care Med. 2016;44(12):2290–2291. doi: 10.1097/CCM.0000000000001982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Su X., Meng Z.T., Wu X.H. Dexmedetomidine for prevention of delirium in elderly patients after non-cardiac surgery: a randomised, double-blind, placebo-controlled trial. Lancet. 2016;388(10054):1893–1902. doi: 10.1016/S0140-6736(16)30580-3. [DOI] [PubMed] [Google Scholar]
- 53.Djaiani G., Silverton N., Fedorko L. Dexmedetomidine versus propofol sedation reduces delirium after cardiac surgery: a randomized controlled trial. Anesthesiology. 2016;124(2):362–368. doi: 10.1097/ALN.0000000000000951. [DOI] [PubMed] [Google Scholar]
- 54.Deiner S., Luo X., Lin H.M. Intraoperative infusion of dexmedetomidine for prevention of postoperative delirium and cognitive dysfunction in elderly patients undergoing major elective noncardiac surgery: a randomized clinical trial. JAMA Surg. 2017;152(8) doi: 10.1001/jamasurg.2017.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li X., Yang J., Nie X.L. Impact of dexmedetomidine on the incidence of delirium in elderly patients after cardiac surgery: a randomized controlled trial. PLoS One. 2017;12(2) doi: 10.1371/journal.pone.0170757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Meagher D.J., O’Hanlon D., O’Mahony E., Casey P.R. The use of environmental strategies and psychotropic medication in the management of delirium. Br J Psychiatry. 1996;168(4):512–515. doi: 10.1192/bjp.168.4.512. [DOI] [PubMed] [Google Scholar]
- 57.Knauert M.P., Yaggi H.K., Redeker N.S., Murphy T.E., Araujo K.L., Pisani M.A. Feasibility study of unattended polysomnography in medical intensive care unit patients. Heart Lung. 2014;43(5):445–452. doi: 10.1016/j.hrtlng.2014.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gupta P., Martin J.L., Needham D.M., Vangala S., Colantuoni E., Kamdar B.B. Use of actigraphy to characterize inactivity and activity in patients in a medical ICU. Heart Lung. 2020;49(4):398–406. doi: 10.1016/j.hrtlng.2020.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schwab K.E., Ronish B., Needham D.M., To A.Q., Martin J.L., Kamdar B.B. Actigraphy to evaluate sleep in the intensive care unit. A systematic review. Ann Am Thorac Soc. 2018;15(9):1075–1082. doi: 10.1513/AnnalsATS.201801-004OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kamdar B.B., Kadden D.J., Vangala S. Feasibility of continuous actigraphy in patients in a medical intensive care unit. Am J Crit Care. 2017;26(4):329–335. doi: 10.4037/ajcc2017660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schwab K.E., To A.Q., Chang J. Actigraphy to measure physical activity in the intensive care unit: a systematic review. J Intensive Care Med. 2020;35(11):1323–1331. doi: 10.1177/0885066619863654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Castro R.A., Angus D.C., Hong S.Y. Light and the outcome of the critically ill: an observational cohort study. Crit Care. 2012;16(4):R132. doi: 10.1186/cc11437. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.