Abstract
A Giardia lamblia antigen detected by the TechLab Giardia Test (TechLab, Inc., Blacksburg, Va.) and the Alexon ProSpecT Giardia microplate assay (Alexon, Inc., Sunnyvale, Calif.) was purified by immunoaffinity chromatography from supernatant fluids of encystment cultures. Two major proteins (Mr 22,000 and 26,000) were observed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Coomassie staining that did not resemble the GSA65 antigen reportedly detected by the Alexon test. These proteins reacted intensely with the monoclonal antibodies used in both commercial enzyme-linked immunosorbent assays (ELISAs). Both proteins had identical N-terminal amino acid sequences and were identified as cyst wall protein 1 (CWP1). The 26-kDa form appeared early during encystment followed by the appearance of the 22-kDa form. Recombinant CWP1 (Mr 26,000) was strongly positive in both commercial tests. CWP1 was stable in human stool specimens, resistant to degradation by proteases and N- and O-glycanases, and unaffected by oxidation with sodium periodate. Two minor proteins with Mrs of 32,000 and 39,000 were detected in CWP1 preparations by using a sensitive fluorescent protein stain. Both were identified as CWP2, and neither reacted with the monoclonal antibodies from the commercial tests. We analyzed 535 stool specimens for CWP1 by using both commercial ELISAs and resolved discrepant results by using routine ova and parasite examination (O&P) and on immunofluorescence antibody assay. The presence of CWP1 correlated well between both ELISAs (98.7% correlation). Our results demonstrate that both commercial ELISAs detect CWP1, which is a useful diagnostic marker because it is highly stable, is secreted in large amounts by encysting trophozoites, and correlates well with O&P.
Giardiasis, caused by the protozoan parasite Giardia lamblia, is the most commonly diagnosed parasitic infection in the United States (5). This parasite exists in two main life forms: trophozoites and cysts. Infection typically occurs following the ingestion of water or foods contaminated with fecal material containing cysts, and the infective dose may be as low as 10 cysts (15). The most common symptoms are diarrhea, abdominal cramps, bloating, flatulence, fatigue, and weight loss resulting from malabsorption.
Several Giardia antigens have been identified and partially characterized (2, 11, 14, 17, 19). GSA65 is a glycoprotein of Mr 65,000 that reportedly is present in trophozoites and cysts (16). It is resistant to proteolysis and heat but is sensitive to sodium periodate oxidation. According to the manufacturer, this is the antigen detected by the Alexon ProSpecT Giardia microplate assay (Alexon, Inc., Sunnyvale, Calif.). Giardia cyst wall proteins 1 and 2 (CWP1 and CWP2, respectively) which have Mrs of 26,000 and 39,000, respectively, are other antigens that have been studied (9, 12). The genes for these proteins have been sequenced, and both proteins contain stretches of leucine-rich repeats. These proteins can form a stable CWP1-CWP2 65-kDa heterodimer (9).
The diagnosis of giardiasis is based on clinical history, symptoms, and the presence of the organism or its antigens in stool specimens from suspected individuals. Several types of tests, including microscopic examination, immunofluorescence, and enzyme-linked immunosorbent assay (ELISA), are utilized as diagnostic aids for testing stool specimens. In the following study, we identified and characterized the antigen detected by two commercial ELISA kits: the TechLab Giardia Test (TechLab, Inc., Blacksburg, Va.) and the Alexon test. Both of these tests are monoclonal antibody (MAb)-based ELISAs used as in vitro diagnostics for giardiasis.
MATERIALS AND METHODS
Preparation of Giardia cysts.
G. lamblia WB (ATCC 30957) trophozoites were axenically cultured in Keister’s modified TYI-33 medium at pH 7.1 and incubated as previously described (7). Growing trophozoites were synchronized by washing a confluent monolayer five times with phosphate-buffered saline (PBS) at pH 7.4. Trophozoites were chilled on ice for 20 min, pelleted by centrifugation, and suspended in TYI-33 medium containing 10 mg of bovine bile per ml (Sigma Chemical Co., St. Louis, Mo.) at pH 7.8 to trigger encystment without a preencystment incubation (4, 6, 7). Cysts were harvested by centrifugation and suspended in deionized water overnight at room temperature to lyse residual trophozoites (7). Cysts were collected by centrifugation and washed with sterile deionized water, and counts were done by trypan blue exclusion (11). Encystment culture supernatants were passed through a 0.2-μm-pore-size filter (Nalgen Co., Rochester, N.Y.) as instructed by the manufacturer, and the culture filtrates were stored at 4°C.
Immunoassays.
The Giardia Test, the ProSpecT Giardia microplate assay, and the Crypto/Giardia IF (immunofluorescence) test (TechLab, Inc.) were performed as instructed by the manufacturer.
Immunoaffinity chromatography.
Giardia antigen was purified by immunoaffinity chromatography with a MAb immobilized on Affi-Gel 15 (Bio-Rad Laboratories, Melville, N.Y.) as instructed by the manufacturer. The MAb used for purification was the same MAb used in the TechLab test. Culture filtrate from Giardia cultures was loaded onto a 2-ml MAb–Affi-Gel 15 column, and the gel was washed with sterile PBS (pH 7.4). Bound antigen was eluted with 100 mM glycine buffer (pH 2.5) containing 10% ethylene glycol and 0.5 M sodium chloride. Purified Giardia antigen was concentrated and washed with PBS by centrifugation with a Centri-plus concentrator (10-kDa size exclusion; Amicon, Beverly, Mass.).
Western blot analysis.
Protein concentration was determined by the method of Bradford (1). Molecular weight was determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) by the method of Laemmli (8). Protein staining was done with Coomassie brilliant blue R-250 and SYPRO orange protein stain (Bio-Rad Laboratories). Immunoblot analysis was done by the method of Towbin et al. (18). The primary antibody consisted of either (i) the TechLab test MAb; (ii) ascites fluid containing the 7D2 MAb, which binds specifically to CWP2 (9); or (iii) the Alexon test MAb conjugate.
N-terminal sequencing.
Samples of purified antigen (1 μg) were transferred to polyvinylidene difluoride membrane for sequencing on an Applied Biosystems Procise sequencer (Perkin-Elmer Corp., Norwalk, Conn.). Sequence homologies were determined with the FASTA program (12).
Assessment of antigen stability.
For stability testing, purified antigen (0.2 μg/ml) was heated at 100°C for 5 min. Serial dilutions were tested by ELISA and immunoblotting. The effect of proteolysis was determined by incubating purified antigen (0.2 μg/ml) with a mixture of trypsin and chymotrypsin (0.2 μg/ml) in 0.1 M Tris-HCl buffer (pH 8.0) and with pronase (2 mg/ml) in 0.1 M Tris-HCl buffer (pH 7.5) containing 0.01 M EDTA and 0.5% SDS. Azocasein was used as a substrate to compare the activities of the protease solutions (3).
Purified antigen (0.2 μg/ml) was treated with N- and O-glycanases as instructed by the manufacturer (Oxford Glycosciences, Inc., Bedford, Mass.) and analyzed by immunoblotting. The effect of oxidation was determined by using purified antigen (0.6 μg) that was first separated by SDS-PAGE, transferred to nitrocellulose, and oxidized with reagents of the GlycoTrack test kit (Oxford Glycosciences, Inc.) as instructed by the manufacturer. Membranes were blocked with 0.5% casein, and residual immunoreactivity was determined by immunoblotting with the TechLab test MAb as the detecting antibody.
Expression and purification of rCWP1.
Recombinant CWP1 (rCWP1) was expressed in a glutathione S-transferase gene fusion system in Escherichia coli BL21 (12) (kindly supplied by the National Institutes of Health). Expression was induced in 6-h (37°C) shaking cultures with 200 μM isopropyl-β-d-thiogalactopyranoside. Cells were lysed by sonication, clarified by centrifugation, and applied to glutathione-Sepharose 4B (Pharmacia Biotech, Piscataway, N.J.). CWP1 was released from the column by cleavage with bovine thrombin (25 U in PBS [pH 7.5]).
Study sites and stool specimens.
Three separate studies evaluating CWP1 as a diagnostic marker of giardiasis were performed. Study 1, performed at Sacred Heart Medical Center (Spokane, Wash.), compared both ELISAs to ova and parasite examination (O&P). Study 2, performed at DeKalb Medical Center (Decatur, Ga.), compared the TechLab test to the Alexon test and included stool samples from SmithKline Laboratory (Atlanta, Ga.). Study 3, performed at the Virus Reference Laboratory (VRL; San Antonio, Tex.) resembled study 1. All specimens were preserved in 10% buffered formalin and submitted for routine O&P. Discrepant results were resolved by repeat testing by ELISA and immunofluorescent antibody (IFA) analysis.
RESULTS
Characterization of purified Giardia antigen.
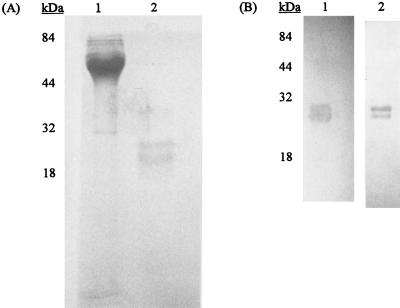
The antigen purified by immunoaffinity chromatography with the MAb used in the TechLab ELISA consisted of two proteins with sizes of 22 and 26 kDa (Fig. 1A) that reacted intensely with both of the MAbs from the TechLab and Alexon tests (Fig. 1B). Preincubation of immunoblots with the TechLab MAb reduced the binding of the Alexon MAb conjugate, demonstrating competitive inhibition by the TechLab MAb. Under nonreducing conditions, a major 50-kDa band and a minor 65-kDa band were detected with both MAbs. Further analysis with a more sensitive fluorescent staining procedure revealed two minor proteins with sizes of 32 and 39 kDa that did not react with these MAbs.
FIG. 1.
(A) Analysis by SDS-PAGE of Giardia CWP1 purified by immunoaffinity chromatography with the immobilized TechLab test MAb. Lanes: 1, starting culture filtrate (5 μg); 2, purified Giardia CWP1 (1 μg). Note the presence of 22- and 26-kDa protein bands in the purified preparation. (B) Analysis of CWP1 by immunoblotting with the TechLab test MAb (lane 1) and Alexon test MAb conjugate (lane 2). Both lanes show the presence of the 22- and 26-kDa protein bands. Molecular mass markers are shown with each gel.
By N-terminal sequencing, we identified the 22- and 26-kDa proteins as CWP1 and the 32- and 39-kDa proteins as CWP2. We compared rCWP1 with native CWP1 and CWP2 by immunoblotting and by ELISA. The rCWP1 reacted with the same intense reaction as native CWP1 (A450 of >2.0) in both ELISAs, and by immunoblotting, rCWP1 showed the same 26-kDa form as native CWP1. We found that MAb 7D2, which is specific for CWP2 (9), reacted with the 32- and 39-kDa proteins, but not with the 22- and 26-kDa proteins (data not shown).
N-terminal studies revealed that the 22- and 26-kDa forms of CWP1 had the same N-terminal amino acids. This observation suggested that the smaller CWP1 form (22 kDa) was derived by limited proteolysis. However, when we treated CWP1 with trypsin, chymotrypsin, or pronase, the 26-kDa protein was not converted into smaller fragments, and it retained 100% of its immunoreactivity in the TechLab test. The 26-kDa form also did not convert to the 22-kDa form or smaller peptides in older cultures with prolonged encystment (30 days). CWP1 was resistant to boiling, glycanase degradation, and oxidation with periodate. The 26-kDa form was not converted to the 22-kDa form by any of these treatments.
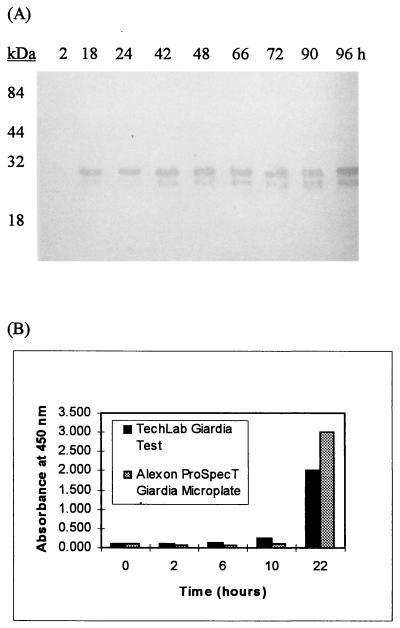
The production of CWP1 in encysting cultures was analyzed by immunoblotting and ELISA. By immunoblot analysis, the 26-kDa CWP1 was detected initially at 18 h, followed by the appearance of the 22-kDa form at 42 h (Fig. 2A). The TechLab and Alexon tests detected CWP1 at 10 h into encystment, and the levels increased through the last sampling at 22 h (Fig. 2B). In synchronized cultures containing nonencysting trophozoites, CWP1 was not detected by either of the commercial tests or by immunoblotting.
FIG. 2.
Appearance of CWP1 during encystment. Culture supernatant fluids were sampled at the time points listed and assayed by immunoblotting and ELISA. (A) Immunoblot analysis of culture supernatant fluids. The MAb from the TechLab test was used as the detecting antibody. The 26-kDa band initially was detected at 18 h, and the 22-kDa band initially was detected at 42 h. (B) Analysis of culture supernatant fluids by the TechLab test and by the Alexon test. CWP1 initially was detected by both ELISAs at 10 h. CWP1 was not detected by ELISA or immunoblotting at any time in cultures of synchronized trophozoites.
Clinical evaluation of CWP1 as a diagnostic marker.
Nine Giardia-positive stool specimens, all of which were positive by both commercial ELISAs, were analyzed by immunoblotting with the MAb from the TechLab test. We detected the 26-kDa CWP1 in eight specimens (data not shown). The stool specimen that did not react by immunoblotting gave an absorbance value of <0.5 in the TechLab test, indicating that it contained only low levels of antigen. Nine stool specimens that were negative by the ELISAs also were negative by immunoblot analysis (data not shown).
The utilization of CWP1 as a diagnostic marker for giardiasis was evaluated by three clinical laboratories using stool specimens preserved in 10% formalin. Stool specimens were examined for CWP1 by the TechLab test and the Alexon test. Results were compared between ELISAs and those from O&P. Discrepant results were retested by ELISA and analyzed by IFA. Of 535 specimens analyzed, there were 93 confirmed positive stools for CWP1 by the TechLab test and 96 confirmed positive stools for CWP1 by the Alexon test. Tables 1 and 2 show the results from each clinical study. Following the resolution of discrepant results, the TechLab test and Alexon test showed overall correlations with microscopic examination of 98.1 and 97.7%, respectively.
TABLE 1.
Clinical evaluation of CWP1 as a diagnostic marker with the TechLab Giardia Testa
| TechLab Giardia Test parameter | Result for:
|
||
|---|---|---|---|
| Study 1 (Sacred Heart Medical Center, Spokane, Wash.) | Study 2 (Dekalb Medical Center, Decatur, Ga.) | Study 3 (VRL, San Antonio, Tex.) | |
| Total no. of stools | 114 | 221 | 200 |
| Total no. positive | 31 | 46 | 16 |
| Total no. negative | 79 | 175 | 180 |
| Total no. of discrepancies | 4 | 0 | 4 |
| % Sensitivity | 91.4 | 100.0 | 100.0 |
| % Specificity | 100.0 | 100.0 | 97.8 |
| Positive predictive value (%) | 100.0 | 100.0 | 80.0 |
| Negative predictive value (%) | 96.3 | 100.0 | 100.0 |
| % Correlation | 97.4 | 100.0 | 98.0 |
The ELISA was performed as instructed by the manufacturer. O&P was done by in-house methods implemented at each study site. All stool specimens were preserved in 10% buffered formalin.
TABLE 2.
Clinical evaluation of CWP1 as a diagnostic marker with the Alexon testa
| Alexon test parameter | Result for:
|
||
|---|---|---|---|
| Study 1 (Sacred Heart Medical Center, Spokane, Wash.) | Study 2 (Dekalb Medical Center, Decatur, Ga.) | Study 3 (VRL, San Antonio, Tex.) | |
| Total no. of stools | 114 | 221 | 200 |
| Total no. positive | 34 | 46 | 16 |
| Total no. negative | 75 | 165 | 177 |
| Total no. of discrepancies | 5 | 10 | 7 |
| % Sensitivity | 100.0 | 100.0 | 100.0 |
| % Specificity | 94.9 | 94.3 | 96.2 |
| Positive predictive value (%) | 89.7 | 82.1 | 69.5 |
| Negative predictive value (%) | 100.0 | 100.0 | 100.0 |
| % Correlation | 96.5 | 95.5 | 96.5 |
The ELISA was performed as instructed by the manufacturer. O&P was done by in-house methods implemented at each study site. All stool specimens were preserved in 10% buffered formalin.
DISCUSSION
The encystment process of G. lamblia remains one of the least understood phases of its life cycle, but during the past decade, researchers have begun to understand some of the intriguing effects of this process. The cysts contain a network of filaments of different sizes that begin at multiple points on the outer membrane of encysting trophozoites known as cap structures (4). These cap structures generate cyst filaments of multiple diameters that elongate and proceed to fully encase the trophozoite. Gas chromatographic and mass spectrometric analyses of the cyst wall indicate the presence of protein and carbohydrate, which is primarily N-acetylgalactosamine (10). Although some progress has been made, actually very little is known about the composition of the cyst wall, the mechanism of construction, and the function of known cyst wall proteins.
The purpose of our study was to identify and characterize the cyst antigen detected by the TechLab test. By utilizing immunoaffinity chromatography, we were able to obtain highly purified antigen and identify it as CWP1. When we examined highly purified CWP1 for cross-reactivity in the Alexon test, we found that CWP1 reacted at roughly the same level in both ELISAs. Supporting these observations was our finding that rCWP1, which has an Mr of 26,000, also reacted strongly in both tests. In addition, the TechLab MAb competitively inhibited the binding of the Alexon MAb conjugate, indicating a similar or identical epitope. Thus, our results demonstrate that both commercial tests detect CWP1, contrary to reports that the Alexon test detects GSA65. Some of the confusion may result from the ability of these proteins to form heterodimers. Heterodimer formation of Giardia cyst wall proteins has been described previously (9), and our findings showing low levels of CWP2 in our CWP1 preparations can lead to heterodimer formation. Whether this explains the confusion with GSA65, however, is not clear, since CWP1 is easily distinguished from GSA65 by SDS-PAGE in the presence of reducing agents (16). In addition, our findings suggest that CWP1 is an unglycosylated protein. This result also is contradictory, based on earlier observations that GSA65 is sensitive to periodate oxidation (16).
The identical homologies of the N-terminal sequences of the 22- and 26-kDa forms of CWP1 suggest that cleavage occurs at the C terminus of the molecule. Both forms appear to be highly stable and retain their immunoreactivity. Interestingly, CWP1 was detected by both ELISAs in cultures undergoing encystment but not in synchronized trophozoite cultures. Synchronization of growing trophozoites is done in vitro by eliminating unattached trophozoites that are beginning the encystment process. During an infection, trophozoites and encysting trophozoites are both present in the small intestine. Thus, the absence of CWP1 in synchronized trophozoite cultures does not diminish the diagnostic value of this antigen. In fact, because of the secretion of CWP1 early in encystment and the high sensitivity of the ELISAs, there may be cases in which the antigen is detected in the absence of mature cysts.
In the clinical evaluation, the presence of CWP1 correlated well with Giardia-positive specimens as determined by ELISA, O&P, and IFA. The correlation between ELISAs was 98.7%, which is not surprising since they both detect the same antigen. Our findings provide additional evidence demonstrating the stability of CWP1 in human feces. The detection of CWP1 in clinical specimens from various study sites suggests that this antigen is conserved among Giardia isolates throughout the United States. These results further support its use as a diagnostic marker.
In conclusion, our results show that CWP1 is a highly stable cyst wall protein produced and released by encysting trophozoites. The combination of encysting Giardia in the gastrointestinal tracts of infected persons, high-level secretion of the antigen early in the encystment process, and a high correlation with O&P in patients suffering from giardiasis makes CWP1 a useful marker of giardiasis.
ACKNOWLEDGMENTS
We thank Carlyn Bruce for maintaining the cultures of Giardia. We are also grateful to Raymond Kaplan (SmithKline Laboratories, Atlanta, Ga.) for providing clinical specimens.
REFERENCES
- 1.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 2.Campbell J D, Faubert G M. Recognition of Giardia lamblia cyst-specific antigens by monoclonal antibodies. Parasite Immunol. 1994;16:211–219. doi: 10.1111/j.1365-3024.1994.tb00342.x. [DOI] [PubMed] [Google Scholar]
- 3.Charney J, Tomarelli R M. A colorimetric method for the determination of the proteolytic activity of duodenal juice. J Biol Chem. 1947;171:501–505. [PubMed] [Google Scholar]
- 4.Erlandsen S L, Macechko P T, Van Keulen H, Jarroll E L. Formation of the Giardia cyst wall: studies on extracellular assembly using immunogold labeling and high resolution field emission SEM. J Eukaryot Microbiol. 1996;43:416–429. doi: 10.1111/j.1550-7408.1996.tb05053.x. [DOI] [PubMed] [Google Scholar]
- 5.Garcia L S, Bruckner D. Diagnostic medical parasitology. Washington, D.C: American Society for Microbiology; 1993. pp. 31–48. [Google Scholar]
- 6.Gillin F D, Reiner D S, Gault M J, Douglas H, Das S, Wunderlich A, Sauch J F. Encystation and expression of cyst antigens by Giardia lamblia in vitro. Science. 1987;235:1040–1043. doi: 10.1126/science.3547646. [DOI] [PubMed] [Google Scholar]
- 7.Keister D B. Axenic culture of Giardia lamblia in TYI-33 medium supplemented with bile. Trans R Soc Trop Med Hyg. 1983;77:487–488. doi: 10.1016/0035-9203(83)90120-7. [DOI] [PubMed] [Google Scholar]
- 8.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 9.Lujan H D, Mowatt M R, Conrad J T, Bowers B, Nash T E. Identification of a novel Giardia lamblia cyst wall protein with leucine-rich repeats. J Biol Chem. 1995;270:29307–29313. doi: 10.1074/jbc.270.49.29307. [DOI] [PubMed] [Google Scholar]
- 10.Manning P, Erlandsen S L, Jarroll E L. Carbohydrate and amino acid analysis of Giardia muris cysts. J Protozool. 1992;39:290–296. doi: 10.1111/j.1550-7408.1992.tb01317.x. [DOI] [PubMed] [Google Scholar]
- 11.Meng T-C, Hetsko M L, Gillin F D. Inhibition of Giardia lamblia excystation by antibodies against cyst walls and by wheat germ agglutinin. Infect Immun. 1996;64:2151–2157. doi: 10.1128/iai.64.6.2151-2157.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mowatt M R, Lujan H D, Cotten D B, Bower B, Yee J, Nash T E, Stibbs H H. Developmentally regulated expression of a Giardia lamblia cyst wall protein. Mol Microbiol. 1995;15:955–963. doi: 10.1111/j.1365-2958.1995.tb02364.x. [DOI] [PubMed] [Google Scholar]
- 13.Pearson W R, Lipman D J. Improved tools for biological sequence comparison. Proc Natl Acad Sci USA. 1988;85:2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reiner D S, Douglas H, Gillin F O. Identification and localization of cyst-specific antigens of Giardia lamblia. Infect Immun. 1989;57:963–968. doi: 10.1128/iai.57.3.963-968.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rendtroff R C. The experimental transmission of human intestinal protozoan parasites. II. Giardia lamblia cysts given in capsules. Am J Hyg. 1954;59:209–220. doi: 10.1093/oxfordjournals.aje.a119634. [DOI] [PubMed] [Google Scholar]
- 16.Rosoff J D, Stibbs H H. Physical and chemical characterization of a Giardia lamblia-specific antigen useful in the coprodiagnosis of giardiasis. J Clin Microbiol. 1986;24:1079–1083. doi: 10.1128/jcm.24.6.1079-1083.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stibbs H H. Monoclonal antibody-based enzyme immunoassay for Giardia lamblia antigen in human stool. J Clin Microbiol. 1989;27:2582–2588. doi: 10.1128/jcm.27.11.2582-2588.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ward H D, Kane A V, Ortega-Barria E, Keusch G T, Pereira M E. Identification of developmentally-regulated Giardia lamblia cyst antigens using GCSA-1, a cyst-specific monoclonal antibody. Mol Microbiol. 1990;4:2095–2102. doi: 10.1111/j.1365-2958.1990.tb00570.x. [DOI] [PubMed] [Google Scholar]