Abstract
Background
Smokers manifest varied phenotypes of pulmonary impairment.
Research Question
Which pulmonary phenotypes are associated with coronary artery disease (CAD) in smokers?
Study Design and Methods
We analyzed data from the University of Pittsburgh COPD Specialized Center for Clinically Oriented Research (SCCOR) cohort (n = 481) and the Genetic Epidemiology of COPD (COPDGene) cohort (n = 2,580). Participants were current and former smokers with > 10 pack-years of tobacco exposure. Data from the two cohorts were analyzed separately because of methodologic differences. Lung hyperinflation was assessed by plethysmography in the SCCOR cohort and by inspiratory and expiratory CT scan lung volumes in the COPDGene cohort. Subclinical CAD was assessed as the coronary artery calcium score, whereas clinical CAD was defined as a self-reported history of CAD or myocardial infarction (MI). Analyses were performed in all smokers and then repeated in those with airflow obstruction (FEV1 to FVC ratio, < 0.70).
Results
Pulmonary phenotypes, including airflow limitation, emphysema, lung hyperinflation, diffusion capacity, and radiographic measures of airway remodeling, showed weak to moderate correlations (r < 0.7) with each other. In multivariate models adjusted for pulmonary phenotypes and CAD risk factors, lung hyperinflation was the only phenotype associated with calcium score, history of clinical CAD, or history of MI (per 0.2 higher expiratory and inspiratory CT scan lung volume; coronary calcium: OR, 1.2; 95% CI, 1.1-1.5; P = .02; clinical CAD: OR, 1.6; 95% CI, 1.1-2.3; P = .01; and MI in COPDGene: OR, 1.7; 95% CI, 1.0-2.8; P = .05). FEV1 and emphysema were associated with increased risk of CAD (P < .05) in models adjusted for CAD risk factors; however, these associations were attenuated on adjusting for lung hyperinflation. Results were the same in those with airflow obstruction and were present in both cohorts.
Interpretation
Lung hyperinflation is associated strongly with clinical and subclinical CAD in smokers, including those with airflow obstruction. After lung hyperinflation was accounted for, FEV1 and emphysema no longer were associated with CAD. Subsequent studies should consider measuring lung hyperinflation and examining its mechanistic role in CAD in current and former smokers.
Key Words: COPD, coronary artery disease, lung hyperinflation, smoking
Abbreviations: CAD, coronary artery disease; COPDGene, Genetic Epidemiology of COPD; FRV, functional residual volume; IPA, index of prediction accuracy; LAA%–950, low attenuation area with a –950-Hounsfield unit cutoff; MI, myocardial infarction; RV, residual volume; SCCOR, University of Pittsburgh COPD Specialized Center for Clinically Oriented Research; TLC, total lung capacity; TLV, total lung volume
In 1989, the Framingham Heart Study identified FEV1 as an important predictor of cardiovascular risk,1 and FEV1 remains the most commonly assessed pulmonary phenotype when investigating cardiovascular risk in smokers. However, it has become increasingly evident that smokers manifest a variety of pulmonary phenotypes besides a reduced FEV1.2, 3, 4 Prominent among these are emphysema characterized by anatomic destruction of the lung parenchyma, airway injury and remodeling, impaired diffusion capacity, and lung hyperinflation characterized by an increase in residual volume relative to total lung capacity (TLC).5 Quantification of these phenotypes in large cohorts demonstrates that systemic manifestations of lung disease may be associated preferentially with a specific pulmonary phenotype. For example, osteoporosis is associated most strongly with the severity of emphysema, rather than FEV1 impairment.6
Nonetheless, an unsupervised search for the pulmonary phenotypes associated with coronary artery disease (CAD) in smokers has not been performed. Identifying such phenotypes will be critical for developing accurate risk prediction models for CAD in smokers and to gain mechanistic insights into coronary vascular injury in smokers. Accordingly, we used data from more than 3,000 smokers enrolled in two exquisitely phenotyped cohorts, the University of Pittsburgh COPD Specialized Center for Clinically Oriented Research (SCCOR) study and the Genetic Epidemiology of COPD (COPDGene) study, to identify the pulmonary phenotypes associated with clinical and subclinical CAD in smokers.
Methods
Study Cohorts
Enrollment criteria for the SCCOR and COPDGene cohorts have been described previously.6,7 In brief, the SCCOR cohort enrolled current and former smokers 40 to 79 years of age with > 10 pack-years of tobacco exposure. Those with prior thoracic surgery, cardiovascular events in the preceding year, or a restrictive pattern on spirometry were excluded. Smokers with cardiovascular events in the prior year were excluded because their diminished survival made them less suitable for inclusion in a multiyear longitudinal cohort. Similarly, the COPDGene cohort enrolled current and former smokers 45 to 80 years of age with > 10 pack-years of tobacco exposure. Those with prior thoracic surgery or lung disease besides asthma or COPD were excluded. The presence of airflow obstruction (FEV1 to FVC ratio, < 0.70) was not required for enrollment in either cohort; therefore, our study included current and former smokers with and without airflow obstruction. Accordingly, the analyses performed in the full cohort were repeated in a predefined subgroup of participants with airflow obstruction. The institutional review boards at all participating sites approved both cohort studies (e-Appendix 1). Written informed consent was obtained from each participant.
Pulmonary Phenotyping
Pulmonary Function
Postbronchodilator spirometry was performed and adjusted to standard population-derived predicted values in both cohorts.8 Diffusing capacity was assessed as the single-breath diffusing capacity for carbon monoxide per standard guidelines in SCCOR only.9
Lung Hyperinflation
In the SCCOR cohort, lung hyperinflation was defined as the ratio of residual volume (RV) to TLC assessed by full-body plethysmography, that is, by the gold standard method. CT-based measures of hyperinflation also have been developed and validated for use in research cohorts.10 One such method, the ratio of lung volume from CT images acquired at full inspiration (total lung volume [TLV]) and at the end of normal expiration (functional residual volume [FRV]), was used in the COPDGene cohort. Ideally, the volume at the end of full expiration (RV), rather than normal expiration (FRV), should be used to calculate radiographic lung hyperinflation. However, many smokers, particularly those with severe airflow limitation, find it difficult to exhale completely and hold their breath long enough for acquisition of high-quality CT images. Therefore, expiratory CT images were captured at normal rather than full expiration. The FRV was highly correlated with RV assessed by plethysmography (rs = 0.81) (e-Fig 1). In addition to published data,10 we validated the FRV to TLV ratio as a correlate of plethysmographically assessed RV to TLC ratio in an unrelated cohort of 196 individuals (e-Figs 2-5). Nonetheless, the two cohorts used different methodologies to assess lung hyperinflation, and the correlation between the two different measures of hyperinflation was insufficient (rs = 0.60) for one measure to be considered the same as the other. Accordingly, the data from these two cohorts were analyzed separately.
Emphysema and Airway Remodeling
In the SCCOR cohort, chest CT images were acquired at full inspiration and were assessed for emphysema and airway remodeling as described previously.11,12 In brief, emphysema was assessed by density mask analysis using a –950-Hounsfield unit cutoff (or low attenuation area a with –950-Hounsfield unit cutoff [LAA%–950]) and by a semiquantitative visual score that increased from 0 to 5 with increasing severity of emphysema (e-Appendix 1).12 Measures of airway remodeling included wall area percentage and lumen perimeter of all airways. Similarly, in the COPDGene cohort, CT images acquired at full inspiration were used to assess emphysema by density mask analysis (LAA%–950) and to measure airway dimensions as described previously.13
CAD
Subclinical CAD
In both cohorts, subclinical CAD was assessed using the coronary artery calcium score measured from the same chest CT images used for pulmonary quantitative analyses. Although such chest CT images lack electrocardiography gating (that reduces cardiac motion), the resulting coronary artery calcium scores are correlated highly with those from electrocardiography-gated cardiac CT sans and independently predict coronary vascular events and mortality in multiple studies.14, 15, 16, 17, 18
In COPDGene, coronary artery calcium was calculated by the method of Agatston et al,19 that is, the volume-area method using chest CT images as described previously. In the SCCOR cohort, coronary artery calcium scores were generated by visual examination of chest CT images by the Weston method, as described previously.16 The Weston score increases from 0 to 12 with increasing amount of coronary artery calcium. Weston scores are divided into four categories (0 = none; 1-3 = mild; 4-7 = moderate; and 8-12 = severe) to match the four standard categories of Agatston scores (0 = none; 1-100 = mild; 101-400 = moderate; > 400 = severe).16 We, and others, previously validated the Weston score as an excellent correlate of Agatston scores.16,20,21 Interobserver and intraobserver agreement on Weston scores were excellent (both κ > 0.90) (e-Appendix 1).
Clinical CAD
In both cohorts, clinical CAD was defined by self-reported history of myocardial infarction (MI), angina, or physician diagnosis of CAD.
Cardiovascular Disease Risk Factors
Major Risk Factors
Major risk factors were defined as age, sex, race, hypertension, diabetes, hyperlipidemia, and tobacco exposure (pack-years of smoking and current smoking status). In both cohorts, hypertension was defined as systolic BP of ≥ 140 mm Hg or diastolic BP of ≥ 90 mm Hg measured during the study visit, patient report of a prior diagnosis of hypertension, or use of antihypertensive medication. Hyperlipidemia and diabetes were defined by the use of a lipid-lowering or diabetes medications, respectively, or patient report of a prior diagnosis.
Additional Risk Factors
Family history of cardiovascular disease, educational status, depression, physical activity level, dietary saturated fat intake, and creatinine clearance were assessed as additional cardiovascular risk factors in the SCCOR cohort, whereas visceral adipose tissue area, a measure of visceral adiposity, was assessed in the COPDGene cohort.
Physical activity level was assessed using the Stanford Brief Activity Survey,22 dietary fat intake was assessed using the Block Dietary Fat Screener,23 and depression was assessed via the Beck Depression Inventory.24 Family history of cardiovascular disease and education status were self-reported. Creatinine levels were measured as described previously and were used to determine the estimates glomerular filtration rate using the Chronic Kidney Disease Epidemiology Collaboration equation.12
In the COPDGene cohort, visceral adipose tissue area was assessed using a single axial CT image at the inferior edge of the transverse process of the first lumbar vertebrae, as described previously.25
Statistical Analyses
Because of the methodological differences between the two cohorts described above, data were not pooled. For descriptive statistics, continuous variables were summarized as mean ± SD if normally distributed and as median with interquartile range if not normally distributed. Scatterplots with Spearman correlation coefficients were used to examine relationships between continuous variables. Phenotypic variables were classified as present or absent only for descriptive analyses. All statistical models included phenotypic variables as continuous, rather than as present or absent.
Logistic regression was used to identify variables associated with subclinical CAD (ordinal category of calcium score was the dependent or outcome variable). Individuals with a history of clinical CAD were excluded from these analyses. Variables associated with a calcium score with P ≤ .10 in univariate models were included in multivariate analyses. Similarly, logistic regression was used to identify variables associated with clinical CAD. The same set of covariates was adjusted for as in the models above. Finally, analyses were repeated with MI as the dependent variable, instead of all forms of clinical CAD.
Analyses were performed using Stata MP version 16 software (StataCorp). Statistical significance was defined as two-tailed P < .05.
Results
Our analysis included 481 participants enrolled in the SCCOR cohort and 2,580 of 10,300 participants enrolled in the COPDGene study. These 2,580 participants were included because they had undergone assessment of coronary artery calcium and CT scan airway and lung volume measurements (e-Fig 1). They were similar to the remaining COPDGene cohort with the exception of more frequently being non-Hispanic White and former smokers and showing lower rates of emphysema (e-Table 1).
The 481 participants in the SCCOR cohort were 65.5 ± 6.2 years of age, 53.4% were male, and 94.8% were non-Hispanic White. Fifty-three percent of the participants demonstrated hypertension and 57.1% demonstrated hyperlipidemia, whereas the prevalence of diabetes was comparatively lower at 9.1%. In comparison, participants in the COPDGene study were significantly younger (60.7 ± 9.1 years; P < .001) with a similar sex distribution (50.5% male) and a higher proportion of other races or ethnicities (76.8% non-Hispanic White; P < .001) compared with the SCCOR cohort.
Pulmonary Phenotypes
In the SCCOR cohort, 49.7% of participants demonstrated airflow obstruction, ie, FEV1 to FVC ratio of < 0.7. The mean severity of pulmonary phenotypic abnormalities was mild (Table 1). Nonetheless, SDs were broad, suggesting a wide severity of phenotypic abnormalities. The prevalence of pulmonary phenotypic abnormalities in those with and without airflow obstruction is summarized in e-Figure 6. Although more frequent, phenotypic abnormalities were not limited to those with airflow obstruction.
Table 1.
Demographics, Cardiovascular Risk Factors, and Pulmonary Phenotypes Stratified by the Extent of Coronary Artery Calcium in the SCCOR Cohort
| Variable | All Patients | Coronary Calcium |
Univariate OR (95% CI)a | P Valuea | |||
|---|---|---|---|---|---|---|---|
| None | Mild | Moderate | Severe | ||||
| No. (%) | 481 | 75 (15.6) | 119 (24.7) | 163 (33.9) | 124 (25.8) | ... | ... |
| Demographics | |||||||
| Age, y | 65.5 ± 6.2 | 61.8 ± 5.0 | 64.2 ± 6.1 | 66.4 ± 6.0 | 67.9 ± 6.0 | 2.7 (2.1-3.6)b | < .001 |
| Male sex | 53.4 | 29.3 | 43.7 | 62.7 | 65.3 | 2.6 (1.8-3.6) | < 001 |
| Non-Hispanic White | 94.8 | 88.0 | 96.6 | 96.9 | 94.3 | 1.8 (0.8-4.0) | .02 |
| Education | ... | ... | ... | ... | ... | 0.9 (0.7-1.1) | .12 |
| High school graduate | 28.1 | 24.0 | 22.0 | 32.5 | 30.6 | ... | ... |
| College graduate | 31.1 | 33.3 | 31.4 | 30.7 | 29.9 | ... | ... |
| Graduate/professional degree | 40.8 | 42.7 | 46.6 | 36.8 | 39.5 | ... | ... |
| Cardiovascular risk factors | |||||||
| Hypertension | 53.0 | 11.0 | 43.7 | 57.0 | 66.1 | 2.1 (1.5-2.9) | < .001 |
| Diabetes mellitus | 9.1 | 1.3 | 7.6 | 10.4 | 13.7 | 2.2 (1.3-3.9) | .004 |
| Hyperlipidemia | 57.1 | 46.7 | 43.7 | 61.3 | 70.9 | 2.1 (1.5-3.0) | < .001 |
| BMI, kg/m2 | 27.9 ± 4.1 | 27.5 ± 4.4 | 27.9 ± 4.1 | 27.9 ± 3.9 | 28.3 ± 4.1 | 1.3 (0.9-2.0)b | .19 |
| Current smoker | 44.5 | 46.7 | 47.0 | 42.9 | 42.7 | 0.9 (0.6-1.2) | .44 |
| Pack-years of smoking | 54.8 ± 30.6 | 44.0 ± 25.2 | 52.2 ± 28.1 | 55.1 ± 28.1 | 63.5 ± 36.3 | 1.1 (1.0-1.3)b | < .001 |
| Cardiovascular medications | |||||||
| Angiotensin converting enzyme inhibitor | 14.7 | 2.7 | 13.4 | 16.6 | 21.0 | 2.1 (1.3-3.3) | .001 |
| Statins | 41.2 | 25.3 | 24.6 | 47.8 | 58.1 | 2.8 (2.0-4.0) | < .001 |
| Aspirin | 40.9 | 26.7 | 36.1 | 41.1 | 54.0 | 1.9 (1.4-2.7) | < .001 |
| Expiratory airflow | |||||||
| FEV1, % predicted | 83.5 ± 20.6 | 89.5 ± 16.9 | 83.6 ± 20.6 | 82.9 ± 21.1 | 80.5 ± 21.3 | 1.2 (1.1-1.4)c | .008 |
| FEV1 to FVC ratio | 0.67 ± 0.13 | 0.72 ± 0.11 | 0.67 ± 0.13 | 0.67 ± 0.13 | 0.65 ± 0.13 | 1.6 (1.2-2.0)c | .001 |
| Postbronchodilator change in FEV1 | 9.1 ± 9.6 | 7.6 ± 8.7 | 9.1 ± 10.3 | 10.0 ± 9.8 | 8.9 ± 9.3 | 0.9 (0.6-1.2)c | .41 |
| Emphysema | |||||||
| Low attenuation area, LAA%-950 | 3.2 ± 6.0 | 1.8 ± 4.0 | 3.5 ± 6.7 | 3.6 ± 6.8 | 3.3 ± 5.0 | 1.4 (0.8-2.3)d | .23 |
| Visual emphysema score | 1.1 ± 1.2 | 0.8 ± 1.0 | 1.1 ± 1.3 | 1.1 ± 1.3 | 1.2 ± 1.2 | 1.1 (0.9-1.3) | .08 |
| Airway remodeling | |||||||
| Mean lumen perimeter, mm | 12.9 ± 1.3 | 12.9 ± 1.3 | 12.7 ± 1.3 | 12.9 ± 1.1 | 13.0 ± 1.5 | 1.1 (1.0-1.3) | .06 |
| Mean wall area | 47.7 ± 5.0 | 46.8 ± 5.0 | 47.7 ± 5.2 | 48.1 ± 5.1 | 47.7 ± 4.7 | 1.0 (0.9-1.1) | .13 |
| Lung hyperinflation | |||||||
| RV to TLC ratio | 0.44 ± 0.09 | 0.39 ± 0.07 | 0.43 ± 0.11 | 0.44 ± 0.10 | 0.45 ± 0.10 | 2.0 (1.4-2.7)d | < .001 |
| Diffusing capacity | |||||||
| Dlco, % predicted | 72.6 ± 18.9 | 77.2 ± 16.5 | 73.0 ± 19.6 | 72.4 ± 19.1 | 69.6 ± 18.8 | 1.3 (1.1-1.5)c | .01 |
Data are presented as percentage or mean ± SD, unless otherwise indicated. Dlco = diffusing capacity for carbon monoxide; % low attenuation area = % of low attenuation areas in density mask analyses of chest CT scans; RV = residual volume; SCCOR = University of Pittsburgh COPD Specialized Center for Clinically Oriented Research; TLC = total lung capacity.
OR and P values are for unadjusted odds of being in a higher category of coronary artery calcium score per unit change for continuous predictors and yes vs no for categorical predictors.
OR is per 10-point increase in the predictor variable.
OR is per 20-point decrease in the predictor variable.
OR is per 20-point increase in the predictor variable.
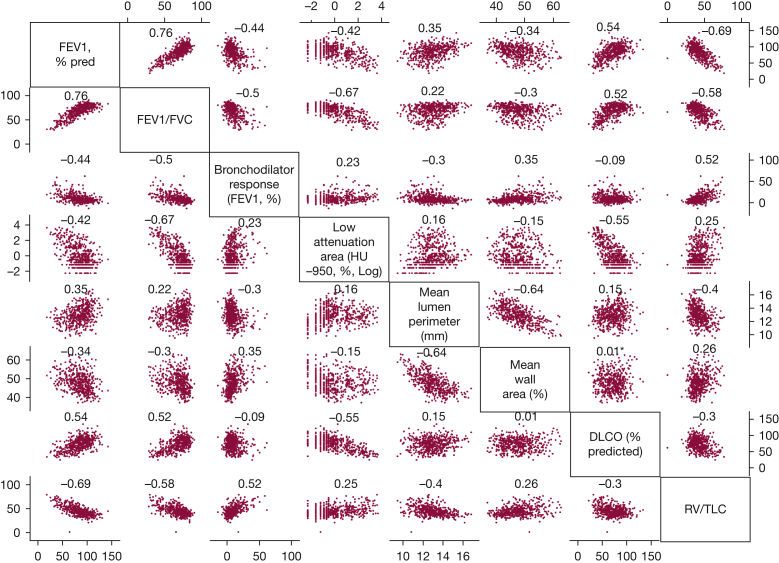
The correlation between various pulmonary phenotypes was weak to moderate (Fig 1), with the exception of the strong correlation of FEV1 % predicted with FEV1 to FVC ratio (r = 0.76) and with RV to TLC ratio (r = –0.69). In those with airflow obstruction, the only strong correlation was between FEV1 % predicted and FEV1 to FVC ratio (r = 0.74).
Figure 1.
Scatterplot matrix depicting the correlations between various pulmonary phenotypes along with Spearman correlation coefficients in the University of Pittsburgh COPD Specialized Center for Clinically Oriented Research cohort. The unit for each pulmonary phenotype is listed along with the name of the phenotype in the diagonal axis of the figure. For ratios, ie, FEV1 to FVC ratio and residual volume to total lung capacity ratio, the units are 0 to 100, rather than 0 to 1. The Spearman correlation coefficient is depicted on the top of each scatterplot.
Similarly, in the COPDGene cohort, pulmonary phenotypic abnormalities on average were mild with wide SDs (Table 2). Although more frequent, phenotypic abnormalities were not limited to those with airflow obstruction (e-Fig 7). Further, the correlation between various pulmonary phenotypes was weak to intermediate in the full cohort (e-Fig 8), in those with FEV1 to FVC ratio of < 0.7, and in those with FEV1 to FVC ratio of < 0.7 and FEV1 < 50% predicted (with the exception of r > 0.70 between FEV1 % predicted and FEV1 to FVC ratio).
Table 2.
Demographics, Cardiovascular Risk Factors, and Pulmonary Phenotypes Stratified by the Extent of Coronary Artery Calcium in the COPDGene Cohort
| Variable | All Patients | Coronary Calcium |
Univariate OR (95% CI)a | P Valuea | |||
|---|---|---|---|---|---|---|---|
| None | Mild | Moderate | Severe | ||||
| No. (%) | 2,580 | 990 (38.3) | 729 (28.2) | 509 (19.7) | 352 (13.6) | . . . | . . . |
| Demographics | |||||||
| Age, y | 60.7 ± 9.1 | 56.3 ± 8.0 | 60.6 ± 8.5 | 64.4 ± 8.1 | 67.7 ± 7.3 | 2.8 (2.5-3.0)b | < .001 |
| Male sex | 50.5 | 42.3 | 48.1 | 58.5 | 66.7 | 1.8 (1.6-2.1) | < .001 |
| Non-Hispanic White | 76.8 | 64.2 | 82.2 | 85.0 | 88.9 | 2.9 (2.4-3.5) | < .001 |
| Cardiovascular risk factors | |||||||
| Hypertension | 52.7 | 22.6 | 35.5 | 46.6 | 57.8 | 2.7 (2.3-3.2) | < .001 |
| Diabetes mellitus | 7.1 | 4.1 | 6.7 | 9.0 | 13.1 | 2.2 (1.7-2.9) | < .001 |
| Hyperlipidemia | 26.4 | 22.3 | 24.8 | 36.1 | 50.1 | 3.1 (2.6-3.6) | < .001 |
| BMI, kg/m2 | 28.4 ± 6.1 | 28.4 ± 6.4 | 28.2 ± 5.9 | 28.1 ± 5.6 | 29.0 ± 6.2 | 1.0 (0.9-1.1)b | .62 |
| Current smoker | 44.5 | 54.5 | 43.9 | 36.1 | 29.6 | 0.5 (0.4-0.6) | < .001 |
| Pack-years of smoking | 44.3 ± 24.3 | 38.9 ± 21.2 | 43.8 ± 23.9 | 47.4 ± 23.4 | 56.0 ± 29.2 | 1.1 (1.1-1.2)b | < .001 |
| Expiratory airflow | |||||||
| FEV1, % predicted | 74.2 ± 27.7 | 78.5 ± 27.1 | 75.6 ± 27.2 | 69.5 ± 28.4 | 65.5 ± 27.1 | 1.2 (1.1-1.3)c | < .001 |
| FEV1 to FVC ratio | 0.64 ± 0.17 | 0.68 ± 0.17 | 0.65 ± 0.17 | 0.60 ± 0.18 | 0.58 ± 0.17 | 1.2 (1.1-1.3)c | < .001 |
| Postbronchodilator change in FEV1 | 6.1 ± 10.3 | 5.4 ± 10.3 | 6.0 ± 10.2 | 6.8 ± 10.0 | 7.1 ± 11.3 | 0.8 (0.7-0.9)c | .003 |
| Emphysema | |||||||
| Low attenuation area LAA%-950 | 8.1 ± 11.3 | 6.4 ± 10.4 | 7.8 ± 11.1 | 10.0 ± 12.1 | 10.9 ± 11.8 | 1.6 (1.4-1.8)d | < .001 |
| Airway remodeling | |||||||
| Mean wall area | 61.2 ± 3.2 | 61.2 ± 3.2 | 61.2 ± 3.3 | 61.3 ± 3.2 | 61.4 ± 3.4 | 1.0 (0.9-1.1) | .15 |
| Lung hyperinflation | |||||||
| FRV to TLV ratioe | 0.58 ± 0.13 | 0.57 ± 0.13 | 0.57 ± 0.12 | 0.60 ± 0.12 | 0.61 ± 0.12 | 1.5 (1.4-1.6)d | < .001 |
Data are presented as percentage or mean ± SD, unless otherwise indicated. COPDGene = Genetic Epidemiology of COPD; FRV = functional residual volume; % low attenuation area = % of low attenuation areas in density mask analyses of chest CT scans; TLV = total lung volume
OR and P values are for unadjusted odds of being in a higher category of coronary artery calcium score per unit change for continuous predictors and yes vs no for categorical predictors.
OR is per 10-point increase in the predictor variable.
OR is per 20-point decrease in the predictor variable.
OR is per 20-point increase in the predictor variable.
FRV was defined as the lung volume assessed on CT images acquired at normal expiration, whereas the TLV was defined as the lung volume assessed on images acquired at full inspiration.
Subclinical CAD
Major cardiovascular risk factors (age, sex, race, hypertension, diabetes, hyperlipidemia, and tobacco exposure) were associated with increased coronary artery calcium scores in both cohorts, as expected (Tables 1, 2). In the SCCOR cohort, FEV1 % predicted, FEV1 to FVC ratio, diffusing capacity for carbon monoxide % predicted, and RV to TLC ratio showed a statistically significant univariate association with coronary artery calcium score (Table 1). In addition to these phenotypes, bronchodilator response and LAA%–950 also were associated with coronary artery calcium score in the COPDGene cohort in univariate analysis (Table 2).
To determine which phenotypes were associated with coronary artery calcification, we performed multivariate analyses whereby any pulmonary phenotype associated with calcium score at P < .1 in univariate analysis was included as a predictor of calcium score along with major cardiovascular risk factors (Tables 3, 4). Lung hyperinflation was the only phenotype associated with calcium score in these multivariate models in either cohort (Tables 3, 4).
Table 3.
Effect Estimates and P Values for the Multivariate Model Predicting Coronary Artery Calcium Scores in the SCCOR Cohort (n = 413; Those With Clinical CAD Were Excluded)
| Variable | Multivariate OR (95% CI) | P Value |
|---|---|---|
| Pulmonary phenotypes | ||
| RV to TLC ratio, per 0.20 increase | 3.2 (1.6-6.4) | .001 |
| FEV1, per 20 % predicted decrease | 1.0 (0.6-1.5) | .92 |
| FEV1 to FVC ratio, per 0.20 decrease | 1.0 (0.3-1.2) | .12 |
| Visual emphysema score, per 1-unit increase | 1.1 (0.9-1.4) | .31 |
| Mean lumen perimeter, per 1-mm increase | 1.1 (0.9-1.3) | .17 |
| Dlco, per 20% predicted decrease | 1.2 (0.9-1.5) | .28 |
| Demographics | ||
| Age, per 10-y increase | 1.8 (1.3-2.6) | .001 |
| Male sex | 3.5 (2.3-5.4) | < .001 |
| Non-Hispanic White | 2.3 (0.9-5.8) | .07 |
| Cardiovascular risk factors | ||
| Hypertension | 1.4 (0.9-2.0) | .09 |
| Diabetes mellitus | 1.6 (0.8-3.2) | .16 |
| Hyperlipidemia | 1.6 (1.1-2.3) | .01 |
| Pack-years of smoking, per 10-y increase | 1.1 (1.0-1.2) | .04 |
| Current smoking | 1.0 (0.7-1.4) | .90 |
CAD = coronary artery disease; Dlco = diffusing capacity for carbon monoxide; RV = residual volume; SCCOR = University of Pittsburgh COPD Specialized Center for Clinically Oriented Research; TLC = total lung capacity.
Table 4.
Effect Estimates and P Values for the Multivariate Model Predicting Coronary Artery Calcium Scores in the COPDGene Cohort (n = 2,377; Those With Clinical CAD Were Excluded)
| Variable | Multivariate OR (95% CI) | P Value |
|---|---|---|
| Pulmonary phenotypes | ||
| FRV to TLV ratio, per 0.20 increase | 1.2 (1.0-1.4) | .01 |
| FEV1, per 20% predicted decrease | 1.1 (0.9-1.2) | .20 |
| FEV1 to FVC, per 0.20 decrease | 1.0 (1.0-1.1) | .85 |
| Low attenuation area LAA%-950, per 10% increase | 1.0 (0.9-1.1) | .61 |
| Postbronchodilator change in FEV1, % | 1.0 (0.9-1.2) | .56 |
| Demographics | ||
| Age, per 10-y increase | 2.4 (2.2-2.7) | < .001 |
| Male sex | 2.3 (2.0-2.7) | < .001 |
| Non-Hispanic White | 1.7 (1.3-2.1) | < .001 |
| Cardiovascular risk factors | ||
| Hypertension | 1.7 (1.4-2.4) | < .001 |
| Diabetes mellitus | 1.3 (1.0-1.7) | .09 |
| Hyperlipidemia | 1.6 (1.3-1.8) | < .001 |
| Pack-years of smoking, per 10-y increase | 1.0 (1.0-1.0) | < .001 |
| Current smoking | 1.3 (1.1-1.6) | .003 |
CAD = coronary artery disease; COPDGene = Genetic Epidemiology of COPD; FRV = functional residual volume; TLC = total lung capacity.
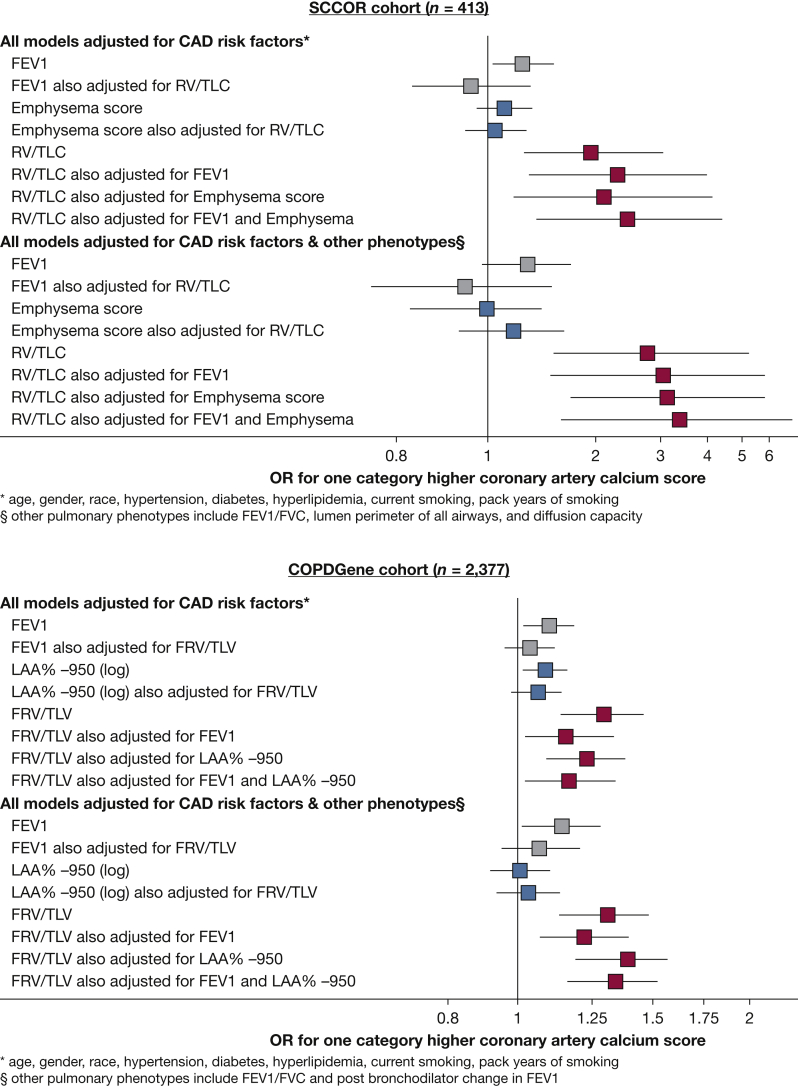
To examine lung hyperinflation further, we compared it directly against FEV1 % predicted and emphysema, which are the most frequently assessed pulmonary phenotypes when investigating cardiovascular disease in smokers.26, 27, 28, 29, 30 In agreement with prior studies, FEV1 % predicted was associated with coronary artery calcium scores in models adjusted for cardiovascular risk factors in both cohorts (Fig 2). However, further adjustment for lung hyperinflation completely attenuated the FEV1 and calcium score association (Fig 2). Emphysema was not associated with calcium score in univariate analysis. In contrast, lung hyperinflation was associated with calcium scores despite accounting for the FEV1, major cardiovascular disease risk factors, and other pulmonary phenotypes in both cohorts (Fig 2). Parsimonious models based on these results also were generated by backward elimination (e-Appendix 1).
Figure 2.
Forrest plots depicting the association of FEV1 (% predicted), emphysema (visual emphysema score), and lung hyperinflation (RV to TLC ratio) with coronary artery calcium score after adjustment for CAD risk factors (top) and further adjustment for other pulmonary phenotypes listed in Tables 3 and 4 (bottom) in the SCCOR (n = 413) and COPDGene (n = 2,377) cohorts. Individuals with history of clinical CAD were excluded from these models. ORs are calculated per 20% decrease in FEV1 % predicted, a 1-point increase in visual emphysema score (SCCOR cohort), q 1-point increase in log LAA%–950 (COPDGene cohort), and a 0.20 increase in RV to TLC ratio (SCCOR cohort) and FRV to TLC ratio (COPDGene cohort). Any association of FEV1 % predicted (gray box) or emphysema (blue box) with calcium score was attenuated after adjustment for lung hyperinflation. In contrast, lung hyperinflation (red box) was associated with the extent of coronary artery calcium despite accounting for the FEV1 % predicted, severity of emphysema, other pulmonary phenotypes, and CAD risk factors in both cohorts. CAD = coronary artery disease; COPDGene = Genetic Epidemiology of COPD; FRV = functional residual volume; LAA%–950 = low attenuation area with a –950-Hounsfield unit cutoff; RV = residual volume; SCCOR = University of Pittsburgh COPD Specialized Center for Clinically Oriented Research; TLC = total lung capacity.
Instead of including both FEV1 and RV to TLC ratio as predictors of coronary artery calcium in the same model, we compared models where only FEV1 or only RV to TLC ratio predicted the presence or absence of coronary calcium in terms of the concordance index (C-statistic or area under receiver operating characteristic curve) and index of prediction accuracy (IPA or Brier score per the method of Kattan and Gerds31). In the SCCOR cohort, the RV to TLC ratio C-statistic was 0.63 as compared with 0.58 for FEV1 when predicting the presence or absence of coronary artery calcium. In agreement, the IPA for RV to TLC ratio (20.1) was greater than for FEV1 (18.8). Results were similar in the COPDGene cohort, although the magnitude of the differences was smaller (RV to TLC ratio C-statistic, 0.77 and IPA, 20.9 vs FEV1 C-statistic, 0.76 and IPA, 20.8).
We also performed a number of analyses to examine the robustness of the association between lung hyperinflation and calcium score. First, further adjustment for additional cardiovascular risk factors and use of cardiovascular medications associated with calcium score with univariate P < .20 did not change the results (e-Appendix 1). Next, using categorical (no vs any) coronary artery calcium as the outcome variable did not alter the results, ie, the findings were not dependent on the use of ordinal categories (none, mild, moderate, severe) of calcium score (e-Appendix 1). The use of ordinal categories of calcium score was supported by nonsignificant P values for likelihood ratio tests for the proportional odds assumption in our multivariate models. Finally, no evidence was found of significant collinearity among the pulmonary phenotypes and other covariates included in statistical models in either cohort (variance inflation factor, < 10). Therefore, the independent association between lung hyperinflation and coronary artery calcium seemed to be robust in both cohorts.
Clinical CAD and MI
Those with a history of cardiovascular events in the preceding year were excluded from the SCCOR cohort; therefore, an insufficient number of participants with a history of myocardial infarction (n = 25) or other forms of clinical CAD (n = 38) was available to allow meaningful multivariate analyses in the SCCOR cohort.
Two hundred two COPDGene participants reported a history of clinical CAD. Increased lung hyperinflation was associated with increased clinical CAD in univariate analyses and in multivariate models adjusted for risk factors, FEV1, and other pulmonary phenotypes (OR, 1.60 per 0.20 increase in FRV to TLV ratio; 95% CI, 1.13-2.26; P = .008; adjusted for age, sex, race, hypertension, diabetes, hyperlipidemia, current smoking, pack years of smoking, FEV1, FEV1 to FVC ratio, postbronchodilator change in FEV1, and LAA%–950). FEV1 was associated with increased odds of clinical CAD (OR, 1.20 per 20% predicted lower FEV1; 95% CI, 1.08-1.32; P = .001); however, similar to calcium scores, adjustment for lung hyperinflation fully attenuated this association (adjusted OR, 1.10 per 20% predicted lower FEV1; 95% CI, 0.96-1.26; P = .15). In the fully adjusted model, lung hyperinflation was the only pulmonary phenotype with a significant association with clinical CAD.
Lung hyperinflation also was associated with a history of MI similar to history of clinical CAD in the COPDGene cohort (76 COPDGene participants reported a history of MI) (e-Appendix 1).
Results in Participants With Airflow Obstruction
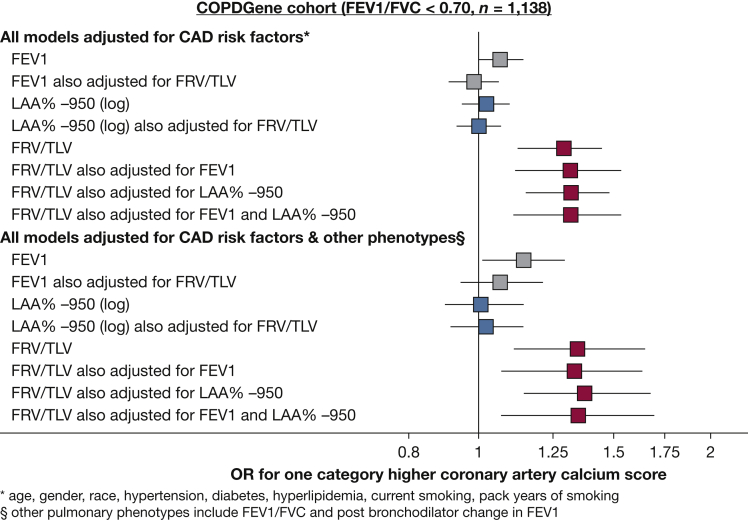
In the COPDGene cohort, lung hyperinflation also was associated with subclinical and clinical CAD in those with airflow obstruction, ie, FEV1 to FVC of < 0.70 (n = 1,248) (Fig 3). Specifically, lung hyperinflation was associated with calcium score in univariate analyses and in multivariate models adjusted for risk factors, FEV1, and other pulmonary phenotypes (Fig 3). Reduced FEV1 was associated with calcium score independent of cardiovascular risk factors; however, adjustment for lung hyperinflation attenuated this association (Fig 3). Similarly, lung hyperinflation was associated with increased clinical CAD and MI in participants with airflow limitation in univariate analyses and in multivariate models adjusted for risk factors, FEV1, and all other pulmonary phenotypes (e-Appendix 1).
Figure 3.
Forrest plots depicting the association of FEV1 (% predicted), emphysema (log LAA%–950), and lung hyperinflation (FRV to TLV ratio) with coronary artery calcium score after adjustment for CAD risk factors (top) and further adjustment for other pulmonary phenotypes (bottom) in participants with airflow obstruction in the COPDGene cohort (n = 1,138). Individuals with a history of clinical CAD were excluded from these models. ORs are calculated per 20% decrease in FEV1 % predicted, a 1-point increase in log LAA%–950, and a 0.20 increase in FRV to TLV ratio. Any association of FEV1 percent predicted (gray box) or emphysema (blue box) with calcium score was attenuated after adjustment for lung hyperinflation. In contrast, lung hyperinflation (red box) was associated with the extent of coronary artery calcium, despite accounting for the FEV1 % predicted, severity of emphysema, other pulmonary phenotypes, and CAD risk factors. CAD = coronary artery disease; COPDGene = Genetic Epidemiology of COPD; FRV = functional residual volume; LAA%–950 = low attenuation area with a –950-Hounsfield unit cutoff; TLV = total lung volume.
In the SCCOR cohort, no pulmonary phenotype showed a statistically significant association with calcium score in individuals with airflow limitation. Specifically, neither lung hyperinflation (P = .36), FEV1 (P = .83), nor emphysema (P = .83) were associated with coronary artery calcium score in models adjusted for other phenotypes and major risk factors. This was possibly because of the relatively low number of individuals with airflow obstruction (n = 239), leading to reduced statistical power in multivariate models.
Discussion
We compared the association of various pulmonary phenotypes with CAD using data from more than 3,000 smokers. Our results suggest that pulmonary phenotypes have a weak to moderate correlation with one another independent of airflow limitation. Our findings indicate that lung hyperinflation is associated with CAD in a manner that may be independent of FEV1 or emphysema. In fact, after lung hyperinflation was accounted for, FEV1 and emphysema were not associated with CAD. These findings were applicable to clinical and subclinical CAD and to current and former smokers with airflow obstruction and were replicated in an unrelated community-based cohort.
If the association between lung hyperinflation and CAD is causal, we can gain insight into mechanisms of coronary vascular injury in smokers. First, lung hyperinflation is an independent predictor of increased left ventricular mass.32 Increase in left ventricular mass in turn has been associated independently with increased coronary artery calcium, clinical CAD, and death in independent studies.33, 34, 35, 36 Some have proposed that increased left ventricular end-diastolic pressure resulting from an increase in left ventricular mass compromises subendocardial blood flow, leading to vascular inflammation and atherosclerosis.36 Alternatively, the coronary circulation may fail to meet increased myocardial oxygen demand because of muscular hypertrophy, resulting in chronic vascular inflammation and atherosclerotic vascular injury.37 Second, lung hyperinflation may impact systemic endothelial function, a well-recognized precursor for coronary atherosclerosis. The high degree of improvement of lung hyperinflation, as compared with FEV1, after lung volume reduction surgery predicts improvement in systemic endothelial function after surgery.38 Finally, lung hyperinflation is associated with decreased sleep efficiency. Decreased sleep efficiency and sleep apnea are highly prevalent in smokers with lung disease and also have been associated with coronary atherosclerosis.39 Our findings suggest that these mechanisms for coronary vascular injury in smokers with lung hyperinflation should be investigated further.
Prior studies of the association between lung and CAD have produced inconsistent results. For example, some have reported an association between calcium score and emphysema.26, 27, 28 In contrast, calcium score was associated with FEV1 % predicted, but not with emphysema, in the Evaluation of COPD Longitudinally to Identify Predictive Surrogate End-points (ECLIPSE) cohort.29 Still others have reported an association between calcium score and emphysema as well as FEV1. Finally, neither emphysema nor FEV1 was associated with calcium score in the Multi-Ethnic Study of Athersclerosis (MESA Lung) Study.30 Our study advances the literature by explaining the contradictory findings of previous reports, ie, all prior studies focused on FEV1 and emphysema and did not assess lung hyperinflation. Specifically, our results suggest that emphysema is associated weakly with calcium score. For example, emphysema was associated with coronary artery calcium in the COPDGene dataset, but not in the SCCOR dataset (Figs 2, 3). Although FEV1 was associated more consistently with calcium scores than emphysema, this association was relatively weak and was fully attenuated after adjusting for lung hyperinflation (Figs 2, 3). In contrast, lung hyperinflation showed highly significant associations with calcium scores in both datasets in all models. Therefore, our findings refine and clarify the association between lung impairment and CAD in smokers. Our study also advances the literature because it is the first study on this topic to demonstrate the reproducibility of results in an independent cohort. A strength of our study is the use of different methodologies to assess lung hyperinflation (RV to TLC ratio in SCCOR vs radiographic FRV to TLV ratio in COPDGene) and calcium scores (Weston score in SCCOR vs Agatston score in COPDGene) in the two cohorts. An association that persists despite using different measures of both the exposure and the outcome likely is more robust than one that is reproducible only when the same methodology has to be used in a second cohort.
For potential clinical use as a novel risk factor for CAD in smokers, lung hyperinflation has some advantages and disadvantages. Its advantages are that the infrastructure to measure lung hyperinflation already exists, is widely available, and is subject to regular quality control per standardized guidelines. Also, its assessment is noninvasive and safe, and the results are understood conceptually by most clinicians. Its major disadvantage is that assessment of lung hyperinflation is more time- and resource-intensive compared with that of FEV1. Therefore, the measurement of lung hyperinflation may be better suited to those with intermediate CAD risk based on current risk stratification algorithms, rather than to all individuals at risk for CAD. Identifying the appropriate subgroup of individuals will require the assessment of lung hyperinflation in cardiovascular cohorts where CAD risk stratification and outcomes have been assessed longitudinally.
Our study has limitations. First, we did not have data to investigate the causality of the association between lung hyperinflation and CAD; longitudinal analysis is needed to demonstrate an association between progression of coronary calcium and hyperinflation. Second, we used two cohorts with some differences in assessment methodology for calcium scores and hyperinflation, which meant that the data could not be pooled and do not confound one another. Next, our cohorts included individuals with mild disease, and our findings should be replicated in those with severe airflow limitation. Similarly, our cohorts did not include never smokers. Further, self-reported history of CAD was assessed, rather than incident coronary vascular events. However, results were identical for calcium scores, suggesting that our findings were not the result of recall bias. Also, individuals who had experienced a cardiovascular event in the preceding year were excluded from the SCCOR cohort, resulting in our inability to analyze cardiovascular events as an end point in this cohort because of the inadequate sample size. Finally, one of the definitions of hypertension was having elevated BP on a single measurement during one study visit. Therefore, we may have overestimated the prevalence of hypertension in our cohorts.
The intent of our analysis is not to suggest that FEV1 should be replaced by lung hyperinflation in the investigation of CAD in smokers. Our results suggest that lung hyperinflation may play a larger role in CAD in smokers than previously appreciated, and therefore, the association between lung hyperinflation and CAD warrants a role in future clinical and translational investigation.
Interpretation
We identified that lung hyperinflation measured via CT scan or via plethysmography is associated with clinical and subclinical CAD in smokers, including those with airflow obstruction. Subsequent studies should consider assessing lung hyperinflation and its associated mechanisms for coronary vascular injury, such as left ventricular remodeling, when investigating CAD in smokers.
Take-home Points.
Study Question: What is the relationship between lung hyperinflation and CAD in smokers?
Results: In two cohort studies that measure hyperinflation using different methodology, lung hyperinflation was associated strongly with clinical and subclinical CAD in smokers, including those with airflow obstruction.
Interpretation: Subsequent studies should consider assessing lung hyperinflation and its associated mechanisms for coronary vascular injury when investigating CAD in smokers.
Acknowledgments
Author contributions: D. C. contributed to conception, data acquisition, design, analysis, and writing; A. G., G. L. K., J. K. L., J. B., G. W., and M. B. contributed to data acquisition and writing; C. R. F., and R. G. B. contributed to data acquisition. J. H. contributed to data acquisition, design, and writing. F. C. S. contributed to conception, data acquisition, design, and writing. D. C. had full access to all the data in this study and takes responsibility for the integrity of the data and accuracy of the data analysis.
Financial/nonfinancial disclosures: None declared.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
∗COPDGene Investigators: Core Units—Administrative Center: James D. Crapo, MD (principal investigator); Edwin K. Silverman, MD, PhD (principal investigator); Barry J. Make, MD; Elizabeth A. Regan, MD, PhD. Genetic Analysis Center: Terri Beaty, PhD; Ferdouse Begum, PhD; Adel R. Boueiz, MD; Peter J. Castaldi, MD, MSc; Michael Cho, MD; Dawn L. DeMeo, MD, MPH; Marilyn G. Foreman, MD, MS; Eitan Halper-Stromberg; Lystra P. Hayden, MD, MMSc; Craig P. Hersh, MD, MPH; Jacqueline Hetmanski, MS, MPH; Brian D. Hobbs, MD, MMSc; John E. Hokanson, MPH, PhD; Nan Laird, PhD; Christoph Lange, PhD; Sharon M. Lutz, PhD; Merry-Lynn McDonald, PhD; Margaret M. Parker, PhD; Dmitry Prokopenko, PhD; Dandi Qiao, PhD; Elizabeth A. Regan, MD, PhD; Phuwanat Sakornsakolpat, MD; Edwin K. Silverman, MD, PhD; Emily S. Wan, MD; Sungho Won, PhD. Imaging Center: Mustafa Al Qaisi, MD; Harvey O. Coxson, PhD; Teresa Gray; MeiLan K. Han, MD, MS; Eric A. Hoffman, PhD; Stephen Humphries, PhD; Francine L. Jacobson, MD, MPH; Philip F. Judy, PhD; Ella A. Kazerooni, MD; Alex Kluiber; David A. Lynch, MB; John D. Newell, Jr., MD; Elizabeth A. Regan, MD, PhD; James C. Ross, PhD; Raul San Jose Estepar, PhD; Joyce Schroeder, MD; Jered Sieren; Douglas Stinson; Berend C. Stoel, PhD; Juerg Tschirren, PhD; Edwin Van Beek, MD, PhD; Bram van Ginneken, PhD; Eva van Rikxoort, PhD; George Washko, MD; Carla G. Wilson, MS. Pulmonary Function Test Quality Assurance Center (Salt Lake City, UT): Robert Jensen, PhD. Data Coordinating Center and Biostatistics (National Jewish Health, Denver, CO): Jim Crooks, PhD; Douglas Everett, PhD; Camille Moore, PhD; Matt Strand, PhD; Carla G. Wilson, MS. Epidemiology Core (University of Colorado Anschutz Medical Campus, Aurora, CO): John E. Hokanson, MPH, PhD; John Hughes, PhD; Gregory Kinney, MPH, PhD; Sharon M. Lutz, PhD; Katherine Pratte, MSPH; Kendra A. Young, PhD. Mortality Adjudication Core: Surya Bhatt, MD; Jessica Bon, MD; MeiLan K. Han, MD, MS; Barry J. Make, MD; Carlos Martinez, MD, MS; Susan Murray, ScD; Elizabeth A. Regan, MD, PhD; Xavier Soler, MD; Carla G. Wilson, MS. Biomarker Core: Farnoush Banaei-Kashani, PhD; Russell P. Bowler, MD, PhD; Katerina Kechris, PhD. Clinical Centers—Ann Arbor VA (Ann Arbor, MI): Jeffrey L. Curtis, MD; Perry G. Pernicano, MD; Baylor College of Medicine (Houston, TX): Nicola Hanania, MD, MS; Mustafa Atik, MD; Aladin Boriek, PhD; Kalpatha Guntupalli, MD; Elizabeth Guy, MD; Amit Parulekar, MD; Brigham and Women’s Hospital (Boston, MA): Dawn L. DeMeo, MD, MPH; Alejandro A. Diaz, MD, MPH; Lystra P. Hayden, MD; Brian D. Hobbs, MD; Craig Hersh, MD, MPH; Francine L. Jacobson, MD, MPH; George Washko, MD; Columbia University (New York, NY): R. Graham Barr, MD, DrPH; John Austin, MD; Belinda D’Souza, MD; Byron Thomashow, MD; Duke University Medical Center (Durham, NC): Neil MacIntyre, Jr., MD; H. Page McAdams, MD; Lacey Washington, MD; HealthPartners Research Institute (Minneapolis, MN): Charlene McEvoy, MD, MPH; Joseph Tashjian, MD; Johns Hopkins University (Baltimore, MD): Robert Wise, MD; Robert Brown, MD; Nadia N. Hansel, MD, MPH; Karen Horton, MD; Allison Lambert, MD, MHS; Nirupama Putcha, MD, MHS; Los Angeles Biomedical Research Institute at Harbor UCLA Medical Center (Torrance, CA): Richard Casaburi, PhD, MD; Alessandra Adami, PhD; Matthew Budoff, MD; Hans Fischer, MD; Janos Porszasz, MD, PhD; Harry Rossiter, PhD; William Stringer, MD; Michael E. DeBakey; VAMC (Houston, TX): Amir Sharafkhaneh, MD, PhD; Charlie Lan, DO; Minneapolis VA (Minneapolis, MN): Christine Wendt, MD; Brian Bell, MD; Ken M. Kunisaki, MD, MS; Morehouse School of Medicine (Atlanta, GA): Marilyn G. Foreman, MD, MS; Eugene Berkowitz, MD, PhD; Gloria Westney, MD, MS; National Jewish Health (Denver, CO): Russell Bowler, MD, PhD; David A. Lynch, MB; Reliant Medical Group (Worcester, MA): Richard Rosiello, MD; David Pace, MD; Temple University (Philadelphia, PA): Gerard Criner, MD; David Ciccolella, MD; Francis Cordova, MD; Chandra Dass, MD; Gilbert D’Alonzo, DO; Parag Desai, MD; Michael Jacobs, PharmD; Steven Kelsen, MD, PhD; Victor Kim, MD; A. James Mamary, MD; Nathaniel Marchetti, DO; Aditi Satti, MD; Kartik Shenoy, MD; Robert M. Steiner, MD; Alex Swift, MD; Irene Swift, MD; Maria Elena Vega-Sanchez, MD; University of Alabama (Birmingham, AL): Mark Dransfield, MD; William Bailey, MD; Surya P. Bhatt, MD; Anand Iyer, MD; Hrudaya Nath, MD; J. Michael Wells, MD; University of California, San Diego (La Jolla, CA): Joe Ramsdell, MD; Paul Friedman, MD; Xavier Soler, MD, PhD; Andrew Yen, MD; University of Iowa (Iowa City, IA): Alejandro P. Comellas, MD; Karin F. Hoth, PhD; John Newell, Jr., MD; Brad Thompson, MD; University of Michigan (Ann Arbor, MI): MeiLan K. Han, MD, MS; Ella Kazerooni, MD; Carlos H. Martinez, MD, MPH; University of Minnesota (Minneapolis, MN): Joanne Billings, MD; Abbie Begnaud, MD; Tadashi Allen, MD; University of Pittsburgh (Pittsburgh, PA): Frank Sciurba, MD; Jessica Bon, MD; Divay Chandra, MD, MSc; Carl Fuhrman, MD; Joel Weissfeld, MD, MPH; University of Texas Health Science Center at San Antonio (San Antonio, TX): Antonio Anzueto, MD; Sandra Adams, MD; Diego Maselli-Caceres, MD; Mario E. Ruiz, MD.
Other contributions: The authors thank Subashan Perera and Seyed Mehdi Nouraie for their insightful comments regarding the analysis of the datasets included in this article.
Additional information: The e-Appendix, e-Figures, and e-Table can be found in the Supplemental Materials section of the online article.
Footnotes
Drs Chandra and Gupta contributed equally to this manuscript.
FUNDING/SUPPORT: This research was supported in part by the National Institutes of Health [Grants 1K23HL126912 and R01 HL153400-01 (D. C.), HL084948 (F. C. S.), HHSN268201100019C (F. C. S.), 7RC2HL101715 (F. C. S.), and SAP 4100062224 (F. C. S.)]. The COPDGene study is supported by the National Heart, Lung, and Blood Institute [Grants R01HL089897 and R01HL089856]. The COPDGene project is also supported by the COPD Foundation through contributions made to an Industry Advisory Board comprising AstraZeneca, Boehringer Ingelheim, Novartis, Pfizer, Siemens, Sunovion, and GlaxoSmithKline. Coronary artery calcium scoring in the COPDGene study is supported by the Tobacco-Related Disease Research Program [Grant 20XT-0014].
Contributor Information
Frank C. Sciurba, Email: sciurbafc@upmc.edu.
COPDGene Investigators:
James D. Crapo, Edwin K. Silverman, Barry J. Make, Elizabeth A. Regan, Terri Beaty, Ferdouse Begum, Adel R. Boueiz, Peter J. Castaldi, Michael Cho, Dawn L. DeMeo, Marilyn G. Foreman, Eitan Halper-Stromberg, Lystra P. Hayden, Craig P. Hersh, Jacqueline Hetmanski, Brian D. Hobbs, John E. Hokanson, Nan Laird, Christoph Lange, Sharon M. Lutz, Merry-Lynn McDonald, Margaret M. Parker, Dmitry Prokopenko, Dandi Qiao, Elizabeth A. Regan, Phuwanat Sakornsakolpat, Edwin K. Silverman, Emily S. Wan, Sungho Won, Mustafa Al Qaisi, Harvey O. Coxson, Teresa Gray, MeiLan K. Han, Eric A. Hoffman, Stephen Humphries, Francine L. Jacobson, Philip F. Judy, Ella A. Kazerooni, Alex Kluiber, David A. Lynch, John D. Newell, Jr., Elizabeth A. Regan, James C. Ross, Raul San Jose Estepar, Joyce Schroeder, Jered Sieren, Douglas Stinson, Berend C. Stoel, Juerg Tschirren, Edwin Van Beek, Bram van Ginneken, Eva van Rikxoort, George Washko, Carla G. Wilson, Robert Jensen, Jim Crooks, Douglas Everett, Camille Moore, Strand, Carla G. Wilson, John E. Hokanson, John Hughes, Gregory Kinney, Sharon M. Lutz, Katherine Pratte, Kendra A. Young, Surya Bhatt, Jessica Bon, MeiLan K. Han, Barry J. Make, Carlos Martinez, Susan Murray, Elizabeth A. Regan, Xavier Soler, Carla G. Wilson, Farnoush Banaei-Kashani, Russell P. Bowler, Katerina Kechris, Jeffrey L. Curtis, Perry G. Pernicano, Nicola Hanania, Mustafa Atik, Aladin Boriek, Kalpatha Guntupalli, Elizabeth Guy, Amit Parulekar, Dawn L. DeMeo, Alejandro A. Diaz, Lystra P. Hayden, Brian D. Hobbs, Craig Hersh, Francine L. Jacobson, George Washko, R. Graham Barr, John Austin, Belinda D’Souza, Byron Thomashow, Neil MacIntyre, Jr., H. Page McAdams, Lacey Washington, Charlene McEvoy, Joseph Tashjian, Robert Wise, Robert Brown, Nadia N. Hansel, Karen Horton, Allison Lambert, Nirupama Putcha, Richard Casaburi, Alessandra Adami, Matthew Budoff, Hans Fischer, Janos Porszasz, Harry Rossiter, William Stringer, Michael E. DeBakey, Amir Sharafkhaneh, Charlie Lan, Christine Wendt, Brian Bell, Ken M. Kunisaki, Marilyn G. Foreman, Eugene Berkowitz, Gloria Westney, Russell Bowler, David A. Lynch, Richard Rosiello, David Pace, Gerard Criner, David Ciccolella, Francis Cordova, Chandra Dass, Gilbert D’Alonzo, Parag Desai, Michael Jacobs, Steven Kelsen, Victor Kim, A. James Mamary, Nathaniel Marchetti, Aditi Satti, Kartik Shenoy, Robert M. Steiner, Alex Swift, Irene Swift, Maria Elena Vega-Sanchez, Mark Dransfield, William Bailey, Surya P. Bhatt, Anand Iyer, Hrudaya Nath, J. Michael Wells, Joe Ramsdell, Paul Friedman, Xavier Soler, Andrew Yen, Alejandro P. Comellas, Karin F. Hoth, John Newell, Jr., Brad Thompson, MeiLan K. Han, Ella Kazerooni, Carlos H. Martinez, Joanne Billings, Abbie Begnaud, Tadashi Allen, Frank Sciurba, Jessica Bon, Divay Chandra, Carl Fuhrman, Joel Weissfeld, Antonio Anzueto, Sandra Adams, Diego Maselli-Caceres, and Mario E. Ruiz
Supplementary Data
References
- 1.Sorlie P.D., Kannel W.B., O’Connor G. Mortality associated with respiratory function and symptoms in advanced age. The Framingham Study. Am Rev Respir Dis. 1989;140(2):379–384. doi: 10.1164/ajrccm/140.2.379. [DOI] [PubMed] [Google Scholar]
- 2.Han M.K., Agusti A., Calverley P.M., Celli B.R., Criner G., Curtis J.L. Chronic obstructive pulmonary disease phenotypes: the future of COPD. Am J Respir Crit Care Med. 2010;182(5):598–604. doi: 10.1164/rccm.200912-1843CC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miravitlles M., Calle M., Soler-Cataluna J.J. Clinical phenotypes of COPD: identification, definition and implications for guidelines. Arch Bronconeumol. 2012;48(3):86–98. doi: 10.1016/j.arbres.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 4.Vestbo J. COPD: definition and phenotypes. Clin Chest Med. 2014;35(1):1–6. doi: 10.1016/j.ccm.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 5.Hogg J.C., Chu F., Utokaparch S. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(26):2645–2653. doi: 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- 6.Bon J, Fuhrman CR, Weissfeld JL, et al. Radiographic emphysema predicts low bone mineral density in a tobacco exposed cohort. Am J Respir Crit Care Med. 2011;183(7):885-890. [DOI] [PMC free article] [PubMed]
- 7.Regan E.A., Hokanson J.E., Murphy J.R. Genetic epidemiology of COPD (COPDGene) study design. COPD. 2010;7(1):32–43. doi: 10.3109/15412550903499522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hankinson J.L., Odencrantz J.R., Fedan K.B. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159(1):179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 9.Macintyre N., Crapo R.O., Viegi G. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J. 2005;26(4):720–735. doi: 10.1183/09031936.05.00034905. [DOI] [PubMed] [Google Scholar]
- 10.Yamashiro T., Matsuoka S., Bartholmai B.J. Collapsibility of lung volume by paired inspiratory and expiratory CT scans: correlations with lung function and mean lung density. Acad Radiol. 2010;17(4):489–495. doi: 10.1016/j.acra.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng B., Leader J.K., McMurray J.M. Automated detection and quantitative assessment of pulmonary airways depicted on CT images. Med Phys. 2007;34(7):2844–2852. doi: 10.1118/1.2742777. [DOI] [PubMed] [Google Scholar]
- 12.Chandra D., Stamm J.A., Palevsky P.M. The relationship between pulmonary emphysema and kidney function in smokers. Chest. 2012;142(3):655–662. doi: 10.1378/chest.11-1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim V., Desai P., Newell J.D. Airway wall thickness is increased in COPD patients with bronchodilator responsiveness. Respir Res. 2014;15:84. doi: 10.1186/s12931-014-0084-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim Y.K., Sung Y.M., Cho S.H., Park Y.N., Choi H.Y. Reliability analysis of visual ranking of coronary artery calcification on low-dose CT of the thorax for lung cancer screening: comparison with ECG-gated calcium scoring CT. Int J Cardiovasc Imaging. 2014;30(suppl 2):81–87. doi: 10.1007/s10554-014-0507-8. [DOI] [PubMed] [Google Scholar]
- 15.Budoff M.J., Nasir K., Kinney G.L. Coronary artery and thoracic calcium on noncontrast thoracic CT scans: comparison of ungated and gated examinations in patients from the COPD Gene cohort. J Cardiovasc Comput Tomogr. 2011;5(2):113–118. doi: 10.1016/j.jcct.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kirsch J., Buitrago I., Mohammed T.L., Gao T., Asher C.R., Novaro G.M. Detection of coronary calcium during standard chest computed tomography correlates with multi-detector computed tomography coronary artery calcium score. Int J Cardiovasc Imaging. 2012;28(5):1249–1256. doi: 10.1007/s10554-011-9928-9. [DOI] [PubMed] [Google Scholar]
- 17.Watts J.R., Jr., Sonavane S.K., Snell-Bergeon J., Nath H. Visual scoring of coronary artery calcification in lung cancer screening computed tomography: association with all-cause and cardiovascular mortality risk. Coron Artery Dis. 2015;26(2):157–162. doi: 10.1097/MCA.0000000000000189. [DOI] [PubMed] [Google Scholar]
- 18.Einstein A.J., Johnson L.L., Bokhari S. Agreement of visual estimation of coronary artery calcium from low-dose CT attenuation correction scans in hybrid PET/CT and SPECT/CT with standard Agatston score. J Am Coll Cardiol. 2010;56(23):1914–1921. doi: 10.1016/j.jacc.2010.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agatston A.S., Janowitz W.R., Hildner F.J., Zusmer N.R., Viamonte M., Jr., Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15(4):827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 20.Chandra D., Gupta A., Leader J.K. Assessment of coronary artery calcium by chest CT compared with EKG-gated cardiac CT in the multicenter AIDS cohort study. PLoS One. 2017;12(4) doi: 10.1371/journal.pone.0176557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhatt S.P., Kazerooni E.A., Newell J.D., Jr. Visual estimate of coronary artery calcium predicts cardiovascular disease in COPD. Chest. 2018;154(3):579–587. doi: 10.1016/j.chest.2018.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taylor-Piliae R.E., Norton L.C., Haskell W.L. Validation of a new brief physical activity survey among men and women aged 60-69 years. Am J Epidemiol. 2006;164(6):598–606. doi: 10.1093/aje/kwj248. [DOI] [PubMed] [Google Scholar]
- 23.Block G., Gillespie C., Rosenbaum E.H., Jenson C. A rapid food screener to assess fat and fruit and vegetable intake. Am J Prev Med. 2000;18(4):284–288. doi: 10.1016/s0749-3797(00)00119-7. [DOI] [PubMed] [Google Scholar]
- 24.Beck A.T., Steer R.A., Ball R., Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J Pers Assess. 1996;67(3):588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- 25.Diaz A.A., Young T.P., Kurugol S. Abdominal visceral adipose tissue is associated with myocardial infarction in patients with COPD. Chronic Obstr Pulm Dis (Miami) 2015;2(1):8–16. doi: 10.15326/jcopdf.2.1.2015.0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O’Hare P.E., Ayres J.F., O’Rourke R.L. Coronary artery calcification on computed tomography correlates with mortality in chronic obstructive pulmonary disease. J Comput Assist Tomogr. 2014;38(5):753–759. doi: 10.1097/RCT.0000000000000119. [DOI] [PubMed] [Google Scholar]
- 27.Alhaj E.K., Alhaj N.E., Bergmann S.R. Coronary artery calcification and emphysema. Can J Cardiol. 2008;24(5):369–372. doi: 10.1016/s0828-282x(08)70598-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhatt S.P., Nath H.P., Kim Y.I. Centrilobular emphysema and coronary artery calcification: mediation analysis in the SPIROMICS cohort. Respir Res. 2018;19(1):257. doi: 10.1186/s12931-018-0946-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williams M.C., Murchison J.T., Edwards L.D. Coronary artery calcification is increased in patients with COPD and associated with increased morbidity and mortality. Thorax. 2014;69(8):718–723. doi: 10.1136/thoraxjnl-2012-203151. [DOI] [PubMed] [Google Scholar]
- 30.Barr R.G., Ahmed F.S., Carr J.J. Subclinical atherosclerosis, airflow obstruction and emphysema: the MESA Lung Study. Eur Respir J. 2012;39(4):846–854. doi: 10.1183/09031936.00165410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kattan M.W., Gerds T.A. The index of prediction accuracy: an intuitive measure useful for evaluating risk prediction models. Diagn Progn Res. 2018;2:7. doi: 10.1186/s41512-018-0029-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith B.M., Kawut S.M., Bluemke D.A. Pulmonary hyperinflation and left ventricular mass: the Multi-Ethnic Study of Atherosclerosis COPD Study. Circulation. 2013;127(14):1503–1511. doi: 10.1161/CIRCULATIONAHA.113.001653. 1511e1501-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tang W., Arnett D.K., Province M.A. Racial differences in the association of coronary calcified plaque with left ventricular hypertrophy: the National Heart, Lung, and Blood Institute Family Heart Study and Hypertension Genetic Epidemiology Network. Am J Cardiol. 2006;97(10):1441–1448. doi: 10.1016/j.amjcard.2005.11.076. [DOI] [PubMed] [Google Scholar]
- 34.Altunkan S., Erdogan N., Altin L., Budoff M.J. Relation of coronary artery calcium to left ventricular mass and geometry in patients with essential hypertension. Blood Press Monit. 2003;8(1):9–15. doi: 10.1097/00126097-200302000-00002. [DOI] [PubMed] [Google Scholar]
- 35.Mehta S.K., Rame J.E., Khera A. Left ventricular hypertrophy, subclinical atherosclerosis, and inflammation. Hypertension. 2007;49(6):1385–1391. doi: 10.1161/HYPERTENSIONAHA.107.087890. [DOI] [PubMed] [Google Scholar]
- 36.Houghton J.L., Prisant L.M., Carr A.A., von Dohlen T.W., Frank M.J. Relationship of left ventricular mass to impairment of coronary vasodilator reserve in hypertensive heart disease. Am Heart J. 1991;121(4 Pt 1):1107–1112. doi: 10.1016/0002-8703(91)90669-9. [DOI] [PubMed] [Google Scholar]
- 37.O’Gorman D.J., Sheridan D.J. Abnormalities of the coronary circulation associated with left ventricular hypertrophy. Clin Sci (Lond) 1991;81(6):703–713. doi: 10.1042/cs0810703. [DOI] [PubMed] [Google Scholar]
- 38.Clarenbach C.F., Sievi N.A., Brock M., Schneiter D., Weder W., Kohler M. LVRS improves endothelial function and blood pressure in patients with COPD: a randomized-controlled trial. Am J Respir Crit Care Med. 2015;192(3):307–314. doi: 10.1164/rccm.201503-0453OC. [DOI] [PubMed] [Google Scholar]
- 39.Kwon J.S., Wolfe L.F., Lu B.S., Kalhan R. Hyperinflation is associated with lower sleep efficiency in COPD with co-existent obstructive sleep apnea. COPD. 2009;6(6):441–445. doi: 10.3109/15412550903433000. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.