Abstract
Background
Metabolic syndrome and insulin resistance are associated with worsened outcomes of chronic lung disease. The triglyceride-glucose index (TyG), a measure of metabolic dysfunction, is associated with metabolic syndrome and insulin resistance, but its relationship to lung health is unknown.
Research Question
What is the relationship of TyG to respiratory symptoms, chronic lung disease, and lung function?
Study Design and Methods
This study analyzed data from the National Health and Nutrition Examination Survey from 1999 to 2012. Participants included fasting adults age ≥ 40 years (N = 6,893) with lung function measurements in a subset (n = 3,383). Associations of TyG with respiratory symptoms (cough, phlegm production, wheeze, and exertional dyspnea), chronic lung disease (diagnosed asthma, chronic bronchitis, and emphysema), and lung function (FEV1, FVC, and obstructive or restrictive spirometry pattern) were evaluated, adjusting for sociodemographic variables, comorbidities, and smoking. TyG was compared vs insulin resistance, represented by the homeostatic model assessment of insulin resistance (HOMA-IR), and vs the metabolic syndrome.
Results
TyG was moderately correlated with HOMA-IR (Spearman ρ = 0.51) and had good discrimination for metabolic syndrome (area under the receiver-operating characteristic curve, 0.80). A one-unit increase in TyG was associated with higher odds of cough (adjusted OR [aOR], 1.28; 95% CI, 1.06-1.54), phlegm production (aOR, 1.20; 95% CI, 1.01-1.43), wheeze (aOR, 1.18; 95% CI, 1.03-1.35), exertional dyspnea (aOR, 1.21; 95% CI, 1.07-1.38), and a diagnosis of chronic bronchitis (aOR, 1.21; 95% CI, 1.02-1.43). TyG was associated with higher relative risk of a restrictive spirometry pattern (adjusted relative risk ratio, 1.45; 95% CI, 1.11-1.90). Many associations were maintained with additional adjustment for HOMA-IR or metabolic syndrome.
Interpretation
TyG was associated with respiratory symptoms, chronic bronchitis, and a restrictive spirometry pattern. Associations were not fully explained by insulin resistance or metabolic syndrome. TyG is a satisfactory measure of metabolic dysfunction with relevance to pulmonary outcomes. Prospective study to define TyG as a biomarker for impaired lung health is warranted.
Key Words: dyslipidemia, insulin resistance, lung health, metabolic disease, spirometry, triglyceride-glucose index
Abbreviations: HOMA-IR, homeostatic model assessment of insulin resistance; NHANES, National Health and Nutrition Examination Survey; PIR, poverty-income ratio; TyG, triglyceride-glucose index
FOR EDITORIAL COMMENT, SEE PAGE 801
The role of metabolism in chronic lung disease is an area of active scrutiny.1 Diabetes, dyslipidemia, and the metabolic syndrome, a cluster of comorbidities underpinned by insulin resistance, have been variously associated with increased incidence, prevalence, or severity of COPD, asthma, and pulmonary fibrosis, leading to speculation that they may directly affect the lung.2, 3, 4, 5, 6, 7 Accurate characterization of metabolic dysfunction is important in delineating its role in precipitating and worsening chronic lung disease.
Both insulin resistance and metabolic syndrome are challenging to measure outside of the research setting. The gold standard assessment of insulin resistance is the hyperinsulinemic-euglycemic clamp, a time-consuming and invasive procedure.8 Laboratory surrogates, such as the homeostatic model assessment of insulin resistance (HOMA-IR), require direct measurement of insulin, which is often impractical to apply in epidemiologic settings. Similarly, accurate detection of metabolic syndrome requires the integration of a matrix of several laboratory and physical examination measurements and, as a dichotomous measure, does not allow investigation into degrees of risk.
The triglyceride-glucose index (TyG) is a measure of metabolic dysfunction calculated by a spot measurement of triglycerides and glucose from fasting blood.9 These assays are standardized and routinely ordered in clinical settings. In several studies, TyG performed similarly to HOMA-IR in measuring insulin resistance compared with the hyperinsulinemic-euglycemic clamp, but it was more accurate than HOMA-IR in the diagnosis of metabolic syndrome, highlighting its value as a more general measure of metabolic disease.10, 11, 12 TyG has been shown to strongly associate with cardiovascular outcomes and incident diabetes, but its relationship to the lung has never been investigated.13, 14, 15, 16
We therefore sought to examine the association between TyG and lung health, which we defined as a spectrum of symptoms and outcomes that may relate to impaired lung function. We examined the association of TyG with: (1) respiratory symptoms; (2) presence of self-reported chronic lung disease; (3) and lung function determined by spirometry within a cross-sectional study of adult participants recruited from the general population. We hypothesized that TyG would be associated with a higher prevalence of respiratory symptoms, chronic lung disease, and impaired lung function. The performance of TyG vs HOMA-IR and metabolic syndrome for these same outcomes was also compared.
Methods
Study Population
This was an observational population-based study using the National Health and Nutrition Examination Survey (NHANES), a national survey of children and adults in the United States conducted by the National Center for Health Statistics of the Centers for Disease Control and Prevention.17 All noninstitutionalized adults residing in the United States, excluding active-duty military or their families, were eligible for inclusion. Invited participants underwent physical examination, laboratory assessment, and answered questionnaires related to health and nutrition. The sampling design allows estimates to be generalizable to the US population. NHANES was conducted with approval by the National Center for Health Statistics Ethics Review Board, and all participants gave written informed consent. This analysis was determined as not human subjects research by the Baylor College of Medicine Institutional Review Board.
The current iteration of NHANES, NHANES Continuous, consists of 2-year sampling cycles beginning in 1999. Fasting blood was collected from a subsample of participants who were scheduled for the morning examination session. Respiratory symptom questionnaires were administered to participants ≥ 40 years old, and spirometry was performed from 2007 to 2012.
The current study included all adult participants ≥ 40 years old from NHANES 1999 to 2012 who were in the fasting subsample (e-Fig 1). Analysis of spirometry data was restricted to the years in which this test was done (2007-2012) with spirometry-specific exclusion criteria as described later.
Exposure Definitions
Plasma glucose and serum triglyceride levels were measured enzymatically by using an autoanalyzer. Serum insulin was measured by radioimmunoassay (1999-2002), chemiluminescent enzyme immunoassay (2003-2004 and 2009-2012), and enzyme-linked immunosorbent assay (2005-2009). We applied all provided equations to correct for measurement drift from any technique or instrumentation changes between cycles. Laboratory measurements were performed under centralized quality assurance and control protocols.17
TyG was calculated as ln[fasting glucose × fasting triglycerides/2], both measured in milligrams per deciliter,9 and HOMA-IR as (fasting insulin [in milliunits per liter] × fasting glucose [in millimoles per liter])/22.5.18 Metabolic syndrome was defined by using the criteria harmonized in 2009 by an international consortium of organizations, including the International Diabetes Federation, the National Heart, Lung, and Blood Institute, and the American Heart Association.19 These required the presence of at least three of five components inclusive of elevated abdominal circumference, hypertension, hyperglycemia, elevated triglycerides, and reduced high-density lipoprotein. Operationalized definitions within NHANES have been described elsewhere.20
Outcome Definitions
We examined questions related to respiratory symptoms (cough, phlegm production, wheeze, and exertional dyspnea) and chronic lung disease (asthma, bronchitis, and emphysema). Presence of cough was determined by “Do you usually cough on most days for 3 consecutive months or more during the year?” Phlegm production was determined by “Do you bring up phlegm on most days for 3 consecutive months or more during the year?” Wheeze was determined by “In the past 12 months have you had wheezing or whistling in your chest?” Exertional dyspnea was assessed by “Have you had shortness of breath either when hurrying on the level or walking up a slight hill?” With exception of wheeze, these questions were only asked in adults ≥ 40 years old. A symptom count variable was created based on the number of distinct respiratory symptoms reported.
With respect to the presence of chronic lung disease, participants were asked “Has a doctor or other health professional ever told you that you had asthma?” This was repeated for chronic bronchitis and emphysema.
Lung Function Measures
Participants aged 6 to 79 years from NHANES 2007 to 2012 were invited to undergo spirometry. Major exclusion criteria included use of supplemental oxygen; recent eye, chest, or abdominal surgery; and recent heart attack or stroke. Inclusion and exclusion criteria are described in greater detail elsewhere.21 Pre-bronchodilator spirometry was performed by using Ohio 822/827 dry-rolling volume seal spirometers according to American Thoracic Society guidelines.22 The FEV1 and FVC were measured. Only maneuvers of American Thoracic Society quality grade C or above (at least partially meeting standards) were used. Raw values were converted to percent-predicted based on the Hankinson equations.23 An obstructive spirometry pattern was defined by an FEV1/FVC < 0.70 and a restrictive spirometry pattern by an FVC < 80% of predicted with FEV1/FVC ≥ 0.70.24
Covariate Definitions
We obtained demographic data, including age, sex, race/ethnicity, and poverty-income ratio (PIR). The PIR ranged from 0 (no family income) to 5 (family income at least five times the federal poverty level). We determined the presence of cardiovascular disease by patient-reported history of heart attack, stroke, congestive heart failure, coronary artery disease, or angina. In addition, measures of physical examination were obtained, including height, weight, abdominal circumference, and BP. Because smoking may strongly confound the relationship between TyG and lung health, smoking status (never, former, and current) and years of smoking were examined. Few participants (n = 7) could not recall if they smoked > 100 cigarettes in their lifetime and were assumed to be nonsmokers; exclusion of these participants did not meaningfully modify the results.
Data Analysis
Population-weighted descriptive statistics were calculated. TyG was compared vs HOMA-IR by using the Spearman correlation and vs the metabolic syndrome by calculation of the area under a receiver-operating characteristic curve generated without covariate adjustment. Due to skewness, HOMA-IR was subsequently log-transformed for modeling.
We assumed that data were missing at random and performed multiple imputation of missing covariates by chained equations. Predictor variables included all covariates from the main analysis, survey weight, and unique groupings of sampling strata and primary sampling unit. Ten imputed datasets were generated and pooled to obtain the overall result.
Logistic and linear models were used to estimate associations of TyG, HOMA-IR, and presence of metabolic syndrome with dichotomous and continuous outcomes, respectively. Test for trend in the association of respiratory symptom count and TyG was performed by treating symptom count as a continuous variable in a multivariable linear regression model in which TyG was the dependent variable. Multinomial logistic regression was used to estimate the relative risk ratio between TyG and an obstructive or restrictive spirometry pattern, with a normal pattern as the referent group. Analyses were adjusted for age, sex, race/ethnicity, BMI, PIR, cardiovascular disease, smoking status, smoking years,25 and fasting duration. These covariates were selected because we considered them to be clinically relevant confounders of the association between metabolism and respiratory outcomes. Subgroup analyses according to sex and race/ethnicity (Hispanic, non-Hispanic White, and non-Hispanic Black) were performed. All regressions accounted for survey weights. Statistical significance was assessed at a two-sided P value < .05. Analysis was performed in Stata 15 (StataCorp).
Results
Study Population
A total of 6,893 participants met inclusion criteria and had available exposure and outcome data (e-Fig 1). Multiple imputation was applied to 536 (8%) participants who were missing the PIR, 272 (4%) who were missing number of smoking years, and 18 (< 1%) who were missing BMI data. Population-weighted mean age was 56.9 years, 52.7% were female, and approximately three-fourths were white (Table 1). There was a high proportion of overweight/obesity. More than one-half of the population was hypertensive or had prediabetes and diabetes (fulfilling metabolic syndrome’s hyperglycemia criteria). Overall, 55.4% of the population had metabolic syndrome.
Table 1.
Participant Characteristics and Study Measurements (N = 6,893)
| Characteristic | Value |
|---|---|
| Age, mean (95% CI), y | 56.9 (56.5-57.4) |
| Female sex, % (SE) | 52.7 (0.65) |
| Ethnicity, % (SE) | |
| Mexican American | 5.8 (0.68) |
| Other Hispanic | 3.7 (0.50) |
| Non-Hispanic White | 75.0 (1.44) |
| Non-Hispanic Black | 9.9 (0.79) |
| Other | 5.6 (0.47) |
| Poverty-income ratio, mean (95% CI) | 3.18 (3.09-3.27) |
| BMI, mean (95% CI), kg/m2 | 29.0 (28.8-29.3) |
| Smoking status, % (SE) | |
| Never smoker | 49.7 (0.95) |
| Former smoker | 31.2 (0.85) |
| Current smoker | 19.2 (0.84) |
| Years smoked, mean (95% CI) | 13.2 (12.6-13.8) |
| Spirometry, mean (95% CI)a | |
| FEV1pp, % | 94.5 (93.5-95.6) |
| FVCpp, % | 97.7 (96.8-98.7) |
| FEV1/FVC, % | 75.3 (74.8-75.7) |
| Spirometry pattern, % (SE)a | |
| Obstructive | 21.2 (1.1) |
| Restrictive | 6.9 (0.7) |
| Metabolic parameters, % (SE) | |
| Elevated waist circumference | 62.5 (0.88) |
| Hypertriglyceridemia | 49.4 (0.88) |
| Decreased high-density lipoprotein | 43.2 (0.80) |
| Hypertension | 60.4 (1.00) |
| Hyperglycemia | 56.4 (1.07) |
| Metabolic syndrome, % (SE) | 55.4 (1.04) |
| TyG, mean (95% CI) | 8.76 (8.74-8.79) |
| HOMA-IR, mean (95% CI) | 3.22 (3.05-3.38) |
| Cardiovascular disease, % (SE) | 12.8 (0.60) |
| Respiratory symptoms, % (SE) | |
| Cough | 11.4 (0.63) |
| Phlegm production | 9.6 (0.44) |
| Wheeze | 15.6 (0.63) |
| Exertional dyspnea | 34.2 (1.03) |
| No. of distinct symptoms, % (SE) | |
| 1 | 25.9 (0.75) |
| 2 | 11.2 (0.64) |
| 3 | 4.3 (0.35) |
| 4 | 2.4 (0.23) |
| Chronic lung disease, % (SE) | |
| Asthma | 12.8 (0.56) |
| Chronic bronchitis | 7.4 (0.58) |
| Emphysema | 2.9 (0.25) |
FEV1pp = percent-predicted FEV1; FVCpp = percent-predicted FVC; HOMA-IR = homeostatic model assessment of insulin resistance; TyG = triglyceride-glucose index.
n = 3,383.
There was a substantial prevalence of cough (11.4%), phlegm production (9.6%), wheeze (15.6%), and exertional dyspnea (34.2%). With respect to chronic lung disease, 12.8% of participants reported a history of asthma. The prevalence of self-reported chronic bronchitis (7.4%) and emphysema (2.9%) was lower than the prevalence of airway obstruction detected by spirometry (21.2%). A minority of participants (6.9%) had a restrictive pattern on spirometry.
Comparison of TyG to HOMA-IR and Metabolic Syndrome
Within the analytic sample, TyG ranged from 6.79 to 12.55, with a mean ± SD of 8.8 ± 0.64. TyG was moderately correlated with HOMA-IR, corresponding to a population-weighted Spearman ρ of 0.51. e-Figure 2 shows participant-level TyG measurements plotted against log-transformed HOMA-IR.
Conversely, TyG exhibited good-to-excellent discrimination for the metabolic syndrome. e-Figure 3 shows the population-weighted receiver-operating characteristic curve with an area under the curve (95% CI) of 0.80 (0.78-0.81). In this study, a TyG of 8.65 had 74% sensitivity and 70% specificity for the metabolic syndrome.
Associations of TyG, HOMA-IR, and Metabolic Syndrome With Study Outcomes
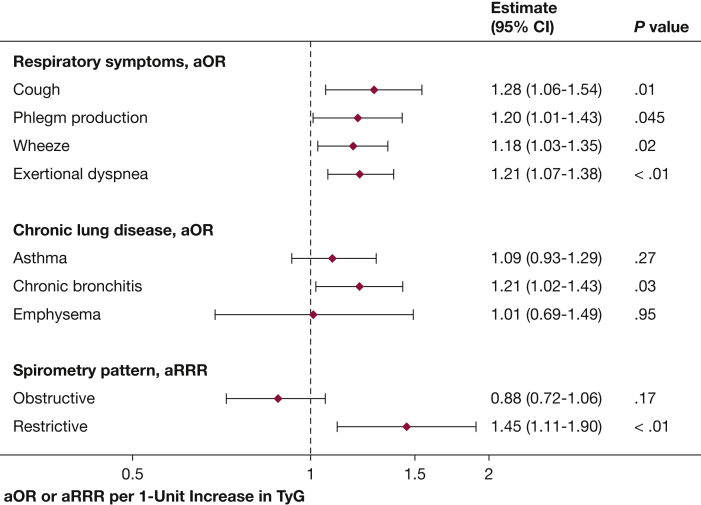
With respect to self-reported respiratory symptoms, in adjusted analysis, associations were identified between TyG with cough, phlegm, wheeze, and exertional dyspnea (Fig 1). For every one-unit increase in TyG, the odds of cough were 28% higher, phlegm production 20% higher, wheezing 18% higher, and exertional dyspnea 21% higher. Higher TyG was also associated with a higher number of distinct respiratory symptoms (P value for linear trend, < .01) (e-Fig 4). With respect to self-reported chronic lung disease, higher TyG was associated with 21% higher odds of a diagnosis of chronic bronchitis but not of emphysema or asthma.
Figure 1.
Association of TyG with study outcomes. Shown are prevalence ORs or relative risk ratios for each outcome for every one-unit increase in TyG, adjusted for age, sex, race/ethnicity, BMI, poverty-income ratio, cardiovascular disease, smoking status, smoking years, and fasting time. aOR = adjusted OR; aRRR = adjusted relative risk ratio; TyG = triglyceride-glucose index.
A subset of 3,383 participants provided acceptable pre-bronchodilator spirometry data. In adjusted analysis, a one-unit increase in TyG was associated with lower percent predicted FEV1 (adjusted mean difference, –1.5%; 95% CI, –2.8 to –0.26) and percent predicted FVC (adjusted mean difference, –2.2%; 95% CI, –3.4 to –1.0). Consequently, higher TyG was associated with a higher relative risk of a restrictive spirometry pattern (adjusted relative risk ratio, 1.45; 95% CI, 1.11 to 1.90) but not with obstruction (adjusted relative risk ratio, 0.88; 95% CI, 0.72 to 1.06). Adjustment for waist circumference instead of BMI did not meaningfully alter the association of TyG with higher relative risk of restrictive spirometry (adjusted relative risk ratio, 1.34; 95% CI, 1.02 to 1.76).
We found no consistent evidence of effect measure modification by sex (e-Table 1) or race/ethnicity (e-Table 2). Compared with non-Hispanic White participants, associations of TyG with study outcomes were less apparent in Hispanic and non-Hispanic Black participants, but CIs were wide and overlapping, which precluded any meaningful inference.
Comparisons with HOMA-IR and metabolic syndrome are included in e-Table 3. Log-transformed HOMA-IR was only associated with higher odds of exertional dyspnea, and metabolic syndrome was associated with higher odds of cough, wheeze, and exertional dyspnea but not of phlegm production. There were no associations between HOMA-IR and metabolic syndrome with any of the queried conditions or with spirometry pattern, although the relationship between metabolic syndrome and restrictive spirometry was marginally statistically insignificant.
Associations of TyG With Study Outcomes Independent of HOMA-IR or the Metabolic Syndrome
Because TyG seemed more strongly associated with study outcomes than either HOMA-IR or the metabolic syndrome, we next tested if associations of TyG with study outcomes were independent of these other measures. With additional adjustment for HOMA-IR, TyG continued to be associated with respiratory symptoms but not spirometry (Table 2). With additional adjustment for metabolic syndrome, TyG continued to be associated with cough and a restrictive spirometry pattern.
Table 2.
Association of TyG With Study Outcomes, With Concurrent Adjustment for Insulin Resistance (HOMA-IR) or the Metabolic Syndrome∗
| Variable | TyG With Concurrent Adjustment for |
|
|---|---|---|
| log(HOMA-IR) | Metabolic Syndrome | |
| Symptom, aOR (95% CI) | ||
| Cough | 1.24 (1.02 to 1.51) | 1.22 (1.01 to 1.47) |
| Phlegm production | 1.19 (0.98 to 1.44) | 1.17 (0.97 to 1.42) |
| Wheeze | 1.21 (1.04 to 1.41) | 1.13 (0.98 to 1.31) |
| Exertional dyspnea | 1.17 (1.02 to 1.35) | 1.14 (0.99 to 1.32) |
| Condition, aOR (95% CI) | ||
| Asthma | 1.10 (0.92 to 1.31) | 1.05 (0.88 to 1.26) |
| Chronic bronchitis | 1.19 (0.98 to 1.44) | 1.19 (0.98 to 1.46) |
| Emphysema | 1.00 (0.65 to 1.54) | 0.98 (0.63 to 1.52) |
| Spirometry, aMD (95% CI) | ||
| FEV1pp | –0.76 (–2.26 to 0.73) | –0.68 (–2.41 to 1.04) |
| FVCpp | –1.24 (–2.76 to 0.28) | –1.56 (–2.99 to –0.13) |
| FEV1/FVC | 0.32 (–0.27 to 0.90) | 0.67 (0.01 to 1.33) |
| Spirometry pattern, aRRR (95% CI) | ||
| Obstructive | 0.87 (0.70 to 1.08) | 0.83 (0.66 to 1.06) |
| Restrictive | 1.37 (0.98 to 1.92) | 1.33 (1.02 to 1.74) |
aMD = adjusted mean difference; aOR = adjusted OR; aRRR = adjusted relative risk ratio; FEV1pp = percent-predicted FEV1; FVCpp = percent-predicted FVC; HOMA-IR = homeostatic model assessment of insulin resistance; TyG = triglyceride-glucose index.
Adjusted for age, sex, race/ethnicity, BMI, poverty-income ratio, cardiovascular disease, smoking status, smoking years, and fasting time.
Discussion
In this population-based study, we showed that the TyG was associated with respiratory symptoms, diagnosed chronic bronchitis, and a restrictive spirometry pattern. Associations with symptoms seemed largely independent of insulin resistance as well as BMI and self-reported cardiovascular disease. TyG was identified as a marker of metabolic dysfunction with relevancy to pulmonary outcomes. To our knowledge, this examination is the first of the relationship of this index to the lung.
Accurate identification of metabolic dysfunction is important to characterizing its potential role in lung health. Numerous mechanisms, reviewed separately, have been proposed to link metabolic disease and the lung.26,27 At a basic level, elevations in the TyG reflect dyslipidemia and hyperglycemia, derangements that affect pulmonary structure and function in translational and experimental studies. Changes in carbohydrate and fat intake can alter surfactant composition.28 Hypertriglyceridemia and circulating free fatty acids induce inflammation in macrophages and other immune cells.26 Signaling by modified lipoproteins, especially low-density lipoprotein, and accumulation of intracellular cholesterol have also been shown to drive lipotoxicity within the innate immune system.29,30 Diabetes and hyperglycemia increase risk and severity of pulmonary infections through impaired host immunity and enhanced virulence of infectious microorganisms.31 Hyperinsulinemia and insulin resistance, which frequently underlie dyslipidemia and diabetes, can precipitate bronchial hyperreactivity through alterations in parasympathetic signaling and may also promote subepithelial fibrosis.32,33 These intermediate findings, although not directly examinable within this study, outline an evolving conceptualization of dysmetabolism as a potentially modifiable risk factor for impaired lung health.
The current study complements others that have reported epidemiologic associations of metabolic dysregulation with impaired respiratory outcomes in the general population. In the third iteration of NHANES, low-density lipoprotein was negatively associated and high-density lipoprotein was positively associated with FEV1, and HOMA-IR and fasting glucose were negatively associated with FEV1 and FVC.24,34 A study in the Spanish population identified associations of low-density lipoprotein and triglycerides with wheeze.35 Metabolic syndrome has been related to lower FEV1 and FVC in several population studies.26 Our investigation extends these results to a novel parameter of metabolic function and with more comprehensive investigation of relevant outcomes.
We identified a consistent association of TyG with respiratory symptoms. Although NHANES did not ask questions related to the frequency of wheezing or severity of exertional dyspnea, affirmative answers to questions of cough and phlegm production, which require the presence of these symptoms on most days, imply an intensity of symptoms that have clinical relevancy. TyG also continued to be associated with symptoms but not spirometry after mutual adjustment for HOMA-IR, suggesting that changes in lung function attributable to insulin resistance do not fully explain the potential effects of TyG on respiratory outcomes. Because physiological relationships between insulin resistance and lipid and glucose disorders are complex and bidirectional, longitudinal study is necessary to better understand interactions between these two measures.36
Notably, TyG was associated with a higher risk of a restrictive spirometry pattern, mirroring findings reported for insulin resistance.26,37 Insulin resistance and a restrictive spirometry pattern are both, in turn, associated with a higher prospective risk of obstructive lung disease.38,39 The mechanisms that explain a transition from restrictive spirometry to obstructive lung disease are not well defined but may reflect independent processes that act on lung parenchyma vs the airway or misattribution of such symptoms to obstructive lung disease in the absence of true airflow limitation. Indeed, although TyG was associated with higher odds of a diagnosis of chronic bronchitis, it was not associated with an obstructive spirometry pattern. Taken together, however, these data suggest that elevated TyG may define a group of individuals distinct from those with insulin resistance who may be susceptible to incident respiratory disease. Continued associations with spirometry with concurrent adjustment for waist circumference suggest that mass loading due to obesity is not a sufficient explanation of these findings.
Finally, TyG continued to be associated with some outcomes when mutually adjusted for metabolic syndrome. Although this finding may implicate disorders of lipid and glucose handling as significant drivers of harms associated with metabolic syndrome, it is likely at least partially explained by efficiencies gained by using a continuous rather than dichotomous measure of metabolic syndrome. This highlights the additive value of TyG in the investigation of metabolic risk factors for impaired lung health.
Some limitations are noted. NHANES was a cross-sectional study, and we thus are unable to comment on the temporal relationship of TyG with study outcomes. Lung volumes were not performed, preventing any comparisons between a restrictive spirometry pattern and a restrictive ventilatory defect. Spirometry was also not obtained from individuals on supplemental oxygen, limiting its applicability to those with more severe respiratory disease, and maneuvers that only partially met American Thoracic Society criteria were included. Residual confounding due to misclassification of study covariates, especially smoking and cardiovascular disease, are possible as these were obtained through self-report. There was a low prevalence of self-reported chronic bronchitis and emphysema overall, which may represent undiagnosed disease or lack of a survey question directly using the term COPD.40 Finally, because the study population was restricted to adults aged ≥ 40 years, our results are not generalizable to younger individuals.
The current study identified TyG as a risk factor for impaired lung health. Future research is necessary to determine its performance as a prognostic and predictive biomarker, with specific attention to subgroup differences according to ethnicity and sex. Longitudinal designs examining temporal relationships between changes in TyG and changes in respiratory outcomes are warranted. Research is also necessary to characterize the association of TyG with patient-reported outcomes, lung function, and exacerbations among populations with prevalent chronic lung disease.2, 3, 4, 5, 6
Interpretation
In a nationally representative study of adults aged ≥ 40 years, the TyG was associated with respiratory symptoms, self-reported chronic bronchitis, and a restrictive spirometry pattern. Findings were not fully explained by insulin resistance or a dichotomous definition of metabolic syndrome. The index is a potential epidemiologic tool to quantify the role of metabolic dysfunction within lung disease, which may have prognostic value as a biomarker for lung health.
Take-home Points.
Study Question: What is the cross-sectional association of the TyG to respiratory symptoms, chronic lung disease, and spirometry?
Results: Higher TyG was associated with higher odds of cough, phlegm production, exertional dyspnea, wheeze, a prevalent diagnosis of chronic bronchitis, and a restrictive spirometry pattern.
Interpretation: The TyG is a valid epidemiologic biomarker of metabolic dysfunction with relevancy to lung health.
Acknowledgments
Author contributions: T. D. W. is the guarantor of the content of the manuscript and takes responsibility for the integrity of the data analysis. T. D. W. conceived of the study design. All authors contributed substantially to data interpretation, critical revision of the manuscript, and approval for submission in its final form.
Financial/nonfinancial disclosures: None declared.
Role of sponsors: The funding source had no role in the development of this study or the manuscript.
Other contributions: The views described herein are those of the authors and do not necessarily reflect those of the Department of Veterans Affairs, the National Institutes of Health, or the United States Government.
Additional information: The e-Figures and e-Tables can be found in the Supplemental Materials section of the online article.
Footnotes
FUNDING/SUPPORT: T. D. W. is supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Center for Innovations in Quality, Effectiveness, and Safety (CIN 13-413), and by the National Heart, Lung, and Blood Institute (K23HL151669). A. F. is supported by the National Heart, Lung, and Blood Institute (K23HL151758). M. C. M. is supported by the National Institute of Environmental Health Sciences (P50ES018176) and by the United States Environmental Protection Agency (83615201 and 83615001).
Supplementary Data
References
- 1.Suratt B.T., Ubags N.D.J., Rastogi D. An official American Thoracic Society Workshop Report: obesity and metabolism. An emerging frontier in lung health and disease. Ann Am Thorac Soc. 2017;14(6):1050–1059. doi: 10.1513/AnnalsATS.201703-263WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cebron Lipovec N., Beijers R.J.H.C.G., van den Borst B., Doehner W., Lainscak M., Schols A.M.W.J. The prevalence of metabolic syndrome in chronic obstructive pulmonary disease: a systematic review. COPD. 2016;13(3):399–406. doi: 10.3109/15412555.2016.1140732. [DOI] [PubMed] [Google Scholar]
- 3.Serafino-Agrusa L., Spatafora M., Scichilone N. Asthma and metabolic syndrome: current knowledge and future perspectives. World J Clin Cases. 2015;3(3):285–292. doi: 10.12998/wjcc.v3.i3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thuesen B.H., Husemoen L.L.N., Hersoug L.G., Pisinger C., Linneberg A. Insulin resistance as a predictor of incident asthma-like symptoms in adults. Clin Exp Allergy. 2009;39(5):700–707. doi: 10.1111/j.1365-2222.2008.03197.x. [DOI] [PubMed] [Google Scholar]
- 5.Ehrlich S.F., Quesenberry C.P., Van Den Eeden S.K., Shan J., Ferrara A. Patients diagnosed with diabetes are at increased risk for asthma, chronic obstructive pulmonary disease, pulmonary fibrosis, and pneumonia but not lung cancer. Diabetes Care. 2010;33(1):55–60. doi: 10.2337/dc09-0880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Breyer M.K., Spruit M.A., Hanson C.K. Prevalence of metabolic syndrome in COPD patients and its consequences. PloS One. 2014;9(6) doi: 10.1371/journal.pone.0098013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang D., Ma Y., Tong X., Zhang Y., Fan H. Diabetes mellitus contributes to idiopathic pulmonary fibrosis: a review from clinical appearance to possible pathogenesis. Front Public Health. 2020;8:196. doi: 10.3389/fpubh.2020.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh B., Saxena A. Surrogate markers of insulin resistance: a review. World J Diabetes. 2010;1(2):36–47. doi: 10.4239/wjd.v1.i2.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simental-Mendía L.E., Rodríguez-Morán M., Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndrome Related Dis. 2008;6(4):299–304. doi: 10.1089/met.2008.0034. [DOI] [PubMed] [Google Scholar]
- 10.Khan S.H., Sobia F., Niazi N.K., Manzoor S.M., Fazal N., Ahmad F. Metabolic clustering of risk factors: evaluation of triglyceride-glucose index (TyG index) for evaluation of insulin resistance. Diabetol Metab Syndr. 2018;10(1):74. doi: 10.1186/s13098-018-0376-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guerrero-Romero F., Simental-Mendía L.E., González-Ortiz M. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab. 2010;95(7):3347–3351. doi: 10.1210/jc.2010-0288. [DOI] [PubMed] [Google Scholar]
- 12.Vasques A.C.J., Novaes F.S., de Oliveira M. da S. TyG index performs better than HOMA in a Brazilian population: a hyperglycemic clamp validated study. Diabetes Res Clin Pract. 2011;93(3):e98–e100. doi: 10.1016/j.diabres.2011.05.030. [DOI] [PubMed] [Google Scholar]
- 13.Navarro-González D., Sánchez-Íñigo L., Pastrana-Delgado J., Fernández-Montero A., Martinez J.A. Triglyceride–glucose index (TyG index) in comparison with fasting plasma glucose improved diabetes prediction in patients with normal fasting glucose: the Vascular-Metabolic CUN cohort. Preven Med. 2016;86:99–105. doi: 10.1016/j.ypmed.2016.01.022. [DOI] [PubMed] [Google Scholar]
- 14.Irace C., Carallo C., Scavelli F.B. Markers of insulin resistance and carotid atherosclerosis. A comparison of the homeostasis model assessment and triglyceride glucose index. Int J Clin Pract. 2013;67(7):665–672. doi: 10.1111/ijcp.12124. [DOI] [PubMed] [Google Scholar]
- 15.Zhang M., Wang B., Liu Y. Cumulative increased risk of incident type 2 diabetes mellitus with increasing triglyceride glucose index in normal-weight people: the Rural Chinese Cohort Study. Cardiovasc Diabetol. 2017;16(1):30. doi: 10.1186/s12933-017-0514-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jin J.L., Cao Y.X., Wu L.G. Triglyceride glucose index for predicting cardiovascular outcomes in patients with coronary artery disease. J Thorac Dis. 2018;10(11):6137. doi: 10.21037/jtd.2018.10.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zipf G., Chiappa M., Porter K., Ostchega Y., Lewis B.G., Dostal J. National Health and Nutrition Examination Survey: plan and operations, 1999-2010. Vital Health Stat 1. 2013;56:1–37. [PubMed] [Google Scholar]
- 18.Wallace T.M., Levy J.C., Matthews D.R. Use and abuse of HOMA modeling. Diabetes Care. 2004;27(6):1487–1495. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]
- 19.Alberti K., Eckel R.H., Grundy S.M. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention Metabolic syndrome prevalence by race/ethnicity and sex in the United States, National Health and Nutrition Examination Survey, 1988–2012. Preventing Chronic Disease. https://www.cdc.gov/pcd 2016;(2017/16_0287a). Accessed December 1, 2020. [DOI] [PMC free article] [PubMed]
- 21.National Center for Health Statistics (U.S.) National Health and Nutrition Examination Survey, Spirometry Procedures Manual, 2011-2012. https://www.cdc.gov/nchs/data/nhanes/nhanes_11_12/spirometry_procedures_manual.pdf
- 22.Miller M.R., Hankinson J., Brusasco V. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 23.Hankinson J.L., Odencrantz J.R., Fedan K.B. Spirometric reference values from a sample of the general US population. Am J Respir Crit Care Med. 1999;159(1):179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 24.Cirillo D.J., Agrawal Y., Cassano P.A. Lipids and pulmonary function in the Third National Health and Nutrition Examination Survey. Am J Epidemiol. 2002;155(9):842–848. doi: 10.1093/aje/155.9.842. [DOI] [PubMed] [Google Scholar]
- 25.Bhatt S.P., Kim Y.I., Harrington K.F. Smoking duration alone provides stronger risk estimates of chronic obstructive pulmonary disease than pack-years. Thorax. 2018;73(5):414–421. doi: 10.1136/thoraxjnl-2017-210722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baffi C.W., Wood L., Winnica D. Metabolic syndrome and the lung. Chest. 2016;149(6):1525–1534. doi: 10.1016/j.chest.2015.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Papaioannou O., Karampitsakos T., Barbayianni I. Metabolic disorders in chronic lung diseases. Front Med (Lausanne) 2017;4:246. doi: 10.3389/fmed.2017.00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schipke J., Jütte D., Brandenberger C. Dietary carbohydrates and fat induce distinct surfactant alterations in mice. Am J Respir Cell Mol Biol. 2021;64(3):379–390. doi: 10.1165/rcmb.2020-0335OC. [DOI] [PubMed] [Google Scholar]
- 29.Tall A.R., Yvan-Charvet L. Cholesterol, inflammation and innate immunity. Nature Rev Immunol. 2015;15(2):104–116. doi: 10.1038/nri3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fessler M.B. A new frontier in immunometabolism. Cholesterol in lung health and disease. Ann Am Thorac Soc. 2017;14(suppl 5):S399–S405. doi: 10.1513/AnnalsATS.201702-136AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klekotka R.B., Mizgała E., Król W. The etiology of lower respiratory tract infections in people with diabetes. Pneumonol Alergol Pol. 2015;83(5):401–408. doi: 10.5603/PiAP.2015.0065. [DOI] [PubMed] [Google Scholar]
- 32.Nie Z., Jacoby D.B., Fryer A.D. Hyperinsulinemia Potentiates Airway Responsiveness to Parasympathetic Nerve Stimulation in Obese Rats. Am J Respir Cell Mol Biol. 2014;51(2):251–261. doi: 10.1165/rcmb.2013-0452OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee H., Kim S.R., Oh Y., Cho S.H., Schleimer R.P., Lee Y.C. Targeting insulin-like growth factor-I and insulin-like growth factor-binding protein-3 signaling pathways. A novel therapeutic approach for asthma. Am J Respir Cell Mol Biol. 2014;50(4):667–677. doi: 10.1165/rcmb.2013-0397TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McKeever T.M., Weston P.J., Hubbard R., Fogarty A. Lung function and glucose metabolism: an analysis of data from the Third National Health and Nutrition Examination Survey. Am J Epidemiol. 2005;161(6):546–556. doi: 10.1093/aje/kwi076. [DOI] [PubMed] [Google Scholar]
- 35.Fenger R.V., Gonzalez-Quintela A., Linneberg A. The relationship of serum triglycerides, serum HDL, and obesity to the risk of wheezing in 85,555 adults. Respir Med. 2013;107(6):816–824. doi: 10.1016/j.rmed.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 36.Vergès B. Pathophysiology of diabetic dyslipidaemia: where are we? Diabetologia. 2015;58(5):886–899. doi: 10.1007/s00125-015-3525-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Godfrey M.S., Jankowich M.D. The vital capacity is vital: epidemiology and clinical significance of the restrictive spirometry pattern. Chest. 2016;149(1):238–251. doi: 10.1378/chest.15-1045. [DOI] [PubMed] [Google Scholar]
- 38.Wan E.S., Fortis S., Regan E.A. Longitudinal phenotypes and mortality in preserved ratio impaired spirometry in the COPDGene Study. Am J Respir Crit Care Med. 2018;198(11):1397–1405. doi: 10.1164/rccm.201804-0663OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wijnant S.R.A., De Roos E., Kavousi M. Trajectory and mortality of preserved ratio impaired spirometry: the Rotterdam Study. Eur Respir J. 2020;55(1) doi: 10.1183/13993003.01217-2019. [DOI] [PubMed] [Google Scholar]
- 40.Martinez C.H., Mannino D.M., Jaimes F.A. Undiagnosed obstructive lung disease in the United States. Associated factors and long-term mortality. Ann Am Thorac Soc. 2015;12(12):1788–1795. doi: 10.1513/AnnalsATS.201506-388OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.