Abstract
Background
Understanding how health outcomes differ for patients with advanced cystic fibrosis (CF) lung disease living in the United States compared with Canada has health policy implications.
Research Question
What are rates of lung transplant (LTx) and rates of death without LTx in the United States and Canada among individuals with FEV1 < 40% predicted?
Study Design and Methods
This was a retrospective population-based cohort study, 2005 to 2016, using the US CF Foundation, United Network for Organ Sharing, and Canadian CF registries. Individuals with CF and at least two FEV1 measurements < 40% predicted within a 5-year period, age ≥ 6 years, without prior LTx were included. Multivariable competing risk regression for time to death without LTx (LTx as a competing risk) and time to LTx (death as a competing risk) was performed.
Results
There were 5,899 patients (53% male) and 905 patients (54% male) with CF with FEV1 < 40% predicted living in the United States and Canada, respectively. Multivariable competing risk regression models identified an increased risk of death without LTx (hazard ratio [HR], 1.79; 95% CI, 1.52-2.1) and decreased LTx (HR, 0.66; 95% CI, 0.58-0.74) among individuals in the United States compared with Canada. More pronounced differences were seen in the patients in the United States with Medicaid/Medicare insurance compared with Canadians (multivariable HR for death without LTx, 2.24 [95% CI, 1.89-2.64]; multivariable HR for LTx, 0.54 [95% CI, 0.47-0.61]). Patients of nonwhite race were also disadvantaged (multivariable HR for death without LTx, 1.56 [95% CI, 1.32-1.84]; multivariable HR for LTx, 0.47 [95% CI, 0.36-0.62]).
Interpretation
There are lower rates of LTx and an increased risk of death without LTx for US patients with CF with FEV1 < 40% predicted compared with Canadian patients. Findings are more striking among US patients with CF with Medicaid/Medicare health insurance, and nonwhite patients in both countries, raising concerns about underuse of LTx among vulnerable populations.
Key Words: access to transplantation, advanced lung disease, cystic fibrosis, lung transplantation
Abbreviations: CF, cystic fibrosis; CFF, Cystic Fibrosis Foundation; HR, hazard ratio; IQR, interquartile range; LTx, lung transplantation; SES, socioeconomic status; UNOS, United Network for Organ Sharing
Cystic fibrosis (CF) is a progressive, fatal genetic disease that affects more than 90,000 individuals worldwide, 35,000 of whom live in the United States.1, 2, 3 Survival is heterogeneous in advanced CF lung disease, with some individuals dying quickly after reaching a low FEV1 threshold and others living for several years.4,5 The primary cause of death for most individuals with CF is respiratory failure.1 The median age of survival for individuals with CF was estimated at 41 years in the United States and 51 years in Canada, a contrast in survival that was most evident among US patients with public health insurance (Medicaid or Medicare).6 A recent comparison of the United States and Canada showed that differences in post-lung transplant survival and transplant waiting list deaths account for one-third of the survival gap between the countries, with notable survival disadvantages for US patients with public health insurance.7 Lung transplant (LTx) is a potential therapeutic option for individuals with advanced CF lung disease and offers a median survival of 10 years posttransplant for adults with CF8,9 and improved health-related quality of life within months of LTx for recipients with CF.10,11 Despite the potential for long-term survival with LTx, individuals with FEV1 < 30% predicted living in the United States are more likely to die without LTx than undergo the procedure.4 Several socioeconomic factors in the United States are associated with decreased referral for LTx, including Medicaid insurance status, lack of high school graduate education, and lower median household income.12, 13, 14
In this study, we used data from 2005 to 2016 to determine whether use of LTx and rates of death without LTx for individuals with advanced CF differ between the United States and Canada. We hypothesized that individuals with advanced CF lung disease (defined as FEV1 < 40% predicted),15 and otherwise similar disease severity (eg, supplemental oxygen use, FEV1 % predicted), have decreased survival and rates of LTx in the United States compared with Canada. In addition, we postulated that disparities in access to LTx would be seen for individuals with public health insurance (Medicaid or Medicare) in the United States, a proxy for socioeconomic status (SES).16
Methods
This study was approved by St. Michael’s Hospital (Toronto, ON, Canada; Research Ethics Board, No. 14-148), the University of Washington (Seattle, WA; Institutional Review Board, No. 2270), and Seattle Children’s Hospital (Institutional Review Board, No. 15294).
Data Source
This study used CF records from the US Cystic Fibrosis Foundation (CFF) Patient Registry and LTx records from the United Network for Organ Sharing (UNOS) provided by the Organ Procurement and Transplantation Network. The US registry merge is described further in the online article (e-Appendix 1 and e-Fig 1). The Canadian CF Registry contains pre- and posttransplant data for nearly all patients with CF monitored at CF centers in Canada,17 and these data were combined with US data; the registry variables were harmonized to ensure consistent comparisons between the countries, as previously described.6 The available data spanned 1990 to 2016, but analyses were limited to a cohort eligible from January 1, 2005 through December 31, 2016 to capture findings relevant to a modern era of CF care and current LTx practices in the setting of the lung allocation score (implemented in May 2005 in the United States).18
Participants
Individuals who were 6 years or older on or after January 1, 2005 were included in analyses if they had two FEV1 measurements < 40% predicted within a 5-year period based on annual lung function assessments, with survival time beginning at the time of the first FEV1 < 40% predicted measurement (the second measurement was considered necessary for confirmation of low lung function). Multiple FEV1 measurements per year have been collected in the Canadian CF Registry only since 2011, and primary analyses used annual FEV1 values in both countries; a sensitivity analysis included patients with two FEV1 < 40% predicted measurements within 1 year (2011-2016). Lung function measurements after LTx were excluded from all analyses.
Definitions of Key Variables
FEV1 % predicted was calculated using the Global Lung Function Initiative reference equations.19,20 Insurance status was defined by insurance type recorded during the 3 years before the first FEV1 < 40% predicted measure: (1) Medicaid/Medicare, if present during this 3-year period; (2) “Other” (predominantly private insurance), if no Medicaid/Medicare present; (3) “None/Missing” for patients who did not have any health insurance type recorded. Eligibility for Medicaid and Medicare is described in the online article. Universal health care coverage exists to provide access to health care for Canada’s population.21 There were no markers of SES available in the Canadian CF Registry. Values for covariates that change over time (eg, age, BMI, noninvasive ventilation) were obtained from the year before entry into the cohort. Other variable classifications are described in the online article.
Statistical Analysis and Outcomes
Descriptive statistics are summarized for patients with CF living in the two countries. Comparisons between the two countries were made, using the Mann-Whitney test for continuous variables and the χ2 test for proportions. Standardized differences were compared, with a difference of > 10 deemed clinically important.22 The difference in proportion of individuals transplanted between countries was assessed using the two-proportion z-test.
Probability of death without LTx after the first FEV1 < 40% predicted measurement was modeled using a cumulative incidence curve with LTx as a competing risk. Gray’s test was used to compare the curves.23 Multivariable competing risk regression for time to death without LTx (with LTx as a competing risk) and time to LTx (with death as a competing risk) was performed. Covariates were chosen for the multivariable model on the basis of a priori variable selection.24 Multiple imputation of missing data (described further in the online article) was used for the primary competing risk regression model, and a sensitivity analysis used complete case analysis. The LTx-to-death ratio was calculated by dividing the number of LTx by the number of deaths without LTx in the two countries; the risk of transplant-to-risk of death ratio over time was obtained from the cumulative incidence curve. Secondary analyses compared all patients in Canada with patients with Medicaid/Medicare in the United States and with patients with “Other” (predominantly private) insurance in the United States. A sensitivity analysis limited the US cohort to those with Medicaid insurance (without Medicare) for comparison with patients in Canada. Additional sensitivity analyses were limited to individuals in both countries with the following: FEV1 < 30% predicted, and age 18 years and older.
Patients who were not transplanted and were still alive at the end of the study period were censored on December 31, 2016. “Lost to follow-up” was defined as the last clinical encounter occurring in 2014 or earlier without a reported outcome of death or LTx, and those patients were censored at their last encounter date.
All analyses were performed with R version 3.6.2 (R Foundation for Statistical Computing). All P values are two-sided and assessed for significance at P < .05.
Results
There were 5,899 patients (53% male) and 905 patients (54% male) with CF with FEV1 < 40% predicted living in the United States and Canada, respectively (Table 1). In both countries, 95% of patients had their second (confirmatory) FEV1 < 40% predicted measurement within 3 years of annual measurements from the first reported FEV1 < 40% predicted measurement (e-Table 1). In both countries, 30% of patients had their first FEV1 < 40% predicted measurement observed in 2005, due to left censoring of data before 2005. The median (interquartile range, IQR) follow-up time was 4.2 years (2.5-6.5 years) in the United States and 4.3 years (2.4-7.3 years) in Canada. Individuals with advanced CF lung disease living in the United States were younger, more likely to be pancreatic insufficient, less likely to have Burkholderia cepacia complex present in sputum culture, and more likely to have cystic fibrosis-related diabetes compared with those with advanced CF lung disease living in Canada (Table 1). Patients with CF living in the United States had more frequent supplemental oxygen use, more frequent noninvasive ventilation use, and more frequent exacerbations compared with patients with CF living in Canada. Patients with CF living in the United States had higher BMI compared with those in Canada. In the United States, 3,428 patients (58%) reported Medicaid or Medicare health insurance; 75% of nonwhite patients reported Medicaid or Medicare insurance. It was rare (< 5% of US patients) for insurance type to change between the first and confirmatory FEV1 measurements.
Table 1.
Characteristics of US and Canadian Cystic Fibrosis Cohorts With Two Measurements of FEV1 < 40% Predicted Within 5 Years, 2005 to 2016
| Characteristic | United States (N = 5,899) | Canada (N = 905) | P Valuea | Standardized Differencea |
|---|---|---|---|---|
| Age at year end, median (IQR), y | 24.2 (19.0-32.6) | 25.4 (19.9-33.7) | < .001 | 13 |
| Sex (male), No. (%) | 3,111 (52.7) | 490 (54.1) | .45 | 2.8 |
| Pancreatic insufficiency, No. (%) | 5,786 (98.1) | 826 (91.3) | < .001 | 30.7 |
| Genotype | ||||
| Homozygous F508del, No. (%) | 2,833 (48) | 489 (54) | .24 | 5.7 |
| Heterozygous F508del, No. (%) | 2,072 (35.1) | 324 (35.8) | 3.4 | |
| Other, No. (%) | 599 (10.2) | 87 (9.6) | 4 | |
| Missing, No. (%) | 395 (6.7) | 5 (0.6) | 33.3 | |
| Race | ||||
| White, No. (%) | 5,585 (94.7) | 859 (94.9) | .37 | 3.6 |
| Nonwhite, No. (%) | 308 (5.2) | 40 (4.4) | 3.6 | |
| Missing, No. (%) | 6 (0.1) | 6 (0.7) | 9.1 | |
| Burkholderia cepacia complex (ever), No. (%) | 342 (5.8) | 182 (20.1) | < .001 | 43.6 |
| CF-related diabetes in year before first low FEV1, No. (%) | 2,048 (34.7) | 212 (23.4) | < .001 | 25.6 |
| CF-related diabetes ever, pretransplant, No. (%) | 3,483 (59) | 375 (41.4) | < .001 | 35.8 |
| FEV1 % predicted, median (IQR) | 43.7 (39.9-49.5) | 43.7 (40.5-48.4) | .59 | 6.3 |
| BMI, median (IQR), kg/m2 | 20.4 (18.8-22.6) | 19.9 (18.0-22.1) | < .001 | 24.8 |
| Normal weight, No. (%) | 3,243 (55) | 505 (55.8) | .43 | 4.5 |
| Overweight, No. (%) | 423 (7.2) | 68 (7.5) | 0 | |
| Underweight, No. (%) | 1,178 (20) | 206 (22.8) | 4.9 | |
| Missing, No. (%) | 1,055 (17.9) | 126 (13.9) | 10.8 | |
| Supplemental oxygen, No. (%) | 724 (12.3) | 56 (6.2) | < .001 | 21.1 |
| Noninvasive ventilation with bilevel support, No. (%) | 56 (4.9) | 0 (0) | .005 | 32.2 |
| Pulmonary exacerbations, median (IQR) | 1 (0-3) | 1 (0-2) | < .001 | 30.7 |
| 0 exacerbations, No. (%) | 2,017 (34.2) | 414 (45.7) | < .001 | 23.8 |
| 1 or 2 exacerbations, No. (%) | 2,008 (34) | 315 (34.8) | 1.6 | |
| 3+ exacerbations, No. (%) | 1,874 (31.8) | 176 (19.4) | 28.5 | |
| Health insurance | ||||
| Medicaid/Medicare, No. (%) | 3,428 (58.1) | N/A | N/A | N/A |
| Other insurance, No. (%) | 2,412 (40.9) | N/A | ||
| None/missing, No. (%) | 59 (1.0) | N/A |
Values for covariates that change over time (eg, age, BMI, number of pulmonary exacerbations, noninvasive ventilation [NIV], FEV1 % predicted) were obtained from the year before cohort entry unless otherwise noted. BMI was classified as (1) underweight if BMI percentile was < 12% for pediatrics (defined as age < 19 years) or BMI < 18.5 kg/m2 for adults, (2) normal weight if their BMI percentile was 12% to 84% for pediatrics or BMI 18.5 to 24.9 kg/m2 for adults, or (3) overweight if their BMI percentile was > 84% for pediatrics or BMI > 24.9 kg/m2 for adults. NIV with bilevel support was included only for individuals with NIV data from the year before cohort entry, beginning in 2011 in both countries, because these data were not available in the Canadian CF Registry before 2011; calculation of proportion receiving bilevel support was out of 1,139 people in the United States and 177 in Canada. CF = cystic fibrosis; IQR = interquartile range; N/A = not applicable.
Boldface P value indicates a value < .05, a statistically significant result; boldface standardized difference indicates a difference of > 10, deemed clinically significant.
Risk of Death Without LTx and LTx
Cumulative Incidence
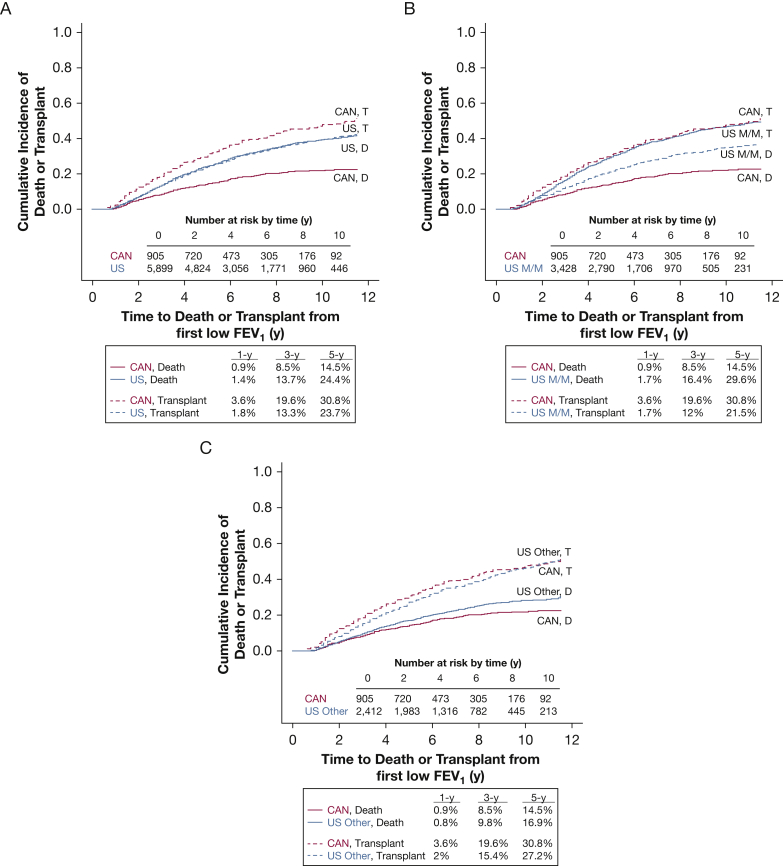
There were 1,842 LTx (91.6% in adults) and 353 LTx (94.3% in adults) in the United States and Canada, respectively (Table 2). Within the United States, individuals with advanced lung disease were equally as likely to die without transplant as they were to undergo LTx, whereas in Canada individuals were more likely to be transplanted than die (Fig 1). LTx or death without LTx, when considered together, occurred more often in the United States (N = 3,693, 63%) than in Canada (N = 516, 57%), a difference of 6% in the combined outcome (95% CI, 2.1%-9.1%; P = .003). Compared with Canadians, US individuals with advanced lung disease were more likely to die without LTx (univariate hazard ratio [HR], 2.06; 95% CI, 1.71-2.48). Individuals in the United States were less likely to receive LTx (univariate HR, 0.74; 95% CI, 0.65-0.84). Rates of death on the waitlist were higher in the United States than in Canada (see the online article). Individuals with Medicaid/Medicare insurance in the United States were even less likely to undergo LTx compared with Canadians (univariate HR, 0.64; 95% CI, 0.56-0.74). Loss to follow-up was similar in the United States (N = 354, 6%) and Canada (N = 61, 7%). Rates of LTx and death without LTx from sensitivity analyses are available in the online article (e-Figs 2-7).
Table 2.
Survival and Lung Transplant Outcomes Among Individuals With FEV1 < 40% Predicted, by Country, 2005 to 2016
| Outcome | United States (N = 5,899) | Canada (N = 905) |
|---|---|---|
| Alive without lung transplant | 1,852 (31%) | 328 (36%) |
| Underwent lung transplant | 1,842 (31%) | 353 (39%) |
| Died without lung transplant | 1,851 (31%) | 163 (18%) |
| Lost to follow-up | 354 (6%) | 61 (7%) |
Figure 1.
Cumulative incidence curves with 1-, 3-, and 5-year rates of lung transplantation (T) and death without lung transplantation (D) among individuals with cystic fibrosis and FEV1 < 40% predicted living in Canada compared with: A, patients in the United States; B, patients with Medicaid/Medicare insurance in the United States; C, patients with “Other” insurance (primarily private health insurance) in the United States. CAN = Canada; D = death; M/M = Medicaid/Medicare; T = lung transplantation; US = United States.
Lung Transplant-to-Death Ratio
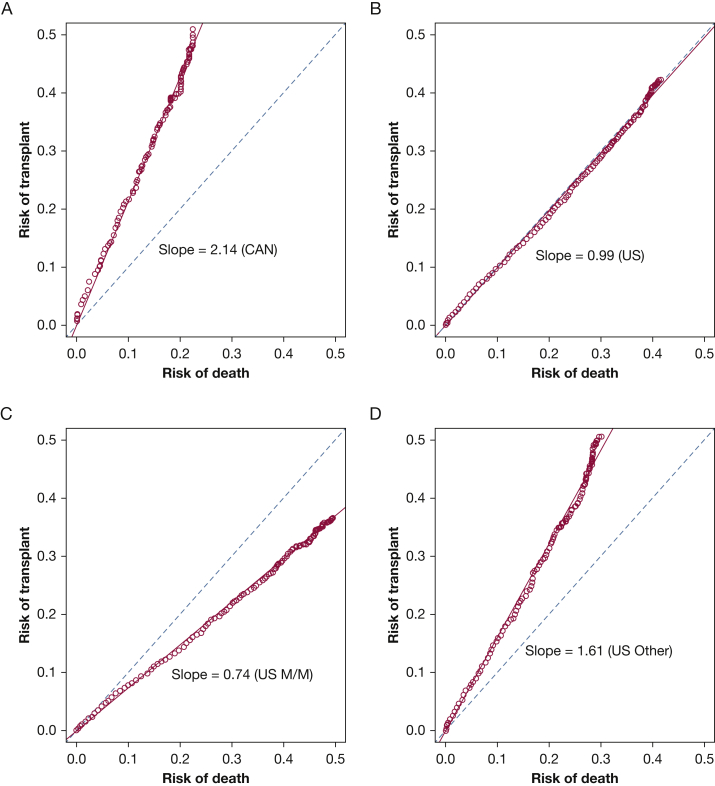
The LTx-to-death ratio was consistently greater than 2:1 in Canada after reaching FEV1 < 40% predicted, but was approximately 1:1 in the United States (Fig 2). The ratio was approximately 1.6:1 for patients with “Other” insurance, and 0.74:1 for patients with Medicaid/Medicare in the United States (Fig 2).
Figure 2.
The risk of lung transplantation (LTx) was linearly related to the risk of death without LTx, which generated a slope that was equivalent to the LTx-to-death ratio (higher is favorable) for individuals with cystic fibrosis and FEV1 < 40% predicted: A, in Canada; B, in the United States; C, for patients with Medicaid/Medicare in the United States; D, patients with “Other” insurance (primarily private health insurance) in the United States. With a slope of 1.0 (dotted reference line), LTx and death without LTx occur at the same rate; a slope greater than 1.0 means LTx occurs more often than death without LTx and a slope less than 1.0 means death without LTx occurs more often than LTx. CAN = Canada; M/M = Medicaid/Medicare; US = United States.
Competing Risk Regression
Multivariable competing risk regression identified an increased risk of death without LTx (HR, 1.79; 95% CI, 1.52-2.11; P < .001) and decreased risk of LTx (HR, 0.66; 95% CI, 0.58-0.74; P < .001) among individuals in the United States compared with Canada (Table 3). Among the subset of individuals with Medicaid/Medicare insurance, increased risk of death without LTx (multivariable HR, 2.24; 95% CI, 1.89-2.64; P < .001) and decreased risk of LTx (multivariable HR, 0.54; 95% CI, 0.47-0.61; P < .001) were more pronounced (e-Table 2). Sensitivity analysis limited to patients 18 years and over with two measurements of FEV1 < 40% predicted in a 5-year period produced similar findings (e-Table 3). US patients with FEV1 < 30% predicted twice within 1 year, 2011 to 2016, had an increased risk of death without LTx (multivariable HR, 1.53; 95% CI, 1.13-2.06; P = .006) and decreased risk of LTx (multivariable HR, 0.53; 95% CI, 0.43-0.65) compared with those in Canada (e-Table 4). Results from models using complete case analysis were not substantially different from results using the multiple imputation method (e-Table 5).
Table 3.
Multivariable Competing Risk Regression, Time to Death Without LTx (LTx as a Competing Risk), and Time to LTx (Death as a Competing Risk), 2005 to 2016: Multiple Imputation Analysisa
| Variable | Time to Death Without LTx |
Time to Lung Transplant |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P Valueb | HR | 95% CI | P Valueb | |
| Country (United States vs Canada) | 1.79 | 1.52-2.11 | < .001 | 0.66 | 0.58-0.74 | < .001 |
| Age | 1.01 | 1.00-1.02 | < .001 | 0.99 | 0.99-1.00 | < .001 |
| Sex (female vs male) | 1.03 | 0.94-1.13 | .49 | 1.04 | 0.95-1.13 | .42 |
| Age at diagnosis (≥ 2 y vs < 2 y) | 1.00 | 0.91-1.11 | .94 | 0.86 | 0.78-0.95 | .004 |
| CF-related diabetes | 1.25 | 1.14-1.37 | < .001 | 0.96 | 0.87-1.06 | .4 |
| Burkholderia cepacia complex | 1.17 | 1-1.37 | .054 | 0.65 | 0.54-0.77 | < .001 |
| Race (nonwhite vs white) | 1.56 | 1.32-1.84 | < .001 | 0.47 | 0.36-0.62 | < .001 |
| BMI categories | ||||||
| Overweight vs adequate weight | 1.02 | 0.86-1.22 | .79 | 0.68 | 0.55-0.83 | .002 |
| Underweight vs adequate weight | 1.22 | 1.09-1.36 | .002 | 0.97 | 0.88-1.07 | .53 |
| FEV1 % predicted | 1.00 | 0.99-1.00 | .54 | 0.98 | 0.98-0.99 | < .001 |
| No. of pulmonary exacerbations | ||||||
| 1 or 2 vs 0 | 1.18 | 1.06-1.32 | .004 | 1.14 | 1.03-1.26 | .013 |
| 3+ vs 0 | 1.57 | 1.39-1.76 | < .001 | 1.29 | 1.15-1.45 | < .001 |
| Supplemental oxygen | 1.29 | 1.13-1.46 | < .001 | 1.26 | 1.10-1.45 | .001 |
CF = cystic fibrosis; HR = hazard ratio; LTx = lung transplantation.
N = 6,804 observations. These observations include 2,014 deaths without LTx and 2,195 transplants. All variables were assessed before the first low lung function (FEV1 < 40% predicted) measurement. BMI was classified as (1) underweight if BMI percentile was < 12% for pediatrics (defined as age < 19 years) or BMI < 18.5 kg/m2 for adults, (2) normal weight if their BMI percentile was 12% to 84% for pediatrics or BMI 18.5 to 24.9 kg/m2 for adults, or (3) overweight if their BMI percentile was > 84% for pediatrics or BMI > 24.9 kg/m2 for adults.
Boldface P value indicates a value < .05, a statistically significant result.
Across the primary and all sensitivity analyses, nonwhite race was significantly associated with a decreased risk of LTx and increased risk of death without LTx (Table 3, e-Tables 2-5).
Discussion
In this North American population-based study from 2005 to 2016, individuals with advanced CF lung disease (FEV1 < 40% predicted) living in the United States were at a significantly higher risk of death without LTx compared with individuals with advanced CF lung disease living in Canada, even after adjusting for important confounders. Within the United States, individuals were equally as likely to die after reaching this lung function threshold as undergo LTx. By contrast, in Canada, patients with CF with FEV1 < 40% predicted were transplanted at twice the rate of death without LTx.
A prior CFF Patient Registry study demonstrated that rates of death without LTx were high for individuals with CF with FEV1 < 30% predicted living in the United States,4 the previously recognized threshold for transplant consideration.25 The current study confirms that the risk of death is present even earlier in the course of the disease. The US CFF established LTx referral guidelines in 2019 focused on risk stratification and earlier referral of individuals with advanced lung disease, targeting individuals with FEV1 < 40% predicted.26 We hypothesize that using a strategy that leads to earlier referral and transplantation of patients with advanced CF living in the United States, comparable to the Canadian pattern of higher rates of LTx within 1 year of reaching FEV1 < 40% predicted, could potentially avoid deaths without LTx. The increased rate of the combined outcome of LTx or death in the United States (driven by the higher rate of death without LTx in the United States) speaks against the notion that the Canadians are transplanting patients too early. Further, in the setting of earlier LTx for CF in Canada, posttransplant median survival for Canadian recipients with CF is longer than the median survival after LTx for US patients with CF.7,27, 28, 29 If individuals with CF living in the United States experienced similar waitlist survival and posttransplant survival as those in Canada, the survival gap between the two countries would narrow.7
Differences in the US and Canadian health care systems have been implicated as potential drivers of differential survival for individuals with CF in the two countries.6 The impact of the different health care systems on survival for those with lower SES in the United States was shown for patients with cancer30, 31, 32 and for kidney transplant recipients33 in the two countries. In the current study, we have identified a substantial increased risk of death without LTx for individuals with public health insurance in the United States compared with Canada and an increased risk of death without LTx for nonwhite patients adjusted for country. We assert that although outcomes are worse for these individuals, it is not the health insurance or race that causes worsened outcomes, but the underlying disadvantages and social inequities that drive differences in access and outcomes. In 2007, the US Department of Health and Human Services created the Final Rule, which established expectations for high-quality transplant service delivery and emphasized that access to transplant should be based on common medical criteria with a stated goal of “fair and nondiscriminatory distribution of organs.”34 The Final Rule recommended psychosocial evaluation and access to qualified social workers for all individuals being considered for transplantation,34 but it did not give specific recommendations for addressing psychosocial issues identified during an evaluation. In fact, it is commonplace for transplant programs to deny access to transplant for candidates with a perceived lack of social support35 or the financial resources36 to have a successful transplant. However, access to LTx as a procedure is not enough on its own; lifelong access to necessary medications and specialist health care services is also vitally important for a successful LTx.37 Posttransplant survival outcomes for individuals with CF with public health insurance in the United States lag behind outcomes for CF LTx recipients in the United States who have private health insurance or individuals transplanted for CF in the United Kingdom.28 For LTx recipients with all diagnoses (including CF), health insurance status is associated with long-term posttransplant survival but not short-term posttransplant outcomes,38 pointing to the important role of continued access to health care over time. Deeper issues include the role of the social determinants of health (eg, food insecurity, poor housing conditions, domestic violence, high stress levels) in health care outcomes, and these force us to consider how we treat vulnerable patient populations in the United States, Canada, and beyond. The current study adds to the evidence base demonstrating inequities related to income and race in the United States, which is exaggerated in this population with a chronic health condition that requires an expensive life-saving treatment. Hence, universal health insurance in Canada may be an additional factor positively affecting survival for individuals with CF in Canada in this study.
This study had several strengths. First, the use of a merged registry that contains data from the US CFF Patient Registry and UNOS improved outcome ascertainment beyond the use of US CFF data alone, particularly related to dates of LTx and death dates for individuals who were listed for LTx. The CFF Registry contains individuals who are not present in the UNOS dataset, capturing those who were potentially eligible for LTx but who were never listed for transplant. Second, this study employed the use of competing risk regression, instead of Cox proportional hazards modeling, in the setting of the informative censoring that occurs for patients who undergo LTx. This rigorous methodology is often not used despite the potential for erroneous inferences when statistical assumptions are violated in the Cox model.39 Third, the international comparison of individuals with advanced CF lung disease, a phenotypic extreme, allows for exploration of differences in clinical and social practices between the countries for individuals at high risk for poor outcomes.
Limitations
This study has limitations. First, because of the nature of working with registries, nuances of data collection impact our analysis options. Because we lacked a unique identifier that is confirmed by UNOS and the CFF Patient Registry, there is the potential for false matches despite the availability of several identifiers in the datasets. Our team identified a match score of 10 or higher (described in the online article) to minimize the number of false matches while retaining the largest number of true matches. The registries did not include dates of exacerbations, the indication for hospitalization, or noninvasive ventilation use for the entirety of the study period. As such, there is a potential for misclassification of individuals’ markers of increased disease severity. Specifically, the frequency of pulmonary exacerbations may be overestimated in both countries, due to double counting exacerbations treated partially at home and partially during a hospitalization; hospitalizations for reasons other than exacerbation falsely raise the number of exacerbations in both countries. In addition, there is a potential for residual confounding by factors unavailable in the two countries’ registries, or inadvertently excluded from our models, which could otherwise explain the differences in health outcomes between the two countries. Given the size of the effect (nearly two-fold increased risk of death without LTx attributed to country), the plausibility of the relationship, and the fact that we captured key variables used for prognostication in CF, we feel the likelihood of residual confounding is low. Further, measures of quality of life were not included in the registries, but reduced quality of life is likely an important factor for people with advanced CF lung disease who are considering LTx. In addition, dates of lung transplant referral, reason for and date of waitlist removal, and presence of absolute contraindications to transplant are not available for the entirety of the study period in both countries. These data could inform our inferences about patients who missed the opportunity to consider LTx. Second, public health insurance as a proxy for SES in the United States is imperfect. However, several other studies in CF, and broader populations, used public health insurance as a proxy for low SES.12,13,16,38,40 Furthermore, there is no direct measure of SES in the Canadian CF registry. A prior study of patients with CF living in Canada showed no association of SES with hospitalization rates for pulmonary exacerbations in Ontario, which the authors concluded may have been due to the availability of universal health care, the national network of CF centers, and drug and travel coverage that is available in Ontario regardless of SES.41 It is, however, still possible that additional unavailable variables, including more accurate markers of SES in both countries, social support, adherence, medical literacy, or implicit bias (of health care systems and/or medical providers), would explain the results. Future studies should attempt to leverage more extensive paradigms for measuring SES, including Singh’s Area Deprivation Index,42 a deprivation index developed in Canada,43 or a new model that incorporates SES data from multiple countries. Third, these data do not include the significant proportions of patients receiving highly effective CF transmembrane conductance regulator (CFTR) modulator therapy, with less than 5% of patients with access to ivacaftor in both countries during the study period.44,45 These novel medications may change the landscape of advanced CF lung disease and modify the population’s need for LTx. As of October 2019, highly effective modulator therapy is available for more than 90% of the US CF population over 12 years old, but the Canadian population has limited access to these medications at present. It is possible that the rates of death without LTx will decline more rapidly in the United States than in Canada because of earlier access to this highly effective therapeutic. Finally, although earlier access to LTx for US individuals with CF could benefit this patient population, the availability of donor organs, use of lungs after cardiac death, level of illness warranting listing and/or LTx, and approach to organ allocation are factors that were not addressed in the current study. Importantly, many of these factors are transplant program-specific, and some of these key elements are guided by international guidelines.25 Publicly available international organ donation data suggest that rates of lung donation may be as much as 30% higher in Canada than in the United States,46 an issue that could also impact access to LTx in the two countries.
Interpretation
There are lower rates of LTx and increased risk of death without LTx for patients with CF with FEV1 < 40% predicted living in the United States compared with Canada. Identifying differences in the use of LTx for individuals with advanced CF living in the United States compared with Canada underscores the importance of improving access to LTx in the United States, including a focus on earlier referral for consideration of LTx and attention to systemic biases that may contribute to exclusion and/or worsened health outcomes.
Take-home Points.
Study Question: Do individuals with advanced cystic fibrosis (CF) have different rates of lung transplant in the United States compared with Canada?
Results: From 2005 to 2016, individuals with CF and FEV1 < 40% predicted were 34% less likely to undergo transplant and twice as likely to die without transplant in the United States compared with Canada. These findings were more pronounced for individuals on Medicaid/Medicare in the United States and for nonwhite patients.
Interpretation: Differential use of lung transplant for patients with CF with FEV1 < 40% predicted living in the United States is associated with worse health outcomes compared with Canada.
Acknowledgments
Author contributions: K. J. R. is the guarantor of the content of the manuscript, including the data and analysis. K. J. R., J. S., S. S., A. Fink, B. S. Q., B. C. M., A. Faro, C. H. G., and A. L. S., made substantial contributions to the conception and design of the work; K. J. R., J. S., X. M., J. S. O., A. Fink, K. P., A. E., C. H. G., and A. L. S. were responsible for acquisition of the data; J. S., S. S., and X. M. were responsible for analysis of the data; all of the co-authors made substantial contributions to the interpretation of data. K. J. R. and A. L. S. wrote the first draft of the manuscript, and all of the co-authors critically revised it for important intellectual content. All of the co-authors approved the final version submitted for publication and agree to be accountable for all aspects of the work. All of the co-authors ensure that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: J. S. O., A. Fink, B. C. M., A. Faro, K. P., and A. E. work at the Cystic Fibrosis Foundation (CFF) in the United States. K. J. R., C. H. G., and A. L. S. receive grant funding from the CFF, and A. L. S. receives funding from CF Canada. These financial relationships did not influence the interpretation or reporting of the current study. K. J. R. receives funding from the CFF [RAMOS17A0, RAMOS20A0-KB] and the National Institutes of Health [K23HL138154]. C. H. G. was supported by grants from the Cystic Fibrosis Foundation, the NIH [UM1 HL119073, P30 DK089507, U01 HL114589, UL1 TR000423], and the FDA [R01 FD003704, R01 FD006848].
Role of sponsors: The sponsors had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Disclaimer: Some of the data reported here have been supplied by UNOS as the contractor for the Organ Procurement and Transplantation Network (OPTN). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the OPTN or the US government.
Other contributions: The authors thank the individuals with cystic fibrosis, care providers, and clinic coordinators at CF centers throughout the United States and Canada for their contributions to their respective cystic fibrosis patient registries.
Additional information: The e-Appendix, e-Tables, and e-Figures can be found in the Supplemental Materials section of the online article.
Footnotes
FUNDING/SUPPORT: K. J. R. receives funding from the CFF [RAMOS17A0, RAMOS20A0-KB] and the National Institutes of Health [K23HL138154]. C. H. G. was supported by grants from the CFF, the NIH [UM1 HL119073, P30 DK089507, U01 HL114589, UL1 TR000423], and the FDA [R01 FD003704, R01 FD006848].
Supplementary Data
References
- 1.Cystic Fibrosis Foundation Cystic Fibrosis Foundation Patient Registry: Annual Data Report 2019. https://www.cff.org/Research/Researcher-Resources/Patient-Registry/2019-Patient-Registry-Annual-Data-Report.pdf
- 2.Knapp E.A., Fink A.K., Goss C.H. The Cystic Fibrosis Foundation Patient Registry: design and methods of a national observational disease registry. Ann Am Thorac Soc. 2016;13(7):1173–1179. doi: 10.1513/AnnalsATS.201511-781OC. [DOI] [PubMed] [Google Scholar]
- 3.Bell S.C., Mall M.A., Gutierrez H. The future of cystic fibrosis care: a global perspective. Lancet Respir Med. 2020;8(1):65–124. doi: 10.1016/S2213-2600(19)30337-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramos K.J., Quon B.S., Heltshe S.L. Heterogeneity in survival in adult patients with cystic fibrosis with FEV1 < 30% of predicted in the United States. Chest. 2017;151(6):1320–1328. doi: 10.1016/j.chest.2017.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.George P., Banya W., Pareek N. Improved survival at low lung function in cystic fibrosis: cohort study from 1990 to 2007. BMJ. 2011;342:d1008. doi: 10.1136/bmj.d1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stephenson A.L., Sykes J., Stanojevic S. Survival comparison of patients with cystic fibrosis in Canada and the United States: a population-based cohort study. Ann Internal Med. 2017;166(8):537–546. doi: 10.7326/M16-0858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stephenson A.L., Ramos K.J., Sykes J. Bridging the survival gap in cystic fibrosis: an investigation of lung transplant outcomes in Canada and the United States. J Heart Lung Transplant. 2021;40(3):201–209. doi: 10.1016/j.healun.2020.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chambers D.C., Cherikh W.S., Harhay M.O. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: thirty-sixth adult lung and heart-lung transplantation report—2019; focus theme: donor and recipient size match. J Heart Lung Transplant. 2019;38(10):1042–1055. doi: 10.1016/j.healun.2019.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.International Society for Heart and Lung Transplantation. International Thoracic Organ Transplant (TTX) Registry data slides: 2019 slides. https://ishltregistries.org/registries/slides.asp?yearToDisplay=2019
- 10.Singer J.P., Katz P.P., Soong A. Effect of lung transplantation on health-related quality of life in the era of the lung allocation score: a US prospective cohort study. Am J Transplant. 2017;17(5):1334–1345. doi: 10.1111/ajt.14081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singer L.G., Chowdhury N.A., Faughnan M.E. Effects of recipient age and diagnosis on health-related quality-of-life benefit of lung transplantation. Am J Respir Crit Care Med. 2015;192(8):965–973. doi: 10.1164/rccm.201501-0126OC. [DOI] [PubMed] [Google Scholar]
- 12.Quon B.S., Psoter K., Mayer-Hamblett N., Aitken M.L., Li C.I., Goss C.H. Disparities in access to lung transplantation for patients with cystic fibrosis by socioeconomic status. Am J Respir Crit Care Med. 2012;186(10):1008–1013. doi: 10.1164/rccm.201205-0949OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramos K.J., Quon B.S., Psoter K.J. Predictors of non-referral of patients with cystic fibrosis for lung transplant evaluation in the United States. J Cyst Fibros. 2016;15(2):196–203. doi: 10.1016/j.jcf.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lehr C.J., Fink A.K., Skeans M. Impact of socioeconomic position on access to the US lung transplant waiting list in a matched cystic fibrosis cohort. Ann Am Thorac Soc. 2020;17(11):1384–1392. doi: 10.1513/AnnalsATS.202001-030OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kapnadak S.G., Dimango E., Hadjiliadis D. Cystic Fibrosis Foundation consensus guidelines for the care of individuals with advanced cystic fibrosis lung disease. J Cyst Fibros. 2020;19(3):344–354. doi: 10.1016/j.jcf.2020.02.015. [DOI] [PubMed] [Google Scholar]
- 16.Schechter M.S., Shelton B.J., Margolis P.A., FitzSimmons S.C. The association of socioeconomic status with outcomes in cystic fibrosis patients in the United States. Am J Respir Crit Care Med. 2001;163(6):1331–1337. doi: 10.1164/ajrccm.163.6.9912100. [DOI] [PubMed] [Google Scholar]
- 17.Corey M., Farewell V. Determinants of mortality from cystic fibrosis in Canada, 1970-1989. Am J Epidemiol. 1996;143(10):1007–1017. doi: 10.1093/oxfordjournals.aje.a008664. [DOI] [PubMed] [Google Scholar]
- 18.Egan T.M., Murray S., Bustami R. Development of the new lung allocation system in the United States. Am J Transplant. 2006;6(5 part 2):1212–1227. doi: 10.1111/j.1600-6143.2006.01276.x. [DOI] [PubMed] [Google Scholar]
- 19.Stanojevic S., Bilton D., McDonald A. Global Lung Function Initiative equations improve interpretation of FEV1 decline among patients with cystic fibrosis. Eur Respir J. 2015;46(1):262–264. doi: 10.1183/09031936.00187314. [DOI] [PubMed] [Google Scholar]
- 20.Quanjer P.H., Stanojevic S., Cole T.J., ERS Global Lung Function Initiative Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40(6):1324–1343. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin D., Miller A.P., Quesnel-Vallée A., Caron N.R., Vissandjée B., Marchildon G.P. Canada’s universal health-care system: achieving its potential. Lancet. 2018;391(10131):1718–1735. doi: 10.1016/S0140-6736(18)30181-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Normand S.-L.T., Landrum M.B., Guadagnoli E. Validating recommendations for coronary angiography following acute myocardial infarction in the elderly: a matched analysis using propensity scores. J Clin Epidemiol. 2001;54(4):387–398. doi: 10.1016/s0895-4356(00)00321-8. [DOI] [PubMed] [Google Scholar]
- 23.Fine J.P., Gray R.J. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. [Google Scholar]
- 24.Lederer D.J., Bell S.C., Branson R.D. Control of confounding and reporting of results in causal inference studies: guidance for authors from editors of respiratory, sleep, and critical care journals. Ann Am Thorac Soc. 2019;16(1):22–28. doi: 10.1513/AnnalsATS.201808-564PS. [DOI] [PubMed] [Google Scholar]
- 25.Weill D., Benden C., Corris P.A. A consensus document for the selection of lung transplant candidates: 2014—an update from the Pulmonary Transplantation Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2015;34(1):1–15. doi: 10.1016/j.healun.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 26.Ramos K.J., Smith P.J., McKone E.F. Lung transplant referral for individuals with cystic fibrosis: Cystic Fibrosis Foundation consensus guidelines. J Cyst Fibros. 2019;18(3):321–333. doi: 10.1016/j.jcf.2019.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stephenson A.L., Sykes J., Berthiaume Y. Clinical and demographic factors associated with post-lung transplantation survival in individuals with cystic fibrosis. J Heart Lung Transplant. 2015;34(9):1139–1145. doi: 10.1016/j.healun.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 28.Merlo C., Clark S., Arnaoutakis G. National healthcare delivery systems influence lung transplant outcomes for cystic fibrosis. Am J Transplant. 2015;15(7):1948–1957. doi: 10.1111/ajt.13226. [DOI] [PubMed] [Google Scholar]
- 29.Ramos K.J., Kapnadak S.G., Bradford M.C. Underweight patients with cystic fibrosis have acceptable survival following lung transplantation: a united network for organ sharing registry study. Chest. 2020;157(4):898–906. doi: 10.1016/j.chest.2019.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gorey K.M., Holowaty E.J., Fehringer G. An international comparison of cancer survival: Toronto, Ontario, and Detroit, Michigan, metropolitan areas. Am J Public Health. 1997;87(7):1156–1163. doi: 10.2105/ajph.87.7.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gorey K.M., Kliewer E., Holowaty E.J., Laukkanen E., Ng E.Y. An international comparison of breast cancer survival: Winnipeg, Manitoba and Des Moines, Iowa, metropolitan areas. Ann Epidemiol. 2003;13(1):32–41. doi: 10.1016/s1047-2797(02)00259-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gorey K.M. Breast cancer survival in Canada and the USA: meta-analytic evidence of a Canadian advantage in low-income areas. Int J Epidemiol. 2009;38(6):1543–1551. doi: 10.1093/ije/dyp193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim S., Schaubel D., Fenton S., Leichtman A., Port F. Mortality after kidney transplantation: a comparison between the United States and Canada. Am J Transplant. 2006;6(1):109–114. doi: 10.1111/j.1600-6143.2005.01141.x. [DOI] [PubMed] [Google Scholar]
- 34.Centers for Medicare & Medicaid Services (CMS), Department of Health and Human Services. Medicare program; hospital conditions of participation: requirements for approval and re-approval of transplant centers to perform organ transplants. Final rule. Fed Regist. 2007;72(61):15197–15280. [PubMed] [Google Scholar]
- 35.Berry K.N., Daniels N., Ladin K. Should lack of social support prevent access to organ transplantation? Am J Bioethics. 2019;19(11):13–24. doi: 10.1080/15265161.2019.1665728. [DOI] [PubMed] [Google Scholar]
- 36.Laurentine K.A., Bramstedt K.A. Too poor for transplant: finance and insurance issues in transplant ethics. Prog Transplant. 2010;20(2):178–185. doi: 10.1177/152692481002000213. [DOI] [PubMed] [Google Scholar]
- 37.Simmerling M. Beyond scarcity: poverty as a contraindication for organ transplantation. Virtual Mentor. 2007;9(6):441–445. doi: 10.1001/virtualmentor.2007.9.6.pfor1-0706. [DOI] [PubMed] [Google Scholar]
- 38.Allen J.G., Arnaoutakis G.J., Orens J.B. Insurance status is an independent predictor of long-term survival after lung transplantation in the United States. J Heart Lung Transplant. 2011;30(1):45–53. doi: 10.1016/j.healun.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 39.Wu M.C., Carroll R.J. Estimation and comparison of changes in the presence of informative right censoring by modeling the censoring process. Biometrics. 1988;44:175–188. [Google Scholar]
- 40.Bradley C.J., Given C.W., Roberts C. Race, socioeconomic status, and breast cancer treatment and survival. J Natl Cancer Inst. 2002;94(7):490–496. doi: 10.1093/jnci/94.7.490. [DOI] [PubMed] [Google Scholar]
- 41.Stephenson A., Hux J., Tullis E., Austin P.C., Corey M., Ray J. Socioeconomic status and risk of hospitalization among individuals with cystic fibrosis in Ontario, Canada. Pediatr Pulmonol. 2011;46(4):376–384. doi: 10.1002/ppul.21368. [DOI] [PubMed] [Google Scholar]
- 42.Singh G.K. Area deprivation and widening inequalities in US mortality, 1969-1998. Am J Public Health. 2003;93(7):1137–1143. doi: 10.2105/ajph.93.7.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pampalon R., Hamel D., Gamache P., Raymond G. A deprivation index for health planning in Canada. Chronic Dis Can. 2009;29(4):178–191. [PubMed] [Google Scholar]
- 44.Middleton P.G., Mall M.A., Dřevínek P., VX17-445-102 Study Group Elexacaftor-tezacaftor-ivacaftor for cystic fibrosis with a single Phe508del allele. N Engl J Med. 2019;381(19):1809–1819. doi: 10.1056/NEJMoa1908639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heijerman H.G., McKone E.F., Downey D.G., VX17-445-103 Trial Group Efficacy and safety of the elexacaftor plus tezacaftor plus ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: a double-blind, randomised, phase 3 trial. Lancet. 2019;394(10212):1940–1948. doi: 10.1016/S0140-6736(19)32597-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.International Registry in Organ Donation and Transplantation (IRODaT). Database: donation activity charts. https://www.irodat.org/?p=database#data
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.