Introduction
Accurate and consistent disease definition and diagnostic criteria are essential for high-quality clinical care and research regarding burning mouth syndrome (BMS). Unfortunately, many randomized controlled trials (RCTs) are inconsistent in their definitions and diagnostic criteria for enrolling BMS participants. This has contributed to heterogeneity in participant selection, which has contributed to uncertainty in study outcomes and limitations in the interpretability of prior results [3,14,39,40,42]. Variation in definition and diagnostic criteria between studies also hampers understanding of the epidemiology and etiology of BMS [27] and the reliability and validity of future research. The International Classification of Diseases (ICD), overseen by the World Health Organization (WHO) in its 11th revision (ICD-11), expected to be implemented in January 2022, proposesa a new definition and diagnostic criteria for BMS.[45] The International Headache Society (IHS) [2], and International Association for the Study of Pain (IASP) [29] also have revisited the classification of orofacial pain, yet the diagnostic criteria for BMS amongst these entities continue to differ. More recently, a committee from the International Network for Orofacial Pain and Related Disorders Methodology (INfORM) developed a Beta version of the research diagnostic criteria for BMS (RDC/BMS) [9] using the ICOP definition. However, the new RDC have not been tested and serve a unique role compared with the ICD-11.
Over the past three decades, there have been attempts to describe and more accurately name “Burning Mouth Syndrome” [19,33]. The current term, “BMS,” implies that the condition be classified as a “syndrome” [1,45], but does it fulfill the criteria to be a syndrome? Stedman’s Medical Dictionary defines a syndrome as an “aggregate of symptoms and signs associated with any morbid process, together constituting the picture of the disease” [36]. Although a broad range of symptoms have been reported with BMS (i.e., allodynia and other varying pain descriptors [6], taste changes [16,38] and subjective dry mouth [23]) whether these symptoms contribute consistently to BMS remains unclear. The only consistent symptom required for diagnosis is a sensation of oral burning or dysesthesia [4,26,28,34]. Thus, experts have suggested re-examining the nomenclature and disease definition of BMS [18,28,29].
The Delphi technique is a mixed qualitative-quantitative research method developed to reach systematically the most reliable consensus among experts on issues that lack clear and consistent empirical data [46]. The technique encourages expert independent thought, considers equality in ideas proposed by all experts, and decreases conformity due to group dynamics [10,12]. In as much as consensus is lacking on the disease definition and nomenclature for BMS, this study aimed to use the Delphi technique to 1) determine if revision in nomenclature and alternative names for “BMS” are warranted; and 2) identify areas of consensus among experts for changes to the disease description and proposeda diagnostic criteria of “BMS” as described in the ICD-11 (WHO).
Materials and Methods
This study sought to develop consensus for the diagnostic criteria, disease description and nomenclature of BMS as well as areas of convergent and divergent thought by using the Delphi technique. This study was approved by Case Western Reserve University Institutional Review Board (STUDY20190366) as exempt and was conducted between April 2019 and January 2020. The research was conducted in accordance with the Declaration of the World Medical Association and informed consent was obtained from participants at each survey round.
Participant recruitment
Since the success of the Delphi technique depends on the participation of committed experts interested in the topic of study, purposive expert sampling was used to invite an international group of clinicians and researchers with expertise in BMS to participate in the study. There is no standard for selecting the sample size of experts for a Delphi study, however, a minimum of 10–18 experts have been recommended [30]. Lists of potential participants were identified by the research team (i.e., the authors of this manuscript) to represent different continents including North and South America, Europe, Asia, and Australia. Individuals were subsequently excluded from the list if they had not published on BMS in the last 15 years. The diversity achieved from purposive sampling was critical to 1) represent true consensus in the field, and 2) develop a definition and nomenclature that is appropriate for diagnostic and research purposes in communities across the globe. Participants were eligible if they were able to read and write in English, held a post-graduate degree (MS, DMD, DDS, PhD or an equivalent), and provided at least five years of clinical care and/or research in BMS. A standard recruitment e-mail was sent by the principal investigator (MC) to invite the identified experts and only those who had participated in the preceding round were eligible to participate in subsequent survey rounds (Fig. 1).
Fig. 1.
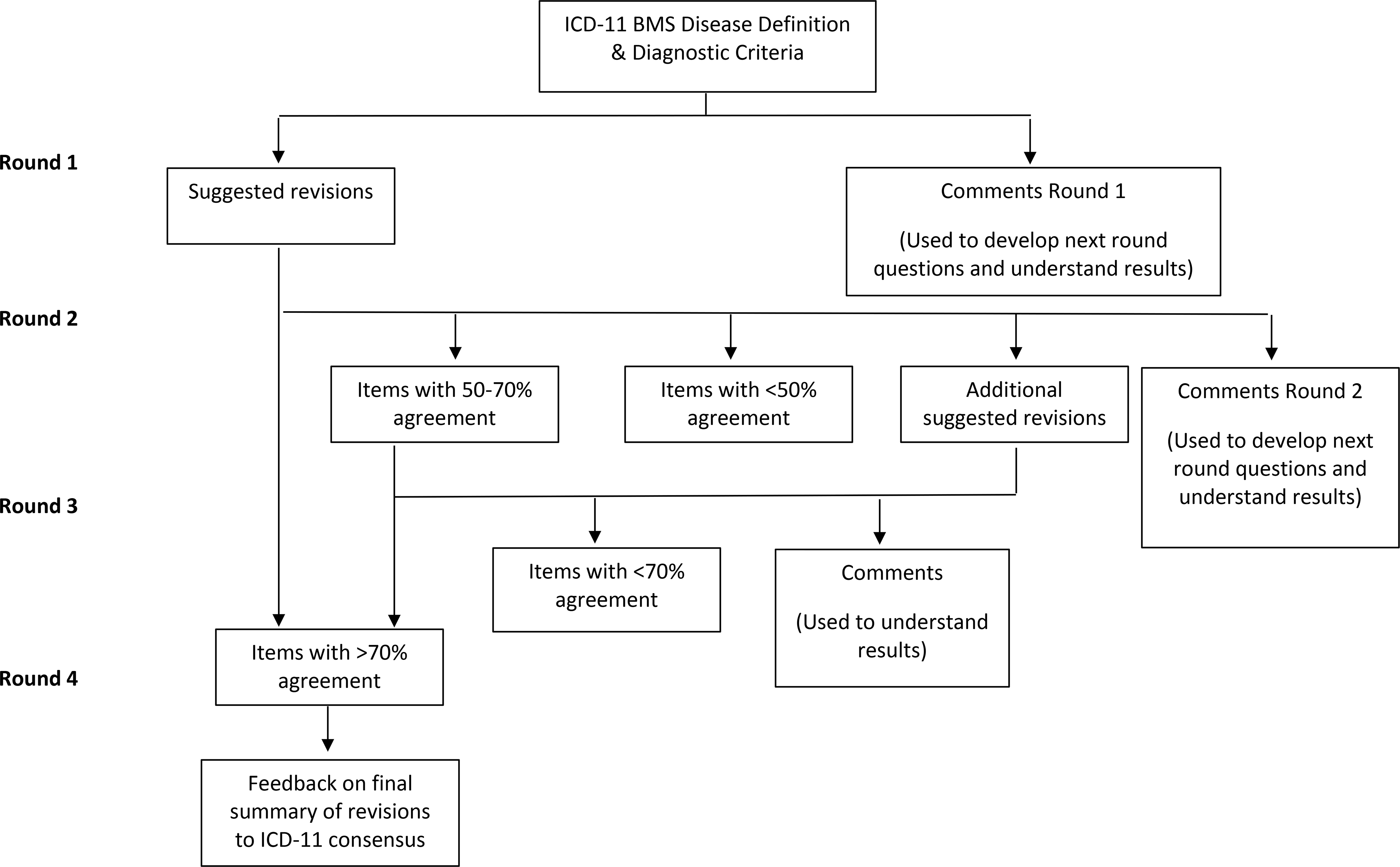
Delphi Survey Rounds
Survey development & data collection
The Delphi technique is well suited to electronic surveys [10,35,37,46], and thus Qualtrics.xm® was used to create electronic self-administered surveys by team members trained in survey design who have published peer-reviewed research using survey methodology. To reduce potential bias from team members who have previously published in the field of BMS, a member (EAS) with expertise in mixed-methods research without experience in orofacial pain oversaw the development of the survey rounds and data analysis/interpretation. Participants were sent a personalized link to the survey by e-mail that allowed for tracking of participants across each round. Four rounds of surveys were used and each survey was iteratively developed based on responses from the previous round. Each of the surveys was reviewed by individuals with knowledge in the topic area for readability, and appropriateness of response options prior to dissemination. The items presented in each survey round are shown in Fig. 1. The first survey presented proposed diagnostic criteria from the ICD-11 maintenance platform (Table 1) and the published ICD-11 disease description (Table 2A) and experts were asked if statements should be changed and to suggest changes. Additionally, they were asked questions whether or not BMS is a “syndrome,” if it should be renamed, and for suggested new names. The second round presented all of the suggested changes from round 1 grouped by topic area, and participants were asked about their agreement with the proposed changes as well as to rank their preference for a new name. Round 3 presented select changes that had not reached consensus by Round 2. Finally, Round 4 presented the revisions to the nomenclature, ICD-11 disease description and proposed diagnostic criteria that met consensus and asked five questions about satisfaction with the results of the study. Close-ended questions were ranked on a 5-point Likert-scale (strongly disagree to strongly agree) in the first round and participants were asked to only select statements with which they agree in subsequent rounds. Although no standard definition for consensus has been established, we followed best practices by pre-defining consensus, set as a minimum of 70% of the respondents being in agreement for quantitative questions (response of “yes”, “agree”, or “strongly agree” or “no”, “disagree”, or “strongly disagree” for a given question) [11,30,32,44]. If a question reached 70% agreement, the results were presented back to participants in the subsequent round but were no longer open for response. Open-ended questions gave participants the opportunity to make suggested changes and provide a rationale for their choices. Subsequent survey rounds included a summary of the quantitative and qualitative data for the participants to review and inform their responses in the current survey round. However, participants and their responses remained anonymous to one another to limit influence and to give equal weight to all members involved [37].
Table 1.
Proposed BMS diagnostic criteria and the resulting changes suggested based on the Delphi method
| Diagnostic category | Proposed† BMS diagnostic criteria | Consensus results of changes to proposed BMS diagnostic criteria |
|---|---|---|
| Chronicity | Chronic oral pain (persisting or recurring for more than 3 months) is present. | Chronic oral pain (persisting or recurring for more than 3 months) is present. |
| Temporality | Pain recurs daily for >2 hours on more than 50% of the days. | Pain recurs daily for more than 50% of the days. |
| Symptom quality | Pain is of a burning quality. | Pain or dysesthesia is of a burning quality. |
| Location | Pain is felt superficially in the oral mucosa. | Pain is felt superficially in the oral mucosa. |
| Functional properties | Pain is characterized by at least one of the following: 1. significant emotional distress 2. functional disability (in particular with orofacial function such as eating, yawning, speaking etc.) |
Functional properties were removed from criteria. |
| Examination findings | Oral mucosa is of normal appearance and no local or systemic causes explains the pain. | No local or systemic causes explains the pain in the oral mucosa. |
| The pain is not better accounted for by another chronic pain condition | The pain is not better accounted for by another chronic pain condition. |
Proposed diagnostic criteria were from the ICD-11 maintenance platform, which is a work in progress between published revisions of ICD-11 (Last accessed from https://icd.who.int/dev11/l-m/en on 6.4.2020)
BMS: Burning mouth syndrome
Table 2.
BMS disease description
| 2A. ICD-11 BMS disease description. | 2B. Revisions to ICD-11 BMS disease description that met consensus in the Delphi study. | 2C. ICD-11 BMS disease description with merged revisions that met consensus in the Delphi study. |
|---|---|---|
| Chronic burning mouth pain is chronic orofacial pain with an intraoral burning or dysesthetic sensation that recurs for more than two hours per day on 50 % of the days over more than three months, without evident causative lesions on clinical investigation and examination. It is characterized by significant emotional distress (anxiety, anger/frustration or depressed mood) or interference with orofacial functions such as eating, yawning, speaking etc. Chronic burning mouth pain is multifactorial: biological, psychological and social factors contribute to the pain condition. The diagnosis is appropriate independently of identified biological or psychological contributors unless another diagnosis would better account for the presenting symptoms. Other chronic headache or orofacial pain diagnoses to be considered are listed under chronic secondary headache and orofacial pain. | • Use term “burning mouth disorder” instead of “burning mouth syndrome” and “chronic burning mouth pain.” • Add expanded description of intraoral location affected including: ○ “Multiple intraoral sites may be affected” ○ “The most common site affected is the tongue” ○ “Symptoms are often bilateral” • Remove that symptoms recur “for more than two hours per day” • Revised “causative lesions” to “causes” • Added “laboratory findings” to clinical investigation/examination. • Added “associated symptoms may include dysgeusia and/or xerostomia (subjective dry mouth).” • Revised “It is characterized by significant emotional distress […]” to “It can be associated with emotional distress […]” • Removed “yawning” from orofacial functions affected by BMS. • Added “with neuropathic characteristics” to statement “[…] biological, psychological and social factors contribute to the pain condition.” • Added as footnote “the following local and systemic causes of oral burning should be evaluated: oral mucosal disease, parafunctional habit of the tongue, hyposalivation, oral candidiasis, anemia, B12 and B9 deficiency, diabetes mellitus, Angiotensin Converting Enzyme (ACE) inhibitor medication. When one of these conditions is found it should be treated and its contribution to oral burning symptoms should be made before a diagnosis of Burning Mouth Disorder is considered. |
Burning mouth disorder is chronic orofacial pain with an intraoral1,2,3 burning or dysaesthetic sensation that recurs on 50 % of the days over more than three months, without evident causes4 on clinical investigation/ examination and laboratory findings. Associated symptoms may include dysgeusia and/or xerostomia (subjective dry mouth). It may be associated with emotional distress (anxiety, anger/frustration or depressed mood) or interference with orofacial functions such as eating, speaking etc. Burning mouth disorder is multifactorial: biological, psychological and social factors contribute to the pain condition with neuropathic characteristics. The diagnosis is appropriate independently of identified biological or psychological contributors unless another diagnosis would better account for the presenting symptoms. Other chronic headache or orofacial pain diagnoses to be considered are listed under chronic secondary headache and orofacial pain. 1Multiple intraoral sites may be affected. 2The most common site affected is the tongue. 3Symptoms are often bilateral. 4The following local and systemic causes of oral burning should be evaluated: oral mucosal disease, parafunctional habit of the tongue, hyposalivation, oral candidiasis, anemia, vitamin B12 and B9 deficiency, diabetes mellitus, Angiotensin Converting Enzyme (ACE) inhibitor medication. When present, these other conditions should be treated and their contribution to oral burning symptoms should be evaluated before a diagnosis of Burning Mouth Disorder is considered. |
Data analysis
Data were collected using Qualtrics.xm and exported into NVivo12 (QSR International Pty Ltd.), software used for mixed-methods research. All participant identifying information was removed from the data prior to analysis thus blinding the study team members to the participants and their responses to reduce bias when analyzing data. The response rate of each round, along with frequency of responses for all nominal data were calculated. If a topic did not reach consensus after Round 3, it was determined that consensus could not be reached on the topic at this time. Individuals who dropped out of the study were counted towards the responses of the rounds for which they participated.
Template analysis, a qualitative technique to organize thematically and analyze textual data was used to code open-ended responses in each survey round and a code book was developed [8,21]. Each transcript was coded by two members of the research team (MC, EAS) trained in qualitative methods, one of whom is a non-clinician, thus providing an external perspective on the participant suggestions and discussion on BMS in the study data. Inter-coder reliability (ICR) was calculated using the Kappa statistic and percent agreement; members of the research team met to resolve coding discrepancies when a code’s Kappa was less than 0.5 and/or percent-agreement was less than 80% [43]. The final mean Kappa across all codes and surveys of the coded data was 0.92 [range,0.522–1.0], demonstrating near perfect coding agreement [43]. The research team then looked within each code to determine themes and specific topics where there was a difference in participants’ responses. A summary of the qualitative data (Supplemental Table 1) referenced in the results is available in the supplemental digital content.
Results
Thirty-one international experts were identified by the research team and sent a recruitment e-mail. Figure 2 describes the recruitment, participation and drop-out rates for the study. A greater than 82% response rate was obtained in each iterative round. All participants (n=19/19, 100%) had either a professional background in oral medicine, orofacial pain, or both. Ten participants identified North America and seven identified Europe as their work location; the remainder identified South America (n=2), Asia (n=1) and Australia (n=1) with three individuals identifying more than one continent as their work place. Fifteen (79%) worked in an academic setting with educational, clinical and research responsibilities. The majority had >20 years of experience (n=12, 63%). Participant demographics are found in Table 3.
Fig. 2.
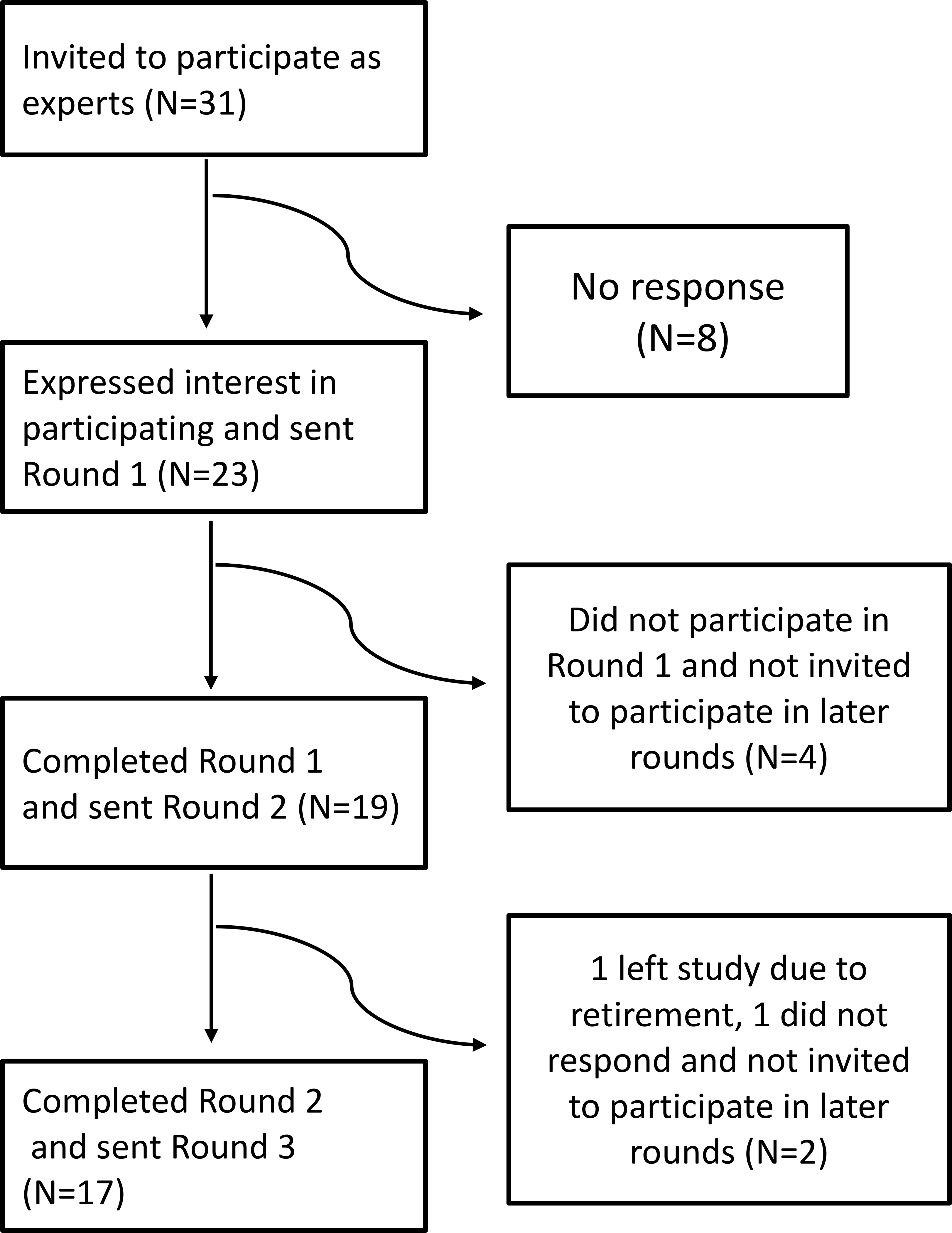
Recruitment and Attrition of Participants
Table 3.
Participant demographics
| Professional background | N=19 (%) |
|
| |
| Oral medicine | 4 (21) |
| Orofacial pain | 3 (16) |
| Both oral medicine and orofacial pain | 12 (63) |
| Professional experience (years) | |
|
| |
| 10 to <15 | 4 (21) |
| 15 to <20 | 3 (16) |
| >20 | 12 (63) |
| Self-reported estimate of BMS patients managed during career | |
|
| |
| <50 | 1 (5) |
| 50 to <100 | 3 (16) |
| >100 to 500 | 7 (37) |
| >500 | 6 (32) |
| Work location by continent † | |
|
| |
| North America | 10 |
| Europe | 7 |
| South America | 2 |
| Asia | 1 |
| Australia | 1 |
| Work setting | |
|
| |
| Academic (research/teaching/clinic) | 15 (79) |
| Academic (research/teaching) | 1 (5) |
| Hospital or outpatient clinic only | 2 (11) |
| Clinic and research | 1 (5) |
Two participants identified more than one continent as their work place.
BMS: Burning Mouth Syndrome
Nomenclature
The results of the assessment for renaming BMS are shown in Table 4. Consensus was reached that BMS is not a syndrome (n=15/17, 88%) and should be renamed (n=15/19, 79%). “Burning Mouth” (n=13/17, 76%) and “Disorder” (n=13/17, 76%) were the terms selected to describe and classify the condition. The rationale provided by participants to rename BMS was that 1) symptoms did not fit the definition of a “syndrome,” 2) symptoms reported in BMS may represent different disease entities rather than a single “syndrome” and that 3) a new name could improve communication, research and understanding. Participants who expressed not wanting to rename BMS tended to suggest that BMS is not well defined to be renamed.
Table 4.
Delphi survey participant suggested names* for “Burning Mouth Syndrome.”
| Qualifier terms | N=22† (%) | Descriptor terms | N=17 (%) | Classification terms‡ | N=17 (%) |
|---|---|---|---|---|---|
| No qualifier needed | 13 (59) | Burning Mouth | 13 (76) | Disorder | 13 (76) |
|
| |||||
| Primary | 8 (36) | Oral Burning | 10 (59) | Disturbance | 5 (29) |
|
| |||||
| Chronic | 4 (18) | None of the Above | 3 (17) | Dysesthesia | 3 (17) |
|
| |||||
| Persistent | 2 (9) | Oral mucosal pain | 2 (12) | Symptoms | 3 (17) |
|
| |||||
| Complex | 2 (9) | Intraoral mucosal sensory | 1 (6) | Disease | 2 (12) |
|
| |||||
| Idiopathic | 1 (5) | Oral Sensitivity | 1 (6) | Symptom complex | 1 (6) |
|
| |||||
| Tropical Mouth | 1 (6) | Syndrome | 1 (6) | ||
|
| |||||
| Orofacial pain | 0 | Dysfunction | 1 (6) | ||
|
| |||||
| None of the above | 1 (6) | ||||
Items in bold reached consensus.
The following names were suggested by participants; Burning Mouth, Burning Mouth Disease, Burning Mouth Disorder, Burning Mouth Dysfunction, Burning Mouth Symptoms, Burning Mouth Symptom Complex, Chronic Persistent Oral Mucosa Dysesthesia, Complex Oral Sensitivity Disorder, Intraoral Mucosal Sensory Disorder, Intraoral Mucosal Sensory Disturbance, Persistent Oral Mucosal Dysesthesia, Persistent Idiopathic Orofacial Pain, Primary And Secondary Burning Mouth Pain, Tropical Mouth Disturbance, Tropical Mouth Dysfunction.
Names were divided into their components of a qualifier, descriptor or classification term. Voting in subsequent Rounds 2 and 3 occurred at the level of the components (qualifier, descriptor, and classification term).
Individuals were given the option to select up to two classification terms.
After round 2 there was no preference among experts for a qualifier, thus in Round 3 individuals were asked to select their top two choices for a qualifier term ranking them by preference, the top choice option 1 was weighted double the value of option 2. The question had a total of 11 responders and 4 non-responders, thus the maximum score any option could receive was 22.
Disease definition and diagnostic criteria
Quality of symptoms
The ICD-11 describes one symptom for the diagnosis of burning mouth disorder (BMD) “the pain is of a burning quality.” Experts suggested six new symptom descriptors beyond “burning” to be considered for the diagnostic criterion (Table 5). Participants were asked to classify the six symptoms as “primary symptom,” “secondary symptom,” “not a symptom of BMS,” or “unsure.” A primary symptom was one that would be sufficient by itself for the diagnosis of BMD, while a secondary symptom may be present in BMD, but by itself is not sufficient for the diagnosis of BMD. All agreed (n=15/15, 100%) that “burning” is a primary symptom of BMD and is sufficient for diagnosis. The majority agreed that “throbbing” is not a symptom of BMD (n=11/15, 73%). Symptom descriptors “stinging,” “scalded” and “hot” were selected by 73% (n=11/15) of participants as either primary or secondary symptoms, however, no consensus was reached for their role in BMD diagnosis (Table 5).
Table 5.
Symptom descriptions and their role in BMD diagnosis.
| Symptom description | Classification | N=15 (%) |
|---|---|---|
| Burning | Primary | 15 (100) |
| Stinging | Primary Secondary Not a symptom Unsure |
6 (40) 8 (53) 1 (6) 0 |
| Scalded | Primary Secondary Not a symptom Unsure |
5 (33) 6 (40) 3 (20) 1 (6) |
| Hot | Primary Secondary Not a symptom Unsure |
4 (27) 7 (47) 3 (20) 1 (6) |
| Tingling | Primary Secondary Not a symptom Unsure |
3 (20) 7 (47) 3 (20) 2 (13) |
| Throbbing | Primary Secondary Not a symptom Unsure |
1 (6) 3 (20) 11 (73) 0 |
A primary symptom was defined as sufficient by itself for the diagnosis of BMD. A secondary symptom was defined as may be present in BMD, but by itself is not sufficient for the diagnosis of BMD.
Expert consensus agreed that the term “dysesthesia” (n=12/17, 71%) also can be used to describe the experience of BMD resulting in the following revision to the proposed diagnostic criterion: “the pain or dysesthesia is of a burning quality.” In the ICD-11 disease description, the experience is already described as “pain or dysesthesia” and experts highlighted that not all patients experience burning as painful but rather as an altered or unpleasant oral sensation.
Consensus (n=12/17, 71%) was reached to add ‘associated symptoms’ to the disease description of BMD (Table 2B), which include “dysgeusia” (altered taste sensation) (n=11/12, 92%)b and “xerostomia (subjective dry mouth)” (n=12/12, 100%). Participants commented that although there is more to learn about the associated symptoms experienced in BMD it is commonly known that altered taste and xerostomia do not occur in all individuals.
Chronicity
The proposed ICD-11 diagnostic criteria define chronicity as “[…] more than three months” and is the length of time symptoms must be present before a definitive diagnosis of BMD is considered. There was consensus (n=13/15, 87%) that chronicity criterion should not be changed. While there was a suggestion to consider changing chronicity to >1 month, reasoning that earlier diagnosis and treatment may result in improved prognosis for patients, this suggestion did not reach consensus (n=6/17, 35%).
Duration of symptoms (Temporality)
Table 6 describes the results of the Delphi Method findings regarding the proposed duration of symptoms criterion. The diagnostic criterion states, “the pain recurs daily for >2 hours on more than 50% of the days.” Consensus was reached (n=11/15, 73%) that the number of hours (>2 hours) symptoms occur should not be used as criterion for the clinical diagnosis of BMD, but consensus was not reached for how the percentage of days that symptoms last should be used. The rationale for changing the criterion included: 1) there is no evidence for the criteria; 2) it is difficult for patients to report accurately; and 3) the daily symptom duration varies among patients. These same explanations emerged when participants were asked what makes the duration of symptom criterion difficult to define.
Table 6.
Summary of the BMS duration of symptoms diagnostic criteria results
| Duration of symptoms | Responses | N (%) |
|---|---|---|
| Should the duration of symptom criteria "The pain recurs daily for >2 hours on more than 50% of the days” be changed? |
Yes No |
15/17 (88) 2/17 (12) |
| Duration of symptoms as core diagnostic criteria. | ||
| Do you think a daily time should be used as a cut off for the clinical diagnosis of BMS? | Yes No Unsure |
3/15 (20) 11/15 (73) 1/15 (7) |
| Do you think the percentage of days should be used as a cut off for the clinical diagnosis of BMS? | Yes No Unsure |
5/15 (33) 9/15 (60) 1/15 (7) |
| How should the percentage of days change? | ||
| Do not change the current criteria, “Symptoms should occur on >50% of the days” | Agree | 6/17 (35) |
| Symptoms should occur daily | Agree | 2/17 (12) |
| Simplify the statement to “Pain is present on most days” | Agree | 7/17 (41) |
| Other, please specify† | 2/17 (12) |
One individual did not specify, one wrote in chronicity rather than duration of symptom criteria
Items in bold reached consensus.
Additionally, we sought to understand how this duration criterion is perceived to affect clinical diagnosis. Participants responded by describing that the duration of symptoms criterion is likely ignored in clinical and/or research settings. Some experts predicted symptom duration could lead to misdiagnosis while others felt symptom duration accurately captures most patients and is unlikely to lead to misdiagnosis. Experts also noted that symptom duration may serve a broader purpose beyond core diagnostic criterion, such as to help phenotype patients, predict patient prognosis, and evaluate treatment efficacy.
Location
Consensus was not reached regarding whether the proposed wording for the ICD-11 diagnostic criterion for location: “the pain is felt superficially in the oral mucosa” should change (n=7/19, 37% agreed the statement should be changed). Three statements 1) multiple intraoral sites can be affected (n=15/17, 88%); 2) the most common site affected is the tongue (n=13/15, 87%); and 3) symptoms are often bilateral (n=11/15, 73%) met consensus to be added as a footnote to enhance the disease description of symptom location in the ICD-11.
Emotional and functional disability
The proposed diagnostic criteria of ICD-11 states, “the pain is characterized by at least one of the following: 1. significant emotional distress, 2. functional disability (in particular with orofacial function such as eating, yawning, speaking etc.).” There was consensus (n=14/15, 93%) that the proposed criteria on emotional distress and functional disability should not be required for diagnosis. Additionally, the terms “significant” (n=15/15, 100%) and “yawning” (n=12/15, 80%) should be removed from the statement. Experts noted that emotional distress is not present in all patients, and although it may help guide patient management, it should not be a requirement for diagnosis. Another concern was that “significant emotional distress” is not operationalized or defined and thus left to the subjective interpretation of each clinician. Experts therefore agreed (n=16/17, 94%) there is a need to validate measures on emotional distress in BMD patients. Themes emerged on functional disability including that; 1) it is not present in all patients; 2) disability may not accurately capture the patient’s experience; and 3) there may be relief of symptoms or exacerbation of symptoms when eating, which may depend on the individual and/or type of food/drink consumed.
Examination findings
The proposed ICD-11 diagnostic criteria state that the examination findings include “the oral mucosa is of normal appearance and no local or systemic causes explains the pain.” Consensus was reached to remove this diagnostic criterion (n=13/15, 87%) and instead utilize the statement “no local or systemic causes explain the pain in the oral mucosa.” The rationale for changing the ICD-11 examination criterion statement was to clarify that independent developmental alterations of the oral mucosa can exist in the oral mucosa of those with BMD and the term “normal mucosa” may be interpreted to exclude such cases from a BMD diagnosis. For example, fissured tongue may be present concurrent with BMD and some clinicians may consider this individual to not meet the original criterion statement of “the oral mucosa is of normal appearance.”
Consensus was reached (n=14/15, 93%) to replace “causative lesions” with “causes” and add “laboratory findings” to the ICD-11 disease description resulting in the following statement, “[…] without causes on clinical investigation/examination and laboratory findings.” There was consensus (n=14/17, 82%) for the disease definition to list local and systemic factors to be evaluated (at a minimum) in the diagnosis of BMD. The following factors met consensusc for evaluation: oral mucosal disease (n=12/14, 86%), parafunctional habit of the tongue (n=10/14, 71%), hyposalivation (n=11/14, 79%), oral candidiasis (n=12/14, 86%), anemia (n=12/14, 86%), B12 deficiency (n=13/15, 87%) and folate deficiency (n=12/15, 80%), diabetes mellitus (n=12/14, 86%), and medications (angiotensin converting enzyme inhibitors) (n=12/14, 86%). Further, the disease definition should specify (n=15/17, 88%) that if a local or systemic condition(s) is found, this abnormality should be treated and its contribution to oral burning symptoms should be made before a diagnosis of BMD is considered.
Pathophysiology
There was consensus (n=13/15, 87%) to add that BMD has “neuropathic characteristics” into the disease description statement, “burning mouth disorder is multifactorial: biological, psychological and social factors contribute to the pain condition with neuropathic characteristics.”
Triangulation of findings and member checking
Round 4 collected participant feedback about the study. Eighty percent (n=12/15) felt the Delphi survey was a “very effective” method and the remaining three (n=3/15, 20%) felt it was “moderately effective” to synthesize expert opinion. The majority felt the resulting suggested revisions to the ICD-11 diagnostic criteria and disease definition were a “very accurate” (n=12/15, 80%) representation of BMD with the remaining three individuals (n=3/15, 20%) reporting it was “moderately accurate.” All participants (n=15/15, 100%) reported that the suggested revisions resulted in an overall improvement in clarity of the disease definition and diagnostic criteria.
Discussion
This study sought to examine expert consensus to determine if revision in nomenclature and alternative names for BMS are warranted. Additionally, we sought to identify areas of consensus for changes to the ICD-11 disease description and proposed diagnostic criteria for BMS. The most important revisions recommended in this study include; 1) revising the established name of “Burning Mouth Syndrome” (BMS) to “Burning Mouth Disorder” (BMD); 2) removing “significant emotional distress” and “functional disability” from the proposed diagnostic criteria; and 3) adding a list of local and systemic causes to investigate in the diagnostic work up of oral burning symptoms. These findings could assist the WHO by more clearly defining BMD.
Nomenclature
This international expert group identified the term “syndrome” as inappropriate. A syndrome is associated with a collection of features, however ‘oral burning or dysesthesia’ is the only consistent feature amongst all patients; the other symptoms vary in their association with the condition. Consensus was achieved to revise the name to “Burning Mouth Disorder,” which has prior support [28,34]. This is consistent with an ontological approach and the IASP’s new classification for chronic pain as either a disease entity (chronic primary pain syndrome) or a symptom of a secondary disease (chronic secondary pain syndrome) [41]. However, since the classification by IASP continues to use the term “syndrome,” the results of this study suggest that modification should be reconsidered. It is unclear if other reported symptoms (i.e., xerostomia, dysgeusia) in BMD represent the same disease process or are the result of differing etiology and pathophysiology from the oral burning [7,20,23]. Other experts have proposed descriptive terminology that omits the word “syndrome” [18,29]. Future work is needed to determine the ramifications of a name change to “BMD” and receive input from stakeholders including non-specialists and patients.
Core Diagnostic Criteria
Core diagnostic criteria should include the signs and symptoms as well as the findings that must be present in all cases to classify correctly patients as having the disease of interest [15]. The core diagnostic criteria should be non-controversial and applied consistently to establish a diagnosis. Below is a detailed discussion of select proposed ICD-11 diagnostic criteria.
Emotional distress and functional disability
The proposed ICD-11 diagnostic criteria use the presence of either emotional distress or functional disability as a threshold for diagnosis, requiring at least one to be present. In this study experts agreed that emotional distress and functional disability should not be diagnostic criteria since not all individuals with the condition present with affective changes or functional disability. This finding is consistent with the beta version of the RDC/BMS.[9] Moreover, the proposed ICD-11 criteria regarding emotional distress and functional disability lack clear operationalized definitions and thresholds for diagnosis. In the proposed criteria, functional disability is narrowly defined to oral function such as “eating, yawning, and speaking.” However, “disability,” is conceptualized by the International Classification of Functioning Health and Disease (ICF) as the impairments, limitations, and restriction of not only body functions and structures, but also of activities and participation [31]. Psychological constructs and disability should be measured for the purpose of phenotyping, patient management [13] and understanding outcomes in therapeutic trials [12] but not to establish a diagnosis.
Duration of symptoms (Temporality)
The duration of symptoms, as written in the proposed ICD-11 diagnostic criterion, states that “the pain recurs daily for >2 hours on more than 50% of the days.” This criterion is separate from chronicity (i.e., the total length of time an individual has presented with symptoms) and describes the temporal daily pattern of the condition. In this study, 88% of experts agreed this criterion should change, and came to consensus to remove the daily number of hours. However, there was no agreement on how or if the percentage of days should change. Contrary to our finding, the IHS and subsequently RDC/BMS, state the symptoms should be “recurring daily” [2,9]. Although large cohort studies in BMD do not exist, smaller epidemiological studies found that the majority of patients with BMD report continuous burning symptoms but do not specify how “continuous” is defined [25]. It is therefore unlikely that removing the threshold of >2 hours will exclude true cases, however, it is important to note that this criterion requires further testing in large cohort studies, as well as refinement and revision before a final version is published [2,9].
The duration of symptoms criterion has the potential to result in under diagnosis of BMD and to skew the diagnosis towards severe cases by using the threshold of “daily” or even“50% of the days.” Benoliel et al [8], has previously suggested using the duration of symptoms criterion for classification of orofacial pain, rather than as a diagnostic threshold, in a similar approach that has been used to classify headache disorders. They described [5] a classification scheme using both the number of hours per day (>4 or ≤4 hours) and the number of days per month (<15 days per month and ≥15 days per month) in which symptoms occur. Consideration should be given to tracking symptom patterns in BMD that could help disease subtyping and be used to form homogenous groups in order to better understand the pathophysiology and prognosis of the condition, as well as treatment outcomes.
Disease definition
All suggested revisions to the proposed diagnostic criteria that met consensus were also mirrored in the ICD-11 disease description for consistency. Below is a discussion of select changes that were unique to the ICD-11 disease description.
The experts suggested local and systemic factors to be evaluated before a diagnosis of BMD is established. These included oral mucosal disease, parafunctional habit of the tongue, hyposalivation, oral candidiasis, anemia, B12 deficiency and folate deficiency, diabetes mellitus, and medications (e.g., angiotensin converting enzyme inhibitors). This list is consistent with the beta version of RDC/BMS, albeit a few differences in semantics exist. The RDC/BMS reduces some ambiguity of the umbrella term “oral mucosal disease” by listing types of “mucosal disease”, “trauma” and “metal/other allergies” to be excluded [9]. While in our study experts specified “hyposalivation” which may be secondary to a broad range of causes as compared to the term “salivary gland disorders”[9]. The ramifications of using different terminology may warrant further investigation. Both lists are considered required for the exclusion of secondary causes of oral burning and each should be further field tested and refined. The RDC/BMS also has provided a comprehensive list of laboratory investigations, which was not the focus of our study [9].
Experts in this study also recommended adding that BMD has neuropathic characteristics into the disease definition. Features of BMS such as burning, onset after trauma, and findings from quantitative sensory tests (QST) and brain imaging suggest a neuropathic pathophysiology in many cases.[17,22,24] However, not all individuals with BMD exhibit findings of neuropathic pain on clinical or more advanced sensory testing [22,41] and thus further investigation is needed to understand if those with “BMD” and somatosensory changes suggestive of a neuropathy represent the same disease as those without somatosensory changes [22,41]. The IASP takes this into consideration and has suggested two separate phenotypes for BMD based on the presence or absence of somatosensory alterations [29]. Due to the scope of this study, additional recommendations or the usefulness of tests such as quantitative sensory testing in the clinical diagnosis of BMD was not explored.
Strengths and Limitations
This study systematically collected and synthesized international expert knowledge using the Delphi survey method of consensus to modify the ICD-11 description and diagnostic criteria of BMD. Anonymity of experts and their responses encouraged freedom of thought, and equalized the voices of all experts while reducing conformity towards the most senior or outspoken expert as reflected by the wide range of suggestions and discussion items generated by participants. The methods used in this study are unique compared to previous attempts to modify diagnostic criteria for BMS. However, several limitations and biases should be noted. First, participants represent a select group of global experts in BMS with a skewed geographic distribution (United States and Europe). Additionally, there is potential to over represent the opinions of experts who believe the BMS nomenclature and diagnostic criteria need modification. Therefore, additional feedback from worldwide orofacial pain experts on the findings of this study is warranted. Although we were unable to reach consensus or discuss all aspects of BMD, consensus was reached on several critical areas of previous ambiguity or disagreement.
Conclusions
This international Delphi study demonstrated that experts agree that revisions should be made to the ICD-11 nomenclature, disease description, and proposed diagnostic criteria for BMS, and to rename this condition as BMD. There are however, items that remain controversial such as the duration of symptoms, symptom descriptors, and the role of subjective symptoms (i.e., xerostomia, dysgeusia), thus additional research efforts are required. Ultimately, in order to establish the gold standard for BMD diagnosis validation of diagnostic criteria is essential and continued re-appraisal based upon emerging evidence is warranted.
Supplementary Material
Acknowledgements
Research described in this publication was supported by award R25CA057711 and 2T32CA057711–26 from the National Cancer Institute of the National Institutes of Health. The funding organization had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Footnotes
Drs. Chmieliauskaite, Farag, Albuquerque, Ariyawardana, Carlson, Klasser, Nasri-Heir, Sardella, and Miller have previously published an editorial titled “Is burning mouth a syndrome or a disorder? A commentary.” The authors have no conflicts of interest to declare.
Proposed diagnostic criteria are not yet published but are available for review on the ICD-11 maintenance platform, a work in progress between released versions of ICD-11.
Only individuals who answered “Yes” to associated symptoms were added (n=12). Since only 12 participants answered the question on which associated symptoms were added the denominator for those questions was set at 12.
In Round 2 only the 14 experts who agreed that the disease definition should list the local and systemic factors were asked to select the specific factors that should be evaluated. However, vitamin B9 was suggested in the free responses and added to the voting in Round 3, which had 15 experts.
References
- [1].Headache classification committee of the international headache society (ihs) the international classification of headache disorders, 3rd edition. Cephalalgia 2018;38(1):1–211. [DOI] [PubMed] [Google Scholar]
- [2].International classification of orofacial pain, 1st edition (icop). Cephalalgia 2020;40(2):129–221. [DOI] [PubMed] [Google Scholar]
- [3].Ariyawardana A, Chmieliauskaite M, Farag AM, Albuquerque R, Forssell H, Nasri-Heir C, Klasser GD, Sardella A, Mignogna MD, Ingram M, Carlson CR, Miller CS. World workshop on oral medicine vii: Burning mouth syndrome: A systematic review of disease definitions and diagnostic criteria utilized in randomized clinical trials. Oral Dis 2019;25Suppl 1:141–156. [DOI] [PubMed] [Google Scholar]
- [4].Bender SD. Burning mouth syndrome. Dent Clin North Am 2018;62(4):585–596. [DOI] [PubMed] [Google Scholar]
- [5].Benoliel R, Eliav E, Sharav Y. Classification of chronic orofacial pain: Applicability of chronic headache criteria. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology 2010;110(6):729–737. [DOI] [PubMed] [Google Scholar]
- [6].Braud A, Touré B, Agbo-Godeau S, Descroix V, Boucher Y. Characteristics of pain assessed with visual analog scale and questionnaire in burning mouth syndrome patients: A pilot study. J Orofac Pain 2013;27(3):235–242. [DOI] [PubMed] [Google Scholar]
- [7].Ceusters W, Michelotti A, Raphael KG, Durham J, Ohrbach R. Perspectives on next steps in classification of oro-facial pain – part 1: Role of ontology. J Oral Rehabil 2015;42(12):926–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Crabtree BMW. Using codes and coding manuals: A template organizing style of interpretation. In: Crabtree B, editor. Doing qualitative research 2nd ed. Thousand Oaks, CA: Sage Publication, 1999. [Google Scholar]
- [9].Currie CC, Ohrbach R, De Leeuw R, Forssell H, Imamura Y, Jääskeläinen SK, Koutris M, Nasri-Heir C, Huann T, Renton T, Svensson P, Durham J. Developing a research diagnostic criteria for burning mouth syndrome: Results from an international delphi process. J Oral Rehabil 2020. [DOI] [PubMed] [Google Scholar]
- [10].Dalkey NHO. An experimental application of the delphi method to the use of experts. RAND; 1962. [Google Scholar]
- [11].Diamond IR, Grant RC, Feldman BM, Pencharz PB, Ling SC, Moore AM, Wales PW. Defining consensus: A systematic review recommends methodologic criteria for reporting of delphi studies. J Clin Epidemiol 2014;67(4):401–409. [DOI] [PubMed] [Google Scholar]
- [12].Dworkin RH, Turk DC, Farrar JT, Haythornthwaite JA, Jensen MP, Katz NP, Kerns RD, Stucki G, Allen RR, Bellamy N, Carr DB, Chandler J, Cowan P, Dionne R, Galer BS, Hertz S, Jadad AR, Kramer LD, Manning DC, Martin S, McCormick CG, McDermott MP, McGrath P, Quessy S, Rappaport BA, Robbins W, Robinson JP, Rothman M, Royal MA, Simon L, Stauffer JW, Stein W, Tollett J, Wernicke J, Witter J. Core outcome measures for chronic pain clinical trials: Immpact recommendations. Pain 2005;113(1–2):9–19. [DOI] [PubMed] [Google Scholar]
- [13].Edwards RR, Dworkin RH, Turk DC, Angst MS, Dionne R, Freeman R, Hansson P, Haroutounian S, Arendt-Nielsen L, Attal N, Baron R, Brell J, Bujanover S, Burke LB, Carr D, Chappell AS, Cowan P, Etropolski M, Fillingim RB, Gewandter JS, Katz NP, Kopecky EA, Markman JD, Nomikos G, Porter L, Rappaport BA, Rice ASC, Scavone JM, Scholz J, Simon LS, Smith SM, Tobias J, Tockarshewsky T, Veasley C, Versavel M, Wasan AD, Wen W, Yarnitsky D. Patient phenotyping in clinical trials of chronic pain treatments: Immpact recommendations. Pain 2016;157(9):1851–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Femiano F, Gombos F, Scully C, Busciolano M, De Luca P. Burning mouth syndrome (bms): Controlled open trial of the efficacy of alpha-lipoic acid (thioctic acid) on symptomatology. Oral Dis 2000;6(5):274–277. [DOI] [PubMed] [Google Scholar]
- [15].Fillingim RB, Bruehl S, Dworkin RH, Dworkin SF, Loeser JD, Turk DC, Widerstrom-Noga E, Arnold L, Bennett R, Edwards RR, Freeman R, Gewandter J, Hertz S, Hochberg M, Krane E, Mantyh PW, Markman J, Neogi T, Ohrbach R, Paice JA, Porreca F, Rappaport BA, Smith SM, Smith TJ, Sullivan MD, Verne GN, Wasan AD, Wesselmann U. The acttion-american pain society pain taxonomy (aapt): An evidence-based and multidimensional approach to classifying chronic pain conditions. J Pain 2014;15(3):241–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Formaker BK, Frank ME. Taste function in patients with oral burning. Chem Senses 2000;25(5):575–581. [DOI] [PubMed] [Google Scholar]
- [17].Forssell H, Jääskeläinen S, List T, Svensson P, Baad-Hansen L. An update on pathophysiological mechanisms related to idiopathic oro-facial pain conditions with implications for management. J Oral Rehabil 2015;42(4):300–322. [DOI] [PubMed] [Google Scholar]
- [18].Fortuna G, Di Lorenzo M, Pollio A. Complex oral sensitivity disorder: A reappraisal of current classification of burning mouth syndrome. Oral Dis 2013;19(7):730–732. [DOI] [PubMed] [Google Scholar]
- [19].Grushka M, Sessle BJ. Burning mouth syndrome: A historical review. The Clinical Journal of Pain 1986;2(4):245–252. [Google Scholar]
- [20].Hershkovich O, Nagler RM. Biochemical analysis of saliva and taste acuity evaluation in patients with burning mouth syndrome, xerostomia and/or gustatory disturbances. Arch Oral Biol 2004;49(7):515–522. [DOI] [PubMed] [Google Scholar]
- [21].Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res 2005;15(9):1277–1288. [DOI] [PubMed] [Google Scholar]
- [22].Jaaskelainen SK. Is burning mouth syndrome a neuropathic pain condition? Pain 2018;159(3):610–613. [DOI] [PubMed] [Google Scholar]
- [23].Jääskeläinen SK, Woda A. Burning mouth syndrome. Cephalalgia 2017;37(7):627–647. [DOI] [PubMed] [Google Scholar]
- [24].Khan SA, Keaser ML, Meiller TF, Seminowicz DA. Altered structure and function in the hippocampus and medial prefrontal cortex in patients with burning mouth syndrome. Pain 2014;155(8):1472–1480. [DOI] [PubMed] [Google Scholar]
- [25].Kohorst JJ, Bruce AJ, Torgerson RR, Schenck LA, Davis MDP. A population-based study of the incidence of burning mouth syndrome. Mayo Clinic proceedings 2014;89(11):1545–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lamey PJ. Burning mouth syndrome. Dermatol Clin 1996;14(2):339–354. [DOI] [PubMed] [Google Scholar]
- [27].McMillan R, Forssell H, Buchanan JA, Glenny AM, Weldon JC, Zakrzewska JM. Interventions for treating burning mouth syndrome. Cochrane Database Syst Rev 2016;11:Cd002779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Miller CS, Farag AM, Chmieliauskaite M, Ariyawardana A, Albuquerque R, Carlson CR, Forssell H, Klasser GD, Nasri-Heir C, Mignogna MD, Sardella A. Is burning mouth a syndrome or a disorder? A commentary. Oral Surg Oral Med Oral Pathol Oral Radiol 2019;127(5):361–363. [DOI] [PubMed] [Google Scholar]
- [29].Nicholas M, Vlaeyen JWS, Rief W, Barke A, Aziz Q, Benoliel R, Cohen M, Evers S, Giamberardino MA, Goebel A, Korwisi B, Perrot S, Svensson P, Wang SJ, Treede RD. The iasp classification of chronic pain for icd-11: Chronic primary pain. Pain 2019;160(1):28–37. [DOI] [PubMed] [Google Scholar]
- [30].Okoli C, Pawlowski SD. The delphi method as a research tool: An example, design considerations and applications. Information & Management 2004;42(1):15–29. [Google Scholar]
- [31].World Health Organization. ‘Towards a common language for functioning, disability and health icf.’ https://www.who.int/classifications/icf/icfbeginnersguide.pdf?ua=1. April 15, 2020.
- [32].Page A, Potter K, Clifford R, McLachlan A, Etherton-Beer C. Prescribing for australians living with dementia: Study protocol using the delphi technique. BMJ Open 2015;5(8):e008048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Perier JM, Boucher Y. History of burning mouth syndrome (1800–1950): A review. Oral Dis 2019;25(2):425–438. [DOI] [PubMed] [Google Scholar]
- [34].Rhodus NL, Carlson CR, Miller CS. Burning mouth (syndrome) disorder. Quintessence Int 2003;34(8):587–593. [PubMed] [Google Scholar]
- [35].Rotondi A GD. Theoretical, methodological and practical issues arising out of the delphi method. In: Ziglio E, editor. Gazing into the oracle: The delphi method and its application to social policy and public health. London: Jessica Kingsley Publisher, 1996. [Google Scholar]
- [36].Stedman TL. Stedman’s medical dictionary for the health professions and nursing. Phildelphia: Lippincott Williams & Wilkins, 2005. [Google Scholar]
- [37].Steurer J The delphi method: An efficient procedure to generate knowledge. Skeletal Radiol 2011;40(8):959–961. [DOI] [PubMed] [Google Scholar]
- [38].Su N, Poon R, Liu C, Dewan C, Darling M, Grushka M. Taste and pain response in burning mouth syndrome with and without geographic tongue. J Oral Facial Pain Headache 2020;34(3):217–221. [DOI] [PubMed] [Google Scholar]
- [39].Sugaya NN, da Silva EFP, Kato IT, Prates R, Gallo CD, Pellegrini VD. Low intensity laser therapy in patients with burning mouth syndrome: A randomized, placebo-controlled study. Brazilian Oral Research 2016;30(1):9. [DOI] [PubMed] [Google Scholar]
- [40].Tammiala-Salonen T, Forssell H. Trazodone in burning mouth pain: A placebo-controlled, double-blind study. J Orofac Pain 1999;13(2):83–88. [PubMed] [Google Scholar]
- [41].Treede RD, Rief W, Barke A, Aziz Q, Bennett MI, Benoliel R, Cohen M, Evers S, Finnerup NB, First MB, Giamberardino MA, Kaasa S, Korwisi B, Kosek E, Lavandʼhomme P, Nicholas M, Perrot S, Scholz J, Schug S, Smith BH, Svensson P, Vlaeyen JWS, Wang SJ. Chronic pain as a symptom or a disease: The iasp classification of chronic pain for the international classification of diseases (icd-11). Pain 2019;160(1):19–27. [DOI] [PubMed] [Google Scholar]
- [42].Valenzuela S, Pons-Fuster A, Lopez-Jornet P. Effect of a 2% topical chamomile application for treating burning mouth syndrome: A controlled clinical trial. J Oral Pathol Med 2016;45(7):528–533. [DOI] [PubMed] [Google Scholar]
- [43].Viera AJ, Garrett JM. Understanding interobserver agreement: The kappa statistic. Fam Med 2005;37(5):360–363. [PubMed] [Google Scholar]
- [44].Vogel C, Zwolinsky S, Griffiths C, Hobbs M, Henderson E, Wilkins E. A delphi study to build consensus on the definition and use of big data in obesity research. International Journal of Obesity 2019;43(12):2573–2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].World Health Organization. ‘Icd-11 for mortality and morbidity statistics - burning mouth syndrome.’ http://id.who.int/icd/entity/618998878 June 1, 2020. [Google Scholar]
- [46].Ziglio E The delphi method and its contribution to decision-making. In: Adler M, editor. Gazing into the oracle : The delphi method and its application to social policy and public health. London: Jessica Kingsley Publishers, 1996. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


