Abstract
Objectives
The aim of the study was to formulate and characterize the farnesol loaded niosomes comprising gel formulation and perform their in vitro–in vivo evaluation for applications in the treatment of oral candidiasis infections.
Methods
Various gelling systems were evaluated for their rheological and stability properties. The formulation was statistically optimized using experimental design method (Box-Behnken). Transmission electron microscopy (TEM) and Atomic force microscopy (AFM) were used to observe the niosomal surface morphology. Centrifugation method and dialysis method were used to find out the % entrapment efficiency (%EE) and in-vitro release of Farnesol, respectively. In-vitro antifungal effect and cell biocompatibility of the Farnesol loaded niosomal gel was also performed using Candida albicans (C. albicans) as the model organism and epithelial cell line (SW480) by MTT cytotoxicity assay. In-vivo skin irritation test was performed on rabbit skin.
Key findings
Farnesol loaded niosomes were integrated into polymeric gel solution. The optimized formulation demonstrated acceptable % EE (>80%) and an optimum particle size (168.8 nm) along with a sustained release and a long-term storage stability for up to a period of 6 months. TEM and AFM observations displayed a spherical niosome morphology. Farnesol niosomal gel showed a higher antifungal efficacy, ex-vivo skin permeation and deposition in comparison to plain farnesol solution. The niosomal gel also showed negligible cytotoxicity to normal cells citing biocompatibility and was found to be non-toxic and non-irritant to rabbit skin.
Conclusions
This novel niosome loaded gel-based formulation could make the oral candidiasis healing process more efficient while improving patient compliance. With the optimized methodology used in this work, such formulation approaches can become an efficient, industrially scalable, and cost-effective alternatives to the existing conventional formulations.
Keywords: Niosomes, Gel, Farnesol, Candida albicans, Electron microscopy, Atomic Force Microscopy, Nano carrier
Niosomes; Gel; Farnesol; Candida albicans; Electron microscopy; Atomic Force Microscopy; Nano carrier.
1. Introduction
Candia albicans microorganism is a part of the normal oral flora without causing any harm. Moreover, it is a commensal microorganism and considered as a one of the foremost species responsible for candidiasis infection which can cause numerous oral health issues [1, 2]. Oral candidiasis infections induced by C. albicans have been reported in humans with poor oral health, human immunodeficiency virus (HIV), post-radiation cancer patients and smokers [3]. Various antifungal drugs such as fluconazole, nystatin etc. are available in tablet form for the treatment of Oral candidiasis infections. Although they are effective in eradication of candida infections, their availability as oral gel is scarce due to instability of the drug in aqueous solutions and tissue irritation. Farnesol is a novel drug that has shown effective antifungal activity against candida infection. However, Farnesol is not available in any form of treatment for candida infections [4, 5].
The common oral microbial flora is considered as a primary causative factor for various oral infectious diseases. Oral candidiasis is one of the most prevalent infections caused due to candida species mainly C. albicans species [6]. As per the earlier reports, Farnesol can be can be utilized for oral candidiasis treatment due to its potent antifungal properties [7, 8]. Moreover, inclusion of an antifungal drug such as Farnesol in lipidic nanocarrier such as niosomes allows sustained release of the drug hence, ultimately decreasing the active dose needed for the treatment and thereby reducing the toxicity and irritation to the oral mucosa layer [9, 10, 11].
Niosomes represent one of the promising options for entrapment of drugs with hydrophilic and hydrophobic solubility [12, 13]. Niosomes are self-assembly based amphiphilic structures which are formed when a polar lipid (such as cholesterol) and a non-ionic surfactant are mixed in a specific ratio. Niosomes as a nano-carrier have offered various benefits such as economical fabrication, high stability for nano drug delivery, low systemic toxicity etc. [14, 15, 16, 17]. Niosomes have also shown improved bioavailability, high biocompatibility, reduction in dose concentration, and a sustained drug release [16]. Moreover, addition of niosomes in oral gel formulation can increases the bioavailability and decrease the dosage of the drug and provide a sustained release action [17].
Gelling agents are known to create a three-dimensional network and can be used as a beneficial carrier for the localized drug delivery in the treatment of oral fungal infections. Integration of farnesol loaded niosomes in the gel formulation facilitates an even distribution and migration of the niosomes [18, 19]. Furthermore, gels provide hydration to the skin by retaining a significant amount of trans-epidermal water and hence enabling drug transport [20].
In this study, niosomes were used as a nano carrier for entrapment of farnesol in fabrication of a gel-based formulation for applications in the treatment of oral candidiasis. Niosomal surface morphology were evaluated using AFM and TEM along with stability and rheological properties of the gel system. In vitro antifungal activity of the optimized niosomal gel was examined alongside plain farnesol drug solution using C. albicans as an in-vitro surrogate model. The topical delivery and skin irritation study of niosomal gel containing farnesol was studied in vitro using a Franz diffusion cell and rabbit skin respectively. Cell biocompatibility was assessed in vitro using epithelial cell line (SW480) by MTT cytotoxicity assay.
2. Materials and methods
2.1. Materials
Farnesol was procured from Sigma Aldrich (St. Louis, MO, USA). Cholesterol, Span 20 ethanol, triethanolamine, sodium chloride, disodium hydrogen phosphate, sodium dihydrogen phosphate, and Carbopol polymer 934P were attained from Sisco Research Laboratories (SRL) (Maharashtra, India). The dialysis bag (MW 12–14 kDa) was acquired from Serva, Heidelberg, Germany. All the reagents were of analytical grade and were used without any additional purification. Deionized water was used for all the experiments.
2.2. Methods
2.2.1. Preparation and optimization of Farnesol loaded niosomes
Ethanol injection (EI) method was used for the preparation of farnesol loaded niosomes [16, 17, 18]. The Span 20 (non-ionic surfactant) and the cholesterol (lipid) were dissolved in ethanol in a ratio of 1:1 (36.4: 36.4 mg). The hydrophobic drug FAR was added to ethanol in the above beaker in 0.5 (18.2 mg) ratio and subjected to bath sonication until a clear solution was obtained (organic phase). In a separate beaker, 100 ml deionized water to prepare the aqueous phase. The organic phase containing FAR was added to the water containing aqueous phase dropwise at a rate of 1 ml/min using a fine-bore syringe. This solution was then subjected to high-speed homogenization at 8500 rpm for an hour, maintained at 60 °C until the solvent evaporated. Solvent evaporation and the succeeding temperature difference created between the phases leads to vesicular formation characteristic of niosomes. The resultant niosomes were kept under refrigeration at 4–8 °C for further studies [21, 22].
Experimental design (Box-Behnken) was used to perform statistical optimization of the FAR loaded niosomes using Design Expert software (version 10.0, USA) with 3 independent and 2 dependent variables. The Independent variables were-X1-Cholesterol: Span 20 ratio; X2- Farnesol concentration (mg) and; X3- Homogenization speed (rpm) and the dependent variables were Y1-Particle size (nm) and Y2- % Entrapment efficiency of Farnesol. Due to a provision of 15 trials which Box-Behnken method provides, a better control over the optimization parameters can be done giving an accurate estimation of formulation model selection (Table 1).
Table 1.
Box-Behnken design variables for preparing Farnesol loaded niosomes.
| Factor | Level used (actual coded) |
|||
|---|---|---|---|---|
| Low (-1) | Medium (0) | High (1) | ||
| Independent variables | ||||
| A | Cholesterol: Span 20 (ratio) | 0.75:0.5 | 01:01 | 1.25:1.5 |
| B | Farnesol (mg) | 0.25 | 0.375 | 0.5 |
| C |
Homogenization speed (rpm) |
7000 |
8500 |
10000 |
| Dependent variables | ||||
| A | Particle size(nm) | |||
| B | % Entrapment efficiency | |||
| (Farnesol) | ||||
2.3. Characterization studies of Farnesol loaded niosomes
2.3.1. Niosome particle size and zeta potential measurements
Mean average particle size (nm), Polydispersity index (PDI) of FAR loaded niosomes were measured using Dynamic Light Scattering (DLS), by means of a Malvern Zetasizer Nano ZS (Malvern Instruments, UK) provided with a backscattered light detector at 173°. Similarly, zeta potential values (mV) were measured using laser Doppler anemometry. Triplicate measurements were performed at 25 °C [23].
2.3.2. Surface morphological studies
Atomic Force Microscopy (AFM, Nanosurf C3000, Switzerland) was used to evaluate the niosomal surface morphology (blank and FAR loaded niosomes). Ten microliters of the niosomes solution were placed on the mica plate, air dried, and analysed in tapping mode for the analysis. Low-stress silicon nitride cantilevers were used for both the experiments [24]. Optimised FAR loaded niosomes were examined using Transmission Electronic Microscopy (TEM) (Tecnai 20, Philips, Holland) at 200 kV of accelerating voltage. Samples were stained using 1% phosphotungstic acid by thorough mixing in order to stain the niosomes. The samples were air dried following placing them gently over a carbon coated copper grid. The grids were then mounted in the instrument, and pictures were taken at various magnifications [24].
2.3.3. Fourier transform infrared spectroscopy – FTIR and differential scanning calorimeter (DSC) measurements
For FTIR analysis, blank niosomes, FAR loaded niosomes and plain FAR were selected for measurement (Jasco FT/IR 4700, Shimadzu, Japan). Methanol and kim-wipe paper were used to clean the Attenuated total reflectance (ATR) crystal and the pressing pin respectively. Small quantities of samples were put on the crystal located in the centre of dwell of the instrument to record the spectra in reflectance mode. An overlay was also taken to investigate FAR presence in niosomes. For the DSC analysis, 2 mg of dried samples of niosomes were selected and for the measurement, a 50 °C–450 °C temperature range along with a 20 ml/min N2 flow rate and a constant heating rate of 20 °C were employed [25, 26].
2.3.4. Entrapment efficiency (% EE) of Farnesol loaded niosomes
The Entrapment Efficiency (% EE) of FAR loaded niosomes was calculated using an indirect method [27]. The niosomes were separated via centrifugation at 15000 rpm and at 4 °C for 15 min. The free drug in the supernatant were measured using UV spectrophotometry at 228 nm (Jasco, Model UV-2400, Japan). The %EE was calculated by plotting standard curve using difference between the total amount of drug (FAR) used to prepare niosomes and the amount found in the supernatant [27]. Measurements were done in triplicates and the % EE of FAR from niosomes were determined using the following Eq. (1) [27]:
| Equation 1 |
2.4. Preparation of gel formulation
To prepare the control and FAR loaded niosome gel, 1.5% w/w of Carbopol 934 was slowly dissolved in 100 mL deionized water under constant stirring for an hour to avoid agglomeration and the pH (6.4) was adjusted using triethanolamine. Prior to Carbopol 934 addition, Propyl paraben and Methyl paraben were added to water. In another beaker, required quantity of FAR loaded niosomes or plain FAR were taken and subsequently dispersed in the Carbopol formulation containing beaker while stirring until a homogeneous product was formed. The remaining volume were made up with distilled water [28]. Then, all the resultant gels were evaluated in order to select the most optimum one. Different gel formulations and their composition are listed in Table 2.
Table 2.
Composition of Farnesol loaded niosomal gel formulation.
| Material | Quantity (% w/w) |
|---|---|
| Farnesol (g) | 0.75–2.5 |
| Carbopol 934 (g) | 1.5 |
| Propylene Glycol (ml) | 15 |
| Glycerine (ml) | 5 |
| Methyl Paraben (g) | 0.18 |
| Propyl Paraben (g) | 0.02 |
| Triethanolamine (ml) | To adjust pH upto 6.4 |
| Deionized water | q. s |
2.4.1. Evaluation of Farnesol loaded niosomes gel formulations
Clarity, pH, and viscosity of the gel formulations were assessed, along with spreadability, drug content, gel strength, and extrudability. The experiments were repeated four times.
-
(a)
Clarity of formulations
Visual inspection of gel formulations were done to assess the clarity under a black and white background.
-
(b)
pH and Viscosity of gel
The pH of the niosomal gels was determined via a pH meter (Mettler Toledo, Switzerland) calibrated using a standard buffer prior to the analysis. A viscometer was used to measure the gel's viscosity at 25 °C with a 0.3 rpm spindle speed.
-
(c)
Spreadability test
The spreadability test of the FAR loaded niosomal gel was investigated by a previously reported method [29]. For analysis, 1 g of niosomal gel was deposited in a pre-marked of 1.2 cm on a glass plate, and was covered with a second glass plate subsequently. The upper glass plate was subjected to 73 g of weight placed on it for 1 min. The dispersion of the gels is caused by the weight on top of the plate, which was hence recorded for the test.
-
(d)
Drug content
1ml of FAR loaded niosomal gel was extracted with 10 ml phosphate buffer at pH 6.4 by vertexing (vortex shaker, HICON, India). Further dilutions were made with pH 6.4, phosphate buffer and analysed spectrophotometrically at 228 nm.
-
(e)
Gel strength
FAR loaded niosomal gel formulation (50g) was placed in a 100 ml graduated cylinder and gelled at 37 °C to determine the gel strength. A 35 g weight was placed on the gelled solution and allowed to penetrate 5 cm. The weight's sinking time up to 5 cm was recorded for measurement [30].
-
(f)
Extrudability
FAR loaded niosomal gel formulations were evaluated for its extrudability using a modified tester. 15 g of prepared niosomal formulation was placed in the aluminium tube and the plunger was used to keep the tube in place. Then, a pressure of 1 kg/cm2 was applied for 30 s forcing the extrusion of the gel from the tube. It was then weighed and the measurement was repeated at 3 equidistance places of the tube [31].
2.4.2. In vitro drug release studies
FAR loaded niosomes were soaked in aqueous medium (PBS; pH 7.4) containing sodium salicylate (sink medium for released Farnesol) [32]. To investigate the release profile, samples were taken into dialysis membrane bag (MWCO: 12–14 kDa) at 37 ± 1 °C in a hatchery shaker. Samples were stored in a sealed environment to prevent evaporation. Release medium was stirred at 100 rpm and 3 ml of samples were extracted and replaced with an equal amount of fresh medium at fixed time points. The amount of FAR released from the niosomes was evaluated spectrophotometrically at 228 nm and release profiles were plotted (Jasco, Model UV-2400, Japan). Mathematical evaluation of the release data from niosomes was kinetically analysed using multiple models (zero order, first order, Korsmeyer-Peppas, and Higuchi model) [33, 34].
A modified Franz diffusion cell apparatus was used for evaluating the release of FAR from niosomal gel. Between the donor and receptor compartments, a 100 mg equivalent FAR loaded niosomal gel was placed on the dialysis membrane and PBS (pH 7.4) was used to fill the receptor compartment kept at 37 ± 1 °C and magnetically stirred at 50 rpm. The FAR content was evaluated by taking 1mL of receptor fluid at fixed time intervals. The withdrawn amount was replaced with the same amount of freshly prepared buffer and measured spectrophotometrically at 228 nm (Jasco, Model UV-2400, Japan) [35].
2.4.3. Stability studies
FAR loaded niosomes and FAR loaded niosomal gels were stored for 6 months in firmly sealed autoclavable transparent glass vials at 4 ± 2 °C, 25 ± 2 °C/60 % RH (relative humidity) ± 5% RH, and 45 ± 2 °C/60 % RH 5% RH (in humidity chamber) according to ICH (international conference on harmonisation) guidelines, Q1A (R2). At the end of each month, samples from each vial were collected and analysed for particle size (nm) and % EE of FAR. Samples of FAR loaded niosomal gels were also tested for pH, viscosity, and drug release [36].
2.4.4. Antifungal activity of the Farnesol loaded niosomal gel against Candida yeasts
Using the agar diffusion method, the antifungal activity of FAR loaded niosomal gel and plain FAR drug was evaluated in vitro against C. albicans strains (ATCC 18804) under aseptic conditions. C. albicans was cultivated on sabouraud dextrose agar (SDA) for 48 h and a suspension of 1 × 103 CFU/ml was prepared. 1 mL suspension was added to 9 mL SDA medium and evenly dispersed on plates at 45 °C. Then, two wells with 5 mm diameter were created in the medium. Finally, wells were filled with plain FAR drug and FAR loaded Niosomal gel (at 2.5 % w/w concentration) and incubated for 24 h at 35 °C. After that, a digital calliper was used to measure the inhibitory zone diameter in mm [37].
2.4.5. Minimal inhibitory concentrations (MICs) of Farnesol loaded niosomal gel
Following their antifungal activities, the MICs of plain FAR and FAR loaded niosomal gel were assessed. The CLSI (Clinical and Laboratory Standards Institute) criteria were used to calculate the MICs against C. albicans strains [38]. Using a Mueller-Hinton broth, two-fold serial dilutions of both plain FAR drug and FAR loaded niosomal gel were prepared for the MIC study. The wells were inoculated with diluted broth culture to provide an initial concentration of 105 CFU/ml and incubated at 37 ± 1 °C for 24–72 h at 100 rpm in a shaking incubator. The concentrations showing no visible growth were recorded which corresponds to C. albicans growth inhibition. C. albicans strains suspended in broth were utilized as positive controls, and formulations without FAR were used as the blank [39].
2.4.6. Cytotoxicity assay
MTT assay was used for evaluating the cytotoxicity of the plain FAR drug and FAR loaded niosomal gel [38]. The epithelial cell line SW480 was cultured in 500 mL of complete cell culture medium (DMEM) supplemented with 10% foetal bovine serum, 50 mL penicillin/streptomycin (PS), and 250 mL L-glutamine for 24–72 h at 37 °C with 5% CO2. The cells were then exposed for 24–72 h to plain FAR drug and FAR loaded niosomal gel. The viability of the cells was determined using an ELISA reader (Star Sate, Germany) and the MTT test at 570 nm. The % cell viability was calculated by dividing the absorbance of the treated samples by the absorbance of the control samples (taken as 100 %). Triplicate measurements were performed and compared to the control group (cells treated without FAR drug and FAR loaded niosomal gel) [40, 41, 42].
2.5. Ex-vivo and in-vivo analysis
2.5.1. Ex-vivo drug permeation and skin deposition studies
Ex-vivo drug permeation studies were conducted using goat oral mucosal skin obtained from a nearby slaughterhouse as a dialysis membrane. Ex-vivo drug release study was performed using a modified permeation apparatus that consists of donor compartment and the receptor compartment cell. The donor compartment has two open ends, containing one previously covered with animal skin and soaked in phosphate buffer (pH 7.4). Then, in the centre of the oral mucosal skin, 1g of control gel containing plain FAR drug and samples of optimum FAR loaded niosomal gel were placed and attached to a specially made glass cylinder. The receptor compartment was filled with 50 mL of phosphate buffer (pH 7.4) in a beaker kept at 37 °C and with continual stirring. To ensure optimal sink conditions, the samples were withdrawn at regular intervals for 24 h and replaced with fresh buffer solution. Spectrophotometry was used to evaluate the absorbance of the samples at 228 nm [35, 43]. For skin deposition studies, after 24 h, the treated skin was cleansed with distilled water, separated and cut into small pieces, then sonicated to extract methanol. The extracts were filtered using a 0.45 μm filter and the FAR concentration was evaluated using UV spectroscopy at 228 nm [44].
2.5.2. Preparations for the in-vivo skin irritation test
Rabbit skin irritation test, acute dermal irritation tests were performed using three rabbits according to OECD 404 guidelines with little modifications after obtaining the Institutional Animal Ethics Committee Approval [IAEC/IRD/GFSU/2019–20/09]. The animal's fur was removed 24 h before the test by trimming the dorsal portion of the trunk using an electric clipper at various locations. Two sites received 0.5 g of Farnesol-loaded niosomal gel, while the other site served as control. Sites were observed critically at 1 h after removal of test substance. The observations were repeated for a period of 24–72 h. The scoring system was used to evaluate erythema and edema skin reactions [45, 46].
For each rabbit, the Score of Primary Irritation (SPI) was calculated. Scores for erythema and edema were summed for the treated sites for 24–72 h and divided by the number of the observations. The SPI for the control sites were calculated in a similar way. The differences between the summary scores from the control and treated sites were calculated. The Primary Irritation Index (PII) was calculated as the arithmetical mean of the SPI values. The irritation degree was categorized based on the PII as negligible, or slight, moderate or severe irritation (Table 3A and B).
Table 3.
A) The scoring system for skin reactions (skin irritation test); B) The Score of Primary Irritation (SPI) index.
| (A) The scoring system for skin reactions (skin irritation test) | |
|---|---|
| Reaction | Score |
| Erythema | |
| No erythema | 0 |
| Very slight erythema (barely perceptible) | 1 |
| Well-defined erythema | 2 |
| Moderate to severe erythema | 3 |
| Severe erythema (beet redness) to eschar formation |
4 |
| Edema | |
| No edema | 0 |
| Very slight edema (barely perceptible) | 1 |
| Well-defined edema (edges of the area well defined by definite raising) | 2 |
| Moderate edema (raising approximately 1 mm) | 3 |
| Severe edema (raised more than 1 mm and extending beyond the area of exposure) | 4 |
| Total possible score for primary irritation |
8 |
| (B) The Score of Primary Irritation (SPI) index | |
|
Category |
Primary Irritation Index (PII) |
| Negligible | 0–0.4 |
| Slight irritation | 0.5–1.9 |
| Moderate irritation | 2–4.9 |
| Severe irritation | 5–8 |
2.6. Statistical analysis
All experiments were performed in triplicate and values were expressed as average standard deviation. Statistical analyses were performed using the software package (Version 6.01, GraphPad, San Diego, CA, USA). Differences were considered statistically significant at p < 0.05.
3. Results
3.1. Characterisations of Farnesol loaded niosomes
3.1.1. Particle size, zeta potential and niosome surface morphology measurements
The average particle size of the niosomes were in the range of 150–170 nm showing a homogenous dispersion. PDI for blank and FAR loaded niosomes were 0.120 and 0.182 respectively. A polydispersity index value < 0.2 indicates uniformity in the formulations [47, 48]. The zeta potential was found to be -32.3 ± 3 mV and -33.5 ± 2 mV for blank and FAR loaded niosomes, respectively. From the surface morphology studies, both AFM and TEM evaluation displayed a spherical shape of the niosome vesicles indicating a homogenous distribution. The particle size of the vesicles were also found in the range of 150–170 nm which were in accordance with the DLS results. In the AFM spectra, some visible larger shaped clustered vesicles were probably due to the reaction of the lipidic vesicle with the mica substrate of the sample plate (Figure 1) [17].
Figure 1.
A, B) Particle size, zeta potential measurements and C) Atomic Force Microscopy D) Transmission Electron Microscope images of optimized Farnesol loaded niosomes.
3.1.2. Formulation optimization using Box-Behnken experimental design
From the results, a significant interaction between the independent and dependent variables was evident. For particle size, as the values of X1 (Cholesterol: Span 20 ratio) and X2 (FAR concentration) variables increased, the particle size also increased which was deemed as unfavourable for the study. Similar results were observed for the dependent variable Y2- % EE of Farnesol, where an increase in the concentrations of X1 and X2 caused a significant decrease in the % EE (Table 4).
Table 4.
Box-Behnken design responses observed for Farnesol loaded niosomes (Mean ± S.D) (n = 3).
| Independent variables |
Dependent variables |
||||
|---|---|---|---|---|---|
| Run | A: Cholesterol: Span 20 (ratio) | B: Farnesol (mg) | C: Homogenization speed (rpm) | Particle size (nm) | % Entrapment efficiency (Farnesol) |
| 1 | 0 | 0 | 0 | 230.12 ± 2.21 | 69.62 ± 0.10 |
| 2 | 0 | 1 | -1 | 310.13 ± 1.12 | 58.83 ± 0.44 |
| 3 | 1 | 0 | -1 | 342.42 ± 4.91 | 49.74 ± 1.12 |
| 4 | 0 | -1 | 1 | 289.32 ± 0.54 | 70.32 ± 0.42 |
| 5 | -1 | -1 | 0 | 154.12 ± 0.54 | 79.93 ± 0.41 |
| 6 | 0 | 0 | 0 | 231.13 ± 4.71 | 69.61 ± 3.22 |
| 7 | -1 | 1 | 0 | 168.36 ± 2.32 | 83.53 ± 0.22 |
| 8 | 0 | 0 | 0 | 230.34 ± 1.21 | 69.94 ± 0.34 |
| 9 | 1 | -1 | 0 | 325.43 ± 3.22 | 61.22 ± 0.41 |
| 10 | -1 | 0 | 1 | 210.14 ± 2.12 | 67.41 ± 1.91 |
| 11 | 1 | 0 | 1 | 388.31 ± 5.24 | 45.23 ± 0.15 |
| 12 | 1 | 1 | 0 | 356.26 ± 3.73 | 56.42 ± 0.31 |
| 13 | -1 | 0 | -1 | 250.33 ± 1.72 | 65.44 ± 0.22 |
| 14 | 0 | 1 | 1 | 386.09 ± 6.24 | 52.73 ± 1.41 |
| 15 | 0 | -1 | -1 | 264.31 ± 1.11 | 65.24 ± 0.14 |
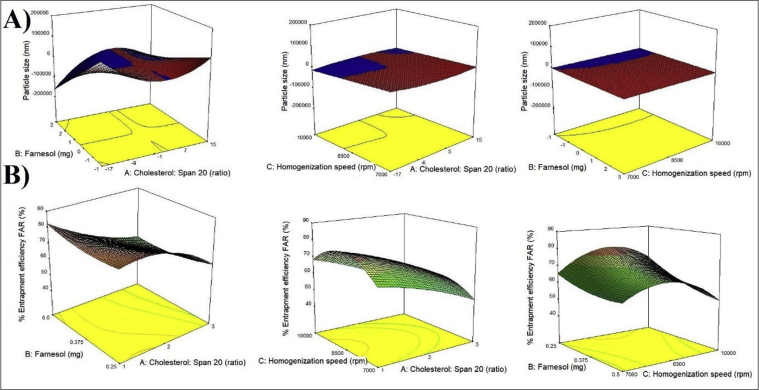
Whereas, for the variable X3, slightly different results were observed. As values of X3 increased, the particle size (Y1) decreased and Y2 increased which was favourable for the study. However, these results were observed only until a certain limit of speed i.e. up to 8500 rpm. A homogenization speed above this caused a rapid increase and a decrease in the particle size and % EE of the niosomes respectively. The effects of the independent variables on the dependent variables have been depicted in the 3-D response surface graphs in Figure 2 (Table 5).
Figure 2.
Three-dimensional response plots displaying the effects of independent variables on A) Particle size and, B) Entrapment efficiency of Farnesol. (n = 3).
Table 5.
A) Responses of regression analysis for Y1 (Particle size), Y2 (%EE FAR); B) Optimized niosomal formula.
| A) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Response | Model | Adequate precision | R2 | Adjusted R2 | Predicted R2 | SD | % CV | p-value |
| Y1 (Particle size) | Linear, Quadratic | 43.96 | 0.99 | 0.99 | 0.83 | 7.27 | 2.59 | <0.0001 |
|
Y2 (%EE FAR) |
Quadratic |
31.1 |
0.99 |
0.98 |
0.92 |
0.79 |
2.22 |
0.0004 |
| B) | ||||||||
| Factor | Optimized level | |||||||
| X1: Cholesterol: Span20 (ratio) | 1:1 | |||||||
| X2: Drug (mg) | 0.5 | |||||||
| X3: Stirring speed (rpm) | 8500 | |||||||
| Response | Expected | Observed | Residual | |||||
| Y1 (particle size nm) | 168 | 168.87 | -0.87 | |||||
| Y2 (%EE Farnesol) | 83.5 | 85.67 | 2.17 | |||||
Whereas, for the variable X3-Homogenization speed, slightly different results were observed. As the homogenization speed increased, the particle size decreased and the % entrapment efficiency increased which was favourable for the study. However, these results were observed only until a certain limit of speed i.e. up to 8500 rpm. A homogenization speed above this caused a sudden increase and a decrease in the particle size and % entrapment efficiency of the niosomes respectively. The effects of the independent variables on the dependent variables have been depicted in the 3-D response surface graphs in Figure 2 (Table 5).
3.1.3. FTIR and DSC analysis of niosomes
FTIR spectroscopy with ATR extension was used to study the compatibility between the excipients used and drug FAR. The FTIR peaks of FAR were absent in the spectra of FAR loaded niosomes indicating its successful entrapment into niosome vesicles (Figure 3). The blank and drug-loaded niosomes spectra showed characteristic peaks of cholesterol and span 20. FTIR vibrational peaks in 3450–1096 cm−1 range shows the vibrations of hydroxyl band in stretching and bending motion (3445 cm−1) and vibrations of –CH3 asymmetric and symmetric stretching at 2935 cm−1 [49]. FTIR vibrational peaks at 1056 and 1173 cm−1 visible in the niosomes corresponds to -C-OH stretching due to Cholesterol and Span 20 respectively. Some characteristic vibration peaks at 1453 cm−1, 1730 cm−1 corresponding to ketone and carboxylic acid groups confirms the presence of Span 20 [26]. A specific vibration band at 3405 cm−1 was considerably wider due to the additional –OH groups contributed by the water and cholesterol used during the niosome vesicle assembly. The FTIR spectra of the drug farnesol showed their distinguishing characteristic peaks at 2917 and 2972 cm−1 (C–H stretching) and 1442 cm−1 (CH bending(alkane) stretch), 1378 cm−1 (Figure 3) [50].
Figure 3.
A-E) FTIR graphs overlaid- Blank niosomes, Farnesol loaded niosomes, Farnesol, Cholesterol and Span 20 respectively.
DSC thermograms of niosomal excipients (Cholesterol, Span 20), Farnesol, blank niosomes and FAR loaded niosomes are shown in Figure 4. The melting values in the DSC spectra for cholesterol, span 20 and the drug FAR were 147 °C, 178 °C and 159 °C respectively. For the blank niosomes and FAR loaded niosomes, a distinct exothermic peak was visible at 238 °C (Figure 4) [51].
Figure 4.
DSC spectras (overlaid) of A) Cholesterol, Span 20, Farnesol, Blank niosomes and Farnesol loaded niosomes.
3.1.4. In vitro drug release study
The release of FAR from niosomes and niosomal gel showed biphasic drug release as shown in Figure 5. The use of niosomes as a carrier enhanced the FAR entrapment in niosomes thereby increasing the dissolution by providing hydrophilicity. Moreover, the niosomal size increased the surface area and ultimately resulting in dissolution enhancement. In initial phase, a burst release was reported where more than 50% of the drug released within an hour followed by a sustained release pattern due to slow release of FAR from the bilayer. In the 12 h of study, 80–100% drug was released which satisfies the criterion for sustained release vehicles. In the case of FAR loaded niosomal gel, the drug release was observed until 24 h, where 50 % of FAR was released after 4 h compared to 1h for FAR release from niosomes. This was expected as release from gel becomes slower as the dispersion medium is more viscous. However, in both instances an initial burst release followed by steady release over the time of study was confirmed. From the drug release kinetic studies, FAR release from niosomes and niosomal gel showed a first order and Higuchi model fit with R2 of 0.98 and 0.95 respectively indicating the best fit for both the samples (Figure 5, Table 6). These results suggest that the FAR loaded niosomal gel may be used as sustained release vehicles [52, 53].
Figure 5.
A) Drug release profile Farnesol at pH 7.4 from the niosomes and niosomal gel (n = 3); B) Drug release kinetics of Farnesol from the niosome and niosomal gel samples fitted to the first order and Higuchi model respectively.
Table 6.
Release rate constant (k) and correlation coefficient (R) after fitting data into different models for the optimized farnesol loaded niosomes and niosomal gel.
| Drug | Zero order model |
First order model |
Higuchi model |
Korsmeyer-Peppas model |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intercept | R2 | K | Intercept | R2 | K | Intercept | R2 | K | Intercept | R2 | N | |
| FAR (pH 7.4) -from niosomes | 32.00 | 0.73 | 6.94 | 1.92 | 0.98 | -0.35 | 12.72 | 0.88 | 28.82 | 1.41 | 0.23 | 0.55 |
| -from niosomal gel | 17.67 | 0.83 | 3.85 | 2.00 | 0.91 | -0.15 | 0.22 | 0.95 | 21.46 | 1.05 | 0.65 | 0.77 |
3.2. Gel characterisations
3.2.1. Clarity and colour
Colour of gels was predominantly clear medium brown in colour.
3.2.2. Determination of pH and viscosity
The niosomal gel formulation displayed increase in viscosity with increase in concentration of FAR as all other additives remained constant. Viscosity of 1.5 % gel (F8) was optimum and used for further evaluation.
3.2.3. Determination of spreadability and extrudability
In the spreadability test, the gel containing 1.5 % Carbopol was found optimum due to its suitable physiochemical property. The spreadability was found to be 66.25 ± 0.04 mm for FAR loaded niosomal formulation. The gel formulation was found smooth and easy to spread on the plate. Moreover, the extrudability was found to be excellent overall for prepared formulation. (Extrudability: >90%, excellent; >80%, good; and >70%, fair). Based on the net content, F8 was (2.5% FAR) selected for further studies (Table 7).
Table 7.
Evaluation parameters for Farnesol loaded niosomes gel formulation.
| Code | Farnesol (% (w/w)) | Viscosity∗ (poise) | pH∗ | Spreadability∗ (g.cm/sec) | Net content∗ (% w/w) | Extrudability∗ |
|---|---|---|---|---|---|---|
| F1 | 0.75 | 0.36 | 6.42 | 31.23 | 98.89 | Excellent |
| F2 | 1.00 | 0.375 | 6.35 | 39.56 | 99.65 | Good |
| F3 | 1.25 | 0.372 | 6.39 | 49.63 | 101.65 | Excellent |
| F4 | 1.50 | 0.382 | 6.40 | 56.12 | 102.26 | Excellent |
| F5 | 1.75 | 0.383 | 6.47 | 59.32 | 105.48 | Good |
| F6 | 2.00 | 0.381 | 6.42 | 63.54 | 103.24 | Excellent |
| F7 | 2.25 | 0.382 | 6.35 | 65.89 | 104.16 | Excellent |
| F8 | 2.50 | 0.385 | 6.48 | 66.25 | 105.28 | Excellent |
indicates value of five determinations.
3.2.4. Determination of antifungal activity
FAR loaded niosomal gel exhibited an almost identical zone of inhibition compared to plain FAR drug indicating no loss of FAR activity after incorporation into a polymeric gel. MIC of most of the samples containing Farnesol were between 0.9-14 mg/ml. The results show that the MIC values of the niosomes samples were lower than plain FAR. The growth rate of C. albicans was determined by optical density (OD) measurements at 360 nm. Antifungal activity of plain FAR drug and FAR loaded niosomal gel was found out by the pure plate method. The findings revealed that at 2.5% plain FAR concentration (control gel), the inhibition zone was detected at 7.0–7.2 mm and 8.9–9.0 mm for FAR loaded niosomal gel [54, 55, 56].
3.2.5. Cytotoxicity assay of Farnesol loaded Niosomes gel
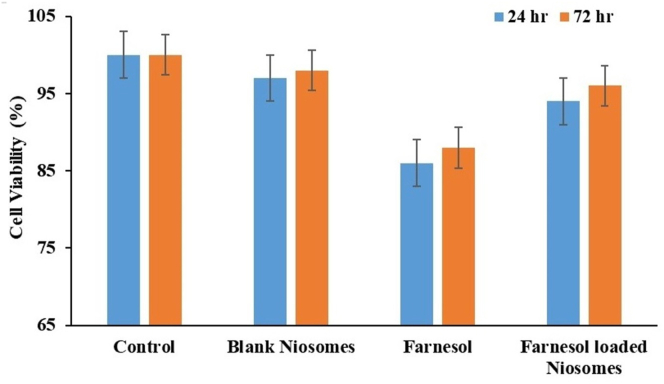
MTT assay was employed to evaluate the cytotoxicity of plain FAR drug and FAR loaded niosomal gel against SW480 cells. Cell viability (%) was estimated statistically and the results are shown in Figure 6. Plain FAR drug and FAR loaded niosomal gel failed to show any significant cytotoxicity on SW480 cell lines compared to untreated cells (p < 0.05). Cell viability for FAR loaded niosomal gel was found to be 98% compared to plain FAR (88%) (Figure 6) [56].
Figure 6.
Cytotoxicity evaluation of Farnesol and Farnesol-loaded niosomes on SW480 cells by MTT assay during 24–72 h treatment.
3.3. Ex-vivo and in-vivo experiments
3.3.1. Ex-vivo drug permeation and skin deposition studies
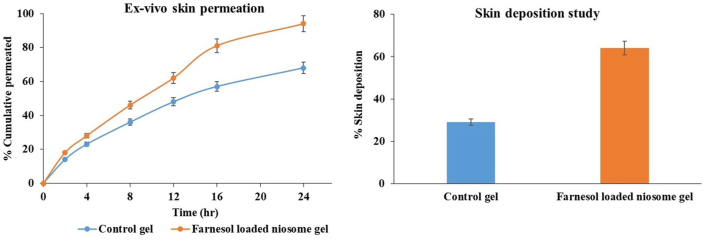
Ex vivo permeation studies were studied for the samples-control and optimized FAR loaded niosomal gel. Cumulative drug permeation (%) from the optimized niosomal gel formulation at 24 h was found to be 94.45 ± 0.248% compared to 68.34 ± 0.114% for the control gel showing a greater drug permeation from the niosomal gel and indicating a greater sustained drug release effect than the control [57]. The aim of the FAR loaded niosomal gel was to enhance the FAR deposition through the skin. From the skin deposition studies, a 2-fold increase was found for FAR loaded niosomal gel (64.23 ± 0.24%) compared to 29.35 ± 0.31% deposition for plain FAR gel signifying an increase in FAR deposition (Figure 7) [44].
Figure 7.
Ex-vivo skin permeation and skin deposition of Control and Farnesol loaded niosomal gel (n = 3).
3.3.2. In-vivo skin irritation test
The results from the study were graded as per standards shown in Table 3. The prepared FAR loaded niosomal gel formulations were applied on the rabbit skin. Set 1 and 3 failed to show any signs of erythema or edema, whereas set 2 showed slight erythema at the end of 72 h and was graded as 0.5. A primary skin irritation study was performed on each rabbit and calculated and the average PII was found to be 0.0166. The results from the study showed a high suitability of the optimized FAR loaded niosomal formulations for topical applications including candida infection treatment [43].
3.4. Stability studies
The FAR loaded niosomes and FAR loaded niosomal gel were evaluated for stability studies for 6 months. The results confirm no significant changes in particle size (nm) of FAR loaded niosomes and % EE of FAR at 4–8 °C. Similarly, there were no changes in the pH, visual appearance, and FAR release from the niosomal gel, confirming higher physical and chemical stability of the niosomal gel formulation throughout the entire course of study at 4–8 °C. At other storage conditions, both the samples were found to sediment and aggregate (Figure 8) [58].
Figure 8.
Stability of the FAR loaded niosomes at 4–8 °C.
4. Discussion
There are numerous therapeutic treatment available for oral candidiasis which are mainly caused by C. albicans species. The rapid cure for oral candidiasis remains challenging due to a constant salivary secretion in oral cavity which lessens the bioavailability of therapeutic drug at the lesion site. Using novel formulation approaches such as bilayer lipid vesicles (niosomes, liposomes, solid lipid nanoparticles etc.) have been found to improve upon those problems by providing sustained release effect, lesser side effect profile and increased drug efficacies. The significance of using these high functioning vesicles in an oral gel as in the current study reduces the need for additional excipients during gel preparation which provides a solution to the above problems discussed. Furthermore, the use of drug loaded niosomal gel may increase its bioavailability as it facilitates the rapid release of the drug at the lesion site. The enhanced drug absorption can provide a sustained drug release thereby reducing its repeated application. Moreover, the drug loaded niosomes acts as a sustained release lipidic nanocarrier itself, and a potentiation of the inherent release of the gel which will ultimately help in maintaining higher drug therapeutic levels upon oral application [17, 23, 26].
The inclusion of non-ionic surfactants and lipids in niosomes helps in providing interesting drug release properties. Spans like Span 20 have a higher transition temperature and a long alkyl chain length which allows a higher drug-loaded concentration into its cores due to increased hydrophobicity which is related to strong –H2 bonding formation with the lipid to provide a sustained drug release from its cores. Additionally, the inclusion of a polar lipid like cholesterol imparts rigidity to the bilayer of the niosomes hence allowing a slow erosion of the bilayer membrane giving a sustained drug release [17, 20]. With the use of niosomal lipidic nanocarrier, potent antifungal properties of FAR could be used for the treatment of oral candidiasis. Moreover, due to the sustained release, the active dose needed for the treatment decreases, thereby reducing the toxicity and irritation to the oral mucosa layer [13, 17].
In this study, FAR loaded niosomes were statistically optimized using experimental design method showing an acceptable particle size of 168.8 nm, a PDI of 0.1 that confirms its homogeneity as per previous reports along with a satisfactory %EE of 80% for FAR [23, 26]. The high % EE of FAR may be followed by suitable interaction between the drugs and even distribution within the lipidic matrix. This experimental design method displayed interesting results which were in accordance with previous reports [16, 17, 18]. The positive and negative relation of the dependent and independent variables affects the particle size and %EE of the drug in the formulation. The significant interaction between the dependent and independent variables can be attributed to the increased competition between the drug FAR and niosomal components to accommodate in the available space for bilayer formation, failing which weak H2 bonding results in breaks in the membrane and hence an increased aggregation and particle size. In case of homogenization speed, as the speed increased, the particle size decrease, and % EE increased to a certain limit, after which opposite results were obtained again, proving a direct relation of the variables. A homogenization speed above 8500 rpm can cause a sudden increase and a decrease in the particle size and % EE of the niosomes, respectively. This could be because increased agitation in the form of stirring gives less time for vesicle homogenization, leading to increased membrane fluidity and rapid drug release. This eventually leads to the aggregation and a failure of the niosomal nanostructure. Drug-excipient compatibility studies using FT-IR analysis revealed the presence of only peaks corresponding to Cholesterol and Span 20 in the FAR loaded niosomes spectra, confirming the successful entrapment of the drug into the niosomal bilayer. These findings confirm the absence of any non-covalent interaction between FAR and the niosomal excipients, implying that the membrane is intact. Similarly, DSC studies displayed that the peaks corresponding to Span 20, cholesterol and the drug FAR were absent from the niosomes spectra, indicating a strong interaction between the drug and niosomal excipients, imparting rigidity to the bilayer formed since the drug is in an amorphous state. This also demonstrates the compatibility of nanocarrier with the drugs, indicating stability, sustained release and high drug entrapment [25, 51].
After the optimization, FAR loaded niosomes were successfully incorporated into a gel formulation. From the prepared gel formulations (F1–F8), F8 was found to be the most optimum as per the pH, drug content, and other gelling properties. Carbopol 934 was optimum for gel preparation (1.5% w/w) as it provided superior results over other gelling agents tested during preliminary studies. The colloidal gel network of FAR loaded niosomal gel formulation demonstrated pseudoplastic behaviour, which eases the oral, topical application. The in-vitro results suggested a greater antifungal effect for FAR loaded niosomes. Furthermore, compared to plain gel, FAR loaded niosomal gel did not cause any significant dermal irritation on rabbit skin over 72 h. The addition of span 20 as a surfactant in the formulation enhances drug penetration through the mucosal skin in ex-vivo drug permeation and skin deposition studies of the FAR loaded niosomal gel. Using the MTT assay, the cell biocompatibility of the FAR loaded niosomal gel demonstrated high cell viability. It was found to be non-toxic to the cells, indicating the safety and biocompatibility of the formulation.
5. Conclusion
In conclusion, the findings here show that Farnesol loaded niosomal gel is a suitable drug delivery approach for applications in Candida albicans treatment. Moreover, the use of Farnesol as a drug itself and loaded into a nanocarrier form could be seen as a potential option over conventional treatments to improve patient compliance and become industry scalable and cost-effective for other oral infections. Future studies could be seen to utilize this approach for combinative drug delivery following robust animal model studies.
Declarations
Author contribution statement
Tejas Barot: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Deepak Rawtani: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Pratik Kulkarni: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
No data was used for the research described in the article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Zhang H., Zhai Y., Wang J., Zhai G. New progress and prospects: the application of nanogel in drug delivery. Mater. Sci. Eng. C. 2016 Mar 1;60:560–568. doi: 10.1016/j.msec.2015.11.041. [DOI] [PubMed] [Google Scholar]
- 2.Sultana F., Manirujjaman M., Imran-Ul-Haque M.A., Sharmin S. An overview of nanogel drug delivery system. J. Appl. Pharmaceut. Sci. 2013 Sep;3(8):95–105. [Google Scholar]
- 3.Tiwari B.K., Valdramidis V.P., O’Donnell C.P., Muthukumarappan K., Bourke P., Cullen P.J. Application of natural antimicrobials for food preservation. J. Agric. Food Chem. 2009 Jul 22;57(14):5987–6000. doi: 10.1021/jf900668n. [DOI] [PubMed] [Google Scholar]
- 4.Nickerson K.W., Atkin A.L., Hornby J.M. Quorum sensing in dimorphic fungi: farnesol and beyond. Appl. Environ. Microbiol. 2006 Jun 1;72(6):3805–3813. doi: 10.1128/AEM.02765-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mosel D.D., Dumitru R., Hornby J.M., Atkin A.L., Nickerson K.W. Farnesol concentrations required to block germ tube formation in Candida albicans in the presence and absence of serum. Appl. Environ. Microbiol. 2005 Aug 1;71(8):4938–4940. doi: 10.1128/AEM.71.8.4938-4940.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kruppa M. Quorum sensing and Candida albicans. Mycoses. 2009 Jan;52(1):1. doi: 10.1111/j.1439-0507.2008.01626.x. 0. [DOI] [PubMed] [Google Scholar]
- 7.Ramage G., Saville S.P., Wickes B.L., López-Ribot J.L. Inhibition of Candida albicans biofilm formation by farnesol, a quorum-sensing molecule. Appl. Environ. Microbiol. 2002 Nov 1;68(11):5459–5463. doi: 10.1128/AEM.68.11.5459-5463.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hornby J.M., Kebaara B.W., Nickerson K.W. Farnesol biosynthesis in Candida albicans: cellular response to sterol inhibition by zaragozic acid B. Antimicrob. Agents Chemother. 2003 Jul 1;47(7):2366–2369. doi: 10.1128/AAC.47.7.2366-2369.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shirtliff M.E., Krom B.P., Meijering R.A., Peters B.M., Zhu J., Scheper M.A., Harris M.L., Jabra-Rizk M.A. Farnesol-induced apoptosis in Candida albicans. Antimicrob. Agents Chemother. 2009 Jun 1;53(6):2392–2401. doi: 10.1128/AAC.01551-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Valenta C. The use of mucoadhesive polymers in vaginal delivery. Adv. Drug Deliv. Rev. 2005 Nov 3;57(11):1692–1712. doi: 10.1016/j.addr.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 11.Kammona O., Kiparissides C. Recent advances in nanocarrier-based mucosal delivery of biomolecules. J. Contr. Release. 2012 Aug 10;161(3):781–794. doi: 10.1016/j.jconrel.2012.05.040. [DOI] [PubMed] [Google Scholar]
- 12.Naderinezhad S., Amoabediny G., Haghiralsadat F. Co-delivery of hydrophilic and hydrophobic anticancer drugs using biocompatible pH-sensitive lipid-based nano-carriers for multidrug-resistant cancers. RSC Adv. 2017;7(48):30008–30019. [Google Scholar]
- 13.Sharma V., Anandhakumar S., Sasidharan M. Self-degrading niosomes for encapsulation of hydrophilic and hydrophobic drugs: an efficient carrier for cancer multi-drug delivery. Mater. Sci. Eng. C. 2015 Nov 1;56:393–400. doi: 10.1016/j.msec.2015.06.049. [DOI] [PubMed] [Google Scholar]
- 14.Abdelbary A.A., AbouGhaly M.H. Design and optimization of topical methotrexate loaded niosomes for enhanced management of psoriasis: application of Box–Behnken design, in-vitro evaluation and in-vivo skin deposition study. Int. J. Pharm. 2015 May 15;485(1-2):235–243. doi: 10.1016/j.ijpharm.2015.03.020. [DOI] [PubMed] [Google Scholar]
- 15.Ravalika V., Sailaja A. Formulation and evaluation of etoricoxib niosomes by thin film hydration technique and ether injection method. Nano Biomed Eng. 2017 Sep 1;9(3):242–248. [Google Scholar]
- 16.Kulkarni P., Rawtani D., Barot T. Formulation and optimization of long acting dual niosomes using box-Behnken experimental design method for combinative delivery of ethionamide and D-cycloserine in tuberculosis treatment. Colloid. Surface. Physicochem. Eng. Aspect. 2019 Mar 20;565:131–142. [Google Scholar]
- 17.Kulkarni P., Rawtani D. Application of box-behnken design in the preparation, optimization, and in vitro evaluation of self-assembly–based tamoxifen-and doxorubicin-loaded and dual drug–loaded niosomes for combinatorial breast cancer treatment. J. Pharmaceut. Sci. 2019 Aug 1;108(8):2643–2653. doi: 10.1016/j.xphs.2019.03.020. [DOI] [PubMed] [Google Scholar]
- 18.Kulkarni P., Rawtani D., Barot T. Design, development and in-vitro/in-vivo evaluation of intranasally delivered Rivastigmine and N-Acetyl Cysteine loaded bifunctional niosomes for applications in combinative treatment of Alzheimer’s disease. Eur. J. Pharm. Biopharm. 2021 Jun 1;163:1–5. doi: 10.1016/j.ejpb.2021.02.015. [DOI] [PubMed] [Google Scholar]
- 19.Schmolka I.R. Artificial skin I. Preparation and properties of pluronic F-127 gels for treatment of burns. J. Biomed. Mater. Res. 1972 Nov;6(6):571–582. doi: 10.1002/jbm.820060609. [DOI] [PubMed] [Google Scholar]
- 20.Joshi M.U., Bolmal U.D., Dandagi P.A. Formulation and evaluation of cefuroxime axetil sol gel for periodontits. Int. J. Pharm. Pharmaceut. Sci. 2014;6(7):498–503. https://innovareacademics.in/journals/index.php/ijpps/article/view/1748/10435 [Google Scholar]
- 21.Somjid S., Krongsuk S., Johns J.R. Cholesterol concentration effect on the bilayer properties and phase formation of niosome bilayers: a molecular dynamics simulation study. J. Mol. Liq. 2018 Apr 15;256:591–598. [Google Scholar]
- 22.El-Say K.M., Abd-Allah F.I., Lila A.E., Hassan A.E., Kassem A.E. Diacerein niosomal gel for topical delivery: development, in vitro and in vivo assessment. J. Liposome Res. 2016 Jan 2;26(1):57–68. doi: 10.3109/08982104.2015.1029495. [DOI] [PubMed] [Google Scholar]
- 23.Roger E., Lagarce F., Garcion E., Benoit J.P. Biopharmaceutical parameters to consider in order to alter the fate of nanocarriers after oral delivery. Nanomedicine. 2010 Feb;5(2):287–306. doi: 10.2217/nnm.09.110. [DOI] [PubMed] [Google Scholar]
- 24.Jadon P.S., Gajbhiye V., Jadon R.S., Gajbhiye K.R., Ganesh N. Enhanced oral bioavailability of griseofulvin via niosomes. AAPS PharmSciTech. 2009 Dec;10(4):1186–1192. doi: 10.1208/s12249-009-9325-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guinedi A.S., Mortada N.D., Mansour S., Hathout R.M. Preparation and evaluation of reverse-phase evaporation and multilamellar niosomes as ophthalmic carriers of acetazolamide. Int. J. Pharm. 2005 Dec 8;306(1-2):71–82. doi: 10.1016/j.ijpharm.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 26.Sultan A.A., El-Gizawy S.A., Osman M.A., El Maghraby G.M. Niosomes for oral delivery of nateglinide: in situ–in vivo correlation. J. Liposome Res. 2018 Jul 3;28(3):209–217. doi: 10.1080/08982104.2017.1343835. [DOI] [PubMed] [Google Scholar]
- 27.Moghddam S.R., Ahad A., Aqil M., Imam S.S., Sultana Y. Formulation and optimization of niosomes for topical diacerein delivery using 3-factor, 3-level Box-Behnken design for the management of psoriasis. Mater. Sci. Eng. C. 2016 Dec 1;69:789–797. doi: 10.1016/j.msec.2016.07.043. [DOI] [PubMed] [Google Scholar]
- 28.Ammar H.O., Ghorab M., El-Nahhas S.A., Higazy I.M. Proniosomes as a carrier system for transdermal delivery of tenoxicam. Int. J. Pharm. 2011 Feb 28;405(1-2):142–152. doi: 10.1016/j.ijpharm.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 29.Borghetti G.S., Knorst M.T. Development and evaluation of physical stability from O/W lotions containing sunscreens. Rev. Bras. Ciencias Farm. 2006 Dec;42(4):531–537. [Google Scholar]
- 30.Singh N.K., Lee D.S. In situ gelling pH-and temperature-sensitive biodegradable block copolymer hydrogels for drug delivery. J. Contr. Release. 2014 Nov 10;193:214–227. doi: 10.1016/j.jconrel.2014.04.056. [DOI] [PubMed] [Google Scholar]
- 31.Bora A., Deshmukh S., Swain K. Recent advances in semisolid dosage form. Int. J. Pharmaceut. Sci. Res. 2014 Sep 1;5(9):3596. [Google Scholar]
- 32.Förster M., Bolzinger M.A., Fessi H., Briançon S. Topical delivery of cosmetics and drugs. Molecular aspects of percutaneous absorption and delivery. Eur. J. Dermatol. 2009 Jul 1;19(4):309–323. doi: 10.1684/ejd.2009.0676. [DOI] [PubMed] [Google Scholar]
- 33.Rinaldi F., Hanieh P.N., Chan L.K., Angeloni L., Passeri D., Rossi M., Wang J.T., Imbriano A., Carafa M., Marianecci C. Chitosan glutamate-coated niosomes: a proposal for nose-to-brain delivery. Pharmaceutics. 2018 Jun;10(2):38. doi: 10.3390/pharmaceutics10020038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Q.Y., Ho P.Y., Tu M.J., Jilek J.L., Chen Q.X., Zeng S., Yu A.M. Lipidation of polyethylenimine-based polyplex increases serum stability of bioengineered RNAi agents and offers more consistent tumoral gene knockdown in vivo. Int. J. Pharm. 2018 Aug 25;547(1-2):537–544. doi: 10.1016/j.ijpharm.2018.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Amores S., Domenech J., Colom H., Calpena A.C., Clares B., Gimeno Á., Lauroba J. An improved cryopreservation method for porcine buccal mucosa in ex vivo drug permeation studies using Franz diffusion cells. Eur. J. Pharmaceut. Sci. 2014 Aug 18;60:49–54. doi: 10.1016/j.ejps.2014.04.017. [DOI] [PubMed] [Google Scholar]
- 36.Allam A., El-Mokhtar M.A., Elsabahy M. Vancomycin-loaded niosomes integrated within pH-sensitive in-situ forming gel for treatment of ocular infections while minimizing drug irritation. J. Pharm. Pharmacol. 2019 Aug;71(8):1209–1221. doi: 10.1111/jphp.13106. [DOI] [PubMed] [Google Scholar]
- 37.Katragkou A., McCarthy M., Alexander E.L., Antachopoulos C., Meletiadis J., Jabra-Rizk M.A., Petraitis V., Roilides E., Walsh T.J. In vitro interactions between farnesol and fluconazole, amphotericin B or micafungin against Candida albicans biofilms. J. Antimicrob. Chemother. 2015 Feb 1;70(2):470–478. doi: 10.1093/jac/dku374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Natrajan D., Srinivasan S., Sundar K., Ravindran A. Formulation of essential oil-loaded chitosan–alginate nanocapsules. J. Food Drug Anal. 2015 Sep 1;23(3):560–568. doi: 10.1016/j.jfda.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qin C., Li H., Xiao Q., Liu Y., Zhu J., Du Y. Water-solubility of chitosan and its antimicrobial activity. Carbohydr. Polym. 2006 Mar 3;63(3):367–374. [Google Scholar]
- 40.Şenel S., İkinci G.Ü., Kaş S., Yousefi-Rad A., Sargon M.F., Hıncal A.A. Chitosan films and hydrogels of chlorhexidine gluconate for oral mucosal delivery. Int. J. Pharm. 2000 Jan 5;193(2):197–203. doi: 10.1016/s0378-5173(99)00334-8. [DOI] [PubMed] [Google Scholar]
- 41.Goyal G., Garg T., Malik B., Chauhan G., Rath G., Goyal A.K. Development and characterization of niosomal gel for topical delivery of benzoyl peroxide. Drug Deliv. 2015 Nov 17;22(8):1027–1042. doi: 10.3109/10717544.2013.855277. [DOI] [PubMed] [Google Scholar]
- 42.Shaker D.S., Shaker M.A., Hanafy M.S. Cellular uptake, cytotoxicity and in-vivo evaluation of Tamoxifen citrate loaded niosomes. Int. J. Pharm. 2015 Sep 30;493(1-2):285–294. doi: 10.1016/j.ijpharm.2015.07.041. [DOI] [PubMed] [Google Scholar]
- 43.Kamkaen N., Phuntuwate W., Samee W., Boonrod A., Treesak C. The investigation of the rabbit and human skin irritation of herbal anti-wrinkle cream. Thai Pharm Health SciJ. 2007;2(1):20–25. [Google Scholar]
- 44.Wavikar P., Vavia P. Nanolipidgel for enhanced skin deposition and improved antifungal activity. AAPS PharmSciTech. 2013 Mar;14(1):222–233. doi: 10.1208/s12249-012-9908-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Organisation for Economic Co-operation and Development (OECD) 2002. Testing Guideline 404: OECD Guideline for Testing of Chemicals, Adopted Guideline 404: Acute Dermal Irritation/Corrosion. [Google Scholar]
- 46.OECD . OECD, Publishing; Paris: 2015. Test No. 404: Acute Dermal Irritation/Corrosion; p. 8. [Google Scholar]
- 47.Ravaghi M., Sinico C., Razavi S.H., Mousavi S.M., Pini E., Fadda A.M. Proniosomal powders of natural canthaxanthin: preparation and characterization. Food Chem. 2017 Apr 1;220:233–241. doi: 10.1016/j.foodchem.2016.09.162. [DOI] [PubMed] [Google Scholar]
- 48.Zhang J., Zhang X., Wang X., Huang Y., Yang B., Pan X., Wu C. The influence of maltodextrin on the physicochemical properties and stabilization of beta-carotene emulsions. AAPS PharmSciTech. 2017 Apr;18(3):821–828. doi: 10.1208/s12249-016-0572-5. [DOI] [PubMed] [Google Scholar]
- 49.Lee H., Finckbeiner S., Jose S.Y., Wiemer D.F., Eisner T., Attygalle A.B. Characterization of (E, E)-farnesol and its fatty acid esters from anal scent glands of nutria (Myocastor coypus) by gas chromatography–mass spectrometry and gas chromatography–infrared spectrometry. J. Chromatogr. A. 2007 Sep 21;1165(1-2):136–143. doi: 10.1016/j.chroma.2007.06.041. [DOI] [PubMed] [Google Scholar]
- 50.Barot T., Rawtani D., Kulkarni P., Hussain C.M., Akkireddy S. Physicochemical and biological assessment of flowable resin composites incorporated with farnesol loaded halloysite nanotubes for dental applications. J. Mechan. Behav. Biomed. Mater. 2020 Apr 1;104:103675. doi: 10.1016/j.jmbbm.2020.103675. [DOI] [PubMed] [Google Scholar]
- 51.Patel P., Barot T., Kulkarni P. Formulation, characterization and in-vitro and in-vivo evaluation of capecitabine loaded niosomes. Curr. Drug Deliv. 2020 Mar 1;17(3):257–268. doi: 10.2174/1567201817666200214111815. [DOI] [PubMed] [Google Scholar]
- 52.Auda S.H., Fathalla D., Fetih G., El-Badry M., Shakeel F. Niosomes as transdermal drug delivery system for celecoxib: in vitro and in vivo studies. Polym. Bull. 2016 May 1;73(5):1229–1245. [Google Scholar]
- 53.Shirsand S.B., Para M.S., Nagendrakumar D., Kanani K.M., Keerthy D. Formulation and evaluation of Ketoconazole niosomal gel drug delivery system. Int. J. Pharma. Invest. 2012 Oct;2(4):201. doi: 10.4103/2230-973X.107002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fetih G. Fluconazole-loaded niosomal gels as a topical ocular drug delivery system for corneal fungal infections. J. Drug Deliv. Sci. Technol. 2016 Oct 1;35:8–15. [Google Scholar]
- 55.Magaldi S., Mata-Essayag S., De Capriles C.H., Perez C., Colella M.T., Olaizola C., Ontiveros Y. Well diffusion for antifungal susceptibility testing. Int. J. Infect. Dis. 2004 Jan 1;8(1):39–45. doi: 10.1016/j.ijid.2003.03.002. [DOI] [PubMed] [Google Scholar]
- 56.Nikoomanesh F., Roudbarmohammadi S., Roudbary M. Investigation of bcr1 Gene Expression in Candida albicans isolates by RT PCR technique and its impact on biofilm formation. IEM. 2016;2:22–24. [Google Scholar]
- 57.Barse R., Kokare C., Tagalpallewar A. Influence of hydroxypropylmethylcellulose and poloxamer composite on developed ophthalmic in situ gel: ex vivo and in vivo characterization. J. Drug Deliv. Sci. Technol. 2016 Jun 1;33:66–74. [Google Scholar]
- 58.Shilakari Asthana G., Asthana A., Singh D., Sharma P.K. Etodolac containing topical niosomal gel: formulation development and evaluation. J. Drug Deliv. 2016;2016 doi: 10.1155/2016/9324567. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.