Abstract
Spotty liver disease (SLD) is a serious condition affecting extensively housed laying hens. The causative bacterium was described in 2015 and characterized in 2016 and named Campylobacter hepaticus. Antibiotics are the only tool currently available to combat SLD. However, antimicrobial resistance has already been detected, so finding therapeutic alternatives is imperative. Isoquinoline alkaloids (IQA), such as sanguinarine and chelerythrine, have been shown to have immunomodulatory effects. It has been hypothesized that IQA could ameliorate some of the deleterious effects of SLD. This study aimed to address that hypothesis in an experimental disease induction model. Birds were fed with diets containing 2 different doses of an IQA containing product, 100 mg of product/kg of feed (0.5 ppm of sanguinarine) and 200 mg of product/kg of feed (1.0 ppm of sanguinarine). Two additional groups remained untreated (a challenged positive control and an unchallenged negative control). After 4 wk of treatment, birds from all groups except the negative control group were exposed to C. hepaticus strain HV10. The IQA treated groups showed a reduction in the number of miliary lesions on the liver surface and reduced lesion scores compared with untreated hens. A significant reduction of egg mass was detected 6 d after exposure to C. hepaticus in the untreated group (P = 0.02). However, there was not a significant drop in egg-mass in the IQA groups, especially those fed with a high dose of IQA (P = 0.93). IQA supplementation did not produce significant changes in intestinal villus height and crypt depth but did result in a significant reduction in the proinflammatory cytokine, interleukin-8, in the blood (P < 0.01). Microbiota analysis showed that IQA treatment did not alter the alpha diversity of the cecal microbiota but did produce changes in the phylogenetic structure, with the higher dose of IQA increasing the Firmicutes/Bacteroidetes ratio.
Other minor changes in production indicators included an increase in feed consumption (P < 0.01) and an increase in body weight of the treated hens (P < 0.0001). The present study has demonstrated that IQA confers some protection of chickens from the impact of SLD.
Key words: Spotty liver disease, Campylobacter hepaticus, isoquinolone alkaloid, sanguinarine, feed additive
INTRODUCTION
Spotty liver disease (SLD) is characterized by increased mortality, particularly around the time of peak egg production, the occurrence of multiple gray/white lesions in the liver, and reduction in egg output. It is prevalent within the layer industry in Australia, especially within the free-range sector of the industry (Grimes and Reece, 2011). It is also a prevalent disease in the United Kingdom (Crawshaw, 2019), and is an emerging disease in other parts of the world with recent reports from the United States, New Zealand, and Jordan (Gregory et al., 2018; Crawshaw et al., 2021; Hananeh and Ababneh, 2021). It has also been described as an emerging disease in layers in Costa Rica (Dr Esteban Cornejo, personal communication). This reflects that SLD is becoming a global poultry issue, related to an increase in the number of farms producing eggs from free-range hens, driven by consumer concerns about the welfare of hens.
The clinical signs include a brief period of depression in laying birds (usually in good body condition and “in-lay”). Often birds are found dead without any prior clinical signs of disease. The disease is less commonly observed in barn and cage birds and parent stock (Scott et al., 2016). The causative agent has been recently identified and isolated (Crawshaw et al., 2015), and then further characterized and named Campylobacter hepaticus (Van et al., 2016). The development of an experimental disease induction method (Van et al., 2017) provided the tools to facilitate the study of disease pathogenesis and the evaluation of experimental vaccines, feed additives, and other potential methods to control of SLD. The disease responds to therapeutic antibiotics although resistance has been reported (Grimes and Reece, 2011; Van et al., 2019). Control or prevention has not been observed with any of the typical growth promoter products used in broiler chickens.
The use of phytobiotics in general has been extensively studied in chickens as they exhibit a range of interesting properties, such as antibacterial, antiviral, antifungal, antioxidative, insecticidal, improvement in feed conversion and growth promoting activities. Their use for the control of other Campylobacter spp. in poultry has been reviewed by Vidanarachchi et al. (2005). They have also been demonstrated to have anticoccidial activity, to stimulate the immune system, and to alter gut morphology, among other functions (Prabakar et al., 2016). Broiler chickens fed with a diet containing inulin and oligofructose compounds extracted from chicory roots (Cichorium intybus) reduced the total Campylobacter count in the large intestine (Yusrizal and Chen, 2003). The use of isoquinoline alkaloid (IQA) contained in Macleaya cordata (MC) extract in the diets of chicken has also been studied. Previous studies have indicated that IQAs may influence the composition of the gut microbiota. (Sugiharto, 2016; Huang et al., 2018; Liu et al., 2020). The presence of low doses of these MC extracts in the diet (15 mg/kg) had positive effects in the cecal metabolism in broilers, changing the content of monounsaturated fatty acids (Juskiewicz et al., 2011). The addition of these alkaloids in poultry and swine diets increased their feed intake and weight gain, improve growth performance, and produce changes in their innate immune response (Ni et al., 2016; Kikusato et al., 2021). The use of allicin, an extract from garlic, showed an effect against C. jejuni under in vitro conditions, but not in vivo (Robyn et al., 2013). The use of essential oils (compounds extracted form plants or plants parts such as marigold, ginger root, jasmine, patchouli, gardenia, cedarwood, carrot seed, celery seed, mugwort, spikenard, and orange bitter oils) has reduced C. jejuni counts, measured as median bactericidal activity. Among these compounds, those that demonstrated more activity against C. jejuni were cinnamaldehyde, estragole, carvacrol, benzaldehyde citral, thymol, eugenol, perillaldehyde, and estragole (Friedman et al., 2002). The phytobiotics tested to date against C. hepaticus have been reported as having limited success (Scott et al., 2018; Moore et al., 2019). However, the role of some phytobiotics is yet to be tested, such as those containing IQA.
In the present study, the recently developed model to experimentally induce SLD in laying hens was used to test whether a standardized phytogenic feed additive containing IQA could modify the progression of SLD in hens exposed to C. hepaticus. Also, changes in gut microbiota may in turn influence the health and productivity of the birds (Stanley et al., 2014). Therefore, in this study the cecal microbiotas of birds challenged with C. hepaticus, with and without IQA pretreatment, were investigated.
MATERIALS AND METHODS
Animal Ethics Approval
This study had the approval from the Wildlife and Small Institutions Animal Ethics Committee (Approval #19.17).
General Procedure
One hundred and thirty-two Hy-Line laying hens were included in the study. The hens, sourced from an SLD-free commercial layer operation, were brought into the research facility at 21 wk of age. After an acclimation period of 24 h, they were distributed into 8 treatment groups, with 4 birds per cage and replicate cages (Table 1). The groups consisted of a negative control (NC, no in-feed IQA and no exposure to C. hepaticus, n = 28) and a positive control groups (PC, no in-feed IQA and exposed to C. hepaticus, n = 36), and 2 treatment groups (feed treated with IQA and exposed to C. hepaticus); high dose (n = 36) and low dose (n = 32). The NC and PC groups received a regular layer diet, consisting of wheat, soybean meal, and meat meal as base ingredients. The 2 treated groups received the same diet supplemented with different concentrations of IQA. A low dose IQA (LD-IQA) group (100 mg of Sangrovit Extra [Phytobiotics Futterzusatzstoffe GmbH, Germany]/kg of feed, equivalent to 0.5 ppm of the active ingredient, sanguinarine]) and a high dose IQA (HD-IQA) group (200 mg of Sangrovit Extra/kg of feed, equivalent to 1 ppm of the active ingredient, sanguinarine). Hens were fed with the IQA supplemented diets for a period of 28 d before their exposure to C. hepaticus. An additional division of treatment groups involved the time of euthanasia postexposure; “Short” group hens were euthanized 6 days after exposure (DAE) to C. hepaticus (at 25 wk and 4 d of age). The number of hens per treatment in this group were 8 NC, 16 PC, 16 LD-IQA and 16 HD-IQA), while in the “Long” groups, hens were euthanized 29 DAE (at 28 wk and 5 d of age. The number of hens per treatment in this group was 20 NC, 20 PC, 16 LD-IQA and 20 HD-IQA). Short groups were used to assess the postmortem changes of the hens, particularly pathological changes of the liver. Typically, birds challenged with C. hepaticus develop liver lesions similar to those observed in the field within 5 to 7 DAE (Van et al., 2017). Long groups were used to compare production parameters between groups, such as egg production, egg mass, weight gain of the hens, feed consumption, and FCE.
Table 1.
Distribution by group of the hens included in the study and the treatment received.
| Group | Treatment | Exposure to CH | Animals per group |
|---|---|---|---|
| A | Non-exposed - no treatment (Short)*- NC | No | 8 |
| B | Non-exposed - no treatment (Long)*- NC | No | 20 |
| C | Exposed - no treatment (Short) - PC | Yes | 16 |
| D | Exposed - no treatment (Long) - PC | Yes | 20 |
| E | Low dose IQA (LD-IQA)-100 mg/kg (Short) | Yes | 16 |
| F | Low dose IQA (LD-IQA)-100 mg/kg (Long) | Yes | 16 |
| G | High dose IQA (HD-IQA)-200 mg/kg (Short) | Yes | 16 |
| H | High dose IQA (HD-IQA)-200 mg/kg (Long) | Yes | 20 |
Abbreviation: CH, Campylobacter hepaticus.
Short group autopsy conducted at 7 d after exposure. Long group autopsy conducted 29 d after exposure.
Twenty-eight days after the start of treatment, the hens were orally exposed to C. hepaticus grown in Brucella broth (1 mL of a 1 × 109 CFU/mL per bird). The NC group received sterile Brucella broth. Throughout the experiment, hens were inspected 2 or 3 times a day. Water was delivered by lines of nipple drinkers, using town water. Water and feed were administered ad libitum.
Diet Composition
Hens in all groups were fed with a commercial laying hen diet. The main ingredients contained in the diet, from the highest to the lowest inclusion rate, were wheat (49.1%), soybean (19.8%), lime grit (8.4%), meat and bone meal (7%), lentils/peas blend (6.8%), vegetable acid oil (4.7%), and canola meal (2.8%), representing 98.6% of the diet. The remaining 1.4% corresponded to micronutrients and other ingredients. In the treated groups, the diet was supplemented with 2 different doses of a feed additive as described above.
Hen Weight
The weights of the hens were recorded on the day of arrival to the animal facility and at the end of the study, 6 DAE for Short groups and 29 DAE for Long groups.
Egg Production and Egg Mass
During the experiment, the egg production per cage was recorded daily. At 3, 6, and 14 DAE, all eggs were marked with the date and cage of origin and were individually weighed. The average egg mass per group was calculated, excluding 30% of the eggs with mass furthest from the mean.
Feed Consumption
The amount of feed administered per cage/pair of cages was recorded during each feeding. In some cases, the same feed trough was shared by 2 cages (6 hens), while other separated cages had their own individual trough (3 hens). On the day of exposure of the hens to C. hepaticus, the residual amount of feed per trough was collected using a vacuum machine, and the amount of residual feed per cage/pair of cages was recorded. The residual feed was collected once again and recorded at 6 DAE for the Short groups, and at 29 DAE for the Long groups.
Feed Conversion Efficiency
The feed conversion efficiency (FCE) was calculated using the data collected on 3, 6, and 14 DAE. The average feed consumption per cage, during the period, was divided by the mass of the eggs collected from that cage. To reduce the noise in the data analysis outliers were excluded; calculated FCEs that were more than the mean ± 3 standard deviations (SD) of their own group were excluded from the calculations.
Necropsy and Scoring of Liver Lesions
During the necropsy of the hens of the Short groups, the number of lesions found on the surface of the liver was recorded per hen. An SLD liver scoring system was also applied, utilizing the number of lesions found on the liver of the hens. This score is based on a logarithmic scale from 0 to 4, where 0 = no visible lesions, 1 = 1–9 lesions, 2 = 10–99 lesions, 3 = 100–999 lesions and 4 = more than 1,000 lesions. A similar scoring system has been previously applied to quantify liver damage caused by C. hepaticus (Van et al., 2017).
Gut Histology
At the necropsy at 6 DAE, tissue samples were taken from the distal end of the jejunum of each bird and preserved in formalin. The tissue samples were processed by Gribbles Pathology (Melbourne, Australia) for embedding, sectioning, and hematoxylin and eosin stained. The mounted tissue sections were examined and scored for villus height and crypt depth, with 10 measurements taken for each bird and mean group scores calculated. Villus height was measured from the top of villus to the crypt opening, and crypt depth was measured from the base of the crypt to the level of crypt opening (Saki et al., 2012).
Cytokine Quantitation
Pro-inflammatory cytokine levels in the blood of hens were assayed using commercial kits from Cusabio (Wuhan, China). The interleukin-6 (IL-6) (Cat. No. CSB-E08549Ch-96T), interleukin-8 (IL-8) (Cat. No. CSB-E14191C-96T, and lipopolysaccharide-induced tumor necrosis factor-alpha factor (LITAF) (Cat. No. CSB-Elo12985CH-96T) enzyme-linked immunosorbent assay (ELISA) kits were used according to the manufacturer's instructions.
Microbiota Assessment
Total DNA was extracted and purified from cecal content samples from the Short PC, LD-IQA, and HD-IQA groups, using a DNeasy PowerSoil Pro Kit (Qiagen, Hilden, Germany). Amplicons of the V3-V4 region of 16S rRNA genes were produced with Q5 high fidelity polymerase (New England BioLabs, Ipswich, MA) using forward (ACTCCTACGGGAGGCAGCAG) and reverse (GGACTACHVGGGTWTCTAAT) primers containing barcodes, spacer sequences, and Illumina sequencing linkers (Fadrosh et al., 2014). The sequencing of the amplicons was performed on the Illumina MiSeq platform using 2 × 300 bp paired-end reads (Caporaso et al., 2010). Paired-end sequences were combined using Fastq-Join algorithm allowing no mismatch within overlapped regions (Edgar, 2010). The reads with the Phred quality scores of 20 or above were used for analysis. The operational taxonomic unit (OTUs) was picked at 97% similarity using UCLUST. Chimera sequences were checked using Pintail (Ashelford et al., 2005) and taxonomy assignment was performed against the Green Genes database (v 2013_8). Further taxonomic assignment of OTUs was done by comparing representative sequences to the GenBank database. After quality trimming, rare sequences that represented OTUs present at less than 0.01% of the total sequences were removed from further analysis. The data set consisted of 1.19 million sequences, with an average of 24,305 sequences per sample.
Statistical Analyses
For the productivity data multiple comparisons were done using one-way or two-way analysis of variance (ANOVA). Tukey's multiple comparisons test was used for parametric data (such as weights), while Kruskal–Wallis test was used for nonparametric data, such as scores. The proportions of positives and negatives were compared using a contingency table, and the Chi-square (χ2) and Fisher's exact test. All statistical calculations were performed using GraphPad Prism software package, version 8.4.2 (GraphPad Software, La Jolla, CA). For the histology information data were analyzed using one-way ANOVA in Minitab 18. The Dunnett test was used to compare every mean to a control mean.
For the microbiota analysis, data visualization and statistical analysis were performed using the MicrobiomeAnalyst online service (Chong et al., 2020). Alpha diversity was investigated using several indices, including Observed, Ace, Chao1, Simpson, Shannon, to compare species richness and evenness across the three groups of samples (Kim et al., 2017). The difference in microbial community composition between the sample types was visualized using principal coordinate analysis (PCoA), using the Bray–Curtis distance metric. The overall difference in microbial community between the sample types for the challenged and unchallenged groups was investigated using permutational multivariate analysis of variances (PERMANOVA) on the Bray–Curtis distance metrics. Linear discriminant analysis effect size (LEfSe) was performed to find the most discriminatory features between the sample types.
RESULTS
Egg Mass
The egg mass results are presented in Table 2. In the negative control group (unexposed to C. hepaticus), the mass of the eggs increased over time. In contrast, the mass of the eggs collected from the hens of the positive control group (exposed to C. hepaticus) significantly decreased between 3 and 6 DAE (P = 0.02). Even though there was a recovery in the mass of the eggs at 14 DAE (4.12%), egg mass was not significantly higher compared with 3 and 6 DAE (P = 0.86 and 0.07, respectively). In the LD-IQA group, the mass of the eggs did not significantly change between 3, 6, and 14 DAE. There was a slight decrease from 3 to 6 DAE, but it was not statistically significant (P = 0.67). Finally, the eggs from the HD-IQA group increased in mass between 3 and 6 DAE and exhibited a significant increase at 14 DAE compared with 3 DAE (P = 0.01) and 6 DAE (P = 0.03), respectively.
Table 2.
Average egg mass in grams per group and their percentage of change between different sampling days.
| Days from challenge | Negative control | Change (%) | Positive control | Change (%) | LD-IQA | Change (%) | HD-IQA | Change (%) |
|---|---|---|---|---|---|---|---|---|
| 3 | 55.64a | 57.14a | 57.65 | 56.74a | ||||
| 6 | 57.97ab | 4.19% | 54.38b | −4.83% | 56.70 | −1.65% | 57.11a | 0.64% |
| 14 | 59.70b | 2.98% | 56.62ab | 4.12% | 58.55 | 3.27% | 59.72b | 4.58% |
Abbreviations: HD-IQA, high dose IQA; LD-IQA, low dose IQA.
Numbers with different superscript letters in the same column differ significantly (P < 0.05).
Egg Production
A significant reduction in egg production was observed in the untreated and challenged group compared with the negative controls (Table 3, P = 0.0014). Egg production in the LD-IQA group was lower compared with its production before the challenge, and it was significantly lower compared to the negative control group (P < 0.0001), and not significantly different compared with the positive control group (P = 0.13). The egg production of the hens in the HD-IQA group was higher after exposure compared with the period pre-exposure to C. hepaticus. The results of the HD-IQA group were not different compared with the negative control group (P = 0.49), but significantly higher compared with the positive control group (P = 0.01).
Table 3.
Number of eggs produced in each group 20 d before the exposure to C. hepaticus (expected number of eggs) and the difference between the expected production and the actual production 20 d after exposure.
| I. | II. NC | III. PC | IV. LD-IQA | HD-IQA |
|---|---|---|---|---|
| Expected | 379 | 383 | 310 | 367 |
| Difference | 20 | 4 | −9 | 15 |
| Significance* | a | b | b | a |
Abbreviations: HD-IQA, high dose IQA; LD-IQA, low dose IQA; NC, negative control; PC, positive control.
P < 0.01
Severity of Spotty Liver Disease Lesions: Clinical Signs and Postmortem Findings
During the experiment, and after the hens were exposed to C. hepaticus, no evident clinical signs were observed in any of the hens.
The Short groups were necropsied 6 DAE to C. hepaticus. The number of SLD lesions on the surface of the livers was assessed, an estimate was made of the number of lesions and each liver was assigned a lesion score, from 0 to 4, based on the number of visible lesions. The results are summarized in Figures 1A and 1B.
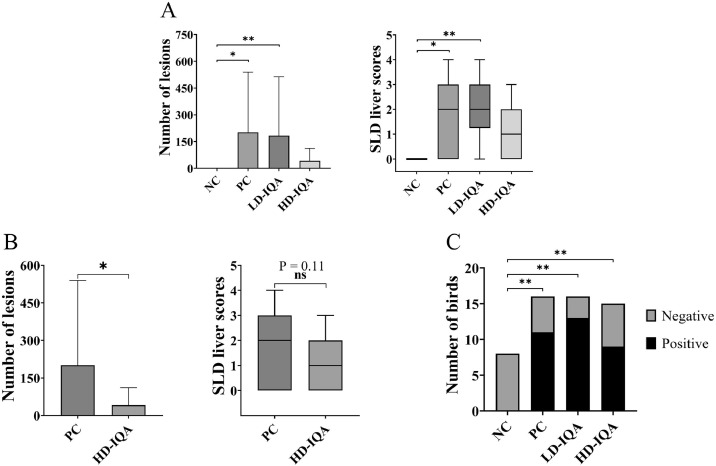
Figure 1.
Number of liver lesions, liver lesion scores calculated, and the number of birds diagnosed positive to SLD during the postmortem of the hens included in the present study. The number of lesions is a rough calculation based on the observation of all the surfaces of the liver. Columns represent the average number of lesions, and the error bars the SD. The liver lesion scores were calculated using the number of lesions observed on the surface of the liver. 0, no lesions detected; 1, 1 to 9 lesions; 2, 10 to 99 lesions; 3, 100 to 999 lesions; 4, more than 1,000 lesions. The central line on each box shows the median score and the margins of the box represent the interquartile range, and the external lines show the minimum and maximum values. (A) data obtained from all the Short group, euthanized 6 DAE to C. hepaticus (number of lesions on the surface of the livers and box plot showing the SLD liver scores). (B) Data obtained from PC and HD-IQA Short groups only (number of lesions on the surface of the livers and box plot showing the SLD liver scores). (C) Proportion of hens positive and negative to SLD by group (Short groups). The positive and negative status was determined by the presence or absence of miliary lesions in the surface of the liver. Abbreviations: NC, negative control; HD-IQA, high dose IQA; LD-IQA, low dose IQA; PC, positive control.
There were no lesions present in any of the hens examined from the NC group. The average number of lesions in the PC and LD-IQA groups was 201.1 ± 327.6 and 183.6 ± 318.4, respectively, statistically significantly higher compared to the NC group (P = 0.01 and 0.003, respectively). The average number of lesions in the HD-IQA group was 41.7 ± 67.3., which was not significantly higher than the NC (P = 0.22), however, because of the high within group variances, it was not significantly lower than the average number in the PC group (P > 0.9999; Figure 1A).
The results were similar for liver lesion scores, with the median scores of both the PC and LD-IQA groups statistically higher compared to the NC, and the median liver lesion score of the hens from the HD-IQA groups not significantly higher than the NC group, but also not significantly lower compared with the PC group.
However, when the NC and LD-IQA groups were excluded from the calculations, and only the HD-IQA and PC groups compared, the difference between the number of lesions was statistically significant (P = 0.04), but not the SLD liver scores (P = 0.11; Figure 1B). The proportions of hens positive to SLD in all the exposed groups were significantly higher to that of the NC group, but they were not different from each other at a statistically significant level (Figure 1C).
Gut Histology
The gut histology measurements were assessed for all birds from each group. The group mean measurements of villus heights and villus/crypt height ratios are shown in Table 4. No statistically significant differences between groups were observed for either villus height or villus/crypt ratios.
Table 4.
Villus height and villus/crypt ratios.
| Group | N | Mean ± SD villus height (µm) | Villus height 95% CI (µm) | Mean ± SD villus/crypt ratio | Villus/crypt ratio 95% CI |
|---|---|---|---|---|---|
| NC | 8 | 285.7 ± 36 | (250.7, 320.7) | 8.80 ± 1.11 | (7.845, 9.764) |
| PC | 15 | 308.9 ± 60 | (283.4, 334.5) | 9.16 ± 1.42 | (8.464, 9.865) |
| LD-IQA | 15 | 326.1 ± 51 | (300.5, 351.7) | 9.92 ± 1.66 | (9.220, 10.621) |
| HD-IQA | 16 | 298.4 ± 41 | (273.6, 323.2) | 8.27 ± 1.04 | (7.591, 8.947) |
Abbreviations: HD-IQA, high dose IQA; LD-IQA, low dose IQA; NC, negative control; PC, positive control.
Cytokine Levels
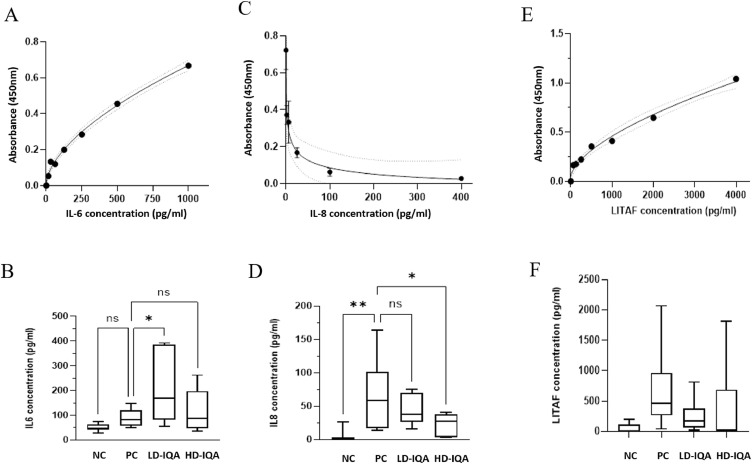
IL-6, IL-8, and LITAF levels were assessed in the blood of Short group birds collected at the necropsy 6 DAE to C. hepaticus. The standard curves that were obtained showed satisfactory fit (R2 > 0.95), resolution, and kit performance (Figures 2A, 2C and 2E). The cytokine levels recorded for each treatment group are shown graphically in Figures 2B, 2D and 2F.
Figure 2.
Cytokine results. Panels A, C, and E are the standard curves for the IL-6, IL-8, and LITAF ELISA assays, respectively. Panels B, D, and F present the blood concentrations of IL-6, IL-8, and LITAF, calculated from the absorbance reading of each sample compared to the standard curve. * P < 0.05. ** P < 0.01. Abbreviations: HD-IQA, high dose IQA, DAE6; LD-IQA, low dose IQA; NC, negative control, DAE6; n.s., not significant; PC, positive control.
Comparison of the NC and PC groups showed that C. hepaticus infection significantly increased the circulating levels of IL-8 in the blood. The average levels of IL-6 and LITAF were numerically greater in the PC groups compared to NC groups, but these differences were not statistically significant. A significant reduction (P < 0.05) in the level of the proinflammatory cytokine IL-8 was noted in the HD-IQA treated group compared with the PC group and this trend was also seen in the LD-IQA treatment group but did not reach statistical significance (Figure 2D). The IL-6 level in the LD-IQA group was significantly higher (P < 0.05) than in the PC group (Figure 2B). No significant differences were observed in the concentrations of LITAF between groups (Figure 2F).
Microbiota Analysis
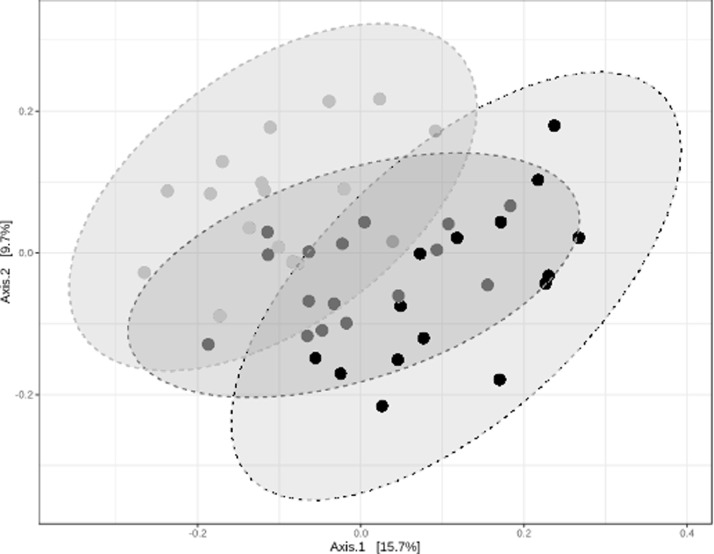
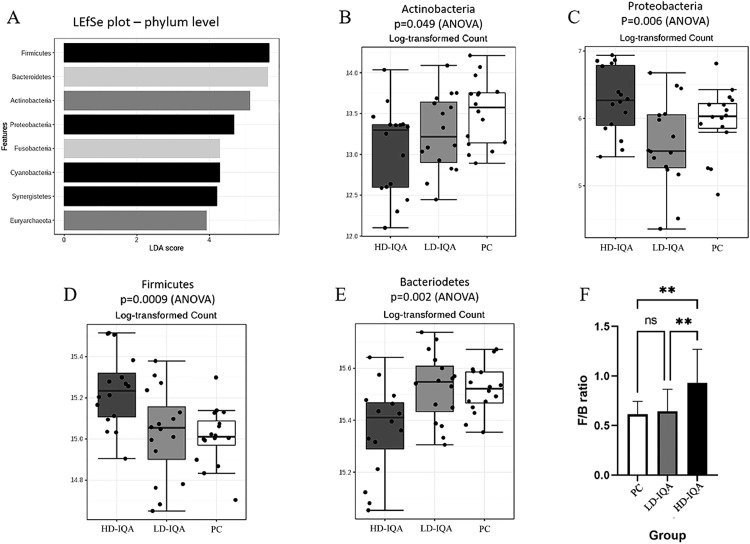
The microbiota composition was analyzed in cecal samples taken 6 d postexposure to C. hepaticus from the PC, LD-IQA and HD-IQA groups to determine if IQA influenced the phylogenetic structure of the microbiota. A total of 1,177,880 sequences were generated from the 48 samples from the 3 groups: an average of 24,539 sequences per sample. The rarefaction curves for all the samples were plateauing, indicating that most of the microbial diversity was captured by the depth of sampling. Eleven phyla were represented in the data set with Bacteroides (53%) and Firmicutes (36%) dominant. Alpha diversity analysis, using a variety of indices, indicated that there was no statistically significant difference between the alpha diversity of groups with and without IQA treatment. Beta diversity analysis showed differentiation between the groups, demonstrating that IQA did affect the composition of the cecal microbiota. A PCoA plot is shown in Figure 3. LEfSe was used to identify taxonomic groups of bacteria that had the most influence on the differentiation of the treatment groups (Figure 4A) and the differences between groups were visualized in abundance plots, with examples shown in Figures 4B–4E. At the phylum taxonomic level, it was found that the HD-IQA group microbiota was significantly enriched for Firmicutes and depleted in Bacteroidetes abundance compared to the PC and LD-IQA groups. This resulted in a significantly higher Firmicutes/Bacteroidetes (F/B) ratio (Figure 4F). IQA treatment reduced the relative abundance of Actinobacteria within the cecal microbiota (Figure 4B) whereas the relative abundance of Proteobacteria was reduced in the LD-IQA group but increased in the HD-IQA group (Figure 4C).
Figure 3.
The PCoA plot of the microbiota composition of the 48 characterized samples shows differentiation between the three groups. Analysis of group similarities (ANOSIM) R: 0.29627; P-value < 0.001. Black, high-dose of IQA group; mid-gray, low dose of IQA group; light-gray, positive control group.
Figure 4.
Taxonomic groups that is differentially abundant between groups. (A) LEfSe analysis identified a number of phyla that were differentially abundant. Plots show the relative abundance of Actinobacteria (B), Proteobacteria (C), Firmicutes (D), and Bacteroidetes (E). The F/B ratio in the HD-IQA group was significantly different to that of the other groups (F), **P < 0.01. Abbreviations: HD-IQA, high dose IQA; LD-IQA, low dose IQA; PC, positive control.
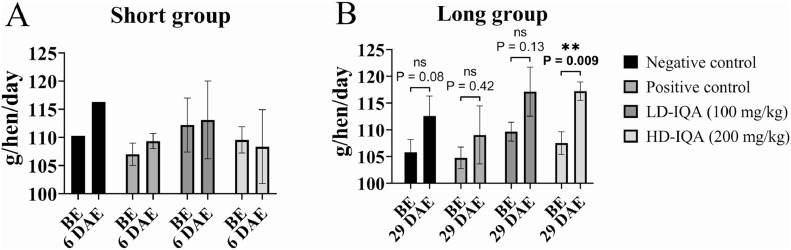
Feed Consumption
As can be seen in Figure 5, there were modest, nonstatistically significant changes in the average feed consumption of each group in the first week after exposure to C. hepaticus. In contrast, after 29 d postexposure to C. hepaticus, all groups showed numerical increases in average feed consumption but only the change in the HD-IQA group feed consumption was statistically significant compared to before exposure and 6 DAE (Figure 5).
Figure 5.
Average feed consumption by group and sampling period. Each column represents the average feed consumption from each group, and the black lines represent the SD. Abbreviations: BE, before exposure; DAE, days after exposure; HD-IQA, high dose of IQA; LD-IQA, low dose of IQA.
Differences in final weights
When the initial weights of the hens were compared between groups, there was no significant difference (Table 5). However, when the final weights were compared, they were significantly higher in hens from the HD-IQA group compared with the PC and NC groups (P < 0.05).
Table 5.
Weights of the hens from the Long groups at the beginning (Initial) and the end (Final) of the study.
| Group | Initial weights ± SD | Final weights ± SD |
|---|---|---|
| NC | 1.76 ± 0.13 | 1.96 ± 0.13a |
| PC | 1.80 ± 0.12 | 1.96 ± 0.16a |
| LD-IQA | 1.84 ± 0.14 | 2.02 ± 0.13ab |
| HD-IQA | 1.86 ± 0.11 | 2.06 ± 0.13b |
Abbreviations: HD-IQA, high dose IQA; LD-IQA, low dose IQA; NC, negative control; PC, positive control.
Numbers with different superscript letters in the same column differ significantly (P < 0.05, two-way ANOVA and Tukey's multiple comparison's test).
Feed Conversion Efficiency
Data presented in Table 6 shows FCE, expressed as feed weight/egg mass ratio (grams of feed needed to produce 1 g of egg). Hens with lower FCE were more efficient, as it required less feed to produce egg mass. There were no statistically significant differences in FCE between groups, across the experiment. The trends within the data suggested FCE improved over time in the hens from the NC group, with a decrease of the feed weight/egg mass ratio from 1.96 ± 0.13 to 1.86 ± 0.14. Similar results were seen in the LD-IQA group, decreasing from 2.01 ± 0.19 to 1.91 ± 0.12 However, changes in these groups were not statistically significant (P = 0.1 and 0.06, respectively). In the PC group there was a decrease in the FCE, with an increase in the feed weight/egg mass ratio between 3 and 6 DAE (from 1.86 ± 0.14 to 1.96 ± 0.10), but this difference was not statistically significant (P = 0.3). Finally, in the HD-IQA group, the efficiency of feed conversion into egg mass improved over time. Larger group sizes or a reduction in the data dispersal would be required in future experiments to determine if these apparent differences could reach statistical significance.
Table 6.
Feed conversion efficiency (± SD) calculated using the Long groups' egg production data and egg mass.
| DAE | NC | PC | LD-IQA (100 mg/Kg) | HD-IQA (200 mg/Kg) |
|---|---|---|---|---|
| 3 | 1.96 ± 0.13 | 1.86 ± 0.14 | 2.01 ± 0.19 | 2.06 ± 0.16 |
| 6 | 1.94 ± 0.11 | 1.96 ± 0.10 | 1.99 ± 0.18 | 2.03 ± 0.25 |
| 14 | 1.86 ± 0.11 | 1.86 ± 0.10 | 1.91 ± 0.12 | 1.95 ± 0.23 |
Abbreviations: DAE, days after exposure; HD-IQA, high dose IQA; LD-IQA, low dose IQA; NC, negative control; PC, positive control.
DISCUSSION
SLD has been increasing in importance in some countries, including Australia and United Kingdom, derived principally from an increase in the presence of cage-free production systems. The only measure currently available to control disease outbreaks is the use of antibiotics, as there is not a commercial vaccine currently available. However, C. hepaticus have already started to exhibit antimicrobial resistance, highlighting the importance of finding alternative therapies to reduce the negative effects of this disease for the egg-laying industry. This study examined the effect of a standardized blend of plant-derived isoquinoline alkaloids on the severity of disease in SLD challenged laying hens. Products containing Macleaya cordata extracts contain isoquinoline alkaloids, of which sanguinarine is one (Hao et al., 2015).
It was found that SLD caused a significant decrease in the average mass of the eggs in the affected hens approximately one week after challenge, in agreement with previous experiments where laying hens were challenged with C. hepaticus (Scott et al., 2020). This decline in egg mass due to C. hepaticus infection has not been previously described in the scientific literature. Other infectious agents have been noted to produce a reduction in egg mass, such as infectious bronchitis virus (Sevoian and Levine, 1957) and Mycoplasma synoviae (Catania et al., 2010). The results also demonstrated that this decrease in mean egg mass can be prevented with the administration of IQA especially in the HD-IQA group. The increase in the mass of the eggs seen in the HD-IQA group could be explained by an increase in the thickness of the eggshell. Results published after the completion of this trial reported that the addition of Macleaya cordata extracts in the diet of laying hens produced thicker eggshells (Guo et al., 2021). In previous studies comprising other feed additives, the administration of essential oils contained in Citrullus lanatus extracts in the diet of laying hens showed a significant increase in the mass of the eggs compared with an untreated control group (Marume et al., 2020). Another study that supplemented the diet of laying hens with an essential oil mixture showed an increase in the mass of the eggs laid by the hens (Bozkurt et al., 2012). A study using 3 different feed additives (a competitive exclusion product, an acidifier and an oregano extract) did not show a significant effect on the mass of eggs (De Asis, 2002).
The hens infected during our studies showed a reduction in the production of eggs after challenge when compared with the production of the same hens before challenge, which has been described in previous publications. Courtice et al. (2018) reported a reduction in total egg production of up to 35% in C. hepaticus infected hens, while other authors have reported egg production losses slightly lower, ranging from 10 to 25% (Grimes and Reece, 2011; Crawshaw and Irvine, 2012; Crawshaw, 2019; Crawshaw et al., 2021). In the current study, the addition of IQA at high dose prevented that reduction in egg production, while the LD-IQA group had the opposite effect in egg production, but the reasons for that reduction in egg production remain unknown. There is very limited information available in the scientific literature about the effect of IQA, sanguinarine, or extracts from Macleaya cordata extracts on egg production in terms of number of eggs laid. To the best our knowledge, this study is the first to describe an improvement in the number of eggs laid in hens challenged with C. hepaticus fed on diets supplemented with these alkaloids or any other feed additive.
Even though the feed additive used in the present study did not reduce the number of birds positive to SLD, it was possible to see an amelioration of the clinical signs in the affected hens, reducing the number of lesions found on the surface of the liver. The number of liver lesions in the HD-IQA treated group was significantly lower than in the PC group. Concomitantly, the same group showed a significant reduction in IL-8 levels, which is probably due to IQA inducing a reduction in the inflammatory response, and the reduction of IL-8 could be related to a reduction in liver damage. Results from the study of hepatitis B virus infection have demonstrated that the liver damage produced by HBV was correlated with upregulation of liver inflammation cytokines, such as IL-8, which induce liver damage through NK cells (Wang et al., 1999; Dunn et al., 2007).
Experiments in human beings showed that sanguinarine compounds had an inhibitory effect in the expression of cytochrome P450 enzymes (group that includes IL-8) in liver cells, suggesting that this compounds could be hepatoprotective (Qi et al., 2013). Other studies demonstrated that the addition of sanguinarine to the diet of broiler chickens challenged with C. perfringens significantly reduced the amount of the liver enzymes aspartate aminotransferase, alanine aminotransferase and alkaline phosphatase compared with the challenged and untreated group (El-Sheikh et al., 2018). Other feed additives have shown a similar hepatoprotection in experiments with rats and mice (Kathirvel et al., 2010; Motawi et al., 2011; Badolati et al., 2020).
Results from the present study showed that infections with C. hepaticus produced a significant increase in the production of IL-8 in the blood of the infected hens studied in the PC group, and a significant reduction in IL-8 level in the HD-IQA treated group compared with the PC group. This reduction in the inflammatory response, induced by IQA-containing products has been observed before (Soler-Vasco et al., 2016), while other studies have shown that addition of sanguinarine-containing products upregulated the relative expression of IL-8, although that increase was not statistically significant (Palócz et al., 2019). IL-8 is a homodimer recognized as a chemokine, and its main role in mammalians is to recruit neutrophils to the inflammation site (Akdis et al., 2011). In birds, the IL-8-like chemokine would have a similar role recruiting, in this case, heterophils (Kogut, 2002). This decrease in the proinflammatory response could have a positive effect in the hens. The immune system requires a significant energy input for normal function and this energy requirement increases when excessive inflammatory responses are induced, for example by pathogen infection (Broom and Kogut, 2018). The apparent suppression of inflammatory responses by the IQA may have reduced the diversion of energy to the immune system, meaning that more energy was available for the maintenance of performance.
Augmentation of feed with IQA induced changes in the cecal microbiota. Most notably the F/B ratio was higher in the HD-IQA group compared to the PC and LD-IQA groups. The F/B ratio has been widely investigated as marker of obesity in humans and animals. In early work in a mouse model, higher F/B ratios were seen in obese mice and a similar finding was then found in humans (Ley et al., 2005; Ley et al., 2006). More recent analysis has indicated that there is unlikely to be a reliable correlation between F/B ratio and obesity in humans (Magne et al., 2020). Several studies in chickens have noted a correlation between increased F/B ratio and productivity (Singh et al., 2012; Salaheen et al., 2017; Hong et al., 2019). In the current study the HD–IQA treatment group had the highest weight gain, the highest egg mass, and the highest F/B. The improved disease protection and productivity outcomes induced by IQA may be related to both its immunomodulating effects and the changes induced in gut microbiota. This microbiota shift is likely to be related with the anti-inflammatory that the IQA have in the gut.
CONCLUSIONS
A blend of plant-derived IQA at a dose rate of 200 mg/kg in feed reduced some of the negative impacts of SLD in layer hens. These improvements include a better egg mass, a reduction in the number of liver lesions, an improvement in the weight of the hens and feed consumption, and a reduction in the expression of proinflammatory cytokines. Further field and laboratory exposure studies should be undertaken to better define both the pathogenesis of the disease and the benefits of this feed-additive in ameliorating SLD.
ACKNOWLEDGMENTS
This work was supported by a grant from the Australian Government's Innovation Connections scheme (grant number ICF002547), with contributions from Phytobiotics Futterzusatzstoffe GmbH (Germany) and National Feed Solutions Pty Ltd (Australia). The funding sources were not involved in the study design, collection, analysis, and interpretation of the data.
DISCLOSURES
All the authors of this paper declare that they do not have any conflict of interest associated with the paper.
REFERENCES
- Akdis M., Burgler S., Crameri R., Eiwegger T., Fujita H., Gomez E., Klunker S., Meyer N., O'Mahony L., Palomares O. Interleukins, from 1 to 37, and interferon-γ: receptors, functions, and roles in diseases. J. Allergy Clin. Immunol. 2011;127:701–721. doi: 10.1016/j.jaci.2010.11.050. e770. [DOI] [PubMed] [Google Scholar]
- Ashelford K.E., Chuzhanova N.A., Fry J.C., Jones A.J., Weightman A.J. At least 1 in 20 16S rRNA sequence records currently held in public repositories is estimated to contain substantial anomalies. Appl. Environ. Microbiol. 2005;71:7724–7736. doi: 10.1128/AEM.71.12.7724-7736.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badolati N., Masselli R., Sommella E., Sagliocchi S., Di Minno A., Salviati E., Campiglia P., Dentice M., Tenore G.C., Stornaiuolo M., Novellino E. The hepatoprotective effect of taurisolo, a nutraceutical enriched in resveratrol and polyphenols, involves activation of mitochondrial metabolism in mice liver. Antioxidants. 2020;9:410. doi: 10.3390/antiox9050410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozkurt M., Küçükyilmaz K., Pamukçu M., çabuk M., Alçiçek A., çatli A.U. Long-term effects of dietary supplementation with an essential oil mixture on the growth and laying performance of two layer strains. Ital. J. Anim. Sci. 2012;11:e5. [Google Scholar]
- Broom L.J., Kogut M.H. Inflammation: friend or foe for animal production? Poult. Sci. 2018;97:510–514. doi: 10.3382/ps/pex314. [DOI] [PubMed] [Google Scholar]
- Caporaso J.G., Kuczynski J., Stombaugh J., Bittinger K., Bushman F.D., Costello E.K., Fierer N., Peña A.G., Goodrich J.K., Gordon J.I., Huttley G.A., Kelley S.T., Knights D., Koenig J.E., Ley R.E., Lozupone C.A., McDonald D., Muegge B.D., Pirrung M., Reeder J., Sevinsky J.R., Turnbaugh P.J., Walters W.A., Widmann J., Yatsunenko T., Zaneveld J., Knight R. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catania S., Bilato D., Gobbo F., Granato A., Terregino C., Iob L., Nicholas R.A. Treatment of eggshell abnormalities and reduced egg production caused by Mycoplasma synoviae infection. Avian Dis. 2010;54:961–964. doi: 10.1637/9121-110309-Case.1. [DOI] [PubMed] [Google Scholar]
- Chong J., Liu P., Zhou G., Xia J. Using MicrobiomeAnalyst for comprehensive statistical, functional, and meta-analysis of microbiome data. Nat. Protoc. 2020;15:799–821. doi: 10.1038/s41596-019-0264-1. [DOI] [PubMed] [Google Scholar]
- Courtice J.M., Mahdi L.K., Groves P.J., Kotiw M. Spotty liver disease: a review of an ongoing challenge in commercial free-range egg production. Vet. Microbiol. 2018;227:112–118. doi: 10.1016/j.vetmic.2018.08.004. [DOI] [PubMed] [Google Scholar]
- Crawshaw T. A review of the novel thermophilic Campylobacter, Campylobacter hepaticus, a pathogen of poultry. Transbound. Emerg. Dis. 2019;66:1481–1492. doi: 10.1111/tbed.13229. [DOI] [PubMed] [Google Scholar]
- Crawshaw T., Irvine R. Spotty liver syndrome in poultry in Great Britain. Vet. Rec. 2012;170:317–318. doi: 10.1136/vr.e2201. [DOI] [PubMed] [Google Scholar]
- Crawshaw T.R., Chanter J.I., Young S.C.L., Cawthraw S., Whatmore A.M., Koylass M.S., Vidal A.B., Salguero F.J., Irvine R.M. Isolation of a novel thermophilic Campylobacter from cases of spotty liver disease in laying hens and experimental reproduction of infection and microscopic pathology. Vet. Microbiol. 2015;179:315–321. doi: 10.1016/j.vetmic.2015.06.008. [DOI] [PubMed] [Google Scholar]
- Crawshaw T.R., Hunter S., Wilkinson D.A., Rogers L.E., Christensen N.H., Midwinter A.C. Isolation of Campylobacter hepaticus from free-range poultry with spotty liver disease in New Zealand. N. Z. Vet. J. 2021;69:58–64. doi: 10.1080/00480169.2020.1801532. [DOI] [PubMed] [Google Scholar]
- De Asis R. P. G. (2002). Effects of nutraceuticals on the performance of broilers and layers. Accessed May, 2021, https://agris.fao.org/agris-search/search.do?recordID=PH2003001466
- Dunn C., Brunetto M., Reynolds G., Christophides T., Kennedy P.T., Lampertico P., Das A., Lopes A.R., Borrow P., Williams K., Humphreys E., Afford S., Adams D.H., Bertoletti A., Maini M.K. Cytokines induced during chronic hepatitis B virus infection promote a pathway for NK cell–mediated liver damage. J. Exp. Med. 2007;204:667–680. doi: 10.1084/jem.20061287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26:2460–2461. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- El-Sheikh S.M., Khairy M.H., Eleiwa N.Z., Abdalla O.E., El-Monsef A.G.A. Effect of sanguinarine phytobiotic, sodium butyrate compared to ampicillin on controlling necrotic enteritis in broiler chickens. Slo. Vet. Zb. 2018;55:405–414. [Google Scholar]
- Fadrosh D.W., Ma B., Gajer P., Sengamalay N., Ott S., Brotman R.M., Ravel J. An improved dual-indexing approach for multiplexed 16S rRNA gene sequencing on the Illumina MiSeq platform. Microbiome. 2014;2:6. doi: 10.1186/2049-2618-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman M., Henka P.R., Mandrell R.E. Bactericidal activities of plant essential oils and some of their isolated constituents against Campylobacter jejuni, Escherichia coli, Listeria monocytogenes, and Salmonella enterica. J. Food Prot. 2002;65:1545–1560. doi: 10.4315/0362-028x-65.10.1545. [DOI] [PubMed] [Google Scholar]
- Gregory M., Klein B., Sahin O., Girgis G. Isolation and characterization of Campylobacter hepaticus from layer chickens with spotty liver disease in the United States. Avian Dis. 2018;62:79–85. doi: 10.1637/11752-092017-Reg.1. [DOI] [PubMed] [Google Scholar]
- Grimes T., Reece R. Spotty liver disease–an emerging disease in free range egg layers in Australia. Paper presented at the Proceedings of the 16th Western Poultry Disease Conference. Sacramento, CA; Sacramento, CA; 2011. [Google Scholar]
- Guo S., Lei J., Liu L., Qu X., Li P., Liu X., Guo Y., Gao Q., Lan F., Xiao B., He C., Zou X. Effects of Macleaya cordata extract on laying performance, egg quality and serum indices in Xuefeng Black-Bone Chicken. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hananeh W., Ababneh M. Spotty liver disease in Jordan: an emerging disease. Vet. Med-Czech. 2021;66:94–98. [Google Scholar]
- Hao D. C., Gu X.-J., & Xiao P. G. 2015. 5 - Phytochemical and biological research of Chelidonieae pharmaceutical resources. In D. C. Hao, X.-J. Gu, & P. G. Xiao (Eds.), Medicinal Plants (pp. 171-216): Woodhead Publishing.
- Hong Y., Cheng Y., Li Y., Li X., Zhou Z., Shi D., Li Z., Xiao Y. Preliminary study on the effect of Bacillus amyloliquefaciens TL on cecal bacterial community structure of broiler chickens. Biomed Res. Int. 2019;2019 doi: 10.1155/2019/5431354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P., Zhang Y., Xiao K., Jiang F., Wang H., Tang D., Liu D., Liu B., Liu Y., He X., Liu H., Liu X., Qing Z., Liu C., Huang J., Ren Y., Yun L., Yin L., Lin Q., Zeng C., Su X., Yuan J., Lin L., Hu N., Cao H., Huang S., Guo Y., Fan W., Zeng J. The chicken gut metagenome and the modulatory effects of plant-derived benzylisoquinoline alkaloids. Microbiome. 2018;6:211. doi: 10.1186/s40168-018-0590-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juskiewicz J., Gruzauskas R., Zdunczyk Z., Semaskaite A., Jankowski J., Totilas Z., Jarule V., Sasyte V., Zdunczyk P., Raceviciute-Stupeliene A. Effects of dietary addition of Macleaya cordata alkaloid extract on growth performance, caecal indices and breast meat fatty acids profile in male broilers. J. Anim. Physiol. Anim. Nutr. (Berl.) 2011;95:171–178. doi: 10.1111/j.1439-0396.2010.01037.x. [DOI] [PubMed] [Google Scholar]
- Kathirvel E., Morgan K., Nandgiri G., Sandoval B.C., Caudill M.A., Bottiglieri T., French S.W., Morgan T.R. Betaine improves nonalcoholic fatty liver and associated hepatic insulin resistance: a potential mechanism for hepatoprotection by betaine. Am. J. Physiol. Gastrointest. Liver Physiol. 2010;299:G1068–G1077. doi: 10.1152/ajpgi.00249.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikusato M., Xue G., Pastor A., Niewold T.A., Toyomizu M. Effects of plant-derived isoquinoline alkaloids on growth performance and intestinal function of broiler chickens under heat stress. Poult. Sci. 2021;100:957–963. doi: 10.1016/j.psj.2020.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B.R., Shin J., Guevarra R., Lee J.H., Kim D.W., Seol K.H., Lee J.H., Kim H.B., Isaacson R. Deciphering diversity indices for a better understanding of microbial communities. J. Microbiol. Biotechnol. 2017;27:2089–2093. doi: 10.4014/jmb.1709.09027. [DOI] [PubMed] [Google Scholar]
- Kogut M.H. Dynamics of a protective avian inflammatory response: the role of an IL-8-like cytokine in the recruitment of heterophils to the site of organ invasion by Salmonella enteritidis. Comp. Immunol. Microbiol. Infect. Dis. 2002;25:159–172. doi: 10.1016/s0147-9571(01)00035-2. [DOI] [PubMed] [Google Scholar]
- Ley R.E., Bäckhed F., Turnbaugh P., Lozupone C.A., Knight R.D., Gordon J.I. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. U. S. A. 2005;102:11070–11075. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley R.E., Turnbaugh P.J., Klein S., Gordon J.I. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- Liu Z.Y., Wang X.L., Ou S.Q., Hou D.X., He J.H. Sanguinarine modulate gut microbiome and intestinal morphology to enhance growth performance in broilers. PLoS One. 2020;15 doi: 10.1371/journal.pone.0234920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magne F., Gotteland M., Gauthier L., Zazueta A., Pesoa S., Navarrete P., Balamurugan R. The Firmicutes/Bacteroidetes ratio: a relevant marker of gut dysbiosis in obese patients? Nutrients. 2020;12:1474. doi: 10.3390/nu12051474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marume U., Mokagane J.M., Shole C.O., Hugo A. Citrullus lanatus essential oils inclusion in diets elicit nutraceutical effects on egg production, egg quality, and physiological characteristics in layer hens. Poult. Sci. 2020;99:3038–3046. doi: 10.1016/j.psj.2020.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore R.J., Scott P.C., Van T.T.H. Spotlight on avian pathology: Campylobacter hepaticus, the cause of spotty liver disease in layers. Avian Pathol. 2019;48:285–287. doi: 10.1080/03079457.2019.1602247. [DOI] [PubMed] [Google Scholar]
- Motawi T.K., Hamed M.A., Shabana M.H., Hashem R.M., Aboul Naser A.F. Zingiber officinale acts as a nutraceutical agent against liver fibrosis. Nutr. Metab. (Lond.) 2011;8:40. doi: 10.1186/1743-7075-8-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni H., Martínez Y., Guan G., Rodríguez R., Más D., Peng H., Valdivié Navarro M., Liu G. Analysis of the impact of isoquinoline alkaloids, derived from Macleaya cordata extract, on the development and innate immune response in swine and poultry. BioMed Res. Int. 2016;2016 doi: 10.1155/2016/1352146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palócz O., Szita G., Csikó G. Alteration in inflammatory responses and cytochrome P450 expression of porcine jejunal cells by drinking water supplements. Mediators Inflamm. 2019;2019 doi: 10.1155/2019/5420381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabakar G., Gopi M., Karthik K., Shanmuganathan S., Kirubakaran A., Pavulraj S. Phytobiotics: could the greens inflate the poultry production. Asian J. Anim. Vet. Adv. 2016;11:383–392. [Google Scholar]
- Qi X.-Y., Liang S.-C., Ge G.-B., Liu Y., Dong P.-P., Zhang J.-W., Wang A.-X., Hou J., Zhu L.-L., Yang L., Tu C.-X. Inhibitory effects of sanguinarine on human liver cytochrome P450 enzymes. Food Chem. Toxicol. 2013;56:392–397. doi: 10.1016/j.fct.2013.02.054. [DOI] [PubMed] [Google Scholar]
- Robyn J., Rasschaert G., Hermans D., Pasmans F., Heyndrickx M. Is allicin able to reduce Campylobacter jejuni colonization in broilers when added to drinking water? Poult. Sci. 2013;92:1408–1418. doi: 10.3382/ps.2012-02863. [DOI] [PubMed] [Google Scholar]
- Saki A., Abbasinezhad M., Ghazi S., Tabatabai M., Ahmadi A., Zaboli K. Intestinal characteristics, alkaline phosphatase and broilers performance in response to extracted and mechanical soybean meal replaced by fish meal. J. Agric. Sci. Technol. 2012;14:105–114. [Google Scholar]
- Salaheen S., Kim S.-W., Haley B.J., Van Kessel J.A.S., Biswas D. Alternative growth promoters modulate broiler gut microbiome and enhance body weight gain. Front. Microbiol. 2017;8:2088. doi: 10.3389/fmicb.2017.02088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott P.C., Moore R., Wilson G. Australian Egg Corporation Limited; North Sydney, NSW, Australia: 2016. Determining the Cause and Methods of Control for Spotty Liver Disease. Publication No 1SX091. [Google Scholar]
- Scott P.C., Moore R., Wilson T., Anwar A., Van T.T.H. Australian Egg Corporation Limited; North Sydney, NSW, Australia: 2018. Final Report on the AE Funded Project to Examine the Effect of Feed Additives on Spotty Liver Disease. Publication No 1BS804a. [Google Scholar]
- Scott, P. C., T. Wilson, J. A. Quinteros, A. M. Anwar, T. Scott , T. T. H. Van, and R. Moore. 2020. Assessment of the efficacy of autogenous vaccines in spotty liver disease control, 1BSO3SX. Accessed 1st May 2021. https://www.australianeggs.org.au/assets/research/documents/Final-Report-Autogenous-SLD-Vaccine-project-final-version-PUBLISHED-v2.pdf.
- Sevoian M., Levine P.P. Effects of infectious bronchitis on the reproductive tracts, egg production, and egg quality of laying chickens. Avian Dis. 1957;1:136–164. [Google Scholar]
- Singh K.M., Shah T., Deshpande S., Jakhesara S.J., Koringa P.G., Rank D.N., Joshi C.G. High through put 16S rRNA gene-based pyrosequencing analysis of the fecal microbiota of high FCR and low FCR broiler growers. Mol. Biol. Rep. 2012;39:10595–10602. doi: 10.1007/s11033-012-1947-7. [DOI] [PubMed] [Google Scholar]
- Soler-Vasco L., Hermes R., Niewold T.A. Macleaya cordata extract reduces inflammatory responses of intestinal epithelial cells in vitro. Am. J. Plant Sci. 2016;07:1531–1537. [Google Scholar]
- Stanley D., Hughes R.J., Moore R.J. Microbiota of the chicken gastrointestinal tract: influence on health, productivity and disease. Appl. Microbiol. Biotechnol. 2014;98:4301–4310. doi: 10.1007/s00253-014-5646-2. [DOI] [PubMed] [Google Scholar]
- Sugiharto S. Role of nutraceuticals in gut health and growth performance of poultry. J. Saudi Soc. Agric. Sci. 2016;15:99–111. [Google Scholar]
- Van T.T.H., Elshagmani E., Gor M.-C., Anwar A., Scott P.C., Moore R.J. Induction of spotty liver disease in layer hens by infection with Campylobacter hepaticus. Vet. Microbiol. 2017;199:85–90. doi: 10.1016/j.vetmic.2016.12.033. [DOI] [PubMed] [Google Scholar]
- Van T.T.H., Elshagmani E., Gor M.C., Scott P.C., Moore R.J. Campylobacter hepaticus sp. nov., isolated from chickens with spotty liver disease. Int. J. Syst. Evol. Microbiol. 2016;66:4518–4524. doi: 10.1099/ijsem.0.001383. [DOI] [PubMed] [Google Scholar]
- Van T.T.H., Lacey J.A., Vezina B., Phung C., Anwar A., Scott P.C., Moore R.J. Survival mechanisms of Campylobacter hepaticus identified by genomic analysis and comparative transcriptomic analysis of in vivo and in vitro derived bacteria. Front. Microbiol. 2019;10:107. doi: 10.3389/fmicb.2019.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidanarachchi J.K., Mikkelsen L., Sims I., Iji P., Choct M. Phytobiotics: alternatives to antibiotic growth promoters in monogastric animal feeds. Rec. Adv. Anim. Nutr. Australia. 2005;15:131–144. [Google Scholar]
- Wang J.-Y., Wang X.-L., Liu P. Detection of serum TNF-alpha, IFN-beta, IL-6 and IL-8 in patients with hepatitis B. World J. Gastroenterol. 1999;5:38–40. doi: 10.3748/wjg.v5.i1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusrizal, and T. C Chen. 2003. Effect of adding chicory fructans in feed on fecal and intestinal microflora and excreta volatile ammonia. Int. J. Poult. Sci. 2:188–194.