Highlights
-
•
We performed WES in 10 patients with presumed genetic epilepsy/epileptic encephalopathy.
-
•
Causative genetic variants found in 9 epilepsy-related genes.
-
•
Genes involved were AMT, EPM2A, GABRG2, GRIN2B, PDHX, SCN1A, SCN2A, SLC2A1, STXBP1.
-
•
In half of our patients: genetic cause with potential management implications.
Keywords: Epilepsy, Genetics, Whole exome sequencing, Next-generation sequencing, Inherited epilepsy
Abstract
We describe a cohort of 10 unrelated Greek patients (4 females, 6 males; median age 6.5 years, range 2–18 years) with heterogeneous epilepsy syndromes with a genetic basis. In these patients, causative genetic variants, including two novel ones, were identified in 9 known epilepsy-related genes through whole exome sequencing. A patient with glycine encephalopathy was a compound heterozygote for the p.Arg222Cys and the p.Ser77Leu AMT variant. A patient affected with Lafora disease carried the homozygous p.Arg171His EPM2A variant. A de novo heterozygous variant in the GABRG2 gene (p.Pro282Thr) was found in one patient and a pathogenic variant in the GRIN2B gene (p.Gly820Val) in another patient. Infantile-onset lactic acidosis with seizures was associated with the p.Arg446Ter PDHX gene variant in one patient. In two additional epilepsy patients, the p.Ala1662Val and the novel non-sense p.Phe1330Ter SCN1A gene variants were found. Finally, in 3 patients we observed a novel heterozygous missense variant in SCN2A (p.Ala1874Thr), a heterozygous splice site variant in SLC2A1 (c.517-2A>G), as a cause of Glut1 deficiency syndrome, and a pathogenic variant in STXBP1 (p.Arg292Leu), respectively. In half of our cases (patients with variants in the GRIN2B, SCN1A, SCN2A and SLC2A1 genes), a genetic cause with potential management implications was identified.
1. Introduction
An increasing number of genes is being associated with epilepsy, leading to a deeper understanding of epileptogenesis [1], [2], [3], [4], [5]. In genetically determined epilepsies, genotype-phenotype correlations are complex since variants in different genes can cause the same epilepsy syndrome and, inversely, pathogenic variants in the same gene can cause heterogeneous phenotypes [3], [6]. Thus, based on the electroclinical, imaging and laboratory findings, it is not always feasible to predict the gene implicated in a genetic epilepsy syndrome and guide targeted sequencing. Next generation sequencing technologies, including whole exome sequencing (WES), have revolutionized the clinical practice of neurology, and especially epileptology, by allowing affordable concurrent sequencing of thousands of genes [4].
This improved ability for an accurate genetic diagnosis is important in clinical decision-making [1], [7]. For example, patients diagnosed with the Glut1 deficiency syndrome due to variants in the SCL2A1 gene could benefit from receiving the ketogenic diet [8]. Also, patients with Dravet syndrome due to SCN1A gene variants should avoid sodium channel blocking antiseizure medication, which, in contrast, are useful in early-onset encephalopathy due to variants in the SCN8A gene [8]. Furthermore, even when a treatable genetic cause of epilepsy is not identified, diagnostic certainty spares the patients and their families from a host of non-diagnostic tests, allows prognosis estimation, and ultimately improves overall patient care.
Our aim was to study the contribution, practicality, and efficiency of WES, as a straightforward approach, in the diagnosis and management of a Greek cohort of children and young adults with epilepsy/epileptic encephalopathy (epilepsy manifesting as developmental and/or epileptic encephalopathy). The results in our cohort extend further the genotypic and phenotypic spectrum of pediatric epilepsies, by identifying two novel causative variants (in the SCN1A and SCN2A genes, respectively), adding new phenotypic information on already described pathogenic variants, and showing that in half of our cases (patients with variants in the GRIN2B, SCN1A, SCN2A and SLC2A1 genes) genetic findings were associated with potential management implications.
2. Patients and methods
2.1. Study subjects
Out of 44 consecutive patients with epilepsy/epileptic encephalopathy (21 females, 23 males, median age 6.5 years, range 0.5–30 years), with or without concurrent developmental delay, referred to the Neurology/Neurogenetics Laboratory of the University of Crete, Medical School, we included in this study 10 patients from 10 unrelated non-consanguineous families with a diagnostic result through WES. All 44 patients were suspected to suffer from genetic epilepsies, based on their clinical presentation, electrophysiological studies, and absence of known acquired causes of epilepsy. Patients were recruited from different clinical centers across Greece (Thessaloniki, Heraklion Crete, Athens). Informed consent was obtained from the patients and/or their legal guardians. The study protocol was approved by the Institutional Review Board of the University Hospital of Heraklion, Crete, Greece.
2.2. Whole exome sequencing
Since extensive work-up in our patients failed to identify an acquired cause of epilepsy, and genetic origin of the epilepsy syndrome was suspected, we proceeded to WES for these patients as the preferred approach. Due to the multiplicity of genes associated with epilepsy, the sequential targeted gene sequencing approach would be expensive and inefficient. Furthermore, an epilepsy gene panel approach would not allow identification, future re-analysis of the data, and new gene variants not included in panels.
Genomic DNA was extracted from peripheral blood. WES and initial bioinformatics analysis were performed in a CLIA-certified laboratory (Otogenetics Corporation, Norcross, GA, USA). Exome target enrichment was performed with the Agilent V5 (51 Mb) Sure-Select Target Enrichment System. For exome sequencing, a HiSeq 2500 (Illumina, USA) platform was used, with paired-ended reads of 100–125 bp and estimated average coverage of 50X.
The data were further analyzed using the Ingenuity Variant Analysis (IVA) software (Qiagen, USA), at the Neurology Laboratory, University of Crete. Initially, variants were filtered for minor allele frequencies < 0.1%. We then focused on the protein-altering variants that matched the patient's phenotype, based on literature and online database search. Confirmatory Sanger sequencing was performed for identified variants.
3. Cohort description (Tables 1-3, supplemental Information)
In this Greek cohort of 10 patients with epilepsy/epileptic encephalopathy (4 females, 6 males; median age 6.5 years, range 2–18 years) and a broad range of phenotypes, with or without developmental delay, we identified causative variants in the AMT, EPM2A, GABRG2, GRIN2B, PDHX, SCN1A (2 patients), SCN2A, SLC2A1 and STXBP1 genes, respectively. The types of seizures in our patient cohort were very heterogeneous, both between patients and even between time periods for the same patient (Table 1). Concerning additional clinical features and outcomes, 8 of the 10 patients showed developmental delay (Table 1), while of the remaining 2 patients, one with Lafora disease developed cognitive decline after normal development. In 8 of the patients, we observed hypotonia and movement disorders, including extrapyramidal syndrome, choreoathetosis and ocular motility disorder (Table 1). Only the patient with the SCN2A variant had a past family history of a similar neurologic disorder in his brother (Table 1). All 10 patients had received multiple antiseizure medications and in 4 of them the ketogenic diet was attempted, with mixed treatment results (Table 1).
Table 1.
Summary of clinical features and family history of patients included in the study.
| Patient # | Sex | Age at WES diagnosis | Age at onset of epilepsy | Type of seizures | Frequency of Seizures | Additional clinical features | Epilepsy/epilepsy syndrome | Family History | Anti-seizure medications used | Outcome (seizures) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 6 mo. | Neonate | Tonic spasms in clusters | Multiple per day | Encephalopathy, hypotonia | Ohtahara syndrome | No | Levetiracetam, clobazam, valproic acid, phenobarbital, zonisamide | Developmental delay (improvement of seizures) |
| 2 | F | 18 y. | 14 y. | Generalized tonic clonic, absences, myoclonic | Multiple per day | Visual hallucinations, myoclonus | Progressive myoclonus epilepsy | No | Levetiracetam, valproate, lamotrigine, clobazam, topiramate, ketogenic diet, zonisamide, phenobarbital | Dementia (partial response of seizures) |
| 3 | M | 18 mo. | 4 mo. | Infantile spasms | Multiple per day | Encephalopathy, hypotonia, abnormal ocular motility, nystagmus, extrapyramidal syndrome | Early infantile epileptic encephalopathy | No | Vigabatrin, steroids, valproic acid, pyridoxal phosphate | Developmental delay (improvement of seizures) |
| 4 | F | 17.5 y. | Neonate | Tonic spasms, myoclonic | Multiple per day | Encephalopathy, hypotonia | Early infantile epileptic encephalopathy | No | Vigabatrin, ACTH, Valproate | Developmental delay (improvement of seizures) |
| 5 | F | 2 y. | Neonate | Early spasms | Multiple per day | Encephalopathy, hypotonia | Early infantile epileptic encephalopathy | No | Vigabatrin, high dose prednisone, ketogenic diet | Developmental delay (seizure free with ketogenic diet (KD) |
| 6 | M | 18 mo. | 5.5 mo. | Generalized tonic clonic | Variable | Mild early hypotonia | Generalized | No | Phenobarbital, oxcarbazepine, levetiracetam, valproate | Normal development (partial seizure control) |
| 7 | M | 15 y. | 8 mo. | Generalized, focal to bilateral tonic clonic | High | - | Unclassified | No | Valproate, tpopiramate, lamotrigine, ketogenic diet | Developmental delay (poor outcome of seizure control) |
| 8 | M | 13 y. | 5 mo. | Focal seizures, tonic, myoclonic | High | Hypotonia | Early infantile epileptic encephalopathy | Yes (brother) | Levetiracetam, valproic acid, ketogenic diet | Developmental delay (poor outcome of seizure control) |
| 9 | M | 15 y. | 3 mo. | Non motor behavior arrest, myoclonic, atonic | High | - | Myoclonic-astatic epilepsy (Doose syndrome) | No | Valproate, levetiracetam,topiramate, lamotrigine, vigabatrin, clobazam, rufinamide, ethosuximide, ketogenic diet | Developmental delay (poor outcome of seizure control) |
| 10 | M | 5 y. | Unknown | Focal tonic spasms, myoclonic, generalized tonic clonic | Multiple per day | Macrosomia, axial hypotonia, limb hypertonia, movement disorder (dyskinesia, choreoathetosis) | Early infantile epileptic encephalopathy | No | ACTH, prednisone, valproate, topiramate | Developmental delay (poor seizure control) |
Abbreviations: mo.: months, y.: years, ACTH: adrenocorticotropic hormone.
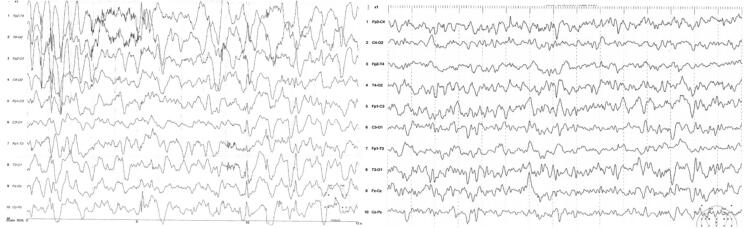
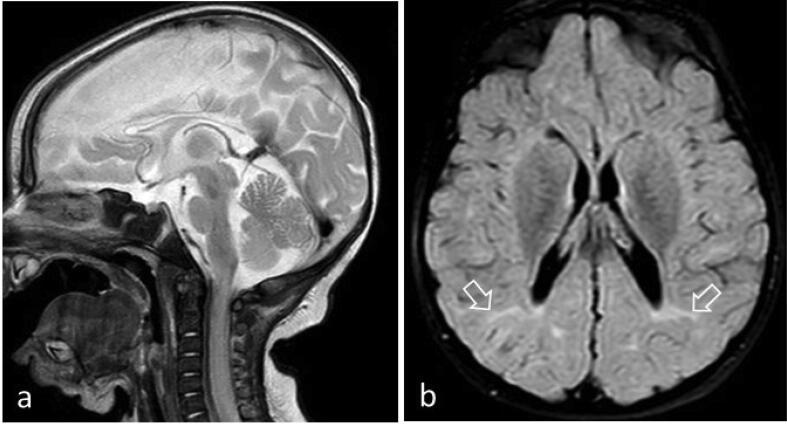
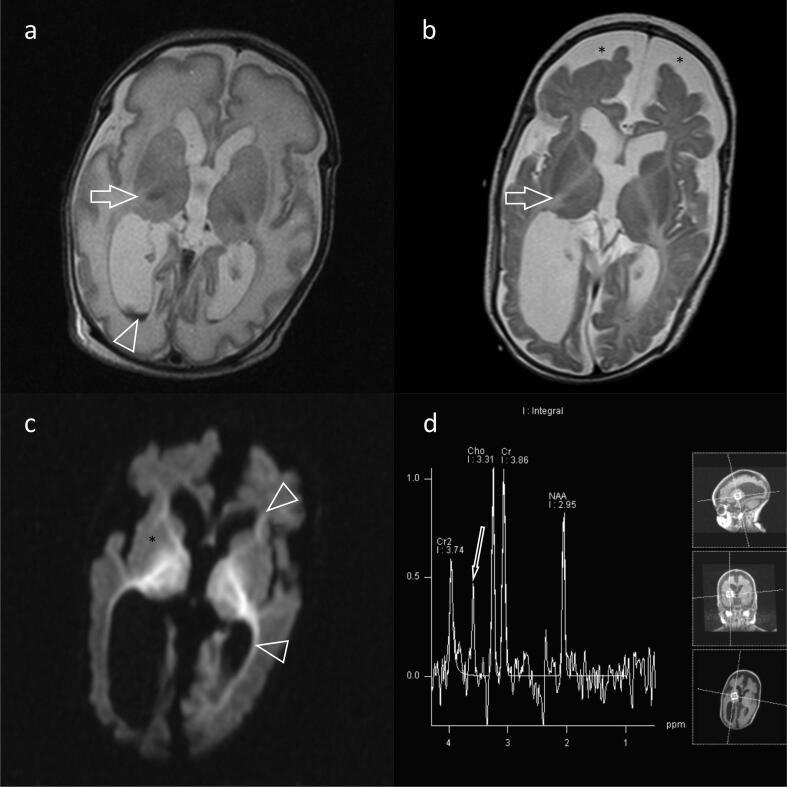
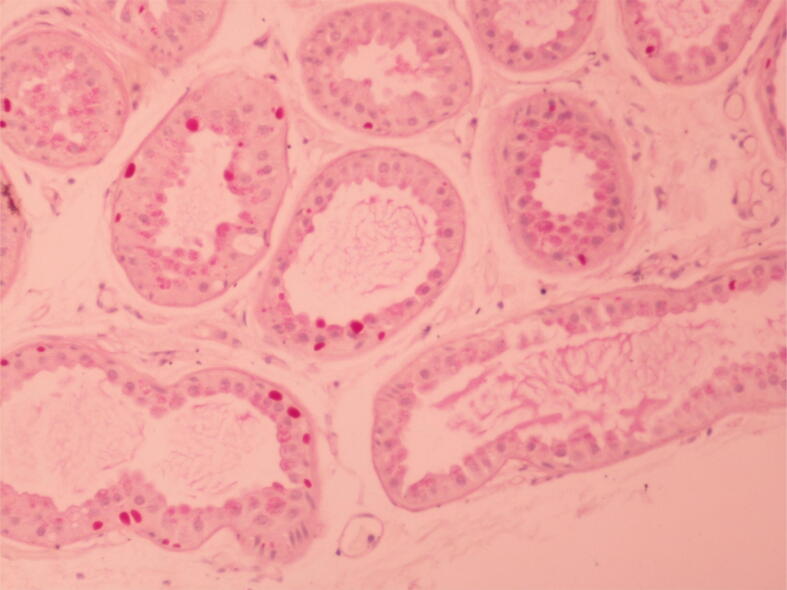
EEG findings were quite variable, both between patients and in the same patient at different ages and under different treatment modalities (Table 2). For example, as shown in Fig. 1 for patient #5 (with the PDHX pathogenic variant) the EEG features of hypsarrhythmia improved after the initiation of the ketogenic diet. In three of the patients, MRI brain scans were unrevealing. In the remaining patients, imaging findings ranged from mild brain atrophy to severe structural lesions (e.g. hematomas) and developmental abnormalities (e.g. delayed myelination and dysgenesis of corpus callosum; Table 2). For instance, brain MRI of patient # 5 (harboring the p.Arg446Ter PDHX gene pathogenic variant in homozygous state) showed corpus callosum dysgenesis, mild dilatation of the occipital horns bilaterally and adjacent leukoencephalopathy (Fig. 2). Brain MRI for patient #1 (with the AMT gene variants) showed intraventricular hemorrhage, subdural hematomas, ventriculomegaly, delayed myelination, bi-thalamic lesions and atrophy and abnormal signal intensity at the internal capsule, as well as a glycine peak in MR spectroscopy (Fig. 3). In 2 of the patients, prominent metabolic abnormalities were noted: elevated CSF/plasma glycine and lactic acidosis in the patients with the AMT and the PDHX variants, respectively (Table 2). Finally, for patient #2, the diagnosis of Lafora disease due to the pathogenic p.Arg171His EPM2A gene variant, was verified by an axillary biopsy that revealed Lafora bodies (PAS positive intracytoplasmic inclusions) in the cells lining the apocrine sweat glands (Fig. 4).
Table 2.
Summary of EEG, MRI and lab/biopsy results of patients included in the study.
| Patient # | EEG | MRI | Lab/biopsy results |
|---|---|---|---|
| 1 | Burst suppression pattern | Intraventricular hemorrhage, subdural hematomas, ventriculomegaly. delayed myelination, bi-thalamic lesions, abnormal signal intensity/restricted diffusion at myelinated areas including the posterior limb of the internal capsule | Elevated plasma and CSF glycine |
| 2 | Encephalopathic pattern, generalized spike-wave complexes | Normal | Lafora bodies in axillary skin biopsy |
| 3 | Encephalopathic pattern | Cerebral atrophy | - |
| 4 | Poor organization, generalized sharp/wave complexes and slow sharp delta waves of high voltage, hypsarrhythmia | Delay of myelination, decrease in cerebral volume | - |
| 5 | Hypsarrhythmia | Corpus callosum dysgenesis, colpocephaly with dilatation of the occipital horns bilaterally | Lactic acidosis |
| 6 | Generalized sharp wave complexes | Unrevealing | - |
| 7 | Compatible with generalized epilepsy | Generalized atrophy, especially at frontal lobes | - |
| 8 | Interictal epileptic activity on left dorsal frontocentral region, midline | Abnormal periventricular and occipital white matter, old infarct of the left basal ganglia | - |
| 9 | Generalized sharp wave complexes | Hypoplasia of posterior part of corpus callosum. | - |
| 10 | Burst suppression pattern, hypsarrhythmia, bilateral occipital slow waves, focal epileptic discharges | Normal | - |
Abbreviations: mo.: months, y.: years, MRI: Magnetic resonance Imaging, EEG: Electroencephalogram, CSF: cerebrospinal fluid.
Fig. 1.
EEG recordings of patient # 5. A. Hypsarrhythmia pattern before initiation of ketogenic diet (KD). B. Improvement of the hypsarrhythmia pattern 2 years after initiation of KD. WES revealed the p.Arg446Ter (c.1336C>T) PDHX gene pathogenic variant in homozygous state.
Fig. 2.
MRI imaging of patient # 5 at 3 years of age. a. Sagittal T2-WI showing corpus callosum dysgenesis. b. Axial T2-FLAIR showing colpocephaly with mild dilatation of the occipital horns bilaterally and adjacent restricted leukoencephalopathy (arrows). WES revealed the p.Arg446Ter (c.1336C>T) PDHX gene pathogenic variant in homozygous state.
Fig. 3.
MRI of patient #1 at various ages. a. T2-weighted sequence, axial image at age 13 days, shows “very white” white matter and a rather rounded cortex, consistent with a diffuse encephalopathy. Intraventricular haemorrhage (arrowhead) is a change frequently encountered in the vulnerable neonatal brain, especially in premature babies. Absence of hypointense posterior limb of internal capsule (PLIC) (arrow) was an early indication of leukoencephalopathy. b. T2-weighted sequence, axial image at age 3.5 months and with reduced head circumference, shows increased extra-axial cerebrospinal fluid space frontally (*) with dilated ventricular system, consistent with reduced brain volume. Note small hyperintense thalami and lack of myelination with persisting absence of PLIC (arrow). C. Diffusion sequence, axial plane at age 3.5 months. There is restricted diffusion at areas where myelinated white matter should exist. This is evident as hyperintensity at the internal capsules (*) and at periventricular white matter around the frontal and occipital horns (arrowheads). D. Spectroscopy at 145 TE shows a glycine peak at 3.35 ppm (arrow). The clinical, imaging and biochemical diagnosis of glycine encephalopathy was confirmed by the identification of the p.Arg222Cys (c.664C>T) and p.Ser77Leu (c.230C>T) AMT gene variants in compound heterozygosity.
Fig. 4.
Lafora bodies in axillary skin biopsy from patient #2. Lafora bodies (PAS positive intracytoplasmic inclusions) are found in the cells lining the apocrine sweat glands. In this patient, we identified by WES a homozygous pathogenic EPM2A variant (p.Arg171His; c.512G>A).
4. Discussion
In this work, we identified the cause of epilepsy/epileptic encephalopathy in a Greek cohort of 10 pediatric patients, across a broad range of phenotypes. Specifically, we found pathogenic variants in the following genes: AMT, EPM2A, GABRG2, GRIN2B, PDHX, SCN1A (2 patients), SCN2A, SLC2A1 and STXBP1, as the cause of epilepsy in this cohort with heterogeneous phenotypic manifestations Table 3. Two variants, the p.Phe1330Ter SCN1A and the p.Ala1874Thr SCN2A gene variants were novel, adding to the genotypic spectrum of genetic epilepsy. Even though there is evidence [9] that in a sizeable proportion of patients analyzed with WES there could be multiple molecular diagnoses (i.e. pathogenic variants in more than one gene underlying the disease phenotype), causative variants in a single gene were identified in our patients. In the patients with homozygous causative genetic variants (in the EPM2A and PDHX genes, respectively), consanguinity was considered as a possibility; however, we were not able to identify parental relationships using family history.
Table 3.
Summary of causative genetic variants included in this study.
| Patient # | Gene | Variants | rs | Inheritance | CADD score | gnomAD frequency (%) | Pathogenicity evidence (ACMG criteria) | Epilepsy Type (Genetic Diagnosis) | Previously reported or novel |
|---|---|---|---|---|---|---|---|---|---|
| 1 | AMT | p.Ser77Leu (c.230C>T)/p.Arg222Cys (c.664C>T) | 386833680/781466698 | AR | 24.0/32.0 | ≤0.001/≤0.001 | PS4, PM2, PM3, PP3, PP5/PS4, PM1, PM3, PM5, PP3, PP5 | Glycine encephalopathy (OMIM 605899) | [18], [19] |
| 2 | EPM2A | p.Arg171His (c.512G>A)- homozygosity | 137852916 | AR | 31.0 | 0.002 | PS4, PM1, PM2, PP3, PP5 | Epilepsy, progressive myoclonic 2A (Lafora) (OMIM 254780) | [24], [25] |
| 3 | GABRG2 | p.Pro282Thr (c.844C>A) | 796052508 | AD (de novo) | 27.2 | 0.000 | PM2, PP3 | Early infantile epileptic encephalopathy type 74 (OMIM 618396) | [32] |
| 4 | GRIN2B | p.Gly820Val (c.2459G>T) | 797044849 | AD | 26.1 | 0.000 | PM2, PP2, PP3 | Epileptic encephalopathy, early infantile, 27 (OMIM 616139) | [35], [41] |
| 5 | PDHX | p.Arg446Ter (c.1336C>T)- homozygosity | 1135402725 | AR | 38.0 | ≤0.001 | PS4, PM2, PP3 | Lactic acidemia due to PDX1 deficiency (OMIM 245349) | [44], [45] |
| 6 | SCN1A | p.Ala1662Val (c.4985C>T) | 794726839 | AD | 28.5 | 0.000 | PM1, PM2, PM6, PP3, PP5 | SCN1A gene (OMIIM 182389) related epilepsy | [55] |
| 7 | SCN1A | p.Phe1330Ter (c.3988_3989insGAGGTGATGGGATACCTTACCC) | - | AD | - | 0.000 | PVS1, PM2 | SCN1A gene (OMIIM 182389) related epilepsy | Novel |
| 8 | SCN2A | p.Ala1874Thr (c.5620G>A) | 753977894 | AD | 22.1 | 0.002 | PP2, PP3/BS1 | Epileptic encephalopathy, early infantile, 11 (OMIM 613721) | Novel |
| 9 | SLC2A1 | c.517-2A>G (Splice site) | - | AD | 34.0 | 0.000 | PVS1, PM2 | Glut1 deficiency syndrome (OMIM 612126, 606777) | [66] |
| 10 | STXBP1 | p.Arg292Leu (c.875G>T) | 796053361 | AD | 25.4 | 0.000 | PS4, PM1, PM2, PM5, PM6, PP3 | Epileptic encephalopathy, early infantile, 4 (OMIM 612164) | [70], [71] |
PS4 - The prevalence of the variant in affected individuals is significantly increased compared with the prevalence in controls.
PVS1 - Null variant in a gene where loss of function is a known mechanism of disease.
PM1 - Located in a mutational hot spot.
PM2 - Absent from controls (or at extremely low frequency if recessive) in gnomAD.
PM3 - For recessive disorders, detected in trans with a pathogenic variant.
PM5 - Novel missense change at an amino acid residue where a different missense change determined to be pathogenic has been seen before.
PM6 - Assumed de novo, but without confirmation of paternity and maternity.
PP2 - Missense variant in a gene that has a low rate of benign missense variation and in which missense variants are a common mechanism of disease.
PP3 - Multiple lines of computational evidence support a deleterious effect on the gene or gene product.
PP5 - Reputable source recently reports variant as pathogenic.
BS1 - Allele frequency is greater than expected for disorder.
In most cases, we were able to reach a diagnosis in children and adolescents that had not been formally diagnosed, despite several non-diagnostic tests, including metabolic studies, EEG recordings, brain imaging and cerebrospinal fluid analyses. In addition, in all patients, genetic diagnosis led to management adjustments, including genetic counseling, abandonment of unnecessary diagnostic tests and treatments and establishment of targeted therapies and follow-up.
There have been several recent studies using a WES approach to investigate patients with presumed genetic epilepsy, either isolated or with concomitant developmental/neurocognitive delay [10], [11], [12]. The diagnostic yield in these studies ranges from 10 to 25% [12], [13], [14], [15], similarly to the 22.7% observed in our study (10 out of 44 patients diagnosed with a causative variant). Among the NGS methods used in the diagnostic investigation of genetic epilepsy, WES has the advantage of more extended coverage compared to gene panels, without significant increase in cost, allowing the detection of variants in not yet described epilepsy genes in future re-analyses [10]. Also, it has a greater diagnostic potential compared to chromosomal micro-arrays, that target exclusively copy number variants [10]. With the wider availability and the decreasing cost of whole genome sequencing in the future, it is predicted that it will surpass WES for the genetic diagnosis of epilepsy; however, at present WES remains the preferred diagnostic method for most patients with epilepsy.
The clinical and biochemical diagnosis of glycine encephalopathy was confirmed by the identification of the p.Ser77Leu and p.Arg222Cys AMT gene variants in compound heterozygosity in patient #1. Pathogenic variants in the AMT gene cause epileptic encephalopathy associated with nonketotic hyperglycinemia [16]. In the typical neonatal form of the disease, patients show decreased level of consciousness, hypotonia, respiratory insufficiency, drug-resistant seizures, and increased CSF glycine. Infantile and late-onset forms of nonketotic hyperglycinemia convey a better prognosis. The AMT gene encodes for an aminomethyltransferase that forms part of the mitochondrial tetrameric glycine cleavage system [17]. Concerning the AMT gene variants of our patient, the p.Ser77Leu variant has previously been found in two patients with neonatal nonketotic hyperglycinemia [18]. Moreover, the p.Arg222Cys AMT gene variant has also been found in two other patients with neonatal nonketotic hyperglycinemia [19].
WES identified the homozygous p.Arg171His EPM2A variant in patient #2 with drug-resistant progressive myoclonic epilepsy and dementia. The diagnosis of Lafora disease was later confirmed by axillary biopsy and spared the patient from unnecessary treatment modalities. Lafora disease causes progressive myoclonic epilepsy presenting in adolescence and leading to death usually within a decade from symptom onset [20], [21]. As in our patient, antiepileptic medications are of limited value in controlling intractable seizures [21]. The loss of function of laforin (encoded by the EPM2A gene) leads to the accumulation of poorly branched dysfunctional glycogen, forming Lafora bodies within neurons and other cells, such as apocrine gland cells in the axilla (Fig. 4). The p.Arg171His EPM2A variant found in our patient has been repeatedly described in the literature [22], [23], [24], [25], [26]. This variant resides in the dual-specificity phosphatase domain of laforin, leads to cytoplasmic clump formation, and decreases phosphatase activity against glycogen [22], [27], [28].
WES linked the heterozygous de novo p.Pro282Thr GABRG2 pathogenic variant to epileptic encephalopathy in patient #3. Variants in this gene are associated with autosomal dominant generalized epilepsy with febrile seizures plus and early infantile epileptic encephalopathy [29], [30]. The GABRG2 gene encodes for the γ subunit of the GABAA heteromeric ion channel receptors, composed of five subunits surrounding a chloride channel [31]. The GABAA receptors are the main inhibitory human brain neurotransmitter receptors. They are the target of barbiturates and benzodiazepines, used as antiepileptics, sedatives and anxiolytics [31]. The p.Pro282Thr variant, as a de novo occurrence, has recently been described in a 12-year-old girl with drug-resistant Lennox-type epilepsy, developmental delay, dysmorphic features, hypotonia, nystagmus and cerebral atrophy on MRI [32], a clinical picture similar to that of our patient. The p.Pro282Thr variant, which results in a non-conservative amino acid substitution, is located in a conserved position across species, in the pore region of the GABAA receptor and is predicted in silico to damage protein function (CADD score 27.2). Also, a pathogenic variant at the same residue (p.Pro282Ser), with proven profound functional consequences on the GABAA receptor, has been described in a 10-year old female patient with global developmental delay, hypotonia and drug-resistant seizures [30].
The p.Gly820Val GRIN2B pathogenic variant was identified in patient #4. Pathogenic GRIN2B variants cause autosomal dominant epileptic encephalopathy and other phenotypes, collectively known as GRIN2B-related neurodevelopmental disorders [33], [34], [35], [36], [37], [38]. For patients with pathogenic variants in GRIN2B, therapy with N-methyl-D-aspartate (NMDA) receptor inhibitors e.g. by memantine may be of value [39]. The GRIN2B gene encodes for the glutamate binding NR2B subunit of the NMDA receptors, which are the main mediators of excitatory neurotransmission in human brain, being especially important for neuronal development and memory formation [34], [35]. The p.Gly820Val GRIN2B variant found in our patient has already been described in the literature as a cause of epileptic encephalopathy [35], [40], [41]. Also, other changes at the same residue (p.Gly820Glu, p.Gly820Ala) have similar phenotypic manifestations [41], [42], [43]. The Gly820 residue lies in the 4th helix of the transmembrane domain of the NMDA receptor, in a highly conserved area where changes are expected to be functionally harmful.
Τhe p.Arg446Ter PDHX pathogenic variant was found in patient # 5 with lactic acidosis and seizures since birth. Variants in the PDHX gene have been associated with congenital lactic acidosis [44], [45], [46], [47]. The PDHX gene encodes for component X of the pyruvate dehydrogenase (PDH) complex, a highly regulated complex interconnecting glycolytic processes with the tricarboxylic acid cycle, and thus playing a crucial role in energy homeostasis. The PDH complex catalyzes the oxidative decarboxylation of pyruvate to acetyl-CoA, CO2 and NADH(H+) through the sequential action of pyruvate dehydrogenase (E1), dihydrolipoamide acetyltransferase (E2) and dihydrolipoamide dehydrogenase (E3) [48], with component X functionally interconnecting the E2 and E3 enzymes. The p.Arg446Ter pathogenic variant results in a truncated protein product and has been initially described in two siblings from the UK [49] and a patient from France [47] showing neurodevelopmental delay and lactic acidosis. Later, it has been found in patients with congenital lactic acidosis, psychomotor delay, progressive encephalopathy and seizures from Bulgaria, Hungary, Romania and Slovakia showing that this is a common variant across Europe [50].
In patients #6 and #7, WES revealed the p.Ala1662Val and p.Phe1330Ter SCN1A gene variants, respectively. SCN1A is the gene most commonly associated with genetic epilepsy, with more than 1200 pathogenic variants described thus far [51]. Variants in the SCN1A gene are associated with generalized epilepsy with febrile seizures plus, early infantile epileptic encephalopathy or Dravet syndrome, familial hemiplegic migraine and autism, among other phenotypes [52], [53]. Also, the identification of a loss of function SCN1A variant could have therapeutic implications, since in this case sodium channel blockers should be avoided [39], [54]. The SCN1A gene encodes for the large α-subunit of the neuronal voltage-gated sodium channel type 1 (NaV1.1) [52]. The six transmembrane domains of this α-subunit form the pore of the sodium channel, whereas the auxiliary β-subunits assist in the regulation of the channel properties and mediate its interaction with other proteins [52]. The p.Ala1662Val SCN1A variant found in our patient has already been described in a patient with severe myoclonic epilepsy of infancy [55]. This variant is in a mutational hot spot, with no benign variation. Also, it is part of a critical functional domain and in silico evidence suggests it is pathogenic (CADD = 28.5). In patient #7, the p.Phe1330Ter SCN1A gene variant was identified, that has not been previously described in the literature. However, it is predicted in silico to be deleterious. Also, it leads to a truncated protein product in a gene that loss of function due to similar variants is a known pathogenetic mechanism. In addition, the p.Phe1330Ter variant has not been found in normal individuals.
The p.Ala1874Thr SCN2A gene variant was identified in patient # 8. Variants in the SCN2A gene are known to cause benign familial neonatal infantile seizures, epilepsy of infancy with migrating focal seizures, epileptic encephalopathies, including Ohtahara syndrome, and other epileptic and non-epileptic phenotypes [56], [57], [58]. It is thought that gain of function mutations lead to early-onset (<3 months) epilepsy or epileptic encephalopathy, whereas loss of function variants are the cause of late-onset epilepsy or intellectual disability syndromes [54], [59]. There is evidence that favors the aggressive use of sodium-channel blockers in patients with gain of function SCN2A variants and, inversely, the avoidance of these anti-epileptics in the case of loss of function variants [39], [54], [60]. The SCN2A gene encodes for the neuronal voltage-gated sodium channel NaV1.2, a paralog of the NaV1.1 channel described above, with which it shares a similar structure and role in neuronal action potential propagation [59]. The α-subunit encoded by the SCN2A gene forms the pore of the sodium channel, being assisted in its function by the β-subunits [59]. Concerning the SCN2A variant (c.5620G>A; p.Ala1874Thr) identified in our patient, it has not been previously described. However, it is a missense variant in a gene with a low rate of benign missense variants and strong computational evidence suggests a deleterious effect on the protein product (CADD = 22.1).
The heterozygous c.517-2A>G splice site variant in the SLC2A1 gene was found in patient #9 with myoclonic astatic epilepsy. Variants in the SLC2A1 gene are associated with the Glut1 deficiency syndrome (infantile seizures, developmental delay, microcephaly, and ataxia), paroxysmal dyskinesia, absence epilepsies, myoclonic astatic epilepsy, episodic choreoathetosis, spasticity, and focal epilepsy [61], [62], [63]. In these Glut1 deficiency syndromes, ketogenic diet, when initiated early, leads to a favorable therapeutic response[39], [62]. However, there have been cases where the ketogenic diet failed, either due to poor tolerability or true inefficiency, as was probably the case in our patient [64]. The SLC2A1 gene encodes for Glut1 (glucose transporter type 1), which transports glucose across the blood-brain barrier. Since brain metabolism relies on glucose utilization, Glut1 is the most important energy carrier across the blood brain barrier. Glut1 deficiency due to SLC2A1 pathogenic variants leads to poor availability of glucose, causing early-onset encephalopathy [65]. The c.517-2A>G SLC2A1 gene variant leads to splice-site loss, in a gene that loss of function is a known pathogenetic mechanism. In addition, it is predicted in silico to be functionally deleterious (CADD = 34.0). The SLC2A1 c.517-2A>G variant has been described before, again in heterozygous state, in a 4-year old patient with recurrent generalized tonic seizures and reversible brain white matter lesions [66].
Finally, WES identified the pathogenic p.Arg292Leu variant in the STXBP1 gene as the cause of epileptic encephalopathy in patient #10 [67], [68]. The STXB1 gene encodes for the syntaxin binding protein, which belongs to the SEC1 family of membrane trafficking proteins, contributes to synaptic vesicle release and may play a role in neurodegenerative disorders [69]. The p.Arg292Leu variant found in our patient has been described before as occurring de novo in several patients with epilepsy [70], [71], [72]. This variant has strong computational evidence favoring its pathogenicity (CADD = 25.4) and is located in a mutational hotspot, at an amino acid residue where another missense change has been characterized as pathogenic (p.Arg292His, ClinVar VCV000207424.2).
5. Conclusions
Overall, we identified disease causing variants in 9 epilepsy-related genes in 10 pediatric patients affected with epilepsy/epileptic encephalopathy, including two variants (one each in the SCN1A and SCN2A genes, respectively) that have not been previously described as causative for epilepsy, but have strong evidence favoring their pathogenicity. These 10 patients were part of a larger cohort of 44 patients with epilepsy/epileptic encephalopathy referred to us for WES, yielding a diagnostic rate of 22.7%. In half of our 10 diagnosed patients (5/10), bearing pathogenic variants in the GRIN2B, SCN1A, SCN2A, and SLC2A1 genes, this improved diagnostic accuracy carried potential implications for their management. The final diagnosis obtained by WES, being either confirmatory or contributing to the diagnosis, spared all the patients and their families from unnecessary further investigations, inappropriate treatment approaches and offered the possibility of appropriate genetic counseling. Our results, in addition to those from other studies, further attest to the practicality and real-world utility of a straightforward approach involving WES, instead of targeted gene-sequencing or gene panel testing, as the initial genetic test in patients with epilepsy/epileptic encephalopathy of presumed genetic etiology.
Declarations of interest
None.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethics statement
All authors meet the criteria for authorship stated in the Uniform Requirements for Manuscripts Submitted to Biomedical Journals. Also, the authors have no relevant conflicts of interest and all contributors have read and approved the manuscript before submission.
Informed consent was obtained from the patients and/or their legal guardians. The study protocol was approved by the Institutional Review Board of the University Hospital of Heraklion, Crete, Greece.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebr.2021.100477.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Berkovic S.F. Genetics of epilepsy in clinical practice. Epilepsy Curr. 2015;15:192–196. doi: 10.5698/1535-7511-15.4.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Devinsky O., Vezzani A., O'Brien T.J., Jette N., Scheffer I.E., de Curtis M. Epilepsy. Nat Rev Dis Primers. 2018;4:18024. doi: 10.1038/nrdp.2018.24. [DOI] [PubMed] [Google Scholar]
- 3.Helbig I., Heinzen E.L., Mefford H.C., Commission T.I.G. Primer Part 1—The building blocks of epilepsy genetics. Epilepsia. 2016;57:861–868. doi: 10.1111/epi.13381. [DOI] [PubMed] [Google Scholar]
- 4.Myers K.A., Johnstone D.L., Dyment D.A. Epilepsy genetics: current knowledge, applications, and future directions. Clin Genet. 2019;95:95–111. doi: 10.1111/cge.13414. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y., Du X., Bin R., Yu S., Xia Z., Zheng G. Genetic variants identified from epilepsy of unknown etiology in chinese children by targeted exome sequencing. Sci Rep. 2017;7:40319. doi: 10.1038/srep40319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nolan D., Fink J. In: Handbook of clinical neurology. Geschwind D.H., Paulson H.L., Klein C., editors. Elsevier; 2018. Chapter 30 - Genetics of epilepsy; pp. 467–491. [Google Scholar]
- 7.Oates S., Tang S., Rosch R., Lear R., Hughes E.F., Williams R.E. Incorporating epilepsy genetics into clinical practice: a 360°evaluation. NPJ Genomic Med. 2018;3:13. doi: 10.1038/s41525-018-0052-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Orsini A., Zara F., Striano P. Recent advances in epilepsy genetics. Neurosci Lett. 2018;667:4–9. doi: 10.1016/j.neulet.2017.05.014. [DOI] [PubMed] [Google Scholar]
- 9.Raththagala M., Brewer M.K., Parker M.W., Sherwood A.R., Wong B.K., Hsu S. Structural mechanism of laforin function in glycogen dephosphorylation and Lafora disease. Mol Cell. 2015;57:261–272. doi: 10.1016/j.molcel.2014.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dunn P., Albury C.L., Maksemous N., Benton M.C., Sutherland H.G., Smith R.A. Next generation sequencing methods for diagnosis of epilepsy syndromes. Front Genet. 2018;9 doi: 10.3389/fgene.2018.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunn P.J., Maher B.H., Albury C.L., Stuart S., Sutherland H.G., Maksemous N. Tiered analysis of whole-exome sequencing for epilepsy diagnosis. Mol Genet Genomics. 2020;295:751–763. doi: 10.1007/s00438-020-01657-x. [DOI] [PubMed] [Google Scholar]
- 12.Swanger Sharon A., Chen W., Wells G., Burger Pieter B., Tankovic A., Bhattacharya S. Mechanistic insight into NMDA receptor dysregulation by rare variants in the GluN2A and GluN2B agonist binding domains. Am J Human Genetics. 2016;99:1261–1280. doi: 10.1016/j.ajhg.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li J., Gao K., Yan H., Xiangwei W., Liu N., Wang T. Reanalysis of whole exome sequencing data in patients with epilepsy and intellectual disability/mental retardation. Gene. 2019;700:168–175. doi: 10.1016/j.gene.2019.03.037. [DOI] [PubMed] [Google Scholar]
- 14.Perucca P., Scheffer I.E., Harvey A.S., James P.A., Lunke S., Thorne N. Real-world utility of whole exome sequencing with targeted gene analysis for focal epilepsy. Epilepsy Res. 2017;131:1–8. doi: 10.1016/j.eplepsyres.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 15.Wu C., Sun D. GABA receptors in brain development, function, and injury. Metab Brain Dis. 2015;30:367–379. doi: 10.1007/s11011-014-9560-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vyklicky, V., Krausova, B., Cerny, J., Ladislav, M., Smejkalova, T., Kysilov, B., Korinek, M., Danacikova, S., Horak, M., Chodounska, H., Kudova, E., Vyklicky, L., 2018. Surface expression, function, and pharmacology of disease-associated mutations in the membrane domain of the human GluN2B subunit. Front Mol Neurosci 11. [DOI] [PMC free article] [PubMed]
- 17.Kikuchi G., Motokawa Y., Yoshida T., Hiraga K. Glycine cleavage system: reaction mechanism, physiological significance, and hyperglycinemia. Proc Japan Acad B. 2008;84:246–263. doi: 10.2183/pjab/84.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kure S., Kato K., Dinopoulos A., Gail C., deGrauw T.J., Christodoulou J. Comprehensive mutation analysis of GLDC, AMT, and GCSH in nonketotic hyperglycinemia. Hum Mutat. 2006;27:343–352. doi: 10.1002/humu.20293. [DOI] [PubMed] [Google Scholar]
- 19.Azize N.A.A., Ngah W.Z.W., Othman Z., Md Desa N., Chin C.B., Md Yunus Z. Mutation analysis of glycine decarboxylase, aminomethyltransferase and glycine cleavage system protein-H genes in 13 unrelated families with glycine encephalopathy. J Hum Genet. 2014;59:593. doi: 10.1038/jhg.2014.69. [DOI] [PubMed] [Google Scholar]
- 20.Kecmanović M., Keckarević-Marković M., Keckarević D., Stevanović G., Jović N., Romac S. Genetics of Lafora progressive myoclonic epilepsy: current perspectives. Appl Clin Genet. 2016;9:49–53. doi: 10.2147/TACG.S57890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nitschke F., Ahonen S.J., Nitschke S., Mitra S., Minassian B.A. Lafora disease — from pathogenesis to treatment strategies. Nat Rev Neurol. 2018;14:606–617. doi: 10.1038/s41582-018-0057-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ganesh S., Delgado-Escueta A.V., Suzuki T., Francheschetti S., Riggio C., Avanzini G. Genotype–phenotype correlations for EPM2A mutations in Lafora's progressive myoclonus epilepsy: exon 1 mutations associate with an early-onset cognitive deficit subphenotype. Hum Mol Genet. 2002;11:1263–1271. doi: 10.1093/hmg/11.11.1263. [DOI] [PubMed] [Google Scholar]
- 23.Ianzano L, Zhang J, Chan EM, Zhao X-C, Lohi H, Scherer SW, Minassian BA. 2005. Lafora progressive myoclonus epilepsy mutation database-EPM2A and NHLRC1 (EMP2B) genes. Human Mutation 26, 397-397. [DOI] [PubMed]
- 24.Minassian B.A., Ianzano L., Meloche M., Andermann E., Rouleau G.A., Delgado-Escueta A.V. Mutation spectrum and predicted function of laforin in Lafora’s progressive myoclonus epilepsy. Neurology. 2000;55:341–346. doi: 10.1212/wnl.55.3.341. [DOI] [PubMed] [Google Scholar]
- 25.Shen D., Hernandez C.C., Shen W., Hu N., Poduri A., Shiedley B. De novo GABRG2 mutations associated with epileptic encephalopathies. Brain. 2017;140:49–67. doi: 10.1093/brain/aww272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Snoeijen-Schouwenaars F.M., van Ool J.S., Verhoeven J.S., van Mierlo P., Braakman H.M.H., Smeets E.E. Diagnostic exome sequencing in 100 consecutive patients with both epilepsy and intellectual disability. Epilepsia. 2019;60:155–164. doi: 10.1111/epi.14618. [DOI] [PubMed] [Google Scholar]
- 27.Ganesh S., Agarwala K.L., Ueda K., Akagi T., Shoda K., Usui T. Laforin, defective in the progressive myoclonus epilepsy of Lafora type, is a dual-specificity phosphatase associated with polyribosomes. Hum Mol Genet. 2000;9:2251–2261. doi: 10.1093/oxfordjournals.hmg.a018916. [DOI] [PubMed] [Google Scholar]
- 28.Reynolds C., King M.D., Gorman K.M. The phenotypic spectrum of SCN2A-related epilepsy. Eur J Paediatric Neurol. 2020;24:117–122. doi: 10.1016/j.ejpn.2019.12.016. [DOI] [PubMed] [Google Scholar]
- 29.Baulac S., Huberfeld G., Gourfinkel-An I., Mitropoulou G., Beranger A., Prud'homme J.-F. First genetic evidence of GABAA receptor dysfunction in epilepsy: a mutation in the γ2-subunit gene. Nat Genet. 2001;28:46–48. doi: 10.1038/ng0501-46. [DOI] [PubMed] [Google Scholar]
- 30.Shiohama T., Fujii K., Takahashi S., Nakamura F., Kohno Y. Reversible white matter lesions during ketogenic diet therapy in glucose transporter 1 deficiency syndrome. Pediatr Neurol. 2013;49:493–496. doi: 10.1016/j.pediatrneurol.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 31.Singh S., Ganesh S. Phenotype variations in Lafora progressive myoclonus epilepsy: possible involvement of genetic modifiers? J Hum Genet. 2012;57:283. doi: 10.1038/jhg.2012.29. [DOI] [PubMed] [Google Scholar]
- 32.Komulainen-Ebrahim J., Schreiber J.M., Kangas S.M., Pylkäs K., Suo-Palosaari M., Rahikkala E. Novel variants and phenotypes widen the phenotypic spectrum of GABRG2-related disorders. Seizure. 2019;69:99–104. doi: 10.1016/j.seizure.2019.03.010. [DOI] [PubMed] [Google Scholar]
- 33.Allen A.S., Berkovic S.F., Cossette P., Delanty N., Dlugos D., Eichler E.E. De novo mutations in epileptic encephalopathies. Nature. 2013;501:217–221. doi: 10.1038/nature12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Endele S., Rosenberger G., Geider K., Popp B., Tamer C., Stefanova I. Mutations in GRIN2A and GRIN2B encoding regulatory subunits of NMDA receptors cause variable neurodevelopmental phenotypes. Nat Genet. 2010;42:1021. doi: 10.1038/ng.677. [DOI] [PubMed] [Google Scholar]
- 35.Hu C., Chen W., Myers S.J., Yuan H., Traynelis S.F. Human GRIN2B variants in neurodevelopmental disorders. J Pharmacol Sci. 2016;132:115–121. doi: 10.1016/j.jphs.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lemke J.R., Hendrickx R., Geider K., Laube B., Schwake M., Harvey R.J. GRIN2B mutations in west syndrome and intellectual disability with focal epilepsy. Ann Neurol. 2014;75:147–154. doi: 10.1002/ana.24073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Platzer, K., Lemke, J.R., 2018. GRIN2B-Related Neurodevelopmental Disorder, in: Adam, M.P., Ardinger, H.H., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Stephens, K., Amemiya, A. (Eds.), GeneReviews(®). University of Washington, Seattle Copyright © 1993-2020, University of Washington, Seattle. GeneReviews is a registered trademark of the University of Washington, Seattle. All rights reserved, Seattle (WA).
- 38.Platzer K., Yuan H., Schütz H., Winschel A., Chen W., Hu C. GRIN2B encephalopathy: novel findings on phenotype, variant clustering, functional consequences and treatment aspects. J Med Genet. 2017;54:460–470. doi: 10.1136/jmedgenet-2016-104509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kearney H., Byrne S., Cavalleri G.L., Delanty N. Tackling Epilepsy with high-definition precision medicine: a review tackling epilepsy with high-definition precision medicinetackling epilepsy with high-definition precision medicine. JAMA Neurol. 2019 doi: 10.1001/jamaneurol.2019.2384. [DOI] [PubMed] [Google Scholar]
- 40.Posey J.E., Harel T., Liu P., Rosenfeld J.A., James R.A., Coban Akdemir Z.H. Resolution of disease phenotypes resulting from multilocus genomic variation. N Engl J Med. 2016;376:21–31. doi: 10.1056/NEJMoa1516767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tajir M., Arnoux J.B., Boutron A., Elalaoui S.C., De Lonlay P., Sefiani A. Pyruvate dehydrogenase deficiency caused by a new mutation of PDHX gene in two Moroccan patients. Eur J Med Genetics. 2012;55:535–540. doi: 10.1016/j.ejmg.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 42.Hamdan F.F., Srour M., Capo-Chichi J.-M., Daoud H., Nassif C., Patry L. De novo mutations in moderate or severe intellectual disability. PLoS Genet. 2014;10 doi: 10.1371/journal.pgen.1004772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang J.-W., Shi X.-Y., Kurahashi H., Hwang S.-K., Ishii A., Higurashi N. Prevalence of SCN1A mutations in children with suspected Dravet syndrome and intractable childhood epilepsy. Epilepsy Res. 2012;102:195–200. doi: 10.1016/j.eplepsyres.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 44.Aral B., Benelli C., Ait-Ghezala G., Amessou M., Fouque F., Maunoury C. Mutations in PDX1, the human lipoyl-containing component X of the pyruvate dehydrogenase-complex gene on chromosome 11p1, in congenital lactic acidosis. Am J Human Genetics. 1997;61:1318–1326. doi: 10.1086/301653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ling M., McEachern G., Seyda A., MacKay N., Scherer S.W., Bratinova S. Detection of a homozygous four base pair deletion in the protein X gene in a case of pyruvate dehydrogenase complex deficiency. Hum Mol Genet. 1998;7:501–505. doi: 10.1093/hmg/7.3.501. [DOI] [PubMed] [Google Scholar]
- 46.Patel K.P., O'Brien T.W., Subramony S.H., Shuster J., Stacpoole P.W. The spectrum of pyruvate dehydrogenase complex deficiency: Clinical, biochemical and genetic features in 371 patients. Mol Genet Metab. 2012;106:385–394. doi: 10.1016/j.ymgme.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tang M., Park S.H., De Vivo D.C., Monani U.R. Therapeutic strategies for glucose transporter 1 deficiency syndrome. Ann Clin Transl Neurol. 2019;6:1923–1932. doi: 10.1002/acn3.50881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Patel M.S., Nemeria N.S., Furey W., Jordan F. The pyruvate dehydrogenase complexes: structure-based function and regulation. J Biol Chem. 2014;289:16615–16623. doi: 10.1074/jbc.R114.563148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brown RM, Head RA, Morris AA, Raiman JJA, Walter JH, Whitehouse WP, Brown GK, 2006. Pyruvate dehydrogenase E3 binding protein (protein X) deficiency. Develop Med Child Neurol 48, 756-760. [DOI] [PubMed]
- 50.Ivanov I.S., Azmanov D.N., Ivanova M.B., Chamova T., Pacheva I.H., Panova M.V. Founder p.Arg 446* mutation in the PDHX gene explains over half of cases with congenital lactic acidosis in Roma children. Mol Genet Metab. 2014;113:76–83. doi: 10.1016/j.ymgme.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 51.Brunklaus A., Schorge S., Smith A.D., Ghanty I., Stewart K., Gardiner S. SCN1A variants from bench to bedside—improved clinical prediction from functional characterization. Hum Mutat. 2020;41:363–374. doi: 10.1002/humu.23943. [DOI] [PubMed] [Google Scholar]
- 52.Parihar R., Ganesh S. The SCN1A gene variants and epileptic encephalopathies. J Hum Genet. 2013;58:573. doi: 10.1038/jhg.2013.77. [DOI] [PubMed] [Google Scholar]
- 53.Serratosa J.M., Gómez-Garre P., Gallardo M.E., Anta B., de Bernabé D.B.-V., Lindhout D. A novel protein tyrosine phosphatase gene is mutated in progressive myoclonus Epilepsy of the Lafora Type (EPM2) Hum Mol Genet. 1999;8:345–352. doi: 10.1093/hmg/8.2.345. [DOI] [PubMed] [Google Scholar]
- 54.Musto E., Gardella E., Møller R.S. Recent advances in treatment of epilepsy-related sodium channelopathies. Eur J Paediatric Neurol. 2020;24:123–128. doi: 10.1016/j.ejpn.2019.12.009. [DOI] [PubMed] [Google Scholar]
- 55.Wang J., Lin Z.-J., Liu L., Xu H.-Q., Shi Y.-W., Yi Y.-H. Epilepsy-associated genes. Seizure - Eur J Epilepsy. 2017;44:11–20. doi: 10.1016/j.seizure.2016.11.030. [DOI] [PubMed] [Google Scholar]
- 56.Martin H.C., Kim G.E., Pagnamenta A.T., Murakami Y., Carvill G.L., Meyer E. Clinical whole-genome sequencing in severe early-onset epilepsy reveals new genes and improves molecular diagnosis. Hum Mol Genet. 2014;23:3200–3211. doi: 10.1093/hmg/ddu030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ogiwara I., Ito K., Sawaishi Y., Osaka H., Mazaki E., Inoue I. De novo mutations of voltage-gated sodium channel α<sub>II</sub> gene <em>SCN2A</em> in intractable epilepsies. Neurology. 2009;73:1046–1053. doi: 10.1212/WNL.0b013e3181b9cebc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saitsu H., Kato M., Mizuguchi T., Hamada K., Osaka H., Tohyama J. De novo mutations in the gene encoding STXBP1 (MUNC18-1) cause early infantile epileptic encephalopathy. Nat Genet. 2008;40:782. doi: 10.1038/ng.150. [DOI] [PubMed] [Google Scholar]
- 59.Scheffer I.E., Nabbout R. SCN1A-related phenotypes: epilepsy and beyond. Epilepsia. 2019;60:S17–S24. doi: 10.1111/epi.16386. [DOI] [PubMed] [Google Scholar]
- 60.Perucca P., Perucca E. Identifying mutations in epilepsy genes: impact on treatment selection. Epilepsy Res. 2019;152:18–30. doi: 10.1016/j.eplepsyres.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 61.De Vivo D.C., Trifiletti R.R., Jacobson R.I., Ronen G.M., Behmand R.A., Harik S.I. Defective glucose transport across the blood-brain barrier as a cause of persistent hypoglycorrhachia, seizures, and developmental delay. N Engl J Med. 1991;325:703–709. doi: 10.1056/NEJM199109053251006. [DOI] [PubMed] [Google Scholar]
- 62.Koch H., Weber Y.G. The glucose transporter type 1 (Glut1) syndromes. Epilepsy Behav. 2019;91:90–93. doi: 10.1016/j.yebeh.2018.06.010. [DOI] [PubMed] [Google Scholar]
- 63.Toone JR, Applegarth DA, Coulter-Mackie MB, James ER, 2001. Identification of the first reported splice site mutation (IVS7-1G→A) in the aminomethyltransferase (T-protein) gene (AMT) of the glycine cleavage complex in 3 unrelated families with nonketotic hyperglycinemia. Human Mutation 17, 76-76. [DOI] [PubMed]
- 64.Bekker Y.A.C., Lambrechts D.A., Verhoeven J.S., van Boxtel J., Troost C., Kamsteeg E.-J. Failure of ketogenic diet therapy in GLUT1 deficiency syndrome. Eur J Paediatric Neurol. 2019;23:404–409. doi: 10.1016/j.ejpn.2019.02.012. [DOI] [PubMed] [Google Scholar]
- 65.De Giorgis V., Veggiotti P. GLUT1 deficiency syndrome 2013: current state of the art. Seizure. 2013;22:803–811. doi: 10.1016/j.seizure.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 66.Sigel E., Steinmann M.E. Structure, function, and modulation of GABAA receptors. J Biol Chem. 2012;287:40224–40231. doi: 10.1074/jbc.R112.386664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hamdan F.F., Piton A., Gauthier J., Lortie A., Dubeau F., Dobrzeniecka S. De novo STXBP1 mutations in mental retardation and nonsyndromic epilepsy. Ann Neurol. 2009;65:748–753. doi: 10.1002/ana.21625. [DOI] [PubMed] [Google Scholar]
- 68.Sanders S.J., Campbell A.J., Cottrell J.R., Moller R.S., Wagner F.F., Auldridge A.L. Progress in understanding and treating SCN2A-mediated disorders. Trends Neurosci. 2018;41:442–456. doi: 10.1016/j.tins.2018.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lanoue, V., Chai, Y.J., Brouillet, J.Z., Weckhuysen, S., Palmer, E.E., Collins, B.M., Meunier, F.A., 2019. <em>STXBP1</em> encephalopathy. Connecting neurodevelopmental disorders with α-synucleinopathies? 93, 114-123. [DOI] [PubMed]
- 70.Farwell K.D., Shahmirzadi L., El-Khechen D., Powis Z., Chao E.C., Tippin Davis B. Enhanced utility of family-centered diagnostic exome sequencing with inheritance model–based analysis: results from 500 unselected families with undiagnosed genetic conditions. Genet Med. 2014;17:578. doi: 10.1038/gim.2014.154. [DOI] [PubMed] [Google Scholar]
- 71.Geisheker M.R., Heymann G., Wang T., Coe B.P., Turner T.N., Stessman H.A.F. Hotspots of missense mutation identify neurodevelopmental disorder genes and functional domains. Nat Neurosci. 2017;20:1043. doi: 10.1038/nn.4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Helbig K.L., Farwell Hagman K.D., Shinde D.N., Mroske C., Powis Z., Li S. Diagnostic exome sequencing provides a molecular diagnosis for a significant proportion of patients with epilepsy. Genet Med. 2016;18:898. doi: 10.1038/gim.2015.186. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.