Abstract
Prostate cancer is one of the most common malignancies among males and commonly metastasizes to bone in advanced stages. Although such osseous metastases are typically osteoblastic, osteolytic lesions are also seen. Here, we present a case of an 81-year-old male with known prostate cancer who presented due to a pathologic right humerus fracture. After skeletal survey and further workup, he was found to have two osteolytic lesions within his right femur. Bone curettage of the right femur revealed metastatic adenocarcinoma from a prostate primary. This case exemplifies the importance of recognizing the potential for prostate cancer metastases to present as osteolytic lesions.
Keywords: Prostate cancer, Osteolytic, Bone metastasis
Introduction
Prostate cancer is the second most common cause of cancer and the fourth most common cause of mortality due to cancer among males worldwide [1]. Advanced-stage prostate cancer is known to metastasize to many locations; pulmonary, hepatic, and pleural sites are among the most frequently observed [2]. Most commonly, however, prostate cancer spreads to bone, occurring in up to 29.2% of all diagnosed prostate cancers, and in 88.74 % of metastatic tumors [3,4]. Bone metastases are typically located in the spine but more rarely can be found in the long bones, ribs, or skull [2].
Conventional wisdom holds osseous prostate cancer metastases to be osteoblastic, appearing as densely sclerotic lesions on imaging [5]. Atypical osteolytic metastases may also be observed but are much rarer. Descriptions of such osteolytic prostatic metastases are consequently sparse, with only a few examples of reported cases in the literature [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17]. As such, lytic-appearing osseous lesions in the setting of suspected metastatic prostate cancer may confound an otherwise straightforward imaging diagnosis. Here, we present a case of a patient with confirmed osteolytic metastases in the right femur from a primary prostate adenocarcinoma.
Case report
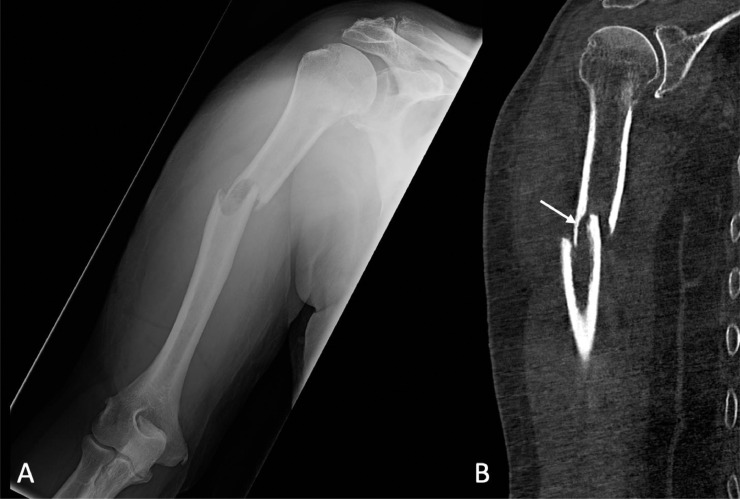
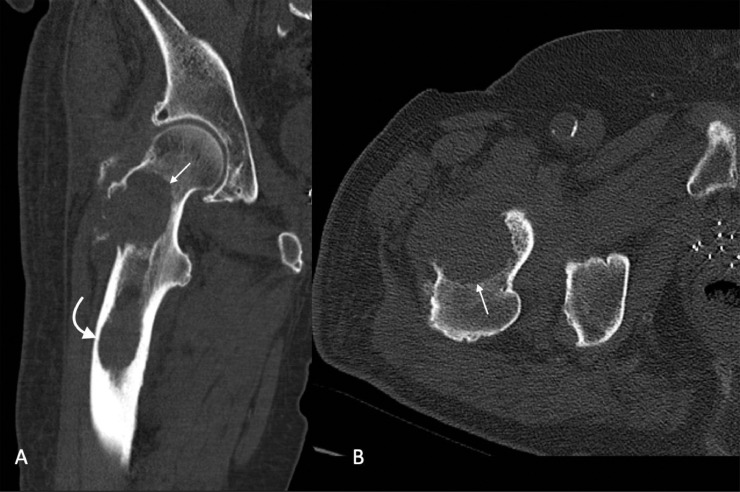
An 81-year-old man with known metastatic prostate adenocarcinoma presented to an emergency department with right arm pain. He reported that as he was shifting positions in bed one night ago, he felt and heard two loud popping sounds in his right arm with associated immediate pain. Initial imaging demonstrated a pathologic fracture involving the mid humerus associated with an intramedullary lesion (Fig. 1). Because of the patient's history of metastatic prostate cancer, he was admitted for further management. A subsequnt skeletal survey identified two osteolytic lesions in the right femur, which were then further characterized with CT (Fig. 2).
Fig. 1.
Plain film (A) and coronal non-contrast CT (B) of the right upper extremity demonstrated a pathologic fracture involving the mid humerus with an intramedullary lesion of the mid humerus. Both anterior and lateral displacement of the distal fracture fragment were observed as was endosteal scalloping of the proximal fracture fragment at the fracture site (arrow).
Fig. 2.
Coronal (A) and axial (B) non-contrast CT of the lower extremity revealed a lytic osseous lesion (straight arrows) in the proximal femur involving the femoral neck and a portion of the greater trochanter. Also noted is a second lytic lesion (curved arrow) in the proximal femoral diaphysis with endosteal erosion.
Bone curreatage of the right femur lesions were obtained and immunohistochemical analysis was performed. Neoplastic cells in the specimen stained positive for NKX3.1 and PSA, compatible with metastatic adenocarcinoma with orgin from a prostate primary. The patient was evaluated by orthopedics and underwent right humerus intramedullary nailing as well as right hip intertrochanteric nailing. He was referred to follow up with oncology for further management. The patient passed away soon after his discharge from the hospital.
Discussion
This case illustrates an atypical appearance of osseous prostatic cancer metastases. Despite the typical osteoblastic phenotype of prostate cancer bone metastasis, this patient was found to have osteolytic lesions in his right femur. Although it is unusual, osteolytic prostate metastases are certainly known entities. In addition to the reported cases in the literature [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], Cheville et al. found that 16.4% of osseous prostatic cancer metastatic lesions were lytic and 12.7% were mixed [18]. Furthermore, Cereceda Flechon and Droz examined vertebral metastases in prostate cancer and reported that as many as 26% of vertebral osseous lesions were mixed and 19% were lytic [19]. Therefore, while osteolytic prostate metastases are not the dominant phenotype, they do occur more frequently than often considered. This case serves to highlight the importance of recognizing that osseous prostatic cancer metastases can present as lytic lesions and this appearance should not dissuade radiologists from considering prostate metastases in their differential diagnosis.
The differing appearances of osseous lesions on imaging can help to differentiate what the primary source of the metastases may be. Most commonly, osteoblastic metastases are from prostate, breast, or carcinoid cancers while lytic lesions are from lung, renal, or thyroid cancers [20]. CT allows for easy differentiation of osteoblastic and osteolytic lesions since the lesions can be visualized based on their degree of intralesional mineralization [21]. Osteoblastic lesions have increased intralesional mineralization which results in higher attenuation while osteolytic lesions will exhibit decreased attenuation. However, on MR images, distinguishing between lytic and blastic lesions can be difficult using standard MRI sequences. This is because both lytic and blastic lesions can appear hypointense on T1-weighted images while on T2-weighted images they can appear heterogeneous [21]. Use of susceptibility-weighted MR images can be helpful in differentiating between the two types of lesions since osteoblastic lesions appear hyperintense on inverted magnitude images and phase images while osteolytic lesions appear hypointense on inverted magnitude images and phase images [21]. Bone scintigraphy is normally used as a screening method in the assessment of bone metastases. Radiotracer uptake is demonstrated in both lytic and blastic metastases however, purely lytic lesions can be missed since they will appear as a region of low radiotracer uptake [22]. 11Choline PET/CT is another method used to visualize and identify areas of metastases by measuring radiotracer uptake. In prostatic bone metastases, the measured maximum standard uptake value is higher in osteolytic lesions than osteoblastic lesions [23].
Both lytic and sclerotic osseous lesions result from a disturbance in the balance between resorption and ossification. Under normal circumstances, bone is constantly remodeled via osteoclasts, which control resorption, and osteoblasts, which control ossification. Disruption of this homeostasis by metastatic lesions results in osteoblastic, osteolytic, or mixed lesions: osteoblastic lesions result from decreased osteoclast activity and/or heightened osteoblast activity, and osteolytic lesions are due to stimulation of osteoclast activity and decreased osteoblastic activity [24]. Prostate cancer cells exhibit a predominant osteoblastic effect through producing several factors including the peptides endothelin-1, PSA, and bone morphogenic protein 4 which all ultimately lead to bone deposition [25]. Generally, it is thought that PSA exerts an osteoblastic effect by cleaving parathyroid-hormone related peptide. Bone resorption by osteoclasts is stimulated by parathyroid-hormone related peptide but its cleavage by PSA results in decreased osteoclast activity and net bone deposition [26].
One possible mechanism by which the observed osteolytic lesions in this case may have occurred is related to the cytokine nuclear factor kappa-B ligand (RANKL) and the receptor osteoprotegerin (OPG). RANKL is an activator of osteoclasts, and therefore leads to greater levels of bone resorption. OPG, conversely, acts as a decoy receptor for RANKL [27]. In the setting of prostate cancer, OPG is often elevated, thereby suppressing the effect of RANKL on osteoclasts, and leading to less osteolytic activity. Some authors have opined that OPG may be produced by prostate cancer cells, ultimately resulting in the observed osteoblastic phenotype of prostate metastases [28]. In this patient, it is possible that the patient's tumor manifested relatively low levels of OPG, leading to the formation of lytic rather than blastic lesions.
Conclusions
It is important for clinicians to recognize that prostate cancer can produce metastatic osteolytic lesions. Even though prostate cancer primarily leads to sclerotic osseous lesions, lytic lesions may also be observed. The presence of lytic osseous lesions should therefore not automatically exclude a diagnosis of prostate cancer.
Patient consent
The patient provided written informed consent for publication of our case report prior to his passing.
Footnotes
Competing interests: No conflicts of interest.
References
- 1.Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144(8):1941–1953. doi: 10.1002/ijc.31937. [DOI] [PubMed] [Google Scholar]
- 2.Bubendorf L, Schöpfer A, Wagner U, Sauter G, Moch H, Willi N. Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients. Hum Pathol. 2000;31(5):578–583. doi: 10.1053/hp.2000.6698. [DOI] [PubMed] [Google Scholar]
- 3.Hernandez RK, Wade SW, Reich A, Pirolli M, Liede A, Lyman GH. Incidence of bone metastases in patients with solid tumors: analysis of oncology electronic medical records in the United States. BMC Cancer. 2018;18(1):44. doi: 10.1186/s12885-017-3922-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang J-F, Shen J, Li X, Rengan R, Silvestris N, Wang M. Incidence of patients with bone metastases at diagnosis of solid tumors in adults: a large population-based study. Ann Transl Med. 2020;8(7):482. doi: 10.21037/atm.2020.03.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Macedo F, Ladeira K, Pinho F, Saraiva N, Bonito N, Pinto L. Bone metastases: an overview. Oncol Rev. 2017;11(1):321. doi: 10.4081/oncol.2017.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agheli A, Patsiornik Y, Chen Y, Chaudhry MR, Gerber H, Wang JC. Prostate carcinoma, presenting with a solitary osteolytic bone lesion to the right hip. Radiol Case Rep. 2009;4(4):288. doi: 10.2484/rcr.v4i4.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alabed YZ. Prostate Cancer Lytic Bone Metastases Imaged With 18F-Fluorocholine PET/CT. Clin Nucl Med. 2018;43(3):220–221. doi: 10.1097/RLU.0000000000001973. [DOI] [PubMed] [Google Scholar]
- 8.Ansari MS, Nabi G, Aron M. Solitary radial head metastasis with wrist drop: a rare presentation of metastatic prostate cancer. Urol Int. 2003;70(1):77–79. doi: 10.1159/000067696. [DOI] [PubMed] [Google Scholar]
- 9.Bakhsh MU, Lee S, Ahmad S, Takher J, Pareek A, Syed U. Should prostate cancer be considered as a differential diagnosis in patients with osteolytic bone lesions? Eur Rev Med Pharmacol Sci. 2015;19(24):4791–4794. [PubMed] [Google Scholar]
- 10.Carmichael FA, Mitchell DA, Dyson DP. Case report. Two contrasting radiological presentations of prostatic adenocarcinoma in the jaws. Dentomaxillofac Radiol. 1996;25(5):283–286. doi: 10.1259/dmfr.25.5.9161183. [DOI] [PubMed] [Google Scholar]
- 11.Gupta A, Gahlot N, Elhence P. Complete paraplegia with diffuse osteolytic skeletal metastases: an uncommon presentation of carcinoma of the prostate. Spinal Cord Ser Cases. 2020;6(1):25. doi: 10.1038/s41394-020-0276-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Idowu BM. Prostate carcinoma presenting with diffuse osteolytic metastases and supraclavicular lymphadenopathy mimicking multiple myeloma. Clin Case Rep. 2018;6(2):253–257. doi: 10.1002/ccr3.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maharaj B, Kalideen JM, Leary WP, Pudifin DJ. Carcinoma of the prostate with multiple osteolytic metastases simulating multiple myeloma. A case report. S Afr Med J. 1986;70(4):227–228. [PubMed] [Google Scholar]
- 14.Okamura S, Fujiwara Y, Nagata K. Multiple osteolytic bone and lung metastases from prostate cancer including small cell carcinoma with marked increases in CEA and Pro-GRP. Urol Case Rep. 2019;24 doi: 10.1016/j.eucr.2019.100883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park Y, Oster MW, Olarte MR. Prostatic cancer with an unusual presentation: polymyositis and mediastinal adenopathy. Cancer. 1981;48(5):1262–1264. doi: 10.1002/1097-0142(19810901)48:5<1262::aid-cncr2820480535>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 16.Segamwenge IL, Mgori NK, Abdallahyussuf S, Mukulu CN, Nakangombe P, Ngalyuka PK. Cancer of the prostate presenting with diffuse osteolytic metastatic bone lesions: a case report. J Med Case Rep. 2012;6:425. doi: 10.1186/1752-1947-6-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tabrizipour AI, Dunne M. The role of 18F-flourocholine PET/CT in biochemically relapsed prostate cancer: a case of osteolytic prostate metastasis. Clin Nucl Med. 2015;40(5):e285–e286. doi: 10.1097/RLU.0000000000000681. [DOI] [PubMed] [Google Scholar]
- 18.Cheville JC, Tindall D, Boelter C, Jenkins R, Lohse CM, Pankratz VS. Metastatic prostate carcinoma to bone. Cancer. 2002;95(5):1028–1036. doi: 10.1002/cncr.10788. [DOI] [PubMed] [Google Scholar]
- 19.Cereceda LE, Flechon A, Droz JP. Management of vertebral metastases in prostate cancer: a retrospective analysis in 119 patients. Clin Prostate Cancer. 2003;2(1):34–40. doi: 10.3816/cgc.2003.n.010. [DOI] [PubMed] [Google Scholar]
- 20.Bernard S, Walker E, Raghavan M. An approach to the evaluation of incidentally identified bone lesions encountered on imaging studies. AJR Am J Roentgenol. 2017;208(5):960–970. doi: 10.2214/AJR.16.17434. [DOI] [PubMed] [Google Scholar]
- 21.Böker SM, Adams LC, Bender YY, Fahlenkamp UL, Wagner M, Hamm B. Differentiation of predominantly osteoblastic and osteolytic spine metastases by using susceptibility-weighted MRI. Radiology. 2019;290(1):146–154. doi: 10.1148/radiol.2018172727. [DOI] [PubMed] [Google Scholar]
- 22.Barragán-Campos HM, Jiménez-Zarazúa O, Mondragón JD. Diagnosis and treatment options of spinal metastases. Rev Invest Clin. 2015;67(3):140–157. [PubMed] [Google Scholar]
- 23.Ceci F, Castellucci P, Graziani T, Schiavina R, Chondrogiannis S, Bonfiglioli R. 11C-choline PET/CT identifies osteoblastic and osteolytic lesions in patients with metastatic prostate cancer. Clin Nucl Med. 2015;40(5):e265–e270. doi: 10.1097/RLU.0000000000000783. [DOI] [PubMed] [Google Scholar]
- 24.Maccauro G, Spinelli MS, Mauro S, Perisano C, Graci C, Rosa MA. Physiopathology of spine metastasis. Int J Surg Oncol. 2011;2011 doi: 10.1155/2011/107969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang M, Xia F, Wei Y, Wei X. Molecular mechanisms and clinical management of cancer bone metastasis. Bone Research. 2020;8(1):30. doi: 10.1038/s41413-020-00105-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roodman GD. Mechanisms of bone metastasis. N Engl J Med. 2004;350(16):1655–1664. doi: 10.1056/NEJMra030831. [DOI] [PubMed] [Google Scholar]
- 27.Sottnik JL, Keller ET. Understanding and targeting osteoclastic activity in prostate cancer bone metastases. Curr Mol Med. 2013;13(4):626–639. doi: 10.2174/1566524011313040012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ohtaka M, Kawahara T, Mochizuki T, Takamoto D, Hattori Y, Teranishi JI. RANK/RANKL expression in prostate cancer. Int J Surg Case Rep. 2017;30:106–107. doi: 10.1016/j.ijscr.2016.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]