Abstract
Schizotypy and psychotic‐like experiences (PLE) form part of the wider psychosis continuum and may have brain structural correlates in nonclinical cohorts. This study aimed to compare the effects of differential schizotypy dimensions, PLE, and their interaction on hippocampal subfields and amygdala volumes in the absence of clinical psychopathology. In a cohort of 367 psychiatrically healthy individuals, we assessed schizotypal traits using the Oxford‐Liverpool Inventory of Life Experiences (O‐LIFE) and PLE using the short form of the Prodromal Questionnaire (PQ‐16). Based on high‐resolution structural MRI scans, we used automated segmentation to estimate volumes of limbic structures. Sex and total intracranial volume (Step 1), PLE and schizotypy dimensions (Step 2), and their interaction terms (Step 3) were entered as regressors for bilateral amygdala and hippocampal subfield volumes in hierarchical multiple linear regression models. Positive schizotypy, but not PLE, was negatively associated with left amygdala and subiculum volumes. O‐LIFE Impulsive Nonconformity, as well as the two‐way interaction between positive schizotypy and PLE, were associated with larger left subiculum volumes. None of the estimators for right hemispheric hippocampal subfield volumes survived correction for multiple comparisons. Our findings support differential associations of hippocampus subfield volumes with trait dimensions rather than PLE, and support overlap and interactions between psychometric positive schizotypy and PLE. In a healthy cohort without current psychosis risk syndromes, the positive association between PLE and hippocampal subfield volume occurred at a high expression of positive schizotypy. Further studies combining stable, transient, and genetic parameters are required.
Keywords: amygdala, hippocampus, neuroimaging, psychosis proneness, schizotypy
This study examined structural variation of the hippocampal subfields and the amygdala associated with subclinical dimensions of schizotypy, and psychotic‐like experiences (PLE). Volume alterations were associated with schizotypal traits, rather than PLE. In the left subiculum, the expression of PLE at higher positive schizotypy was associated with larger volumes.
1. INTRODUCTION
Psychotic‐like experiences (PLE) signify psychosis risk, yet only a considerably small portion of persons reporting such transient expressions of psychosis proneness will go on to develop a psychotic disorder (Linscott & van Os, 2013). PLE are elevated in individuals displaying schizotypal traits, which are behavioral, emotional, and cognitive characteristics resembling the core symptoms of psychotic disorders along a health‐illness spectrum (Claridge & Beech, 1995; Grant, Green, & Mason, 2018; Kwapil & Barrantes‐vidal, 2015). Schizotypy encompasses the positive, negative, and disorganized dimensions (Debbané & Barrantes‐Vidal, 2015) found in psychotic disorders, with each trait dimension showing differential associations with psychopathology (Kwapil, Gross, Silvia, & Barrantes‐Vidal, 2013), affective states (Kemp, Gross, Barrantes‐Vidal, & Kwapil, 2018), and perceptual and cognitive outcomes (Ettinger et al., 2015; Ettinger, Meyhöfer, Steffens, Wagner, & Koutsouleris, 2014).
Past research highlights the multifaceted nature of schizotypy and its value in the detection of clinical high risk (CHR) states (Barrantes‐Vidal et al., 2013; Flückiger et al., 2016). For example, increased PLE levels are especially observed in positive schizotypy (Barrantes‐Vidal, Chun, Myin‐Germeys, & Kwapil, 2013; Kwapil et al., 2020), as well as depression and anxiety (Varghese et al., 2011), demonstrating that the emergence of psychopathology, PLE, and schizotypal traits are intertwined in a dynamic fashion (Barrantes‐Vidal, Grant, & Kwapil, 2015). The fully dimensional conceptualization of schizotypy also accounts for the non‐pathological phenotypes (Nelson, Seal, Pantelis, & Phillips, 2013), such as “benign schizotypes”, that is individuals characterized by high positive schizotypy, but low negative and disorganized traits (Mohr & Claridge, 2015). Hence, the positive schizotypy facet (together with low negative and disorganized facets) is related to higher PLE levels independently of induced stress states (Grant & Hennig, 2020), while the emergence of distressing PLE outside of familiar positive traits may convey increased psychosis vulnerability (Debbané & Barrantes‐Vidal, 2015). PLE distress is reduced at higher schizotypy in nonclinical subjects (Kline et al., 2012), suggesting that PLE occurring in the context of specific trait dimensions could relate to health or resilience in schizotypy.
Previous studies demonstrated that trait schizotypy and PLE correlate with cortical changes in areas consistently observed in clinical psychosis. They found brain structural variation in prefrontal (Pfarr & Nenadić, 2020) and parietal regions (Meller et al., 2020; Modinos et al., 2010), and cortical surface variation in parietal and temporal regions (Evermann, Gaser, Besteher, Langbein, & Nenadić, 2020) associated with psychosis phenotypes. Further alterations in the nonclinical psychosis continuum also include hippocampal activity (Modinos et al., 2018). These findings suggest that subclinical psychosis prone phenotypes show brain correlates in regions affected in clinical psychosis, which are not necessarily a sign of vulnerability but could also indicate compensatory processes (Kühn, Schubert, & Gallinat, 2012; Mohr & Claridge, 2015). Investigating brain regions involved in psychosis pathophysiology may facilitate the demarcation of vulnerable or disease progressive states.
Abnormalities of medial temporal lobe hippocampal (HC) and amygdala structures observed in schizophrenia (van Erp et al., 2016), propose neuroanatomical targets for psychosis spectrum research (Lieberman et al., 2018). Hippocampal subfield analyses point to volume reductions in the cornu ammonis (CA) and dentate gyrus (DG) sections (Haukvik, Tamnes, Söderman, & Agartz, 2018; Nakahara, Matsumoto, & van Erp, 2018), which are paralleled by functional studies indicating CA1 and possibly also subiculum hyperactivity (operationalized as increased cerebral blood volume) in patients (Schobel et al., 2013, 2009; Talati et al., 2014). Volume reductions in total hippocampal volume and subfields might already be present at disease onset (Briend et al., 2020) and, more importantly, already at CHR stages preceding disease onset (Ganzola, Maziade, & Duchesne, 2014; Wood et al., 2010), although findings are not entirely consistent across cohorts (for a review see Walter et al., 2016).
Post mortem studies in schizophrenia show differential involvement of CA1, CA3, and DG subfields (Bobilev, Perez, & Tamminga, 2020; Perez et al., 2020), which is supported by differential associations between HC segments and positive and negative clinical symptoms in in vivo studies. Left CA2/3 and CA4/DG (Kawano et al., 2015) and subiculum (Haukvik et al., 2015) volumes show inverse associations with negative symptom severity in schizophrenia. Further studies report CA1 and CA2/3 (Kühn et al., 2012) and subiculum (Mathew et al., 2014) volume deficits in association with positive symptoms of psychosis. Mathew et al. (2014) found negative correlations between both the total positive symptoms and the hallucinations scale scores based on the positive and negative syndrome scale (PANSS, Kay, Fiszbein, & Opler, 1987) for schizophrenia and CA4/DG, presubiculum, subiculum, and whole HC volumes. A noticeable asymmetry in clinical subjects (Baglivo et al., 2018; Velakoulis et al., 2006; Wood et al., 2010) also suggests that such pathological alterations are more readily observable in the left hemisphere.
Further examinations of HC volumes as potential biological markers have emerged in the nonclinical part of the psychosis spectrum, too. A developmental study demonstrated flattened bilateral hippocampal volume trajectories in adolescents with elevated psychometric disorganized schizotypy (Derome et al., 2020). Recently we reported that HC subfields are altered by the interaction of negative and disorganized schizotypy dimensions, which predicted volumetric reductions in anterior and whole left HC (Sahakyan et al., 2021). Structural effects in schizotypy and ultra‐high risk (UHR) states are also paralleled by functional alterations, such as augmented right hippocampal perfusion in high positive schizotypy (Modinos et al., 2018) and increased hippocampal perfusion in UHR (Allen et al., 2018, 2015; Bossong et al., 2019). Hypermetabolism spreading from the CA1 subregion could explain gradual hippocampal atrophy (Schobel et al., 2013, 2009).
Contemporary automated segmentation methods also provide high‐resolution structural delineation of the amygdala. In the wider limbic system investigations show bilateral whole amygdala volume reductions in first‐episode psychosis (FEP) (Watson et al., 2012), as well as smaller amygdala subnuclei in CHR and FEP (Armio et al., 2020). Superimposed organizational patterns suggest demarcated ventral HC (CA1 and subiculum) connectivity with the amygdala (Strange, Witter, Lein, & Moser, 2014). Emotion recognition is a functional amygdala substrate showing alterations in schizophrenia (Mier et al., 2014), adolescents at UHR (Bartholomeusz et al., 2014), and schizotypy (Statucka & Walder, 2017; Wang et al., 2018). Other studies have shown significant negative relationships between blunted affect and left amygdala activation in schizophrenia patients during positive affect processing (Rahm et al., 2015), and left amygdala and hippocampal volumes and parent‐rated negative schizotypal traits in typically developing children and adolescents (Evans et al., 2016). Additionally, asymmetric amygdalar surface volumes in CHR with violent ideation (Feng et al., 2019) implicate a relationship with aggression and impulsivity.
This study aims to explore interactions between schizotypy dimensions and PLE in a general population cohort in association with HC subfields and amygdala volumes. We hypothesize that left medial temporal lobe structures show differential associations with schizotypy dimensions and PLE, as well as their interaction. Based on previously described patterns of volume reductions in incipient and early psychosis patients, we predict that positive schizotypy and PLE are associated with left CA1 volume reduction. Further, we expect PLE, positive and negative schizotypy dimensions to associate with subiculum, CA2/3 and CA4/DG volume reductions, and amygdala volume to vary as a function of impulsive and negative schizotypy dimensions. Based on a previous study (Sahakyan et al., 2021), we predict that interactions between PLE and the disorganized schizotypy dimension are associated with left medial temporal lobe volume reductions. Associations in volumes of the right hemisphere are explored without hypotheses.
2. METHODS
2.1. Study cohort
This study included 367 German language proficient individuals (aged 18 to 40) from the general community, volunteering in response to university‐based email circulation, local and online advertisements. Participants were screened by phone using structured clinical interview for DSM‐IV (Wittchen, Wunderlich, Gruschwitz, & Zaudig, 1997), and selected for study inclusion if no history of mental health, neurological or chronic medical conditions were present. This study protocol was in agreement with the Declaration of Helsinki (World Medical Association, 2013) and approved by the local ethics committee of the Medical School of the Philipps‐University of Marburg. Participants provided written informed consent, completed phenotype self‐report measures online, and received financial compensation upon study completion. Overall, 383 participants initially participated. Following exclusion of 16 individuals due to insufficient T1‐image quality or incompleteness of survey data, full phenotyping and HC volume estimates were available from 367 (238 [64.85%] females, 129 males [35.15%]) healthy adults (Mean age = 23.85, SD = 3.75 years, min = 18, max = 39) included in the analysis. In this study, we extended the sample previously described in Sahakyan et al. (2021). Seven (1.91%) participants scored PLE equal to or above the CHR screening criteria applied in previous studies (Chen et al., 2016; Ising et al., 2012). CHR was ruled out in all four out of seven (51.14%) participants who also agreed to follow‐up assessments with Schizophrenia Proneness Instrument (Adult version) (Schultze‐Lutter, Addington, Ruhrmann, & Klosterkötter, 2007). The mean laterality quotient according to the Edinburgh Handedness Inventory (Oldfield, 1971) was 78.65 (SD = 53.22), and mean intelligence quotient (IQ) estimated by the Mehrfachwahl‐Wortschatz‐Test B (Lehrl, 2005) was 116.38 (SD = 14.02, min = 92, max = 145), a comparatively brief estimate of general cognitive capacity mainly employed to rule out IQ < 80 in this sample.
2.2. Imaging data acquisition and preprocessing
T1‐weighed brain images were obtained with a 3‐T Siemens Tim Trio magnetic resonance scanner (Siemens, Erlangen, Germany) using a 12‐channel quadrature head coil and MPRAGE sequence with a duration of 4:26 min (TE = 2.26 ms, TI = 900 ms, TR = 1900 ms). Homogeneity bias correction and tissue segmentation were conducted using Computational Anatomy Toolbox for SPM, (CAT12.7, r1598, Gaser, Dahnke, Kurth, & Luders, in review) in SPM12 (version 12, v7771, Statistical Parametric Mapping, Wellcome Trust Centre for Neuroimaging, London, UK) running in Matlab (R2017a, The Mathworks Inc.). Images were both visually checked for artifacts as well as undergoing the standard quality assurance protocol for image homogeneity implemented in CAT12 software. Hippocampal regions of interest volumes were estimated in unsmoothed native gray matter images.
2.3. Assessment of trait schizotypy
Schizotypal traits were measured using the German version of the Oxford‐liverpool inventory of feelings and experiences (O‐LIFE, Mason, Claridge, & Jackson, 1995). Based on 104 items the O‐LIFE measures scores on four individual dimensions, which reflect the heterogeneous positive (UnEx), negative (IntAn), disorganized (CogDis), as well as behaviorally odd (ImpNon) facets of schizotypy. UnEx corresponds to perceptual anomalies and magical thinking, while CogDis taps into attention and thought aberrances reflecting disorganized symptoms of psychosis. The IntAn dimension measures anhedonic phenomena related to social and physical activities, and ImpNon refers to impulsive and socially non‐conforming behavior (Mason et al., 1995; Mason & Claridge, 2006). Descriptive statistics of sample characteristics and dimensional internal consistencies are shown in Table 1.
TABLE 1.
Descriptive statistics of psychotic‐like experiences (PLE) and schizotypy dimensions with Spearman correlation coefficients
| Mean | SD | Min | Max | Skew | Kurtosis | UnEx | CogDis | IntAn | ImpNon | Total | Crobach's α | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| O‐LIFE scale | ||||||||||||
| UnEx | 1.86 | 2.46 | 0 | 16 | 2.31 | 7.16 | 0.45** | 0.08 | 0.33** | 0.60** | .75 | |
| CogDis | 5.21 | 4.31 | 0 | 21 | 0.96 | 0.56 | 0.35** | 0.19** | 0.81** | .84 | ||
| IntAn | 4.06 | 3.51 | 0 | 19 | 1.59 | 3.02 | 0.02 | 0.58** | .77 | |||
| ImpNon | 6.10 | 2.88 | 0 | 15 | 0.44 | 0.09 | 0.53** | .58 | ||||
| Total | 17.23 | 8.69 | 3 | 54 | 0.88 | 0.82 | .85 | |||||
| PQ‐16 | ||||||||||||
| PLE | 1.08 | 1.51 | 0 | 9 | 1.97 | 4.88 | 0.54** | 0.46** | 0.11* | 0.30** | 0.52** | .62 |
| PLE distress | 1.17 | 1.89 | 0 | 15 | 2.66 | 10.59 | 0.52** | 0.45** | 0.10 | 0.29** | 0.51** | .60 |
Abbreviations: CogDis, cognitive disorganization; ImpNon, impulsive nonconformity; IntAn, introvertive anhedonia; O‐LIFE, Oxford‐Liverpool Inventory of Life Experiences; PQ‐16, Prodromal Questionnaire; UnEx, unusual experiences.
pFDR<.001.
pFDR < .05 (two‐tailed).
2.4. Assessment of PLE
PLE were assessed using the 16‐item version of the Prodromal Questionnaire (PQ‐16) (Ising et al., 2012), which provides a total PLE score on a two‐point scale (answers “true” =1, “false” = 0), and a measure of distress severity induced by PLE (“none” =0 to “severe” =3). Cut‐off scores of 6 on the total PLE scale and 9 on the distress scale have been identified as sufficient detection criteria for psychosis proneness (Chen et al., 2016; Ising et al., 2012). All questionnaires were completed online (www.soscisurvey.de, Leiner, 2019) and inspected for PLE above the recommended screening cut‐off after study completion.
2.5. Hippocampal subfield volume estimation and extraction
We selected six bilateral limbic regions that were of a priori interest. These included the HC subfields labeled subiculum, cornus ammonis (CA)1, CA2/3, CA4/dentate gyrus (DG), SR/SL/SM (stratum radiatum [SR], stratum lacunosum [SL], stratum moleculare [SM]) as well as the whole amygdala. We used the novel segmentation tool implemented in CAT12.7, which uses the CoBra (Computational Brain Anatomy Laboratory at the Douglas Institute, Verdun, Canada) atlas (Winterburn et al., 2013) based on high‐resolution (1 mm isotropic voxel size) images of HC subfields and amygdala (manual segmentation described in Entis, Doerga, Feldman, & Dickerson, 2012; atlas described in Treadway et al., 2015). Figure 1 displays subfield segmentations and Table 2 shows summarized average volumes across all subjects. Merging HC subfields, such as CA2/3, into a single label circumvents reliability issues related to particularly small subfields sizes. This offers a robustness advantage when anatomical segmentation is based on T1 images only.
FIGURE 1.
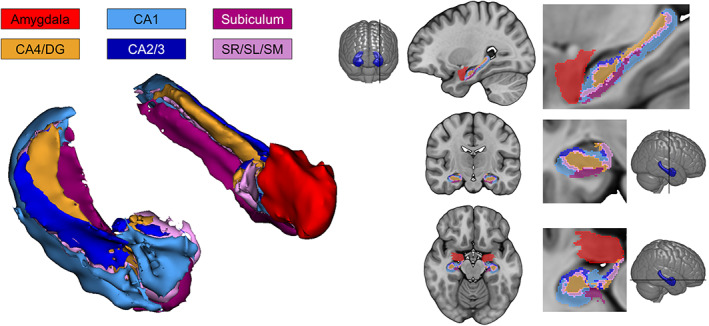
Visualization of hippocampal subfield and amygdala segmentation using CoBra atlas (Winterburn et al., 2013) implemented in computational anatomy toolbox (CAT12.7, Gaser et al., in review). For display purposes the model on the left shows smoothed volumes of the hippocampal formation with (left hemisphere) and without (right hemisphere) the amygdala. CA, cornu ammonis; DG, dentate gyrus; SR, stratum radiatum; SL, stratum lacunosum (SL); SM, stratum moleculare. This figure was prepared with 3D Slicer (https://www.slicer.org) and MRIcroGL (https://www.mccauslandcenter.sc.edu/mricrogl/)
TABLE 2.
Means and standard deviations (SD) for left and right hemispheric subfield volumes
| Mean volume (SD) (mm3) | ||
|---|---|---|
| Left hemisphere | Right hemisphere | |
| Amygdala | 1,733.22 (186.06) | 1,733.30 (180.93) |
| CA1 | 1,016.14 (107.16) | 1,074.99 (118.91) |
| CA2/3 | 208.21 (29.01) | 236.46 (30.55) |
| CA4/DG | 728.15 (79.10) | 731.99 (82.62) |
| Subiculum | 512.98 (56.91) | 537.91 (59.56) |
| SR/SL/SM | 548.60 (58.76) | 562.14 (64.55) |
Abbreviations: CA, cornu ammonis; DG, dentate gyrus; SL, stratum lacunosum; SM, stratum moleculare; SR, stratum radiatum.
3. STATISTICAL ANALYSIS
3.1. Phenotype associations with amygdala and hippocampal subfield volumes
HC and amygdala volumes were analyzed with hierarchical linear regression models in R (Version 3.6.3, R Core Team, 2020) in RStudio (Version 1.1.463, RStudio Team, 2016). We conducted 12 separate models, using the six bilateral volumes as dependent variables. Two‐tailed correlations between subfield volumes and variables sex and total intracranial volume (TIV) (all p' s < .05) were significant, but nonsignificant for age (all p' s > .05). Sex (0 = male, 1 = female) and TIV were entered at the first step for the covariate models. In the second step, trait schizotypy dimensions (UnEx, CogDis, IntAn, ImpNon) and PLE (PQ‐16) scores were entered simultaneously (main effects models), followed by the phenotype interaction terms of PLE × schizotypy dimension in the third step. We standardized dependent and independent variables, compared models using analysis of variance (ANOVA), and examined two‐way interactions using the Johnson‐Neyman method through the PROCESS macro (Hayes, 2018). Since phenotype scales correlated (Table 1), multicollinearity at each step was controlled for by observation of variance inflation factor (>5 criterion) and tolerance (<0.1 criterion) using the olsrr package (Hebbali, 2020) in R. We chose to test left medial temporal lobe regions (hippocampal subfields and amygdala), since our recent investigation on schizotypy (Sahakyan et al., 2021) indicated effects in the left hemisphere. Since we did not have an a priori hypothesis for right hemispheric subfields, we applied false detection rate (FDR) correction for multiple comparisons for right‐sided models.
4. RESULTS
4.1. Main analysis
In our analysis of the differential effects of trait schizotypy and PLE on HC subfield volumes, we observed significant effects for single schizotypy dimensions as well as a two‐way interaction among trait and PLE scales. To facilitate comparisons between scales, we report standardized regression coefficients (β) with their individual p‐values (Table 3).
TABLE 3.
Standardized regression coefficients and significance values of hierarchical regression models
| Step 1 | Step 2 | Step 3 | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Covariates | O‐LIFE dimension | PQ‐16 | Two‐way interactions | |||||||||||||||||||
| Sex | TIV | UnEx | CogDis | IntAn | ImpNon | PLE score | PLE × UE | PLE × CD | PLE × IA | PLE × IN | ||||||||||||
| β | p | β | p | β | p | β | p | β | p | β | p | β | p | β | p | β | p | β | p | β | p | |
| Left hemisphere | ||||||||||||||||||||||
| Amygdala | −.197 | <.001 | .599 | <.001 | −.109 | .029 | .063 | .172 | −.071 | .073 | .061 | .135 | .077 | .130 | .012 | .582 | .000 | .991 | −.002 | .945 | −.008 | .824 |
| CA1 | −.007 | .880 | .662 | <.001 | −.036 | .510 | .009 | .862 | −.038 | .377 | .079 | .073 | .032 | .566 | .040 | .077 | −.020 | .534 | .019 | .599 | .045 | .259 |
| CA2/3 | −.042 | .449 | .392 | <.001 | .030 | .648 | −.054 | .378 | .023 | .659 | .030 | .577 | .045 | .501 | −.024 | .383 | −.039 | .324 | .002 | .964 | −.027 | .586 |
| CA4/DG | −.027 | .586 | .593 | <.001 | −.020 | .725 | .009 | .864 | −.033 | .472 | .076 | .106 | .027 | .641 | .020 | .400 | −.029 | .391 | −.003 | .930 | .009 | .826 |
| Subiculum | .001 | .979 | .728 | <.001 | −.112 | .024 | .058 | .201 | −.052 | .186 | .088 | .030 | .051 | .307 | .053 | .011 | .015 | .617 | .014 | .669 | .022 | .547 |
| SR/SL/SM | −.052 | .260 | .638 | <.001 | −.063 | .247 | .013 | .797 | −.042 | .330 | .064 | .146 | .075 | .172 | .017 | .454 | −.028 | .385 | .005 | .884 | .021 | .604 |
Note: Bold face indicates significance at α < .05 or less.
Abbreviations: CogDis (CD), cognitive disorganization; ImpNon (IN), impulsive nonconformity; IntAn (IA), introvertive anhedonia; PLE, psychotic‐like experiences; TIV, total intracranial volume; UnEx (UE), unusual experiences.
The main effect of positive schizotypy (UnEx) showed a significant association with left amygdala and subiculum volume reductions (Table 4). ImpNon was also positively associated with left subicular volume (Table 3). We did not find any effect of the negative (IntAn) and disorganized (CogDis) dimensions on HC subfield volumes. For the sake of completeness, results of exploratory analyses of right medial temporal lobe volumes are shown in Table S3. In summary, model regressors of right hemispheric HC subfield models did not survive FDR‐correction.
TABLE 4.
Summary of regression models predicting volume of the left amygdala and subiculum
| a | b | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Left amygdala | Left subiculum | |||||||||||
| Step 1 (covariates) | Step 2 (Main effects) | Step 3 (two‐way interaction) | Step 1 (covariates) | Step 2 (Main effects) | Step 3 (two‐way interaction) | |||||||
| β | SE | β | SE | β | SE | β | SE | β | SE | β | SE | |
| Intercept | 469.02*** | 99.52 | 456.30*** | 102.31 | 451.53*** | 102.77 | 24.62 | 30.18 | 16.58 | 31.01 | 9.92 | 30.88 |
| Sex | −76.65*** | 16.55 | −77.60*** | 17.63 | −76.68*** | 17.72 | .13 | 5.02 | 1.31 | 5.34 | 2.60 | 5.32 |
| TIV | 8.80 × 10−4 *** | 6.24 × 10−5 | 8.77 × 10−4 *** | 6.28 × 10−5 | 8.80 × 10−4 *** | 6.32 × 10−5 | 3.27 × 10−4 *** | 1.89 × 10−5 | 3.26 × 10−4 *** | 1.90 × 10−5 | 3.31 × 10−4 *** | 1.90 × 10−5 |
| UnEx | −8.25* | 3.77 | −9.99* | 4.92 | −2.58* | 1.14 | −5.02*** | 1.12 | ||||
| CogDis | 2.70 | 1.97 | 2.95 | 2.03 | .77 | 0.60 | 1.12 | 0.61 | ||||
| IntAn | −3.77 | 2.09 | −3.87 | 2.10 | −.84 | 0.63 | −0.98 | 0.63 | ||||
| ImpNon | 3.93 | 2.62 | 4.16 | 2.66 | 1.73* | 0.79 | 2.06* | 0.80 | ||||
| PLE | 9.49 | 6.26 | 6.97 | 7.76 | 1.94 | 1.90 | −1.58 | 2.33 | ||||
| PLE × UnEx | .58 | 1.05 | 0.81* | 0.32 | ||||||||
| df | 2, 364 | 7, 359 | 8, 358 | 2, 364 | 7, 359 | 8, 358 | ||||||
| Adj. R 2 | .52 | .53 | .53 | .53 | .53 | .54 | ||||||
| R 2 | .52 | .54 | .54 | .53 | .54 | .55 | ||||||
| ∆R 2 | .01 | .00 | .01 | .01 | ||||||||
| F | 197.60*** | 59.02 | 51.58 | 204.20*** | 61.06 | 55.08 | ||||||
| ∆F | 2.24* | .30 | 2.35* | 6.57* | ||||||||
Abbreviations: Adj.R 2, adjusted R 2; CogDis, cognitive disorganization; ImpNon, impulsive nonconformity; IntAn, introvertive anhedonia; PLE, psychotic‐like experiences; SE, standard error; TIV, total intracranial volume; UnEx, unusual experiences.
p < .001, **p < .01,
p < .05.
Left subiculum subfield volume increase was associated with the two‐way interaction of positive schizotypy and PLE, which significantly explained volume variability beyond main effects (Table 4b). Examination of regression slopes showed that PLE levels were significantly associated with a predicted subiculum volume increase at higher positive schizotypy levels. This moderation effect occurred in high positive schizotypy (observed UnEx score ≥ 6.95 equalling UnExmean + 2.07 × SD) (Figure 2).
FIGURE 2.
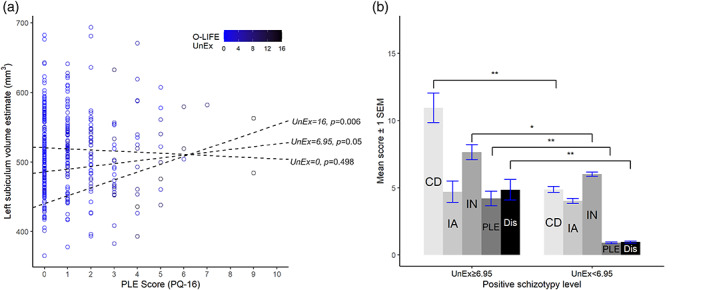
Prediction of left subicular volume by psychotic‐like experiences [PLE; assessed by prodromal questionnaire (PQ‐16)] is moderated by high levels of positive trait schizotypy (scores ≥6.95) as measured by the Unusual Experiences (UnEx) scale of the Oxford‐Liverpool Inventory of Life Experiences (O‐LIFE) (a). Side B displays mean levels of negative (IA), disorganized (CD), impulsive (IN) traits, PLE and PLE distress severity (Dis) in a subgroup (n = 20) with positive schizotypy levels ≥6.95 compared with the rest of the sample (n = 347). Bar graphs show statistically significant group differences based on the Mann–Whitney U test, *p FDR < .01, **p FDR < 0.001, SEM = standard error of the mean. This figure was preprared with the ggplot2 package (Wickham, 2016)
4.2. Exploratory analyses
Based on the two‐way interaction's significance interval, we used UnEx ≥ 6.95 as a cut‐off to conduct an exploratory subgroup comparison of state and trait profiles (Figure 2). The high positive schizotypy subgroup (n = 20) showed significantly higher trait levels in all other schizotypy facets, PLE, and PLE associated distress (Table S1). UnEx and PLE showed the largest correlation in our sample (r = .54, p < .001). Hence, within the entire sample, the possibility of a covert nonlinear association was explored with a polynomial regression model (Belzak & Bauer, 2019), exchanging the interaction term for a quadratic UnEx term, which produced a comparable model (Table S2).
5. DISCUSSION
The aim of this study was to investigate state and trait psychosis prone phenotypes within the nonclinical section of a putative psychosis spectrum of neurobiological abnormalities (Nelson et al., 2013; Siever & Davis, 2004; Taylor, Calkins, & Gur, 2020). By examining individuals considered psychiatrically healthy rather than at CHR, we aimed to decouple HC variability from psychopathological states. This objective also underlines the importance of finding psychosis biomarkers applicable to the entire psychosis spectrum. For this purpose, we chose schizotypy, which represents stable personality dimensions, and PLE that are putatively transitory in nature (Barrantes‐Vidal et al., 2015).
In the main effect analyses, UnEx, that is, positive schizotypy, was a significant estimator of left amygdala and subiculum volume decrease. Additionally, left subicular volume was positively associated with impulsive nonconformity (ImpNon). The modest internal consistency of ImpNon was comparable to previous reports from an online community sample, which also suggested that ImpNon does not dilute the classical three‐factor model of schizotypy (Polner et al., 2019). Our findings support the utility of ImpNon as a separate psychosis phenotypic estimator for brain structural variation. Consistent with previous findings suggesting that failure to distinguish between positive and negative schizotypy dimensions could result in reduced estimator robustness (Barrantes‐Vidal, Gross, et al., 2013; Barrantes‐Vidal, Lewandowski, & Kwapil, 2010), we found differences in the direction between the regressors that reflect the unique explanatory contribution of each schizotypy dimension. The significant main effect of the positive dimension is not confirmed by our previous study (Sahakyan et al., 2021) with the Multidimensional Schizotypy Scale (MSS; Kwapil, Gross, Silvia, Raulin, & Barrantes‐Vidal, 2018). This inconsistency may be attributed to differences between psychometric instruments that provide three (MSS) or four (O‐LIFE) phenotype dimensions entered as model predictors. If the positive O‐LIFE dimension reflects the core components of psychosis as supported by associations with dopamine regulating gene variants (Grant, Gabriel, Kuepper, Wielpuetz, & Hennig, 2014), its impact on hippocampal volume estimation would be expectedly higher. A topographical organization of effects for dopamine‐related schizotypy facets may also be linked to anterior–posterior differences in the density of D2 dopamine receptors in hippocampal subfields (Dubovyk & Manahan‐Vaughan, 2019). A clinical study showed that hippocampal hypertrophy in FEP responds to antipsychotic treatment, notably in, for example, CA3 and CA4 (Li et al., 2018). Thus, although positive schizotypy did not consistently associate with subfield volumes, variations in specific hippocampal regions may be featured in positive schizotypy to a higher degree. This is supported by findings for the specific effect of polygenic risk on the volume of the left CA2/3 (Alnæs et al., 2019), suggesting a link between hippocampal subfields, genetics, and possibly schizophrenia endophenotypes.
While the main effect of positive schizotypy on left subiculum volume was negative, the interaction with PLE was associated with larger volumes. Variations of PLE may signify a dynamic state within the positive schizotypy dimension. A longitudinal behavioral study found that the expression of transient subclinical psychotic features is influenced by time‐invariant traits (Rössler, Hengartner, Ajdacic‐Gross, Haker, & Angst, 2013). Against a backdrop of a schizotypal predisposition for stress response (Grattan & Linscott, 2019; Soliman et al., 2011), PLE could indicate an ongoing susceptibility to latent states and stressors (Barrantes‐Vidal, Chun, et al., 2013; Rössler et al., 2013), genetic and environmental influences (Barkhuizen, Pain, Dudbridge, & Ronald, 2020; Brambilla et al., 2014). Extending this to neurobiological measures demonstrated that the positive relationship between PLE and left subicular volume depended on increased positive trait schizotypy. In those individuals with PLE at higher positive schizotypy predicting enlarged subiculum volumes, levels of disorganized and impulsive traits, and distress severity were augmented. Thus, expressions consistent with “benign” or “happy schizotypy” (Grant & Hennig, 2020; Mohr & Claridge, 2015), which could explain the observed positive correlation between PLE and schizotypy do not match the phenotype presented in this study, but rather point toward increased psychosis proneness.
The amygdala, together with the subiculum, showed an unexpected negative association with positive schizotypy. Based on rodent studies, a linkage between these two regions is supported by substantial inputs to the amygdala from the (temporal end of the) CA1, and the subiculum (Pitkänen, Pikkarainen, Nurminen, & Ylinen, 2000). With anterior–posterior functional differentiation, the anterior subregion of the subiculum is especially connected to the ventral striatum, midbrain, and amygdala (Chase et al., 2015). This hippocampus‐midbrain‐striatum‐network shows reduced connectivity in schizophrenia (Gangadin, Cahn, Scheewe, Hulshoff Pol, & Bossong, 2021). Volume changes in both subfields could be related to functional findings, and more multimodal imaging studies in the psychosis spectrum are required to investigate such linkages (Liu et al., 2020; Wang et al., 2015; Wang et al., 2020).
We did not find the association between positive schizotypy and CA1 region. CA1 volume change may especially demarcate CHR trajectories as it was the only HC subfield bilaterally associated with progressive global symptomatic deterioration in UHR individuals (Ho et al., 2017). An association between the anterior HC, which includes CA1, and negative schizotypy was dependent on high disorganized schizotypy measured by the MSS (Sahakyan et al., 2021). Still, in alignment with our findings, no main effect of the positive dimension was present. This may reflect results from nonclinical individuals displaying persistent PLE, suggesting that cognitive deficits may be more relevant for poorer outcomes than merely positive PLE (Brett, Peters, & McGuire, 2015). In UHR individuals, CA1 and subiculum volumes were positively correlated with verbal performance (Vargas et al., 2018), and subicular volume was also associated with negative symptoms in schizophrenia and cognitive deficits in bipolar disorder (Haukvik et al., 2015). Examining how cognitive endophenotypes (Siddi, Petretto, & Preti, 2017) relate to medial temporal lobe structures (Antoniades et al., 2018) in the nonclinical psychosis spectrum may help close a gap in the literature.
Contrary to expectations, we did not find that amygdala volume is related to negative or impulsive trait expressions. Previous studies investigating the amygdala across the psychosis spectrum connote mixed evidence including no volume abnormalities in unaffected and affected relatives of patients with bipolar disorder (Hajek et al., 2009), but also variable volume abnormalities across samples of adults, and children and adolescents at high risk of schizophrenia (Ganzola et al., 2014). Moreover, amygdala enlargements were associated with negative symptoms and depressive symptoms in prodromal syndromes (Bartholomeusz et al., 2014), and also found in first‐episode affective psychosis compared to controls (Velakoulis et al., 2006), while other studies report volume reduction in FEP (Armio et al., 2020), and first episode schizophrenia (but not bipolar disorder) (Watson et al., 2012). Notably, some studies demonstrate left amygdalar volume reductions in FEP but not in high‐risk individuals compared with controls (Bois et al., 2015; Witthaus et al., 2010). The null finding adds to these inconsistencies, suggesting that a relationship with negative schizotypy in children and adolescents (Evans et al., 2016) is not confirmed in young adults. This study instead indicates an inverse relationship with specifically positive features in nonclinical adults, while also being the first to suggest that, contrary to predictions, amygdala volume is not associated with the impulsive trait dimension. Building on previous clinical studies that assessed psychotic symptoms using the PANSS (Kawano et al., 2015; Kühn, Musso, et al., 2012; Mathew et al., 2014), we could neither confirm associations between negative schizotypy and CA2/3 or CA4/DG. Other studies report functional specialization in these regions compatible with cognitive impairments (Haukvik et al., 2018; Vargas et al., 2018). One study of first‐episode schizophrenia did not confirm HC subfield volume correlations with negative or total PANSS scores, but instead found a positive relationship between right CA1 volume and positive PANSS score (Hýža, Kuhn, Češková, Ustohal, & Kašpárek, 2016). Across the psychosis continuum, relationships between different symptom domains and HC subfields are emerging—especially in the nonclinical part—with variable consistency. There is a likely dependency between structural and functional brain changes associated with behavioral and clinical characteristics, but causal mechanisms are subject to longitudinal studies. These are required to explain the occurrence of larger regional hippocampal volumes in the healthy part of the psychosis spectrum.
The main effects of disorganized and negative schizotypy dimensions on subfield volumes were nonsignificant, partially supporting the explanation that high positive schizotypy is the main driver. In the light of previous findings demonstrating that prediction of prodromal outcomes was explained by additive effects of positive and negative schizotypy (Barrantes‐Vidal, Gross, et al., 2013), this may suggest that within individuals displaying increased positive schizotypy, these proneness profiles are not wholly enough expressed to effect noticeable differences across all longitudinal volumes utilized in this study. Consistent with this explanation, interactions between positive, negative, and disorganized dimensions reach significance in the anterior, but not the posterior portion of the hippocampus (Sahakyan et al., 2021), supporting an anterior–posterior gradient of pathological hippocampal volume changes in clinical subjects (McHugo et al., 2018). This may also explain the lack of expected associations for the interaction between the disorganized dimension and PLE in left hippocampal subfields. Apart from longitudinal, opposed to anterior/posterior subdivisions, usage of different automated segmentation methods may further explain inconsistencies.
In this study, HC subfield volume correlates corresponded to psychosis phenotypes absent of a clinically manifest vulnerability. Notably, this does not necessitate exemption from vulnerability in the form of genotypes associated with PLE and schizotypy (Legge et al., 2019; Meller et al., 2019), or hippocampal subfield volumes (Alnæs et al., 2019; van der Meer et al., 2020). As indicated by moderation, in healthy participants with PLE not indicative of CHR, the effect on HC subfield volume was trait schizotypy driven. The present findings imply a sensitivity of the limbic structures to time‐invariant traits rather than PLE. It was also shown that the PLE‐trait interaction effect is captured by the quadratic trait term. This finding suggests that peak expressions of PLE featured in higher positive schizotypy correlate with hippocampal volume variation in healthy individuals. Nonetheless, unusual experiences in the context of positive schizotypy and PLE might not be interchangeable phenomenological entities. We advocate a phenotype distinction based on transience (PLE) and stability (traits) (Fonseca‐Pedrero & Debbané, 2017; Seiler, Nguyen, Yung, & O'Donoghue, 2020). However, to our best knowledge, no assessment of the discriminant validity between positive psychometric schizotypy and PLE so far exists.
This study has some limitations. The first one being that a lack of associations between PLE and medial temporal structures above the schizotypal traits may be explained by comparatively small effect sizes or reduced PLE persistence (Dominguez, Wichers, Lieb, Wittchen, & van Os, 2011; Hanssen, Bak, Bijl, Vollebergh, & van Os, 2005; Nelson, Fusar‐Poli, & Yung, 2012). Since the PQ‐16 does not provide a measure of PLE persistence, longitudinal investigations are required to address this issue. There was a considerable overrepresentation of females and an absence of psychopathology in the present cohort, limiting comparability with other studies reporting expectedly higher CHR screening rates in the general population (McDonald et al., 2018).
This study was also the first to use CAT12 automated segmentation for HC subfield delineation. This achieves an alternative route to limbic subfield characterization compared to anterior and posterior HC subdivisions applied elsewhere (McHugo et al., 2018; Sahakyan et al., 2021). Our findings from a novel toolbox call for replication so that results from different HC subfield volumetry methods will expectedly accumulate. We propose that future studies involving PLE could explore (and control for) variance explained by positive schizotypy, PLE distress, or persistence. Etiological studies involving endophenotypes capturing genetic psychosis liability, especially in association with medial lobe structures, could benefit from incorporating individual differences.
CONFLICT OF INTERESTS
All authors declare no conflicts of interest.
Supporting information
Appendix S1: Supporting information
ACKNOWLEDGMENTS
This work was supported by a Research Grant of the University Medical Center Giessen and Marburg (UKGM) (project 11/2017 MR to Igor Nenadić; project 05/2018 MR to Sarah Grezellschak and Igor Nenadić). The study sponsor had no role in study design, analysis, or interpretation. Data and analyses shown here have not been published previously. We thank Simon Schmitt, and our student research assistants Aliénor Bergmann, Yvonne Schröder, Daniela Hohmann, Franziska Hildesheim, Julia Hoffmann, Ulrike Louis, and Paulina Schweickert for their support. Open Access funding enabled and organized by Projekt DEAL.
Evermann, U., Gaser, C., Meller, T., Pfarr, J.‐K., Grezellschak, S., & Nenadić, I. (2021). Nonclinical psychotic‐like experiences and schizotypy dimensions: Associations with hippocampal subfield and amygdala volumes. Human Brain Mapping, 42(15), 5075–5088. 10.1002/hbm.25601
Funding information Uniklinikum Giessen und Marburg, Grant/Award Numbers: 05/2018 MR and, 11/2017 MR
Contributor Information
Ulrika Evermann, Email: ulrika.evermann@staff.uni-marburg.de.
Igor Nenadić, Email: nenadic@staff.uni-marburg.de.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- Allen, P., Azis, M., Modinos, G., Bossong, M. G., Bonoldi, I., Samson, C., … McGuire, P. (2018). Increased resting hippocampal and basal ganglia perfusion in people at ultra high risk for psychosis: Replication in a second cohort. Schizophrenia Bulletin, 44(6), 1323–1331. 10.1093/schbul/sbx169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen, P., Chaddock, C. A., Egerton, A., Howes, O. D., Bonoldi, I., Zelaya, F., … McGuire, P. (2015). Resting Hyperperfusion of the hippocampus, midbrain, and basal ganglia in people at high risk for psychosis. American Journal of Psychiatry, 173(4), 392–399. 10.1176/appi.ajp.2015.15040485 [DOI] [PubMed] [Google Scholar]
- Alnæs, D., Kaufmann, T., Van Der Meer, D., Córdova‐Palomera, A., Rokicki, J., Moberget, T., … Westlye, L. T. (2019). Brain heterogeneity in schizophrenia and its association with polygenic risk. JAMA Psychiatry, 76(7), 739–748. 10.1001/jamapsychiatry.2019.0257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniades, M., Schoeler, T., Radua, J., Valli, I., Allen, P., Kempton, M. J., & McGuire, P. (2018). Verbal learning and hippocampal dysfunction in schizophrenia: A meta‐analysis. Neuroscience and Biobehavioral Reviews, 86, 166–175. 10.1016/j.neubiorev.2017.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armio, R. L., Laurikainen, H., Ilonen, T., Walta, M., Salokangas, R. K. R., Koutsouleris, N., … Tuominen, L. (2020). Amygdala subnucleus volumes in psychosis high‐risk state and first‐episode psychosis: Amygdala subnuclei and psychosis. Schizophrenia Research, 215, 284–292. 10.1016/j.schres.2019.10.014 [DOI] [PubMed] [Google Scholar]
- Baglivo, V., Cao, B., Mwangi, B., Bellani, M., Perlini, C., Lasalvia, A., … Brambilla, P. (2018). Hippocampal subfield volumes in patients with first‐episode psychosis. Schizophrenia Bulletin, 44(3), 552–559. 10.1093/schbul/sbx108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkhuizen, W., Pain, O., Dudbridge, F., & Ronald, A. (2020). Genetic overlap between psychotic experiences in the community across age and with psychiatric disorders. Translational Psychiatry, 10(1), 86. 10.1038/s41398-020-0765-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrantes‐Vidal, N., Chun, C. A., Myin‐Germeys, I., & Kwapil, T. R. (2013). Psychometric schizotypy predicts psychotic‐like, paranoid, and negative symptoms in daily life. Journal of Abnormal Psychology, 122, 1077–1087. 10.1037/a0034793 [DOI] [PubMed] [Google Scholar]
- Barrantes‐Vidal, N., Grant, P., & Kwapil, T. R. (2015). The role of schizotypy in the study of the etiology of schizophrenia spectrum disorders. Schizophrenia Bulletin, 41(2), S408–S416. 10.1093/schbul/sbu191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrantes‐Vidal, N., Gross, G. M., Sheinbaum, T., Mitjavila, M., Ballespí, S., & Kwapil, T. R. (2013). Positive and negative schizotypy are associated with prodromal and schizophrenia‐spectrum symptoms. Schizophrenia Research, 145(1–3), 50–55. 10.1016/j.schres.2013.01.007 [DOI] [PubMed] [Google Scholar]
- Barrantes‐Vidal, N., Lewandowski, K. E., & Kwapil, T. R. (2010). Psychopathology, social adjustment and personality correlates of schizotypy clusters in a large nonclinical sample. Schizophrenia Research, 122(1–3), 219–225. 10.1016/j.schres.2010.01.006 [DOI] [PubMed] [Google Scholar]
- Bartholomeusz, C. F., Whittle, S. L., Pilioussis, E., Allott, K., Rice, S., Schäfer, M. R., … Paul Amminger, G. (2014). Relationship between amygdala volume and emotion recognition in adolescents at ultra‐high risk for psychosis. Psychiatry Research: Neuroimaging, 224(3), 159–167. 10.1016/j.pscychresns.2014.10.005 [DOI] [PubMed] [Google Scholar]
- Belzak, W. C. M., & Bauer, D. J. (2019). Interaction effects may actually be nonlinear effects in disguise: A review of the problem and potential solutions. Addictive Behaviors, 94, 99–108. 10.1016/j.addbeh.2018.09.018 [DOI] [PubMed] [Google Scholar]
- Bobilev, A. M., Perez, J. M., & Tamminga, C. A. (2020). Molecular alterations in the medial temporal lobe in schizophrenia. Schizophrenia Research, 217, 71–85. 10.1016/j.schres.2019.06.001 [DOI] [PubMed] [Google Scholar]
- Bois, C., Levita, L., Ripp, I., Owens, D. C. G., Johnstone, E. C., Whalley, H. C., & Lawrie, S. M. (2015). Hippocampal, amygdala and nucleus accumbens volume in first‐episode schizophrenia patients and individuals at high familial risk: A cross‐sectional comparison. Schizophrenia Research, 165(1), 45–51. 10.1016/j.schres.2015.03.024 [DOI] [PubMed] [Google Scholar]
- Bossong, M. G., Antoniades, M., Azis, M., Samson, C., Quinn, B., Bonoldi, I., … McGuire, P. (2019). Association of Hippocampal Glutamate Levels with adverse outcomes in individuals at clinical high risk for psychosis. JAMA Psychiatry, 76(2), 199–207. 10.1001/jamapsychiatry.2018.3252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambilla, P., Fagnani, C., Cecchetto, F., Medda, E., Bellani, M., Salemi, M., … Stazi, M. A. (2014). Genetic and environmental bases of the interplay between magical ideation and personality. Psychiatry Research, 215(2), 453–459. 10.1016/j.psychres.2013.11.021 [DOI] [PubMed] [Google Scholar]
- Brett, C. M. C., Peters, E. R., & McGuire, P. K. (2015). Which psychotic experiences are associated with a need for clinical care? European Psychiatry, 30(5), 648–654. 10.1016/j.eurpsy.2014.12.005 [DOI] [PubMed] [Google Scholar]
- Briend, F., Nelson, E. A., Maximo, O., Armstrong, W. P., Kraguljac, N. V., & Lahti, A. C. (2020). Hippocampal glutamate and hippocampus subfield volumes in antipsychotic‐naive first episode psychosis subjects and relationships to duration of untreated psychosis. Translational Psychiatry, 10(1), 137. 10.1038/s41398-020-0812-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase, H. W., Clos, M., Dibble, S., Fox, P., Grace, A. A., Phillips, M. L., & Eickhoff, S. B. (2015). Evidence for an anterior‐posterior differentiation in the human hippocampal formation revealed by meta‐analytic parcellation of fMRI coordinate maps: Focus on the subiculum. NeuroImage, 113, 44–60. 10.1016/j.neuroimage.2015.02.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, F., Wang, L., Wang, J., Heeramun‐Aubeeluck, A., Yuan, J., & Zhao, X. (2016). Applicability of the Chinese version of the 16‐item prodromal questionnaire (CPQ‐16) for identifying attenuated psychosis syndrome in a college population. Early Intervention in Psychiatry, 10(4), 308–315. 10.1111/eip.12173 [DOI] [PubMed] [Google Scholar]
- Claridge, G., & Beech, T. (1995). Fully and quasi‐dimensional constructions of schizotypy. In Raine A., Lencz T., & Mednick S. A. (Eds.), Schizotypal personality (pp. 192–216). Cambridge: Cambridge University Press. [Google Scholar]
- Debbané, M., & Barrantes‐Vidal, N. (2015). Schizotypy from a developmental perspective. Schizophrenia Bulletin, 41(2), S386–S395. 10.1093/schbul/sbu175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derome, M., Zöller, D., Modinos, G., Schaer, M., Eliez, S., & Debbané, M. (2020). Developmental trajectories of subcortical structures in relation to dimensional schizotypy expression along adolescence. Schizophrenia Research, 218, 76–84. 10.1016/j.schres.2020.02.005 [DOI] [PubMed] [Google Scholar]
- Dominguez, M. D. G., Wichers, M., Lieb, R., Wittchen, H.‐U., & van Os, J. (2011). Evidence that onset of clinical psychosis is an outcome of progressively more persistent subclinical psychotic experiences: An 8‐year cohort study. Schizophrenia Bulletin, 37(1), 84–93. 10.1093/schbul/sbp022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubovyk, V., & Manahan‐Vaughan, D. (2019). Gradient of expression of dopamine D2 receptors along the dorso‐ventral axis of the hippocampus. Frontiers in Synaptic Neuroscience, 11(October), 1–11. 10.3389/fnsyn.2019.00028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entis, J. J., Doerga, P., Feldman, L., & Dickerson, B. C. (2012). NeuroImage a reliable protocol for the manual segmentation of the human amygdala and its subregions using ultra‐high resolution MRI. NeuroImage, 60(2), 1226–1235. 10.1016/j.neuroimage.2011.12.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettinger, U., Meyhöfer, I., Steffens, M., Wagner, M., & Koutsouleris, N. (2014). Genetics, cognition, and neurobiology of schizotypal personality: A review of the overlap with schizophrenia. Frontiers in Psychiatry, 5(18). 10.3389/fpsyt.2014.00018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettinger, U., Mohr, C., Gooding, D. C., Cohen, A. S., Rapp, A., Haenschel, C., & Park, S. (2015). Cognition and brain function in Schizotypy: A selective review. Schizophrenia Bulletin, 41(suppl 2), S417–S426. 10.1093/schbul/sbu190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans, D. W., Michael, A. M., Ularević, M., Lusk, L. G., Buirkle, J. M., & Moore, G. J. (2016). Neural substrates of a schizotypal spectrum in typically‐developing children: Further evidence of a normal‐pathological continuum. Behavioural Brain Research, 315, 141–146. 10.1016/j.bbr.2016.08.034 [DOI] [PubMed] [Google Scholar]
- Evermann, U., Gaser, C., Besteher, B., Langbein, K., & Nenadić, I. (2020). Cortical gyrification, psychotic‐like experiences, and cognitive performance in nonclinical subjects. Schizophrenia Bulletin, 46(6), 1524–1534. 10.1093/schbul/sbaa068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng, X., Provenzano, F., Appelbaum, P. S., Masucci, M. D., Brucato, G., Lieberman, J. A., & Girgis, R. R. (2019). Amygdalar volume and violent ideation in a sample at clinical high‐risk for psychosis. Psychiatry Research: Neuroimaging, 287(April), 60–62. 10.1016/j.pscychresns.2019.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flückiger, R., Ruhrmann, S., Debbané, M., Michel, C., Hubl, D., Schimmelmann, B. G., … Schultze‐Lutter, F. (2016). Psychosis‐predictive value of self‐reported schizotypy in a clinical high‐risk sample. Journal of Abnormal Psychology, 125(7), 923–932. 10.1037/abn0000192 [DOI] [PubMed] [Google Scholar]
- Fonseca‐Pedrero, E., & Debbané, M. (2017). Schizotypal traits and psychotic‐like experiences during adolescence: An update. Psicothema, 29(1), 5–17. 10.7334/psicothema2016.209 [DOI] [PubMed] [Google Scholar]
- Gangadin, S. S., Cahn, W., Scheewe, T. W., Hulshoff Pol, H. E., & Bossong, M. G. (2021). Reduced resting state functional connectivity in the hippocampus‐midbrain‐striatum network of schizophrenia patients. Journal of Psychiatric Research, 138, 83–88. 10.1016/j.jpsychires.2021.03.041 [DOI] [PubMed] [Google Scholar]
- Ganzola, R., Maziade, M., & Duchesne, S. (2014). Hippocampus and amygdala volumes in children and young adults at high‐risk of schizophrenia: Research synthesis. Schizophrenia Research, 156(1), 76–86. 10.1016/j.schres.2014.03.030 [DOI] [PubMed] [Google Scholar]
- Gaser, C., Dahnke, R., Kurth, K., & Luders, E. (2020). Alzheimer's disease neuroimaging initiative. A computational anatomy toolbox for the analysis of structural MRI data. NeuroImage (in review). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant, P., Gabriel, F., Kuepper, Y., Wielpuetz, C., & Hennig, J. (2014). Psychosis‐proneness correlates with expression levels of dopaminergic genes. European Psychiatry, 29(5), 304–306. 10.1016/j.eurpsy.2013.12.002 [DOI] [PubMed] [Google Scholar]
- Grant, P., Green, M. J., & Mason, O. J. (2018). Models of Schizotypy: The importance of conceptual clarity. Schizophrenia Bulletin, 44(2), S556–S563. 10.1093/schbul/sby012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant, P., & Hennig, J. (2020). Schizotypy, social stress and the emergence of psychotic‐like states ‐ a case for benign schizotypy? Schizophrenia Research, 216, 435–442. 10.1016/j.schres.2019.10.052 [DOI] [PubMed] [Google Scholar]
- Grattan, R. E., & Linscott, R. J. (2019). Components of schizophrenia liability affect the growth of psychological stress sensitivity following major life events. Schizophrenia Research, 212, 134–139. 10.1016/j.schres.2019.07.056 [DOI] [PubMed] [Google Scholar]
- Hajek, T., Gunde, E., Slaney, C., Propper, L., MacQueen, G., Duffy, A., & Alda, M. (2009). Amygdala and hippocampal volumes in relatives of patients with bipolar disorder: A high‐risk study. Canadian Journal of Psychiatry, 54(11), 726–733. 10.1177/070674370905401102 [DOI] [PubMed] [Google Scholar]
- Hanssen, M., Bak, M., Bijl, R., Vollebergh, W., & van Os, J. (2005). The incidence and outcome of subclinical psychotic experiences in the general population. British Journal of Clinical Psychology, 44(2), 181–191. 10.1348/014466505x29611 [DOI] [PubMed] [Google Scholar]
- Haukvik, U. K., Tamnes, C. K., Söderman, E., & Agartz, I. (2018). Neuroimaging hippocampal subfields in schizophrenia and bipolar disorder: A systematic review and meta‐analysis. Journal of Psychiatric Research, 104, 217–226. 10.1016/j.jpsychires.2018.08.012 [DOI] [PubMed] [Google Scholar]
- Haukvik, U. K., Westlye, L. T., Mørch‐Johnsen, L., Jørgensen, K. N., Lange, E. H., Dale, A. M., … Agartz, I. (2015). In vivo hippocampal subfield volumes in schizophrenia and bipolar disorder. Biological Psychiatry, 77(6), 581–588. 10.1016/j.biopsych.2014.06.020 [DOI] [PubMed] [Google Scholar]
- Hayes, A. F. (2018). Introduction to mediation, moderation, and conditional Process analysis: A regression‐based approach (2nd ed.). New York, NY: Guilford Press. [Google Scholar]
- Hebbali, A. (2020). olsrr: Tools for Building OLS Regression Models. R package version 0.5.3. Retrieved from https://cran.r-project.org/package=olsrr
- Ho, N. F., Holt, D. J., Cheung, M., Iglesias, J. E., Goh, A., Wang, M., … Zhou, J. (2017). Progressive decline in hippocampal CA1 volume in individuals at ultra‐high‐risk for psychosis who do not remit: Findings from the longitudinal youth at risk study. Neuropsychopharmacology, 42(6), 1361–1370. 10.1038/npp.2017.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hýža, M., Kuhn, M., Češková, E., Ustohal, L., & Kašpárek, T. (2016). Hippocampal volume in first‐episode schizophrenia and longitudinal course of the illness. World Journal of Biological Psychiatry, 17(6), 429–438. 10.1080/15622975.2016.1199893 [DOI] [PubMed] [Google Scholar]
- Ising, H. K., Veling, W., Loewy, R. L., Rietveld, M. W., Rietdijk, J., Dragt, S., … Van Der Gaag, M. (2012). The validity of the 16‐item version of the prodromal questionnaire (PQ‐16) to screen for ultra high risk of developing psychosis in the general help‐seeking population. Schizophrenia Bulletin, 38(6), 1288–1296. 10.1093/schbul/sbs068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano, M., Sawada, K., Shimodera, S., Ogawa, Y., Kariya, S., Lang, D. J., … Honer, W. G. (2015). Hippocampal subfield volumes in first episode and chronic schizophrenia. PLoS One, 10(2), 1–14. 10.1371/journal.pone.0117785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay, S. R., Fiszbein, A., & Opler, L. A. (1987). The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophrenia Bulletin, 13(2), 261–276. 10.1093/schbul/13.2.261 [DOI] [PubMed] [Google Scholar]
- Kemp, K. C., Gross, G. M., Barrantes‐Vidal, N., & Kwapil, T. R. (2018). Association of positive, negative, and disorganized schizotypy dimensions with affective symptoms and experiences. Psychiatry Research, 270, 1143–1149. 10.1016/j.psychres.2018.10.031 [DOI] [PubMed] [Google Scholar]
- Kline, E., Wilson, C., Ereshefsky, S., Nugent, K. L., Pitts, S., Reeves, G., & Schiffman, J. (2012). Schizotypy, psychotic‐like experiences and distress: An interaction model. Psychiatry Research, 200(2–3), 647–651. 10.1016/j.psychres.2012.07.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn, S., Musso, F., Mobascher, A., Warbrick, T., Winterer, G., & Gallinat, J. (2012). Hippocampal subfields predict positive symptoms in schizophrenia: First evidence from brain morphometry. Translational Psychiatry, 2(6), e127. 10.1038/tp.2012.51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn, S., Schubert, F., & Gallinat, J. (2012). Higher prefrontal cortical thickness in high schizotypal personality trait. Journal of Psychiatric Research, 46(7), 960–965. 10.1016/j.jpsychires.2012.04.007 [DOI] [PubMed] [Google Scholar]
- Kwapil, T. R., & Barrantes‐vidal, N. (2015). Schizotypy: Looking back and moving forward. Schizophrenia Bulletin, 41(2), S366–S373. 10.1093/schbul/sbu186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwapil, T. R., Gross, G. M., Silvia, P. J., & Barrantes‐Vidal, N. (2013). Prediction of psychopathology and functional impairment by positive and negative schizotypy in the Chapmans' ten‐year longitudinal study. Journal of Abnormal Psychology, 122(3), 807–815. 10.1037/a0033759 [DOI] [PubMed] [Google Scholar]
- Kwapil, T. R., Gross, G. M., Silvia, P. J., Raulin, M. L., & Barrantes‐Vidal, N. (2018). Development and psychometric properties of the multidimensional Schizotypy scale: A new measure for assessing positive, negative, and disorganized schizotypy. Schizophrenia Research, 193, 209–217. 10.1016/j.schres.2017.07.001 [DOI] [PubMed] [Google Scholar]
- Kwapil, T. R., Kemp, K. C., Mielock, A., Sperry, S. H., Chun, C. A., Gross, G. M., & Barrantes‐Vidal, N. (2020). Association of multidimensional schizotypy with psychotic‐like experiences, affect, and social functioning in daily life: Comparable findings across samples and schizotypy measures. Journal of Abnormal Psychology, 129(5), 492–504. 10.1037/abn0000522 [DOI] [PubMed] [Google Scholar]
- Legge, S. E., Jones, H. J., Kendall, K. M., Pardiñas, A. F., Menzies, G., Bracher‐Smith, M., … Walters, J. T. R. (2019). Association of Genetic Liability to psychotic experiences with Neuropsychotic disorders and traits. JAMA Psychiatry, 76(12), 1256–1265. 10.1001/jamapsychiatry.2019.2508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrl, S. (2005). Mehrfachwahl‐Wortschatz‐Intelligenztest MWT‐B. Balingen: Spitta Verlag. [Google Scholar]
- Leiner, D. J. (2019). SoSci Survey (Version 3.1.06) [Computer software]. Retrieved from https://www.soscisurvey.de
- Li, W., Li, K., Guan, P., Chen, Y., Xiao, Y., Lui, S., … Gong, Q. (2018). Volume alteration of hippocampal subfields in first‐episode antipsychotic‐naïve schizophrenia patients before and after acute antipsychotic treatment. NeuroImage: Clinical, 20(April), 169–176. 10.1016/j.nicl.2018.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman, J. A., Girgis, R. R., Brucato, G., Moore, H., Provenzano, F., Kegeles, L., … Small, S. A. (2018). Hippocampal dysfunction in the pathophysiology of schizophrenia: A selective review and hypothesis for early detection and intervention. Molecular Psychiatry, 23(8), 1764–1772. 10.1038/mp.2017.249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linscott, R. J., & van Os, J. (2013). An updated and conservative systematic review and meta‐analysis of epidemiological evidence on psychotic experiences in children and adults: On the pathway from proneness to persistence to dimensional expression across mental disorders. Psychological Medicine, 43(6), 1133–1149. 10.1017/S0033291712001626 [DOI] [PubMed] [Google Scholar]
- Liu, S., Li, A., Liu, Y., Yan, H., Wang, M., Sun, Y., … Liu, B. (2020). Polygenic effects of schizophrenia on hippocampal grey matter volume and hippocampus‐medial prefrontal cortex functional connectivity. The British Journal of Psychiatry: the Journal of Mental Science, 216(5), 267–274. 10.1192/bjp.2019.127 [DOI] [PubMed] [Google Scholar]
- Mason, O., & Claridge, G. (2006). The Oxford‐Liverpool inventory of feelings and experiences (O‐LIFE): Further description and extended norms. Schizophrenia Research, 82(2–3), 203–211. 10.1016/j.schres.2005.12.845 [DOI] [PubMed] [Google Scholar]
- Mason, O., Claridge, G., & Jackson, M. (1995). New scales for the assessment of schizotypy. Personality and Individual Differences, 18(1), 7–13. 10.1016/0191-8869(94)00132-C [DOI] [Google Scholar]
- Mathew, I., Gardin, T. M., Tandon, N., Eack, S., Francis, A. N., Seidman, L. J., … Keshavan, M. S. (2014). Medial temporal lobe structures and hippocampal subfields in psychotic disorders: Findings from the bipolar‐schizophrenia network on intermediate phenotypes (B‐SNIP) study. JAMA Psychiatry, 71(7), 769–777. 10.1001/jamapsychiatry.2014.453 [DOI] [PubMed] [Google Scholar]
- McDonald, M., Christoforidou, E., Van Rijsbergen, N., Gajwani, R., Gross, J., Gumley, A. I., … Uhlhaas, P. J. (2018). Using online screening in the general population to detect participants at clinical high‐risk for psychosis. Schizophrenia Bulletin, 45(3), 600–609. 10.1093/schbul/sby069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugo, M., Talati, P., Woodward, N. D., Armstrong, K., Blackford, J. U., & Heckers, S. (2018). Regionally specific volume deficits along the hippocampal long axis in early and chronic psychosis. NeuroImage: Clinical, 20(June), 1106–1114. 10.1016/j.nicl.2018.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meller, T., Schmitt, S., Ettinger, U., Grant, P., Stein, F., Brosch, K., … Nenadić, I. (2020). Brain structural correlates of schizotypal signs and subclinical schizophrenia nuclear symptoms in healthy individuals. Psychological Medicine, 1–10. 10.1017/S0033291720002044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meller, T., Schmitt, S., Stein, F., Brosch, K., Mosebach, J., Yüksel, D., … Nenadić, I. (2019). Associations of schizophrenia risk genes ZNF804A and CACNA1C with schizotypy and modulation of attention in healthy subjects. Schizophrenia Research, 208, 67–75. 10.1016/j.schres.2019.04.018 [DOI] [PubMed] [Google Scholar]
- Mier, D., Lis, S., Zygrodnik, K., Sauer, C., Ulferts, J., Gallhofer, B., & Kirsch, P. (2014). Evidence for altered amygdala activation in schizophrenia in an adaptive emotion recognition task. Psychiatry Research: Neuroimaging, 221(3), 195–203. 10.1016/j.pscychresns.2013.12.001 [DOI] [PubMed] [Google Scholar]
- Modinos, G., Egerton, A., Mcmullen, K., McLaughlin, A., Kumari, V., Barker, G. J., … Zelaya, F. (2018). Increased resting perfusion of the hippocampus in high positive schizotypy: A pseudocontinuous arterial spin labeling study. Human Brain Mapping, 39(10), 4055–4064. 10.1002/hbm.24231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modinos, G., Mechelli, A., Ormel, J., Groenewold, N. A., Aleman, A., & McGuire, P. K. (2010). Schizotypy and brain structure: A voxel‐based morphometry study. Psychological Medicine, 40(9), 1423–1431. 10.1017/S0033291709991875 [DOI] [PubMed] [Google Scholar]
- Modinos, G., Şimşek, F., Azis, M., Bossong, M., Bonoldi, I., Samson, C., … McGuire, P. (2018). Prefrontal GABA levels, hippocampal resting perfusion and the risk of psychosis. Neuropsychopharmacology, 43(13), 2652–2659. 10.1038/s41386-017-0004-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr, C., & Claridge, G. (2015). Schizotypy ‐ Do not worry, it is not all worrisome. Schizophrenia Bulletin, 41(2), S436–S443. 10.1093/schbul/sbu185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahara, S., Matsumoto, M., & van Erp, T. G. M. (2018). Hippocampal subregion abnormalities in schizophrenia: A systematic review of structural and physiological imaging studies. Neuropsychopharmacology Reports., 38, 156–166. 10.1002/npr2.12031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson, B., Fusar‐Poli, P., & Yung, A. R. (2012). Can we detect psychotic‐like experiences in the general population? Current Pharmaceutical Design, 18(4), 376–385. 10.2174/138161212799316136 [DOI] [PubMed] [Google Scholar]
- Nelson, M. T., Seal, M. L., Pantelis, C., & Phillips, L. J. (2013). Evidence of a dimensional relationship between schizotypy and schizophrenia: A systematic review. Neuroscience and Biobehavioral Reviews, 37(3), 317–327. 10.1016/j.neubiorev.2013.01.004 [DOI] [PubMed] [Google Scholar]
- Oldfield, R. C. (1971). The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia, 9(1), 97–113. 10.1016/0028-3932(71)90067-4 [DOI] [PubMed] [Google Scholar]
- Perez, J. M., Berto, S., Gleason, K., Ghose, S., Tan, C., Kim, T. K., … Tamminga, C. A. (2020). Hippocampal subfield transcriptome analysis in schizophrenia psychosis. Molecular Psychiatry. , 10.1038/s41380-020-0696-6 [DOI] [PubMed] [Google Scholar]
- Pfarr, J.‐K., & Nenadić, I. (2020). A multimodal imaging study of brain structural correlates of schizotypy dimensions using the MSS. Psychiatry Research: Neuroimaging, 302, 111104. 10.1016/j.pscychresns.2020.111104 [DOI] [PubMed] [Google Scholar]
- Pitkänen, A., Pikkarainen, M., Nurminen, N., & Ylinen, A. (2000). Reciprocal connections between the amygdala and the hippocampal formation, perirhinal cortex, and postrhinal cortex in rat: A review. Annals of the New York Academy of Sciences, 911, 369–391. 10.1111/j.1749-6632.2000.tb06738.x [DOI] [PubMed] [Google Scholar]
- Polner, B., Faiola, E., Urquijo, M. F., Meyhöfer, I., Steffens, M., Rónai, L., … Ettinger, U. (2019). The network structure of schizotypy in the general population. European Archives of Psychiatry and Clinical Neuroscience, 271, 635–645. 10.1007/s00406-019-01078-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team . (2020). R: A language and environment for statistical computing. Vienna, Austria. Retrieved from: R Foundation for Statistical Computing. https://www.r-project.org/ [Google Scholar]
- Rahm, C., Liberg, B., Reckless, G., Ousdal, O., Melle, I., Andreassen, O. A., & Agartz, I. (2015). Negative symptoms in schizophrenia show association with amygdala volumes and neural activation during affective processing. Acta Neuropsychiatrica, 27(4), 213–220. 10.1017/neu.2015.11 [DOI] [PubMed] [Google Scholar]
- Rössler, W., Hengartner, M. P., Ajdacic‐Gross, V., Haker, H., & Angst, J. (2013). Deconstructing sub‐clinical psychosis into latent‐state and trait variables over a 30‐year time span. Schizophrenia Research, 150(1), 197–204. 10.1016/j.schres.2013.07.042 [DOI] [PubMed] [Google Scholar]
- RStudio Team . (2016). RStudio: Integrated development for R. Boston, MA: RStudio, Inc. Retrieved from. http://www.rstudio.com/ [Google Scholar]
- Sahakyan, L., Meller, T., Evermann, U., Schmitt, S., Pfarr, J.‐K., Sommer, J., … Nenadić, I. (2021). Anterior vs posterior hippocampal subfields in an extended psychosis phenotype of multidimensional Schizotypy in a nonclinical sample. Schizophrenia Bulletin, 47(1), 207–218. 10.1093/schbul/sbaa099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schobel, S. A., Chaudhury, N. H., Khan, U. A., Paniagua, B., Styner, M. A., Asllani, I., … Small, S. A. (2013). Imaging patients with psychosis and a mouse model establishes a spreading pattern of hippocampal dysfunction and implicates glutamate as a driver. Neuron, 78(1), 81–93. 10.1016/j.neuron.2013.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schobel, S. A., Lewandowski, N. M., Corcoran, C. M., Moore, H., Brown, T., Malaspina, D., & Small, S. A. (2009). Differential targeting of the CA1 subfield of the hippocampal formation by schizophrenia and related psychotic disorders. Archives of General Psychiatry, 66(9), 938–946. 10.1001/archgenpsychiatry.2009.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultze‐Lutter, F., Addington, J., Ruhrmann, S., & Klosterkötter, J. (2007). Schizophrenia proneness instrument, Adult Version (SPI‐A). Roma: Giovanni Fioriti Editore. [Google Scholar]
- Seiler, N., Nguyen, T., Yung, A., & O'Donoghue, B. (2020). Terminology and assessment tools of psychosis: A systematic narrative review. Psychiatry and Clinical Neurosciences, 74(4), 226–246. 10.1111/pcn.12966 [DOI] [PubMed] [Google Scholar]
- Siddi, S., Petretto, D. R., & Preti, A. (2017). Neuropsychological correlates of schizotypy: A systematic review and meta‐analysis of cross‐sectional studies. Cognitive Neuropsychiatry, 22(3), 186–212. 10.1080/13546805.2017.1299702 [DOI] [PubMed] [Google Scholar]
- Siever, L. J., & Davis, K. L. (2004). The pathophysiology of schizophrenia disorders: Perspectives from the spectrum. The American Journal of Psychiatry, 161(3), 398–413. 10.1176/appi.ajp.161.3.398 [DOI] [PubMed] [Google Scholar]
- Soliman, A., O'Driscoll, G. A., Pruessner, J., Joober, R., Ditto, B., Streicker, E., … Dagher, A. (2011). Limbic response to psychosocial stress in schizotypy: A functional magnetic resonance imaging study. Schizophrenia Research, 131(1), 184–191. 10.1016/j.schres.2011.05.016 [DOI] [PubMed] [Google Scholar]
- Statucka, M., & Walder, D. J. (2017). Facial affect recognition and social functioning among individuals with varying degrees of schizotypy. Psychiatry Research, 256, 180–187. 10.1016/j.psychres.2017.06.040 [DOI] [PubMed] [Google Scholar]
- Strange, B. A., Witter, M. P., Lein, E. S., & Moser, E. I. (2014). Functional organization of the hippocampal longitudinal axis. Nature Reviews. Neuroscience, 15(10), 655–669. 10.1038/nrn3785 [DOI] [PubMed] [Google Scholar]
- Talati, P., Rane, S., Kose, S., Blackford, J. U., Gore, J., Donahue, M. J., & Heckers, S. (2014). Increased hippocampal CA1 cerebral blood volume in schizophrenia. NeuroImage: Clinical, 5, 359–364. 10.1016/j.nicl.2014.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor, J. H., Calkins, M. E., & Gur, R. E. (2020). Markers of psychosis risk in the general population. Biological Psychiatry, 88(4), 337–348. 10.1016/j.biopsych.2020.02.002 [DOI] [PubMed] [Google Scholar]
- Treadway, M. T., Waskom, M. L., Dillon, D. G., Holmes, A. J., Park, M. T. M., Chakravarty, M. M., … Pizzagalli, D. A. (2015). Archival report illness progression, recent stress, and morphometry of hippocampal sub fi elds and medial prefrontal cortex in major depression. Biological Psychiatry, 77(3), 285–294. 10.1016/j.biopsych.2014.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meer, D., Rokicki, J., Kaufmann, T., Córdova‐Palomera, A., Moberget, T., Alnæs, D., … Westlye, L. T. (2020). Brain scans from 21,297 individuals reveal the genetic architecture of hippocampal subfield volumes. Molecular Psychiatry, 25(11), 3053–3065. 10.1038/s41380-018-0262-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Erp, T. G. M., Hibar, D. P., Rasmussen, J. M., Glahn, D. C., Pearlson, G. D., Andreassen, O. A., … Turner, J. A. (2016). Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA consortium. Molecular Psychiatry, 21(4), 547–553. 10.1038/mp.2015.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas, T., Dean, D. J., Osborne, K. J., Gupta, T., Ristanovic, I., Ozturk, S., … Mittal, V. A. (2018). Hippocampal subregions across the psychosis spectrum. Schizophrenia Bulletin, 44(5), 1091–1099. 10.1093/schbul/sbx160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varghese, D., Scott, J., Welham, J., Bor, W., Najman, J., O'Callaghan, M., … McGrath, J. (2011). Psychotic‐like experiences in major depression and anxiety disorders: A population‐based survey in young adults. Schizophrenia Bulletin, 37(2), 389–393. 10.1093/schbul/sbp083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velakoulis, D., Wood, S. J., Wong, M. T. H., McGorry, P. D., Yung, A., Phillips, L., … Pantelis, C. (2006). Hippocampal and amygdala volumes according to psychosis stage and diagnosis: A magnetic resonance imaging study of chronic schizophrenia, first‐episode psychosis, and ultra‐high‐risk individuals. Archives of General Psychiatry, 63(2), 139–149. 10.1001/archpsyc.63.2.139 [DOI] [PubMed] [Google Scholar]
- Walter, A., Suenderhauf, C., Harrisberger, F., Lenz, C., Smieskova, R., Chung, Y., … Vogel, T. (2016). Hippocampal volume in subjects at clinical high‐risk for psychosis: A systematic review and meta‐analysis. Neuroscience and Biobehavioral Reviews, 71, 680–690. 10.1016/j.neubiorev.2016.10.007 [DOI] [PubMed] [Google Scholar]
- Wang, Y., Li, Z., Liu, W.‐H., Wei, X.‐H., Jiang, X.‐Q., Lui, S. S. Y., … Chan, R. C. K. (2018). Negative Schizotypy and altered functional connectivity during facial emotion processing. Schizophrenia Bulletin, 44(suppl_2), S491–S500. 10.1093/schbul/sby036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y., Yan, C., Yin, D. Z., Fan, M. X., Cheung, E. F. C., Pantelis, C., & Chan, R. C. K. (2015). Neurobiological changes of schizotypy: Evidence from both volume‐based morphometric analysis and resting‐state functional connectivity. Schizophrenia Bulletin, 41(suppl 2), S444–S454. 10.1093/schbul/sbu178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y. M., Cai, X.‐L., Zhang, R.‐T., Zhang, Y.‐J., Zhou, H.‐Y., Wang, Y., … Chan, R. C. K. (2020). Altered brain structural and functional connectivity in schizotypy. Psychological Medicine, 1–10. 10.1017/S0033291720002445 [DOI] [PubMed] [Google Scholar]
- Watson, D. R., Bai, F., Barrett, S. L., Turkington, A., Rushe, T. M., Mulholland, C. C., & Cooper, S. J. (2012). Structural changes in the hippocampus and amygdala at first episode of psychosis. Brain Imaging and Behavior, 6(1), 49–60. 10.1007/s11682-011-9141-4 [DOI] [PubMed] [Google Scholar]
- Wickham, H. (2016). ggplot2: Elegant graphics for data analysis. New York: Springer‐Verlag, New York. [Google Scholar]
- Winterburn, J. L., Pruessner, J. C., Chavez, S., Schira, M. M., Lobaugh, N. J., Voineskos, A. N., & Chakravarty, M. M. (2013). A novel in vivo atlas of human hippocampal subfields using high‐resolution 3T magnetic resonance imaging. NeuroImage, 74, 254–265. 10.1016/j.neuroimage.2013.02.003 [DOI] [PubMed] [Google Scholar]
- Wittchen, H.‐U., Wunderlich, U., Gruschwitz, S., & Zaudig, M. (1997). SKID‐I. Strukturiertes Klinisches Interview für DSM‐IV. Göttingen: Hogrefe. [Google Scholar]
- Witthaus, H., Mendes, U., Brüne, M., Ozgürdal, S., Bohner, G., Gudlowski, Y., … Juckel, G. (2010). Hippocampal subdivision and amygdalar volumes in patients in an at‐risk mental state for schizophrenia. Journal of Psychiatry & Neuroscience, 35(1), 33–40. 10.1503/jpn.090013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood, S. J., Kennedy, D., Phillips, L. J., Seal, M. L., Yücel, M., Nelson, B., … Pantelis, C. (2010). Hippocampal pathology in individuals at ultra‐high risk for psychosis: A multi‐modal magnetic resonance study. NeuroImage, 52(1), 62–68. 10.1016/j.neuroimage.2010.04.012 [DOI] [PubMed] [Google Scholar]
- World Medical Association . (2013). World medical association declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA, 310(20), 2191–2194. 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting information
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


