Abstract
Anorexia nervosa (AN) is a complex psychiatric disorder with poorly understood etiology. Numerous voxel‐based morphometry (VBM) and resting‐state functional imaging studies have provided strong evidence of abnormal brain structure and intrinsic and functional activities in AN, but with inconsistent conclusions. Herein, a whole‐brain meta‐analysis was conducted on VBM (660 patients with AN, and 740 controls) and resting‐state functional imaging (425 patients with AN, and 461 controls) studies that measured differences in the gray matter volume (GMV) and intrinsic functional activity between patients with AN and healthy controls (HCs). Overall, patients with AN displayed decreased GMV in the bilateral median cingulate cortex (extending to the bilateral anterior and posterior cingulate cortex), and left middle occipital gyrus (extending to the left inferior parietal lobe). In resting‐state functional imaging studies, patients with AN displayed decreased resting‐state functional activity in the bilateral anterior cingulate cortex and bilateral median cingulate cortex, and increased resting‐state functional activity in the right parahippocampal gyrus. This multimodal meta‐analysis identified reductions of gray matter and functional activity in the anterior and median cingulate in patients with AN, which contributes to further understanding of the pathophysiology of AN.
Keywords: anorexia nervosa, meta‐analysis, multimodal, resting‐state functional imaging, voxel‐based morphometry
This meta‐analysis demonstrated a significant reduction in the functional activity and gray matter in the cingulate cortex in patients with AN, particularly in the ACC and MCC, which imply that structural changes may underlie functional alterations. These results expand the current understanding of functional and structural brain abnormalities in AN patients, which would provide additional potential targets for therapeutic intervention.
1. INTRODUCTION
Anorexia nervosa (AN) is a psychiatric disorder characterized by severe restriction of energy intake leading to significantly low body weight, intense fear of gaining weight or persistent behavior that interferes with weight gain, and distortion in the perception of one's body weight or shape (Washington, 2013). It is an important cause of physical and psychosocial morbidity in adolescent girls and young adult women, but is much less frequent in men (Fairburn & Harrison, 2003). AN is a disorder with relatively high rates of morbidity and mortality, as the lifetime prevalence of AN (diagnosed by fifth edition of The Diagnostic and Statistical Manual of Mental Disorders [DSM‐5]) among women might be up to 4% (Smink, van Hoeken, & Hoek, 2013; Washington, 2013), and the crude rate of mortality due to all causes for AN was up to 5.9% (Sullivan, 1995). No body system is spared from the adverse sequelae of AN (Westmoreland, Krantz, & Mehler, 2016), and the long‐term prognosis is relatively poor (Steinhausen, 2002). However, effective treatments for AN remain limited (Zhang et al., 2013; Zipfel, Giel, Bulik, Hay, & Schmidt, 2015), and the neural underpinnings of this disease are not well understood.
Multi‐modal neuroimaging has been recently applied to investigate the structural and functional abnormalities of the brain in AN (Cha et al., 2016; Gaudio, Wiemerslage, Brooks, & Schioth, 2016). Several meta‐analyses have indicated brain structural alterations in AN. A meta‐analysis and qualitative review of morphological changes in the brain showed reduced gray matter volume (GMV) in the hippocampal, cingulate, midbrain, cerebellar regions, and the lateral occipital cortex, and increased GM in the dorsolateral prefrontal cortex and medial orbitofrontal cortex in AN (Seitz et al., 2014). Another meta‐analysis of voxel‐based morphometry (VBM) study (228 patients with AN; 240 controls) demonstrated decreased GMVs of the left hypothalamus, left inferior parietal lobe, right lentiform nucleus, and the right caudate, suggesting that excessive restrained eating as found in patients with AN, correlates with structural changes in the brain analogous to clinical symptoms (Titova, Hjorth, Schioth, & Brooks, 2013). Zhang et al. studied 389 patients with AN and 410 controls, and identified decreased GMV in the bilateral median and posterior cingulate cortices extending to the bilateral precuneus, and the supplementary motor area in the AN patients (Zhang et al., 2018). Given that some VBM studies were not included in the previous meta‐analyses and several VBM studies have been conducted in recent years (Lazaro et al., 2013; Mainz, Schulte‐Ruther, Fink, Herpertz‐Dahlmann, & Konrad, 2012; Muhlau et al., 2007; Nickel et al., 2017; Oliva et al., 2020; Seitz et al., 2015; Wagner et al., 2006), an updated meta‐analysis is needed to extend and/or modify previous structural findings in AN.
Functional magnetic resonance imaging (fMRI) has been used to identify abnormalities of regional brain function evoked by specific tasks or in the resting state. In contrast to task‐based fMRI studies, resting‐state functional magnetic resonance imaging (rs‐fMRI) removes some performance‐related confounders, and therefore could measure task‐independent changes in brain function in humans (Guerra‐Carrillo, Mackey, & Bunge, 2014). Meta‐analyses of resting‐state functional imaging studies have been conducted in psychiatric disorders such as major depressive disorder, attention‐deficit/hyperactivity disorder, and autism spectrum disorder, obsessive–compulsive disorder, internet gaming disorder (Gray, Muller, Eickhoff, & Fox, 2020; Lukito et al., 2020; Norman et al., 2016; Yao et al., 2017), and so forth. However, there is no meta‐analysis of resting‐state functional imaging studies on AN. Resting‐state imaging studies including regional homogeneity (ReHo) (Seidel et al., 2019; Yue, 2013), (fractional) amplitude of low frequency fluctuations (fALFF/ALFF) (Cicerale et al., 2013; Lai et al., 2020; Seidel et al., 2019), together with brain blood flow studies using arterial spin labeling (ASL) (Sheng, Lu, Liu, Thomas, & McAdams, 2015; Wierenga et al., 2017), positron emission tomography (PET) (Gordon et al., 2001; Pasanisi et al., 2013; Zhang et al., 2013) and single‐photon emission computed tomography (SPECT) (Jinjue et al., 2007; Kojima et al., 2005; Naruo et al., 2001; Takano et al., 2001; Yonezawa, Otagaki, Miyake, Okamoto, & Yamawaki, 2008) have contributed to understanding the regional brain function in AN. However, these studies have shown relatively inconsistent results for AN, such as abnormal neural activity in the frontal lobe, hippocampus/parahippocampus (Seidel et al., 2019; Takano et al., 2001; Yue, 2013), parietal lobe (Kojima et al., 2005; Zhang et al., 2013), cingulate cortex (Cicerale et al., 2013; Kojima et al., 2005; Naruo et al., 2001; Wierenga et al., 2017), subcallosal gyrus (Yonezawa et al., 2008; Zhang et al., 2013), and so forth. The reasons for the inconsistent or conflicting findings, may include small sample sizes, age ranges, clinical symptoms, medical comorbidity, duration of illness, and the technical methods of data acquisition and analysis. Therefore, it is important to conduct a meta‐analysis to identify convergent abnormal regional function of AN in the resting‐state functional imaging studies.
Therefore, a multimodal coordinate‐based meta‐analysis of VBM and resting‐state functional studies was conducted to investigate the structural and functional alterations in the brain in AN, and to explore whether there is a structural basis for the functional impairment in specific brain regions of AN. Based on the previous empirical studies investigating VBM and resting‐state functional activity in AN, we speculated that structural and functional changes in AN would be primarily located in the cingulate cortex, and parietal frontal lobe. Furthermore, the present study used Seed‐based d Mapping with Permutation of Subject Images (SDM‐PSI), a software with stricter correction than Anisotropic effect‐size version of Seed‐based d Mapping (AES‐SDM), to explore localized changes of neural function and GMV between AN patients and controls. SDM‐PSI is a novel method with a less biased estimation of the population effect size and the use of random‐effects models and threshold‐free cluster enhancement (TFCE) statistics, which identifies brain regions consistently altered by testing for spatial convergence across findings from published neuroimaging studies (Samartsidis, Montagna, Nichols, & Johnson, 2017; Tench, Tanasescu, Constantinescu, Cottam, & Auer, 2020). The present study offered a more precise understanding of the pathophysiology of AN, and provided potential targets for neuromodulation and other interventions.
2. METHODS AND MATERIALS
2.1. Data source
A comprehensive and systematic literature search of the following databases: Web of Science, Embase, PubMed, Sinomed, China National Knowledge Infrastructure, and WanFang databases for VBM and resting‐state functional imaging studies published between January 1990 and December 2020, was conducted. We used the following MESH terms and keywords for the search: (1) (“Voxel‐based morphometry” OR “VBM” OR “morphometry”) AND (“eating disorders” OR “anorexia nervosa” OR “anorexia”). (2) (“neuroimag*” OR “fMRI” OR “cerebral blood flow” OR “CBF” OR “positron emission tomography” OR “PET” OR “single photon emission computed tomography” OR “SPECT” OR “arterial spin labeling” OR “ASL” “ALFF” OR “ReHo” OR “resting‐state”) AND (“eating disorders” OR “anorexia nervosa” OR “anorexia”). Besides synonyms of these terms were used, we also manually conducted an additional search within the references lists of the included articles and prior AN‐related meta‐analyses studies for additional qualified studies.
2.2. Study selection
Eligible VBM or resting‐state functional imaging studies fulfilled the following inclusion criteria: (a) they compared GMV or resting‐state functional imaging between AN and HCs at the whole brain level; (b) the study was an original article limited to study of human subjects (not as an abstract or a review) in a Chinese or English language journal of peer‐reviewed; (c) articles reporting peak coordinates of significant clusters in standard MNI or Talairach space; (d) When the original manuscripts' details were not reported, it could be available by making a reasonable request to the corresponding author.
Studies were excluded based on the following criteria: (a) analysis focus on specific regions of interest (ROI); (b) sample size was less than three either in the AN group or the HC group; (c) studies reported significant results without listing the three‐dimensional coordinates; (d) the authors did not provide neuroanatomical coordinates after contacting with them by telephone or email; (e) Data were used repeatedly among much paper (Figure 1).
FIGURE 1.
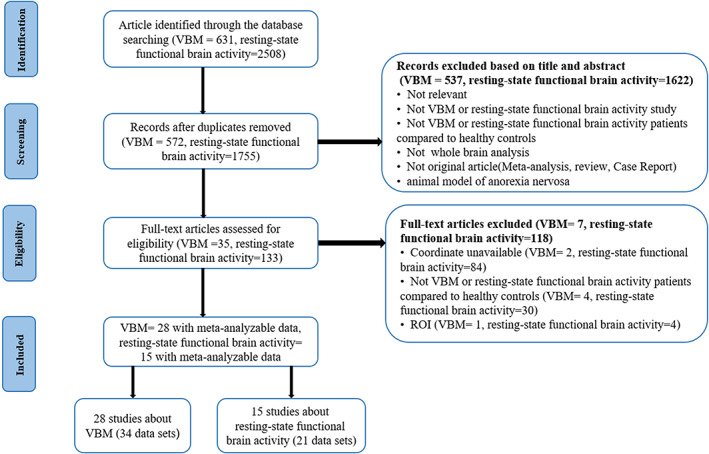
Flow chart of meta‐analysis of voxel‐based morphometry (VBM) and resting‐state functional imaging studies of patients with anorexia nervosa (AN)
2.3. Quality assessment and data extraction
We used a 10‐point checklist to assess individual study quality for meta‐analyses, which focused on the clinical and demographic aspects of the study samples and the imaging methodology (Table S9). This checklist was based on previous meta‐analytic studies (Chen et al., 2015; Gong et al., 2020; Gong et al., 2020). Three investigators (J. Y. G, S. J. Q, and T. S.) searched, evaluated, and selected the retrieved studies, extracted, and cross‐checked data independently following guidelines for neuroimaging meta‐analyses promoted by Müller and colleagues (Muller et al., 2018). For each study, the following data were extracted: the first author's last name, year of publication, demographic information (age and gender), sample size, illness variables (Body Mass Index [BMI], AN type and AN stage (acute vs. recovered), age at onset, illness duration), imaging parameters. The peak coordinates showing differences between subjects and controls were extracted by the SDM method (Radua et al., 2014). Inconsistent information was resolved by a fourth investigator (Y. W.) after comparing the results.
2.4. Data analysis
2.4.1. Main meta‐analysis
VBM
The whole‐brain VBM meta‐analysis of structural imaging studies was used to determine the structural substrates of altered cerebral volume in AN, which was conducted using SDM‐PSI software package (version 6.21 for Windows) in a standard process (www.sdmproject.com). The SDM‐PSI approach uses effect sizes to combine reported peak coordinates that are extracted from databases with statistical parametric maps, and it imputes the brain maps of the effect size of GMV differences between patients and HCs for each study and then conducts a standard random‐effects meta‐analysis (Dugre et al., 2020; Radua et al., 2012; Sheng et al., 2020). The SDM‐PSI uses standard statistical procedures to control the family wise error rate (FWE) (Albajes‐Eizagirre, Solanes, Vieta, & Radua, 2019). We performed the analysis as described in the SDM‐PSI tutorial and related publications (Lim, Radua, & Rubia, 2014; Radua et al., 2014) and used MRIcron software (www.mricro.com/mricron/) to visualize SDM‐PSI maps.
In order to identify abnormal brain intrinsic and functional activity in AN, the SDM‐PSI approach is briefly described here. The coordinates and height of the peaks (its t‐value or z‐value, but a p‐value is also useful) with poorer and greater hemodynamic activity for each study compared with HC were extracted separately. A standard MNI map of the GMV differences was then separately recreated for each data set using an anisotropic Gaussian kernel. The mean map was finally generated by voxel‐wise calculation of the random‐effects mean of the data set maps, weighted by the sample size, intra‐data set variability, and between‐data set heterogeneity. To optimally balance false positives and negatives, we used the default SDM kernel size and thresholds (full anisotropy = 1, isotropic full width at half maximum [FWHM] = 20 mm, and voxel = 2 mm). FWE correction for multiple comparisons using common permutation tests; and use of TFCE in statistical thresholding (p < .05 and cluster extent = 10 voxels) (Albajes‐Eizagirre et al., 2019). It should be noted that this FWHM kernel is intended to assign indicators of proximity to reported coordinates but not to smooth any different images. We conducted the meta‐analysis in total patients and the subgroup analysis of AN with no psychiatric comorbidities within VBM studies.
Resting‐state functional imaging
Consistent with the meta‐analysis of VBM studies, a similar procedure was performed to select studies related to functional brain activity analysis. We extracted peak coordinates and effect size (e.g., t‐values) of differences in functional brain activity between patients with AN and HCs from each data set. Functional brain activity meta‐analysis was also performed with the above‐mentioned SDM analysis algorithm.
2.4.2. Subgroup meta‐analysis
VBM
Subgroup meta‐analysis was conducted when the number of data sets was sufficient (n ≥ 10). Analysis would be performed in five subgroups, including (a) adult, (b) female, (c) acute patients with AN, and (d) AN with no psychiatric comorbidities. We did not perform the subgroup analysis of untreated patients because of the small amount of data sets (n < 10).
Resting‐state functional imaging
Subgroup meta‐analysis was conducted when the number of data sets was sufficient (n ≥ 10). Subgroup would be performed in AN with no psychiatric comorbidities. We did not perform the following subgroup analysis because of the small number of data sets (n < 10): (a) adult, (b) acute, and (c) untreated patients.
2.4.3. Jackknife sensitivity analysis
To test the repeatability of the results, we performed a jackknife sensitivity analysis, repeating the same analysis K times iteratively (K represents the number of data sets included), leaving out one data set each time (Radua et al., 2014; Radua & Mataix‐Cols, 2009). If the previous major brain regions remain significant in all or most of the combinations of studies, then the findings are considered to be highly repeatable.
2.4.4. Analysis of heterogeneity and publication bias
A heterogeneity analysis was conducted using a random effects model with I 2 statistics to explore unexplained between‐study variability for every significant cluster with a peak MNI coordinate reported in the main meta‐analysis results. Heterogeneous brain regions were obtained using the default SDM kernel size and thresholds (FWHM = 20 mm, p = .005, uncorrected for FDR, peak height Z = 1, cluster extent = 10 voxels) (Lim et al., 2014), with I 2 < 50% indicating low heterogeneity.
To inspect the publication bias, Egger's tests were performed for quantitative examination and funnel plots were created for visual inspection by extracting the values from significant peaks (Albajes‐Eizagirre et al., 2019). p < .05 and an asymmetric plot were considered statistically significant.
2.4.5. Meta‐regression analyses
Meta‐regression analyses were carried out to explore the associations between the analytic results and clinical variables (e.g., illness duration, age, and BMI). The results were weighted by the square root of the sample size. To minimize the reporting of spurious relationship, we selected a more conservative threshold of p = .05, TFCE correction, cluster extent = 10 as suggested by the authors of the SDM‐PSI method, requiring abnormalities to be detected both in the slope and in one of the extremes of the regressor, and discarding findings in regions other than those detected in the main analyses.
2.5. Multimodal conjunction of abnormal GMV and resting‐state functional activity in AN
To localize brain regions with both GMV and resting‐state functional activity abnormalities in AN, between‐group contrasts of multimodal brain imaging were summarized in a meta‐analytic map (Radua, Romeo, Mataix‐Cols, & Fusar‐Poli, 2013). We identified multimodally affected brain regions by computing the overlapping p values of the GMV and resting‐state functional activity. The method we implemented in SDM considered the underlying noise of the meta‐analytic estimation. The voxel‐level threshold was p < .0025 because of four tails.
3. RESULTS
3.1. General overview of studies
We identified 28 included VBM studies with 34 data sets, recruited a total of 660 AN patients (99.3% female; mean age = 22.22 years; illness duration = 37.81 months; mean BMI = 16.61) and 740 HCs (99.4% female; mean age = 22.92 years; mean BMI = 21.42); and 15 resting‐state functional imaging studies reporting 21 data sets comprised 425 AN patients (all are females; mean age = 21.23 years; mean illness duration = 23.74 months; mean BMI = 15.95) and 461 HCs (all are females; mean age = 22.26 years; mean BMI = 20.91). AN and HC groups were mainly composed of female.
We found no significant differences between participants with AN and their HC counterparts with respect to age in VBM studies (t = −0.508; p = .613) and resting‐state functional imaging studies (t = −0.951; p = .347). In the VBM studies, the sex distribution of AN patients and HC was matched (χ2 = 0.047; p = 0.828). Tables 1 and 2 are briefly summarized the demographic data, clinical and imaging characteristics of the studies.
TABLE 1.
Demographic, clinical, and imaging characteristics of the included studies of VBM
| Study | Demographic characteristics | Clinical characteristics (AN patients) | Imaging characteristics | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Participants (female), n | Mean age ± SD, year | Age group | AN state | Comorbid | Illness duration (months) | Age at onset (years) | BMI | Scanner | Software | FWHM (mm) | Threshold | Quality scorea | ||||
| AN | HC | AN | HC | AN | HC | |||||||||||
| (Wagner et al., 2006)b | 14(14) | 31(31) | 23.7±5.3 | 26.8±7.3 | Adult | Recovered | NO | 28.7±20.4 | 18.2±4.5 | 21.2±2.0 | 21.9±2.0 | 1.5T MRI | SPM2 | 12 | pFDR <.05 | 10 |
| 16(16) | 27.4±7.2 | Adult | Recovered | NO | 39.5±52.7 | 15.7±2.7 | 21.2±1.5 | NA | ||||||||
| (Muhlau et al., 2007) | 22(22) | 37(37) | 27.2 | 27.4 | Adult | Recovered | NO | 116 | 17.6 | 19.8 | 21.1 | 1.5 T MRI | SPM2 | 14 | puncorrected <.01 | 10 |
| (Castro‐Fornieles et al., 2009) | 12(11) | 9(8) | 14.5±1.5 | 14.6±3.2 | Adolescent | NA | NO | NA | NA | 14.8±2.0 | NA | 1.5 T MRI | SPM5, FSL | NA | pFWE<.05 | 9 |
| (Suchan et al., 2010) | 13(13) | 14(14) | 26.8±8.4 | 29.5±8.2 | Adult | Chronic | NO | 66.0±60.0 | NA | 16.0±1.3 | 22.0±2.1 | 1.5 T MRI | SPM5 | 12 | pFWE <.05 | 9.5 |
| (Boghi et al., 2011)b | 21(21) | 27(27) | 29.0±10.0 | 30.8±8.7 | Adult | Recovered/acute | NO | 135.6±145.2 | NA | 15.5±1.8 | 21.9±1.5 | 1.0 T MRI | SPM2 | 12 | PFDR <.05 | 9.5 |
| 10(10) | 13(13) | 21.4±2.5 | 22.8±1.5 | Adult | Acute | NO | 22.8±15.6 | NA | 15.0±1.2 | 21.7±1.7 | NA | NA | NA | |||
| 11(11) | 14(14) | 35.9±9.25 | 38.2±5.1 | Adult | Chronic | NO | 237.6±133.2 | NA | 16.0±2.1 | 22.1±1.4 | 1.0 T MRI | NA | puncorrected <.001 | |||
| (Brooks et al., 2011) | 14(14) | 21(21) | 26.0±1.9 | 26±2.1 | Adult | NA | YES | 110.4±22.8 | NA | 15.6±0.4 | 21.4±0.5 | 1.5 T MRI | SPM5 | 12 | NA | 9.5 |
| (Gaudio et al., 2011) | 16(16) | 16(16) | 15.2±1.7 | 15.1±1.5 | Adolescent | Acute | NO | 5.3±3.2 | 14.7±1.7 | 14.2±1.4 | 20.2±1.6 | 1.5 T MRI | SPM2 | NA | puncorrected <.001 | 10 |
| (Joos et al., 2011)b | 5(5) | 18(18) | 29.6±5.1 | 26.9±5.7 | Adult | Recovered | NO | 86.4±72 | NA | 19.9±1.5 | 21.2±2.0 | 3.0 T MRI | SPM8 | 12 | puncorrected <.001 | 9 |
| 12(12) | 25.0±4.8 | Adult | NO | NA | NA | 16.0±1.2 | 21.2±2.0 | |||||||||
| (Friederich et al., 2012)b | 12(12) | 14(14) | 24.3±6.2 | 25.6±3.7 | Adult | Acute | NO | 75.6±52.8 | NA | 15.9±1.6 | 21.1±1.5 | 3.0 T MRI | SPM5 | 8 | pcorrected <.05, p uncorrected <.05 | 9 |
| 13(13) | 25.0±4.8 | Adult | Recovered | NO | 68.4±43.2 | NA | 19.5±1.4 | 21.1±1.5 | ||||||||
| (Mainz et al., 2012) | 19(19) | 19(19) | 15.7±1.5 | 15.6±1.9 | Adolescent | Recovered | YES | NA | NA | 15.3±1.5 | 21.8±2.7 | 3.0 T MRI | SPM5 | NA | FWE corrected | 9.5 |
| (Amianto et al., 2013) | 17(17) | 14(14) | 20.0±4.0 | 24.0±3.0 | Adolescent | Acute | NO | 13.0±8.0 | NA | 16.0±1.0 | 21±2 | 1.5 T MRI | FSL | 7 | pTFCE <.005 | 9.5 |
| (Cicerale et al., 2013) | 10(10) | 8(8) | 22.0±4.0 | 24.0±2.0 | Adult | NA | NA | 16.0±9.0 | 16±9 | 15.9±1.0 | 21.1±1.7 | 1.5 T MRI | FSL | 7 | pTFCE<.005 | 9 |
| (Frank, Shott, Hagman, & Mittal, 2013) | 19(19) | 24(24) | 23.1±5.8 | 27.4±6.3 | Adult | Acute | NO | NA | NA | 16.0±1.1 | 21.6±1.3 | 3.0 T MRI | SPM8 | 8 | pFWE <.05 | 9.5 |
| (Lazaro et al., 2013) | 35(NA) | 17(NA) | 16.3±1.3 | 16.7±1.5 | Adolescent | Recovered | NO | 7.4±5.2 | 14.02±1.6 | 19.3±1.1 | NA | 1.5 T MRI | SPM8 | 12 | p<.05 | 9 |
| (Fonville, Giampietro, Williams, Simmons, & Tchanturia, 2014) | 31(NA) | 31(NA) | 23.0 | 25.0 | Adult | NA | YES | 84.0±120.0 | 16±4.75 | 15.8±1.4 | 21.8±1.8 | 1.5 T MRI | FSL | 7 | pFWE <.05 | 9 |
| (Bar, de la Cruz, Berger, Schultz, & Wagner, 2015) | 26(23) | 26(23) | 23.0±5.0 | 24.0±1.9 | NA | Acute | NO | 22.4±14.8 | NA | 17.0±1.5 | 21.7±1.5 | 1.5 T MRI | SPM8 | 10 | puncorrected<.001 | 9.5 |
| (Bomba et al., 2015) | 11(11) | 8(8) | 13.6±2.8 | 13.3±2.4 | Adolescent | NA | NO | 14.5±10.9 | NA | 12.8±0.8 | 19.9±1.5 | 1.5 T MRI | SPM5 | 8 | pFWE <.05 | 9 |
| (D'Agata et al., 2015) | 21(21) | 17(17) | 21.0±5.0 | 23.0±4.0 | Adolescent | Acute | NO | NA | NA | 16.1±0.9 | 21.5±2.3 | 1.5 T MRI | FSL | NA | puncorrected<.005 | 9.5 |
| (Fujisawa et al., 2015) | 20(20) | 14(14) | 14.2±1.8 | 14.9±1.6 | Adolescent | NA | NO | 23.6±17.0 | 12.6±1.8 | 14.4±2.1 | NA | 3.0 T MRI | SPM8 | 12 | L_IFG: p FWE <.05 R_IFG: p uncorrected <.05 | 9.5 |
| (Seitz et al., 2015) | 56(56) | 50(50) | 15.5±1.7 | 15.8±1.7 | Adolescent | Acute | NO | 11.4±8.3 | 14.5±1.6 | 15.1±1.4 | 21.4±3.3 | 3.0 T MRI | Free surfer | NA | pBonferroni<.01 | 9.5 |
| (van Opstal et al., 2015) | 10(10) | 11(11) | 22.1±3.3 | 20.8±0.5 | Adult | NA | NO | 42.5±27.6 | NA | 15.6±1.0 | 20.3±1.5 | 3.0 T MRI | FSL | NA | NA | 9 |
| (Björnsdotter et al., 2017) | 25(25) | 25(25) | 20.3±2.2 | 21.3±2.1 | Adolescent | NA | NO | 49.7±42.5 | NA | 16.3±0.9 | 21.1±2.3 | 3.0 T MRI | SPM8 | 8 | pcorrected <.05 | 9.5 |
| (Kohmura et al., 2017)b | 23(23) | 29(29) | 28.5±6.7 | 28.2±7.0 | Adult | NA | NO | 126±74.4 | 18.0±3.2 | 13.2±1.5 | 21.5±3.3 | 3.0 T MRI | SPM8 | 8 | pFWE <.05 | 9.5 |
| 23(23) | NA | NA | Adult | NA | NO | |||||||||||
| (Martin Monzon et al., 2017) | 26(26) | 20(20) | 16.5±0.3 | 17.3±0.4 | Adolescent | NA | NO | NA | NA | 16.7±0.2 | 22.6±0.9 | 3.0 T MRI | SPM12 | 6 | pFDR <.05 | 9.5 |
| (Nickel et al., 2017) | 34(34) | 41(41) | 23.8±4.3 | 23.6±3.8 | Adult | Acute | NO | 79.2±44.4 | NA | 16.1±1.4 | 22.3±2.4 | 3.0 T MRI | SPM12 | 8 | pFWE <.05 | 9.5 |
| (Olivo et al., 2018) | 22(22) | 38(38) | 14.9±1.6 | 14.7±1.3 | Adolescent | Acute | NO | 7.9±4.8 | NA | 19.3±2.0 | 20.7±2.3 | 3.0 T MRI | SPM12 | 8 | pFWE <.001 | 9.5 |
| (Phillipou et al., 2018) | 26(26) | 27(27) | 22.8±6.7 | 22.5±3.2 | Adolescent | Acute | YES | 6.4±7.4 | 16.0±3.4 | 16.6±1.2 | 22.6±3.5 | 3.0 T MRI | SPM12 | 8 | pFWE<.05 | 9.5 |
| (Oliva et al., 2020) | 15(15) | 15(15) | 25.9±6.2 | 25.2±1.0 | Adult | Recovered | NO | 38.4±38.0 | 16.5±2.3 | 20.1±2.0 | 21.3±2.5 | 1.5 T MRI | SPM12 | 8 | NA | 9.5 |
Abbreviations: AN, anorexia nervosa patients; BMI, body mass index; c, the Threshold‐Free Cluster Enhancement; FDR, false discovery rate; FSL, FMRIB's Software Library, the University of Oxford; FWE, family wise error; FWHM, full width at half maximum; HC, healthy control; IFG, inferior frontal gyrus; L_IFG, left IFG; NA, not available; R_IFG, right IFG; SPM, statistical parametric mapping; VBM, voxel‐based morphometry.
Total score out of 10.
At least two data sets are included.
TABLE 2.
Demographic, clinical, and imaging characteristics of the included studies of resting‐state functional activity
| Study | Demographic characteristics | Clinical characteristics (AN patients) | Imaging characteristics | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Participants (female), n | Mean age±SD, year | Age group | AN state | Comorbid | Illness duration (months) | Age at onset (years) | BMI | Imaging techniquea | Scanner | Software | FWHM (mm) | Threshold | Quality scoreb | ||||
| AN | HC | AN | HC | AN | HC | ||||||||||||
| (Gordon et al., 2001) | 8(8) | 8(8) | 20.1±2.6 | 23.7±2.6 | NA | NA | NO | 33.2±32.4 | NA | 18.0±3.5 | 21.7±1.8 | PET | PET | SPM96 | 6 | NA | 8.5 |
| (Naruo et al., 2001) | 7(7) | 7(7) | NA | NA | NA | NA | NO | NA | NA | 12.8±2.1 | 20.0±1.4 | SPECT | SPECT | SPM96 | 12 | NA | 8.5 |
| (Takano et al., 2001) | 14(14) | 8(8) | 21.2±6.6 | 28.3±3.3 | NA | NA | NO | 16.8±9.6 | NA | 14.0±2.2 | 19.7±0.8 | SPECT | SPECT | SPM96 | 10 | puncorrected <.05 | 9 |
| (Kojima et al., 2005)c | 12(12) | 11(11) | 18.6±3.5 | 21.8±2.1 | NA | Before WG | NO | NA | NA | 12.5±1.7 | 20.1±0.8 | SPECT | SPECT | SPM99 | 12 | puncorrected <.05 | 9.5 |
| 12(12) | 11(11) | 18.8±3.5 | 21.8±2.1 | NA | After WG | NO | NA | NA | 15.6±0.7 | 20.1±0.8 | NA | NA | |||||
| (Jinjue et al., 2007) | 3(3) | 25(25) | 20.3 | 20.4±1.8 | Adult | NA | NO | NA | NA | 16.3 | 21.3±1.2 | SPECT | SPECT | SPM2 | NA | puncorrected <.05 | 9 |
| (Yonezawa et al., 2008)c | 26(26) | 10(10) | 22.2±3.9 | 20.6±1.7 | NA | Acute and chronic | NO | 32.0±22.4 | 19.7±4.3 | 13.5±1.3 | 19.7 ±1.8 | SPECT | SPECT | NA | 8 | NA | 9.5 |
| 13(13) | 10(10) | 22.2±3.6 | 20.6±1.7 | NA | Acute | NO | 30.3±23.7 | 19.6±4.6 | 13.8±1.5 | 19.7 ±1.8 | NA | NA | NA | ||||
| 13(13) | 10(10) | 22.3±4.4 | 20.6±1.7 | NA | Chronic | NO | 33.8±21.9 | 19.7±4.2 | 13.4±1.2 | 19.7 ±1.8 | NA | NA | NA | ||||
| (Cicerale et al., 2013) | 10(10) | 8(8) | 22.0±4.0 | 24.0±2.0 | NA | NA | NO | 16.0±9.0 | 20.0±4.0 | 15.9±1.0 | 21.1 ±1.7 | ALFF | 1.5T MRI | FSL | NA | pTFCE <.005 | 9.0 |
| (Pasanisi et al., 2013)c | 7(7) | 20(20) | 23.4±4.5 | 25.6±3.9 | Adult | Acute | NO | NA | NA | 15.5±0.8 | 22.2±1.5 | PET | PET | NA | NA | NA | 8.5 |
| 3(3) | 20(20) | 21.3±1.5 | 25.6±3.9 | NA | Recovered | NO | NA | NA | 18.8±1.1 | 22.2±1.5 | NA | NA | NA | ||||
| (Yue, 2013) | 8(8) | 8(8) | 20.2±4.8 | 19.7±3.5 | 14‐32 | NA | NO | NA | NA | 16.1±2.8 | 20.6±2.4 | ReHo | 3.0T MRI | SPM8 | 4 | puncorrected <.005 | 8.5 |
| (Zhang et al., 2013) | 6(6) | 12(12) | 17.0±0.8 | 24.0±3.3 | NA | NA | NO | 23.8±10.5 | NA | 12.2±1.0 | NA | PET | PET | SPM2 | 10 | puncorrected <.001 | 9.0 |
| (Sheng et al., 2015)c | 23(23) | 25(25) | 25.8±8.3 | 25.5±6.6 | Adult | Acute | YES | NA | NA | 18.2±1.6 | 22.6±2.7 | ASL | 3.0T MRI | SPM5 | 8 | pFWE<<.001 | 9.5 |
| 19(19) | 25(25) | 30.4±8.4 | 25.5±6.6 | Adult | Recovered | YES | NA | NA | 23.0±3.0 | 22.6±2.7 | |||||||
| (Wierenga et al., 2017) | 21(21) | 16(16) | 27.2±1.7 | 23.9±1.5 | Adult | NA | NO | NA | NA | 21.8±0.3 | 22.4±0.4 | ASL | 3.0T MRI | FSL | 4 | pcorrected <.05 | 9.5 |
| (Seidel et al., 2019)c | 74(74) | 74(74) | 16.0±2.9 | 16.2±2.9 | 12.1‐28.5 | Acute and nonchronic | NO | 17.5±22.7 | NA | 14.6±1.3 | 20.6±2.6 | fALFF | 3.0T MRI | SPM8 | 6 | pFDR<.05 | 9.5 |
| 74(74) | 74(74) | 16.0±2.9 | 16.2±2.9 | NA | NO | 14.5±1.3 | 20.6±2.6 | ReHo | 3.0T MRI | NA | NA | NA | |||||
| (Lai et al., 2020) | 7(7) | 14(14) | 17.5±2.4 | 19.1±3.1 | NA | Acute | NO | 10.3±6.7 | NA | 13.7±1.4 | 19.6±1.8 | fALFF | 3.0T MRI | DPARSF | 4 | pGFR <.05 | 9 |
| (Seidel et al., 2019) | 65(65) | 65(65) | 22.06±3.3 | 22.05±3.3 | NA | Recovered | NO | NA | NA | 20.7±1.8 | 21.61±2 | fALFF | 3.0T MRI | SPM8 | 6 | pFWE<<.05 | 9.5 |
Abbreviations: ALFF, amplitude of low‐frequency fluctuation; AN, anorexia nervosa patients; ASL, arterial Spin Labeling; BMI, body mass index; DPARSF, data processing assistant for resting‐State fMRI software; fALFF, the fractional ALFF; FDR, false discovery rate; fMRI, functional magnetic resonance imaging; FSL, FMRIB's Software Library, the University of Oxford; FWE, family wise error; FWHM, full width at half maximum; HC, healthy control; NA, not available; ReHo, regional homogeneity; SD, standard deviation; SPM, statistical parametric mapping; TFCE, the threshold‐free cluster enhancement; WG, weight gain.
Include ALFF, ALFF, ReHo, PET, SPECT, and ASL.
Total score out of 10.
Represents at least two data sets are included.
3.2. Meta‐analysis
3.2.1. Pooled meta‐analysis
VBM
Structural differences in patients with AN relative to HCs are shown in Table 3 and Figure 2a. AN patients decreased GMV in the bilateral MCC (extending to the bilateral precuneus, posterior cingulate cortex [PCC], ACC, superior frontal gyrus), and left middle occipital gyrus (extending to left inferior parietal lobe). No significantly increased GMV was observed in patients with AN.
TABLE 3.
Meta‐analyses results regarding VBM and resting‐state functional brain activity difference between AN and HCs
| Local maximum | Cluster | |||||||
|---|---|---|---|---|---|---|---|---|
| Region | Peak MNI coordinate (x, y, z) | SDM‐Z value | p value | No. of voxels | Breakdown (no. of voxels) | Egger's test (p value) | Jackknife sensitivity | Heterogeneity |
| VBM | ||||||||
| AN vs. HCs (AN < HCs) bilateral median cingulate/paracingulate gyri, BA 23 | 0,−30,40 | −4.727 | .001 | 5497 |
Left precuneus, BA 5 (860) Right precuneus, BA 7 (503) Left anterior cingulate/paracingulate gyri, BA 32 (434) |
.906 | 35/35 | No (I 2<50%) |
| Right anterior cingulate/paracingulate gyri, BA 32 (225) | ||||||||
| Left median cingulate/paracingulate gyri, BA 24 (1141) | ||||||||
| Right median cingulate/paracingulate gyri, BA 4 (1046) | ||||||||
| Left posterior cingulate gyrus, BA 26 (272) | ||||||||
| Right posterior cingulate gyrus, BA 26 (72) | ||||||||
| Left superior frontal gyrus, medial (168) | ||||||||
| Right superior frontal gyrus, medial (17) | ||||||||
| Left middle occipital gyrus, BA 7 | −32,−72,38 | −3.425 | .027 | 124 | Left inferior parietal gyri, BA 7 (36) | .982 | 35/35 | No (I 2<50%) |
| Left middle occipital gyrus, BA 7 (49) | ||||||||
| Left superior occipital gyrus, BA 7 (15) | ||||||||
| Left superior parietal gyrus, BA 7 (9) | ||||||||
| Resting‐state functional activity | ||||||||
| AN vs. HCs (AN> HCs) right parahippocampal gyrus, BA 28 | 24,2,‐32 | 1.905 | <.001 | 465 |
Right temporal pole, middle temporal gyrus, BA 36 (78) Right parahippocampal gyrus, BA 28 (106) Right amygdala, BA 36 (94) |
.979 | 22/22 | No (I 2<50%) |
| AN vs. HCs (AN < HCs) bilateral anterior cingulate/paracingulate gyri, BA 24 | 0,36,20 | −1.705 | <.001 | 1317 |
Left anterior cingulate/paracingulate gyri, BA 32 (595) Right anterior cingulate/paracingulate gyri, BA 32 (235) Left median cingulate/paracingulate gyri, BA 24 (150) Right median cingulate/paracingulate gyri, BA 24 (99) |
.084 | 19/22 | No (I 2<50%) |
Abbreviations: AN, anorexia nervosa; BA, Brodmann area; HCs, healthy controls; JK, Jackknife sensitivity analysis; MNI, Montreal Neurological Institute; SDM, the seed‐based d mapping; VBM, voxel‐based morphometry.
FIGURE 2.
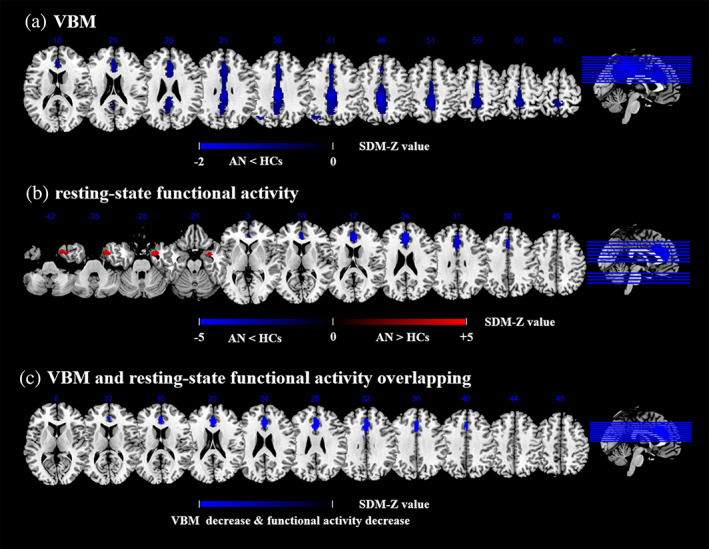
Meta‐analyses results regarding (a) GMV difference between AN and HCs, (b) Resting‐state functional activity difference between AN and HCs, (c) Conjunction of GMV differences and resting‐state functional activity differences. Areas with decreased GMV value or resting‐state functional activity value are displayed in blue, and areas with increased GMV value or resting‐state functional activity value are displayed in red. The color bar indicates the maximum and minimum SDM‐Z values. AN, anorexia nervosa; HCs, healthy controls; SDM, seed‐based d mapping; VBM, voxel‐based morphometry; GMV, gray matter volume
Resting‐state functional imaging
Functional differences in patients with AN relative to HCs are shown in Table 3 and Figure 2b. AN patients displayed decreased resting‐state functional activity in the bilateral anterior cingulate cortex (ACC) (extending to the bilateral superior frontal gyrus) and bilateral median cingulate cortex (MCC), and increased activity in the right parahippocampal gyrus (extending to the right temporal pole, middle temporal gyrus, and amygdala).
3.2.2. Subgroup meta‐analysis
VBM
The adult AN subgroup (included 20 data sets) demonstrated decreased GMV in the bilateral ACC (extending to the bilateral MCC, superior frontal gyrus, and superior frontal gyrus). The female AN patient subgroup (included 29 data sets) showed the same clusters of GMV variation as the adult AN patient subgroup. The acute AN patient subgroup (included 11 data sets) displayed decreased GMV in the bilateral MCC. The subgroup of AN with no psychiatric comorbidities (included 30 data sets) showed decreased GMV in the bilateral MCC (extending to the bilateral ACC, PCC, and the supplementary motor area). These results are in agreement with the pooled meta‐analysis, indicating that the main effects are stable and reliable (Tables S1–S4). However, there were too few resting‐state functional imaging studies to perform the following subgroup: functional brain activity of adult and untreated AN patients.
Resting‐state functional imaging
The subgroup of AN with no psychiatric comorbidities (included 19 data sets) demonstrated decreased resting‐state functional activity in the bilateral ACC (extending to the bilateral MCC and left superior frontal gyrus), and increased activity in the right amygdala (extending to the right temporal pole and parahippocampal gyrus) (Table S6).
3.2.3. Sensitivity analysis
As seen in Table 3, the whole‐brain jackknife sensitivity analysis revealed that, the most replicable findings in patients with AN were the decrease in GMV in the bilateral MCC (extending to the bilateral ACC and PCC) and left inferior parietal lobe (which were replicable in all 34 data sets). In addition, the resting‐state functional activity decrease in the bilateral anterior cingulate cortex and bilateral median cingulate cortex, and increase in the right parahippocampal gyrus (preserved in at least 85% of the combinations).
3.2.4. Analyses of heterogeneity and publication bias
VBM
The low I 2 statistic indicates very small heterogeneity, the funnel plot does not show asymmetries (Figure S1A,B) and the Egger's test did not show any publication bias for these areas (Egger's p = .906, .982).
Resting‐state functional imaging
The I 2 statistic indicates a certain extent heterogeneity. The funnel plot is listed in Figure S1C,D, and the Egger's tests show no publication bias in these areas (Egger's p = .979, .084).
3.2.5. Meta‐regression analyses
Illness duration, age, and BMI were not associated with AN‐related GMV or resting‐state functional activity changes. We were unable to assess the relationship to AN symptom severity because this was reported using a variety of incompatible measures.
3.2.6. Conjunction analyses
A conjunction analysis further found that the ACC and MCC were altered in AN in both VBM and resting‐state functional studies. Specifically, AN patients showed concurrent decreased GMV and hypoactivity in these regions (Figure 2c).
4. DISCUSSION
To the best of our knowledge, this is the first whole‐brain voxel‐based meta‐analysis of all available VBM and resting‐state functional imaging studies on AN. The main findings were as follows: (a) AN patients showed reduced GMV of the bilateral MCC (extending to the ACC and PCC), and left middle occipital gyrus (extending to left inferior parietal lobe). (b) AN patients showed decreased resting‐state functional activity in the bilateral ACC and MCC, and increased activity in the right parahippocampal gyrus. (c) structural and functional reductions overlapped in the bilateral ACC and MCC in the patients with AN. Additionally, meta‐regression analyses demonstrated that the main resting‐state functional activity and GMV changes in AN remained unaffected by the potential confounding variables of illness duration and BMI, which further confirm the robustness of the findings. These findings provide novel insights into the pathophysiology of AN.
This study, found reduced GMV and spontaneous neural activity in the cingulate cortex (particularly in the ACC and MCC) in AN, suggesting both structural and functional impairments in these regions in AN. The findings of reduced GMV in the ACC and MCC are partly consistent with a previous meta‐analysis that showed decreased GMV in the bilateral MCC and PCC (Zhang et al., 2018). The minor difference may be due to the larger number of studies and more accurate methodology used in this meta‐analysis. Additionally, a previous meta‐analysis of task‐based fMRI studies reported abnormal activity in the ACC in AN patients when receiving food stimuli (Zhu et al., 2012), which further supports our findings of abnormal regional function in the brain ACC of AN patients. The ACC is a part of a circuit involved in attention that serves to regulate both cognitive and emotional processing (Bush, Luu, & Posner, 2000), and appears to play a crucial role in initiation, motivation, and goal‐directed behaviors (Devinsky, Morrell, & Vogt, 1995). A task‐based fMRI study showed that increased rCBF in the ACC was associated with anxiety and physiological arousal in AN, and the AN group showed higher level of anxiety along with increased blood flow in the ACC in response to contrasting stimuli (Ellison et al., 1998). However, more studies are needed to confirm the neural activity variations between resting and task‐dependent states of AN. Also, the ACC has been suggested to be specifically involved in body image distortion (Friederich et al., 2010; Gaudio et al., 2015), abnormal reward processing (Kaye, Fudge, & Paulus, 2009; Keating, 2011), impaired cognitive‐behavioral flexibility (Zastrow et al., 2009), excessive cognitive control of appetite (Kim, Ku, Lee, Lee, & Jung, 2012), and perfectionism (Kaye et al., 2009) in AN patients. Given these considerations, the disturbances in this area might seriously alter the perception/conception and emotional regulation in the patients with AN. Additionally, a longitudinal study found reduced ACC volume during active AN, which was normalized with weight restoration, and smaller changes in right dorsal ACC volume prospectively predicted relapse after treatment (McCormick et al., 2008). Therefore, our findings provide a potential brain‐specific marker for the treatment and outcomes of patients with AN.
The MCC is involved in negative cognition and emotion regulation (Jiang, He, Guo, & Gao, 2019; Riemann et al., 2010), especially the emotionally salient stimuli processing (Maddock, 1999; Small, Zatorre, Dagher, Evans, & Jones‐Gotman, 2001). In the present study, the results of the two meta‐analyses overlapped in the MCC (showing both GMV reductions and functional hypoactivity in AN as compared with HCs), suggesting that structural lesions in the MCC might be linked to functional impairment. The aberrant structure and neural activity of the MCC has been hypothesized to be linked to excessive perfectionism (Fassino et al., 2006), cognitive rigidity, and excessive attention to detail (Friederich & Herzog, 2011). This anorectic personality type relates to exaggerated cognitive control, impaired cognitive set‐shifting (i.e., concrete and rigid behaviors to changing rules), and impaired behavioral response shifting (i.e., stereotyped or perseverative behaviors) (Fairburn, Shafran, & Cooper, 1999; Roberts, Tchanturia, Stahl, Southgate, & Treasure, 2007). This might explain the body image distortion of AN patients that leads to excessive concern about weight (even when the patients have seriously low body weight) and body shape (Fairburn & Harrison, 2003). Additionally, the MCC is also related to energy‐sensing. It has been found that different taste activities with different energy contents during starvation differ from those during satiety (van Rijn, de Graaf, & Smeets, 2015). Therefore, the MCC might play an important role in mental representation, body image, and energy‐sensing (Gaudio et al., 2011) in AN. Taken together, the structural and functional disruptions of the ACC and MCC may be a crucial neurobiological feature of AN.
Specific to VBM, we observed decreased GMV in the left middle occipital gyrus (extending to left inferior parietal lobe), and precuneus/PCC in AN, which were partially consistent with the two previous meta‐analyses of VBM studies (Titova et al., 2013; Zhang et al., 2018). However, this was the largest VBM meta‐analysis on AN (including 660 patients with AN, and 740 controls), with stricter correction. The occipital cortex plays an important role in processing visual information that is subsequently transferred to the temporal cortex for further integration (Amianto et al., 2013; Madsen, Bohon, & Feusner, 2013). Specifically, recent investigations have linked body image distortions in AN to the posterior part of the fusiform gyrus (Suchan et al., 2010; Suchan et al., 2013). These findings showed the possibility that deficit in the middle occipital gyrus could be associated with the body image distortion experienced by individuals with AN. Moreover, our meta‐analysis found decreased GMV in the left inferior parietal lobe (which extended from the left middle occipital gyrus) and precuneus/PCC in AN. The inferior parietal lobe and precuneus/PCC are core hubs of the default mode network (DMN), which plays an important role in cognitive performance to balance external stimulus processing and self‐directed processing (Gerlach, Spreng, Madore, & Schacter, 2014; Price & Drevets, 2010). Several rs‐fMRI studies found abnormal functional connectivity in the DMN in acute and recovered patients with AN (Boehm et al., 2014; Cowdrey, Filippini, Park, Smith, & McCabe, 2014; McFadden, Tregellas, Shott, & Frank, 2014), and the DMN dysfunction might be related to the core symptoms of AN, including ruminative preoccupation with eating, body weight and shape, excessive planning and impaired cognitive flexibility (Cowdrey et al., 2014; Kaye et al., 2009). Additionally, the parietal cortex is related to abnormal food intake rewards (e.g., long‐term restricted food intake may lead to abnormal reward response and unpleasant sense of normal eating) and satiation in AN (Decker, Figner, & Steinglass, 2015). Therefore, the findings of gray matter atrophy in the left middle occipital gyrus (extending to left inferior parietal lobe) and precuneus/PCC could be associated with the core symptoms in AN, which suggests that the DMN dysfunction might be a vulnerability marker for the development of AN.
Additionally, the present meta‐analysis found increased resting‐state functional activity in the right parahippocampal gyrus in AN. Several structural MRI studies found reduced parahippocampal volumes in AN (Brooks et al., 2011; Connan et al., 2006). The parahippocampal gyrus is a limbic system structure, located in the medial temporal lobe. It is involved in the formation and regulation of learning, memory, and emotional control of food intake (Aminoff, Kveraga, & Bar, 2013; Rosenbaum, Sy, Pavlovich, Leibel, & Hirsch, 2008; Squire et al., 2010) such as the memory of specific attributes of recently eaten food (Higgs, 2008). Task‐based fMRI studies found abnormal activity in the parahippocampal gyrus in AN patients during viewing their own bodies (Vocks et al., 2010), perception of distorted body images (Miyake et al., 2010), and cognitive flexibility on the Wisconsin Card Sorting Test (WCST) (Sato et al., 2013). Taken together, these findings of spontaneous functional activity abnormalities in the parahippocampal gyrus may contribute to the rigid and strategic cognitive styles associated with AN. However, the findings need to be verified by further homogeneity studies with large samples.
5. LIMITATIONS
First, this study was based on summarized data (e.g., meta‐analyses of peak and effect sizes from published studies) rather than raw data (Radua et al., 2012), and few small or moderate effect size results may have been neglected. Second, the studies included in this meta‐analysis used different statistical thresholds and multiple comparison corrections. Third, some studies included in the present analyses did not rigorously adhere to the guidelines for brain imaging studies in eating disorders (Frank, Favaro, Marsh, Ehrlich, & Lawson, 2018). Therefore, the clinical characteristics such as age (adolescents or adults), BMI, duration of illness, AN status (acute, chronic, and recovery), age of onset, eating disorder‐related cognitions, depression severity, and inclusion of both binge/purge and restricting types of AN (Kaufmann et al., 2020; Seitz, Herpertz‐Dahlmann, & Konrad, 2016), may act as potential confounding factors. Fourth, many subjects were taking psychotropic medicines such as antidepressants, so whether the alteration of cerebral functional activity and GMV in AN participants were caused by medicines or by AN remains unknown. Fifth, there may have heterogeneity in the results of functional analyses, because they were based on several different measures (ReHo, ALFF, ASL, PET, SPECT). Therefore, we have carried out the subgroup analysis of AN patients in SPECT studies (included 8 data sets) (Table S7, Figure S8) and the results (bilateral anterior cingulate/paracingulate gyri) validated the main results. Finally, the results may be generalized to females but not males because the majority of studies included female participants.
6. CONCLUSION
By summarizing functional and structural studies to date, this meta‐analysis demonstrated a significant reduction in the functional activity and gray matter in the cingulate cortex in patients with AN, particularly in the ACC and MCC, which imply that structural changes may underlie functional alterations. These results expand the current understanding of functional and structural brain abnormalities in AN patients, which would provide additional potential targets for therapeutic intervention.
CONFLICT OF INTERESTS
The authors declare that they have no conflict of interests.
AUTHOR CONTRIBUTIONS
Ting Su: conception and design, acquisition of data, analysis and interpretation of data; drafted the article. Jiaying Gong: conception and design, acquisition of data. Guixian Tang: analysis and interpretation of data; drafted the article. Shaojuan Qiu: revised the manuscript critically for important intellectual content. Pan Chen: revised the manuscript critically for important intellectual content. Guanmao Chen: acquisition of data. Junjing Wang: acquisition of data. Li Huang: revised the manuscript critically for important intellectual content. Ying Wang: conception and design, acquisition of data, analysis and interpretation of data; revised the manuscript critically for important intellectual content. All authors gave final approval for the version to be published.
Supporting information
Appendix S1 Supplementary Information.
ACKNOWLEDGMENTS
The study was supported by grants from the National Natural Science Foundation of China (81671670 and 81971597); National Key Research and Development Program of China (2020YFC2005700); Project in Basic Research and Applied Basic Research in General Colleges and Universities of Guangdong, China (2018KZDXM009); Planned Science and Technology Project of Guangzhou, China (201905010003). The funding organizations played no further role in study design, data collection, analysis and interpretation and paper writing.
Su, T., Gong, J., Tang, G., Qiu, S., Chen, P., Chen, G., Wang, J., Huang, L., & Wang, Y. (2021). Structural and functional brain alterations in anorexia nervosa:A multimodal meta‐analysis of neuroimaging studies. Human Brain Mapping, 42(15), 5154–5169. 10.1002/hbm.25602
Ting Su, Jiaying Gong, and Guixian Tang contributed equally to this work.
Funding information National Key Research and Development Program of China, Grant/Award Number: 2020YFC2005700; National Natural Science Foundation of China, Grant/Award Numbers: 81671670, 81671597; Planned Science and Technology Project of Guangzhou, Grant/Award Number: 201905010003; Basic Research and Applied Basic Research in General Colleges and Universities of Guangdong, Grant/Award Number: 2018KZDXM009
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Albajes‐Eizagirre, A., Solanes, A., Vieta, E., & Radua, J. (2019). Voxel‐based meta‐analysis via permutation of subject images (PSI): Theory and implementation for SDM. NeuroImage, 186, 174–184. 10.1016/j.neuroimage.2018.10.077 [DOI] [PubMed] [Google Scholar]
- Amianto, F., Caroppo, P., D'Agata, F., Spalatro, A., Lavagnino, L., Caglio, M., … Fassino, S. (2013). Brain volumetric abnormalities in patients with anorexia and bulimia nervosa: A voxel‐based morphometry study. Psychiatry Research, 213(3), 210–216. 10.1016/j.pscychresns.2013.03.010 [DOI] [PubMed] [Google Scholar]
- Aminoff, E. M., Kveraga, K., & Bar, M. (2013). The role of the parahippocampal cortex in cognition. Trends in Cognitive Sciences, 17(8), 379–390. 10.1016/j.tics.2013.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar, K. J., de la Cruz, F., Berger, S., Schultz, C. C., & Wagner, G. (2015). Structural and functional differences in the cingulate cortex relate to disease severity in anorexia nervosa. Journal of Psychiatry & Neuroscience, 40(4), 269–279. 10.1503/jpn.140193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björnsdotter, M., Davidovic, M., Karjalainen, L., Starck, G., Olausson, H., & Wentz, E. (2017). Grey matter correlates of autistic traits in women with anorexia nervosa. Journal of Psychiatry & Neuroscience, 43(2), 79–86. 10.1503/jpn.170072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm, I., Geisler, D., King, J. A., Ritschel, F., Seidel, M., Deza Araujo, Y., … Ehrlich, S. (2014). Increased resting state functional connectivity in the fronto‐parietal and default mode network in anorexia nervosa. Frontiers in Behavioral Neuroscience, 8, 346. 10.3389/fnbeh.2014.00346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boghi, A., Sterpone, S., Sales, S., D'Agata, F., Bradac, G. B., Zullo, G., & Munno, D. (2011). In vivo evidence of global and focal brain alterations in anorexia nervosa. Psychiatry Research, 192(3), 154–159. 10.1016/j.pscychresns.2010.12.008 [DOI] [PubMed] [Google Scholar]
- Bomba, M., Riva, A., Morzenti, S., Grimaldi, M., Neri, F., & Nacinovich, R. (2015). Global and regional brain volumes normalization in weight‐recovered adolescents with anorexia nervosa: preliminary findings of a longitudinal voxel‐based morphometry study. Neuropsychiatric Disease and Treatment, 11, 637–645. 10.2147/NDT.S73239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks, S. J., Barker, G. J., O'Daly, O. G., Brammer, M., Williams, S. C., Benedict, C., … Campbell, I. C. (2011). Restraint of appetite and reduced regional brain volumes in anorexia nervosa: A voxel‐based morphometric study. BMC Psychiatry, 11, 179. 10.1186/1471-244X-11-179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush, G., Luu, P., & Posner, M. I. (2000). Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences, 4(6), 215–222. 10.1016/s1364-6613(00)01483-2 [DOI] [PubMed] [Google Scholar]
- Castro‐Fornieles, J., Bargallo, N., Lazaro, L., Andres, S., Falcon, C., Plana, M. T., & Junque, C. (2009). A cross‐sectional and follow‐up voxel‐based morphometric MRI study in adolescent anorexia nervosa. Journal of Psychiatric Research, 43(3), 331–340. 10.1016/j.jpsychires.2008.03.013 [DOI] [PubMed] [Google Scholar]
- Cha, J., Ide, J. S., Bowman, F. D., Simpson, H. B., Posner, J., & Steinglass, J. E. (2016). Abnormal reward circuitry in anorexia nervosa: A longitudinal, multimodal MRI study. Human Brain Mapping, 37(11), 3835–3846. 10.1002/hbm.23279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Z. Q., Du, M. Y., Zhao, Y. J., Huang, X. Q., Li, J., Lui, S., … Gong, Q. Y. (2015). Voxel‐wise meta‐analyses of brain blood flow and local synchrony abnormalities in medication‐free patients with major depressive disorder. Journal of Psychiatry & Neuroscience, 40(6), 401–411. 10.1503/jpn.140119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicerale, A., Settanta, C., D'Agata, F., Caglio, M., Caroppo, P., Coriasco, M., … Amianto, F. (2013). Neuroanatomical correlates of state of mind with respect to attachment in patients with anorexia nervosa. Clinical Neuropsychiatry, 10(5), 217–225. 10.9774/GLEAF.978-1-909493-38-4_2 [DOI] [Google Scholar]
- Connan, F., Murphy, F., Connor, S. E., Rich, P., Murphy, T., Bara‐Carill, N., … Treasure, J. (2006). Hippocampal volume and cognitive function in anorexia nervosa. Psychiatry Research, 146(2), 117–125. 10.1016/j.pscychresns.2005.10.006 [DOI] [PubMed] [Google Scholar]
- Cowdrey, F. A., Filippini, N., Park, R. J., Smith, S. M., & McCabe, C. (2014). Increased resting state functional connectivity in the default mode network in recovered anorexia nervosa. Human Brain Mapping, 35(2), 483–491. 10.1002/hbm.22202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Agata, F., Caroppo, P., Amianto, F., Spalatro, A., Caglio, M. M., Bergui, M., … Fassino, S. (2015). Brain correlates of alexithymia in eating disorders: A voxel‐based morphometry study. Psychiatry and Clinical Neurosciences, 69(11), 708–716. 10.1111/pcn.12318 [DOI] [PubMed] [Google Scholar]
- Decker, J. H., Figner, B., & Steinglass, J. E. (2015). On weight and waiting: Delay discounting in anorexia nervosa pretreatment and posttreatment. Biological Psychiatry, 78(9), 606–614. 10.1016/j.biopsych.2014.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinsky, O., Morrell, M. J., & Vogt, B. A. (1995). Contributions of anterior cingulate cortex to behaviour. Brain, 118(Pt 1), 279–306. 10.1093/brain/118.1.279 [DOI] [PubMed] [Google Scholar]
- Dugre, J. R., Radua, J., Carignan‐Allard, M., Dumais, A., Rubia, K., & Potvin, S. (2020). Neurofunctional abnormalities in antisocial spectrum: A meta‐analysis of fMRI studies on five distinct neurocognitive research domains. Neuroscience and Biobehavioral Reviews, 119, 168–183. 10.1016/j.neubiorev.2020.09.013 [DOI] [PubMed] [Google Scholar]
- Ellison, Z., Foong, J., Howard, R., Bullmore, E., Williams, S., & Treasure, J. (1998). Functional anatomy of calorie fear in anorexia nervosa. The Lancet, 352(9135), 1192. 10.1016/s0140-6736(05)60529-6 [DOI] [PubMed] [Google Scholar]
- Fairburn, C. G., & Harrison, P. J. (2003). Eating disorders. Lancet, 361(9355), 407–416. 10.1016/s0140-6736(03)12378-1 [DOI] [PubMed] [Google Scholar]
- Fairburn, C. G., Shafran, R., & Cooper, Z. (1999). A cognitive behavioural theory of anorexia nervosa. Behaviour Research and Therapy, 37(1), 1–13. 10.1016/s0005-7967(98)00102-8 [DOI] [PubMed] [Google Scholar]
- Fassino, S., Piero, A., Gramaglia, C., Daga, G. A., Gandione, M., Rovera, G. G., & Bartocci, G. (2006). Clinical, psychological, and personality correlates of asceticism in anorexia nervosa: From saint anorexia to pathologic perfectionism. Transcultural Psychiatry, 43(4), 600–614. 10.1177/1363461506070785 [DOI] [PubMed] [Google Scholar]
- Fonville, L., Giampietro, V., Williams, S. C., Simmons, A., & Tchanturia, K. (2014). Alterations in brain structure in adults with anorexia nervosa and the impact of illness duration. Psychological Medicine, 44(9), 1965–1975. 10.1017/S0033291713002389 [DOI] [PubMed] [Google Scholar]
- Frank, G. K. W., Favaro, A., Marsh, R., Ehrlich, S., & Lawson, E. A. (2018). Toward valid and reliable brain imaging results in eating disorders. International Journal of Eating Disorders, 51(3), 250–261. 10.1002/eat.22829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank, G. K., Shott, M. E., Hagman, J. O., & Mittal, V. A. (2013). Alterations in brain structures related to taste reward circuitry in ill and recovered anorexia nervosa and in bulimia nervosa. American Journal of Psychiatry, 170(10), 1152–1160. 10.1176/appi.ajp.2013.12101294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederich, H. C., Brooks, S., Uher, R., Campbell, I. C., Giampietro, V., Brammer, M., … Treasure, J. (2010). Neural correlates of body dissatisfaction in anorexia nervosa. Neuropsychologia, 48(10), 2878–2885. 10.1016/j.neuropsychologia.2010.04.036 [DOI] [PubMed] [Google Scholar]
- Friederich, H. C., & Herzog, W. (2011). Cognitive‐behavioral flexibility in anorexia nervosa. Current Topics in Behavioral Neurosciences, 6, 111–123. 10.1007/7854_2010_83 [DOI] [PubMed] [Google Scholar]
- Friederich, H. C., Walther, S., Bendszus, M., Biller, A., Thomann, P., Zeigermann, S., … Herzog, W. (2012). Grey matter abnormalities within cortico‐limbic‐striatal circuits in acute and weight‐restored anorexia nervosa patients. NeuroImage, 59(2), 1106–1113. 10.1016/j.neuroimage.2011.09.042 [DOI] [PubMed] [Google Scholar]
- Fujisawa, T. X., Yatsuga, C., Mabe, H., Yamada, E., Masuda, M., & Tomoda, A. (2015). Anorexia nervosa during adolescence is associated with decreased gray matter volume in the inferior frontal gyrus. PLoS One, 10(6), e0128548. 10.1371/journal.pone.0128548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudio, S., Nocchi, F., Franchin, T., Genovese, E., Cannatà, V., Longo, D., & Fariello, G. (2011). Gray matter decrease distribution in the early stages of anorexia nervosa restrictive type in adolescents. Psychiatry Research, 191(1), 24–30. 10.1016/j.pscychresns.2010.06.007 [DOI] [PubMed] [Google Scholar]
- Gaudio, S., Piervincenzi, C., Beomonte Zobel, B., Romana Montecchi, F., Riva, G., Carducci, F., & Quattrocchi, C. C. (2015). Altered resting state functional connectivity of anterior cingulate cortex in drug naive adolescents at the earliest stages of anorexia nervosa. Scientific Reports, 5, 10818. 10.1038/srep10818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudio, S., Wiemerslage, L., Brooks, S. J., & Schioth, H. B. (2016). A systematic review of resting‐state functional‐MRI studies in anorexia nervosa: Evidence for functional connectivity impairment in cognitive control and visuospatial and body‐signal integration. Neuroscience and Biobehavioral Reviews, 71, 578–589. 10.1016/j.neubiorev.2016.09.032 [DOI] [PubMed] [Google Scholar]
- Gerlach, K. D., Spreng, R. N., Madore, K. P., & Schacter, D. L. (2014). Future planning: Default network activity couples with frontoparietal control network and reward‐processing regions during process and outcome simulations. Social Cognitive and Affective Neuroscience, 9(12), 1942–1951. 10.1093/scan/nsu001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong, J., Wang, J., Luo, X., Chen, G., Huang, H., Huang, R., … Wang, Y. (2020). Abnormalities of intrinsic regional brain activity in first‐episode and chronic schizophrenia: A meta‐analysis of resting‐state functional MRI. Journal of Psychiatry & Neuroscience, 45(1), 55–68. 10.1503/jpn.180245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong, J., Wang, J., Qiu, S., Chen, P., Luo, Z., Wang, J., … Wang, Y. (2020). Common and distinct patterns of intrinsic brain activity alterations in major depression and bipolar disorder: Voxel‐based meta‐analysis. Translational Psychiatry, 10(1), 353. 10.1038/s41398-020-01036-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon, C. M., Dougherty, D. D., Fischman, A. J., Emans, S. J., Grace, E., Lamm, R., … Rauch, S. L. (2001). Neural substrates of anorexia nervosa: A behavioral challenge study with positron emission tomography. Journal of Pediatrics, 139(1), 51–57. 10.1067/mpd.2001.114768 [DOI] [PubMed] [Google Scholar]
- Gray, J. P., Muller, V. I., Eickhoff, S. B., & Fox, P. T. (2020). Multimodal abnormalities of brain structure and function in major depressive disorder: A meta‐analysis of neuroimaging studies. American Journal of Psychiatry, 177(5), 422–434. 10.1176/appi.ajp.2019.19050560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra‐Carrillo, B., Mackey, A. P., & Bunge, S. A. (2014). Resting‐state fMRI: A window into human brain plasticity. The Neuroscientist, 20(5), 522–533. 10.1177/1073858414524442 [DOI] [PubMed] [Google Scholar]
- Higgs, S. (2008). Cognitive influences on food intake: The effects of manipulating memory for recent eating. Physiology & Behavior, 94(5), 734–739. 10.1016/j.physbeh.2008.04.012 [DOI] [PubMed] [Google Scholar]
- Jiang, B., He, D., Guo, Z., & Gao, Z. (2019). Effect‐size seed‐based d mapping of resting‐state fMRI for persistent insomnia disorder. Sleep & Breathing, 24, 653–659. 10.1007/s11325-019-02001-3 [DOI] [PubMed] [Google Scholar]
- Jinjue, Y., Yiyuan, T., Hongbo, F., Qingbao, Y., Ye, Z., … Yanjun, Z. (2007). Exploring the regional cerebral blood flow in a patient with anorexia nervosa using 99mTc‐ECD single photon emission computed tomography. Journal of Biophysics, 23, 116–122. 10.3321/j.issn:1000-6737.2007.02.005 [DOI] [Google Scholar]
- Joos, A., Hartmann, A., Glauche, V., Perlov, E., Unterbrink, T., Saum, B., … Zeeck, A. (2011). Grey matter deficit in long‐term recovered anorexia nervosa patients. European Eating Disorders Review, 19(1), 59–63. 10.1002/erv.1060 [DOI] [PubMed] [Google Scholar]
- Kaufmann, L. K., Hanggi, J., Jancke, L., Baur, V., Piccirelli, M., Kollias, S., … Milos, G. (2020). Age influences structural brain restoration during weight gain therapy in anorexia nervosa. Translational Psychiatry, 10(1), 126. 10.1038/s41398-020-0809-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye, W. H., Fudge, J. L., & Paulus, M. (2009). New insights into symptoms and neurocircuit function of anorexia nervosa. Nature Reviews Neuroscience, 10(8), 573–584. 10.1038/nrn2682 [DOI] [PubMed] [Google Scholar]
- Keating, C. (2011). Sex differences precipitating anorexia nervosa in females: The estrogen paradox and a novel framework for targeting sex‐specific neurocircuits and behavior. Current Topics in Behavioral Neurosciences, 8, 189–207. 10.1007/7854_2010_99 [DOI] [PubMed] [Google Scholar]
- Kim, K. R., Ku, J., Lee, J. H., Lee, H., & Jung, Y. C. (2012). Functional and effective connectivity of anterior insula in anorexia nervosa and bulimia nervosa. Neuroscience Letters, 521(2), 152–157. 10.1016/j.neulet.2012.05.075 [DOI] [PubMed] [Google Scholar]
- Kohmura, K., Adachi, Y., Tanaka, S., Katayama, H., Imaeda, M., Kawano, N., … Ozaki, N. (2017). Regional decrease in gray matter volume is related to body dissatisfaction in anorexia nervosa. Psychiatry Research: Neuroimaging, 267, 51–58. 10.1016/j.pscychresns.2017.07.004 [DOI] [PubMed] [Google Scholar]
- Kojima, S., Nagai, N., Nakabeppu, Y., Muranaga, T., Deguchi, D., Nakajo, M., … Naruo, T. (2005). Comparison of regional cerebral blood flow in patients with anorexia nervosa before and after weight gain. Psychiatry Research, 140(3), 251–258. 10.1016/j.pscychresns.2005.08.002 [DOI] [PubMed] [Google Scholar]
- Lai, J., Xu, T., Zhang, H., Xi, C., Zhou, H., Du, Y., … Xu, D. (2020). Fractional amplitude of low frequency fluctuation in drug‐naive first‐episode patients with anorexia nervosa: A resting‐state fMRI study. Medicine (Baltimore), 99(9), e19300. 10.1097/MD.0000000000019300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazaro, L., Andres, S., Calvo, A., Cullell, C., Moreno, E., Plana, M. T., … Castro‐Fornieles, J. (2013). Normal gray and white matter volume after weight restoration in adolescents with anorexia nervosa. International Journal of Eating Disorders, 46(8), 841–848. 10.1002/eat.22161 [DOI] [PubMed] [Google Scholar]
- Lim, L., Radua, J., & Rubia, K. (2014). Gray matter abnormalities in childhood maltreatment: A voxel‐wise meta‐analysis. American Journal of Psychiatry, 171(8), 854–863. 10.1176/appi.ajp.2014.13101427 [DOI] [PubMed] [Google Scholar]
- Lukito, S., Norman, L., Carlisi, C., Radua, J., Hart, H., Simonoff, E., & Rubia, K. (2020). Comparative meta‐analyses of brain structural and functional abnormalities during cognitive control in attention‐deficit/hyperactivity disorder and autism spectrum disorder. Psychological Medicine, 50(6), 894–919. 10.1017/S0033291720000574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddock, R. J. (1999). The retrosplenial cortex and emotion: New insights from functional neuroimaging of the human brain. Trends in Neurosciences, 22(7), 310–316. 10.1016/s0166-2236(98)01374-5 [DOI] [PubMed] [Google Scholar]
- Madsen, S. K., Bohon, C., & Feusner, J. D. (2013). Visual processing in anorexia nervosa and body dysmorphic disorder: Similarities, differences, and future research directions. Journal of Psychiatric Research, 47(10), 1483–1491. 10.1016/j.jpsychires.2013.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainz, V., Schulte‐Ruther, M., Fink, G. R., Herpertz‐Dahlmann, B., & Konrad, K. (2012). Structural brain abnormalities in adolescent anorexia nervosa before and after weight recovery and associated hormonal changes. Psychosomatic Medicine, 74(6), 574–582. 10.1097/PSY.0b013e31824ef10e [DOI] [PubMed] [Google Scholar]
- Martin Monzon, B., Henderson, L. A., Madden, S., Macefield, V. G., Touyz, S., Kohn, M. R., … Hay, P. (2017). Grey matter volume in adolescents with anorexia nervosa and associated eating disorder symptoms. European Journal of Neuroscience, 46(7), 2297–2307. 10.1111/ejn.13659 [DOI] [PubMed] [Google Scholar]
- McCormick, L. M., Keel, P. K., Brumm, M. C., Bowers, W., Swayze, V., Andersen, A., & Andreasen, N. (2008). Implications of starvation‐induced change in right dorsal anterior cingulate volume in anorexia nervosa. International Journal of Eating Disorders, 41(7), 602–610. 10.1002/eat.20549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden, K. L., Tregellas, J. R., Shott, M. E., & Frank, G. K. (2014). Reduced salience and default mode network activity in women with anorexia nervosa. Journal of Psychiatry & Neuroscience, 39(3), 178–188. 10.1503/jpn.130046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake, Y., Okamoto, Y., Onoda, K., Kurosaki, M., Shirao, N., Okamoto, Y., & Yamawaki, S. (2010). Brain activation during the perception of distorted body images in eating disorders. Psychiatry Research, 181(3), 183–192. 10.1016/j.pscychresns.2009.09.001 [DOI] [PubMed] [Google Scholar]
- Muhlau, M., Gaser, C., Ilg, R., Conrad, B., Leibl, C., Cebulla, M. H., … Nunnemann, S. (2007). Gray matter decrease of the anterior cingulate cortex in anorexia nervosa. American Journal of Psychiatry, 164(12), 1850–1857. 10.1176/appi.ajp.2007.06111861 [DOI] [PubMed] [Google Scholar]
- Muller, V. I., Cieslik, E. C., Laird, A. R., Fox, P. T., Radua, J., Mataix‐Cols, D., … Eickhoff, S. B. (2018). Ten simple rules for neuroimaging meta‐analysis. Neuroscience and Biobehavioral Reviews, 84, 151–161. 10.1016/j.neubiorev.2017.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naruo, T., Nakabeppu, Y., Deguchi, D., Nagai, N., Tsutsui, J., Nakajo, M., & Nozoe, S. I. (2001). Decreases in blood perfusion of the anterior cingulate gyri in anorexia nervosa Restricters assessed by SPECT image analysis. BMC Psychiatry, 1, 1. 10.1186/1471-244X-1-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickel, K., Joos, A., Tebartz van Elst, L., Matthis, J., Holovics, L., Endres, D., … Maier, S. (2017). Recovery of cortical volume and thickness after remission from acute anorexia nervosa. International Journal of Eating Disorders, 51(9), 1056–1069. 10.1002/eat.22918 [DOI] [PubMed] [Google Scholar]
- Norman, L. J., Carlisi, C., Lukito, S., Hart, H., Mataix‐Cols, D., Radua, J., & Rubia, K. (2016). Structural and functional brain abnormalities in attention‐deficit/hyperactivity disorder and obsessive‐compulsive disorder: A comparative meta‐analysis. JAMA Psychiatry, 73(8), 815–825. 10.1001/jamapsychiatry.2016.0700 [DOI] [PubMed] [Google Scholar]
- Oliva, R., Baiano, M., Salvo, P., Cereser, L., Castiello, U., & Begliomini, C. (2020). Metacognition in individuals recovered from anorexia nervosa: A voxel‐based morphometry study. Psychiatry Research: Neuroimaging, 304, 111138. 10.1016/j.pscychresns.2020.111138 [DOI] [PubMed] [Google Scholar]
- Olivo, G., Solstrand Dahlberg, L., Wiemerslage, L., Swenne, I., Zhukovsky, C., Salonen‐Ros, H., … Schioth, H. B. (2018). Atypical anorexia nervosa is not related to brain structural changes in newly diagnosed adolescent patients. International Journal of Eating Disorders, 51(1), 39–45. 10.1002/eat.22805 [DOI] [PubMed] [Google Scholar]
- Pasanisi, F., Pace, L., Fonti, R., Marra, M., Sgambati, D., De Caprio, C., … Contaldo, F. (2013). Evidence of brown fat activity in constitutional leanness. The Journal of Clinical Endocrinology and Metabolism, 98(3), 1214–1218. 10.1210/jc.2012-2981 [DOI] [PubMed] [Google Scholar]
- Phillipou, A., Rossell, S. L., Gurvich, C., Castle, D. J., Abel, L. A., Nibbs, R. G., & Hughes, M. E. (2018). Differences in regional grey matter volumes in currently ill patients with anorexia nervosa. European Journal of Neuroscience, 47(2), 177–183. 10.1111/ejn.13793 [DOI] [PubMed] [Google Scholar]
- Price, J. L., & Drevets, W. C. (2010). Neurocircuitry of mood disorders. Neuropsychopharmacology, 35(1), 192–216. 10.1038/npp.2009.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radua, J., & Mataix‐Cols, D. (2009). Voxel‐wise meta‐analysis of grey matter changes in obsessive‐compulsive disorder. British Journal of Psychiatry, 195(5), 393–402. 10.1192/bjp.bp.108.055046 [DOI] [PubMed] [Google Scholar]
- Radua, J., Mataix‐Cols, D., Phillips, M. L., El‐Hage, W., Kronhaus, D. M., Cardoner, N., & Surguladze, S. (2012). A new meta‐analytic method for neuroimaging studies that combines reported peak coordinates and statistical parametric maps. European Psychiatry, 27(8), 605–611. 10.1016/j.eurpsy.2011.04.001 [DOI] [PubMed] [Google Scholar]
- Radua, J., Romeo, M., Mataix‐Cols, D., & Fusar‐Poli, P. J. C. (2013). A general approach for combining voxel‐based meta‐analyses conducted in different neuroimaging modalities, 20(3), 462–466. [PubMed] [Google Scholar]
- Radua, J., Rubia, K., Canales‐Rodriguez, E. J., Pomarol‐Clotet, E., Fusar‐Poli, P., & Mataix‐Cols, D. (2014). Anisotropic kernels for coordinate‐based meta‐analyses of neuroimaging studies. Frontiers in Psychiatry, 5, 13. 10.3389/fpsyt.2014.00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riemann, D., Spiegelhalder, K., Feige, B., Voderholzer, U., Berger, M., Perlis, M., & Nissen, C. (2010). The hyperarousal model of insomnia: A review of the concept and its evidence. Sleep Medicine Reviews, 14(1), 19–31. 10.1016/j.smrv.2009.04.002 [DOI] [PubMed] [Google Scholar]
- Roberts, M. E., Tchanturia, K., Stahl, D., Southgate, L., & Treasure, J. (2007). A systematic review and meta‐analysis of set‐shifting ability in eating disorders. Psychological Medicine, 37(8), 1075–1084. 10.1017/S0033291707009877 [DOI] [PubMed] [Google Scholar]
- Rosenbaum, M., Sy, M., Pavlovich, K., Leibel, R. L., & Hirsch, J. (2008). Leptin reverses weight loss‐induced changes in regional neural activity responses to visual food stimuli. Journal of Clinical Investigation, 118(7), 2583–2591. 10.1172/JCI35055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samartsidis, P., Montagna, S., Nichols, T. E., & Johnson, T. D. (2017). The coordinate‐based meta‐analysis of neuroimaging data. Statistical Science, 32(4), 580–599. 10.1214/17-sts624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato, Y., Saito, N., Utsumi, A., Aizawa, E., Shoji, T., Izumiyama, M., … Fukudo, S. (2013). Neural basis of impaired cognitive flexibility in patients with anorexia nervosa. PLoS One, 8(5), e61108. 10.1371/journal.pone.0061108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidel, M., Borchardt, V., Geisler, D., King, J. A., Boehm, I., Pauligk, S., … Ehrlich, S. (2019). Abnormal spontaneous regional brain activity in young patients with anorexia nervosa. Journal of the American Academy of Child and Adolescent Psychiatry, 58(11), 1104–1114. 10.1016/j.jaac.2019.01.011 [DOI] [PubMed] [Google Scholar]
- Seitz, J., Buhren, K., von Polier, G. G., Heussen, N., Herpertz‐Dahlmann, B., & Konrad, K. (2014). Morphological changes in the brain of acutely ill and weight‐recovered patients with anorexia nervosa. A meta‐analysis and qualitative review. Zeitschrift für Kinder‐ und Jugendpsychiatrie und Psychotherapie, 42(1), 7–17; quiz 17‐18. 10.1024/1422-4917/a000265 [DOI] [PubMed] [Google Scholar]
- Seitz, J., Herpertz‐Dahlmann, B., & Konrad, K. (2016). Brain morphological changes in adolescent and adult patients with anorexia nervosa. Journal of Neural Transmission (Vienna), 123(8), 949–959. 10.1007/s00702-016-1567-9 [DOI] [PubMed] [Google Scholar]
- Seitz, J., Walter, M., Mainz, V., Herpertz‐Dahlmann, B., Konrad, K., & von Polier, G. (2015). Brain volume reduction predicts weight development in adolescent patients with anorexia nervosa. Journal of Psychiatric Research, 68, 228–237. 10.1016/j.jpsychires.2015.06.019 [DOI] [PubMed] [Google Scholar]
- Sheng, L., Zhao, P., Ma, H., Radua, J., Yi, Z., Shi, Y., … Pan, P. (2020). Cortical thickness in Parkinson disease: A coordinate‐based meta‐analysis. Medicine (Baltimore), 99(31), e21403. 10.1097/MD.0000000000021403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng, M., Lu, H., Liu, P., Thomas, B. P., & McAdams, C. J. (2015). Cerebral perfusion differences in women currently with and recovered from anorexia nervosa. Psychiatry Research, 232(2), 175–183. 10.1016/j.pscychresns.2015.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small, D. M., Zatorre, R. J., Dagher, A., Evans, A. C., & Jones‐Gotman, M. (2001). Changes in brain activity related to eating chocolate: From pleasure to aversion. Brain, 124(Pt 9), 1720–1733. 10.1093/brain/124.9.1720 [DOI] [PubMed] [Google Scholar]
- Smink, F. R., van Hoeken, D., & Hoek, H. W. (2013). Epidemiology, course, and outcome of eating disorders. Current Opinion in Psychiatry, 26(6), 543–548. 10.1097/YCO.0b013e328365a24f [DOI] [PubMed] [Google Scholar]
- Squire, L. R., van der Horst, A. S., McDuff, S. G., Frascino, J. C., Hopkins, R. O., & Mauldin, K. N. (2010). Role of the hippocampus in remembering the past and imagining the future. Proceedings of the National Academy of Sciences of the United States of America, 107(44), 19044–19048. 10.1073/pnas.1014391107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhausen, H. C. (2002). The outcome of anorexia nervosa in the 20th century. American Journal of Psychiatry, 159(8), 1284–1293. 10.1176/appi.ajp.159.8.1284 [DOI] [PubMed] [Google Scholar]
- Suchan, B., Bauser, D. S., Busch, M., Schulte, D., Gronemeyer, D., Herpertz, S., & Vocks, S. (2013). Reduced connectivity between the left fusiform body area and the extrastriate body area in anorexia nervosa is associated with body image distortion. Behavioural Brain Research, 241, 80–85. 10.1016/j.bbr.2012.12.002 [DOI] [PubMed] [Google Scholar]
- Suchan, B., Busch, M., Schulte, D., Gronemeyer, D., Herpertz, S., & Vocks, S. (2010). Reduction of gray matter density in the extrastriate body area in women with anorexia nervosa. Behavioural Brain Research, 206(1), 63–67. 10.1016/j.bbr.2009.08.035 [DOI] [PubMed] [Google Scholar]
- Sullivan, P. F. (1995). Mortality in anorexia nervosa. The American Journal of Psychiatry, 152(7), 1073–1074. 10.1176/ajp.152.7.1073 [DOI] [PubMed] [Google Scholar]
- Takano, A., Shiga, T., Kitagawa, N., Koyama, T., Katoh, C., Tsukamoto, E., & Tamaki, N. (2001). Abnormal neuronal network in anorexia nervosa studied with I‐123‐IMP SPECT. Psychiatry Research: Neuroimaging, 107(1), 45–50. 10.1016/S0925-4927(01)00093-2 [DOI] [PubMed] [Google Scholar]
- Tench, C. R., Tanasescu, R., Constantinescu, C. S., Cottam, W. J., & Auer, D. P. (2020). Coordinate based meta‐analysis of networks in neuroimaging studies. NeuroImage, 205, 116259. 10.1016/j.neuroimage.2019.116259 [DOI] [PubMed] [Google Scholar]
- Titova, O. E., Hjorth, O. C., Schioth, H. B., & Brooks, S. J. (2013). Anorexia nervosa is linked to reduced brain structure in reward and somatosensory regions: A meta‐analysis of VBM studies. BMC Psychiatry, 13, 110. 10.1186/1471-244X-13-110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Opstal, A. M., Westerink, A. M., Teeuwisse, W. M., van der Geest, M. A., van Furth, E. F., & van der Grond, J. (2015). Hypothalamic BOLD response to glucose intake and hypothalamic volume are similar in anorexia nervosa and healthy control subjects. Frontiers in Neuroscience, 9, 159. 10.3389/fnins.2015.00159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rijn, I., de Graaf, C., & Smeets, P. A. (2015). Tasting calories differentially affects brain activation during hunger and satiety. Behavioural Brain Research, 279, 139–147. 10.1016/j.bbr.2014.11.019 [DOI] [PubMed] [Google Scholar]
- Vocks, S., Busch, M., Gronemeyer, D., Schulte, D., Herpertz, S., & Suchan, B. (2010). Neural correlates of viewing photographs of one's own body and another woman's body in anorexia and bulimia nervosa: An fMRI study. Journal of Psychiatry & Neuroscience, 35(3), 163–176. 10.1503/jpn.090048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner, A., Greer, P., Bailer, U. F., Frank, G. K., Henry, S. E., Putnam, K., … Kaye, W. H. (2006). Normal brain tissue volumes after long‐term recovery in anorexia and bulimia nervosa. Biological Psychiatry, 59(3), 291–293. 10.1016/j.biopsych.2005.06.014 [DOI] [PubMed] [Google Scholar]
- Washington, D. (2013). Diagnostic and statistical manual of mental disorders: DSM‐5 (5th ed.). American Psychiatric Association, 51, 338. [Google Scholar]
- Westmoreland, P., Krantz, M. J., & Mehler, P. S. (2016). Medical complications of anorexia nervosa and bulimia. American Journal of Medicine, 129(1), 30–37. 10.1016/j.amjmed.2015.06.031 [DOI] [PubMed] [Google Scholar]
- Wierenga, C. E., Bischoff‐Grethe, A., Rasmusson, G., Bailer, U. F., Berner, L. A., Liu, T. T., & Kaye, W. H. (2017). Aberrant cerebral blood flow in response to hunger and satiety in women remitted from anorexia nervosa. Frontiers in Nutrition, 4, 32. 10.3389/fnut.2017.00032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao, Y. W., Liu, L., Ma, S. S., Shi, X. H., Zhou, N., Zhang, J. T., & Potenza, M. N. (2017). Functional and structural neural alterations in internet gaming disorder: A systematic review and meta‐analysis. Neuroscience and Biobehavioral Reviews, 83, 313–324. 10.1016/j.neubiorev.2017.10.029 [DOI] [PubMed] [Google Scholar]
- Yonezawa, H., Otagaki, Y., Miyake, Y., Okamoto, Y., & Yamawaki, S. (2008). No differences are seen in the regional cerebral blood flow in the restricting type of anorexia nervosa compared with the binge eating/purging type. Psychiatry and Clinical Neurosciences, 62(1), 26–33. 10.1111/j.1440-1819.2007.01769.x [DOI] [PubMed] [Google Scholar]
- Yue, L. (2013). A study on brain function of inhibitory control in patients with anorexia nervosa. Shanghai Jiaotong University School of Medicine. [Google Scholar]
- Zastrow, A., Kaiser, S., Stippich, C., Walther, S., Herzog, W., Tchanturia, K., … Friederich, H. C. (2009). Neural correlates of impaired cognitive‐behavioral flexibility in anorexia nervosa. The American Journal of Psychiatry, 166(5), 608–616. 10.1176/appi.ajp.2008.08050775 [DOI] [PubMed] [Google Scholar]
- Zhang, H. W., Li, D. Y., Zhao, J., Guan, Y. H., Sun, B. M., & Zuo, C. T. (2013). Metabolic imaging of deep brain stimulation in anorexia nervosa: A 18F‐FDG PET/CT study. Clinical Nuclear Medicine, 38(12), 943–948. 10.1097/RLU.0000000000000261 [DOI] [PubMed] [Google Scholar]
- Zhang, S., Wang, W., Su, X., Kemp, G. J., Yang, X., Su, J., … Gong, Q. (2018). Psychoradiological investigations of gray matter alterations in patients with anorexia nervosa. Translational Psychiatry, 8(1), 277. 10.1038/s41398-018-0323-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, Y., Hu, X., Wang, J., Chen, J., Guo, Q., Li, C., & Enck, P. (2012). Processing of food, body and emotional stimuli in anorexia nervosa: A systematic review and meta‐analysis of functional magnetic resonance imaging studies. European Eating Disorders Review, 20(6), 439–450. 10.1002/erv.2197 [DOI] [PubMed] [Google Scholar]
- Zipfel, S., Giel, K. E., Bulik, C. M., Hay, P., & Schmidt, U. (2015). Anorexia nervosa: Aetiology, assessment, and treatment. Lancet Psychiatry, 2(12), 1099–1111. 10.1016/s2215-0366(15)00356-9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supplementary Information.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


