Abstract
People living with human immunodeficiency virus (PLWH) often have neurocognitive impairment. However, findings on HIV‐related differences in brain network function underlying these impairments are inconsistent. One principle frequently absent from these reports is that brain function is largely emergent from brain structure. PLWH commonly have degraded white matter; we hypothesized that functional communities connected by degraded white matter tracts would show abnormal functional connectivity. We measured white matter integrity in 69 PLWH and 67 controls using fractional anisotropy (FA) in 24 intracerebral white matter tracts. Then, among tracts with degraded FA, we identified gray matter regions connected to these tracts and measured their functional connectivity during rest. Finally, we identified cognitive impairment related to these structural and functional connectivity systems. We found HIV‐related decreased FA in the corpus callosum body (CCb), which coordinates activity between the left and right hemispheres, and corresponding increases in functional connectivity. Finally, we found that individuals with impaired cognitive functioning have lower CCb FA and higher CCb functional connectivity. This result clarifies the functional relevance of the corpus callosum in HIV and provides a framework in which abnormal brain function can be understood in the context of abnormal brain structure, which may both contribute to cognitive impairment.
Keywords: corpus callosum, diffusion imaging, fMRI, fractional anisotropy, functional connectivity, HIV, neuropsychological functioning
People living with human immunodeficiency virus have degraded white matter in the body of the corpus callosum. This is associated with decreased functional connectivity among regions connected to the body of the corpus callosum. These brain differences are both associated with impaired cognition.
Abbreviations
- CCb
corpus callosum body
- GDS
global deficit score
- PLWH
persons living with human immunodeficiency virus
- rs‐fMRI
resting‐state fMRI
1. INTRODUCTION
Up to half of people living with human immunodeficiency virus (PLWH) have neurological and neurocognitive impairment, even with sustained viral suppression on antiretroviral therapy (Heaton et al., 2010). Neurocognitive impairment in HIV disease has been associated with structural and functional brain abnormalities (Schouten, Cinque, Gisslen, Reiss, & Portegies, 2011). HIV enters the central nervous system during early infection and viral reservoirs can linger even after successful antiretroviral treatment, which leads to an early and persistent immune/inflammatory response that causes neuronal damage (Schouten et al., 2011). Thus, initiation of early treatment is critical for optimal long‐term neurocognitive outcomes: early treatment is associated with better outcomes among perinatally acquired HIV, sexually transmitted HIV, and other modes of transmission (Brahmbhatt et al., 2017; Hamilton et al., 1992; May et al., 2011; Willen, 2006).
Although in vivo changes in functional brain network connectivity have been detected in PLWH, findings are inconsistent. Some papers present increases in connectivity in PLWH, including our own, using data that constitutes a subsample of the current sample (e.g., Hall, Lalee, Bell, Towe, & Meade, 2021), others present decreases (e.g., Thomas, Brier, Snyder, Vaida, & Ances, 2013) and others show no group differences (e.g., Guha et al., 2016). These studies have typically considered structural and functional measures independently, but it is widely understood that structural and functional connectivity are strongly related (Honey et al., 2009; Honey, Thivierge, & Sporns, 2010) with cognitive ramifications (Daselaar et al., 2015; Persson et al., 2012; Sarwar, Tian, Yeo, Ramamohanarao, & Zalesky, 2021). Furthermore, multiple neurocognitive conditions associated with degraded white matter have associated large‐scale functional network organization abnormalities (Fornito, Zalesky, Pantelis, & Bullmore, 2012; Griffis, Metcalf, Corbetta, & Shulman, 2020). Because this broad white matter/functional connectivity relationship is well‐documented, we hypothesized that structural abnormalities would be associated with abnormalities in brain network organization in PLWH that have not been previously identified.
Brain white matter is made of connective fibers that facilitate communication among gray matter regions for overall brain function. White matter damage is typical in chronic HIV disease. Over 60% of PLWH have white matter lesions (Haddow et al., 2014), and PLWH are more than twice as likely to have cerebral small‐vessel disease and other white matter damage than people without HIV (Moulignier et al., 2018; Soontornniyomkij et al., 2014; Trentalange et al., 2020). HIV‐associated decreases in white matter integrity measured by fractional anisotropy (FA) are found diffusely across commissural, projection, and association fibers (Bell et al., 2018; Jones et al., 2018; Leite et al., 2013; Pomara, Crandall, Choi, Johnson, & Lim, 2001; Su et al., 2016; van Zoest et al., 2017; Wu et al., 2006), with decreased FA, frequently found in the corpus callosum (Ragin et al., 2015; Su et al., 2016; van Zoest et al., 2017). This white matter damage is associated with impaired functioning in various cognitive domains (Gongvatana et al., 2009; Underwood et al., 2017), but information connecting these structural declines with the functional system supporting cognition is lacking.
While the effects of HIV disease on white matter integrity are relatively well‐established, the effects on functional connectivity in large‐scale brain networks are less clear. Some work reports HIV‐related increases in connectivity (A. Z. Abidin, DSouza, Schifitto, & Wismüller, 2020; Hall et al., 2021), while others report decreases (Thomas et al., 2013; Thomas, Brier, Ortega, Benzinger, & Ances, 2015). Further, there is a sizeable literature reporting no difference in functional brain network connectivity between PLWH and controls (A.Z. Abidin et al., 2018; Egbert et al., 2018; Egbert et al., 2019; Guha et al., 2016; Janssen et al., 2017; Wang et al., 2011). These studies typically investigate connectivity patterns across the whole brain or among canonical resting‐state networks such as the default mode network, frontoparietal network, and the salience networks. This approach ignores the considerable white matter declines in PLWH, and therefore defining functional communities as anatomically constrained systems is an unconventional approach that may reveal novel large‐scale functional abnormalities.
Few studies have investigated the relationship between abnormal structure and function in HIV disease, and most of the existing work has focused on the abnormal structure and function of single regions. This small body of work suggests that regions typically involved in higher‐level cognitive functioning show increased blood oxygenation level‐dependent (BOLD) activity or connectivity when the underlying structural integrity is damaged (D. Liu et al., 2020; Sui et al., 2020; Zhou et al., 2017), whereas sensory regions show decreased BOLD activity, theta activity, and functional connectivity (D. Liu et al., 2020; Sui et al., 2020; Wilson et al., 2015), though this pattern is not consistent across all studies (e.g., Casagrande et al., 2021). To our knowledge, only one study has investigated large‐scale brain network organization. Zhuang et al. (2021), who used graph theory, did not find a relationship between white matter abnormalities and whole‐brain network organization. These studies support the idea that structural abnormalities are related to abnormal brain function in PLWH but also suggest the whole‐brain network organization may not be as strongly impacted. Because structural abnormalities appear to be most strongly associated with function in those same regions, we hypothesize that large‐scale communities defined by connectivity to a shared white matter tract with compromised integrity will have abnormal functional connectivity.
The current study sought to apply a common functional and structural connectivity framework to examine macroscale brain differences in PLWH compared to HIV‐negative controls. First, we identified white matter tracts with reduced FA in PLWH. We then defined functional communities as collections of gray matter regions connected to a shared white matter tract. In functional communities connected to degraded white matter tracts, we examined group differences in functional connectivity strength. Finally, we examined the relationship between impaired cognition and structural/functional connectivity strength. This multimodal approach therefore may elucidate a multiplexed mechanism through which abnormal brain function is likely to develop in PLWH.
2. METHODS
2.1. Participant recruitment and eligibility
The study was open to adults with and without HIV aged 18–55. Participants in the PLWH group were included if they had a confirmed diagnosis of HIV disease of at least 3 months. Control participants were included if they had confirmed HIV‐negative status, verified using an OraQuick rapid test. To increase the generalizability of our results, the use of alcohol, marijuana, and nicotine was permitted in both groups due to the high prevalence of use in PLWH. For other substances, including cocaine, methamphetamine, heroin, and controlled medications that were not prescribed, participants were excluded for any lifetime dependence, regular use for >2 years, and regular use in the past 90 days. Additional exclusion criteria were: nonfluency in English; illiteracy; <8th‐grade education; severe learning disability; severe mental illness; current use of antipsychotic or mood‐stabilizing medications; serious neurological disorders or history of neuroinfections; severe head trauma with loss of consciousness >30 min and persistent functional decline; magnetic resonance imaging (MRI) contraindications; and/or impaired mental status (i.e., not acutely psychotic, manic, delirious, or intoxicated). All exclusion criteria were assessed using the screening measures described below.
2.2. Procedures
We recruited participants through advertisements in local newspapers, websites, and community‐based organizations. After a short prescreener to identify individuals with obvious exclusion criteria, individuals did an in‐person screening. Eligible participants returned for the MRI scan and additional assessments, including clinical interviews and drug screening (see below). All participants provided written informed consent, and procedures were approved by the institutional review board at Duke University Health System. Participants were compensated up to $180 for their participation.
2.2.1. Screening measures
All participants had a confirmed blood alcohol level of 0.00 before participating in any study activities. If blood alcohol levels were above 0.00, participants were asked to wait to begin testing until it was at 0.00 or returned another day. Participants completed clinical interviews, computerized surveys, urine drug screening, and pregnancy testing. Module E of the Structured Clinical Interview for DSM‐IV‐TR identified substance use disorders, psychotic disorders, and bipolar disorder (First, Spitzer, Gibbon, & Williams, 1996), and the Addiction Severity Index‐Lite assessed current and lifetime substance use and associated impairments (McLellan et al., 1992). A medical history interview was used to assess history of neurologic disorders, like stroke. Healthcare records were reviewed to ensure no exclusionary medical history, including substance abuse. Participants reported demographic characteristics, including age, gender, race, and education.
2.2.2. Neuropsychological assessment
A neuropsychological battery was used to assess global cognitive functioning impairment. Because HIV‐associated neurocognitive disorder is diagnosed based on impaired cognitive function, we identified individuals with such impairment using a dichotomous neuropsychological impairment score. To calculate global impairment, functioning was assessed in seven cognitive domains: working memory/attention, executive function, memory, learning, verbal fluency, motor skills, and processing speed, as described by Sui et al. (2020). Standardized T scores on each cognitive test were converted to deficit scores ranging from 0 to 5 (Carey et al., 2004). Domain deficit scores were calculated by averaging the deficit scores for each domain test, which were then averaged to create the global deficit score (GDS). A GDS cutoff of 0.5 yields the optimal balance between sensitivity and specificity in detecting HIV‐related neurocognitive impairment (Carey et al., 2004; Heaton et al., 2004); therefore, a GDS ≥0.5 was categorized as impaired and GDS <0.5 was categorized as not impaired.
2.3. MRI data acquisition
MRI data were acquired on a 3.0 T GE Discovery MR750 whole‐body scanner using an eight‐channel head coil. High‐resolution T1‐weighted (T1w) images were recorded using a spoiled echo sequence (repetition time [TR] = 8.10 ms, echo time [TE] = 3.18 ms, voxel size = 1 mm × 1 mm × 1 mm, field of view [FOV] = 256 mm2, 256 × 256 matrix, 12° flip angle, 166 interleaved slice data acquisition). Diffusion‐weighted imaging (DWI) data were acquired in the axial plane using a diffusion sensitized parallel echo‐planar sequence (FOV = 256 mm2, voxel size = 2 mm × 2 mm × 2 mm, 128 × 128 matrix, 90° flip angle, interleaved slice data acquisition). Data were collected during three studies. Several parameters differed slightly between protocol 1 (b‐factor = 900 s/mm2, TR/TE = 10,000/min: 79.4 ms, 73 slices; 16 PLWH, 13 controls), protocol 2 (b‐factor = 800 s/mm2, TR/TE = 8,000/min: 77.9 ms, 67 slices; 16 PLWH, 18 controls), and protocol 3 (b‐factor = 800 s/mm2, TR/TE = 8,000/min: 77.9 ms, 67 slices; 37 PLWH, 36 controls). Data were acquired in 30 directions for the first 2 protocols and 64 directions for the third protocol. To harmonize the data, the 64‐direction protocol was downsampled using a MATLAB dot product function that identified the diffusion‐encoding direction that was most similar to those in the 30‐direction protocol (https://github.com/nankueichen/DWI_downsampling).
Whole‐brain BOLD images were collected using T2*‐weighted echo‐planar imaging (TR = 2,000 ms, voxel size = 3.75 mm × 3.75 mm × 3.8 mm, FOV = 240 mm2, 64 × 64 matrix, interleaved data acquisition). During resting‐state (rs‐fMRI) scanning, participants were asked to fixate on a crosshair with their eyes open. Additional parameters differed slightly between the first protocol (TE = 27 ms, 77° flip angle, 39 slices) and the other two protocols (TE = 25 ms, 90° flip angle, 35 slices). For data harmonization, each scan included only the first 150 volumes.
2.4. MRI data processing/analysis
2.4.1. Structural data processing
T1‐weighted images (T1w) were skull‐stripped using custom thresholds in FSL's Brain Extraction Tool (BET) for registration to diffusion‐weighted images. The DWI data were denoised (Chen, Chang, Bilgin, Bernstein, & Trouard, 2018), motion and eddy‐corrected using DTIPrep (Oguz et al., 2014). FA images were created by fitting a tensor model to the raw diffusion data using FMRIB's Diffusion Toolbox (Jenkinson, Beckmann, Behrens, Woolrich, & Smith, 2012), and then brain‐extracted using BET (Smith, 2002). The FA data were then aligned into a common space using the nonlinear registration tool FNIRT (Andersson, Jenkinson, & Smith, 2007a, 2007b), which uses a b‐spline representation of the registration warp field (Rueckert et al., 1999).
2.4.2. Functional data processing
The first six volumes of the rs‐fMRI data were excluded to ensure that steady state had been reached. The processing pipeline was run using FSL v5.0 and included the following steps: motion correction using rigid‐body transformation, slice‐timing correction, high‐pass temporal filtering using a Gaussian filter with 0.01 Hz cutoff, intensity normalization across volumes (grand mean scaling = 1,000), spatial smoothing with a Gaussian kernel with a full width at half maximum (FWHM) of 6 mm, and nuisance signal regression of mean white matter and cerebral spinal fluid (Smith et al., 2004). Additional denoising to remove motion and physiological‐related components was conducted through ICA‐AROMA (Pruim et al., 2015). T1w images were registered to the 2 mm MNI template using nonlinear registration using FSL's FNIRT (Andersson et al., 2007b), followed by registration of the rs‐fMRI data to the T1w acquisition using a 12‐parameter affine transformation. A motion threshold of relative mean displacement was set at 0.3 mm, measured using FSL's MCFLIRT using the middle volume as the reference. This threshold was chosen as it is a commonly used threshold, including in populations at‐risk of having high motion (Fair et al., 2020; Y. He, Byrge, & Kennedy, 2020; Hernandez‐Alvarez et al., 2020; Y. Liu et al., 2017; Van Dijk, Sabuncu, & Buckner, 2012). All participants had a mean relative motion of <0.2 mm and were retained in the analysis.
2.4.3. Identifying structural abnormalities
We extracted the mean FA values from 24 intracerebral tracts, defined by the Illinois Institute of Technology white matter atlas (Qi & Konstantions, 2020; Zhang & Arfanakis, 2018) using FSL v. 5.0.9 (Jenkinson et al., 2012; Smith et al., 2004). A smoothing kernel of 4 mm FWHM was applied using FSL. We compared mean FA between groups using analysis of covariances (ANCOVAs) with age (mean‐centered) and protocol as covariates of no interest using SPSS 26. FDR correction was performed to control for multiple comparisons across 24 tracts.
2.4.4. Construction of functional connectivity matrices
We used an atlas containing 392 regions: 322 cortical, 27 subcortical, and 43 cerebellar/brain stem regions (Craddock, James, Holtzheimer, Hu, & Mayberg, 2012). We obtained the timecourse of brain activity from each region across 144 time points using nilearn (Abraham et al., 2014). We then computed partial correlations between all possible pairs of regions, creating a 392 × 392 partial correlation matrix. The partial correlation between two regions is the correlation between two regions, regressing out all other time series (Smith et al., 2013). Partial correlations theoretically measure only direct connections between two regions and do not include indirect connections (e.g., the mediating effect of region C on the connectivity between regions A and B; Marrelec, Kim, Doyon, & Horwitz, 2009; Marrelec et al., 2006; Varoquaux & Craddock, 2013). Partial correlations are more accurate than Pearson's correlations at identifying true network connections (Smith et al., 2011), and they are more closely related to structural connections (which are also direct connections) than full correlation (Liegeois, Santos, Matta, Van De Ville, & Sayed, 2020). Correlations between regions whose anatomical centers were less than 20 mm apart were excluded because connectivity between close regions may be artificially inflated due to motion (Power, Barnes, Snyder, Schlaggar, & Petersen, 2012). The partial correlation matrices were then Fisher‐transformed.
2.4.5. Defining gray matter tract groups
Following the methods described in Davis, Szymanski, Boms, Fink, and Cabeza (2019), gray matter tract groups were defined as regions connected to a common white matter tract. Regions were considered to be connected to the white matter tract if there were at least 10 overlapping voxels. We calculated the average functional connectivity strength between each region in the tract group and every other region in the tract group for each participant.
2.4.6. Identifying abnormal gray matter tract group functional connectivity
The average tract group connectivity strength was compared between groups using ANCOVAs with age (mean‐centered), protocol, and in‐scanner relative mean displacement as covariates of no interest. We compared functional connectivity strength among the tract groups that had significantly different FA between groups. We further investigated functional connectivity strength between regions in each hemisphere separately to identify whether group differences were driven by within‐ and between‐hemisphere connectivity together or whether within‐hemisphere connectivity alone also differed. To identify the specific regions with the greatest group differences in connectivity, we separately measured the functional connectivity between each region in the tract group and the rest of the tract group using ANCOVAs with the same covariates.
2.4.7. Relationship between HIV‐related group‐level structural abnormalities and HIV‐related group‐level functional abnormalities
Next, we determined whether tracts with larger group differences in FA also have larger group differences in tract group functional connectivity strength. Group differences in FA and functional connectivity strength were quantified with effect size (partial η 2), controlling for age (mean‐centered), protocol, and in‐scanner relative mean displacement. We performed a Pearson correlation between the effect size measuring group differences in FA and the effect size measuring group differences in functional connectivity strength. A positive partial η 2 indicates higher FA or functional connectivity in the PLWH group, whereas negative values indicate lower values in the PLWH.
2.4.8. Relationship between tract FA and functional connectivity strength
To determine whether there was a relationship between tract FA and mean functional connectivity strength, we ran a regression using functional connectivity strength as the dependent variable and FA as an independent variable. This was run for tracts that had a group difference in FA as well as functional connectivity strength. Age (mean‐centered), protocol, and in‐scanner relative mean displacement were included as covariates. As a follow‐up, we calculated the mean connectivity strength of the regions that had significant group differences in functional connectivity strength with the rest of their tract. These are referred to as group differentiating regions.
2.4.9. Relationship between brain and behavior
Finally, separate logistic regression models were used to examine the association of mean tract FA and tract group mean connectivity strength to GDS impairment. Age, protocol, and in‐scanner relative mean displacement were included as covariates of no interest. As a follow‐up analysis, we ran a logistic regression using only the mean functional connectivity strength of the high connectivity regions. FDR‐correction was used to control for multiple comparisons.
3. RESULTS
3.1. Demographic data
The sample comprised 136 participants: 69 PLWH and 67 controls. These groups did not differ on key demographic variables (all p's > .05; see Table 1). Most of the PLWH (84.84%) had suppressed viral load at <50 copies. The median most recent CD4 count was 678.50 (interquartile range [IQR] = 422), and the median lowest CD4 count was 256 (IQR = 332). The average years since HIV diagnosis was 9.89 (SD = 7.67). All PLWH were in HIV care, and almost all (97%) were currently on antiretroviral medication. Additionally, 94.20% (65 individuals) contracted HIV through sexual contact, 1.45% (1 individual) contracted HIV through a blood transfusion, 1.45% (1 individual) contracted HIV perinatally, and 2.90% (2 individuals) through an unknown source. A greater proportion of PLWH had an impaired GDS compared to controls (additional details in Table 1).
TABLE 1.
Demographic and neuropsychological information
| HIV (N = 69) | Control (N = 67) | Statistic | p‐Value | |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Male sex, %, N | 73.91%, 51 | 64.18%, 43 | χ2(2) = 2.85 | .241 |
| Cisgender, %, N | 98.55%, 68 | 100%, 67 | χ2(1) = 0.98 | .323 |
| Age in years, M (SD) | 39.43 (9.46) | 37.42 (9.03) | t(134) = 1.28 | .203 |
| Black race, %, N | 73.91%, 51 | 61.20%, 41 | χ2(1) = 2.51 | .113 |
| Hispanic ethnicity, %, N | 4.35%, 3 | 1.59%, 1 | χ2(1) = 0.97 | .324 |
| Education in years, M (SD) | 13.93 (2.11) | 14.57 (2.08) | t(134) = −1.78 | .078 |
| Marital status | χ2(3) = 0.83 | .843 | ||
| Married, partnered, %, N | 17.39%, 12 | 22.39%, 15 | ||
| Separated, %, N | 2.90%, 2 | 1.49%, 1 | ||
| Divorced, %, N | 15.94%, 11 | 16.42%, 11 | ||
| Never married, %, N | 63.77%, 44 | 59.70%, 40 | ||
| In‐scanner mean displacement M (SD) | 0.06 (0.03) | 0.05 (0.03) | t(134) = 0.69 | .494 |
| Daily cigarette smoker, N, % | 31.88%, 22 | 23.88%, 16 | χ2(1) = 1.08 | .298 |
| Alcohol use past 90 days, M (SD) | 5.32 (7.48) | 8.16 (14.71) | t(134) = −1.43 | .156 |
| Alcohol lifetime dependence, %, N | 13.04%, 9 | 9.00%, 6 | χ2(2) = 0.58 | .447 |
| Cannabis use past 90 days, M (SD) | 22.23 (36.00) | 21.42 (36.65) | t(134) = 0.13 | .896 |
| Cannabis lifetime dependence, %, N | 18.84%, 13 | 22.39%, 15 | χ2(2) = 0.26 | .609 |
| Neuropsychological impairment | ||||
| Global deficit score impairment, % | 59.42% | 32.83% | χ2(1) = 9.66 | .002 |
Abbreviations: HIV, human immunodeficiency virus; IQR, interquartile range; M, mean; SD, standard deviation
3.2. HIV‐related decreases in FA
In Table 2, we show significantly decreased FA in the HIV group compared to controls in eight white matter tracts: the middle of the corpus callosum, the corpus callosum, the left and right frontal aslant tracts, the left cingulum, the left superior longitudinal fasciculus, the left middle longitudinal fasciculus, and the left arcuate. It is notable that every tract shows an HIV‐related decrease in FA, even when the difference is not significant after FDR correction (see Supporting Information Table 1 for information about the other tracts).
TABLE 2.
IIT tracts with significant group differences in FA (FDR‐corrected)
| Tract | HIV, mean (SD), N = 69 | Control, mean (SD), N = 67 | F‐statistic (df: 1,131) | p‐Value | Adjusted p‐value | Partial η 2 |
|---|---|---|---|---|---|---|
| Left frontal aslant tract | 0.335 (0.016) | 0.344 (0.012) | 10.773 | .001 | .024 | −0.076 |
| Middle of corpus callosum | 0.433 (0.020) | 0.443 (0.015) | 7.985 | .005 | .048 | −0.057 |
| Left superior longitudinal fasciculus | 0.305 (0.014) | 0.312 (0.013) | 7.609 | .007 | .048 | −0.055 |
| Corpus callosum | 0.421 (0.019) | 0.430 (0.014) | 7.054 | .009 | .048 | −0.051 |
| Left cingulum | 0.445 (0.022) | 0.456 (0.019) | 6.798 | .010 | .048 | −0.049 |
| Right frontal aslant tract | 0.334 (0.015) | 0.341 (0.012) | 6.539 | .012 | .048 | −0.048 |
| Left middle longitudinal fasciculus | 0.383 (0.017) | 0.392 (0.017) | 5.918 | .016 | .048 | −0.043 |
| Left arcuate fasciculus | 0.350 (0.016) | 0.357 (0.015) | 5.911 | .016 | .048 | −0.043 |
Abbreviations: FA, fractional anisotropy; HIV, human immunodeficiency virus; IIT, Illinois Institute of Technology.
3.3. Group differences in tract group functional connectivity strength
We compared functional connectivity strength between groups within the gray matter tract groups associated with the eight tracts that had significant HIV‐related decreases in FA. There was a significant group difference in functional connectivity strength in the middle of the corpus callosum tract group (see Table 3), which corresponds to the body of the corpus callosum (CCb), excluding the genu and splenium regions. In Figure 1, we show that of the 24 tracts, the CCb has nearly the lowest effect size indicating FA degradation in PLWH, and also has the highest effect size indicating functional hyperconnectivity in PLWH.
TABLE 3.
Group differences in functional connectivity strength within tract groups associated with tracts with HIV‐related FA degradation
| Tract | HIV functional connectivity strength, mean (SD) | Control functional connectivity strength, mean (SD) | F‐statistic (df: 1,133) | p‐Value | Partial η 2 |
|---|---|---|---|---|---|
| Middle of corpus callosum | 0.142 (0.070) | 0.112 (0.066) | 5.916 | .016 | 0.044 |
| Right frontal aslant tract | 0.163 (0.089) | 0.136 (0.095) | 2.362 | .127 | 0.018 |
| Corpus callosum | 0.121 (0.062) | 0.107 (0.061) | 1.440 | .232 | 0.011 |
| Left cingulum | 0.132 (0.090) | 0.114 (0.075) | 1.396 | .239 | 0.011 |
| Left frontal aslant tract | 0.154 (0.104) | 0.133 (0.107) | 1.174 | .281 | 0.009 |
| Left superior longitudinal fasciculus | 0.160 (0.110) | 0.142 (0.079) | 0.758 | .386 | 0.006 |
| Left middle longitudinal fasciculus | 0.132 (0.090) | 0.117 (0.124) | 0.701 | .404 | 0.005 |
| Left arcuate fasciculus | 0.137 (0.111) | 0.144 (0.121) | 0.255 | .614 | −0.002 |
Abbreviations: FA, fractional anisotropy; HIV, human immunodeficiency virus.
FIGURE 1.
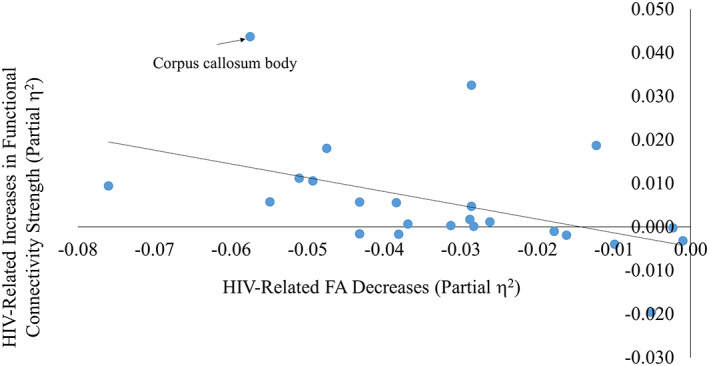
Human immunodeficiency virus (HIV)‐related changes in fractional anisotropy (FA) versus HIV‐related changes in functional connectivity. The effect size of HIV‐related FA degradation in all tracts (measured by partial η 2) is plotted against the effect size of HIV‐related increases in functional connectivity strength in all tracts (measured by partial η 2). Each point represents one of the 24 white matter tract groups. Positive values represent HIV‐related increases in FA and functional connectivity strength, and negative values represent HIV‐related decreases in the same. The corpus callosum body is on the extreme end of both measures
We then compared functional connectivity strength of CCb tract group regions within the right and left hemispheres separately. There were no significant group differences in functional connectivity strength among left hemisphere regions, but there was increased functional connectivity strength in the HIV group in the CCb tract among right hemisphere regions (see Supporting Information Tables 1 and 2).
Further, across all 24 tracts, there was a significant correlation between HIV‐related FA degradation effect size and HIV‐related increases in functional connectivity, as measured by effect size (r(22) = −.47, p = .022). This shows that in all intracerebral tracts, greater HIV‐related FA degradation is related to more HIV‐related increases in functional connectivity in the corresponding tract group (see Supporting Information Table 3 for partial η 2 values for all tracts).
3.4. Gray matter regions in the CCb tract group
There were 92 gray matter subregions linked to the CCb. Regions in the tract group are listed in Supporting Information Table 4. In order to assess whether there was a discernible pattern of increased or decreased connectivity across the tract group, we further investigated which of the subregions in this tract group showed the largest HIV‐related differences in functional connectivity. Among the 92 subregions, 15 group differentiating regions had significantly increased functional connectivity to the rest of the tract group in PLWH compared to controls (p's < .05, uncorrected; see Supporting Information Table 4 for details), and one had significantly decreased functional connectivity to the rest of the tract group in PLWH. These subregions include the right superior parietal lobule and the right and left superior frontal gyri. Further, medial regions were consistently negatively connected to the rest of the tract group, with a significant group difference in the posterior cingulate cortex. Gray matter tract subregions are shown in Figure 2.
FIGURE 2.
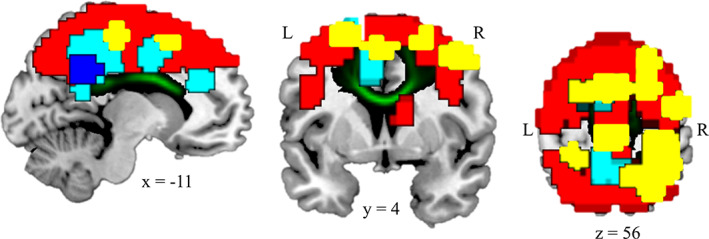
Corpus callosum body (CCb) and its corresponding gray matter tract group. Green: CCb white matter tract. Yellow: CCb tract group regions with significantly greater connectivity in people living with human immunodeficiency virus (PLWH) than controls at p < .05. These are referred to as group differentiating regions. Red: CCb tract group regions with nonsignificantly greater connectivity in PLWH than controls at p > .05. Dark blue: CCb tract group regions with significantly lower connectivity in PLWH than controls at p < .05. Light blue: CCb tract group regions with nonsignificantly lower connectivity in PLWH than controls at p > .05. These data show largely increased connectivity in PLWH in most regions but decreased connectivity in medial regions
3.5. Correlation between CCb FA and functional connectivity strength
Using linear regression, there was a negative but nonsignificant relationship between CCb FA and mean functional connectivity strength (β = −.108, p = .264). We then calculated the mean connectivity strength for the regions that had significantly higher connectivity strength in PLWH (indicated in yellow in Figure 2 and listed in Supporting Information Table 4). This yielded a significant negative correlation (β = −.190, p = .045; Figure 3), indicating that decreased FA is associated with increased functional connectivity strength in the CCb tract group.
FIGURE 3.
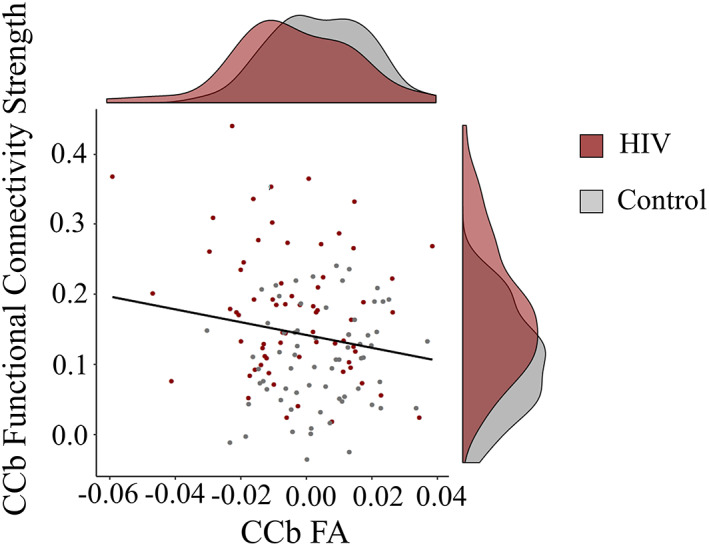
Relationship between corpus callosum body (CCb) fractional anisotropy (FA) and CCb functional connectivity strength. CCb functional connectivity strength is the mean connectivity strength for the group differentiating regions (i.e., those with significantly greater connectivity with the rest of the tract group in people living with human immunodeficiency virus (PLWH) than controls, indicated in yellow in Figure 2)
3.6. CCb FA and CCb tract group functional connectivity strength relationship with behavior
Finally, we used logistic regression models to determine whether the observed group differences in CCb FA and functional connectivity were related to cognitive impairment. For FA, lower FA was associated with increased likelihood of cognitive impairment (β = −20.883, Wald χ2(1) = 3.610, p = .057, adjusted p = .086). For CCb tract group functional connectivity strength, higher connectivity strength was associated with an increased likelihood of cognitive impairment (β = 4.079, Wald χ2(1) = 2.476, p = .116, adjusted p = .116), indicating that people with higher functional connectivity are more likely to have impaired global functioning. We then investigated the relationship between the mean functional connectivity strength of the high connectivity regions (see Figure 2 and Supporting Information Table 4) and again found that increased connectivity strength was associated with an increased likelihood of cognitive impairment (β = 5.083, Wald χ2(1) = 5.664, p = .017, adjusted p = .051; see Figure 4).
FIGURE 4.
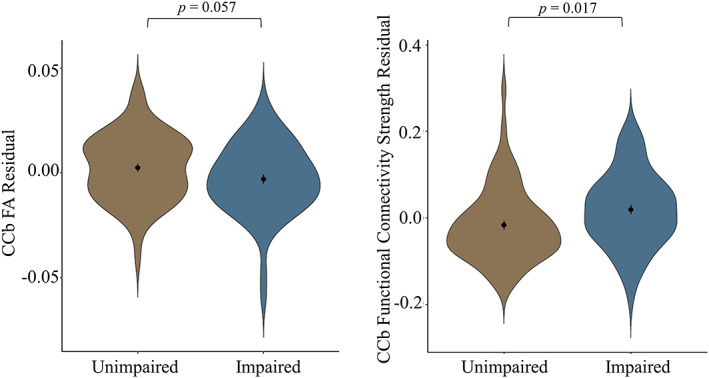
Relationship between brain structure/function residuals and global deficit score (GDS). There is a relationship between corpus callosum body (CCb) fractional anisotropy (FA) and GDS (left) and CCb functional connectivity strength and GDS (right). Note that functional connectivity strength is measured in group differentiating regions. Residuals are obtained by regressing functional connectivity/FA against age (mean centered), protocol, and relative mean displacement. The mean and SE for each group are indicated by the point in the middle of each violin plot. Lower FA and higher functional connectivity are associated with a higher incidence of GDS impairment
4. DISCUSSION
We present results from a multimodal analysis of canonical fiber systems, their concurrent functional connectivity, and their behavioral relevance in the context of HIV disease. We had four primary findings. The first was decreased FA in PLWH in eight white matter tracts, including the CCb. Second, we found that gray matter regions connected to the CCb had increased functional connectivity in PLWH. The regions with the most altered HIV‐related connectivity in this tract group included superior frontal and parietal regions, and midline regions. Third, we found a significant negative correlation between CCb FA and CCb tract group functional connectivity in regions with the greatest degree of HIV‐related hyperconnectivity. Fourth, we found that lower FA and higher functional connectivity were related to global cognitive impairment across both groups, confirming expectations of the role of structural and functional connectivity in neuroHIV.
Lower white matter integrity has been commonly observed in the body of the CC in PLWH (Davies et al., 2019; Li, Sun, Tang, Zhang, & Li, 2018; Su et al., 2016). It has been suggested that the CC is especially vulnerable to viral invasion due to its proximity to cerebrospinal fluid, which is an HIV reservoir that can carry the virus within 8 days of infection (Li et al., 2018; Shiramizu et al., 2005; Valcour et al., 2012). Consistent with this early impact on the brain, white matter degradation has been found within the first 100 days after HIV transmission (Ragin et al., 2015). This early cerebral infiltration of the virus can occur before antiretroviral medications are administered, exposing the surrounding white matter to the neurotoxic effects before effective viral suppression. Our finding of decreased FA in PLWH in the CCb is consistent with this body of work.
Our finding of increased functional connectivity in the CCb tract group is also consistent with previous research showing HIV‐related increases in localized functional connectivity. In PLWH, regions in the frontal cortex and some posterior midline regions become more globally central (i.e., more connections with the rest of the brain; A.Z. Abidin et al., 2018; Thomas et al., 2015) and our previous work showing HIV‐related increases in local connectivity across the brain (Hall et al., 2021). Further, a longitudinal study examining PLWH with mild cognitive impairment showed increasing intranetwork connectivity over time in networks that contained superior parietal, superior frontal, posterior midline, and motor regions (Correa et al., 2017), similar regions to those found in the CCb tract group. Further, midline regions had relatively consistent HIV‐related hypoconnectivity with the rest of the tract group, which may reflect an exaggeration of the anti‐correlation typically found between the default mode network regions and other task‐related networks (Fox et al., 2005; Raichle et al., 2001; Uddin, Kelly, Biswal, Castellanos, & Milham, 2009). The decrease in connectivity between midline regions and the rest of the CCb tract group is consistent with other work done in PLWH showing decreased connectivity between regions in the default mode network (which encompasses similar medial regions) and other networks (Samboju et al., 2018; Thomas et al., 2013). This suggests that decreased CCb integrity in PLWH may enhance the connections that exist: positive connections that become more positive and negative connections that become more negative. Furthermore, these results extend this previous work by providing greater anatomical specificity.
Although there is work done on PLWH showing increased connectivity, there is also a relatively sizeable literature reporting no significant abnormalities in connectivity in canonical functional brain networks in PLWH (A.Z. Abidin et al., 2018; Egbert et al., 2018; Egbert et al., 2019; Guha et al., 2016; Janssen et al., 2017; Wang et al., 2011); our work provides a potential explanation. Structural degradation can impact brain function (Honey et al., 2010), but the functional connectivity within canonical functional networks does not have a one‐to‐one relationship with structural connectivity (Misic et al., 2016). In fact, structural connectivity has a stronger relationship with the functional connectivity in hub regions than with canonical functional networks (Misic et al., 2016). The increased connectivity we found in the CCb tract group was largely driven by strong HIV‐related hyperconnectivity in the superior parietal cortex, and lateral superior frontal cortex, as well as hypoconnectivity between midline regions and the rest of the tract group. These are all regions typically considered to be hub regions (van den Heuvel & Sporns, 2011, 2013). Therefore, white matter degradation in PLWH may not have a strong impact on functional connectivity within canonical functional networks but rather, it may impact the functional connectivity of the hub regions directly connected to it, as hub regions typically have longer connections that may be more susceptibility to white matter damage (Fornito, Zalesky, & Breakspear, 2015). The significant relationship between white matter degradation and functional connectivity strength of the regions with the strongest hyperconnectivity supports that these hub nodes may be particularly susceptible to the effects of white matter damage. Follow‐up work investigating dysfunction in hub nodes, particularly in networks containing these regions, like the dorsal attention network (Behrmann, Geng, & Shomstein, 2004; Corbetta & Shulman, 2002), the executive control network (Seeley et al., 2007), and default mode network (Raichle et al., 2001), may illustrate how structural degradation impacts canonical functional network organization. It is possible that as HIV disease progresses and white matter degradation becomes more severe, which occurs even in individuals successfully treated with cART (Schouten et al., 2011; Su et al., 2016), abnormal connectivity in canonical functional networks may become more apparent.
HIV is not the only condition in which there is increased functional connectivity associated with callosal damage. Individuals born without a CC or whose CC has been severed can have increased functional connectivity between temporal lobe regions and their cross‐hemispheric counterpart, as well as increased intrahemispheric functional connectivity (Koeda et al., 1995; Roland et al., 2017). Further, 2 years post‐commissurotomy, the average functional path length between any two regions is shorter, with increased cross‐hemispheric functional connectivity (Liang, Kim, Ko, Kim, & Lee, 2018). Although individuals with callosal agenesis or who have had commissurotomies typically have a general decrease in interhemispheric functional connectivity (Johnston et al., 2008; Mancuso, Uddin, Nani, Costa, & Cauda, 2019; Pizoli et al., 2011; Roland et al., 2017; Uddin et al., 2008), the increased functional connectivity may be driven by localized increases in connectivity. In older adults, in which the CC is intact but damaged, there are both decreases and increases in functional connectivity in response to degraded white matter in different regions (Davis et al., 2019). This suggests that in PLWH, the specific damage done to the CCb may lead to an increase in functional connectivity between regions with direct CCb connections.
Given that some of the abnormal functional connectivity in PLWH may be tied to CCb white matter degradation, understanding the role played by the CC in facilitating functional connectivity can help explain the mechanism through which such abnormalities in functional connectivity exist. The CC regulates cross‐hemispheric connectivity (Bogen, Fisher, & Vogel, 1965). It contains both glutamatergic (i.e., excitatory) connections, which facilitate cross‐hemispheric connectivity, and GABAergic (i.e., inhibitory) connections, which constrain cross‐hemispheric connectivity (Fabri & Manzoni, 2004; Gonchar, Johnson, & Weinberg, 1995; Peters, Payne, & Josephson, 1990; Rock, Zurita, Lebby, Wilson, & Apicella, 2018). There is evidence to suggest that HIV can excite glutamatergic functioning and can inhibit GABAergic functioning (Musante et al., 2010), which would produce heightened cross‐hemispheric connectivity. Furthermore, there is also evidence to suggest that patients with callosotomies have increased intrahemispheric connectivity compared to the pre‐collosotomy state (Mancuso et al., 2019; Roland et al., 2017), consistent with our finding of increased connectivity in PLWH among regions in the right hemisphere. There is also evidence that there can be increased connectivity among regions contralateral to a site of structural damage (Almeida et al., 2017; Maccotta et al., 2013). Although the CCb is a bilateral white matter tract, five of the six lateralized white matter tracts that had structural degradation were left‐lateralized, which may have led to compensatory increases in right‐lateralized functional connectivity. Such compensatory activity may have occurred because lesions have been associated with decreased connectivity between regions laying on the lesioned pathway but increased connectivity between parallel connections (Alstott, Breakspear, Hagmann, Cammoun, & Sporns, 2009). Future work examining the relationship between glutamate/GABA and functional connectivity in PLWH will clarify this issue.
The behavioral consequences of HIV‐related abnormalities in brain structure and function are critical for identifying potential intervention targets. PLWH in our sample had a greater incidence of impaired cognitive functioning compared to controls, which is consistent with a broader literature showing HIV‐related impairments in multiple cognitive domains (for a review, see Woods, Moore, Weber, & Grant, 2009). In our sample, individuals with lower FA and with higher functional connectivity in group differentiating regions were more likely to have impaired global functioning (trending toward significance for FA). Callosal damage is associated with impaired cognitive function (Hinkley et al., 2012; Huang et al., 2015; Zahr, Rohlfing, Pfefferbaum, & Sullivan, 2009) and a variety of other neurocognitive conditions like autism, bipolar disorder, attention disorders, mild cognitive disorder, and Alzheimer's disease (Anstey et al., 2007; Di Paola et al., 2010; van der Knaap & van der Ham, 2011). Furthermore, increased functional connectivity may be maladaptive due to the reduced selectivity of neural responses. Interhemispheric fibers play an important role in maintaining segregation, especially within early sensorimotor regions, ensuring that lateralized functions operate without interference from the contralateral system (Meyer, Roricht, & Woiciechowsky, 1998). Additionally, right hemisphere intrahemispheric connectivity may be inflated in PLWH to compensate for white matter degradation and corresponding deficits in lateralized cognitive functions, like motor function, or aspects of working memory (Bernal & Altman, 2009; Celeghin et al., 2017; Crowell et al., 2020; Davis, Kragel, Madden, & Cabeza, 2012; Lotze et al., 2000; Lv et al., 2014; Vanderhasselt, De Raedt, & Baeken, 2009), which are included in our measure of global cognitive function. Dedifferentiation, which describes the reduction in the selectivity of neural response, has been used to explain why hyperactivity is related to cognitive decline (Dennis & Cabeza, 2011; Fling, Peltier, Bo, Welsh, & Seidler, 2011). Dedifferentiation has been shown in PLWH: longitudinal work shows increasing activity underlying similar task performance over time in PLWH (Ernst et al., 2009). Therefore, HIV‐related increased functional connectivity strength may reflect a hyperconnected, inefficient state in which information is conveyed to regions that would not typically receive such information.
It is also possible that increased connectivity is compensatory (for a review, see Fornito et al., 2015), allowing some PLWH to maintain cognitive function. Although we find that increased connectivity is associated with cognitive decline, dedifferentiation is typically observed during task performance. However, resting‐state brain function tends to be negatively correlated with task‐based brain function (Cole, Bassett, Power, Braver, & Petersen, 2014; B. J. He, 2013) so increased resting‐state functional connectivity may be related to better task performance. Increased activity associated with better task performance or cognitive functioning has been found in resting‐state and task performance in PLWH (Chang et al., 2004; Chang, Holt, Yakupov, Jiang, & Ernst, 2013; Egbert et al., 2018; Meade et al., 2016). Additional work investigating tract group functional connectivity in PLWH during task performance will be necessary to clarify whether this increased connectivity is adaptive or not.
4.1. Limitations
It is important to acknowledge the limitations in this work. First, we have a relatively modest sample, which may have been underpowered to detect small effects. Second, while PLWH demonstrated poorer cognitive functioning, the overall level of impairment was mild/moderate, consistent with epidemiological studies of HIV‐associated neurological disorders (Heaton et al., 2010; Heaton et al., 2011). This means that the impact of HIV disease on brain function may have been minimized, and there may not have been enough variance in neuropsychological function to identify strong relationships with the brain. Third, we focused on global neuropsychological function as our measure of behavioral function. However, it is possible that in less severe cases of HIV disease, the behavioral impacts may require measures that focus on specific cognitive processes rather than broad cognitive categories. Fourth, our design was cross‐sectional, so we cannot draw conclusions about causation. Fifth, it is possible that cART medication may have neurotoxic effects (Gannon, Khan, & Kolson, 2011), which would be confounded with HIV disease and could not be teased apart in the current analysis. Finally, we relied on one white matter tract parcellation and one gray matter parcellation. To confirm whether these results are robust, future work will be necessary to replicate these results using different parcellations, including parcellations that include smaller tracts, which may differ in across‐subject variance.
4.2. Conclusions
This work shows that HIV‐related degradation in white matter tracts is related to increased functional connectivity between regions directly connected by these tracts. The CCb is most strongly impacted by these abnormalities in HIV disease. This suggests a framework for neuroHIV disease in which functional connectivity abnormalities are underlain by structural degradation. Future work is needed to investigate the specific impact of CCb degradation on cross‐hemispheric connectivity, as well as on dysfunction in hub versus non‐hub regions within the CCb tract group, and to identify the impact of HIV disease progression on the structure/function relationship will further this work. This line of research has strong potential to explain the discrepancies in the HIV‐related functional connectivity literature.
CONFLICT OF INTERESTS
The authors declare no conflicts of interest.
Supporting information
Appendix S1. Supporting Information.
ACKNOWLEDGMENTS
The authors would like to thank all participants for their time and help with our research. This work was supported by the National Institutes of Health (grant number R01‐DA045565).
Hall, S. A., Bell, R. P., Davis, S. W., Towe, S. L., Ikner, T. P., & Meade, C. S. (2021). Human immunodeficiency virus‐related decreases in corpus callosal integrity and corresponding increases in functional connectivity. Human Brain Mapping, 42(15), 4958–4972. 10.1002/hbm.25592
Funding information National Institutes of Health, Grant/Award Number: R01‐DA‐45565
REFERENCES
- Abidin, A. Z., DSouza, A. M., Nagarajan, M. B., Wang, L., Qiu, X., Schifitto, G., & Wismüller, A. (2018). Alteration of brain network topology in HIV‐associated neurocognitive disorder: A novel functional connectivity perspective. NeuroImage: Clinical, 17, 768–777. 10.1016/j.nicl.2017.11.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abidin, A. Z., DSouza, A. M., Schifitto, G., & Wismüller, A. (2020). Detecting cognitive impairment in HIV‐infected individuals using mutual connectivity analysis of resting state functional MRI. Journal of Neurovirology, 26(2), 188–200. 10.1007/s13365-019-00823-1 [DOI] [PubMed] [Google Scholar]
- Abraham, A., Pedregosa, F., Eickenberg, M., Gervais, P., Mueller, A., Kossaifi, J., … Varoquaux, G. (2014). Machine learning for neuroimaging with scikit‐learn. Frontiers in Neuroinformatics, 8, 14. 10.3389/fninf.2014.00014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida, S. R. M., Vicentini, J., Bonilha, L., De Campos, B. M., Casseb, R. F., & Min, L. L. (2017). Brain connectivity and functional recovery in patients with ischemic stroke. Journal of Neuroimaging, 27(1), 65–70. 10.1111/jon.12362 [DOI] [PubMed] [Google Scholar]
- Alstott, J., Breakspear, M., Hagmann, P., Cammoun, L., & Sporns, O. (2009). Modeling the impact of lesions in the human brain. PLoS Computational Biology, 5(6), e1000408. 10.1371/journal.pcbi.1000408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson, J. L. R., Jenkinson, M., & Smith, S. (2007a). Non‐linear optimisation FMRIB technical report TR07JA1. Oxford, England: FMRIB Centre. [Google Scholar]
- Andersson, J. L. R., Jenkinson, M., & Smith, S. (2007b). Non‐linear registration, aka spatial normalisation. Retrieved from http://www.fmrib.ox.ac.uk/analysis/techrep/tr07ja2/tr07ja2.pdf
- Anstey, K. J., Mack, H. A., Christensen, H., Li, S. C., Reglade‐Meslin, C., Maller, J., … Sachdev, P. (2007). Corpus callosum size, reaction time speed and variability in mild cognitive disorders and in a normative sample. Neuropsychologia, 45(8), 1911–1920. 10.1016/j.neuropsychologia.2006.11.020 [DOI] [PubMed] [Google Scholar]
- Behrmann, M., Geng, J. J., & Shomstein, S. (2004). Parietal cortex and attention. Current Opinion in Neurobiology, 14(2), 212–217. 10.1016/j.conb.2004.03.012 [DOI] [PubMed] [Google Scholar]
- Bell, R. P., Barnes, L. L., Towe, S. L., Chen, N. K., Song, A. W., & Meade, C. S. (2018). Structural connectome differences in HIV infection: Brain network segregation associated with nadir CD4 cell count. Journal of Neurovirology, 24(4), 454–463. 10.1007/s13365-018-0634-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernal, B., & Altman, N. (2009). Neural networks of motor and cognitive inhibition are dissociated between brain hemispheres: An fMRI study. International Journal of Neuroscience, 119(10), 1848–1880. 10.1080/00207450802333029 [DOI] [PubMed] [Google Scholar]
- Bogen, J. E., Fisher, E. D., & Vogel, P. J. (1965). Cerebral commissurotomy. A second case report. Jama, 194(12), 1328–1329. [PubMed] [Google Scholar]
- Brahmbhatt, H., Boivin, M., Ssempijja, V., Kagaayi, J., Kigozi, G., Serwadda, D., … Gray, R. H. (2017). Impact of HIV and atiretroviral therapy on neurocognitive outcomes among school‐aged children. JAIDS‐Journal of Acquired Immune Deficiency Syndromes, 75(1), 1–8. 10.1097/QAI.0000000000001305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey, C. L., Woods, S. P., Gonzalez, R., Conover, E., Marcotte, T. D., Grant, I., & Heaton, R. K. (2004). Predictive validity of global deficit scores in detecting neuropsychological impairment in HIV infection. Journal of Clinical and Experimental Neuropsychology, 26(3), 307–319. 10.1080/13803390490510031 [DOI] [PubMed] [Google Scholar]
- Casagrande, C. C., Lew, B. J., Taylor, B. K., Schantell, M., O'Neill, J., May, P. E., … Wilson, T. W. (2021). Impact of HIV‐infection on human somatosensory processing, spontaneous cortical activity, and cortical thickness: A multimodal neuroimaging approach. Human Brain Mapping, 42, 2851–2861. 10.1002/hbm.25408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celeghin, A., Diano, M., de Gelder, B., Weiskrantz, L., Marzi, C. A., & Tamietto, M. (2017). Intact hemisphere and corpus callosum compensate for visuomotor functions after early visual cortex damage. Proceedings of the National Academy of Sciences of the United States of America, 114(48), E10475–E10483. 10.1073/pnas.1714801114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, L., Holt, J. L., Yakupov, R., Jiang, C. S., & Ernst, T. (2013). Lower cognitive reserve in the aging human immunodeficiency virus‐infected brain. Neurobiology of Aging, 34, 1240–1253. 10.1016/j.neurobiolaging.2012.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, L., Tomasi, D., Yakupov, R., Lozar, C., Arnold, S., Caparelli, E., & Ernst, T. (2004). Adaptation of the attention network in human immunodeficiency virus brain injury. Annals of Neurology, 56(2), 259–272. 10.1002/ana.20190 [DOI] [PubMed] [Google Scholar]
- Chen, N. K., Chang, H. C., Bilgin, A., Bernstein, A., & Trouard, T. P. (2018). A diffusion‐matched principal component analysis (DM‐PCA) based two‐channel denoising procedure for high‐resolution diffusion‐weighted MRI. PLoS One, 13(4), e0195952. 10.1371/journal.pone.0195952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole, M. W., Bassett, D. S., Power, J. D., Braver, T. S., & Petersen, S. E. (2014). Intrinsic and task‐evoked network architectures of the human brain. Neuron, 83(1), 238–251. 10.1016/j.neuron.2014.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta, M., & Shulman, G. L. (2002). Control of goal‐directed and stimulus‐driven attention in the brain. Nature Reviews Neuroscience, 3(3), 201–215. 10.1038/nrn755 [DOI] [PubMed] [Google Scholar]
- Correa, D. G., Zimmermann, N., Ventura, N., Tukamoto, G., Doring, T., Leite, S. C., … Gasparetto, E. L. (2017). Longitudinal evaluation of resting‐state connectivity, white matter integrity and cortical thickness in stable HIV infection: Preliminary results. The Neuroradiology Journal, 30(6), 535–545. 10.1177/1971400917739273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craddock, R. C., James, G. A., Holtzheimer, P. E., Hu, X. P. P., & Mayberg, H. S. (2012). A whole brain fMRI atlas generated via spatially constrained spectral clustering. Human Brain Mapping, 33(8), 1914–1928. 10.1002/hbm.21333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowell, C. A., Davis, S. W., Beynel, L., Deng, L., Lakhlani, D., Hilbig, S. A., … Cabeza, R. (2020). Older adults benefit from more widespread brain network integration during working memory. NeuroImage, 218. 10.1016/j.neuroimage.2020.116959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daselaar, S. M., Iyengar, V., Davis, S. W., Eklund, K., Hayes, S. M., & Cabeza, R. E. (2015). Less wiring, more firing: Low‐performing older adults compensate for impaired white matter with greater neural activity. Cerebral Cortex, 25(4), 983–990. 10.1093/cercor/bht289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies, O., Haynes, B. I., Casey, S. J., Gerbase, S., Barker, G. J., Pitkanen, M., … Kopelman, M. D. (2019). Clinical and neuroimaging correlates of cognition in HIV. Journal of Neurovirology, 25(6), 754–764. 10.1007/s13365-019-00763-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, S. W., Kragel, J. E., Madden, D. J., & Cabeza, R. (2012). The architecture of cross‐hemispheric communication in the aging brain: Linking behavior to functional and structural connectivity. Cerebral Cortex, 22(1), 232–242. 10.1093/cercor/bhr123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, S. W., Szymanski, A., Boms, H., Fink, T., & Cabeza, R. (2019). Cooperative contributions of structural and functional connectivity to successful memory in aging. Network Neuroscience, 3(1), 173–194. 10.1162/netn_a_00064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis, N. A., & Cabeza, R. (2011). Age‐related dedifferentiation of learning systems: An fMRI study of implicit and explicit learning. Neurobiology of Aging, 32(12), 2318.e2317. 10.1016/j.neurobiolaging.2010.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Paola, M., Di Iulio, F., Cherubini, A., Blundo, C., Casini, A. R., Sancesario, G., … Spalletta, G. (2010). When, where, and how the corpus callosum changes in MCI and AD: A multimodal MRI study. Neurology, 74(14), 1136–1142. 10.1212/WNL.0b013e3181d7d8cb [DOI] [PubMed] [Google Scholar]
- Egbert, A. R., Biswal, B., Karunakaran, K., Gohel, S., Pluta, A., Wolak, T., … Lojek, E. (2018). Age and HIV effects on resting state of the brain in relationship to neurocognitive functioning. Behavioural Brain Research, 344, 20–27. 10.1016/j.bbr.2018.02.007 [DOI] [PubMed] [Google Scholar]
- Egbert, A. R., Biswal, B., Karunakaran, K. D., Pluta, A., Wolak, T., Rao, S., … Lojek, E. (2019). HIV infection across aging: Synergistic effects on intrinsic functional connectivity of the brain. Progress in Neuro‐Psychopharmacology and Biological Psychiatry, 88, 19–30. 10.1016/j.pnpbp.2018.06.006 [DOI] [PubMed] [Google Scholar]
- Ernst, T., Yakupov, R., Nakama, H., Crocket, G., Cole, M., Watters, M., … Chang, L. (2009). Declined neural efficiency in cognitively stable human immunodeficiency virus patients. Annals of Neurology, 65(3), 316–325. 10.1002/ana.21594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabri, M., & Manzoni, T. (2004). Glutamic acid decarboxylase immunoreactivity in callosal projecting neurons of cat and rat somatic sensory areas. Neuroscience, 123(2), 557–566. 10.1016/j.neuroscience.2003.09.011 [DOI] [PubMed] [Google Scholar]
- Fair, D. A., Miranda‐Dominguez, O., Snyder, A. Z., Perrone, A., Earl, E. A., Van, A. N., … Dosenbach, N. U. F. (2020). Correction of respiratory artifacts in MRI head motion estimates. NeuroImage, 208. 10.1016/j.neuroimage.2019.116400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- First, M. B., Spitzer, R. L., Gibbon, M., & Williams, J. B. W. (1996). Structured clinical interview for DSM‐IV Axis I disorders, research version, patient/non‐patient edition. New York, NY: Biometrics Research, New York State Psychiatric Institute. [Google Scholar]
- Fling, B. W., Peltier, S. J., Bo, J., Welsh, R. C., & Seidler, R. D. (2011). Age differences in interhemispheric interactions: Callosal structure, physiological function, and behavior. Frontiers in Neuroscience, 5, 38. 10.3389/fnins.2011.00038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornito, A., Zalesky, A., & Breakspear, M. (2015). The connectomics of brain disorders. Nature Reviews Neuroscience, 16(3), 159–172. 10.1038/nrn3901 [DOI] [PubMed] [Google Scholar]
- Fornito, A., Zalesky, A., Pantelis, C., & Bullmore, E. T. (2012). Schizophrenia, neuroimaging and connectomics. NeuroImage, 62(4), 2296–2314. 10.1016/j.neuroimage.2011.12.090 [DOI] [PubMed] [Google Scholar]
- Fox, M. D., Snyder, A. Z., Vincent, J. L., Corbetta, M., Van Essen, D. C., & Raichle, M. E. (2005). The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences of the United States of America, 102(27), 9673–9678. 10.1073/pnas.0504136102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon, P., Khan, M. Z., & Kolson, D. L. (2011). Current understanding of HIV‐associated neurocognitive disorders pathogenesis. Current Opinion in Neurology, 24(3), 275–283. 10.1097/WCO.0b013e32834695fb [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonchar, Y. A., Johnson, P. B., & Weinberg, R. J. (1995). Gaba‐immunopositive neurons in rat neocortex with contralateral projections to S‐I. Brain Research, 697(1–2), 27–34. 10.1016/0006-8993(95)00746-D [DOI] [PubMed] [Google Scholar]
- Gongvatana, A., Schweinsburg, B. C., Taylor, M. J., Theilmann, R. J., Letendre, S. L., Alhassoon, O. M., … Grant, I. (2009). White matter tract injury and cognitive impairment in human immunodeficiency virus‐infected individuals. Journal of Neurovirology, 15(2), 187–195. 10.1080/13550280902769756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffis, J. C., Metcalf, N. V., Corbetta, M., & Shulman, G. L. (2020). Damage to the shortest structural paths between brain regions is associated with disruptions of resting‐state functional connectivity after stroke. NeuroImage, 210. 10.1016/j.neuroimage.2020.116589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guha, A., Wang, L., Tanenbaum, A., Esmaeili‐Firidouni, P., Wendelken, L. A., Busovaca, E., … Valcour, V. (2016). Intrinsic network connectivity abnormalities in HIV‐infected individuals over age 60. Journal of Neurovirology, 22(1), 80–87. 10.1007/s13365-015-0370-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddow, L. J., Dudau, C., Chandrashekar, H., Cartledge, J. D., Hyare, H., Miller, R. F., & Jager, H. R. (2014). Cross‐sectional study of unexplained white matter lesions in HIV positive individuals undergoing brain magnetic resonance imaging. AIDS Patient Care and STDs, 28(7), 341–349. 10.1089/apc.2013.0230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall, S. A., Lalee, Z., Bell, R. P., Towe, S. L., & Meade, C. S. (2021). Synergistic effects of HIV and marijuana use on functional brain network organization. Progress in Neuro‐Psychopharmacology and Biological Psychiatry, 104. 10.1016/j.pnpbp.2020.110040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton, J. D., Hartigan, P. M., Simberkoff, M. S., Day, P. L., Diamond, G. R., Dickinson, G. M., … Zollapazner, S. B. (1992). A controlled trial of early versus late treatment with zidovudine in symptomatic human‐immunodeficiency‐virus infection—Results of the veterans affairs cooperative study. New England Journal of Medicine, 326(7), 437–443. 10.1056/Nejm199202133260703 [DOI] [PubMed] [Google Scholar]
- He, B. J. (2013). Spontaneous and task‐evoked brain activity negatively interact. Journal of Neuroscience, 33(11), 4672–4682. 10.1523/Jneurosci.2922-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, Y., Byrge, L., & Kennedy, D. P. (2020). Nonreplication of functional connectivity differences in autism spectrum disorder across multiple sites and denoising strategies. Human Brain Mapping, 41(5), 1334–1350. 10.1002/hbm.24879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton, R. K., Clifford, D. B., Franklin, D. R., Jr., Woods, S. P., Ake, C., Vaida, F., … Grant, I. (2010). HIV‐associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology, 75(23), 2087–2096. 10.1212/WNL.0b013e318200d727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton, R. K., Franklin, D., Ellis, R., McCutchan, J., Letendre, S., LeBlanc, S., … Grant, I. (2011). HIV‐associated neurocognitive disorders before and during the era of combination antiretroviral therapy: Differences in rates, nature, and predictors. Journal of Neurovirology, 17(1), 3–16. 10.1007/s13365-010-0006-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton, R. K., Marcotte, T. D., Mindt, M. R., Sadek, J., Moore, D. J., Bentley, H., … Grant, I. (2004). The impact of HIV‐associated neuropsychological impairment on everyday functioning. Journal of the International Neuropsychological Society, 10(3), 317–331. 10.1017/S1355617704102130 [DOI] [PubMed] [Google Scholar]
- Hernandez‐Alvarez, D. M., Pacheco, L., Velasco‐Segura, R., de la Mora, M. P., Tejeda‐Romero, C., & Gonzalez‐Garcia, N. (2020). Default mode network efficiency is correlated with deficits in inhibition in adolescents with inhalant use disorder. Frontiers in Psychiatry, 11. 10.3389/fpsyt.2020.00209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkley, L. B., Marco, E. J., Findlay, A. M., Honma, S., Jeremy, R. J., Strominger, Z., … Sherr, E. H. (2012). The role of corpus callosum development in functional connectivity and cognitive processing. PLoS One, 7(8), e39804. 10.1371/journal.pone.0039804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honey, C. J., Sporns, O., Cammoun, L., Gigandet, X., Thiran, J. P., Meuli, R., & Hagmann, P. (2009). Predicting human resting‐state functional connectivity from structural connectivity. Proceedings of the National Academy of Sciences of the United States of America, 106(6), 2035–2040. 10.1073/pnas.0811168106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honey, C. J., Thivierge, J. P., & Sporns, O. (2010). Can structure predict function in the human brain? NeuroImage, 52(3), 766–776. 10.1016/j.neuroimage.2010.01.071 [DOI] [PubMed] [Google Scholar]
- Huang, X., Du, X., Song, H., Zhang, Q., Jia, J., Xiao, T., & Wu, J. (2015). Cognitive impairments associated with corpus callosum infarction: A ten cases study. International Journal of Clinical and Experimental Medicine, 8(11), 21991–21998. [PMC free article] [PubMed] [Google Scholar]
- Janssen, M. A. M., Hinne, M., Janssen, R. J., van Gerven, M. A., Steens, S. C., Goraj, B., … Kessels, R. P. C. (2017). Resting‐state subcortical functional connectivity in HIV‐infected patients on long‐term cART. Brain Imaging and Behavior, 11(5), 1555–1560. 10.1007/s11682-016-9632-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson, M., Beckmann, C. F., Behrens, T. E., Woolrich, M. W., & Smith, S. M. (2012). FSL. NeuroImage, 62(2), 782–790. 10.1016/j.neuroimage.2011.09.015 [DOI] [PubMed] [Google Scholar]
- Johnston, J. M., Vaishnavi, S. N., Smyth, M. D., Zhang, D. Y., He, B. J., Zempel, J. M., … Raichle, M. E. (2008). Loss of resting interhemispheric functional connectivity after complete section of the corpus callosum. Journal of Neuroscience, 28(25), 6453–6458. 10.1523/Jneurosci.0573-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, J. D., Kuhn, T., Mahmood, Z., Singer, E. J., Hinkin, C. H., & Thames, A. D. (2018). Longitudinal intra‐individual variability in neuropsychological performance relates to white matter changes in HIV. Neuropsychology, 32(2), 206–212. 10.1037/neu0000390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeda, T., Knyazeva, M., Njiokiktjien, C., Jonkman, E. J., De Sonneville, L., & Vildavsky, V. (1995). The EEG in acallosal children. Coherence values in the resting state: Left hemisphere compensatory mechanism? Electroencephalography and Clinical Neurophysiology, 95(6), 397–407. 10.1016/0013-4694(95)00171-9 [DOI] [PubMed] [Google Scholar]
- Leite, S. C. B., Correa, D. G., Doring, T. M., Kubo, T. T. A., Netto, T. M., Ferracini, R., … Gasparetto, E. L. (2013). Diffusion tensor MRI evaluation of the corona radiata, cingulate gyri, and corpus callosum in HIV patients. Journal of Magnetic Resonance Imaging, 38(6), 1488–1493. 10.1002/jmri.24129 [DOI] [PubMed] [Google Scholar]
- Li, R. L., Sun, J., Tang, Z. C., Zhang, J. J., & Li, H. J. (2018). Axonal chronic injury in treatment‐naive HIV plus adults with asymptomatic neurocognitive impairment and its relationship with clinical variables and cognitive status. BMC Neurology, 18, 66. 10.1186/s12883-018-1069-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang, J. G., Kim, N. Y., Ko, A., Kim, H. D., & Lee, D. (2018). Changes in functional brain network topology after successful and unsuccessful corpus callosotomy for Lennox‐Gastaut syndrome. Scientific Reports, 8(1), 1–10. 10.1038/s41598-018-21764-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liegeois, R., Santos, A., Matta, V., Van De Ville, D., & Sayed, A. H. (2020). Revisiting correlation‐based functional connectivity and its relationship with structural connectivity. Network Neuroscience, 4(4), 1235–1251. 10.1162/netn_a_00166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, D., Zhao, C., Wang, W., Wang, Y. Y., Li, R. L., Sun, J., … Li, H. J. (2020). Altered gray matter volume and functional connectivity in human immunodeficiency virus‐infected adults. Frontiers in Neuroscience, 14. 10.3389/fnins.2020.601063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y., Wang, J. Q., Zhang, J. S., Wen, H. W., Zhang, Y., Kang, H. Y., … Peng, Y. (2017). Altered spontaneous brain activity in children with early Tourette syndrome: A resting‐state fMRI study. Scientific Reports, 7(1), 1–8. 10.1038/s41598-017-04148-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotze, M., Erb, M., Flor, H., Huelsmann, E., Godde, B., & Grodd, W. (2000). fMRI evaluation of somatotopic representation in human primary motor cortex. NeuroImage, 11(5), 473–481. 10.1006/nimg.2000.0556 [DOI] [PubMed] [Google Scholar]
- Lv, Z. X., Huang, D. H., Ye, W., Chen, Z. R., Huang, W. L., & Zheng, J. O. (2014). Alteration of functional connectivity within visuospatial working memory‐related brain network in patients with right temporal lobe epilepsy: A resting‐state fMRI study. Epilepsy & Behavior, 35, 64–71. 10.1016/j.yebeh.2014.04.001 [DOI] [PubMed] [Google Scholar]
- Maccotta, L., He, B. J., Snyder, A. Z., Eisenman, L. N., Benzinger, T. L., Ances, B. M., … Hogan, R. E. (2013). Impaired and facilitated functional networks in temporal lobe epilepsy. NeuroImage: Clinical, 2, 862–872. 10.1016/j.nicl.2013.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancuso, L., Uddin, L. Q., Nani, A., Costa, T., & Cauda, F. (2019). Brain functional connectivity in individuals with callosotomy and agenesis of the corpus callosum: A systematic review. Neuroscience and Biobehavioral Reviews, 105, 231–248. 10.1016/j.neubiorev.2019.07.004 [DOI] [PubMed] [Google Scholar]
- Marrelec, G., Kim, J., Doyon, J., & Horwitz, B. (2009). Large‐scale neural model validation of partial correlation analysis for effective connectivity investigation in functional MRI. Human Brain Mapping, 30(3), 941–950. 10.1002/hbm.20555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrelec, G., Krainik, A., Duffau, H., Pelegrini‐Issac, M., Lehericy, S., Doyon, J., & Benali, H. (2006). Partial correlation for functional brain interactivity investigation in functional MRI. NeuroImage, 32(1), 228–237. 10.1016/j.neuroimage.2005.12.057 [DOI] [PubMed] [Google Scholar]
- May, M., Gompels, M., Delpech, V., Porter, K., Post, F., Johnson, M., … Sabin, C. (2011). Impact of late diagnosis and treatment on life expectancy in people with HIV‐1: UK Collaborative HIV Cohort (UK CHIC) Study. BMJ‐British Medical Journal, 343. 10.1136/bmj.d6016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan, A. T., Kushner, H., Metzger, D., Peters, R., Smith, I., Grissom, G., … Argeriou, M. (1992). The fifth edition of the addiction severity index. Journal of Substance Abuse Treatment, 9(3), 199–213. 10.1016/0740-5472(92)90062-S [DOI] [PubMed] [Google Scholar]
- Meade, C. S., Cordero, D. M., Hobkirk, A. L., Metra, B. M., Chen, N.‐K., & Huettel, S. A. (2016). Compensatory activation in fronto‐parietal cortices among HIV‐infected persons during a monetary decision‐making task. Human Brain Mapping, 37(7), 2455–2467. 10.1002/hbm.23185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer, B. U., Roricht, S., & Woiciechowsky, C. (1998). Topography of fibers in the human corpus callosum mediating interhemispheric inhibition between the motor cortices. Annals of Neurology, 43(3), 360–369. 10.1002/ana.410430314 [DOI] [PubMed] [Google Scholar]
- Misic, B., Betzel, R. F., de Reus, M. A., van den Heuvel, M. P., Berman, M. G., McIntosh, A. R., & Sporns, O. (2016). Network‐level structure‐function relationships in human neocortex. Cerebral Cortex, 26(7), 3285–3296. 10.1093/cercor/bhw089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulignier, A., Savatovsky, J., Assoumou, L., Lescure, F. X., Lamirel, C., Godin, O., … Microvascular Brain Retina and Kidney (MicroBREAK) Study Group . (2018). Silent cerebral small‐vessel disease is twice as prevalent in middle‐aged individuals with well‐controlled, combination antiretroviral therapy‐treated human immunodeficiency virus (HIV) than in HIV‐uninfected individuals. Clinical Infectious Diseases, 66(11), 1762–1769. 10.1093/cid/cix1075 [DOI] [PubMed] [Google Scholar]
- Musante, V., Summa, M., Neri, E., Puliti, A., Godowicz, T. T., Severi, P., … Pittaluga, A. (2010). The HIV‐1 viral protein Tat increases glutamate and decreases GABA exocytosis from human and mouse neocortical nerve endings. Cerebral Cortex, 20(8), 1974–1984. 10.1093/cercor/bhp274 [DOI] [PubMed] [Google Scholar]
- Oguz, I., Farzinfar, M., Matsui, J., Budin, F., Liu, Z., Gerig, G., … Styner, M. (2014). DTIPrep: Quality control of diffusion‐weighted images. Frontiers in Neuroinformatics, 8, 4. 10.3389/fninf.2014.00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson, J., Pudas, S., Lind, J., Kauppi, K., Nilsson, L. G., & Nyberg, L. (2012). Longitudinal structure‐function correlates in elderly reveal MTL dysfunction with cognitive decline. Cerebral Cortex, 22(10), 2297–2304. 10.1093/cercor/bhr306 [DOI] [PubMed] [Google Scholar]
- Peters, A., Payne, B. R., & Josephson, K. (1990). Transcallosal non‐pyramidal cell projections from visual cortex in the cat. Journal of Comparative Neurology, 302(1), 124–142. 10.1002/cne.903020110 [DOI] [PubMed] [Google Scholar]
- Pizoli, C. E., Shah, M. N., Snyder, A. Z., Shimony, J. S., Limbrick, D. D., Raichle, M. E., … Smyth, M. D. (2011). Resting‐state activity in development and maintenance of normal brain function. Proceedings of the National Academy of Sciences of the United States of America, 108(28), 11638–11643. 10.1073/pnas.1109144108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomara, N., Crandall, D. T., Choi, S. J., Johnson, G., & Lim, K. O. (2001). White matter abnormalities in HIV‐1 infection: A diffusion tensor imaging study. Psychiatry Research: Neuroimaging, 106(1), 15–24. 10.1016/S0925-4927(00)00082-2 [DOI] [PubMed] [Google Scholar]
- Power, J. D., Barnes, K. A., Snyder, A. Z., Schlaggar, B. L., & Petersen, S. E. (2012). Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage, 59(3), 2142–2154. 10.1016/j.neuroimage.2011.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruim, R. H., Mennes, M., van Rooij, D., Llera, A., Buitelaar, J. K., & Beckmann, C. F. (2015). ICA‐AROMA: A robust ICA‐based strategy for removing motion artifacts from fMRI data. NeuroImage, 112, 267–277. 10.1016/j.neuroimage.2015.02.064 [DOI] [PubMed] [Google Scholar]
- Qi, X., & Konstantions, A. (2020). Regionconnect: Rapidly extracting standardized brain connectivity information in voxel‐wise neuroimaging studies. NeuroImage, 225, 117462. 10.1016/j.neuroimage.2020.117462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragin, A. B., Wu, Y., Gao, Y., Keating, S., Du, H., Sammet, C., … Epstein, L. G. (2015). Brain alterations within the first 100 days of HIV infection. Annals of Clinical Translational Neurology, 2(1), 12–21. 10.1002/acn3.136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle, M. E., MacLeod, A. M., Snyder, A. Z., Powers, W. J., Gusnard, D. A., & Shulman, G. L. (2001). A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America, 98(2), 676–682. 10.1073/pnas.98.2.676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock, C., Zurita, H., Lebby, S., Wilson, C. J., & Apicella, A. J. (2018). Cortical circuits of callosal GABAergic neurons. Cerebral Cortex, 28(4), 1154–1167. 10.1093/cercor/bhx025 [DOI] [PubMed] [Google Scholar]
- Roland, J. L., Snyder, A. Z., Hacker, C. D., Mitra, A., Shimony, J. S., Limbrick, D. D., … Leuthardt, E. C. (2017). On the role of the corpus callosum in interhemispheric functional connectivity in humans. Proceedings of the National Academy of Sciences of the United States of America, 114(50), 13278–13283. 10.1073/pnas.1707050114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueckert, D., Sonoda, L. I., Hayes, C., Hill, D. L. G., Leach, M. O., & Hawkes, D. J. (1999). Non‐rigid registration using free‐form deformations: Application to breast MR images. IEEE Transactions on Medical Imaging, 18(8), 712–721. 10.1109/42.796284 [DOI] [PubMed] [Google Scholar]
- Samboju, V., Philippi, C. L., Chan, P., Cobigo, Y., Fletcher, J. L. K., Robb, M., … Teams, R. P. (2018). Structural and functional brain imaging in acute HIV. NeuroImage: Clinical, 20, 327–335. 10.1016/j.nicl.2018.07.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarwar, T., Tian, Y., Yeo, B. T. T., Ramamohanarao, K., & Zalesky, A. (2021). Structure‐function coupling in the human connectome: A machine learning approach. NeuroImage, 226. 10.1016/j.neuroimage.2020.117609 [DOI] [PubMed] [Google Scholar]
- Schouten, J., Cinque, P., Gisslen, M., Reiss, P., & Portegies, P. (2011). HIV‐1 infection and cognitive impairment in the cART era: A review. AIDS, 25(5), 561–575. 10.1097/QAD.0b013e3283437f9a [DOI] [PubMed] [Google Scholar]
- Seeley, W. W., Menon, V., Schatzberg, A. F., Keller, J., Glover, G. H., Kenna, H., … Greicius, M. D. (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. The Journal of Neuroscience, 27(9), 2349–2356. 10.1523/jneurosci.5587-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiramizu, B., Gartner, S., Williams, A., Shikuma, C., Ratto‐Kim, S., Watters, M., … Valcour, V. (2005). Circulating proviral HIV DNA and HIV‐associated dementia. AIDS, 19(1), 45–52. 10.1097/00002030-200501030-00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, S. M. (2002). Fast robust automated brain extraction. Human Brain Mapping, 17(3), 143–155. 10.1002/hbm.10062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, S. M., Jenkinson, M., Woolrich, M. W., Beckmann, C. F., Behrens, T. E., Johansen‐Berg, H., … Matthews, P. M. (2004). Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage, 23(Suppl 1), S208–S219. 10.1016/j.neuroimage.2004.07.051 [DOI] [PubMed] [Google Scholar]
- Smith, S. M., Miller, K. L., Salimi‐Khorshidi, G., Webster, M., Beckmann, C. F., Nichols, T. E., … Woolrich, M. W. (2011). Network modelling methods for FMRI. NeuroImage, 54(2), 875–891. 10.1016/j.neuroimage.2010.08.063 [DOI] [PubMed] [Google Scholar]
- Smith, S. M., Vidaurre, D., Beckmann, C. F., Glasser, M. F., Jenkinson, M., Miller, K. L., … Van Essen, D. C. (2013). Functional connectomics from resting‐state fMRI. Trends in Cognitive Sciences, 17(12), 666–682. 10.1016/j.tics.2013.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soontornniyomkij, V., Umlauf, A., Chung, S. A., Cochran, M. L., Soontornniyomkij, B., Gouaux, B., … Achim, C. L. (2014). HIV protease inhibitor exposure predicts cerebral small vessel disease. AIDS, 28(9), 1297–1306. 10.1097/Qad.0000000000000262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su, T., Caan, M. W., Wit, F. W., Schouten, J., Geurtsen, G. J., Cole, J. H., … AGEhIV Cohort Study . (2016). White matter structure alterations in HIV‐1‐infected men with sustained suppression of viraemia on treatment. AIDS, 30(2), 311–322. 10.1097/QAD.0000000000000945 [DOI] [PubMed] [Google Scholar]
- Sui, J., Li, X., Bell, R. P., Towe, S. L., Gadde, S., Chen, N.‐K., & Meade, C. S. (2020). Structural and functional brain abnormalities in human inmunodeficiency virus disease revealed by multimodal magnetic resonance imaging fusion: Association with cognitive function. Clinical Infections Diseases. 10.1093/cid/ciaa1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas, J. B., Brier, M. R., Ortega, M., Benzinger, T. L., & Ances, B. M. (2015). Weighted brain networks in disease: Centrality and entropy in human immunodeficiency virus and aging. Neurobiology of Aging, 36(1), 401–412. 10.1016/j.neurobiolaging.2014.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas, J. B., Brier, M. R., Snyder, A. Z., Vaida, F. F., & Ances, B. M. (2013). Pathways to neurodegeneration: Effects of HIV and aging on resting‐state functional connectivity. Neurology, 80(13), 1186–1193. 10.1212/WNL.0b013e318288792b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trentalange, A., Prochet, A., Imperiale, D., Cusato, J., Tettoni, M., Nunnari, G., … Calcagno, A. (2020). Cerebral white matter hyperintensities in HIV‐positive patients. Brain Imaging and Behavior, 14(1), 10–18. 10.1007/s11682-018-9966-1 [DOI] [PubMed] [Google Scholar]
- Uddin, L. Q., Kelly, A. M. C., Biswal, B. B., Castellanos, F. X., & Milham, M. P. (2009). Functional connectivity of default mode network components: Correlation, anticorrelation, and causality. Human Brain Mapping, 30(2), 625–637. 10.1002/hbm.20531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin, L. Q., Mooshagian, E., Zaidel, E., Scheres, A., Margulies, D. S., Kelly, A. M., … Milham, M. P. (2008). Residual functional connectivity in the split‐brain revealed with resting‐state functional MRI. Neuroreport, 19(7), 703–709. 10.1097/WNR.0b013e3282fb8203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underwood, J., Cole, J. H., Caan, M., De Francesco, D., Leech, R., van Zoest, R. A., … Sharp, D. J. (2017). Grey and white matter abnormalities in treated HIV‐disease and their relationship to cognitive function. Clinical Infectious Diseases, 65(3), 422–432. 10.1093/cid/cix301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valcour, V., Chalermchai, T., Sailasuta, N., Marovich, M., Lerdlum, S., Suttichom, D., … Grp, R. S. S. (2012). Central nervous system viral invasion and inflammation during acute HIV infection. Journal of Infectious Diseases, 206(2), 275–282. 10.1093/infdis/jis326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel, M. P., & Sporns, O. (2011). Rich‐club organization of the human connectome. The Journal of Neuroscience, 31(44), 15775–15786. 10.1523/JNEUROSCI.3539-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel, M. P., & Sporns, O. (2013). Network hubs in the human brain. Trends in Cognitive Sciences, 17(12), 683–696. 10.1016/j.tics.2013.09.012 [DOI] [PubMed] [Google Scholar]
- van der Knaap, L. J., & van der Ham, I. J. (2011). How does the corpus callosum mediate interhemispheric transfer? A review. Behavioural Brain Research, 223(1), 211–221. 10.1016/j.bbr.2011.04.018 [DOI] [PubMed] [Google Scholar]
- Van Dijk, K. R. A., Sabuncu, M. R., & Buckner, R. L. (2012). The influence of head motion on intrinsic functional connectivity MRI. NeuroImage, 59(1), 431–438. 10.1016/j.neuroimage.2011.07.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zoest, R. A., Underwood, J., De Francesco, D., Sabin, C. A., Cole, J. H., Wit, F. W., … Comorbidity in Relation to AIDS (COBRA) Collaboration . (2017). Structural brain abnormalities in successfully treated HIV infection: Associations with disease and cerebrospinal fluid biomarkers. Journal of Infectious Diseases, 217(1), 69–81. 10.1093/infdis/jix553 [DOI] [PubMed] [Google Scholar]
- Vanderhasselt, M. A., De Raedt, R., & Baeken, C. (2009). Dorsolateral prefrontal cortex and Stroop performance: Tackling the lateralization. Psychonomic Bulletin & Review, 16(3), 609–612. 10.3758/Pbr.16.3.609 [DOI] [PubMed] [Google Scholar]
- Varoquaux, G., & Craddock, R. C. (2013). Learning and comparing functional connectomes across subjects. NeuroImage, 80, 405–415. 10.1016/j.neuroimage.2013.04.007 [DOI] [PubMed] [Google Scholar]
- Wang, X., Foryt, P., Ochs, R., Chung, J. H., Wu, Y., Parrish, T., & Ragin, A. B. (2011). Abnormalities in resting‐state functional connectivity in early human immunodeficiency virus infection. Brain Connectivity, 1(3), 207–217. 10.1089/brain.2011.0016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willen, E. J. (2006). Neurocognitive outcomes in pediatric HIV. Mental Retardation and Developmental Disabilities Research Reviews, 12(3), 223–228. 10.1002/mrdd.20112 [DOI] [PubMed] [Google Scholar]
- Wilson, T. W., Heinrichs‐Graham, E., Becker, K. M., Aloi, J., Robertson, K. R., Sandkovsky, U., … Swindells, S. (2015). Multimodal neuroimaging evidence of alterations in cortical structure and function in HIV‐infected older adults. Human Brain Mapping, 36(3), 897–910. 10.1002/hbm.22674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods, S. P., Moore, D. J., Weber, E., & Grant, I. (2009). Cognitive neuropsychology of HIV‐associated neurocognitive disorders. Neuropsychology Review, 19(2), 152–168. 10.1007/s11065-009-9102-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, Y., Storey, P., Cohen, B. A., Epstein, L. G., Edelman, R. R., & Ragin, A. B. (2006). Diffusion alterations in corpus callosum of patients with HIV. American Journal of Neuroradiology, 27(3), 656–660. [PMC free article] [PubMed] [Google Scholar]
- Zahr, N. M., Rohlfing, T., Pfefferbaum, A., & Sullivan, E. V. (2009). Problem solving, working memory, and motor correlates of association and commissural fiber bundles in normal aging: A quantitative fiber tracking study. NeuroImage, 44(3), 1050–1062. 10.1016/j.neuroimage.2008.09.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, S. W., & Arfanakis, K. (2018). Evaluation of standardized and study‐specific diffusion tensor imaging templates of the adult human brain: Template characteristics, spatial normalization accuracy, and detection of small inter‐group FA differences. NeuroImage, 172, 40–50. 10.1016/j.neuroimage.2018.01.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, Y. W., Li, R. L., Wang, X. X., Miao, H., Wei, Y. R., Ali, R. W., … Li, H. J. (2017). Motor‐related brain abnormalities in HIV‐infected patients: A multimodal MRI study. Neuroradiology, 59(11), 1133–1142. 10.1007/s00234-017-1912-1 [DOI] [PubMed] [Google Scholar]
- Zhuang, Y., Zhang, Z., Tivarus, M., Qiu, X., Zhong, J., & Schifitto, G. (2021). Whole‐brain computational modeling reveals disruption of microscale brain dynamics in HIV infected individuals. Human Brain Mapping, 42(1), 95–109. 10.1002/hbm.25207 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting Information.


