Summary
Physical confinement, or restraint, is a psychological stressor used in rodent studies. A single restraint episode elevates blood corticosterone levels, a hallmark of stress responses. Repeated restraint results in habituation (or desensitization), whereas chronic exposure to unpredictable stressors fails to induce habituation. Here, we provide our protocols and guidelines in using three mouse restraint models, namely prolonged restraint stress, repeated restraint stress, and chronic variable stress, to examine immunological homeostasis/competence, or lack thereof, under stress with or without habituation.
For complete information on the generation and use of these protocols, please refer to Rudak et al. (2021).
Subject areas: Immunology, Model Organisms, Neuroscience, Behavior
Graphical abstract

Highlights
-
•
Three physical restraint mouse models to study the impact of long-term stress on immunity
-
•
A model of prolonged restraint stress altering immune homeostasis/competence
-
•
A model of repeated daily restraint stress resulting in habituation in animals
-
•
An optimized protocol for chronic variable stress circumventing habituation
Physical confinement, or restraint, is a psychological stressor used in rodent studies. A single restraint episode elevates blood corticosterone levels, a hallmark of stress responses. Repeated restraint results in habituation (or desensitization), whereas chronic exposure to unpredictable stressors fails to induce habituation. Here, we provide our protocols and guidelines in using three mouse restraint models, namely prolonged restraint stress, repeated restraint stress, and chronic variable stress, to examine immunological homeostasis/competence, or lack thereof, under stress with or without habituation.
Before you begin
Housing and preparation of experimental mice
Timing: [1–2 weeks]
-
1.
Experimental protocols must be approved first by local or institutional animal use/care committees. All experimental procedures described herein were approved by Animal Care and Veterinary Services at Western University.
-
2.
House 4 mice per cage with free access to food and water in a quiet room with controlled temperature and humidity and a split light-dark cycle (e.g., light on at 07:00; light off at 19:00). If mice were transported from elsewhere, allow at least one week for them to acclimate to their new environment and housing conditions before proceeding with experiments.
-
3.
House stressed mice and non-stressed controls in separate cages. Returning stressed mice to cages containing control animals will stress the latter, due partly to odor cues (Sterley et al., 2018).
CRITICAL: In our protocols and recent publications (Matovic et al., 2020; Rudak et al., 2019, 2021; Salter et al., 2018), we have primarily used male C57BL/6 mice, at 8–16 weeks of age, before introducing the initial stressor. However, the effects of stress can vary by mouse strain (Mozhui et al., 2010), sex (Nair et al., 2021), and age (Ferrari et al., 2001), and so can immune responses. Therefore, we have also used female animals and other mouse strains (e.g., BALB/c) in some of our studies to date on stress (Rudak et al., 2021).
CRITICAL: For group housing, it is critical to use a consistent group size (e.g., 4 mice per cage) (Prendergast et al., 2014). If necessary, different group sizes (e.g., 2–3 per cage) can be set but need to be matched between stress and control cohorts.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Fluorescein isothiocyanate (FITC)-conjugated anti-mouse TCRβ monoclonal antibody (mAb) (clone H57-597) | Thermo Fisher Scientific | Cat # 11-5961-85; RRID: AB_465324 |
| Phycoerythrin-cyanine7 (PE-Cy7)-conjugated anti-mouse TCRβ mAb (clone H57-597) | Thermo Fisher Scientific | Cat # 25-5961-82; RRID: AB_2573507 |
| Phycoerythrin (PE)-conjugated anti-mouse NK1.1 mAb (clone PK136) | Thermo Fisher Scientific | Cat # 12-5941-82; RRID: AB_466050 |
| Phycoerythrin-cyanine7 (PE-Cy7)-conjugated anti-mouse NK1.1 mAb (clone PK136) | Thermo Fisher Scientific | Cat # 25-5941-82; RRID: AB_469665 |
| Chemicals, peptides, and recombinant proteins | ||
| Allophycocyanin (APC)-conjugated PBS-57-loaded mouse CD1d tetramers | NIH Tetramer Core Facility | N/A |
| Critical commercial assays | ||
| Corticosterone ELISA Kit | Arbor Assays | K014-H1 |
| CaspaTag Pan-Caspase In Situ Assay Kit | EMD Millipore | Cat # APT420 |
| Experimental models: organisms/strains | ||
| Mouse: C57BL/6: C57BL/6NCrl | Charles River Laboratories | Strain code: 027 |
| Mouse: BALB/c: BALB/cAnNCrl | Charles River Laboratories | Strain code: 028 |
| Other | ||
| 4°C Refrigerator with glass door or cold room | N/A | N/A |
| Small Mouse Holder (for mice weighing up to 25 g) | Kent Scientific | HLD-MS-T |
| Medium Mouse Holder (for mice weighing 25–50 g) | Kent Scientific | HLD-MM-T |
| Blood-collecting capillary tubes | Fisher Scientific | 22-260950 |
| Everlast Rocker 247 | Benchmark Scientific | BR5000 |
| Falcon 50-mL conical centrifuge tubes | Fisher Scientific | 14-432-22 |
| Glue gun | N/A | N/A |
| Plastic container for forced swim, 20 cm (diameter) × 25 cm (height) | N/A | N/A |
| Plastic disc (e.g., a button, 2.5 cm in diameter) | N/A | N/A |
| Plastic popsicle sticks | N/A | N/A |
| Virox 5 Concentrated Surface Cleaner & Disinfectant General Virucide | Diversey Global | 2963741 |
Materials and equipment
Alternatives: Mouse restrainers are commercially available from a variety of sources. To build a makeshift restraining device, drill ∼80 ventilation holes into the side of a 50-mL conical tube, cut a small opening for a plug at the bottom end of the tube, and a hole/slit for the animal’s tail in the cap. Make a plug by gluing a plastic popsicle stick to a plastic disc (e.g., a button with a diameter of 2.5 cm) (Figure 1). The plug can be used to adjust the available space within the tube and the restrained animal’s back-and-forth movements. To prevent the restraining device from rolling, glue two plastic sticks to the sides of the conical tube.
Figure 1.
A makeshift restraining device for mouse studies on psychological stress
A suitable restraint tube should contain ventilation holes on its sides, a small hole for a plug at its bottom end, and a hole/slit for the animal’s tail in its cap. Mice are to be held in restraining devices horizontally, as shown, without being physically compressed. This figure is a composite of three independent images.
Step-by-step method details
Prolonged restraint stress
Timing: [12 or 15 h]
Physical restraint is among the most commonly used stressors for the evaluation of immune responses or other physiological functions in stressed animals (Glaser and Kiecolt-Glaser, 2005). Accordingly, mice are confined in small tubes that restrict their movement without causing pain, constriction, or suffocation (Buynitsky and Mostofsky, 2009). Overnight restraint during the nocturnal phase, in which mice are active, provides a model of prolonged psychological stress (Glaser and Kiecolt-Glaser, 2005).
-
1.
Transfer mice to a procedure room separate from the original housing room. Physically confine each mouse for 12 h during the light-off period in a commercial or makeshift restrainer (Figure 1). During the confinement period, place the restraining device(s) inside a new cage(s). For 12 h of prolonged restraint stress, begin at 19:00 and end at 07:00 on the following day.
-
2.
Transfer the stressed mice back to their original home cage.
Optional: The duration of the confinement period can be extended to 15 h, starting at 19:00 and ending at 10:00, with high circulating levels of corticosterone readily detectable during this period (Figure 3).
-
3.
The non-stressed control cohort should remain undisturbed in their home cages in the original housing room with free access to food and water.
Optional: Prolonged restraint stress results in food and water deprivation, which is a strong physiological stressor on its own. To isolate the psychological effects of restraint from the animals’ inability to access food and water, separate non-stressed cohorts can be deprived of food and water for 12 or 15 h as applicable.
Optional: Animals can be subjected to additional interventions or receive agents or reagents of interest, such as those triggering select immune responses, before, during, and/or after the prolonged restraint period.
Optional: Monitor physiological functions and/or disease outcomes, for instance by collecting blood and/or tissue specimens (e.g., primary and/or secondary lymphoid organs) at appropriate time points. Venous blood can be collected after snipping the tip of the tail using glass capillary tubes. Alternatively, blood can be drawn from the saphenous vein into a 1.5-mL microcentrifuge tube.
Optional: Soluble factors and mediators of interest (cytokines, chemokines, enzymes, stress hormones, antibodies, etc.) can be quantified in the serum/plasma by enzyme-linked immunosorbent assays (ELISA) or similar assays. For example, α-galactosylceramide (αGC), a glycolipid agonist of invariant natural killer T (iNKT) cells, can be administered (100 μg/kg, i.p.) at the end of prolonged restraint stress, and blood cytokine levels can be measured at various time points post-αGC injection (Rudak et al., 2021).
Optional: Immune cells can be isolated from various tissues for downstream analyses (e.g., immunophenotyping, transcriptomic studies, functional assays). Fluorochrome-labeled antibodies and tetramers used for cell staining must be titrated to determine optimal working dilutions.
-
4.
Upon the completion of the confinement period, soak restraining tubes in a 1:125 solution of Virox 5 (e.g., 8 mL Virox 5 in 1 L of water) for 10 min. Subsequently, rinse the tubes in water and air-dry them for future use.
CRITICAL: It is recommended to house stressed and control mice in separate cages. Returning stressed animals to cages that contain control cage-mates will stress the latter due, in part, to odor cues (Sterley et al., 2018). Of note, however, in our recent prolonged restraint stress experiments (Rudak et al., 2019, 2021), in which we used cage-mates to fully control for housing conditions, we found restrained mice to exhibit a far greater stress response and significantly poorer innate-like invariant T cell responses than did control cage-mates. This suggests that the impact of social transmission of stress can be relatively mild compared to that of physical confinement. Removing one mouse from a cage that accommodates only two animals can cause stress, due to isolation, and should be avoided (Ieraci et al., 2016; Senst et al., 2016).
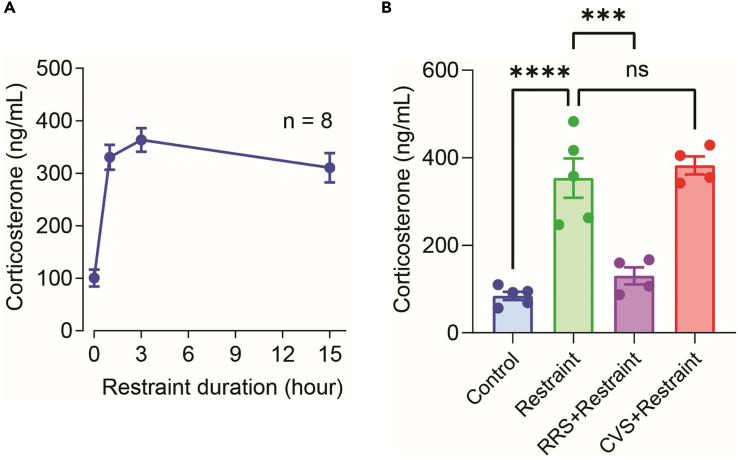
Figure 3.
Blood corticosterone levels in mice subjected to restraint stress with or without habituation
(A) Eight-to-ten-week old male C57BL/6 mice (n=8) were serially bled at indicated time points during a 15-h period of prolonged restraint stress in the nocturnal phase, followed by plasma corticosterone quantification by ELISA.
(B) In a separate experiment, blood corticosterone levels were measured immediately after a 1-h episode of restraint stress for the first time (Restraint), one day after a 21-day-long repeated restraint stress study was concluded (RRS + Restraint), or one day after a 21-day-long chronic variable stress was ended (CVS + Restraint). Data from stress-naïve mice are also provided. Each symbol represents an individual mouse, and error bars represent standard error of the mean. Statistical analyses were conducted using one-way ANOVA with Tukey’s correction. ∗∗∗∗ and ∗∗∗ denote significant statistical differences with p<0.0001 and p<0.001, respectively. ns, not significant
Repeated restraint stress
Timing: [once daily for 3 weeks]
In the repeated restraint stress (RRS) model, animals are exposed to the same stressor in a predictable manner, thus enabling habituation to the stressor. Repeatedly subjecting mice to restraint results in a gradual decrease in stress-induced neural activity (Matovic et al., 2020) and glucocorticoid release (Grissom and Bhatnagar, 2009). This entails daily restraint at a fixed time (e.g., 11:00–12:00) for 21 days.
-
1.
Place each mouse inside a commercial or makeshift restrainer made from a 50-mL conical tube (Figure 1) for 1 h. Place restrainers in a new cage(s).
-
2.
Transfer mice back to their home cages between restraint cycles and leave them undisturbed for the rest of the day.
Optional: Animals can be subjected to additional interventions and/or injected with agents or reagents of interest, such as those prompting immune responses, before, during, and/or after the RRS procedure.
CRITICAL: Stress levels in animals can be affected by a multitude of confounding factors, including but not limited to environmental noise and vibration, unstable room temperatures, and poor breeding conditions. It is therefore critical to include unstressed control cohorts in each experiment. The animals’ body weight and appearance should be monitored daily. A body weight loss of up to 10% can be expected. A loss of over 15% relative to the starting weight is considered too severe, and the animal should be withdrawn from the experiment.
CRITICAL: Restraint should be applied at the same time of the day. This is because the effects of stress have been reported to differ between active and inactive phases (Bartlang et al., 2012; Rybkin et al., 1997).
Chronic variable stress
Timing: [twice daily for 3 weeks]
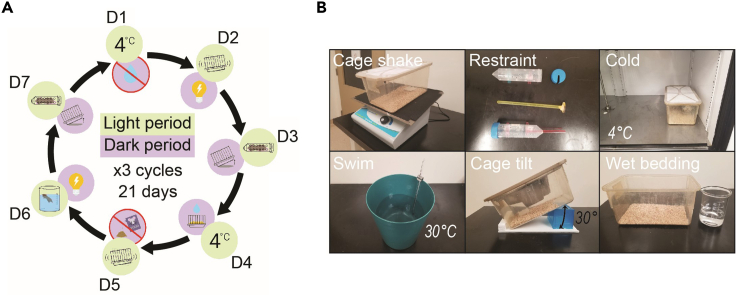
Chronic variable stress (CVS) is designed to minimize habituation by applying different stressors in an unpredictable fashion (Franco et al., 2016; Herman et al., 1995). Accordingly, mice are subjected to heterotypic psychological and physical stressors (including restraint), once daily and once nightly, for 21 days (Figure 2). The daytime stressors, which are of brief duration, are each randomized to be introduced anytime between 08:00–18:00. The nighttime stressors can be started anytime between 17:00–19:00 and terminated anytime between 08:00–10:00 on the following day. Below, we describe several daytime and nighttime stressors, which we routinely use in our optimized protocol.
Figure 2.
A typical mouse model of chronic variable stress (CVS)
(A) A weekly cycle in which various stressors are introduced is schematically illustrated and needs to be repeated thrice for a 21-day CVS study.
(B) Materials and/or conditions employed to apply various stressors include cage shaking (80 rpm for 1 h), physical restraint (1 h), cold exposure (at 4°C for 1 h), forced swimming (for 15 min in a bucket containing 30°C water), cage tilting overnight (at a 30-to-45-degree angle), and wet bedding overnight. Figure 2B is a composite of six independent images.
Daytime stressors:
-
1.Cage shake:
-
a.Place cages on a horizontal rocking laboratory shaker (Figure 2) set to a speed of 80 revolutions per minute (rpm) for a one-hour duration.
-
a.
- 2.
-
3.Cold exposure:
-
a.Place cages inside a laboratory refrigerator maintained at 4°C (Figure 2) for 1 h.
-
a.
-
4.Forced swim:
-
a.Fill a plastic bucket (20 cm in diameter at the top) with water to create a depth of at least 15 cm (Figure 2).
-
b.Adjust the water temperature to 30° ± 1°C using a thermometer. Place a mouse inside the water-filled bucket for 15 min. Dry the animal using paper towels before returning it to its home cage.
-
a.
CRITICAL: The water temperature commonly used for forced swim is 24°C–30°C (National Research Council (US) Committee on Guidelines for the Use of Animals in Neuroscience and Behavioral Research, 2003). Experimenters should be aware that water temperature even within this range (e.g., 25°C vs 30°C) can have different effects on the stress response and behavior (Bächli et al., 2008; Drugan et al., 2005). Therefore, using a consistent water temperature is important. Switching to different temperatures requires re-evaluation of the end points.
Nighttime stressors:
-
5.Light on:
-
a.Leave the light on in the room where mice are housed overnight.
-
a.
-
6.Cage tilt:
-
a.Elevate one side of the cage until it rests at a 30–45-degree angle (Figure 2). Leave the cage tilted overnight.
-
a.
CRITICAL: Make sure the water bottle is full and mice have access to water.
-
7.Wet bedding:
-
a.Pour tap water into home cages. The amount to be used should be sufficient to soak the bedding but not too large to create a pool of free-floating water in the cage. For a standard mouse cage (26 cm × 16.5 cm × 14 cm), approximately 200 mL of room temperature water is needed (Figure 2).
-
b.Leave mice in their wet cage(s) overnight.
-
c.Transfer mice to a new cage(s) with fresh bedding.
-
a.
-
8.Food deprivation:
-
a.Remove access to food overnight by transferring mice into a new cage(s) without food. Using new cage(s) is important because mice can potentially feed on small pieces of food pellets that may fall onto the bedding of their original cage(s). Overnight food deprivation can start anytime between 17:00–19:00 and end anytime between 8:00–10:00 on the following day.
-
a.
-
9.Water deprivation:
-
a.Withdraw access to water overnight by removing drinking bottles from home cages. Overnight water deprivation can start anytime between 17:00–19:00 and end anytime between 8:00–10:00 on the following day.
-
a.
Optional: Animals may receive additional interventions and/or agents or reagents of interest before, during, and/or after the CVS procedure.
CRITICAL: Stress levels in animals can be affected by a multitude of confounding factors, including but not limited to environmental noise and vibration, unstable room temperatures, and poor breeding conditions. It is therefore critical to include unstressed control cohorts in each experiment. The animals’ body weight and appearance should be monitored daily. A weight loss of up to 10% can be expected. A loss of over 15% relative to the starting weight is considered too severe, which warrants the animal’s withdrawal from the experiment.
CRITICAL: In the case of overnight food deprivation and water deprivation, body weight needs to be measured 24 h after food/water is reintroduced to allow for recovery from a transient weight loss. Nighttime food deprivation and water deprivation should not be introduced consecutively. Moreover, their application, each, should be limited to once a week to avoid severe physiological challenges, otherwise introducing adaptive changes in addition to stress responses.
Expected outcomes
Prolonged restraint stress is expected to induce a sustained increase in circulating corticosterone levels (Figure 3A). The effectiveness of a stressor may be assessed by quantitating corticosterone in the plasma or serum immediately after restraint.
A one-hour episode of restraint stress alone should raise the plasma concentrations of corticosterone. After 21 days of RRS, however, mice should demonstrate habituation as evidenced by a drastically blunted corticosterone response to a subsequent one-hour episode of physical restraint. In contrast, after 21 days of CVS, mice should remain sensitive to restraint and should be able to mount an impeccable or near-impeccable corticosterone response to this stressor (Radley et al., 2013) (Figure 3B).
Body weight can also be a simple but valuable readout. Control mice will continue to gain weight in lengthy experiments. CVS will typically cause a weight loss of about 5% that will persist over the 21-day period. In contrast, RRS-induced transient weight loss will be gradually reversed towards the final week of the 21-day-long experiment (Figure 4).
Figure 4.
Body weight changes in mice subjected to RRS, CVS and control conditions
Adult mice were weighed daily before and after they were subjected to RRS or CVS. For control mice, body weights were recorded weekly at the time of cage cleaning. Error bars represent standard error of the mean.
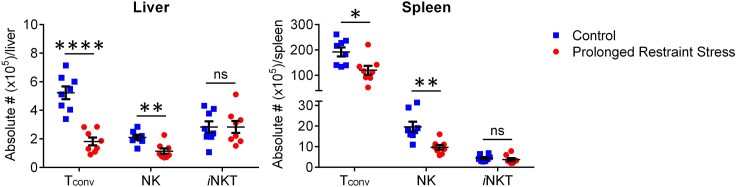
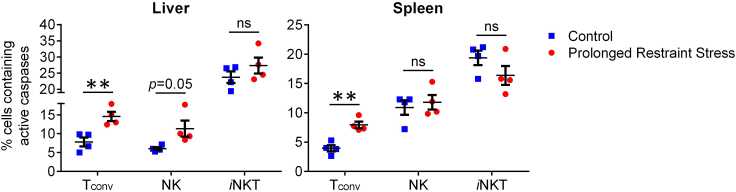
Psychological stress is known to alter many aspects of immune homeostasis and host responses in health and disease, and restraint models have proven useful in addressing such effects. For instance, restraint stress raises circulating levels of certain inflammatory cytokines, including interleukin (IL)-6 (Nukina et al., 2001; Qing et al., 2020). As another example, we recently reported that prolonged restraint stress decreases the absolute numbers of hepatic and splenic conventional T (Tconv) cells, natural killer (NK) cells, and B cells in mice (Rudak et al., 2021 and Figure 5). Our cytofluorimetric analyses also revealed increases in intracellular active caspase levels in Tconv and hepatic NK cells (Rudak et al., 2021 and Figure 6). Furthermore, the observed numerical reductions in NK and B cells were reversible upon treatment with RU486, a glucocorticoid receptor (GR) antagonist, and diminished Tconv cell counts were not evident in T cell-specific GR knockout mice (Rudak et al., 2021), indicating a requirement for GR signaling in stress-elicited death in the above compartments. In stark contrast, iNKT cells were unusually resistant to stress-induced apoptosis (Rudak et al., 2021 and Figures 5 and 6) although they became unresponsive or hyporesponsive to cognate glycolipid antigens and select cytokine stimuli (Rudak et al., 2021).
Figure 5.
The numerical abundance of Tconv, NK and iNKT cells following prolonged restraint stress
Adult C57BL/6 mice were subjected to 12 h of prolonged restraint stress. Control mice were left undisturbed without access to food and water. Immediately afterward, non-parenchymal hepatic mononuclear cells and splenocytes were isolated and stained with PBS-57-loaded CD1d tetramers and mAbs against NK1.1 and TCRβ. TCRβ+PBS-57-loaded CD1d tetramer- Tconv cells, NK1.1+TCRβ- NK cells, and TCRβ+PBS-57-loaded CD1d tetramer+iNKT cells were then enumerated in each organ by flow cytometry. Each symbol represents an individual mouse. Error bars represent standard error of the mean. ∗, ∗∗ and ∗∗∗∗ denote differences with p<0.05, p<0.01 and p<0.0001, respectively, using unpaired Student’s t-tests. ns, not significant
Figure 6.
The intracellular active caspase contents of Tconv, NK and iNKT cells after prolonged restraint stress
Adult male C57BL/6 mice were subjected to restraint stress for 12 h. Control cohorts remained undisturbed but were deprived of food and water. Non-parenchymal hepatic mononuclear cells and splenocytes were then stained using the CaspaTag Pan-Caspase In Situ Assay Kit before the levels of intracellular active caspases in Tconv, NK and iNKT cell populations were quantified by flow cytometry. Each symbol represents an individual mouse, and error bars represent standard error of the mean. ∗∗ denotes a difference with p<0.01 using unpaired Student’s t-tests. ns, not significant
Limitations
Host responses to both acute and chronic stress can be influenced by various factors, including housing conditions (e.g., building noise and air temperature fluctuations), past exposure to various other stressors (e.g., transportation), and early life events. It is critical to include a control group in each independent experiment. If any signs or indications of an overt stress response (e.g., high blood levels of corticosterone or a dramatic weight loss) are noticed in control mice, the experimental results should be discarded or interpreted with an abundance of caution at the very least.
Animal handling, anesthesia, injections, and blood collection, which are regarded as inherent stressors, should be performed consistently for both restrained and control cohorts. Blood draws for the measurement of corticosterone and other hormones or mediators should be conducted within 5 min of touching home cages, before potential manipulation-associated corticosterone release starts to affect the blood levels.
It is conceivable that mice subjected to prolonged restraint consume more metabolic resources as they attempt to escape from physical confinement. Therefore, restraint may not always represent a merely psychological stressor (Harris, 2015).
The CVS model involves the application of both psychological and physical stressors whose relative contributions to immunological outcomes are difficult to discern or interpret.
Troubleshooting
Problem 1
Baseline blood corticosterone levels in the absence of stress should be approximately 50 ng/mL (Rudak et al., 2021). Levels higher than 100 ng/mL in control mice may indicate their exposure to uncontrolled stressors (step 3 under prolonged restraint stress).
Potential solution
One common reason behind elevated blood corticosterone levels in control mice is animal handling prior to or during blood collection. To determine baseline corticosterone levels, blood should be withdrawn within 5 min of manipulating home cages, before potential manipulation-associated corticosterone release starts to affect the blood levels of this hormone. By the same token, the home cages of experimental mice should not be touched (e.g., for cage cleaning) on the day of blood collection. Home cages should be kept in a room with minimal traffic that is solely designated for the experiment.
Problem 2
If mice subjected to RRS fail to habituate to restraint, they will respond to a subsequent restraint episode of one hour in duration on day 22. The blood corticosterone levels found in these animals will be similar to those detectable in mice exposed to one-hour restraint for the first time (steps 1 and 2 under repeated restraint stress).
Potential solution
Presentation of an unexpected stressor can disrupt or reverse habituation (Grissom and Bhatnagar, 2009). Therefore, one should investigate any possible incidences or sources of uncontrolled stress (e.g., building construction noise, lack of food or water, cage flooding). Also of note, restraint should be applied by the same experimenter(s), no more than two individuals, throughout the RRS study.
Problem 3
Male mice housed together may show aggressive behavior, which would require the isolation of aggressive and/or injured animals. Consequently, the entire cage needs to be withdrawn from the experiment (steps 1–4 under prolonged restraint stress, steps 1 and 2 under repeated restraint stress, and steps 1–9 under chronic variable stress).
Potential solution
Aggression at the cage level is a multifaceted phenomenon, for which no established solutions currently exist (Weber et al., 2017). However, it may be mitigated by: i) establishing stable groups, by keeping siblings or familiar mice together; ii) transferring the nest site, but not soiled bedding, during cage changes; iii) keeping the ambient temperature at 20°C–22°C while providing abundant nesting materials; iv) avoiding exposure to female scent that can trigger aggression (Hurst, 2005), for instance through housing male and female mice in separate rooms.
Problem 4
Larger mice may be difficult to fit inside a standard commercially available mouse restrainer, which can cause unintended physical stress (hyperthermia due to reduced ventilation, suffocation, and physical pain) (step 1 under prolonged restraint stress, step 1 under repeated restraint stress, and step 2 under chronic variable stress).
Potential solution
Obtain or set up larger restrainers appropriate for the animals’ body size.
Problem 5
Large experiment-to-experiment variability is possible in how stress affects physiological functions (e.g., immune responses, neuroendocrine activities, body weight changes) or disease outcomes (steps 1–4 under prolonged restraint stress, steps 1 and 2 under repeated restraint stress, and steps 1–9 under chronic variable stress).
Potential solution
Stress responses and adaptation are influenced by numerous factors. Review all steps and potential variables across different experiments in detail. For example, differences in animal handling, which can vary between experimenters or animal care technicians, have been shown to affect stress-related physiological and behavioral changes (Gouveia and Hurst, 2017; Mertens et al., 2019).
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, S.M. Mansour Haeryfar (Mansour.Haeryfar@schulich.uwo.ca).
Materials availability
This study did not generate new unique reagents.
Acknowledgments
This work was funded through a Discovery Grant (04706-2019 RGPIN) from the Natural Sciences and Engineering Research Council of Canada (NSERC; to S.M.M.H.) and a Project Grant (PJT-148707) from the Canadian Institutes of Health Research (CIHR; to W.I.). Mouse behavioral work was supported by the BrainsCAN rodent core. P.T.R. was a recipient of an Alexander Graham Bell Canada Graduate Scholarship (CGS-Doctoral) from NSERC.
Author contributions
J.X.D. performed experiments and wrote the initial manuscript draft. P.T.R. performed experiments, analyzed and interpreted data, and edited the manuscript. W.I. developed and characterized chronic stress protocols, obtained funding, analyzed and interpreted data, and edited the final manuscript. S.M.M.H. obtained funding, administered the project, designed experiments, analyzed and interpreted data, and edited the final manuscript.
Declaration of interests
The authors declare no competing interests.
Contributor Information
Wataru Inoue, Email: winoue@uwo.ca.
S.M. Mansour Haeryfar, Email: mansour.haeryfar@schulich.uwo.ca.
Data and code availability
The results of plasma corticosterone measurements in control mice and those subjected to CVS (Figure 3) are available in our recently published article (Rudak et al., 2021). Data from animals subjected to prolonged restraint or to RRS (Figure 3) have not been published but can be made available by the corresponding author upon request. The body weight data illustrated in Figure 4 are unpublished but available from the corresponding author. The absolute numbers (Figure 5) and the active caspase content (Figure 6) of Tconv and iNKT cells were recently published (Rudak et al., 2021). Similar values for NK cells (Figures 5 and 6) have not been published but can be obtained from the corresponding author.
References
- Bächli H., Steiner M.A., Habersetzer U., Wotjak C.T. Increased water temperature renders single-housed C57BL/6J mice susceptible to antidepressant treatment in the forced swim test. Behav. Brain Res. 2008;187:67–71. doi: 10.1016/j.bbr.2007.08.029. [DOI] [PubMed] [Google Scholar]
- Bartlang M.S., Neumann I.D., Slattery D.A., Uschold-Schmidt N., Kraus D., Helfrich-Förster C., Reber S.O. Time matters: pathological effects of repeated psychosocial stress during the active, but not inactive, phase of male mice. J. Endocrinol. 2012;215:425–437. doi: 10.1530/JOE-12-0267. [DOI] [PubMed] [Google Scholar]
- Buynitsky T., Mostofsky D.I. Restraint stress in biobehavioral research: recent developments. Neurosci. Biobehav. Rev. 2009;33:1089–1098. doi: 10.1016/j.neubiorev.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Drugan R.C., Eren S., Hazi A., Silva J., Christianson J.P., Kent S. Impact of water temperature and stressor controllability on swim stress-induced changes in body temperature, serum corticosterone, and immobility in rats. Pharmacol. Biochem. Behav. 2005;82:397–403. doi: 10.1016/j.pbb.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Ferrari E., Cravello L., Muzzoni B., Casarotti D., Paltro M., Solerte S.B., Fioravanti M., Cuzzoni G., Pontiggia B., Magri F. Age-related changes of the hypothalamic-pituitary-adrenal axis: pathophysiological correlates. Eur. J. Endocrinol. 2001;144:319–329. doi: 10.1530/eje.0.1440319. [DOI] [PubMed] [Google Scholar]
- Franco A.J., Chen C., Scullen T., Zsombok A., Salahudeen A.A., Di S., Herman J.P., Tasker J.G. Sensitization of the hypothalamic-pituitary-adrenal Axis in a male rat chronic stress model. Endocrinology. 2016;157:2346–2355. doi: 10.1210/en.2015-1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser R., Kiecolt-Glaser J.K. Stress-induced immune dysfunction: implications for health. Nat. Rev. Immunol. 2005;5:243–251. doi: 10.1038/nri1571. [DOI] [PubMed] [Google Scholar]
- Gouveia K., Hurst J.L. Optimising reliability of mouse performance in behavioural testing: the major role of non-aversive handling. Sci. Rep. 2017;7:1–12. doi: 10.1038/srep44999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grissom N., Bhatnagar S. Habituation to repeated stress: get used to it. Neurobiol. Learn. Mem. 2009;92:215–224. doi: 10.1016/j.nlm.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris R.B.S. Chronic and acute effects of stress on energy balance: are there appropriate animal models? Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015;308:R250–R265. doi: 10.1152/ajpregu.00361.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman J.P., Adams D., Prewitt C. Regulatory changes in neuroendocrine stress-integrative circuitry produced by a variable stress paradigm. Neuroendocrinology. 1995;61:180–190. doi: 10.1159/000126839. [DOI] [PubMed] [Google Scholar]
- Hurst J. National Centre for the Replacement, Refinement and Reduction of Animals in Research; London, UK: 2005. Making Sense of Scents: Reducing Aggression and Uncontrolled Variation in Laboratory Mice; pp. 1–8. [Google Scholar]
- Ieraci A., Mallei A., Popoli M. Social isolation stress induces anxious-depressive-like behavior and alterations of neuroplasticity-related genes in adult male mice. Neural Plast. 2016;2016:6212983. doi: 10.1155/2016/6212983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matovic S., Ichiyama A., Igarashi H., Salter E.W., Sunstrum J.K., Wang X.F., Henry M., Kuebler E.S., Vernoux N., Martinez-Trujillo J. Neuronal hypertrophy dampens neuronal intrinsic excitability and stress responsiveness during chronic stress. J. Physiol. 2020;598:2757–2773. doi: 10.1113/JP279666. [DOI] [PubMed] [Google Scholar]
- Mertens S., Vogt M.A., Gass P., Palme R., Hiebl B., Chourbaji S. Effect of three different forms of handling on the variation of aggression-associated parameters in individually and group-housed male C57BL/6NCrl mice. PLoS One. 2019;14:e0215367. doi: 10.1371/journal.pone.0215367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozhui K., Karlsson R.-M., Kash T.L., Ihne J., Norcross M., Patel S., Farrell M.R., Hill E.E., Graybeal C., Martin K.P. Strain differences in stress responsivity are associated with divergent amygdala gene expression and glutamate-mediated neuronal excitability. J. Neurosci. 2010;30:5357–5367. doi: 10.1523/JNEUROSCI.5017-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair B.B., Aung Z.K., Porteous R., Prescott M., Glendining K.A., Jenkins D.E., Augustine R.A., Silva M.S.B., Yip S.H., Bouwer G.T. Impact of chronic variable stress on neuroendocrine hypothalamus and pituitary in male and female C57BL/6J mice. J. Neuroendocrinol. 2021;33:e12972. doi: 10.1111/jne.12972. [DOI] [PubMed] [Google Scholar]
- National Research Council (US) Committee on Guidelines for the Use of Animals in Neuroscience and Behavioral Research . National Academies Press (US); 2003. Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research. [PubMed] [Google Scholar]
- Nukina H., Sudo N., Aiba Y., Oyama N., Koga Y., Kubo C. Restraint stress elevates the plasma interleukin-6 levels in germ-free mice. J. Neuroimmunol. 2001;115:46–52. doi: 10.1016/s0165-5728(01)00260-0. [DOI] [PubMed] [Google Scholar]
- Prendergast B.J., Onishi K.G., Zucker I. Female mice liberated for inclusion in neuroscience and biomedical research. Neurosci. Biobehav. Rev. 2014;40:1–5. doi: 10.1016/j.neubiorev.2014.01.001. [DOI] [PubMed] [Google Scholar]
- Qing H., Desrouleaux R., Israni-Winger K., Mineur Y.S., Fogelman N., Zhang C., Rashed S., Palm N.W., Sinha R., Picciotto M.R. Origin and function of stress-induced IL-6 in murine models. Cell. 2020;182:372–387.e14. doi: 10.1016/j.cell.2020.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley J.J., Anderson R.M., Hamilton B.A., Alcock J.A., Romig-Martin S.A. Chronic stress-induced alterations of dendritic spine subtypes predict functional decrements in an hypothalamo-pituitary-adrenal-inhibitory prefrontal circuit. J. Neurosci. 2013;33:14379–14391. doi: 10.1523/JNEUROSCI.0287-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudak P.T., Gangireddy R., Choi J., Burhan A.M., Summers K.L., Jackson D.N., Inoue W., Haeryfar S.M.M. Stress-elicited glucocorticoid receptor signaling upregulates TIGIT in innate-like invariant T lymphocytes. Brain Behav. Immun. 2019;80:793–804. doi: 10.1016/j.bbi.2019.05.027. [DOI] [PubMed] [Google Scholar]
- Rudak P.T., Choi J., Parkins K.M., Summers K.L., Jackson D.N., Foster P.J., Skaro A.I., Leslie K., McAlister V.C., Kuchroo V.K. Chronic stress physically spares but functionally impairs innate-like invariant T cells. Cell Rep. 2021;35:108979. doi: 10.1016/j.celrep.2021.108979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybkin I.I., Zhou Y., Volaufova J., Smagin G.N., Ryan D.H., Harris R.B.S. Effect of restraint stress on food intake and body weight is determined by time of day. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1997;273:R1612–R1622. doi: 10.1152/ajpregu.1997.273.5.R1612. [DOI] [PubMed] [Google Scholar]
- Salter E., Sunstrum J., Matovic S., Inoue W. Chronic stress dampens excitatory synaptic gain in the paraventricular nucleus of the hypothalamus. J. Physiol. 2018;596:4157–4172. doi: 10.1113/JP275669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senst L., Baimoukhametova D., Sterley T.-L., Bains J.S. Sexually dimorphic neuronal responses to social isolation. Elife Sci. 2016;5:e18726. doi: 10.7554/eLife.18726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterley T.-L., Baimoukhametova D., Füzesi T., Zurek A.A., Daviu N., Rasiah N.P., Rosenegger D., Bains J.S. Social transmission and buffering of synaptic changes after stress. Nat. Neurosci. 2018;21:393–403. doi: 10.1038/s41593-017-0044-6. [DOI] [PubMed] [Google Scholar]
- Weber E.M., Dallaire J.A., Gaskill B.N., Pritchett-Corning K.R., Garner J.P. Aggression in group-housed laboratory mice: why can’t we solve the problem? Lab. Anim. 2017;46:157–161. doi: 10.1038/laban.1219. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The results of plasma corticosterone measurements in control mice and those subjected to CVS (Figure 3) are available in our recently published article (Rudak et al., 2021). Data from animals subjected to prolonged restraint or to RRS (Figure 3) have not been published but can be made available by the corresponding author upon request. The body weight data illustrated in Figure 4 are unpublished but available from the corresponding author. The absolute numbers (Figure 5) and the active caspase content (Figure 6) of Tconv and iNKT cells were recently published (Rudak et al., 2021). Similar values for NK cells (Figures 5 and 6) have not been published but can be obtained from the corresponding author.