Abstract
Antimicrobial resistance (AMR) is a major threat to humans and animals globally. Antimicrobial stewardship has been acknowledged as a primary strategy to tackle AMR. An important first step for antimicrobial stewardship is to quantify antimicrobial use (AMU). In Fiji, there are currently no data on AMU in livestock farms. This study aimed to quantify AMU in different livestock enterprises (beef, dairy, broiler, and layer) and farming systems (backyard, semi-commercial and commercial) in Central and Western divisions of Viti Levu, Fiji. A survey with 210 livestock farmers and 26 managers representing 276 enterprises was conducted between May and September 2019. The difference in AMU between different livestock enterprises and farming systems was investigated using ANOVA. In Fiji, the estimated annual antibiotic use in livestock was lower than the global average (44 compared with 118 mg/PCU). However, this use was concentrated in 56% of participant farms (the remaining 44% did not use antimicrobials). Total estimated quarterly anthelmintic use (20,797 mg) was not affected by farming systems but was highest (P < 0.001) in dairy enterprises (24,120 mg) and lowest in broiler enterprises (4 mg). Quarterly antibiotic use was different between the enterprises regardless of the metrics used to quantify the use (P < 0.05). Total estimated quarterly mg/PCU of antibiotic use was highest (P < 0.001) in broiler enterprises (12.4 mg/PCU) and lowest in beef enterprises (0.2 mg/PCU). For all other ESVAC metrics, total estimated antibiotic use was higher in poultry and lower in cattle enterprises. Backyard systems used less antibiotics (total mg) than commercial systems, but for other metrics, the trend was reversed. The use of both antibiotics and anthelmintics (rather than antibiotics or anthelmintics alone, or no AMU) was associated with dairy enterprises (Χ2 = 123, P < 0.001). Further studies should be conducted to quantify and evaluate the drivers of AMU in Fijian livestock farms. In addition, differences in AMU between different enterprises and farming systems suggest that strategies to reduce AMU should be tailored to specific settings.
Keywords: Quantification, Antimicrobial use, Farm enterprises, Farming systems, Fiji
Highlights
-
•
A little over half (56%) of 276 livestock enterprises surveyed in Fiji used antimicrobials.
-
•
22% of these enterprises used only antibiotics, 16% used only anthelmintics, 18% used both antibiotics and anthelmintics.
-
•
Highest quarterly antibiotic use (12.4 mg/PCU) was in broiler enterprises and anthelmintics use (24,120 mg) in dairy enterprises.
-
•
Estimated annual antibiotic use of 44 mg/PCU in Fijian livestock farms (beef, dairy, broiler, and layer) is lower than the global average of 118 mg/PCU.
-
•
Evidence that antibiotic use (mg/PCU) tended to be higher in Fijian backyard systems compared with semi-commercial and commercial systems
1. Introduction
Antimicrobial Resistance (AMR) is a major threat to human health globally [1,2]. Although a direct link between antimicrobial use (AMU) in livestock and increasing AMR in humans has yet to be established, there is an acknowledgement of the need to reduce the use of antimicrobials in livestock farms [[3], [4], [5], [6], [7]]. Many farm level AMU monitoring studies have highlighted the AMU in livestock such as pigs, broiler flocks and cattle globally [4,5,[8], [9], [10]]. Livestock serve as a major source of protein and essential nutrition for human development and also provide social and economic security to the livelihoods of millions of livestock keepers [11,12]. Livestock are raised for domestic consumption (backyard), domestic consumption and sales (semi-commercial) and for sales only (commercial) [12]. Ensuring the safety of animal-based foods while securing the livelihoods of livestock keepers is therefore an important consideration [13,14].
Globally, the World Health Organization (WHO), World Organization of Animal Health (OIE) and Food and Agricultural Organization of United Nations (FAO) have agreed to promote the prudent use of antimicrobials in livestock through the One Health approach [1,15]. Surveillance systems such as the European Surveillance Veterinary Antimicrobial Consumption (ESVAC) project [16,17], WHO GLASS (Global Antimicrobial Surveillance System) [15] and the OIE surveillance of veterinary medicines use [18] are now in place in many developed countries such as the United States, European Union, Canada, Japan, and United Kingdom. The prudent use of antimicrobials has been prioritized to combat the global risks of AMR [1,2] whilst maintaining access to antimicrobials to combat the emerging zoonotic diseases [19].
In the Oceania region, there is surveillance data on AMU in the livestock sector in the Australian [8,20] and New Zealand settings [21], but not in Fiji [22]. In tropical developing countries, the majority of the population depend on backyard farming [11] where multiple species of livestock are kept for domestic use and sold when required [12,23]. In Fiji, the majority of the population live and raise livestock in Viti Levu [24,25] but as yet, information on livestock farmers, enterprises and farming systems is unknown. The relationship between the farming systems (commercial, semi-commercial or backyard) and AMU in Fijian livestock production is unclear.
To date, data on AMR patterns in the Fijian human health sector have been published [26] but there is no equivalent data in the livestock sector. There is currently no data on farm market orientation and livestock production distribution by economic models (farming systems) and antimicrobial sales and use by livestock. At a species level, there is limited information on annual animal census information and livestock performance data; the most recent accessible National Animal Census data is from 2009 [25] and the recently published 2020 Agricultural Census [24]. In addition, the gaps in diagnostic capability and lack of trained professionals in Fijian human health and animal health sectors have been highlighted [22]. There are also policy gaps on the regulation and importation of veterinary medicinal products in Fiji [22].
Globally, antibiotic use in livestock is reported to be 118 mg/PCU [18,27], where PCU is ‘population correction unit’ and takes into account the size of the animal population and the animal's liveweight at the time of treatment. As global food demand is increasing, it is predicted that livestock production will further intensify both locally and globally, resulting in further increases in AMU in livestock [23,28]. Within species globally, dairy farming systems tend to use more antibiotics than beef farming systems due to the frequent treatment of mammary gland infections [29]. Even though antibiotic use is relatively low in beef farms, anthelmintic use may be high in beef cattle because most beef enterprises rely on extensive grazing, resulting in greater exposure of beef cattle to parasites [30,31]. In terms of mg/PCU, antibiotic use is higher in poultry compared with cattle production because of the higher stocking density of poultry [20]. Furthermore, antibiotic use in poultry enterprises could be expected to be higher compared with cattle production because of flock level rather than individual administration of antibiotics in poultry [21,29,32]. Within poultry enterprises, antibiotic use is higher in commercial broiler enterprises compared with laying hens because the former are young, fast growing birds that are more vulnerable than adult laying hens [[33], [34], [35], [36]]. In a Fijian setting, therefore, it was hypothesized that antibiotic use would be higher in poultry compared with cattle enterprises, and that commercial enterprises would use more antimicrobials than semi-commercial and backyard farming systems. The aim of this study was therefore to quantify AMU on Fijian livestock farms and determine the effect of farming systems (commercial, semi-commercial or backyard) and farm enterprises (beef, dairy, broiler, or layer) on AMU.
2. Material and methods
2.1. Study design, study area, and recruitment
A cross-sectional researcher-administered survey was conducted in livestock farms raising cattle and/or poultry from May to August 2019. The survey was conducted in the Central and Western divisions of Viti Levu, which is the largest island of Fiji. Using purposive and snowball sampling methods, livestock farmers and managers were recruited using the inclusion criterion {farmer or manager >18 years old, raised livestock (dairy, beef, broiler, or layer), located in Central or Western division, farm accessible by road}. Livestock farms were recruited from a list of farms provided by the Animal Health and Production division of the Fijian Ministry of Agriculture, local network of farmers and local markets. The eligibility of participants was assessed using the inclusion criteria by XK. Participants were then contacted via phone or visited in person if their contact details were unknown or unavailable. The purpose and scope of the survey was explained. Informed consent was taken prior to data collection. All collected information was anonymized prior to analysis.
2.2. Data collection
A survey was developed to collect information on farm population and production, AMU, other medicine use and feed and feeding practices. The ESVAC guide and literature review guided the development of the survey [17]. The parameters needed for the quantification of AMU was incorporated in the survey (see Supplementary Table 1). A single researcher (XK) visited participants at their farm and collected data using a paper copy of the survey. All data was collected in a single farm visit. Where available and accessible, farm records were used to collect information on AMU for the last three calendar months [32]. For farms without any records, the farmers and managers were asked to provide their best recall of estimated AMU for the past three calendar months [37]. Information from farm records, medicine labels, and verbal recollections of farmers were recorded directly onto paper copies of the survey. The survey was piloted with 10 farmers. Most of the farm visits lasted approximately 30 min and were conducted in English, however, where participants did not understand English, data collection was conducted in their spoken language (Itaukei, Hindi) and translated back to English by the researcher (XR). Ethical approval was granted by the Ethical Committee (Ref #: 00772P) at School of Agriculture, Policy and Development, University of Reading.
2.3. Data analysis
Data were transferred into Microsoft Excel (Microsoft Corporation, Washington, USA) for analysis. This study reports data relevant to the quantification of antimicrobials (antibiotics and anthelmintics) only. AMU was quantified using ESVAC metrics; namely milligrams (mg), milligrams of antibiotics used per population correction unit (mg/PCU), number of Defined Daily Doses (nDDDvet), number of Defined Course Doses (nDCDvet) [16,17,37,38], and those reported in the current literature; mg/kg (milligrams of antibiotics administered per kg of body surface area of animals treated), Treatment Frequency per day (TF per day), percentage treated (% treated), number of doses per animal per day (dose-animal per day) [17,[39], [40], [41]] (see Supplementary Table 1). Only oral, parenteral and intramammary antimicrobials were included in the quantification. In the absence of a Fijian national reference for DDDvet and DCDvet, the ESVAC DDDvet and DCDvet were used for benchmarking [38]. Since there is little seasonal variation in climate or livestock disease incidence in Fiji, the annual mg/PCU of antibiotics was estimated as four times that of quarterly use in cattle enterprises (mg/PCU × 4/1), four times that of quarterly use in broiler enterprises per four batches of flock (mg/PCU × 4/4) and four times that of quarterly use in layer enterprises per two batches of flock (mg/PCU × 4/2). We considered that cattle herds remained the same over a year, and on average, four batches of broiler were produced, and two batches of layer flocks were raised over a year. The ArcGIS Pro (ESRI) was used for mapping the enterprises on the island of Viti Levu, Fiji.
2.4. Statistical analysis
The SPSS Software V27 (IBM SPSS Statistics for Windows Version 27, Armonk, NY; IBM Corp) was used for descriptive and inferential analysis. Continuous data on AMU were log transformed and categorised into types of antimicrobial use (antibiotics only, anthelmintic only, combination of both, and no antimicrobial used). General linear model was adopted for hypothesis testing and the arithmetic mean of continuous data was reported. A Chi-square test was used for analysis of association between enterprise types and types of antimicrobial use. AMU data were analysed using analysis of variance (ANOVA) to investigate the effect of farming system and farm enterprise on AMU and mean separation was done by Tukey's test. Statistical significance was considered at P < 0.05.
3. Results and discussion
3.1. Farm, enterprises, and farming system characteristics
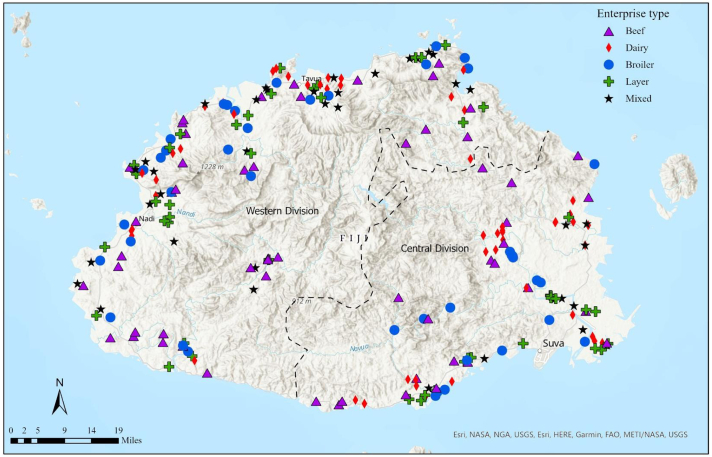
A total of 236 farms (94%) of the 250 farms contacted were recruited. Most farms were managed by farmers (89%) rather than farm managers (11%). The majority of participant farms (85%) raised single livestock species (i.e., single enterprise), but a small proportion of farms (15%) had more than one livestock species (i.e., mixed enterprises) (Fig. 1). Therefore, the 236 farms represented 276 enterprises in total, comprising of dairy (n = 74, 27%), layer (n = 73, 26%), beef (n = 72, 26%) and broiler (n = 57, 21%). The majority of enterprises were located in the Western division (n = 174, 63%) and raised livestock in semi-commercial enterprises (n = 166, 60%) (Table 1). Farm records were maintained by 122 farms (52%), but only 38 farms (16%) maintained antimicrobial use records. The median cattle herd size was 70 and 35 for beef (range 2 to 4500 cattle) and dairy (range 6 to 487 cattle) enterprises, respectively. Broiler and layer enterprises had a median flock size of 100 (range 5 to 192,000 chickens) and 160 (range 3 to 195,963 hens), respectively. Participating farms represented 14 and 15% of Fijian cattle herds and poultry flocks, respectively [24].
Fig. 1.
Distribution of livestock enterprises in Viti Levu, Fiji (n = 276).Dotted line represents the divisional border.
Table 1.
Description of enterprises by farming systems in Central and Western divisions of Viti Levu, Fiji.
| Enterprises by division | Farming system |
|||||||
|---|---|---|---|---|---|---|---|---|
| Backyard |
Semi commercial |
Commercial |
Total |
|||||
| n | % | n | % | n | % | n | % | |
| Central division | ||||||||
| Beef | 1 | 4 | 14 | 61 | 8 | 35 | 23 | 100 |
| Dairy | 2 | 6 | 15 | 48 | 14 | 45 | 31 | 100 |
| Broiler | 4 | 19 | 9 | 43 | 8 | 38 | 21 | 100 |
| Layer | 3 | 11 | 11 | 41 | 13 | 48 | 27 | 100 |
| Western division | ||||||||
| Beef | 2 | 4 | 35 | 71 | 12 | 24 | 49 | 100 |
| Dairy | 10 | 23 | 30 | 70 | 3 | 7 | 43 | 100 |
| Broiler | 2 | 6 | 25 | 69 | 9 | 25 | 36 | 100 |
| Layer | 11 | 24 | 27 | 59 | 8 | 17 | 46 | 100 |
| Total | ||||||||
| Beef | 3 | 4 | 49 | 68 | 20 | 28 | 72 | 100 |
| Dairy | 12 | 16 | 45 | 61 | 17 | 23 | 74 | 100 |
| Broiler | 6 | 11 | 34 | 60 | 17 | 30 | 57 | 100 |
| Layer | 14 | 19 | 38 | 52 | 21 | 29 | 73 | 100 |
The counts(n) and percentage (%) are presented in the columns.
3.2. Types of antimicrobials used in different enterprises
To the best of our knowledge, this study is the first to estimate the AMU in Fijian livestock enterprises. Antimicrobials were used by a little over half (56%) of the 276 participant livestock enterprises. Of these, 22% of 276 enterprises used antibiotics only, 16% anthelmintics only, and 18% used both antibiotics and anthelmintics (Table 2). The types of antimicrobials used in a livestock enterprise was associated with the enterprise type (P < 0.001, Table 2). Relatively large proportions of dairy (84%) and beef (60%) enterprises used antimicrobials, but only 42% of broiler and 36% of layer enterprises used antimicrobials. Antimicrobials were sourced from agricultural veterinary clinics (85%) and 87% of all AMU were self-prescribed by the farmers or managers. Given that there is higher misuse of antimicrobials when self-prescribed [13], identification of factors that drive AMU in different enterprises is necessary, so that more targeted interventions could be developed. No significant interactions were observed between farming system and enterprise (P > 0.05) for any measure of antimicrobial use.
Table 2.
Chi-Square analysis of association of enterprise type and type of antimicrobials used in 276 enterprises located in Central and Western division of Viti Levu, Fiji.
| Enterprise type | Antibiotics only |
Anthelmintics only |
Botha |
No AMUb |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | Χ2 | n | % | Χ2 | n | % | Χ2 | n | % | Χ2 | |
| Beef (n = 72) | 10 | 14 | 2 | 25 | 35 | 16 | 8 | 11 | 2 | 29 | 40 | 0 |
| Dairy (n = 74) | 11 | 15 | 2 | 14 | 19 | 0 | 37 | 50 | 42 | 12 | 16 | 13 |
| Broiler (n = 57) | 23 | 40 | 9 | 0 | 0 | 9 | 1 | 2 | 8 | 33 | 58 | 3 |
| Layer (n = 73) | 17 | 23 | 0 | 5 | 7 | 4 | 4 | 5 | 6 | 47 | 64 | 7 |
Both denotes both antibiotics and anthelmintics used in enterprises.
No AMU denotes no antimicrobials were used, n denotes the frequency, % denotes percentage observed, Χ2 denotes contribution to Chi-square, Chi-square statistics, Χ2 = 123, p < 0.001.
3.3. Quarterly anthelmintic use
3.3.1. Total mg
The present study is the first study to quantify anthelmintic use in different livestock enterprises and farming systems in Fiji. The quarterly anthelmintic use (mg) was not different between farming systems but was highest in the dairy enterprise followed by beef, layer, and broiler (Table 3). Both beef and dairy enterprises used more anthelmintics compared to broiler and layer enterprises, but the anthelmintic use was not different between the broiler and layer enterprises. As hypothesized above, a larger total amount (mg) of anthelmintic use could be expected in cattle compared with poultry. This is partly because cattle are much bigger than chickens, and partly because grazing cattle would be exposed to a greater helminth burden compared with poultry that are housed some or all of the time. Most interestingly, anthelmintics were not used at all in both backyard and commercial broiler enterprises. Although there are no Fijian past data for meaningful evaluation of anthelmintic use over time, our finding of high anthelmintic use in cattle is consistent with findings reported in other countries [39,42]. Despite lower amounts of anthelmintic use in poultry enterprises participating in the current study, overall anthelmintic use requires further attention due to the growing risks of anthelmintic resistance [30] and the presence of residues in animal products [43] and environment (groundwater) [31]. Given that a framework for the quantification of anthelmintic use was unavailable, the ‘mg’ metric was used [39]. The data from the current study can be used as a reference point for further longitudinal studies to quantify anthelmintic use. To implement anthelmintic stewardship programmes in the Fijian livestock sector similar to those in other countries [39], further studies are required to explore and understand the drivers for anthelmintic use.
Table 3.
Effects of farming systems and enterprises on quarterly antimicrobial use in Fijian livestock farms.
| Enterprise |
P |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Farming system | Beef | Dairy | Broiler | Layer | SEM | FS | E | FS*E | |
| Anthelmintic use (mg) | |||||||||
| Backyard | 900 | 7158 | 0 | 230 | 5522.1 | 0.104 | < 0.001 | 0.058 | |
| Semi commercial | 32,856 | 9363 | 6 | 701 | |||||
| Commercial | 112,111 | 75,157 | 0 | 3333 | |||||
| Antibiotic use: ESVAC metrics | |||||||||
| mg | Backyard | 0 | 23,065 | 698 | 17,278 | 8877.9 | 0.017 | < 0.001 | 0.215 |
| Semi commercial | 2696 | 11,702 | 3571 | 26,264 | |||||
| Commercial | 3075 | 51,073 | 211,699 | 80,120 | |||||
| mg/PCU | Backyard | 0 | 5 | 10 | 250 | 12.5 | 0.062 | < 0.001 | 0.950 |
| Semi commercial | <0.1 | 1 | 17 | 9 | |||||
| Commercial | <0.1 | 2 | 4 | 72 | |||||
| nDDDvet | Backyard | 0 | 0.1 | 0.2 | 8 | 0.4 | 0.001 | < 0.001 | 0.791 |
| Semi commercial | <0.1 | 0.1 | 0.5 | 0.3 | |||||
| Commercial | <0.1 | <0.1 | 0.2 | 2 | |||||
| nDCDvet | Backyard | 0 | 0.1 | <0.1 | 1.3 | 0.1 | 0.001 | 0.002 | 0.770 |
| Semi commercial | <0.1 | <0.1 | 0.1 | <0.1 | |||||
| Commercial | <0.1 | <0.1 | <0.1 | 0.4 | |||||
| Antibiotic use: non-ESVAC metrics | |||||||||
| mg/kg administered | Backyard | 0 | 14 | 7 | 148 | 9.5 | 0.640 | 0.040 | 0.384 |
| Semi commercial | 3 | 7 | 62 | 36 | |||||
| Commercial | 7 | 18 | 11 | 51 | |||||
| Treatment Frequency per day | Backyard | 0 | <0.1 | <0.1 | 0.1 | <0.1 | 0.025 | < 0.001 | 0.384 |
| Semi commercial | <0.1 | <0.1 | <0.1 | <0.1 | |||||
| Commercial | <0.1 | <0.1 | <0.1 | <0.1 | |||||
| Percentage treated (%) | Backyard | 0 | 36 | 30 | 27 | 2.7 | 0.762 | 0.002 | 0.583 |
| Semi commercial | 2 | 21 | 26 | 16 | |||||
| Commercial | 2 | 10 | 38 | 16 | |||||
| Dose-animal per day | Backyard | 0 | 0.2 | 2.1 | 3.4 | 0.1 | 0.022 | < 0.001 | 0.749 |
| Semi commercial | <0.1 | 0.3 | 0.8 | 0.7 | |||||
| Commercial | <0.1 | 0.2 | 1.3 | 0.4 | |||||
Note: FS denotes farming systems; E denotes the enterprises. ESVAC denotes European Surveillance of Veterinary antimicrobials consumption, SEM represent the standard error of mean of interaction of FS and E for each metric. 0 indicates no antimicrobials used. <0.1 indicates the number was smaller than 0.1. The arithmetic means are presented, and statistical analysis was conducted using log transformed data. Metrics: mg = the amount of antimicrobial use expressed as milligrams, mg/PCU = milligrams of antibiotics used per population correction unit of animals at risk (PCU- population correction unit- weight in kilograms of all animals at risk), nDDDvet = number of defined daily doses of antibiotics, nDCDvet = number of doses of antibiotics per treatment course, mg/kg = amount of antibiotics used in milligrams per kilograms weight of animals administered antibiotics per body surface area, TF per day = treatment frequency per day, Percentage treated = proportions (%) of animals treated with antibiotics, dose-animal per day = defined doses per total animal/days at risk (calculated per day).
3.4. Annual antibiotic use
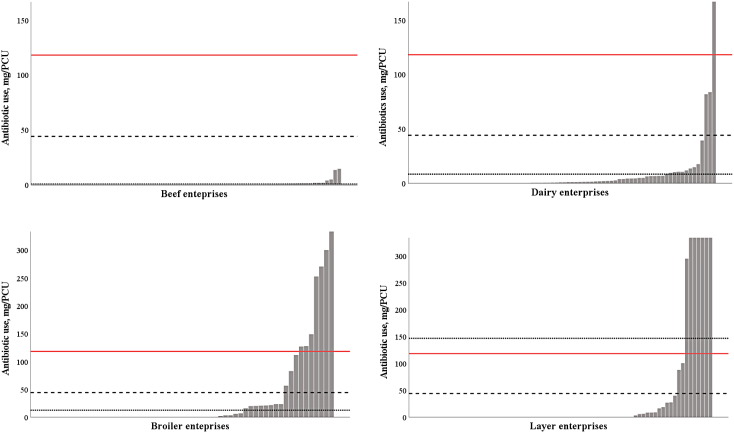
The estimated annual average antibiotic use across all enterprise types (beef, dairy, broiler, and layer) was 44 mg/PCU, which is lower than the global average of annual antibiotic use in livestock (118 mg/PCU) [27]. The antibiotic use estimated in the current study was similar to that reported for Solomon Islands (43.9 mg/PCU) and Papua New Guinea (44.1 mg/PCU) [27], but was considerably higher than that in New Zealand (10.2 mg/PCU in different food enterprises) [44]. Only a small proportion (4.7%) of the 276 enterprises participating in the current study used antibiotics above the global average of 118 mg/PCU. Most interestingly, antibiotic use was not greater than the global average in any beef enterprises (Fig. 2). 7 and 11% of broiler and layer enterprises, respectively, while some dairy enterprises used greater than the global annual average of 118 mg/PCU antibiotics (Fig. 2). Ironically, backyard systems used more antibiotics (mg/PCU) than global average compared to other systems; 14 vs 4 (semi-commercial) vs 1% (commercial). We suggest further studies are needed to quantify antibiotic use in all livestock enterprises as in the case in other countries [7,44] so that a more accurate country level estimation could be achieved, and the types of enterprise associated with higher levels of antibiotic use could be better identified.
Fig. 2.
Distribution of estimated yearly antibiotic use in total mg/PCU in different enterprises in Central and Western divisions of Viti Levu, Fiji. Note solid red line represents the global average of 118 mg/PCU of antibiotics. Dashed black line represents the estimated annual average (all enterprises) of 44 mg/PCU, SEM ± 25.2 mg/PCU) in all Fijian livestock farms. Dotted black line represents the estimated annual average mg/PCU of individual Fijian enterprise. (Beef = 0.7 mg/PCU, SEM ± 0.28 mg/PCU), (Dairy = 8.3 mg/PCU, SEM ± 3.28 mg/PCU), (Broiler = 12.4 mg/PCU. SEM ± 5.43 mg/PCU), (Layer = 146.37 mg/PCU, SEM ± 94.62 mg/PCU). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.5. Quarterly antibiotic use: ESVAC metrics
3.5.1. Total mg
Overall, total antibiotic use (mg) was highest in dairy and broiler with much lower use recorded in layer, and beef enterprises (Table 3). Farming system also affected use, being higher in commercial farming systems but with no significant difference between backyard and semi-commercial farming systems. High antibiotic use in dairy cattle is consistent with the findings from other countries and this trend could be attributed to higher disease prevalence in dairy cows because of environmental [29] and physiological stress [21]. The remarkably high use of antibiotics in broilers reared in commercial farming systems was because of larger flock sizes and higher numbers of production cycles per year. This is one of the limitations of using total ‘mg’ as a metric of antibiotic use, which has been acknowledged previously [37]. However, the use of this metric requires less effort in data collection and thus could generate useful antibiotic use data especially in countries where the framework for AMU surveillance does not exist. We further recommend studies quantifying antibiotics sold for use in livestock so that assessments could be done based on overall use and by enterprise types as has been done in other countries [7,44].
3.5.2. Total mg/PCU
Antibiotic use was higher in poultry (broiler followed by layer) enterprises compared with cattle (dairy followed by beef) enterprises (Table 3), in contrast to the total mg metric (4.5.1), reflecting the greater liveweight of cattle compared with poultry. Use within the cattle (beef vs dairy) or poultry (broiler vs layer) enterprises was not significantly different. There was no significant difference between systems (backyard, semi-commercial and commercial) in antibiotic use, but there was a tendency (P = 0.062) for a higher use in backyard systems particularly with laying hens. This is not what was hypothesized, and the reason for the high use in low-intensity, backyard systems is unclear. It may reflect the high value that is attributed to individual animals or birds in a backyard system, with a greater willingness to invest in their health as demonstrated in other studies [36]. It is also worth noting that a high usage was recorded on just four farms, and that the turnover of birds in a backyard system is much lower than in a commercial setting so that the size of PCU is lower (making the calculated mg/PCU higher) in a backyard system. Very limited availability of data on the comparison of antibiotic use between different farming systems makes it difficult to compare our findings with others [9,[34], [35], [36]]. Therefore, we recommend further studies quantifying antibiotic use in different farming systems.
3.5.3. Number of DDDvet and DCDvet
For the remaining two ESVAC metrics (nDDDvet and nDCDvet), antibiotic use was highest in the layer enterprise and lowest in the dairy enterprise. The broiler enterprise was the second highest user of antibiotics with the nDDDvet metric while the beef enterprise, the second highest user of antibiotics with the nDCDvet metric. The number of DDDvet was higher in both broiler and layer enterprises compared with the dairy enterprise and the nDCDvet tended to be higher in the layer enterprise compared with the dairy enterprise. The number of DCDvet was higher in layer and beef enterprises compared with the dairy enterprise, but the broiler and dairy enterprises had similar DCDvet. In the current study, the greater number of DDDvet and DCDvet in poultry compared with cattle is likely a consequence of blanket antibiotic administration via drinking water to the whole poultry flock as opposed to antibiotic treatment of individual cattle for therapeutic purposes [21,32,34]. In addition, the assessment of dose and course of intramammary units is necessary because of non-compliance to instructions noted in developing countries [45]. The reference DDDvet and DCDvet differ greatly between antibiotic classes, the dosage forms, and type of livestock [38] and thus, might have contributed to the difference in the antibiotic use between cattle and poultry enterprises.
Both nDDDvet and nDCDvet were higher in backyard and semi-commercial farming systems compared with commercial farming systems. However, they were not different between backyard and semi-commercial farming systems. These results suggest that there was a higher number of daily doses of antibiotics and a higher number of courses of antibiotics used in smaller herds and flocks (backyard and semi-commercial) compared with larger herds or flocks found in commercial farming systems [35,36]. Therefore, we recommend development of national reference for standardized daily and course doses of antimicrobials and standardized body weight of livestock similar to ESVAC in order to conduct further studies assessing the drivers for high antibiotic use in smaller herds of cattle and flocks of poultry.
3.6. Quarterly antibiotic use: Non-ESVAC metrics
3.6.1. Total mg/kg administered
For the non-ESVAC metric mg/kg administered per unit of body surface area, highest users of antibiotics were dairy enterprises, with much lower use recorded in beef cattle (Table 3). As with mg/PCU, the mg/kg metric may help in estimating and comparing antibiotic use within enterprises and therefore, we recommend the use of mg/PCU and mg/kg metrics to quantify antibiotic use in future studies so that national reference benchmarks could be developed at the enterprise level [16,46].
3.6.2. Treatment frequency (TF)
The treatment frequency per day of antibiotics use was highest in layer followed by broiler, beef, and dairy enterprises. The treatment frequency was presumed to be higher in layers because of administration of antibiotics at flock level compared with administration to individual birds or animals. The smaller herd size might also have contributed to lower use of antibiotics in cattle compared to poultry. Antibiotic use was higher in beef enterprises compared to dairy. However, similar AMU studies in other countries have demonstrated higher antibiotic use in dairy rather than beef enterprises [40]. It is unclear why this difference between Fiji and other countries has arisen, or whether such a difference is real.
3.6.3. Percentage treated (%)
The percentage treated with antibiotics was much lower in beef cattle compared with other enterprises. The flock level administration of antibiotics might explain the higher antibiotic use in poultry enterprises, but not in dairy farms. The likelihood of prophylactic use of antibiotics is higher in poultry [35,46], however, we were unable to establish if prophylactic use contributed to the high use of antibiotics in our study. Therefore, we suggest that future studies should evaluate on-farm practices so that necessary interventions could be developed to promote prudent antibiotic use.
3.6.4. Dose -animal per day
The number of dose-animal per day of antibiotics use was higher in poultry enterprises and in backyard farming systems. For all non-ESVAC metrics, the difference in antibiotic use was greater between cattle and poultry enterprises (being higher in poultry enterprises) than within poultry or cattle enterprises. Antibiotic use was higher in dairy compared with beef enterprises for mg/kg administered and percentage treated metrics. We presume the high antibiotic use in dairy enterprises may be because of the greater susceptibility of dairy cows to bacterial infections associated with lactation and poor hygiene at milking [29]. The use of antibiotics for growth promotion in beef cattle have been reported in other developing countries and therefore, we believe it may have contributed to some of the antibiotic use in beef enterprises in the current study [45]. The high use of antibiotics in poultry may, as has been noted, be in large part a consequence of flock level administration. The high use of antibiotics in backyard systems is of concern because of the large number of small-scale producers in a setting such as Fiji [24,25]. It is very important that the drivers for this high usage in backyard settings is understood, so that appropriate policies and interventions may be developed to address this.
3.7. Overall trends
In the current study, differences in AMU were observed between enterprises and farming systems. Some of these differences arise from the choice of metric and the different parameters used in the quantification of AMU by ESVAC and non-ESVAC metrics (liveweight, reference defined dose, course dose and population) [37]. Anthelmintic use was highest in cattle while antibiotic use was higher in poultry. Although there was no effect of farming system on anthelmintic use, with many of the metrics used to determine antibiotic use there was a significant difference between farming systems. The highest use of antibiotics was associated with backyard systems of production. This is despite these systems having a lower stocking density and, usually, lower levels of performance compared with commercial systems.
The mg/PCU metric has been commonly used for quantification and comparison of AMU in other countries [7] and our results based on the mg/PCU metric suggest that targeted interventions are required in poultry enterprises (both broiler and layer) which had higher antibiotic use than the global average (Fig. 2); many of these enterprises are backyard systems. Overall, Fijian annual antibiotic use is lower than the global average (44 mg/PCU vs 118 mg/PCU). However, anthelmintic and antibiotic use monitoring is required to reduce the use in a small proportion of livestock farm enterprises and promote the prudent use of antimicrobials. It is also important to evaluate and understand the livestock production and management factors which may have contributed to AMU, as studies in other countries have demonstrated [47]. To better explain the data reported in this paper, we suggest further studies are conducted to evaluate, explore, and understand the determinants of antibiotic use practice.
4. Limitations and future research
There was unequal participation per sub-category of farming system and enterprises in our study. We recommend a stratified approach to the recruitment of participant farms with different systems. Nevertheless, the distribution of enterprises across the island of Viti Levu, Fiji gives some confidence that the sample was illustrative of the type and range of farming enterprises and systems in Fiji. The lack of livestock registers, the National Livestock Census data (2020) only being made available in 2021 [24] and the numerous information gaps in the Fiji setting, means we adopted a 3-month survey approach as used in other studies [32]. Although ESVAC guidelines and other studies suggest a 12-month period survey, only a 3-month survey was feasible for our study [17,37,48] because we considered most farmers would not be able to recall information for more than the past 3 months. Data collection period was also constrained by practical consideration of time and resources.
A probability based random sampling method was impossible however inclusion and exclusion criteria were used to reduce bias. Nevertheless, since convenience sampling was done, and the 3-month survey data was extrapolated to annual use (mg/PCU), caution must be taken when extrapolating the data to determine national annual antibiotic use (mg/PCU). We extrapolated assuming that herds of cattle were the same over 12 months, the average of 4 batches of broiler flock were raised with batch in/out approach and 2 batches of layer flocks were raised with batch in/out approach. We also assumed that quarterly antibiotic use practice would be similar over the year and thus multiplying quarterly antibiotic use (mg/PCU) by 4 seemed justified. To enable more accurate estimation of annual AMU, we recommend yearlong studies to consider all seasons since weather could affect AMU as demonstrated in other studies [24,25,29,33,37,49]. The data source for quantification has limitations of its own. The accuracy of the farm's records or recollections of the antibiotic use for the past three months cannot be guaranteed, a limitation that has also been reported in other studies [37,50]. There were no published data or information available on the prescription and sales data of antimicrobials for livestock and therefore, farm record and verbal recollection of AMU by farmers was most feasible, a method which has also been used in other similar studies [32,37]. The sales data would have provided the sales of antimicrobials, however it does not provide an insight into actual use and purpose of AMU. Estimations and accuracy of liveweights of local livestock, required for the calculation of antibiotic use in many of the metrics, is a limitation but a farmer's estimation was considered more reflective of the actual weights of livestock rather than ESVAC estimates (see Supplementary Table 1).
Estimated antibiotic use and its precision cannot be confirmed, but it provides an estimate of relative antibiotic use for different enterprises and farming systems, which could be useful to prioritise the enterprise/system for which interventions need to be developed. Care must be taken when comparing mg/PCU of antibiotic use with other countries since different countries report consolidated values representing different enterprises [33]. There are limited studies that have quantified AMU using non-ESVAC metrics, thus we suggest more studies using a range of metrics for better evaluation and benchmarking of AMU within and between countries. Different studies have used different metrics to calculate the AMU which limits comparisons; however, all metrics assist in explaining relative AMU at the country level. In addition, the AMU in feed was unquantifiable as feed labels lacked information on the formulation and farmers were unaware of the content of feeds. Therefore, we suggest further studies to evaluate and understand the feed use practice so that antibiotic use in feed can be quantified in the future.
5. Conclusions
This study shows that quarterly anthelmintic use (mg) was high in dairy enterprises and antibiotic use (mg/PCU) in broiler enterprises. The overall annual antibiotic use in all enterprises was lower than the global average (44 vs 118 mg/PCU). The current study also shows that a small proportion of poultry and dairy enterprises used more antibiotics than the global average. There was some evidence that AMU was affected by farming system, with AMU being higher in backyard systems compared with commercial ones. This study suggests the need for follow up AMU longitudinal studies in all enterprises and studies evaluating and understanding drivers for AMU so that targeted policy recommendations can be made to reduce AMU in Fijian livestock farms.
The following is the supplementary data related to this article.
Metrics and parameters for quantification of antimicrobial use.
Authors' contribution
XK contributed to conceptualization, methodology, software, data collection, analysis, writing original draft preparation, writing, reviewing, and editing. CR, PR, RL contributed to conceptualization, methodology, analysis, reviewing and editing, interpretation, and supervision. All reviewed and approved the final version of the manuscript.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial or non-for -profit sectors.
Declaration of Competing Interest
None.
Acknowledgements
Our acknowledgment goes to the livestock farmers and managers who participated in the survey. Also, we thank the team of Animal Health and Production division of the Fijian Ministry of Agriculture who helped in identifying the livestock farms.
References
- 1.OIE Antimicrobial Resistance (AMR) 2021. http://www.oie.int/en/for-the-media/amr/ (Accessed 8 February 2021)
- 2.WHO Antimicrobial Resistance. 2021. http://www.who.int/en/news-room/fact-sheets/detail/antimicrobial-resistance (Accessed 8 February 2021)
- 3.Carrique-Mas J.J., Rushton J. Integrated interventions to tackle antimicrobial usage in animal production systems: the ViParc project in Vietnam. Front. Microbiol. 2017;8:1062. doi: 10.3389/fmicb.2017.01062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koo M., Cho A.R., Jeong A.R., Kim H.J., Park Y.H., Kwak H.S. Antibiotic susceptibility and molecular typing of enterococcus faecalis from retail pork meat products in Korea. J. Korean Soc. Appl. Biol. Chem. 2013;56:295–299. doi: 10.1007/s13765-012-3212-0. [DOI] [Google Scholar]
- 5.Strom G., Boqvist S., Albihn A., Fernstrom L.L., Djurfeldt A.A., Sokerya S. Antimicrobials in small-scale urban pig farming in a lower middle-income country - arbitrary use and high resistance levels. Antimicrob. Resist. Infect. Control. 2018;7 doi: 10.1186/s13756-018-0328-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andersen V.D., Aarestrup F.M., Munk P., Jensen M.S., de Knegt L.V., Bortolaia V. Predicting effects of changed antimicrobial usage on the abundance of antimicrobial resistance genes in finisher’ gut microbiomes. Prevent. Vet. Med. 2020;174:104853. doi: 10.1016/j.prevetmed.2019.104853. [DOI] [PubMed] [Google Scholar]
- 7.VMD UK Veterinary Antibiotic Resistance and Sales Surveillance Report (UK-VARSS 2019) 2020. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/950126/UK-VARSS_2019_Report__2020-TPaccessible.pdf (Accessed 8 July 2021)
- 8.Jordan D., Chin J.J., Fahy V.A., Barton M.D., Smith M.G., Trott D.J. Antimicrobial use in the Australian pig industry: results of a national survey. Aust. Vet. J. 2009;87:222–229. doi: 10.1111/j.1751-0813.2009.00430.x. [DOI] [PubMed] [Google Scholar]
- 9.Bryan M., Hea S.Y. A survey of antimicrobial use in dairy cows from farms in four regions of New Zealand. N. Z. Vet. J. 2017;65:93–98. doi: 10.1080/00480169.2016.1256794. [DOI] [PubMed] [Google Scholar]
- 10.Mitema E.S., Kikuvi G.M., Wegener H.C., Stohr K. An assessment of antimicrobial consumption in food producing animals in Kenya. J. Vet. Pharmacol. Ther. 2001;24:385–390. doi: 10.1046/j.1365-2885.2001.00360.x. [DOI] [PubMed] [Google Scholar]
- 11.Baiphethi M.N., Jacobs P.T. The contribution of subsistence farming to food security in South Africa. Agrekon. 2009;48:459–482. doi: 10.1080/03031853.2009.9523836. [DOI] [Google Scholar]
- 12.Adhikari J., Timsina J., Khadka S.R., Ghale Y., Ojha H. COVID-19 impacts on agriculture and food systems in Nepal: implications for SDGs. Agric. Syst. 2021;186:1–7. doi: 10.1016/j.agsy.2020.102990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ayukekbong J.A., Ntemgwa M., Atabe A.N. The threat of antimicrobial resistance in developing countries: causes and control strategies. Antimicrob. Resist. Infect. Control. 2017;6:1–8. doi: 10.1186/s13756-017-0208-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaurivi Y.B., Laven R.A., Parkinson T., Hickson R., Stafford K. Assessing extensive semi-arid rangeland beef cow–calf welfare in Namibia: part 1: comparison between farm production System’s effect on the welfare of beef cows. Animals. 2021;11:165. doi: 10.3390/ani11010165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.WHO Global Antimicrobial Resistane Surveillance System(GLASS) 2021. www.who.int/drugresistance (Accessed 20 February 2021)
- 16.EMA Principles on Assignment of Defined Daily Dose for Animals (DDDvet) and Defined Course Dose for Animals (DCDvet) 2015. https://www.ema.europa.eu/en/documents/scientific-guideline/principles-assignment-defined-daily-dose-animals-dddvet-defined-course-dose-animals-dcdvet_en.pdf (Accessed 8 February 2021)
- 17.EMA Guidance on Collection and Provision of National Data on Antimicrobial use by Animal Species/ Categories 2018. 2018. https://www.ema.europa.eu/en/documents/scientific-guideline/guidance-collection-provision-national-data-antimicrobial-use-animal-species/categories_en.pdf (Accessed 8 February 2021)
- 18.OIE OIE Annual Report on Antimicrobial Agents Intended for Use in Animal (fourth report) 2020. https://www.oie.int/scientific-expertise/veterinary-products/antimicrobials/ (Accessed 8 February 2021) [DOI] [PMC free article] [PubMed]
- 19.Córdoba-Aguilar A., Ibarra-Cerdeña C.N., Castro-Arellano I., Suzan G. Tackling zoonoses in a crowded world: lessons to be learned from the COVID-19 pandemic. Acta Trop. 2021;214:105780. doi: 10.1016/j.actatropica.2020.105780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Langham F., Cheng A.C. Antibiotic use in animals and humans in Australia. Med. J. Aust. 2019;211:159. doi: 10.5694/mja2.50258. [DOI] [PubMed] [Google Scholar]
- 21.McDougall S., Niethammer J., Graham E.M. Antimicrobial usage and risk of retreatment for mild to moderate clinical mastitis cases on dairy farms following on-farm bacterial culture and selective therapy. N. Z. Vet. J. 2018;66:98–107. doi: 10.1080/00480169.2017.1416692. [DOI] [PubMed] [Google Scholar]
- 22.Loftus M., Stewardson A.J., Naidu R., Coghlan B., Jenney A., Kepas J. Antimicrobial resistance in the Pacific Island countries and territories. BMJ Glob. Health. 2020;5 doi: 10.1136/bmjgh-2020-002418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thornton P.K. Livestock production: recent trends, future prospects. Philos. Trans. R. Soc. B. 2010;365:2853–2867. doi: 10.1098/rstb.2010.0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ministry of Agriculture 2020 Fiji Agriculture Census. 2021. https://www.agriculture.gov.fj/documents/census/VOLUMEI_DESCRIPTIVEANALYSISANDGENERALTABLEREPORT.pdf (Accessed 15 July 2021)
- 25.DOA Fiji National Agriculture Census 2009. 2009. http://www.fao.org/fileadmin/templates/ess/ess_test_folder/World_Census_Agriculture/Country_info_2010/Reports/Reports_3/FJI_ENG_REP_2009.pdf (Accessed 8 February 2021)
- 26.Kumar S., Graham S.M., Varman S., Kado J., Viney K. Resistance of bacterial isolates from neonates with suspected sepsis to recommended first-line antibiotics in Fiji. Pediatr. Infect. Dis. J. 2015;34:915–916. doi: 10.1097/inf.0000000000000764. [DOI] [PubMed] [Google Scholar]
- 27.Ritchie H. How do We Reduce Antibotic Resistance from Livestock? 2017. https://ourworldindata.org/antibiotic-resistance-from-livestock
- 28.Van Boeckel Thomas P., Brower Charles, Gilbert Marius, Grenfell Bryan T., Levin Simon A., Robinson Timothy P. Global trends in antimicrobial use in food animals. Proc. Natl. Acad. Sci. 2015;112:5649–5654. doi: 10.1073/pnas.1503141112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sinha R., Sinha B., Kumari R., Vineeth M.R., Verma A., Gupta I.D. Effect of season, stage of lactation, parity and level of milk production on incidence of clinical mastitis in Karan Fries and Sahiwal cows. Biol. Rhythm. Res. 2021;52:593–602. doi: 10.1080/09291016.2019.1621064. [DOI] [Google Scholar]
- 30.Learmount J., Stephens N., Boughtflower V., Barrecheguren A., Rickell K. The development of anthelmintic resistance with best practice control of nematodes on commercial sheep farms in the UK. Vet. Parasitol. 2016;229:9–14. doi: 10.1016/j.vetpar.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 31.Mooney D., Richards K.G., Danaher M., Grant J., Gill L., Mellander P.E. An analysis of the spatio-temporal occurrence of anthelmintic veterinary drug residues in groundwater. Sci. Total Environ. 2021;769:144804. doi: 10.1016/j.scitotenv.2020.144804. [DOI] [PubMed] [Google Scholar]
- 32.Redding L.E., Bender J., Baker L. Quantification of antibiotic use on dairy farms in Pennsylvania. J. Dairy Sci. 2019;102:1494–1507. doi: 10.3168/jds.2018-15224. [DOI] [PubMed] [Google Scholar]
- 33.Martin H., Manzanilla E.G., More S.J., O’Neill L., Bradford L., Carty C.I. Current antimicrobial use in farm animals in the Republic of Ireland. Ir. Vet. J. 2020;73:11. doi: 10.1186/s13620-020-00165-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cuong N.V., Phu D.H., Van N.T.B., Dinh Truong B., Kiet B.T., Hien B.V. High-resolution monitoring of antimicrobial consumption in Vietnamese small-scale chicken farms highlights discrepancies between study metrics. Front. Vet. Sci. 2019;6:174. doi: 10.3389/fvets.2019.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carrique-Mas J.J., Trung N.V., Hoa N.T., Mai H.H., Thanh T.H., Campbell J.I. Antimicrobial usage in chicken production in the Mekong Delta of Vietnam. Zoonoses Public Health. 2015;62:70–78. doi: 10.1111/zph.12165. [DOI] [PubMed] [Google Scholar]
- 36.Barroga T.R.M., Morales R.G., Benigno C.C., Castro S.J.M., Caniban M.M., Cabullo M.F.B. Antimicrobials used in backyard and commercial poultry and swine farms in the Philippines: a qualitative pilot study. Front. Vet. Sci. 2020;7:329. doi: 10.3389/fvets.2020.00329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mills H.L., Turner A., Morgans L., Massey J., Schubert H., Rees G. Evaluation of metrics for benchmarking antimicrobial use in the UK dairy industry. Vet. Rec. 2018;182:379. doi: 10.1136/vr.104701. [DOI] [PubMed] [Google Scholar]
- 38.EMA Defined Daily Doses for Animals (DDDvet) and Defined Course doses for Animals (DCDvet) 2016. https://www.ema.europa.eu/en/documents/other/defined-daily-doses-animals-dddvet-defined-course-doses-animals-dcdvet-european-surveillance_en.pdf (Accessed 8 February 2021)
- 39.RUMA Measuring Antibiotic Use - Dairy, Beef, Poultry. 2020. https://www.ruma.org.uk/measuring-antibiotic-use/ (Accessed 8 February 2021)
- 40.Hommerich K., Ruddat I., Hartmann M., Werner N., Käsbohrer A., Kreienbrock L. Monitoring antibiotic usage in German dairy and beef cattle farms—a longitudinal analysis. Front. Vet. Sci. 2019;6:244. doi: 10.3389/fvets.2019.00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Collineau L., Belloc C., Stärk K.D.C., Hémonic A., Postma M., Dewulf J. Guidance on the selection of appropriate indicators for quantification of antimicrobial usage in humans and animals. Zoonoses Public Health. 2017;64:165–184. doi: 10.1111/zph.12298. [DOI] [PubMed] [Google Scholar]
- 42.Gemeda B.A., Amenu K., Magnusson Ul., Dohoo I., Hallenberg G.S., Alemayehu G. Antimicrobial use in extensive smallholder livestock farming systems in Ethiopia: knowledge, attitudes, and practices of livestock keepers. Front. Vet. Sci. 2020;7:55. doi: 10.3389/fvets.2020.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Adesiyun A.A., Fasina F.O., Abafe O.A., Mokgoatlheng-Mamogobo M., Adigun O., Mokgophi T. Occurrence and concentrations of residues of tetracyclines, polyether ionophores, and anthelmintics in livers of chickens sold in the informal market in Gauteng Province, South Africa. J. Food Prot. 2021;84:655–663. doi: 10.4315/JFP-20-312. [DOI] [PubMed] [Google Scholar]
- 44.Hillerton J.E., Bryan M., Beattie B., Scott D., Millar A., French N. Use of antimicrobials for food animals in New Zealand: updated estimates to identify a baseline to measure targeted reductions. N. Z. Vet. J. 2021;69:180–185. doi: 10.1080/00480169.2021.1890648. [DOI] [PubMed] [Google Scholar]
- 45.Alhaji N.B., Aliyu M.B., Ghali-Mohammed I., Odetokun I.A. Survey on antimicrobial usage in local dairy cows in North-Central Nigeria: drivers for misuse and public health threats. PLoS One. 2019;14 doi: 10.1371/journal.pone.0224949Editor:Arda. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.De Campos J.L., Kates A., Steinberger A., Sethi A., Suen G., Shutske J. Quantification of antimicrobial usage in adult cows and preweaned calves on 40 large Wisconsin dairy farms using dose-based and mass-based metrics. J. Dairy Sci. 2021;104:4727–4745. doi: 10.3168/jds.2020-19315. [DOI] [PubMed] [Google Scholar]
- 47.Kramer T., Jansen L.E., Lipman L.J.A., Smit L.A.M., Heederik D.J.J., Dorado-Garcia A. Farmers’ knowledge and expectations of antimicrobial use and resistance are strongly related to usage in Dutch livestock sectors. Prevent. Vet. Med. 2017;147:142–148. doi: 10.1016/j.prevetmed.2017.08.023. [DOI] [PubMed] [Google Scholar]
- 48.Lardé Hélène, Francoz David, Roy Jean-Philippe, Massé Jonathan, Archambault Marie, Paradis Marie-Ève. Comparison of quantification methods to estimate farm-level usage of antimicrobials other than in medicated feed in dairy farms from Québec, Canada. Microorganisms. 2021;9:1106. doi: 10.3390/microorganisms9051106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Waret-Szkuta A., Coelho V., Collineau L., Hémonic A., Buy C., Treff M. How input parameters and calculation rules influence on-farm antimicrobial use indicators in animals. Front. Vet. Sci. 2019;6:438. doi: 10.3389/fvets.2019.00438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rees G.M., Barrett D.C., Sánchez-Vizcaíno F., Reyher K.K. Measuring antimicrobial use on dairy farms: a method comparison cohort study. J. Dairy Sci. 2021;104:4715–4726. doi: 10.3168/jds.2020-18690. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Metrics and parameters for quantification of antimicrobial use.