Abstract
Background: The recent newly appeared Coronavirus disease (COVID-19), caused by an enveloped RNA virus named “severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)”, is associated with severe respiratory morbidity and mortality. Recent studies have shown that lymphopenia and a cytokine mass release represent important pathogenic features, with clinical evidence of dyspnea and hypoxemia, often leading to acute respiratory distress syndrome (ARDS), in severely ill patients, with a high death toll. Currently, stem cells are actively being investigated for their potential use in many “untreatable” diseases. In this regard and in particular, Mesenchymal Stem Cells (MSC), due to their intrinsic features, including either ability to impact on regulation of the immune system, or association with both anti-viral and anti-inflammatory properties, or potential for differentiation into several cell lineages, have become a promising tool for cell and molecular-based therapies. On this background, we wished to explore whether human umbilical cord-derived mesenchymal stem cells (hUCMS) would represent a potential viable therapeutic approach for the management of critically ill COVID19 patients. Methods: We tested the hUCMS effects on peripheral blood mononuclear cell (PBMCs) retrieved from patients with COVID19 (Ethical Committee CEAS Umbria, Italy CER N°3658/20 7, May, 2020), both as free cell monolayers and after envelopment in sodium alginate microcapsules. Both cell systems, after priming with IFN-γ, proved able to produce several immunomodulatory molecules such as IDO1 and HLAG5, although only the microencapsulated hUCMS were associated with massive and dose-dependent production of these factors. Results: The microencapsulated hUCMS improved allo-suppression in mixed lymphocytes reactions (MLRs), while also blunting T helper 1 and T helper 17 responses, that are involved with the cytokine storm and greatly contribute to the patient death. Moreover, we observed that both free and microencapsulated hUCMS permitted 5 days survival of in vitro culture maintained PBMCs extracted from very ill patients. Conclusion: We have provided evidence that microencapsulated hUCMS in vitro, seem to represent a powerful tool to impact on several immune pathways, clearly deranged in COVID19 patients. Further study is necessary to begin in vivo assessment of this experimental system, upon determining both, the most appropriate time of the disease onset for intervention, and cell dosage/patient of our experimental product.
Keywords: Sars-CoV-2, COVID-19, Th17 skewing, cytokine storm, lymphopenia, hUCMS, sodium alginate, in vitro therapy, immunomodulation
Introduction
The novel contagious primary atypical pneumonia epidemic, which broke out in December 2019, is now formally named Coronavirus Disease 2019 (COVID19), with the causative virus identified as Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). Recent studies have shown that in addition to dyspnea and hypoxemia, possibly progressing into ARDS, lymphopenia and a cytokine mass release syndrome also represent relevant features in patients with severe SARS-CoV-2 infection. Eventually, impairment of the immune system homeostasis plays an important role in the development of COVID19 pneumonia [1]. The affected patients usually exhibit lymphopenia (often associated with unfavorable disease outcome, if present on admission to the hospital), lymphocyte activation and dysfunction, granulocyte and monocyte abnormalities, high cytokine levels, and an increase in total antibodies, with special regard to immunoglobulins G (IgG) [2]. Lymphocytes count decline below 20% in severe cases, including decrease of memory helper T cells as compared with less severe cases. Additional typical immune markers are: marked depletion of CD4+ T, CD8+ T, Natural Killers (NK), and B cells [3-5]. Finally, in COVID19 acutely ill patients, cytokine production is relevant, frequently leading to a “cytokine storm” on top of a series of adverse reactions. Upon infection with SARS-CoV-2, CD4+ T cells can rapidly be activated into pathogenic T helper (Th) 1 cells that secrete granulocyte-macrophage colony-stimulating factor (GM-CSF) [6]. This event triggers further expansion of CD14+CD16+ monocytes with production of high interleukin 6 (IL-6) levels, and accelerates inflammation. So far, several pharmacologic agents, such as Tocilizumab, Baricitinib, high dose corticosteroids and others [7] have been successfully employed at different stages of the disease, although severe involvement of the lungs leading into ARDS has resulted in high death toll in many instances.
Mesenchymal stem cells (MSC) hold natural propensity to fight viral infections due to their powerful anti-viral, coupled to their anti-inflammatory and immunomodulatory, properties, all potentially useful for preventing or attenuating the cytokine storm in COVID19 patients [8]. As a matter of fact, MSC-based cell therapy trials for COVID19 have recently been reported, especially applied to patients with severe pulmonary complications [9]. The rationale for these preliminary studies rested on the fact that MSC promote generation and expansion of regulatory immune cell subsets, such as CD4+CD25+FOXP3+ T cells, CD8+CD28- T cells, interleukin-10 (IL-10) producing B cells, and IL-10 -producing dendritic cells (DC) with immunoregulatory effects [10-13]. Additional molecular bases are that human adult Umbilical Cord Matrix Stem Cells (hUCMS), one of the youngest available mesenchymal stem cells, produce soluble non-classical histocompatibility antigen complexes, such as soluble human leukocyte antigen HLA-G5 (sHLA-G5) which was reported to contribute to MSC tolerogenic potential [14,15]. These immune-active features fostered application of MSC-based cell therapy to an array of autoimmune diseases including Systemic Lupus Erythematosus (SLE) [16], type 1 diabetes mellitus (T1D) and Sjogren Syndrome (SSJ), which led to restoration of a state of immune tolerance [17,18].
The above-described properties, all together, constitute a powerful immunomodulatory and anti-inflammatory complex stimulating the switch from an aggressive to a host’s immune tolerogenic state. All together, these actions constitute an anti-inflammatory complex which inhibits activation, proliferation and differentiation of allogeneic T cells into T helper 1 (Th1) and 17 (Th17) subsets, and stimulate their differentiation into T helper 2 (Th2) and T regulatory (Treg) cells [19]. In particular, immunosuppressive effects of IDO1 are based on the conversion of tryptophan into kynurenine (Kyn) [20].
Study rationale
We aimed to establish efficacy of an in vitro model in elucidating the possible impact of our hUCMS, upon microencapsulation in alginate-based (AG) microspheres, on PBMCs retrieved from patients with overt, but initial COVID19 disease that had not received any pharmacological therapy. AG microcapsules, generated in our laboratory decades ago, and subsequently finely implemented, had been used, upon approval by the national Regulatory Agency, in pilot human clinical trials of pancreatic islet transplantation grafts in patients with Type 1 Diabetes (T1D) [21].
We wished to assess the in vitro immunomodulatory effects of microencapsulated hUCMS on: (a) inhibition of blood mononuclear cell proliferation; (b) identification of the lymphomonocytes induced into apoptosis; (c) quantification of tolerogenic T cell populations (Treg) to the detriment of inflammatory T cell subsets (Th17 and Th1); (d) increase in survival and number of T cells in spite of COVID19-induced lymphopenia. Our idea was that this in vitro biohybrid model could help interpretation of the immunopathogenesis of COVID19 as well as development of a dose-response curve system, possibly suitable for exploring an in vivo cell therapy approach for the disease [8].
Materials and methods
Demographics and PBMC donor patient inclusion criteria
We enrolled in our study 18 patients between 29 and 80, 12 men and 6 women, hospitalized at the COVID19 Unit, University of Perugia Hospital (Table 1). Patient enrollment followed these criteria:
Table 1.
Demographics of the enrolled patients
| N° | Year of bird | Gender | % Lymphocytes (20.5-51.1) | % Monocytes (1.0-10.1) | % Neutrophils (42.0-75.0) | % Eosinophils (0.0-5.0) | % Basophils (0.0-1.0) |
|---|---|---|---|---|---|---|---|
| COV#1 | 1961 | M | 24.7 | 14.8 | 55.6 | 4.2 | 0.7 |
| COV#2 | 1944 | M | 13.9 | 8.6 | 75.2 | 1.7 | 0.6 |
| COV#3 | 1969 | F | 34.9 | 6.1 | 58.8 | 0 | 0.2 |
| COV#4 | 1943 | M | 8.9 | 5.2 | 84.4 | 1.3 | 0.2 |
| COV#5 | 1968 | M | 32.9 | 9 | 56.1 | 1.5 | 0.5 |
| COV#6 | 1960 | M | 8.6 | 5.4 | 85.8 | 0 | 0.2 |
| COV#7 | 1942 | M | 63.5 | 5.6 | 30.2 | 0.5 | 0.2 |
| COV#8 | 1991 | M | 19.2 | 9.6 | 70.5 | 0.5 | 0.2 |
| COV#9 | 1991 | F | 26 | 8.9 | 63.5 | 1.1 | 0.5 |
| COV#10 | 1945 | M | 4.5 | 5.8 | 88.6 | 0.9 | 0.2 |
| COV#11 | 1951 | F | 47.2 | 18.5 | 33.3 | 0.5 | 0.5 |
| *COV#12 | 1934 | M | 7 | 4.5 | 88.4 | 0 | 0.1 |
| COV#13 | 1986 | F | 5.9 | 1.8 | 92 | 0 | 0.3 |
| COV#14 | 1954 | F | 23.7 | 7.3 | 68.8 | 0 | 0.2 |
| COV#15 | 1957 | M | 17.4 | 6.5 | 74.8 | 1.1 | 0.2 |
| COV#16 | 1950 | M | 3.9 | 2.3 | 93 | 0.4 | 2.7 |
| COV#17 | 1976 | F | 30.1 | 8.7 | 60.9 | 0 | 0.3 |
| COV#18 | 1943 | M | 19.1 | 14 | 66.3 | 0.2 | 0.4 |
Patients were highly heterogeneous and some of them showed severe lymphopenia.
This patient died for complications related to the disease.
Inclusion criteria
1. Informed consent prior to performing study procedures. Written consent from patient or representatives was obtained. 2. Adult patients ≥18 years of age at the time of enrollment. 3. Laboratory-confirmed SARS-CoV-2 infection as determined by Polymerase Chain Reaction (PCR), in oropharyngeal swabs or any other relevant specimen obtained during the course of the disease. 4. Moderate to severe ARDS: PaO2/FiO2 ratio equal to or lower than 200 mmHg, for less than 96 hours at the time of randomization. 5. Eligibility of patients requiring invasive ventilation within 72 hours of intubation.
Exclusion criteria
1. Imminent and unavoidable progression to death within 24 hours, irrespective of the applied treatments (as decided as by the clinical team). 2. “No Attempt of Resuscitation” order in place. 3. History of autoimmune/allergic disorders. 4. Ongoing corticosteroid or immunosuppressive therapy. 5. Chronic renal failure. 6. Any end-stage organ disease or condition, which in the investigator’s opinion, makes the patient an unsuitable candidate. 7. History of a moderate/severe lung disorder requiring home-based oxygen therapy. 8. Patients requiring Extracorporeal Membrane Oxygenation (ECMO), hemodialysis or hemofiltration by the time of enrollment. 9. Current diagnosis of pulmonary embolism. 10. Active neoplasm, except carcinoma in situ or basalioma. 11. Current pregnancy or lactation. 12. Current participation in a clinical trial with an experimental treatment for COVID19. 13. Any circumstances that in the investigator’s opinion compromises the patient’s ability to participate in the study.
Fourteen age and sex matched healthy subjects served as controls.
Blood samples (10 ml to 25 ml) were drawn upon the patient admission to the clinical ward, before starting any type of therapy. In all patients, SARS-CoV-2 was detected by assaying a nasopharyngeal swab specimen by real-time PCR. Some patients showed lung involvement under chest X-Ray examination, others had no fever and the respiratory frequency (RF) ranged on 12 to 30 acts/min. Marked heterogeneity in the white blood cell counts between patients was evident (Table 1). In fact, some subjects showed an almost normal count, while others showed severe lymphopenia with inversion of the neutrophils/lymphocyte percentage ratio. The IL-6 values ranged on 5.7 to 31.3 pg/ml; the C-reactive protein (CRP) ranged on 0.4 to 21.8 mg/dl; procalcitonin (PCT) on <0.12 to 4.95 ng/ml; and ferritin on 22 to 1551 ng/ml. Of the enrolled patients, only one (COV#12) died for complications related to the disease.
The study was approved by the local ethical committee (CEAS Umbria, Italy CER N°3658/20 7, May, 2020) and a written informed consent was obtained from participants in accordance with the Declaration of Helsinki.
hUCMS procurement, isolation and culture
The hUCMS isolation procedure from the post-partum umbilical cords, retrieved by caesarean section or at natural childbirth, followed our established method [21], based on the cord tissue enzymatic digestion, mechanical disruption and gradient centrifugation. At the end of the isolation/purification process, the cells were seeded, at a concentration of 6000 to 8000/cm2 per culture flask and incubated at 37°C in humidified 95% air. Cell expansion throughout 80% confluence was achieved by treatment with 0.05% trypsin/EDTA (Gibco, Invitrogen, Milan, Italy) for 3 minutes at 37°C. To characterize the isolated hUCMS, the following fluorochrome-conjugated monoclonal antibodies against CD29, CD44, CD45, CD90, CD105, CD117, SCF. OCT4, SOX2, NANOG were used. All cytofluorimetric assays were performed on the Attune® Acoustic Focusing Cytometer (Thermo Fischer) and analyzed by FlowJo software (Tree Star Inc.). The purpose of the procedure was to procure sufficient amounts of highly purified hUCMS that could be used either fresh, or thawed, after the initial freezing.
AG procurement and purification for microencapsulation
Powdered alginate (AG) was purchased from Monsanto-Kelco (San Diego, CA) featuring the following properties: molecular weight =120,000-190,000 kDa; main AG polymers: mannuronic acid (M) and guluronic acid (G) patterned as following; M fraction (FM) 61%; G fraction (FG) 39%. AG ultra-purification was conducted in-house under good laboratory practice (GLP), according to methods developed in our laboratory (patent no. WO 2009093184 A139); endotoxin levels were <0.5 EU/mL, protein content <0.45%, while viscosity was 100-300 cps.
Microencapsulation of hUCMS
Briefly, hUCMS were thoroughly mixed with a 1.8% AG solution, until a homogenous suspension was obtained. The cell/AG ratio was 3×106 hUCMS/1.2 ml AG. The suspension was then mechanically extruded through a microdroplet generator [21], and the AG/cell microdroplets were collected on a 1.2% CaCl2 bath which immediately converted the microdroplets into gel microbeads. The microbeads were soaked in the gelling solution, at r.t. for 10 minutes, thereafter washed in saline twice. Upon coating with 0.05% poly-L-ornithine chloride for 10 minutes (Sigma-Aldrich), the microbeads were partially de-gelled by 55 mM sodium citrate for 20 minutes at r.t. The microcapsules were finally coated with 0.1% AG for 10 minutes, and culture maintained at 37°C and 95% air/CO2. These microcapsules were similar but not identical to those used in early pilot clinical trials in T1D [23]. In fact, here the longer exposure of the microbeads to sodium citrate resulted in better aggregation of the enveloped cells, and eventually facilitated the microcapsules breakage to retrieve the cells. On this purpose, the capsules were forced through G22 needles and the suspension through 180 μm stainless mesh. Microencapsulated (CpS-hUCMS) or free hUCMS were exposed overnight to Interferon-γ (IFN-γ) (from 840 U/106 to 3640 U/106 cells) (Sigma-Aldrich, St Louis, MO) that was removed prior to starting the co-culture incubation system with PBMCs. Cps-hUCMS were tested for viability, after overnight priming with IFN-γ, by ethidium bromide and fluorescein diacetate (Sigma-Aldrich) under fluorescence microscopy, using appropriate filter sets. The cell monolayers viability was examined by phase contrast microscopy.
PBMC isolation, co-culture and phenotypic analysis
PBMCs were isolated by standard gradient separation on LymphoprepTM. For cell proliferation studies, the cells were labeled by the CellTrace Violet Cell Proliferation Kit for flow cytometry (Thermo Fisher Scientific) following the manufacturer’s instructions. PBMCs were co-cultured for 5 days with CPS-hUCMS or free hUCMS after priming the preparations with IFN-γ. Unstimulated and anti-CD3/CD28-coated beads (Dynabeads, Invitrogen, Carlsbad, CA) triggered PBMCs served for negative and positive controls, respectively. Cytofluorimetric assessment was performed using fluorochrome-conjugated monoclonal antibodies against CD3, CD4, CD45RA, CD25, IL17A, IFN-γ. All antibodies were provided by Thermo Fisher Scientific. As for IL17A and IFN-γ the cells were pre-treated with protein transport inhibitor cocktail (500×) (Thermo Fisher Scientific) following vendor’s recommendations. Before intracellular staining, the cells were fixed with 0.5% formaldehyde and permeabilized with 0.1% saponin. PBMCs: CPS-hUCMS or PBMCs: hUCMS ratio was 50:1. Hence for 106 PBMCs aliquots, placed in a well of an untreated 24 multiwell, an adequate number of microcapsules (an average 60 CPS) containing hUCMS cell aggregates was added.
Transcriptional expression analysis by quantitative PCR
Total cellular RNA was extracted from the cells using Direct-zol (Zymo Research corp). cDNA was synthesized using the iScript cDNA Synthesis Kit (Bio-Rad Laboratories) and was used as a template for quantitative PCR (qPCR) (ACE2 F-5’gggatcagagatcggaagaagaaa R-5’aggaggtctgaacatcatcagtg, ANGPT1 F-5’tactcagtggctgcaaaaacttg R-5’catggtagccgtgtggttct, FGF7 F-5’gctgttagcaacaaaacaaaagtca R-5’tgctctttccaaattgttagcctt, HLAG5 F-5’ctcaccttcacctcctttccca R-5’caatctgagctcttctttctccaca, HPRT1 F-5’ggtcaggcagtataatccaaag R-5’ggactccagatgtttccaaac, IDO1 F-5’gccttgcacgtctagttct R-5’tcttggagagttggcagtaag, IL6 F-5’acagccactcacctcttc R-5’cctcaaactccaaaagaccag, IL10 F-5’gcctaacatgcttcgagatc R-5’ggtcttcaggttctcccc, TGFβ1 F-5’ggacaccaactattgcttcag R-5’cgggttatgctggttgtac, TLR2 F-5’ggagttctcccagtgtttggt R-5’tcatcaacctgcctcacacc, TLR3 F-5’ggctagcagtcatccaacagaa R-5’tgaagttggcggctggtaat, TLR4 F-5’aaaatccccgacaacctccc R-5’ttgtctggatttcacacctgga, TLR9 F-5’cccccagcatgggtttctg R-5’gaagtggggcacagacttca). qPCR amplifications were performed using the SsoAdvanced Universal SYBR Green Supermix (Bio-Rad Laboratories) and Agilent AriaMx (Stratagene, La Jolla, CA). PCR products were demonstrated to be a single PCR product by melting curve and electrophoresis analysis.
Western blotting
Protein samples (40 μg) were analyzed on 10% SDS-PAGE and transferred onto nitrocellulose membrane (Bio-Rad Laboratories). The used detection antibody was rabbit antintibodynd transferreThermo Scientific). Immunodetection was performed by Clarity Chemiluminescent kit (Bio-Rad Laboratories).
hUCMS differentiation pathways
To confirm hUCMS stemness nature, we embarked on in vitro trans-differentiation experiments. Adipogenic or osteogenic differentiation was induced in the sub-confluent cultured hUCMS as previously described [22]. Briefly, hUCMS were incubated with “ad hoc” differentiation tissue cell culture media following specific culture-driven differentiation pathways. Final staining with Oil Red-O and Alizarin Red-S were used to identify the differentiated (adipose and osteocytic, respectively) tissue.
Statistical analysis
To evaluate the differences between groups, p values were calculated using ordinary one-way ANOVA (Tukey) test. In the evaluation of two single groups the unpaired Student t test was performed. The significance level was two-sided and set at P<0.05. All statistical analyses were conducted using Prism software (GraphPad Software).
Results
PBMCs yield
PBMCs isolation by centrifugation on LymphoprepTM gradients, confirmed lymphopenia as previously mentioned. In fact, in these Covid 19 (COV) patients, the average cellular yield was <0.8×106 PBMC/ml as compared with 1.2×106 PBMC/ml of controls (CTR) (Figure 1).
Figure 1.
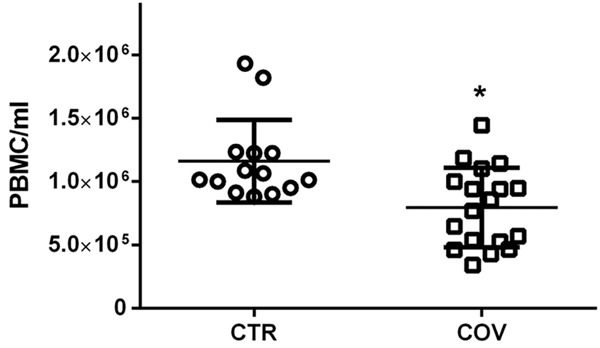
Comparison in peripheral blood mononuclear cell (PBMC) yield upon gradient separation between COVID19 patients (COV) and controls (CTR); COV n=18 and controls CTR n=14. *P<0.05.
hUCMS characterization
Flow cytometry showed that our isolated hUCMS (cultured for 5 passages in 10% FBS supplemented CMRL) marked positive for CD29, CD44, CD90 e CD105 and negative for CD45. The cells were 70% positive for SCF and NANOG showing low positivity for CD117, SOX2 and OCT4 (Figure 2A). Hence these hUCMS were consistent with adult mesenchymal stem cells of not hematopoietic origin. Our hUCMS maintained their differentiation potential throughout the fifth culture passage as demonstrated by their ability to differentiate into osteocytic and adipocytic cell phenotypes upon appropriate treatment (Figure 2B). Furthermore, hUCMS did not show basal expression of ACE2 and treatment with IFN-γ (2400 U/106 cells) did not induce expression of the relative messenger. Cellular aggregation induced by microcapsules and the subsequent treatment with IFN-γ at the same concentration did not induce ACE2 expression (Figure 2C) (normal thyroid tissue extracts were used as a positive control according to Hikmet [24]).
Figure 2.
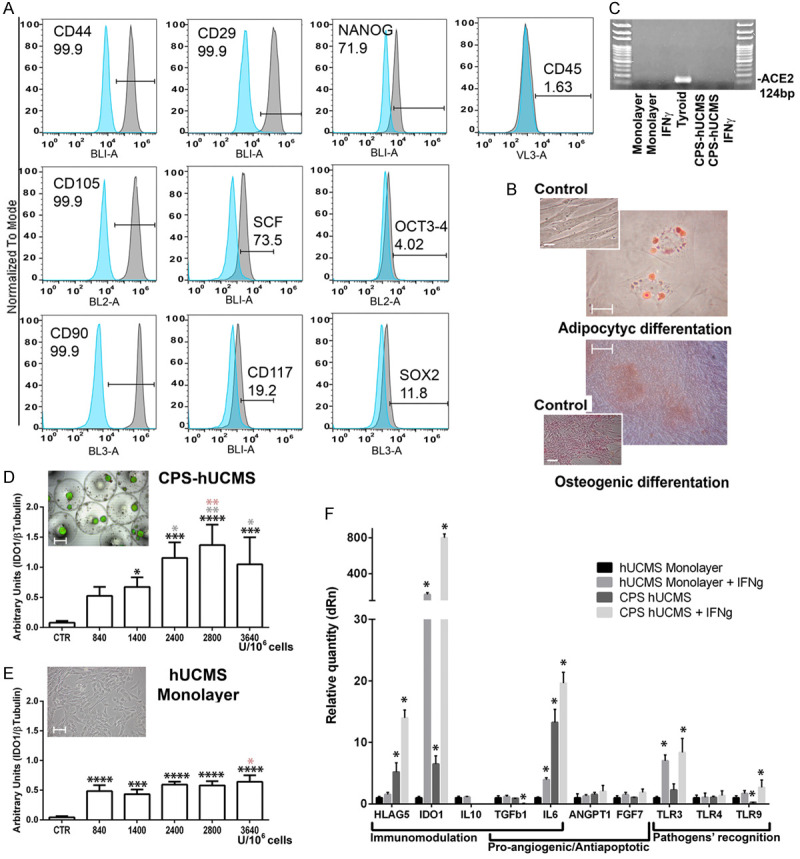
hUCMS characterization. A. Cytofluorimetric panel representative for the indicated markers. B. hUCMS in vitro retained their differentiation potential. Osteocytic and adipocytic differentiation capacity of hUCMS were confirmed by staining with Oil-Red-O and Alizarin Red-S, respectively, after 3 weeks of specific in vitro induction procedures. Magnification bars are 100 μm (osteocytic differentiation) and 10 μm (adipocytic differentiation). C. Angiotensin-Converting Enzyme 2 (ACE2) messenger expression on hUCMS monolayers and upon microencapsulation, treated or not with IFN-γ, as compared with positive controls (human thyroid tissue extract). D. Dose-response curve for Indoleamine 2,3-Dioxygenase 1 (IDO1) protein expression in microencapsulated hUCMS upon o/n treatment with increasing IFN-γ concentrations as compared to untreated encapsulated cells. Viability was assessed by staining the microencapsulated cells with ethidium bromide/fluorescein diacetate. Magnification bars are 300 μm. E. Dose-response curve for IDO1 protein expression in hUCMS flask-plated (shown in the microphotograph. Magnification bars are 100 μm) upon o/n treatment with increasing IFN-γ concentrations as compared to untreated cells. (*P<0.05, **P<0.01, ***P<0.001, ****P<0.0001; black asterisks indicate comparison of CTR vs treated, gray asterisks 840 U/106 cells vs other doses; orange asterisks 1400 U/106 cells vs other doses). F. Messengers involved in the immunomodulatory, pro-angiogenic/anti-apoptotic action or in the recognition of pathogens carried out by hUCMS microencapsulated or monolayered, treated or not with IFN-γ 2400 U/106 cells. The expression of each marker was calculated by assigning hUCMS monolayers with the arbitrary value 1. HPRT1 served for control. All results were expressed as mean ± SD of three independent experiments (*P<0.05).
IDO1 appeared as one of the main molecules secreted by hUCMS, upon IFN-γ priming, which helped both their immunomodulatory and antiviral activities. As far as IDO1 protein was concerned, we confirmed that IFN-γ concentration of 2400 U/106 cells (300 U/ml), previously used in our experiments [17,18], induced IDO1 production from both, unencapsulated and microencapsulated hUCMS.
For similar IFN-γ concentrations/106 cells, the aggregated cells responded with greater protein production as compared to the cell monolayers; the cell aggregates showed a regular dose-response curve throughout high IFN-γ concentrations, with significant differences between control and treated samples. The free cell monolayers treated with IFN-γ were all different from controls but not from each other. Therefore, microenvironment provided by the microcapsules, by favoring cell aggregation, greatly enhanced the production capacity of IDO1 in response to comparable IFN-γ concentrations.
Assessment of main immunoregulatory, anti-apoptotic and pro-angiogenic factors, and Toll-Like Receptor (TLRs) for recognition of pathogens such as viruses or bacteria was performed by qPCR (Figure 2F). From this analysis, IDO1 messenger was greatly induced by IFN-γ in cells aggregated within the microcapsules. Interestingly, the simple aggregation process enabled induction of IDO1. Actually, immunomodulation markers such as HLAG5 and IL-6 were already induced by cell aggregation, and the IFN-γ priming further potentiated their expression. IDO1 also affected viruses and, along with this, TLRs. In particular, TLR4 did not show changes, while TLR9 was negatively modulated by the aggregation, and positively by the IFN-γ, after aggregation, as compared to controls. TLR3 messenger was induced by IFN-γ treatment on both monolayers and microencapsulated cells. TLR2 was never expressed by hUCMS [8]. Angiopoietin 1 (ANGPT1) and FGF7 (KGF) were among the pro-angiogenic factors secreted by mesenchymal stem cells; these were present in monolayered hUCMS cells, treated or not with IFN-γ, and in treated/untreated aggregated cells, although IFN-γ did not induce significant changes in their expression. TGFβ1 messenger decreased upon treatment with IFN-γ on cells, aggregated within microcapsules, while IL10 messenger disappeared after the cell aggregation process, and IFN-γ treatment did not reactivate it. CPS-hUCMS, treated with IFN-γ o/n retained an optimal viability as compared to the untreated control (Figure 2D).
PBMCs features and typing
After 5 days of co-culture, the PBMCs underwent live/dead staining with 7ADD/po-pro1 (Figure 3A). Analysis of the relative morphological plots, where patient-derived PBMCs were co-incubated with microencapsulated or free hUCMS, was associated with higher percentage of lymphocytes as compared to culture of PBMCs in medium alone. For example, in COV patient #15 (Figure 3A), lymphocytes averaged 0.73% and reached 2.26% upon co-culture with free, and 2.58% with microencapsulated hUCMS. In particular, in severely ill patients such as COV#4 or COV#12 (Table 1), it was not possible to find lymphocytes after 5 days of culture under any circumstances. Free, and even more microencapsulated hUCMS showed similar effects on control lymphocytes with an increase of cells percentage into the lymphocyte gate after 5 days of co-culture, with free or microencapsulated hUCMS (Figure 3A).
Figure 3.
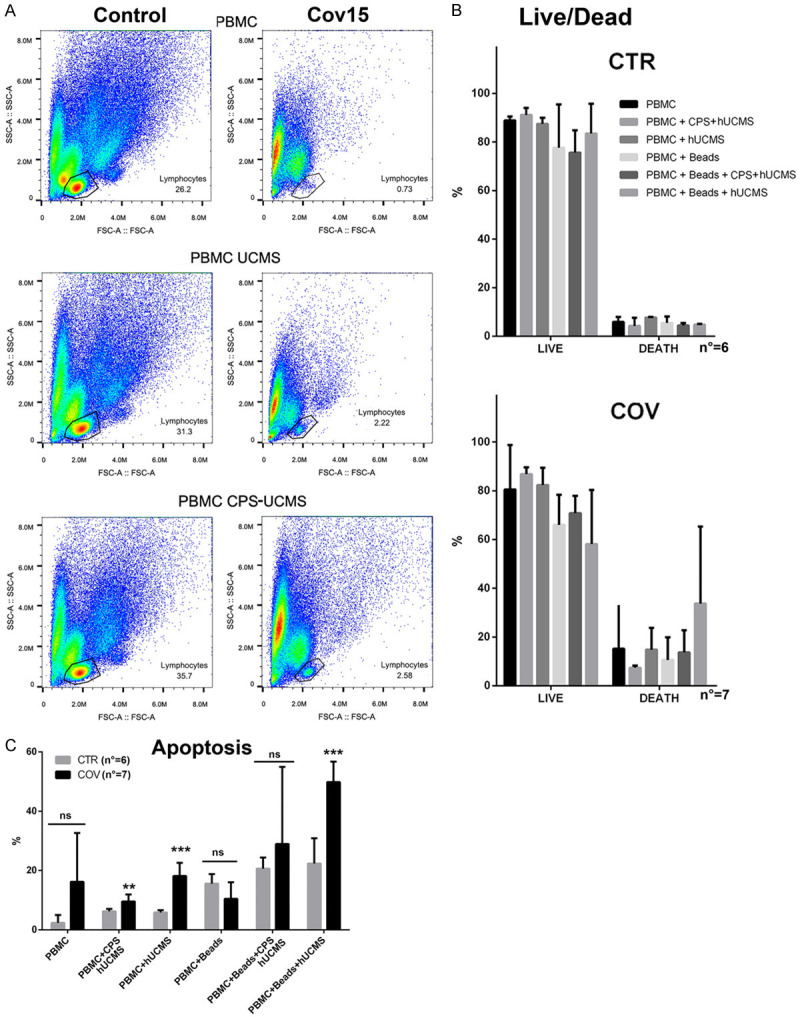
Peripheral blood mononuclear cell (PBMC) examination. A. Morphological plot of a representative control and a patient (COV#15) with respect to either lymphocytes percentage after 5 days of culture, or co-culture with monolayered or microencapsulated hUCMS (CPS-hUCMS). B. Means of life-death data in controls as compared to COVID19 patients. C. Assessment of apoptosis with propidium Iodide. **P<0.01; ***P<0.001 in controls vs. patients within each individual condition.
The life/death assay showed that, after 5 days of culture maintenance, the percentage of live lymphocytes was 90% in controls vs. 80% of COV patients (Figure 3B). In COV patients, co-culture with free, but mostly microencapsulated hUCMS, was associated with an increase of percentage of both lymphocytes and live cells. In these experiments, the addition of anti-CD3/CD28 beads provided for a physiological-like stimulus for T cells, mimicking the activation process induced in vivo by the antigen presenting cells. In these experiments, retention of PBMC responsiveness was assessed. In the COV patients, as well as in controls, the presence of anti-CD3/CD28 beads decreased the percentage of live vs. dead cells; moreover, the simultaneous presence of microencapsulated, but not free hUCMS, mitigated this effect. In controls, free hUCMS did not change the percentage of live vs. dead treated as compared to untreated PBMCs; on the contrary, microencapsulated hUCMS seemed to increase the percentage of the live cells. Contrary to COV patients, in controls simultaneous treatment with anti-CD3/CD28 beads and free cells seemed to improve the percentage of live cells as compared to microencapsulated cells.
To clarify behavior of PBMCs in the various experimental settings, we employed propidium iodide (PI) for the analysis of the cell cycle and apoptosis (Figure 3C). After 5 days of culture, the cell cycle showed that most of cells were in the G1/G0 phase (data not shown) in all conditions. As for apoptosis assessment, while for controls the analyses were easy, for COV patients (PBMCs cultured in medium for 5 days or with anti-CD3CD28 activation beads, with and without hUCMS) no lymphocytes were found on the morphological plot. COV patient lymphocytes always remained detectable only in the presence of free or microencapsulated hUCMS. This confirmed the beneficial effects of hUCMS. However incomplete data analysis prevented definitive conclusions while confirming lymphocytes frailty in the COV patients. By examining COV patients vs. controls, PBMCs apoptosis resulted higher in the COV patients: in fact, percentage of the apoptotic cells significantly raised upon co-culture with both, microencapsulated and hUCMS free cells, as compared to controls. The presence of anti-CD3/CD28 beads increased the apoptosis of the PBMCs in both, controls and patients, as compared to baseline. Presence of microencapsulated or free hUCMS did not change results in the controls, but in the patients the percentage of apoptotic cells increased greatly when the hUCMS were free. From this data we decided to continue our studies by using only microencapsulated hUCMS.
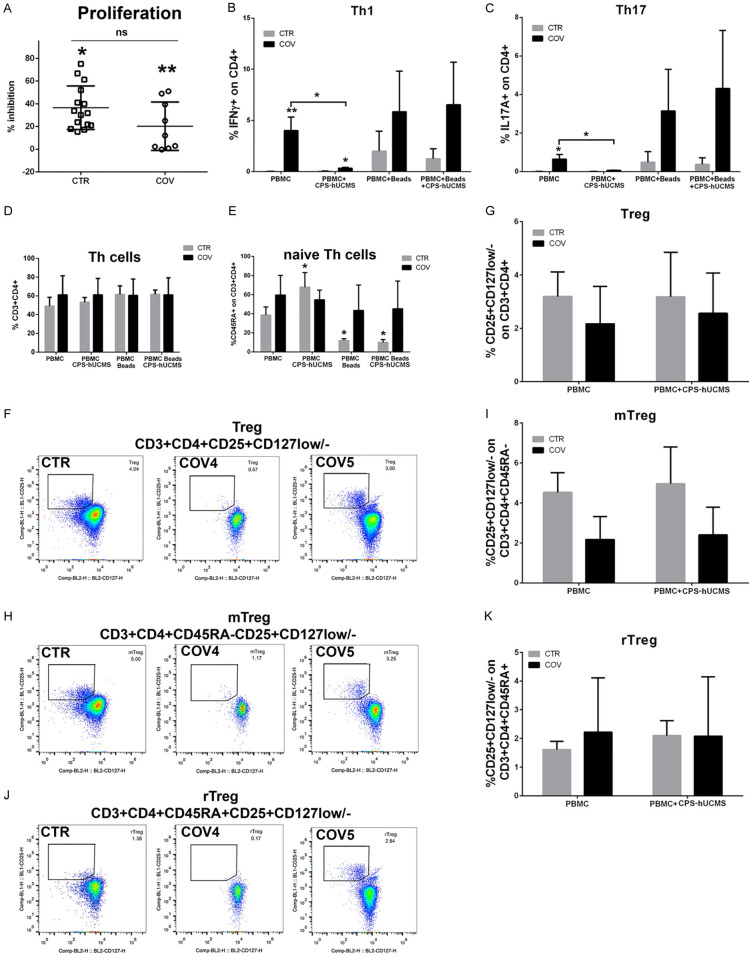
Microencapsulated hUCMS significantly inhibited proliferation of PBMC activated with anti-CD3/CD28 beads in both, COV patients and controls (Figure 4A). In COV patients, the percentage of inhibition was lower than in controls. In severely ill COV patients, with specific regard to the number of isolated lymphocytes, PBMCs were not activated by anti-CD3/CD28 beads, thereby impeding completion of the assay.
Figure 4.
Peripheral blood mononuclear cell (PBMC) typing. A. Proliferation inhibition induced by anti-CD3/CD28 beads by microencapsulated hUCMS on PBMC from control (CTR) group or COVID19 (COV) patients. The inhibition effects were significantly different from bead-activated PBMC in both groups CTR, *P<0.05, COV, **P<0.01; CTR and COV were not statistically different from each other. B. Percentage of IFN-γ lymphocytes in CD4+ in controls and patients. *P<0.05, **P<0.01. C. Percentage of IL17A+ lymphocytes in CD4+ in controls and patients. *P<0.05. D. Percentage of CD3+CD4+ cells in controls and patients; in patients this percentage was always above the normal threshold regardless of treatment. E. Percentage of CD45RA+ in CD3+CD4+ in controls and patients at the various experimental points. F. Representative dot plots of regulatory T (Treg) cells of CD25highCD127Low/- cells in CD3+CD4+ of one control and two patients with COVID19; COV#4 was more compromised than COV#5 regarding the total number of lymphocytes. G. Percentage of Treg of CD25highCD127Low/- cells in CD3+CD4+ of the controls (n=6) and COV patients (n=6) compared for the different experimental points. H. Activated Memory Regulatory T cells (mTreg) in untreated 5 days cultured lymphocytes from a representative control and from two COV patients. I. Percentage of mTreg CD45RA-CD25highCD127Low/- cells in CD3+CD4+ of controls and COV patients 5 days cultured lymphocytes with or without CPS-hUCMS. J. Resting Memory Regulatory T cells (rTreg) in untreated 5 days cultured lymphocytes from a control and from two COV patients. K. Percentage of rTreg CD45RA+CD25highCD127Low/- cells in CD3+CD4+ of controls and COV patients 5 days cultured lymphocytes with or without CPS-hUCMS.
Microencapsulated hUCMS induced decrease of Th1 and Th17 subsets in patients where they basically were strongly expressed as compared to controls (Figure 4B, 4C). The presence of the anti-CD3/CD28 beads increased their percentage while the addition of microencapsulated hUCMS (CPS-hUCMS) did not change the results. In the controls, Th1 and Th17 were basically very low and remained so after the addition of microencapsulated hUCMS; the presence of anti-CD3/CD28 beads raised percentage of these populations which was, in this case, reduced by microencapsulated hUCMS.
In controls, CD3+CD4+ (helper T lymphocytes) (Figure 4D) increased after treatment with microencapsulated hUCMS and even more, with anti-CD3/CD28 beads; there were no differences between anti-CD3/CD28 beads and CPS-hUCMS and anti-CD3/CD28 beads alone. In COV patients, the percentage of T cells did not vary in all conditions and remained above the upper limit of normality. The anti-CD3/CD28 beads are supposed to increase the CD4+: in controls this occurred, while in the COV patients their percentage was already high and did not increase further.
In controls, microencapsulated hUCMS significantly increased naive T helper (Th) cells (CD3+CD4+CD45RA+) as compared to PBMCs cultured alone for 5 days. On the contrary, addition of the stimulation beads anti-CD3/CD28 significantly decreased the naive T helper cells both in the presence or absence of microencapsulated hUCMS. In COV patients, the various treatments did not statistically affect the percentage of naive Th cells as compared to PBMCs. Percentage of this class of cells was higher as compared with the values of healthy subjects (Figure 4E).
Thanks to the expression of CD127 (low) and CD25 (high), we were able to identify putative populations of T regulatory cells: CD3+CD4+CD25highCD127low/-. Figure 4F shows plots for lymphocytes, after 5 days of culture, labeled for Treg of a control as compared to that of two COV patients. The percentage of this class of lymphocytes was about 3.2% in healthy subjects, vs. 1.4% of our COV patients (especially those with severe illness). Figure 4G shows that percentage of Treg seemed to increase after co-culture with CPS-hUCMS although on a not statistically significant manner.
In COVID19 patients, microencapsulated hUCMS corrected the Th17/Treg ratio, bringing it from 0.299 to 0.026, by decreasing the Th17 without increasing the Treg significantly, in support to the anti-inflammatory properties of CPS-hUCMS. In controls, the Th17/Treg ratio did not vary significantly (from 0.0019 to 0.0023).
Through CD45RA expression it was possible to split the Treg in Activated Memory Regulatory T cells (mTreg) (CD3+CD4+CD45RA-CD25+CD127low/-) (an average 4.53% in healthy), and Resting Memory Regulatory T cells (rTreg) (CD3+CD4+CD45RA+CD25+CD127low/-) (an average 1.61% in healthy). As per Figure 4H, 4J, 4I, 4K, the rTregs showed no differences as compared to healthy ones while the mTregs were halved. Co-culture with microencapsulated hUCMS did not significantly change their percentage either in controls or COV patients.
Discussion
Severe SARS-CoV-2 viral infection seems to result from a hyperinflammatory state, coupled with an immune system’s overreaction, throughout “cytokine storm” and immune-thrombosis. Patients with severe COVID19 frequently develop ARDS, which is associated with high death toll [25,26]. Mortality in patients with COVID19 complicated by ARDS has been described to average 52.4% [27].
As previously reported, in COVID19 patients, there are concomitant events consisting of immune inhibition, activation, exhaustion and complex alterations in cells at different stages of differentiation, with huge production/release of a variety of pro-inflammatory cytokines [28]. SARS-CoV-2 seems to cause, quite rapidly, a massive activation of the host’s immune response. In particular, increase of Th17 exacerbates the inflammatory response leading to massive secretion of cytokines, overall defined “cytokine storm”. IL-17 is crucial for recruitment and activation of neutrophils, cells that can migrate to the lungs and are heavily involved in the pathogenesis of the lung disease.
Over the past decade, hUCMS have been proved to represent not only adult multipotent stem cells with promising applications in regenerative medicine [29], but also a powerful tool to regulate the immune system. Moreover, hUCMS procurement is almost unlimited, by employing a rapid isolation method [22]. These cells can be cultured, frozen-thawed, and expanded in vitro, with favorable impact on their retrieved mass yield. hUCMS may suppress proliferation and modulate function of both the innate and adaptive immune system. On the basis of our previous experience with hUCMS in autoimmune disorders, we showed that, in vitro, hUCMS were associated with a positive impact on Treg expansion and Th1/Th2/Th17 rebalance induction [17,18], while in vivo, hUCMS enabled reversal of hyperglycemia upon graft in non-obese diabetic (NOD) mice with spontaneous autoimmune diabetes if administered early on the disease course [30].
At this time, in the middle of the SARS-CoV-2 pandemic, mesenchymal stem cells of different origin [9,31] have been tentatively applied to the treatment of COVID19 patients, mostly by direct intra-venous administration in an attempt to cope with ARDS. In fact, hUCMS may exert beneficial effects in patients with severe COVID19 by modulating the immune responses and altering immunopathogenesis of the cytokine storm [13,33].
From this background, we wished to determine if either free or microencapsulated hUCMS would be able, in vitro, to positively impact on lymphocytes retrieved from COVID19 patients, at an early disease stage, when no therapy had been initiated yet. In fact, concomitant use of conventional drugs and MSC therapy would clearly cloud interpretation of the obtained results, with special regard to side effects, and effectiveness of the latter.
In this work, we have preliminarily observed that microencapsulated or free hUCMS, upon pre-incubation with IFN-γ at an appropriate concentration, significantly increased expression of key soluble molecules, associated with immunomodulatory properties, such as IDO1, and HLA-G5. These are important tolerogenic factors normally situated at the maternal-fetal interface, and deeply involved in immunoregulatory pathways, and pro-angiogenic, anti-apoptotic, anti-fibrotic and possibly anti-microbial activities. In particular, IDO1 has been shown to hold strong antiviral properties [34-37]. Moreover, tryptophan depletion induced by IDO1 leads to a surrounding concentration of its catabolite, kynurenine, which seems to be especially toxic for T cells [21,38]. IDO1-expressing MSC also induce monocytes to differentiate into M2-like macrophages [10]. Furthermore, IDO1 and PGE2 induced a synergistic inhibition of NK and Th17 cells, B cell inhibition via cell cycle arrest, decreased immunoglobulins (IgM, IgG and IgA) production [8] and diminished expression of CXCR4, CXCR5, and CXCR7 in the same cells [10,11,38]. These features, all together, constitute an anti-inflammatory complex which inhibits activation, proliferation and differentiation of allogeneic T cells into Th1, Th17 subsets, and stimulate their differentiation into tolerogenic Th2 and Treg cells [17,18].
COVID19 patients develop lymphopenia, to a different extent, often related to clinical course of the disease, with unbalanced expression of the lymphocyte populations. In fact, PBMCs of the examined patients showed a lower number of total lymphocytes. In some patients, PBMCs were not activated by anti-CD3/CD28 beads, hence they were anergic, with blunted immune responsiveness. In other cases, activation took place, but to a lower extent, upon exposure to hUCMS, as compared to controls. In some instances, the cells were not found after 5 days of culture, confirming the presence of a severe lymphocytic damage. Th and naive Th cells were higher, in percentage, in COV patients than in controls, confirming severe inflammation.
According to recent reports in COVID19 patients [28], a significant skewing of T cell activation towards Th17 functional phenotype has been observed. This may suggest that blocking IL-17 pathways by biological drugs or immunomodulatory molecules could be a novel, additional approach for treating patients affected by SARS-COV-2 disease. On this purpose, microencapsulated hUCMS represent potential optimal candidates, since they greatly reduce Th17 and Th1 percentages. In our patients these lymphocytes were well present as compared to controls, but upon five days exposure to microencapsulated hUCMS, percentage of cells declined by over 90% in both instances (Figure 4B, 4C). In COVID19 patients, we observed a decline of regulatory T cells, and consequently higher Th17/Treg ratio (0.299). Treg cells, a subset of T helper cells, here identified by detecting the high expression of the IL-2 receptor α-chain (CD25) and low/null expression of IL-7 receptor α-chain (CD127), play a crucial role by negatively regulating activation, proliferation, and effector functions of a wide range of immune cells, addressed to the maintenance of self-tolerance and immune homeostasis. They suppress autoimmune phenomena, dampen allergic reactions, or block transplant rejection, but they can also inhibit a protective immune response against invading pathogens or tumors [39,40]. Here we showed that the percentages of Tregs did not decline in PBMCs of COVID19 patients upon co-culture with microencapsulated hUCMS. However, significant decline of Th17 led to an evident decrease of Th17/Treg ratio on PBMCs of patients (0.026) never reaching, by the way, control values (0.0023).
Percentage of rTreg did not vary as compared to controls, while mTreg halved as compared to controls (presence of CPS-hUCMS does not alter the situation). During a chronic viral infection, mTreg are associated with significant peripheral clonal expansion to regulate memory T effector response and prevent collateral tissue damage [41]. Here we did not observe mTreg expansion, possibly due to early onset of the disease.
Our previous work showed that the 3D microenvironment provided by our microcapsules enhances the cells survival/viability/differentiation potential, possibly due to mechano-transduction effects. In fact, within microcapsules, the cells form vital 3D aggregates continue production of immunomodulatory and trophic molecules. Alginate hydrogels also constitute a physical barrier to intracapsular ingress of pro-inflammatory cytokines. Our recent study showed that microencapsulated rodent hybridoma cells released monoclonal antibodies that were of higher quality and quantity as compared to those secreted from free cells [42]. Upon days of culture maintenance, intracapsular proteins were released on a drug delivery-like mode. We showed that for identical IFN-γ concentrations which both, free or microencapsulated hUCMS, were exposed to, IDO1, HLAG5, and IL6 production were significantly higher for the latter, in terms of secretory dynamics. On the contrary, as for hUCMS monolayers, at same cell numbers, production of these molecules was lower. In terms of immunity, hUCMS may interact with the majority of immune cell types, via cell-to-cell contact. Such interaction leads to inhibition of effector T lymphocytes and stimulation of regulatory T cells, with the TLRs presence helping anti-viral responsiveness. However, cellspons immunity, hUCMS are associated with some caveats: for instance, we had observed in vitro that if hUCMS were at direct contact with lymphocytes, isolated from patients with primary Sjögren’s syndrome or those with T1D [17,18], their immunomodulatory capacity was crippled. On the other hand, if physical contact between hUCMS and immune cells was prevented (ie, by means of microcapsules), immune activity of the cells was preserved, seemingly thanks to release of soluble mediators. In our studies, both free and microencapsulated cells enabled preservation of cells that would otherwise disappear, upon 5 days of co-culture, in immunocompromised COVID19 patients. Nevertheless, also in this work free hUCMS showed defects in immunomodulatory activity. In fact, the cells induced apoptosis. In light of the actual lymphopenia, this action could be unfavorable.
As mentioned, MSCs have become an exciting candidate for treating COVID19-induced ARDS due to their immunomodulatory effects, regenerative properties, control of the oxidative damage, protection of the endothelium, and epithelium and their anti-microbial effects [43]. Despite early promising results, caveats related to MSC therapy [31,44] include:
1) in vitro mechanistic effects require further study;
2) the route of administration deserves cautiousness. In fact, intravascular coagulation and thromboembolism may cause mortality in patients with COVID19 and MSCs express pro-coagulant tissue factors (TF/CD142) [8]. The latter might significantly weaken direct MSCs application to the disease treatment [45]. Moreover, in COVID19 patients, concomitant use of conventional drugs and MSC-based therapy would prevent straight forward interpretation of the obtained effects. Therefore, results obtained by cell therapy might not be ascribed to the effects of MSCs alone [46]. Finally, the lack of a standardized therapeutic protocol introduces further variables from sourcing of MSC, stem cell pre-conditioning, route of administration, dose and frequency of MSC transplantation and identification of the appropriate stage of disease suitable for MSC-based therapy. All of these mentioned factors may impact on MSC cell therapy effects. This is why a cell therapy based on MSCs requires a detailed study rationale/design and in vitro assessment of the targeted expressed effects.
In conclusion and just preliminarily, we have provided preliminary evidence that microencapsulated, over free, hUCMS in vitro, seem to represent a powerful tool to condition several immune pathways clearly compromised in COVID19 patients. Further study is necessary to begin in vivo assessment of this experimental system, upon identification of most appropriate timing of the disease onset, as well as product dosing/patients, suitable for intervention.
Acknowledgements
Authors thank Dr Onelia Bistoni, Division of Rheumatology, Department of Medicine and Surgery, School of Medicine, University of Perugia Hospital, Italy, for her precious help; authors thank Dr Filippo Figorilli, Division of Internal Medicine, Department of Medicine and Surgery, Perugia Hospital, Italy, for his valuable help. Support of Altucell Inc., Astor Court 3, Dix Hills, New York, NY (USA) 11746 is gratefully acknowledged.
Disclosure of conflict of interest
None.
Abbreviations
- IDO1
Indoleamine 2,3-Dioxygenase 1
- HLAG5
Human Leukocyte Antigen-G5
- IFN-γ
Interferon g
- Th1
T helper 1 cells
- Th17
T helper 17 cells
- SCF
Stem Cell Factor
- OCT4
Octamer-binding Transcription factor 4
- SOX2
Sex determining region Y-box 2
- NANOG
Homeobox protein NANOG
- ACE2
Angiotensin-Converting Enzyme 2
- qPCR
real time polymerase chain reaction
References
- 1.Yang L, Liu S, Liu J, Zhang Z, Wan X, Huang B, Chen Y, Zhang Y. COVID-19: immunopathogenesis and Immunotherapeutics. Signal Transduct Target Ther. 2020;5:128. doi: 10.1038/s41392-020-00243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang FS. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tan M, Liu Y, Zhou R, Deng X, Li F, Liang K, Shi Y. Immunopathological characteristics of coronavirus disease 2019 cases in Guangzhou, China. Immunology. 2020;160:261–268. doi: 10.1111/imm.13223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, Xie C, Ma K, Shang K, Wang W, Tian DS. Dysregulation of immune response in patients with Coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 2020;71:762–768. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smail SW, Saeed M, Twana A, Khudhur ZO, Younus DA, Rajab MF, Abdulahad WH, Hussain HI, Niaz K, Safdar M. Inflammation, immunity and potential target therapy of SARS-COV-2: a total scale analysis review. Food Chem Toxicol. 2021;150:112087. doi: 10.1016/j.fct.2021.112087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.REMAP-CAP Investigators. Gordon AC, Mouncey PR, Al-Beidh F, Rowan KM, Nichol AD, Arabi YM, Annane D, Beane A, van Bentum-Puijk W, Berry LR, Bhimani Z, Bonten MJM, Bradbury CA, Brunkhorst FM, Buzgau A, Cheng AC, Detry MA, Duffy EJ, Estcourt LJ, Fitzgerald M, Goossens H, Haniffa R, Higgins AM, Hills TE, Horvat CM, Lamontagne F, Lawler PR, Leavis HL, Linstrum KM, Litton E, Lorenzi E, Marshall JC, Mayr FB, McAuley DF, McGlothlin A, McGuinness SP, McVerry BJ, Montgomery SK, Morpeth SC, Murthy S, Orr K, Parke RL, Parker JC, Patanwala AE, Pettila V, Rademaker E, Santos MS, Saunders CT, Seymour CW, Shankar-Hari M, Sligl WI, Turgeon AF, Turner AM, van de Veerdonk FL, Zarychanski R, Green C, Lewis RJ, Angus DC, McArthur CJ, Berry S, Webb SA, Derde LPG. Interleukin-6 receptor antagonists in critically ill patients with COVID-19. N Engl J Med. 2021;384:1491–1502. doi: 10.1056/NEJMoa2100433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sadeghi S, Soudi S, Shafiee A, Hashemi SM. Mesenchymal stem cell therapies for COVID-19: current status and mechanism of action. Life Sci. 2020;262:118493. doi: 10.1016/j.lfs.2020.118493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rocha JLM, de Oliveira WCF, Noronha NC, Dos Santos NCD, Covas DT, Picanco-Castro V, Swiech K, Malmegrim KCR. Mesenchymal stromal cells in viral infections: implications for COVID-19. Stem Cell Rev Rep. 2021;17:71–93. doi: 10.1007/s12015-020-10032-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi Y, Wang Y, Li Q, Liu K, Hou J, Shao C, Wang Y. Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nat Rev Nephrol. 2018;14:493–507. doi: 10.1038/s41581-018-0023-5. [DOI] [PubMed] [Google Scholar]
- 11.Naji A, Eitoku M, Favier B, Deschaseaux F, Rouas-Freiss N, Suganuma N. Biological functions of mesenchymal stem cells and clinical implications. Cell Mol Life Sci. 2019;76:3323–3348. doi: 10.1007/s00018-019-03125-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Atluri S, Manchikanti L, Hirsch JA. Expanded Umbilical Cord Mesenchymal Stem Cells (UC-MSCs) as a therapeutic strategy in managing critically ill COVID-19 patients: the case for compassionate use. Pain Physician. 2020;23:E71–E83. [PubMed] [Google Scholar]
- 13.Leng Z, Zhu R, Hou W, Feng Y, Yang Y, Han Q, Shan G, Meng F, Du D, Wang S, Fan J, Wang W, Deng L, Shi H, Li H, Hu Z, Zhang F, Gao J, Liu H, Li X, Zhao Y, Yin K, He X, Gao Z, Wang Y, Yang B, Jin R, Stambler I, Lim LW, Su H, Moskalev A, Cano A, Chakrabarti S, Min KJ, Ellison-Hughes G, Caruso C, Jin K, Zhao RC. Transplantation of ACE2(-) mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging Dis. 2020;11:216–228. doi: 10.14336/AD.2020.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Selmani Z, Naji A, Zidi I, Favier B, Gaiffe E, Obert L, Borg C, Saas P, Tiberghien P, Rouas-Freiss N, Carosella ED, Deschaseaux F. Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+ regulatory T cells. Stem Cells. 2008;26:212–222. doi: 10.1634/stemcells.2007-0554. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, Chen X, Cao W, Shi Y. Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nat Immunol. 2014;15:1009–1016. doi: 10.1038/ni.3002. [DOI] [PubMed] [Google Scholar]
- 16.Chen C, Liang J, Yao G, Chen H, Shi B, Zhang Z, Zhao C, Zhang H, Sun L. Mesenchymal stem cells upregulate Treg cells via sHLA-G in SLE patients. Int Immunopharmacol. 2017;44:234–241. doi: 10.1016/j.intimp.2017.01.024. [DOI] [PubMed] [Google Scholar]
- 17.Alunno A, Montanucci P, Bistoni O, Basta G, Caterbi S, Pescara T, Pennoni I, Bini V, Bartoloni E, Gerli R, Calafiore R. In vitro immunomodulatory effects of microencapsulated umbilical cord Wharton jelly-derived mesenchymal stem cells in primary Sjogren’s syndrome. Rheumatology (Oxford) 2015;54:163–168. doi: 10.1093/rheumatology/keu292. [DOI] [PubMed] [Google Scholar]
- 18.Montanucci P, Alunno A, Basta G, Bistoni O, Pescara T, Caterbi S, Pennoni I, Bini V, Gerli R, Calafiore R. Restoration of t cell substes of patients with type 1 diabetes mellitus by microencapsulated human umbilical cord Wharton jelly-derived mesenchymal stem cells: an in vitro study. Clin Immunol. 2016;163:34–41. doi: 10.1016/j.clim.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 19.Luz-Crawford P, Kurte M, Bravo-Alegria J, Contreras R, Nova-Lamperti E, Tejedor G, Noel D, Jorgensen C, Figueroa F, Djouad F, Carrion F. Mesenchymal stem cells generate a CD4+CD25+Foxp3+ regulatory T cell population during the differentiation process of Th1 and Th17 cells. Stem Cell Res Ther. 2013;4:65. doi: 10.1186/scrt216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hayaishi O. Properties and function of indoleamine 2,3-dioxygenase. J Biochem. 1976;79:13P–21P. doi: 10.1093/oxfordjournals.jbchem.a131115. [DOI] [PubMed] [Google Scholar]
- 21.Calafiore R, Basta G, Luca G, Lemmi A, Racanicchi L, Mancuso F, Montanucci MP, Brunetti P. Standard technical procedures for microencapsulation of human islets for graft into nonimmunosuppressed patients with type 1 diabetes mellitus. Transplant Proc. 2006;38:1156–1157. doi: 10.1016/j.transproceed.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 22.Montanucci P, Basta G, Pescara T, Pennoni I, Di Giovanni F, Calafiore R. New simple and rapid method for purification of mesenchymal stem cells from the human umbilical cord Wharton jelly. Tissue Eng Part A. 2011;17:2651–2661. doi: 10.1089/ten.TEA.2010.0587. [DOI] [PubMed] [Google Scholar]
- 23.Calafiore R, Basta G, Luca G, Lemmi A, Montanucci MP, Calabrese G, Racanicchi L, Mancuso F, Brunetti P. Microencapsulated pancreatic islet allografts into nonimmunosuppressed patients with type 1 diabetes: first two cases. Diabetes Care. 2006;29:137–138. doi: 10.2337/diacare.29.1.137. [DOI] [PubMed] [Google Scholar]
- 24.Hikmet F, Mear L, Edvinsson A, Micke P, Uhlen M, Lindskog C. The protein expression profile of ACE2 in human tissues. Mol Syst Biol. 2020;16:e9610. doi: 10.15252/msb.20209610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berlin DA, Gulick RM, Martinez FJ. Severe COVID-19. N Engl J Med. 2020;383:2451–2460. doi: 10.1056/NEJMcp2009575. [DOI] [PubMed] [Google Scholar]
- 26.McElvaney OJ, McEvoy NL, McElvaney OF, Carroll TP, Murphy MP, Dunlea DM, Ni Choileain O, Clarke J, O’Connor E, Hogan G, Ryan D, Sulaiman I, Gunaratnam C, Branagan P, O’Brien ME, Morgan RK, Costello RW, Hurley K, Walsh S, de Barra E, McNally C, McConkey S, Boland F, Galvin S, Kiernan F, O’Rourke J, Dwyer R, Power M, Geoghegan P, Larkin C, O’Leary RA, Freeman J, Gaffney A, Marsh B, Curley GF, McElvaney NG. Characterization of the inflammatory response to severe COVID-19 illness. Am J Respir Crit Care Med. 2020;202:812–821. doi: 10.1164/rccm.202005-1583OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, Huang H, Zhang L, Zhou X, Du C, Zhang Y, Song J, Wang S, Chao Y, Yang Z, Xu J, Zhou X, Chen D, Xiong W, Xu L, Zhou F, Jiang J, Bai C, Zheng J, Song Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Biasi S, Meschiari M, Gibellini L, Bellinazzi C, Borella R, Fidanza L, Gozzi L, Iannone A, Lo Tartaro D, Mattioli M, Paolini A, Menozzi M, Milic J, Franceschi G, Fantini R, Tonelli R, Sita M, Sarti M, Trenti T, Brugioni L, Cicchetti L, Facchinetti F, Pietrangelo A, Clini E, Girardis M, Guaraldi G, Mussini C, Cossarizza A. Marked T cell activation, senescence, exhaustion and skewing towards TH17 in patients with COVID-19 pneumonia. Nat Commun. 2020;11:3434. doi: 10.1038/s41467-020-17292-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barry F, Murphy M. Mesenchymal stem cells in joint disease and repair. Nat Rev Rheumatol. 2013;9:584–594. doi: 10.1038/nrrheum.2013.109. [DOI] [PubMed] [Google Scholar]
- 30.Montanucci P, Pescara T, Alunno A, Bistoni O, Basta G, Calafiore R. Remission of hyperglycemia in spontaneously diabetic NOD mice upon transplant of microencapsulated human umbilical cord Wharton jelly-derived mesenchymal stem cells (hUCMS) Xenotransplantation. 2019;26:e12476. doi: 10.1111/xen.12476. [DOI] [PubMed] [Google Scholar]
- 31.Li Z, Niu S, Guo B, Gao T, Wang L, Wang Y, Wang L, Tan Y, Wu J, Hao J. Stem cell therapy for COVID-19, ARDS and pulmonary fibrosis. Cell Prolif. 2020;53:e12939. doi: 10.1111/cpr.12939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Can A, Coskun H. The rationale of using mesenchymal stem cells in patients with COVID-19-related acute respiratory distress syndrome: what to expect. Stem Cells Transl Med. 2020;9:1287–1302. doi: 10.1002/sctm.20-0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kane M, Zang TM, Rihn SJ, Zhang F, Kueck T, Alim M, Schoggins J, Rice CM, Wilson SJ, Bieniasz PD. Identification of interferon-stimulated genes with antiretroviral activity. Cell Host Microbe. 2016;20:392–405. doi: 10.1016/j.chom.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Obojes K, Andres O, Kim KS, Daubener W, Schneider-Schaulies J. Indoleamine 2,3-dioxygenase mediates cell type-specific anti-measles virus activity of gamma interferon. J Virol. 2005;79:7768–7776. doi: 10.1128/JVI.79.12.7768-7776.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mao R, Zhang J, Jiang D, Cai D, Levy JM, Cuconati A, Block TM, Guo JT, Guo H. Indoleamine 2,3-dioxygenase mediates the antiviral effect of gamma interferon against hepatitis B virus in human hepatocyte-derived cells. J Virol. 2011;85:1048–1057. doi: 10.1128/JVI.01998-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meisel R, Brockers S, Heseler K, Degistirici O, Bulle H, Woite C, Stuhlsatz S, Schwippert W, Jager M, Sorg R, Henschler R, Seissler J, Dilloo D, Daubener W. Human but not murine multipotent mesenchymal stromal cells exhibit broad-spectrum antimicrobial effector function mediated by indoleamine 2,3-dioxygenase. Leukemia. 2011;25:648–654. doi: 10.1038/leu.2010.310. [DOI] [PubMed] [Google Scholar]
- 37.Qian F, Villella J, Wallace PK, Mhawech-Fauceglia P, Tario JD Jr, Andrews C, Matsuzaki J, Valmori D, Ayyoub M, Frederick PJ, Beck A, Liao J, Cheney R, Moysich K, Lele S, Shrikant P, Old LJ, Odunsi K. Efficacy of levo-1-methyl tryptophan and dextro-1-methyl tryptophan in reversing indoleamine-2,3-dioxygenase-mediated arrest of T-cell proliferation in human epithelial ovarian cancer. Cancer Res. 2009;69:5498–5504. doi: 10.1158/0008-5472.CAN-08-2106. [DOI] [PubMed] [Google Scholar]
- 38.Zhou Y, Yamamoto Y, Xiao Z, Ochiya T. The immunomodulatory functions of mesenchymal stromal/stem cells mediated via paracrine activity. J Clin Med. 2019;8:1025. doi: 10.3390/jcm8071025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10:490–500. doi: 10.1038/nri2785. [DOI] [PubMed] [Google Scholar]
- 40.Sakaguchi S. Regulatory T cells: key controllers of immunologic self-tolerance. Cell. 2000;101:455–458. doi: 10.1016/s0092-8674(00)80856-9. [DOI] [PubMed] [Google Scholar]
- 41.Garcia-Broncano P, Medrano LM, Berenguer J, González-García J, Jiménez-Sousa MÁ, Carrero A, Hontañón V, Guardiola JM, Crespo M, Quereda C, Sanz J, García-Gómez AB, Jimenez JL, Resino S GESIDA 3603b Study Group. Dysregulation of the immune system in HIV/HCV-coinfected patients according to liver stiffness status. Cells. 2018;7:196. doi: 10.3390/cells7110196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cari L, Montanucci P, Basta G, Petrillo MG, Ricci E, Pescara T, Greco A, Cipriani S, Shimizu J, Migliorati G, Nocentini G, Calafiore R, Riccardi C. Microencapsulated G3C hybridoma cell graft delays the onset of spontaneous diabetes in NOD mice by an expansion of Gitr(+) Treg cells. Diabetes. 2020;69:965–980. doi: 10.2337/db19-0087. [DOI] [PubMed] [Google Scholar]
- 43.Hayes M, Curley G, Ansari B, Laffey JG. Clinical review: stem cell therapies for acute lung injury/acute respiratory distress syndrome-hope or hype? Crit Care. 2012;16:205. doi: 10.1186/cc10570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Musial-Wysocka A, Kot M, Majka M. The pros and cons of mesenchymal stem cell-based therapies. Cell Transplant. 2019;28:801–812. doi: 10.1177/0963689719837897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moll G, Drzeniek N, Kamhieh-Milz J, Geissler S, Volk HD, Reinke P. MSC therapies for COVID-19: importance of patient coagulopathy, thromboprophylaxis, cell product quality and mode of delivery for treatment safety and efficacy. Front Immunol. 2020;11:1091. doi: 10.3389/fimmu.2020.01091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lanzoni G, Linetsky E, Correa D, Messinger Cayetano S, Alvarez RA, Kouroupis D, Alvarez Gil A, Poggioli R, Ruiz P, Marttos AC, Hirani K, Bell CA, Kusack H, Rafkin L, Baidal D, Pastewski A, Gawri K, Lenero C, Mantero AMA, Metalonis SW, Wang X, Roque L, Masters B, Kenyon NS, Ginzburg E, Xu X, Tan J, Caplan AI, Glassberg MK, Alejandro R, Ricordi C. Umbilical cord mesenchymal stem cells for COVID-19 acute respiratory distress syndrome: a double-blind, phase 1/2a, randomized controlled trial. Stem Cells Transl Med. 2021;10:660–673. doi: 10.1002/sctm.20-0472. [DOI] [PMC free article] [PubMed] [Google Scholar]