Abstract
Acute respiratory distress syndrome is a heterogenous syndrome with many etiologies for which there are no definitive pharmacologic treatments, despite decades of research. We explore some adjunctive pharmacologic therapies, including neuromuscular blockade, corticosteroids, and inhaled pulmonary vasodilators. Additionally, we explore some investigative therapies, including Vitamin C, beta-agonists, statins, mesenchymal stromal cells, and granulocyte–macrophage colony stimulating factor. We do discuss the potential role of steroids in acute respiratory distress syndrome with severe acute respiratory syndrome coronavirus 2 as a trigger. The standard of care, however, remains supportive care.
Keywords: ARDS, Neuromuscular blockade, Corticosteroids, Pulmonary vasodilators
Key points
-
•
The management of acute respiratory distress syndrome is primarily supportive.
-
•
No pharmacologic intervention has yet demonstrated a clear mortality benefit in acute respiratory distress syndrome.
-
•
Improvement in surrogate end points does not necessarily translate into improved survival.
-
•
There is a growing body of evidence suggesting that corticosteroids may improve outcomes in certain subgroups of patients with acute respiratory distress syndrome.
-
•
Further research is needed to assess the impact of investigational therapies such as vitamin C, mesenchymal stromal cells, and granulocyte–macrophage colony stimulating factor.
Introduction
Over 50 years have passed since the acute respiratory distress syndrome (ARDS) was first described.1 Despite the passage of more than one-half of a century, almost all of ARDS care continues to be supportive in nature, and mortality remains high at 34% to 45%.2 As our understanding of ARDS pathophysiology has improved, a number of pharmacologic interventions have been tested, including those targeting ventilator-associated lung injury (VALI), dead space ventilation, inflammation, alveolar epithelial and capillary endothelial injury, and dysfunctional fluid clearance. Although no medications have yet demonstrated a clear mortality benefit in ARDS, a number of therapies are currently being evaluated in clinical trials (Table 1 ). In this article, we review selected pharmacologic treatments for ARDS.
Table 1.
Pharmacologic therapies in ARDS
| Therapy | Mechanism of Action | Findings to Date |
|---|---|---|
| Neuromuscular blockade11,13 | Possible ↓ VALI occurrence via ↓ patient-ventilator interaction | No impact on mortality in moderate-to-severe ARDS No impact on incidence of ICU-acquired weakness (specifically with short-term use of cisatracurium) |
| Corticosteroids19, 20, 21, 22, 23, 24, 25, 26 | ↓ Synthesis of proinflammatory mediators | Possible ↓ mortality in early ARDS (<14 d since onset) Possible ↑ mortality in late ARDS (>14 d since onset) ↑ Ventilator-free days |
| Inhaled pulmonary vasodilators44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54 | ↓ Ventilation–perfusion mismatch | ↑ Oxygenation (short term) No known impact on mortality Possible ↑ risk of renal failure (iNO) |
| Vitamin C55, 56, 57, 58, 59, 60, 61 | ↓ Expression of proinflammatory mediators ↓ Microvascular thrombosis Enhancement of epithelial barrier function |
Possible ↓ mortality, ICU and hospital days in patients with ARDS + sepsis (exploratory finding) |
| Beta-agonists63, 64, 65, 66, 67 | ↑ Alveolar fluid clearance ↓ Lung vascular permeability |
↑ Mortality and ICU days Clinical trial assessing role of inhaled beta agonists for prevention of acute respiratory failure ongoing (NCT04193878) |
| Statins68, 69, 70, 71, 72, 73 | ↓ Synthesis of proinflammatory mediators | No impact on mortality or ventilator-free days Possible benefit in hyperinflammatory subphenotype (exploratory finding) |
| Mesenchymal Stromal Cells74, 75, 76, 77 | Enhanced epithelial/endothelial repair Improved phagocytosis ↑ Alveolar fluid clearance |
Safety established in phase I and IIA studies Multiple clinical trials ongoing (NCT02444455, NCT03608592, NCT03042143, NCT04367077) |
| GM-CSF24,78, 79, 80 | ↓ Oxidative epithelial cell injury Enhanced phagocyte function | Possible ↓ hypoxia and severity of illness (exploratory finding) Clinical trial of inhaled GM-CSF ongoing (NCT02595060) |
| Surfactant87,88 | Replacement of deficient or dysfunctional endogenous surfactant ↓ Alveolar surface tension ↓ Hydrostatic force driving pulmonary edema |
No impact on mortality No impact on oxygenation |
| Interferon β-1a89 | Prevention of vascular leakage Inhibition of leukocyte recruitment |
No impact on mortality No impact on ventilator-free days |
Commonly used therapies
Widely available therapies, and those studied in large clinical trials, include neuromuscular blockade, corticosteroids, and inhaled pulmonary vasodilators. These therapies are frequently used in the clinical setting, despite a lack of strong evidence favoring their use in ARDS. We examine each here, in the context of underlying pathophysiology.
Neuromuscular Blockade
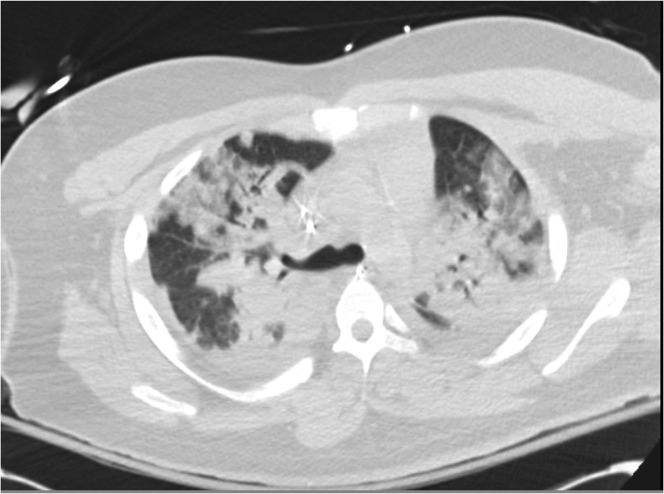
Much of ARDS care is supportive in nature, with lung protective mechanical ventilation as its mainstay. VALI is a well-known occurrence, and approaches to mechanical ventilation are aimed at minimizing it and avoiding its complications. The use of neuromuscular blockade is rationalized as a measure that may decrease the occurrence of VALI, which can be caused by both overdistension (volutrauma, and to a lesser extent, barotrauma) and repeated opening and closing of underrecruited alveoli (atelectrauma). Patients with heterogenous consolidation (Fig. 1 ), often seen in ARDS, are at particularly high risk for both.
Fig. 1.
Heterogenous consolidation in ARDS seen on a computed tomography scan.
The impact of large tidal volumes on lung injury and the protective effect of positive end-expiratory pressure (PEEP) are well-illustrated in a murine model. Dreyfuss and colleagues3 examined the effects of 5 different ventilator strategies in rats: low pressure–low tidal volume, high pressure–high tidal volume, high pressure–high volume with PEEP, high pressure–low volume (achieved by banding the chest wall), and low pressure–high volume. The rats ventilated with high volumes all had evidence of pulmonary edema.
However, edema was markedly decreased by PEEP. The low-volume groups had an essentially normal lung structure, including the high pressure–low volume group. Intuitively, one would predict that high airway pressures would play a significant role in VALI, but these findings demonstrated that it is actually the transalveolar gradient (intra-alveolar minus pleural pressure) that plays a more substantial role. Additionally, the protective effect of PEEP seen in this model may have been mediated by a decrease in atelectrauma.
Dysfunctional surfactant and gravitational forces resulting in the nonuniform distribution of edema can result in a difficult to recruit lung, regional overdistension, and thus atelectrauma. The application of PEEP can help to mitigate this factor by stenting open alveoli and preventing the shear forces associated with repeated alveolar opening and collapse.3
Large changes in volume can also lead to a disruption of the alveolar epithelial and capillary endothelial interface, with the resultant release of inflammatory mediators, worsening pulmonary edema, further compromise in gas exchange, and, in turn, a perpetual loop of worsening lung injury.4 Although lung protective ventilator strategies involve low tidal volume ventilation and application of relatively high levels of PEEP,5 , 6 current sedation strategies emphasizing daily awakenings and/or light sedation,7 leading to a fair amount of patient–ventilator interaction. Patients with a high metabolic demand and respiratory drive often attempt to generate higher tidal volumes, which may result in ventilator asynchronies, such as double triggering and even active exhalation, leading to the collapse of alveoli. Repeated collapse and hyperinflation can then in turn lead to worsening lung injury. As such, it may follow that, if a patient is breathing completely passively on the ventilator, there may be a decrease in VALI and an increased potential for healing. Neuromuscular blockade has been used for decades with this rationale in mind.
Although a number of neuromuscular blocking agents have been used for the management of ARDS, the use of older nondepolarizing neuromuscular blocking agents (pancuronium, vecuronium, or atracurium), especially in conjunction with systemic corticosteroids, in critically ill, mechanically ventilated patients has become increasingly limited owing to concerns for critical illness weakness.8 Hepatic or renal failure, common in these patients, seems to increase the likelihood of persistent weakness substantially. Cisatracurium is a newer nondepolarizing agent that is metabolized via Hoffman degradation to metabolites without neuromuscular blocking activity. It is also associated with a decrease in both pulmonary and systemic inflammatory cytokines,9 which may be a result not only of minimizing VALI, but also a direct effect of the cisatracurium itself.10 It is not associated with intensive care unit (ICU)-acquired weakness11 and, in comparison with vecuronium, cisatracurium is associated with fewer ICU and ventilator days,12 suggesting that it may be a preferable neuromuscular blocking agent for patients with ARDS.
The usefulness of cisatracurium for the management of ARDS has been assessed in multiple clinical trials (Table 2 ). In the ACURASYS trial, Papazian and colleagues13 randomized 340 patients with moderate-to-severe ARDS (P/F ≤ 150) to either cisatracurium, or placebo (with deep sedation) for 48 hours in a blinded fashion. Although the crude 90-day mortality was not different, after adjustment for baseline oxygenation, Simplified Acute Physiology Score II, and plateau pressure, mortality did seem to be decrease with a hazard ratio of 0.68 (95% confidence interval, 0.48–0.98; P = .04). Additionally, there was an increase in the number of ventilator free days both at 28 and 90 days.13 This trial was criticized because it was underpowered, a mortality benefit was only seen after statistical adjustment, and the control arm consisted of deep sedation to maintain blinding (in contrast with modern practices of daily awakenings or light sedation). Additionally, the assessment of critical care weakness, although performed, was inadequate, and thus remained mostly unresolved. Despite these issues, a prospective, multicenter epidemiologic study showed that 22% of all patients with ARDS and 38% of patients with severe ARDS received neuromuscular blockade.2 Additionally, a survey of academic intensivists showed that 97% of respondents used paralytics in managing patients with ARDS, and that 40% of intensivists use neuromuscular blockade in more than one-half of their patients.14 In light of the limitations of ACURASYS, the usefulness of cisatracurium for the management of ARDS was recently reexamined in the Reevaluation of Systemic Early Neuromuscular Blockade (ROSE), a multicenter, randomized, controlled trial comparing early neuromuscular blockade to light sedation in ARDS in 1006 patients. One of the biggest differences between the 2 trials were the control arms—a strategy of daily awakenings or light sedation—is known to result in improved outcomes when compared with deep sedation.7 , 15 In the ROSE trial, no difference was seen between the 2 groups in hospital mortality, ventilator-free days, ICU-free days, or hospital-free days. The cisatracurium group was less mobile while in the hospital and did have more adverse cardiovascular events. Importantly, the ROSE trial, in more robust fashion than in ACURASYS, also confirmed that cisatracurium was not associated with ICU-acquired weakness. Although longer term outcomes require further research, the use of neuromuscular blockade was not associated with differences in survival, disability, cognitive function or psychiatric symptoms in the ROSE study. Despite the results of these trials, neuromuscular blockade may have therapeutic value in carefully selected patients with severe ARDS, substantial ventilator asynchrony, and refractory hypoxemia, especially with a Pao 2/Fio 2 of less than 100 mm Hg. However, its routine use cannot be recommended.
Table 2.
Comparison of findings from 2 landmark studies on the use of cisatracurium in ARDS
| ACURASYS13 | ROSE11 | Importance |
|---|---|---|
| Multicenter, randomized trial of 340 patients | Multicenter, randomized trial of 1006 patients | ACURASYS was underpowered to detect a mortality difference without statistical adjustment |
| Double-blind—control group required deep sedation | Unblinded—control group sedation strategy was either to target RASS of –1 to 0, or to perform daily awakenings | Daily awakenings and light sedation have been shown to improve outcomes compared with deep sedation, so there is concern that the control arm in ACURASYS fared more poorly than they would have with modern sedation strategies |
| Patients enrolled with P/F of <150 on a PEEP of ≥5 cm H2O | Patients enrolled with P/F of <150 on a PEEP of ≥8 cm H2O | ROSE may have initially enrolled a more hypoxic cohort |
| Enrollment allowed up to 48 h after meeting criteria. Median time to enrollment was 16 hours [interquartile range, 6–29 hours] | Enrollment allowed up to 48 h after meeting criteria. Median time to enrollment was 8 hours [interquartile range, 4–16 hours] | ROSE may have enrolled patients who may not have survived to be enrolled in ACURASYS |
| Ventilatory strategy was low-tidal volume coupled with a conventional PEEP table | Ventilatory strategy was low tidal volume coupled with a moderately high PEEP table | Unclear |
Corticosteroids
The pathologic features of ARDS include a marked acute inflammatory response precipitated by alveolar epithelial and capillary endothelial injury.16 Processes that trigger ARDS, such as sepsis and pancreatitis, are frequently inflammatory themselves, as is VALI, often a consequence of supportive care for ARDS with mechanical ventilation.
Corticosteroids are anti-inflammatory and immunosuppressive. They act by binding to cell-surface receptors, and then translocate to the cell nucleus, where they inhibit the synthesis of proinflammatory mediators, such as cytokines, chemokines, inflammatory enzymes, receptors, and proteins.17 Corticosteroids are widely administered to patients with ARDS, both for the management of ARDS or for concurrent conditions such as septic shock or pneumonia. In a large US cohort, 44% of patients with moderate-to-severe ARDS received corticosteroids.18 However, the benefit of steroids in ARDS remains unclear, despite a number of studies assessing their use in ARDS and ARDS-related conditions (Table 3 ).
Table 3.
Evidence base for the use of corticosteroids in ARDS and ARDS-related conditions
| Patient Population | Findings to Date |
|---|---|
| ARDS (all causes)19, 20, 21, 22, 23, 24, 25, 26 | Possible ↓ mortality in early ARDS (<14 d since onset) Possible ↑ mortality in late ARDS (>14 d since onset) ↑ Ventilator-free days |
| Community-acquired pneumonia27, 28, 29, 30, 31, 32, 33 | ↓ Treatment failure ↓ Hospital length of stay ↓ Risk of ARDS |
| Influenza34 | Possible ↑ mortality Possible ↑ hospital-acquired infection Delayed viral clearance |
| Middle Eastern respiratory syndrome36 | No impact on mortality Delayed viral clearance |
| Severe acute respiratory syndome35 | Unclear impact on mortality Delayed viral clearance |
| COVID-1937, 38, 39, 40 | ↓ Mortality ↑ Ventilator-free days |
There has been substantial heterogeneity in steroid dosing, timing, and duration in clinical trials studying steroids in ARDS, and results have been mixed. In 1 trial comparing the early administration of very high-dose methylprednisolone (120 mg/kg over 24 hours in divided doses) with placebo in 99 patients with ARDS, no difference was found in the 45-day mortality rate, or in the reversal of ARDS.19 However, a small subsequent placebo-controlled trial in 24 patients assessing a prolonged course of lower dose methylprednisolone (2 mg/kg/d with tapering over a maximum of 32 days) in unresolving ARDS (≥7 days of mechanical ventilation) reported that steroids were associated with improvements in both physiologic parameters and mortality (ICU and hospital), although there was substantial cross-over in this trial.20 Similar findings were reported in a larger multicenter, randomized, placebo-controlled trial using even more modest doses of methylprednisolone (1 mg/kg/d with taper, for ≤28 days) for early ARDS (within 72 hours of onset), which demonstrated decreased duration of mechanical ventilatory support, ICU length of stay, and mortality (20.6% vs 42.9%; P = .03) in patients receiving steroids.21 Unfortunately, these mortality benefits were not replicated in the Late Steroids Rescue Study (LaSRS) performed by the ARDS Clinical Trials Network.22 Despite an increase in the number of ventilator-free days and shock-free days in patients with ARDS for more than7 days, the overall mortality rate was not decreased, and there was a concerning increase in 60- and 180-day mortality rates in the subset of patients given steroids more than 2 weeks after onset of ARDS. Some meta-analyses have suggested that steroids may reduce mortality and increase ventilator-free days in ARDS, particularly in patients treated within 14 days of onset,23, 24, 25 indicating that the role of steroids should be further evaluated in early ARDS. Very recently, an unblinded, randomized, controlled trial of 277 patients comparing a 10-day regimen of dexamethasone with placebo in early ARDS (within 30 hours of meeting the Berlin Criteria) was conducted, demonstrating a significant decrease in the primary end point of ventilator-free days at 28 days (12.3 ± 9.9 vs 7.5 ± 9.0 days; P<.0001), and a secondary end point of all-cause mortality at 60 days (21% vs 36%; P<.0047).26 However, some criticisms of this trial included slow enrollment occurring over 5 years, an inability to complete the planned enrollment, and the exclusion of a large number of patients.
Consistent with the hypothesis that steroids may be beneficial in lung injury, the role of steroids has also been tested in community-acquired pneumonia. Multiple clinical trials have found that steroids improve outcomes in severe community-acquired pneumonia,27, 28, 29 primarily with regard to the resolution of pneumonia, including two multicenter, randomized, double-blind, placebo-controlled trials. The first trial included 304 patients with community-acquired pneumonia and found that dexamethasone decreased the hospital length of stay by 1 day compared with placebo.30 A subsequent trial of 120 patients with community-acquired pneumonia and high inflammatory response (C-reactive protein of >150 mg/L at admission), found that the use of methylprednisolone decreased treatment failure.31 These findings have been redemonstrated in recent meta-analyses.32 , 33 Similarly, the use of corticosteroids in viral pneumonias has also been evaluated, but with mixed results. Although prior studies evaluating the use of steroids in viral pneumonias have been associated with delayed viral clearance and possible harm,34, 35, 36 there has been marked heterogeneity in these trials. A retrospective review of 774 patients with COVID-19, 90% of whom were receiving oxygen by simple nasal cannula, found that corticosteroid therapy was associated with harm, particularly in those who received high-dose steroids (>200 mg of hydrocortisone or the equivalent) and who received them in the first 3 days of hospitalization.37 Other clinical trials, however, have found that corticosteroids improved outcomes in patients with COVID-19 with acute hypoxemic respiratory failure.38, 39, 40 The RECOVERY group randomized 2104 patients with COVID-19 to dexamethasone (6 mg/d for ≤10 days), and 4321 to usual care. They showed that the use of dexamethasone in these patients resulted in a lower 28-day mortality in those requiring invasive mechanical ventilation (29% vs 41%), as well as in patients receiving supplemental oxygen without invasive mechanical ventilation (23% vs 26%).40 Patients with COVID-19 ARDS were the focus of the multicenter CoDEX trial, which randomized 299 patients with moderate-to-severe ARDS to high-dose dexamethasone (20 mg/d for 5 days, followed by 10 mg/d for 5 days) or usual care. Patients receiving dexamethasone had more ventilator-free days (6.6 vs 4.0; P = .04), although the 28-day mortality was not significantly different (56% vs 62%; P = .83).38 Potential reasons for differences between RECOVERY, CoDEX, and other studies are very possibly related to dosage of steroids, the timing of the administration (early administration may decrease the ability to impair clearance of virus), and severity of illness (increased benefit in those requiring more ventilatory support).41 A reevaluation of the use of steroids in other viral pneumonias may be warranted, with careful attention to trial design (dose, timing, and severity of illness); this would likely impact the body of evidence for steroids in ARDS.
Inhaled Pulmonary Vasodilators
Vasculopathy in ARDS can contribute to worsening ventilation–perfusion matching and increased dead space ventilation. On a microvascular level, both thromboembolic and endothelial cell injury can result in a decrease in pulmonary blood flow, and hypoxic vasoconstriction also occurs.42 Together, these phenomena lead to a ventilatory–perfusion mismatch and increased dead space ventilation. An increased dead space fraction is associated with higher mortality in patients with ARDS.43 Inhaled pulmonary vasodilators such as nitric oxide and prostacyclins are sometimes used as therapeutic agents in ARDS with the goal of improving oxygenation and decreasing dead space.
Inhaled nitric oxide (iNO) diffuses through the alveoli into pulmonary vascular smooth muscle cells where it causes smooth muscle relaxation via an increase in cyclic GMP.44 Vasodilation results in improved perfusion to ventilated areas of lung, and thus better ventilation–perfusion matching. In a well-designed, multicenter, randomized, blinded, placebo-controlled study of iNO (5 ppm) in 385 patients with ARDS (P/F ≤ 250), oxygenation did indeed improve significantly with the use of iNO compared with placebo. Despite this finding, iNO had no impact on either the primary end point of days alive and off assisted ventilation, or the secondary end point of mortality.45 A subsequent meta-analysis of iNO treatment for ARDS has similarly demonstrated that iNO is associated with improvements in oxygenation, but no significant change in mortality, duration of mechanical ventilation, or ICU length of stay. Improvements in oxygenation also tend to be small and transient, and iNO is associated with an increased incidence of renal failure.46
Prostacyclins act on G-protein–coupled receptors in the pulmonary vasculature to increase cyclic adenosine monophosphate, and ultimately vascular smooth muscle relaxation.47 Like iNO, inhaled prostacyclins have also been shown to improve oxygenation. However, no trials have yet demonstrated an effect on mortality in ARDS. 48, 49, 50, 51
Despite the paucity of evidence supporting their use, inhaled pulmonary vasodilators are used in a small but significant number of patients with ARDS. Their use was reported in 13% of patients with severe ARDS in a large global cohort.2 Although they may improve right heart function, decrease ventilation–perfusion mismatch, and improve oxygenation,52, 53, 54 pulmonary vasodilators have yet to demonstrate any impact on mortality or other patient-centered outcomes, so their routine use in ARDS cannot be recommended. However, they may be of some usefulness in patients with concurrent right ventricular failure, as a temporizing measure for patients requiring transportation, or as a short-term rescue therapy for patients with refractory hypoxia before the initiation of extracorporeal support. Their utility in other situations requires further research.
Investigational Therapies: Vitamin C
In animal models of sepsis and lung injury, vitamin C has been shown to act on a number of physiologic derangements present in ARDS, including attenuating inflammation, improving epithelial–endothelial function, speeding resolution of pulmonary edema fluid, and decreasing coagulopathy.55, 56, 57, 58 Intravenous vitamin C has also been evaluated in clinical trials of the critically ill. Administration of high-dose vitamin C at 66 mg/kg/h in burn patients has been found to decrease the need for intravenous fluid resuscitation, and potentially decrease respiratory dysfunction.59 In critically ill patients with sepsis, a phase I study of vitamin C versus placebo demonstrated that high-dose infused vitamin C was safe, and potentially beneficial as reflected in an improved modified Sequential Organ Failure Assessment (mSOFA) scores, a decrease in inflammatory, and a nonstatistically significant decrease in ICU length of stay.60 Because of these preliminary findings, a multicenter, randomized, double-blind, placebo-controlled trial of 167 patients was conducted to examine the effects of high-dose intravenous vitamin C (50 mg/kg every 6 hours for 96 hours) on the primary outcome measures of mSOFA score and plasma markers of inflammation and vascular injury in patients with ARDS and sepsis. Although the mSOFA scores, biomarkers of inflammation, and vascular injury were not significantly decreased, the secondary outcomes of 28-day mortality, ICU-free days, and hospital-free days did favor the use of vitamin C (Table 4 ).61 One criticism of the trial was that the mSOFA scores of patients who died or were discharged before the conclusion of the 96-hour study period were excluded from the analysis. In a post hoc analysis incorporating maximum mSOFA scores (20) for patients who were deceased, and minimum scores (0) for those who were discharged alive, a statistically significant improvement of mSOFA score at 96 hours was seen in patients receiving vitamin C.62 These findings are exploratory and require confirmation in larger randomized controlled trials.
Table 4.
Key end points in CITRUS-ALI trial61
| Variable | Vitamin C | Placebo |
|---|---|---|
| Change in mSOFA at 96 h, meana | 3 | 3.5 |
| All-cause mortality to day 28, %b | 29.8% | 46.3% |
| Ventilator-free days to day 28, medianb | 13.1 | 10.6 |
| ICU-free days to day 28, medianb | 10.7 | 7.7 |
| Hospital-free days to day 28, medianb | 22.6 | 15.5 |
Primary end point.
Secondary end point.
Beta-Agonists
The end result of inflammation, alveolar epithelial–capillary endothelial dysfunction, and dysfunctional fluid clearance is flooding of the alveolar spaces with fluid and pulmonary edema formation. Beta-agonists stimulate alveolar fluid clearance and have been explored as therapeutic agents for ARDS. Their mechanism of action may occur through vectorial transport of sodium from the alveolar space via apical amiloride-sensitive Na channels on alveolar type II cells, and then egress via basolateral Na, K-ATPase pumps.63 Additionally, beta-agonists may decrease lung vascular permeability.64
Despite their theoretic advantages, in a multicenter, randomized controlled trial comparing aerosolized albuterol with placebo in 282 patients with ARDS,65 patients receiving albuterol had significantly more ICU days but no significant difference in mortality or days of mechanical ventilation. A subsequent randomized controlled trial of 236 patients assessing the effects of intravenous salbutamol on patients with ARDS found that treatment was poorly tolerated and was associated with increased mortality (34% vs 23%; relative risk, 1.47; 95% confidence interval, 1.03–2.08).66 Routine treatment of established ARDS with beta-agonists should thus be avoided, as it may be associated with harm. However, the combination of beta-agonists with corticosteroids, may have a role in preventing the progression of at-risk patients to ARDS.67 In a pilot study, 61 patients at risk of developing ARDS based on the presence of at least 1 risk factor and a Lung Injury Prevention Score of 4 or higher were randomized to either placebo or inhaled budesonide and formoterol. Those randomized to the intervention demonstrated improved oxygenation based on the S/F ratio. Additional trials are ongoing, including the Arrest RESpiraTory Failure from PNEUMONIA (ARREST) trial, which examines whether inhaled beta-agonists and corticosteroids can prevent acute respiratory failure in patients with hypoxemia and pneumonia (NCT04193878).
Statins
By inhibiting the conversion of 3-hydroxy-3-methylglutaryl-coenzyme A to l-mevalonate, statins not only inhibit cholesterol synthesis in the liver, but also the production of multiple downstream signaling molecules, which may be the etiology of their anti-inflammatory effects.68 Statins decrease inflammation and lung injury in murine and human models,69 , 70 suggesting a possible role for their use in ARDS. However, both rosuvastatin and simvastatin have been evaluated in multicenter, double-blinded, randomized controlled trials, and neither drug has been shown to decrease mortality or days of mechanical ventilation.71 , 72 A secondary analysis of the simvastatin study did, however, reveal a differential response to statins in patients with hypoinflammatory and hyperinflammatory subphenotypes, suggesting its possible value for subsets of patients with a hyperinflammatory ARDS phenotype.73 Prospective confirmation of these findings is needed in a randomized controlled trial.
Mesenchymal Stromal Cells
Preclinical studies suggest that human mesenchymal stromal cells (MSCs) may have the ability to attenuate inflammation, enhance resolution of lung injury, and facilitate bacterial clearance, and thus hold promise for the treatment of ARDS. MSCs have been found to improve mortality in murine lung injury models, and in ex vivo human lungs.74 On the basis of these findings, preliminary trials assessing the use of MSCs in patients with moderate-to-severe ARDS have been performed, which have demonstrated that that MSCs are safe and well-tolerated.74, 75, 76 Additionally, an initial report of results from the MUST-ARDS trial, which assessed the use of bone marrow–derived MSCs in patients with moderate to severe ARDS in 30 patients, suggested that the use of MSCs may be associated with decreased mortality, as well as increased ventilator-free days and ICU-free days. However, the primary end point of this study was safety; it was not powered to assess these secondary end points. Although this initial report is promising, further research is needed to determine the efficacy of MSCs, and multiple clinical trials are ongoing (NCT02444455, NCT03608592, NCT03042143, NCT04367077).77
Granulocyte–Macrophage Colony Stimulating Factor
Granulocyte–macrophage colony stimulating factor (GM-CSF) plays important roles in surfactant clearance, pulmonary innate immunity, and the growth and survival of alveolar epithelial cells. In animal models, it has been found to prevent hyperoxia-induced lung injury, possibly by limiting epithelial cell injury.78 Higher concentrations of GM-CSF in the bronchoalveolar lavage fluid from patients with ARDS may also be associated with increased survival.79 Its use as a therapeutic remains unclear at this time. In a randomized, double-blind, placebo-controlled trial of intravenous GM-CSF in 130 patients with ARDS, there were trends toward lower mortality rates and increased organ failure-free days, but these differences did not achieve statistical significance.80 A small study of inhalational GM-CSF suggested that its use may improve oxygenation and severity of illness81; these findings are being further assessed in a multicenter trial (NCT02595060).
Challenges and Future Directions
The potential reasons why no single pharmacologic treatment has definitively been found to be beneficial in ARDS are manifold. The pathogenesis of ARDS is complex, and involves multiple mechanisms of injury, possibly rendering interventions targeted to single mediators ineffective. There is also substantial heterogeneity in this syndrome—a multitude of etiologies exist, as does a wide spectrum of severity. Variability exists even in the management of ARDS,18 creating challenges in the evaluation of any single therapy.
In light of these issues, there has been growing interest in the personalization of care in ARDS. A number of strategies have been used to derive homogenous subgroups in ARDS, including categorization based on physiology, etiology, biomarkers, or gene expression.82 Identifying distinct subsets may increase the likelihood of either response or adverse outcome associated with specific interventions, and as such provide an opportunity for improving patient selection for clinical trials on therapeutics for ARDS.83 , 84 As an example, subphenotypes of ARDS with variable levels of inflammation have been identified by using latent class analysis to examine clinical and biological data.85 These subphenotypes have exhibited divergent clinical outcomes and differential response to specific supportive and pharmacologic therapies.73 , 86 Although targeting ARDS therapies to subphenotypes holds promise, another challenge lies in the capability of rapidly identifying such subphenotypes at the bedside. Methods for doing so must first be developed before personalized approaches can be assessed in a meaningful manner.
Additionally, the COVID-19 pandemic has spawned numerous clinical trials, including those involving steroids and immunomodulatory therapies such as IL-1 inhibitors, anti–IL-6 receptor monoclonal antibodies, and interferons. To date, aside from dexamethasone, no agent has yet demonstrated a clear benefit in COVID-19-related ARDS. However, many trials are still ongoing and their impact on ARDS care remains to be seen.
Summary
No pharmacologic interventions have yet demonstrated a clear mortality benefit in ARDS, although some have been associated with improvement in surrogate end points. There is a growing body of evidence suggesting that corticosteroids may be beneficial. Additionally, a number of investigational therapies such as vitamin C, mesenchymal stem cells, and GM-CSF may be promising, but further research is needed.
Clinics care points
-
•
The cornerstone of care for ARDS remains lung protective ventilation, because no definitive pharmacologic therapies have yet been found.
-
•
Currently, the most promising pharmacologic therapy for ARDS is corticosteroid. Timing of administration, however, matters because the benefit has only been seen when initiated in early ARDS. There is no benefit to starting systemic steroid therapy at 7 or more days after the onset of ARDS.
-
•
Although there is no impact on survival, neuromuscular blockade can be of usefulness in carefully selected patients with moderate to severe ARDS.
-
•
We cannot recommend any other pharmacologic interventions at this time given the lack of evidence, although investigations are ongoing for high-dose vitamin C, MSCs, and other potential therapies.
Acknowledgments
Disclosure
N. Qadir reports no significant conflicts of interest; S.Y. Chang was an advisor for a COVID trial to PureTech Pharmaceuticals in 2020; and was a speaker for La Jolla Pharmaceuticals in 2018.
References
- 1.Ashbaugh D.G., Bigelow D.B., Petty T.L., et al. Acute respiratory distress in adults. Lancet. 1967;2:319–323. doi: 10.1016/s0140-6736(67)90168-7. [DOI] [PubMed] [Google Scholar]
- 2.Bellani G., Laffey J.G., Pham T., et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. J Am Med Assoc. 2016;315:788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 3.Dreyfuss D., Soler P., Basset G., et al. High inflation pressure pulmonary edema. Respective effects of high airway pressure, high tidal volume, and positive end-expiratory pressure. Am Rev Respir Dis. 1988;137:1159–1164. doi: 10.1164/ajrccm/137.5.1159. [DOI] [PubMed] [Google Scholar]
- 4.Matthay M.A., Ware L.B., Zimmerman G.A. The acute respiratory distress syndrome. J Clin Invest. 2012;122:2731–2740. doi: 10.1172/JCI60331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Acute Respiratory Distress Syndrome N, Brower R.G., Matthay M.A., et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 6.Meade M.O., Cook D.J., Guyatt G.H., et al. Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end-expiratory pressure for acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. J Am Med Assoc. 2008;299:637–645. doi: 10.1001/jama.299.6.637. [DOI] [PubMed] [Google Scholar]
- 7.Kress J.P., Pohlman A.S., O'Connor M.F., et al. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med. 2000;342:1471–1477. doi: 10.1056/NEJM200005183422002. [DOI] [PubMed] [Google Scholar]
- 8.Hansen-Flaschen J., Cowen J., Raps E.C. Neuromuscular blockade in the intensive care unit. More than we bargained for. Am Rev Respir Dis. 1993;147:234–236. doi: 10.1164/ajrccm/147.1.234. [DOI] [PubMed] [Google Scholar]
- 9.Forel J.M., Roch A., Marin V., et al. Neuromuscular blocking agents decrease inflammatory response in patients presenting with acute respiratory distress syndrome. Crit Care Med. 2006;34:2749–2757. doi: 10.1097/01.CCM.0000239435.87433.0D. [DOI] [PubMed] [Google Scholar]
- 10.Fanelli V., Morita Y., Cappello P., et al. Neuromuscular blocking agent cisatracurium attenuates lung injury by inhibition of nicotinic acetylcholine receptor-alpha1. Anesthesiology. 2016;124:132–140. doi: 10.1097/ALN.0000000000000907. [DOI] [PubMed] [Google Scholar]
- 11.National Heart L, Blood Institute PCTN, Moss M., et al. Early neuromuscular blockade in the acute respiratory distress syndrome. N Engl J Med. 2019;380:1997–2008. doi: 10.1056/NEJMoa1901686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sottile P.D., Kiser T.H., Burnham E.L., et al. An observational study of the efficacy of cisatracurium compared with vecuronium in patients with or at risk for acute respiratory distress syndrome. Am J Respir Crit Care Med. 2018;197:897–904. doi: 10.1164/rccm.201706-1132OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Papazian L., Forel J.M., Gacouin A., et al. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med. 2010;363:1107–1116. doi: 10.1056/NEJMoa1005372. [DOI] [PubMed] [Google Scholar]
- 14.Dodia N.N., Richert M.E., Deitchman A.R., et al. A survey of academic intensivists' Use of neuromuscular blockade in subjects with ARDS. Respir Care. 2020;65:362–368. doi: 10.4187/respcare.07026. [DOI] [PubMed] [Google Scholar]
- 15.Girard T.D., Kress J.P., Fuchs B.D., et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet. 2008;371:126–134. doi: 10.1016/S0140-6736(08)60105-1. [DOI] [PubMed] [Google Scholar]
- 16.Ware L.B., Matthay M.A. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 17.Barnes P.J. How corticosteroids control inflammation: quintiles prize lecture 2005. Br J Pharmacol. 2006;148:245–254. doi: 10.1038/sj.bjp.0706736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qadir N., Bartz R.R., Cooter M.L., et al. Variation in Early Management Practices in Moderate-to-Severe Acute Respiratory Distress Syndrome in the United States. Chest. 2021 doi: 10.1016/j.chest.2021.05.047. [DOI] [Google Scholar]
- 19.Bernard G.R., Luce J.M., Sprung C.L., et al. High-dose corticosteroids in patients with the adult respiratory distress syndrome. N Engl J Med. 1987;317:1565–1570. doi: 10.1056/NEJM198712173172504. [DOI] [PubMed] [Google Scholar]
- 20.Meduri G.U., Headley A.S., Golden E., et al. Effect of prolonged methylprednisolone therapy in unresolving acute respiratory distress syndrome: a randomized controlled trial. J Am Med Assoc. 1998;280:159–165. doi: 10.1001/jama.280.2.159. [DOI] [PubMed] [Google Scholar]
- 21.Meduri G.U., Golden E., Freire A.X., et al. Methylprednisolone infusion in early severe ARDS: results of a randomized controlled trial. Chest. 2007;131:954–963. doi: 10.1378/chest.06-2100. [DOI] [PubMed] [Google Scholar]
- 22.Steinberg K.P., Hudson L.D., Goodman R.B., et al. Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N Engl J Med. 2006;354:1671–1684. doi: 10.1056/NEJMoa051693. [DOI] [PubMed] [Google Scholar]
- 23.Peter J.V., John P., Graham P.L., et al. Corticosteroids in the prevention and treatment of acute respiratory distress syndrome (ARDS) in adults: meta-analysis. BMJ. 2008;336:1006–1009. doi: 10.1136/bmj.39537.939039.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lewis S.R., Pritchard M.W., Thomas C.M., et al. Pharmacological agents for adults with acute respiratory distress syndrome. Cochrane Database Syst Rev. 2019;7:Cd004477. doi: 10.1002/14651858.CD004477.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meduri G.U., Bridges L., Shih M.C., et al. Prolonged glucocorticoid treatment is associated with improved ARDS outcomes: analysis of individual patients' data from four randomized trials and trial-level meta-analysis of the updated literature. Intensive Care Med. 2016;42:829–840. doi: 10.1007/s00134-015-4095-4. [DOI] [PubMed] [Google Scholar]
- 26.Villar J., Ferrando C., Martinez D., et al. Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respir Med. 2020 doi: 10.1016/S2213-2600(19)30417-5. [DOI] [PubMed] [Google Scholar]
- 27.Confalonieri M., Urbino R., Potena A., et al. Hydrocortisone infusion for severe community- acquired pneumonia: a preliminary randomized study. Am J Respir Crit Care Med. 2005;171:242–248. doi: 10.1164/rccm.200406-808OC. [DOI] [PubMed] [Google Scholar]
- 28.Fernandez-Serrano S., Dorca J., Garcia-Vidal C., et al. Effect of corticosteroids on the clinical course of community-acquired pneumonia: a randomized controlled trial. Crit Care. 2011;15:R96. doi: 10.1186/cc10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mikami K., Suzuki M., Kitagawa H., et al. Efficacy of corticosteroids in the treatment of community-acquired pneumonia requiring hospitalization. Lung. 2007;185:249–255. doi: 10.1007/s00408-007-9020-3. [DOI] [PubMed] [Google Scholar]
- 30.Meijvis S.C., Hardeman H., Remmelts H.H., et al. Dexamethasone and length of hospital stay in patients with community-acquired pneumonia: a randomised, double-blind, placebo-controlled trial. Lancet. 2011;377:2023–2030. doi: 10.1016/S0140-6736(11)60607-7. [DOI] [PubMed] [Google Scholar]
- 31.Torres A., Sibila O., Ferrer M., et al. Effect of corticosteroids on treatment failure among hospitalized patients with severe community-acquired pneumonia and high inflammatory response: a randomized clinical trial. J Am Med Assoc. 2015;313:677–686. doi: 10.1001/jama.2015.88. [DOI] [PubMed] [Google Scholar]
- 32.Siemieniuk R.A., Meade M.O., Alonso-Coello P., et al. Corticosteroid therapy for patients hospitalized with community-acquired pneumonia: a systematic review and meta-analysis. Ann Intern Med. 2015;163:519–528. doi: 10.7326/M15-0715. [DOI] [PubMed] [Google Scholar]
- 33.Wan Y.D., Sun T.W., Liu Z.Q., et al. Efficacy and safety of corticosteroids for community-acquired pneumonia: a systematic review and meta-analysis. Chest. 2016;149:209–219. doi: 10.1378/chest.15-1733. [DOI] [PubMed] [Google Scholar]
- 34.Lansbury L.E., Rodrigo C., Leonardi-Bee J., et al. Corticosteroids as adjunctive therapy in the treatment of influenza: an updated Cochrane systematic review and meta- analysis. Crit Care Med. 2020;48:e98–e106. doi: 10.1097/CCM.0000000000004093. [DOI] [PubMed] [Google Scholar]
- 35.Lee N., Allen Chan K.C., Hui D.S., et al. Effects of early corticosteroid treatment on plasma SARS- associated Coronavirus RNA concentrations in adult patients. J Clin Virol. 2004;31:304–309. doi: 10.1016/j.jcv.2004.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arabi Y.M., Mandourah Y., Al-Hameed F., et al. Corticosteroid therapy for critically ill patients with Middle East respiratory syndrome. Am J Respir Crit Care Med. 2018;197:757–767. doi: 10.1164/rccm.201706-1172OC. [DOI] [PubMed] [Google Scholar]
- 37.Liu J., Zhang S., Dong X., et al. Corticosteroid treatment in severe COVID-19 patients with acute respiratory distress syndrome. J Clin Invest. 2020;130:6417–6428. doi: 10.1172/JCI140617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tomazini B.M., Maia I.S., Cavalcanti A.B., et al. Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID- 19: the CoDEX randomized clinical trial. J Am Med Assoc. 2020;324:1307–1316. doi: 10.1001/jama.2020.17021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sterne J.A.C., Murthy S., Diaz J.V., et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. J Am Med Assoc. 2020;324:1330–1341. doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Horby P., Lim W.S., Emberson J.R., et al. Dexamethasone in hospitalized patients with Covid-19 - preliminary report. N Engl J Med. 2020 doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matthay M.A., Wick K.D. Corticosteroids, COVID-19 pneumonia, and acute respiratory distress syndrome. J Clin Invest. 2020;130:6218–6221. doi: 10.1172/JCI143331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tomashefski J.F., Jr., Davies P., Boggis C., et al. The pulmonary vascular lesions of the adult respiratory distress syndrome. Am J Pathol. 1983;112:112–126. [PMC free article] [PubMed] [Google Scholar]
- 43.Nuckton T.J., Alonso J.A., Kallet R.H., et al. Pulmonary dead-space fraction as a risk factor for death in the acute respiratory distress syndrome. N Engl J Med. 2002;346:1281–1286. doi: 10.1056/NEJMoa012835. [DOI] [PubMed] [Google Scholar]
- 44.Yu B., Ichinose F., Bloch D.B., et al. Inhaled nitric oxide. Br J Pharmacol. 2019;176:246–255. doi: 10.1111/bph.14512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taylor R.W., Zimmerman J.L., Dellinger R.P., et al. Low-dose inhaled nitric oxide in patients with acute lung injury: a randomized controlled trial. J Am Med Assoc. 2004;291:1603–1609. doi: 10.1001/jama.291.13.1603. [DOI] [PubMed] [Google Scholar]
- 46.Gebistorf F., Karam O., Wetterslev J., et al. Inhaled nitric oxide for acute respiratory distress syndrome (ARDS) in children and adults. Cochrane Database Syst Rev. 2016:CD002787. doi: 10.1002/14651858.CD002787.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Del Pozo R., Hernandez Gonzalez I., Escribano-Subias P. The prostacyclin pathway in pulmonary arterial hypertension: a clinical review. Expert Rev Respir Med. 2017;11:491–503. doi: 10.1080/17476348.2017.1317599. [DOI] [PubMed] [Google Scholar]
- 48.Afshari A., Bastholm Bille A., Allingstrup M. Aerosolized prostacyclins for acute respiratory distress syndrome (ARDS) Cochrane Database Syst Rev. 2017;7:CD007733. doi: 10.1002/14651858.CD007733.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dahlem P., van Aalderen W.M., de Neef M., et al. Randomized controlled trial of aerosolized prostacyclin therapy in children with acute lung injury. Crit Care Med. 2004;32:1055–1060. doi: 10.1097/01.ccm.0000120055.52377.bf. [DOI] [PubMed] [Google Scholar]
- 50.Walmrath D., Schneider T., Schermuly R., et al. Direct comparison of inhaled nitric oxide and aerosolized prostacyclin in acute respiratory distress syndrome. Am J Respir Crit Care Med. 1996;153:991–996. doi: 10.1164/ajrccm.153.3.8630585. [DOI] [PubMed] [Google Scholar]
- 51.Fuller B.M., Mohr N.M., Skrupky L., et al. The use of inhaled prostaglandins in patients with ARDS: a systematic review and meta-analysis. Chest. 2015;147:1510–1522. doi: 10.1378/chest.14-3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hill N.S., Preston I.R., Roberts K.E. Inhaled therapies for pulmonary hypertension. Respir Care. 2015;60:794–802. doi: 10.4187/respcare.03927. discussion -5. [DOI] [PubMed] [Google Scholar]
- 53.Siobal M. Aerosolized prostacyclins. Respir Care. 2004;49:640–652. [PubMed] [Google Scholar]
- 54.Hsu C.W., Lee D.L., Lin S.L., et al. The initial response to inhaled nitric oxide treatment for intensive care unit patients with acute respiratory distress syndrome. Respiration. 2008;75:288–295. doi: 10.1159/000101478. [DOI] [PubMed] [Google Scholar]
- 55.Mohammed B.M., Fisher B.J., Kraskauskas D., et al. Vitamin C: a novel regulator of neutrophil extracellular trap formation. Nutrients. 2013;5:3131–3151. doi: 10.3390/nu5083131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fisher B.J., Seropian I.M., Kraskauskas D., et al. Ascorbic acid attenuates lipopolysaccharide-induced acute lung injury. Crit Care Med. 2011;39:1454–1460. doi: 10.1097/CCM.0b013e3182120cb8. [DOI] [PubMed] [Google Scholar]
- 57.Fisher B.J., Kraskauskas D., Martin E.J., et al. Attenuation of sepsis-induced organ injury in mice by vitamin C. JPEN J Parenter Enteral Nutr. 2014;38:825–839. doi: 10.1177/0148607113497760. [DOI] [PubMed] [Google Scholar]
- 58.Fisher B.J., Kraskauskas D., Martin E.J., et al. Mechanisms of attenuation of abdominal sepsis induced acute lung injury by ascorbic acid. Am J Physiol Lung Cell Mol Physiol. 2012;303:L20–L32. doi: 10.1152/ajplung.00300.2011. [DOI] [PubMed] [Google Scholar]
- 59.Tanaka H., Matsuda T., Miyagantani Y., et al. Reduction of resuscitation fluid volumes in severely burned patients using ascorbic acid administration: a randomized, prospective study. Arch Surg. 2000;135:326–331. doi: 10.1001/archsurg.135.3.326. [DOI] [PubMed] [Google Scholar]
- 60.Fowler A.A., 3rd, Syed A.A., Knowlson S., et al. Phase I safety trial of intravenous ascorbic acid in patients with severe sepsis. J Transl Med. 2014;12:32. doi: 10.1186/1479-5876-12-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fowler A.A., 3rd, Truwit J.D., Hite R.D., et al. Effect of vitamin C infusion on organ failure and biomarkers of inflammation and vascular injury in patients with sepsis and severe acute respiratory failure: the CITRIS-ALI randomized clinical trial. J Am Med Assoc. 2019;322:1261–1270. doi: 10.1001/jama.2019.11825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fowler A.A., 3rd, Fisher B.J., Kashiouris M.G. Vitamin C for sepsis and acute respiratory failure- reply. J Am Med Assoc. 2020;323:792–793. doi: 10.1001/jama.2019.21987. [DOI] [PubMed] [Google Scholar]
- 63.Groshaus H.E., Manocha S., Walley K.R., et al. Mechanisms of beta-receptor stimulation- induced improvement of acute lung injury and pulmonary edema. Crit Care. 2004;8:234–242. doi: 10.1186/cc2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Basran G.S., Hardy J.G., Woo S.P., et al. Beta-2-adrenoceptor agonists as inhibitors of lung vascular permeability to radiolabelled transferrin in the adult respiratory distress syndrome in man. Eur J Nucl Med. 1986;12:381–384. doi: 10.1007/BF00252194. [DOI] [PubMed] [Google Scholar]
- 65.Matthay M.A., Brower R.G., Carson S., et al. Randomized, placebo-controlled clinical trial of an aerosolized β₂-agonist for treatment of acute lung injury. Am J Respir Crit Care Med. 2011;184:561–568. doi: 10.1164/rccm.201012-2090OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gao Smith F., Perkins G.D., Gates S., et al. Effect of intravenous β-2 agonist treatment on clinical outcomes in acute respiratory distress syndrome (Balti-2): a multicentre, randomised controlled trial. Lancet. 2012;379:229–235. doi: 10.1016/S0140-6736(11)61623-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Festic E., Carr G.E., Cartin-Ceba R., et al. Randomized clinical trial of a combination of an inhaled corticosteroid and beta agonist in patients at risk of developing the acute respiratory distress syndrome. Crit Care Med. 2017;45:798–805. doi: 10.1097/CCM.0000000000002284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Oesterle A., Laufs U., Liao J.K. Pleiotropic effects of statins on the cardiovascular system. Circ Res. 2017;120:229–243. doi: 10.1161/CIRCRESAHA.116.308537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jacobson J.R., Barnard J.W., Grigoryev D.N., et al. Simvastatin attenuates vascular leak and inflammation in murine inflammatory lung injury. Am J Physiol Lung Cell Mol Physiol. 2005;288:L1026–L1032. doi: 10.1152/ajplung.00354.2004. [DOI] [PubMed] [Google Scholar]
- 70.Shyamsundar M., McKeown S.T., O'Kane C.M., et al. Simvastatin decreases lipopolysaccharide- induced pulmonary inflammation in healthy volunteers. Am J Respir Crit Care Med. 2009;179:1107–1114. doi: 10.1164/rccm.200810-1584OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Truwit J.D., Bernard G.R., Steingrub J., et al. Rosuvastatin for sepsis-associated acute respiratory distress syndrome. N Engl J Med. 2014;370:2191–2200. doi: 10.1056/NEJMoa1401520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McAuley D.F., Laffey J.G., O'Kane C.M., et al. Simvastatin in the acute respiratory distress syndrome. N Engl J Med. 2014;371:1695–1703. doi: 10.1056/NEJMoa1403285. [DOI] [PubMed] [Google Scholar]
- 73.Calfee C.S., Delucchi K.L., Sinha P., et al. Acute respiratory distress syndrome subphenotypes and differential response to simvastatin: secondary analysis of a randomised controlled trial. Lancet Respir Med. 2018;6:691–698. doi: 10.1016/S2213-2600(18)30177-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Matthay M.A. Therapeutic potential of mesenchymal stromal cells for acute respiratory distress syndrome. Ann Am Thorac Soc. 2015;12(Suppl 1):S54–S57. doi: 10.1513/AnnalsATS.201406-254MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wilson J.G., Liu K.D., Zhuo H., et al. Mesenchymal stem (stromal) cells for treatment of ARDS: a phase 1 clinical trial. Lancet Respir Med. 2015;3:24–32. doi: 10.1016/S2213-2600(14)70291-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yip H.K., Fang W.F., Li Y.C., et al. Human umbilical cord-derived mesenchymal stem cells for acute respiratory distress syndrome. Crit Care Med. 2020;48:e391–e399. doi: 10.1097/CCM.0000000000004285. [DOI] [PubMed] [Google Scholar]
- 77.Jacono F., Bannard-Smith J., Brealey D., et al. Primary analysis of a phase 1/2 study to assess MultiStem® cell therapy, a regenerative advanced therapy medicinal product (ATMP), in acute respiratory distress syndrome (MUST-ARDS) Am J Respir Crit Care Med. 2020;201:A7353. [Google Scholar]
- 78.Paine R., 3rd, Wilcoxen S.E., Morris S.B., et al. Transgenic overexpression of granulocyte macrophage-colony stimulating factor in the lung prevents hyperoxic lung injury. Am J Pathol. 2003;163:2397–2406. doi: 10.1016/S0002-9440(10)63594-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Matute-Bello G., Liles W.C., Radella F., 2nd, et al. Modulation of neutrophil apoptosis by granulocyte colony-stimulating factor and granulocyte/macrophage colony-stimulating factor during the course of acute respiratory distress syndrome. Crit Care Med. 2000;28:1–7. doi: 10.1097/00003246-200001000-00001. [DOI] [PubMed] [Google Scholar]
- 80.Paine R., 3rd, Standiford T.J., Dechert R.E., et al. A randomized trial of recombinant human granulocyte-macrophage colony stimulating factor for patients with acute lung injury. Crit Care Med. 2012;40:90–97. doi: 10.1097/CCM.0b013e31822d7bf0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Herold S., Hoegner K., Vadasz I., et al. Inhaled granulocyte/macrophage colony-stimulating factor as treatment of pneumonia-associated acute respiratory distress syndrome. Am J Respir Crit Care Med. 2014;189:609–611. doi: 10.1164/rccm.201311-2041LE. [DOI] [PubMed] [Google Scholar]
- 82.Sinha P., Calfee C.S. Phenotypes in acute respiratory distress syndrome: moving towards precision medicine. Curr Opin Crit Care. 2019;25:12–20. doi: 10.1097/MCC.0000000000000571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Matthay M.A., Arabi Y.M., Siegel E.R., et al. Phenotypes and personalized medicine in the acute respiratory distress syndrome. Intensive Care Med. 2020;46:2136–2152. doi: 10.1007/s00134-020-06296-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ware L.B., Matthay M.A., Mebazaa A. Designing an ARDS trial for 2020 and beyond: focus on enrichment strategies. Intensive Care Med. 2020;46:2153–2156. doi: 10.1007/s00134-020-06232-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sinha P., Churpek M.M., Calfee C.S. Machine learning classifier models can identify acute respiratory distress syndrome phenotypes using readily available clinical data. Am J Respir Crit Care Med. 2020;202:996–1004. doi: 10.1164/rccm.202002-0347OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Famous K.R., Delucchi K., Ware L.B., et al. Acute respiratory distress syndrome subphenotypes respond differently to randomized fluid management strategy. Am J Respir Crit Care Med. 2017;195:331–338. doi: 10.1164/rccm.201603-0645OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Spragg R.G., Taut F.J., Lewis J.F., et al. Recombinant surfactant protein C-based surfactant for patients with severe direct lung injury. Am J Respir Crit Care Med. 2011;183:1055–1061. doi: 10.1164/rccm.201009-1424OC. [DOI] [PubMed] [Google Scholar]
- 88.Willson D.F., Truwit J.D., Conaway M.R., et al. The adult calfactant in acute respiratory distress syndrome trial. Chest. 2015;148:356–364. doi: 10.1378/chest.14-1139. [DOI] [PubMed] [Google Scholar]
- 89.Ranieri V.M., Pettila V., Karvonen M.K., et al. Effect of intravenous interferon beta-1a on death and days free from mechanical ventilation among patients with moderate to severe acute respiratory distress syndrome: a randomized clinical trial. J Am Med Assoc. 2020 doi: 10.1001/jama.2019.22525. [DOI] [PMC free article] [PubMed] [Google Scholar]