Abstract
Corticotropin-releasing factor (CRF) signaling in the mesocorticolimbic system is known to modulate anxiety-like behavior and alcohol consumption, behaviors that also have been associated with the hyper-glutamatergic state of the lateral habenula (LHb) neurons in rats. However, the role of CRF signaling in the LHb on the glutamate transmission, anxiety-like behaviors and alcohol consumption is unknown. Here, we used male rats that had been consuming alcohol for three months to address this gap in the literature. First, using electrophysiological techniques, we evaluated CRF's effects on the glutamate transmission in LHb neurons in brain slices. CRF facilitated glutamate transmission. The facilitation was greater in neurons of alcohol-withdrawing rats than in those of naïve rats. The facilitation was mimicked by the activation of CRF receptor 1 (CRF1R) but attenuated by the activation of CRF receptor 2 (CRF2R). This facilitation was mediated by upregulating CRF1R-protein kinase A signaling. Conversely, protein kinase C blockade attenuated CRF's facilitation in neurons of naïve rats but promoted it in neurons of alcohol-withdrawing rats. Next, using site-direct pharmacology, we evaluated the role of CRF signaling in the LHb on anxiety-like behaviors and alcohol consumption. Intra-LHb inhibition of CRF1R or activation of CRF2R ameliorated the anxiety-like behaviors in alcohol-withdrawing rats and reduced their alcohol intake when drinking was resumed. These observations provide the first direct behavioral pharmacological and cellular evidence that CRF signaling in the LHb modulates glutamate transmission, anxiety-like behaviors and alcohol consumption, and that adaptation occurs in CRF signaling in the LHb after chronic alcohol consumption.
Keywords: Ethanol withdrawal, Glutamate, Stress, Lateral habenula, CRF receptors
Highlights
-
•
CRF regulates glutamate transmission in the lateral habenula of male rats.
-
•
CRF1R blockage or CRF2R activation in the LHb reduces anxiety in male rats.
-
•
CRF1R blockage/CRF2R activation in the LHb reduces alcohol consumption in male rats.
-
•
Acute ethanol facilitates LHb glutamate transmission involving CRF signaling.
1. Introduction
Increased anxiety during withdrawal from chronic alcohol (ethanol) misuse is a negative reinforcing property of ethanol (Becker, 2012). Corticotropin-releasing factor (CRF), a 41 amino acid peptide, plays a major role in behavioral responses to stress (Vale et al., 1981). CRF is also implicated in the pathophysiology of affective disorders (Steckler and Holsboer, 1999), such as anxiety and depression. Compelling evidence indicates that CRF systems play critical roles in the transition toward problematic ethanol drinking. Acute ethanol administration increases CRF synthesis (Rivier and Lee, 1996) and release (Lam and Gianoulakis, 2011) in the brain (see review Rivier (2014)). Chronic alcohol exposure promotes less consistent changes in CRF signaling, including decreases, no changes, or increases in CRF transcripts/peptide, mRNA level, and secretion (Quadros et al., 2016) in a brain region-dependent manner. CRF exerts its effects via interaction with two G-protein coupled receptors: CRF receptor 1 (CRF1R) and CRF2R. CRF is released in response to stress (Binder and Nemeroff, 2010), and has an 8-fold higher affinity to CRF1R than to CRF2R (Lovenberg et al., 1995). Thus, higher doses of CRF are necessary to activate both receptor subtypes (Chalmers et al., 1995). Generally speaking, CRF1R blockade dampens the physiological stress response, reducing ethanol consumption and negative states associated with ethanol withdrawal (Funk et al., 2007; Gehlert et al., 2007); these effects could also be achieved through CRF2R activation (Lowery et al., 2010; Valdez et al., 2004).
Previous studies on the CRF's role in alcohol related behaviors have been focused on the mesocorticolimbic regions associated with emotional regulation (Gilpin et al., 2015). Accumulating evidence indicates that lateral habenula (LHb), a brain region strongly associated with psychiatric disorders, plays a critical role in the negative affects induced by abused drugs (Graziane et al., 2018). The LHb receives innervation from several major CRF sources in the brain. Both CRF1R and CRF2R are expressed within the LHb (Chappell et al., 1986; Coen et al., 2015). Intracerebroventricular injection of CRF or exposure to stress increases habenula c-fos mRNA expression (Imaki et al., 1993). CRF increases LHb neuronal excitability by activating intracellular CRF1R-protein kinase A (PKA) signaling and decreasing presynaptic GABAergic inhibitory synaptic transmission (Authement et al., 2018). Moreover, maternal deprivation is shown to abolish CRF's excitatory action without affecting gene expression of corticotropin-releasing hormone Crh/Crhr1 (Authement et al., 2018). These findings suggest that CRF signaling could contribute to LHb hyperactivity. Previously, we have shown that LHb neurons in ethanol withdrawing rats are hyperactive (Kang et al., 2017; Li et al., 2019), partly due to enhanced glutamate transmission (Kang et al., 2018; Zuo et al., 2017a). However, we know nothing about either the role of CRF signaling in the LHb in the anxiety-like behaviors during ethanol withdrawal, or the contribution of CRF1R/CRF2R to hyper-glutamate state of LHb neurons of ethanol withdrawing rats. This study tests the hypothesis that dysregulations in the CRF signaling in the LHb elicited by chronic ethanol administration contribute to the hyper-glutamate state of LHb neurons, as well as to the anxiety-like and ethanol consumption behaviors.
2. Materials and methods
2.1. Animals
All animal experiments were carried out in accordance with National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978). All procedures were approved by the Animal Care and Utilization Committee of Rutgers University, the State University of New Jersey. All animal studies were complied with the ARRIVE guidelines. All efforts were made to minimize animal suffering, to reduce the number of animals used, and to utilize alternatives to in vivo techniques, if available. Adult male Sprague-Dawley rats (Envigo, NJ) used in the experiments upon arrival on postnatal days 58–65. Rats were singly housed with food and water available ad libitum unless otherwise indicated in a room with a reversed 12-h light/dark cycle: light off at 11:00 a.m. Electrophysiology studies were performed a half-hour after the end of the light cycle (11:30h-19:00h). Behavioral tests occurred during the dark period, and rats were habituated to the recording room and lighting conditions for at least 1 h.
2.2. Experimental outline
Rats were assigned randomly to the water group (Naïve, Nrats = 69) or the ethanol group (EtOH-WD, Nrats = 166). Rats in the latter group drank ethanol in the intermittent access to 20% ethanol two bottle free choice (IA2BC) paradigm (see below for details). At 13 weeks of drinking sessions, behavioral studies were started with one test per week as follows: first the ethanol preference test (APT), followed by elevated plus maze (EPM) and marble burying test (MBT).
2.3. Intermittent access to 20% ethanol two-bottle free choice (IA2BC) drinking procedure
The IA2BC was performed as described (Li et al., 2016). Animals were given 24 h concurrent access to one bottle of 20% (v/v) ethanol in water and one bottle of water, on Mondays, Wednesdays, and Fridays. After 24 h, the ethanol bottle was replaced with a second bottle of plain water. On all other days, the rats had unlimited access to two bottles of water. To prevent the development of a side preference or bias, water and ethanol bottle positions were counterbalanced across days. One water bottle and one ethanol bottle were placed on an empty cage for the same period to adjust for leakage and evaporation. Animal body weight was determined every Wednesday. The amount of ethanol or water consumed was determined by weighing the bottles before access and after 24 h of access. Ethanol intake was measured by calculating grams of ethanol consumed per kilogram of body weight.
2.4. Brain slice preparation and electrophysiology
Electrophysiological recordings were carried out as previously described (Zuo et al., 2016). Rats were sacrificed under deep anesthesia with ketamine/xylazine (80 mg/5 mg/kg, i.p.). The brain was removed and placed in artificial cerebrospinal fluid (aCSF) carbogenated (95% O2/5% CO2) and containing the following (in mM): 126 NaCl, 2.5 KCl, 1.25 NaH2PO4, 1 MgCl2, 2 CaCl2, 25 NaHCO3, 0.3 L-ascorbate, and 11 glucose. Coronal slices (250 μm thick) containing the LHb were cut with a Compresstome VF-200 slicer (Precisionary Instruments Inc., Greenville, NC, USA), then immediately transferred to a holding chamber and incubated in carbogenated aCSF for 1 h at 32 °C, then in carbogenated aCSF at room temperature (24–25 °C). A single slice was transferred to a submersion-type recording chamber and mechanically stabilized with a platinum ring.
LHb neurons were visualized using infrared differential contrast and fluorescence microscopy (Leica Microsystems). Electrical signals were recorded with an Axon 700B amplifiers, a Digidata 1440A A/D converter, and Clampfit 10.4 software (Molecular Devices Co., Union City, CA, USA). Data were filtered at 2 kHz and sampled at 5 kHz. Throughout the experiments, the bath was continually perfused with warm (33 °C) carbogenated aCSF (2.0 ml/min). Patch pipettes (6–8 MΩ) were filled with internal solutions of (in mM) 140 cesium methanesulfonate, 5 KCl, 2 MgCl2, 10 HEPES, 2 MgATP, 0.2 GTP for recordings under voltage-clamp. Each experimental group contained neurons from a minimum of five rats. The excitatory postsynaptic currents (EPSCs) were recorded at a holding potential (VH) of −60 mV in the presence of gabazine (10 μM), SCH50911 (20 μM) and strychnine (0.5 μM), which block GABAA, GABAB and glycine receptors respectively. These events were blocked by DNQX (20 μM), an antagonist of α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptors, indicating that they were mediated by AMPA receptors. Electrical stimuli (100–200 μs in duration, 0.05 Hz) elicited EPSCs (eEPSCs) via a nichrome wire bipolar electrode positioned within 200 μm of the soma. Near the start of the recording an input/output curve was obtained and the stimulation was then set to 20–30% of the maximum, an intensity that resulted in stable responses with no failures. Paired eEPSCs were elicited with a pair of identical stimuli separated by an interval of 50 ms. Miniature EPSCs (mEPSCs) were recorded in 0.5 μM TTX.
2.5. Reagents and drugs
We purchased CRF, NBI27914, Stressin I, Astressin 2b,Urocortin, from Tocris Bioscience (Ellisville, MO, USA). These chemicals were dissolved in vehicle (aCSF + DMSO) shortly before their use. Ethanol (made from grains, 190 proof, stored in glass bottle) was purchased from Pharmco Products (Brookfield, CT). Forskolin, Phorbol 12-myristate 13-acetate (PMA), Rp-cAMPS, Gӧ 6976 and all other reagents were from Sigma-Aldrich.
2.6. Implantation of cannula
Stereotaxic surgery was performed on rats as described (Kang et al., 2017). Bilateral guide cannulas (FIT 5 MM C232G-1.5W-1 MM PROJ, 22 gauge; Plastics One, Roanoke, VA) were inserted dorsally to the LHb (mm) (−3.9 AP, ±0.75 ML, −5.2 DV). Histological verification was performed as described (Kang et al., 2017). In five rats, the cannula tips were outside the LHb, and their data were excluded from analysis.
2.7. Intra-LHb injection of drugs
Separate cohorts of rats received intra-LHb infusions of drugs, including vehicle (500 nl/side), NBI27914 (1.5 mM/500 nl/side), Stressin I (1 μM/500 nl/side), Astressin 2B (20 μM/500 nl/side) Urocortin (40 pmol/500 nl/side) and CRF (0.5–2.5 μg/500 nl/side). The injector extended 1.0 mm beyond the guide cannula tip; the infusion lasted 60 s; and the injector was left in place for an additional 60 s to allow for diffusion. A given compound was infused into the LHb 20 min before behavioral tests.
2.8. Quantitative real-time PCR analysis for CRF1R and CRF2R gene expression
Both naïve rats and rats at 24 h withdrawal from chronic ethanol administration (Post-EtOH) (N = 6/group) were sacrificed under deep anesthesia (pentobarbital, 80 mg/kg, i.p.) and the LHb region was quickly dissected and stored in RNAlater RNA Stabilization Reagent (Sigma-Aldrich) at −20 °C. Total mRNAs were extracted from RPTCs using TRIzol RNA extract reagent (Thermo Fisher Scientific, Waltham, MA) according to manufacturer specifications. Concentration of total extracted RNA was determined spectrophotometrically at 260 nm. Reverse transcription was performed using a High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific) according to manufacturer specifications. Real-time PCR was performed using TaqMan Gene Expression Master Mix (Thermo Fisher Scientific). The TaqMan gene expression assay probes used were the CRF1R (probe ID: Rn00578611 m1), CRF2R (Rn00575617 m1) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH; Rn01775763 g1). The real-time PCR was performed in duplicate for each sample. Differences in amplification were determined using the delta-Ct method. GAPDH was used as an endogenous control to normalize expression levels between samples.
2.9. Elevated plus maze (EPM) test
Anxiety-like behaviors were assessed using a standard EPM apparatus, as previously described (Kang et al., 2017). Briefly, both naïve rats and rats at 24 h withdrawal from chronic ethanol administration were placed in the central platform of the EPM with their heads directed toward the open arms. Their behavior was then recorded for 5 min using Smart 3.0 (Pan lab Harvard Apparatus, Barcelona, Spain). The percentages of open-arm entries (100 × open/total entries) and of time spent in the open arms (100 × open/total time) were calculated for each rat as standard anxiety indices. Travel distances were used as indices of locomotor activity.
2.10. Marble burying test (MBT)
The MBT test was used to depict anxiety or obsessive–compulsive disorder behavior. The test was conducted as previously described (Li et al., 2019). Briefly, animals were individually housed in Plexiglas cages (47 × 25 × 30 cm) and were left undisturbed prior to the marble-burying test. During the test, the animals were transferred to a fresh cage of the same size that contained 20 glass marbles (1.5 cm in diameter, arranged in a 4 × 5 grid) on top of the 10-cm thick bedding. After a 30-min test, the animals were returned to their home cage and the number of marbles buried (to 2/3 their depth) with bedding was counted.
2.11. Data analysis and statistics
All data are presented as mean ± standard error of the mean. All statistical calculations were carried out using SigmaPlot 14.0 (SYSTAT Software, USA). Behavioral data were analyzed with one-way (Treatment) analysis of variance (ANOVA). For all data, n/N represents the number of recorded cells/rats. Electrophysiological data were analyzed with two-way ANOVA (Group × Concentration or Group × Treatment) or two-way repeated ANOVA followed by Turkey post hoc comparisons, one-sample t-test, independent-samples t-test, or a Kolmogorov-Smirnov (K–S) test, as appropriate. Data recorded for mEPSCs during the initial control period were averaged and normalized to 100%. p < 0.05 was considered significant.
3. Results
3.1. CRF modulation of glutamate transmission to LHb neurons is enhanced in rats withdrawing from chronic intermittent ethanol drinking
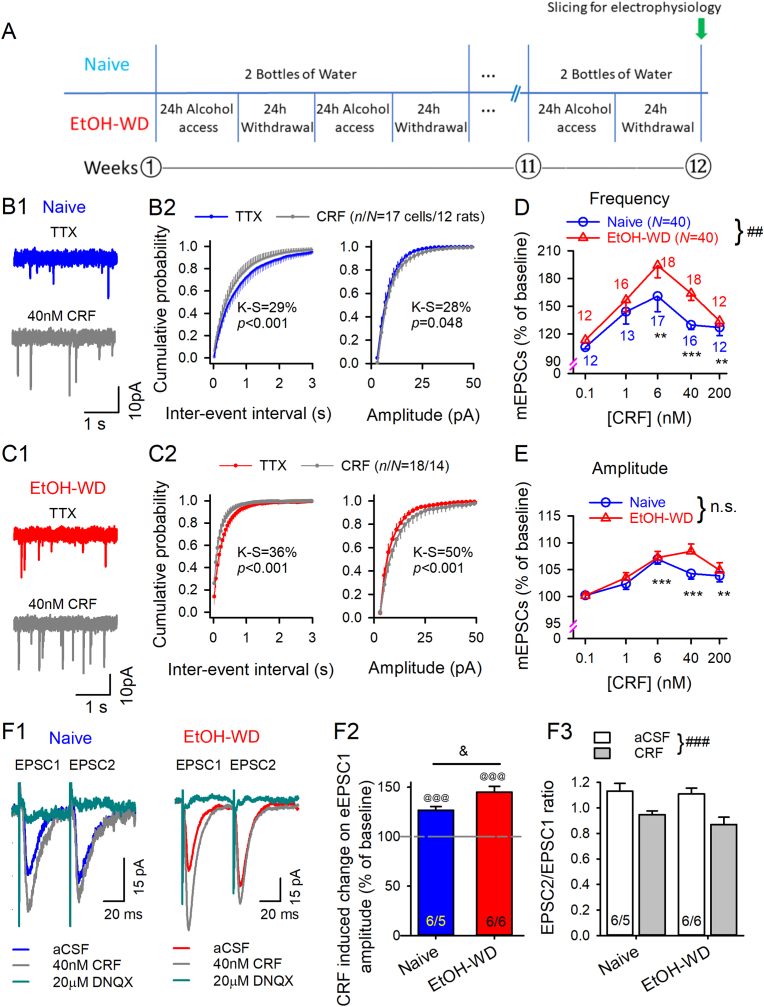
Rats in the IA2BC paradigm increased their ethanol intake over the course of 12 weeks and reached an averaged level of 4.2 g/kg/24 h. We recorded electrophysiological events of LHb neurons in brain slices of rats at 24 h withdrawal of ethanol (EtOH-WD, Nrats = 40), and naïve controls (Nrats = 40) (Fig. 1A). Basal frequency of miniature excitatory postsynaptic currents (mEPSC) mediated by AMPA receptor(AMPAR) was higher in LHb neurons of EtOH-WD (1.52 ± 0.16 Hz, ncells/Nrats 90/40; 90 cells from 40 rats) than in those from naïve rats (1.05 ± 0.1 Hz, ncells/Nrats = 93/40)(t181 = 2.5, p = 0.013, naïve vs. EtOH-WD), in keeping with our previous reports (Gregor et al., 2019; Li et al., 2019).
Fig. 1.
Corticotropin releasing factor (CRF) induced potentiation of glutamate transmission is stronger in LHb neurons of ethanol withdrawing (EtOH-WD) rats than those of ethanol-naïve rats. (A) Experimental timeline. (B–C) mEPSCs recorded in TTX. Representative traces showing CRF (40 nM)-induced enhancement of mEPSCs in LHb neurons from a Naïve (B1) or an EtOH-WD (C1) rat. (B2, C2) Cumulative probability plots show CRF increased incidence of shorter interval as well as mEPSC amplitude. n/N represents the number of recorded cells/rats. Concentration dependence of increases in mEPSC frequency (D) and amplitude (E). **p < 0.01, ***p < 0.001 relative to 0.1 nM CRF, ##p < 0.01 Naïve in comparison with EtOH-WD rats. n.s.; no significant difference. Data were analyzed with two-way ANOVA and Tukey post hoc comparison. Numbers of cells are indicated. (F1) When two EPSCs were evoked (50 msec apart) by paired-pulse stimuli, CRF increased the first (EPSC1) and second (EPSC2) of each pair. The current was abolished by DNQX. (F2) CRF increased eEPSC1 amplitude. @@@p < 0.001, Student's paired t-test for CRF vs. baseline. &p < 0.05 between Naïve and EtOH-WD rats. (F3) CRF reduced paired-pulse ratio (PPR = EPSC2/EPSC1) of eEPSCs in the LHb neurons of Naïve and EtOH-WD rats. ###p < 0.001 aCSF vs. CRF, two-way repeated ANOVA followed by Tukey post hoc test.
To determine whether CRF signaling plays a role in the glutamate transmission on the LHb neurons, we used CRF, the endogenous ligands for CRF1Rs and CRF2Rs, along with selective agonists and antagonists for CRF1Rs and CRF2Rs. Bath application of CRF (0.1–200 nM) significantly increased mEPSC frequency (F4,136 = 10.6, p < 0.001; Fig. 1B–D), and shifted the cumulative interevent interval distribution towards shorter intervals (p < 0.001, K–S test; Fig. 1B2, C2). The effect of CRF depended on its concentration with an inverted U-shaped concentration-response curve, reaching a maximal effect at 6 nM (p < 0.001). CRF induced enhancement of mEPSC frequency (p < 0.05, K–S test; Fig. 1B2, C2) was greater on LHb neurons of EtOH-WD than those of naïve rats (F1,136 = 7.5, p = 0.007), without a significant group × dose interaction (F4,136 = 0.8, p = 0.53). The increase in mEPSC frequency was accompanied by a higher incidence of larger mEPSCs in a concentration-dependent manner (also an “inverted-U″ Trajectory, F1,136 = 12.6, p < 0.001; Fig. 1B, C, E). No significant difference was detected between groups (F1, 136 = 3.5, p = 0.062) or group × dose interaction (F4, 136 = 1.28, p = 0.28).
CRF (40 nM) also significantly enhanced the amplitudes of EPSC elicited by the paired pulse in LHb neurons of both groups of rats (both p < 0.001 vs. aCSF; Fig. 1, Fig. 2, Fig. 3), and decreased the PPRs (PPRs = EPSC2/EPSC1) (main effect of Treatment: F1,10 = 80.76, p < 0.001; Group: F1,10 = 0.58, p = 0.47; Treatment × Group interaction: F1,10 = 1.39, p = 0.27; Fig. 1F3). Notably, the change in the PPR indicate changes in transmitter release. These results suggest that CRF increases presynaptic glutamate release. LHb neurons of EtOH-WD rats had a higher sensitivity to CRF than that of naïve rats (t10 = 2.7, p = 0.022; Fig. 1F2). These results suggest that CRF is a critical modulator of excitatory drive onto LHb neurons, and CRF signaling is altered after chronic EtOH administration.
Fig. 2.
Activation of CRF1R/CRF2R induces stronger changes in glutamate transmission in LHb neurons from EtOH-WD rats. Representative traces of mEPSCs in the absence (TTX) and presence of CRF1R agonist (Stressin I, A1) or CRF2R agonist (Urocortin, B1). Time course of Stressin I (A2) or Urocortin (B2) -induced changes in mEPSC frequency in Naïve (○) and EtOH-WD (△) rats. Summary of Stressin I (A3) and Urocortin (B3) induced changes in the frequency and amplitude of mEPSCs. @p < 0.05, @@p < 0.01, @@@p < 0.001, Student's paired t-test for agonist vs. baseline. &p < 0.05 between Naïve and EtOH-WD rats.
Fig. 3.
Effects of antagonists of CRF1R/CRF2R, PKA inhibitors of PKA/PKC on glutamate transmission in LHb neurons of EtOH-WD and naïve rats. Time course of NBI27914 (CRF1R antagonist, A1) or Astressin 2b (CRF2R antagonist, B1) -induced changes in mEPSC frequency in Naïve (○) and EtOH-WD (△) rats. Summary of NBI27914 (A2) and Astressin 2b (B2) -induced changes on mEPSCs. @p < 0.05, @@p < 0.01, Student's paired t-test for antagonist vs. baseline, &p < 0.05 between Naïve and EtOH-WD rats, t-test. Mean % changes of mEPSC frequency (C1) and amplitude (C2) induced by 40 nM CRF in the absence and presence of NBI27914 or Astressin 2b. Summary of change of mEPSC frequency (D1) and amplitude (D2) induced by 40 nM CRF in the absence and presence of PKA or PKC inhibitors. *p < 0.05, ***p < 0.001 vs. relative to 40 nM CRF. ^^^p < 0.001 among treatments. Two-way ANOVA. (E) Forskolin (FSK) or PMA produced changes in mEPSC frequency. @p < 0.05, @@p < 0.01, @@@p < 0.001, Student's paired t-test for agonist vs. baseline. &p < 0.05 between Naïve and EtOH-WD rats.
3.2. Activation of CRF1R or CRF2R has opposite effect on LHb glutamate release
The mRNA levels of the CRF1R but not of the CRF2R in the LHb of EtOH-WD rats were significantly increased relative to controls (Suppl Fig. 1). To identify the CRFR subtype which was responsible for CRF induced enhancement of LHb glutamatergic activity, we examined the effect of Stressin I and Urocortin, respectively the selective CRF1R and CRF2R agonists, on mEPSCs. Bath application of Stressin I (5 nM) significantly increased mEPSC frequency and amplitude (p < 0.01 vs. baseline; Fig. 2, Fig. 3) with greater enhancement in EtOH-WD than in naïve rats (p = 0.043; Fig. 2A2-A3). Conversely, Urocortin (5 nM) induced reduction (p < 0.001; Fig. 2, Fig. 3) of mEPSC frequency was greater in EtOH-WD rats than in naïve rats (p = 0.043, naïve vs. EtOH-WD). These data indicate CRF1R and CRF2R play opposite roles in the glutamatergic transmission on LHb neurons, and their effects were enhanced in the LHb of EtOH-WD rats.
3.3. Blockade of CRF1R or CRF2R respectively reduces or increases glutamate release in LHb neurons of EtOH-withdrawing rats
To further examine the role of CRFRs in glutamate transmission on LHb neurons, we examined the effects of NBI27914 (a selective CRF1R antagonist) and Astressin 2b (a CRF2R antagonist) on mEPSCs in LHb neurons. Bath application of NBI27914 (1 μM) reduced mEPSC frequency and amplitude in slices of EtOH-WD rats (p < 0.05; Fig. 3A1-2), but not in those of naïve rats. In contrast, Astressin 2b (20 nM), increased mEPSC frequency and amplitude of EtOH-WD but not of naïve rats (both p < 0.05; Fig. 3B1-2). These results further confirmed that CRF1R and CRF2R in the LHb mediate a positive and negative tone, respectively, on glutamate transmission, and that CRF1R and CRF2R are tonically activated in the LHb of EtOH-WD rats.
3.4. CRF1R, cAMP-PKA and PKC signaling mediates the effects of CRF
We next determined whether CRF's effects on glutamate release involve CRF1R and CRF2R by comparing CRF's effect (40 nM) in the absence and presence of CRF1R antagonist NBI27914 or CRF2R antagonist Astressin 2b. NBI27914 (1 μM), but not Astressin 2b (20 nM), completely blocked CRF (40 nM)-induced facilitation on mEPSC frequency (Treatment: F2,96 = 30.3, p < 0.001; Fig. 3C1), and NBI27914's effect was stronger (Treatment × Group interaction: F2,96 = 7.5, p < 0.001) in slices of EtOH-WD rats (post hoc p < 0.001) than in those of naïve rats (p = 0.036). NBI27914 tended to attenuate CRF's potentiation of mEPSC amplitude (main effect on Treatment: F2,96 = 4.6, p = 0.012, post hoc p = 0.065 CRF vs. NBI + CRF; Fig. 3C2). This result indicates that CRF's facilitation of glutamate transmission is mediated by CRF1R.
To determine whether CRF activation of CRF1R involves the protein kinase A (PKA) or PKC pathways, we employed Rp-cAMP (10 μM), a selective membrane-permeable antagonist of cAMP-dependent signaling. Preincubation with Rp-cAMP reduced CRF-induced enhancement of mEPSC frequency (Group × Treatment interaction: F2,94 = 12.9, p < 0.001; Fig. 3D1); post hoc test indicated that the reduction was stronger in EtOH-WD rats (p < 0.001) than in naïve rats (p = 0.031). Also, PKA blocker (F2,94 = 2.8, p = 0.066; Fig. 3D2) tended to attenuate CRF's potentiation of mEPSC amplitude. Conversely, bath application of a PKC inhibitor Gӧ6976 (200 nM) reduced CRF-induced potentiation of EPSC frequencies in naïve rats (p = 0.04) but increased it in EtOH-WD (p = 0.049) rats (Fig. 3D1), indicating that PKC is involved in CRF's enhancement of glutamate release.
Additionally, forskolin (FSK, 10 μM), an adenylyl cyclase activator, enhanced mEPSC frequency with a greater effect in EtOH-WD rats than in naïve rats (t27 = 2.1, p = 0.042; Fig. 3E). Also, phorbol 12-myristate 13-acetate (PMA, 100 nM), a PKC activator, enhanced glutamate release in naïve rats but reduced it in EtOH-WD rats (t38 = 2.2, p = 0.033, Naïve vs. EtOH; Fig. 3E). These results indicate that CRF, acting at presynaptic CRF1Rs in the LHb neurons, preferentially activates PKA to increase glutamate release. CRF also activates the CRF2R-PKC pathway to decrease glutamate transmission in the LHb of EtOH-WD rats.
3.5. Acute cellular exposure to ethanol facilitates mEPSCs in LHb neurons involving CRF signaling
We previously reported that acute cellular exposure of ethanol signficantly facilitates mEPSCs in LHb neurons (Zuo et al., 2017b, 2019). To determine whether CRF is involved, we compared the effects of acute ethanol in the absence and presence of CRFR antagonists. In keeping with our previous reports (Zuo et al., 2017b, 2019), bath-perfusion of ethanol alone robustly increased glutamate release on LHb neurons (naïve: 153.4 ± 10.56% of baseline, EtOH-WD: 128.5 ± 3.3%, ncells/Nrats = 19/15, 19/17, respectively). Ethanol's facilitation of mEPSC frequency in slices of both naïve and EtOH-WD rats was similarly attenuated in the presence of CRF1R blocker NBI27914 (Treatment: F1,17 = 14.9, p = 0.001; Group: F1,17 = 1.78, p = 0.203; Treatment × Group interaction: F1,17 = 0.64, p = 0.436; Fig. 4A1–2), whereas blocking CRF2R with Astressin 2b potentiated ethanol-induced facilitation in LHb neurons of EtOH-WD rats (Treatment × Group interaction: F1,17 = 5.59, p = 0.03, pos hoc p = 0.007; Fig. 4B1–2) but not in those of naïve rats. These data indicate that the action of acute ethanol involves activating CRF1R and inhibiting CRF2R at the glutamatergic terminals on LHb neurons.
Fig. 4.
CRF1Rs mediate the potentiation of mEPSCs induced by acute ethanol. Sample traces of mEPSCs of LHb neurons in response to acute ethanol (11 mM) in the absence and presence of NBI27914, a CRF1R antagonist (A1) or Astressin 2b, a CRF2R blocker (B1) on LHb neurons of Naïve and EtOH-WD rats. Mean % changes of mEPSC frequency induced by 11 mM ethanol in the absence and presence of NBI27914 (A2) or Astressin 2b (B2). ##p < 0.01 ethanol vs. antagonist plus ethanol, two-way RM ANOVA.
3.6. Manipulation of LHb CRFR function alters ethanol consumption and anxiety-like behaviors
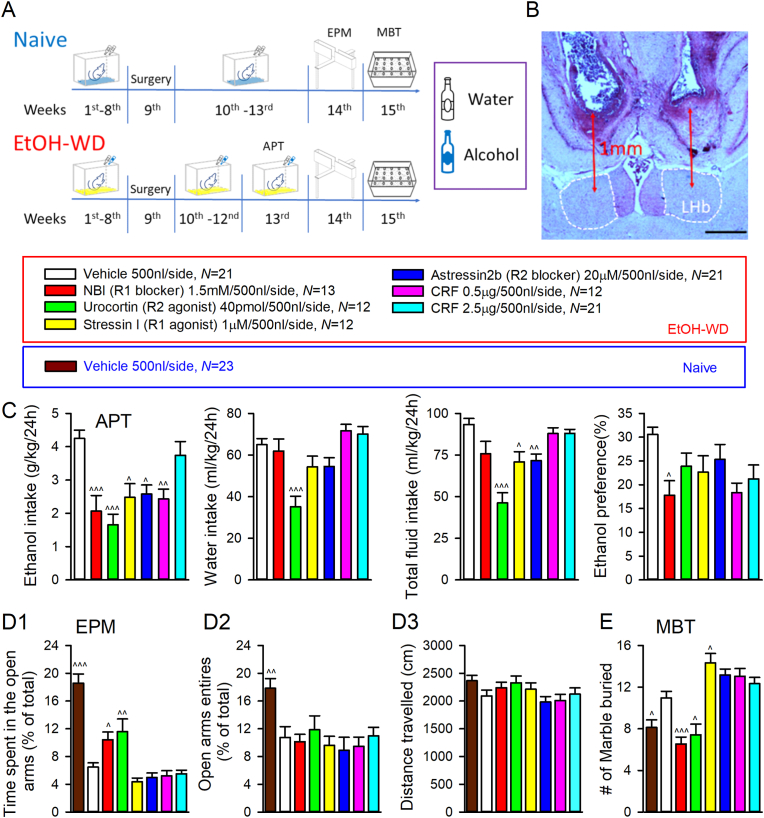
CRFRs and downstream signaling pathways are known to regulate several ethanol-related behaviors in animal studies (de Guglielmo et al., 2019; Quadros et al., 2016; Valdez et al., 2004). However, it is unknown whether LHb CRFRs play a role in anxiety during ethanol withdrawal and in relapse drinking. To address this question, we used site-direct pharmacology to selectively activate/inactivate CRFRs in the LHb (Fig. 5A). Cannula placements in the LHb (Fig. 5B) were verified at the end of the experiments. Intra-LHb infusion of CRF1R antagonist NBI27914 significantly decreased ethanol intake (F6,105 = 7.32, p < 0.001; post hoc p < 0.001 vs. vehicle), without affecting water intake at 24 h (F6,105 = 7.43, p < 0.001; post hoc p > 0.5), thus reducing ethanol preference (F6,105 = 2.61, p = 0.021; post hoc p = 0.028; Fig. 5C). Interestingly, intra-LHb Urocortin (CRF2R agonist) decreased the intake of both ethanol and water (p < 0.001). Intra-LHb Stressin I (CRF1R agonist) or Astressin 2b (CRF2R antagonist) decreased ethanol and total fluid consumption (F6,105 = 11.04, p < 0.001). Intra-LHb infusion of CRF dose-dependently decreased ethanol drinking without altering water intake (Fig. 5C). These data indicate that CRF signaling in the LHb regulates ethanol and liquid drinking behaviors.
Fig. 5.
Blockage of CRF1R or activation of CRF2R reduce ethanol consumption and produce anxiolytic-like effects in EtOH-WD rats. (A) Timeline representation of animal experimentation process from water (Naïve)/ethanol consumption (EtOH-WD) to anxiety-like behavioral tests. Cannula was implanted to the LHb at the end of after 8 weeks of drinking (week 9). Rats resumed drinking ethanol at week 10. Behavior tests on the effects of intra-LHb infusion of chemicals were conducted at week 13–15. Abbreviations: APT, Alcohol preference test; EPM, Elevated plus maze; MBT, marble burying test. (B) Cannula injection sites. Scale bar: 500 mm. (C) Effects of injection of chemicals into the LHb on ethanol intake (g/kg), water intake, total fluid intake and ethanol preference on 24h after the onset of drinking in EtOH-WD rats. Effects of intra-LHb infusion of vehicle in naïve rats, and intra-LHb chemicals in rats withdrawn from ethanol on the time spent on open arms (D1), percentage of open arms entries (D2), and locomotion activity (D3) at EPM test. (E) Effects of intra-LHb infusion of vehicle or chemicals on the number of marbles buried in naïve and EtOH-WD rats. ^p < 0.05, ^^p < 0.01, ^^^p < 0.001 vs. vehicle in EtOH-WD rats, ANOVA.
We next examined the role of LHb CRFRs in the anxiety-like behaviors. In the elevated plus maze (EPM) test, EtOH-WD rats spent either shorter time overall on the open arms (F7,127 = 29.08, p < 0.001, post hoc p < 0.001 naïve vs. EtOH-WD on Vehicle treatment; Fig. 5D1) or entered less to open arms (F7,127 = 4.1, p < 0.001, post hoc p = 0.003; Fig. 5D2) in EPM, compared to ethanol-naive counterparts, consistent with our previous reports (Kang et al., 2017; Li et al., 2019). Also, EtOH-WD rats buried more marbles in MBT (F7,127 = 14.89, p < 0.001, post hoc p = 0.033 naïve vs. EtOH-WD on Vehicle treatment; Fig. 5E). In EtOH-WD rats, intra-LHb infusion of NBI27914 or Urocortin increased (post hoc p < 0.05 vs. Vehicle in EtOH-WD rats) whereas Stressin I tended to decrease (p = 0.16) the percentage of time spent on the open arms in the EPM (Fig. 5D1). No significant difference in percentage of open-arm entries (all p > 0.5 vs. Vehicle in EtOH-WD rats; Fig. 5D2) or locomotion (F7,117 = 1.78, p = 0.1; Fig. 5D3) was observed between treatment groups. These data indicate that CRF signaling promotes anxiety-like behavior in the EPM. Following EPM testing, the same cohort of rats treated with NBI27914 or Urocortin buried fewer marbles, compared to the vehicle-infused group (both p < 0.05) (Fig. 5E). Conversely, intra-LHb infusion of Stressin I significantly increased marble burying activity (p = 0.036). These data indicate that CRF systems in the LHb play critical roles in behavioral responses to stress.
4. Discussion
The central finding of this study is that CRF, via activating CRF1R, enhanced glutamate transmission on the LHb neurons, regulated anxiety-like behaviors, and these effects were stronger in rats with a chronic drinking history than in ethanol-naïve rats. Although previous studies have shown ethanol administration alters CRF signaling in mesocorticolimbic areas in the brain (Roberto et al., 2017), this is the first report showing ethanol ingestion alters CRF signaling in the LHb.
In the present study, we showed that CRF increased mEPSC frequency and amplitude. Interestingly, a recent study (Authement et al., 2018) showed that CRF (250 nM) alters neither mEPSC amplitude nor frequency in LHb neurons. The mechanism underlying the difference is unclear but may be caused by the different experimental conditions. In our study, CRF produced strongest effects at ∼6 nM, which reduced with increase of CRF concentrations. A previous study in nucleus locus coeruleus neurons reported that CRF at 50 nM increased, but at 200 nM, decreased sEPSCs (Prouty et al., 2017). A previous study using microdialysis measured CRF-like immunoreactivity (CRF-IR) in the amygdala of awake adult male Wistar rats reported that the CRF levels are around 1.2 fmol/50 μl (0.06 nM) in the basal conditions and ∼11 fmol/50 μl (0.55 nM) during withdrawal from chronic alcohol administration (Merlo Pich et al., 1995). However, the in vivo concentrations of CRF in the LHb is unknown and is an important area of investigation. The age of the rats tested may also contribute to the difference, given that localization and expression of CRF1R in the LHb vary with age (Rosinger et al., 2017). Whereas Authement used juvenile rats (postnatal days 21–28), we utilized adult rats (∼4 months old).
CRF is widely distributed in the brain, including the LHb (Chappell et al., 1986), and CRFRs are in both the pre- and post-synaptic membrane of glutamatergic synapses. The LHb receives glutamatergic afferents from several brain regions involving stress response (Hu et al., 2020). Notably, whereas CRF induced increase in mEPSC frequency was greater in LHb neurons of rats with an ethanol drinking history than in those of naïve rats, there is no difference in CRF-induced facilitation of mEPSC frequency in the central amygdala neurons between naïve rats and ethanol-dependent rats (Varodayan et al., 2017). Thus, CRF's effects on glutamate transmission and on the response to ethanol exposure may vary with brain regions. This kind of variation may contribute in part to the disappointing effect in clinical practice regarding pharmacological interventions related to CRF in the field of ethanol-related research.
Withdrawal from chronic ethanol exposure activates CRF positive neurons in the brain (de Guglielmo et al., 2019), and CRF release peaks at ∼10–12 h into withdrawal (Merlo Pich et al., 1995). However, CRF levels decrease after animals resume drinking (Olive et al., 2002). Growing evidence suggests that an upregulation of the CRF system underlies anxiety- and depression-like phenotypes during drug withdrawal (Quadros et al., 2016). The results of the current study showed that CRF1R is involved in the increase in glutamate transmission in LHb neurons of rats withdrawing from EtOH; we therefore propose that the increased glutamate transmission in the LHb of ethanol-withdrawing rats is mediated at least in part by the elevated CRF activity.
The result of CRF1R and CRF2R mRNA expressions shows that CRF1R gene expression was upregulated in the LHb of ethanol withdrawing rats. Interestingly, LHb CRF2R mRNA level was not altered although their function was enhanced as reflected by the greater effect of the CRF2R agonist on the mEPSCs. The different sensitivity could be driven by changes in receptor function that are not reflected in the total gene expression. Collectively, it is likely that CRF (40 nM) predominately activates CRF1R, which leads to facilitation of glutamate release.
Ethanol dysregulates numerous signaling cascades. CRFRs couple to either Gsα or Gq proteins, stimulating cAMP generation, phospholipase C activity, and intracellular calcium mobilization, which in turn influences neuronal excitability. We previously reported that ethanol-stimulated vesicular glutamate release depends on cAMP-PKA and Ca2+/calmodulin-dependent kinase II (CaMKII) in the LHb (Kang et al., 2017; Zuo et al., 2019). In this report, we showed an upregulation of CRF1R-PKA, and/or reversal of CRF2R-PKC signaling pathways on glutamate transmission on the LHb neurons of ethanol-withdrawing rats, indicating that CRF's action involves cAMP-PKA/PKC signal pathways. The results of this study support the idea that the elevated intracellular cAMP levels are a molecular mechanism underlying anxiety and ethanol-drinking behaviors (for review, see Ref (Mons and Beracochea, 2016).). Also, the observation that CRF1R and PKA pathways are involved in the increased LHb glutamate transmission induced by both CRF and ethanal suggests that CRF and ethanol may share a common mechanism in enhancing glutamate transmission on LHb neurons.
Anxiety-like behaviors are commonly seen during ethanol withdrawal. Pharmacological manipulations of the CRF system have been employed to ameliorate drug-taking and withdrawal-related behaviors (Quadros et al., 2016; Robinson et al., 2019; Valdez et al., 2002). Although previous behavioral studies have demonstrated a key role for the LHb in the ethanol withdrawal syndrome in rats (Kang et al., 2018; Li et al., 2019; Zuo et al., 2019), how the LHb CRF system may contribute to these behaviors remains unknown. Based on our electrophysiological results, we propose that CRF1R and CRF2R in the LHb may play opposing roles in ethanol-related behaviors. As expected, intra-LHb application of a CRF1R antagonist or a CRF2R agonist attenuated but a CRF1R agonist increased the anxiety-like behaviors in ethanol withdrawing rats. Thus, manipulating LHb CRF system can regulate anxiety-like behaviors in ethanol-withdrawing rats. We also showed that intra-LHb infusion of a CRF1R antagonist decreased ethanol consumption and preference. Unexpectedly, ethanol intake was also reduced when a CRF1R agonist was infused into the LHb. Thus, CRF1R agonist/antagonist produced the same effects on ethanol intake. This observation is intriguing, but the underlying mechanisms are unclear. Notably, the LHb receives several afferents and projects to many different brain areas. Distinct LHb circuits may contribute to different behaviors. Thus, modulating LHb CRFRs may elicit a variety of behavioral outcomes through distinct neurocircuits. Taken together, our behavioral studies confirm that biological responses involving LHb CRF signaling are wide and complex, and recruitment of specific CRFRs will vary according to each particular behavior and/or pathology(Pomrenze et al., 2017).
A limitation of this study is that it does not include female rats. Since there are significant sex differences in CRF signaling and in response to drugs of abuse (Agoglia et al., 2020), female data may be very different from what we have shown in the current study using males. Thus, our findings are limited to 50% of the population, and it is essential to include females in future studies.
5. Conclusion
The results of electrophysiology and behavioral tests show that CRF systems in the LHb play important roles in the glutamate transmission and in anxiety, as well as in relapse-like drinking behaviors in male rats. These results reveal a cellular mechanism linking ethanol use disorder to executive dysfunction via LHb-CRF-protein kinase signaling pathways, indicating that CRF regulates the excitatory drive to LHb neurons, which is altered by chronic ethanol administration. These findings might have clinical implications in the treatment of ethanol misuse in the male. Thus, ethanol consumption and anxiety-like behaviors in the male could be treated by manipulating LHb CRF signaling.
CRediT authorship contribution statement
Wanhong Zuo: Formal analysis, Data curation, Writing – original draft. Qikang Zuo: Visualization, Data curation, Writing – original draft, Writing – review & editing. Liangzhi Wu: Formal analysis. Qinghua Mei: Formal analysis. Manan Shah: Writing – review & editing. Jiayi Zheng: Formal analysis. Ding Li: Data curation. Ying Xu: Writing – review & editing. Jiang-Hong Ye: Funding acquisition, Project administration, Supervision, Writing – review & editing, final approval of the revision to be submitted.
Declaration of competing interest
All authors declare no financial and personal relationships with other people or organizations that could inappropriately influence (bias) this work.
Acknowledgements
We thank Aman Garg and Alyssa Lillian for reading over the manuscript. This work is made possible by National Institutes of Alcohol Abuse and Alcoholism (NIAAA) grants.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ynstr.2021.100395.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Suppl Fig. 1.
Experimental timeline (top) and relative ratio of mRNA levels of CRF1R/ CRF2R genes and GAPDH in the LHb of Naive rats and rats withdrawing from chronic ethanol administration (EtOH-WD) (bottom) (Subtypes × Group interection: F1,20 = 6.2, p = 0.022; post hoc test *p = 0.014 naïve vs. EtOH-WD rats within CRF1R subtype. Two-way ANOVA.
Data availability
Data will be made available on request.
References
- Agoglia A.E., Crofton E.J., Herman M.A. Biological intersection of sex, age, and environment in the corticotropin releasing factor (CRF) system and alcohol. Neuropharmacology. 2020;170:108045. doi: 10.1016/j.neuropharm.2020.108045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Authement M.E., Langlois L.D., Shepard R.D., Browne C.A., Lucki I., Kassis H., Nugent F.S. A role for corticotropin-releasing factor signaling in the lateral habenula and its modulation by early-life stress. Sci. Signal. 2018;11 doi: 10.1126/scisignal.aan6480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker H.C. Effects of alcohol dependence and withdrawal on stress responsiveness and alcohol consumption. Alcohol Res. 2012;34:448–458. [PMC free article] [PubMed] [Google Scholar]
- Binder E.B., Nemeroff C.B. The CRF system, stress, depression and anxiety-insights from human genetic studies. Mol. Psychiatr. 2010;15:574–588. doi: 10.1038/mp.2009.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmers D.T., Lovenberg T.W., De Souza E.B. Localization of novel corticotropin-releasing factor receptor (CRF2) mRNA expression to specific subcortical nuclei in rat brain: comparison with CRF1 receptor mRNA expression. J. Neurosci. 1995;15:6340–6350. doi: 10.1523/JNEUROSCI.15-10-06340.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell P.B., Smith M.A., Kilts C.D., Bissette G., Ritchie J., Anderson C., Nemeroff C.B. Alterations in corticotropin-releasing factor-like immunoreactivity in discrete rat brain regions after acute and chronic stress. J. Neurosci. 1986;6:2908–2914. doi: 10.1523/JNEUROSCI.06-10-02908.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen C.W., Kalamatianos T., Oosthuizen M.K., Poorun R., Faulkes C.G., Bennett N.C. Sociality and the telencephalic distribution of corticotrophin-releasing factor, urocortin 3, and binding sites for CRF type 1 and type 2 receptors: a comparative study of eusocial naked mole-rats and solitary Cape mole-rats. J. Comp. Neurol. 2015;523:2344–2371. doi: 10.1002/cne.23796. [DOI] [PubMed] [Google Scholar]
- de Guglielmo G., Kallupi M., Pomrenze M.B., Crawford E., Simpson S., Schweitzer P., Koob G.F., Messing R.O., George O. Inactivation of a CRF-dependent amygdalofugal pathway reverses addiction-like behaviors in alcohol-dependent rats. Nat. Commun. 2019;10:1238. doi: 10.1038/s41467-019-09183-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk C.K., Zorrilla E.P., Lee M.J., Rice K.C., Koob G.F. Corticotropin-releasing factor 1 antagonists selectively reduce ethanol self-administration in ethanol-dependent rats. Biol. Psychiatr. 2007;61:78–86. doi: 10.1016/j.biopsych.2006.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehlert D.R., Cippitelli A., Thorsell A., Le A.D., Hipskind P.A., Hamdouchi C., Lu J., Hembre E.J., Cramer J., Song M., McKinzie D., Morin M., Ciccocioppo R., Heilig M. 3-(4-Chloro-2-morpholin-4-yl-thiazol-5-yl)-8-(1-ethylpropyl)-2,6-dimethyl-imidazo [1,2-b]pyridazine: a novel brain-penetrant, orally available corticotropin-releasing factor receptor 1 antagonist with efficacy in animal models of alcoholism. J. Neurosci. 2007;27:2718–2726. doi: 10.1523/JNEUROSCI.4985-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin N.W., Herman M.A., Roberto M. The central amygdala as an integrative hub for anxiety and alcohol use disorders. Biol. Psychiatr. 2015;77:859–869. doi: 10.1016/j.biopsych.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziane N.M., Neumann P.A., Dong Y. A focus on reward prediction and the lateral habenula: functional alterations and the behavioral outcomes induced by drugs of abuse. Front. Synaptic Neurosci. 2018;10:12. doi: 10.3389/fnsyn.2018.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregor D.M., Zuo W., Fu R., Bekker A., Ye J.H. Elevation of transient receptor potential vanilloid 1 function in the lateral habenula mediates aversive behaviors in alcohol-withdrawn rats. Anesthesiology. 2019;130:592–608. doi: 10.1097/ALN.0000000000002615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H., Cui Y., Yang Y. Circuits and functions of the lateral habenula in health and in disease. Nat. Rev. Neurosci. 2020;21:277–295. doi: 10.1038/s41583-020-0292-4. [DOI] [PubMed] [Google Scholar]
- Imaki T., Shibasaki T., Hotta M., Demura H. Intracerebroventricular administration of corticotropin-releasing factor induces c-fos mRNA expression in brain regions related to stress responses: comparison with pattern of c-fos mRNA induction after stress. Brain Res. 1993;616:114–125. doi: 10.1016/0006-8993(93)90199-w. [DOI] [PubMed] [Google Scholar]
- Kang S., Li J., Bekker A., Ye J.H. Rescue of glutamate transport in the lateral habenula alleviates depression- and anxiety-like behaviors in ethanol-withdrawn rats. Neuropharmacology. 2018;129:47–56. doi: 10.1016/j.neuropharm.2017.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S., Li J., Zuo W., Fu R., Gregor D., Krnjevic K., Bekker A., Ye J.H. Ethanol withdrawal drives anxiety-related behaviors by reducing M-type potassium channel activity in the lateral habenula. Neuropsychopharmacology. 2017;42:1813–1824. doi: 10.1038/npp.2017.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam M.P., Gianoulakis C. Effects of acute ethanol on corticotropin-releasing hormone and beta-endorphin systems at the level of the rat central amygdala. Psychopharmacology (Berl) 2011;218:229–239. doi: 10.1007/s00213-011-2337-x. [DOI] [PubMed] [Google Scholar]
- Li J., Zuo W., Fu R., Xie G., Kaur A., Bekker A., Ye J.H. High frequency electrical stimulation of lateral habenula reduces voluntary ethanol consumption in rats. Int. J. Neuropsychopharmacol. 2016;19:1–8. doi: 10.1093/ijnp/pyw050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Zuo W., Wu W., Zuo Q.K., Fu R., Wu L., Zhang H., Ndukwe M., Ye J.H. Activation of glycine receptors in the lateral habenula rescues anxiety- and depression-like behaviors associated with alcohol withdrawal and reduces alcohol intake in rats. Neuropharmacology. 2019;157:107688. doi: 10.1016/j.neuropharm.2019.107688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovenberg T.W., Liaw C.W., Grigoriadis D.E., Clevenger W., Chalmers D.T., De Souza E.B., Oltersdorf T. Cloning and characterization of a functionally distinct corticotropin-releasing factor receptor subtype from rat brain. Proc. Natl. Acad. Sci. U. S. A. 1995;92:836–840. doi: 10.1073/pnas.92.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery E.G., Spanos M., Navarro M., Lyons A.M., Hodge C.W., Thiele T.E. CRF-1 antagonist and CRF-2 agonist decrease binge-like ethanol drinking in C57BL/6J mice independent of the HPA axis. Neuropsychopharmacology. 2010;35:1241–1252. doi: 10.1038/npp.2009.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlo Pich E., Lorang M., Yeganeh M., Rodriguez de Fonseca F., Raber J., Koob G.F., Weiss F. Increase of extracellular corticotropin-releasing factor-like immunoreactivity levels in the amygdala of awake rats during restraint stress and ethanol withdrawal as measured by microdialysis. J. Neurosci. 1995;15:5439–5447. doi: 10.1523/JNEUROSCI.15-08-05439.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mons N., Beracochea D. Behavioral neuroadaptation to alcohol: from glucocorticoids to histone acetylation. Front. Psychiatr. 2016;7:165. doi: 10.3389/fpsyt.2016.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive M.F., Koenig H.N., Nannini M.A., Hodge C.W. Elevated extracellular CRF levels in the bed nucleus of the stria terminalis during ethanol withdrawal and reduction by subsequent ethanol intake. Pharmacol. Biochem. Behav. 2002;72:213–220. doi: 10.1016/s0091-3057(01)00748-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomrenze M.B., Fetterly T.L., Winder D.G., Messing R.O. The corticotropin releasing factor receptor 1 in alcohol use disorder: still a valid drug target? Alcohol Clin. Exp. Res. 2017;41:1986–1999. doi: 10.1111/acer.13507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prouty E.W., Waterhouse B.D., Chandler D.J. Corticotropin releasing factor dose-dependently modulates excitatory synaptic transmission in the noradrenergic nucleus locus coeruleus. Eur. J. Neurosci. 2017;45:712–722. doi: 10.1111/ejn.13501. [DOI] [PubMed] [Google Scholar]
- Quadros I.M., Macedo G.C., Domingues L.P., Favoretto C.A. An update on CRF mechanisms underlying alcohol use disorders and dependence. Front. Endocrinol. 2016;7:134. doi: 10.3389/fendo.2016.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivier C. Role of hypothalamic corticotropin-releasing factor in mediating alcohol-induced activation of the rat hypothalamic-pituitary-adrenal axis. Front. Neuroendocrinol. 2014;35:221–233. doi: 10.1016/j.yfrne.2013.10.005. [DOI] [PubMed] [Google Scholar]
- Rivier C., Lee S. Acute alcohol administration stimulates the activity of hypothalamic neurons that express corticotropin-releasing factor and vasopressin. Brain Res. 1996;726:1–10. [PubMed] [Google Scholar]
- Roberto M., Spierling S.R., Kirson D., Zorrilla E.P. Corticotropin-releasing factor (CRF) and addictive behaviors. Int. Rev. Neurobiol. 2017;136:5–51. doi: 10.1016/bs.irn.2017.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S.L., Perez-Heydrich C.A., Thiele T.E. Corticotropin releasing factor type 1 and 2 receptor signaling in the medial prefrontal cortex modulates binge-like ethanol consumption in C57BL/6J mice. Brain Sci. 2019;9:171. doi: 10.3390/brainsci9070171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosinger Z.J., Jacobskind J.S., Park S.G., Justice N.J., Zuloaga D.G. Distribution of corticotropin-releasing factor receptor 1 in the developing mouse forebrain: a novel sex difference revealed in the rostral periventricular hypothalamus. Neuroscience. 2017;361:167–178. doi: 10.1016/j.neuroscience.2017.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steckler T., Holsboer F. Corticotropin-releasing hormone receptor subtypes and emotion. Biol. Psychiatr. 1999;46:1480–1508. doi: 10.1016/s0006-3223(99)00170-5. [DOI] [PubMed] [Google Scholar]
- Valdez G.R., Roberts A.J., Chan K., Davis H., Brennan M., Zorrilla E.P., Koob G.F. Increased ethanol self-administration and anxiety-like behavior during acute ethanol withdrawal and protracted abstinence: regulation by corticotropin-releasing factor. Alcohol Clin. Exp. Res. 2002;26:1494–1501. doi: 10.1097/01.ALC.0000033120.51856.F0. [DOI] [PubMed] [Google Scholar]
- Valdez G.R., Sabino V., Koob G.F. Increased anxiety-like behavior and ethanol self-administration in dependent rats: reversal via corticotropin-releasing factor-2 receptor activation. Alcohol Clin. Exp. Res. 2004;28:865–872. doi: 10.1097/01.alc.0000128222.29875.40. [DOI] [PubMed] [Google Scholar]
- Vale W., Spiess J., Rivier C., Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and β-endorphin. Science. 1981:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- Varodayan F.P., Correia D., Kirson D., Khom S., Oleata C.S., Luu G., Schweitzer P., Roberto M. CRF modulates glutamate transmission in the central amygdala of naive and ethanol-dependent rats. Neuropharmacology. 2017;125:418–428. doi: 10.1016/j.neuropharm.2017.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo W., Fu R., Hopf F.W., Xie G., Krnjevic K., Li J., Ye J.H. Ethanol drives aversive conditioning through dopamine 1 receptor and glutamate receptor-mediated activation of lateral habenula neurons. Addict Biol. 2017;22:103–116. doi: 10.1111/adb.12298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo W., Fu R., Hopf F.W., Xie G., Krnjević K., Li J., Ye J.H. Ethanol drives aversive conditioning through dopamine 1 receptor and glutamate receptor-mediated activation of lateral habenula neurons. Addict Biol. 2017;22:103–116. doi: 10.1111/adb.12298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo W., Wu L., Mei Q., Zuo Q., Zhou Z., Fu R., Li W., Wu W., Matthew L., Ye J.H. Adaptation in 5-HT2 receptors-CaMKII signaling in lateral habenula underlies increased nociceptive-sensitivity in ethanol-withdrawn rats. Neuropharmacology. 2019;158 doi: 10.1016/j.neuropharm.2019.107747. [DOI] [PubMed] [Google Scholar]
- Zuo W., Xiao C., Gao M., Hopf F.W., Krnjevic K., McIntosh J.M., Fu R., Wu J., Bekker A., Ye J.H. Nicotine regulates activity of lateral habenula neurons via presynaptic and postsynaptic mechanisms. Sci. Rep. 2016;6 doi: 10.1038/srep32937. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.