Abstract
Although primary cardiac tumours are extremely uncommon, secondary tumours or cardiac metastasis are not. We present a 68-year-old gentleman with squamous cell carcinoma of the right lower lobe with bony metastasis to the right clavicle who was treated with radiotherapy to the lung and clavicle as well as combination immunotherapy (Pembrolizumab) and chemotherapy (Carboplatin/Paclitaxel). Despite completing the above treatment regime, 18F-FDG PET/CT scan showed progression with two new sites of metastasis including a focus in the lateral wall of the right ventricle which correlate to a soft tissue density mass on CT as well as a FDG avid mass in the left masseter. Identification of cardiac lesions with 18F-FDG PET/CT maybe challenging with routine preparation due high physiological FDG uptake in the myocardium and significant variability, nevertheless, focal FDG uptake in the heart should be carefully assessed for the possibility of cardiac metastasis.
Keywords: FDG, PET/CT, Lung cancer, Cardiac metastasis
Background
Although primary cardiac tumours are extremely uncommon, secondary tumours or cardiac metastasis are not. In theory, any malignant cancer with metastatic potential can spread to the heart and the incidence of cardiac metastasis seems to be not as low as expected, with reported incidence of 1.5%-20% of autopsies of cancer patients.[1] Primary lung cancer represents about one third of cardiac metastasis followed by breast cancer and haematologic malignancies.[2], [3], [4] 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) is well established as an important imaging modality in oncology for tumor staging, restaging, detection of recurrence, and monitoring treatment response. However, due to high physiological FDG uptake in the myocardium and significant inter-and intra-individual variability, focal cardiac uptake not confined to normal pattern can be a real challenge for interpretation. Although there is existing literature on cardiac metastasis from uterine cervical carcinoma[5], vesical carcinoma[6], renal cell carcinoma[7] and squamous cell carcinoma of the tongue[8], there is few literature on cardiac metastasis from lung cancer[9,10], in particular squamous cell carcinoma of the lung.
Case presentation
A 68-year-old gentleman with a background medical history of hypertension, hypercholesterolaemia and osteoporosis presented to his general practitioner with one month history of right shoulder pain. He is an ex-smoker and has an approximately 30 pack year smoking history. He also have previous asbestos exposure from truck brake linings. A subsequent X-ray of his shoulder revealed a distal right clavicle lesion (Fig. 1, white arrow) and a mass lesion of the right lung (Fig. 1, black arrow). He denied any shortness of breath, cough or haemoptysis prior to presentation. On examination, there was no focal tenderness of the right clavicle and his chest was clear to auscultation. A subsequent computer tomography (CT) scan of the chest showed a right lower lobe pulmonary mass measuring approximately 50 mm with invasion into the adjacent pleura. This was associated with a right perihilar speculated lesion with infiltration of the right upper pulmonary arteries and veins and associated right lower and upper paratracheal lymphadenopathy consistent with primary lung malignancy with ipsilateral lung and nodal metastasis. There was also CT evidence consistent with asbestos related pleural disease.
Fig. 1.
X-ray of the right shoulder of a 68-year-old man who was referred with right shoulder pain revealed presence of a right lung mass (white arrow) with bulky pulmonary hilum associated with a destructive lesion in the distal right clavicle (white arrow)
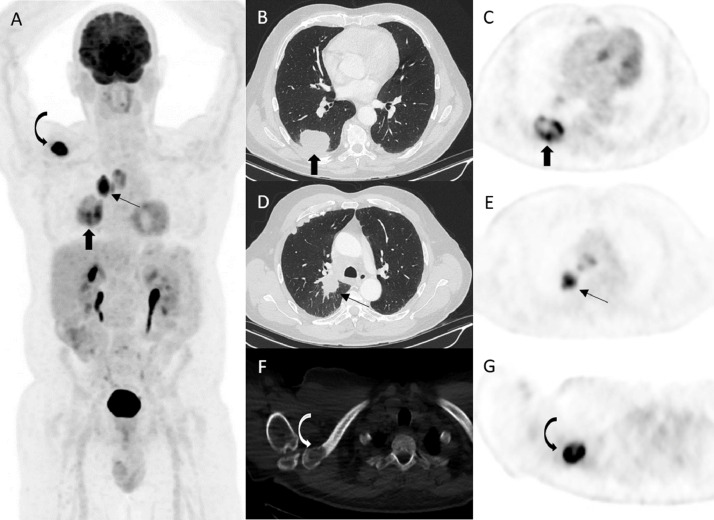
Endobronchial ultrasound and biopsy of right lower paratracheal lymph node as well as the right clavicle were performed confirming the diagnosis of metastatic squamous cell carcinoma of the lung. 18F- FDG PET/CT scan was performed for further evaluation which showed FDG-avid lesions in the right lower lobe with central photopenia suggestive of necrosis (SUVmax 9.7) (Fig. 2, block arrows) and right hilar lesion (SUVmax 10.2) (Fig. 2, thin arrows) with FDG avid metastasis to upper and lower paratracheal lymph nodes (SUVmax 6.4) as well as the right lateral clavicle corresponding to a destructive soft tissue lesion (SUVmax 17.1) (Fig. 2, curved arrows). He was started on chemotherapy (Carboplatin and Paclitaxel) with immunotherapy (Pembrolizumab) in addition to high dose radiotherapy to the right lung as well as the clavicle following discussion at a Lung Multidisciplinary Team Meeting.
Fig. 2.
Patient was referred for 18F- FDG PET/CT scan for staging of squamous cell carcinoma of the right lower lobe. Maximal Intensity Projection (A), axial CT (B, D and F) and PET (C, E and G) images demonstrated FDG-avid lesion in the right lower lobe (block arrows) associated with a speculated perihilar mass (thin arrows) and metastatic spread to paratracheal lymph nodes and the right clavicle corresponding to a lytic lesion on CT (curved arrows).
After completing four cycles of chemotherapy and immunotherapy, a repeat PET/CT scan was performed which demonstrated that the previously treated right lower lobe mass as well as the right upper lobe masses have decreased in size, however the right lower lobe mass had significantly increased in FDG avidity (SUVmax 53 compared to 9.7) (Fig. 3, thin arrows). There was also anatomical and metabolic progression of the right clavicular lesion (Fig. 3, curved arrows). There was also interval increase in size and avidity in the right upper and lower paratracheal lymph nodes. Two new FDG avid disease were also discovered including the lateral wall of the right ventricle corresponding to a low density lesion on CT (SUVmax 16.1) (Fig. 3, black block arrows) and the left masseter muscle (SUVmax 28.0) (Fig. 3, white block arrows).
Fig. 3.
Maximal Intensity Projection (A) and axial CT (B, D and F) and PET (C, E and G) images of repeat FDG-PET/CT nine months later following treatment with radiotherapy to the lung and clavicle as well as combination immunotherapy (Pembrolizumab) and chemotherapy (Carboplatin/Paclitaxel) showed increase in FDG avidity in the known pulmonary mass, paratracheal lymph nodes and right clavicle; two new FDG avid sites of metastasis were discovered including the lateral wall of the right ventricle (SUVmax 16.1) represented by black block arrows and the left masseter muscle region (SUVmax 28.0) represented by white block arrow.
He received radiotherapy to the left masseter muscle and was placed on clinical trial treatment for targeted therapy. However, further disease progression was demonstrated on follow up imaging with new metastasis to the right 7-9 ribs and right femur. The right ventricular mass lesion had progressed in size and extent, seen to cause extrinsic compression of the right ventricular cavity as demonstrated on CT scan 6 months following the repeat FDG-PET/CT (Fig. 4, block arrows).
Fig. 4.
Axial diagnostic CT image 6 months following the repeat FDG-PET/CT study shows progression of the right ventricular lesion (block arrows) causing extrinsic compression of the right ventricular cavity and enlargement of the pulmonary mass.
Discussion
Primary cardiac tumors are extremely uncommon whereas secondary cardiac tumors or cardiac metastases are 20 to 132 times more common than primary cardiac tumors.[2,3] Previous studies have shown that up to 20% of oncology patients have metastases to the heart at autopsy.[4] Tumors can spread to the heart via 4 pathways: direct hematogenous spread, lymphatic spread, transvenous extension, and direct extension. Lung cancer is reported to be the most common cause of cardiac metastasis representing 36%-39% of all cardiac metastasis and is thought to spread to the pericardium and epicardium most commonly via the lymphatic route.[11,12]
18F-FDG PET/CT is a useful modality for detecting sites of metastatic disease in cancer patients, however 18F-FDG PET/CT does not yet have an established role in routine evaluation of cardiac metastasis probably due to high physiological FDG uptake in the myocardium and significant inter- and intra-individual variability.[13] As a result, guidelines have been published which recommend patients to follow a specific preparation with high-fat/low-carbohydrate diet and fasting to shift myocardium metabolism toward fatty acid consumption in both oncology imaging and imaging for myocardial inflammation.[14,15] However, in a series of 24 patients with cardiac tumors who underwent 18F-FDG PET/CT with standard oncological preparation (fasting period of at least 6 h), Rahbar and Colleagues found that malignant primary and cardiac metastasis can be determined from benign cardiac tumors with a sensitivity of 100% and specificity of 86% using a SUVmax threshold of 3.5 for benign lesions. [16] Liu suggested that SUVmax ratio between focal cardiac uptake and surrounding background uptake may help to differentiate malignant lesions from benign cardiac uptake on FDG PET/CT as true positive lesions had a SUVmax ratio of 4.5 ± 1.5 vs 2.3 ± 0.44 that of negative cardiac lesions (P < .05).[17]
The heart can be overlooked due variable physiological myocardial uptake, particularly on the MIP images, however missed cardiac metastasis can result in significant management changes for patients. This emphasizes that careful examination of the heart is required for oncological patients undergoing 18F-FDG PET/CT with routine preparation and focal uptake should be carefully assessed for the possibility of cardiac metastasis. Future studies are awaited to further define and validate 18F-FDG PET/CT in the assessment of cardiac tumors.
Conclusion
The incidence of cardiac metastasis may not be as low as expected and lung cancer constitutes about one third of all cases. Identification of cardiac lesions with 18F-FDG PET/CT maybe challenging with routine preparation due high physiological FDG uptake in the myocardium and significant variability, nevertheless, as shown in this case focal FDG uptake should be carefully assessed for the possibility of cardiac metastasis.
Footnotes
Competing interests: none declared.
Patient consent and permission for publication of this case was obtained.
References
- 1.Al Mamgani A, Baartman L, Baaujens M. Int J Clin Onclol. 2008;13(4):369–372. doi: 10.1007/s10147-007-0749-8. [DOI] [PubMed] [Google Scholar]
- 2.Lam KY, Dickens P, Chan ACL. Tumors of the heart: a 20-year experience with a review of 12485 consecutive autopsies. Arch Pathol Lab Med. 1993;117:1027–1031. [PubMed] [Google Scholar]
- 3.Butany J, Leong SW, Carmichael K, Komeda M. A 30-year analysis of cardiac neoplasms at autopsy. Can J Cardiol. 2005;21:675–680. [PubMed] [Google Scholar]
- 4.Bussani R, De-Giorgio F, Abbate A, Silvestri F. Cardiac metastases. J Clin Pathol. 2007;60(1):27–34. doi: 10.1136/jcp.2005.035105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shimotsu Y, Ishida Y, Fukuchi K, Hayashida K, Toba M, Hamada S. Fluorine-18-fluorodeoxyglucose PET identification of cardiac metastasis arising from uterine cervical carcinoma. J Nucl Med. 1998;39(12):2084–2087. [PubMed] [Google Scholar]
- 6.Biancheri I, Zsigmond R, Angoué O, Blagosklonov O, Klingelschmitt S, Legalery P. F-18 FDG PET identification of right atrium metastasis from a vesical carcinoma. Clin Nucl Med. 2007;32(10):812–815. doi: 10.1097/RLU.0b013e318148b1fd. [DOI] [PubMed] [Google Scholar]
- 7.Pinnamaneni N, Muthukrishnan A. Left ventricular myocardium metastasis in a patient with primary renal cell carcinoma detected by 18F-FDG PET/CT. Clin Nucl Med. 2012;37(7):e181–e183. doi: 10.1097/RLU.0b013e31824c5def. [DOI] [PubMed] [Google Scholar]
- 8.Delabie P, Evrard D, Zouhry I, Ou P, Rouzet F, Benali K. Squamous cell carcinoma of the tongue with cardiac metastasis on 18F-FDG PET/CT: A case report and literature review. Medicine (Baltimore) 2021;100(15):e25529. doi: 10.1097/MD.0000000000025529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim JH, Jung JY, Park YL, Hwang SI, Jung CS, Lee SH. Non-small cell lung cancer initially presenting with intracardiac metastasis. Korean J Intern Med. 2005;20(1):86–89. doi: 10.3904/kjim.2005.20.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Orcurto MV, Delaloye AB, Letovanec I, Favre MM, Prior JO. Detection of an asymptomatic right-ventricle cardiac metastasis from a small-cell lung cancer by F-18-FDG PET/CT. J Thorac Oncol. 2009;4(1):127–130. doi: 10.1097/JTO.0b013e318189f60e. [DOI] [PubMed] [Google Scholar]
- 11.Goldberg AD, Blankstein R, Padera RF. Tumors metastatic to the heart. Circulation. 2013;15(16):1790–1794. doi: 10.1161/CIRCULATIONAHA.112.000790. 128. [DOI] [PubMed] [Google Scholar]
- 12.Yusuf SW, Bathina JD, Qureshi S, Kaynak HE, Banchs J, Trent JC. Cardiac tumors in a tertiary care cancer hospital: clinical features, echocardiographic findings, treatment and outcomes. Heart Int. 2012;7(1):e4. doi: 10.4081/hi.2012.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saponara M, Ambrosini V, Nannini M, Gatto L, Astolfi A, Urbini M. 18F-FDG-PET/CT imaging in cardiac tumors: illustrative clinical cases and review of the literature. Ther Adv Med Oncol. 2018;10:1–9. doi: 10.1177/1758835918793569. 1758835918793569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boellaard R, Delgado-Bolton R, Oyen WJ, Giammarile F, Tatsch K, Eschner W. European Association of Nuclear Medicine (EANM). FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging. 2015;42(2):328–354. doi: 10.1007/s00259-014-2961-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dorbala S, Di Carli MF, Delbeke D, Abbara S, DePuey EG, Dilsizian V. SNMMI/ASNC/SCCT guideline for cardiac SPECT/CT and PET/CT 1.0. J Nucl Med. 2013;54(8):1485–1507. doi: 10.2967/jnumed.112.105155. [DOI] [PubMed] [Google Scholar]
- 16.Rahbar K, Seifarth H, Schäfers M, Stegger L, Hoffmeier A, Spieker T. Differentiation of malignant and benign cardiac tumors using 18F-FDG PET/CT. J Nucl Med. 2012;53(6):856–863. doi: 10.2967/jnumed.111.095364. [DOI] [PubMed] [Google Scholar]
- 17.Liu Y. Focal mass-like cardiac uptake on oncologic FDG PET/CT: Real lesion or atypical pattern of physiologic uptake? J Nucl Cardiol. 2019;26(4):1205–1211. doi: 10.1007/s12350-018-01524-8. [DOI] [PubMed] [Google Scholar]