Highlights
-
•
PD patients with ICD behave like controls in proactive and reactive inhibition.
-
•
PD patients with ICD recruit different mechanisms depending on the inhibition type.
-
•
Proactive inhibition is executed hyperactivating the stopping network bilaterally.
-
•
Restrained inhibition is accomplished with the coactivation of attentional areas.
-
•
In restrained inhibition, connectivity between right STN and precuneus is reduced.
Abbreviations: FC, functional connectivity; HC, healthy control; ICD, Impulse Control Disorder; IFG, inferior frontal gyrus; IPC, inferior parietal cortex; fMRI, functional Magnetic Resonance Imaging; MNI, Montreal Neurological Institute; preSMA, presupplementary motor cortex; PD, Parkinson’s Disease; PD-ICD, Parkinson’s Disease with Impulse Control Disorder; PD-noICD, Parkinson’s Disease without Impulse Control Disorder; QUIP-RS, Questionnaire for Impulsive-Compulsive Disorders in Parkinson’s Disease Rating Scale; ROI, region-of-interest; RT, response time; SMG, supramarginal gyrus; SST, Stop Signal Task; STN, subthalamic nucleus
Keywords: Impulse control disorders, Parkinson’s disease, Inhibitory control, Attention
Abstract
Impulse control disorder is a prevalent side-effect of Parkinson’s disease (PD) medication, with a strong negative impact on the quality of life of those affected. Although impulsivity has classically been associated with response inhibition deficits, previous evidence from PD patients with impulse control disorder (ICD) has not revealed behavioral dysfunction in response inhibition. In this study, 18 PD patients with ICD, 17 PD patients without this complication, and 15 healthy controls performed a version of the conditional Stop Signal Task during functional magnetic resonance imaging. Whole-brain contrasts, regions of interest, and functional connectivity analyses were conducted. Our aim was to investigate the neural underpinnings of two aspects of response inhibition: proactive inhibition, inhibition that has been prepared beforehand, and restrained inhibition, inhibition of an invalid inhibitory tendency. We observed that, in respect to the other two groups, PD patients with ICD exhibited hyperactivation of the stopping network bilaterally while performing proactive inhibition. When engaged in restrained inhibition, they showed hyperactivation of the left inferior frontal gyrus, an area linked to action monitoring. Restrained inhibition also resulted in changes to the functional co-activation between inhibitory regions and left inferior parietal cortex and right supramarginal gyrus. Our findings indicate that PD patients with ICD completed the inhibition task correctly, showing altered engagement of inhibitory and attentional areas. During proactive inhibition they showed bilateral hyperactivation of two inhibitory regions, while during restrained inhibition they showed additional involvement of attentional areas responsible for alerting and orienting.
1. Introduction
The ability to appropriately stop an ongoing motor response or refrain from inhibiting that response enables us to adapt to the environment. Inhibitory control is the cognitive function responsible for those adaptive behaviors. More specifically, response inhibition refers to a motor form of inhibitory control. Its neural underpinnings are a right-lateralized network consisting of the anatomically connected (Aron et al., 2007a) presupplementary motor area (preSMA), inferior frontal gyrus (IFG), and subthalamic nucleus (STN) (Aron, 2011). Despite the right-lateralization of the stopping network, some left hemisphere recruitment, particularly of the left IFG, is typically observed (Aron et al., 2014, Criaud and Boulinguez, 2013, Swick et al., 2008, Swick et al., 2011).
Response inhibition also requires attention and monitoring resources, which can easily be confounded with inhibitory mechanisms. For this reason, a number studies have aimed to segregate the effects of response inhibition and attention (Criaud and Boulinguez, 2013, Hampshire et al., 2010, Meffert et al., 2016). Working alongside the stopping network, attentional mechanisms managed by the dorsal and ventral networks ensure a task can be completed without distractions. The dorsal network, comprising mostly bilateral areas, such as dorsal fronto-parietal regions (Corbetta et al., 2008), is responsible for maintaining alertness, through the top-down allocation of attention. The ventral network, comprising right-lateralized frontoparietal areas, such as the IFG (Cabeza et al., 2008, Corbetta et al., 2008), is responsible for shifting attention when reorienting from one stimulus to another (Fan et al., 2005). Although segregated, the dorsal and ventral attentional networks interact to meet task demands. This flexible collaboration is orchestrated by the IFG and the middle frontal gyrus (Vossel et al., 2014).
When the stopping network functions deficiently, it can affect impulse control abilities (Aron et al., 2007b), leaving patients unable to manage urges. This, in turn, can lead to socioeconomic, personal, and family problems that affect quality of life. This is the struggle that Parkinson’s disease (PD) patients experience if they develop Impulse Control Disorder (ICD). In PD patients, ICD is an adverse effect of dopaminergic treatment, which leads patients to experience problems regulating behaviors (American Psychiatric Association, 2013). Large cross-sectional studies estimate that between 13.6% and 40% of PD patients under dopaminergic medication will experience ICD (Antonini et al., 2017, Garcia-Ruiz et al., 2014, Sharma et al., 2015, Weintraub et al., 2010). The most common addictive behaviors that patients develop are compulsive buying, compulsive eating, pathological gambling, and compulsive sexual behaviors (Martini et al., 2018, Weintraub et al., 2009). Investigating how treatments affect the stopping network will help us better understand how response inhibition mechanisms function in PD, especially PD- ICD patients, and in impulsive pathologies more generally.
The effects of PD pathology on motor regions and movement initiation have led to multiple studies examining response inhibition in PD patients without ICD (PD-noICD). Different aspects of inhibition have been studied, including reactive inhibition, the ability to stop once instructed, and proactive inhibition, the ability to prepare for inhibition beforehand. PD-noICD patients show inhibitory deficits in reactive and proactive inhibition (Gauggel et al., 2004, Mirabella et al., 2017, Obeso et al., 2014). However, early-stage PD patients show heterogeneous impairment, with evidence for impaired (Di Caprio et al., 2020) as well as unimpaired (Vriend et al., 2015) reactive inhibition, and normal proactive inhibition (Di Caprio et al., 2020). Results do not seem driven by the effects of medication, with patients showing impairment while they are in both ON and OFF states (Obeso et al., 2011). Yet, it is still unclear whether inhibitory deficits are linked to disease progression or to prolonged medication intake. The lateralization of the stopping network could also be altered by PD pathology, implicating bilateral structures to a greater extent (Mirabella et al., 2017).
Going beyond the effects of PD pathology, the case of PD with ICD (PD-ICD) provides a study sample in which continuous dopamine intake alters patients’ ability to regulate behavior, triggering ICDs. Yet, few studies have assessed response inhibition in PD-ICD patients. Behaviorally, studies with PD-ICD patients have shown mixed results. It has been reported that PD-ICD patients i) stop initiated movements faster than matched PD-noICD patients and healthy controls (Claassen et al., 2015); ii) behave like their control counterparts (Filip et al., 2018); and iii) respond more impulsively than PD-noICD controls (Meyer et al., 2020). Meyer and colleagues (2020) also found abnormal beta activity in the SMA and precuneus during proactive inhibition in their PD-ICD group. To the best of our knowledge, the only functional magnetic resonance imaging (fMRI) study to date that has examined response inhibition in PD-ICD (Filip et al., 2018) found that, despite observing no differences in reactive inhibition between PD-ICD and PD-noICD groups ON dopaminergic medication, PD-ICD patients showed hypoactivation in frontal areas and the left caudate compared to PD-noICD and healthy control (HC) groups. Furthermore, compared to the other groups, the PD-ICD group showed reduced functional connectivity (FC) between the caudate nuclei and both the superior parietal lobe and insula. These alterations of co-activation strengthened the authors’ claim that changes associated with PD-ICD go beyond the stopping network. Remarkably, this study did not show functional activation or connectivity differences in areas of the stopping network. This could be due to a reactive inhibition paradigm that was not sufficiently challenging to detect changes in this population.
Although contradictory, most evidence leans towards a lack of behavioral impairment in PD-ICD compared to PD-noICD individuals in response inhibition (Claassen et al., 2015, Filip et al., 2018) and cognitive inhibition (Paz‐Alonso et al., 2020), suggesting compensatory mechanisms in PD-ICD patients. Thus, in the present work, our goal was to investigate the neural correlates of response inhibition in patients with medication-induced increased impulsivity. We utilized a naturalistic version of the demanding conditional Stop Signal Task (SST) in which we did not increase the level of difficulty to artificially force participants to fail. The SST paradigm is particularly challenging and differs from other classical response inhibition tasks because it requires the participant to stop an action that has already been initiated. This makes the SST particularly demanding for PD-ICD subjects, in contrast to the task used by Filip and colleagues in which a non-initiated action had to be inhibited (Filip et al., 2018). An fMRI study using the SST in healthy adults showed activation of the right IFG, right STN, and right preSMA (Aron et al., 2007a). We focused on two critical aspects of response inhibition – proactive and restrained inhibition – in PD patients with and without ICD.
Proactive inhibition refers to the ability to prepare to inhibit (Aron, 2011), which involves recruiting the stopping network before inhibition occurs. It has been proposed as a more valid measure of response inhibition (Aron, 2011, Meyer et al., 2020) than reactive inhibition which does not involve preparation. Because of the taxing nature of our task, we expected PD-ICD patients would show changes in inhibitory regions as they strove to perform correctly. We also expected greater co-activation between the stopping network and the dorsal attention network responsible for maintaining an alerting state, since PD-ICD patients would need to maintain greater focus to perform successfully.
Restrained inhibition is the ability to disengage from an invalid cue signaling inhibition and perform the motor response despite the incongruence of the cue. While performing our task, participants encountered trials containing the salient stimulus they had learned to associate with inhibition. However, during restrained inhibition trials, they had to refrain from the learnt inhibition and execute the task (a button-press). This aspect of response inhibition is understood as the inhibition of an inhibitory action. Restrained inhibition has not been previously examined in PD-ICD patients. However, the fact that this group shows increased difficulty in regulating behavior (i.e., switching between rules, redirecting attention, and processing incongruent stimuli) suggests they may be particularly affected when attempting to restrain inhibition. We expected PD-ICD patients to show alterations in the stopping network as well as co-activation with areas in the ventral attentional network involved in reorienting attention and disengaging from invalid cues, and regions of the dorsal network that provide optimal focus on a task. The IFG might also be vital for restrained inhibition: the right IFG for stopping and reorienting attention to relevant stimuli (Corbetta et al., 2008); the left for filtering out irrelevant actions (Chong et al., 2008).
2. Materials and methods
2.1. Participants
The final sample comprised fifty participants: 18 PD-ICD patients, 17 PD-noICD, and 15 HC matched on age, sex, education, and premorbid intelligence (see supplementary materials for details on original sample). Nine additional participants were excluded: three participants (one PD-ICD and two HC) due to outlier performance (>2 SDs) on the conditional SST task; three (two PD-ICD and one HC) for excessive head motion during fMRI scanning (see MRI data analysis for more details); and three (one PD-noICD and two HC) for problems related to constructing a functional mask due to motion during structural data acquisition.
All PD patients were diagnosed according to the UK Parkinson’s Disease Society Brain Bank criteria. They were recruited from the Movement Disorders Unit of the Hospital Universitario Donostia, Spain. Inclusion criteria for the PD-ICD group included at least one ICD not present either at the time of PD diagnosis or before the initiation of dopamine replacement therapy. The ICD diagnosis was confirmed by a neurologist and a psychiatrist based on the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (American Psychiatric Association, 2013) criteria and the Questionnaire for Impulsive-Compulsive Disorders in PD (Weintraub et al., 2009). Every PD-ICD patient scored above the cut-off for their ICD subtype in the Questionnaire for Impulsive-Compulsive Disorders in PD Rating Scale (QUIP-RS) (Weintraub et al., 2012). Exclusion criteria for PD patients were dementia (Emre et al., 2007) and mild cognitive impairment according to the Movement Disorders Society Task Force criteria (Level II) (Litvan et al., 2012), presence of dyskinesias, brain surgery, or previous diagnosis of PD-ICD that had been resolved at recruitment. HC participants were recruited from the Basque Center on Cognition, Brain and Language (BCBL)’s participants’ pool. Exclusion criteria for HC participants was the presence of any neurological condition or any type of cognitive impairment.
The three groups underwent a comprehensive neuropsychological assessment (see supplementary Table 1 for more details). This study was approved by the Gipuzkoa Clinical Research Ethics Committee. All participants were right-handed and provided written informed consent before joining the experiment.
2.2. MRI data acquisition
Functional and structural images were collected at the BCBL’s 3T Siemens Magnetom TIM Trio MRI scanner using a 32-channel head coil. PD participants underwent scanning under the effect of dopaminergic medication. See Supplementary Data for additional information on MRI data acquisition.
2.3. Functional magnetic resonance imaging paradigm: Conditional Stop-Signal task
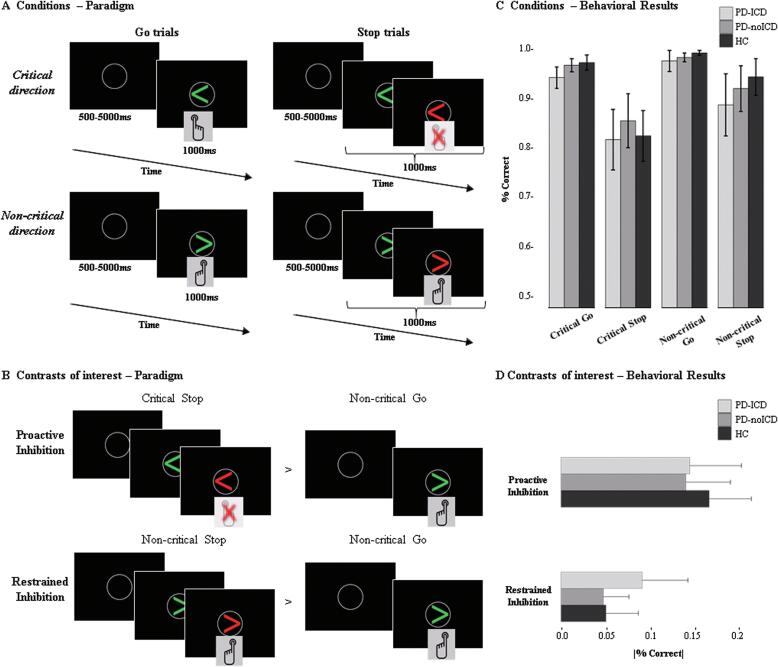
In the scanner, participants completed a conditional variation of the traditional SST (Logan et al., 1984). This conditional SST (see Fig. 1A) comprised 75% Go and 25% Stop trials. All trials began with a grey fixation circle. After 500–5000 ms, a green arrow appeared, pointing either left or right. Participants were instructed to press the button corresponding to the direction of the arrow, as quickly as possible. Prior to the scanning, each participant had completed a practice session, learning that one direction (left or right) was non-critical, while the other was critical (directions counterbalanced across participants). In Stop trials, a red arrow, appeared 100–250 ms (varying by 50 ms intervals) after – always pointing in the same direction as the green arrow. Importantly, on critical Stop trials, participants had to inhibit their initiated response as soon as the red arrow appeared, while on non-critical Stop trials, they had to ignore the red arrow and respond normally. After each trial, a fixation cross appeared on screen for 500 ms. To examine PD-ICD patients’ impulsivity in a naturalistic setting where they were not forced to fail, all participants were presented with the same inter-stimulus-intervals between the green and red arrows in Stop trials. This constitutes a more naturalistic measure of response inhibition. Nevertheless, because we did not adjust the inter-stimulus-interval to accommodate participants’ individual performance levels, we could not calculate the stop signal response time, a measure typically employed in the SST literature. Instead, we were used accuracy measures to determine performance impairments. The task design allowed us to measure two aspects of inhibition (see Fig. 1B): Proactive Inhibition was calculated by subtracting correct non-critical Go trials, which entailed neither preparation to inhibit nor inhibition, from critical Stop trials, which required both preparation to inhibit and inhibition [critical Stop minus non-critical Go]. To execute proactive inhibition, participants needed to flexibly switch between the rules for critical and non-critical trials, prepare for the possible need to inhibit if a green arrow pointed in the critical direction, and stop the motor execution when the red arrow appeared in critical Stop trials. Restrained inhibition was measured by subtracting correct non-critical Go trials from correct non-critical Stop trials, in which participants had to ignore an invalid inhibitory stimulus [non-critical Stop minus non-critical Go]. To execute restrained inhibition correctly, participants needed to flexibly switch between the rules for critical and non-critical trials. Further, in non-critical Stop trials they needed to refrain from inhibiting the button-press, this is, stop the inhibitory action, and finish executing the movement as quickly as possible.
Fig. 1.
Schematic representation of the conditional SST task by (A) conditions and (B) contrasts of interests; and percentage of correct responses by (C) conditions and (D) contrasts of interests. Error bars represent 95% confidence interval. PD-ICD = Parkinson’s Disease with Impulse Control Disorder, PD-noICD = Parkinson’s Disease patients with no Impulse Control Disorder, HC = healthy controls.
2.4. Behavioral data analyses
Behavioral results were analysed with a 3 (Group: PD-ICD, PD-noICD, HC) by 2 (Direction: critical, non-critical) by 2 (Condition: Go, Stop) mixed-model analysis of variance (ANOVA), run on the percent of correct responses in each condition on the SST task. Given our specific interest in focusing on proactive and restrained inhibition, we ran separate one-way ANOVAs with the factor Group and the absolute values of the subtraction between trials for Proactive Inhibition (|critical Stop - non-critical Go|), and Restrained Inhibition (|non-critical Stop - non-critical Go|), with accuracy as the dependent measure.
Participants had to withhold their response in one of the main conditions of interest–critical Stop. Analyzing response time (RT) data without including that key condition either in the whole-experimental design or the Proactive/Restrained Inhibition analyses would have given us an incomplete, possibly biased, result. Therefore, we focused exclusively on accuracy results. However, to ensure there were no differential speed-accuracy trade-off between groups that could bias our results, with one group applying a different strategy than the other groups, we additionally computed an adapted version of the Balanced Integration Score (BIS) (Liesefeld et al., 2015, Liesefeld and Janczyk, 2019). The BIS removes any trade-off effect from the accuracy scores. For information on RT see supplementary Table 2.
2.5. MRI data analyses
SPM8 was used to perform standard preprocessing routines and analysis. See Supplementary Data for further details on MRI data preprocessing and analyses.
Statistical analyses were performed on individual participant’s data applying the general linear model. The fMRI time series data were modeled as a series of events convolved with a canonical hemodynamic response function. Five fMRI experimental conditions were modeled (critical Go correct, non-critical Go correct, critical Stop correct, non-critical Stop correct, and critical Stop incorrect), with each trial modeled as an event and time locked to the presentation of the first stimulus in each trial. The study followed a 3 (Group: PD-ICD, PD-noICD, HC) by 5 (Conditions: critical Go, non-critical Go, critical Stop, non-critical stop, Critical stop incorrect) experimental design. We had two main comparisons of interest comprising correct responses: Proactive Inhibition (i.e., critical Stop – non-critical Go), and Restrained Inhibition (i.e., non-critical Stop – non-critical Go). These contrasts were examined by means of whole-brain contrasts, region-of-interest (ROI) analyses, and FC analyses. Depiction of FC analyses was done with BrainNet Viewer (Xia et al., 2013).
3. Results
3.1. Demographic and clinical data
There were no differences between groups in demographic data (see Table 1). PD-noICD and PD-ICD groups did not differ in disease duration, dopaminergic medication, motor severity and cognitive outcomes (see supplementary Table 1).
Table 1.
Demographics and clinical characteristics of the sample.
| PD-ICD | PD-noICD | HC | p | |
|---|---|---|---|---|
| n = 18 | n = 17 | n = 15 | ||
| Age | 63.33 (8.24) | 61.65 (9.21) | 61.87 (9.77) | 0.837a |
| Sex, male (%) | 16 (88.90%) | 14 (82.40%) | 14 (93.30%) | 0.837b |
| Education (years) | 12.50 [7–20] | 11 [7–20] | 20 [5–20] | 0.319c |
| Premorbid IQ (WAIS-III Vocabulary) | 42.94 (10.13) | 44.82 (10.18) | 50.2 (7.46) | 0.319a |
| Disease duration (years) | 7.13 (3.96) | 7.5 (4.52) | – | 0.837d |
| UPDRS-III | 21.50 [10–46] | 18.00 [11–30] | – | 0.837e |
| H&Y stage | 2 [1.5–3] | 2 [1–3] | – | 0.837b |
| LEDTOTAL (mg) | 970 [450–2660] | 792 [250–1664] | – | 0.837e |
| LEDDA (mg) | 194.83 (165.99) | 211.76 (144.88) | – | 0.837d |
| HADS score | 6.50 [1–25] | 4 [1–10] | 6 [1–16] | 0.148c |
| QUIP-RS score | 16.50 [7–46] | 0 [0–0] | – | >0.001e |
Note: Values expressed in Mean (SD) for normally distributed variables, in Median [Range] for other variables. P-values represent FDR-adjusted p-values.
Abbreviations: IQ = Intelligence Quotient; WAIS-III = Wechsler Adult Intelligence Scale-III; UPDRS = Unified Parkinson's Disease Rating Scale; H&Y = Hoehn and Yahr scale; LEDTOTAL = Total levodopa equivalent daily dose was calculated according to the formula described by Tomlinson et al. (2010); LEDDA = Levodopa equivalent daily dose of dopamine agonist was calculated using the same formula; HADS = Hospital Anxiety and Depression Scale; QUIP-RS = Questionnaire for Impulsive-Compulsive Disorders in Parkinson’s Disease-Rating Scale.
One factor ANOVA.
Chi-Square Likelihood Ratio.
Kruskall-Wallis.
Two-sample T-test.
U Mann Whitney.
3.2. Behavioral results
The 3 (Group) by 2 (Direction) by 2 (Condition) mixed-model ANOVA on the percentage of correct responses revealed main effects of Direction, (F1,43 = 37.36, p < 0.001, ηp2 = 0.46) and Condition, (F1,43 = 117.79, p < 0.001, ηp2 = 0.73), subsumed by a statistically significant Direction by Condition interaction, (F1,43 = 11.42, p = 0.002, ηp2 = 0.21). There were no main or interactive effects of Group (Fs < 1.1, p > 0.1, ηp2 ≤ 0.05). Simple-effect post-hoc analyses examining the Direction by Condition interaction showed trial differences associated with task difficulty (non-critical Go = critical Go > non-critical Stop > critical Stop, ps < 0.02) (see Fig. 1C). Separate one-way ANOVAs for the contrasts Proactive and Restrained inhibition did not reveal Group effects (Fs < 2, p > 0.1, ηp2 < 0.1) (see Fig. 1D). We re-ran the previously described analyses with the BIS of the conditions and contrasts but again found no main or interactive effects of the factor Group (Fs < 2.2, p > 0.12, ηp2 ≤ 0.09).
3.3. MRI results
3.3.1. Whole-brain analysis
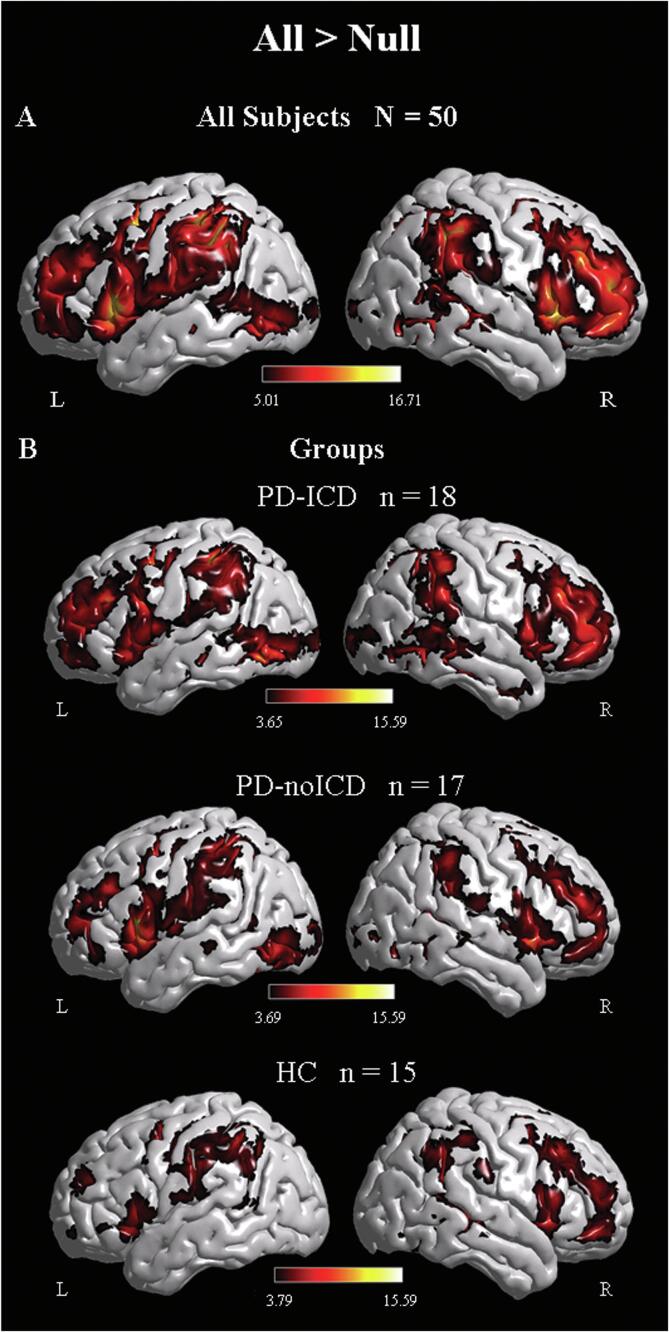
We computed a whole-brain functional contrast for all the task conditions versus the baseline condition (All Conditions > Null) to identify regions activated during the task across all participants (see Fig. 2A). We observed recruitment of right-lateralized nodes of the stopping network (IFG, preSMA, STN) as well as their contralateral homologues in all participants. We computed the same contrast separately for each group and observed that the two PD groups tended to show left-lateralized extended activation (see Fig. 2B). The bilateral recruitment during inhibitory control in PD patients resembles a previous claim (Mirabella et al., 2017) and highlights the importance of examining not only the right-lateralized nodes from the classical inhibitory control network, but also their left-lateralized homologues.
Fig. 2.
Brain renderings showing activations for the whole brain contrast All > Null for (A) all participants at a statistical threshold of p < 0.05 voxel FWE-corrected, and for each study group (B), p < 0.001 voxel-level extent threshold, p < 0.05 cluster-level FWE corrected.
3.3.2. ROI analyses
3.3.2.1. Proactive inhibition
The 3 (Group) by 2 (Hemisphere) mixed-model ANOVA showed a main effect of Group for the percent signal change of the bilateral preSMA (F2,46 = 6.74, q = 0.009, ηp2 = 0.23) and bilateral IFG (F2,46 = 3.85, q = 0.043, ηp2 = 0.14). Post-hoc tests revealed hyperactivation of the preSMA for the PD-ICD group relative to both the PD-noICD (t33 = 3.29, q = 0.007, d = 0.47) and the HC (t31 = 3.01, q = 0.008, d = 0.43) groups, as well as hyperactivation of the IFG for the PD-ICD group compared to the PD-noICD group (t33 = 2.59, q = 0.043, d = 0.37). No group differences were observed for the STN (see Fig. 3A).
Fig. 3.
ROI analyses for Proactive and Restrained Inhibition. (A) Proactive inhibition showed a group effect for preSMA and IFG. (B) Restrained inhibition showed a group by hemisphere interaction. Significance of group effects shown in graphs. Error bars represent 95% confidence interval.
This analysis also showed a main effect of Hemisphere for the percent signal change of the preSMA (F2,46 = 21.65, q < 0.001, ηp2 = 0.32), the IFG (F2,46 = 62.98, q < 0.001, ηp2 = 0.58), and the STN (F2,46 = 4.51, q = 0.039, ηp2 = 0.09), with right hemisphere ROIs showing greater activation than their left hemisphere homologues.
For PD-ICD patients, we observed a significant negative correlation between preSMA activation and their QUIP score (rho = -0.49, p = 0.041). However, this correlation did not survive multiple comparisons correction (q = 0.082). We found no other significant correlations.
3.3.2.2. Restrained inhibition
The analysis revealed a statistically significant Group by Hemisphere interaction for the percent signal change of the IFG (F2,46 = 7.92, q < 0.001, ηp2 = 0.26). To follow-up on this interaction we performed between-group ANOVAs separately for the left and right IFG. We found an effect only for the left IFG (F2,46 = 5.87, q = 0.01, ηp2 = 0.2), with post-hoc tests revealing hyperactivation of the left IFG in the PD-ICD group compared to the PD-noICD (t32 = 2.78, q = 0.013, d = 0.95) and HC groups (t30 = 3.13, q = 0.013, d = 1.02) (see Fig. 3B).
3.3.3. Functional connectivity analysis
A previous study suggested that activation changes during inhibition are present beyond the stopping network in PD-ICD patients (Filip et al., 2018). To examine the FC of inhibitory regions and their homologues with other brain areas, we conducted whole-brain functional connectivity analyses seeding the nodes of the traditional stopping network and their left counterparts for the contrasts of interest: Critical Stop vs Null and Non-critical Stop vs Null.
3.3.3.1. Proactive inhibition
No between-group differences in FC emerged using the IFG, preSMA, and STN left and right seeds.
3.3.3.2. Restrained inhibition
Stronger left IFG-inferior parietal (IPC)/supramarginal (SMG) FC (maxima at MNI coordinates –33, −52, 43, t = 5.99) was observed for the PD-ICD compared to the PD-noICD group (see Fig. 4A). The left IPC and SMG are areas associated with the dorsal attention network. Also, tighter right IFG-postcentral/SMG FC (maxima at 51, −10, 28, t = 6.17) was found for the PD-ICD compared to the PD-noICD group (see Fig. 4B). The right SMG has been linked to the ventral attention network.
Fig. 4.
Functional whole-brain connectivity during Restrained Inhibition. Compared to PD-noICD participants, PD-ICD patients showed increased functional coupling of the left IFG (A) and right IFG (B) with parietal regions, plus decreased functional co-activation of the right preSMA (C) with putamen/insula and right STN (D) with precuneus.
We also found reduced right preSMA-putamen/insula (maxima at 33, −13, 1, t = 5.06) (see Fig. 4C) and right STN-precuneus (maxima at 9, −49, 37, t = 4.49) FC for the PD-ICD compared to the PD-noICD group (see Fig. 4D).
4. Discussion
In the current study, we employed a demanding response inhibition task, with sustained natural difficulty levels. By not forcing participants to fail, we were able to observe unbiased performance. PD-ICD patients presented no behavioral impairments relative to their PD-noICD and HC counterparts, as expected from previous evidence (Claassen et al., 2015, Filip et al., 2018). However, functional correlates linked to proactive and restrained inhibition suggested that PD-ICD resolved inhibitory demands differently from their PD-noICD and HC counterparts.
While performing proactive inhibition, regions of the right-lateralized stopping network showed the highest activation across all groups. However, the PD-ICD group showed strong right-lateralized hyperactivation as well as hyperactivation of left-lateralized homologues, previously not considered to form part of the stopping network. This bilateral involvement might indicate that PD-ICD subjects had to exert greater effort during proactive inhibition than the other groups but, since this was not entirely resolved by right-lateralized hyperactivation, they then recruited the stopping network bilaterally. Together with the lack of behavioral impairment and the absence of differences in functional connectivity, these patterns of hyperactivation suggest that PD-ICD participants engage in proactive inhibition by strongly activating the bilateral stopping network and that this challenge is resolved at the regional level without increasing recruitment of attentional resources.
One of these bilaterally hyperactivated areas is the IFG. The right IFG forms part of both the stopping network (Aron et al., 2007a) and the ventral attention network (Corbetta et al., 2008). As stated in the Introduction, a role for the left IFG – alongside other regions – in inhibition is still challenged by some authors (Aron et al., 2014) but accepted by others (Criaud and Boulinguez, 2013, Meffert et al., 2016, Swick and Chatham, 2014). The left IFG is known to be responsible for evaluating and selecting appropriate actions (Pobric et al., 2006, Swick et al., 2008). This attentional function could be recruited when inhibition must be prepared in a critical trial and then applied if a stop signal appears. The other hyperactive region, the preSMA, is known to be involved in action selection (Mueller et al., 2007, Tanji, 1994), which is important when deciding whether a motor action should be halted or not. The role of both IFG and preSMA in implementing inhibition was previously underscored by a transcranial magnetic stimulation study (Obeso et al., 2013a). In this study, PD patients showed contralateral recruitment as a compensatory mechanism for malfunction of the stopping network (Obeso et al., 2013b). Specifically, the authors reported evidence for contralateral compensatory engagement of the left IFG and left preSMA – but not the STN. Similarly, we did not observe differential activation of the STN in the PD-ICD group. While these patients exhibited evidence of increased demand in regions responsible for detecting the need to inhibit (Aron, 2011) (i.e., preSMA, IFG), they were still able to execute motor inhibition correctly, showing normal demand in the region that executed the order (i.e., STN). In fact, PD-ICD patients are known to stop faster than controls (Claassen et al., 2015), and therefore execute inhibitory commands correctly. Taken together with our findings, this suggests that PD-ICD patients find preparation to inhibit (not necessarily inhibition per se), challenging. Thus, it is regions responsible for detecting and sending the inhibitory command to the STN that become hyperactivated.
As opposed to proactive inhibition, restrained inhibition did not differentially activate any of the classical components of the right-lateralized stopping network as a function of group. Nonetheless, in PD-ICD patients, relative to PD-noICD and HCs, restrained inhibition was associated with hyperactivation of the left IFG. As discussed above, the left IFG has been linked to monitoring relevant and irrelevant actions (Milham et al., 2001, Swick et al., 2008). Patients with ICD might be particularly challenged when they are presented with invalid stop cues and have to decide whether to respond or instead withhold movement. This need to supervise the action of responding or withholding could explain the hyperactivation of the left IFG.
We found functional connectivity changes were only associated with restrained inhibition. Compared to the PD-noICD group, PD-ICD patients showed greater co-activation between the IFG, relevant not only for inhibition but also for monitoring and reorienting attention, and areas related to: alerting (left IPC and SMG) (Cabeza et al., 2008); reorienting attention (right SMG) (Chica et al., 2013, Corbetta et al., 2008) and motor sensation (postcentral gyrus). Moreover, it has been suggested that components of the dorsal and ventral networks interact with each other, that this interaction is led by frontal areas such as the IFG, and that both networks are required to shift attention (Vossel et al., 2014). The role of attention in response inhibition tasks is an important issue. Many experimental paradigms could lead to confounds between activation related to attention and that related to inhibition per se. The IFG has been at the center of this debate as it is likely involved in both functions (Hampshire and Sharp, 2015, Padmala and Pessoa, 2010). In contrast to PD-noICD patients, PD-ICD patients show increased FC between the IFG and components of the dorsal and ventral networks. This suggests they recruit additional attentional resources to perform restrained inhibition when task demands increase. PD-ICD patients asked to make a motor decision while also coping with a contradictory irrelevant stimulus likely experience greater difficulty maintaining attention, shifting focus when disengaging from the invalid stop stimulus to return to the button-press task, and processing feedback on that motor response.
However, relative to PD-noICD patients, PD-ICD patients showed weaker functional co-activation between the right preSMA, involved in motor planning, and both the right posterior insula and putamen – tightly connected areas (Chikama et al., 1997), associated with somatosensory awareness (Chang et al., 2013) and motor control (Lehéricy et al., 2006), respectively. This reduced functional co-activation for PD-ICD relative to PD-noICD patients could reflect PD-noICD patients’ well-established difficulty in initiating movement (Dietz et al., 1990), which is known to alter preSMA activity even at an early stage of PD disease manifestation (Eckert et al., 2006). PD-ICD patients’ impulsivity might alleviate movement initiation difficulties, eliminating the need for strong co-activation of areas involved in planning a movement and sensorimotor processing. Yet, further studies would be needed to test this claim. Finally, PD-ICD patients showed weaker co-activation than PD-noICD subjects between the right STN and the right precuneus. A previous study suggested increased co-activation between those regions –right STN and right precuneus– for PD-noICD compared to HC participants during resting state (Fernández-Seara et al., 2015). We found, however, this abnormal connectivity was reduced in PD-ICD patients. This discrepancy might be due to the increased impulsivity of PD-ICD patients and the fact that the BOLD signal was not obtained at rest but during the inhibition of an inhibitory signal, in sum, facilitation of the action (the button press). Such facilitation is associated with reduced FC between the STN and the precuneus, which is responsible for both integration of perceptual information (Cavanna and Trimble, 2006) and cue reactivity (Starcke et al., 2018). This finding is in keeping with the observation that STN stimulation, which reduces STN activity and therefore its FC, speeds up patients' decisions under conflict conditions (Ballanger et al., 2009, Frank et al., 2007) such as restrained inhibition. In fact, although not significant, in our experiment, PD-ICD patients showed higher accuracy in restrained inhibition, probably due to facilitated inhibition of the irrelevant stop signal, whereas the three groups behaved similarly during proactive inhibition (Fig. 1D). This interesting finding indicates that it is easier for PD patients with abnormal impulsivity to ignore inhibition signals than to obey them, an effect linked to the STN. This effect would also help explain why PD patients make rapid and impulsive decisions under high-conflict conditions during subthalamic stimulation (Ballanger et al., 2009, Frank et al., 2007); with impulsivity appearing to be a side-effect of such stimulation (Mosley et al., 2020).
To our knowledge, no previous study has examined the functional correlates of different aspects of response inhibition in PD-ICD. However, our results should be considered bearing some limitations in mind. Firstly, the small sample size might have decreased the sensitivity of our analysis to detect further between-group differences. Secondly, all PD patients were deliberately assessed under medication to ensure optimal scanning – this was important because ICD in PD is associated with dopaminergic treatment. There were, however, no differences in the dosage of antiparkinsonian medication between PD groups. Future studies should examine different aspects of response inhibition and attention in similar samples, for instance, manipulating task difficulty and/or including distractor stimuli across conditions. This could answer remaining questions, such as: Which aspects of inhibition impose higher demands on PD-ICD than PD-noICD patients? Do these demands force PD-ICD patients to recruit additional attentional resources during response inhibition? What influence do alerting and orienting mechanisms exert on PD-ICD patients’ ability to inhibit a response? And, does task difficulty play a role in the performance of PD-ICD patients?
4.1. Conclusions
This study is the first to address functional alterations in PD-ICD patients by examining different aspects of inhibition. Subjects performed a taxing response inhibition task, in which participants were required to stop an ongoing action or instead to finalize a movement. The PD-ICD group performed these tasks normally, but they used different mechanisms than either of the control groups to execute both aspects of inhibition. During proactive inhibition, PD-ICD patients hyperactivated a more bilateral network than their control counterparts. During restrained inhibition, PD-ICD patients activated the left IFG to a greater extent and recruited additional attentional resources, while also showing reduced co-activation between the right STN and precuneus. Therefore, the two aspects of inhibition assessed here presented specific challenges for the PD-ICD group, as reflected by differences in the functional correlates of inhibition.
CRediT authorship contribution statement
Teresa Esteban-Peñalba: Formal analysis, Data curation, Writing – original draft, Visualization. Pedro M. Paz-Alonso: Conceptualization, Methodology, Software, Data curation, Writing – original draft, Supervision. Irene Navalpotro-Gómez: Investigation, Resources, Writing – review & editing. María C. Rodríguez-Oroz: Conceptualization, Methodology, Writing – review & editing, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments and Funding sources
This work was supported by grants from the Carlos III Institute of Health (PI11/02109) and the ERA-Neuron program (PIM2010ERN-0033). Additionally, the authors received the following grants and honoraria: T.E.-P. received a grant from the Spanish Ministry of Economy and Competitiveness (BES-2016-079489). P.M.P.-A. was supported by grants from the Spanish Ministry of Economy and Competitiveness (RYC-2014-15440), the Spanish Ministry of Science and Innovation (PGC2018-093408-B-I00), and the Fundación Tatiana Pérez de Guzmán el Bueno. I.N.-G. was the recipient of a Rio Hortega grant (CM16/00033) from the Carlos III Institute of Health. I.N.-G. received honoraria from Zambon and TEVA for travel and accommodation to attend scientific meetings. M.C.R.-O. received financial support for her research from national and local government institutions in Spain (Carlos III Institute of Health, Basque Country Government, Diputacion Foral Guipuzcoa, and CIBERNED). M.C.R.-O. received honoraria from Zambon, Bial, and Boston Scientific for lectures, travel, and accommodation to attend scientific meetings. BCBL acknowledges support from the Basque Government through the BERC 2018-2021 program.
None of these funding bodies influenced the content of the manuscript or the decision to publish in any way.
Footnotes
Supplementary data (Table 1 Neuropsychological results of the sample. P-values represent FDR-adjusted p-values. Table 2 Response times – Conditional Stop Signal Task.) to this article can be found online at https://doi.org/10.1016/j.nicl.2021.102822.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Table 1 Neuropsychological results of the sample. P-values represent FDR-adjusted p-values. Table 2 Response times – Conditional Stop Signal Task.
References
- American Psychiatric Association, editor. Diagnostic and Statistical Manual of Mental Disorders. Fifth Edition. American Psychiatric Association; 2013. [DOI] [Google Scholar]
- Antonini A., Barone P., Bonuccelli U., Annoni K., Asgharnejad M., Stanzione P. ICARUS study: Prevalence and clinical features of impulse control disorders in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry. 2017;88(4):317–324. doi: 10.1136/jnnp-2016-315277. [DOI] [PubMed] [Google Scholar]
- Aron A.R. From reactive to proactive and selective control: Developing a richer model for stopping inappropriate responses. Biol. Psychiatry. 2011;69(12):e55–e68. doi: 10.1016/j.biopsych.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron A.R., Behrens T.E., Smith S., Frank M.J., Poldrack R.A. Triangulating a cognitive control network using diffusion-weighted magnetic resonance imaging (MRI) and functional MRI. J. Neurosci. 2007;27(14):3743–3752. doi: 10.1523/JNEUROSCI.0519-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron A.R., Durston S., Eagle D.M., Logan G.D., Stinear C.M., Stuphorn V. Converging evidence for a fronto-basal-ganglia network for inhibitory control of action and cognition. J. Neurosci. 2007;27(44):11860–11864. doi: 10.1523/JNEUROSCI.3644-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron A.R., Robbins T.W., Poldrack R.A. Inhibition and the right inferior frontal cortex: One decade on. Trends Cogn. Sci. 2014;18(4):177–185. doi: 10.1016/j.tics.2013.12.003. [DOI] [PubMed] [Google Scholar]
- Ballanger B., Eimeren T.V., Moro E., Andres M., Hamani C., Boulinguez P., Houle S., Poon Y.Y., Lang A.E., Strafella A. Stimulation of the STN and impulsivity: Release your horses. Ann. Neurol. 2009;66:817–824. doi: 10.1002/ana.21795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R., Ciaramelli E., Olson I.R., Moscovitch M. The parietal cortex and episodic memory: an attentional account. Nat. Rev. Neurosci. 2008;9(8):613–625. doi: 10.1038/nrn2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanna A.E., Trimble M.R. The precuneus: A review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Chang L.J., Yarkoni T., Khaw M.W., Sanfey A.G. Decoding the role of the insula in human cognition: Functional parcellation and large-scale reverse inference. Cereb. Cortex. 2013;23(3):739–749. doi: 10.1093/cercor/bhs065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chica A.B., Paz-Alonso P.M., Valero-Cabre A., Bartolomeo P. Neural bases of the interactions between spatial attention and conscious perception. Cereb. Cortex. 2013;23(6):1269–1279. doi: 10.1093/cercor/bhs087. [DOI] [PubMed] [Google Scholar]
- Chikama M., McFarland N.R., Amaral D.G., Haber S.N. Insular cortical projections to functional regions of the striatum correlate with cortical cytoarchitectonic organization in the primate. J. Neurosci. 1997;17(24):9686–9705. doi: 10.1523/JNEUROSCI.17-24-09686.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong T.-J., Williams M.A., Cunnington R., Mattingley J.B. Selective attention modulates inferior frontal gyrus activity during action observation. NeuroImage. 2008;40(1):298–307. doi: 10.1016/j.neuroimage.2007.11.030. [DOI] [PubMed] [Google Scholar]
- Claassen D.O., Van Den Wildenberg W.P.M., Harrison M.B., Van Wouwe N.C., Kanoff K., Neimat J.S., Wylie S.A. Proficient motor impulse control in Parkinson disease patients with impulsive and compulsive behaviors. Pharmacol. Biochem. Behav. 2015;129:19–25. doi: 10.1016/j.pbb.2014.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M., Patel G., Shulman G.L. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58(3):306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criaud M., Boulinguez P. Have we been asking the right questions when assessing response inhibition in go/no-go tasks with fMRI? A meta-analysis and critical review. Neurosci. Biobehav. Rev. 2013;37(1):11–23. doi: 10.1016/j.neubiorev.2012.11.003. [DOI] [PubMed] [Google Scholar]
- Di Caprio V., Modugno N., Mancini C., Olivola E., Mirabella G. Early-stage Parkinson’s patients show selective impairment in reactive but not proactive inhibition. Mov. Disord. 2020;35(3):409–418. doi: 10.1002/mds.27920. [DOI] [PubMed] [Google Scholar]
- Dietz M.A., Goetz C.G., Stebbins G.T. Evaluation of a modified inverted walking stick as a treatment for Parkinsonian freezing episodes. Mov. Disord. 1990;5(3):243–247. doi: 10.1002/mds.870050311. [DOI] [PubMed] [Google Scholar]
- Eckert T., Peschel T., Heinze H.J., Rotte M. Increased pre-SMA activation in early PD patients during simple self-initiated hand movements. J. Neurol. 2006;253(2):199–207. doi: 10.1007/s00415-005-0956-z. [DOI] [PubMed] [Google Scholar]
- Emre M., Aarsland D., Brown R., Burn D.J., Duyckaerts C., Mizuno Y., Broe G.A., Cummings J., Dickson D.W., Gauthier S., Goldman J., Goetz C., Korczyn A., Lees A., Levy R., Litvan I., McKeith I., Olanow W., Poewe W., Quinn N., Sampaio C., Tolosa E., Dubois B. Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov. Disord. 2007;22(12):1689–1707. doi: 10.1002/mds.21507. [DOI] [PubMed] [Google Scholar]
- Fan J., McCandliss B., Fossella J., Flombaum J., Posner M. The activation of attentional networks. NeuroImage. 2005;26(2):471–479. doi: 10.1016/j.neuroimage.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Fernández-Seara M.A., Mengual E., Vidorreta M., Castellanos G., Irigoyen J., Erro E., Pastor M.A. Resting state functional connectivity of the subthalamic nucleus in Parkinson’s disease assessed using arterial spin-labeled perfusion fMRI. Hum. Brain Mapp. 2015;36(5):1937–1950. doi: 10.1002/hbm.22747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filip P., Linhartová P., Hlavatá P., Šumec R., Baláž M., Bareš M., Kašpárek T. Disruption of multiple distinctive neural networks associated with impulse control disorder in Parkinson’s disease. Front. Hum. Neurosci. 2018;12:462. doi: 10.3389/fnhum.2018.00462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank M.J., Samanta J., Moustafa A.A., Sherman S.J. Hold your horses: Impulsivity, deep brain stimulation, and medication in Parkinsonism. Science. 2007;318(5854):1309–1312. doi: 10.1126/science:1146157. [DOI] [PubMed] [Google Scholar]
- Garcia-Ruiz P.J., Martinez Castrillo J.C., Alonso-Canovas A., Herranz Barcenas A., Vela L., Sanchez Alonso P., Mata M., Olmedilla Gonzalez N., Mahillo Fernandez I. Impulse control disorder in patients with Parkinson’s disease under dopamine agonist therapy: A multicentre study. J. Neurol. Neurosurg. Psychiatry. 2014;85(8):840–844. doi: 10.1136/jnnp-2013-306787. [DOI] [PubMed] [Google Scholar]
- Gauggel S., Rieger M., Feghoff T.-A. Inhibition of ongoing responses in patients with Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry. 2004;75(4):539–544. doi: 10.1136/jnnp.2003.016469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampshire A., Chamberlain S.R., Monti M.M., Duncan J., Owen A.M. The role of the right inferior frontal gyrus: inhibition and attentional control. NeuroImage. 2010;50(3):1313–1319. doi: 10.1016/j.neuroimage.2009.12.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampshire A., Sharp D.J. Contrasting network and modular perspectives on inhibitory control. Trends Cogn. Sci. 2015;19(8):445–452. doi: 10.1016/j.tics.2015.06.006. [DOI] [PubMed] [Google Scholar]
- Lehéricy S., Bardinet E., Tremblay L., Van De Moortele P.F., Pochon J.B., Dormont D., Kim D.S., Yelnik J., Ugurbil K. Motor control in basal ganglia circuits using fMRI and brain atlas approaches. Cereb. Cortex. 2006;16:149–161. doi: 10.1093/cercor/bhi089. [DOI] [PubMed] [Google Scholar]
- Liesefeld H.R., Fu X., Zimmer H.D. Fast and careless or careful and slow? Apparent holistic processing in mental rotation is explained by speed-accuracy trade-offs. J. Exp. Psychol. Learn. Mem. Cogn. 2015;41:1140–1151. doi: 10.1037/xlm0000081. [DOI] [PubMed] [Google Scholar]
- Liesefeld H.R., Janczyk M. Combining speed and accuracy to control for speed-accuracy trade-offs(?) Behav. Res. Methods. 2019;51(1):40–60. doi: 10.3758/s13428-018-1076-x. [DOI] [PubMed] [Google Scholar]
- Litvan I., Goldman J.G., Tröster A.I., Schmand B.A., Weintraub D., Petersen R.C., Mollenhauer B., Adler C.H., Marder K., Williams-Gray C.H., Aarsland D., Kulisevsky J., Rodriguez-Oroz M.C., Burn D.J., Barker R.A., Emre M. Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: Movement Disorder Society Task Force guidelines. Mov. Disord. 2012;27(3):349–356. doi: 10.1002/mds.24893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan G.D., Cowan W.B., Davis K.A. On the ability to inhibit simple and choice reaction time responses: A model and a method. J. Exp. Psychol. Hum. Percept. Perform. 1984;10:276–291. doi: 10.1037/0096-1523.10.2.276. [DOI] [PubMed] [Google Scholar]
- Martini A., Ellis S.J., Grange J.A., Tamburin S., Dal Lago D., Vianello G., Edelstyn N.M.J. Risky decision-making and affective features of impulse control disorders in Parkinson’s disease. J. Neural Transm. 2018;125(2):131–143. doi: 10.1007/s00702-017-1807-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meffert H., Hwang S., Nolan Z.T., Chen G., Blair J.R. Segregating attention from response control when performing a motor inhibition task. Segregating attention from response control. NeuroImage. 2016;126:27–38. doi: 10.1016/j.neuroimage.2015.11.029. [DOI] [PubMed] [Google Scholar]
- Meyer, G.M., Spay, C., Beliakova, A., Gaugain, G., Pezzoli, G., Cilia, R., 2020. Inhibitory control dysfunction in parkinsonian impulse control disorders. Brain 1–14. https://doi.org/10.1093/brain/awaa318. [DOI] [PubMed]
- Milham M.P., Banich M.T., Webb A., Barad V., Cohen N.J., Wszalek T., Kramer A.F. The relative involvement of anterior cingulate and prefrontal cortex in attentional control depends on nature of conflict. Cogn. Brain Res. 2001;12(3):467–473. doi: 10.1016/S0926-6410(01)00076-3. [DOI] [PubMed] [Google Scholar]
- Mirabella G., Fragola M., Giannini G., Modugno N., Lakens D. Inhibitory control is not lateralized in Parkinson’s patients. Neuropsychologia. 2017;102:177–189. doi: 10.1016/j.neuropsychologia.2017.06.025. [DOI] [PubMed] [Google Scholar]
- Mosley P.E., Paliwal S., Robinson K., Coyne T., Silburn P., Tittgemeyer M., Stephan K.E., Perry A., Breakspear M. The structural connectivity of subthalamic deep brain stimulation correlates with impulsivity in Parkinson’s disease. Brain. 2020;143:2235–2254. doi: 10.1093/brain/awaa148. [DOI] [PubMed] [Google Scholar]
- Mueller V.A., Brass M., Waszak F., Prinz W. The role of the preSMA and the rostral cingulate zone in internally selected actions. NeuroImage. 2007;37(4):1354–1361. doi: 10.1016/j.neuroimage.2007.06.018. [DOI] [PubMed] [Google Scholar]
- Obeso I., Cho S.S., Antonelli F., Houle S., Jahanshahi M., Ko J.H., Strafella A.P. Stimulation of the pre-SMA influences cerebral blood flow in frontal areas involved with inhibitory control of action. Brain Stimulation. 2013;6(5):769–776. doi: 10.1016/j.brs.2013.02.002. [DOI] [PubMed] [Google Scholar]
- Obeso I., Robles N., Marrón E.M., Redolar-Ripoll D. Dissociating the role of the pre-SMA in response inhibition and switching: A combined online and offline TMS approach. Front. Hum. Neurosci. 2013;7:1–9. doi: 10.3389/fnhum.2013.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeso I., Wilkinson L., Casabona E., Speekenbrink M., Luisa Bringas M., Álvarez M., Álvarez L., Pavón N., Rodríguez-Oroz M.C., Macías R., Obeso J.A., Jahanshahi M. The subthalamic nucleus and inhibitory control: impact of subthalamotomy in Parkinson’s disease. Brain. 2014;137:1470–1480. doi: 10.1093/brain/awu058. [DOI] [PubMed] [Google Scholar]
- Obeso I., Wilkinson L., Jahanshahi M. Levodopa medication does not influence motor inhibition or conflict resolution in a conditional stop-signal task in Parkinson’s disease. Exp. Brain Res. 2011;213(4):435–445. doi: 10.1007/s00221-011-2793-x. [DOI] [PubMed] [Google Scholar]
- Padmala S., Pessoa L. Interactions between cognition and motivation during response inhibition. Neuropsychologia. 2010;48(2):558–565. doi: 10.1016/j.neuropsychologia.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz‐Alonso P.M., Navalpotro‐Gomez I., Boddy P., Dacosta‐Aguayo R., Delgado‐Alvarado M., Quiroga‐Varela A., Jimenez‐Urbieta H., Carreiras M., Rodriguez‐Oroz M.C. Functional inhibitory control dynamics in impulse control disorders in Parkinson’s disease. Mov. Disord. 2020;35(2):316–325. doi: 10.1002/mds.27885. [DOI] [PubMed] [Google Scholar]
- Pobric, G., Hamilton, A.F. d. C., 2006. Action understanding requires the left inferior frontal cortex. Curr. Biol. 16, 524–529. https://doi.org/10.1016/j.cub.2006.01.033. [DOI] [PubMed]
- Sharma A., Goyal V., Behari M., Srivastva A., Shukla G., Vibha D. Impulse control disorders and related behaviours (ICD-RBs) in Parkinson’s disease patients: Assessment using “questionnaire for impulsive-compulsive disorders in Parkinson’s disease” (QUIP) Ann. Indian Acad. Neurol. 2015;18:49–59. doi: 10.4103/0972-2327.144311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starcke K., Antons S., Trotzke P., Brand M. Cue-reactivity in behavioral addictions: A meta-analysis and methodological considerations. J. Behav. Addict. 2018;7(2):227–238. doi: 10.1556/2006.7.2018.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swick D., Ashley V., Turken A.U. Left inferior frontal gyrus is critical for response inhibition. BMC Neurosci. 2008;9:1–11. doi: 10.1186/1471-2202-9-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swick D., Ashley V., Turken U. Are the neural correlates of stopping and not going identical? Quantitative meta-analysis of two response inhibition tasks. NeuroImage. 2011;56(3):1655–1665. doi: 10.1016/j.neuroimage.2011.02.070. [DOI] [PubMed] [Google Scholar]
- Swick D., Chatham C.H. Ten years of inhibition revisited. Front. Hum. Neurosci. 2014;8:115–116. doi: 10.3389/fnhum.2014.00329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanji J. The supplementary motor area in the cerebral cortex. Neurosci. Res. 1994;19(3):251–268. doi: 10.1016/0168-0102(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Vossel S., Geng J.J., Fink G.R. Dorsal and ventral attention systems: Distinct neural circuits but collaborative roles. Neuroscientist. 2014;20(2):150–159. doi: 10.1177/1073858413494269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vriend C., Gerrits N.J.H.M., Berendse H.W., Veltman D.J., van den Heuvel O.A., van der Werf Y.D. Failure of stop and go in de novo Parkinson’s disease—a functional magnetic resonance imaging study. Neurobiol. Aging. 2015;36(1):470–475. doi: 10.1016/j.neurobiolaging.2014.07.031. [DOI] [PubMed] [Google Scholar]
- Weintraub D., Hoops S., Shea J.A., Lyons K.E., Pahwa R., Driver-Dunckley E.D., Adler C.H., Potenza M.N., Miyasaki J., Siderowf A.D., Duda J.E., Hurtig H.I., Colcher A., Horn S.S., Stern M.B., Voon V. Validation of the questionnaire for impulsive-compulsive disorders in Parkinson’s disease. Mov. Disord. 2009;24(10):1461–1467. doi: 10.1002/mds.22571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub D., Koester J., Potenza M.N., Siderowf A.D., Stacy M., Voon V., Whetteckey J., Wunderlich G.R., Lang A.E. Impulse control disorders in Parkinson Disease: a cross-sectional study of 3090 patients. Arch. Neurol. 2010;67:589–595. doi: 10.1001/archneurol.2010.65. [DOI] [PubMed] [Google Scholar]
- Weintraub, D., Mamikonyan, E., Papay, K., Shea, J.A., Xie, S.X., Siderowf, A., 2012. Questionnaire for impulsive-compulsive disorders in Parkinson’s Disease-Rating Scale. Mov. Disord. 27, 242–247. https://doi.org/10.1002/mds.24023. [DOI] [PMC free article] [PubMed]
- Xia M., Wang J., He Y., Csermely P. BrainNet Viewer: A network visualization tool for human brain connectomics. PLoS ONE. 2013;8(7):e68910. doi: 10.1371/journal.pone.0068910. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table 1 Neuropsychological results of the sample. P-values represent FDR-adjusted p-values. Table 2 Response times – Conditional Stop Signal Task.