Highlights
-
•
Chemotherapy should be suggested to patients with high-risk characteristics.
-
•
Chemotherapy should be discussed for tumor downsizing before excisional surgery.
-
•
Further studies are needed to identify potential predictive biomarkers in PO patients.
Abbreviations: PO, periosteal osteosarcoma; OST, osteosarcoma
Keywords: Osteosarcoma, Periosteal osteosarcoma, Chemotherapy, Sarcoma, Bone tumors
Abstract
Periosteal osteosarcoma (PO), an intermediate-grade chondroblastic osteosarcoma (OST) arising from the surface of the bones, is a rare histological subtype among primary bone sarcomas, most commonly diagnosed in young patients. It is characterized by distinct specific radiological and pathological features. The current management strategy is based on several case reports and series, without any solid international recommendations. Most sarcoma experts agree on the crucial role of an optimal complete surgical approach. However, with the paucity of available reports, the role of systemic treatment and its timing remains debatable. With this paper, we will review the available data on the actual impact of chemotherapy in PO patients with emphasis on the radiological, pathological, and therapeutic characteristics of this rare entity.
1. Introduction
Osteosarcomas (OSTs) are the most common primary malignant tumors of the bones with an overall incidence of approximately 0.2–3 per 100 000 cases, yearly [1], [2]. This group of malignant tumors, with aggressive local behavior and an increased tendency for distant metastasis, are most commonly found in the young population [3]. They share common histological features of high osteoid formation with the presence of malignant mesenchymal cells [4]. Although the conventional subgroup is the most common among the high-grade OSTs, there are other diverse subgroups, including the osteoblastic, chondroblastic, and fibroblastic subgroups [5].
On the other hand, surface OSTs (also commonly called “juxtacortical osteosarcomas”) represent 4%–10% of all the diagnosed OST cases. Based on their location, radiological aspects, and histopathological features, surface OSTs can be subclassified as parosteal, periosteal, and high-grade surface OSTs [6], [7], [8], [9], [10]. Although parosteal OSTs are treated with surgical excision only, patients with high-grade surface OSTs may be managed with additional treatments, including chemotherapy and/or radiation therapy [11], [12], [13]. The treatment strategy for periosteal osteosarcomas (POs) usually includes a complete surgical removal with clean margins; however, the role of systemic therapy remains debatable [13], [14].
Although POs were first identified as distinct entities in 1939 by Ewing and in 1959 by Lichtenstein, their detailed pathological characteristics and oncological outcomes were reported initially by Unni et al in 1976 [15], [16], [17]. PO is a rare variant that represents approximately 1% of all of the diagnosed OSTs and approximately 25% of juxtacortical OSTs [18]. PO, an intermediate-grade chondroblastic OST arising from the surface of the bones, is most commonly diagnosed among young patients in their second and third decades of life with a median age of 18 years [12], [19], [20]. In contrast to conventional OSTs where only 5%–10% of cases occur in the diaphyseal region, it usually affects the periosteum of long bones of the femur and tibia [15], [16], [21], [22]. Distant metastases occur more commonly in the lungs and pleura; however, isolated cases of bone skip lesions and diaphragmatic metastases have also been reported [23], [24]. Even though they have distinct specific radiological and pathological features, the current management strategies are based on only several case reports and series, without any solid recommendations [25], [26], [27], [28]. Nonetheless, most experts agree on the crucial role of an optimal complete surgical approach. With the paucity of available reports, the role of chemotherapy and its timing remain debatable. In this review paper, we aimed to review the available studies on the role of chemotherapy in POs focusing on potential predictive biomarkers.
2. Discussion
2.1. Diagnostic features
2.1.1. Pathology
Sarcomas arising adjacent to the outer surface of cortical bone are composed mainly of periosteal chondrosarcomas and surface OSTs. POs as a subclass of surface OSTs are mostly located in the femur or the tibia, but any bone in the body can be affected, including both flat and long bones [29], [30], [31], [32], [33], [34], [35], [36], [37], [38]. PO is a predominantly chondroblastic, bone-forming malignant neoplasm, which arises mainly underneath the periosteum. The tumor appears grossly as a well-demarcated lobulated broad-based mass, attached to the surface of the cortex, with a cartilaginous cut surface [39]. The incidence of medullary involvement, a common feature of high-grade surface OSTs, varied among the different reports of POs. In many reports, medullary involvement was described as rare; however, Cesari et al. reported this type of involvement in almost 70% of cases [14]. It is not yet clear whether the presence of intra-medullary involvement conferred a poorer prognosis. However, in some reports, intra-medullary involvement seemed to predict a more aggressive clinical course with the propensity to develop distant metastases [14].
Histopathology has indicated that PO is composed of variably sized lobules of well-differentiated and cytologically atypical hyaline cartilage with intervening bands of bone-forming malignant mesenchymal cells in which fibroblastic appearing areas and undifferentiated areas may be seen [40]. An accurate diagnosis is necessary for adequate treatment with surface OSTs being divided into three different categories: Parosteal OST (low grade), PO (intermediate grade) and high-grade surface OST [6], [7], [8], [9], [10]. On one hand, parosteal OST carries the best prognosis, and on the other hand, a PO has a lower propensity for metastasis in comparison to a high-grade surface OST and a conventional OST. Microscopic examinations have indicated that parosteal OST consists of well-formed bone trabeculae with fibroblastic backgrounds where cartilaginous differentiation has been described in only half of the cases. In contrast to PO, parosteal OST display cartilaginous differentiation as cartilaginous nodules or in the form of a cartilaginous cap. Histologically, high-grade surface OSTs are identical to conventional OSTs and display high-grade anaplastic features [40]. There are no reliable immunohistochemical features that allow surface chondroblastic OSTs to be distinguished from chondrosarcomas or surface OSTs from conventional OSTs [41]. Also, no amplification of mouse double minute 2 oncogene (MDM2) at the RNA or protein levels (commonly found in low-grade and parosteal OST) or point mutations in TP53 (20% of conventional OST), have been described in PO [42]. Fig. 1 demonstrates the pathological findings of PO.
Fig. 1.
Microscopic findings in periosteal osteosarcoma showing (A) nodules with predominant neoplastic hyaline cartilage delineated by a layer of fibrous tissue (hematoxylin and eosin stain – H&E, ×4 magnification). (B) Neoplastic cartilage is hyaline and hypercellular (asterisk) with tumor bone having a coarse and lace-like appearance (arrow) (H&E, ×10 magnification). (C) Irregular purple tumor bone merges with cartilage (H&E, ×20 magnification). (D) Proliferating malignant cells with Moderate to severe cytologic atypia are seen (arrowhead) (H&E, ×20 magnification). (E) Hypercellular areas are composed of atypical tumor cells that contain hyperchromatic nuclei. Tumor cells are in intimate contact with unmineralized lace-like tumor bone (H&E, ×40 magnification). (F) short fascicles of malignant spindle cells may be seen (H&E, ×40 magnification). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
2.1.2. Radiology
Despite the lack of population studies, the radiological characteristics of POs constitute an essential pillar in their diagnosis, in addition to their specific pathologic features, [43]. In the largest series on POs, Murphy et al. reported the association between the pathological and radiological features of 40 patients with PO [8].
In plain radiographs, the main characteristic appearance of a PO is a broad-based surface soft tissue mass attached to the bone cortex, causing cortical thickening and/or erosion/scalloping with a perpendicular periosteal reaction extending to the soft-tissue component. Furthermore, additional areas of mineralization were also identified in almost 68% of the PO cases. These characteristics were also identified on CT (computed tomography)-scan and MRI (magnetic resonance imaging), but plain radiographs were more specific for these features. Additionally, the soft-tissue mass component was identified in almost all the cases of PO that surrounded a median of 50%–55% of the bone cortex. From bone scintigraphy, the tumor demonstrated increased eccentric uptake in all the patients with PO, with a homogenous radionuclide activity in almost 70% of patients. In comparison to a PO, a parosteal OS is usually without a perpendicular periosteal reaction affecting the metaphysis, whereas a high-grade OST affects a larger circumference of the bones with a medullary invasion, but without the high-water content soft tissue mass [8].
Conversely, the available CT-scan images of the majority of cases showed a low attenuation of the non-mineralized component of the soft tissue mass in comparison with muscles with well-defined lesion margins as well as the absence of pseudo capsules. Additionally, there were increased areas of calcifications within the soft-tissue mass component. The MRI showed a high signal heterogeneous intensity on the T2-weighted images with a predominant similar signal intensity for the muscle on the T1-weighted images. Focal areas of marrow replacement without continuity with the soft-mass component were identified; these were mostly reactive, which is essential for the determination of the exact area of resection. Also, the lesion margins were predominantly well-defined without pseudo-capsules. These are most likely related to the high water content within the chondroblastic lesions of the POs [8]. Fig. 1 shows the radiological aspect of PO.
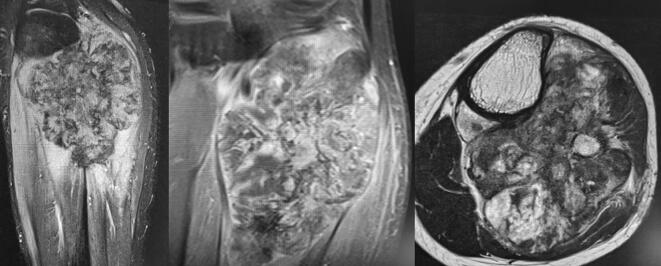
Fig. 2.
MRI Findings of periosteal osteosarcoma: a high signal of heterogeneous intensity with a mass of the posterior fossa of the leg in contact with the posterior and medial cortical bone of the proximal metaphysis of the tibia; hypersignal T2-weighted images with a predominant similar signal intensity for the muscle on the T1-weighted images and heterogeneous enhancement. Focal areas of marrow replacement without continuity with the soft-mass component and the lesion margins are poly-lobulated.
2.2. Therapeutic management
2.2.1. The general approach for the treatment of OST
The current approach for the management of patients with localized OSTs relies on a multimodal approach with peri-operative systemic chemotherapy, which has drastically changed the prognosis of OST patients [3]. Although the incorporation of neoadjuvant or induction chemotherapy in the treatment sequence has largely improved the functional outcomes of patients (limb-salvage approach and decreased rate of amputations), it has also decreased the risk of developing distant metastases [44], [45], [46]. Also, the multidisciplinary surgical approach (plastic, vascular and orthopedic surgery) has drastically changed the functional outcomes after optimal surgery in PO. The achievement of a high pathological response after neoadjuvant chemotherapy (>90% of tumor necrosis) was associated with improved oncological outcomes and prolonged survival [47], [48]. The current standard of care is to administer different combinations of chemotherapy, including doxorubicin, cisplatin, high dose methotrexate, ifosfamide, and etoposide in both neoadjuvant and adjuvant settings [2], [3]. These different approaches have achieved a substantial improvement in outcomes (recurrence-free survival and overall survival) and quality of life [3], [49]; however, it is still not yet clear which chemotherapy protocol is optimal. Some oncologists have tended to omit high-dose methotrexate in the adult population and have limited its use to younger patients (<18 years), whereas others have opted to modify the adjuvant chemotherapeutic regimen based on the histological responses to neoadjuvant chemotherapy (>or < 90% of tumor necrosis) [49]. In patients with relapsed disease, data are sparse on the use of the different active targeted therapies, mainly anti-angiogenic agents, including regorafenib, cabozantinib, and sorafenib as well as different chemotherapeutic combinations [50], [51], [52]. By extrapolation from the available data on the treatment of conventional OSTs, the same chemotherapeutic protocols and approaches are currently applied to patients with PO.
2.2.2. Available data on the management of PO
With the lack of strict guidelines and recommendations on the management of POs, the mainstay of therapy depends on complete surgical excisions with clear margins; however, controversy remains regarding the role of chemotherapy in this subset of patients [13], [18], [53]. Different surgical approaches were evaluated in POs to preserve limb function and maintain a good quality of life. The diaphyseal location of these tumors facilitated a limb-salvage approach surgery without affecting the joint surface [8]. The current understanding of the pathophysiology of the PO has led to major modifications in the surgical approach with a significant decrease in amputation rates; currently limited to patients in which resection en bloc was not feasible.
Wide surgical excision with clear margins is the currently accepted approach for patients with PO, more particularly with advances in reconstructive surgery leading to limb and function preservation. The role of systemic chemotherapy in localized POs is not yet clear as there are discordant results in the literature. Different chemotherapeutic protocols were used and reported in few case reports and small case series without any clear indications, primary endpoints, and outcomes. Studies on POs and the use of systemic chemotherapy are summarized in Table 1.
Table 1.
Patients characteristics.
| Author | Year | N | Study design | Grade | Grade (High) | MI (%) | CT (%) | LOE |
|---|---|---|---|---|---|---|---|---|
| Unni et al. | 1976 | 23 | Retrospective | High - intermediate | NA | NA | 0 | VI |
| Bertoni et al. | 1982 | 20 | Retrospective | All | NA | NA | 2 (10%) | VI |
| Hall et al. | 1985 | 6 | Retrospective | NA | NA | 3 (50%) | 3 (50%) | VI |
| Ritts et al. | 1985 | 22 | Retrospective | NA | NA | 0 | 2 (9.1%) | VI |
| Revell et al. | 2002 | 17 | Retrospective | High - intermediate | NA | 4 (24%) | 14 (82%) | VI |
| Grimer et al. | 2005 | 119 | Retrospective | All | 44 (86%) | NA | 81 (69%) | VI |
| Rose et al. | 2006 | 29 | Retrospective | High - intermediate | 18 (62%) | NA | 9 (35%) | VI |
| Cesari et al. | 2011 | 33 | Retrospective | All | 14 (42%) | 16 (69%) | 14 (42%) | VI |
| Giulia et al. | 2014 | 18 | Retrospective | NA | NA | 7 (44%) | 16 (89%) | VI |
| Chan et al. | 2018 | 18 | Retrospective | All | 12 (66.6%) | 10 (50%) | 11 (61%) | VI |
Abbreviations: NA: Not assessed; LOE: LevelOf Evidence; MI: medullary involvement; CT: chemotherapy
2.2.3. Management without chemotherapy
In one of the first available studies on PO, Unni et al. reported the outcomes of 23 patients with PO; however, the administration of chemotherapy was not part of the treatment strategy, despite all patients exhibiting high-grade features. Of these 23 patients, 13 were managed with amputations (three died from distant metastases), whereas the remaining had resections or excisions of their primary tumors. Overall, seven patients had a local recurrence (30%), four had confirmed distant metastases (17%), and the overall survival was 65% [16] (Table 2).
Table 2.
Chemotherapy outcomes in PO patients.
| Author | Year | N | Chemotherapy protocol | Indication | NeoA (%) | N > 90% (%) | LR | DM | OS | Second malignancy |
|---|---|---|---|---|---|---|---|---|---|---|
| Unni et al. (1976) | 1976 | 23 | NA | NA | NA | NA | 7 (30%) | 4 (17%) | 65% | 2 (AML and LPS) |
| Bertoni et al. (1982) | 1982 | 20 | NA | NA | 0 | NA | 8 (40%) | 3 (15%) | 10y OS = 85% | |
| Hall et al. (1985) | 1985 | 6 | Adrimaycine / methotrextate + vincristine | NA | 0 | NA | 1 (17%) | 0 | 84% | |
| Ritts et al. (1985) | 1985 | 22 | NA | NA | 0 | NA | 3 (13.6%) | 3 (13.6%) | 10y OS = 71.3% | 2 (AML and LPS) |
| Revell et al. (2002) | 2002 | 17 | Doxorubicin + Cisplatin | High grade or MI | 10 (59%) | 2 (20%) | 1 (6%) | 0 | 88% at last FU | 2 (AML/brain) |
| Grimer et al. (2005) | 2005 | 119 | Doxorubicin-based | NA | 50 (42%) | 10 (32%) | 8 (6.7%) | 17 (14%) | 10y OS = 83% | 3 (AML/colon cancer/ Brain tumor) |
| Rose et al. (2006) | 2006 | 29 | MAP + I (Various) | All patients | 2 (7%) | 0 (2 pts) | NA | NA | NA | |
| Cesari et al. (2011) | 2011 | 33 | MAP + I (Various) | Grade 3 | 5 (15%) | 3 (75%) | 7 (21%) | 3 (9%) | 10y OS = 84% | 3 (AML, breast cancer, MCS) |
| Giulia et al. (2014) | 2014 | 18 | NA | All patients | 16 (89%) | 4 (40%) | 2 (11%) | 4 (22%) | 5y OS = 83.3% | |
| Chan et al. (2018) | 2018 | 18 | NA | HIgh grade | 5 (28%) | 3 (75%) | 1 (5.3%) | 2 (10.5%) | 10y OS = 77% | 1 (MCS) |
OS: Overall survival; DM: Distant metastasis; FU:Follow-up; MI:medulary involvement; LR: local relapse; NeoA (%): neoadjuvant approach; MCS: Mesenchymal Chondrosarcoma; LPS: liposarcoma; AML: acute myeloid leukemia; N: necrosis
Additional data on 20 patients by Bertoni et al. demonstrated a high rate of local recurrence in seven patients (35%) with a further three patients (15%) dying from lung metastases within 1 year of diagnosis. Seven patients underwent amputations, including those with recurrent disease, whereas seven out of eight patients with marginal excisions had local recurrences of their diseases. Nevertheless, chemotherapy was only administered in two patients (10%) and the 10-year overall survival was 85% [54].
Further data on 22 patients showed a 10-year overall survival of 71.3%; however, chemotherapy had not become a part of the therapeutic regimen as only two patients received chemotherapy for their metastatic disease [20]. Surgical management included 15 amputations and two patients with local surgical therapy had local recurrence.
Another small series by Hall et al. had six patients who were treated successfully with surgery with no distant metastases observed at a longer follow-up and only one local relapse. Interestingly, the authors did not recommend adjuvant chemotherapy after surgery even though three patients (50%) had received chemotherapy (two had systemic therapy [one with adriamycin and the other a combination of methotrexate and vincristine for microscopic intramedullary involvement] and one with a superficial femoral artery administration of adriamycin) [55].
In these case series, amputation was performed on the majority of PO patients, whereas local surgery (excision or wide resection) was associated with a non-negligible rate of local recurrence, thus affecting the functional outcomes of the limbs. Systemic therapy was not considered as a standard approach either for a higher chance of limb-preserving surgery or for improving survival outcomes. Nevertheless, these data are based on old data when access to optimal radiological evaluation was limited, thus largely affecting the functional and surgical outcomes.
2.2.4. Management with chemotherapy
With the rare occurrence of POs, few case reports and series were in favor of using chemotherapy to improve the functional outcomes and to delay local recurrences of the disease [32], [33], [53], [56], [57], [58]. In the case series by Revell et al, 17 patients with localized POs were included in the analysis. Systemic chemotherapy was offered to all the patients with high-grade features (high-grade and medullary involvement). All the patients had their tumors removed surgically, whereas 14 patients (82%) received chemotherapy with doxorubicin and cisplatin, similar to conventional OST; of these, ten patients (59%) received systemic therapy preoperatively. Overall, none developed distant metastases, two patients were considered good responders (>90% of necrosis) and there were no tumor-related deaths. Only one patient exhibited a local recurrence after adjuvant therapy alone and that patient opted for an elective amputation. The authors attributed their outcomes to the radical excisions of the tumors, which reduced local recurrences, and second, to the use of systemic therapy. They also concluded that it would be prudent to treat patients with high-grade tumors and medullary involvement with a neoadjuvant approach [53].
Moreover, with the paucity of available data on neoadjuvant chemotherapy, individual cases treated successfully with preoperative therapy followed by local surgery might point to the important role of chemotherapy in Pos, despite their limitations. For instance, two siblings with the Li-Fraumeni syndrome and a confirmed diagnosis of PO (including a multifocal PO) were successfully treated with neoadjuvant chemotherapy (MAP + ifosfamide and etoposide) (60%–70% necrosis) followed by surgery and adjuvant chemotherapy with no evidence of disease after a long follow up [59]. An interesting approach was the use of Bone SPECT/CT (single-photon emission computed tomography) to evaluate its role as a potential tool in the detection of the response to neoadjuvant chemotherapy (two cycles of MAP) in a young patient [60]. Moreover, two young PO patients underwent successful tibial marginal resections of two tibias after neoadjuvant chemotherapy using two different protocols. In the first patient, two preoperative 2-week cycles with ifosfamide (2 g/m2, high dose methotrexate [8–12 g/m2] and doxorubicin [30 mg/m2], then six adjuvant 2-week cycles of the same regimen was used), whereas in the second patient, cisplatin (120 mg/m2) was administered instead of methotrexate) [61].
Notwithstanding these findings, and despite some evidence of activity, mostly in the preoperative setting, several case series concluded against a solid role for chemotherapy in POs, partly because of their chondroblastic pathological feature which might confer greater resistance to chemotherapy [61]. A collaborative report by the EMSOS (European Musculo-Skeletal Oncology Society) discussed the outcomes of 119 patients with a median age of 18 years diagnosed with POs. Almost all the patients had successful limb-sparing surgery, whereas only nine patients had amputations with a 10-year overall survival of 83%. Local relapse was the only factor related to reduced survival (eight patients [7%] had a local relapse), but not age, size of the tumor, chemotherapy administration, degree of necrosis, or inadequate margins. Seventeen patients developed distant metastases with lungs being the most common site of the disease (14%). Concerning systemic chemotherapy, 81 patients received doxorubicin-based systemic therapy, among which 50 patients received it in the neoadjuvant setting. Among the 38 patients with documented necrosis, 32% were found to be good responders (necrosis > 90%) with no significant differences among the different chemotherapeutic protocols (a combination of doxorubicin, cisplatin, ifosfamide, and high-dose methotrexate). There was no local recurrence occurring in those with very good histological responses. Since the administration of chemotherapy in POs was not a prognostic factor and did not affect survival in this study, the authors did not recommend the systematic use of chemotherapy in this rare group of patients. However, it is important to note that not only were the patients who received chemotherapy younger (mean age 19.4 vs. 28.6 years; P = 0.003) but that different selection criteria for the chemotherapy as well as different chemotherapeutic regimens were used between the different centers affecting the generalization of the results. Another limitation for this analysis was that a central review was not properly conducted and few cases of parosteal OS or high-grade OS may have been included [13].
Rose et al. evaluated the long-term outcomes of 29 patients with POs in an attempt to overcome the lack of longer follows up within other studies. Importantly, local or distant relapses and death occurred in the first 3 years following the initial diagnosis, and the disease-free survival (DFS) after 15.8 years of follow-up was 83%. There were no differences in the local relapse in terms of anatomic locations (proximal or distal), tumor grades, or types of surgery (amputation or limb salvage surgery). Due to the long duration of inclusion, only nine patients (35%) were offered chemotherapy, among which only two received neoadjuvant therapy since they amended their protocols to include neoadjuvant chemotherapy in all PO patients in the 1990 s. In those who received preoperative therapy, tumor necrosis was poor (10%–20%); however, the small sample size, non-standardized chemotherapeutic protocols, and the long period of follow-up precluded any meaningful conclusion, regardless of the efficacy of systemic chemotherapy.
In another report by Giulia et al., 18 patients with localized POs were treated in a systematically similar manner to high-grade OSTs with neoadjuvant chemotherapy followed by surgery and adjuvant chemotherapy. All the patients had undergone limb-preserving surgery, except for the one patient who had an amputation. Overall, four out of ten patients exhibited good pathological responses after neoadjuvant chemotherapy (40%) and the five-year overall survival was 83.3%. Two patients had local relapses (11%), whereas four patients had distant metastases (22%). In those with distant metastasis, two were good responders to chemotherapy (99% of necrosis). There was a trend toward poor survival at 5 years in those with a medullary involvement (90% vs. 75%, P = 0.32); however, there was no impact on survival between the good and poor responders to neoadjuvant chemotherapy [62].
In addition, Cesari et al. reported that 33 patients with localized POs were evaluated, and among these 14 pts (42%) received chemotherapy for grade 3 tumors. There was no difference in the OS of those who received chemotherapy versus chemotherapy-free patients (86% vs. 83%; P = 0.73). Only four patients received neoadjuvant chemotherapy (with various protocols) with their tumor necrosis ranging from 70% to 95%. Three of these patients had no evidence of disease, at the last follow-up. The medullary extension was an important prognostic factor with a 10-year DFS of 61% versus 86% when confirmed. The ten-year DFS was 65% of all the recurrent diseases in the first 3 years from diagnosis. Despite different chemotherapeutic protocols, a small sample size, and the low rate of neoadjuvant therapy, the authors concluded that chemotherapy should not be administered in this population [14].
Recently, Chan et al using 18 patients with PO, demonstrated a 10-year overall survival of 77.1% and a 10-year recurrence-free survival of 66.4%. The indication for chemotherapy in this population included high-grade features (12 patients, 66%) and it was offered to 11 patients (61.1%). No prognostic factors were identified, including the use of chemotherapy; nevertheless, there was no difference in outcomes between those who received chemotherapy and those who did not. Neoadjuvant chemotherapy in five patients demonstrated a potential sensitivity to chemotherapy (three patients [75%] were good responders), in particular in those with high-grade tumors [24].
Building evidence for rare diseases such as PO constitutes a true challenge in the medical community. The design of dedicated clinical trials is often complicated by the low number of patients but also by financial issues at the pharmaceutical level or policymakers. Several strategies have been suggested to overcome this hurdle such as the use of systematic observation forms gathered from experts or ad hoc qualitative date, or registry or indirect data through extrapolation from common diseases [63]. Current guidelines and recommendations do not seem to share common ground about the role of chemotherapy in POs. In the ESMO–PaedCan–EURACAN Clinical Practice Guidelines on bone sarcomas, chemotherapy for surface OSTs, including parosteal and periosteal OSTs, with its lack of benefit in this subset of patients, is not recommended and should not be routinely used [2]. However, in the National Comprehensive Cancer Network guidelines, chemotherapy is considered in the neoadjuvant setting of POs followed by postoperative adjuvant systemic therapy in case of high-grade tumors with the same chemotherapy approach in high-grade OSTs [64].
3. Conclusions
In summary, the management of rare malignancies constitutes a true challenge, mainly because of the complexity of conducting randomized controlled trials to draw solid conclusions and recommendations [65]. Reporting individual experiences from large centers is mandatory to support the role of systemic therapy in POs. The earlier series reported a high incidence of radical surgery with amputations and a higher risk of local recurrences, thus highlighting a potential role for systemic therapy to improve functional outcomes, mostly in the neoadjuvant setting [16], [54]. The authors have also expressed their concerns regarding the risk of secondary malignancies; nevertheless, causality cannot be confirmed with essentially the time bias related to the occurrence of POs at a younger age as well as the incidence of secondary malignancies in the pre-chemotherapy era [14], [16], [20].
Available data are in favor of administering chemotherapy only to patients with high-risk characteristics, including high grade and medullary involvement thus excluding those with low-grade tumors. Also, chemotherapy plays a major role in high-risk patients in need of tumor downsizing before excisional surgery, except those with low-grade, chemo-resistant tumors. Encouraging data in this regard has been reported in the preoperative setting. There is an unmet need for better identification of potentially predictive biomarkers in PO patients who may benefit from systemic chemotherapy to improve functional and survival outcomes.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
None.
Funding source
None.
Conflicts of interest statement
Dr. Mir has received consultancy fees from AstraZeneca, Blueprint Medicines, Bristol-Myers Squibb, Eli Lilly, Ipsen, Merck Sharpe & Dohme, Pfizer, Roche, Servier, and Vifor Pharma. He is a shareholder of Amplitude Surgical, Ipsen, and Transgene.
Dr. Le Cesne has received consultancy fees from Amgen, Bayer, Eli-Lilly, Novartis, Pfizer, and PharmaMar.
Professor Court has received consultancy fees from Medtronics and Safeorthopaedic. He is a shareholder of NeuroFrance and SpineGuard.
Other authors have no conflict of interest to disclose.
References
- 1.Mirabello L., Troisi R.J., Savage S.A. Osteosarcoma incidence and survival rates from 1973 to 2004: data from the Surveillance, Epidemiology, and End Results Program. Cancer Interdiscip Int J Am Cancer Soc. 2009;115(7):1531–1543. doi: 10.1002/cncr.24121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Casali P.G., Bielack S., Abecassis N., Aro H.T., Bauer S., Biagini R. Bone sarcomas: ESMO–PaedCan–EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Supplement_4):79–95. doi: 10.1093/annonc/mdy310. [DOI] [PubMed] [Google Scholar]
- 3.Luetke A., Meyers P.A., Lewis I., Juergens H. Osteosarcoma treatment–where do we stand? A state of the art review. Cancer Treat Rev. 2014;40(4):523–532. doi: 10.1016/j.ctrv.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 4.Raymond A.K., Jaffe N. Pediatric and Adolescent Osteosarcoma. Springer; 2009. Osteosarcoma multidisciplinary approach to the management from the pathologist’s perspective; pp. 63–84. [DOI] [PubMed] [Google Scholar]
- 5.Gorlick R. Pediatric and Adolescent Osteosarcoma. Springer; 2009. Current concepts on the molecular biology of osteosarcoma; pp. 467–478. [DOI] [PubMed] [Google Scholar]
- 6.Seeger L.L., Yao L., Eckardt J.J. Surface lesions of bone. Radiology. 1998;206(1):17–33. doi: 10.1148/radiology.206.1.9423647. [DOI] [PubMed] [Google Scholar]
- 7.Murphey M.D., Robbin M.R., McRae G.A., Flemming D.J., Temple H.T., Kransdorf M.J. The many faces of osteosarcoma. Radiographics. 1997;17(5):1205–1231. doi: 10.1148/radiographics.17.5.9308111. [DOI] [PubMed] [Google Scholar]
- 8.Murphey M.D., Jelinek J.S., Temple H.T., Flemming D.J., Gannon F.H. Imaging of periosteal osteosarcoma: radiologic-pathologic comparison. Radiology. 2004;233(1):129–138. doi: 10.1148/radiol.2331030326. [DOI] [PubMed] [Google Scholar]
- 9.Huvos A.G. Osteoid osteoma. Bone Tumors Diagn Treat Progn. 1991;2:49–66. [Google Scholar]
- 10.Mirra J.M. Bone tumors. Clin Radiol Pathol Correl. 1989 [Google Scholar]
- 11.Hang J.-F., Chen P.-C.-H. Parosteal osteosarcoma. Arch Pathol Lab Med. 2014;138(5):694–699. doi: 10.5858/arpa.2013-0030-RS. [DOI] [PubMed] [Google Scholar]
- 12.Okada K., Unni K.K., Swee R.G., Sim F.H. High grade surface osteosarcoma: a clinicopathologic study of 46 cases. Cancer. 1999;85(5):1044–1054. doi: 10.1002/(sici)1097-0142(19990301)85:5<1044::aid-cncr6>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 13.Grimer R.J., Bielack S., Flege S., Cannon S.R., Foleras G., Andreeff I., Sokolov T., Taminiau A., Dominkus M., San-Julian M., Kollender Y., Gosheger G. Periosteal osteosarcoma–a European review of outcome. Eur J Cancer. 2005;41(18):2806–2811. doi: 10.1016/j.ejca.2005.04.052. [DOI] [PubMed] [Google Scholar]
- 14.Cesari M., Alberghini M., Vanel D., Palmerini E., Staals E.L., Longhi A., Abate M., Ferrari C., Balladelli A., Ferrari S. Periosteal osteosarcoma: a single-institution experience. Cancer. 2011;117(8):1731–1735. doi: 10.1002/cncr.25718. [DOI] [PubMed] [Google Scholar]
- 15.Lichtenstein L. Tumors of periosteal origin. Cancer. 1955;8(5):1060–1069. doi: 10.1002/1097-0142(1955)8:5<1060::aid-cncr2820080533>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 16.Unni K.K., Dahlin D.C., Beabout J.W. Periosteal osteogenic sarcoma. Cancer. 1976;37(5):2476–2485. doi: 10.1002/1097-0142(197605)37:5<2476::aid-cncr2820370541>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 17.Ewing W. A review of the classification of bone tumor. Surg Gynecol Obstet. 1939;69:971–976. [Google Scholar]
- 18.Rose P.S., Dickey I.D., Wenger D.E., Unni K.K., Sim F.H. Periosteal osteosarcoma: long-term outcome and risk of late recurrence. Clin Orthop Relat Res. 2006;1976–2007(453):314–317. doi: 10.1097/01.blo.0000229341.18974.95. [DOI] [PubMed] [Google Scholar]
- 19.Liu X.-W., Zi Y., Xiang L.-B., Han T.-Y. Periosteal osteosarcoma: a review of clinical evidence. Int J Clin Exp Med. 2015;8(1):37. [PMC free article] [PubMed] [Google Scholar]
- 20.Ritts G.D., Pritchard D.J., Unni K.K., Beabout J.W., Eckardt J.J. Periosteal osteosarcoma. Clin Orthop. 1987;219:299–307. [PubMed] [Google Scholar]
- 21.Campanacci M., Giunti A. Periosteal osteosarcoma. Review of 41 cases, 22 with long-term follow-up. Ital J Orthop Traumatol. 1976;2(1):23–35. [PubMed] [Google Scholar]
- 22.Campanacci M. Springer; New York: 1999. Bone and Soft Tissue Tumors. Padova: Piccin Nuova Libraria and Wien. [Google Scholar]
- 23.Zhao L., Qin Y., Ma D., Wang W., Li S., Liu H. Isolated diaphragmatic metastasis from periosteal osteosarcoma of the humerus: a case report. J Cardiothorac Surg. 2020;15(1):1–3. doi: 10.1186/s13019-020-01103-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chan C.M., Lindsay A.D., Spiguel A.R.V., Gibbs C.P., Scarborough M.T. Periosteal osteosarcoma: a single-institutional study of factors related to oncologic outcomes. Sarcoma. 2018;2018:1–6. doi: 10.1155/2018/8631237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barakat M.J., Collins C., Dixon J.H. Distal recurrence of periosteal osteosarcoma after complete excision of proximal primary tumour with good excision margins. Sarcoma. 2003;7(2):79–80. doi: 10.1080/13577140310001607310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chano T., Matsumoto K., Ishizawa M., Morimoto S., Hukuda S., Okabe H. Periosteal osteosarcoma and parosteal chondrosarcoma evaluated by double immunohistochemical staining: report of 2 cases. Acta Orthop Scand. 1994;65(3):355–358. doi: 10.3109/17453679408995471. [DOI] [PubMed] [Google Scholar]
- 27.Dash H., Little J.R., Zaino R., Colao D.J., Chaurushiya P., Schoolwerth A.C., O'Neill M.J., Hart J.C., Martin G.B. Metastatic periosteal osteosarcoma causing cardiac and renal failure. Am J Med. 1983;75(1):145–149. doi: 10.1016/0002-9343(83)91178-6. [DOI] [PubMed] [Google Scholar]
- 28.Hoshi M., Matsumoto S., Manabe J., Tanizawa T., Shigemitsu T., Takeuchi K., Kawaguchi N. Three cases with periosteal osteosarcoma arising from the femur. J Orthop Sci. 2006;11(2):224–228. doi: 10.1007/s00776-005-0981-x. [DOI] [PubMed] [Google Scholar]
- 29.Kalil R., Santini E., Kalil R., Bertoni F., Park Y. Springer, London; London: 2015. Tumors and Tumor-Like Lesions of Bone: For Surgical Pathologists, Orthopedic Surgeons and Radiologists [Internet] [Google Scholar]
- 30.Mohammadi A., Porghasem J., Noroozinia F., Ilkhanizadeh B., Ghasemi-rad M., Khenari S. Periosteal osteosarcoma of the fifth metatarsal: a rare pedal tumor. J Foot Ankle Surg. 2011;50(5):620–622. doi: 10.1053/j.jfas.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 31.Patterson LTC.A., Greer R.O., Howard D. Periosteal osteosarcoma of the maxilla: a case report and review of literature. J Oral Maxillofac Surg. 1990;48(5):522–526. doi: 10.1016/0278-2391(90)90246-x. [DOI] [PubMed] [Google Scholar]
- 32.Singh D., Sen R., Chaudhary S., Tripathy S.K. Periosteal osteosarcoma of the calcaneum: a case report. Foot Ankle Spec. 2012;5(2):121–123. doi: 10.1177/1938640011434510. [DOI] [PubMed] [Google Scholar]
- 33.Robertson N.J., Newman P.L. Periosteal osteosarcoma of the cranium. Ann Diagn Pathol. 2000;4(5):300–302. doi: 10.1053/adpa.2000.17887. [DOI] [PubMed] [Google Scholar]
- 34.Wang G.D., Zhao Y.F., Liu Y., Jiang L., Jiang X.Z. Periosteal osteosarcoma of the mandible: case report and review of the literature. J Oral Maxillofac Surg Off J Am Assoc Oral Maxillofac Surg. 2011;69(6):1831–1835. doi: 10.1016/j.joms.2010.07.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Piattelli A., Favia G.F. Periosteal osteosarcoma of the jaws: report of 2 cases. J Periodontol. 2000;71(2):325–329. doi: 10.1902/jop.2000.71.2.325. [DOI] [PubMed] [Google Scholar]
- 36.Yoon J.H., Yook J.I., Kim H.J., Ho Cha I., Kim J. Periosteal osteosarcoma of the mandible. J Oral Maxillofac Surg. 2005;63(5):699–703. doi: 10.1016/j.joms.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 37.Muir T.M., Lehman T.P., Meyer W.H. Periosteal osteosarcoma in the hand of a pediatric patient: a case report. J Hand Surg. 2008;33(2):266–268. doi: 10.1016/j.jhsa.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 38.Minić A.J. Periosteal osteosarcoma of the mandible. Int J Oral Maxillofac Surg. 1995;24(3):226–228. doi: 10.1016/s0901-5027(06)80133-5. [DOI] [PubMed] [Google Scholar]
- 39.WHO Classification of Tumours of Soft Tissue and Bone. Fourth Edition - WHO - OMS - [Internet]. [cited 2018 Oct 14]. Available from: http://apps.who.int/bookorders/WHP/detart1.jsp?sesslan=1&codlan=1&codcol=70&codcch=4005.
- 40.Vigorita V.J. Lippincott Williams & Wilkins; 2008. Orthopaedic pathology. [Google Scholar]
- 41.Franchi A, Ultrastruct Pathol. 1999; 23(4):233-40 - Google Search [Internet]. [cited 2021 Jan 28]. Available from: https://www.google.com/search?safe=off&sxsrf=ALeKk03_PU6umCEk-Jq2tf8X6pP2D0ERIQ%3A1611849058651&ei=Yt0SYLGtJ6qe1fAPy5SvoAY&q=Franchi+A%2C+Ultrastruct+Pathol.++1999%3B+23%284%29%3A233-40+&oq=Franchi+A%2C+Ultrastruct+Pathol.++1999%3B+23%284%29%3A233-40+&gs_lcp=CgZwc3ktYWIQAzIECAAQRzIECAAQRzIECAAQRzIECAAQRzIECAAQRzIECAAQRzIECAAQRzIECAAQR1DkFVjkFWCIF2gAcAJ4AIABAIgBAJIBAJgBAKABAaoBB2d3cy13aXrIAQjAAQE&sclient=psy-ab&ved=0ahUKEwix5snU_b7uAhUqTxUIHUvKC2QQ4dUDCA0&uact=5. [DOI] [PubMed]
- 42.deSantos L.A., Murray J.A., Barnett Finklestein J., Spjut H.J., Ayala A.G. The radiographic spectrum of periosteal osteosarcoma. Radiology. 1978;127(1):123–129. doi: 10.1148/127.1.123. [DOI] [PubMed] [Google Scholar]
- 43.Bielack S.S., Kempf-Bielack B., Delling Günter, Exner G.U., Flege S., Helmke K., Kotz R., Salzer-Kuntschik M., Werner M., Winkelmann W., Zoubek A., Jürgens H., Winkler K. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol. 2002;20(3):776–790. doi: 10.1200/JCO.2002.20.3.776. [DOI] [PubMed] [Google Scholar]
- 44.Whelan J.S., Bielack S.S., Marina N., Smeland S., Jovic G., Hook J.M. EURAMOS-1, an international randomised study for osteosarcoma: results from pre-randomisation treatment. Ann Oncol. 2015;26(2):407–414. doi: 10.1093/annonc/mdu526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Le Deley M.-Cécile., Guinebretière J.-M., Gentet J.-C., Pacquement Hélène, Pichon F., Marec-Bérard P. SFOP OS94: a randomised trial comparing preoperative high-dose methotrexate plus doxorubicin to high-dose methotrexate plus etoposide and ifosfamide in osteosarcoma patients. Eur J Cancer. 2007;43(4):752–761. doi: 10.1016/j.ejca.2006.10.023. [DOI] [PubMed] [Google Scholar]
- 46.Ferrari S., Bacci G., Picci P., Mercuri M., Briccoli A., Pinto D. Long-term follow-up and post-relapse survival in patients with non-metastatic osteosarcoma of the extremity treated with neoadjuvant chemotherapy. Ann Oncol. 1997;8(8):765–771. doi: 10.1023/a:1008221713505. [DOI] [PubMed] [Google Scholar]
- 47.Ferrari S., Ruggieri P., Cefalo G., Tamburini A., Capanna R., Fagioli F. Neoadjuvant chemotherapy with methotrexate, cisplatin, and doxorubicin with or without ifosfamide in nonmetastatic osteosarcoma of the extremity: an Italian sarcoma group trial ISG/OS-1. J Clin Oncol. 2012;30(17):2112–2118. doi: 10.1200/JCO.2011.38.4420. [DOI] [PubMed] [Google Scholar]
- 48.Piperno‐Neumann S., Ray‐Coquard I., Occean B., Laurence V., Cupissol D., Perrin C. Results of API–AI based regimen in osteosarcoma adult patients included in the French OS2006/Sarcome-09 study. Int J Cancer. 2020;146(2):413–423. doi: 10.1002/ijc.32526. [DOI] [PubMed] [Google Scholar]
- 49.Duffaud F., Mir O., Boudou-Rouquette P., Piperno-Neumann S., Penel N., Bompas E. Efficacy and safety of regorafenib in adult patients with metastatic osteosarcoma: a non-comparative, randomised, double-blind, placebo-controlled, phase 2 study. Lancet Oncol. 2019;20(1):120–133. doi: 10.1016/S1470-2045(18)30742-3. [DOI] [PubMed] [Google Scholar]
- 50.Italiano A., Mir O., Mathoulin-Pelissier S., Penel N., Piperno-Neumann S., Bompas E. Cabozantinib in patients with advanced Ewing sarcoma or osteosarcoma (CABONE): a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2020;21(3):446–455. doi: 10.1016/S1470-2045(19)30825-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grignani G., Palmerini E., Dileo P., Asaftei S.D., D’Ambrosio L., Pignochino Y. A phase II trial of sorafenib in relapsed and unresectable high-grade osteosarcoma after failure of standard multimodal therapy: an Italian Sarcoma Group study. Ann Oncol. 2012;23(2):508–516. doi: 10.1093/annonc/mdr151. [DOI] [PubMed] [Google Scholar]
- 52.Revell M.P., Deshmukh N., Grimer R.J., Carter S.R., Tillman R.M. Periosteal osteosarcoma: a review of 17 cases with mean follow-up of 52 months. Sarcoma. 2002;6(4):123–130. doi: 10.1080/1357714021000066368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bertoni F., Boriani S., Laus M., Campanacci M. Periosteal chondrosarcoma and periosteal osteosarcoma. Two distinct entities. J Bone Joint Surg Br. 1982;64-B(3):370–376. doi: 10.1302/0301-620X.64B3.7096408. [DOI] [PubMed] [Google Scholar]
- 54.Bruce Hall R., Robinson L.H., Malawar M.M., Dunham W.K. Periosteal osteosarcoma. Cancer. 1985;55(1):165–171. doi: 10.1002/1097-0142(19850101)55:1<165::aid-cncr2820550126>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 55.Suehara Y., Yazawa Y., Hitachi K., Yazawa M. Periosteal osteosarcoma with secondary bone marrow involvement: a case report. J Orthop Sci. 2004;9(6):646–649. doi: 10.1007/s00776-004-0834-z. [DOI] [PubMed] [Google Scholar]
- 56.Hutagalung E.U., Librianto D., Gumay S., Muthalib A., Budiatmoko B. Periosteal osteosarcoma: a case report. Med J Indones. 2003;12(3):166–170. [Google Scholar]
- 57.Dhaliwal J., Sumathi V.P., Grimer R.J. Radiation-induced periosteal osteosarcoma. Gd Rounds. 2010;10:13–18. [Google Scholar]
- 58.Maheshwari A.V., Jelinek J.S., Seibel N.L., Meloni-Ehrig A.M., Kumar D., Henshaw R.M. Bilateral synchronous tibial periosteal osteosarcoma with familial incidence. Skeletal Radiol. 2012;41(8):1005–1009. doi: 10.1007/s00256-012-1376-7. [DOI] [PubMed] [Google Scholar]
- 59.Kitajima K., Futani H., Fujiwara M., Minakawa G., Osugi Y., Tsuchitani T. Usefulness of quantitative bone single photon emission computed tomography/computed tomography for evaluating response to neoadjuvant chemotherapy in a patient with periosteal osteosarcoma. Cureus. 2018;10(11) doi: 10.7759/cureus.3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen Y., Xu S.-F., Xu M., Yu X.-C. Intentional marginal resection of periosteal osteosarcoma in combination with neoadjuvant chemotherapy: a report of two cases and a review of the literature. Oncol Lett. 2017;13(3):1343–1347. doi: 10.3892/ol.2017.5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tsagozis P., Laitinen M.K., Stevenson J.D., Jeys L.M., Abudu A., Parry M.C. Treatment outcome of patients with chondroblastic osteosarcoma of the limbs and pelvis. Bone Jt J. 2019;101-B(6):739–744. doi: 10.1302/0301-620X.101B6.BJJ-2018-1090.R1. [DOI] [PubMed] [Google Scholar]
- 62.Puri A., Pruthi M., Desai S., Gulia A. Oncological and functional outcome of periosteal osteosarcoma. Indian J Orthop. 2014;48:279–284. doi: 10.4103/0019-5413.132518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pai M., Yeung C.H.T., Akl E.A., Darzi A., Hillis C., Legault K. Strategies for eliciting and synthesizing evidence for guidelines in rare diseases. BMC Med Res Methodol. 2019;19(1):1–10. doi: 10.1186/s12874-019-0713-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.NCCN Guideline Version 2020.1 Bone Cancer.
- 65.Casali P.G., Bruzzi P., Bogaerts J., Blay J.-Y. Rare Cancers Europe (RCE) methodological recommendations for clinical studies in rare cancers: a European consensus position paper. Ann Oncol. 2015;26(2):300–306. doi: 10.1093/annonc/mdu459. [DOI] [PMC free article] [PubMed] [Google Scholar]