Highlights
-
•
Optimal dosing interval of bone-targeting agents (BTAs) has not been fully defined.
-
•
Study of 4 vs 12-weekly BTAs in breast or prostate cancer pts with bone metastases.
-
•
Study arms showed no significant differences SSE rates, time to SSEs or toxicity.
-
•
There were however significant differences in cost-effectiveness results.
-
•
On study SSE (12-weekly arm) associated with slight increase in subsequent SSEs.
Keywords: Bisphosphonates, Denosumab, Breast cancer, Prostate cancer
Abstract
Background
We present the 2-year results of a randomised trial comparing 4- versus 12-weekly bone-targeting agents (BTAs) in patients with bone metastases from breast or castration-resistant prostate cancer (CRPC).
Patients and Methods
Patients with bone metastases from breast or CRPC, who were going to start or were already receiving BTAs, were randomised to 4- or 12-weekly BTA treatment for 2 years. The endpoints were: symptomatic skeletal events (SSE) rates, time to SSEs, toxicity and cost-effectiveness.
Results
Of 263 patients (160 breast cancer, 103 CRPC), 133 (50.6%) and 130 (49.4%) were randomised to the 4- and 12-weekly groups, respectively. BTAs included denosumab (56.3%), zoledronate (24.0%) and pamidronate (19.8%). After 2 years, the cumulative incidence rate (95% CI) of SSEs was 32.7% (24.6% to 41.1%) and 28.1% (20.3% to 36.4%) for the 4- and 12-weekly intervention groups respectively. The hazard ratio for time to first SSE was 0.96 (95% CI = 0.63 to 1.47). However, in a post hoc analysis, those patients who had an on-study SSE, there was a small non-statistical increased risk of subsequent SSEs among patients on the 12-weekly dosing arm (HR = 1.14; 95% CI – 0.90–1.44). BTA-related toxicity rates were similar between study arms. A cost-utility analysis showed that 12-weekly BTA is cost-effective from a public payer’s perspective.
Conclusion
These results in addition to those previously reported for de-escalating zoledronate, would support that de-escalation of commonly used BTAs is a reasonable and economically valid treatment option. While not statistically significant, the increase in subsequent SSEs in the 12-weekly arm requires further exploration.
1. Introduction
In patients with bone metastases from breast and castration resistant prostate cancer (CRPC) bone-targeted agents (BTAs), such as bisphosphonates and denosumab are associated with improvements in symptomatic skeletal event (SSE) rates, time to first SSE, morbidity, pain, quality of life (QoL)[1]. As they have shown no improvement in either progression-free or overall survival they remain supportive care drugs, and questions about their optimal dosing schedules remain pertinent [2].
Previous published randomised trials evaluating de-escalation of pamidronate [3], zoledronate [4], [5], [6], and denosumab [7] from traditional 3 to 4-weekly dosing to less frequent dosing have shown similar efficacy outcomes across a broad range of endpoints. Subsequent systematic reviews in both breast [8], [9] and CRPC [10] have confirmed these results. However, despite these findings and evidence-based guidelines stating the efficacy of 12-weekly zoledronate [11], surveys of both patients and oncologists [12], [13] confirmed that significant clinical equipoise still exists with patients receiving different BTAs at different dosing intervals [14], [15].
We previously published the 1-year results of a randomised trial in which patients with bone metastases from breast or CRPC, who were going to start or were already on BTAs, were randomised to 4-weekly or 12-weekly BTA treatment for two years [16]. The 1-year results showed no statistically significant differences between the study arms for any of the trial endpoints of; health-related quality of life (HRQoL), pain, global health status or SSEs rates. In this analysis, we present the 2-year results for frequency of SSE, time to first SSE, toxicity and cost-effectiveness. Additionally, as the occurrence of an SSE increases the risk of a subsequent SSE, the frequency of recurrent SSEs was evaluated in an exploratory analysis.
2. Patients and methods
2.1. Study Population
We previously presented the primary results of the REaCT-BTA trial [16]. This was a pragmatic, randomised, open-label, non-inferiority trial at five Canadian centres. In this study 263 patients with bone metastases from either metastatic breast (160 patients), or CRPC (103 patients), who were either going to start or were already receiving BTAs (either denosumab, pamidronate, or zoledronate) were eligible and were randomised to the 4- (133, 50.6%) or 12-weekly (130, 49.4%) arms for 2-years. BTAs included denosumab (56.3%), zoledronate (24.0%) and pamidronate (19.8%). There were no study-mandated changes in the type of BTA the patient received, no prior maximum duration of BTA use and patients could have had prior SSEs. All patients provided verbal consent following the integrated consent model [17], The study was approved by the Ontario Research Ethics Board and registered on clinicaltrials.gov (NCT02721433).
2.2. Trial Design and Treatment
Eligible and consented patients were randomised in a 1:1 ratio using a permuted block design with variable block sizes of 2 and 4 to either 4-weekly or 12-weekly BTAs for 2- year via a web-based randomization system. Stratification was based on tumour type (breast vs. CRPC) and centre. After enrolment, neither investigators nor participants were masked to treatment allocation.
2.3. Procedures
The choice of BTA (either denosumab, pamidronate, zoledronate) was made before randomisation and was left to the patient and treating physician. Patients were instructed to take calcium and vitamin D as per guidelines. As the trial was pragmatic in nature, if a patient was receiving another systemic therapy (e.g. trastuzumab) every 3 weeks and therefore randomisation to the 4-weekly BTA arm would be more inconvenient, then the BTA could be administered every 3 or 6 weeks. During year 1, endpoint data were collected at baseline and weeks 12, 24, 36 and 48 and included self-completed patient questionnaires. During year 2 there were no patient questionnaires, however, follow up emails to the patients treating physician, which gathered information on patient toxicities, treatment changes or outcome events at weeks 60, 72, 84 and 96. Study data were also obtained and verified through the patient’s electronic medical record. Patients were assessed at their usual clinic visits. No radiological assessments beyond conventional practice were mandated.
2.4. Outcomes
Primary outcome was time to first SSE. Secondary outcomes were; time to first SSEs, toxicity and incremental net benefit (INB). A post hoc analysis of the incidence of subsequent SSEs in patients who had an on study SSE was also performed. SSEs were defined as: radiotherapy to bone, new symptomatic pathological bone fractures, tumour-related orthopedic surgery, spinal cord compression, and symptomatic hypercalcaemia. Time to development of SSEs was calculated from the date of randomization until the first date a patient experienced an SSE and presented as a cumulative incidence rate. Any patient not experiencing an SSE was censored on the last date that they were confirmed to be SSE-free. Total number of SSEs and time to subsequent on-study SSE were used to calculate the Skeletal Morbidity Rates (SMR, mean number of SSEs per year).
2.5. Toxicity
Treatment-related adverse events such as; renal toxicity (defined as changes in renal function leading to dose de-escalation/discontinuation), documented symptomatic hypocalcaemia and confirmed ONJ.
2.6. Sample Size and Statistical Analysis
The sample size of the original trial was based on there being no difference between the 12-weekly and 4-weekly treatment arms, and a non-inferiority bound of 5 points on the C30 Physical Subdomain at 48 weeks. This 5-point inferiority margin has previously been shown to be clinically meaningful in patients receiving BTAs [18], [19], [20]. A sample size of 224 patients was required to have 80% statistical power in ensuring the lower limit of a one-sided 95% confidence interval will be above the non-inferiority limit. To allow for an expected 10% non-compliance rate and account for stratification, enrolment of 250 total patients was planned.
2.7. Analytic Plan
Baseline characteristics are summarized using means and standard deviations (continuous measures) or proportions (categorical data). As a non-inferiority study, the primary analysis of time to first SSE was based on a per protocol dataset, defined as those patients who completed all 48 weeks of allocated treatment. All remaining analyses were based on the intent-to-treat population, that is using data from all patients according to the allocated treatment, irrespective of missing or incomplete data.
Time to first SSE was analysed as a cumulative incidence analysis, accounting for the competing risk of death. As the primary statistical analysis for this study was conducted previously, no statistical testing was performed for this analysis. Hazard ratios were calculated using the Fine and Gray method. The proportion of patients experiencing an outcome event were calculated for each intervention group, and the absolute risk difference along with their 95% confidence intervals were calculated and presented.
As patients could experience multiple events, the number of recurrent events were calculated along with the mean number per patient. The skeletal morbidity rate (SMR) was calculated as the number of events pre patient divided by the length of follow-up. The Andersen-Gill method was used to estimate the hazard ratio for experiencing a recurrent SSE event or death between the two interventions. Subgroup analyses were performed to explore differences based on disease, type of BTA and prior BTA exposure.
All analyses were performed using SAS v9.2 (SAS Institute, North Carolina, USA). All confidence intervals were two-sided and set at the α = 0.05 level.
2.8. Health economic evaluation
We performed a cost-utility analysis utilising a decision tree and patient-level data from the trial over a two-year period. We did not use a lifetime horizon because BTAs are supportive care therapies without survival benefits. SSE incidence data were taken from the trial. Health outcome was shown as quality-adjusted life year (QALY), that was estimated based on health utility data reported in the published Year 1 BTA data [21]. Costs were estimated from the Ontario Ministry of Health’s perspective and expressed in 2021 Canadian dollars (C$), as previously described [21]. Costs were sought from Cancer Care Ontario and the Physician Schedule of Benefits and updated, where applicable, to 2021 values. Our model included the costs of BTA (denosumab, pamidronate and zoledronate), drug administration, physicians, bone metastases-related hospitalisation and outpatient SSE management. An annual discount rate of 1.5 % was applied to cost and outcome data. We presented a cost-effectiveness result as an incremental benefit (INB). We converted incremental QALYs into a net monetary benefit by multiplying incremental QALYs with a willingness to pay (WTP) value of $50,000 per QALY. The INB is equal to difference in the net monetary value of the QALYs gain (ΔQALYs × WTP) and the cost difference between 12-weekly and 4-weekly BTA. The intervention is cost-effective if the INB is greater than zero. We performed a series of deterministic and probabilistic sensitivity analyses to assess the robustness of study results.
3. Results
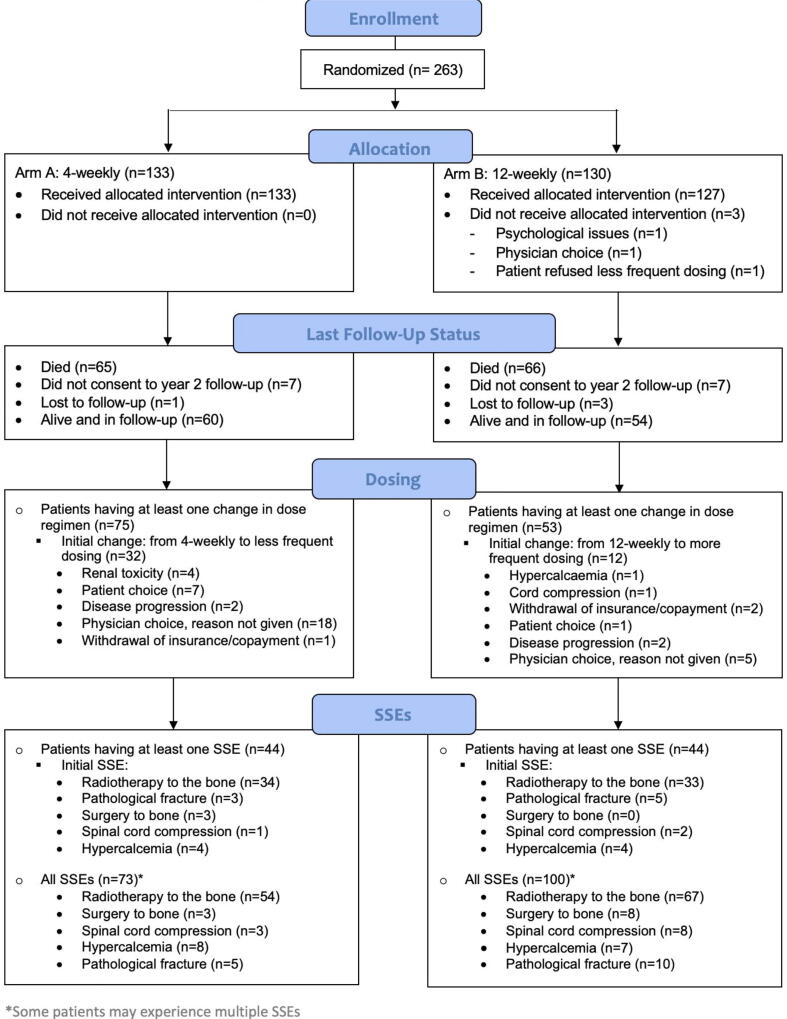
Between August 3, 2016 and June 5, 2018, 263 patients were enrolled and 133 (50.6%) randomised to 4- and 130 (49.4%) to 12-weekly therapy (Consort diagram, Fig. 1). Baseline characteristics are in shown in Table 1, 60.8% (1 6 0) had breast cancer and 39.2% (1 0 3) CRPC. Median patient age, cancer type and tumour hormone receptor status (breast cancer patients) were well balanced between the study arms. Participants in the 4-weekly treatment arm had had bone metastases for longer (median 15.3 vs 7.0 months) and were more likely to be on a BTA prior to randomisation (54.9% vs 41.5%) than participants in the 12-weekly arm. For the patients with breast cancer and CRPC the 4- and 12-weekly arms were well balanced for the frequency and types of SSEs experienced prior to randomisation. Patients received denosumab (n = 148, 56.3%), zoledronate (n = 63, 24.0%) or pamidronate (n = 52, 19.8%). There were more CRPC patients on denosumab (85.4%) compared with breast cancer (37.5%) (Table 1). Baseline anti-cancer treatments (evaluated at the Ottawa site only) were well balanced (Table 1).
Fig. 1.
CONSORT flow diagram.
Table 1.
Patient baseline disease and treatment characteristics. Values are n (%) unless otherwise indicated.
| Statistic | 4 weekly BTA | 12 weekly BTA | |
|---|---|---|---|
| N | 133 | 130 | |
| Age | Median (range) | 67 (26, 97) | 68 (30, 92) |
| Sex | N (%) Female | 81 (60.9) | 79 (60.8) |
| Baseline serum creatinine, mg/dL | Mean (sd) | 0.83 (0.22) | 0.90 (0.30) |
| Disease characteristics | |||
| Cancer type | N (%) Breast | 81 (60.9) | 79 (60.8) |
| CRPC | 52 (39.1) | 51 (39.2) | |
| Hormonal Status, Breast Patients (n = 160) | N (%) ER | 76 (93.8) | 73 (92.4) |
| PR | 64 (79.0) | 55 (69.6) | |
| HER2 | 12 (14.8) | 12 (15.2) | |
| Months from initial bone metastases diagnosis to randomisation | Median (range) | 15.3 (0, 93.5) | 7.0 (0, 86.8) |
| N (%) < 3 | 31 (23.3) | 42 (32.3) | |
| 3–5.9 | 10 (7.5) | 19 (14.6) | |
| 6–11.9 | 13 (9.8) | 13 (10.0) ) |
|
| 12–23.9 | 36 (27.1) | 21 (16.2 | |
| ≥24 | 43 (32.3) | 35 (26.9) | |
| Months from initial bone metastases diagnosis to randomisation, prostate cancer patients | Median (range) | 22.4 (1.0, 81.0) | 19.2 (0, 86.8) |
| N (%) < 3 | 1 (1.9) | 9 (17.7) | |
| 3–5.9 | 5 (9.6) | 3 (5.9) | |
| 6–11.9 | 5 (9.6) | 7 (13.7) | |
| 12–23.9 | 18 (34.6) | 11 (21.6) | |
| ≥24 | 23 (44.2) | 21 (41.2) | |
| Months from initial bone metastases diagnosis to randomisation, breast cancer patients | Median (range) | 8.9 (0, 93.5) | 4.1 (0, 71.8) |
| N (%) < 3 | 30 (37.0) | 33 (41.8) | |
| 3–5.9 | 5 (6.2) | 16 (20.3) | |
| 6–11.9 | 8 (9.9) | 6 (7.6) | |
| 12–23.9 | 18 (22.2) | 10 (12.7) | |
| ≥24 | 20 (24.7) | 14 (17.7) | |
| Prior SSEs | N (%) Any | 60 (45.1) | 54 (41.5) |
| RT to bone to reduce fracture risk | 0 (0.0) | 2 (1.5) | |
| RT to bone to bone for pain | 51 (38.4) | 46 (35.4) | |
| RT to bone to bone, other | 1 (0.8) | 2 (1.5) | |
| Pathological fracture | 6 (4.5) | 6 (4.6) | |
| Surgery to bone | 3 (2.3) | 2 (1.5) | |
| Spinal cord compression | 4 (3.0) | 3 (2.3) | |
| Hypercalcaemia | 1 (1.7) | 1 (1.9) | |
| Treatment characteristics | |||
| Type of BTA | N (%) Denosumab | 77 (57.9) | 71 (54.6) |
| Pamidronate | 25 (18.8) | 27 (20.8) | |
| Zoledronate | 31 (23.3) | 32 (24.6) | |
| Prior use of parenteral BTA | N (%) Yes | 73 (54.9) | 54 (41.5) |
| If yes, number of prior parenteral BTA injections | Median (IQR), maximum | 1 (0, 7), 48 | 0 (0, 5), 46 |
| Mean (sd) | 5.0 (8.5) | 4.5 (8.9) | |
| Baseline Anti-Cancer Treatment and SSEs characteristics by tumour type* | |||
| Breast cancer patients | N | 55 | 54 |
| Endocrine therapy | N (%) | 33 (60.0) | 28 (51.9) |
| Chemotherapy** | N (%) | 18 (32.7) | 20 (37.0) |
| Trastuzumab-based anti-her2 therapy alone | N (%) | 9 (16.4) | 11 (20.4) |
| Number of pre-randomization SREs | N (%) 0 | 30 (54.6) | 31 (57.4) |
| 1 | 17 (30.9) | 16 (29.6) | |
| 2 | 2 (3.6) | 5 (9.3) | |
| 3 | 4 (7.3) | 2 (3.7) | |
| 4 | 2 (3.6) | 0 (0) | |
| Type of pre-randomization SREs | Radiotherapy to the bone | 34 | 27 |
| Spinal Cord Compression | 3 | 3 | |
| Surgery to bone | 1 | 1 | |
| Hypercalcaemia | 1 | 1 | |
| Pathologic Fracture | 2 | 0 | |
| Prostate cancer patients | N | 27 | 26 |
| Androgen Receptor Antagonists | N (%) | 24 (88.9) | 22 (84.6) |
| Chemotherapy | N (%) | 1 (3.7) | 0 (0) |
| Radium-223 | N (%) | 3 (11.1) | 4 (15.4) |
| Number of pre-randomization SREs | N (%) 0 | 10 (37.0) | 11 (42.3) |
| 1 | 13 (48.2) | 9 (34.6) | |
| 2 | 4 (14.8) | 4 (15.4) | |
| 3 | 0 (0) | 1 (3.9) | |
| 4 | 0 (0) | 1 (3.9) | |
| Type of pre-randomization SREs | Radiotherapy to the bone | 19 | 23 |
| Spinal Cord Compression | 2 | 0 | |
| Surgery to bone | 0 | 1 | |
sd = standard deviation, IQR = interquartile range, *for Ottawa site only, **includes patients receiving chemotherapy and concurrent anti-her2 therapy
3.1. First on-study SSE results
SSE data is presented in Table 2. After 2 years, the cumulative incidence rate (95% CI) for experiencing a SSE was 32.7% (24.6% to 41.1%) and 28.1% (20.3% to 36.4%) for the 4- and 12-weekly intervention groups respectively (Fig. 2). The hazard ratio for time to first SSE was 0.96 (95% CI = 0.63 to 1.47). Forty-four patients in each arm experienced at least one SSE, with the most common first SSE experienced being radiotherapy to the bone..
Table 2.
Clinical endpoint data.
| 4-weekly | 12-weekly | Estimated Difference (95% CI) | |
|---|---|---|---|
| N | 133 | 130 | |
| First SSE | |||
| N (%) SSE | 44 (33.1) | 44 (33.9) | 0.8 (-10.6, 12.2) |
| Radiotherapy to bone | 34 (25.6) | 33 (25.4) | −0.2 (-10.7, 10.4) |
| Pathological fracture | 3 (2.3) | 5 (3.8) | 1.6 (-2.6, 5.8) |
| Surgery to bone | 2 (1.5) | 0 (0) | −1.5 (-3.6, 0.6) |
| Spinal cord compression | 1 (0.8) | 2 (1.5) | 0.8 (-1.8, 3.4) |
| Hypercalcaemia | 4 (3.0) | 4 (3.1) | 0.1 (-4.1, 4.2) |
| Time to first study SSE | |||
| 1-year cumulative incidence of SSE (95% CI) | 17.7 (11.7, 24.8) | 16.7 (10.8, 23.8) | 0.96 (0.63, 1.47)** |
| 2-year cumulative incidence of SSE (95% CI) | 32.7 (24.6, 41.1) | 28.1 (20.3, 36.4) | |
| Toxicity | |||
| Osteonecrosis of the jaw | 1 (0.8) | 1 (0.8) | 0.0 (-2.1, 2.1) |
| Renal impairment | 7 (5.3) | 8 (6.2) | 0.9 (-4.7, 6.5) |
| Symptomatic hypocalcaemia | 4 (3.0) | 3 (2.3) | −0.7 (-4.6, 3.2) |
| Any Above Toxicity | 11 (8.3) | 12 (9.2) | 1.0 (3.5, 7.8) |
| Hospitalization due to bone metastases | |||
| Hospitalization due to bone metastases | 9 (6.8) | 5 (3.9) | −2.9 (-8.3, 2.5) |
| Change in BTA dosing*** | |||
| N (%) Any Change | 63 (47.4) | 32 (24.6) | −22.8 (-34.0, −11.5) |
| Discontinuation | 33 (24.8) | 23 (17.7) | −7.1 (-17.0, 2.7) |
| Change to 4-weekly dosing | – | 5 (3.9) | – |
| Change to 12-weekly dosing | 28 (21.1) | – | – |
| Pamidronate to denosumab | 0 (0) | 1 (0.8) | 0.8 (-0.7, 2.2) |
| Pamidronate to zoledronate | 3 (2.3) | 0 (0) | −2.3 (-4.8, 0.3) |
| Zoledronate to pamidronate | 1 (0.8) | 0 (0) | −0.8 (-2.2, 0.7) |
| Zoledronate to denosumab | 1 (0.8) | 0 (0) | −0.8 (-2.2, 0.7) |
| Missed Dose(s) | 15 (11.3) | 6 (4.6) | −6.7 (-13.1, −0.2) |
SSE = symptomatic skeletal related event, SMR = skeletal mobility rate, * = risk difference,** = hazard ratio for 12-weekly versus 4-weekly
NOTE: that the number of patients with at least 1 SSE is over any time period (including beyond 2 years), hence does not equal the number of patients with a SSE within 2 years
*** Patients may have experienced multiple changes in dosing
Fig. 2.
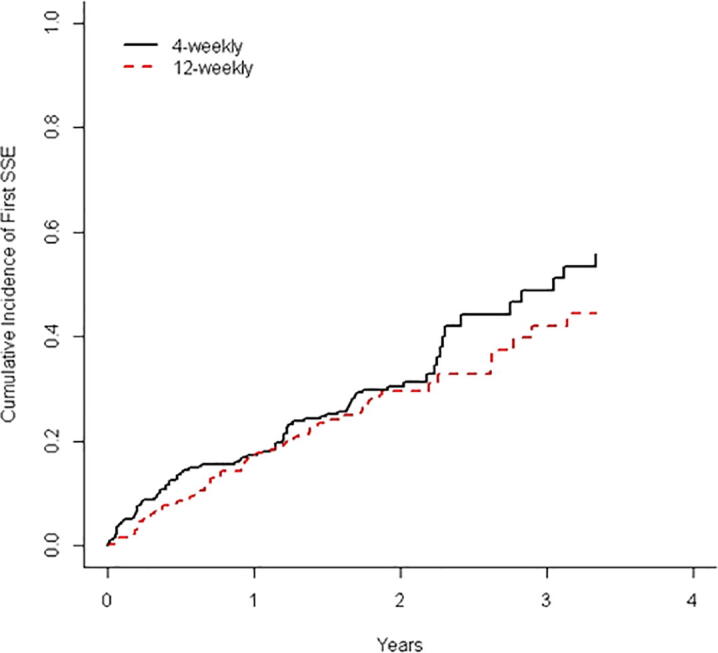
Cumulative incidence of first SSE.
3.2. All on-study SSE events
At the final analysis, 65 (48.9%) and 66 (50.8%) patients had died, and maximum follow-up was 3.7 and 3.6 years for those alive at last follow-up, for patients in the 4-weekly and 12-weeky intervention groups respectively. During this time, there were 73 SSEs experienced by patients in the 4-weekly arm, with the most common SSE being radiotherapy to the bone (54 times), compared with 100 SSEs experienced by patients in the 12-weekly arm (67 with radiotherapy to the bone). Details of SSEs are in Table 3 with a Forest plot in Fig. 3.
Table 3.
Results of Analysis of Recurrent Events.
| 4-weekly | 12-weekly | |
|---|---|---|
| Median (maximum) Follow-Up (Years) | ||
| Alive at Last Follow-Up | 1.96 (3.70) | 1.96 (3.62) |
| Deceased at Last Follow-Up | 1.38 (3.45) | 1.23 (3.49) |
| All SSE | 73 | 100 |
| Radiotherapy to bone | 54 | 67 |
| Pathological fracture | 5 | 10 |
| Surgery to bone | 3 | 8 |
| Spinal cord compression | 3 | 8 |
| Hypercalcaemia | 8 | 7 |
| Number of SSE Per Patient | ||
| N (%) ≥ 1 | 44 (33.9) | 44 (33.1) |
| 0 1 2 3 4 5 6 7 8 |
89 (66.9) 29 (21.8) 7 (5.3) 5 (3.8) 1 (0.8) 1 (0.8) 1 (0.8) 0 (0) 0 (0) |
86 (66.2) 21 (16.2) 10 (7.7) 6 (4.6) 3 (2.3) 0 (0) 1 (0.8) 1 (0.8) 2 (1.5) |
| Mean (sd) Events per Patient | Mean (sd) Events per Patient | |
| All | 0.55 (1.03) | 0.77 (1.52) |
| BTA Naïve | 0.50 (1.02) | 0.84 (1.71) |
| Prior exposure | 0.59 (1.04) | 0.67 (1.21) |
| Denosumab | 0.52 (0.94) | 0.85 (1.48) |
| Pamidronate | 0.48 (0.65) | 0.89 (2.06) |
| Zoledronate | 0.68 (1.42) | 0.50 (1.02) |
| Breast cancer | 0.58 (1.07) | 0.62 (1.38) |
| Prostate cancer | 0.50 (0.96) | 1.00 (1.71) |
| Mean (sd) per patient | Mean (sd) per patient | |
| Skeletal Morbidity Rates (SSE / year) | 0.41 (1.20) | 0.45 (0.86) |
Fig. 3.
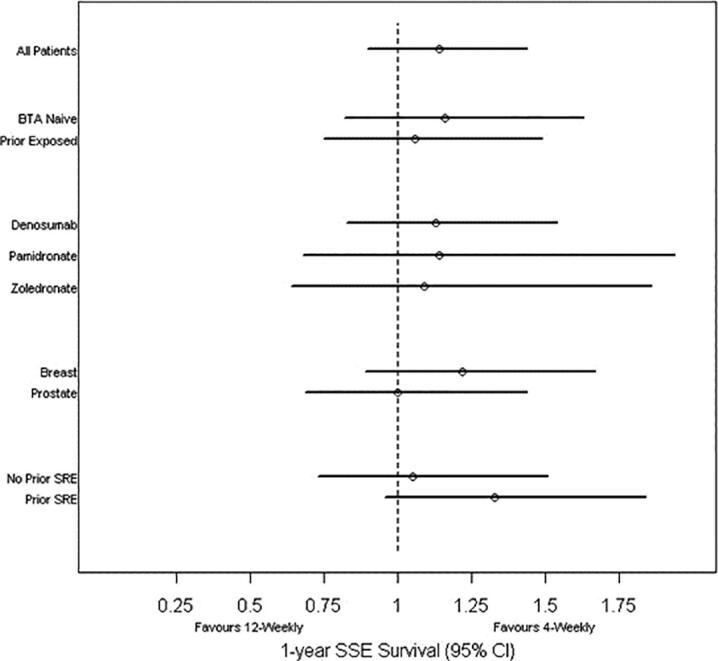
One-year (95% CI) SSE-free survival by study group, bone-targeted agent and tumour type. SSE, symptomatic skeletal event.
Across patients in the 4-weekly arm, there were a mean (sd) of 0.55 (1.03) SSE events experienced per patient, compared with 0.77 (1.52) SSEs per patient in the 12-weekly arm. Accounting for length of follow-up, the SMR is a mean (sd) of 0.41 (1.20) SSE events per patient per year for the 4-weekly arm and was 0.45 (0.86) for the 12-weekly arm. The Andersen-Gill method was used to estimate the hazard ratio for experiencing a SSE event or death between the two interventions. The Andersen-Gill hazard ratio was 1.14 (95% CI = 0.90 to 1.44), indicating an increased hazard of experiencing a SSE or death of 14% for patients in the 12-weekly arm (Fig. 4a and b).
Fig. 4.
SSE events in the 4-weekly (Fig a) and 12-weekly (Fig b) arms.
Results were similar across all subgroups evaluated with the possible exception of having a prior SSE at the time of randomization (Supplemental table 1). Of the 149 patients who did not have a prior SSE at the time of randomization, the mean number of SSEs per patient was similar for the 4-weekly and 12-weekly intervention arms (rates of 0.41 and 0.37 respectively, hazard ratio = 1.05, 95% CI = 0.73 to 1.51). However, of those 114 patients who had a prior SSE, the mean number of SSEs was 0.72 per patient amongst those receiving 4-weekly treatment, compared with 1.33 per patient amongst those receiving 12-weekly treatment. The hazard ratio for patients on the 12-weekly intervention arm was 1.33 (95% CI = 0.96 to 1.84). An interaction term between prior BTA and intervention arm was not statistically significant (p-value = 0.59).
As the 4-weekly treatment arm had had bone metastases for longer (median 15.3 vs 7.0 months) and duration of bone metastases is associated with SSE risk, we looked at the effect of time from bone diagnosis to randomization on the effect of intervention. For time to first SSE, there was no interaction observed between randomization arm and time from bone diagnosis to randomization (p-value = 0.71). After adjusting for time from bone diagnosis to randomization, the effect for 12-weekly versus 4-weekly intervention remained virtually the same (hazard ratio = 0.98, 95% CI = 0.64 to 1.49). Similarly, for all SSEs on-study as the outcome, the interaction term was not significant (p-value = 0.62) and after adjustment, the Andersen-Gill model was similar (hazard ratio = 1.17, 95% CI = 0.92 to 1.48).
3.3. Toxicity
Overall 19 (14.3%) of patients receiving 4-weekly dosing experienced a BTA-related toxicity, compared with 15 (11.5%) of patients receiving 12-weekly dosing, a difference of −2.8% (95% CI = -10.8% to 5.4%). Patients receiving 4-weekly dosing were more likely to have a dosing change (47.4% versus 24.6%, a difference of –22.8%, 95% CI = -34.0% to −11.5%), with the most frequent reasons being discontinuation (33 or 24.8% of patients), a change to 12-weekly dosing (28 or 21.1%) and missed doses (15 or 11.3%) (Table 2 and Fig. 1).
3.4. Economic Evaluation Results
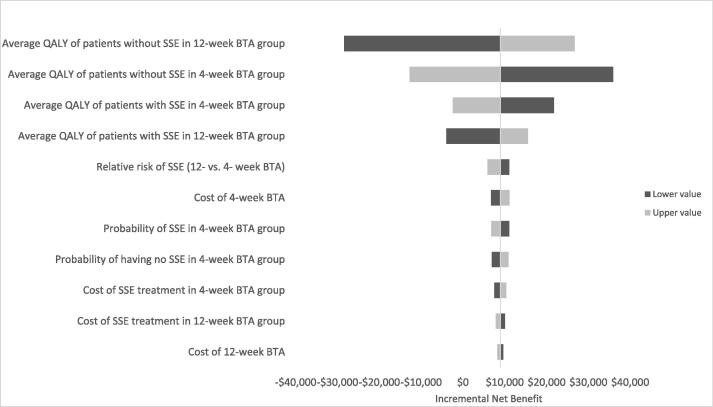
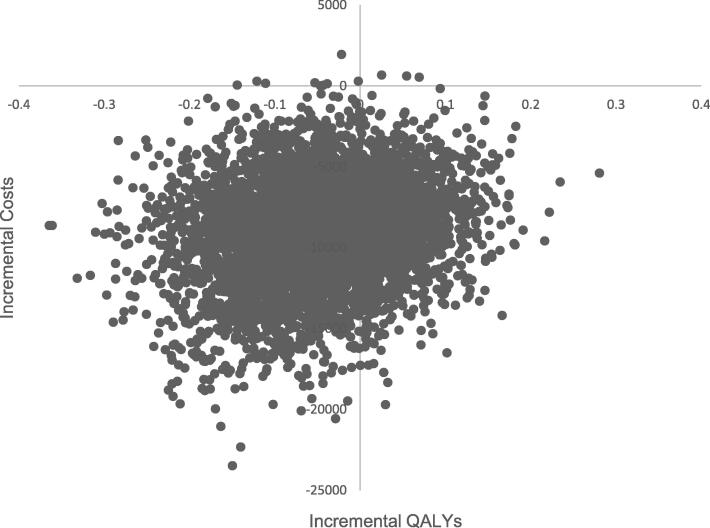
Overall 12-weekly BTA was associated with lower cost (C$9,104 vs. C$18,191) and slightly fewer QALYs (1.21 vs. 1.25) compared to 4-weekly BTA, with an estimated INB of $6,792.39 (Supplemental table 2). The cost-effectiveness results were highly sensitive to changes in QALY of patients in both treatment groups (Fig. 5). The lower the QALY in patients receiving 12-weekly BTA, the less likely that 12-weekly BTA would be cost-effective relative to 4-weekly BTA. Results of the probabilistic sensitivity analysis showed that 12-weekly BTA was associated with decreased cost in the thousands of dollars with minimal reduction in QALY in the majority of the 5,000 simulations (Fig. 6). Regardless of the willingness-to-pay values, 12-weekly BTA always had the greater probability of being a cost-effective option than 4-weekly BTA due to overall reduced drug and administration costs.
Fig. 5.
Deterministic sensitivity analysis results.
Fig. 6.
Probabilistic sensitivity analysis results.
4. Discussion
This is the final analysis of the first prospective randomised, open label, clinical trial involving patients with bone metastases from either breast or CRPC, comparing 4- versus 12-weekly dosing of three commonly used BTAs. Similar to the year-1 data, at 2-years there was no statistically significant difference in SSE rates or time to first SSE observed between the two intervention arms. These results are consistent with those previously reported for de-escalating zoledronate [4], [6], [22] and add to the literature about the acceptability of de-escalating denosumab [7] and pamidronate [3], [5]. These finding are especially pertinent at the moment due to the Covid-19 pandemic, when both patients and physicians are trying to safely reduce the number of visits patients make to health care providers. They are also important because there is increasing data suggesting that due to advances in the systemic treatment of breast and CRPC that the benefits of BTAs with prolonged use may well be reduced while treatment-related toxicities increase [23].
Of note, while the current study demonstrated no significant difference in the percentage of patients who have on-study SSE between the 4-weekly and 12-weekly interventions, there was a slight (∼14% increased hazard risk), but not statistically significant increase in subsequent recurrent SSEs experienced by those in the 12-weekly arm compared to 4-weekly arm. This observation suggests that while the 12-weekly intervention does not statistically increase the risk of having a first on study SSE, there may be an increase in the rate of recurrent events in the 12-weekly intervention after a patient experiences their first SSE (a potential reflection of more aggressive subsequent treatment refractory disease). Thus, while one might recommend starting patients on 12-weekly BTA treatment, consideration to switching to the 4-weekly regimen could be made when the patient experiences a first SSE. Another option could be switching to more potent BTA however data to inform the choice of treatment in such patients are limited and inconclusive [24], [25], [26]. We evaluated whether this finding could have also been related to the denosumab patients and the shorter half life of this agent, the number of patients receiving each agent was small and is therefore inconclusive. The results do indicate that the greatest risk of recurrent events occurred amongst patients receiving pamidronate but again interpretation is limited by the small sample size and the reduced ability to detect a difference if one exists. As the 4-weekly treatment arm had had bone metastases for longer (median 15.3 vs 7.0 months) and duration of bone metastases is associated with SSE risk, we looked at the effect of time from bone diagnosis to randomization on the effect of intervention. This analysis showed that the interaction term was not significant (p-value = 0.62) and after adjustment, the Andersen-Gill model was similar (hazard ratio = 1.17, 95% CI = 0.92 to 1.48).
Consistent with a previous economic evaluation of de-escalation of bone-targeted agents [21], the current study showed that 12-weekly BTA is cost-effective compared to 4-weekly BTA at the WTP value of $50,000 per QALY. However, 12-weekly BTA led to a slight decrease in QALYs compared to 4-weekly BTA; fewer QALYs could be a result of higher on-study SSE events among patients receiving 12-weekly BTA.
This study has both strengths and limitations. While there is considerable heterogeneity in the study subgroups (2 different tumor types, 3 different drugs, patients with and without prior SSEs, 2 different settings with BTA pretreated and BTA-naive patients) the study was designed to be broadly applicable to real-world practice by including patients with breast and CRPC. These differences in baseline characteristics could in have led to differences in the results that could only be answered by a significantly larger sample size. For example, patients who have been on BTAs might be assumed to have a different risk profile for SSEs compared to BTA-naive subjects. The pragmatic nature of the trial with very few restrictive inclusion criteria also means that the study population more readily reflects that in clinical practice. This reality of real world practice is noticeable in the number of patients who died during the study period (n = 131) as well as the number of patients who changed from 12-weekly to 4-weekly dosing (3.9%) and from 4-weekly to 12-weekly (21.1%) dosing (see Consort Fig. 1 and Table 2). There were also differences in the rates of SSEs between the study arms, with 73 SSEs experienced by patients in the 4-weekly arm, with the most common SSE being radiotherapy to the bone (54), compared with 100 SSEs experienced by patients in the 12-weekly arm (67 with radiotherapy to the bone). In addition, there were numerically different rates of pathologic fractures in the 2 arms. Given the unblinded nature of this study as well as it’s small sample size it is not possible to tell if these differences are clinically meaningful. These questions will be answered by the REDUSE trial [28]. Another limitation could be viewed as the inclusion of hypercalcaemia of malignancy as an SSE in the current study. However, as hypercalcaemia can lead to hospitalisations and incurs costs to both the patient and the health care system we felt it was important to include it as an endpoint. Given the lack of any clinically meaningful difference in the primary analysis, even with greater sample size, it is highly unlikely to affect the study results substantially. Finally, the study follow-up was only for 1 year; however, the protocol has been updated to allow patients to remain on study for 2 years, which will be analysed at a later date.
Despite nearly 3 decades of use, questions remain about the optimal use of BTAs. Future studies could evaluate the effect of de-escalation after a standardised period of 4-weekly treatment. This would be similar to the ZOOM trial where de-escalation of zoledronate was performed after 12 to 15-months of 4-weekly treatment [4]. Future prospective studies could also assess the findings of a recent systematic review on the risk–benefit of BMA use for > 2 years in breast cancer or castrate-resistant prostate cancer was conducted [23]. Evidence informing the use of BMA beyond 2 years is heterogeneous and based on retrospective analysis. Prospective randomized studies with greater emphasis on quality of life are needed. Indeed we are currently performing such a study [27]. It would make some sense to start on the 12-weekly intervention, but if patients start experiencing SSEs, maybe increase at that point. In addition, studies also need to report on more than first on study SSE rates and time to SSEs. It is important that we evaluate alternative means of assessing multiple events.
5. Conclusion
In this pragmatically designed randomized clinical trial for metastatic breast or CRPC, analysis at 2-years confirms that 12-weekly BTAs were non-inferior to 4-weekly BTAs for SSE rates and time to first SSE. This trial’s results are consistent with those previously reported for de-escalating zoledronate and add to the literature about the acceptability of de-escalating denosumab and pamidronate. The standard incorporation of 12-weekly dosing of BTAs into routine clinical practice could substantially benefit both patients and the health care system. While awaiting the results of the REDUSE trial [28], which will definitively answer the question of de-escalating 4-weekly to 12-weekly denosumab in our opinion de-escalation of all commonly used bone-targeted agents is a reasonable clinical decision. Questions however, do remain about rates of subsequent SSEs. Larger datasets need to be interrogated to see if de-escalation while having no effect on first SSEs, they may not be as effective as 4-weekly treatment in preventing additional events.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
We are grateful for patients and their families for their assistance with this study, as well as physicians for approaching patients. Main accrual by physician was: Clemons (83), Ong (21), Ernst (17), Booth (15), Canil (14), Mates (10), Reaume (9), Joy (9), Robinson (8), Hilton (7), Verma (7), Aseyev (6), Chan (6), Dent (6), Gertler (6), Blanchette (5) and Fernandes (5).
Declarations:
Role of the funding source: This work was supported by the Rethinking Clinical Trials Program (REaCT), the Canadian Institute of Health Research (Patient Oriented Research grant), Cancer Care Ontario – Government of Ontario (Clinical Programs and Quality Initiatives grant 2017 and 2018 competitions), the Ottawa Hospital Foundation and its generous donors, and the Canadian Cancer Clinical Trials Network (3CTN).
Conflict of Interest Statement: Mark Clemons is a co-author on both the American Society of Clinical Oncology - Cancer Care Ontario Focused Guideline on the role of Bone-Modifying Agents in Metastatic Breast and the UpToDate chapter on Osteoclast inhibitors for patients with bone metastases from breast, prostate, and other solid tumors. GRP reports personal fees from Astra-Zeneca and Merck outside the submitted work, as well as a family member who works for Roche and owns stock in Roche. All other authors declare no competing interests.
Ethics Committee Approval: All procedures performed in this study involving human participants were in accordance with the ethical standards of the institution, the Ontario Cancer Research Ethics Board and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all trial participants included in the study.
Consent to Participate: Integrated verbal consent was obtained from all trial participants included in the study.
Consent for Publication: All participants consented to de-identified publication of aggregate study results.
Availability of Data and Material: De-identified dataset is available upon request and approval by the Ontario Cancer Research Ethics Board (OCREB 1-866-678-6427 Ext 6649).
Code availability: N/A.
Author Contributions: Conceptualization: MC, LV, and DF Data curation: MC, ML, CS, SE, CB, MM, PB, AAJ, and OA Formal analysis: GP, MT and KT Funding acquisition: MC Methodology: MC and DF Project administration: ML, CS and LV Supervision: MC Writing - original draft: MC, ML, CS, MJA, LV, MT Writing - review & editing: all authors.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jbo.2021.100388.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Southcott D., Awan A., Ghate K., Clemons M., Fernandes R. Practical Update for the Use of Bone-Targeted Agents in Patients with Bone Metastases from Metastatic Breast Cancer or Castration-Resistant Prostate Cancer. Curr Oncol. 2020;27(4):220–224. doi: 10.3747/co.27.6631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hutton B., Mazzarello S., Clemons M. Dosing Strategies of Bone-Targeting Agents. JAMA internal medicine. 2015;175(11):1864–1865. doi: 10.1001/jamainternmed.2015.4789. [DOI] [PubMed] [Google Scholar]
- 3.Addison C.L., Bouganim N., Hilton J., Vandermeer L., Dent S., Amir E., Hopkins S., Kuchuk I., Segal R., Song X., Gertler S., Mazzarello S., Dranitsaris G., Ooi D., Pond G., Clemons M. A phase II, multicentre trial evaluating the efficacy of de-escalated bisphosphonate therapy in metastatic breast cancer patients at low-risk of skeletal-related events. Breast Cancer Res. Treat. 2014;144(3):615–624. doi: 10.1007/s10549-014-2906-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amadori D., Aglietta M., Alessi B., Gianni L., Ibrahim T., Farina G., Gaion F., Bertoldo F., Santini D., Rondena R., Bogani P., Ripamonti C.I. Efficacy and safety of 12-weekly versus 4-weekly zoledronic acid for prolonged treatment of patients with bone metastases from breast cancer (ZOOM): a phase 3, open-label, randomised, non-inferiority trial. Lancet Oncol. 2013;14(7):663–670. doi: 10.1016/S1470-2045(13)70174-8. [DOI] [PubMed] [Google Scholar]
- 5.Amir E., Freedman O., Carlsson L., Dranitsaris G., Tomlinson G., Laupacis A., Tannock I.F., Clemons M. Randomized feasibility study of de-escalated (every 12 wk) versus standard (every 3 to 4 wk) intravenous pamidronate in women with low-risk bone metastases from breast cancer. Am. J. Clin. Oncol. 2013;36(5):436–442. doi: 10.1097/COC.0b013e3182568f7a. [DOI] [PubMed] [Google Scholar]
- 6.Himelstein A.L., Foster J.C., Khatcheressian J.L., Roberts J.D., Seisler D.K., Novotny P.J., Qin R., Go R.S., Grubbs S.S., O'Connor T., Velasco M.R., Jr., Weckstein D., O'Mara A., Loprinzi C.L., Shapiro C.L. Effect of Longer-Interval vs Standard Dosing of Zoledronic Acid on Skeletal Events in Patients With Bone Metastases: A Randomized Clinical Trial. JAMA. 2017;317(1):48–58. doi: 10.1001/jama.2016.19425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lipton A., Steger G.G., Figueroa J., Alvarado C., Solal-Celigny P., Body J.J., de Boer R., Berardi R., Gascon P., Tonkin K.S., Coleman R., Paterson A.H., Peterson M.C., Fan M., Kinsey A., Jun S. Randomized active-controlled phase II study of denosumab efficacy and safety in patients with breast cancer-related bone metastases. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007;25(28):4431–4437. doi: 10.1200/JCO.2007.11.8604. [DOI] [PubMed] [Google Scholar]
- 8.Awan A.A., Paterson A., Clemons M. De-Escalation of Bone-Modifying Agents in Patients With Bone Metastases: The Best of Times and the Worst of Times? Journal of oncology practice. 2018;14(8):465–467. doi: 10.1200/JOP.18.00393. [DOI] [PubMed] [Google Scholar]
- 9.Awan A.A., Hutton B., Hilton J., Mazzarello S., Van Poznak C., Vandermeer L., Bota B., Stober C., Sienkiewicz M., Fergusson D., Shorr R., Clemons M. De-escalation of bone-modifying agents in patients with bone metastases from breast cancer: a systematic review and meta-analysis. Breast Cancer Res Treat. 2019;176(3):507–517. doi: 10.1007/s10549-019-05265-1. [DOI] [PubMed] [Google Scholar]
- 10.Hong B.Y., Ibrahim M.F., Fernandes R., Mazzarello S., Hutton B., Shorr R., Clemons M. De-escalation of bone-targeted agents for metastatic prostate cancer. Current oncology. 2016;23(1):e77–e78. doi: 10.3747/co.23.2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dhesy-Thind S., Fletcher G.G., Blanchette P.S., Clemons M.J., Dillmon M.S., Frank E.S., Gandhi S., Gupta R., Mates M., Moy B., Vandenberg T., Poznak C.H.V. Use of Adjuvant Bisphosphonates and Other Bone-Modifying Agents in Breast Cancer: A Cancer Care Ontario and American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2017;35(18):2062–2081. doi: 10.1200/JCO.2016.70.7257. [DOI] [PubMed] [Google Scholar]
- 12.AlZahrani M., Clemons M., Vandermeer L., Sienkiewicz M., Awan A.A., Hutton B., Pond G.R., Ng T.L. Real-world practice patterns and attitudes towards de-escalation of bone-modifying agents in patients with bone metastases from breast and prostate cancer: A physician survey. Journal of bone oncology. 2021;26 doi: 10.1016/j.jbo.2020.100339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alzahrani M., Clemons M., Sienkiewicz M., Shrem N.S., McGee S.F., Vandermeer L., Sehdev S., Savard M.F., Awan A., Canil C., Hutton B., Pond G., Saunders D., Ng T. Perceptions around bone-modifying agent use in patients with bone metastases from breast and castration resistant prostate cancer: a patient survey. Support Care Cancer. 2021:1–10. doi: 10.1007/s00520-021-06238-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hutton B., Addison C., Mazzarello S., Joy A.A., Bouganim N., Fergusson D., Clemons M. De-escalated administration of bone-targeted agents in patients with breast and prostate cancer-A survey of Canadian oncologists. Journal of bone oncology. 2013;2(2):77–83. doi: 10.1016/j.jbo.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hutton B., Morretto P., Emmenegger U., Mazzarello S., Kuchuk I., Addison C.L., Crawley F., Canil C., Malone S., Berry S., Fergusson D., Clemons M. Bone-targeted agent use for bone metastases from breast cancer and prostate cancer: A patient survey. Journal of bone oncology. 2013;2(3):105–109. doi: 10.1016/j.jbo.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clemons M., Ong M., Stober C., Ernst S., Booth C., Canil C., Mates M., Robinson A., Blanchette P., Joy A.A. A randomised trial of 4-versus 12-weekly administration of bone-targeted agents in patients with bone metastases from breast or castration-resistant prostate cancer. Eur. J. Cancer. 2021;142:132–140. doi: 10.1016/j.ejca.2020.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Basulaiman B., Awan A.A., Fergusson D., Vandermeer L., Arnaout A., Hilton J., Hutton B., Joy A.A., Robinson A., Califaretti N., Stober C., Sienkiewicz M., Thavorn K., Clemons M. REaCT); Breast cancer research and treatment: 2019. Creating a pragmatic trials program for breast cancer patients: Rethinking Clinical Trials. [DOI] [PubMed] [Google Scholar]
- 18.Chow E., Hird A., Velikova G., Johnson C., Dewolf L., Bezjak A., Wu J., Shafiq J., Sezer O., Kardamakis D., van der Linden Y., Ma B., Castro M., Arnalot P.F., Ahmedzai S., Clemons M., Hoskin P., Yee A., Brundage M., Bottomley A. The European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire for patients with bone metastases: the EORTC QLQ-BM22. Eur J Cancer. 2009;45(7):1146–1152. doi: 10.1016/j.ejca.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 19.Raman S., Ding K., Chow E., Meyer R.M., van der Linden Y.M., Roos D., Hartsell W.F., Hoskin P., Wu J.S., Nabid A. Minimal clinically important differences in the EORTC QLQ-C30 and brief pain inventory in patients undergoing re-irradiation for painful bone metastases. Qual. Life Res. 2018;27(4):1089–1098. doi: 10.1007/s11136-017-1745-8. [DOI] [PubMed] [Google Scholar]
- 20.Cramarossa G., Zeng L., Zhang L., Tseng L.-M., Hou M.-F., Fairchild A., Vassiliou V., Jesus-Garcia R., Alm El-Din M.A., Kumar A. Predictive factors of overall quality of life in advanced cancer patients using EORTC QLQ-C30. Expert review of pharmacoeconomics & outcomes research. 2014;14(1):139–146. doi: 10.1586/14737167.2014.864560. [DOI] [PubMed] [Google Scholar]
- 21.Tu M.M., Clemons M., Stober C., Jeong A., Vandermeer L., Mates M., Blanchette P., Joy A.A., Aseyev O., Pond G. Cost-Effectiveness Analysis of 12-Versus 4-Weekly Administration of Bone-Targeted Agents in Patients with Bone Metastases from Breast and Castration-Resistant Prostate Cancer. Current Oncology. 2021;28(3):1847–1856. doi: 10.3390/curroncol28030171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hortobagyi G.N., Van Poznak C., Harker W.G., Gradishar W.J., Chew H., Dakhil S.R., Haley B.B., Sauter N., Mohanlal R., Zheng M., Lipton A. Continued Treatment Effect of Zoledronic Acid Dosing Every 12 vs 4 Weeks in Women With Breast Cancer Metastatic to Bone: The OPTIMIZE-2 Randomized Clinical Trial. JAMA oncology. 2017;3(7):906–912. doi: 10.1001/jamaoncol.2016.6316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ng T.L., Tu M.M., Ibrahim M.F.K., Basulaiman B., McGee S.F., Srikanthan A., Fernandes R., Vandermeer L., Stober C., Sienkiewicz M., Jeong A., Saunders D., Awan A.A., Hutton B., Clemons M.J. Long-term impact of bone-modifying agents for the treatment of bone metastases: a systematic review. Support Care Cancer. 2020 doi: 10.1007/s00520-020-05556-0. [DOI] [PubMed] [Google Scholar]
- 24.Fizazi K., Lipton A., Mariette X., Body J.-J., Rahim Y., Gralow J.R., Gao G., Wu L., Sohn W., Jun S. Randomized phase II trial of denosumab in patients with bone metastases from prostate cancer, breast cancer, or other neoplasms after intravenous bisphosphonates. J. Clin. Oncol. 2009;27(10):1564–1571. doi: 10.1200/JCO.2008.19.2146. [DOI] [PubMed] [Google Scholar]
- 25.Clemons M.J., Dranitsaris G., Ooi W.S., Yogendran G., Sukovic T., Wong B.Y., Verma S., Pritchard K.I., Trudeau M., Cole D.E. Phase II trial evaluating the palliative benefit of second-line zoledronic acid in breast cancer patients with either a skeletal-related event or progressive bone metastases despite first-line bisphosphonate therapy. J. Clin. Oncol. 2006;24(30):4895–4900. doi: 10.1200/JCO.2006.05.9212. [DOI] [PubMed] [Google Scholar]
- 26.Jacobs C., Kuchuk I., Bouganim N., Smith S., Mazzarello S., Vandermeer L., Dranitsaris G., Dent S., Gertler S., Verma S. A randomized, double-blind, phase II, exploratory trial evaluating the palliative benefit of either continuing pamidronate or switching to zoledronic acid in patients with high-risk bone metastases from breast cancer. Breast Cancer Res. Treat. 2016;155(1):77–84. doi: 10.1007/s10549-015-3646-2. [DOI] [PubMed] [Google Scholar]
- 27.Clinicaltrials.gov, A Randomised Trial Comparing Continuation or De-escalation of Bone Modifying Agents (BMA) in Patients Treated for Over 2 Years for Bone Metastases From Either Breast or Castration-resistant Prostate Cancer (REaCT-Hold BMA), 2019. https://www.clinicaltrials.gov/ct2/show/NCT04549207. (Accessed June 9 2021).
- 28.Templeton A.J., Stalder L., Bernhard J., Brauchli P., Gillessen S., Hayoz S., Klingbiel D., Matter-Walstra K., Thurlimann B.J., Von Moos R. American Society of Clinical Oncology; REDUSE): 2014. Prevention of symptomatic skeletal events with denosumab administered every 4 weeks versus every 12 weeks: A noninferiority phase III trial (SAKK 96/12. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.