Abstract
Background
Vaccine‐induced immune thrombotic thrombocytopenia (VITT) is a rare distinctive syndrome characterized by unusual site thrombosis accompanied by thrombocytopenia after ChAdOx1 nCoV‐19 vaccination. Platelet‐activating anti–platelet factor 4–dependent antibodies (anti‐PF4 Abs) were detected in most cases of VITT. To date, data from Asian countries are lacking.
Objectives
To determine the prevalence of thrombocytopenia, anti‐PF4 Abs, and D‐dimer elevation in Thai people administered the ChAdOx1 vaccine.
Patients/Methods
A total of 521 vaccinated and 146 nonvaccinated subjects were enrolled. Blood samples were collected to determine platelet counts, anti‐PF4 Abs using ELISA and D‐dimer levels 5 to 30 days after the first vaccination.
Results
None of the participants developed thrombocytopenia or had significantly decreased platelet counts from baseline after ChAdOx1 vaccination. The frequencies of anti‐PF4 Abs between vaccinated (16/521; 3.1%; 95% confidence interval [CI], 1.8‐4.9) and nonvaccinated Thai people (6/146; 4.1%; 95% CI, 1.5‐8.7) were similar. None of the detectable anti‐PF4 Abs activated platelets in vitro. The average D‐dimer levels between vaccinated and control groups were similar (282.2 ± 286.3 vs 267.8 ± 219.3 ng/mL; P = 0.58). Four vaccinated and one nonvaccinated participants had markedly elevated D‐dimer levels >2000 ng/mL without detectable anti‐PF4 Abs. Imaging studies of these asymptomatic subjects revealed incidental pulmonary embolism in a vaccinated elderly woman.
Conclusions
This study demonstrated a low prevalence of thrombocytopenia and pathogenic anti‐PF4 Abs after ChAdOx1 vaccination. D‐dimer testing revealed no significant coagulation activation. Routine tests for platelet counts, anti‐PF4 Abs, and D‐dimer levels are not recommended for VITT screening without clinical suspicion.
Keywords: platelet factor 4, prevalence, thrombocytopenia, thrombosis, vaccine
Essentials.
Low platelet counts were not observed in 521 Thai people after ChAdOx1 vaccination.
The frequency of platelet‐activating antibodies was low after ChAdOx1 vaccination.
No significant activation of blood clotting was observed after ChAdOx1 vaccination.
Blood tests are not recommended in asymptomatic people administered the ChAdOx1 vaccine.
1. INTRODUCTION
Vaccines against severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) are key medical interventions that offer a global hope to end the coronavirus disease 2019 (COVID‐19) pandemic by controlling the spread of the virus. The ChAdOx1 nCoV‐19 adenoviral vector vaccine (AstraZeneca, Cambridge, England) has demonstrated its efficacy in reducing the risk of COVID‐19 and hospitalization after the national mass rollout.1, 2 Additionally, it has shown excellent safety in large clinical trials and real‐world studies without increasing the risk of venous thromboembolism, stroke, and myocardial infarction.1, 2, 3 However, there have been reports of rare thrombosis, especially in cerebral veins, accompanied by thrombocytopenia and disseminated intravascular coagulation 5 to 30 days after ChAdOx1 vaccination.4, 5, 6, 7, 8 This syndrome is known as vaccine‐induced immune thrombotic thrombocytopenia (VITT).4, 5 Although the pathogenesis of VITT is largely unknown, platelet‐activating anti–platelet factor 4–dependent antibodies (anti‐PF4 Abs) have been identified as pathogenic antibodies using ELISA, detecting anti‐PF4/polyanion IgG antibodies in almost all patients.4, 5, 6, 7, 8 Markedly elevated D‐dimer levels have also been observed in all VITT cases.4, 5, 6, 7, 8 VITT was subsequently documented in patients administered the Ad26.COV2.S adenoviral vector vaccine (Johnson & Johnson, New Brunswick, NJ, USA) in the United States.9 Therefore, VITT may be a distinctive complication of the adenoviral vector vaccines against SARS‐CoV‐2.
A meta‐analysis has estimated the pooled incidence of VITT across 10 countries (8 from Europe, Canada, and Australia) after ChAdOx1 vaccination of 0.73 per 100 000 persons.10 Despite its rarity, many European countries have restricted or even terminated the ChAdOx1 vaccine from their national COVID‐19 vaccine campaigns. There have been no reports of confirmed VITT from Asian countries. In Thailand, the ChAdOx1 vaccine is the cornerstone vaccine against SARS‐CoV‐2 and will be administered to at least 30 million Thai people in the second half of 2021. To date, the prevalence of anti‐PF4 Abs after ChAdOx1 vaccination have never been evaluated in Asian cohorts.
This study aimed to estimate the prevalence of thrombocytopenia, anti‐PF4 Abs, and evaluated D‐dimer levels within the first 30 days after ChAdOx1 vaccination among Thai people.
2. MATERIALS AND METHODS
2.1. Subjects
Thai health care workers from King Chulalongkorn Memorial Hospital and faculty members of the Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand, were enrolled. Participants who were administered the first dose of the ChAdOx1 vaccine between 5 and 30 days were eligible for the study. Blood samples were collected between April 16 and April 30, 2021. Unvaccinated health care workers and faculty members were recruited for comparison. Participants with documented thrombocytopenia within 12 months, receiving high‐dose immunosuppressive drugs (such as prednisolone ≥30 mg/day) and/or heparin within 3 months before enrollment were excluded. Informed consent was obtained from all participants. The study was approved by the Institutional Review Board (IRB) of the Faculty of Medicine, Chulalongkorn University (IRB No. 323/64) and was performed in accordance with the Declaration of Helsinki.
Participants with detectable anti‐PF4 Abs and/or markedly elevated D‐dimer levels (>1000 ng/mL) were evaluated for symptoms and signs related to thrombosis, and relevant laboratory and imaging studies were performed.
2.2. Questionnaire
Demographic data and medical history, including data on underlying diseases, current medications, previous vaccination, and solicited and unsolicited adverse reactions developed after ChAdOx1 vaccination were recorded in the questionnaire. Any symptoms and signs suggestive of thromboembolic or hemorrhagic events were assessed and investigated according to standard practice.
2.3. Laboratory measurement
Blood samples were collected in EDTA and citrated tubes, as appropriate. Specimens were processed within 60 minutes of collection. Complete blood count (CBC) was assessed using a Sysmex XN‐9000 hematology analyzer (Sysmex, Kobe, Japan). Platelet‐poor plasma was stored at 80℃ until it was tested.
The following assay kits were purchased: anti‐PF4/heparin Abs of the IgG isotype using Zymutest HIA IgG (Hyphen Biomed, Neuville‐sur‐Oise, France) and Vidas D‐dimer (Biomerieux, Marcy‐l'Étoile, France). All assays were performed according to the manufacturer’s instructions. The negative cutoff optical density (OD) for anti‐PF4 IgG Abs was ≤0.25. The OD values of >0.25 to 0.5, >0.5 to 1.0, and >1.0 were assigned as weakly, moderately, and strongly positive for anti‐PF4 Abs, respectively.
Samples with positive anti‐PF4 Abs ELISA results were tested for platelet‐activating antibodies using in‐house platelet aggregometry to measure heparin‐induced platelet aggregation (HPA), with a few modifications.11 Briefly, 250 µL of normal platelet‐rich plasma (PRP) was incubated with 200 µL (250 µL in cuvettes without heparin) of patient’s plasma for 3 minutes at 37℃ in the siliconized cuvettes of a Chrono‐log Model 700 aggregometer (Chrono‐log Corp., Havertown, PA, USA). Fifty microliters of heparin with final concentrations of 0.5 and 100 U/mL was added to each sample. Plasma samples were tested in duplicate with PRP from healthy donors having known reactive platelets. HPA was also performed using PRP preincubated with the ChAdOx1 vaccine at 1:1000–1:2000 dilution under conditions similar to those described above.
2.4. Statistical analysis
The normality of the data was tested using the Shapiro‐Wilk test. Continuous data were presented as mean (± standard deviation) or median (interquartile range [IQR]), as appropriate. Comparisons between continuous and categorical data were analyzed using Student’s t‐test and the chi‐square test, respectively, as appropriate. Statistical significance was considered when P values were <0.05. All statistical parameters were analyzed using SPSS version 22.0, for Windows (SPSS Inc., Chicago, IL, USA).
3. RESULTS AND DISCUSSION
A total of 521 ChAdOx1‐vaccinated and 146 nonvaccinated Thai subjects were enrolled in the study. The nonvaccinated group had a higher proportion of younger and female participants than the vaccinated group (Table 1). The vaccinated group had a higher proportion of patients with hypertension and dyslipidemia, but a lower proportion of iron deficiency anemia and thyroid diseases, than the nonvaccinated group. The other baseline characteristics were similar. The median time of blood collection was 19 days (IQR, 15‐21 days) after vaccination. Myalgia (58.2%), fever (55.5%), headache (26.7%), nausea (7.1%), and diarrhea (3.8%) were the most common symptoms reported after vaccination. Neurological symptoms such as numbness (1.9%), weakness (1%), or blurred vision (0.2%) were also reported. All symptoms were mild and spontaneously resolved within 72 hours after vaccination. None of the participants sought medical attention.
TABLE 1.
Demographic data among participants vaccinated with the ChAdOx1 nCoV‐19 vaccine and nonvaccinated participants
| Variable |
Vaccinated cohort (N = 521) |
Nonvaccinated control (N = 146) |
P value |
|---|---|---|---|
| Age, y | 42.7 ± 13.4 (range, 22‐83) | 34.5 ± 9.4 (range, 21‐63) | <.001 |
| Age < 60 y, n (%) | 453 (88.5) | 144 (98.6) | <.001 |
| Sex, female, n (%) | 379 (72.7) | 118 (80.8) | .05 |
| Days after vaccination | 19 (IQR, 15‐21; range, 6‐30) | NA | NA |
| Underlying diseases, n (%) | 186 (35.7) | 40 (27.4) | .06 |
| Hypertension | 50 (9.8) | 4 (2.7) | .007 |
| Diabetes mellitus | 13 (2.5) | 1 (0.7) | .18 |
| Dyslipidemia | 76 (14.8) | 8 (5.5) | 0.003 |
| Ischemic stroke | 3 (0.6) | 0 | 0.36 |
| Myocardial infarction | 2 (0.4) | 0 | 0.45 |
| Venous thromboembolism | 0 | 0 | NA |
| Peripheral arterial disease | 0 | 1 (0.7) | 0.06 |
| Cirrhosis | 1 (0.2) | 1 (0.7) | 0.34 |
| Chronic lung disease | 4 (0.8) | 0 | 0.23 |
| Autoimmune disease | 10 (2.0) | 1 (0.7) | 0.30 |
| Others (eg, iron deficiency anemia, allergy, thyroid diseases, depression, etc) | 42 (8.2) | 26 (17.8) | 0.001 |
| Antiplatelet therapy, n (%) | 11 (2.1) | 0 | 0.08 |
| Immunosuppressive therapy, n (%) | 4 (0.8) | 1 (0.7) | 0.36 |
| Other vaccines within 3 months before ChAdOx1 nCoV‐19 vaccine (eg, influenza, HBV, HPV, etc.) | 15 (2.9) | 1 (0.7) | 0.13 |
Abbreviations: HBV, hepatitis B virus; HPV, human papillomavirus; NA, not applicable.
Recent prevaccinated platelet counts (within 3 months) were available for 494 participants in the vaccinated group. The mean platelet counts increased slightly after vaccination. None of the participants were observed to have decreased platelet counts >30% of their baseline values. The mean platelet counts did not differ between the vaccinated and nonvaccinated groups. Three participants had mild thrombocytopenia (platelet count, 100‐150 × 109/L) before vaccination. Their platelet counts after vaccination remained unchanged. The Norwegian cohort reported mild thrombocytopenia (platelet count, 100‐150 × 109/L) in 8 (1.6%) of 492 after ChAdOx1 vaccination.12 However, there were no baseline platelet counts for comparison. Therefore, it is unclear whether mild thrombocytopenia develops after ChAdOx1 vaccination.
In this study, a similar prevalence of anti‐PF4 Abs was observed among vaccinated (16/521; 3.1%, 95% confidence interval [CI], 1.8%‐4.9%) and nonvaccinated participants (6/146; 4.1%; 95% CI, 1.5%‐8.7%) (Figure 1). The manufacturer cutoff OD value of 0.25 was used. Of the 16 anti‐PF4 Abs that were detected after vaccination, 10, 4, and 2 were mildly, moderately, and strongly reactive, respectively. Thirteen (81%) participants were tested between 11 and 21 days after vaccination, and three (19%) were tested between 22 and 30 days after vaccination. Only one nonvaccinated participant had a moderate OD value of 0.80. HPA did not detect platelet‐activating anti‐PF4 Abs. In the Norwegian and German cohorts, the frequencies of anti‐PF4 Abs after ChAdOx1 vaccination were 1.2% (6/492 using OD ≥ 0.4, LIFECODES PF4 IgG ELISA, Immucor, Waukesha, WI, USA) and 8.0% (11 of 138 using OD ≥ 0.5, an in‐house IgG‐specific PF4/polyanion enzyme immunoassay).12, 13 The anti‐PF4 Abs in most VITT cases had very high OD values (>2.0).4, 5, 6, 7, 8 However, a few of them had low OD values (<0.4) (OD cutoff: 0.238, Asserachrom HPIA IgG immunoassay; Stago, Asnières‐sur‐Seine, France).6 Recent studies demonstrated that different ELISA assays (Zymutest, LIFECODES, and Asserachrom) provided excellent VITT diagnostic performance with a sensitivity of >90%.14, 15 Although the frequencies of anti‐PF4 Abs varied among studies after ChAdOx1 vaccination because of different ELISA assays and cutoff OD values, all anti‐PF4 Abs were nonpathogenic in all cohorts. Additionally, the frequencies of anti‐PF4 Abs detected in the vaccinated group were not higher than those detected in the nonvaccinated group, suggesting a very low seroconversion rate after ChAdOx1 vaccination. The German cohort revealed a low frequency (2/138; 1.4%) of seroconversion after ChAdOx1 vaccination.13 Furthermore, the prevalence of anti‐PF4 Abs in healthy blood donors was reported to be ≈6%.16 Therefore, the detection of anti‐PF4 Abs, especially at low OD values, in asymptomatic individuals after ChAdOx1 vaccination is clinically insignificant.
FIGURE 1.
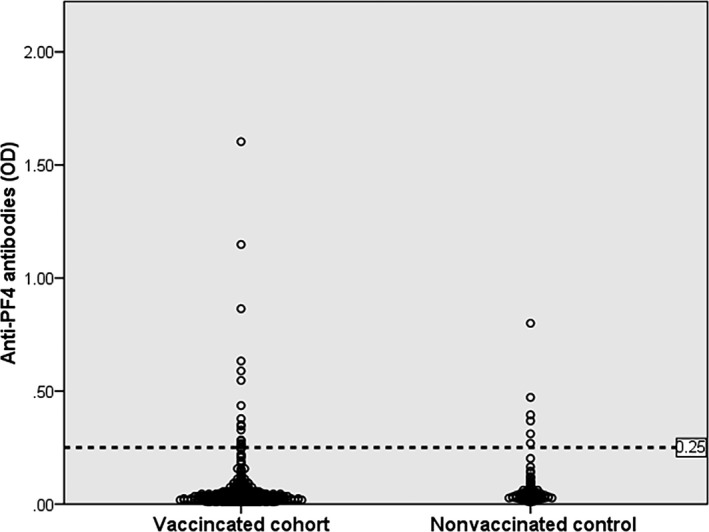
The frequencies of anti–platelet factor 4 (anti‐PF4) antibodies using ELISA (cutoff optical density [OD] value >0.25) detected in 521 Thai participants after ChAdOx1 nCoV‐19 vaccination and in 146 nonvaccinated controls
D‐dimer levels are markedly elevated in almost all documented VITT cases.4, 5, 6, 7, 8 International guidelines recommend D‐dimer testing for VITT screening.17, 18 The British guidance has suggested that VITT is very unlikely when D‐dimer levels are <2000 ng/mL.18 In this study, the average D‐dimer levels between the vaccinated and nonvaccinated cohorts were similar (282.2 ± 286.3 vs. 267.8 ± 219.3 ng/mL; P = 0.58) (Figure 2). Additionally, there were no significant differences in the proportions of elevated D‐dimer levels using different cutoff values between both groups (Table 2). These findings suggested no significant coagulation activation after ChAdOx1 vaccination.
FIGURE 2.
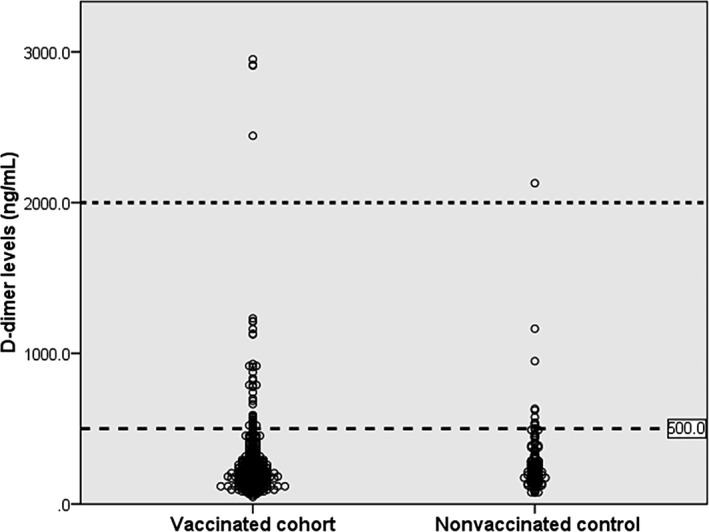
D‐dimer levels in 521 vaccinated Thai participants after ChAdOx1 nCoV‐19 vaccination and in 146 nonvaccinated controls
TABLE 2.
Laboratory data among participants vaccinated with the ChAdOx1 nCoV‐19 vaccine and nonvaccinated participants
| Laboratory variables |
Vaccinated cohort (N = 521) |
Nonvaccinated control (N = 146) |
P value |
|---|---|---|---|
| Prevaccinated platelet count (× 109/L; N = 494) | 278.0 ± 62.9 | NA | <0.001a |
| Platelet count (× 109/L) | 285.3 ± 61.5 | 284.8 ± 62.3 | 0.93b |
| Anti‐PF4 Abs, n (%; 95% CI) | |||
| OD > 0.25 | 16 (3.1; 1.8‐4.9) | 6 (4.1; 1.5‐8.7) | 0.39 |
| OD > 0.5 | 6 (1.2; 0.4‐2.5) | 1 (0.7; 0.02‐3.8) | 0.76 |
| OD > 1.0 | 2 (0.4; 0.05‐1.4) | 0 (0; 0‐0.3) | 0.45 |
| OD > 2.0 | 0 (0%; 0‐0.7) | 0 (0; 0‐0.3) | NA |
| D‐dimer (ng/mL) | 282.2 ± 286.3 | 267.8 ± 219.3 | 0.58 |
| > 500 (ng/mL), n (%; 95% CI) | 41 (7.9; 5.7‐10.5) | 10 (6.8; 3.3‐12.2) | 0.68 |
| > 1000 (ng/mL), n (%; 95% CI) | 10 (1.9; 0.9‐3.5) |
2 (1.4; 0.2‐4.9) |
0.70 |
| > 2000 (ng/mL), n (%; 95% CI) | 4 (0.8; 0.2‐2.0) | 1 (0.7; 0.02‐3.8) | 0.91 |
| > 4000 (ng/mL), n (%; 95% CI) | 0 (0; 0‐0.7) | 0 (0; 0‐0.3) | NA |
Anti‐PF4 Abs, anti–platelet factor 4–dependent antibodies; CI, confidence interval; OD, optical density.
Prevaccinated platelet counts versus postvaccinated platelets count (paired‐sample t test).
Postvaccinated platelet counts versus platelet counts in the nonvaccinated group.
Four participants in the vaccinated cohort and one participant in the control group had markedly elevated D‐dimer levels of >2000 ng/mL. CBC and D‐dimer testing were repeated in all participants with elevated D‐dimer levels of >1000 ng/mLl. None of the 12 participants (10 in the vaccinated group and 2 in the nonvaccinated group) with D‐dimer levels of >1000 ng/mL had symptoms or signs indicating thrombosis. All participants had normal platelet counts and anti‐PF4 Ab levels, with OD values of <0.25. Among the 12 participants with D‐dimer levels >1000 ng/mL, 5 had persistently elevated D‐dimer levels for >1 month after vaccination. One nonvaccinated young man aged 30 years with persistently elevated D‐dimer levels (2129.04 ng/mL) was recently diagnosed with tuberculous pleuritis and had received antituberculous therapy. Four vaccinated participants with persistently elevated D‐dimer levels underwent imaging studies. Doppler ultrasonography of both lower extremities of a 37‐year‐old man (D‐dimer level: 1350.81 ng/mL) yielded negative results. Three elderly participants aged 61 (D‐dimer level: 2212.84 ng/mL), 76 (3721.87 ng/mL), and 83 (2819.09 ng/mL) years underwent computed tomography of the chest and abdomen for screening of occult cancers and thrombosis. Only one elderly 76‐year‐old woman with obesity and diabetes was diagnosed with acute bilateral segmental and subsegmental pulmonary embolism with radiographic evidence of pulmonary hypertension. Although she remained asymptomatic and had normal oxygen saturation levels, antithrombotic therapy was prescribed. Elevated D‐dimer levels are associated with thrombus formation and increased risk of venous thromboembolism in healthy population. However, marked elevation in D‐dimer levels could be observed in healthy people without thromboembolism, especially in the geriatric population.19, 20 Routine D‐dimer testing without any clinical suspicion of thrombosis may lead to unnecessary investigations and may not be cost effective.
This study has some limitations. Although the prevalence of anti‐PF4 Abs in the vaccinated and control groups was comparable, comparisons between two groups with different baseline characteristics should be carefully interpreted. Due to the rarity of VITT, it is difficult to be documented in a small cohort. Additionally, the confirmatory assay using HPA for testing functional anti‐PF4 Abs may not be as sensitive as modified heparin‐induced platelet activation or serotonin release assays. However, all participants with detectable anti‐PF4 Abs were periodically evaluated for signs and symptoms related to thrombosis, CBC and D‐dimer levels up to 12 weeks to ascertain their nonfunctionality.
In conclusion, this is the first Asian cohort study that demonstrated a low prevalence of thrombocytopenia and pathogenic anti‐PF4 Abs after ChAdOx1 vaccination. The evidence did not suggest significant activation of platelets and the coagulation system after ChAdOx1 vaccination. One case of incidental pulmonary embolism observed in an elderly vaccinated woman was probably not related to vaccination. Routine screening tests for CBC, anti‐PF4 Abs, and D‐dimer levels after ChAdOx1 vaccination are not recommended for VITT screening without clinical suspicion.
RELATIONSHIP DISCLOSURE
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AUTHOR CONTRIBUTIONS
NU, PW, LP, and PR designed the study. RV, ST, and WJ collected data. BA, and AS performed laboratory tests. TT performed interpretation of imaging studies. NU analyzed data and wrote the first draft of manuscript. All authors discuss data, revised the manuscript, and approved the final version for publication.
ACKNOWLEDGEMENTS
This study was funded by Jaikrating Foundation, King Chulalongkorn Memorial Hospital, Research Funding of Division of Hematology, Chulalongkorn University, and Thai Society of Hematology.
Uaprasert N, Watanaboonyongcharoen P, Vichitratchaneekorn R, et al. Prevalence of thrombocytopenia, anti–platelet factor 4 antibodies and D‐dimer elevation in Thai people After ChAdOx1 nCoV‐19 vaccination. Res Pract Thromb Haemost. 2021;5:e12580. 10.1002/rth2.12580
Handling Editor: Cihan Ay
REFERENCES
- 1.Menni C, Klaser K, May A, et al. Vaccine side‐effects and SARS‐CoV‐2 infection after vaccination in users of the COVID Symptom Study app in the UK: a prospective observational study. Lancet Infect Dis. 2021;21:939‐949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vasileiou E, Simpson CR, Shi T, et al. Interim findings from first‐dose mass COVID‐19 vaccination roll‐out and COVID‐19 hospital admissions in Scotland: a national prospective cohort study. Lancet. 2021;397:1646‐1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Voysey M, Costa Clemens SA, Madhi SA, et al. Single‐dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV‐19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet. 2021;397:881‐891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schultz NH, Sørvoll IH, Michelsen AE, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV‐19 vaccination. N Engl J Med. 2021;384:2124‐2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov‐19 vaccination. N Engl J Med. 2021;384:2092‐2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scully M, Singh D, Lown R, et al. Pathologic antibodies to platelet factor 4 after ChAdOx1 nCoV‐19 vaccination. N Engl J Med. 2021;384:2202‐2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tiede A, Sachs UJ, Czwalinna A, et al. Prothrombotic immune thrombocytopenia after COVID‐19 vaccine. Blood. 2021;138:350–353. 10.1182/blood.2021011958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Althaus K, Möller P, Uzun G, et al. Antibody‐mediated procoagulant platelets in SARS‐CoV‐2‐ vaccination associated immune thrombotic thrombocytopenia. Haematologica. 2021;106:2170–2179. 10.3324/haematol.2021.279000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.See I, Su JR, Lale A, et al. US case reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S vaccination, March 2 to April 21, 2021. JAMA. 2021;325:2448‐2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan BTB, Bobos P, Odutayo A, Pai M. Meta‐analysis of risk of vaccine‐induced immune thrombotic thrombocytopenia following ChAdOx1‐S recombinant vaccine. https://www.medrxiv.org/content/ 10.1101/2021.05.04.21256613v1 [DOI] [Google Scholar]
- 11.Isenhart CE, Brandt JT. Platelet aggregation studies for the diagnosis of heparin‐induced thrombocytopenia. Am J Clin Pathol. 1993;99:324‐330. [DOI] [PubMed] [Google Scholar]
- 12.Sørvoll IH, Horvei KD, Ernstsen SL, et al. An observational study to identify the prevalence of thrombocytopenia and anti‐PF4/polyanion antibodies in Norwegian health care workers after COVID‐19 vaccination. J Thromb Haemost. 2021;19:1813‐1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thiele T, Ulm L, Holtfreter S, et al. Frequency of positive anti‐PF4/polyanion antibody tests after COVID‐19 vaccination with ChAdOx1 nCoV‐19 and BNT162b2. Blood. 2021;138(4):299‐303. 10.1182/blood.2021012217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Platton S, Bartlett A, MacCallum P, et al. Evaluation of laboratory assays for anti‐platelet factor 4 antibodies after ChAdOx1 nCOV‐19 vaccination. J Thromb Haemost. 2021;19(8):2007‐2013. 10.1111/jth.15362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sachs UJ, Cooper N, Czwalinna A, et al. PF4‐dependent immunoassays in patients with vaccine‐induced immune thrombotic thrombocytopenia (VITT): results of an inter‐laboratory comparison. Thromb Haemost. 2021. 10.1055/a-1535-9002 [DOI] [PubMed] [Google Scholar]
- 16.Hursting MJ, Pai PJ, McCracken JE, et al. Platelet factor 4/heparin antibodies in blood bank donors. Am J Clin Pathol. 2010;134:774‐780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nazy I, Sachs UJ, Arnold DM, et al. Recommendations for the clinical and laboratory diagnosis of VITT against COVID‐19: communication from the ISTH SSC Subcommittee on Platelet Immunology. J Thromb Haemost. 2021;19:1585‐1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guidance from the Expert Haematology Panel (EHP) on Covid‐19 vaccine‐induced immune thrombocytopenia and thrombosis (VITT). https://b‐s‐h.org.uk/media/19590/guidance‐version‐17‐on‐mngmt‐of‐vitt‐20210420.pdf Accessed 25 May 2021. [Google Scholar]
- 19.Cushman M, Folsom AR, Wang L, et al. Fibrin fragment D‐dimer and the risk of future venous thrombosis. Blood. 2003;15(101):1243‐1248. [DOI] [PubMed] [Google Scholar]
- 20.Currie MS, Rao MK, Blazer DG, Cohen HJ. Age and functional correlations of markers of coagulation and inflammation in the elderly: functional implications of elevated crosslinked fibrin degradation products (D‐dimers). J Am Geriatr Soc. 1994;42:738‐742. [DOI] [PubMed] [Google Scholar]


