Significance
For centuries, it has been recognized that asthma severity shows a daily rhythm, with the worst symptoms at night. However, it is unclear to what degree this is driven by everyday behaviors, such as sleep, physical activity, and body posture changes, versus intrinsic rhythms generated by the internal body clock (i.e., the circadian system). To distinguish the contribution of the circadian system, patients with asthma underwent two complementary gold-standard circadian protocols: a constant routine and a forced desynchrony protocol. These highly standardized protocols revealed that the endogenous circadian system plays a significant role in modulating pulmonary function and asthma severity independent of sleep and other daily behavioral or environmental cycles. Moreover, these circadian influences summate with daily behavioral and environmental effects to drive asthma to be worst at night.
Keywords: circadian rhythms, asthma, sleep, circadian misalignment, bronchodilation
Abstract
Asthma often worsens at night. To determine if the endogenous circadian system contributes to the nocturnal worsening of asthma, independent of sleep and other behavioral and environmental day/night cycles, we studied patients with asthma (without steroid use) over 3 wk in an ambulatory setting (with combined circadian, environmental, and behavioral effects) and across the circadian cycle in two complementary laboratory protocols performed in dim light, which separated circadian from environmental and behavioral effects: 1) a 38-h “constant routine,” with continuous wakefulness, constant posture, 2-hourly isocaloric snacks, and 2) a 196-h “forced desynchrony” incorporating seven identical recurring 28-h sleep/wake cycles with all behaviors evenly scheduled across the circadian cycle. Indices of pulmonary function varied across the day in the ambulatory setting, and both laboratory protocols revealed significant circadian rhythms, with lowest function during the biological night, around 4:00 AM, uncovering a nocturnal exacerbation of asthma usually unnoticed or hidden by the presence of sleep. We also discovered a circadian rhythm in symptom-based rescue bronchodilator use (β2-adrenergic agonist inhaler) whereby inhaler use was four times more likely during the circadian night than day. There were additive influences on asthma from the circadian system plus sleep and other behavioral or environmental effects. Individuals with the lowest average pulmonary function tended to have the largest daily circadian variations and the largest behavioral cycle effects on asthma. When sleep was modeled to occur at night, the summed circadian, behavioral/environmental cycle effects almost perfectly matched the ambulatory data. Thus, the circadian system contributes to the common nocturnal worsening of asthma, implying that internal biological time should be considered for optimal therapy.
Asthma is characterized by bronchial hyperreactivity leading to airway inflammation, bronchoconstriction, and symptoms of “chest tightness.” It has been recognized for hundreds of years that asthma severity generally increases at night, producing what had historically been termed “nocturnal asthma.” For instance, in 1698, the physician Sir John Floyer recognized from his own experience that “at first waking, about one or two of the Clock in the Night, the Fit of the Asthma more evidently begins” (1). Nocturnal worsening of asthma occurs in as many as 75% of patients with asthma, equating to 20 million people in the United States alone (2–6). The variation across the day and night in peak expiratory flow (PEF) can be as much as 50% (7, 8). Also, the highest rate of asthma exacerbations leading to respiratory failure or death occurs across the night (3, 9, 10). Therefore, understanding the mechanisms underlying the daily variability in asthma severity could have major diagnostic and therapeutic implications.
Behavioral and environmental factors are known to affect asthma severity, including exercise, air temperature, pollution, the sleep/wake cycle (11), changes in posture (12), and the sleeping environment (13). However, it is also possible that the endogenous circadian timing system contributes to nocturnal worsening of asthma (14). The circadian system—composed of the central circadian pacemaker in the suprachiasmatic nucleus of the hypothalamus and circadian oscillators in most organs and tissues of the body—orchestrates ∼24-h rhythms in physiology and behavior (15). This endogenous timing system may influence the pulmonary and inflammatory system via the autonomic nervous system (16), humoral factors (17), and/or local molecular clocks (18, 19).
We tested the hypothesis that the circadian system, independent of sleep and other behavioral and environmental factors, contributes to the nocturnal worsening of asthma in humans.
Results
To determine the independent effect of the endogenous circadian system on asthma, we studied participants with asthma without steroid use (to prevent the influence of timed exogenous steroid intake from affecting the circadian pattern of pulmonary function) and control participants throughout two intensive and complementary in-laboratory protocols (Fig. 1): 1) a 38-h “constant routine” (CR) protocol to estimate circadian rhythmicity in asthma severity in the absence of environmental and behavioral changes (by maintaining continuous wakefulness during semirecumbent rest in dim light conditions and with fixed-interval isocaloric intakes) (14), and 2) a 196-h “forced desynchrony” (FD) protocol with all behaviors scheduled evenly across the circadian cycle in dim light conditions, enabling quantification of independent circadian effects on asthma severity (while accounting for all behaviors) and behavioral effects on asthma severity (while controlling for circadian phase) (20). Collecting data in two different circadian protocols increases the rigor of the approach and would add to the robustness of any circadian findings if similar results are obtained. The FD also permits determination of any interaction between environmental/behavioral effects and circadian effects (20). In addition, to compare circadian rhythms in asthma severity with daily patterns observed under typical behavioral and environmental conditions in the home, measurements of pulmonary function were recorded across each day of a 3-wk ambulatory protocol. We also assessed daily patterns of symptom-based rescue bronchodilator use (β2-adrenergic agonist inhaler) in ambulatory and laboratory protocols. So that rescue inhaler use did not interfere with measurements of asthma severity, all pulmonary function and airway resistance measurements within 6 h following beta2-adrenergic agonist inhaler use were excluded from all analyses (21).
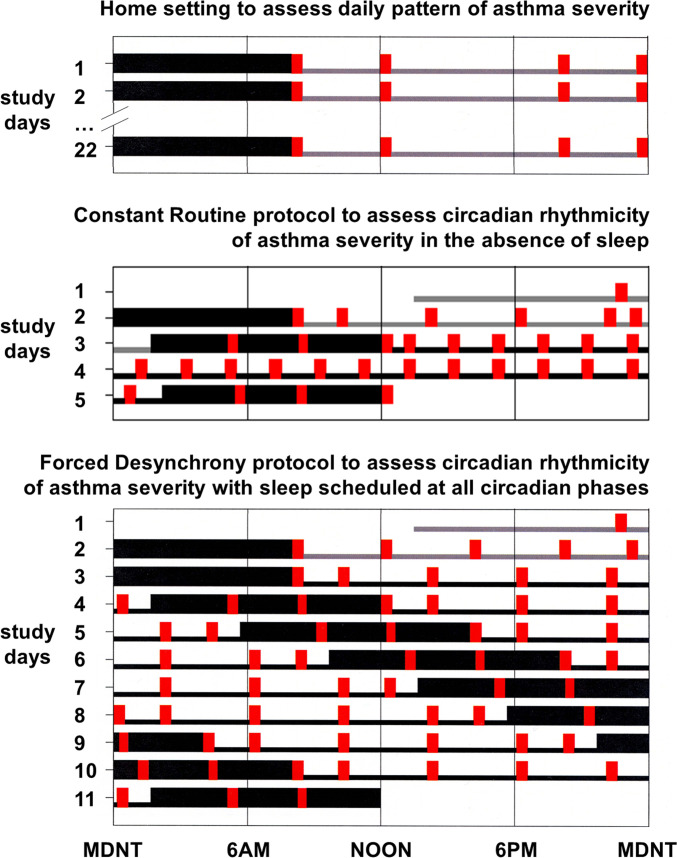
Fig. 1.
Ambulatory, CR, and FD protocols. Wide black bars represent sleep episodes; narrow gray bars represent wakefulness in normal lighting conditions; narrow black bars represent wake episodes in dim light (∼2 lx); and red bars represent recordings of FEV1 (plus airway resistance when in the laboratory) and breathing discomfort every 2 to 4 h, including immediately after regularly scheduled awakenings from sleep when in the laboratory. Times of any rescue medication use were also recorded. The ∼3 wk (median 22 d) ambulatory protocol (Top) assessed the daily pattern of asthma in the home. Participants maintained a self-selected regular 8-h sleep schedule. The CR protocol (Middle) assessed the circadian rhythm of asthma without sleep while minimizing masking effects of the environment and behaviors. The CR lasted 5 d with 2 baseline days and nights and 38 h of wakefulness in a semirecumbent posture in an individual laboratory suite in dim light, with 2-hourly isocaloric snacks. The FD protocol (Bottom) assessed the separate effects of the circadian system and the behavioral sleep/wake schedule on asthma. The FD lasted 11 d with 2 baseline days and nights and then seven × 28-h “days” in an individual laboratory suite in dim light.
Other physiologic aspects and detailed specific methodologies of these studies have been previously published, including descriptions of circadian rhythmicity in cardiovascular and metabolic variables, circulating adipokines, nitrogen regulation, blood pressure, cardiac and physical activity dynamics, and sleep inertia (20, 22–27).
Participants.
The demographics of the participants and the demarcation of which protocols they completed are presented in SI Appendix, Table S1. Seventeen participants with asthma completed the ambulatory protocol (10 women and 7 men; mean age 26.5 y, range 20 to 41 y; mean body mass index [BMI] 24.3 kg/m2, range 19.0 to 28.9 kg/m2), 13 of whom completed the FD protocol and 11 of whom completed the CR protocol (10 participants with asthma completed all three protocols). Ten control participants without asthma completed both the ambulatory and CR protocols (8 women and 2 men; mean age 28.0 y, range 19 to 44 y; mean BMI 25.3 kg/m2, range 23.0 to 26.7 kg/m2), and 5 of these 10 control participants completed the FD protocol. There were no significant differences between the asthma and control participants in age or in BMI. There was a significantly higher ratio of males versus females in the control group as compared with the asthma group in both the CR and FD protocol (both P < 0.001; χ2 analysis). As expected, an indirect estimate of airway inflammation, fractional exhaled nitric oxide (FeNO), was significantly higher in participants with asthma (mean 25.0 ppb, range 8.2 to 65.3 ppb) than in control participants (mean 8.5 ppb, range 4.5 to 14.7 ppb; P < 0.001).
Circadian Phases, Circadian Periods, and Habitual Timing of Sleep.
As assessed utilizing the FD protocol, there were no significant differences between asthma and control participants in endogenous circadian period (the duration of one circadian cycle; asthma participants: 24.14 ± 0.08 h; 23.60 to 24.44 h; n = 13; control participants: 24.29 ± 0.13 h; 23.78 to 24.51 h; n = 5), time of core body temperature (CBT) minimum (asthma participants: 4:53 AM ± 23 min; 3:22 AM to 8:42 AM; n = 13; control participants: 4:54 AM ± 45 min; 2:10 AM to 6:24 AM; n = 5), habitual waketime (asthma participants: 8:31 AM ± 23 min; 6:00 AM to 11:00 AM; n = 13; control participants: 7:48 AM ± 44 min; 5:00 AM to 9:00 AM; n = 5), or phase angle of entrainment (the time between CBT minimum and habitual waketime; asthma participants: 3.62 ± 0.28 h; 1.95 to 5.47 h; n = 13; control participants: 2.90 ± 0.48 h; 1.60 to 4.54 h; n = 5). However, when including all participants who completed the ambulatory protocol, an incidental finding was that there was a significant 1 h and 16 min average difference in habitual waketime between asthma participants and control (asthma participants: mean ± SEM: 8:31 AM ± 19 min; range: 6:00 AM to 11:00 AM; n = 17; control participants: 7:15 AM ± 26 min; 5:00 AM to 9:00 AM; n = 10; P = 0.023, two-tailed unpaired t test). For each individual, CBT was used as the circadian phase marker and the time of CBT minimum was assigned as 0 circadian degrees (0°), with one full circadian cycle spanning 360°. All dependent variable data were then assigned corresponding circadian phases.
Average Differences in Pulmonary Function between Participants with Asthma and Matched Controls across All Protocols.
Across 3 wk during the ambulatory protocol, the 17 participants with asthma had an average forced expired volume in 1 s (FEV1) of 75% ± 15% (mean ± SD) of the predicted pulmonary function value expected in asymptomatic participants based on height, age, and sex (28). FEV1 was significantly worse is asthma participants than in the 10 control participants, in whom FEV1 was 100% ± 10% predicted (P < 0.0001; two-tailed t test; Fig. 2A). Similarly, in both laboratory protocols, the asthma participants’ average FEV1 was always lower than in control participants (CR: 82% ± 16% versus 101% ± 10% predicted, respectively, P = 0.003; FD: 80% ± 15% versus 99% ± 9% predicted, respectively, P = 0.018; Fig. 3 B, D, and F) and airway resistance was always higher in participants with asthma than in control participants (CR: 0.51 ± 0.17 versus 0.33 ± 0.10 kP/L/s, respectively, P = 0.008; FD: 0.53 ± 0.17 versus 0.35 ± 0.08 kP/L/s, respectively, P = 0.036; Fig. 3 A, C, and E).
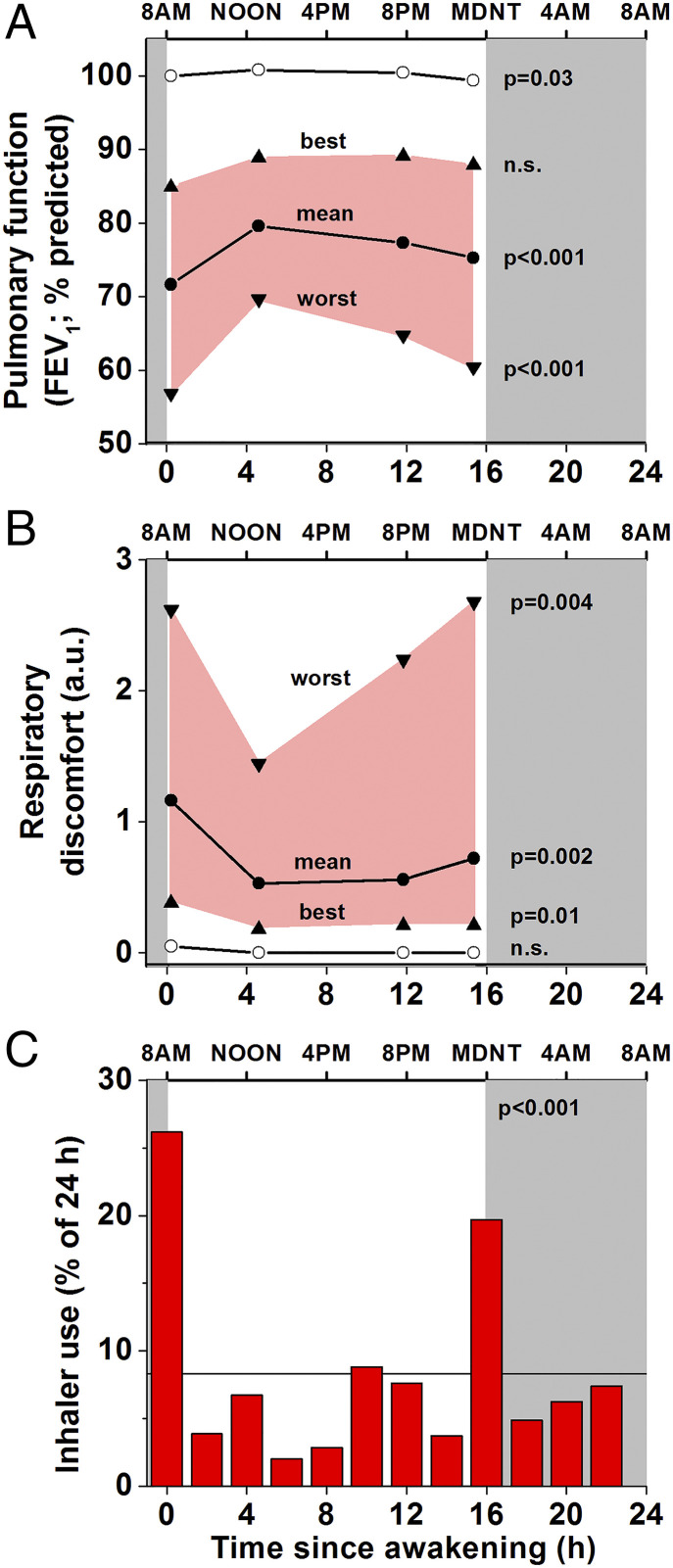
Fig. 2.
Ambulatory protocol: asthma is more severe at awakening and at bedtime compared with the middle of the day. Group data with individuals aligned according to time of scheduled sleep (gray shading). The within-participant ranges of asthma severity were averaged for the group (n = 17; worst [downward triangles], mean, and best [upward triangles] at each measurement time). The most severe asthma (worst FEV1 [A] and respiratory discomfort [B]) occurred before bedtime and upon awakening. Similar, although less striking, daily patterns were seen for mean and best FEV1 and for mean and least discomfort. These times coincided with the highest frequency of (unscheduled) use of bronchodilator rescue medication (red columns, C). Note: all data within 6 h following bronchodilator use were excluded. There were large significant differences between control participants (open circles, n = 10) and asthma participants in FEV1 and respiratory discomfort at all times of day. For controls, only group averages of individuals’ mean FEV1 and discomfort are shown (because FEV1 was close to 100% predicted without respiratory discomfort at all times). The bottom plot shows significant evening and morning peaks in the average distribution of beta2-adrenergic agonist use in those participants (n = 15) who needed to use their inhaler (horizontal line indicates expected frequency assuming no daily variation). P values indicate significance levels for variations relative to time since awakening.
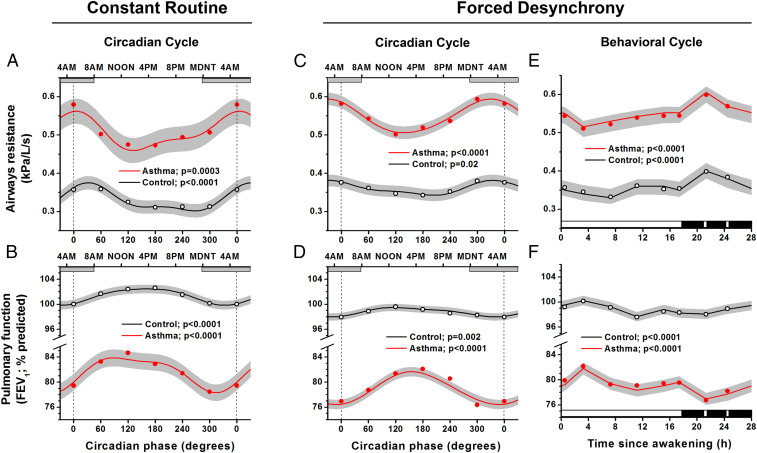
Fig. 3.
Independent effects of circadian system and behavioral sleep/wake cycle on pulmonary function. The CR (A and B: 11 asthma, 10 control) and FD protocols (C and D: 13 asthma, 5 control) show expected mean differences in pulmonary function (FEV1) and airway resistance between asthma and control participants and revealed significant circadian rhythms for both groups in airway resistance and FEV1. Cosinor models, which make use of the precise circadian phase of each measurement for each participant, are shown (red lines represent asthma; black lines represent controls; gray areas represent 95% CI). Circles indicate raw 60° bin-averaged data and are added primarily to illustrate that the model fits the data. X-axes show the circadian phases at the bottom, with corresponding clock times at the top. Gray horizontal bars represent group-average timing of habitual sleep episodes. Both the CR and FD revealed that pulmonary function was worst during the biological night (highest airway resistance and lowest FEV1) around the CBT minimum (vertical dotted lines at 0° = ∼5:00 AM). Right panels (E and F) show independent effects on pulmonary function of the 28-h sleep/wake schedule (averaged across all circadian phases). Black bars represent sleep episode (interspersed with two awakenings for pulmonary function measurements). There were significant changes across the sleep/wake cycle in asthma and control groups, with pulmonary function always best during the wake episode and almost invariably worst at the time of the first scheduled awakening from sleep. P values indicate significance levels for circadian (A–D) and sleep/wake cycle (E and F) variations. Note: for panels A through D, the first and last data points and the beginning and end of the model curves are double-plotted to make it easier to visualize rhythmicity.
Ambulatory Protocol: Daily Pattern of Asthma Severity.
One of the hallmarks of asthma is its variable severity across time. The ambulatory protocol was used to assess the habitual daily pattern of asthma severity, which presumably includes summed and/or interacting behavioral, environmental, and circadian influences on asthma. The within-day pattern was evident, as there was significant variability in FEV1, respiratory discomfort, and rescue inhaler use across the day in participants with asthma (Fig. 2). For the ambulatory data, we report the group-average of each individual’s mean at each of the four measurement times per day across all days of the assessment. In addition, to capture potential clinical relevance, the single worst measurement for a person at each timepoint was determined, and the group averages for each of the four measurement times are shown in Fig. 2. The same was done for the best pulmonary function at each time of day. Focusing on the times of worst FEV1 and worst symptoms across the days, as these are the most clinically relevant, the two scheduled measurement times with most severe asthma were immediately before bedtime and immediately upon awakening (FEV1: 60% ± 19% predicted and 57% ± 18% predicted, respectively [Fig. 2A]; subjective respiratory discomfort: 2.7 ± 1.8 units and 2.6 ± 1.6 units on a Modified 0 to 10 Borg scale, respectively [Fig. 2B]). We note that in the ambulatory condition we did not attempt to assess asthma severity across the night, as this would have required disrupting sleep, although participants were always permitted to use their symptom-based beta2-adrenergic agonist rescue inhaler if needed across the night. Of the 17 asthma participants who participated in the ambulatory setting, one participant did not use rescue medication, and we later learned that one other participant used their beta2-adrenergic agonist inhaler based on a preset schedule rather than symptoms. The 15 remaining participants used their rescue inhalers a total of 331 occasions, which averaged one inhaler use per day per participant (median = 22 uses per participant [range 1 to 54 uses]; median duration of measurements = 22 d per participant [range 14 to 41 d]). Dividing the ambulatory 24-h day into twelve 2-h bins (awakening ± 1 h, etc.), the chance for asthma inhaler use had a significant day/night pattern (P < 0.0001; Friedman) with the highest peak at awakening (odds ratio: 3.1 [95% CI: 1.7 to 4.7]) and a second peak at bedtime (odds ratio: 2.4 [1.4 to 3.3]) (Fig. 2C). Thus, the times of unscheduled rescue inhaler uses (Fig. 2C) coincided with the times of worst FEV1 (Fig. 2A) and maximal discomfort (Fig. 2B).
CR Protocol: Endogenous Circadian Rhythm in Asthma Severity.
In the CR protocol, there were highly significant endogenous circadian rhythms in indices of pulmonary function in participants with asthma, with lowest function during the biological night (i.e., the half of the circadian cycle centered around the circadian CBT minimum, corresponding to ∼11:00 PM to 11:00 AM, or the circadian phase span of 270° to 90°; Fig. 3 A and B). This finding suggests that the circadian variation in pulmonary function would contribute to nocturnal worsening of asthma, including the worsening asthma severity before bedtime and upon awakening in the morning that was revealed by the scheduled measurements in the ambulatory data. From cosinor analyses, the peak-to-trough circadian differences in airway resistance were 0.10 kPa/L/s (20% of the mean) in asthma participants and 0.07 kPa/L/s (22% of the mean) in control participants, and the peak-to-trough circadian differences for FEV1 were 5.6% predicted (7% of the mean) in asthma participants and 2.6% predicted (3% of the mean) in control participants, in whom these indices of pulmonary function also were lowest during the biological night.
FD Protocol: Endogenous Circadian Rhythm in Asthma Severity.
The FD protocol, which uncovers underlying circadian rhythmicity while averaging out all behavioral/environmental factors, also revealed significant endogenous circadian rhythms in airway resistance and FEV1 both in asthma and control participants (Fig. 3 C and D) that were very similar to those observed in the CR (Fig. 3 A and B). Again, the indices of pulmonary function were lowest during the biological night. The peak-to-trough differences in airway resistance were 0.07 kPa/L/s (14% of the mean) in asthma participants and 0.04 kPa/L/s (11%) in control participants, and peak-to-trough differences in FEV1 were 4.7% predicted (6% of the mean) in asthma participants and 1.8% predicted (2%) in control participants. The very similar timing of the cosinor-fitted peaks in these variables between the complementary FD and CR protocols (e.g., for airway resistance at ∼330° in the FD and at ∼360° in the CR) suggests that these are robust circadian influences (Fig. 3 A and B versus C and D). Furthermore, from individual cosinor analyses, 11 of the 13 individuals with asthma who completed the FD protocol had significant circadian rhythms of FEV1, with all 11 demonstrating worst pulmonary function during the biological night, with the range between 320° and 30° (Fig. 4 A and B). Across individuals with asthma, there was a clear negative relationship between the average FEV1 and the amplitude of the circadian rhythmicity in FEV1, such that those individuals with the lowest FEV1 had the largest circadian amplitudes in FEV1 (r = −0.66, P = 0.027; Fig. 4C). To exclude the possibility that the significant circadian rhythms in pulmonary function could have been caused by varying amounts of sleep across the circadian cycle, we also performed analyses wherein all data from the scheduled sleep episodes were excluded. From these data collected during scheduled wakefulness alone, we found in both groups (asthma and control) that the circadian rhythms of FEV1 and airway resistance persisted with virtually identical phases, amplitudes, and significance levels (Fig. 3 C and D versus SI Appendix, Fig. S1 A and B).
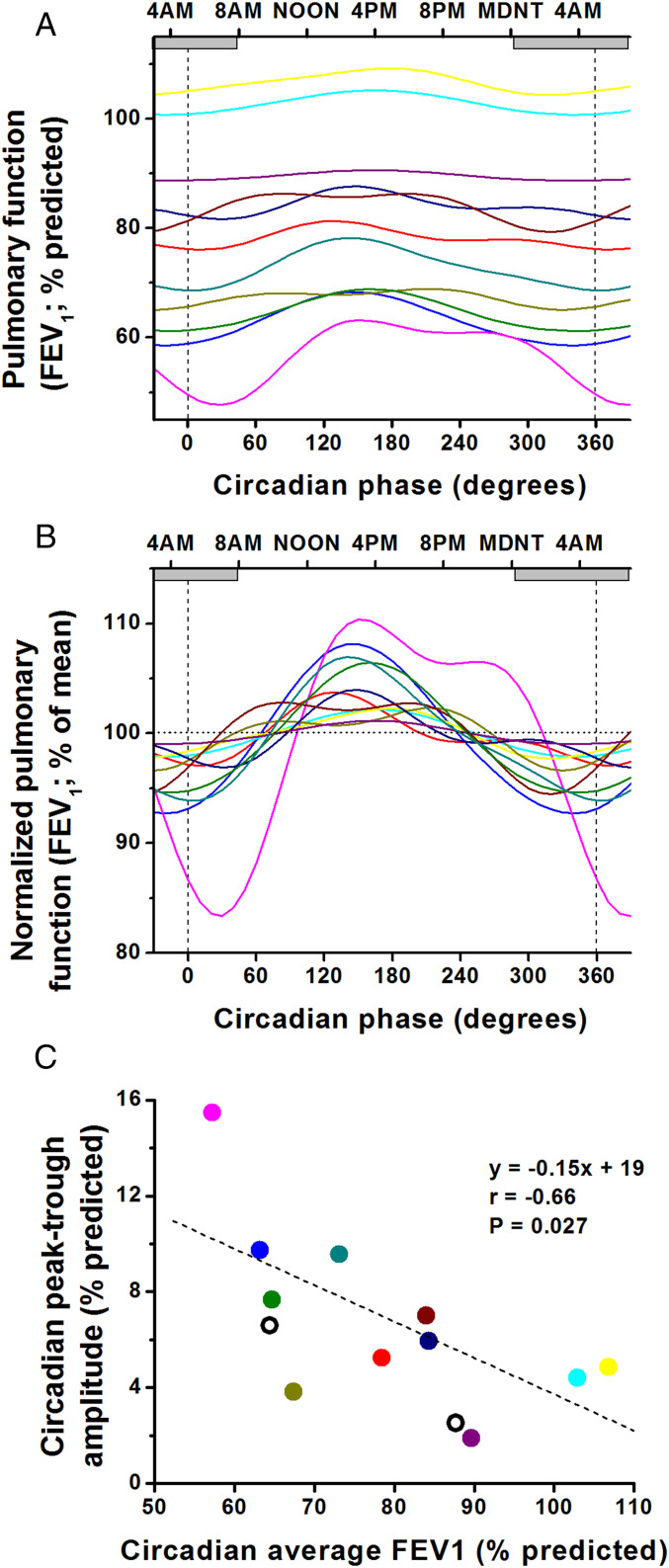
Fig. 4.
More severe asthma associated with greater circadian amplitude in pulmonary function. During the FD protocol, 11 of 13 participants with asthma had a significant endogenous circadian rhythm in FEV1 (shown by individual colors): absolute values (A), normalized to percent deviations around means (B). X-axes for A and B show the circadian phase, with corresponding clock times at the top. Note: the first and last data points and the beginning and end of the cosine curves are double-plotted to make it easier to visualize rhythmicity. All 11 individuals had their circadian trough in FEV1 during the biological night (circadian phases 270° to 90°; gray horizontal bars show average habitual sleep episodes across the biological night). C shows the relationship between overall severity of asthma (x-axis; lower values indicating more severe asthma) and amplitude of circadian rhythm in FEV1 (y-axis). Colors of lines and symbols are matched for individuals across all three panels. It can be seen that those individuals with more severe asthma (lower circadian average FEV1) had greater amplitudes of circadian rhythms in FEV1 and therefore much lower circadian nocturnal troughs in FEV1. Thus, overall, the average FEV1 predicts the extent of the circadian nocturnal trough in FEV1. The two individuals without statistically significant circadian rhythms in FEV1 are superimposed on C (open circles) and appear to fit within the group trend.
FD Protocol: Behavioral Cycle Influences on Asthma Severity.
Using the FD protocol, we also assessed the effect of the behavioral sleep/wake cycle on pulmonary function independent of circadian effects. There were eight measurements of FEV1 and airway resistance made across each of the identical seven recurring 28-h behavioral cycles for each participant, such that by the end of the protocol, these 28-h behavioral cycles had spanned all circadian phases. Thus, the average 28-h profiles of pulmonary function effectively averaged out (i.e., controlled for) circadian phases, as shown in Fig. 3 E and F. Throughout all scheduled wake episodes, participants were mostly seated and at rest, and there were fixed schedules for the timing of awakening and of other behaviors including meals, taking a shower, cognitive computer tasks, and pulmonary function measures. Exercise was not permitted. In addition, to specifically assess the effect of sleep (or prior sleep) on pulmonary function, measurements were performed immediately following three standardized awakenings equally spaced throughout each sleep episode, thereby again spanning the entire circadian cycle. For both groups (asthma and control), there were highly significant systematic effects of the behavioral sleep/wake cycle (independent from the circadian effects) on both FEV1 and airway resistance (P < 0.0001 for all; Fig. 3 E and F). From Fig. 3 E and F, it is evident that there was some variability in pulmonary function across the waking episode with FEV1 increasing and airway resistance decreasing across the first few hours following wakefulness. Furthermore, the most striking finding was that the worst pulmonary function occurred during the scheduled sleep episodes, especially at the end of the first third of the sleep period (i.e., upon the first standardized awakening). We note that in the individuals with asthma, there was a clear negative relationship between the average FEV1 and the extent of the changes in FEV1 associated with the behavioral cycle (r = −0.69, P < 0.01). This is a similar relationship as observed for the circadian rhythm amplitude and average FEV1, as noted. Indeed, the circadian amplitudes and behavioral ranges across participants were highly significantly correlated (r = +0.85; P < 0.001). Thus, those individuals with asthma with the lowest average FEV1 have the largest circadian amplitude as well as the largest behavioral effects on FEV1.
Although all sleep opportunities were strictly scheduled in the protocols, the actual presence of sleep versus wakefulness during sleep opportunities was outside the control of the experimenters. Thus, in additional post hoc analyses, we assessed the role of (prior) sleep on pulmonary function by pairing measurements immediately following polysomnographically (PSG)-confirmed sleep with control measurements during PSG-confirmed wakefulness at equivalent circadian phases (see Materials and Methods). In participants with asthma, prior sleep was systematically associated with a slightly decreased FEV1 (−0.75% predicted; P = 0.026) and a trend for increased airway resistance (0.013 kPa/L/s; P = 0.062) when compared with wakefulness. This latter effect on airway resistance was also significant in control participants (0.015 kPa/L/s; P = 0.006), whereas there was no significant effect of sleep on FEV1 in controls.
The variations across the day and night caused by the behavioral cycle (Fig. 3 E and F) were of similar magnitudes to the circadian variations (Fig. 3 C and D). There were no significant interactions between sleep/wake state and circadian phase on asthma severity (P > 0.05). Thus, while our analyses enable us to statistically separate behavioral effects and circadian effects, the lack of statistical interaction between these effects suggests that they could be summed to predict asthma severity across the day and night in real life when these behavioral and circadian effects continuously co-occur (see Composite Models of Summed Circadian, Behavioral, and Environmental Factors Contributing to Nocturnal Worsening of Asthma).
FD Protocol: Endogenous Circadian and Behavioral Influences on Asthma Rescue Inhaler Use.
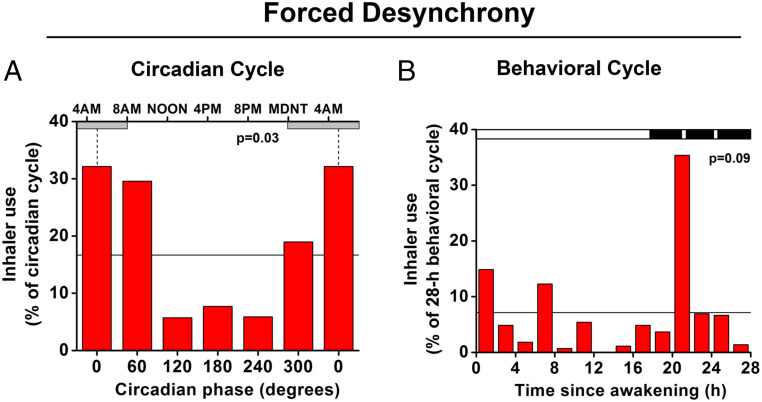
Eight of the 13 individuals with asthma who completed the FD protocol used their β2-adrenergic agonist inhaler medication based on symptoms at least once during the FD part of the protocol. In these eight participants, the median inhaler use was 6 times throughout the FD (range 1 to 17 times), which equates to approximately once every 33 h. The timing of inhaler use in this group of participants had a significant circadian rhythm (Fig. 5A). Overall, bronchodilator rescue medication use was four times more likely to occur during the biological night (81%) than during the biological day (19%) (P = 0.03; Friedman ANOVA). Thus, the circadian phases with the worst pulmonary function (Fig. 3) coincided with the circadian phases with the highest incidence of symptom-driven rescue inhaler use (Fig. 5). Fig. 5 also shows the pattern of inhaler use across the behavioral sleep/wake cycle, independent of the circadian cycle. With this limited dataset, the variation across the behavioral cycle did not reach conventional statistical significance (P = 0.09), but there was a distinct peak of inhaler use at the time of the first scheduled awakening, which coincided with the lowest FEV1 and highest airway resistance in the asthma group (see statistics mentioned previously and Fig. 3 E and F).
Fig. 5.
Endogenous circadian rhythm in rescue medication. (A) Average distribution of beta2-adrenergic agonist inhaler use across the circadian cycle. Data were derived during the FD protocol from the eight asthma participants who used bronchodilator rescue medication during this protocol. There was a significant rhythm in bronchodilator rescue medication use, which was four times more likely to occur during the biological night (circadian bins 300°, 0°, and 60°; CBT minimum shown by vertical dotted lines at 0° = ∼5:00 AM; gray horizontal bars represent average habitual sleep episodes) than during the biological day (bins 120°, 180°, and 240°). The horizontal line indicates expected relative frequency of inhaler use assuming no circadian rhythm (100/6 circadian phase bins = 16.7%). The P value represents the significance of the circadian effect from nonparametric ANOVA. Note: the first and last bars are double-plotted to make it easier to visualize rhythmicity. B shows effects on inhaler use of the 28-h sleep/wake schedule, averaged across all circadian phases. The black bar represents the sleep period interspersed with three awakenings for pulmonary function measurements. The horizontal line indicates expected relative frequency of inhaler use assuming no effect of time (100/14 2-h bins = 7.1%). There was no overall significant effect of time into the sleep/wake schedule (P = 0.09), yet there was a sharp peak in rescue-inhaler use at the time of the first awakening from sleep, coincident with worst pulmonary function (Fig. 3 E and F).
To begin to understand the potential role of the autonomic nervous system in affecting pulmonary function across the day and night, we correlated all pulmonary function variables (FEV1, PEF, and airway resistance by interrupter technique [Rint]) against a number of autonomic parameters as estimated from heart rate variability (root mean square of successive differences [RMSSD]; power of high frequency heart rate variability [HF]; power of low frequency over power of high frequency heart rate variability [LF/HF]) in each person across the FD protocol (see SI Appendix, Table S2). We found that very few individuals had significant correlations between autonomic and pulmonary function variables across the FD study.
Composite Models of Summed Circadian, Behavioral, and Environmental Factors Contributing to Nocturnal Worsening of Asthma.
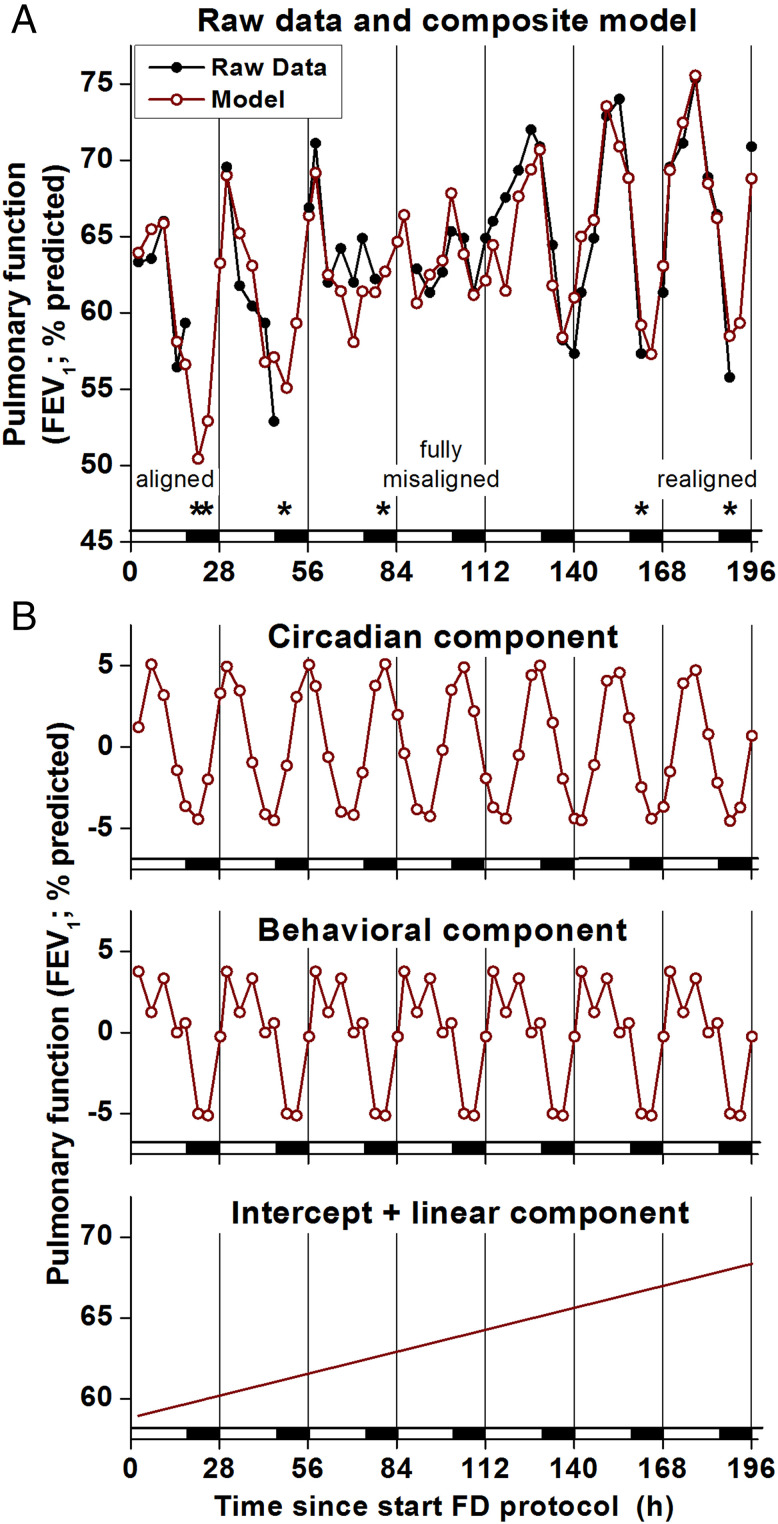
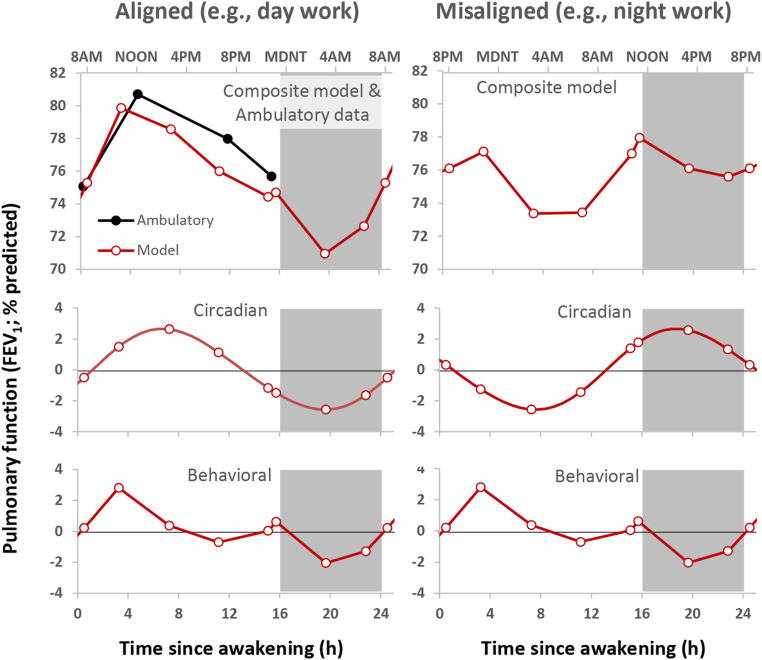
Fig. 6 shows a cosinor regression model of FEV1 data from a representative individual with asthma with data modeled as separate additive effects of the circadian cycle (recurring ∼24-h cycles), scheduled behavioral cycle (recurring 28-h cycles), and environmental conditions (modeled as time since admission to the laboratory) across the seven sequential 28-h wake/sleep cycles of the FD protocol (196 h). The composite model in this participant fits the data well (Fig. 6A; R2 = 0.82), and Fig. 6B shows that the average model variation was comprised of a ∼10% circadian variation (lowest FEV1 during the biological night), a ∼9% behavioral cycle variation (lowest FEV1 during the scheduled sleep period), and a ∼9% linear increase across the 196-h laboratory protocol. Looking in more detail at specific times of worst pulmonary function across the 196 h, the participant used the symptom-based rescue inhaler six times across the FD protocol, with five occurring when sleep was close to its normal alignment with the circadian system (inhaler events denoted by * in Fig. 6A). Thus, the symptom-based rescue inhaler use coincides with the reduction in FEV1 caused by sleep summed with the circadian reduction in FEV1 during the biological night. Similar analysis of the group data from the FD protocol showed that there were apparently additive influences on asthma severity from circadian, sleep, and other behavioral or environmental effects. The additive model derived from data in the laboratory with the normal nocturnal timing of sleep almost perfectly matched the ambulatory pulmonary function data collected over weeks at home in the same patients (Fig. 7). Overall, this group model had a mean ∼4% circadian variation (lowest FEV1 during the biological night) plus a ∼4% behavioral/environmental cycle variation (lowest FEV1 during the sleep period). This group model predicts that FEV1 would be lowest during the normal sleep episode (a time window when measurements were not taken when at home) due to the summed adverse effects of sleep and the biological night on FEV1. Thus, we speculate that the ambulatory data greatly underestimate the actual day/night variation in pulmonary function as the trough is missed (hidden) because of sleep at night. We also note that the data suggest that while the 24-h average FEV1 does not change during circadian misalignment, the peak and trough are less pronounced because the modulation of FEV1 by sleep and by the circadian system partially cancel each other out.
Fig. 6.
Summed circadian, behavioral, and environmental effects on pulmonary function. (A) FEV1 data (black closed circles and lines) and composite regression model (red open circles and lines) from one individual with asthma across seven sequential 28-h wake/sleep cycles of the FD protocol (196 h). The x-axis represents time into FD; black bars represent scheduled sleep. Scheduled waketime varied, ranging from the individual’s habitual waketime (“aligned”) to 12 h out of synchrony (“fully misaligned”). The model fit the data well (R2 = 0.816). * denotes beta2-adrenergic agonist rescue inhaler use (followed by 6-h gaps in FEV1 data that were excluded from analyses because of effects of inhaler on FEV1). Most inhaler uses and lowest FEV1 occurred when aligned (at the beginning of the FD protocol) and when realigned (at the end of the FD protocol) due to summed effects of sleep and circadian cycle on pulmonary function (i.e., sleeping during the circadian night). In contrast, when fully misaligned (in the middle of the FD protocol), there were less severe drops in FEV1 and much less bronchodilator use. (B) Circadian, behavioral, and linear FEV1 model components derived from FD. Circadian and behavioral components each contributed recurring ∼10% variations per day with lowest FEV1 during the biological night (circadian component: ∼24-h cycle) and during sleep (behavioral component: 28-h cycle). An additional steady increase in FEV1 was evident across the protocol (196-h linear component), which is likely attributable to living in the clean laboratory environment. The intercept represents this participant’s average FEV1 upon entering the FD.
Fig. 7.
Summed circadian and behavioral components derived from FD protocol versus ambulatory data. (Left) Modeled effects (red lines) of the behavioral cycle (Bottom), circadian cycle (Middle), and combined effects (Top) on FEV1 compared with actual averaged ambulatory data (black lines) for the same 13 participants who completed the FD. For comparison with ambulatory data, the linear component across the FD was extrapolated back to the day of laboratory admission. The circadian and behavioral components each contributed ∼4% variation, with lowest FEV1 during the biological night (circadian component) and during sleep (behavioral component). The composite summed model fit the ambulatory data well, albeit with slightly lower FEV1 across the daytime (compare red and black lines in top left panel). Notably, during normal nocturnal alignment of sleep (Left), the model predicts FEV1 would be lowest during sleep (a time window when measurements were not taken when at home) due to summed reductions in FEV1 caused by sleep (behavioral component) and the endogenous biological night (circadian component). The right panels show the same modeled data but where sleep occurs 12 h misaligned compared with their habitual sleep time, as may occur during night work. During this circadian misalignment, the peak and trough are less pronounced than during circadian alignment because the modulation of FEV1 by sleep and by the circadian system partially cancel each other out. Note: in each panel, the first and last data points and the beginning and end of the curves are double-plotted to make it easier to visualize rhythmicity.
Discussion
Summary of Findings.
The current study represents perhaps the most comprehensive long-term monitoring of patients with asthma under carefully controlled laboratory conditions. Moreover, by use of two complementary circadian protocols that either excluded or systematically scheduled all behaviors across all phases of the circadian cycle, we were able to distinguish the contribution to pulmonary function variations of the endogenous circadian system from those of the environmental and behavioral factors that normally cycle in synchrony with the circadian system. In patients with asthma, we found an independent influence of the circadian system on indices of pulmonary function, including airway resistance and FEV1. The circadian system caused the worst function during the biological night (equivalent to 11:00 PM through 11:00 AM). The similarity of timing of circadian peaks of indices of pulmonary function in the FD and CR protocols as well between the asthma and control participants shows that these effects are very robust. It also appeared that the sleep phase of the behavioral cycle itself increases airway resistance, independent of and without interaction with any underlying circadian effects. We were able to compare these systematic circadian and behavioral factors revealed in the laboratory with daily patterns occurring in the home environment and found they were almost identical when sleep occurred at the normal time across the night. Furthermore, when aligned, the summation of the circadian and behavioral influences resulted in a large variation in pulmonary function across the sleep–wake cycle. In contrast, during maximum misalignment, the effects of behavioral cycle and the circadian system partially cancel each other out, resulting in less pronounced variation in pulmonary function across the sleep/wake cycle. Only by using circadian protocols that scheduled wakefulness across the night was it possible to uncover the severity of nocturnal worsening of asthma, whereas this worsening could be masked by sleep (and lack of measurements during sleep) in the normal daily routine (e.g., compare paucity of inhaler use during scheduled sleep at home; Fig. 2C versus peak in inhaler use during the biological night in the FD protocol [Fig. 5A]). Overall, in this selective group of participants with asthma, without steroid use and otherwise healthy, the summation of the circadian and behavioral effects (which occurs continuously in real life) appear to be clinically meaningful, both in terms of rescue inhaler use (Figs. 5 and 6) and pulmonary function (Fig. 7).
Potential Mechanisms.
There are numerous external and behavioral factors that exhibit changes across the day and night that can affect asthma severity, including environment, body position, sleep, exercise, and physical activity. While these may be important factors, they were all controlled in the current in-laboratory studies. The remaining underlying endogenous mechanisms that could contribute to the circadian rhythmicity in asthma severity include circadian variation in autonomic nervous system activity (29–31); humoral factors such as epinephrine, histamine, corticosteroids (17, 32), and melatonin (33); cell-autonomous oscillations in pulmonary tissues and immune cells (18, 34); and/or airway inflammation (35). Autonomic nervous system effects on asthma include the bronchodilating adrenergic effects via circulating catecholamines and the bronchoconstricting cholinergic influences via the vagus nerve (17, 29, 30). Furthermore, circulating catecholamines suppress mast cell degranulation and histamine release (36). We have previously published autonomic function data from the current CR and FD study (20, 23) and an independent FD study (37) and found that sympathetic markers (urinary and plasma epinephrine and norepinephrine) are at their lowest and that vagal markers (from heart rate variability measures) are at their highest during the middle of the biological nighttime, while during the biological daytime, sympathetic markers are at their highest and vagal markers at their lowest. However, based on correlation analyses between markers of pulmonary function (FEV1, PEF, and Rint) and indirect estimates of autonomic function (RMSSD, HF, and LF/HF), we found no support for the hypothesis that the autonomic nervous system contributes to the variation in pulmonary function across the FD protocol. Concerning the effect of sleep on asthma severity, we detected a small average decline in pulmonary function attributable to prior sleep, with the greatest decrease across the first third of the night (Fig. 3 E and F) when sleep continuity and depth are likely to be greatest during an FD protocol (38) and as shown in the current FD protocol (22, 39). It is possible that we underestimated the effect of sleep on pulmonary function because arousal from sleep might rapidly improve pulmonary function before our pulmonary function assessments occurred. Nonetheless, our sleep results are consistent with results from a more invasive study that used an esophageal pressure technique to estimate airway resistance during sleep (11).
Limitations of Previous Studies Addressed by the Current Study.
To investigate day/night rhythms of pulmonary function in patients with asthma, previous studies have used epidemiological exploration of the time of occurrence of asthma episodes (2, 3), shift work (40), waking participants from sleep (7, 30), or total sleep deprivation (7, 41). While these studies have clearly documented a day/night rhythm in asthma severity, virtually all of these studies have ignored or have failed to distinguish the relative contributions of endogenous circadian effects versus the behavioral plus environmental effects on asthma. These studies generally have limitations regarding assessment of underlying circadian control of asthma severity because of 1) lack of control of environment (e.g., temperature and light) or behaviors (e.g., sleep, posture, physical activity, food intake) during protocols; 2) daytime steroid use; 3) measurements occurring at only a few times during the 24-h day (e.g., 4:00 AM and 4:00 PM or only measuring during the habitual waking hours) with likelihood of peaks and troughs being missed; 4) absence of markers enabling assessment of the endogenous circadian phase; and 5) an inability to determine the interaction between the circadian effects and the behavioral plus environmental effects on asthma. Our comprehensive experiments were designed to overcome these limitations.
Limitations of Current Study.
Our findings must be viewed in light of a number of limitations that include 1) the small number of participants, which does not permit assessment of the effect of participant characteristics such as age, sex, and different asthma phenotypes on the outcomes (42), thus future studies will be needed to test generalizability of our results to patients with different characteristics; 2) the multiple repeated measurements necessitated only noninvasive techniques (e.g., excluding repeated bronchoconstrictor challenges, sputum inductions, or bronchoalveolar lavages), and rather than the gold-standard body plethysmography for assessment of airway resistance, we used the simpler interrupter technique, which lacks thorough validation in adults; 3) in the ambulatory study, we relied on the participants to note down their rescue inhaler uses, which could have led to some inadvertent data loss (this was less of an issue in the laboratory, as participants were continuously monitored, even during sleep); 4) the effect of prior sleep on pulmonary function that we assessed may underestimate the full effects of sleep itself (note: awakenings from sleep were required for the pulmonary function assessments); and 5) our protocols were primarily designed to study circadian effects while controlling or accounting for behavioral and environmental factors. We acknowledge that there is a large array of known behavioral and environmental effects on asthma, such as exercise and ambient temperature changes, that we did not investigate in this laboratory protocol.
Integration of Findings and Clinical Implications.
The FD and CR protocols both revealed decreased pulmonary function during the biological night, i.e., attributable to the circadian timing system. Furthermore, the FD revealed an additional adverse effect upon pulmonary function of prior sleep itself. Overall, when sleep occurred during the biological night, it appeared that both circadian and behavioral sleep/wake cycle effects summate to contribute to the characteristic worsening of asthma severity at night. Moreover, those individuals with asthma with the lowest average FEV1 had the largest circadian amplitude as well as the largest behavioral cycle effects on FEV1. During the ambulatory part of the study when our participants slept at their usual circadian phase (Fig. 2), we found that the timing of worst pulmonary function, symptoms, and inhaler use occurred during the biological night, that is, immediately before bedtime in the evening and immediately upon awakening in the morning but with no measurements made during sleep. There are many reports of asthma worsening during the normal sleep episode (43, 44), presumably in patients who awoke due to impaired pulmonary function or other underlying problems, such as insomnia. However, in our population under ambulatory conditions, inhaler use was not significantly elevated during the scheduled sleep episode, presumably because the degree of pulmonary function impairment did not reach the threshold to trigger arousal from sleep. This finding suggests there may be a degree of “silent bronchoconstriction” (i.e., unnoticed or hidden) during sleep when at home. It is possible that long episodes of impaired pulmonary function during the night can be deleterious, even if they do not lead to awakening, because it is hypothesized that bronchoconstriction itself can lead to airway remodeling and more severe asthma over time (45). In the future, perhaps the advent of technological advances will enable us to assess airway resistance during sleep to uncover the degree of any “silent bronchoconstriction,” which is likely to be of high clinical relevance.
Our studies have enabled us to statistically separate the independent contributions of circadian versus behavioral and environmental influences on pulmonary function. These results may be important, as they could point to separate targets for therapy. Specifically, therapeutics directed at the circadian system can potentially include timed light exposure or timed pharmaceuticals to improve effectiveness, reduce side effects, or influence central or peripheral circadian clocks themselves (46). First, in terms of timed medication, short-acting drugs could be targeted to counter the circadian time of worst pulmonary function and reduce side effects rather than simply giving patients the maximum tolerated dose of medications across the entire day. We found that the interindividual range of the phase angle of entrainment (i.e., the timing of the central circadian cycle relative to the habitual sleep episode) was ∼4 h in our study, which is consistent with published data from healthy participants (47). This phase angle of entrainment varies even more dramatically with shift work, jet lag, and sleep disorders (47–49). Given this variability between people, it seems that any chronotherapy that targets the circadian system or factors that have endogenous circadian rhythmicity ought to consider a patient’s endogenous circadian phase (rather than simply considering the time of day or the time of a behavior e.g., upon awakening or before sleep). Second, as an example of therapy directed at the circadian system, it was recently discovered that cardiac surgery has a worse outcome when performed in the morning, that this outcome correlated with increased expression of the core clock gene Rev-Erbα, and that drugs blocking this clock gene reduced injury at the start of the active phase in a rodent model (50). Third, therapeutics directed at the behavioral or environmental factors to influence asthma severity could include avoiding or changing behaviors (e.g., exercise, sleep) or certain environments (e.g., specific allergens) at particular circadian phases. Further studies are required to determine if circadian rhythms in the peripheral tissues, including the airways (18), contribute to asthma severity, and if so, whether these clocks ever dissociate from the timing of the endogenous central circadian pacemaker in the suprachiasmatic nucleus (51) due to the actions of, for instance, medications or specific behaviors. In summary, our study helps to provide the critical foundation needed for the optimization of asthma therapy by exploiting knowledge of internal body clock time, so-called “circadian precision medicine” (52).
Materials and Methods
This study was approved by the Institutional Review Board of Brigham and Women’s Hospital, and written informed consent was provided by all participants for the screening procedures and separately for the monitoring procedures.
Study Participants.
Participant demographics and characteristics are presented in SI Appendix, Table S1.
Participants with asthma but no other current medical conditions and healthy control participants were recruited via newspaper, poster and internet advertisements, and mailings via Brigham and Women’s Hospital's Asthma Research Center. Consecutive participants who met all of the inclusion criteria and none of the exclusion criteria were studied over a 3-y period. Eighty-seven potential participants were screened, and the principle study–related details of the 17 participants with asthma and 10 controls who completed the protocols are presented in the Results.
Presence of asthma was confirmed by medical history plus either daytime laboratory assessment of reversible bronchoconstriction (>12% increase in FEV1 or PEF after 200 μg β2-adrenergic receptor agonist inhaler [Albuterol]) or hyperreactivity to inhaled methacholine (provocative concentration of methacholine causing a 20% fall in FEV1 [PC20] <8 mg/mL). Participants with nonreversible airway obstruction such as chronic obstructive pulmonary disease were excluded (i.e., FEV1, PEF, or forced vital capacity [FVC] <80% of predicted or FEV1/FVC <95% predicted that were not normalized by administration of inhaled β2-agonists). Only participants with asthma who used an inhaled β2-agonist as their sole rescue medication were recruited. Additional exclusion criteria for participants with asthma were use of inhaled or topical steroids in the past 8 wk, use of oral steroids in the past year, or acute asthma exacerbation in the past 6 wk.
Absence of asthma was confirmed in healthy control participants by normal pulmonary function (FEV1, PEF, or FVC >80% predicted and FEV1/FVC >95% predicted), daytime laboratory confirmation of absence of reversible bronchoconstriction (<12% increase in FEV1 or PEF after 200 μg Albuterol), and no evidence of hyperreactivity to inhaled methacholine (PC20 >8 mg/mL). As an indirect estimate of underlying airway inflammation, FeNO was assessed five times across the baseline day in the laboratory (53).
To ensure that all participants were completely healthy apart from their asthma and had stable circadian rhythms and no habits or conditions that affected the variables of interest, the following additional exclusion criteria were employed for all participants: any acute or chronic psychiatric or medical conditions (other than asthma) as detected by history, physical, 12-lead electrocardiogram, chest X-ray, complete blood count, blood and urine comprehensive metabolic panel analysis, and overnight polysomnography; periodic limb movements of sleep (periodic limb movement index of >15/h); sleep apnea (apnea/hypopnea index of >10/h); obesity (BMI ≥ 30 kg/m2); age <18 and >45 y; irregular menstrual cycle or current pregnancy in women; smoking history in the past year or >10 pack-year history of smoking (absence of current smoking confirmed during screening and on admission to the laboratory by urine cotinine assay); habitual use of >20 units of alcohol per week or >500 mg caffeine per day (no alcohol or caffeine for 3 wk prior to the laboratory phase of the study); risk of deconditioning during the study (i.e., habitual heavy exercise such as >20 miles per week jogging); unstable circadian rhythms (recent shift work or >2 h time zone difference due to transmeridian flight in prior 3 mo); or use of any current medication (other than oral contraceptives in females and β2-adrenergic receptor agonist inhaler in asthma participants).
Study Design.
All participants underwent an initial ambulatory protocol in the home environment, immediately followed by either an FD and/or a CR protocol (Fig. 1).
The Ambulatory Protocol.
The Ambulatory Protocol (Fig. 1, Top) was used to assess the daily pattern of asthma severity during regular sleep/wake cycles in a home environment. The protocol lasted ∼3 wk (median 22 d; 14 to 41 d) and was performed in all participants with asthma and all control participants. Following appropriate training, participants maintained a regular self-selected sleep–wake cycle (8 h sleep opportunity and 16 h wakefulness) and recorded their FEV1 via a handheld spirometer (Micromedical Ltd.) as well as their respiratory symptoms at four scheduled times per day: immediately after awakening, at 4 h and at 12 h after awakening, and immediately before bedtime. In addition, the unscheduled times of use of their Albuterol (100 to 200 μg) rescue inhaler were recorded in participants with asthma. Finally, for at least 1 wk prior to admission, participants wore a wrist-worn activity monitor (Actiwatch, Mini Mitter) to verify adherence to the regular sleep/wake schedule.
The Laboratory Protocols.
The CR and FD protocols were performed while the participants stayed in individual laboratory suites in an environment free from external time cues such as clocks, radios, televisions, personal computers, visitors, and sunlight (i.e., no windows) but maintained contact with staff members. The room temperature was maintained at 23 ± 1 °C. Suites were equipped with a hospital bed, a desk with computer console, a bathroom including toilet and shower, and a closet. Suites also contained handheld terminals for online recording of events, a porthole for 24-h blood sample collection without disruption of sleep, a voice-activated audio system, and two video cameras for monitoring participants from outside of the suite. Experimenters were present 24 h per day to carry out the protocol, make measurements, collect biological specimens, and administer meals. Balanced meals were designed to keep the participant in energy balance and were composed of 25% fat, 25% protein, and 50% carbohydrates. Fluid intake was 3.5 L/day. The precise and predetermined times of showers, meals, other behavioral events, specimen collections, and protocol markers were carried out via a centralized event-recording system and recorded via handheld terminals (Termiflex) connected to a computer network.
The CR Protocol.
The CR protocol (Fig. 1, Middle) was used to assess the endogenous circadian rhythm of asthma severity during constant environmental and behavioral conditions, including continued relaxed wakefulness (54). The overall CR protocol lasted 5 d, during which participants completed 2 acclimation days and nights in normal room-lighting conditions (∼90 lx during wake periods and <1 lx during sleep episodes), followed by the CR portion of the study consisting of 37 h 40 min of wakefulness in a semirecumbent posture (upper body at 35° and legs horizontal) in dim light (∼1.8 lx) to minimize any modulating effects of light on the circadian timing system, with 2-hourly isocaloric snacks (to minimize the effects of larger sporadic meals on measured variables). Airway resistance, pulmonary function, and asthma symptom assessments (as described in Materials and Methods) were made every 3 to 4 h during acclimation days and nights (including after scheduled awakenings across the sleep episodes) and taken every 2 h during the CR portion of the protocol. Participants with asthma were instructed to take their Albuterol inhaler (200 μg) based on symptoms alone, and these unscheduled times of rescue medication use were recorded. The protocol ended after a night of recovery sleep.
The FD Protocol.
The complementary FD protocol (Fig. 1, Lower) was used to assess any separate and interacting effects of the endogenous circadian system and the behavioral sleep/wake schedule on asthma severity. The protocol lasted 11 d, during which participants completed 2 acclimation days and nights in normal room-lighting conditions (∼90 lx during wake periods and <1 lx during sleep periods), followed by the FD portion of the study consisting of 7 recurring 28-h “days” in dim light (∼1.8 lx) to minimize any modulating effects of light on the circadian timing system. In each 28-h sleep/wake cycle, a total of 9 h 20 min was allotted for sleep, and 18 h 40 min was allotted for wakefulness, which included two evenly spaced 30-min awakenings from sleep for pulmonary function testing. A 28-h sleep–wake cycle under such dim light conditions is outside the range of entrainment of the human circadian pacemaker, allowing the independent investigation of the influence of the circadian pacemaker and the behavioral sleep/wake cycle (55). Airway resistance, pulmonary function, and asthma symptom assessments (as described in Materials and Methods) were made every 3 to 4 h, including during the baseline days and immediately following the scheduled awakenings from sleep. In addition, the unscheduled times of rescue medication use (200 μg Albuterol) were recorded in participants with asthma. Participants were instructed to take their Albuterol inhaler based on symptoms alone. The protocol ended after a recovery night of sleep. Of the 27 participants, 15 (10 asthma and 5 control participants) completed both an FD protocol and a CR protocol—in these cases, the CR immediately followed the FD without additional acclimation days between protocols.
Independent Variables.
During the ambulatory protocol, the independent variable was time since wake. During the FD protocol in the laboratory, the independent variables were circadian phase (fitted CBT minimum assigned as phase 0°), time since wake, and time into the protocol (in hours). During the in-patient parts of the study, CBT was recorded every minute via a rectal thermistor and used for circadian phase assessment (see Statistical Analysis). The biological night was defined as the 180° surrounding body temperature minimum.
Dependent Variables (Indices of Asthma Severity).
All laboratory measurements were made with the participant in the semirecumbent posture on a bed with the back raised to a 35° angle. The following primary outcome variables were measured in sequence every 2 to 4 h throughout both laboratory protocols:
-
1)
Participants rated the severity of symptoms of respiratory discomfort using the modified Borg scale (56) by assigning a number between 0 (no respiratory discomfort) and 10 (most severe respiratory discomfort imaginable).
-
2)
FEV1 was assessed by forced spirometry on a handheld spirometer using standardized procedures (57) utilizing a turbine flowmeter (ambulatory study: MicroDL Spirometer [MS08]; laboratory study: SuperSpiro [SU6000]; both Micromedical Ltd.).
-
3)
Airway resistance was measured during tidal exhalation using an interrupter technique (MicroRint, Micromedical Ltd.). The instrument consisted of a handheld pressure/flow meter connected to a portable computer. The participant breathed through the instrument while wearing a noseclip and while squeezing their cheeks against the teeth to minimize influences on this measurement from the oral cavity. The participant then breathed normally through the mouthpiece for 30 s to 2 min, while at a preset expiratory flow rate the airflow was automatically interrupted and pressure at the mouth was measured. Resistance was calculated from the interpolated flow estimate at this pressure (58, 59). The average of seven airway resistance determinations was recorded for each session.
-
4)
The use of bronchodilator medication in the participants with asthma was based on symptoms alone. The timing and symptom scores at the time of bronchodilator rescue medication use (Albuterol) were recorded. Because of limited data, the timing of inhaler use was grouped according to 60° circadian phase bins, irrespective of whether this occurred during scheduled wake or sleep episodes.
Statistical Analysis.
The main comparisons for these studies were within asthma participants. However, to provide further perspective, we included a healthy control group. For the Ambulatory Protocol, the main comparisons were across time of day. For the CR protocol, the main comparisons were across circadian phase. For the FD protocol, the main comparisons were across circadian phase during scheduled wakefulness, controlling for behaviors that were evenly spaced across the circadian cycle. Furthermore, we performed comparisons across the behavioral sleep/wake cycle controlling for circadian phase and between measurements following awakening from PSG-confirmed sleep and following PSG-confirmed wake, matched for circadian phase bins (see Materials and Methods).
For both the CR protocol and the FD protocol, the endogenous circadian phase was estimated from CBT data using a nonorthogonal spectral analysis technique. For the CR protocol, the first 5 h of data after awakening were excluded to allow for stabilization. For the FD protocol, the CBT data during the seven 28-h cycles were fitted simultaneously with periodic components corresponding to both the forced period of the imposed sleep–wake cycle and the sought-for period of the endogenous circadian rhythm, together with their harmonics. The composite minimum of the fit was assigned the circadian phase of 0° (CBT minimum).
No measurements of asthma severity could be made during sleep. For purposes of estimating the influence of prior sleep as compared to prior wake on the outcome variables for the FD protocol, only measurements during the scheduled sleep episodes in the 28-h “day” were used (immediately before sleep and every 3 to 4 h into the scheduled sleep episode, totaling 28 per participant) and in post hoc analyses were defined as either following PSG-confirmed wake or following PSG-confirmed sleep (the mode of the last ten 30-s epochs identified as sleep, regardless of sleep stage, as there were insufficient data available to be able to assess the separate effects of different sleep stages). Participants were awakened three times during each sleep episode in a standard fashion (alarm clock for 2 min), and all measurements were completed within 15 min of each awakening. In case participants were already awake at the time of the scheduled awakenings, those data were used as control-awake data. Data were segregated into prior-sleep or prior-wakefulness and matched within each participant according to the 60° circadian phase bin during which the measurements took place, whereby the effect of sleep independent of the effect of circadian phase could be determined. Using mixed model analysis, in which circadian bin (six 60°-bins) and PSG-confirmed sleep/wake state immediately prior to the pulmonary function measurements were included as fixed factors, the effect of prior sleep was thus only compared with prior wake between similar circadian phases.
For inhaler rescue medication use in the FD protocol, individual frequency distributions of medication use according to circadian phase were computed prior to group analysis to ensure that each participant contributed equally to the group distribution. For this normalization, the proportion of inhaler use in each 60° circadian bin was calculated relative to that during the full circadian cycle (set as 100%) for each individual. To compensate for the reoccurrence of a similar alignment between the 28-h sleep–wake cycle and the circadian rhythm during the first and seventh 28-h “day,” we assigned any medication use on the first and seventh 28-h cycle as half the weight of that occurring on any other 28-h day (see also Fig. 1). This process ensured that each combination of the circadian cycle and the 28-h sleep–wake cycle was represented equally (with average tau = 24.18 h, drift was only ∼1 h across seven 24-h days).
As noted, to avoid rescue inhaler use affecting measurements of asthma severity, all pulmonary function and airway resistance measurements within 6 h following inhaler use were excluded from all analyses (21). For purposes of analysis of a circadian rhythm in the outcome measures, for the CR protocol and the FD protocol, we applied cosinor analyses including both the fundamental (∼24-h) and second harmonic (∼12-h) of circadian rhythmicity and two-factor or three-factor mixed model ANOVA (circadian phase in degrees, time since wake in hours [only for FD], and time into the circadian protocol in hours to account for any linear changes) with restricted maximum likelihood estimates of the variance components (JMP; SAS Institute) (23). The frequency distribution of inhaler and asthma symptoms use was tested by Friedman ANOVA (Statistica, StatSoft, Inc.).
Supplementary Material
Acknowledgments
We thank the research participants for their participation in the study. We also thank all the staff of the Center for Clinical Investigation for all their help in running the studies. We thank Kun Hu, PhD, and Mariya Kogan, MBiotech, for their help with the analyses of heart rate variability presented in SI Appendix, Table S2. This work was supported by NIH Grants R01HL064815, R35HL155681, R01HL118601, M01RR02635, and UL1TR002541, and by the Oregon Institute of Occupational Health Sciences at Oregon Health and Science University via funds from the Division of Consumer and Business Services of the State of Oregon (ORS 656.630).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2018486118/-/DCSupplemental.
Data Availability
All data needed to evaluate the conclusions in the paper are present in the paper and/or the SI Appendix. As per the NIH Policy on Data Sharing, we will make the datasets available to other investigators following the publication of the final study results. Such datasets will not contain identifying information per the regulations outlined in HIPPA. Per standard Partners HealthCare System policies, we will require from any investigator or entity requesting the data a data-sharing agreement that provides for 1) a commitment to using the data only for research purposes and not to identify any individual participant, 2) a commitment to securing the data using appropriate computer technology, and 3) a commitment to destroying or returning the data after analyses are completed.
References
- 1.Floyer J., A Treatise of the Asthma (Wilkin, London, 1698). [Google Scholar]
- 2.Dethlefsen U., Repgas R., Ein neues therapieprinzip bei nachtlichen asthma. Klin. Med. (Mosk.) 80, 44–47 (1985). [Google Scholar]
- 3.Turner-Warwick M., Epidemiology of nocturnal asthma. Am. J. Med. 85, 6–8 (1988). [DOI] [PubMed] [Google Scholar]
- 4.Martin R. J., Banks-Schlegel S., Chronobiology of asthma. Am. J. Respir. Crit. Care Med. 158, 1002–1007 (1998). [DOI] [PubMed] [Google Scholar]
- 5.Bagg L. R., Hughes D. T., Diurnal variation in peak expiratory flow in asthmatics. Eur. J. Respir. Dis. 61, 298–302 (1980). [PubMed] [Google Scholar]
- 6.National Center for Health Statistics and Centers for Disease Control and Prevention , Asthma (2021) https://www.cdc.gov/nchs/fastats/asthma.htm. Accessed 24 June 2021.
- 7.Hetzel M. R., Clark T. J., Does sleep cause nocturnal asthma? Thorax 34, 749–754 (1979). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hetzel M. R., Clark T. J., Comparison of normal and asthmatic circadian rhythms in peak expiratory flow rate. Thorax 35, 732–738 (1980). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cochrane G. M., Clark J. H., A survey of asthma mortality in patients between ages 35 and 64 in the Greater London hospitals in 1971. Thorax 30, 300–305 (1975). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hetzel M. R., Clark T. J., Branthwaite M. A., Asthma: Analysis of sudden deaths and ventilatory arrests in hospital. BMJ 1, 808–811 (1977). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ballard R. D., Saathoff M. C., Patel D. K., Kelly P. L., Martin R. J., Effect of sleep on nocturnal bronchoconstriction and ventilatory patterns in asthmatics. J Appl Physiol (1985) 67, 243–249 (1989). [DOI] [PubMed] [Google Scholar]
- 12.Jönsson E., Mossberg B., Impairment of ventilatory function by supine posture in asthma. Eur. J. Respir. Dis. 65, 496–503 (1984). [PubMed] [Google Scholar]
- 13.Chen W. Y., Chai H., Airway cooling and nocturnal asthma. Chest 81, 675–680 (1982). [DOI] [PubMed] [Google Scholar]
- 14.Spengler C. M., Shea S. A., Endogenous circadian rhythm of pulmonary function in healthy humans. Am. J. Respir. Crit. Care Med. 162, 1038–1046 (2000). [DOI] [PubMed] [Google Scholar]
- 15.Kalsbeek A., et al., SCN outputs and the hypothalamic balance of life. J. Biol. Rhythms 21, 458–469 (2006). [DOI] [PubMed] [Google Scholar]
- 16.Lewis M. J., Short A. L., Lewis K. E., Autonomic nervous system control of the cardiovascular and respiratory systems in asthma. Respir. Med. 100, 1688–1705 (2006). [DOI] [PubMed] [Google Scholar]
- 17.Barnes P., FitzGerald G., Brown M., Dollery C., Nocturnal asthma and changes in circulating epinephrine, histamine, and cortisol. N. Engl. J. Med. 303, 263–267 (1980). [DOI] [PubMed] [Google Scholar]
- 18.Gibbs J. E., et al., Circadian timing in the lung; a specific role for bronchiolar epithelial cells. Endocrinology 150, 268–276 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Durrington H. J., Farrow S. N., Loudon A. S., Ray D. W., The circadian clock and asthma. Thorax 69, 90–92 (2014). [DOI] [PubMed] [Google Scholar]
- 20.Scheer F. A., Hilton M. F., Mantzoros C. S., Shea S. A., Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc. Natl. Acad. Sci. U.S.A. 106, 4453–4458 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pearlman D. S., et al., A comparison of salmeterol with albuterol in the treatment of mild-to-moderate asthma. N. Engl. J. Med. 327, 1420–1425 (1992). [DOI] [PubMed] [Google Scholar]
- 22.Scheer F. A., Shea T. J., Hilton M. F., Shea S. A., An endogenous circadian rhythm in sleep inertia results in greatest cognitive impairment upon awakening during the biological night. J. Biol. Rhythms 23, 353–361 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shea S. A., Hilton M. F., Hu K., Scheer F. A., Existence of an endogenous circadian blood pressure rhythm in humans that peaks in the evening. Circ. Res. 108, 980–984 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ivanov P. Ch., Hu K., Hilton M. F., Shea S. A., Stanley H. E., Endogenous circadian rhythm in human motor activity uncoupled from circadian influences on cardiac dynamics. Proc. Natl. Acad. Sci. U.S.A. 104, 20702–20707 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jeyaraj D., et al., Klf15 orchestrates circadian nitrogen homeostasis. Cell Metab. 15, 311–323 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shea S. A., Hilton M. F., Orlova C., Ayers R. T., Mantzoros C. S., Independent circadian and sleep/wake regulation of adipokines and glucose in humans. J. Clin. Endocrinol. Metab. 90, 2537–2544 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu K., et al., Non-random fluctuations and multi-scale dynamics regulation of human activity. Physica A 337, 307–318 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hankinson J. L., Odencrantz J. R., Fedan K. B., Spirometric reference values from a sample of the general U.S. population. Am. J. Respir. Crit. Care Med. 159, 179–187 (1999). [DOI] [PubMed] [Google Scholar]
- 29.Soutar C. A., Carruthers M., Pickering C. A., Nocturnal asthma and urinary adrenaline and noradrenaline excretion. Thorax 32, 677–683 (1977). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morrison J. F., Pearson S. B., Dean H. G., Parasympathetic nervous system in nocturnal asthma. Br. Med. J. (Clin. Res. Ed.) 296, 1427–1429 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bando H., et al., Vagal regulation of respiratory clocks in mice. J. Neurosci. 27, 4359–4365 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barnes P. J., Fitzgerald G. A., Dollery C. T., Circadian variation in adrenergic responses in asthmatic subjects. Clin. Sci. (Lond.) 62, 349–354 (1982). [DOI] [PubMed] [Google Scholar]
- 33.Sutherland E. R., Martin R. J., Ellison M. C., Kraft M., Immunomodulatory effects of melatonin in asthma. Am. J. Respir. Crit. Care Med. 166, 1055–1061 (2002). [DOI] [PubMed] [Google Scholar]
- 34.Keller M., et al., A circadian clock in macrophages controls inflammatory immune responses. Proc. Natl. Acad. Sci. U.S.A. 106, 21407–21412 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bousquet J., Jeffery P. K., Busse W. W., Johnson M., Vignola A. M., Asthma. From bronchoconstriction to airways inflammation and remodeling. Am. J. Respir. Crit. Care Med. 161, 1720–1745 (2000). [DOI] [PubMed] [Google Scholar]
- 36.Barnes P. J., Endogenous catecholamines and asthma. J. Allergy Clin. Immunol. 77, 791–795 (1986). [DOI] [PubMed] [Google Scholar]
- 37.Scheer F. A. J. L., et al., Impact of the human circadian system, exercise, and their interaction on cardiovascular function. Proc. Natl. Acad. Sci. U.S.A. 107, 20541–20546 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dijk D. J., Czeisler C. A., Paradoxical timing of the circadian rhythm of sleep propensity serves to consolidate sleep and wakefulness in humans. Neurosci. Lett. 166, 63–68 (1994). [DOI] [PubMed] [Google Scholar]
- 39.Morris C. J., Aeschbach D., Scheer F. A., Circadian system, sleep and endocrinology. Mol. Cell. Endocrinol. 349, 91–104 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guberan E., Williams M. K., Walford J., Smith M. M., Circadian variation of F.E.V. in shift workers. Br. J. Ind. Med. 26, 121–125 (1969). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Catterall J. R., et al., Effect of sleep deprivation on overnight bronchoconstriction in nocturnal asthma. Thorax 41, 676–680 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Busse W. W., et al., Biomarker profiles in asthma with high vs low airway reversibility and poor disease control. Chest 148, 1489–1496 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Janson C., Gislason T., Boman G., Hetta J., Roos B. E., Sleep disturbances in patients with asthma. Respir. Med. 84, 37–42 (1990). [DOI] [PubMed] [Google Scholar]
- 44.Strunk R. C., Sternberg A. L., Bacharier L. B., Szefler S. J., Nocturnal awakening caused by asthma in children with mild-to-moderate asthma in the childhood asthma management program. J. Allergy Clin. Immunol. 110, 395–403 (2002). [DOI] [PubMed] [Google Scholar]
- 45.Lazaar A. L., Panettieri R. A. Jr., Airway smooth muscle: A modulator of airway remodeling in asthma. J. Allergy Clin. Immunol. 116, 488–495, quiz 496 (2005). [DOI] [PubMed] [Google Scholar]
- 46.Laing E. E., et al., Blood transcriptome based biomarkers for human circadian phase. eLife 6, 6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wright K. P. Jr, Gronfier C., Duffy J. F., Czeisler C. A., Intrinsic period and light intensity determine the phase relationship between melatonin and sleep in humans. J. Biol. Rhythms 20, 168–177 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sack R. L., et al., Circadian rhythm sleep disorders: Part I, basic principles, shift work and jet lag disorders. An American Academy of Sleep Medicine review. Sleep 30, 1460–1483 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maidstone R. J., et al., Night shift work is associated with an increased risk of asthma. Thorax 76, 53–60 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Montaigne D., et al., Daytime variation of perioperative myocardial injury in cardiac surgery and its prevention by Rev-Erbα antagonism: A single-centre propensity-matched cohort study and a randomised study. Lancet 391, 59–69 (2018). [DOI] [PubMed] [Google Scholar]
- 51.Yamazaki S., et al., Resetting central and peripheral circadian oscillators in transgenic rats. Science 288, 682–685 (2000). [DOI] [PubMed] [Google Scholar]
- 52.Ruben M. D., Smith D. F., FitzGerald G. A., Hogenesch J. B., Dosing time matters. Science 365, 547–549 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Deykin A., Massaro A. F., Coulston E., Drazen J. M., Israel E., Exhaled nitric oxide following repeated spirometry or repeated plethysmography in healthy individuals. Am. J. Respir. Crit. Care Med. 161, 1237–1240 (2000). [DOI] [PubMed] [Google Scholar]
- 54.Duffy J. F., Dijk D. J., Getting through to circadian oscillators: Why use constant routines? J. Biol. Rhythms 17, 4–13 (2002). [DOI] [PubMed] [Google Scholar]
- 55.Czeisler C. A.et al., Stability, precision, and near-24-hour period of the human circadian pacemaker. Science 284, 2177–2181 (1999). [DOI] [PubMed] [Google Scholar]
- 56.Moy M. L., Lantin M. L., Harver A., Schwartzstein R. M., Language of dyspnea in assessment of patients with acute asthma treated with nebulized albuterol. Am. J. Respir. Crit. Care Med. 158, 749–753 (1998). [DOI] [PubMed] [Google Scholar]
- 57.Gardner R. M., Hankinson J. L., Standardization of spirometry—1987 ATS update (American Thoracic Society). J. Occup. Med. 30, 272–273 (1988). [PubMed] [Google Scholar]
- 58.Fontanari P., Zattara-Hartmann M. C., Burnet H., Jammes Y., Nasal eupnoeic inhalation of cold, dry air increases airway resistance in asthmatic patients. Eur. Respir. J. 10, 2250–2254 (1997). [DOI] [PubMed] [Google Scholar]
- 59.Fontana G. A., et al., Handgrip-induced airway dilation in asthmatic patients with bronchoconstriction induced by MCh inhalation. J Appl Physiol (1985) 93, 1723–1730 (2002). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data needed to evaluate the conclusions in the paper are present in the paper and/or the SI Appendix. As per the NIH Policy on Data Sharing, we will make the datasets available to other investigators following the publication of the final study results. Such datasets will not contain identifying information per the regulations outlined in HIPPA. Per standard Partners HealthCare System policies, we will require from any investigator or entity requesting the data a data-sharing agreement that provides for 1) a commitment to using the data only for research purposes and not to identify any individual participant, 2) a commitment to securing the data using appropriate computer technology, and 3) a commitment to destroying or returning the data after analyses are completed.