Significance
P2X1 receptors are important for neurogenic contraction of smooth muscle, thrombus formation, and regulation of neutrophil migration. Knockdown of P2X1 causes a 90% reduction in male fertility, and up-regulation of P2X1 receptors is associated with inflammatory bladder conditions, such as interstitial cystitis and idiopathic detrusor instability. This study identifies EPAC as a negative regulator of P2X1 receptors, but a positive regulator of P2X2 receptors. Substitution of residues 21 to 23 on P2X1 receptors, for the equivalent residues on P2X2, protected P2X1 receptors from the inhibitory effects of EPAC activation. An inhibitor of the Rho GTPase, Rac1, also prevented the inhibitory effects of EPAC activation on P2X1 currents. Our findings demonstrate regulation of P2X1 and P2X2 receptors by EPAC.
Keywords: P2X1, EPAC, cAMP
Abstract
P2X1 receptors are adenosine triphosphate (ATP)-gated cation channels that are functionally important for male fertility, bladder contraction, and platelet aggregation. The activity of P2X1 receptors is modulated by lipids and intracellular messengers such as cAMP, which can stimulate protein kinase A (PKA). Exchange protein activated by cAMP (EPAC) is another cAMP effector; however, its effect on P2X1 receptors has not yet been determined. Here, we demonstrate that P2X1 currents, recorded from human embryonic kidney (HEK) cells transiently transfected with P2X1 cDNA, were inhibited by the highly selective EPAC activator 007-AM. In contrast, EPAC activation enhanced P2X2 current amplitude. The PKA activator 6-MB-cAMP did not affect P2X1 currents, but inhibited P2X2 currents. The inhibitory effects of EPAC on P2X1 were prevented by triple mutation of residues 21 to 23 on the amino terminus of P2X1 subunits to the equivalent amino acids on P2X2 receptors. Double mutation of residues 21 and 22 and single mutation of residue 23 also protected P2X1 receptors from inhibition by EPAC activation. Finally, the inhibitory effects of EPAC on P2X1 were also prevented by NSC23766, an inhibitor of Rac1, a member of the Rho family of small GTPases. These data suggest that EPAC is an important regulator of P2X1 and P2X2 receptors.
P2X1 receptors are adenosine triphosphate (ATP)-gated cation channels with a relatively high permeability to Ca2+ (1). As with all members of the P2X receptor family, they are trimeric proteins, whereby each subunit consists of a large extracellular domain, two transmembrane domains, and intracellular amino and carboxyl termini (2–5). Ca2+ influx via P2X1 receptors in smooth muscle cells takes the form of localized Ca2+ transients that have the capacity to modulate neighboring intracellular Ca2+ release channels, as well as plasmalemmal voltage-dependent Ca2+ channels (VDCCs) and Ca2+-activated K+ channels (6). Functionally, P2X1 activation can induce action potentials in bladder myocytes (7) and potentiate responses to adrenergic stimulation in pressurized arteries (8). Knockdown of P2X1 channels in male mice results in a 90% reduction in fertility as a result of vas deferens dysfunction (9), whereas up-regulation of P2X1 receptors is associated with inflammatory bladder conditions, such as interstitial cystitis, idiopathic detrusor instability, and overactive bladder (OAB) syndrome (10, 11). Therefore, P2X1 receptors are regarded as potential therapeutic targets for OAB and the development of male contraceptives.
It has been postulated that P2X1 receptors form macromolecular complexes with kinases and phosphatases (6, 12) in a similar fashion to VDCCs, which are associated with protein kinase C (PKC), AKAP150, calcineurin, and the Ca2+-dependent transcription factor NFAT (13, 14). Regulation of P2X channels by protein kinases is complex and varied. Activation of PKC with phorbol 12-myristate 13-acetate potentiates P2X1 and P2X3 currents (15–19), but not P2X4 or P2X7 currents (18). Protein kinase A (PKA) positively modulates P2X3 and P2X4 currents (20–23) but inhibits P2X2 receptors (24, 25). The effect of PKA on P2X1 responses is less well characterized; however, functional experiments on vas deferens and detrusor preparations indicate that inhibition of cyclic adenosine monophosphate (cAMP) hydrolysis by the phosphodiesterase inhibitor rolipram reduced the amplitude of contractions induced by application of ATP and by stimulation of purinergic nerves (26, 27). Therefore, it appears that a rise in intracellular cAMP levels can inhibit native P2X1 responses, but it is not clear whether these effects were mediated by a direct effect on P2X1 receptors.
Although cAMP is typically thought to exert its effects via activation of PKA, there is now growing appreciation that activation of alternative cAMP effectors, such as exchange protein directly activated by cAMP (EPAC), can mediate cAMP-dependent effects independently of PKA (28). Wang et al. (20) found that P2X3 currents were potentiated by the EPAC activator 007-AM (20), whereas Brown and Yule (23) reported that P2X4 currents were unaffected by EPAC activation (23). The effects of EPAC on P2X1 receptors has not yet been established; however Fong et al. (29) found that ATP-evoked currents in murine detrusor myocytes were inhibited by the EPAC activator 007-AM. Their data suggested that EPAC may exert an inhibitory effect on P2X1 currents (29); however, it is not yet known whether this resulted from direct modulation of P2X1 channels. P2X1 receptors are also known to be modulated by cholesterol, as cholesterol-depleting agents reduced P2X1 currents by ∼90% (30), and Allsopp et al. (31) found that these effects were prevented by mutation of residues at positions 20 to 23 on the N terminus region of P2X1 receptors to the equivalent residues on P2X2 receptors, suggesting that this site is important for regulation of P2X1 receptors (31).
The purpose of the present study was to investigate whether activation of EPAC reduced the amplitude of P2X1 currents overexpressed in human embryonic kidney (HEK) cells and, if so, to identify the residues involved with this effect.
Materials and Methods
Electrophysiology.
ATP-induced currents were recorded from HEK cells, transiently transfected with P2X1 or P2X2 receptors, using the perforated patch configuration of the whole cell patch clamp technique as described previously (29, 32). Electrical access between the pipette and cell interior was achieved by inclusion of the pore-forming compound amphotericin B (600 mg/mL) in the pipette solution. Voltage clamp commands were delivered via an Axopatch 1D patch clamp amplifier (Molecular Devices) connected to a Digidata 1322A AD/DA converter (Axon Instruments) interfaced to a computer running pClamp software (Axon Instruments). During the experiments, the dish containing the cells was superfused with Hanks’ solution. In addition, the cell under study was continuously superfused by means of a close delivery system consisting of a pipette (tip diameter 200 μm) placed ∼300 μm away. This could be switched, with a dead-space time of <5 s, to a solution containing a drug. All experiments were carried out at room temperature. Cells were held at −60 mV and ATP (10 μM) was applied for 10-s durations. P2X1 currents were reproducible at 8-min intervals and reproducible responses to ATP were obtained under control conditions in each experiment prior to addition of drugs.
Solutions.
Solutions used were of the following composition (in millimoles): Hanks’ solution: NaCl (125.0), KCl (5.4), glucose (10.0), sucrose (2.9), NaHCO3 (4.2), KH2PO4 (0.4), NaH2PO4 (0.3), MgCl20.6H2O (0.5), CaCl20.2H2O (1.8), MgSO4 (0.4), and Hepes (10.0), pH to 7.4 using NaOH; and perforated patch pipette solution: CsCl (133), MgCl2 (1.0), ethylene glycol-bis(2-aminoethylether)-N,N,N',N'-tetraacetic acid (0.5), Hepes (10), pH adjusted to 7.2 with CsOH.
Ion Channel Cloning and Mutagenesis.
Human P2X1 (NM_002558, Origene Technologies) and P2X2 (NM_053656, Addgene) plasmid constructs of 50 ng mL−1 were transiently transfected into HEK293 cells using Lipofectamine 2000 (Invitrogen). Site-directed mutagenesis was achieved using the Phusion kit (Thermo Scientific) and all mutations were confirmed by Sanger sequencing.
Drugs.
ATP (Tocris); 8-pCPT-2′-O-Me-cAMP-AM (007-AM) (Biolog); N6-monobutyryladenosine-3′, 5′-cyclic monophosphate, sodium salt (6-MB-cAMP) (Biolog); NSC23766 (Sigma); isobutylmethylxanthine (IBMX) (Sigma); and RP-8-CPT-cAMPS (RP-cAMPS) (Biolog).
Statistics.
Summary data are presented as mean ± SEM. Statistical analysis was performed using GraphPad Prism 9.0 software and statistical analyses were performed using a Student’s paired t test or one-way ANOVA (with a Bonferonni post hoc test), as appropriate.
Results
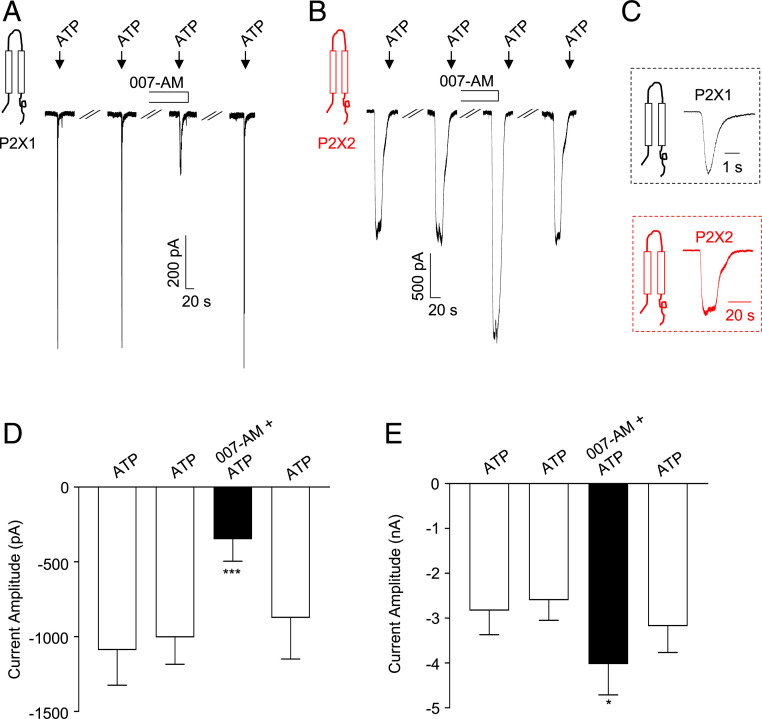
HEK cells overexpressing either P2X1 or P2X2 were voltage clamped at −60 mV and currents were evoked by application of ATP for 10 s. ATP (10 μM) evoked robust P2X1 currents that showed rapid activation and desensitization in the continued presence of ATP (Fig. 1 A and C) and P2X2 currents that were sustained and declined slowly after removal of ATP (Fig. 1 B and C). P2X1 currents were reproducible when ATP was applied at 8-min intervals and this time interval was used throughout the study.
Fig. 1.
Effect of the EPAC activator, 007-AM on P2X1 and P2X2 currents. (A and B) Representative traces demonstrating that 007-AM (10 μM) reversibly inhibited P2X1 currents (A), but enhanced P2X2 currents (B), evoked by application of ATP (10 μM). (C) Control P2X1 and P2X2 currents, on an expanded scale. The summary bar charts in D and E plot mean P2X1 and P2X2 current amplitude, respectively, before, during, and following wash out of 007-AM. Error bars represent SEM. *P < 0.05; ***P < 0.001.
Experiments were performed to examine the effect of the EPAC activator, 007-AM, on P2X1 and P2X2 currents. There are two variants of EPAC, EPAC1 and EPAC2 (28) and the quantitative PCR data shown in SI Appendix, Fig. S1 demonstrate that EPAC2 was dominantly expressed over EPAC1 in HEK cells. The representative trace and summary data in Fig. 1 A and D show that 007-AM (10 μM) reduced the amplitude of P2X1 currents by ∼65% (n = 8, P < 0.001). Similar effects were observed on P2X1 currents evoked by 1 μM ATP (SI Appendix, Fig. S2). 007-AM also slowed the rates of activation and inactivation of P2X1 currents and these data are summarized in SI Appendix, Fig. S3. In contrast, 007-AM enhanced, rather than inhibited, P2X2 currents (Fig. 1B). In seven cells, mean P2X2 current increased by 55% in the presence of 007-AM (Fig. 1E, P < 0.05).
The effect of PKA activation on P2X1 and P2X2 currents was examined in Fig. 2. Unlike the effects of 007-AM, the amplitude of P2X1 currents was unaffected by the PKA activator 6-MB-cAMP [100 μM, Fig. 2 A and C, n = 12, not significant (ns)]. However, P2X2 current amplitude was reduced by 69% in the presence of 6-MB-cAMP (Fig. 2 B and D, n = 14, P < 0.0001). Consistent with an inhibitory effect of EPAC on P2X1 currents, the data in SI Appendix, Fig. S4 show that application of the phosphodiesterase inhibitor IBMX (500 μM), in the presence of the selective PKA inhibitor RP-cAMPS (100 μM) to eliminate effects from elevated PKA levels, reduced the amplitude of P2X1 currents by 41% (n = 6, P < 0.05).
Fig. 2.
Effect of the PKA activator, 6-MB cAMP on P2X1 and P2X2 currents. (A and B) Representative traces demonstrating that 6-MB cAMP (100 μM) did not affect the amplitude of P2X1 currents (A), but inhibited P2X2 currents (B), evoked by application of ATP (1 μM). The summary bar charts in C and D plot mean P2X1 and P2X2 current amplitude, respectively, before, during, and following wash out of 6-MB cAMP. Error bars represent SEM. ***P < 0.0001.
Next, we sought to identify which residues on the P2X1 receptor were involved in mediating the inhibitory effects of EPAC activation. Allsopp et al. (31) showed that mutation of residues 20 to 23 on the intracellular N terminus of P2X1 receptors prevented the inhibitory effects of cholesterol depletion on these channels (31). In addition, Allsopp et al. (33) showed that residues 21 to 23 were also involved in mediating the rapid desensitization kinetics of P2X1 channels (33). Therefore, the effect of 007-AM was examined on P2X1 receptors, containing mutations at positions 21 to 23, in which these residues were substituted for those at equivalent positions on the P2X2 channel, referred to as P2X1-2NT (Fig. 3A). The representative record and summary data in Fig. 3 B and C demonstrate that the inhibitory effects of 007-AM were absent in P2X1-2NT receptors, highlighting the importance of these residues in mediating the effects of EPAC activation on P2X1 channels (n = 6, P > 0.05). Fig. 4 shows that individual mutations at positions 21 (P2X1-M21V) or 22 (P2X1-V22I) to the P2X2 equivalents (Fig. 4 A and B, n = 6 and 7, respectively), did not affect the inhibitory effects of 007-AM. 007-AM reduced the amplitude of P2X1-M21V currents by 75% (P < 0.05, n = 6, Fig. 4 C and E) and P2X1-V22I currents were reduced by 90% (P < 0.01, n = 7, Fig. 4 D and F). However, the representative trace and summary bar chart in Fig. 5 B and C show that double mutation of these residues (P2X1-M21V and V22I) abolished the effects of 007-AM (n = 6, ns). Fig. 6 shows that a single point mutation of leucine to valine at position 23 on the P2X1 receptor (Fig. 6A) also completely prevented the inhibitory effects of 007-AM on P2X1 currents (Fig. 6 B and C, n = 9, ns). A summary of the effect of the point mutations described above on the activation and desensitization rates of P2X1 currents is provided in SI Appendix, Table S1.
Fig. 3.
Effect of the EPAC activator, 007-AM on P2X1 receptors with a triple mutation at residues 21 to 23. (A) Amino acid sequence of the intracellular amino terminus of P2X1 (black) and P2X2 receptors (red). P2X1-2NT shows the sequence of P2X1 receptors in which residues at positions 21 to 23 (M, V, L) were substituted for the equivalent residues on the P2X2 receptor (V, I, V). (B) Representative trace of P2X1-2NT currents before, during, and following wash out of 007-AM. The summary data plotted in C demonstrate that 007-AM did not significantly affect the amplitude of P2X1-2NT currents.
Fig. 4.
Effect of the EPAC activator, 007-AM on P2X1 receptors with single mutations at positions 21 and 22. (A and B) Amino acid sequences of the intracellular amino terminus of P2X1 receptors in which the methionine at position 21 was substituted to a valine (A, P2X1-M21V) and when the valine at position 22 was substituted to an isoleucine (B, P2X1-V22I). (C and D) Representative traces showing that 007-AM inhibited P2X1-M21V (C) and P2X1-V22I (D) currents to a similar degree as P2X1. (E and F) Summary bar charts showing mean peak amplitude of P2X1-V22I (E) and P2X1-M21V (F) currents, respectively, before, during, and following wash out of 6-MB cAMP. Error bars represent SEM. *P < 0.05; **P < 0.01.
Fig. 5.
Effect of the EPAC activator, 007-AM, on P2X1 receptors with a double mutation at positions 21 and 22. (A) Amino acid sequences of the intracellular amino terminus of P2X1 receptors in which the methionine and valine at positions 21 and 22 were substituted to the P2X2 equivalents (valine and isoleucine, respectively). P2X1 V22I-M21V currents were not affected by 007-AM, as shown by the representative trace in B. Summary data plotted in C demonstrate that 007-AM did not significantly affect the amplitude of P2X1 V22I-M21V currents.
Fig. 6.
Effect of the EPAC activator, 007-AM on P2X1 receptors with a single mutation at position 23. (A) Amino acid sequences of the intracellular amino terminus of P2X1 receptors in which the leucine at position 23 was substituted to a valine, equivalent to that on P2X2 receptors (P2X1-L23V). (B) Representative trace showing that P2X1-L23V currents were unaffected by application of 007-AM (10 μM). Summary data for nine similar experiments are plotted in C and show that substitution of this single residue prevented the inhibitory effects of 007-AM. Error bars represent SEM.
Rac1 (Ras-related C3 botulinum toxin substrate 1) belongs to the Rho family of small GTPases and can regulate smooth muscle cell contraction, migration, and angiogenesis (34–36). It has also been identified as an EPAC effector (37) and is involved in EPAC-induced relaxation of airway smooth muscle via a reduction in myosin light chain phosphorylation (38). Fig. 7A shows the effect of 007-AM when applied in the presence of the Rac1 inhibitor NSC23766 (10 μM). Application of NSC23766 did not affect P2X1 current amplitude, but prevented the inhibitory effects of 007-AM (P > 0.05, n = 6, Fig. 7B).
Fig. 7.
Effect of the EPAC activator, 007-AM on P2X1 currents in the presence of the Rac1 inhibitor (NSC23766). The representative trace in A shows that P2X1 currents induced by application of ATP (10 μM) were unaffected by application of NSC23766 (10 μM). However, 007-AM (NSC23766) failed to inhibit P2X1 currents when applied in the presence of NSC23766. (B) Summary bar chart plotting the mean amplitude of P2X1 currents under control conditions, in the presence of NSC23766 and in the presence of NSC23766 plus 007-AM.
HEK cells expressing P2X1 receptors tagged with green fluorescent protein (P2X1-GFP) were imaged with a Nipkow spinning disk confocal microscope to examine whether activation of EPAC with 007-AM affected the membrane expression of P2X1 receptors. SI Appendix, Fig. S5A shows a representative live-confocal image of a single HEK293 cell expressing P2X1-GFP and SI Appendix, Fig. S5B is an intensity trace, plotting P2X1-GFP fluorescence levels before (black trace) and after a 2-min incubation period with the EPAC activator 007-AM (red trace; 10 μM). Summary data in SI Appendix, Fig. S5C show that 007-AM had no significant effect on membrane fluorescence (n = 9, ns).
Discussion
This study demonstrates that activation of the cAMP effector EPAC depressed P2X1 currents, but potentiated P2X2 currents. In contrast, activation of PKA did not affect P2X1 current amplitude, but inhibited P2X2 currents. The inhibitory effects of EPAC on P2X1 currents by 007-AM were prevented by double substitution of residues 21 and 22 (methionine and valine) and by a single mutation of residue 23 (leucine), to the equivalent amino acids (valine, isoleucine, and valine, respectively on the P2X2 receptor. Finally, the inhibitory effects of EPAC activation on P2X1 currents were prevented by the Rac1 inhibitor NSC23766, suggesting that activation of the Rac1 signaling cascade negatively regulates P2X1 receptors.
Activation of P2X1 receptors accounts for the atropine-insensitive component of neurogenic contractions of the detrusor (39). Studies in rodents indicate that this can account for ∼50% of the overall response, although in humans, this component is much smaller (∼2%) (40). However, in tissues taken from patients with bladder disorders leading to OAB, including carcinoma or interstitial cystitis, the purinergic component is enhanced (41). Therefore, the regulatory pathway identified in the present study could be relevant for the development of therapeutics for OAB. Indeed, our findings suggest that this observation may also account for the therapeutic effects of existing OAB pharmacotherapies. β3-adrenoreceptor agonists (β3-AR), such as mirabegron, were approved (in the United States) for treatment of OAB in 2012 (42) and now, according to international guidelines, represent a first line pharmacological treatment for OAB, alongside anticholinergics (43). However, despite their widespread use, their mechanism of action is still not agreed upon (44). Several studies have shown that β3-AR agonists inhibit cholinergic contractions of the detrusor (45–47), but Fong et al. (29) showed that these drugs were much more effective at inhibiting purinergic nerve-mediated responses (29). In addition, Fong et al. (29) also showed that β3-AR agonists inhibited ATP-evoked inward currents in detrusor myocytes and that these effects were mimicked by the EPAC activator 007-AM (29). The results of the present study now demonstrate that P2X1 receptors are directly modulated by activation of the EPAC signaling cascade and that this pathway could involve activation of Rac1.
P2X1 currents are inhibited by cholesterol depletion (31) and these effects are prevented by stabilization of the cytoskeleton (48). Mutation of residues 20 to 23 on the intracellular N terminus of the P2X1 receptor protected P2X1 receptors from the effects of cholesterol depletion, highlighting the importance of this site in the regulation of P2X1 gating. As these residues were also important for mediating the effects of EPAC, it opens up the possibility of convergence in the mechanisms responsible for inhibition of P2X1 currents by EPAC activation, cholesterol depletion, and cytoskeleton disruption. Such an idea is consistent with a role for Rac1, which is known to regulate the cytoskeleton in several cell types (49, 50). Rac1 is also known to regulate ion channel activity. For example, Yin et al. (51) showed that inhibition of Rac1 decreased TRPC1 expression in vascular smooth muscle cells (51), and Lopez-Guerrero et al. (52) demonstrated that Rac1 was involved in translocation of ORAI channels during tumor cell migration (52). Qu et al. (53) indicated that Rac1 could have a direct role in ion channel activation as the Rac1 inhibitor, NSC23766, reduced the amplitude of small conductance Ca2+-activated K+ currents.
The precise mechanisms underlying the reduction in P2X1 currents by activation of EPAC are unclear. Functional expression of P2X1 receptors is regulated by trafficking from the membrane (54) and P2X1 receptors have been reported to internalize following their activation (55–57), opening up a possible regulatory pathway for regulation by EPAC. However, Allsopp et al. (31) showed that surface expression of P2X1 receptors was unaffected by cholesterol-depleting agents (31). Therefore, if EPAC acts via a similar mechanism, it seems unlikely that the diminished amplitude of the P2X1 currents results from reduced membrane expression of P2X1 receptors. Instead, it seems more likely that EPAC signaling affects P2X1 current amplitude via modulation of channel gating involving residues 21 to 23, which have already been identified as an important regulatory site for P2X1 channel activation (31); however, this requires further investigation.
The results of the present study are consistent with earlier studies (24, 25), which found that P2X2 currents were negatively regulated by PKA, but go further, by demonstrating that they are enhanced by EPAC. This illustrates a bimodal regulation of P2X2 receptor activity by cAMP, via activation of EPAC and PKA. P2X2 receptors are expressed in the inner ear and are essential for auditory transduction (58). Several P2X2 mutations are associated with hereditary hearing loss in humans (59–61) and transgenic mice lacking P2X2 receptors demonstrate age-related hearing loss (59). Positive modulators of P2X2 receptors have been proposed as potential treatment targets for hearing loss (62); therefore, the stimulatory effects of EPAC on P2X2 receptors described in the present study represent an attractive pathway for development of novel treatments for hearing disorders.
In summary, the results of the present study demonstrate a regulatory pathway for P2X1 and P2X2 receptors, with implications for development of therapeutics that target disorders associated with dysfunction of these receptors.
Supplementary Material
Acknowledgments
Z.F. received funding from the Irish Research Council (GOIPG/2016/1300) and is also grateful for support from the Higher Education Authority (Ireland) Covid19 Research Relief fund. C.S.G. is funded under the Dundalk Institute of Technology Technological Universities Transformation Fund Postdoctoral Fellowship Scheme. This work was supported by the BREATH project, funded by the European Union INTERREG VA Health and Life Science Programme (INT-VA/045).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2108094118/-/DCSupplemental.
Data Availability
All study data are included in the article and/or supporting information.
References
- 1.Egan T. M., Khakh B. S., Contribution of calcium ions to P2X channel responses. J. Neurosci. 24, 3413–3420 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khakh B. S., et al., International union of pharmacology. XXIV. Current status of the nomenclature and properties of P2X receptors and their subunits. Pharmacol. Rev. 53, 107–118 (2001). [PubMed] [Google Scholar]
- 3.North R. A., Molecular physiology of P2X receptors. Physiol. Rev. 82, 1013–1067 (2002). [DOI] [PubMed] [Google Scholar]
- 4.Coddou C., Yan Z., Obsil T., Huidobro-Toro J. P., Stojilkovic S. S., Activation and regulation of purinergic P2X receptor channels. Pharmacol. Rev. 63, 641–683 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Illes P., et al., Update of P2X receptor properties and their pharmacology: IUPHAR Review 30. Br. J. Pharmacol. 178, 489–514 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hill-Eubanks D. C., Werner M. E., Nelson M. T., Local elementary purinergic-induced Ca2+ transients: From optical mapping of nerve activity to local Ca2+ signaling networks. J. Gen. Physiol. 136, 149–154 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Young J. S., Meng E., Cunnane T. C., Brain K. L., Spontaneous purinergic neurotransmission in the mouse urinary bladder. J. Physiol. 586, 5743–5755 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rummery N. M., Brock J. A., Pakdeechote P., Ralevic V., Dunn W. R., ATP is the predominant sympathetic neurotransmitter in rat mesenteric arteries at high pressure. J. Physiol. 582, 745–754 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mulryan K., et al., Reduced vas deferens contraction and male infertility in mice lacking P2X1 receptors. Nature 403, 86–89 (2000). [DOI] [PubMed] [Google Scholar]
- 10.Kennedy C., ATP as a cotransmitter in the autonomic nervous system. Auton. Neurosci. 191, 2–15 (2015). [DOI] [PubMed] [Google Scholar]
- 11.Andersson K. E., Purinergic signalling in the urinary bladder. Auton. Neurosci. 191, 78–81 (2015). [DOI] [PubMed] [Google Scholar]
- 12.Garcia-Marcos M., Dehaye J. P., Marino A., Membrane compartments and purinergic signalling: The role of plasma membrane microdomains in the modulation of P2XR-mediated signalling. FEBS J. 276, 330–340 (2009). [DOI] [PubMed] [Google Scholar]
- 13.Navedo M. F., et al., AKAP150 is required for stuttering persistent Ca2+ sparklets and angiotensin II-induced hypertension. Circ. Res. 102, e1–e11 (2008). [DOI] [PubMed] [Google Scholar]
- 14.Nieves-Cintrón M., Amberg G. C., Navedo M. F., Molkentin J. D., Santana L. F., The control of Ca2+ influx and NFATc3 signaling in arterial smooth muscle during hypertension. Proc. Natl. Acad. Sci. U.S.A. 105, 15623–15628 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paukert M., et al., Inflammatory mediators potentiate ATP-gated channels through the P2X(3) subunit. J. Biol. Chem. 276, 21077–21082 (2001). [DOI] [PubMed] [Google Scholar]
- 16.Vial C., Tobin A. B., Evans R. J., G-protein-coupled receptor regulation of P2X1 receptors does not involve direct channel phosphorylation. Biochem. J. 382, 101–110 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ase A. R., Raouf R., Bélanger D., Hamel E., Séguéla P., Potentiation of P2X1 ATP-gated currents by 5-hydroxytryptamine 2A receptors involves diacylglycerol-dependent kinases and intracellular calcium. J. Pharmacol. Exp. Ther. 315, 144–154 (2005). [DOI] [PubMed] [Google Scholar]
- 18.Brown D. A., Yule D. I., Protein kinase C regulation of P2X3 receptors is unlikely to involve direct receptor phosphorylation. Biochim. Biophys. Acta 1773, 166–175 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wen H., Evans R. J., Regions of the amino terminus of the P2X receptor required for modification by phorbol ester and mGluR1alpha receptors. J. Neurochem. 108, 331–340 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang C., Gu Y., Li G. W., Huang L. Y., A critical role of the cAMP sensor Epac in switching protein kinase signalling in prostaglandin E2-induced potentiation of P2X3 receptor currents in inflamed rats. J. Physiol. 584, 191–203 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang S., et al., Potentiation of the P2X3 ATP receptor by PAR-2 in rat dorsal root ganglia neurons, through protein kinase-dependent mechanisms, contributes to inflammatory pain. Eur. J. Neurosci. 36, 2293–2301 (2012). [DOI] [PubMed] [Google Scholar]
- 22.Brown D. A., Bruce J. I., Straub S. V., Yule D. I., cAMP potentiates ATP-evoked calcium signaling in human parotid acinar cells. J. Biol. Chem. 279, 39485–39494 (2004). [DOI] [PubMed] [Google Scholar]
- 23.Brown D. A., Yule D. I., Protein kinase A regulation of P2X(4) receptors: Requirement for a specific motif in the C-terminus. Biochim. Biophys. Acta 1803, 275–287 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen C., Bobbin R. P., P2X receptors in cochlear Deiters’ cells. Br. J. Pharmacol. 124, 337–344 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chow Y. W., Wang H. L., Functional modulation of P2X2 receptors by cyclic AMP-dependent protein kinase. J. Neurochem. 70, 2606–2612 (1998). [DOI] [PubMed] [Google Scholar]
- 26.Bergantin L. B., et al., Novel model for “calcium paradox” in sympathetic transmission of smooth muscles: Role of cyclic AMP pathway. Cell Calcium 54, 202–212 (2013). [DOI] [PubMed] [Google Scholar]
- 27.Longhurst P. A., Briscoe J. A., Rosenberg D. J., Leggett R. E., The role of cyclic nucleotides in guinea-pig bladder contractility. Br. J. Pharmacol. 121, 1665–1672 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bos J. L., Epac proteins: Multi-purpose cAMP targets. Trends Biochem. Sci. 31, 680–686 (2006). [DOI] [PubMed] [Google Scholar]
- 29.Fong Z., Griffin C. S., Hollywood M. A., Thornbury K. D., Sergeant G. P., β3-Adrenoceptor agonists inhibit purinergic receptor-mediated contractions of the murine detrusor. Am. J. Physiol. Cell Physiol. 317, C131–C142 (2019). [DOI] [PubMed] [Google Scholar]
- 30.Vial C., Evans R. J., Disruption of lipid rafts inhibits P2X1 receptor-mediated currents and arterial vasoconstriction. J. Biol. Chem. 280, 30705–30711 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Allsopp R. C., Lalo U., Evans R. J., Lipid raft association and cholesterol sensitivity of P2X1-4 receptors for ATP: Chimeras and point mutants identify intracellular amino-terminal residues involved in lipid regulation of P2X1 receptors. J. Biol. Chem. 285, 32770–32777 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bradley E., et al., P2X receptor currents in smooth muscle cells contribute to nerve mediated contractions of rabbit urethral smooth muscle. J. Urol. 186, 745–752 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Allsopp R. C., Evans R. J., The intracellular amino terminus plays a dominant role in desensitization of ATP-gated P2X receptor ion channels. J. Biol. Chem. 286, 44691–44701 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Doanes A. M., Irani K., Goldschmidt-Clermont P. J., Finkel T., A requirement for rac1 in the PDGF-stimulated migration of fibroblasts and vascular smooth cells. Biochem. Mol. Biol. Int. 45, 279–287 (1998). [DOI] [PubMed] [Google Scholar]
- 35.Sawada N., Li Y., Liao J. K., Novel aspects of the roles of Rac1 GTPase in the cardiovascular system. Curr. Opin. Pharmacol. 10, 116–121 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rahman A., et al., The small GTPase Rac1 is required for smooth muscle contraction. J. Physiol. 592, 915–926 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roscioni S. S., Elzinga C. R., Schmidt M., Epac: Effectors and biological functions. Naunyn Schmiedebergs Arch. Pharmacol. 377, 345–357 (2008). [DOI] [PubMed] [Google Scholar]
- 38.Roscioni S. S., et al., Epac as a novel effector of airway smooth muscle relaxation. J. Cell. Mol. Med. 15, 1551–1563 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vial C., Evans R. J., P2X receptor expression in mouse urinary bladder and the requirement of P2X(1) receptors for functional P2X receptor responses in the mouse urinary bladder smooth muscle. Br. J. Pharmacol. 131, 1489–1495 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Burnstock G., Purinergic signalling: Therapeutic developments. Front. Pharmacol. 8, 661 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Palea S., Artibani W., Ostardo E., Trist D. G., Pietra C., Evidence for purinergic neurotransmission in human urinary bladder affected by interstitial cystitis. J. Urol. 150, 2007–2012 (1993). [DOI] [PubMed] [Google Scholar]
- 42.Schena G., Caplan M. J., Everything you always wanted to know about β3-AR * (* but were afraid to ask). Cells 8, 357 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lightner D. J., Gomelsky A., Souter L., Vasavada S. P., Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU Guideline amendment 2019. J. Urol. 202, 558–563 (2019). [DOI] [PubMed] [Google Scholar]
- 44.Igawa Y., Aizawa N., Michel M. C., β3-Adrenoceptors in the normal and diseased urinary bladder-What are the open questions? Br. J. Pharmacol. 176, 2525–2538 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Afeli S. A., Hristov K. L., Petkov G. V., Do β3-adrenergic receptors play a role in guinea pig detrusor smooth muscle excitability and contractility? Am. J. Physiol. Renal Physiol. 302, F251–F263 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Afeli S. A., Rovner E. S., Petkov G. V., BRL37344, a β3-adrenergic receptor agonist, decreases nerve-evoked contractions in human detrusor smooth muscle isolated strips: Role of BK channels. Urology 82, 744.e1–744.e7 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Griffin C. S., et al., β3-adrenoceptor agonists inhibit carbachol-evoked Ca2+ oscillations in murine detrusor myocytes. BJU Int. 121, 959–970 (2018). [DOI] [PubMed] [Google Scholar]
- 48.Lalo U., Roberts J. A., Evans R. J., Identification of human P2X1 receptor-interacting proteins reveals a role of the cytoskeleton in receptor regulation. J. Biol. Chem. 286, 30591–30599 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sells M. A., et al., Human p21-activated kinase (Pak1) regulates actin organization in mammalian cells. Curr. Biol. 7, 202–210 (1997). [DOI] [PubMed] [Google Scholar]
- 50.Birukova A. A., et al., Prostaglandins PGE(2) and PGI(2) promote endothelial barrier enhancement via PKA- and Epac1/Rap1-dependent Rac activation. Exp. Cell Res. 313, 2504–2520 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yin Q., et al., LPS promotes vascular smooth muscle cells proliferation through the TLR4/Rac1/Akt signalling pathway. Cell. Physiol. Biochem. 44, 2189–2200 (2017). [DOI] [PubMed] [Google Scholar]
- 52.Lopez-Guerrero A. M., et al., RAC1-Dependent ORAI1 translocation to the leading edge supports lamellipodia formation and directional persistence. Sci. Rep. 10, 6580 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Qu L., et al., Decreased neuronal excitability in medial prefrontal cortex during morphine withdrawal is associated with enhanced SK channel activity and upregulation of small GTPase Rac1. Theranostics 10, 7369–7383 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lalo U., Allsopp R. C., Mahaut-Smith M. P., Evans R. J., P2X1 receptor mobility and trafficking; regulation by receptor insertion and activation. J. Neurochem. 113, 1177–1187 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dutton J. L., et al., P2X(1) receptor membrane redistribution and down-regulation visualized by using receptor-coupled green fluorescent protein chimeras. Neuropharmacology 39, 2054–2066 (2000). [DOI] [PubMed] [Google Scholar]
- 56.Li G. H., et al., The distribution of P2X receptor clusters on individual neurons in sympathetic ganglia and their redistribution on agonist activation. J. Biol. Chem. 275, 29107–29112 (2000). [DOI] [PubMed] [Google Scholar]
- 57.Ennion S. J., Evans R. J., Agonist-stimulated internalisation of the ligand-gated ion channel P2X(1) in rat vas deferens. FEBS Lett. 489, 154–158 (2001). [DOI] [PubMed] [Google Scholar]
- 58.Mittal R., et al., Molecular structure and regulation of p2x receptors with a special emphasis on the role of P2X2 in the auditory system. J. Cell. Physiol. 231, 1656–1670 (2016). [DOI] [PubMed] [Google Scholar]
- 59.Yan D., et al., Mutation of the ATP-gated P2X(2) receptor leads to progressive hearing loss and increased susceptibility to noise. Proc. Natl. Acad. Sci. U.S.A. 110, 2228–2233 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Faletra F., et al., A novel P2RX2 mutation in an Italian family affected by autosomal dominant nonsyndromic hearing loss. Gene 534, 236–239 (2014). [DOI] [PubMed] [Google Scholar]
- 61.Moteki H., Azaiez H., Booth K. T., Hattori M., Sato A., et al., Hearing loss caused by a P2RX2 mutation identified in a MELAS family with a coexisting mitochondrial 3243AG mutation. Ann. Otol. Rhinol. Laryngol. 124, 177S–183S (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stokes L., Bidula S., Bibič L., Allum E., To inhibit or enhance? Is there a benefit to positive allosteric modulation of P2X receptors? Front. Pharmacol. 11, 627 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or supporting information.