Significance
Gas separation membranes are an emerging energy-efficient alternative toward conventional, energy-intensive separation technologies such as cryogenic distillation. Ladder polymers with intrinsic microporosity show exceptional promise toward redefining state-of-the-art gas separation membranes due to their high permeability (throughput) and selectivity (separation efficiency). However, they are typically inhibited by major reductions in permeability over time due to collapsing membrane free volume (open space between polymer chains), forfeiting their greatest asset. This work explores a route toward enhanced aging resistance and overall separation performance by incorporating pentiptycene (an H-shaped scaffold containing five fused arene rings) into ladder-like polymers to incorporate natural, more permanent “micropores” that aren’t susceptible to densification of polymer chains that occurs over time in traditional microporous polymers.
Keywords: ladder polymers, iptycenes configurational free volume, gas separation membranes, physical aging
Abstract
Polymers of intrinsic microporosity (PIMs) have shown promise in pushing the limits of gas separation membranes, recently redefining upper bounds for a variety of gas pair separations. However, many of these membranes still suffer from reductions in permeability over time, removing the primary advantage of this class of polymer. In this work, a series of pentiptycene-based PIMs incorporated into copolymers with PIM-1 are examined to identify fundamental structure–property relationships between the configuration of the pentiptycene backbone and its accompanying linear or branched substituent group. The incorporation of pentiptycene provides a route to instill a more permanent, configuration-based free volume, resistant to physical aging via traditional collapse of conformation-based free volume. PPIM-ip-C and PPIM-np-S, copolymers with C- and S-shape backbones and branched isopropoxy and linear n-propoxy substituent groups, respectively, each exhibited initial separation performance enhancements relative to PIM-1. Additionally, aging-enhanced gas permeabilities were observed, a stark departure from the typical permeability losses pure PIM-1 experiences with aging. Mixed-gas separation data showed enhanced CO2/CH4 selectivity relative to the pure-gas permeation results, with only ∼20% decreases in selectivity when moving from a CO2 partial pressure of ∼2.4 to ∼7.1 atm (atmospheric pressure) when utilizing a mixed-gas CO2/CH4 feed stream. These results highlight the potential of pentiptycene’s intrinsic, configurational free volume for simultaneously delivering size-sieving above the 2008 upper bound, along with exceptional resistance to physical aging that often plagues high free volume PIMs.
As we continue to strive toward industrial separations that are less energy-intensive, membrane-based gas separations have shown particular promise due to their ability to perform necessary separations without the need for phase changes or a thermal driving force, all while providing operational simplicity and the potential for reduced spatial requirements and a lower overall carbon footprint (1–5). Since Freeman’s theoretical analysis in 1999 of the permeability (gas throughput) and selectivity (separation efficiency) tradeoff relations that challenge polymeric gas separation membranes and predicted strategies to overcome them, an abundance of research efforts have been undertaken to explore these possible structural optimizations that simultaneously increase interchain spacing and rigidity (6). One promising class of polymers arising from these endeavors is that of polymers of intrinsic microporosity (PIMs). First reported in 2004, the archetypical PIM, PIM-1, utilized an inflexible, ladder-like backbone with a spiro center, providing a contortion site to deliver a polymer with high free volume due to inefficient chain packing, leading to exceptional permeability with sufficient selectivities to help redefine the (at the time) upper bound from its 1991 location to its 2008 location (7–9). The birth of this new class of polymers has inspired over 700 publications on PIMs, with PIM-type polymers helping further redefine the upper bounds for hydrogen- and oxygen-based (H2/N2, H2/CH4, and O2/N2) separations in 2015 and for carbon dioxide–based separations (CO2/N2 and CO2/CH4) in 2019, highlighting their continued potential to advance the membrane gas separation field (10–12).
In addition to the tradeoff between permeability and selectivity, gas separation membranes face a significant challenge brought upon by physical aging where over time initial nonequilibrium excess free volume slowly collapses through the segmental motion of polymer chains, reducing gas permeabilities with accompanying increases in selectivity. This challenge is especially relevant for PIMs, which see much of their initial permeability lost over time as it is primarily the result of conformational free volume. PIM-1 itself, after aging 1,380 d, experienced an 80% decrease in its nitrogen permeability for a 102 µm film, underlining the challenges physical aging can provide when trying to advance a polymer from the laboratory into commercial use (13).
Besides the creation of a superrigid polymer backbone, the incorporation of a more permanent, configurational free volume through the integration of hierarchical, shape-persistent molecules has shown promise for both overcoming potential permeability and selectivity tradeoffs while also delivering more graceful aging profiles. One such family of molecules, iptycenes, have seen broad deployment into high performing polymers that have contributed to the redefinition of the Robeson upper bounds to their current location, while also providing unique and exceptional aging-based gas separation enhancements (3, 4, 10, 14–21). Iptycenes, such as triptycene and pentiptycene, deliver a path to instill intrinsic microcavities in similar size regimes to relevant gas pairs, as well as potential for controlling the size and size distribution of these free volume regions. With iptycenes providing internal molecular free volume (IMFV) that is configuration-based and intrinsic to their superrigid molecular structure, in addition to their general bulkiness and pendant arene blades, they allow opportunity for a multipronged approach for taking on both the tradeoff relationship and physical aging challenges simultaneously. Previous research has also shown that the simultaneous incorporation of iptycenes and appropriately placed substituent groups into the polymer backbone offers a route to tunability of the polymer microstructure and separation performance due to the potential of these neighboring pendant groups to partially occupy the natural pores instilled by the molecular architecture of the iptycene unit (14, 20–25).
While triptycene-based polymers and PIMs have seen consistent examination, little exploration has been done on the incorporation of pentiptycene into polymers for gas separation membranes. Here, we report a series of pentiptycene-based ladder polymers incorporated as copolymers with PIM-1, utilizing pentiptycene’s potential for chemical modification to study fundamental structure–property relationships within the series by examining a variety of backbone configurations and substituent groups and their effect on the polymers physical properties and gas separation performance. Even when constituting only ∼17% of the overall polymer, the integration of pentiptycene-based PIMs into PIM-1 enhances gas separation performances under both pure- and mixed-gas conditions, while producing exceptional aging-enhanced separation performance.
Results and Discussion
Synthesis of Pentiptycene-Based Ladder Polymers of Intrinsic Microporosity.
Beginning from commercially available starting materials, 2,3-dimethoxy anthracene was synthesized via a three-step synthesis route in high purity. A Diels–Alder cycloaddition between 2,3-dimethoxy anthracene and p-benzoquinone then provided two tetramethoxy-functionalized pentiptycene quinone isomers (TMPQ-S and TMPQ-C), which were purified and separated (SI Appendix) via column chromatography following a previously reported procedure (26). To polymerize with the common PIM-1 monomer tetrafluoroterephthalonitrile, tetrahydroxyl-functionalization is required. First, the center-ring quinone was converted to a hydroquinone, providing a route to simultaneously protect the center-ring from engaging in the polymerization while also allowing incorporation of a bulky substituent group that can provide additional solubility and tunability of eventual polymer microporosity (20, 21). This was achieved via attachment of either a linear n-propoxy (denoted -np) or branched isopropoxy substituent (denoted -ip). Once the chosen propoxy unit is attached to the center-ring, the four methoxy units are selectively converted to quinones and finally reduced to tetraphenols (THPnP-S, THPiP-S, and THPiP-C; a representative pentiptycene monomer structure shown in Fig. 1 and SI Appendix, Figs. S2–S4).
Fig. 1.
(A) Tetra-functional pentiptycene monomer structures and general synthesis scheme for S- and C-shape isopropoxy and n-propoxy center-ring substituted pentiptycene-based ladder co-PIMs. Energy changes corresponding to deviations in the associated dihedral angles (shown by color on backbone structure) within the representative homopolymer repeating units of PPIM-ip-S (B) and PPIM-ip-C (C).
To explore the effects of substituted pentiptycene units with varied backbone configurations on polymer properties and gas separation performance, the respective monomers were incorporated into copolymers with the commonly studied PIM-1 in a 1:5 ratio of the pentiptycene monomer to the standard spirobisindane unit (5,5′,6,6′-tetrahydroxy-3,3,3′,3′-tetramethyl-1,1′-spirobisindane), leading to ∼17% mol pentiptycene-based PIMs (Fig. 1). For nomenclature, the pentiptycene-based PIMs (PPIMs) have -ip or -np based on the isopropoxy or n-propoxy substituent and S or C based on the configuration of the pentiptycene monomer. For example, PPIM-ip-S refers to the pentiptycene-based PIM with an isopropoxy substituent group at the middle ring and the “S-shape” backbone. The entire synthetic process, film casting, and characterization procedure was repeated a second time for PPIM-ip-S, and values reported here are averages of the measurements of the two respective trials (individual measurements data can be found in the SI Appendix). Additionally, PIM-1 was synthesized as a reference polymer following previously reported procedures (PIM-1; SI Appendix) (7, 27). Thin films of the polymers were cast in a 1.5% wt/vol solution in chloroform with 1 to 3 d of evaporation, followed by a 24 h methanol soak and subsequent vacuum oven drying at 120 °C for 12 h.
Pentiptycene-Based PIMs Polymer Characterization.
To provide a molecular level understanding of the effects of the various backbone configurations and substituent groups on polymer backbone rigidity for PPIMs, molecular modeling was used to analyze the energy changes that occur from the deviation of dihedral angles within the pentiptycene unit. To explore the rigidity of the pentiptycene-based ladder structure relative to that of PIM-1, homopolymer analogs emulating the pentiptycene-PIM regions repeat unit had three different dihedral angles selected, and the energy associated with changes in these angles was calculated (Fig. 1 B and C). Corresponding calculations were done on two dihedral angles chosen from PIM-1, and the results showed good agreement with previously reported molecular modeling of PIM-1 (SI Appendix, Fig. S10) (28). The pentiptycene-PIM and PIM-1 both contain similar dioxane units within their backbones, and this is highlighted by comparable energy wells for the respective dihedral angles encompassing the dioxane units. Relative to the spirobisindane unit, however, two dihedral angles representing the pentiptycene unit exhibit a much narrower energy well, highlighting the inflexibility of the pentiptycene moiety. This enhanced backbone rigidity instilled by the pentiptycene unit, along with pentiptycenes unique architecture providing intrinsic microcavities, highlights the potential of incorporating pentiptycene into a ladder-type polymer.
To understand the structure–property relationships imposed by the unique backbone shapes and pendant groups and their individual effects on polymer properties, polymer powders underwent N2 adsorption testing (SI Appendix, Fig. S13) to explore microporosity via Brunauer–Emmett–Teller (BET) surface area analysis as well as examine pore size distribution (PSD) through the nonlocal density functional theory (NLDFT; SI Appendix, Fig. S14). While varied film histories and potential swelling during N2 adsorption limit true internal surface area analysis within PIMs, BET surface area analysis does provide some insight for comparing between various PIMs (12, 29). For pure PIM-1, literature reports of BET surface areas typically range between 600 and 900 m2/g, which puts the value observed here of 584 m2/g within a reasonable range (7, 30). With pentiptycene present at ∼17% in the PPIM copolymers, similar BET surface areas of 564 to 604 m2/g were observed for all copolymers containing the isopropoxy substituent group, regardless of backbone configuration. The copolymer containing the linear n-propoxy substituent, PPIM-np-S, exhibited a lower BET surface area of 281 m2/g. This is consistent with the results of other iptycene-based PIM series, wherein comparable decreases in BET surface area were observed when changing from branched chain bridgehead substituents to a linear alkyl unit, likely due to greater disruption of polymer chain packing via the less flexible, bulkier branched chain as opposed its linear isomer (15, 16). NLDFT analysis provides a route toward a basic understanding of PSD, as opposed to providing a detailed substructure, and gives some perspective for general comparisons between polymers. PSDs for the series are presented in SI Appendix, Fig. S14 and highlight similar raw NLDFT results as to what is observed in other PIM-1 literature (31). Two primary peaks are observed: one around 7-8 Å and a second peak suspected to be an artifact peak in the 10 to 14 Å regime (31). Similar intensities within the PSD are observed for the isopropoxy-containing polymers for the peak around 7 Å, while a much lower intensity is observed within PPIM-np-S with the linear n-propoxy substituent. Slight shifts in the main peak location are observed in the PSD comparisons, but due to the analysis being done on the polymers in powder form and the challenges already observed in typical NLDFT analysis, such as the previously mentioned presence of artifact peaks, the potential for swelling caused by the N2 adsorption, as well as the different physical state of the polymer due to the cold temperatures (77K) relative to standard permeation conditions, no major conclusions can be drawn from these minor peak shifts (12, 32).
All copolymers were synthesized in sufficient molecular weight (SI Appendix, Table S1) to form robust thin films with thicknesses ranging from 86 to 109 µm (exact thickness of films used for gas permeation testing reported in SI Appendix, Table S2) and 1H-NMR was used to confirm polymer structures (SI Appendix, Figs. S5–S7). Thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) were performed to ascertain the thermal properties of the polymers. All copolymers exhibited good thermal stability, with 10% decomposition occurring above ∼440 °C for all in the series (SI Appendix, Table S1). Similar to what is commonly observed in PIM-1–like ladder polymers, no obvious glass transition temperature is observed prior to the onset of thermal decomposition (33).
Glassy polymer membranes are primarily diffusion-controlled and dependent on the free volume architecture present within the membrane. Density measurements and subsequent fractional free volume (FFV) calculations via the group contribution method were performed to investigate total FFV within the series (SI Appendix, Table S1). PPIM-ip-C exhibited the highest FFV of 25.2%, possibly due to the most tortuous C-shaped backbone configuration of the series, while PPIM-ip-S showed the lowest FFV of 20.1% due to its more symmetric ladder profile, allowing for more efficient chain packing. PPIM-np-S, relative to its isopropoxy substituted counterpart, actually saw a slightly higher FFV of 21.4%, counter to the BET surface area observations that indicated lower surface area from the presence of the more flexible, less bulky substituent unit. PIM-1 displayed a FFV of 21.4%, slightly below the range of reported PIM-1 FFVs, which typically falls from 24 to 26% (34). PPIM-ip-C showed higher FFV than the PIM-1 FFV observed here, with comparable FFVs to PIM-1 for the other copolymers in the series, albeit slightly lower than the reported literature values. This may be due to the presence of the fairly flexible, ether-based substituent groups, which could occupy free volume otherwise unoccupied in pure PIM-1.
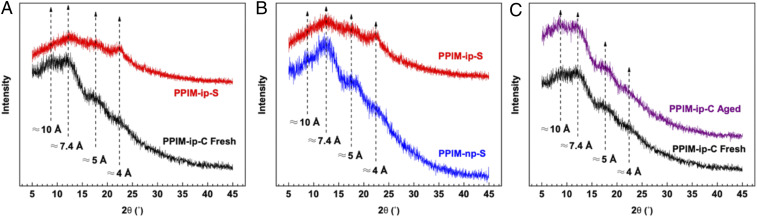
Due to the interrelated nature of interchain distance, FFV, and overall gas permeabilities within polymers, wide-angle X-ray scattering (WAXS) data were collected to further explore the effect of the different backbone configurations and substituents on the overall polymer microstructure (Fig. 2). As is typical for amorphous polymer membranes and PIM-1 type ladder polymers, multiple broad peaks across a range of d-spacing values were observed. For pure PIM-1, up to four peaks are typically observed in WAXS, corresponding to various interchain spacings, and comparable peaks were identified here. The small peak lightly visible around 4 Å is typical of aromatic systems containing π–π stacking interactions, while the peak at ∼5 Å corresponds to regions of more efficient polymer chain packing (35–38). The peak located in the 7 Å range is attributed to the regions of inefficiently packed polymer chains arising from the rigid, contorted ladder structure of the backbone, while the final peak at ∼10 Å relates to the distance between the units containing spiro carbon atoms present in the PIM-1 portions of the polymer chains. Most notable within the series is the slight peak shift toward a region of higher d-spacing for the peak relating to the regions of inefficient chain packing of ladder backbone, which is commonly reported to be around 6.5 to 6.6 Å in pure PIM-1 (35–38). In all pentiptycene-based PPIMs examined here, a shift closer to the 7 to 7.4 Å range is observed, which can likely be attributed to the presence of the bulky pentiptycene unit providing additional configurational rigidity, along with pendant alkyl substituents that protrude off the polymer backbone, potentially driving further distance between the polymer chains. For the copolymers containing the same isopropoxy substituent group but different backbone configurations, small shifts were observed in the inefficiently packed peak regime, as observed in Fig. 2A. The C-shape backbone appears to deliver a shift toward even more inefficient packing, shifting slightly toward higher d-spacing values (slightly greater than ∼7.4 Å), likely due to its more tortuous backbone structure relative to the S-shape. Comparatively, the PPIM-ip-S peak in the inefficient packing range leans closer toward ∼7 Å. PPIM-ip-S and PPIM-np-S were also compared to explore potential packing differences caused by the linear n-propoxy substituent group as opposed to the branched isopropoxy group (Fig. 2B). No significant shifts are observed in the inefficient packing regime around 7.4 Å caused by the two different pendant groups; however, a small shift may be observed in the region attributed to more efficient chain packing where PPIM-ip-S containing the isopropoxy substituent group may be shifted to slightly higher d-spacings (above 5 Å) than PPIM-np-S. This shift can likely be attributed to the stiffer, bulkier isopropoxy unit providing a better disruption of chain packing than its more flexible, linear isomer. Additionally, as physical aging typically has significant effects on the performance of PIMs and glassy polymers in general, an aged sample of PPIM-ip-C was examined as well to divine any effects physical aging may have on the interchain spacing of the polymer. However, no significant differences were observed between the WAXS spectra of the fresh and aged films of PPIM-ip-C, indicating that, on the scale that WAXS can report, no obvious major change within the polymer microstructure occurred (Fig. 2C) though local segmental rearrangements may still be possible.
Fig. 2.
WAXS spectra of fresh films with S- and C-shape backbone configurations and the same branched substituent (A), branched versus linear substituents in fresh films with the S-shape backbone configuration (B), and fresh PPIM-ip-C versus its 150 d aged version (C).
Fresh Film Pure-Gas Separation Performance.
To test the pure-gas permeation properties of H2, CH4, N2, O2, and CO2 within the PPIM series, a constant-volume, variable-pressure pure-gas permeation system was used. As polymers, especially those containing high free volume, are sensitive to a variety of factors within a films processing and thermal history, all freshly cast films were methanol soaked for 24 h then dried in a vacuum oven at 120 °C for 12 h before permeation testing. The permeability and selectivity data for the PPIM series can be seen in Fig. 3 A and B and in SI Appendix, Table S2 with the order of permeabilities being consistent with the trend of pure PIM-1 (PCO2 > PH2 > PO2 > PN2 > PCH4) for each of the pentiptycene-based polymers. While the copolymers reported here only contain around ∼17% of the substituted pentiptycene unit, some clear differences can be observed when compared to pure PIM-1. For all fresh films containing the branched isopropoxy substituent, regardless of the backbone configuration, a few trends emerged (Fig. 3 A and B). PPIM-ip-S and PPIM-ip-C experienced an obvious increase in H2 permeability by 17 to 21% to coincide with either very comparable or slightly reduced permeabilities in CH4, N2, and O2. This led to each of the respective isopropoxy-based PPIMs seeing higher H2/CH4, H2/N2, and O2/N2 selectivities. While PIM-1 still experienced slightly higher CO2 permeabilities than all the respective PPIMs, CO2/CH4 and CO2/N2 selectivities for all isopropoxy-based PPIMs were very comparable to those of PIM-1, with no obvious trend. A starker difference was observed between PPIM-np-S containing the linear n-propoxy substituent group and the isopropoxy-based PPIM-ip series and PIM-1. Consistent with the results from the BET surface area analysis, PPIM-np-S exhibited much lower permeabilities for the fresh film than any other PPIM in the series. These lower permeabilities did coincide with moderately higher selectivities, still providing overall performance of PPIM-np-S above the 2008 upper bound (Fig. 3E). While the presence of the more flexible linear substituent may occupy some of the free volume voids leading to reduced permeabilities, it appears that this has a much greater effect on the larger gases, therefore delivering greatly enhanced selectivities compared to its isopropoxy substituted companions. Regarding the effect of ladder configuration induced by pentiptycene unit (i.e., PPIM-ip-C versus PPIM-ip-S), it appears to have limited impact on gas permeation results, possibly due to the low content of pentiptycene unit. Thus, we cannot yet draw any concrete conclusions on the effect of ladder configuration on gas transport properties. Further study on copolymers with higher pentiptycene contents is ongoing and will provide insights on this topic.
Fig. 3.
Permeabilities at 30 psi (A) and selectivities (B) of freshly cast films for the pentiptycene-based PIMs:PIM-1 1:5 copolymers and PIM-1. Permeabilities (C) and selectivities (D) of fresh and aged PPIM-ip-C (30 psi permeation data) and PPIM-np-S (130 psi permeation data). Robeson upper bound plots for (E) H2/CH4 and (F) O2/N2; all data reported at 30 psi with the exception of PPIM-np-S, which is reported at 130 psi.
Unique Aging-Enhanced Performance Within Pentiptycene-Based PPIMs.
Physical aging is a significant challenge gas separation membranes face in terms of their potential to transition from the laboratory to industry. This is especially relevant for PIM-type microporous polymers, which often experience substantial reductions in permeability over time, usually accompanied by either a corresponding increase in selectivity, but occasionally realizing noteworthy gains in selectivity relative to the permeability loss (3, 13, 39). For example, a 102 µm thick PIM-1 film suffered an 80% decrease in nitrogen permeability after aging 1,380 d (13). To investigate how structure variations (i.e., S-shape versus C-shape and -ip versus -np substitution) may influence physical aging behavior of the pentiptycene-containing ladder polymers, two representative copolymers (i.e., PPIM-np-S [90 µm] and PPIM-ip-C [109 µm]) were aged under ambient conditions for 60 or 120 d, and a piece of the original film was tested after the respective aging period. After 60 or 120 d of aging, both copolymers experienced atypical aging profiles with striking increases in permeability across all gases relative to the fresh films, in sharp contrast to all previously reported microporous ladder polymers, which experience significant reduction in permeability over time (Fig. 3 C and D). Interestingly, the aging-enhanced permeability was accompanied by nearly maintained selectivity for the C-shape configuration (i.e., PPIM-ip-C), and moderately reduced or maintained selectivities coinciding with the greater permeability increases for the S-shape copolymer (i.e., PPIM-np-S). For both polymers, this greatly improves their position relative to the Robeson upper bound after this aging period (Fig. 3 E and F). For a direct, systematic comparison, a PIM-1 polymer was synthesized, purified, cast, and aged following identical procedures to that of the PPIMs, and its gas permeation properties tested after 45 d with a film thickness of 82 µm. Relative to reported fresh PIM-1 permeation data (13, 40), the aged film followed the trend commonly observed in PIM-1, producing much lower permeabilities accompanied by minor selectivity increases after only 45 d. To explore any potential structural changes that may occur in PPIM copolymers during the aging process, 1H-NMR (SI Appendix, Fig. S8) and Fourier-transform infrared spectroscopy (FTIR) (SI Appendix, Fig. S9) were performed on pieces of aged films of PPIM-ip-C. For the 1H-NMR, 88 and 970 d aged films of PPIM-ip-C were analyzed, showing no observable difference in the backbone proton peaks. Using FTIR, films of 88 and 970 d aged PPIM-ip-C samples were examined. To provide further evidence of polymer stability over time, the 88 d aged film was subjected to methanol treatment (soaked in methanol for 24 h, followed by 12 h in the vacuum oven at 120 °C) to revert the aging effect, as frequently applied to PIM-like polymers in the literature. However, between all three FTIR spectra, no significant changes in structure were observed. This, combined with the 1H-NMR data, indicates PPIM copolymers are stable over time. Additionally, no change in the physical appearance and dimensions of the film appeared to occur during aging.
This unique aging performance shown within the pentiptycene-based series can likely be attributed to a few concurrent factors. First, the incorporation of the shape-consistent pentiptycene unit into the ladder backbone along with its respective alkyl side chains may obstruct the polymer chain’s ability to collapse over time, preserving conformational free volume. Second, pentiptycene, like other iptycenes such as triptycene, provides a more permanent intrinsic free volume imbued by the pentiptycene skeleton situated between the pentiptycene blades. Also referred to as IMFV, this is intrinsic to the pentiptycene unit and considered configurational free volume and is therefore not susceptible to collapsing over time through the physical aging process, as conventional conformational free volume may succumb to (3, 4, 12, 41). Lastly, while IMFV of these pentiptycene units is less susceptible to traditional aging, there is potential that these microcavities may be occupied by small substituent groups in close proximity to the pentiptycene unit. The improved permeability over time observed here can potentially be credited to the opening of microcavities initially occupied by isopropoxy or n-propoxy units on neighboring pentiptycene moieties, where polymer chains undergoing their local segmental mobility during the aging process cause these substituent groups to partially or fully vacate IMFV they may have previously been occupying. This mechanism of “partial filling” has been proposed and referenced in other polymers incorporating iptycenes into polymer backbones with various substituent groups, where these groups may partially or fully block these microcavities initially (3, 20, 21, 24, 25). For the PPIM copolymers reported here, this mechanism is further supported by the observation that PPIM-np-S with linear n-propoxy substitution experienced much larger increase in permeability over time than PPIM-ip-C with the bulky isopropoxy substituent because the n-propoxy unit is more readily available to occupy or evacuate the IMFV microvoids due to its linear and more flexible nature as compared to the branched isopropoxy unit. However, further evaluation into the mechanism behind this unusual aging phenomenon is still required for validation, potentially through utilization of microstructure analysis of films before and after aging using positron annihilation lifetime spectroscopy or additional methods of polymer microstructure characterization.
Mixed-Gas Separation Performance of Pentiptycene-Based PIMs.
Another major challenge affecting polymer gas separation membranes is that of plasticization, where condensable gases such as CO2 can cause swelling within the polymer, leading to greater increases in permeability for larger gases than smaller ones, drastically reducing selectivities. While pure-gas permeation data typically delivers ideal separation values, mixed-gas analysis can provide separation performance more comparable to conditions a polymer membrane may actually encounter in industry. To explore membrane performance under mixed-gas conditions, two of the copolymers, PPIM-ip-C (aged 220 d) and PPIM-np-S (aged 10 d) were tested at CO2:CH4 feed ratios [cm3(STP)/min CO2: cm3(STP)/min CH4] of 20:80 and 50:50, with total pressures of 100, 150, and 180 pounds per square inch (psi) measured at each feed ratio (Fig. 4). For both copolymers, CO2 permeability decreased under mixed-gas conditions with increasing CO2 partial pressure due to expected competitive sorption with the cofeed gas of CH4, consistent with reported results for PIM-1 under mixed-gas conditions (32). For membranes with poor resistance to plasticization, this often causes a significant increase in CH4 permeability, therefore greatly reducing CO2/CH4 selectivities. For the pentiptycene-based PPIMs tested here, while substantial reductions in CO2 permeabilities relative to the pure-gas results were observed, they were accompanied by unexpected increases in the CO2/CH4 selectivities under mixed-gas conditions. For example, PPIM-ip-C (aged 220 d) exhibited a CO2/CH4 selectivity of 15.2 in a feed ratio of 20:80 CO2/CH4 at 100 psi, which is more selective than its pure-gas ideal selectivity of ∼10 (for the fresh or aged pure-gas samples). Similar results were observed for PPIM-np-S (aged 10 d), which realized a gain in selectivity from 17.6 in the fresh pure-gas data up to 25.9 when in a feed ratio of 20:80 CO2/CH4 at 100 psi. Additionally, as seen in Fig. 4, neither polymer saw a drastic decrease in selectivity with increasing CO2 partial pressure, each one suffering ∼20% decreases in selectivity when moving from a CO2 partial pressure of ∼2.4 to ∼7.1 atm (atmospheric pressure). Comparatively, PIM-1 sees its mixed-gas CO2 permeability fall from ∼5,500 to ∼5,000 and its mixed-gas CO2:CH4 selectivity fall from ∼14 to ∼10 as it goes from 2 bar to 7 bar, a close to 30% decrease in selectivity in that range (32). This moderate plasticization resistance observed in the PPIM series can likely be attributed to the rigid pentiptycene unit’s ability to take up absorbed gas molecules without excessive swelling of the membrane due to its intrinsic, configurational free volume (23, 42).
Fig. 4.
Mixed-gas CO2 permeability (A) and CO2/CH4 selectivity (B) of two pentiptycene-based PPIM copolymers tested under mixed-gas permeation conditions. Filled points tested at a feed ratio of 20:80 CO2:CH4 at 100, 150, and 180 total psi, while open points represent a 50:50 CO2:CH4 feed ratio also at total pressures of 100, 150, and 180 psi. (C) Robeson upper bound plot presenting pure- and mixed-gas separation performance for PPIM-ip-C and PPIM-np-S.
Conclusions and Future Directions
A series of pentiptycene-based PIM-1–like ladder copolymers utilizing various backbone configurations and substituent groups were synthesized from commercially available starting materials and solution-cast into robust thin films for gas permeation testing. Incorporation of the pentiptycene unit into the ladder backbone of PIM-1, even at only ∼17% integration, delivered gas separation performance enhancement for fresh films within the series. While the branched isopropoxy substituted copolymers generally delivered higher permeabilities with comparable selectivities, the linear n-propoxy unit provided an initial permeability decrease accompanied by corresponding increases in selectivity, with little obvious effect arising from the various backbone configurations at this stage. The presence of the more permanent, configuration-based free volumes coinciding with the proposed unblocking of partially filled microcavities during the aging process yielded moderate permeability increases for PPIM-ip-C and PPIM-np-S with maintained selectivities, delivering unexpected aging-enhanced separation performance. Continued and systematic study of the physical aging behavior of PPIM polymers is undergoing to validate the proposed “partial filling” mechanism. Additionally, mixed-gas permeation testing exhibited surprising mixed-gas selectivity and moderate plasticization resistance again provided by the shape-persistent pentiptycene unit. Pentiptycenes incorporation into PIM-1 yielded promising overall performance, while conveying insight into pentiptycene’s potential to further improve physical aging resistance within PIMs. Further studies will continue to explore the polymer microstructure and unique aging results, as well as incorporate greater amounts of pentiptycene into the polymer backbone.
Materials and Methods
Detailed synthetic procedures for making the S- and C-shaped pentiptycene-based monomers and polymers beginning from commercially available starting materials can be found in the SI Appendix. Also included in the SI Appendix are the full set of characterization methods including 1H-NMR, SEC, TGA, DSC, FTIR, WAXS, density, FFV, N2 adsorption, NLDFT, and molecular modeling of dihedral angle energy deviations, as well as pure- and mixed-gas permeation testing. Appropriate data tables and additional supplemental figures are also included in the SI Appendix.
Supplementary Material
Acknowledgments
This work is supported by the National Science Foundation under Award No. CBET-1603414. T.J.C. acknowledges the support by the National Science Foundation Graduate Research Fellowship Program under Grant No. DGE-1841556. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation. R.G. acknowledges the support by the Division of Chemical Sciences, Biosciences, and Geosciences, Office of Basic Energy Sciences of the US Department of Energy, under Award No. DE-SC0019024. We gratefully acknowledge the Lin laboratory at the University of Buffalo for use of their mixed-gas permeation cell to obtain the mixed-gas CO2:CH4 separation data. We also thank and acknowledge the University of Notre Dame Center for Environmental Science and Technology for use of material characterization equipment.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2022204118/-/DCSupplemental.
Data Availability
All study data are included in the article and/or SI Appendix.
References
- 1.Park H. B., Kamcev J., Robeson L. M., Elimelech M., Freeman B. D., Maximizing the right stuff: The trade-off between membrane permeability and selectivity. Science 356, eaab0530 (2017). [DOI] [PubMed] [Google Scholar]
- 2.Sanders D. F., et al., Energy-efficient polymeric gas separation membranes for a sustainable future: A review. Polymer (Guildf.) 54, 4729–4761 (2013). [Google Scholar]
- 3.Corrado T., Guo R., Macromolecular design strategies toward tailoring free volume in glassy polymers for high performance gas separation membranes. Mol. Syst. Des. Eng. 5, 22–48 (2020). [Google Scholar]
- 4.Weidman J. R., Guo R., The use of iptycenes in rational macromolecular design for gas separation membrane applications. Ind. Eng. Chem. Res. 56, 4220–4236 (2017). [Google Scholar]
- 5.Galizia M., et al., 50th anniversary perspective : Polymers and mixed matrix membranes for gas and vapor separation: A review and prospective opportunities. Macromolecules 50, 7809–7843 (2017). [Google Scholar]
- 6.Freeman B. D., Basis of permeability/selectivity tradeoff relations in polymeric gas separation membranes. Macromolecules 32, 375–380 (1999). [Google Scholar]
- 7.Budd P. M.et al., Polymers of intrinsic microporosity (PIMs): Robust, solution-processable, organic nanoporous materials. Chem. Commun. (Camb.), 230–231 (2004). [DOI] [PubMed] [Google Scholar]
- 8.Robeson L. M., Correlation of separation factor versus permeability for polymeric membranes. J. Membr. Sci. 62, 165–185 (1991). [Google Scholar]
- 9.Robeson L. M., The upper bound revisited. J. Membr. Sci. 320, 390–400 (2008). [Google Scholar]
- 10.Comesaña-Gándara B., et al., Redefining the Robeson upper bounds for CO2/CH4 and CO2/N2 separations using a series of ultrapermeable benzotriptycene-based polymers of intrinsic microporosity. Energy Environ. Sci. 12, 2733–2740 (2019). [Google Scholar]
- 11.Swaidan R., Ghanem B., Pinnau I., Fine-tuned intrinsically ultramicroporous polymers redefine the permeability/selectivity upper bounds of membrane-based air and hydrogen separations. ACS Macro Lett. 4, 947–951 (2015). [DOI] [PubMed] [Google Scholar]
- 12.McKeown N. B., Polymers of intrinsic microporosity (PIMs). Polymer (Guildf.) 202, 122736 (2020). [Google Scholar]
- 13.Swaidan R., Ghanem B., Litwiller E., Pinnau I., Physical aging, plasticization and their effects on gas permeation in “rigid” polymers of intrinsic microporosity. Macromolecules 48, 6553–6561 (2015). [Google Scholar]
- 14.Luo S., et al., Highly selective and permeable microporous polymer membranes for hydrogen purification and CO2 removal from natural gas. Chem. Mater. 30, 5322–5332 (2018). [Google Scholar]
- 15.Swaidan R., Al-Saeedi M., Ghanem B., Litwiller E., Pinnau I., Rational design of intrinsically ultramicroporous polyimides containing bridgehead-substituted triptycene for highly selective and permeable gas separation membranes. Macromolecules 47, 5104–5114 (2014). [Google Scholar]
- 16.Ghanem B. S., Swaidan R., Ma X., Litwiller E., Pinnau I., Energy-efficient hydrogen separation by AB-type ladder-polymer molecular sieves. Adv. Mater. 26, 6696–6700 (2014). [DOI] [PubMed] [Google Scholar]
- 17.Carta M., et al., Triptycene induced enhancement of membrane gas selectivity for microporous Tröger’s base polymers. Adv. Mater. 26, 3526–3531 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rose I., et al., Highly permeable benzotriptycene-based polymer of intrinsic microporosity. ACS Macro Lett. 4, 912–915 (2015). [DOI] [PubMed] [Google Scholar]
- 19.Rose I., et al., Polymer ultrapermeability from the inefficient packing of 2D chains. Nat. Mater. 16, 932–937 (2017). [DOI] [PubMed] [Google Scholar]
- 20.Luo S., et al., Pentiptycene-based polyimides with hierarchically controlled molecular cavity architecture for efficient membrane gas separation. J. Membr. Sci. 480, 20–30 (2015). [Google Scholar]
- 21.Wiegand J. R., et al., Synthesis and characterization of triptycene-based polyimides with tunable high fractional free volume for gas separation membranes. J. Mater. Chem. A Mater. Energy Sustain. 2, 13309–13320 (2014). [Google Scholar]
- 22.Swager T. M., Iptycenes in the design of high performance polymers. Acc. Chem. Res. 41, 1181–1189 (2008). [DOI] [PubMed] [Google Scholar]
- 23.Loianno V., Zhang Q., Luo S., Guo R., Galizia M., Modeling gas and vapor sorption and swelling in triptycene-based polybenzoxazole: Evidence for entropy-driven sorption behavior. Macromolecules 52, 4385–4395 (2019). [Google Scholar]
- 24.Weidman J. R., et al., Analysis of governing factors controlling gas transport through fresh and aged triptycene-based polyimide films. J. Membr. Sci. 522, 12–22 (2017). [Google Scholar]
- 25.Luo S., et al., Molecular origins of fast and selective gas transport in pentiptycene-containing polyimide membranes and their physical aging behavior. J. Membr. Sci. 518, 100–109 (2016). [Google Scholar]
- 26.Zhu X.-Z., Chen C.-F., Iptycene quinones: Synthesis and structure. J. Org. Chem. 70, 917–924 (2005). [DOI] [PubMed] [Google Scholar]
- 27.Song J., et al., Linear high molecular weight ladder polymers by optimized polycondensation of tetrahydroxytetramethylspirobisindane and 1,4-dicyanotetrafluorobenzene †. Macromolecules 41, 7411–7417 (2008). [Google Scholar]
- 28.Carta M., et al., An efficient polymer molecular sieve for membrane gas separations. Science 339, 303–307 (2013). [DOI] [PubMed] [Google Scholar]
- 29.Minelli M., Paul D. R., Sarti G. C., On the interpretation of cryogenic sorption isotherms in glassy polymers. J. Membr. Sci. 540, 229–242 (2017). [Google Scholar]
- 30.Foster A. B., et al., Understanding the topology of the polymer of intrinsic microporosity PIM-1: Cyclics, tadpoles, and network structures and their impact on membrane performance. Macromolecules 53, 569–583 (2020). [Google Scholar]
- 31.Kupgan G., Liyana-Arachchi T. P., Colina C. M., NLDFT pore size distribution in amorphous microporous materials. Langmuir 33, 11138–11145 (2017). [DOI] [PubMed] [Google Scholar]
- 32.Swaidan R., Ghanem B. S., Litwiller E., Pinnau I., Pure- and mixed-gas CO2/CH4 separation properties of PIM-1 and an amidoxime-functionalized PIM-1. J. Membr. Sci. 457, 95–102 (2014). [Google Scholar]
- 33.Yin H., et al., First clear-cut experimental evidence of a glass transition in a polymer with intrinsic microporosity: PIM-1. J. Phys. Chem. Lett. 9, 2003–2008 (2018). [DOI] [PubMed] [Google Scholar]
- 34.Li F. Y., Xiao Y., Chung T.-S., Kawi S., High-performance thermally self-cross-linked polymer of intrinsic microporosity (PIM-1) membranes for energy development. Macromolecules 45, 1427–1437 (2012). [Google Scholar]
- 35.Mei Wu X., et al., Towards enhanced CO2 selectivity of the PIM-1 membrane by blending with polyethylene glycol. J. Membr. Sci. 493, 147–155 (2015). [Google Scholar]
- 36.Li F. Y., Xiao Y., Ong Y. K., Chung T.-S., UV-rearranged PIM-1 polymeric membranes for advanced hydrogen purification and production. Adv. Energy Mater. 2, 1456–1466 (2012). [Google Scholar]
- 37.Du N., et al., Polymers of intrinsic microporosity containing trifluoromethyl and phenylsulfone groups as materials for membrane gas separation †. Macromolecules 41, 9656–9662 (2008). [Google Scholar]
- 38.Yong W. F., Li F. Y., Chung T.-S., Tong Y. W., Highly permeable chemically modified PIM-1/Matrimid membranes for green hydrogen purification. J. Mater. Chem. A Mater. Energy Sustain. 1, 13914–13925 (2013). [Google Scholar]
- 39.Low Z.-X., Budd P. M., McKeown N. B., Patterson D. A., Gas permeation properties, physical aging, and its mitigation in high free volume glassy polymers. Chem. Rev. 118, 5871–5911 (2018). [DOI] [PubMed] [Google Scholar]
- 40.Bezzu C. G., et al., A spirobifluorene-based polymer of intrinsic microporosity with improved performance for gas separation. Adv. Mater. 24, 5930–5933 (2012). [DOI] [PubMed] [Google Scholar]
- 41.Long T. M., Swager T. M., Minimization of free volume: Alignment of triptycenes in liquid crystals and stretched polymers. Adv. Mater. 13, 601–604 (2001). [Google Scholar]
- 42.Loianno V., Luo S., Zhang Q., Guo R., Galizia M., Gas and water vapor sorption and diffusion in a triptycene-based polybenzoxazole: Effect of temperature and pressure and predicting of mixed gas sorption. J. Membr. Sci. 574, 100–111 (2019). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or SI Appendix.