Abstract
In the next decade, separation science will be an important research topic in addressing complex challenges like reducing carbon footprint, lowering energy cost, and making industrial processes simpler. In industrial chemical processes, particularly in petrochemical operations, separation and product refining steps are responsible for up to 30% of energy use and 30% of the capital cost. Membranes and adsorption technologies are being actively studied as alternative and partial replacement opportunities for the state-of-the-art cryogenic distillation systems. This paper provides an industrial perspective on the application of membranes in industrial petrochemical cracker operations. A gas separation performance figure of merit for propylene/propane separation for different classes of materials ranging from inorganic, carbon, polymeric, and facilitated transport membranes is also reported. An in-house–developed model provided insights into the importance of operational parameters on the overall membrane design.
Keywords: membranes, separation, cryogenic distillation, petrochemicals
Separation science has emerged as an active research topic to address energy-related and sustainability challenges. The National Academies of Sciences, Engineering, and Medicine identified the chemical process’s energy intensity as one of the top eight grand challenges for sustainability in the chemical industry (1). In a more recent report, the National Academy of Science outlined a research agenda for transforming separation sciences (2). As stated in the report, industrial separation operations are estimated to be about half the energy used in the US industry and 10–15% of the total US energy consumption. In a separate report, Sholl and Lively (3) reported the top seven separation challenges to change the world. The report highlights separation of alkenes from alkanes as a key energy-intensive processes, with the purification of propylene alone accounting for 0.3% of global energy use (3). For example, in a typical petrochemical steam cracker plant for the production of ethylene, separation units account for almost 30% of the overall energy usage for operating the complex as shown in SI Appendix, Fig. S1 (4). Capital cost savings and debottlenecking are additional value propositions justifying innovation needs in the field of separation science for a hydrocarbon cracker operation.
It is essential to recognize that savings that often appear to be promising from the implementation of new separation technologies on initial review are limited by the practical process and operational limitations. This current paper attempts to provide an industrial perspective on the separation challenges and opportunities for olefin/paraffin separation in a petrochemical cracker operation with specific emphasis on the following topics: 1) overview of current separation unit operation in ethylene/propylene production in a petrochemical cracker operation; 2) alternative separation technologies—materials, the current state of development, potential, and research needs; and 3) applications of olefin–paraffin separation materials in petrochemical process.
Overview of Current Separation Unit Operation in Ethylene/Propylene Production in a Petrochemical Cracker Operation
Petrochemical cracker plant design and the order of the chemical separations depends on the feed being cracked, the age of the plant, and the method of heat integration. These configurations are described in Ullmann’s Encyclopedia of Industrial Chemistry (5). A typical flow sheet for olefin production is provided in Fig. 1. In this flowsheet, the crude product from the cracking furnaces is sent to a quench column to remove water and heavy fractions, and the remaining gas is then compressed. The first column takes C3 stream (propylene and propane) and lighter components in the overhead, and C4 (butane and heavier) components in the tails. The second separation in the sequence is demethanizer, which separates H2 and CH4 from the C2 (ethylene and ethane)/C3 stream. The critical separation in this column is methane/ethylene. The tail stream is then sent to the deethanizer, which separates C2s from C3s. The key separation in this step is ethane/propylene. The final two columns are the C2 splitter, which separates ethylene and ethane, and the C3 splitter that separates propylene and propane. The green-colored sections in Fig. 1 are unit operations where there is potential for application of advanced separation technologies to reduce the energy and capital footprint. Typical compositions of different gas streams in the cracker plant is given in SI Appendix, Table S1 (5). SI Appendix, Table S2 provides a summary of different unit operations with operational characteristics. The unit operations were chosen based on the potential for membranes or other advanced separation technologies to be applied either in conjunction with the current state of the art technology or alone. Understanding the impact of integrating a membrane into an existing chemical process is a critical research area. Process integration plays an essential role in maximizing the benefit of membrane applications (6). Capital and operating costs for having pretreatments, compressors, vacuum pumps, membrane lifetime, and reliability often diminish the returns. SI Appendix, Fig. S1 illustrates the typical breakdown of energy and the capital requirements for a typical ethylene production plant (4, 7, 8). Further details on the capital and energy requirements for separations, unit operations in separations scheme, and integration of membranes in the separation process are discussed in SI Appendix. The potential of olefin/paraffin separation using membranes for olefin/paraffin splitting will be reviewed in this paper in detail.
Fig. 1.
Petrochemical cracker separation general diagram.
Alternative Separation Technologies—Materials, the Current State of Development, Potential, and Research Needs
Several reviews have covered overviews of the advanced separation technology landscape for olefin–paraffin separation in recent years (9, 10). This paper is not an attempt to provide a comprehensive review, but to understand specific challenges and opportunities and the impact of process design/operational parameters on the overall design space. A recent report provided an overview of different thermal separation technologies and ranked them in the order of energy use (2). SI Appendix, Fig. S2 is a schematic overview of different separation technologies ranked according to their energy usage. This report will focus mostly on membrane applications. We propose the initial implementations will likely be a hybrid design of membrane with distillation or membrane with adsorption. Membranes are considered one of the promising technologies for bulk separation in chemical processes. Membrane processes are typically associated with reduced energy and capital footprint, the ability to be modular, thus having the potential to lower capital intensity, use less chemicals, and complement existing processes that enable higher production output. Specifically, Sholl et al. (3) report that membrane-based separation could use 90% less energy than distillation. In the last decade, considerable progress has been made in academic literature in developing new membrane materials for olefin–paraffin separation (9–13). The materials can be classified as conventional polymeric membrane; inorganic (zeolite and metal–organic frameworks [MOFs]); carbon molecular sieves (CMSs); and facilitated transport membranes.
SI Appendix, Fig. S3 provides the gas separation mechanism in each material class. The transport mechanism in polymer matrix is believed to be based on classical solution diffusion theory (14). Polymer chain mobility, fractional free volume, and chemical composition plays a critical role in controlling performance. In facilitated transport membranes, olefins transport by reversible chemical binding with a carrier (π‐complex) along with pure physical diffusion as shown in SI Appendix, Fig. S3B (15). CMS membranes are prepared by pyrolysis of polymeric precursors and the inefficient packing of the turbostratic graphite structure results in polydisperse pore structure as shown in SI Appendix, Fig. S3C (16). The gas molecules separated based on the gas kinetic diameter and pore size of the membranes. Zeolites and MOFs are other crystalline materials with defined pores as shown in SI Appendix, Fig. S3D and separate the gases by molecular sieving similar to the CMS membranes.
Polymeric Membranes.
Materials and gas separation performance.
Polymeric membranes currently dominate industrial gas separation applications compared to other membrane materials because of their low cost, processability, and scalability. Polymer membranes show an upper bound relationship for olefin/paraffin separations similar to other gas pairs as shown by Burns and Koros (17), and Rungta et al. (18) Four main polymer classes have been studied for hydrocarbon separations; rubbery polymers, polyimides, microporous polymers, and thermally rearranged (TR) polymers. Lin and Freeman (19) studied polyethylene oxide for olefin/paraffin separations and observed low selectivity compared to glassy polymers, as shown in Fig. 2. Rubbery polymers can plasticize very easily in the presence of cracked gases compared to glassy polymer membranes because of their framework flexibility. Glassy polymers such as cellulose acetate, poly(phenylene oxide), matrimid, polysulfone, ethylcellulose, and 6FDA-based copolymers showed improvement in hydrocarbon separation performance while displaying improved plasticization resistance (20). Porous polymers, such as polymers of intrinsic microporosity (PIM) and TR polymer membranes, surpassed the Robeson upper bound for most gas pairs (21). Due to the inefficient packing of inflexible and contorted chains, PIM membranes showed promising C3H6 permeability, as shown in Fig. 2. The gas separation performance and plasticization resistance of PIM membranes can be tuned by polymer cross-linking, by increasing the intrachain rigidity and by introducing interchain charge-transfer complexes (22–24). However, they also observed lower separation performance of PIM-1 under mixed gas and at high-pressure conditions because of plasticization. Although PIMs showed excellent gas separation performance, practical performance is still questionable because of expected performance deterioration due to aging (25). It is also known that thin films age faster compared to dense films, and it is important to study the aging of PIM-1 membranes as thin films (26). TR polymers with well‐connected pores and high free volume, showed improved propylene separation performance, but the performance quickly degraded due to plasticization in the presence of mixed gases (27).
Fig. 2.
Comparison of propylene/propane gas separation performance of different polymeric materials (further details on the source of literature data are provided inSI Appendix).
Polymeric membranes were also studied for ethylene/ethane separation. In most cases, polymeric membranes always showed lower ethylene selectivity while the same membrane showed high propylene selectivity (28, 29). The difference in size and condensability properties of ethane and ethylene is very small, and as a result it is difficult to separate C2s based on either diffusivity or solubility. Polymeric chains with more defined pores and rigid chains are needed to differentiate ethylene and ethane based on their molecular size. Asymmetric polyimide hollow fiber membranes with a thin selective layer were also studied for hydrocarbon separations (see Fig. 8). Separation performance of the polymer membranes followed trade-off relations and lower performance compared to other materials, which needs to be addressed.
Fig. 8.
Figure of merit of C3 separation for inorganic, CMS, polymeric, and facilitated membranes (further details on the source of literature data are provided in SI Appendix).
Challenges and outlook.
Currently, polymeric membranes are available commercially for several large-scale gas separation applications, but in the case of hydrocarbon separations, polymeric membranes were only used for small-scale olefin recovery applications (30). Properties of polymeric membranes, such as inherent permeability/selectivity trade-off, plasticization at high pressures, and lower thermal and chemical stability, restrict their application in large-scale cracked gas separations. Novel polymeric membranes, which show good potential for hydrocarbon separations, should be tested under high pressures and in the presence of impurities to study plasticization effects on gas separation performance. It is also important to fabricate industrially useful thin-film asymmetric membranes, which plasticize, and age differently compared to dense films, to evaluate the true potential of these polymers for hydrocarbon separations. The polymeric membranes usually show high ideal selectivity with pure gas testing at low pressures, and the selectivity decreases significantly at high-pressure mixed-gas conditions due to the cooperative diffusion effect, which needs to be addressed. The cost of high-performance polyimides is also high compared to conventional glassy polymers, and research also needs to be focused on synthesizing these polymers using low-cost monomers. It is also important to understand diffusion and sorption mechanism of olefins and paraffins to design the polymer structure such that it can differentiate these gas molecules while having good plasticization resistance.
Facilitated Transport Membranes.
For practical applications, olefin selectivity should be higher than the current polymeric membranes performance to meet the required purity of olefins (>99%) needed for further processing. Higher olefin selectivity in polymer membranes can be achieved by the addition of functional groups that preferentially interact and facilitate the transport of olefin over paraffin across the membrane. In a facilitated transport process, olefins can reversibly bind to the carrier by forming electron donor/acceptor complexes with olefins through interactions of the olefin π-orbitals and diffuse onto the other side of the membrane, resulting in higher separation performance compared to the polymeric membranes (see Fig. 8). Facilitated transport membranes can be fabricated in two main forms: liquid carrier membranes and fixed carrier membranes. The olefins transport in the facilitated transport membranes occurs by either mobile diffusion of the olefin-carrier complex in the case of liquid carrier agents or by hopping mechanism in the case of fixed site carriers along with the solution diffusion mechanism in polymer phase. The separation performance of facilitated transport membranes also depends on the carrier concentration and pressure drop across the membranes.
Materials and gas separation performance.
Several fixed-site carrier-based facilitated transport membranes containing transition metal salts and metal nanoparticles were reported for olefin/paraffin separation. Ag(I) ion was the most studied carrier for olefin/paraffin separation. AgNO3 (31), and AgBF4 (32), salts were dispersed in the polymer matrix and studied for olefin/paraffin separation. Addition of metal nanoparticles (Ag, Au, and Cu) was also studied by several groups and showed that the activity/complex formation ability of metal nanoparticles was very low compared to metal salts, resulting in low separation performance (33). In order to further improve the olefin transport, mobile carriers were immobilized in the pores of the porous polymeric supports and showed high separation performance (34). Both fixed and mobile carriers in polymeric support showed improved performance compared to polymers (see Fig. 8).
Challenges and outlook.
Despite the attractive separation performance of facilitated transport membranes, the long-term stability of carrier is still a major problem restricting its commercial viability. Very high starting selectivity for olefin/paraffin separation has been reported using the mobile carrier membranes, but rapid carrier loss during high pressure operations caused separation performance degradation (35). Fixed carrier site membranes can withstand high pressures without physical loss of the carrier, but chemical deactivation of metal ions is a major challenge (36). Merkel et al. (37) studied different operational parameters such as impact of light exposure, hydrogen, hydrogen sulfide, and acetylene on the silver salt activity in Pebax 2533/AgBF4 membranes and observed that all of them significantly reduced ethylene/ethane selectivity. They also found out that silver ion activity can be regenerated by peroxide/acid treatment, but the continuous variation in performance during the life of membrane and stability in the presence of impurities are still issues that needs to overcome for commercial success. A critical observation was that the olefins can form complexes with silver ions and decrease silver ion activity even in the presence of ideal olefin/paraffin mixtures without any impurities (36), exemplifying the importance of carrier instability as a barrier to application of such membranes to olefin/paraffin separations.
Improving the stability of the carrier is the most important research area to bring the facilitated membranes closer to industrial applications. Impurities in the cracker gas streams such as acetylene, methyl acetylene (MA), propadiene (PD), and sulfur compounds also pose problems by reacting with the carriers and degrading rapidly with significant loss in separation performance (36). Currently, pilot-scale facilitated transport membranes are under investigation in the cracker plants to understand the issues in the presence of impurities. The optimization of binding strength of olefins with the carrier was not studied extensively in the literature and should be a focus area to improve the performance of these membranes by finding stable and optimal electronegative transition metal-based carriers beyond silver. In the olefin/paraffin separation process using facilitated transport membranes, sometimes feed gas needs to be hydrated to achieve high performances (37). However, the costs associated with the addition and removal of water needs to be justified with the improvement in the separation performance.
CMS Membranes.
CMS membranes have been reported to exhibit superior separation properties, potentially surpassing the permeability vs. selectivity trade-off relationship of available polymeric membranes (38). CMS membranes are prepared through pyrolysis of a polymeric membrane precursor under a controlled vacuum or inert environment to form micropores (7–20 Å) and ultramicropores (<7 Å) as described by Koros and coworkers (39). Besides polymer precursors, pyrolysis conditions such as pyrolysis environment, ramp rate, final pyrolysis temperature, and thermal soak time play a critical role to determine the resultant CMS membrane structure and its separation properties. Also, during the carbonization of these precursors, there remains residual oxygen and nitrogen functionality, which both play a role in surface adsorption and carbon texture/microstructure by affecting the bonding that can exist in the carbon domains (39).
Materials and gas separation performance.
Fig. 3 represents an upper bound relationship of propylene–propane separation using CMS membranes that were classified based on the polymer precursor used. Four classes of polymers were reported; copolyimides, Novolac resin, PIMS, and PEEK. Copolyimides were further subclassified based on the structure of dianhydride monomer. Membranes were also classified based on the fabrication method. Unfilled data points represent membranes cast as dense films. Filled data points represent membrane fabricated as hollow fiber or composite. A strong trade-off between membrane permeability and selectivity was observed. Among the classes of polyimides, no significant difference of polymer precursor was observed. One exception was the FDA-based copolyimides, which appear to have a better trade-off from the rest. Differences could be a factor of polymer structure as well as the method of testing, fabrication, and other parameters. Novolac resin-based CMS tends to have a higher permeability than the copolyimides but falls on a similar trade-off line (40).
Fig. 3.
Permeability vs. selectivity plot of C3 separation for CMS membranes with different polymeric precursors (further details on the source of literature data are provided in SI Appendix).
SI Appendix, Fig. S4 represents the selectivity vs. permeability trade-off relationship for ethylene/ethane separation for CMS membrane. The PIM precursor-based CMS membranes showed a higher ethylene selectivity and lower permeability, while the Novolac resin precursor-based CMS membranes were associated with higher permeability and lower selectivity. The polyimides were intermediate in performance between the PIM-based and Novolac-based CMS membranes. Fig. 3 and SI Appendix, Fig. S4 demonstrate a strong olefin/paraffin upper-bound relationship in CMS membrane performance. There is definitely room for developing a new polymeric structure, and research should be focused in that direction. At the same time, the trade-off may suggest that efforts should be pursued in understanding the factors controlling the final CMS structure.
Challenges and outlook.
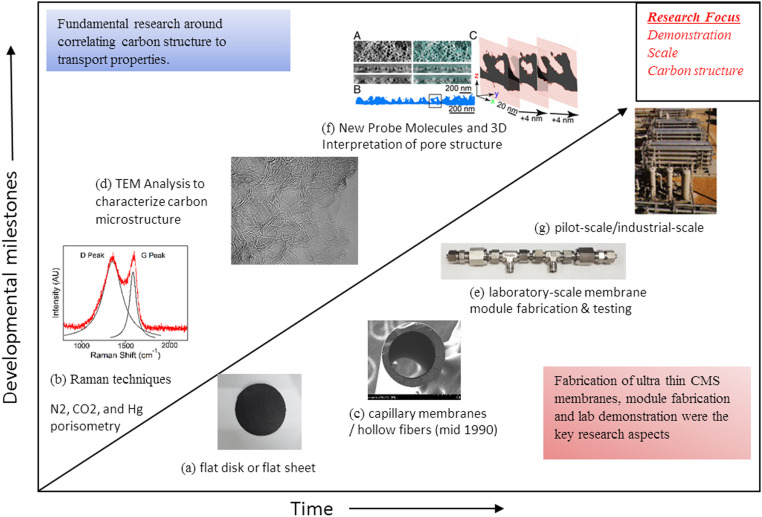
Fig. 4 provides a roadmap of CMS development and outlines the specific CMS membrane research direction for the future. Demonstration and reliability, and structure–property relationships are the two main next-generation research directions for CMS development. Early-stage CMS membranes were developed by coating polymer precursors on the flat disk or sheets and ceramic tube supports, and pyrolyze the composite to obtain CMS membranes. CMS membrane research gained momentum with the development of stand-alone hollow fiber CMS membranes. Due to its high packing density and scalability, considerable progress has been made toward the pilot-scale demonstration of CMS hollow fiber membranes. CMS membranes undergo reduction in permeability during storage and during operations due to physical aging and impact of chemical environment (41). Besides scale-up, reliability issues like membrane aging, long-term performance, and the impact of impurities need to be studied and understood. Unlike polymeric membranes, membrane replacement cost and operating cost at scale are not well understood for CMS membranes.
Fig. 4.
Proposed roadmap for CMS membrane technology development: (A) CMS flat disk membranes; (B) Raman analysis of carbon structure; (C) SEM cross-sectional image of a CMS hollow-fiber membrane; (D) TEM analysis of a pyrolyzed polymer (60) (Reproduced with permission from ref. 60, which is licensed under CC BY 4.0); (E) a CMS hollow-fiber module for permeation testing; (F) TEM tomography of a reverse osmosis membrane (61) (Reproduced with permissions from ref. 61); and (G) photograph of a model demonstration skid.
As material development continues, it is equally important to accelerate application development to fully understand the potential and economics behind the technology. Evaluating gas separation properties are often reported as a method to understand the structure–property relationship owing to unique structural characterization challenges of CMS membranes. The challenges in the development of structure–property relationship are discussed in detail in SI Appendix. As these challenges are overcome, the existing conceptual model of the CMS membrane can be refined, and details of the structure–property relationships can be exploited in the development of next-generation membranes tailored to specific applications. Detailed information of structure–property relationship recommendations is provided in SI Appendix.
Inorganic Membranes.
The main advantage of inorganic membranes compared to other types of membranes is their thermal and chemical resistance along with high gas separation performance, which makes them an attractive choice for petrochemical separations. Two types of inorganic porous membranes that were studied extensively for petrochemical applications are zeolites an MOFs (42). Porous materials showed great potential for petrochemical separations because of their high gas fluxes compared to nonporous materials while having desirable selectivity due to their rigid and uniform pore structures in the range of molecular dimensions.
Materials and gas separation performance.
Several zeolite membranes such as ETS-10 (43), Zeolite X (44), fajusite (45), and Zeolite Y (46) showed higher olefin permeances compared to other membranes under a wide range of temperatures and feed pressures, but the olefin selectivity was still low (see Fig. 8). MOFs are a relatively novel class of porous crystalline materials composed of coordination bonds between metal ions and organic linkers. Compared to traditional porous materials like zeolites, MOFs are advantageous because of their ease of structure and chemistry modification at the molecular level via presynthetic and postsynthetic processes (47). Zeolitic imidazolate framework-8 (ZIF-8), a subclass of MOFs, has been extensively studied for C3H6/C3H8 separation due to its interesting molecular sieving properties and promising gas separation performance (see Figs. 5 and 8). ZIF-8 membranes showed promising separation performance for propylene separation, but ethylene/ethane separation perfomance of the very same membranes was low (48). The inorganic porous materials need to be designed depending on the gas pair of separation with strict and tighter pores to achieve very good olefin sieving properties.
Fig. 5.
Proposed roadmap for inorganic membranes development: (A) zeolite membrane (62) (Reprinted with permission from ref. 62. Copyright 1998 American Chemical Society); (B) rapid thermal processing (49) (From ref. 49. Reprinted with permission from AAAS); (C) ZIF-8 membrane fabrication (63) (Reprinted with permission from ref. 63. Copyright 2009 American Chemical Society); (D) MOF membrane fabrication by flow synthesis (53) (From ref. 53. Reprinted with permission from AAAS); (E) vapor-phase MOF membrane fabrication process (64) (Reprinted with permission from ref. 64. Copyright 2018 American Chemical Society); (F) large-area MOF membrane module fabrication (54) (Reproduced with permission from ref. 54, which is licensed under CC BY 4.0); and (G) crystal engineered MOF membranes (55) (Reprinted with permission from ref. 55. Copyright 2020 American Chemical Society.).
Challenges and outlook.
Significant progress has been made on the fabrication of inorganic membranes over the last two decades. The inorganic membrane representative milestones are provided in Fig. 5. Zeolite membranes were first studied for hydrocarbon separations in 1998 as shown in Fig. 5. Scalability was the biggest issue for commercialization of these membranes. Even though the studies, such as rapid thermal processing (49) and one-step fabrication method (50), showed the potential of scalability, there is still a long way to go to fabricate thin, scalable, and economical zeolite membranes. Significant progress has been made after the introduction of MOF materials because of the ease of synthesis and material design. A scalable counter diffusion-based in situ method was developed for the fabrication of defect-free ZIF-8 (51) and ZIF-67 (52) membranes at the interface of the porous support by flowing metal and organic ligand precursors on either side of the porous support. Brown et al. (53) extended this method for the fabrication of ZIF-8 membranes using cheaper and scalable polymer hollow fiber supports as shown in Fig. 5. Li et al. (54) successfully demonstrated the fabrication of defect-free inorganic membranes on 30 polymeric hollow-fiber supports in a single module, showing the potential for the fabrication of large-scale modules. Controlling the framework flexibility and grain boundary defect elimination should be the focus to further enhance the olefin/paraffin selectivity. Crystal engineering strategy was used to balance the grain boundary structure and framework flexibility and showed high separation performance for ZIF-67 membrane compared with randomly crystallized membranes as shown in Fig. 5 (55). Very little research went into the fabrication of defect-free thin films (<100 nm) in order to obtain high fluxes and this should be the focus to obtain industrial-scale gas fluxes. Another route to utilize the promising features of porous membrane materials is by mixing these materials with polymers that are scalable, but compatibility between polymer and inorganic materials is still a major challenge (56).
Currently, commercially available inorganic membranes are scarce and commercialized only for solvent dehydration applications (57, 58). Research attention toward inorganic membranes is rapidly growing, focusing on several issues such as cost, reproducibility, and thickness as shown in Fig. 5, and if successful, the market demand for these inorganic membranes can grow rapidly. The cost of these membranes can be reduced by using cheaper raw materials and the development of cost-effective fabrication methods. The packing density of the current inorganic membranes is very low compared to polymer hollow fibers and results in a large footprint and high cost. The cost of inorganic membranes on a unit area basis is 10 to 50 times that of a polymer membrane module (42). The olefin separation performance of inorganic membranes needs to be very high to justify the high cost. Inorganic membranes also suffer from a high degree of variability in performance arising from an extreme sensitivity to synthesis and preparation conditions. More robust fabrication techniques that are scalable need to be developed to obtain thinner membranes that can process the large amount of gases coming from the petrochemical plants. These membranes need to be tested at realistic cracked gas conditions, i.e., at high pressures and in the presence of impurities, to evaluate the robustness of these membranes.
Applications of Olefin–Paraffin Separation Materials in Petrochemical Process
In the previous section, the importance of membrane structural parameters and membrane thickness on overall membrane productivity or flux was highlighted. One operational parameter that often decides the economic viability of a membrane process is the partial pressure difference of the permeating gas. Flux is directly proportional to the partial pressure difference. In cases where there is not enough partial pressure gradient for transport, a compressor on the feed side or vacuum pump on the permeate side is used to increase the partial pressure difference. These unit operations increase the overall capital (CAPEX) and operational (OPEX) costs. Various system parameters including pressure ratio (PR), stage cut, purity, and recovery are defined in SI Appendix. A thorough understanding of the relationship between membrane performance and operational parameters is needed to maximize overall system performance. An in-house custom model utilizing Aspen Plus software was developed to simulate the impact of operational parameters, such as pressure ratio and stage cut, on membrane-intrinsic properties. Both single membrane and a hybrid design were evaluated. Membrane permeance for C3 gas pair was varied from 5 to 1,000 GPU, and selectivity was varied from 4 to 1,000. Below is a summary of the findings.
Single-Membrane Case.
The relationship between selectivity, recovery, and pressure ratio across the membrane can be illustrated by a simple single-membrane example. The impact of pressure ratio on selectivity is shown in SI Appendix, Fig. S5, and further details are provided in SI Appendix. Fig. 6 demonstrate the impact of selectivity and pressure ratio on product recovery. For a given membrane selectivity and pressure ratio across the membrane, a stage cut can be calculated, which gives a permeate purity of 95%, provided the selectivity is high enough. For a pressure ratio of 2.5 and a selectivity of 250, the stage cut required to meet the product specification is only 1%. As a result, the recovery is very low. A higher stage cut, which increases recovery, results in a lower product purity. If the membrane selectivity is increased at constant pressure ratio, the recovery increases, but even at 1,000, recovery is still below 20%. An increase in pressure ratio results in increased recovery with diminishing returns after pressure ratio of 5. If the pressure ratio is increased, the product specification can be met at lower selectivity. In addition, much higher recovery can be achieved as selectivity is increased. However, for each pressure ratio there is a limit on the recovery caused by a reduction in driving force across the membrane. These curves show that as selectivity is increased, there is an initial large increase in recovery, but this levels out at high selectivity.
Fig. 6.
Recovery vs. propylene/propane selectivity.
The corresponding membrane area*GPU for each pressure ratio curve is shown in Fig. 7. For a pressure ratio of 5, the recovery is about 80% for a selectivity of 200. The area*GPU is a little over 10,000. If a higher selectivity membrane was available, recovery could be marginally improved at constant pressure, but the required membrane area would also be much higher. At a selectivity of 400, the area*GPU would be over 20,000. Generally, an increase in selectivity results in a decrease in propylene permeance, so the actual membrane area would even more than double. Similar analysis on hybrid membrane/distillation case is provided in SI Appendix, Figs. S6 and S7).
Fig. 7.
Area*permeance (GPU) vs. propylene/propane selectivity.
Conclusions and Recommendations
There have been several applications where membranes are currently used to bring economical value and improve overall sustainability. In many cases, it is not only the membrane performance, but a combination of several metrics that allow the successful commercialization and adaptation to existing and new process (59). The following paragraphs will focus on the critical questions around the pathway to implementation of membrane-based technologies for olefin paraffin separation. In most of the previously reported literature, upper bound relationships for various materials were reported with the gas transport expressed as permeability. The plots provide an approach to compare and guide material design for a given gas pair separation. In most studies, the membranes are cast in the dense membrane form. For practical applications, thinner membranes are desired to maximize the permeance or flux. Permeance information could readily be leveraged for further techno-economic and process integration analysis. Fig. 8 represents the upper bound relationship for various materials as reported for C3 gas pair separation. The data represent membranes fabricated in hollow-fiber or thin-film composites, allowing comparison and a step closer to practicality. Individual trade-offs were observed for a given class of material. Inorganic membranes based on MOFs show higher permeance and selectivity compared to other materials. The trend between CMS and polymeric membranes are similar, as reported in previous literature.
The application section and discussion above were to underline the importance of operating parameters in designing and implementing membranes into a process. A minimum pressure ratio cutoff is required to allow membranes with reasonable selectivity (>20) to achieve desired permeate purity. An increase in selectivity at a given pressure ratio will increase the overall recovery to a certain point before the increase in recovery is offset by corresponding increase in membrane area. Hence a both upper limit and lower limit selectivity guidelines could be drawn for a reasonable pressure ratio. Increasing membrane permeance while having the optimized olefin selectivity will allow an increased CAPEX and OPEX savings. Similar studies need to be conducted for achieving polymer-grade propylene purity. Performance is believed to be one of the key metrics for success, and at the same time it is important to consider all the metrics, such as robustness, sustained long-term performance, in choosing the right technology for the right applications. Reliability both in terms of ability to manufacture reproducibly at a larger scale and achieving long-term sustainable field performance are equally important. The membranes for gas separation applications are expected to show stable performance (i.e., stable permeance and selectivity) for ∼5 y (59). Each technology has its own merits and demerits. SI Appendix, Table S3 summarizes four separate metrics important for overall success of the membrane materials in a process and the current state of each technology.
Hydrocarbon separation performance of polymeric membranes is low compared to other materials, but it has advantages of easy fabrication at industrial scale with low cost as shown in SI Appendix, Table S3. However, the long-term performance along with plasticization and aging in the presence of aggressive cracked gas feed conditions is limiting in terms of their applicability for olefin/paraffin separations. Alternatively, facilitated transport membranes showed desirable olefin/paraffin separation performance with good scalability. Currently pilot-scale facilitated transport membranes are showing promising performance for propylene separation. However, carrier deactivation in the presence of impurities and, in some cases, in the presence of olefins itself is the biggest hurdle for applications with these membranes. Stabilization of carrier would make them excellent candidates for hydrocarbon separations. Pyrolysis of polymers to form CMS membranes improved the separation performance significantly while having the stability under these aggressive conditions. Even though fabrication of the CMS is moderately difficult compared to the polymer membranes (SI Appendix, Table S3), these are potentially scalable, and the added cost of pyrolysis makes them more costly. Porous inorganic membranes garnered significant attention due to their high propylene separation performance as shown in Fig. 8. Currently laboratory-scale membranes are under development for olefin/paraffin separation. While it is clear that the structure of the material that composes the membrane will dictate the performance of the final product, there are several analytical challenges that persist in gaining insight into the structure–property relationship, which will enable the design of new, more effective membranes. Also, the cost of these membranes is higher due to their costly starting materials and fabrication process, which needs to be addressed to be applicable for industrial applications. At the same time, it is important to acknowledge that the application development for olefin/paraffin separation using membranes is in infancy compared to other membrane separation processes. Front-end engineering design will highlight and help to maximize the impact of advanced separation technologies in petrochemical cracker operation. Industrial perspective on the current landscape of olefin/paraffin separation technology focusing on material development, application development, and demonstration at scale was discussed and further recommendations are provided in SI Appendix.
Supplementary Material
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission. M.K. is a guest editor invited by the Editorial Board.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2022194118/-/DCSupplemental.
Data Availability
All study data are included in the article and/or SI Appendix.
References
- 1.National Academies of Sciences, Engineering, and Medicine , Sustainability in the Chemical Industry: Grand Challenges and Research Needs (National Academies Press, 2005). [Google Scholar]
- 2.National Academies of Sciences, Engineering, and Medicine , A Research Agenda for Transforming Separation Science (National Academies Press, 2019). [Google Scholar]
- 3.Sholl D. S., Lively R. P., Seven chemical separations to change the world. Nature 532, 435–437 (2016). [DOI] [PubMed] [Google Scholar]
- 4.Ernst Worrell D. P., Einstein D., Martin N., “Energy use and energy intensity of the U.S. chemical industry” (LBNL-44314, Lawrence Berkeley National Laboratory, 2000. [Google Scholar]
- 5.Zimmermann H., Walzi R., “Ethylene” in Ullmann’s Encyclopedia of Industrial Chemistry (Wiley, 2012), vol. 13, pp. 465–529. [Google Scholar]
- 6.Caballero J. A., Grossmann I. E., Keyvani M., Lenz E. S., Design of hybrid distillation—vapor membrane separation systems. Ind. Eng. Chem. Res. 48, 9151–9162 (2009). [Google Scholar]
- 7.Gajendra Kumar M. A., “Steam cracking of crude oil” (PEP report 29J, IHS Chemical, 2016). [Google Scholar]
- 8.Nielsen R., “Ethylene plant enhancement” (PEP report 29G, IHS Chemical, 2001). [Google Scholar]
- 9.Ren Y., et al., Membrane-based olefin/paraffin separations. Adv. Sci. (Weinh.) 7, 2001398 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanders D. F., et al., Energy-efficient polymeric gas separation membranes for a sustainable future: A review. Polymer (Guildf.) 54, 4729–4761 (2013). [Google Scholar]
- 11.Takht Ravanchi M., Kaghazchi T., Kargari A., Application of membrane separation processes in petrochemical industry: A review. Desalination 235, 199–244 (2009). [Google Scholar]
- 12.Liang C. Z., Chung T.-S., Lai J.-Y., A review of polymeric composite membranes for gas separation and energy production. Prog. Polym. Sci. 97, 101141 (2019). [Google Scholar]
- 13.Azhin M., Kaghazchi T., Rahmani M., A review on olefin/paraffin separation using reversible chemical complexation technology. J. Ind. Eng. Chem. 14, 622–638 (2008). [Google Scholar]
- 14.Wijmans J. G., Baker R. W., The solution-diffusion model: A review. J. Membr. Sci. 107, 1–21 (1995). [Google Scholar]
- 15.Cheng L., Liu G., Jin W., Recent advances in facilitated transport membranes for olefin/paraffin separation. Discover Chem. Eng. 1, 1 (2020). [Google Scholar]
- 16.Bhuwania N., et al., Engineering substructure morphology of asymmetric carbon molecular sieve hollow fiber membranes. Carbon 76, 417–434 (2014). [Google Scholar]
- 17.Burns R. L., Koros W. J., Defining the challenges for C3H6/C3H8 separation using polymeric membranes. J. Membr. Sci. 211, 299–309 (2003). [Google Scholar]
- 18.Rungta M., Zhang C., Koros W. J., Xu L., Membrane-based ethylene/ethane separation: The upper bound and beyond. AIChE J. 59, 3475–3489 (2013). [Google Scholar]
- 19.Lin H., Freeman B. D., Gas solubility, diffusivity and permeability in poly(ethylene oxide). J. Membr. Sci. 239, 105–117 (2004). [Google Scholar]
- 20.Iyer G. M., Liu L., Zhang C., Hydrocarbon separations by glassy polymer membranes. J. Polym. Sci. 58, 2482–2517 (2020). [Google Scholar]
- 21.Robeson L. M., The upper bound revisited. J. Membr. Sci. 320, 390–400 (2008). [Google Scholar]
- 22.Khan M. M., et al., Cross-linking of polymer of intrinsic microporosity (PIM-1) via nitrene reaction and its effect on gas transport property. Eur. Polym. J. 49, 4157–4166 (2013). [Google Scholar]
- 23.Du N., et al., Azide-based cross-linking of polymers of intrinsic microporosity (PIMs) for condensable gas separation. Macromol. Rapid Commun. 32, 631–636 (2011). [DOI] [PubMed] [Google Scholar]
- 24.Swaidan R. J., Ghanem B., Swaidan R., Litwiller E., Pinnau I., Pure- and mixed-gas propylene/propane permeation properties of spiro- and triptycene-based microporous polyimides. J. Membr. Sci. 492, 116–122 (2015). [Google Scholar]
- 25.Bernardo P., et al., Effect of physical aging on the gas transport and sorption in PIM-1 membranes. Polymer (Guildf.) 113, 283–294 (2017). [Google Scholar]
- 26.Huang Y., Paul D. R., Effect of film thickness on the gas-permeation characteristics of glassy polymer membranes. Ind. Eng. Chem. Res. 46, 2342–2347 (2007). [Google Scholar]
- 27.Yerzhankyzy A., Ghanem B. S., Wang Y., Alaslai N., Pinnau I., Gas separation performance and mechanical properties of thermally-rearranged polybenzoxazoles derived from an intrinsically microporous dihydroxyl-functionalized triptycene diamine-based polyimide. J. Membr. Sci. 595, 117512 (2020). [Google Scholar]
- 28.Staudt-Bickel C., Koros W. J., Olefin/paraffin gas separations with 6FDA-based polyimide membranes. J. Membr. Sci. 170, 205–214 (2000). [Google Scholar]
- 29.Smith Z. P., et al., Effect of polymer structure on gas transport properties of selected aromatic polyimides, polyamides and TR polymers. J. Membr. Sci. 493, 766–781 (2015). [Google Scholar]
- 30.Feng X., “Recovery of olefins from polyolefin resin purge streams by membranes” in Frontiers on Separation Science and Technology, Z. Tong, S. H. Kim, Eds. (World Scientific Publishing, 2004), pp. 10–11. [Google Scholar]
- 31.Kang S. W., Kim J. H., Char K., Won J., Kang Y. S., Nanocomposite silver polymer electrolytes as facilitated olefin transport membranes. J. Membr. Sci. 285, 102–107 (2006). [Google Scholar]
- 32.Surya Murali R., Yamuna Rani K., Sankarshana T., Ismail A. F., Sridhar S., Separation of binary mixtures of propylene and propane by facilitated transport through silver incorporated poly(ether-block-amide) membranes. Oil Gas Sci. Technol. Rev. IFP Energies Nouvelles 70, 381–390 (2015). [Google Scholar]
- 33.Jung J. P., et al., Facilitated olefin transport through membranes consisting of partially polarized silver nanoparticles and PEMA-g-PPG graft copolymer. J. Membr. Sci. 548, 149–156 (2018). [Google Scholar]
- 34.Lee J. H., Kang S. W., Song D., Won J., Kang Y. S., Facilitated olefin transport through room temperature ionic liquids for separation of olefin/paraffin mixtures. J. Membr. Sci. 423–424, 159–164 (2012). [Google Scholar]
- 35.Liao J., et al., Fabrication of high-performance facilitated transport membranes for CO2 separation. Chem. Sci. (Camb.) 5, 2843–2849 (2014). [Google Scholar]
- 36.Merkel T. C. B., Blanc R., Zeid J., Suwarlim A., Firat B., Wijmans H., Asaro M., Greene M., “Separation of olefin/paraffin mixtures with carrier facilitated membranes” (Report #DE-FC36-04GO14151, US Department of Energy, 2007). [Google Scholar]
- 37.Merkel T. C., et al., Silver salt facilitated transport membranes for olefin/paraffin separations: Carrier instability and a novel regeneration method. J. Membr. Sci. 447, 177–189 (2013). [Google Scholar]
- 38.Koresh J. E., Sofer A., Molecular sieve carbon permselective membrane. Part I. Presentation of a new device for gas mixture separation. Sep. Sci. Technol. 18, 723–734 (1983). [Google Scholar]
- 39.Rungta M., Xu L., Koros W. J., Structure–performance characterization for carbon molecular sieve membranes using molecular scale gas probes. Carbon 85, 429–442 (2015). [Google Scholar]
- 40.Zhou W., Yoshino M., Kita H., Okamoto K.-i., Carbon molecular sieve membranes derived from phenolic resin with a pendant sulfonic acid group. Ind. Eng. Chem. Res. 40, 4801–4807 (2001). [Google Scholar]
- 41.Xu L., et al., Physical aging in carbon molecular sieve membranes. Carbon 80, 155–166 (2014). [Google Scholar]
- 42.Lin Y. S., Inorganic membranes for process intensification: Challenges and perspective. Ind. Eng. Chem. Res. 58, 5787–5796 (2019). [Google Scholar]
- 43.Tiscornia I., Irusta S., Téllez C., Coronas J., Santamaría J., Separation of propylene/propane mixtures by titanosilicate ETS-10 membranes prepared in one-step seeded hydrothermal synthesis. J. Membr. Sci. 311, 326–335 (2008). [Google Scholar]
- 44.Nikolakis V., et al., Growth of a faujasite-type zeolite membrane and its application in the separation of saturated/unsaturated hydrocarbon mixtures. J. Membr. Sci. 184, 209–219 (2001). [Google Scholar]
- 45.Giannakopoulos I. G., Nikolakis V., Separation of propylene/propane mixtures using faujasite-type zeolite membranes. Ind. Eng. Chem. Res. 44, 226–230 (2005). [Google Scholar]
- 46.Shrestha S., Dutta P. K., Modification of a continuous zeolite membrane grown within porous polyethersulfone with Ag(I) cations for enhanced propylene/propane gas separation. Microporous Mesoporous Mater. 279, 178–185 (2019). [Google Scholar]
- 47.Qian Q., et al., MOF-based membranes for gas separations. Chem. Rev. 120, 8161–8266 (2020). [DOI] [PubMed] [Google Scholar]
- 48.Pan Y., Lai Z., Sharp separation of C2/C3 hydrocarbon mixtures by zeolitic imidazolate framework-8 (ZIF-8) membranes synthesized in aqueous solutions. Chem. Commun. (Camb.) 47, 10275–10277 (2011). [DOI] [PubMed] [Google Scholar]
- 49.Choi J., et al., Grain boundary defect elimination in a zeolite membrane by rapid thermal processing. Science 325, 590–593 (2009). [DOI] [PubMed] [Google Scholar]
- 50.Min B., Yang S., Korde A., Jones C. W., Nair S., Single-step scalable fabrication of zeolite MFI hollow fiber membranes for hydrocarbon separations. Adv. Mater. Interfaces 7, 2000926 (2020). [Google Scholar]
- 51.Kwon H. T., Jeong H.-K., In situ synthesis of thin zeolitic-imidazolate framework ZIF-8 membranes exhibiting exceptionally high propylene/propane separation. J. Am. Chem. Soc. 135, 10763–10768 (2013). [DOI] [PubMed] [Google Scholar]
- 52.Tran N. T., Kwon H. T., Kim W.-S., Kim J., Counter-diffusion-based in situ synthesis of ZIF-67 membranes for propylene/propane separation. Mater. Lett. 271, 127777 (2020). [Google Scholar]
- 53.Brown A. J., et al., Separation membranes. Interfacial microfluidic processing of metal-organic framework hollow fiber membranes. Science 345, 72–75 (2014). [DOI] [PubMed] [Google Scholar]
- 54.Li W., et al., Ultrathin metal-organic framework membrane production by gel-vapour deposition. Nat. Commun. 8, 406 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hou Q., Zhou S., Wei Y., Caro J., Wang H., Balancing the grain boundary structure and the framework flexibility through bimetallic metal-organic framework (MOF) membranes for gas separation. J. Am. Chem. Soc. 142, 9582–9586 (2020). [DOI] [PubMed] [Google Scholar]
- 56.Bachman J. E., Smith Z. P., Li T., Xu T., Long J. R., Enhanced ethylene separation and plasticization resistance in polymer membranes incorporating metal-organic framework nanocrystals. Nat. Mater. 15, 845–849 (2016). [DOI] [PubMed] [Google Scholar]
- 57.Sugita M., Takewaki T., Oshima K., Fujita N., “Inorganic porous support-zeolite membrane composite, production method thereof, and separation method using the composite.” US Patent US8376148B2 (2015).
- 58.Imasaka S., Itakura M., Hasegawa Y., Sato K., “Zeolite membrane, production method thereof, and separation method using same.” European Patent EP3167953B1 (2020).
- 59.He X., Arvid Lie J., Sheridan E., Hägg M.-B., CO2 capture by hollow fibre carbon membranes: Experiments and process simulations. Energy Procedia 1, 261–268 (2009). [Google Scholar]
- 60.Sharma S., Shyam Kumar C. N., Korvink J. G., Kübel C., Evolution of glassy carbon microstructure: In situ transmission electron microscopy of the pyrolysis process. Sci. Rep. 8, 16282 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Culp T. E., et al., Electron tomography reveals details of the internal microstructure of desalination membranes. Proc. Natl. Acad. Sci. U.S.A. 115, 8694–8699 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dong J., Lin Y. S., In situ synthesis of P-type zeolite membranes on porous α-alumina supports. Ind. Eng. Chem. Res. 37, 2404–2409 (1998). [Google Scholar]
- 63.Bux H., et al., Zeolitic imidazolate framework membrane with molecular sieving properties by microwave-assisted solvothermal synthesis. J. Am. Chem. Soc. 131, 16000–16001 (2009). [DOI] [PubMed] [Google Scholar]
- 64.Tanaka S., et al., Vapor-phase synthesis of ZIF-8 MOF thick film by conversion of ZnO nanorod array. Langmuir 34, 7028–7033 (2018). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or SI Appendix.