Significance
Fractal structures and phenomena have existed in nature for hundreds of millions of years. Developing their practical applications in material design is of fundamental importance, but this goal has not yet been reached. In this work, NaCl crystals with a fractal structure are formed between the polyamide active layer and the support during an interfacial polymerization process. The branching of fractal NaCl nanocrystals creates numerous tiny interworking water channels that enable water transport, maximizing the effective permeating area of the polyamide nanofiltration (NF) membrane. The fractal NaCl nanocrystals–templated polyamide NF membrane exhibits an improved desalination performance with a three to four times increase in permeance. Applying fractal structure successfully to the design of artificial materials improves performance.
Keywords: nanofiltration membrane, high flux, interfacial polymerization, fractal structure
Abstract
In this study, we report the emergence of two-dimensional (2D) branching fractal structures (BFS) in the nanoconfinement between the active and the support layer of a thin-film-composite polyamide (TFC-PA) nanofiltration membrane. These BFS are crystal dendrites of NaCl formed when salts are either added to the piperazine solution during the interfacial polymerization process or introduced to the nascently formed TFC-PA membrane before drying. The NaCl dosing concentration and the curing temperature have an impact on the size of the BFS but not on the fractal dimension (∼1.76). The BFS can be removed from the TFC-PA membranes by simply dissolving the crystal dendrites in deionized water, and the resulting TFC-PA membranes have substantially higher water fluxes (three- to fourfold) without compromised solute rejection. The flux enhancement is believed to be attributable to the distributed reduction in physical binding between the PA active layer and the support layer, caused by the exertion of crystallization pressure when the BFS formed. This reduced physical binding leads to an increase in the effective area for water transport, which, in turn, results in higher water flux. The BFS-templating method, which includes the interesting characteristics of 2D crystal dendrites, represents a facile, low-cost, and highly practical method of enhancing the performance of the TFC-PA nanofiltration membrane without having to alter the existing infrastructure of membrane fabrication.
Thin-film composite polyamide (TFC-PA) membranes are widely used in reverse osmosis and nanofiltration (NF), which have extensive and continuously growing applications in water treatment, desalination, and wastewater reuse (1–4). Typical TFC-PA membranes are fabricated using interfacial polymerization (IP), which involves a polymerizing reaction between amine and acid chloride precursors at the water–oil interface (5–9). In a typical IP for producing TFC-PA NF membranes, a polyether sulfone (PES) ultrafiltration membrane is first impregnated with an aqueous solution of piperazine (PIP) and then placed into contact with a hexane solution of trimesoyl chloride (TMC). The PIP monomers diffuse across the water–hexane interface and react with the TMC to form a cross-linked dense PA film that serves as the active layer for water–salt separation (3, 8, 10). This PA film is tightly bound to the underlying PES support layer, and the way they bind to each has a strong impact on the water flux of the resulting TFC-PA membrane (11–15).
Enhancing the water flux of an NF membrane without compromising its solute rejection can potentially lead to substantial savings in treatment cost and has sizable practical impacts due to the broad application of TFC-PA membranes. While extensive research (9, 10, 16–25) has been performed with the goal of performance enhancement, many promising approaches (10, 16–25) reported in the literature require significant modifications of the existing infrastructure or method of manufacturing TFC-PA membranes and thus are prohibitively complex or too expensive to implement. A desirable approach for enhancing TFC-PA membrane performance should be simple, low cost, effective, and readily integrated into the existing method of TFC-PA membrane fabrication.
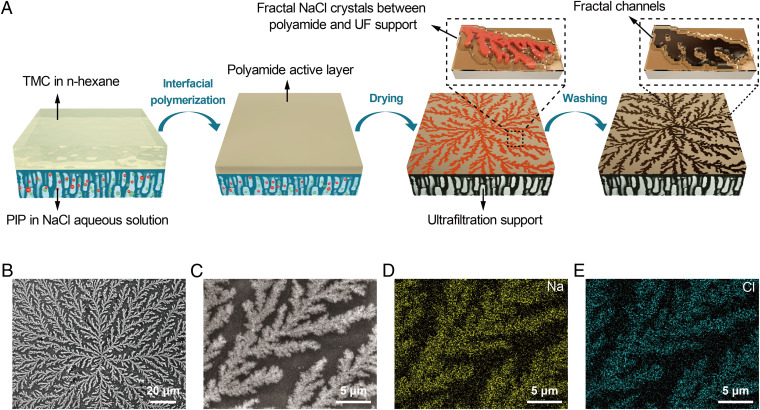
Herein, we report an elegant and highly practical method using two-dimensional (2D) fractal crystal dendrites to dramatically increase the water permeance of the TFC-PA NF membrane while maintaining its solute rejection performance. By adding NaCl to the aqueous PIP solution during the IP process, we observed that NaCl crystal dendrites emerged in the confinement between the PA layer and the PES support when the TFC-PA membrane was cured by heat (Fig. 1 A–C). These spectacular branching fractal structures (BFS) are considered to be 2D because they are less thick when compared with the overall size of the BFS sprawling along the plane parallel to the membrane surface. Dissolving the PES support using dimethylformamide revealed a large number of crystals adhering to the bottom of the PA film (SI Appendix, Figs. S1 and S2), confirming the position of the BFS to be between the PA active layer and the PES support. Elemental analysis using energy-dispersive X-ray spectroscopy (Fig. 1 D and E) and crystal structure analysis using X-ray diffraction (SI Appendix, Fig. S3) confirmed that these 2D BFS were indeed NaCl crystals.
Fig. 1.
Formation process and surface morphology of BFS-templated TFC-PA membrane. (A) Schematic illustration of the process for preparing a BFS-templated TFC-PA membrane via interfacial polymerization. (B) Surface morphology of the BFS-templated TFC-PA membrane. (C) Close-up surface morphology of the BFS-templated TFC-PA membrane. (D and E) Elemental mapping images of Na (D) and Cl (E) on the surface of a BFS-templated TFC-PA membrane. (NaCl concentration in PIP solution: 8 g·L−1; curing temperature: 60 °C).
Results
The presence of NaCl crystal dendrites significantly increased the surface roughness of the TFC-PA membrane (Fig. 2 A and B). The thickness of the BFS is estimated to be 78 ± 10 nm based on an analysis of the surface topography measured using atomic force microscopy (AFM). While the NaCl crystals can be removed simply by dissolving them in deionized water, the BFS formation has lasting impacts on the binding between the PA layer and the underlying PES support. Unlike the tightly integrated interface between the support and active layers in conventional TFC-PA NF membranes (Fig. 2C), voids were created where the BFS existed before NaCl dissolution (Fig. 2D).
Fig. 2.
Properties of conventional and BFS-templated TFC-PA membranes. (A and B) AFM images and corresponding height profiles of conventional (A) and BFS-templated (B) TFC-PA membranes. Here, the BFS-templated TFC-PA membrane was not washed with water to remove the NaCl crystal dendrites. (C and D) Cross-sectional transmission electron microscopy (TEM) images of conventional (C) and BFS-templated (D) TFC-PA membranes. Here, the BFS-templated TFC-PA membrane was washed with water to remove the NaCl crystal dendrites. (E) Zeta potential of conventional and BFS-templated TFC-PA membranes. (F) Rejection of neutral organic solutes (200 ppm) including raffinose, sucrose, and glucose by the conventional and BFS-templated TFC-PA membranes (applied pressure: 4 bar; cross-flow rate: 60 L·h−1). (Inset) The corresponding pore size distributions of TFC-PA membranes derived from the rejection are presented. (G) Rejections of various salt solutions (1,000 ppm) by conventional and BFS-templated TFC-PA membranes (applied pressure: 6 bar; cross-flow rate: 40 L·h−1). (H) Fluxes of conventional and BFS-templated TFC-PA membranes with different salt solutions (1,000 ppm) as the feed solutions (applied pressure: 6 bar; cross-flow rate: 40 L·h−1). (I) Illustration of the increased effective area of the active layer by BFS templating. (NaCl concentration in PIP solution: 8 g·L−1; curing temperature: 60 °C).
Interestingly, the addition of NaCl and the formation of the BFS did not seem to affect the properties of the PA active layer itself. Both the reference TFC-PA membrane (without BFS formation) and the TFC-PA membrane (with BFS dissolved after formation) had practically indistinguishable properties, including zeta potential (Fig. 2E), water contact angle (SI Appendix, Fig. S4), elemental composition (SI Appendix, Fig. S5 and Table S1), distribution of functional groups (SI Appendix, Fig. S6), pore size distribution (Fig. 2F), and rejection of several tested common salts (Fig. 2G). In other words, the addition of NaCl had no impact on the formation of the PA layer via interfacial polymerization.
While the addition of NaCl did not affect the properties of the PA active layer, it substantially improved the overall performance of the TFC-PA membrane. The formation of the BFS resulted in a remarkable enhancement of the water permeance of the membrane. Specifically, a three- to fourfold increase in water flux was measured, depending on the type of solution tested (Fig. 2H). The substantial improvement in water flux cannot be explained merely by the increase in the specific surface area, as the specific surface area of the BFS-templated TFC-PA membrane (after dissolving NaCl) was very similar to that of the conventional TFC-PA membrane (SI Appendix, Fig. S7). Instead, this flux enhancement is caused by the increase in the effective area of the active layer for water transport (Fig. 2I). The PES support layer has a surface porosity of ∼15%, meaning that the PA active layer is in direct contact with ∼85% of the underlying PES support. This fraction of the PA layer area either is not available or is ineffective for water and solute transport.
Such a theory is corroborated by the observation in previous studies (16–18, 20, 26) that a porous interlayer (between the active and the support layer) with a very high porosity can substantially enhance the water flux. In the case of a BFS-templated TFC-PA membrane, the emergence and subsequent removal of the BFS reduced the area of direct adhesion between the PA active layer and the PES substrate, thereby increasing the effective area of the PA active layer for water transport (Fig. 2I). This argument is also well supported by an approximate quantitative analysis that investigated the porosity of the support layer and the surface coverage of the NaCl dendrite (SI Appendix, section 2.7).
The morphology of the BFS depends on the NaCl concentration, as BFS with “wider branches” formed with a high concentration of NaCl dosed in the PIP solution (Fig. 3 A–E). However, regardless of the NaCl concentration, the fractal dimension of the BFS was consistently ∼1.76 as determined by the box-counting method (Fig. 3F and SI Appendix, Figs. S8 and S9), implying that the BFS may form from the nucleation limited aggregation (NLA) model (27, 28). While the branches in the BFS formed with a higher NaCl concentration appeared to be wider, we cannot measure the width of the branches due to the fractal nature of the BFS (i.e., width varies depending on the scale). Instead, we performed a quantitative analysis of the branch size using a metric called characteristic length, defined as the ratio between the area and the perimeter of the BFS and determined by image analysis of the scanning electron microscopy (SEM) images of the BFS (SI Appendix, section 2.9). As the dosing NaCl concentration increased, the characteristic length increased accordingly (Fig. 3F), which is semiquantitatively consistent with the increase in BFS thickness of the BFS-templated PA active layer as measured using AFM (Fig. 3F and SI Appendix, Fig. S10).
Fig. 3.
The effect of NaCl concentration on BFS morphology. (A–E) Surface morphology of BFS-templated TFC-PA membranes prepared with different NaCl concentrations in PIP solutions. (F) Variation of fractal dimension, characteristic length, and thickness of BFS formed with respect to NaCl concentrations.
To better understand how BFS form, we performed a Monte Carlo simulation following NLA (SI Appendix, section 2.11) to emulate the formation of the 2D BFS (Fig. 4A) and obtained structures similar to those shown in Figs. 1B and 3 A–E. The NLA assumes the presence of a central seed (i.e., the nucleus) from which the crystal grows radially, and that existing crystal particles on the fractal structure can serve as new nuclei for the formation of additional crystals. The simulated temporal evolution of an NaCl crystal dendrite resembles that observed using an optical microscope (Fig. 4B). We speculate the formation mechanism of BFS to be as follows (SI Appendix, Figs. S11–S19): a nascent NaCl nucleus is first attached to multiple carboxyl groups of the PA layer and then grows larger with further crystallization. NLA occurs locally around an immobilized nucleus along a thin film of water sandwiched between the PA and the PES support layer. The local growth of nanocrystal particles exerts a crystallization pressure (29–31) that further enlarges the gap between the PA and the support layer, which facilitates the further expansion of the BFS.
Fig. 4.
The Monte Carlo simulation and growth process of BFS. (A) Monte Carlo simulation of the formation process of BFS based on the NLA model. (B) The growth process of BFS as recorded by optical microscopy. (NaCl concentration in PIP solution: 32 g·L−1; curing temperature: 60 °C).
Interestingly, if we soak in an NaCl solution a nascent (and still wet) TFC-PA membrane formed without dosing NaCl in the aqueous PIP solution, fractal NaCl dendrites also form with a high degree of similarity to those formed by adding NaCl to the aqueous PIP solution during interfacial polymerization (SI Appendix, Fig. S20A). In addition, the water permeance of a TFC-PA membrane modified via such an approach of postfabrication BFS templating was also substantially enhanced (SI Appendix, Table S2). However, if we dry a conventional TFC-PA membrane first, and soak the dried membrane in an NaCl solution, neither BFS formation (SI Appendix, Fig. S20B) nor enhancement in water flux is observed (SI Appendix, Table S2). The comparison between these two experiments reaffirms the importance of the presence of a thin film of water in the formation of the 2D BFS. Another key factor that determines whether BFS can form is the pore size of the PES support. When the pore size of the PES support increases to the submicrometer range, NaCl crystals form randomly without distinct fractal characteristics (SI Appendix, Figs. S21–S24).
Discussion
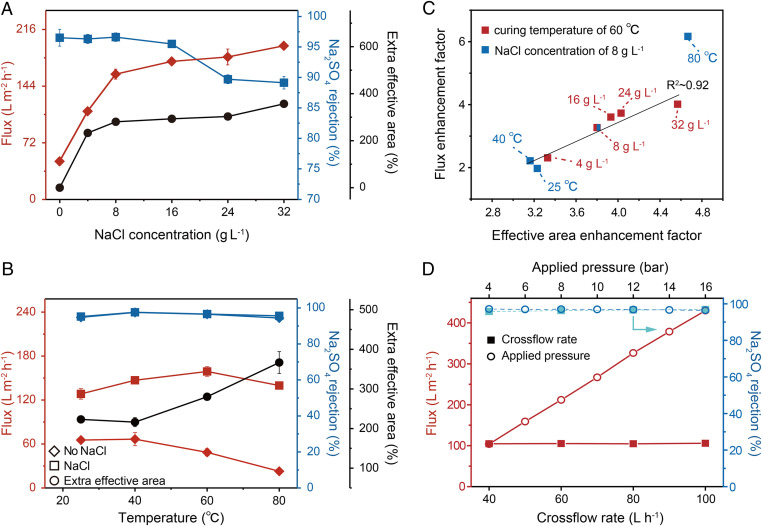
The impact of the NaCl dosing concentration (in the aqueous PIP solution) on the BFS morphology can influence NF performance (SI Appendix, Table S3 and Fig. S25). Using an Na2SO4 solution, for example, the flux increased from ∼48 to ∼198 L·m−2⋅h−1 when the NaCl dosing concentration increased from 0 to 32 g·L−1, which correlates well with the extra effective area created by the BFS templating (Fig. 5A). The Na2SO4 rejection was barely affected by the NaCl dosing concentration up to 16 g·L−1 but became compromised when the NaCl dosing concentration was too high. Evaluating NF performance with four other salts (SI Appendix, Table S3) and neutral solutes (SI Appendix, Fig. S25) also reveals qualitatively similar dependence of performance on the NaCl dosing concentration. The deterioration in solute rejection performance is believed to be caused by the overstretching of the PA active layer due to the crystallization pressure exerted by the relatively thick crystal dendrites formed at a high NaCl dosing concentration (30). The overstretching of the active layer reduced its thickness and resulted in lower solute rejections. Notably, we observed no macroscopic defect from physical piercing by the forming crystals (SI Appendix, Fig. S26).
Fig. 5.
Desalination performance of BFS-templated TFC-PA membranes. (A) Water flux, rejection, and extra effective area variation of BFS-templated TFC-PA membranes with different NaCl concentrations in PIP aqueous solutions (applied pressure: 6 bar; cross-flow rate: 40 L·h−1). (B) Water flux, rejection, and extra effective area variation of BFS-templated TFC-PA membranes with different curing temperatures (applied pressure: 6 bar; cross-flow rate: 40 L·h−1). (C) Correlation between the flux enhancement factor and the effective area enhancement factor calculated based on the measured surface porosity of 15%. Due to the uneven distribution of pores on the membrane surface, the surface porosity is biased for different samples. The flux enhancement factor is defined as the ratio between the water flux measured with a BFS-templated membrane and that measured with a referenced TFC-PA membrane, whereas the effective area enhancement factor is defined as the ratio between the effective area of a BFS-templated membrane and that of a referenced TFC-PA membrane. The R2 for correlation was calculated without using the data represented by the outlier at 80 °C. (D) Water flux and rejection of the Na2SO4 solution by the BFS-templated TFC-PA membrane under varying cross-flow rates (solid squares, applied pressure: 6 bar) and under varying applied pressures (open circles, cross-flow rate: 40 L·h−1).
We also investigated the impact of the membrane curing temperature on the BFS morphology (SI Appendix, Fig. S27) and the NF performance of the BFS-templated TFC-PA membranes (Fig. 5B). Increasing the curing temperature reduced the characteristic length (i.e., the BFS become “slimmer”) but increased the extra effective area (Fig. 5B and SI Appendix, Fig. S28); this differs from the positive correlation between the characteristic length and the extra effective area observed when changing the NaCl dosing concentration. Regardless of the curing temperature, the Na2SO4 rejection was consistently high. However, the water permeance of the conventional TFC-PA membranes without BFS templating dropped when the curing temperature exceeded 40 °C due to the densification of the PA layer (32–34). Because of the extra effective area, BFS templating overcame such a performance deterioration at a high curing temperature and maintained a very high water flux regardless of the curing temperature. Balancing the effects of the extra effective area and the densification of the PA layer, the optimum curing temperature was identified to be ∼60 °C.
Our hypothesis regarding the flux enhancement by distributed reduction of PA adhesion to the support layer is again corroborated by a nice linear correlation between the flux enhancement factor and the effective area enhancement factor in a series of TFC-PA membranes fabricated using different NaCl concentrations and curing temperatures (Fig. 5C). The only outlier in the correlation is the membrane obtained with a curing temperature of 80 °C (Fig. 5C). It has been reported previously that the pores of the support layer will collapse at a high curing temperature (33–35), which results in a very low flux, even with the reference TFC-PA membrane (without BFS templating).
Because water flux enhancement is attributable to the increased effective area of the active layer and the overall weaker binding between the active layer and the PES support, a BFS-templated TFC-PA membrane may face the risk of compromised mechanical integrity and even delamination of the active layer. Fortunately, these detrimental impacts were not observed in our experiments in which the BFS-templated membranes were subject to a wide spectrum of NF operating conditions. Specifically, both the water flux and the Na2SO4 rejection were constantly high, regardless of the cross-flow rate (Fig. 5D and SI Appendix, Figs. S29–S31) and applied pressure (Fig. 5D), and the performance was stable with each set of operating conditions over 90 h of experiments (SI Appendix, Fig. S32).
The sustained mechanical integrity of the BFS-templated TFC-PA membrane is likely attributable to the distributed presence of a strong binding area between the PA and the PES support layer. In theory, the strength of binding is not affected in regions not occupied by the NaCl crystal branches. If the NaCl formed big cubic single crystals instead of 2D BFS such as those we observed, not only does local delamination become more likely, but the active layer may also become nonfunctional due to overstretching or even physical damage. In this regard, BFS templating truly takes advantage of the characteristics of spatially distributed fractals. While it may be argued that it is the 2D distributed structure of the BFS, not the fractality of it, that contributes mainly to enhancing the performance of the TFC-PA membranes without compromising their mechanical integrity, the common chloride salts that we experimented with happen to form beautiful fractal structures that serve the same role.
While we used NaCl as the dosing salt for BFS formation throughout, other inorganic minerals may be used for the same purpose. For example, KCl can also form morphologically similar crystal dendrites (SI Appendix, Fig. S33) with a fractal dimension (SI Appendix, Table S4) similar to that of NaCl dendrites. In addition to NaCl and KCl, RbCl can form BFS, although the fractal characteristic becomes less obvious as the structure boundary becomes blurred at a smaller scale (SI Appendix, Fig. S34). With CsCl, another chloride salt of alkali metal, the 2D crystal still has some scale-invariant similarity but not the branching structures found in NaCl, KCl, and RbCl dendrites (SI Appendix, Fig. S35). In all cases, 2D crystal templating can enhance the water flux of TFC-PA membranes without compromising their salt-rejecting performance (SI Appendix, Table S5).
In summary, our work shows that 2D BFS can form in the confined space between the PA active layer and the porous PES support in a TFC-PA NF membrane. Templating the TFC-PA membrane with these 2D BFS results in interconnecting networks between the two layers and an increased effective area of PA for water and solute transport but does not alter the properties of the PA active layer (except when the concentration of the crystal-forming salt is too high). Consequently, the templated TFC-PA membranes have dramatically higher water flux and uncompromised solute rejection. The mechanism of performance enhancement using this novel BFS-templating approach is similar to that using a fibrous interlayer of nanotubes or nanostrands. Compared with using a physical interlayer, however, BFS templating is substantially more practical due to its low cost and easy implementation by retrofitting the existing infrastructure for roll-to-roll manufacturing of TFC-PA membranes.
Materials and Methods
Materials and methods for membrane preparation, membrane performance evaluation and characterization, Monte Carlo simulation, a detailed description of fractal dimension, and characteristic length calculation can be found in the SI Appendix.
Supplementary Material
Acknowledgments
This study was supported by grants from the National Key Research and Development Plan (Grant 2019YFC1711300), the National Natural Science Funds for Distinguished Young Scholars (Grant 51625306), the National Natural Science Foundation of China (Grants 21988102 and 51873230), the Science and Technology Service Network Initiative program of the Chinese Academy of Sciences (Grant KFJ-STS-QYZD-141), and the Natural Science Foundation of Jiangsu Province (Grant BK20180259). R.W. and S.L. acknowledge partial support from the US NSF via Grant CBET 1739884. We also appreciate Prof. Xing Yang at Katholieke Universiteit Leuven for the computational fluid dynamics (CFD) simulation and Ms. Yi Zhang at Suzhou Institute of Nano-Tech and Nano-Bionics for SEM characterization.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2019891118/-/DCSupplemental.
Data Availability
All study data are included in the article and/or SI Appendix. There are no data underlying this work.
References
- 1.Werber J. R., Osuji C. O., Elimelech M., Materials for next-generation desalination and water purification membranes. Nat. Rev. Mater. 1, 16018 (2016). [Google Scholar]
- 2.Lau W. J., Ismail A. F., Misdan N., Kassim M. A., A recent progress in thin film composite membrane: A review. Desalination 287, 190–199 (2012). [Google Scholar]
- 3.Mohammad A. W., et al., Nanofiltration membranes review: Recent advances and future prospects. Desalination 356, 226–254 (2015). [Google Scholar]
- 4.Culp T. E., et al., Electron tomography reveals details of the internal microstructure of desalination membranes. Proc. Natl. Acad. Sci. U.S.A. 115, 8694–8699 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marchetti P., Jimenez Solomon M. F., Szekely G., Livingston A. G., Molecular separation with organic solvent nanofiltration: A critical review. Chem. Rev. 114, 10735–10806 (2014). [DOI] [PubMed] [Google Scholar]
- 6.Wittbecker E. L., Morgan P. W., Interfacial polycondensation. I. J. Polym. Sci. 40, 289–297 (1959). [Google Scholar]
- 7.Morgan P. W., Kwolek S. L., Interfacial polycondensation. II. Fundamentals of polymer formation at liquid interfaces. J. Polym. Sci. 40, 299–327 (1959). [Google Scholar]
- 8.Liang Y., et al., Polyamide nanofiltration membrane with highly uniform sub-nanometre pores for sub-1 Å precision separation. Nat. Commun. 11, 2015 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tan Z., Chen S., Peng X., Zhang L., Gao C., Polyamide membranes with nanoscale Turing structures for water purification. Science 360, 518–521 (2018). [DOI] [PubMed] [Google Scholar]
- 10.Wang Z., et al., Nanoparticle-templated nanofiltration membranes for ultrahigh performance desalination. Nat. Commun. 9, 2004 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karan S., Jiang Z., Livingston A. G., Sub-10 nm polyamide nanofilms with ultrafast solvent transport for molecular separation. Science 348, 1347–1351 (2015). [DOI] [PubMed] [Google Scholar]
- 12.Peng L. E., Yao Z., Yang Z., Guo H., Tang C. Y., Dissecting the role of substrate on the morphology and separation properties of thin film composite polyamide membranes: Seeing is believing. Environ. Sci. Technol. 54, 6978–6986 (2020). [DOI] [PubMed] [Google Scholar]
- 13.Jiang Z., Karan S., Livingston A. G., Water transport through ultrathin polyamide nanofilms used for reverse osmosis. Adv. Mater. 30, 1705973 (2018). [DOI] [PubMed] [Google Scholar]
- 14.Li X., Li Q., Fang W., Wang R., Krantz W. B., Effects of the support on the characteristics and permselectivity of thin film composite membranes. J. Membr. Sci. 580, 12–23 (2019). [Google Scholar]
- 15.Ramon G. Z., Wong M. C. Y., Hoek E. M. V., Transport through composite membrane, part 1: Is there an optimal support membrane? J. Membr. Sci. 415-416, 298–305 (2012). [Google Scholar]
- 16.Wang J.-J., Yang H.-C., Wu M.-B., Zhang X., Xu Z.-K., Nanofiltration membranes with cellulose nanocrystals as an interlayer for unprecedented performance. J. Mater. Chem. A Mater. Energy Sustain. 5, 16289–16295 (2017). [Google Scholar]
- 17.Zhu Y., et al., Single-walled carbon nanotube film supported nanofiltration membrane with a nearly 10 nm thick polyamide selective layer for high-flux and high-rejection desalination. Small 12, 5034–5041 (2016). [DOI] [PubMed] [Google Scholar]
- 18.Gao S., et al., Ultrathin polyamide nanofiltration membrane fabricated on brush-painted single-walled carbon nanotube network support for ion sieving. ACS Nano 13, 5278–5290 (2019). [DOI] [PubMed] [Google Scholar]
- 19.Gui L., et al., Ultrafast ion sieving from honeycomb-like polyamide membranes formed using porous protein assemblies. Nano Lett. 20, 5821–5829 (2020). [DOI] [PubMed] [Google Scholar]
- 20.Yoon K., Hsiao B. S., Chu B., High flux nanofiltration membranes based on interfacially polymerized polyamide barrier layer on polyacrylonitrile nanofibrous scaffolds. J. Membr. Sci. 326, 484–492 (2009). [Google Scholar]
- 21.Lakhotia S. R., Mukhopadhyay M., Kumari P., Iron oxide (FeO) nanoparticles embedded thin-film nanocomposite nanofiltration (NF) membrane for water treatment. Separ. Purif. Tech. 211, 98–107 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shen J. N., Yu C. C., Ruan H. M., Gao C. J., Van der Bruggen B., Preparation and characterization of thin-film nanocomposite membranes embedded with poly(methyl methacrylate) hydrophobic modified multiwalled carbon nanotubes by interfacial polymerization. J. Membr. Sci. 442, 18–26 (2013). [Google Scholar]
- 23.Lai G. S., et al., Graphene oxide incorporated thin film nanocomposite nanofiltration membrane for enhanced salt removal performance. Desalination 387, 14–24 (2016). [Google Scholar]
- 24.Wang C., et al., Covalent organic framework modified polyamide nanofiltration membrane with enhanced performance for desalination. J. Membr. Sci. 523, 273–281 (2017). [Google Scholar]
- 25.Gong Y., et al., Thin-film nanocomposite nanofiltration membrane with an ultrathin polyamide/UIO-66-NH2 active layer for high-performance desalination. J. Membr. Sci. 600, 117874 (2020). [Google Scholar]
- 26.Zhou Z., et al., High-performance thin-film composite membrane with an ultrathin spray-coated carbon nanotube interlayer. Environ. Sci. Technol. Lett. 5, 243–248 (2018). [Google Scholar]
- 27.Ming N.-B., Wang M., Peng R.-W., Nucleation-limited aggregation in fractal growth. Phys. Rev. E Stat. Phys. Plasmas Fluids Relat. Interdiscip. Topics 48, 621–624 (1993). [DOI] [PubMed] [Google Scholar]
- 28.Liu X.-Y., et al., Nucleation-limited aggregation of crystallites in fractal growth. J. Cryst. Growth 208, 687–695 (2000). [Google Scholar]
- 29.La Iglesia A., González V., López-Acevedo V., Viedma C., Salt crystallization in porous construction materials I estimation of crystallization pressure. J. Cryst. Growth 177, 111–118 (1997). [Google Scholar]
- 30.Steiger M., Crystal growth in porous materials—I: The crystallization pressure of large crystals. J. Cryst. Growth 282, 455–469 (2005). [Google Scholar]
- 31.Steiger M., Crystal growth in porous materials—II: Influence of crystal size on the crystallization pressure. J. Cryst. Growth 282, 470–481 (2005). [Google Scholar]
- 32.Liu M. H., Yu S. C., Zhou Y., Gao C. J., Study on the thin-film composite nanofiltration membrane for the removal of sulfate from concentrated salt aqueous: Preparation and performance. J. Membr. Sci. 310, 289–295 (2008). [Google Scholar]
- 33.Han R., Zhang S., Hu L., Guan S., Jian X., Preparation and characterization of thermally stable poly(piperazine amide)/PPBES composite nanofiltration membrane. J. Membr. Sci. 370, 91–96 (2011). [Google Scholar]
- 34.Zhan Z.-M., Xu Z.-L., Zhu K.-K., Tang Y.-J., How to understand the effects of heat curing conditions on the morphology and performance of polypiperazine-amide NF membrane. J. Membr. Sci. 597, 117640 (2020). [Google Scholar]
- 35.Ghosh A. K., Jeong B.-H., Huang X., Hoek E. M. V., Impacts of reaction and curing conditions on polyamide composite reverse osmosis membrane properties. J. Membr. Sci. 311, 34–45 (2008). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or SI Appendix. There are no data underlying this work.