Abstract
Background
Few studies have investigated the influence of white matter lesions (WMLs) on the prognosis of acute cardioembolic stroke (CES). We aimed to explore the role of WMLs in predicting 3-month prognosis of CES without reperfusion therapy.
Methods
A number of 251 acute CES patients without reperfusion therapy at a single center were retrospectively recruited. The severity of WMLs was evaluated by Fazekas scale and patients were divided into mild WMLs group (188 cases, Fazekas ≤ 2 points) and moderate to severe WMLs group (63 cases, Fazekas ≥ 3 points) accordingly. General data and clinical features of the two groups were compared. Functional outcomes of patients were followed up for 3 months using the modified Rankin scale (mRS) and patients were divided into poor outcome group (mRS ≥ 3) and favorable outcome group (mRS ≤ 2). The effect of WMLs on the prognosis was identified by binary logistic regression.
Results
Patients in moderate to severe WMLs group were older (P < 0.001). Also, they had higher baseline National Institutes of Health Stroke Scale (NIHSS) score (P < 0.001) and elevated incidence of asymptomatic cerebral hemorrhage (P = 0.040) and stroke associated pneumonia (P = 0.001) than those in mild WMLs group. At 3 months, there were 100 cases in the poor outcome group. Patients in poor outcome group had higher baseline NIHSS score, increased proportion of moderate to severe WMLs, and elevated incidence of stroke associated pneumonia than those in favorable outcome group (P < 0.001). Binary logistic regression analysis showed that moderate to severe WMLs (odds ratio [OR] = 4.105, 95 % confidence interval [CI] = 1.447–11.646), baseline NIHSS score (OR = 1.368, 95 % CI = 1.240–1.511), and stroke-associated pneumonia (OR = 4.840, 95 %CI = 1.889–12.400) were independent risk factors for poor outcome.
Conclusions
Moderate to severe WMLs is an independent risk factor for prognosis of CES patients without reperfusion therapy.
Keywords: Acute cardioembolic stroke, White matter lesions, Microcirculation disorder, Functional outcome
Background
White matter lesions (WMLs) characterized by bilateral, mostly symmetrical hyperintensities on T2-weighted and fluid attenuated inversion recovery (FLAIR) MRI sequences are an imaging manifestation of cerebral small vessel disease and an important marker of cerebral microcirculation disorder [1, 2]. Studies have found that WMLs are a risk factor for stroke, dementia and death in the general and high-risk population [3]. In addition, they can determine the severity and hinder the neurological recovery of stroke [4–6]. Cardioembolic stroke (CES) accounts for around 15–30 % of ischemic stroke [7]. As thrombus from heart suddenly blocks the intracranial artery, the status of cerebral microcirculation may affect the establishment of secondary and tertiary collateral vessels, and thus influence the outcomes of CES patients.
However, few studies have focused on the relationship between WMLs and the functional outcomes of CES patients [8, 9]. This study aimed to investigate the effect of WMLs on the 3-month prognosis of CES patients without reperfusion therapy.
Methods
For this single-center, retrospective, observational study, we retrieved data from the stroke center database of Changzhou NO.2 People’s Hospital, Jiangsu Province, China. This study was approved by the Clinical Research Ethics Committee of the Affiliated Changzhou No.2 People’s Hospital of Nanjing Medical University (2018KY032-01). Verbal consents were obtained from all the patients or their legally authorized representatives.
Participants
Consecutive patients with first-ever acute CES within 48 h of stroke onset from June 2014 to September 2019 were included. All patients had known or newly-diagnosed non-valvular atrial fibrillation, and stroke events were confirmed by magnetic resonance imaging (MRI) or computerized tomography (CT). Other inclusion criteria were: (1) 18 ≤ age ≤ 80 years, (2) anterior circulation infarction, and (3) involvement of at least one side of the middle cerebral artery. Patients were excluded if they had reperfusion therapy or had no brain MRI/magnetic resonance angiography (MRA) or CT/computerized tomography angiography (CTA) which would be essential to estimate the stroke subtype.
Diagnosis of cardioembolic stroke
Essential tests like electrocardiogram, transthoracic echocardiography, cervical vascular ultrasound and cranial CTA/MRA were routinely performed. Thus, the known or newly-diagnosed non-valvular atrial fibrillation was confirmed and the stroke subtype of CES defined by the Trial of Org 10,172 in Acute Stroke Treatment (TOAST-criteria) was identified [10].
Grading of white matter lesions
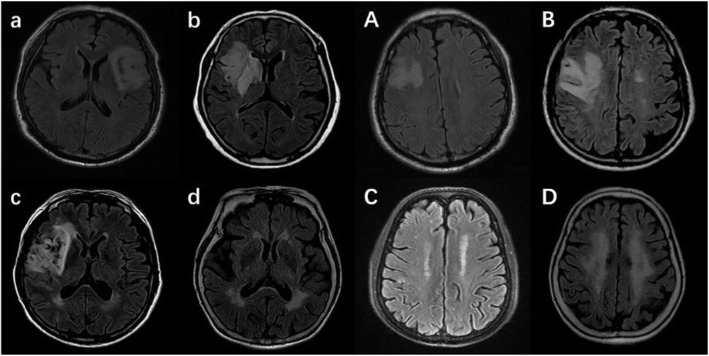
The extent of white matter lesions (WMLs) was graded by the Fazekas scale according to the non-contrast CT scans or FLAIR MRI imaging [11]. Periventricular and deep WMLs were rated separately. Periventricular WMLs were scored as follows: 0 = absence, 1 = caps or pencil-thin lining, 2 = smooth halo, and 3 = irregular periventricular hyperintensities extending into the deep white matter. Deep WMLs were scored as follows: 0 = absence, 1 = punctuate foci, 2 = beginning confluence of foci, and 3 = large confluent areas. A total Fazekas WMLs score, ranging from 0 to 6, was obtained by summing the periventricular and deep white matter scores. Mild WMLs was defined as Fazekas score ≤ 2, and moderate to severe WMLs as Fazekas score ≥ 3 (Fig. 1).
Fig. 1.
Grading of white matter lesions (WMLs) by the Fazekas scale. a, b, c, d indicates 0, 1, 2, and 3 for Fazekas scale score of periventricular WMLs. A, B, C, D indicates 0, 1, 2, and 3 for Fazekas scale score of deep WMLs.
Clinical evaluation and functional follow-up
Baseline neurological deficit was assessed with the National Institutes of Health Stroke Scale (NIHSS) score.
An intracranial hemorrhage was defined as symptomatic (sICH) if patient had clinical deterioration causing an increasement in the NIHSS score by ≥ 4 points and as non-symptomatic (nsICH) otherwise [12].
Stroke-associated pneumonia (SAP) was diagnosed in the presence of fever, purulent sputum, abnormal respiratory examination, and pathologic chest X-ray findings and/or leukocytosis or leukopenia (white blood cells count ≥ 10 × 109 or ≤ 4 × 109/L, respectively) [13].
Functional outcome was evaluated by the modified Rankin Scale (mRS) via telephone or a face-to-face manner at 3 months after stroke onset. A doctor and a trained nurse separately judged the function status of each patient and then achieved a consistent result. Accordingly, patients were divided into poor outcome group (mRS ≥ 3) and favorable outcome group (mRS ≤ 2).
Statistical analysis
Continuous variables were presented as mean ± standard deviation (SD) or median (interquartile range, IQR) and compared between groups using Student’s t-test or Kruskal-Wallis H test. Categorical variables were expressed as percentage and compared between groups with Pearson’s χ2 test. Association between WMLs and 3-month functional outcome was analyzed using binary logistic regression. The results are shown as odds ratio (OR) and 95 % confidence intervals (CI). A two-tailed P < 0.05 was considered statistically significant. All statistical analysis was performed on SPSS 22.0 (IBM Corp., Chicago, IL, USA).
Results
Baseline information
Among the 292 patients who initially met the inclusion criteria, 41 patients were lost to follow-up. Thus, a total of 251 patients were included for final analysis, of whom 245 (97.6 %) underwent at least one MRI scan. The median age was 73 years (IQR 67, 77) and the median admission NIHSS score was 7 (IQR 3,14). Sixty-three participants (25.1 %) had moderate to severe WMLs (Table 1).
Table 1.
Baseline information
| Variable | n = 251 |
|---|---|
| Age, y, median (IQR) | 73 (67, 77) |
| Male sex, n (%) | 138 (55) |
| Smoking, n (%) | 62 (24.7) |
| Hypertension, n (%) | 196 (78.1) |
| Diabetes mellitus, n (%) | 52 (20.7) |
| Hyperlipidemia, n (%) | 43 (17.1) |
| Congestive heart failure, n (%) | 52 (20.7) |
| Baseline SBP (mm Hg, ‾x ± s) | 146.5 ± 19.8 |
| Baseline DBP (mm Hg, ‾x ± s) | 86.1 ± 13.2 |
| Baseline NIHSS score, median (IQR) | 7 (3, 14) |
| Moderate-severe WMLs, n (%) | 63 (25.1) |
| sICH, n (%) | 27 (10.8) |
| nsICH, n (%) | 46 (18.3) |
| SAP, n (%) | 79 (31.5) |
| Anticoagulant therapy, n (%) | 142 (56.6) |
| Stroke recurrence | 8 (3.2) |
| Laboratory findings, mmol/l | |
| Total cholesterol (‾x ± s) | 4.1 ± 0.8 |
| Triglyceride (‾x ± s) | 1.2 ± 0.8 |
| LDL-C (‾x ± s) | 2.2 ± 0.7 |
| Fasting glucose (‾x ± s) | 6.3 ± 2.2 |
Abbreviations: IQR interquartile range; SBP systolic blood pressure; DBP diastolic blood pressure; NIHSS National Institutes of Health Stroke Scale; WMLs white matter lesions; sICH symptomatic intracerebral hemorrhage; nsICH non-symptomatic intracerebral hemorrhage; SAP stroke-associated pneumonia; LDL-C low-density lipoprotein cholesterol
Differences of variables between the two WMLs groups
Sixty-three patients were classified into the moderate to severe WMLs group, and 188 patients were included in the mild WMLs group. Patients in the moderate to severe WMLs group were older (P < 0.005), had higher baseline NIHSS score (P < 0.005) and had higher proportion of hypertension and congestive heart failure (P < 0.005). Incidences of SAP (47.6 % vs. 26.1 %, p = 0.001) and nsICH (27.0 vs. 15.4 %, p = 0.040) in moderate to severe WMLs group were significantly higher than those in mild group. However, there was no significant difference in the incidence of sICH (15.9 vs. 9.0 %, p = 0.130) between the two groups (Table 2).
Table 2.
Differences of variables between the two WMLs groups
| Variable | moderate to severe WMLs group | mild WMLs group | Test Value | P Value |
|---|---|---|---|---|
| (n = 63) | (n = 188) | |||
| Age, y, median (IQR) | 77 (74,79) | 71 (66,75) | 6.241 | < 0.001 |
| Male, n (%) | 36 (57.1) | 102 (54.3) | 0.159 | 0.690 |
| Smoking, n (%) | 14 (22.2) | 48 (25.5) | 0.278 | 0.598 |
| Hypertension, n (%) | 60 (95.2) | 136 (72.3) | 14.459 | < 0.001 |
| Diabetes mellitus, n (%) | 17 (27.0) | 35 (18.6) | 2.011 | 0.156 |
| Hyperlipidemia, n (%) | 12 (19.0) | 31 (16.5) | 0.218 | 0.641 |
| Congestive heart failure, n (%) | 23 (36.5) | 29 (15.4) | 12.769 | < 0.001 |
| Baseline SBP (mm Hg, ‾x ± s) | 152.5 ± 21.0 | 143.7 ± 21.3 | 2.870 | 0.005 |
| Baseline DBP (mm Hg, ‾x ± s) | 88.4 ± 14.0 | 85.4 ± 12.9 | 1.490 | 0.139 |
| Baseline NIHSS score, median (IQR) | 11 (6,16) | 6 (2,13) | 3.514 | < 0.001 |
| Fazekas score, median (IQR) | 3 (3,4) | 2 (1,2) | 12.492 | < 0.001 |
| sICH, n (%) | 10 (15.9) | 17 (9.0) | 2.293 | 0.130 |
| nsICH, n (%) | 17 (27.0) | 29 (15.4) | 4.212 | 0.040 |
| SAP,n (%) | 30 (47.6) | 49 (26.1) | 10.165 | 0.001 |
| Anticoagulant therapy, n (%) | 32 (50.8) | 110 (58.5) | 1.144 | 0.285 |
| Stroke recurrence, n (%) | 3 (4.8) | 5 (2.7) | 0.166 | 0.411 |
| Laboratory findings, mmol/l | ||||
| Total cholesterol (‾x ± s) | 4.1 ± 0.8 | 4.1 ± 0.9 | 0.146 | 0.884 |
| Triglyceride (‾x ± s) | 1.1 ± 0.5 | 1.2 ± 0.9 | -1.234 | 0.219 |
| LDL-C (‾x ± s) | 2.3 ± 0.7 | 2.2 ± 0.7 | 1.232 | 0.221 |
| Fasting glucose (‾x ± s) | 6.3 ± 2.4 | 6.2 ± 2.2 | 0.314 | 0.754 |
Differences of risk factors and clinical findings between the two outcome groups
Totally, 100 (39.8 %) patients were defined as having poor outcome. Compared with patients in the favorable outcome group, those who had poor outcome had much severe WMLs (moderate-severe WMLs: 39.0 % vs. 15.9 %, P < 0.001) and higher baseline NIHSS score (P < 0.001). Also, they were older (P = 0.002)). Additionally, they had significantly higher rates of sICH (17.0 % vs. 6.6 %, P = 0.009), nsICH (30 % vs. 10.6 %, P < 0.001) and SAP (66.0 % vs. 8.6 %, P < 0.001) (Table 3)
Table 3.
Differences of risk factors and clinical findings between the two outcome groups
| Variable | Poor outcome | Favorable outcome | Test Value | P Value |
|---|---|---|---|---|
| (n = 100) | (n = 151) | |||
| Age, y, median (IQR) | 75 (70, 78.75) | 72 (66, 75) | 3.658a | < 0.001 |
| Male, n (%) | 58 (58.0) | 80 (53.0) | 0.612c | 0.434 |
| Smoking, n (%) | 24 (24.0) | 38 (25.2) | 0.044c | 0.834 |
| Hypertension, n (%) | 83 (83.0) | 113 (74.8) | 2.344c | 0.126 |
| Diabetes mellitus, n (%) | 23 (23.0) | 29 (19.2) | 0.527c | 0.468 |
| Hyperlipidemia, n (%) | 14 (14.0) | 29 (19.2) | 1.148c | 0.284 |
| Congestive heart failure, n (%) | 30 (30.0) | 22 (14.6) | 8.721c | 0.003 |
| Baseline SBP (mm Hg, ‾x ± s) | 149.6 ± 24.5 | 143.5 ± 19.0 | 2.113b | 0.036 |
| Baseline DBP (mm Hg, ‾x ± s) | 87.9 ± 14.1 | 85.0 ± 12.6 | 1.707b | 0.089 |
| Baseline NIHSS score, median (IQR) | 15 (11, 19) | 4 (2, 7) | 11.150a | < 0.001 |
| Moderate-severe LA, n (%) | 39 (39.0) | 24 (15.9) | 17.084c | < 0.001 |
| sICH, n (%) | 17 (17.0) | 10 (6.6) | 6.749c | 0.009 |
| nsICH, n (%) | 30 (30.0) | 16 (10.6) | 15.133c | < 0.001 |
| SAP, n (%) | 66 (66.0) | 13 (8.6) | 63.224c | < 0.001 |
| Anticoagulant therapy, n (%) | 56 (56.0) | 64 (42.4) | 0.022c | 0.881 |
| Stroke recurrence, n (%) | 4 (4.0) | 4 (2.6) | 0.004c | 0.953 |
| Laboratory findings, mmol/l | ||||
| Total cholesterol (‾x ± s) | 4.1 ± 0.9 | 4.1 ± 0.8 | 0.703b | 0.482 |
| Triglyceride (‾x ± s) | 1.0 ± 0.4 | 1.3 ± 0.9 | -2.843b | 0.005 |
| LDL-C (‾x ± s) | 2.3 ± 0.7 | 2.2 ± 0.7 | 1.412b | 0.152 |
| Fasting glucose (‾x ± s) | 7.0 ± 2.7 | 5.8 ± 1.7 | 3.844b | < 0.001 |
a Kruskal-Wallis H test; bt test; cχ2 test.
Impact of moderate to severe WMLs on the 3-month prognosis in the regression model
After adjustment for parameters with p values < 0.05 in univariate analysis, multivariate analysis using binary logistic regression with enter method was performed. The results showed that moderate to severe WMLs (OR = 4.105, 95 %CI = 1.447–11.646, P = 0.008), baseline NIHSS (OR = 1.368, 95 %CI = 1.240–1.511, P < 0.001), SAP (OR = 4.840, 95 %CI = 1.889–12.400, P = 0.001) and nsICH (OR = 3.751, 95 %CI = 1.234–11.399, P = 0.020) were independent risk factors for a poor prognosis at 3 months (Table 4).
Table 4.
Influence of moderate to severe WMLs on the 3-month prognosis
| Variable | OR | 95 %CI | P Value |
|---|---|---|---|
| Moderate-severe WMLs | 4.105 | 1.447–11.646 | 0.008 |
| Baseline NIHSS score | 1.368 | 1.240–1.511 | < 0.001 |
| nsICH | 3.751 | 1.234–11.399 | 0.020 |
| SAP | 4.840 | 1.889–12.400 | 0.001 |
Discussion
The present study described the overall distribution of WMLs in CES patients and found that moderate to severe WMLs was an independent risk factor for poor prognosis at 3 months in such patients without reperfusion therapy.
Researches have showed that WMLs are widely distributed in the elderly and stroke patients and are an indicator of cerebral microcirculation disturbance [2, 14]. For example, a study scanned 26 cognitively normal elderly subjects with arterial spin labeling (ASL) sequence, and found that the volume of periventricular WMLs was positively correlated with decreased regional cortical blood flow [15]. Another study indicated that the severity of WMLs was negatively associated with leptomeningeal collateral circulation rating in patients with acute large artery occlusion, which further suggested that WMLs could disrupt collateral circulation compensation and decrease regional cerebral blood flow [16]. In our study, the incidences of sICH and nsICH in moderate to severe WMLs group were higher than those in mild WMLs group, which may be related to the more serious damage of microcirculation caused by extended WMLs.
The essence of CES is acute cerebral vascular occlusion resulted in cardiac embolus shedding. In this instance, the survival of ischemic brain tissue largely depends on the collateral compensation, which is partly conditioned by microcirculation. As a result, the presence of moderate to severe WMLs may indicate insufficient collateral circulation compensation and unfavorable prognosis with respect to neurological recovery [16]. In our study, moderate to severe WMLs was an independent risk factor for 3 months prognosis of CES patients, which is consistent with the previous research results. However, one study has shown that WMLs affects the outcomes of different stroke types differently. In that study, WMLs volume was positively correlated with the probability of poor prognosis in patients with stroke subtype of large artery atherosclerosis and small artery occlusion but not in patients with CES [17]. We speculate that the different research conclusions may be due to unequal neurological deficit, which is the key determinant of stroke outcome [18].
WMLs may affect the prognosis of stroke through other mechanisms [19]. First of all, WMLs could damage the microstructure of white matter tissue and disrupt the brain neural connections and functional network, thus impairing the plasticity and compensatory mechanisms and slow down the brain’s recovery from stroke [20]. Besides, WMLs could disrupt motor/cognitive networks that are important for learning and neurorehabilitation [21, 22].
There are many other factors affecting the outcome of stroke patients, such as the baseline NIHSS score, SAP and age. In our study, patients with higher NIHSS score have much higher risk of developing SAP, which is consistent with previous research [23]. We speculate that the possible reasons are the widespread disturbance of consciousness, indwelling gastric cannula, paralysis and assisted breathing in patients with much severer neurological deficits.
Our study has some limitations. First, this retrospective study adopted a single center database and had a relatively small sample size of CES patients, which may increase the possibility of selection bias. Second, patients who died rapidly after admission were excluded, which may overestimate the impact of WMLs on prognosis.
In conclusion, moderate to severe WMLs was an independent risk factor for poor outcome of CES patients without reperfusion therapy. Measures should be taken on the evaluation and management of WMLs in CES patients.
Acknowledgements
None.
Abbreviations
- ASL
Arterial spin labeling
- CES
Cardioembolic stroke
- CI
Confidence interval
- CT
Computerized tomography
- CTA
Computerized tomography angiography
- FLAIR
Fluid attenuated inversion recovery
- M(IQR)
Median (interquartile range)
- MRI
Magnetic resonance imaging
- MRA
Magnetic resonance angiography
- mRS
Modified Rankin scale
- NIHSS
National Institutes of Health Stroke Scale
- nsICH
Non-symptomatic intracranial hemorrhage
- sICH
Symptomatic intracranial hemorrhage
- OR
Odds ratio
- SAP
Stroke-associated pneumonia
- SD
Standard deviation
- WMLs
White matter lesions
Authors' contributions
YKG participated in the design, data collection, data statistical analysis, data interpretation, and drafting most of the manuscript; ZYC participated in the design and substantively revision of the manuscript; QW: helped with the data collection, analysis, and drafting part of the manuscript; MZ: helped with the design and data analysis; GZD: helped in the data collection and interpretation; WYZ: helped with the data collection and statistical analysis; TY: participated in the data collection; YX: participated in the design, financial support, data statistical analysis, data interpretation and revision of the manuscrpit; All authors have approved the submitted version and have agreed both to be personally accountable for the author’s own contributions.
Funding
None.
Availability of data and materials
The datasets during and/or analyzed during the current study available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was approved by the Clinical Research Ethics Committee of the Affiliated Changzhou No.2 People’s Hospital of Nanjing Medical University (2018KY032-01). Verbal consents were obtained from all the patients or their legally authorized representatives.
Consent for publication
Verbal consents for publication were obtained from all the patients or their legally authorized representatives.
Conflict of interest
None.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12(8):822–38. doi: 10.1016/S1474-4422(13)70124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wardlaw JM, Smith C, Dichgans M. Small vessel disease: mechanisms and clinical implications. Lancet Neurol. 2019;18(7):684–96. doi: 10.1016/S1474-4422(19)30079-1. [DOI] [PubMed] [Google Scholar]
- 3.Debette S, Schilling S, Duperron MG, et al. Clinical Significance of Magnetic Resonance Imaging Markers of Vascular Brain Injury: A Systematic Review and Meta-analysis. JAMA Neurol. 2019;76(1):81–94. doi: 10.1001/jamaneurol.2018.3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Song J, Kim KH, Jeon P, et al. White matter hyperintensity determines ischemic stroke severity in symptomatic carotid artery stenosis. Neurol Sci. 2021. [DOI] [PubMed]
- 5.Gómez-Choco M, Mengual JJ, Rodríguez-Antigüedad J, et al. Pre-Existing Cerebral Small Vessel Disease Limits Early Recovery in Patients with Acute Lacunar Infarct. J Stroke Cerebrovasc Dis. 2019;28(11):104312. doi: 10.1016/j.jstrokecerebrovasdis.2019.104312. [DOI] [PubMed] [Google Scholar]
- 6.Appleton JP, Woodhouse LJ, Adami A, et al. Imaging markers of small vessel disease and brain frailty, and outcomes in acute stroke. Neurology. 2020;94(5):e439–52. doi: 10.1212/WNL.0000000000008881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Celeste F, Muratori M, Mapelli M, et al. The Evolving Role and Use of Echocardiography in the Evaluation of Cardiac Source of Embolism. J Cardiovasc Echogr. 2017;27(2):33–44. doi: 10.4103/jcecho.jcecho_1_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oksala NK, Salonen T, Strandberg T, et al. Cerebral small vessel disease and kidney function predict long-term survival in patients with acute stroke. Stroke. 2010;41(9):1914–20. doi: 10.1161/STROKEAHA.110.587352. [DOI] [PubMed] [Google Scholar]
- 9.Chen X, Wang L, Jiang J, et al. Association of neuroimaging markers of cerebral small vessel disease with short-term outcomes in patients with minor cerebrovascular events. BMC Neurol. 2021;21(1):21. doi: 10.1186/s12883-021-02043-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adams HP, Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24(1):35–41. doi: 10.1161/01.STR.24.1.35. [DOI] [PubMed] [Google Scholar]
- 11.Wahlund LO, Barkhof F, Fazekas F, et al. A new rating scale for age-related white matter changes applicable to MRI and CT. Stroke. 2001;32(6):1318–22. doi: 10.1161/01.STR.32.6.1318. [DOI] [PubMed] [Google Scholar]
- 12.Larrue V, von Kummer RR, Müller A, et al. Risk factors for severe hemorrhagic transformation in ischemic stroke patients treated with recombinant tissue plasminogen activator: a secondary analysis of the European-Australasian Acute Stroke Study (ECASS II) Stroke. 2001;32(2):438–41. doi: 10.1161/01.STR.32.2.438. [DOI] [PubMed] [Google Scholar]
- 13.de Montmollin E, Ruckly S, Schwebel C, et al. Pneumonia in acute ischemic stroke patients requiring invasive ventilation: Impact on short and long-term outcomes. J Infect. 2019;79(3):220–7. doi: 10.1016/j.jinf.2019.06.012. [DOI] [PubMed] [Google Scholar]
- 14.Lin MP, Brott TG, Liebeskind DS, et al. Collateral Recruitment Is Impaired by Cerebral Small Vessel Disease. Stroke. 2020;51(5):1404–10. doi: 10.1161/STROKEAHA.119.027661. [DOI] [PubMed] [Google Scholar]
- 15.Bahrani AA, Powell DK, Yu G, et al. White Matter Hyperintensity Associations with Cerebral Blood Flow in Elderly Subjects Stratified by Cerebrovascular Risk. J Stroke Cerebrovasc Dis. 2017;26(4):779–86. doi: 10.1016/j.jstrokecerebrovasdis.2016.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mark I, Seyedsaadat SM, Benson JC, et al. Leukoaraiosis and collateral blood flow in stroke patients with anterior circulation large vessel occlusion. J Neurointerv Surg. 2020;12(10):942–5. doi: 10.1136/neurintsurg-2019-015652. [DOI] [PubMed] [Google Scholar]
- 17.Ryu WS, Woo SH, Schellingerhout D, et al. Stroke outcomes are worse with larger leukoaraiosis volumes. Brain. 2017;140(1):158–70. doi: 10.1093/brain/aww259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tai MLS, Goh KJ, Kadir KAA, et al. Predictors of functional outcome in patients with stroke thrombolysis in a tertiary hospital in Malaysia. Singapore Med J. 2019;60(5):236–40. doi: 10.11622/smedj.2018150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huo Y-c, Li Q, Zhang W-y, et al. Total Small Vessel Disease Burden Predicts Functional Outcome in Patients With Acute Ischemic Stroke. Front Neurol. 2019;10:808. doi: 10.3389/fneur.2019.00808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quandt F, Fischer F, Schröder J, et al. Higher white matter hyperintensity lesion load is associated with reduced long-range functional connectivity. Brain Commun. 2020;2(2):fcaa111. doi: 10.1093/braincomms/fcaa111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.d’Arbeloff T, Elliott ML, Knodt AR, et al. White matter hyperintensities are common in midlife and already associated with cognitive decline. Brain Commun. 2019;1(1):fcz041. doi: 10.1093/braincomms/fcz041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Puzo C, Labriola C, Sugarman MA, et al. Independent effects of white matter hyperintensities on cognitive, neuropsychiatric, and functional decline: a longitudinal investigation using the National Alzheimer’s Coordinating Center Uniform Data Set. Alzheimers Res Ther. 2019;11(1):64. doi: 10.1186/s13195-019-0521-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang GQ, Lin YT, Wu YM, et al. Individualized Prediction Of Stroke-Associated Pneumonia For Patients With Acute Ischemic Stroke. Clin Interv Aging. 2019;14:1951–62. doi: 10.2147/CIA.S225039. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets during and/or analyzed during the current study available from the corresponding author on reasonable request.