Abstract
Ovarian cancer is a significant clinical challenge as no effective treatments are available to enhance patient survival. Recently, N6-methyladenosine (m6A) RNA modification has been demonstrated to play a pivotal role in tumorigenesis and progression. However, the roles of m6A target genes in ovarian cancer haven’t been clearly illustrated. In this study, we presented a comprehensive bioinformatics and in vitro analysis to evaluate the roles of m6A target genes. Cell division cycle 42 effector protein 3 (CDC42EP3), one probable m6A target gene, was identified to be down-regulated in ovarian cancer tissues and cells. Meanwhile, quantitative PCR (qPCR) and western blot were used to confirm the down-regulated CDC42EP3 in ovarian cancer cells A2780 and TOV112D. The biological function of CDC42EP3 in ovarian cancer was further validated with several algorithms, such as PrognoScan, K-M plotter, LinkedOmics and TISIDB. These findings indicated that lower expression of CDC42EP3 was correlated with poor prognosis in patients with ovarian cancer. In addition, CDC42EP3 expression was significantly associated with a diverse range of tumor-infiltrating immune cells, including natural killer cells (NK), T central memory cells (Tcm), T gamma delta cells (Tgd), etc. Taken together, this study uncovered the potential roles of m6A target gene CDC42EP3 in the regulation of immune microenvironment in the ovarian cancer, and identified CDC42EP3 as a novel prognostic target.
Keywords: CDC42EP3, Immune infiltration, Ovarian cancer, N6-methyladenosine, Prognosis
Introduction
Ovarian cancer is the eighth most frequently diagnosed cancer and the eighth leading cause of cancer death in women, with an estimated 314,000 new cases (3.4% of all cancer cases) and 207,000 deaths (4.7% of all cancer deaths) worldwide in 2020 (Sung et al., 2021). Despite the advances made in cancer screening, diagnosis and treatment methods during the past few decades, the five-year survival rate for ovarian cancer has changed modestly (Torre et al., 2018). Thus, discovering potential diagnostic markers and specific therapeutic targets are of importance to improve survival rate of ovarian cancer patients.
The tumor-infiltrating immune cells in the tumor microenvironment (TME) play pivotal roles in the initiation, progression, invasion, and metastasis of the malignant tumors. Poly (ADP-ribose) polymerase inhibitors (PARPi) have shown promising clinical benefits in ovarian cancer through influencing the TME and immune system response (Turinetto et al., 2021). The cell subtypes characterized by low expression of checkpoints and major histocompatibility complex (MHC) have been proved to be associated with the poor prognosis of ovarian cancer patients (Cong et al., 2020). However, comprehensive research on the tumor immune microenvironment of ovarian cancer is still rare and needs further exploration.
Recent studies have suggested the biofunctional roles of cell division cycle 42 effector protein 3 (CDC42EP3) in regulating actin cytoskeleton re-organization during cell shape changes, including pseudopodia formation (Farrugia & Calvo, 2016). In addition, CDC42EP3 also plays an important role in the occurrence and development of human cancers. Feng’s group revealed that CDC42EP3 was significantly up-regulated in colorectal cancer, and its high expression could accelerate cancer progression through regulating cell proliferation, apoptosis, and migration (Feng et al., 2021). In contrast, the expression of CDC42EP3 was down-regulated in peripheral blood monocyte from patients with chronic lymphocytic leukemia, which may be associated with impairment of phagocytosis (Maffei et al., 2013). However, the detailed function and mechanism of CDC42EP3 in the tumorigenesis and immune infiltration of ovarian cancer have not yet been investigated.
The purpose of this study was to investigate the potential roles of CDC42EP3 in ovarian cancer. The expression of CDC42EP3 was found to be down-regulated in ovarian cancer tissues and cells, and its low level was associated with poor prognosis. The results revealed that CDC42EP3 expression had significant prognostic value and could be a promising target for immune regulation in ovarian cancer.
Material and Methods
Cell culture
The human ovarian epithelial cell IOSE80 and ovarian cancer cells A2780 and TOV112D were obtained from Center for Molecular Medicine, Xiangya Hospital, Central South University. All cell lines were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM, Gibco, USA) with 10% fetal bovine serum (FBS, Gibco, USA) and 1% penicillin/streptomycin (Gibco, USA). The cultures were placed in a sterile incubator maintained at 37 °C with 5% CO2.
Western blot
To begin this procedure, cell lysates were processed with RIPA buffer supplemented with protease inhibitor. The total protein concentration was detected by BCA protein assay kit. Equivalent amounts of protein were loaded in each lane of SDS-polyacrylamide gel electrophoresis. Besides, the bands were transferred to PVDF membranes. Next, the membranes were blocked for 1 h at room temperature with TBST supplemented with 5% non-fat dry milk and then incubated at 4 °C overnight with primary antibodies. The primary antibodies displayed as follows: CDC42EP1 (1:500; Abcam, USA) and β-actin (1:5000; Santa Cruz, USA). Finally, protein bands were detected through the Immobilon Western Chemiluminescent HRP reagents (WBKLS0500; Millipore, USA).
Quantitative PCR (qPCR)
Total RNA was extracted from disparate cell lines using TRIzol reagent (Invitrogen, USA) following the manufacturer’s protocol and then converted to cDNA using a PrimeScriptTM RT reagent kit (6210; Takara, China). The qPCR assay was performed with iTaqTM Universal SYBR green Supermix (1725121, Bio-Rad, USA) to determine the relative RNA levels. β-actin was used as an internal control for quantification of each gene. The sequences of gene primers displayed as follows: CDC42EP3 forward: 5′-AGCAGTCTGTTGGAGAATGGG-3′and reverse: 5′-AGGAGGGAACCTGTAAGGTCAG-3′; β-actin forward: 5′-CATGTACGTTGCTATCCAGGC-3′and reverse, 5′-CTCCTTAATGTC ACGCACGAT-3′. The relative expression levels of RNAs were determined using the 2-ΔΔCT method (Buford et al., 2020; Kiss et al., 2019; Pandelides et al., 2020).
Data acquisition and processing
The integrative bioinformatics analysis of differential expressed genes was obtained from several bioinformatics databases (Table 1).
Table 1. The integrative bioinformatics analyzed in this study.
| Database | URL | Refs. |
|---|---|---|
| Oncomine | https://www.oncomine.org/resource/main.html | Rhodes et al. (2007) |
| RMVar | http://www.rmvar.renlab.org/browse.html | Luo et al. (2021) |
| UALCAN | http://ualcan.path.uab.edu/ | Chandrashekar et al. (2017) |
| PrognoScan | http://dna00.bio.kyutech.ac.jp/PrognoScan/index.html | Mizuno et al. (2009) |
| Kaplan-Meier Plotter | https://kmplot.com/analysis/ | Nagy, Munkacsy & Gyorffy (2021) |
| LinkedOmics | http://linkedomics.org/login.php | Vasaikar et al. (2018) |
| TISIDB | http://cis.hku.hk/TISIDB | Ru et al. (2019) |
| TNMplot | https://tnmplot.com/ | Bartha & Gyorffy (2021) |
The gene expression profiles from two public datasets regarding ovarian cancer were obtained from the Oncomine datasets (Rhodes et al., 2007), Yoshihara Ovarian and Bonome Ovarian. The GEO2R, an R-based web application (Barrett et al., 2013), was applied to assess the co-differential expressed genes in these two datasets. The screening criteria to identify the differential expression genes were as follows: p-value < 0.01 and — log2Fold Change —≥ 1.5. In addition, the N6-methyladenosine (m6A) target genes of ovarian cancer were acquired from the RNA modification-associated variants database (RMVar) (Luo et al., 2021).
UALCAN (Chandrashekar et al., 2017) integrates the RNA-seq data and clinical information of pan-cancer types from The Cancer Genome Atlas (TCGA). This database could enable researchers to explore the expression level of candidate genes between primary tumor and normal tissue samples.
PrognoScan (Mizuno et al., 2009) is utilized to analyze the relation between gene expression and clinical prognosis of patients, including overall survival (OS). In this research, PrognoScan was applied for the survival analysis of differentially expressed genes (DEGs) in patients with ovarian cancer. In addition, the Kaplan Meier (K-M) plotter (Nagy, Munkacsy & Gyorffy, 2021) is capable to assess the effect of candidate genes on the prognosis in pan-cancer. The K-M survival curves were applied to exhibit the prognostic effects of CDC42EP3 in ovarian cancer patients.
LinkedOmics (Vasaikar et al., 2018) provides a unique platform to analyze multi-omics data across pan-cancer types. The DEGs associated with CDC42EP3 were screened from the TCGA-OV cohort through LinkFinder module in this database. The statistical method used in correlation analysis was Pearson correlation and the results of correlation were presented in volcano plot and heat maps, respectively. The LinkInterpreter module was performed to analyze the Gene Ontology molecular function (GO_MF) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway.
The TISIDB (Ru et al., 2019) is a web portal for tumor and immune system interplay, which contains multiple heterogeneous data. Here, TISIDB was employed to investigate the roles of CDC42EP3 in several immune-associated signaling pathways, such as tumor-infiltrating immune cells, immunomodulators, chemokines and chemokine receptors.
Statistical analyses
All experimental findings were shown as mean ± standard deviation (SD). Student’s t-test was used to explore the difference between two groups. P < 0.05 was considered as statistically significant difference.
Results
Identification of differentially expressed genes
To screen for DEGs in ovarian cancer, we compared normal ovary tissues and tumor samples in two datasets from Oncomine, Yoshihara Ovarian and Bonome Ovarian. According to the filtering conditions of p-value < 0.01 and —log2Fold Change—≥1.5, we identified 225 up-regulated genes in Bonome Ovarian and 1085 up-regulated genes in Yoshihara Ovarian, respectively. Meanwhile, 536 genes in Bonome Ovarian and 623 genes in Yoshihara Ovarian have been identified to be significantly down-regulated in ovarian cancer tissues (Table S1), and 757 m6A target genes of ovarian cancer acquired from the RMVar database were shown in Table S2.
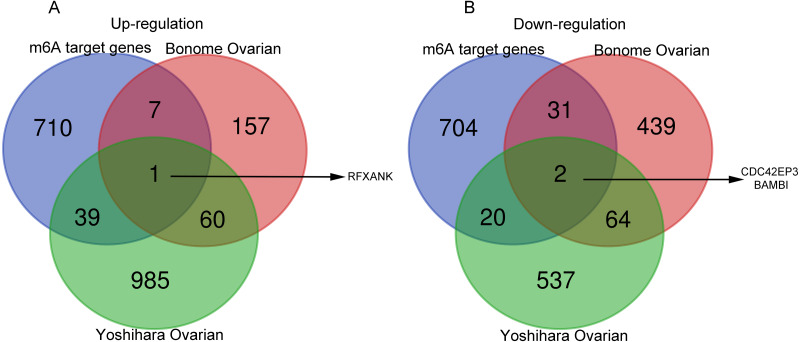
Next, the co-DEGs between Yoshihara Ovarian and Yoshihara Ovarian were analyzed by Venn diagram (http://bioinformatics.psb.ugent.be/webtools/Venn/). Studies have demonstrated that the m6A RNA modification, a reversible and dynamic epigenetic modification, affects the RNA metabolism and homeostasis, and plays critical functions in multiple of important physiological and pathological bioprocesses, including tumorigenesis and progression (Xu et al., 2020a). In order to investigate the possible roles of m6A target genes in ovarian cancer, Venn diagram was used to display the overlapping genes between co-DEGs and m6A target genes. The results showed that one m6A target gene, regulatory factor X subunit B (RFXANK), was highly-expressed, and two m6A target genes, CDC42EP3 and BMP and activin membrane bound inhibitor (BAMBI), were lowly-expressed (Figs. 1A–1B). These three genes were presumed to have a significance impact on the development of ovarian cancer.
Figure 1. Venn diagrams of gene expression profiles.
(A) RFXANK was identified as the only one up-regulated overlapping gene among Yoshihara Ovarian, Bonome Ovarian and m6A target gene set. (B) CDC42EP3 and BAMBI were recognized as the down-regulated overlapping genes among Yoshihara Ovarian, Bonome Ovarian and m6A target gene set. The number in each overlapping area represents the number of co-DEGs.
CDC42EP3 shows the promising prognostic value in ovarian cancer
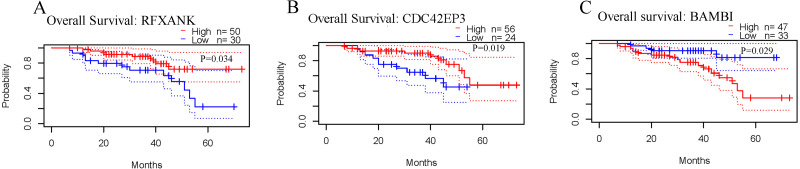
The correlations between the expression levels of RFXANK, CDC42EP3 and BAMBI and prognosis in ovarian cancer patients were analyzed by the PrognoScan database. The results revealed that patients with high level of RFXANK and CDC42EP3 displayed favorable OS (Figs. 2A–2B). However, patients with high level of BAMBI displayed poor OS (Fig. 2C). Given the up-regulated RFXANK and down-regulated CDC42EP3 and BAMBI in two independent ovarian cancer datasets from Oncomine (Figs. 1A–1B), we expected CDC42EP3 as a potential prognostic target that needs further investigation.
Figure 2. Prognostic values of RFXANK, CDC42EP3 and BAMBI in ovarian cancer.
(A–C) PrognoScan database was used to analyze the kaplan-Meier curves of overall survival for patients with high or low expression levels of RFXANK, CDC42EP3 and BAMBI.
Associations between CDC42EP3 expression and clinicopathological parameters in ovarian cancer patients
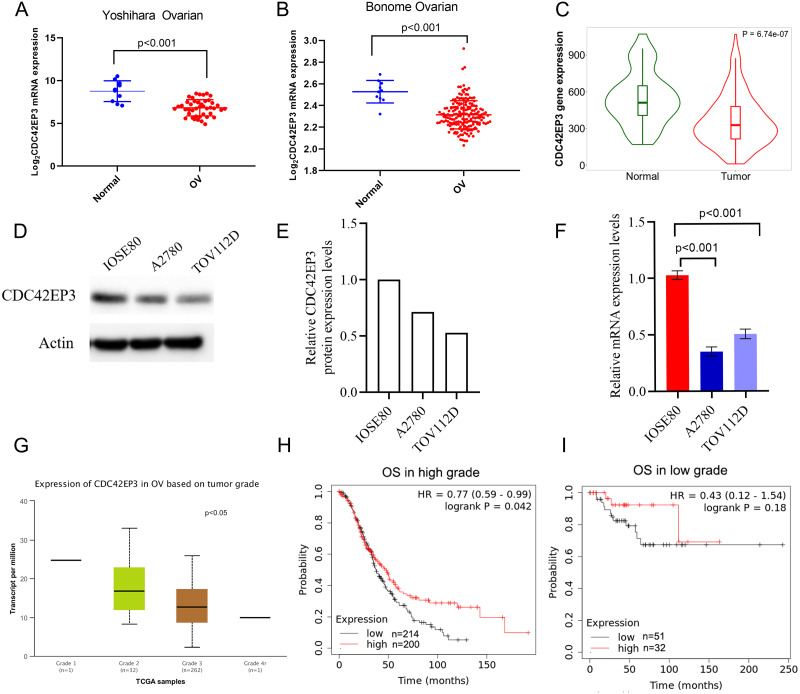
The expression profiles of CDC42EP3 were further confirmed using several independent bioinformatics databases, such as TNM plot (Bartha & Gyorffy, 2021) and UALCAN. Firstly, two above-mentioned datasets, Yoshihara Ovarian and Bonome Ovarian, indicated that CDC42EP3 transcriptional level in ovarian cancer samples was significantly lower than adjacent noncancerous tissues (Figs. 3A–3B). Next, TNM plot revealed that CDC42EP3 mRNA expression levels were significantly down-regulated in ovarian cancer samples (Fig. 3C). In addition, qPCR and western blot were used to confirm the down-regulation of CDC42EP3 expression in ovarian cancer cells A2780 and TOV112D compared with the normal ovarian epithelial cell IOSE80 (Figs. 3D–3F). All these data revealed that the expression of CDC42EP3 was significantly downregulated in ovarian cancer tissues and cell lines.
Figure 3. Associations between down-regulated CDC42EP3 and clinicopathological parameters in ovarian cancer patients.
(A–C) Compared to normal ovary tissues, CDC42EP3 was significantly down-regulated in ovarian cancer tissues. (D–F) qPCR and western blot were used to confirm the down-regulation of CDC42EP3 expression in ovarian cancer cells A2780 and TOV112D compared with the normal ovarian epithelial cell IOSE80. (G) Association of CDC42EP3 level with tumor grade. (H–I) Kaplan–Meier curves was conducted to explore the roles of CDC42EP3 expression on the OS in low-grade and high-grade ovarian cancer patients.
Furthermore, we analyzed the correlation between CDC42EP3 expression levels and clinicopathological features of ovarian cancer patients. According to UCLCAN database, we discovered that the higher tumor grade, the lower expression levels of CDC42EP3 in ovarian cancer (Fig. 3G). However, the Xiantao tool (http://www.xiantao.love/products) was analyzed to reveal that CDC42EP3 expression has no correlation with the other clinical parameters, including tumor status, stage, age and invasion (Table 2). In addition, the K-M plotter uncovered that the expression of CDC42EP3 was significantly positively correlated with OS in high-grade tumor patients (p < 0.05) (Fig. 3H). However, the correlation between CDC42EP3 level and OS in low-grade patients was of no statistical significance (Fig. 3I). Collectively, these results indicate that CDC42EP3 could be a potential prognostic biomarker for ovarian cancer, especially like the high-grade patients.
Table 2. Demographic characteristics of CDC42EP3 expression in ovarian cancer.
| Characteristics | Total(N) | Odds ratio | P value |
|---|---|---|---|
| Stage (Stage III&Stage IV vs. Stage I&Stage II) | 376 | 1.432 (0.624–3.404) |
0.401 |
| Primary therapy outcome (CR&PR vs. PD&SD) | 308 | 1.139 (0.618–2.112) |
0.676 |
| Age (> 60 vs. ≤ 60) | 379 | 0.817 (0.545–1.225) |
0.329 |
| Venous invasion (No vs. Yes) | 105 | 0.754 (0.338–1.658) |
0.484 |
| Lymphatic invasion (No vs. Yes) | 149 | 1.674 (0.839–3.385) |
0.146 |
| Tumor residual (NRD vs. RD) | 335 | 1.391 (0.813–2.400) |
0.230 |
| Tumor status (Tumor free vs. With tumor) | 337 | 1.241 (0.736–2.104) |
0.420 |
CDC42EP3 co-expression network in ovarian cancer
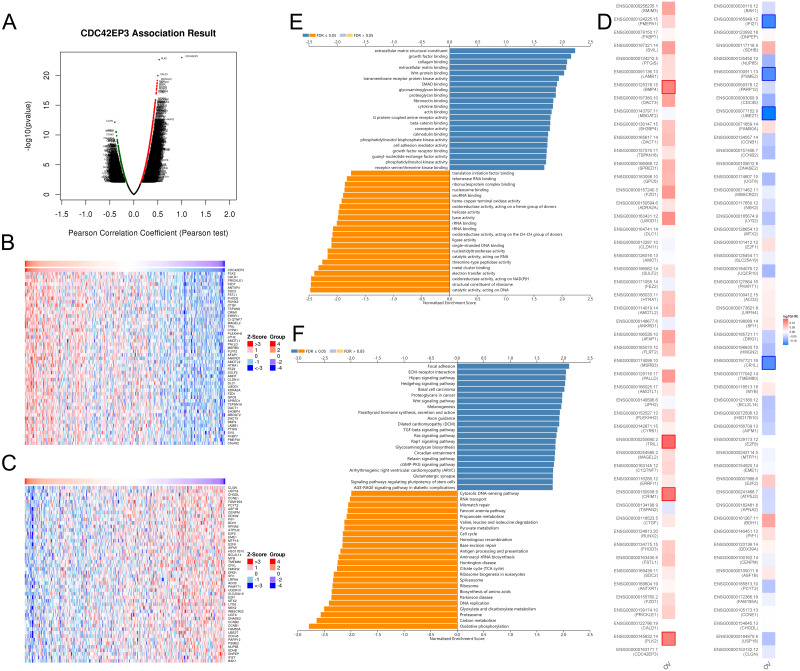
In order to explore the biological functions of CDC42EP3 in ovarian cancer, we performed the co-expression pattern of CDC42EP3 screened from the TCGA-OV cohort by the LinkFinder module of LinkedOmics. As shown in Fig. 4A, 4227 genes (red dots) were positively correlated with CDC42EP3, and 3122 genes (green dots) were negatively correlated with CDC42EP3. Figures 4B–4C showed the heatmaps of top 50 genes positively and negatively associated with CDC42EP3, respectively (Tables S3–S4). 14/50 positively correlated genes had protective hazard ratio (HR), while 13/50 genes negatively correlated with CDC42EP3 had unfavorable HR (Fig. 4D).
Figure 4. The co-expression genes with CDC42EP3 in ovarian cancer by the LinkedOmics database.
(A) The significantly associated genes with CDC42EP3 analyzed by Pearson correlation in ovarian cancer cohort. (B–C) Heatmaps of the top 50 genes positively and negatively correlated with CDC42EP3 in ovarian cancer, respectively. Red dots indicate the positively correlated genes and blue dots indicate the negatively correlated genes. (D) Survival maps depicting the top 50 genes positively and negatively related to CDC42EP3 in ovarian cancer, respectively. (E–F) GO annotations and KEGG pathways of CDC42EP3-associated network in ovarian cancer cohort.
To further elaborate the potential biological roles of CDC42EP3 in ovarian cancer development, we performed GO and KEGG functional enrichment analysis. GO enrichment analysis revealed that co-expression genes of CDC42EP3 mainly participated in extracellular matrix structural constituent, growth factor binding, collagen binding, extracellular matrix binding, Wnt-protein binding, etc (Fig. 4E). KEGG pathway analysis indicated these genes were significantly related to several cancer-associated signaling pathways, such as focal adhesion, extracellular matrix (ECM)-receptor interaction, Hippo signaling pathway, hedgehog signaling pathway, basal cell carcinoma, Wnt signaling pathway, etc (Fig. 4F). These findings suggested that important functions of CDC42EP3-associated network in ovarian cancer.
Relation between CDC42EP3 with immune molecules
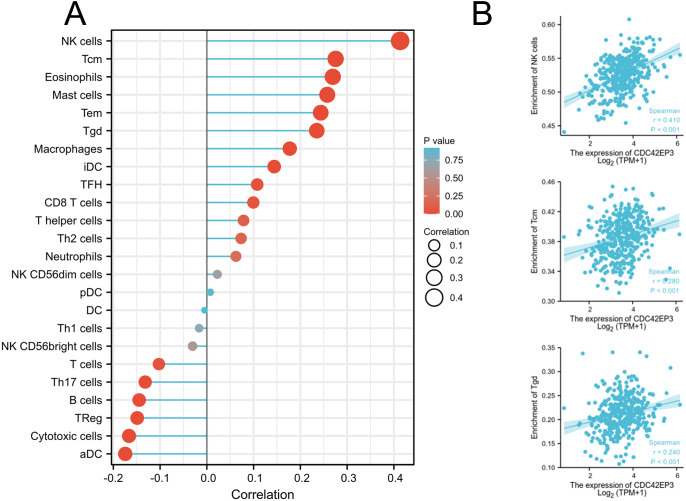
We explored whether CDC42EP3 expression can affect the abundance of tumor-infiltrating lymphocytes (TILs) in ovarian cancer using Xiantao Tool. The significantly positive relations could be obtained between CDC42EP3 expression and several tumor-infiltrating immune cells, including natural killer cells (NK), T central memory cells (Tcm) and T gamma delta cells (Tgd) (Figs. 5A–5B). Similar results were obtained by analyzing the immune-related signatures of 28 TIL types through TISIDB algorithm (Figs. S1A–S1B).
Figure 5. Correlation of CDC42EP3 expression with tumor-infiltrating immune cells in ovarian cancer.
(A) Lollipop of correlation between CDC42EP3 expression and immune cells by the Xiantao Tool. (B) The cross-validated correlation between CDC42EP3 expression and several immune cells, such as NK, Tcm and Tgd cells.
Furthermore, we used TISIDB database to analyze the correlation between CDC42EP3 expression and other immune signatures, including immunostimulators, immunoinhibitors, chemokines and chemokine receptors. Figs. S2A–S2B exhibited the positively correlated immunostimulators with CDC42EP3, including 5′-nucleotidase ecto (NT5E), C-X-C motif chemokine ligand 12 (CXCL12), tumor necrosis factor superfamily member 4 (TNFSF4), and TNF receptor superfamily member 8 (TNFRSF8). Figures S2C–S2D uncovered the negatively correlated immunoinhibitors with CDC42EP3, including galectin 9 (LGALS9), indoleamine 2,3-dioxygenase 1 (IDO1), cluster of differentiation 274 (CD274) and V-set domain containing T cell activation inhibitor 1 (VTCN1). Figures S3A–S3B showed the significant associations between CDC42EP3 expression and chemokines, such as C-C motif chemokine ligand 14 (CCL14). Figures S3C–S3D revealed the associations between CDC42EP3 expression and chemokine receptors, including C-C motif chemokine receptor 1 (CXCR1). Generally, we concluded that CDC42EP3 might participated in modulating diverse of immunoregulatory molecules in ovarian cancer, which could affect the infiltration and functions of immune cells in TME.
Discussion
Our research aimed to explore the m6A target genes in the occurrence and development of ovarian cancer. Intriguingly, through the integrative bioinformatics and experimental analysis, the expression of CDC42EP3 was found to be down-regulated in ovarian cancer tissues and cells, and its low level was associated with poor prognosis.
The m6A modification in messenger RNAs is the most widespread epitranscriptome modification involving in the tumorigenesis and development (Huang et al., 2020a; Zhang et al., 2020). RNA m6A modification is regulated by three major epigenetic modifiers, including methyltransferases (writers), demethylases (erasers) and binding proteins (readers) (Geng et al., 2020). Hua’s group unveiled that methyltransferase-like 3 (METTL3) was up-regulated in ovarian cancer and promoted growth and invasion of ovarian cancer cells via stimulating the receptor tyrosine kinase AXL translation (Hua et al., 2018). In addition, Huang et al. disclosed that the fat mass- and obesity-associated protein (FTO), an m6A demethylase, was down-regulated in ovarian cancer stem cells (CSC) and inhibited the cell self-renewal by blocking cAMP signaling (Huang et al., 2020b). Xu and the colleague revealed that F-box and WD repeat domain-containing 7 (FBW7) suppressed ovarian cancer progression through targeting the m6A binding protein YTH domain family 2 (YTHDF2) (Xu et al., 2021). Nevertheless, more research should be carried out to clarify the detailed mechanisms of m6A modification in ovarian tumorigenesis and development.
Cell division cycle 42 (CDC42), a family member of the Rho GTPase proteins, modulates a variety of essential biological processes, including cell polarization, cytoskeleton remodeling, vesicular trafficking, and pseudopodia formation (Maldonado & Dharmawardhane, 2018). Like other Rho family members, CDC42 possesses two conformational states, GDP-bound inactive state and GTP-bound active state. Activated CDC42 exerts important physiological and pathological functions via interacting with downstream effectors, like CDC42/Rac interactive binding motif (CRIB) (Farrugia & Calvo, 2016). CDC42EP3, one of the CDC42 effector proteins, participates in cell actin cytoskeleton re-organization. The current studies concerning the biological roles of CDC42EP3 have been mainly focused on its regulatory effect on tumor progression. The expression level of CDC42EP3 was up-regulated in primary cultured hepatocellular carcinoma cells and can enhance the cellular migratory ability in vitro (Lin & Chuang, 2012). It has also been shown that the up-regulated CDC42EP3 in colorectal cancer tissues can promote tumor growth by regulating cell proliferation, apoptosis, and migration (Feng et al., 2021). Similarly, Chen et al. (2021) revealed that up-regulated CDC42EP3 in gastric cancer tissues facilitated tumor development and progression. Conversely, down-regulated CDC42EP3 was found in patients with chronic lymphocytic leukemia (Maffei et al., 2013). Likewise, Hsia et al. (2015) reported that CDC42EP3 was down-regulated in cantharidin-treated lung cancer cells. However, the detailed roles of CDC42EP3 in human gynecological tumors, especially ovarian cancer, have been rarely studied. In this research, we firstly indicated the clinical prognostic value and potentially functional roles of CDC42EP3 in ovarian cancer through a comprehensive bioinformatics analysis.
GO and KEGG functional enrichment analysis indicated the co-expression genes of CDC42EP3 mainly involved in several cancer-associated signaling pathways, such as focal adhesion, ECM-receptor interaction and Hippo signaling pathway. Focal adhesion connects cytoskeleton within ECM, participating in the regulation of cell adhesion, proliferation and apoptosis. Focal adhesion kinase (FAK) is a widely expressed non-receptor tyrosine kinase participating in integrin-mediated signal transduction. Its overexpression and activation are frequently identified in multiple cancers, including ovarian cancer, and are associated with poor clinical outcome (Levy et al., 2019). The invasive phenotype of ovarian cancer cells was found to be closely correlated to ECM-receptor interaction (Wang et al., 2020). The Hippo signaling pathway controls organ size by regulating cell proliferation and apoptosis (Yu et al., 2012). Increasingly evidence has confirmed that dysregulation of Hippo pathway is involved in cancer immunosuppressive microenvironment (Yang et al., 2020). Therefore, CDC42EP3 might participate in malignant biological phenotypes of ovarian cancer through the afore-mentioned signaling pathways. However, the specific biological mechanism still needs further experimental verification.
The TME is defined as an intricate and multicellular environment that is clearly associated with cancer characteristic and therapy response (Jia, Liu & Shan, 2020; Wei et al., 2020). The TME typically comprises extracellular matrix, stromal cells, immune cells and blood and lymphatic vascular networks (Horowitz et al., 2020). Recently, accumulating evidence has demonstrated that TME plays a crucial role in tumor progression and therapeutic resistance by facilitating immunosuppression and limiting anticancer immune responses. Immunotherapies, including checkpoint blockade therapy, oncolytic virus, and adoptive cell therapy, are shifting the tumor treatment landscape and outlook (Da Silva et al., 2019; Xu et al., 2020b). Advanced ovarian cancer is the most lethal gynecological tumor with a particularly poor prognosis, which makes it urgent to explore novel therapeutic strategies. In this research, the correlation between CDC42EP3 and immune signatures was cross-validated by the TISIDB database and Xiantao Tool. It was found that CDC42EP3 had the most relevance with several TILs, including NK cell, Tcm_CD4 and Tgd. NK-based immunotherapy strategy, especially cytokine-induced killer (CIK) cells, has been extensively studied for decades (Sarivalasis et al., 2021). The clinical efficacy of CIK cells was evaluated in patients with advanced epithelial ovarian cancer in a phase 3 clinical trial. The results showed an improved progression free survival (PFS) in the treatment (Liu et al., 2014). Furthermore, CDC42EP3 had the highest correlation with immunostimulators, including NT5E, TNFSF4, CXCL12 and TNFRSF8, and immunoinhibitors, including LGALS9, IDO1, CD274 and VTCN1. NT5E, also designated CD73, is an ecto-nucleotidase that catalyzes extracellular ATP to adenosine and plays a critical role in the maintenance of immune homeostasis. High CD73 expression was significantly correlated with shorter OS in ovarian cancer (Jiang et al., 2018). Programmed death-ligand 1 (PD-L1), also known as CD274, is high expressed in ovarian cancer. However, the single agent targeting PD-1/PD-L1 has shown modest efficiency in treating ovarian cancer (Pujade-Lauraine, 2017). Currently, immunotherapy combined with other anticancer therapies, such as chemotherapy, radiotherapy and targeted therapy, are underway. Together, these findings suggest that CDC42EP3, which is correlated with immune-associated molecules, can serve as a potential immunotherapeutic target for treatment of ovarian cancer.
Nonetheless, several limitations in this study need to be acknowledged. First, the data collected from several public database were retrospective. Thus, the results need to be further validated in large-scale prospective studies. Moreover, more functional experiments as well as clinical trials are warranted to explore the clinical value of CDC42EP3 in patients with ovarian cancer.
Conclusion
In conclusion, this is the first study to investigate the clinical prognostic roles of CDC42EP3 in ovarian cancer. CDC42EP3 is down-regulated in ovarian cancer tissues and cells, and may serve as a promising prognostic biomarker for ovarian cancer patients. Furthermore, the expression of CDC42EP3 is obviously associated with TILs, which profoundly affect the prognosis of ovarian cancer. Therefore, our findings indicated that CDC42EP3 might play key roles in TME, and provide a novel target for immunotherapy.
Supplemental Information
(A) Heatmap indicated the correlation between CDC42EP3 expression and immune cells analyzed by TISIDB. (B) The cross-validated correlation between CDC42EP3 expression and several immune cells, such as NK, Tcm and Tgd cells.
(A) The correlation between CDC42EP3 expression and multiple of immunostimulators. (B) The top four immunostimulators highly correlated with CDC42EP3 expression. (C) The correlation between CDC42EP3 expression and multiple of immunoinhibitors. (D) The top four immunoinhibitors highly correlated with CDC42EP3 expression.
(A) The correlation between CDC42EP3 expression and chemokines. (B) The significant associations between CDC42EP3 expression and CCL14. (C) The correlation between CDC42EP3 expression and chemokine receptors. (D) The significant associations between CDC42EP3 expression and CXCR1.
Western blot confirmed the down-regulation of CDC42EP3 expression in ovarian cancer cells A2780 and TOV112D.
qPCR confirmed the down-regulation of CDC42EP3 expression in ovarian cancer cells A2780 and TOV112D.
Funding Statement
This study is supported by grants from the National Natural Science Foundation of China (81803035), the China Postdoctoral Science Foundation (2021T140754, 2020M672521), the Natural Science Foundation of Hunan Province (2020JJ5934, 2019JJ50932), and the Postdoctoral Science Foundation of Central South University (248485). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Contributor Information
Zhijie Xu, Email: xzj1322007@csu.edu.cn.
Qiaoli Yi, Email: yql2019@csu.edu.cn.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Yuanliang Yan conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Qiuju Liang analyzed the data, prepared figures and/or tables, and approved the final draft.
Zhijie Xu and Qiaoli Yi conceived and designed the experiments, performed the experiments, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Data Availability
The following information was supplied regarding data availability:
The raw measurements are available in the Supplementary Files.
References
- Barrett et al. (2013).Barrett T, Wilhite SE, Ledoux P, Evangelista C, Kim IF, Tomashevsky M, Marshall KA, Phillippy KH, Sherman PM, Holko M, Yefanov A, Lee H, Zhang N, Robertson CL, Serova N, Davis S, Soboleva A. NCBI GEO: archive for functional genomics data sets–update. Nucleic Acids Research. 2013;41:D991–D995. doi: 10.1093/nar/gks1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartha & Gyorffy (2021).Bartha A, Gyorffy B. TNMplot.com: a web tool for the comparison of gene expression in normal, tumor and metastatic tissues. International Journal of Molecular Sciences. 2021;22:2622. doi: 10.3390/ijms22052622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buford et al. (2020).Buford TW, Sun Y, Roberts LM, Banerjee A, Peramsetty S, Knighton A, Verma A, Morgan D, Torres GE, Li Q, Carter CS. Angiotensin (1-7) delivered orally via probiotic, but not subcutaneously, benefits the gut-brain axis in older rats. Geroscience. 2020;42:1307–1321. doi: 10.1007/s11357-020-00196-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrashekar et al. (2017).Chandrashekar DS, Bashel B, Balasubramanya SAH, Creighton CJ, Ponce-Rodriguez I, Chakravarthi B, Varambally S. UALCAN: a portal for facilitating tumor subgroup gene expression and survival analyses. Neoplasia. 2017;19:649–658. doi: 10.1016/j.neo.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen et al. (2021).Chen W, Zhu Y, Zhang W, Zhang H, Zhou Y, Sun P, Wu G. CDC42EP3 is a key promoter involved in the development and progression of gastric cancer. Carcinogenesis. 2021 doi: 10.1093/carcin/bgab048. Epub ahead of print 2021 10 June. [DOI] [PubMed] [Google Scholar]
- Cong et al. (2020).Cong S, Guo Q, Cheng Y, He Y, Zhao X, Kong C, Ning S, Zhang G. Immune characterization of ovarian cancer reveals new cell subtypes with different prognoses, immune risks, and molecular mechanisms. Frontiers in Cell and Developmental Biology. 2020;8:614139. doi: 10.3389/fcell.2020.614139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Silva et al. (2019).Da Silva JL, Dos Santos ALS, Nunes NCC, De Moraes Lino da Silva F, Ferreira CGM, De Melo AC. Cancer immunotherapy: the art of targeting the tumor immune microenvironment. Cancer Chemotherapy and Pharmacology. 2019;84:227–240. doi: 10.1007/s00280-019-03894-3. [DOI] [PubMed] [Google Scholar]
- Farrugia & Calvo (2016).Farrugia AJ, Calvo F. The Borg family of Cdc42 effector proteins Cdc42EP1-5. Biochemical Society Transactions. 2016;44:1709–1716. doi: 10.1042/BST20160219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng et al. (2021).Feng Q, Xu D, Zhou M, Wu Z, Wu Z, Wang Z, Bi J, Pei W. CDC42EP3 promotes colorectal cancer through regulating cell proliferation, cell apoptosis and cell migration. Cancer Cell International. 2021;21:169. doi: 10.1186/s12935-021-01845-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng et al. (2020).Geng Y, Guan R, Hong W, Huang B, Liu P, Guo X, Hu S, Yu M, Hou B. Identification of m6A-related genes and m6A RNA methylation regulators in pancreatic cancer and their association with survival. Annals of Translational Medicine. 2020;8:387. doi: 10.21037/atm.2020.03.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz et al. (2020).Horowitz M, Esakov E, Rose P, Reizes O. Signaling within the epithelial ovarian cancer tumor microenvironment: the challenge of tumor heterogeneity. Annals of Translational Medicine. 2020;8:905. doi: 10.21037/atm-2019-cm-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsia et al. (2015).Hsia TC, Yu CC, Hsu SC, Tang NY, Lu HF, Yu CS, Wu SH, Lin JG, Chung JG. cDNA microarray analysis of the effect of cantharidin on DNA damage, cell cycle and apoptosis-associated gene expression in NCI-H460 human lung cancer cells in vitro. Molecular Medicine Reports. 2015;12:1030–1042. doi: 10.3892/mmr.2015.3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua et al. (2018).Hua W, Zhao Y, Jin X, Yu D, He J, Xie D, Duan P. METTL3 promotes ovarian carcinoma growth and invasion through the regulation of AXL translation and epithelial to mesenchymal transition. Gynecologic Oncology. 2018;151:356–365. doi: 10.1016/j.ygyno.2018.09.015. [DOI] [PubMed] [Google Scholar]
- Huang et al. (2020a).Huang H, Bai Y, Lu X, Xu Y, Zhao H, Sang X. N6-methyladenosine associated prognostic model in hepatocellular carcinoma. Annals of Translational Medicine. 2020a;8:633. doi: 10.21037/atm-20-2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang et al. (2020b).Huang H, Wang Y, Kandpal M, Zhao G, Cardenas H, Ji Y, Chaparala A, Tanner EJ, Chen J, Davuluri RV, Matei D. FTO-Dependent N (6)-Methyladenosine modifications inhibit ovarian cancer stem cell self-renewal by blocking cAMP signaling. Cancer Research. 2020b;80:3200–3214. doi: 10.1158/0008-5472.CAN-19-4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia, Liu & Shan (2020).Jia Y, Liu L, Shan B. Future of immune checkpoint inhibitors: focus on tumor immune microenvironment. Annals of Translational Medicine. 2020;8:1095. doi: 10.21037/atm-20-3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang et al. (2018).Jiang T, Xu X, Qiao M, Li X, Zhao C, Zhou F, Gao G, Wu F, Chen X, Su C, Ren S, Zhai C, Zhou C. Comprehensive evaluation of NT5E/CD73 expression and its prognostic significance in distinct types of cancers. BMC Cancer. 2018;18:267. doi: 10.1186/s12885-018-4073-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss et al. (2019).Kiss T, Giles CB, Tarantini S, Yabluchanskiy A, Balasubramanian P, Gautam T, Csipo T, Nyul-Toth A, Lipecz A, Szabo C, Farkas E, Wren JD, Csiszar A, Ungvari Z. Nicotinamide mononucleotide (NMN) supplementation promotes anti-aging miRNA expression profile in the aorta of aged mice, predicting epigenetic rejuvenation and anti-atherogenic effects. Geroscience. 2019;41:419–439. doi: 10.1007/s11357-019-00095-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy et al. (2019).Levy A, Alhazzani K, Dondapati P, Alaseem A, Cheema K, Thallapureddy K, Kaur P, Alobid S, Rathinavelu A. Focal adhesion kinase in ovarian cancer: a potential therapeutic target for platinum and taxane-resistant tumors. Current Cancer Drug Targets. 2019;19:179–188. doi: 10.2174/1568009618666180706165222. [DOI] [PubMed] [Google Scholar]
- Lin & Chuang (2012).Lin ZY, Chuang WL. Genes responsible for the characteristics of primary cultured invasive phenotype hepatocellular carcinoma cells. Biomedicine & Pharmacotherapy. 2012;66:454–458. doi: 10.1016/j.biopha.2012.04.001. [DOI] [PubMed] [Google Scholar]
- Liu et al. (2014).Liu J, Li H, Cao S, Zhang X, Yu J, Qi J, An X, Yu W, Ren X, Hao X. Maintenance therapy with autologous cytokine-induced killer cells in patients with advanced epithelial ovarian cancer after first-line treatment. Journal of Immunotherapy. 2014;37:115–122. doi: 10.1097/CJI.0000000000000021. [DOI] [PubMed] [Google Scholar]
- Luo et al. (2021).Luo X, Li H, Liang J, Zhao Q, Xie Y, Ren J, Zuo Z. RMVar: an updated database of functional variants involved in RNA modifications. Nucleic Acids Research. 2021;49:D1405–D1412. doi: 10.1093/nar/gkaa811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffei et al. (2013).Maffei R, Bulgarelli J, Fiorcari S, Bertoncelli L, Martinelli S, Guarnotta C, Castelli I, Deaglio S, Debbia G, De Biasi S, Bonacorsi G, Zucchini P, Narni F, Tripodo C, Luppi M, Cossarizza A, Marasca R. The monocytic population in chronic lymphocytic leukemia shows altered composition and deregulation of genes involved in phagocytosis and inflammation. Haematologica. 2013;98:1115–1123. doi: 10.3324/haematol.2012.073080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado & Dharmawardhane (2018).Maldonado MDM, Dharmawardhane S. Targeting Rac and Cdc42 GTPases in cancer. Cancer Research. 2018;78:3101–3111. doi: 10.1158/0008-5472.CAN-18-0619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno et al. (2009).Mizuno H, Kitada K, Nakai K, Sarai A. PrognoScan: a new database for meta-analysis of the prognostic value of genes. BMC Medical Genomics. 2009;2:18. doi: 10.1186/1755-8794-2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy, Munkacsy & Gyorffy (2021).Nagy A, Munkacsy G, Gyorffy B. Pancancer survival analysis of cancer hallmark genes. Scientific Reports. 2021;11:6047. doi: 10.1038/s41598-021-84787-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandelides et al. (2020).Pandelides Z, Thornton C, Faruque AS, Whitehead AP, Willett KL, Ashpole NM. Developmental exposure to cannabidiol (CBD) alters longevity and health span of zebrafish (Danio rerio) Geroscience. 2020;42:785–800. doi: 10.1007/s11357-020-00182-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujade-Lauraine (2017).Pujade-Lauraine E. New treatments in ovarian cancer. Annals of Oncology. 2017;28:viii57–viii60. doi: 10.1093/annonc/mdx442. [DOI] [PubMed] [Google Scholar]
- Rhodes et al. (2007).Rhodes DR, Kalyana-Sundaram S, Mahavisno V, Varambally R, Yu J, Briggs BB, Barrette TR, Anstet MJ, Kincead-Beal C, Kulkarni P, Varambally S, Ghosh D, Chinnaiyan AM. Oncomine 3.0: genes, pathways, and networks in a collection of 18, 000 cancer gene expression profiles. Neoplasia. 2007;9:166–180. doi: 10.1593/neo.07112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ru et al. (2019).Ru B, Wong CN, Tong Y, Zhong JY, Zhong SSW, Wu WC, Chu KC, Wong CY, Lau CY, Chen I, Chan NW, Zhang J. TISIDB: an integrated repository portal for tumor-immune system interactions. Bioinformatics. 2019;35:4200–4202. doi: 10.1093/bioinformatics/btz210. [DOI] [PubMed] [Google Scholar]
- Sarivalasis et al. (2021).Sarivalasis A, Morotti M, Mulvey A, Imbimbo M, Coukos G. Cell therapies in ovarian cancer. Therapeutic Advances in Medical Oncology. 2021;13:17588359211008399. doi: 10.1177/17588359211008399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung et al. (2021).Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- Torre et al. (2018).Torre LA, Trabert B, De Santis CE, Miller KD, Samimi G, Runowicz CD, Gaudet MM, Jemal A, Siegel RL. Ovarian cancer statistics, 2018. CA: A Cancer Journal for Clinicians. 2018;68:284–296. doi: 10.3322/caac.21456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turinetto et al. (2021).Turinetto M, Scotto G, Tuninetti V, Giannone G, Valabrega G. The role of PARP inhibitors in the ovarian cancer microenvironment: moving forward from synthetic lethality. Frontiers in Oncology. 2021;11:689829. doi: 10.3389/fonc.2021.689829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasaikar et al. (2018).Vasaikar SV, Straub P, Wang J, Zhang B. LinkedOmics: analyzing multi-omics data within and across 32 cancer types. Nucleic Acids Research. 2018;46:D956–D963. doi: 10.1093/nar/gkx1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al. (2020).Wang J, Zhang T, Yang L, Yang G. Comprehensive genomic analysis of microenvironment phenotypes in ovarian cancer. PeerJ. 2020;8:e10255. doi: 10.7717/peerj.10255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei et al. (2020).Wei CJ, Gu YH, Wang W, Ren JY, Cui XW, Lian X, Liu J, Wang HJ, Gu B, Li QF, Wang ZC. A narrative review of the role of fibroblasts in the growth and development of neurogenic tumors. Annals of Translational Medicine. 2020;8:1462. doi: 10.21037/atm-20-3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu et al. (2021).Xu F, Li J, Ni M, Cheng J, Zhao H, Wang S, Zhou X, Wu X. FBW7 suppresses ovarian cancer development by targeting the N(6)-methyladenosine binding protein YTHDF2. Molecular Cancer. 2021;20:45. doi: 10.1186/s12943-021-01340-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu et al. (2020a).Xu Z, Peng B, Cai Y, Wu G, Huang J, Gao M, Guo G, Zeng S, Gong Z, Yan Y. N6-methyladenosine RNA modification in cancer therapeutic resistance: Current status and perspectives. Biochemical Pharmacology. 2020a;182:114258. doi: 10.1016/j.bcp.2020.114258. [DOI] [PubMed] [Google Scholar]
- Xu et al. (2020b).Xu Z, Zeng S, Gong Z, Yan Y. Exosome-based immunotherapy: a promising approach for cancer treatment. Molecular Cancer. 2020b;19:160. doi: 10.1186/s12943-020-01278-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang et al. (2020).Yang W, Yang S, Zhang F, Cheng F, Wang X, Rao J. Influence of the Hippo-YAP signalling pathway on tumor associated macrophages (TAMs) and its implications on cancer immunosuppressive microenvironment. Annals of Translational Medicine. 2020;8:399. doi: 10.21037/atm.2020.02.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu et al. (2012).Yu FX, Zhao B, Panupinthu N, Jewell JL, Lian I, Wang LH, Zhao J, Yuan H, Tumaneng K, Li H, Fu XD, Mills GB, Guan KL. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell. 2012;150:780–791. doi: 10.1016/j.cell.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang et al. (2020).Zhang X, Wang F, Wang Z, Yang X, Yu H, Si S, Lu J, Zhou Z, Lu Q, Wang Z, Yang H. ALKBH5 promotes the proliferation of renal cell carcinoma by regulating AURKB expression in an m(6)A-dependent manner. Annals of Translational Medicine. 2020;8:646. doi: 10.21037/atm-20-3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Heatmap indicated the correlation between CDC42EP3 expression and immune cells analyzed by TISIDB. (B) The cross-validated correlation between CDC42EP3 expression and several immune cells, such as NK, Tcm and Tgd cells.
(A) The correlation between CDC42EP3 expression and multiple of immunostimulators. (B) The top four immunostimulators highly correlated with CDC42EP3 expression. (C) The correlation between CDC42EP3 expression and multiple of immunoinhibitors. (D) The top four immunoinhibitors highly correlated with CDC42EP3 expression.
(A) The correlation between CDC42EP3 expression and chemokines. (B) The significant associations between CDC42EP3 expression and CCL14. (C) The correlation between CDC42EP3 expression and chemokine receptors. (D) The significant associations between CDC42EP3 expression and CXCR1.
Western blot confirmed the down-regulation of CDC42EP3 expression in ovarian cancer cells A2780 and TOV112D.
qPCR confirmed the down-regulation of CDC42EP3 expression in ovarian cancer cells A2780 and TOV112D.
Data Availability Statement
The following information was supplied regarding data availability:
The raw measurements are available in the Supplementary Files.