Abstract
Varroa destructor is among the greatest biological threats to western honey bee (Apis mellifera L.) health worldwide. Beekeepers routinely use chemical treatments to control this parasite, though overuse and mismanagement of these treatments have led to widespread resistance in Varroa populations. Integrated Pest Management (IPM) is an ecologically based, sustainable approach to pest management that relies on a combination of control tactics that minimize environmental impacts. Herein, we provide an in-depth review of the components of IPM in a Varroa control context. These include determining economic thresholds for the mite, identification of and monitoring for Varroa, prevention strategies, and risk conscious treatments. Furthermore, we provide a detailed review of cultural, mechanical, biological, and chemical control strategies, both longstanding and emerging, used against Varroa globally. For each control type, we describe all available treatments, their efficacies against Varroa as described in the primary scientific literature, and the obstacles to their adoption. Unfortunately, reliable IPM protocols do not exist for Varroa due to the complex biology of the mite and strong reliance on chemical control by beekeepers. To encourage beekeeper adoption, a successful IPM approach to Varroa control in managed colonies must be an improvement over conventional control methods and include cost-effective treatments that can be employed readily by beekeepers. It is our intention to provide the most thorough review of Varroa control options available, ultimately framing our discussion within the context of IPM. We hope this article is a call-to-arms against the most damaging pest managed honey bee colonies face worldwide.
Keywords: honey bee, Varroa destructor, integrated pest management, Apis mellifera, control
Varroa destructor (Anderson & Trueman) is considered by many honey bee researchers as one of the most significant pests of western honey bee (Apis mellifera L.) colonies globally (Carreck et al. 2010, Guzman-Novoa et al. 2010, Le Conte et al. 2010, McMenamin and Genersch 2015). It has had a devastating impact on apiculture since its spread from its natural honey bee host, the eastern or Asian honey bee (Apis cerana (Hymenoptera: Apidae)), to the western honey bee (hereafter called honey bee). Varroa plays a major role in the colony losses observed worldwide (van der Zee et al. 2015, Kulhanek et al. 2017, Beyer et al. 2018, Brown et al. 2018, Brodschneider et al. 2019). With a nearly global distribution (Ellis and Munn 2005, Rosenkranz et al. 2010, Iwasaki et al. 2015, Boncristiani et al. 2021), this parasitic mite will severely weaken or cause the collapse of most honey bee colonies if left untreated (Boecking and Genersch et al. 2008, Thompson et al. 2014, Frey and Rosenkranz 2014).
Collaborative efforts from insect pathologists, acarologists, and apiculturists have yet to yield long-term solutions for Varroa control. Thus, the continuous development of new and innovative control methods for Varroa should remain a priority among honey bee researchers and funding agencies (Dietemann et al. 2012). However, a single control strategy is unlikely to provide a permanent solution to Varroa control. Despite this, beekeepers heavily rely on one primary method to control the mite in most managed honey bee colonies: chemical control (Haber et al. 2019). Consequently, there is a need to review research that supports a combination of multiple strategies available for Varroa control.
Integrated Pest Management (IPM) is an ecologically based, sustainable approach to pest management. It relies on a combination of control tactics and minimizes the impact that controlling a given pest has on the environment (Frisbee and Luna 1989). An effective IPM program consists of identifying economic thresholds, monitoring the pest population, performing a suite of preventative techniques, and applying a step-by-step treatment plan depending on need (Flint 2012). Unfortunately, there has largely been a failure by many beekeepers to adopt IPM principles in their Varroa management programs, primarily due to gaps in knowledge and deficiencies in training (Whitehead 2017). Herein, we discuss the core principles of IPM, how they relate to Varroa management, current Varroa control options, and offer perspectives on sustainable solutions. While other recent reviews on Varroa biology and control offer discussions on various Varroa control strategies (Rosenkranz et al. 2010, Gregorc and Sampson 2019, Noël et al. 2020, Roth et al. 2020), we aim to provide a single, comprehensive review of Varroa control within an IPM framework.
Determining Thresholds
IPM is based on the premise that certain levels of pests and injury are tolerable and do not require eradication (Ostlie and Pedigo 1987). As such, establishing thresholds for the point at which the pest density will cause economic damage and the pest density at which control measures should be applied is really the cornerstone of IPM (Higley and Peterson 2009). These thresholds are indispensable as they direct the course of action to be taken in any management situation.
The first step in IPM is to quantify the pest density that will justify the cost of applying control measures. The economic injury level (EIL) is defined as the lowest population density that will cause economic damage (Stern et al. 1959). The EIL is a simple cost–benefit equation, where the costs associated with management of the pest are balanced with the benefit of preventing losses due to management (Pedigo et al. 1986). The simplest equation used to calculate the EIL is:
where C = cost of management per production unit (example: $/ha), V = market value per unit of produce (example: $/bushel), I = injury units per pest per production unit (example: percent defoliation/insect/acre, expressed as a proportion), D = damage per unit injury (example: bushels lost/ha/injury unit) (Pedigo et al. 1986).
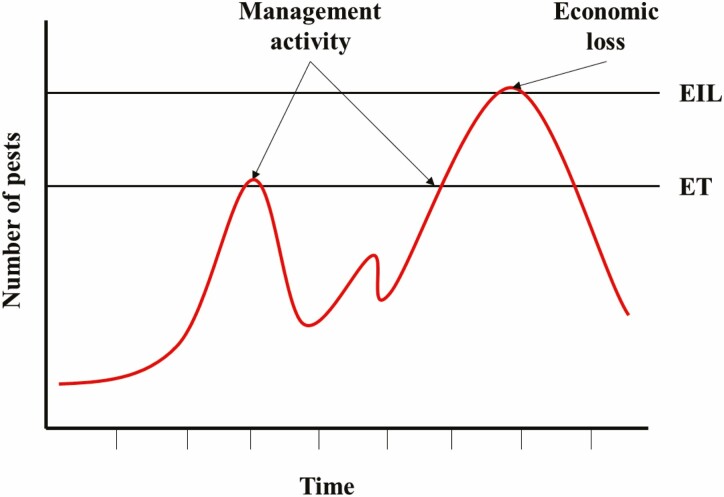
The economic threshold (ET) is the number of pests at which control measures should be initiated in order to avoid reaching the EIL (Stern et al. 1959), sometimes referred to as the action threshold. The ET is a time parameter, with pest numbers used as an index for when to implement management (Pedigo and Rice 2009). Generally, there are no formulas used to quantify ETs because of the variabilities among different management actions (Pedigo et al. 1986). The ET is always set at a lower value than the EIL because the pest population will continue to grow until treatment. It is, therefore, imperative to act as soon as the pest populations reach the ET to reduce populations before they can reach the EIL (Fig. 1). No action is taken at levels below the ET.
Fig. 1.
Graph demonstrating the relationship between the economic threshold (ET) and the economic injury level (EIL). The pest population crosses the ET twice (noted by two arrows). Here, management activity is necessary to prevent the pest population from reaching the EIL. When the EIL is reached (right-most arrow), the colony’s health/productivity decreases to the point that the beekeeper experiences an economic loss.
Challenges Associated with Determining Varroa Thresholds
To determine a Varroa-specific EIL, beekeepers must be able to identify the variables in the given formula specific to their Varroa management situation. The cost of management/hive (C = $/colony) and the market value per unit of produce (V = $/kg of honey, or $/loss of pollination, etc.) are relatively easy to quantify. However, some variables are more difficult to quantify due to the complex nature of honey bee colonies and the lack of information regarding Varroa’s effect on the overall colony. For example, the injury caused per pest, per production unit is hard to quantify. Varroa are primarily perceived as a threat to honey bee colonies due to the risk of transferring viruses (Martin et al. 2012); therefore, quantifying injury (I) in terms of percent of bees with a virus per Varroa per colony is difficult to calculate. According to our knowledge, this has not been determined in honey bee colonies. One may be able to calculate the costs of colony death, including the cost of replacement, opportunity costs from unfulfilled pollination contracts, or unrealized honey production. Still, for the purposes of creating an EIL equation, one cannot include a variable that deals in absolutes such as “alive” or “dead”. Furthermore, without understanding the unit of injury, quantifying the damage (D) per unit injury is impossible. For example, a beekeeper might be able to estimate the loss in kg of honey per colony due to a high infestation (Emsen et al. 2014), reduced pollination efficacy, or reduced ability to make splits, but not at an individual injury unit which is required for an accurate EIL calculation.
Without a clear EIL for Varroa management, it is also difficult to determine a true ET. Several researchers have proposed ETs for Varroa management (Delaplane and Hood 1997, 1999; Strange and Sheppard 2001, Currie and Gatien 2006), but none are based on an EIL calculation. To complicate matters further, treatment efficacies for Varroa management vary by season and location (Currie and Gatien 2006, Gracia et al. 2017). Apiary-level factors, such as the density of honey bee colonies and available forage in the area, can affect a colony’s mite load (Seeley and Smith 2015, Smart et al. 2016). These all play an important role in establishing ETs. Thus, it is necessary that beekeepers determine individual thresholds relevant to their location, management preferences, and management goals.
Previously Derived Varroa Thresholds
Within the U.S., ETs for Varroa have been derived for the southeast region (Georgia and South Carolina) and northwest region (Washington State). Thresholds for both regions were based on 300-bee ether rolls. Delaplane and Hood (1999) reported that early season (February) and late season (August) thresholds were 0.13–0.93 mites/100 bees and 5–12.67 mites/100 bees, respectively. In the northwest, Strange and Sheppard (2001) reported an early season (April) threshold of 3 mites/100 bees, a summer season (August) threshold of 14 mites/100 bees, and a late season (October) threshold of 3 mites/100 bees. In the prairie region of Canada, treatment thresholds were established using mites/100 bees determined from alcohol washes. Currie and Gatien (2006) reported the ETs for Varroa treatment as 2 mites/100 bees in the spring (April) and 4 mites/100 bees in the late season (September).
A thorough search of the literature revealed that ETs are not commonly reported outside of North America. Le Conte et al. (2010) mentioned in their review that beekeepers in Germany are required to treat if their natural mite drop exceeds 10 mites/24 h, but there is no reference to the literature source of this threshold. Likewise, other groups report ~3 mites/100 bees as the ET, though they do not cite their sources (Honey Bee Health Coalition 2018). Nevertheless, it appears that ~2–5 mites/100 bees is a generally accepted ET for Varroa as it is often taught to beekeepers (Goodwin and Van Eaton 2001, Honey Bee Health Coalition 2018, Ontario Ministry of Agriculture, Food and Rural Affairs 2020), though there is a surprising lack of research data to support this number. Establishing ETs for Varroa management has been previously identified as an avenue of critical research needed for appropriate control of the mite (Dietemann et al. 2012). We further emphasize it here given that a successful IPM strategy is built on the back of knowing accurate and actionable EILs and ETs.
Identification and Monitoring
Accurate identification of the pest is a crucial component of IPM, as misidentification can lead to needless treatment, wasting of resources, and potential harm to the agricultural system. Although Varroa infestations are widespread (Ellis and Munns 2005, Boncristiani et al. 2021), proper diagnosis of Varroa in a colony is crucial before making any management decisions.
There are two main things beekeepers may want to know about Varroa: (1) their presence/absence and (2) some sort of estimate of Varroa populations. The standard methods for these are presented in the BEEBOOK (Dietemann et al. 2013). However, we expand on their discussion here.
Identifying Varroa
Physical Characteristics of Varroa destructor
Beekeepers are most likely to see the adult female mites, as they are visible on the bodies of adult bees (Infantidis, 1983). Other review articles describe Varroa anatomy and morphology in much greater detail than we will do here (Dillier et al. 2006, Rosenkranz et al. 2010). Nevertheless, we note key physical characteristics useful for beekeepers to identify the pest as Varroa correctly. While this may seem unnecessary, there is at least one other honey bee commensal that can be mistaken for Varroa, the adult wingless fly Braula coeca (Kulincevic et al. 1991).
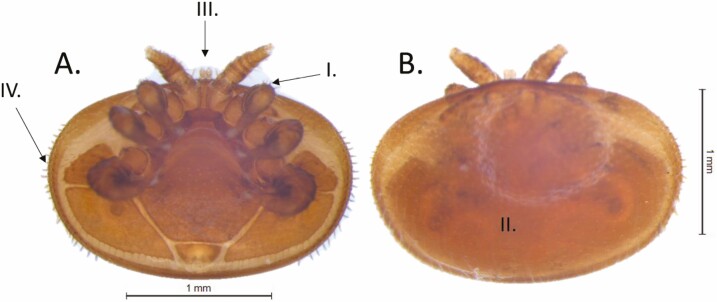
Adult female Varroa are reddish-brown to dark brown in color and shaped like an oval (Fig. 2). They are typically ~1.1 mm long and 1.6 mm wide (Anderson and Trueman 2000) and are visible with the naked eye. As Varroa are arachnids and not insects, they have eight legs (Fig. 2A). They have a large dorsal shield (Fig. 2B), an anterior region called the gnathosoma (Fig. 2A-III), which contains the mouth, and their bodies are almost entirely covered in setae (Fig. 2A-IV).
Fig. 2.
Varroa destructor anatomy: A. Varroa ventral view; B. Varroa dorsal view; (I). Legs, (II). Dorsal shield, (III). Gnathosoma, (IV). Setae. Photo credit: N. Noble, University of Florida.
Honey Bee Brood Examination
Varroa reproduction occurs entirely within the capped cells containing honey bee brood (Ifantidis 1983, Boot et al. 1994, Donze and Guerin 1994, 1997, Martin 1994). In fact, ~>70% of Varroa in a colony are present in capped cells while brood is abundant in the colony (Boot et al. 1995, Frey and Rosenkranz 2014). Varroa demonstrate a preference for drone brood over worker brood (Fuchs 1990, Boot et al. 1995) due to longer periods of time prior to sealing (Ifantidis 1988, Boot et al. 1992), more frequent tending by nurse bees (Calderone and Kuenen 2003) and longer developmental time (Boot et al. 1995) for drones, thus allowing mites more time to reproduce. Therefore, examining drone brood will increase the probability of detecting Varroa within colonies (Dietemann et al. 2013). That said, Varroa also are found within worker brood cells and can be easily detected when Varroa are present in moderate-to-high levels. Hence, brood cells provide a good location to detect Varroa.
One can confirm the presence of the mites on the brood or within the cell by opening the cells and removing the honey bee brood contained within. One method is to flush the honey bee pupae out of their cells with a stream of warm water over a sieve to observe the mites contained within the cells (Dietemann et al. 2013). Once the pupae are removed from the cells, the feces of the mites may also be visible along the cell walls.
Adult Honey Bee Examination
Mature female Varroa also can be detected on adult honey bees (Delfinado-Baker et al. 1992, Kuenen and Calderone 1997, Dietemann et al. 2013). Though one can see Varroa on adult bees with the naked eye, they are difficult to spot on moving bees, especially given their preference for feeding on the underside of the bee’s abdomen (Ramsey et al. 2019). It is best, then, if Varroa are dislodged from adult bees for visualization and quantification purposes.
Debris Examination
Debris from hives equipped with a screened bottom board can be examined for the presence of Varroa (Rosenkranz et al. 1997, Webster et al. 2000, Branco et al. 2006). Bees may groom Varroa from their bodies or the Varroa may naturally fall from the comb and through the screened bottom board (Arechavaleta-Velasco and Guzman-Novoa 2001, Harbo and Harris 2004). Consequently, a sticky board (a thin piece of cardboard or plastic coated with a sticky substance such as vegetable oil, petroleum jelly, or Tanglefoot) can be placed beneath the hive to catch the falling mites and used to quantify mite population, as dead mites can be visualized on the boards (Ostiguy and Sammataro 2000, Calderone and Lin 2003). Similarly, screen-covered sticky boards can be placed in entrances of hives equipped with solid bottom boards. The screen prevents bees from getting stuck on the board.
Quantifying Varroa Populations
Frequent monitoring of the pest population is a crucial part of IPM (Moon and Wilson 2009). In order to make an educated control decision, it is necessary to know the current status of the Varroa population and compare it with the ET. There are many different diagnostic methods that have been used to estimate Varroa populations (Branco et al. 2006, Lee et al. 2010, Flores et al. 2015). However, Varroa populations in honey bee colonies are generally estimated two ways: 1) counting the number of mites on a subsample of adult bees and converting that to a mites/adult bee ratio (usually, a mites/100 adult bees ratio or “infestation rate”), and 2) counting the number of mites that fall naturally to the bottom of a hive where they are collected on a sticky board, using this information to estimate the entire population of mites in a colony.
The mites/adult bee ratio typically is the preferred method, and that is most used by beekeepers, because it gives an index of mite population regardless of the size of the colony. While estimating entire mite populations using sticky boards is useful, especially for scientific purposes, its practical application is limited given you can only use it to estimate the actual number of mites in a colony (see Natural Mite Fall below).
Dislodging Mites from Adult Bees
Multiple strategies can be used to determine the mites/adult bee ratio, all of which require dislodging mites from adult bees. Dietemann et al. (2013) reviewed four different substances that are used frequently to dislodge mites from adult bees: powdered sugar, ether, soapy water, and ethanol. A 2015 study demonstrated that ethanol was more effective at dislodging mites from adult bees than was powdered sugar (Flores et al. 2015); however, the advantage of powdered sugar is that it is non-lethal to bees. Many researchers recommend collecting about 300 adult bees (without the queen) from brood comb samples (Delaplane 1997, Strange and Sheppard 2001, Lee et al. 2010, Dietemann et al. 2013). If more precision is needed, one can take three samples of 300 bees (900 total) and average the counts (Lee et al. 2010), though caution should be taken on over-collecting from weak colonies. By sampling at least eight colonies within an apiary, beekeepers can have an accurate estimate of the average Varroa infestation rate within that apiary (Lee et al. 2010). However, the more colonies sampled per apiary, the more accurate the estimate.
When using alcohol or soapy water to dislodge the mites, fill the jar containing the adult bees with either substance until ½–¾ of the jar is full. Put the lid on the container and shake vigorously for 30 s. One can then dump the contents of the jar through a screened mesh or into a white container to count the mites. Alcohol and soapy water kill the adult bees, but this method allows you to count them to calculate an accurate mite/100 bee ratio. Most beekeepers, though, simply estimate a volume of adult bees that is ~300 bees when collecting them into a jar, without counting them directly. This results in less accurate mite/adult bee ratios but is quicker to do in the field.
When using powdered sugar to dislodge mites from adult bees, place about two tablespoons of powdered sugar (~20g) into a jar of ~300 live bees. Place a lid made of screen mesh on the container and gently shake/roll the jar horizontally so that the powdered sugar is applied evenly to all the bees in the sample. Place the jar on a hard surface, in the shade, for 2 min to allow the mites time to become dislodged from the bees. Hold the jar upside-down and shake lightly over a white tray for 1 min. Count the mites and record the number of mites collected. The mite infestation rate can be determined by dividing the number of mites captured by the estimated number of bees in the sample and multiplying by 100. For example, if you shake out 15 mites from a jar containing ~300 bees, the infestation rate would equal the number of mites (15) divided by the number of bees in the sample (~300) multiplied by 100. The result in this example is ~5 mites/100 bees or a 5% infestation rate.
Natural Mite Fall
Honey bees clean themselves (autogroom) or one another (allogroom) of dust, debris, pollen, and even mites. This behavior involves brushing movements of the legs and biting Varroa with their mandibles (Boecking and Spivak 1999, Andino and Hunt 2011). Varroa may either be groomed off by the bees or naturally fall from the bees or combs through the action of normal hive activity. Consequently, one can sample Varroa by collecting them from below the hive, usually on a sticky board (Fries et al. 1991).
The assessment of natural mite fall from a colony is considered to be an effective method in determining whole colony mite populations (Fries et al. 1991, Harbo and Harris 2004, Branco et al. 2006, Flores et al. 2015). This non-invasive and non-destructive method is commonly used for long-term surveys and for testing the efficacy of treatments used in Varroa control. However, the standardization of the mite fall method when comparing different colonies is somewhat questionable as mite fall is largely determined by the amount of emerging brood within a colony (Dietemann et al. 2013). Unless you know your honey bee colony population, you should be cautious about making treatment decisions based on mite fall. In most cases, beekeepers should make treatment decisions based on the infestation rate (mites/100 adult bees), rather than the entire mite population.
When measuring natural mite fall, place a sticky board underneath a hive equipped with a screened bottom board or adhere the sheet to the underside of a screen, sliding the entire structure, sticky side up, into the entrance of a hive. Remove the sticky board from the hive after 72 h, which ensures a more robust sampling period (Jack et al. 2020a), and count the total number of mites found on the board. The mite population within a colony can be estimated using the formula by substituting the total number of mites captured on a sticky board for in the equation, solving for and dividing by the number of days the sticky board was in the hive (K. Delaplane, personal communication; Jack et al. 2019). For example, if you captured 100 mites on your sticky board after 72 h, the total colony mite population (x) equals 3,208 mites in a colony (3.76 − 100 = –96.24; –96.24/0.01 mites = 9,624; 9,624/# of days in the hive (3) = 3,208).
Delaplane and Hood (1999) described a late season economic threshold for an overnight (20 ± 4 h) mite fall for their location in the southeastern U.S. as 59–187 mites for a mid-sized colony (one deep brood box and one medium super). While this threshold may not be appropriate for all locations and seasons, it can be used as an example of an ET for a colony of “average” strength.
Varroa population estimates can be misleading because an estimate of colony strength is necessary to know if the population estimate determined by mite fall is harmful to the bees (Dietemann et al. 2013). For example, your screen counts may suggest that you have 3,000 mites in the colony. This would be extremely detrimental to a colony of 10,000 bees, but less so to one with 50,000 bees. Thus, making treatment decisions based on the mite infestation rate is more favorable. However, sticky boards used to monitor mite fall provide some information and many beekeepers prefer to monitor Varroa levels this way.
There are other important considerations when using natural mite fall to monitor Varroa populations within a colony. With this method, the fallen mites can be removed from the sticky board by ants or bees, walk off the board (if the board is not sticky enough), etc. Thus, it is necessary to take precautions to limit mite removal from the combs (Dietemann et al. 2013). Furthermore, this sampling method requires multiple visits to be made to the hive (insert screens and remove screens) and additional time to count the mites on the screen. Thus, sticky boards are unlikely to be used by commercial or large-scale beekeepers unless subsamples of the entire apiary are taken. Lee et al. (2010) demonstrated that sampling eight colonies per apiary is enough to give you an accurate estimate of the average Varroa loads within an apiary using methods to dislodge mites from bees; however, apiary-level estimates have not yet been identified using natural mite fall. Stratified sampling procedures can also significantly decrease the time of analysis without sacrificing the accuracy (Ostiguy and Sammataro 2000, Calderone and Lin 2003, Kretzschmar et al. 2015). Sticky boards can be designed with grids and counting pre-designated cells (Ostiguy and Sammataro 2000) or circles (Kretzschmar et al. 2015) within the grids can still give you an accurate estimate of the number of mites falling on the sticky boards.
Dangerous or Ineffective Monitoring Methods
Visual observations of the mites are ineffective. Varroa are difficult to see given they are often hidden underneath the sclerites of honey bees (Ramsey et al. 2019). Instead of monitoring Varroa, some beekeepers choose to look for signs of infestation caused by the mite. However, common signs of infestation, such as spotty brood patterns, are not solely due to Varroa infestation (Boecking and Spivak 1999, Tarpy and Page 2002) and should not be the primary metric used to determine treatment. Additionally, some beekeepers choose to observe the infestation rates of drone brood as they remove them from the hive (Wilkinson and Smith 2002). While robust sampling of capped cells from a brood frame could be informative as an infestation rate, drone brood production is seasonal (Charriere et al. 2003, Branco et al. 2006). Thus, sampling only drone brood would not be effective for most of the year and this method lacks any kind of standardization.
For several decades, ether rolls were used as a common monitoring method. This method is performed similarly to other methods used to dislodge mites from the bodies of the bees. Briefly, ether is sprayed into a jar containing the sample of bees, killing the bees and the mites. The dying bees regurgitate the nectar or honey from their crops. After rolling the jar for about a minute, dead mites will adhere to the sides of the jar, making it possible to count the mites easily (Dietemann et al. 2013). Unfortunately, this method is environmentally unfriendly and dangerous because of the highly flammable nature of ether. Therefore, it is not recommended to use ether rolls to monitor Varroa populations.
Prevention
One aspect of IPM that is often overlooked is prevention. Prevention involves removing the conditions that attract pests or help them to build their populations (Pedigo 1995). As Varroa occurs throughout much of the world (Boncristiani et al. 2021), complete prevention is nearly impossible. Furthermore, Varroa only feed on honey bees and only reproduce in their brood cells (Donzé and Guerin 1994, Rosenkranz et al. 2010); thus, there currently is no way for beekeepers to remove the conditions that attract Varroa. While some beekeepers’ primary goal is to prevent the arrival of Varroa in their area, beekeepers should employ preventative practices to keep Varroa populations from spreading to different areas. Some preventative actions might include reducing drifting and robbing within apiaries, practicing effective swarm control, and regulating the movement of bees between areas.
Preventing the Spread of Varroa
Varroa can spread from colony to colony by a number of mechanisms, some due to the nature of honey bee biology, but others due to the nature of beekeeping. Mites can spread indirectly by moving to a neutral location, such as a flower, then to a new honey bee, and then onto a new colony (Peck et al. 2016). Nevertheless, this mechanism is unlikely to lead to significant dispersal of mites between colonies (Peck and Seeley 2019). Instead, it is more likely that Varroa transmission occurs directly when a honey bee carrying a mite moves from one nest to another through drifting or robbing (Frey et al. 2011). Drifting is when a honey bee leaves its hive and enters into a different colony’s hive. Robbing is when a honey bee enters another colony’s hive to steal honey or nectar and then returns to her own hive. Peck and Seeley (2019) demonstrated that robbing was more important for Varroa transmission than was drifting, given weak, collapsing colonies are robbed by neighboring bee colonies. However, they did observe drifting, especially from drones, which can carry Varroa when flying (Mortensen et al. 2018). Thus, beekeepers ideally should manage colonies so they remain strong (less prone to robbing) and space colonies >300 m within an apiary to prevent the horizontal transmission of Varroa from one colony to another by robbing or drifting (Seeley and Smith 2015, Nolan and Delaplane 2017, Peck and Seeley 2019). Nevertheless, spacing colonies at this distance is not practical for most beekeepers. Painting hives with unique colors and/or patterns can aid in the reduction of drift (Dynes et al. 2019).
Vertical transmission of Varroa is possible as colonies reproduce via swarming, with the swarming bees carrying mites to the new nest site (Wilde et al. 2005). In fact, Wilde et al. (2005) found that about 25% of a colony’s mite population will leave with a swarm, leaving the other 75% of the mites with the parent colony. As untreated colonies are unlikely to stay healthy for long (Frey and Rosenkranz 2014), they pose a risk to nearby (within 1.5 km; Frey et al. 2011) treated/managed colonies (Frey et al. 2011). Thus, effective swarm control should be practiced to prevent the vertical transmission of Varroa from a parent colony to a newly established one (Fries and Camazine 2001).
Role of Government Regulations
As with most pests or diseases, Varroa is much more difficult to eradicate than to prevent from arriving. Regulatory control is often practiced by government agencies to prevent the entry or spread of pests into an area. Typical efforts include inspection, quarantine, and destruction of infested materials (USDA APHIS 2020, BeeAware 2021). This is of critical importance for beekeepers located near seaports or airports as pests and diseases are most likely to invade a new area through these ports of entry. Therefore, intensive monitoring, sanitation, and training are required for beekeepers to protect the welfare of honey bee colonies in their specific regions.
Varroa-Free Locations
Despite the general, widespread occurrence of Varroa globally, there are areas where Varroa do not yet occur (Boncristiani et al. 2021). These include many islands/island nations, Australia, and some remote areas. These areas are beneficial for the fight against Varroa for two primary reasons. First, beekeepers in Varroa-free areas can enact strict regulatory requirements to limit Varroa movement to the area, i.e., prevent their occurrence. Second, and perhaps more importantly, they can serve as a source for Varroa-free bees for those wishing to acquire colonies that do not yet have mites. This was the case when Australia exported packages of bees to the U.S. during the 1990s (Manning 1996). However, there are potential drawbacks associated with using bees from areas where Varroa do not occur. Most notably, the bees cannot be expected to have developed any level of tolerance to the mite, likely making them highly susceptible to mite pressures should they ever encounter Varroa. Nevertheless, acquiring Varroa-less bees and then managing them to prevent infestation remains possible in some areas globally.
Prevention vs. Management
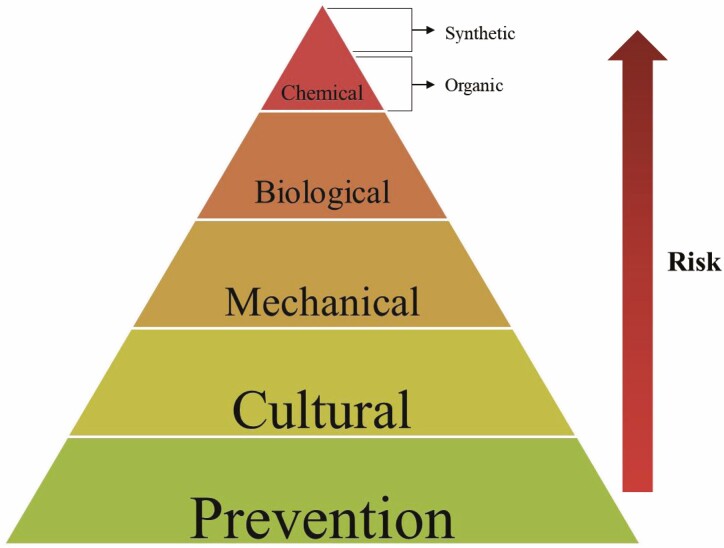
Prevention refers to the measures employed to prevent the arrival of pests into/signs of infestations in an area. This is especially important for destructive pests or those that are the most difficult to control. Management refers to control measures employed after the pest or signs of infestation are detected. Management includes cultural, mechanical, biological, and chemical control (Fig. 3). As Varroa is already present in many areas globally, the greatest focus now must be on its management rather than its prevention. We present a summary of the efficacies of all Varroa treatment strategies in Table 1.
Fig. 3.
IPM Treatment Pyramid. Beekeepers should employ non-chemical or low-risk control methods at the bottom of the pyramid first and move up the pyramid to chemical or high-risk methods as the situation requires.
Table 1.
Efficacy of common treatments used to control Varroa destructor in Apis mellifera colonies
Treatment efficacy may not be the same in all regions of the world; however, the three categories of effectiveness were established by pooling the results found in the literature review. A low rating indicates that literature reported efficacy of a given strategy/control ranges between 0 and 24% efficacy. A moderate rating indicates literature reported efficacies between 25 and 75%. A high rating indicates literature reported efficacies between 76 and 100%.
§ Indicates that there is a lack of scientific literature for this treatment and caution should be exercised before use.
*Varroa has demonstrated some level of resistance to the active ingredient.
Cultural Control
The main goal of cultural control is to change the hive environment to make it less suitable for the pest or disease, while minimally affecting the honey bees. In many instances, cultural controls act as preemptive measures, simply to minimize the impact of the pest or disease on the colony. An example of a cultural control would be the use of a hygienic honey bee stock, which is able to remove pest or disease-infested brood from the nest (Boecking and Spivak 1999). Caging the queen to cause a break in the honey bee brood rearing cycle can disrupt Varroa mating biology and improve the efficacy of chemical treatments (Wagnitz and Ellis 2010, Gregorc et al. 2017). Also, sanitary practices used by the beekeeper, such as comb culling or sterilization of hive equipment, would be considered cultural controls.
Breeding for Varroa Resistance
Breeding for Varroa resistant honey bees has been a focal point for researchers and breeders throughout the world (reviews by Büchler et al. 2010, Rinderer et al. 2010, Guichard et al. 2020, Le Conte et al. 2020). Resistance is most often defined as an organism’s ability to limit parasite burden, while tolerance refers to an organism’s ability to limit the damage caused by a given burden (Råberg et al. 2009). Thus, resistance is the correct term to describe honey bees that keep Varroa infestations at a relatively low level (Danka et al. 2013).
There are obvious advantages of breeding Varroa-resistant honey bees; these include reducing the use of in-hive acaricides and reducing the labor involved in mite control efforts. Varroa resistance, however, does not derive from a single trait, but is the result of successful interactions between the mites and honey bees within the hive (Büchler et al. 2010). Unfortunately, the process of creating suitable resistant stock often takes breeders decades. Furthermore, identifying selectable genetic traits is extremely challenging due to the complex interactions between the two species and the mating biology of honey bees. Nevertheless, genetic research and breeding efforts will continue to be major areas of focus as long as Varroa remains a problem for honey bee colonies.
Selectable Traits
Hygienic Behavior.
The selection of hygienic bees has been practiced for decades. Hygienic worker bees have the ability to detect diseased/infested brood, uncover the wax capping covering the cell containing the diseased/infested individual, and remove the diseased/infested larvae or pupae (Boecking and Spivak 1999). Hygienic behavior was first described by Rothenbuhler (1964) who found workers removing brood infected with the bacterial disease known as American foulbrood (Paenibacillus larvae). Since then, many other studies have emerged describing hygienic behavior as a mechanism for resisting chalkbrood (Milne Jr. 1983, Gilliam et al. 1988), a fungal disease of honey bee brood, European foulbrood (Palacio et al. 2000), and, of course, Varroa (Spivak 1996, Spivak and Reuter 1998, Ibrahim et al. 2007). Hygienic behavior is now considered a social immune response of honey bees (reviewed by Evans and Spivak 2010 and Simone-Finstrom 2017).
Hygienic behavior is effective at reducing Varroa populations in a colony because it disrupts the reproductive cycle of the mite, thus prolonging the less damaging time that the mite spends on adult workers (Spivak and Gilliam 1998). Varroa’s natural host, A. cerana, is typically more hygienic than is A. mellifera, which is one of the main reasons that Varroa populations are lower in A. cerana colonies than in A. mellifera ones (Rath 1999, Rosenkranz et al. 2010). However, A. mellifera colonies selected for heightened hygienic expression have demonstrated the ability to maintain lower mite populations than those not selected for the trait (Kefuss 2004, Danka 2012). This trait is also considered moderately heritable with heritability estimates ranging from 0.17 – 0.65 (Harbo and Harris 1999, Boecking et al. 2000, Stanimirović et al. 2008, Pernal et al. 2012). Additionally, the mode of inheritance of hygienic traits is likely due to maternal effects and is not easily reduced by drones from less hygienic colonies (Unger and Guzman-Novoa 2010).
Standardized methods for identifying hygienic behavior are based on the removal of brood by adult bees (described in Büchler et al. 2014, reviewed by Leclercq et al. 2018a, Spivak and Danka 2021). Common methods include killing capped brood using a pin (Spivak and Downey 1998) and using cuticular hydrocarbons of diseased brood to elicit a response (Wagoner et al. 2020). However, the most common identification method involves placing an open cylinder on a section of comb containing sealed pupae and pouring liquid nitrogen into the cylinder, thus freeze-killing the brood (Leclercq et al. 2018a). The freeze-killed brood is returned to the colony, which then uncaps and removes some fraction of the dead brood over a designated period, usually 48 h. A colony is considered hygienic when it removes at least 95% of the dead brood within 48 h (Spivak and Downey 1998), though there is a stronger correlation between the removal of dead bees and disease resistance when the removal of dead bees within 24 h is considered. While freeze-kill brood assays may not predict Varroa-resistance for unselected stocks (Leclercq et al. 2018b), it has been used quite successfully to identify hygienic behavior in “hygienic” stock (Spivak and Rueter 1998, 2001b; Masterman et al. 2001).
Grooming Behavior.
Grooming is an important social behavior of honey bees. Grooming involves brushing movements of the mesothoracic legs over the body and biting Varroa with their mandibles (Boecking and Spivak 1999). This behavior may injure the mites by mutilating their legs or in some cases, crushing the mite in their mandibles (Ruttner and Hänel 1992). Grooming is thought to be an important resistance mechanism towards Varroa for A. cerana and African subspecies of A. mellifera (Peng et al. 1987, Büchler et al. 1992, Moretto et al. 1993, Rath 1999, Frazier et al. 2010). A. cerana is the most efficient groomer, having been observed to remove and damage 73% of the mites placed upon them (Peng et al. 1987). Büchler et al. (1992) observed that A. cerana workers caught 32% of Varroa on their bodies with their mandibles, while A. mellifera workers caught none. Additionally, they observed that A. cerana ultimately removed 75% of mites from their bodies, while A. mellifera only removed 48%. In another study, Aumeier (2001) observed A. m. scutellata remove 18% of Varroa through vigorous autogrooming behavior.
Grooming is heritable, though it is considered to have low heritability, with heritability estimates ranging from 0.16to0.49 (Stanimirović et al. 2010). To test the practical efficacy of grooming behavior, researchers often perform laboratory assays by collecting bees from specific colonies and specific ages, then placing Varroa onto the thoraces of the worker bees to observe their behavioral responses (Peng et al. 1987, Büchler et al. 1992, Boecking and Ritter 1993). Grooming is often measured as the proportion of damaged mites to undamaged ones found on the bottom board (Guzman-Novoa et al. 2012, Morfin et al. 2020, Smith et al. 2021). The process of analyzing the fallen mites within a colony can be time-consuming and somewhat subjective as mite injuries may be caused by other factors such as other insects like ants and wax moths (Szabo and Walker 1995), temperature, and humidity (Currie and Tahmasbi 2008), or physiological issues with mite development (Davis 2009). Furthermore, measuring bee grooming ability by simply analyzing fallen Varroa may be flawed because some mites may fall to the bottom of the nest during the regular house cleaning activities of bees removing mites that died of natural causes (Büchler et al. 1992, Rinderer et al. 2013). Recent studies have focused on finding better ways to quantify grooming behavior in order to improve the efficacy of selective breeding for resistance to Varroa, such as the age of fallen mites (Rinderer et al. 2013), injuries of fallen mites (Rinderer et al. 2014b) or genetic mapping of bees (Arechavaleta-Velasco et al. 2012). Interestingly, the expression of the gene AmNrx-1 (neurexin-1) is significantly higher in honey bee stock selected for intense grooming, potentially making it a promising tool for marker-assisted selection of grooming behavior (Hamiduzzaman et al. 2017, Morfin et al. 2020).
Other Potential Traits.
Hygienic and grooming behaviors are currently the most common traits selected for in breeding programs (reviewed by Zakar et al. 2014). There are, however, other traits thought to be potentially useful against Varroa, though mechanisms for selecting these traits have not yet been fully identified. One trait that is increasingly being investigated is brood cell uncapping and recapping by workers (Oddie et al. 2018). The resulting reduction in Varroa reproductive success is thought to be from the opening of pupal cells, thereby causing changes in temperature and humidity within the pupal cells and disrupting mite reproduction (Martin et al. 2019, Oddie et al. 2019). The physical removal of mites from the colony by adult bees is another trait that may confer bee resistance to Varroa (Lodesani et al. 1996, Rinderer et al. 2010). Lodesani et al. (1996) measured the amount of damage to mites and found that 46% of mites carried out the front entrance were damaged compared to the 26% found on the bottom boards. Another potential trait was described by Kralj and Fuchs (2006) who suggested that Varroa-infested foragers may not return to their colony in an effort to reduce colony mite levels, though this could be an example of a behavior rigged by the parasite to facilitate horizontal transmission of the mite (Schmid-Hempel 1998). This behavior is difficult to quantify and may not realistically be a trait for which one might select.
The use of polyandrous queens may also support Varroa resistance in synergy with, or instead of, classical trait-based selection. Honey bee queens typically mate with an average of 12 males (Tarpy et al. 2004), though mating with 40 males or more has been observed (Estroup et al. 1994). While researchers have not observed significant reductions in pest or pathogen rates in colonies headed by queens mated with a slightly above average number of drones (16–20) (Delaney et al. 2011, Tarpy et al. 2015), Delaplane et al. (2015) found significantly more brood and a lower proportion of samples positive for Varroa in colonies whose queens were inseminated with 30 or 60 drones. Thus, there may be a colony-level benefit of hyper polyandry on Varroa management, though additional research should confirm these findings.
Breeds of Resistant Stock
Minnesota Hygienic Bees
Minnesota hygienic bees were bred from Italian stock (A.m. ligustica) to have high levels of hygienic behavior, thus reducing the presence of American foulbrood, chalkbrood, and Varroa in colonies (Spivak and Gilliam 1998, Spivak and Reuter 2001, Ibrahim and Spivak 2006). Spivak and Reuter (1998) found that Minnesota hygienic bee colonies removed, on average, 94.2% of freeze-killed brood and had an average Varroa load of 0.6 mites per 100 bees compared to non-hygienic colonies which only removed 82% of dead brood and had an average of 1.0 mites per 100 bees by the end of the experiments. There does not appear to be any negative trade-offs from breeding for hygienic behavior. However, the freeze-kill brood assay is somewhat labor-intensive, which makes the selection process somewhat slow (Spivak and Gilliam 1998).
Varroa Sensitive Hygiene
Breeding efforts by Jeff Harris and John Harbor at the USDA laboratory in Baton Rouge, Louisiana, USA focused on a heritable trait originally called “suppressed mite reproduction” (Harbo and Harris 1999, 2000). Bees with this trait were believed to interfere with Varroa reproduction in the cells. It was later determined that the mite suppression was due to the selective removal by bees of pupae infested with a reproducing Varroa. Brood in cells containing non-reproducing Varroa were ignored by the bees. This led the trait to be called “Varroa Sensitive Hygiene” (VSH—Harbo and Harris 2005). The VSH stock is considered to be more hygienic than the Minnesota hygienic stock of bees (Ibrahim and Spivak 2006). Ibrahim and Spivak (2006) used several metrics to compare the two lines with the most notable finding being that VSH bees removed 85% of infested pupae while the Minnesota hygienic bees removed 66%.
Russian Honey Bees
Researchers at the USDA Honey Bee Research Laboratory in Baton Rouge, Louisiana, USA searched in Asia for a stock of Varroa-resistant A. mellifera that had potentially been exposed to Varroa longer than were A. mellifera colonies elsewhere around the world. The premise was that A. mellifera taken from Europe into Asia decades earlier would have been exposed to Varroa naturally and possibly developed resistance to the mite. They found a promising stock in the Primorski region of the far-eastern side of Russia. These bees (now called “Russian honey bees”) had been exposed to the mite for potentially 45–100 yr longer than had other populations of A. mellifera in Asia (Danka et al. 1995).
Russian honey bees have shown to be more resistant to Varroa and tracheal mites (Acarapis woodii) than are other A. mellifera stock (Rinderer et al. 2001a, de Guzman et al. 2005, Tarpy et al. 2007, Ward et al. 2008, Kirrane et al. 2018). The utility of this honey bee stock for commercial operations has been well documented (Rinderer et al. 2001b, Danka et al. 2012, Rinderer et al. 2014a). The mechanisms of Russian honey bee resistance to Varroa is thought to be due to low brood attractiveness, reduced mite reproduction, and an extended phoretic period (Rinderer et al. 2010). In 2008–2009, Russian honey bees were compared with VSH and Italian-derived honey bees during commercial pollination events (Danka et al. 2012). The Italian-derived honey bees were treated for Varroa infestation twice each year, as per the standard commercial practice. Danka et al. (2012) found that all groups performed similarly, though Russian bee colonies were smaller in size than colonies of the other bee types during the early spring almond pollination season. Nevertheless, they rebounded in size by summer pollination season. The treated Italian bees consistently had the lowest mite counts. Similar comparisons were made in 2010–2012, though control colonies were not treated for mites (Rinderer et al. 2014a). Rinderer et al. (2014a) noted that during periods of honey production and almond pollination, colony sizes were similar among all stocks, though Russian bees had 36–54% lower Varroa infestation than the untreated control colonies.
One major negative to Russian honey bee stock is the high frequency of queen loss when managed commercially (Danka et al. 2012). Danka et al. (2012) observed that nearly 75% of original Russian queens died each year. The Russian Bee Breeders Association has been distributing the stock to the beekeeping industry in the U.S. (Brachman 2009).
Survival Stock
Some honey bee researchers have taken a different approach to develop Varroa resistant bees. Instead of routinely treating their colonies with acaricides, they do nothing to treat against Varroa and allow colonies that cannot combat the mites to die, leaving only a few naturally surviving colonies. An approach known as the “Bond” test (after James Bond: “live and let die”) was first implemented in France by Kefuss et al. (2004) in 1993. After nine years, all but three of the colonies had died (Kefuss et al. 2004). The surviving colonies, a hybrid of local A.m. carnica (bees native to the study area) and A.m. intermissa colonies (imported from Tunisia to France), were selected as breeder colonies based on their hygienic behavior and Varroa infestation levels (colonies with lower levels were favored by the researchers). Kefuss et al. (2009) later reported that about 2/3 of the colonies died, but Varroa infestation remained below 5% in surviving colonies.
The Bond test was applied to 150 colonies located on the Swedish island of Gotland in 1999 (Fries et al. 2006). The colonies were allowed to swarm. Only 10–15 colonies survived after seven years of no Varroa treatment applications. Both Fries and Bommarco (2007) and Locke and Fries (2011) suggested that the mite loads were significantly lower in their selected colonies than in Varroa-susceptible ones, though their results are difficult to interpret. In a later examination of these bees, Locke et al. (2014) observed that the Gotland bees had mite loads >30 mites/100 bees, well above what is typically sustainable, yet the colonies survived the following winter. Le Conte et al. (2020) recently reviewed many other examples of surviving honey bee populations worldwide, including those found in Avignon, France, the Østlandet region of Norway, and the Arnot Forest, NY. Currently, it appears that beekeepers do not have access to these Varroa-tolerant bees for purchase.
The long-term success of survivor stock populations is possible because many beekeepers are averse to chemical treatments and due to the rise in acaricide resistance among many Varroa populations (Lodesani et al. 1995, Elzen and Westervelt 2002). However, the concept of survivor stock leads to many questions. The major issue is that survivor bees are not necessarily selected for Varroa resistance or tolerance, as other pressures may be the main driver of selection in a given season. The pressures include weather, nutrition factors, other pests or diseases, etc. Furthermore, just because a stock of bees can survive Varroa infestation does not necessarily make them bees that you would want to keep. Without selection, the traits that beekeepers desire (gentleness, honey production, spring build-up, etc.) may be lost within a short amount of time. Until survivor bees are able to demonstrate productivity as well as survivability, they will likely not gain much popularity among the world’s commercial beekeepers. While the possibility of developing survivor stock, arguably, has been demonstrated, its practical usefulness has not.
Emerging/Other Varroa-Resistant Stocks
Breeding efforts to obtain a productive, yet Varroa-resistant or tolerant stock can take decades. There are several emerging stocks that, at this time, are not widespread, but may one day be so in the future. One is the Indiana “mite-biter” stock, produced at Purdue University, IN (Hunt et al. 2016). These bees have demonstrated an increased grooming behavior and have been selected for increased mutilation of Varroa (Morfin et al. 2020). There is some evidence that this stock has structural changes in the worker mandibles (Smith et al. 2021) and can reduce mite populations when compared to non-selected stocks (Hunt et al. 2016), with Morfin et al. (2020) reporting a nearly three-fold increase in fallen mites.
Another emerging stock is the POL-line Hygienic Italian honey bee. This bee was bred by scientists at the USDA-ARS laboratory located in Baton Rouge, LA. They are the result of outcrossing VSH queens to U.S. commercial stocks and then selecting for low mite infestations (Danka et al. 2016). To date, there is not much evidence to support that POL-line bees significantly reduce Varroa populations compared to untreated controls (Danka et al. 2016). Additionally, these bees appear to be more sensitive to virus infections (Deformed Wing Virus—Khongphinitbunjong et al. (2016) and Israeli Acute Paralysis Virus infections—Bhatia et al. 2021) and exhibit a low pesticide tolerance in brood (Milone et al. 2020) when compared to other commercial stocks. This suggests that more breeding efforts are needed before this stock will be widely accepted by beekeepers.
In Canada, several new stocks of bees are under development (De la Mora et al. 2020, Maucourt et al. 2020). In Saskatchewan, Canada, the Saskatraz bees were established by crossing a number of different races (A. m. carnica, ligustica, mellifera) with Russian bees in an isolated apiary. The goal was to promote gentleness, productivity, and Varroa-resistance in the stock (Robertson et al. 2014, 2020). From the limited research conducted on this stock, it appears that the Saskatraz bees are successful at reducing brood infestation levels as much as ~68% compared to non-resistant stock (Robertson et al. 2014). They also survive longer and produce more honey than non-resistant stock (Robertson et al. 2020). Nevertheless, more research is needed before use recommendations can be made.
Using Molecular Genetics to Breed for Resistance
Genetic markers can be used to identify the relevant genes or traits that contribute to bee tolerance of Varroa, making this a useful tool for breeding purposes. Navajas et al. (2008) compared pupae from Varroa-resistant and Varroa-susceptible genetic stocks bred in Avignon, France. They found that Varroa infestation did induce changes in gene expression and that Varroa-resistant bees expressed differences in genes regulating neuronal sensitivity and olfaction. Navajas et al. (2008) suggest that bee olfaction and neuronal sensitivity may play an important role in the detection of Varroa-infested brood cells and, therefore, be associated with hygienic and grooming behaviors.
More recently, the location of genes influencing hygienic and grooming behavior have been identified using quantitative trait locus (QTL) mapping (Oxley et al. 2010, Arechavaleta-Velasco et al. 2012, Tsuruda et al. 2012). QTL mapping is used commonly to explain the function of genes within identified regions of DNA. A recent study by Lattorff et al. (2015) compared samples of the Gotland bees before (2000) and after (2007) selection. They found that bee genetic diversity greatly decreased over the selection process and that the genes responsible for the volatiles emitted by bee larvae, which might be essential to trigger oogenesis in Varroa, had changed in the Varroa-resistant Gotland bees. Experiments that identify the main behavioral or physiological mechanisms of Varroa resistance provide a well-defined target for current and future breeding efforts.
Brood Interruption
Brood interruption refers to a process through which beekeepers disrupt the regular Varroa reproductive cycle by causing a colony-level break in the honey bee brood cycle (Lodesani et al. 2014), i.e., a colony goes without brood for a period of time. A beekeeper can cause a break in the brood cycle by placing the queen in a cage and preventing her from laying eggs for a complete brood cycle (about 24 d) or by completely removing the brood from a hive. This interrupts the growth of the Varroa population, which is otherwise closely associated with that of the honey bee (Rosenkranz et al. 2010). Artificial brood interruption is not a sufficient stand-alone treatment strategy for Varroa (Gregorc et al. 2017, Jack et al. 2020a). Giacomelli et al. (2016) observed that caging the queen for 20 d reduced Varroa populations by ~40%. However, the real benefit of imposing a brood interruption is that all mites are forced onto adult bees in the absence of brood in the colony. This makes them vulnerable to grooming behaviors or treatment with an acaricide. Therefore, artificial brood interruption typically is used in conjunction with organic treatments such as formic acid, oxalic acid, and/or thymol (Lodesani et al. 2014, Giacomelli et al. 2016, Gregorc et al. 2017, Büchler et al. 2020). Caging queens to create broodless periods in a hive requires handling the queen, which can be risky. With good beekeeping skills, queen mortality can be low to none after 24 d of caging (Giacomelli et al. 2016, Gregorc et al. 2017, Jack et al. 2020a).
“Failed” Cultural Control Method
Small cell foundation is a cultural control method that, anecdotally, seemed promising initially, but ultimately failed to hold up to experimental rigor, i.e., failed to control Varroa in colonies. Foundation is the part of the frame on which bees build comb. Standard foundation has cell bases ~5.3 mm wide while small cell foundation was composed of cells ~4.9 mm wide (Ellis et al. 2009a). The reduced cell size was originally believed to affect mite behavior inside the cell, squeezing the mite between the brood and the cell wall (Message and Goncalves 1995). Also, it was once noted that small cell foundation resulted in shorter developmental times of honey bee pupae, interfering with Varroa reproduction because adult bees would emerge before the mites reached maturity (Camazine 1986). However, the reduced cell size had no measurable impact on mite population growth in several studies (Taylor et al. 2008, Ellis et al. 2009a, Berry et al. 2010, Coffey et al. 2010, Seeley and Griffin 2011).
Mechanical Control
Mechanical control implies that the pest is controlled using physical methods or mechanical devices such as equipping hives with screen bottom boards, drone brood trapping, or heat treatments. Varroa populations can be reduced significantly via the implementation of certain beekeeping cultural or mechanical practices. These non-chemical approaches are considered essential for long-term, sustainable solutions to Varroa control (Rosenkranz et al. 2010); however, they are rarely sufficient as stand-alone treatments. The effectiveness of some of the mechanical control methods described next is controversial, as many studies have produced conflicting results due to differences in honey bee behavior across the study regions and a general lack of standardization of the studies.
Screened Bottom Boards
The use of a screened bottom board, rather than a solid one, on a colony is a strategy employed by beekeepers to reduce Varroa populations in a hive. Screened bottom boards are believed to work by allowing mites that ordinarily fall from bees or the comb to fall out of a hive rather than landing on the solid bottom board and returning to the hive on bees entering the nest. Researchers testing the efficacy of screened bottom boards found that they indeed reduce Varroa populations (Pettis and Shimanuki 1999, Webster et al. 2000, Ellis et al. 2001, Rinderer et al. 2003, Harbo and Harris 2004, Delaplane et al. 2005), though they only provide a modest impact of about 11–14% (Delaplane 2005) and should not be used as a stand-alone treatment.
Drone Brood Trapping
Drone brood trapping involves removing drone brood from a hive in an attempt to lower Varroa populations. It is based on the principle that Varroa preferentially invade drone cells at a higher rate than they do worker brood cells (Fuchs and Langenbach 1989, Boot et al. 1995). Thus, removing or destroying drone cells in a hive can reduce Varroa populations. Drone brood removal can be achieved in a few ways. First, the beekeeper can simply cut out or remove capped drone cells constructed by the bees from the colony. Second, the beekeeper can place a frame that includes drone foundation into the brood-rearing area of the colony. The bees will construct drone-sized cells on the foundation and the queen lay unfertilized (drone) eggs in the resulting cells. The frame can be removed from the colony once all the cells are capped, frozen (effectively killing all the developing mites and drones contained within), and returned to the colony to allow the bees to abort the dead drones and mites. After this, the queen will lay eggs in the drone cells and the process can start again. This method has been shown to be effective at lowering mite levels as much as 50.3–93.4% (Calis et al. 1999, Wilkinson and Smith 2002, Charriere et al. 2003, Calderone 2005, Wantuch and Tarpy 2009), though it is only useful in the spring and early summer seasons when the colonies actively rear drones (Wantuch and Tarpy 2009). Drawbacks with drone removal include the intensive labor associated with the practice, the required sacrifice of many drones, and the danger of rapid Varroa population growth if one accidentally leaves the drone frames within the hive without killing the mites.
Hyperthermia
Hyperthermia is a mechanical control method whereby Varroa are exposed to a sustained lethal temperature that does not harm the bees. This strategy has been investigated as an avenue of Varroa control since the 1970s and has been used in many countries (reviewed by Tihelka 2016). Several investigators have shown that temperatures ≥40°C are lethal to Varroa, while short exposures to the same temperatures do not affect bees negatively (Hoppe and Ritter 1987, Le Conte et al. 1990, Tabor and Ambrose 2001), though they often become agitated (Goras et al. 2015). Historically, hyperthermia was most often achieved by placing hives in “thermal boxes” (incubators) to raise the nest temperature (Tihelka 2016), though efficacy data was not noted. More recently, devices have been created to either heat-treat the brood chamber electronically (Thermovar, Varroa Terminator, Vatorex, The Victor, Mighty Mite Killer, Silent Future Tec Varroa Kill II) or the hive will include modifications, such as windows, to facilitate heating the colony periodically (Thermosolar Hive). Unfortunately, the efficacies of only a small number of products have been published in peer-reviewed research journals. Goras et al. (2015) found that the Thermovar device killed >90% of mites in a hive after 360 to 480 min of treatment.
A device called the Mite-Zapper combined the concept of drone brood trapping with that of hyperthermia (Huang 2001). The Mite-Zapper is a drone comb embedded with heating elements that can be connected to a 12-volt battery for 1–5 min, causing the combs to reach temperatures of 43°C (Huang 2001). Preliminary results showed 100% efficacy (Huang 2001) but with no peer-reviewed studies available on the product. The use of heat as a Varroa control is promising and many beekeepers and industry partners are eagerly creating new products to sell. However, there is a desperate need for researchers to investigate the efficacies, safety, and practicality of the many devices available.
“Failed” Mechanical Control Methods
One mite treatment that had anecdotal promise, but unproven efficacy, was the use of powdered sugar as colony dust. Some data suggested that dusting colonies with powdered sugar caused the mites to lose their grip on the bees, falling from them to the bottom board (Fakhimzadeh et al. 2011). The sugar also was believed to initiate grooming responses among the bees, leading to increased mite fall. A few initial studies demonstrated the potential effectiveness of mite removal with powdered sugar (Fakhimzadeh 2001, Macedo et al. 2002, Aliano and Ellis 2005, Fakhimzadeh et al. 2011); but long-term, comprehensive field studies failed to achieve any level of mite control (Ellis et al. 2009b, Berry et al. 2012). Thus, dusting colonies with powdered sugar, or other inert dust, is not effective as a Varroa control (Berry et al. 2012).
There are other examples of impractical, failed, or unproven Varroa control strategies. Some of these approaches include the use of ultrasound, electromagnetic fields, and energized water (Rosenkranz et al. 2010). Such strategies should only be adopted after their efficacy against Varroa has been demonstrated so that unsubstantiated claims will not cause beekeepers to lose money implementing a doomed strategy.
Biological Control
The traditional definition of biological control is a pest management tactic that involves the purposeful manipulation of a living agent to reduce a pest’s status (Pedigo and Rice 2009). There are two kinds of biological control: classical—in which a natural enemy is brought to a new location to control the pest; and augmentative—in which the population of a biological control agent is increased or released into an environment where presently there are too few (O’Neil and Obrycki 2009). Researchers have been exploring the idea of biological control of Varroa for decades, testing various pathogens and predators against the mite (Chandler et al. 2001). A successful control requires the biological control agent to focus primarily on the mite while leaving the honey bee unharmed. This is difficult to achieve as the mite is sheltered inside honey bee hives and often within the honey bee brood cells (Rosenkranz et al. 2010). Nevertheless, the discovery of a biological control agent that could effectively reduce Varroa populations within the hive would be of benefit to beekeepers.
Theoretically, biological controls can self-perpetuate as long as a host remains present. The biological control agent even may spread to other nearby colonies, depending on the organism. Nevertheless, honey bee colonies may act as a Varroa refuge where they are protected from potential natural enemies. This could explain why no natural enemies of the mite have been discovered to date (Chandler et al. 2001). This has made the selection of an effective and self-perpetuating biological control agent extremely difficult. That said, some biological control agents have been tested against Varroa, with mixed, but generally low, success.
Entomopathogenic Fungi
Entomopathogenic fungi have been the most heavily researched biological control agent for Varroa and are considered to have the highest potential for success based on their control of other mites (reviewed by Chandler et al. 2001). The two main species of entomopathogenic fungi evaluated have been Metarhizium anisopliae Metschnikoff (Hypocreales: Clavicipitaceae) and Beauveria bassiana Balsamo (Hypocreales: Cordycipitaceae) due to their success controlling other arthropod pests in agricultural systems (Meikle et al. 2012). Both fungi have been tested extensively for the biological control of Varroa (Shaw et al. 2002, Kanga et al 2003, Hamiduzzaman et al. 2012, Sinia and Guzman-Novoa 2018). In the laboratory, Shaw et al. (2002) observed that three isolates of M. anisopliae and one of B. bassiana killed 100% of Varroa within one week postexposure. Similarly, Hamiduzzaman et al. (2012) observed that two isolates of M. anisopliae and one of B. bassiana killed 100% of Varroa that were hand-dipped into the fungal suspensions. The mites were dead one week postexposure, though the honey bee brood was also infected. Initial reports of field trials testing M. anisopliae were promising. Kanga et al. (2003) observed Varroa efficacy equal to that of the miticide Apistan. However, all others have been unsuccessful in field trials (reviewed by Meikle et al. 2012). Sinia and Guzman-Novoa (2018) observed in field trials that an isolate of M. anisopilae killed 62% of Varroa while treatments of B. bassiana killed 41–53% of Varroa.
There does appear, however, to be many challenges with using entomopathogenic fungi to control Varroa. Meikle et al. (2012) suggest that the formulation, duration of application in the hive, risk of contaminating bees and hive products, and the ability to target the different life stages of Varroa all present challenges in the development of effective fungal biopesticides. It may be possible to combine other IPM tactics with M. anisopliae or B. bassiana application to increase efficacy (Sinia and Guzman-Novoa 2018); thus, further explorations to overcome these challenges are warranted.
Predators
One possible avenue for the biological control of Varroa is using predators that feed upon or negatively disrupt the mites. Donovan and Paul (2005) speculated that some chelifers (also known as pseudoscorpions) could feed effectively on Varroa. They also considered the use of pseudoscorpions as a potentially viable option because they have been observed to feed on Varroa within A. cerana colonies (Donovan and Paul 2006) and can be massed reared (Read et al. 2014). It was shown in a laboratory study that a single pseudoscorpion fed on as many as 1–9 Varroa per day (Fagan et al. 2012) and that the predation of Varroa by pseudoscorpions found in honey bee colonies was confirmed by molecular analysis (van Toor et al. 2015). However, feelings towards using pseudoscorpions to control Varroa are mixed as Thapa et al. (2013) observed pseudoscorpions prefer to feed on dead A. cerana larvae and adults rather than Varroa. There has been no evidence that pseudoscorpions have reduced Varroa populations within a colony. It is unlikely that augmenting honey bee colonies with pseudoscorpions would result in any kind of Varroa control.
The Stratiolaelaps scimitus (Mesostigmata: Laelapidae) mite, used as a biological control agent for the sciarid fly Bradysia matogrossensis (Diptera: Sciaridae) in commercial mushroom production (Castilho et al. 2009), has also been examined as a possible Varroa control candidate. In laboratory trials, Rangel and Ward (2018) observed that S. scimitus killed 97% of Varroa housed in the same vials, though in honey bee hives, the predators were completely ineffective against Varroa. Risk assessment by Rondeau et al. (2018) found that S. scimitus will feed on unprotected bee larvae or eggs and that the mites would not attack any Varroa that were attached to adult honey bees. In field studies, Rondeau et al. (2019) also observed that S. scimitus were completely ineffective within the honey bee hive, regardless of season. As S. scimitus has demonstrated risk to honey bee brood and no benefit within the hive, it does not appear likely that this predatory mite will ever be an effective biological control agent for Varroa.
Bacteria
Bacillus thuringiensis (Bt) (Bacillales: Bacillaceae) is considered by some to be the bacterial pathogen with the greatest potential to control Varroa (Chandler et al. 2001). Bt has been deemed safe for use in honey bee colonies, as it has been used as a biological control for the greater wax moth (Galleria mellonella (Lepidoptera: Pyralidae)), another honey bee pest (Vandenberg and Shimanuki 1990). In an in vitro laboratory study, several Bt strains demonstrated promise in controlling Varroa destructor, killing >80% of mites within 48 h (Alquisira-Ramírez et al. 2014). Additional laboratory experiments showed that two of the effective Bt strains were essentially harmless to honey bee adults and larvae (Alquisira-Ramírez et al. 2017), though field testing has not yet occurred.
There are several other bacterial strains that have been shown to be effective against Varroa. Tsagou et al. (2004) found strains of bacteria from both the Micrococcacea and the Bacillaceae families that decreased the amount of time it took mites to reach 50% mortality by several hours, thus demonstrating some effect against the mites. The bacteria Serratia marcescens (Enterobacterales: Yersiniaceae) (GEI strain), an isolate from the gut of the workers of Apis cerana, has been found in the laboratory to degrade chitin and kill 100% of Varroa within a few days (Tu et al. 2010). Still, none of these bacteria have demonstrated an ability to control Varroa within a honey beehive. Thus, future research is needed before a determination can be made about the promise of these bacteria as biological control agents.
Chemical Control of Varroa
Varroa control is most commonly attempted using chemical treatment, though, within an IPM paradigm, chemical control should be used sparingly and in combination with other methods to control damaging populations (Flint 2012). Synthetic compounds, often referred to as “hard chemicals”, are widely used due to the convenience of application, low costs, and generally higher efficacy (Rosenkranz et al. 2010). Organic compounds, sometimes referred to as “soft chemicals,” are frequently used as well, though these substances are not necessarily safer for humans or honey bees despite their “soft” moniker (Budavari 1989). A wide range of chemical products used to control Varroa are available worldwide, though not all products are registered in every country (Table 2). Chemical treatment of Varroa continues to be a complex issue due to concerns of resistance management and in-hive accumulation of residues.
Table 2.
Chemical treatments available to control Varroa destructor in Apis mellifera colonies globally
| Country | Synthetic “Hard” | Natural “Soft” | ||||||
|---|---|---|---|---|---|---|---|---|
| Amitraz (formamidine) | Coumaphos (organophosphate) | Fluvalinate (pyrethroid) | Flumethrin (pyrethroid) | Formic acid | Oxalic acid | Thymol | Hop beta acids | |
| Western Hemisphere | ||||||||
| Argentina | X | X | X | |||||
| Canada | X | X | X | X | X | X | X | |
| Chile | X | |||||||
| Colombia | X | |||||||
| Costa Rica | X | X | X | |||||
| El Salvador | X | |||||||
| Jamaica | X | X | ||||||
| Mexico | X | X | X | |||||
| Nicaragua | X | X | ||||||
| Paraguay | X | |||||||
| Trinidad and Tobago | X | |||||||
| United States | X | X | X | X | X | X | X | |
| Uruguay | X | X | ||||||
| Europe and Eurasia | ||||||||
| Albania | X | X | X | X | X | |||
| Austria | X | X | X | X | X | |||
| Azerbaijan | X | |||||||
| Belgium | X | X | ||||||
| Bosnia and Herzegovina | X | |||||||
| Bulgaria | X | X | X | X | X | |||
| Croatia | X | X | X | |||||
| Cyprus | X | X | X | X | X | |||
| Czech Republic | X | X | X | X | ||||
| Denmark | X | X | ||||||
| Estonia | X | X | X | |||||
| France | X | X | X | X | X | |||
| Georgia | X | |||||||
| Germany | X | X | X | X | ||||
| Greece | X | X | X | X | X | |||
| Hungary | X | X | X | X | X | X | ||
| Ireland | X | X | X | X | ||||
| Italy | X | X | X | X | X | |||
| Latvia | X | X | X | |||||
| Lithuania | X | X | X | X | X | |||
| Luxembourg | X | |||||||
| Macedonia | X | X | ||||||
| Malta | X | X | X | X | ||||
| Moldova | X | X | ||||||
| Montenegro | X | |||||||
| Netherlands | X | X | ||||||
| Poland | X | X | X | |||||
| Portugal | X | X | X | X | X | X | X | |
| Romania | X | X | X | X | X | X | ||
| Russia | X | X | X | |||||
| Serbia | X | X | ||||||
| Slovakia | X | X | X | X | X | X | ||
| Slovenia | X | X | X | X | X | |||
| Spain | X | X | X | X | X | X | ||
| Sweden | X | X | X | X | X | |||
| Switzerland | X | X | X | T | X | |||
| Turkey | X | X | X | X | ||||
| Ukraine | X | X | ||||||
| United Kingdom | T | X | X | X | X | X | ||
| Near Eastern | ||||||||
| Algeria | X | X | X | X | X | |||
| Egypt | X | |||||||
| Iran | X | X | X | |||||
| Iraq | T | X | X | |||||
| Israel | X | |||||||
| Lebanon | X | X | ||||||
| Libya | X | |||||||
| Morocco | X | X | X | |||||
| Oman | X | |||||||
| Saudi Arabia | X | X | ||||||
| Syria | X | X | ||||||
| Tunisia | X | X | X | |||||
| Africa (Sub-Sahara) | ||||||||
| Madagascar | X | |||||||
| Mauritius | T | X | ||||||
| South Africa | X | X | X | |||||
| South and Central Asia | ||||||||
| Afghanistan | T | |||||||
| Uzbekistan | X | X | ||||||
| East Asia and Pacific | ||||||||
| Australia | E | X | X | X | ||||
| Japan | X | X | ||||||
| Korea, South | X | X | ||||||
| New Zealand | X | X | X | X | X | X | ||
| Philippines | X | |||||||
| Thailand | X | X |
Registered, X; Temporary Permit, T; Emergency Permit, E.
Organic Chemicals
Many beekeepers are opposed to administering synthetic chemicals to their honey bee colonies out of a belief that these compounds are harmful to the bees, and thus not safe to use. Other beekeepers simply seek to augment the number of tools available to use against Varroa. In any case, there are several natural compounds shown to be effective at controlling Varroa. These mostly include organic acids such as formic acid (marketed as MAQS, Nassenheider Professional, Varterminator), and oxalic acid (Api-Bioxal), but also include the essential oil thymol (Apiguard, Api Life Var, Thymovar). Additionally, hop beta acids (HopGuard) are becoming an increasingly popular treatment in North America. Organic chemicals typically do not persist within honey bee hives (reviewed by Rademacher and Harz 2006, Gregorc and Sampson 2019) and are applied to colonies differently from one another due to the varying nature of the chemicals, the formulations used, and the labeled use restrictions. Correspondingly, the use and efficacies of natural compounds are highly variable compared to those of synthetic chemicals.
Formic Acid
Formic acid (FA) was investigated as a potential Varroa control and has been used regularly by beekeepers since the mid-1980s (Moosebeckhofer and Derakhshifar 1986). Though the mode of action is not well understood, FA likely inhibits electron transport in the Varroa mitochondria by binding cytochrome c oxidase (reviewed by Johnson et al. 2010). There are several different formulations of FA. They can be applied to honey bee colonies as a gel (MAQS), tablet (Varterminator) or liquid solution (Nassenheider Professional) (Eguaras et al. 2003, Giovenazzo and Dubreuil 2011, Giusti et al. 2017, Pietropaoli and Formato 2019). Performance of FA appears to be somewhat better using slow-release gel formulations (Ostermann and Currie 2004, Pietropaoli and Formato 2019) and it is the only miticide that has demonstrated an ability to kill both phoretic mites and reproductive mites contained within the sealed brood cells (Fries 1991). Most experiments through which the efficacy of formic acid against Varroa has been tested have yielded positive results (Calderone and Nasr 1999, Satta et al. 2005, Vandervalk et al. 2014, Giusti et al. 2017, Pietropaoli and Formato 2019), with the efficacy typically ranging in the 35–75% Varroa mortality range. Factors such as ambient temperature, the amount of brood in a colony, and the distance of the brood from the site of formic acid volatilization can affect treatment efficacy (Eischen 1998, Calderone and Nasr 1999, Skinner et al. 2001, Underwood and Currie 2003). Formic acid can result in the mortality of honey bee brood and queens if the ambient temperature is too warm (Elzen et al. 2004, Giovenazzo and Dubreuil 2011). It can also negatively affect honey bee memory (Gashout et al. 2020). Formic acid is commonly used throughout North America and Europe (Table 2).
Oxalic Acid
Oxalic acid (OA) is permitted for use in the U.S., several European countries, and in New Zealand (Table 2). This compound has been used effectively for several decades (Popov et al. 1989) with no reports of mite resistance (Maggi et al. 2017). While the mode of action for OA is not fully understood, OA kills Varroa upon contact (Aliano et al. 2006, Aliano and Ellis 2008) and is also effective at dislodging mites as it increases honey bee grooming behavior (Schneider et al. 2012). Beekeepers commonly treat their colonies with a ≥3% OA solution by dissolving ~35 g of OA dihydrate (Api-Bioxal) into 1 l of 1:1 sugar: water (weight:volume) solution and trickling 50 ml of the solution between the tops of frames (Charriere and Imdorf 2002, reviewed by Rademacher and Harz 2006). Some also choose to spray 3–4 ml of the solution directly onto one side of the frames of bees (reviewed by Rademacher and Harz 2006). Other beekeepers, especially those in temperate climates, may choose to sublimate OA (or vaporize if using OA dihydrate) crystals inside a colony during the winter so that the colonies do not need to be opened.
Oxalic acid is most effective during broodless periods (Gregorc and Planinc 2001, Gregorc et al. 2016), as the chemical will not kill mites that are inside capped cells; however, some beekeepers treat with oxalic acid once a week for up to three weeks when brood is present in the hive (Gregorc and Planinc 2001, Jack et al. 2021). Recent studies have produced contradicting results regarding which method of oxalic acid application is most effective at controlling Varroa (Al Toufailia et al. 2015, Gregorc et al. 2016). However, all application methods have demonstrated effectiveness, often resulting in >90% Varroa mortality (reviewed by Rademacher and Harz 2006). That efficacy can rise to nearly 100% when colonies are broodless (Gregorc and Planinc 2001, reviewed by Gregorc and Sampson 2019). Negative impacts on honey bee brood development, behavior, and longevity have been observed with the use of OA (Higes et al. 1999, Schneider et al. 2012).
Essential Oils
Thymol is the most commonly used essential oil Varroa treatment and likely works against Varroa by binding to octopamine or GABA receptors (reviewed by Johnson et al. 2010). The commercially available thymol-treatments (Apiguard, Api Life Var, Thymovar) are formulated in different matrices such as gel packets, vermiculite tablets, and cellulose wafers to supply a steady release of the volatile (Melathopoulos and Gates 2003, Gregorc and Planinc 2012, Coffey and Breen 2013). Like formic acid, thymol efficacy is dependent upon temperature and the amount of brood within the colony (Calderone 1999). Additionally, the volume of air above the combs where the treatment is placed can affect the overall efficacy of thymol, with larger air space increasing the rate of sublimation, thus increasing its efficacy (Lodesani and Costa 2008). Temperatures between 20 and 30°C are generally when thymol will be most effective, with it losing its effectiveness below 15°C (Imdorf et al. 1995). The thymol-based treatments generally kill 50–80% of Varroa (Melathopoulos and Gates 2003, Gregorc and Planinc 2012, Coffey and Breen 2013). However, thymol can be quite harmful to honey bee brood and queens when applied during periods of high ambient temperatures (Floris et al. 2004). The use of thymol-based products is permitted nearly worldwide (Table 2).
There are literally hundreds of other essential oils that have been tested against Varroa (Imdorf et al. 1999). The main component of most essential oils are monoterpenes and, like thymol, most of these essential oils act as a fumigant (Imdorf et al. 1999). However, others such as garlic, clove, and menthol have demonstrated contact acaricidal properties against Varroa (Gashout and Guzman-Novoa 2009, Goswami and Khan 2013). The efficacy of essential oils varies greatly, with the large majority providing no or negligible control of Varroa. Perhaps the main obstacle for achieving high levels of consistent mite control, regardless of location or climatic conditions, is the lack of efficient delivery methods and formulations that release constant doses of the oils (Sabahi et al. 2017). However, a few promising essential oils have been discovered. In laboratory studies, menthol, clove, and origanum oil caused 87, 96, and 100% mite mortality, respectively (Gashout and Guzman-Novoa 2009), and rosewood and fennel oil both caused 65% mite mortality (Lin et al. 2020). In the field, garlic oil killed 73% of Varroa (Goswami and Khan 2013), oregano oil delivered with electric vaporizers killed 97% (Sabahi et al. 2017), and neem oil killed 85% (Gómez et al. 2016), though the latter did impact honey bee larvae and queens negatively. Imdorf et al. (1999) reviewed the efficacies of many other different essential oils as Varroa treatments. At this point, considerable essential oil use in honey bee colonies by beekeepers is off-label, with the violations typically going unenforced.
Hop Beta Acids
Beta plant acids, specifically compounds called lupulones derived from hop plants, are the active ingredients in a product called HopGuard. The mode of action of hop beta acids is not fully understood, but lupulones have been shown to have a repellent effect on the two-spotted spider mites (Tetranychus urticae) (Jones et al. 1996). Initially, many North American beekeepers were hopeful that HopGuard would be a valuable product for several reasons. One, it can be applied easily on formulated cardboard strips that are hung between frames, similar to how the synthetic acaricides are applied. Also, HopGuard can be applied to both packages and colonies during the summer when temperatures are high (DeGrandi-Hoffman et al. 2012). Finally, hop beta acids are non-toxic to humans and have demonstrated low toxicity to bees (Rademacher et al. 2015). However, reports on the effects of HopGuard in the field have been quite mixed. Rademacher et al. (2015) observed up to 88% mite mortality in treated colonies while Vandervalk et al. (2014) and Gregorc et al. (2018) observed efficacies of just 43% and 64%, respectively. Currently, HopGuard is only labeled for use in the U.S. and Canada.
Synthetic Chemicals
Of the different synthetic chemical treatments used to control Varroa across the world, there are four common active ingredients (AIs). These include the formamidine amitraz (marketed as Apivar), the organophosphate coumaphos (most common is Checkmite), and two pyrethroids, flumethrin (Bayvarol and PolyVar Yellow) and tau-fluvalinate (Apistan). These acaricides are most commonly administered to honey bee colonies by placing plastic strips impregnated with the chemicals into the brood area. The bees contact the strips as they move about the surface of the combs, thus exposing the mites to the AIs. Large-scale, commercial beekeepers typically prefer to use these compounds as they can be applied rapidly and demonstrate high efficacy against Varroa (Rosenkranz et al. 2010). That said, there have been many reported cases of Varroa resistance to these AIs (Table 3).
Table 3.
List of documented Varroa destructor resistance to synthetic chemicals in countries for which data exist
Formamidines
Amitraz is registered for use in many countries (see Table 2). Formamidines, such as amitraz, are octopamine mimics that block the regular neuromodulating octopamine receptor (Casida and Durkin 2013). Apivar, registered for use in the U.S. in 2013, is formulated amitraz in plastic strips that hang between brood frames, one strip per five frames of brood for 42 d. Many studies have shown amitraz to be a highly effective control (Floris et al. 2001, Semkiw et al. 2013, Vandervalk et al. 2014, Al Naggar et al. 2015, Gregorc et al. 2018), consistently killing 75–90% of Varroa. Recently, amitraz usage among U.S. beekeepers was associated with low winter colony losses from survey data (Haber et al. 2019). Thus, amitraz use has become popular and is frequently used throughout the world to control Varroa (Table 2).
While efficacious, Apivar is not considered affordable by many beekeepers. Often, beekeepers will purchase other products containing amitraz and concoct their own homemade treatments, typically soaking a paper towel with their concoctions and placing it on top of the brood frames. In the U.S., for example, amitraz was registered as a product named Miticure from 1992 to 1994 (reviewed by Johnson et al. 2010). However, it lost its registration for use in colonies, at which time many beekeepers found the AI in another product (Taktic) that was registered for the control of cattle ticks (Chen et al. 2007, Oliver 2014). Taktic is popularly used as an off-label amitraz treatment in the U.S.
Amitraz at high dosages can negatively impact brood survival (Dai et al. 2017, 2018, Tome et al. 2020), drone sperm viability (Fisher and Rangel 2018), honey bee cardiac function, and virus tolerance (O’Neal et al. 2017). Varroa resistance has been reported for decades and in many regions (Elzen et al. 1999, 2000; Rodríguez-Dehaibes et al. 2005, Maggi et al. 2010, Kamler et al. 2016, Rinkevich 2020, Table 3), though mite populations have remained susceptible to amitraz for much longer than they have to fluvalinate and coumaphos.
Organophosphates
Coumaphos is registered for use in Europe as well as the U.S., Canada, and Nicaragua (Table 2). Organophosphates such as coumaphos inhibit acetylcholinesterase, and this prevents the hydrolysis of acetylcholine at synapses (Casida and Durkin 2013). There have been several coumaphos-based products, each with different formulations. Asuntol50 was formulated as a powder and applied by mixing with powdered sugar, and sprinkling between the brood frames (Martel et al. 2007). However, Asuntol50 is not available to many beekeepers. Other products like Perizin, which is formulated as a liquid and applied to colonies by trickling between brood frames (Blacquière et al. 2017) and CheckMite which is formulated as strips that are hung between brood frames (Kast et al. 2020) are widely available. However, products containing coumaphos generally have low efficacies and have largely been abandoned by beekeepers (Haber et al. 2019).
Varroa resistance to coumaphos has been widely reported around the world (Spreafico et al. 2001, Pettis et al. 2004, Maggi et al. 2009, Medici et al. 2016, Table 3), rendering the treatment effectively useless. Furthermore, even when treatment of coumaphos has been absent for a decade, a reversion to coumaphos susceptibility does not appear likely (Mitton et al. 2018). Moreover, many beekeepers do not want to treat with coumaphos because of the harmful negative effects it may cause to honey bees. For instance, coumaphos has been observed reducing honey bee learning and memory (Williamson et al. 2013), brood survival (Dai et al. 2018), queen survival (Haarmann et al. 2002), viability of sperm stored in queens’ spermatheca (Chaimanee 2016), and many honey bee metabolic responses (Boncristiani et al. 2012).
Pyrethroids
Nearly every country where honey bees are managed permits the use of a pyrethroid to control Varroa because of this group’s ability to kill mites at low concentrations with correspondingly low toxicity to honey bees (Perez-Santiago et al. 2000, Johnson et al. 2010) (Table 2). Pyrethroids disrupt the mite’s neurotransmission by blocking sodium transport at the voltage-gated sodium channels (Casida and Durkin 2013), resulting in prolonged channel openings (Dong et al. 2014). The success of these chemicals is mainly due to their ability to initiate repetitive synaptic disturbances, causing the mites to convulse (Casida and Durkin 2013) and fall off their honey bee host.
Both the products Apistan (AI—tau-fluvalinate) and Bayvarol (AI—flumethrin) are formulated as strips impregnated with their respective active ingredients. The strips are hung between brood frames for 6–8 wk. Apistan was widely used in the 1980s in Europe and in the early and mid-1990s in the U.S. and had efficacies >90% (Cabras et al. 1997). However, beekeepers lessened their use of Apistan when resistance issues became widespread (Lodesani et al. 1995, Elzen et al. 1998, Mozes-Koch et al. 2000, Thompson et al. 2002, reviewed by Johnson et al. 2010, Table 3) due to mutations in the mite’s voltage-gated sodium channels (González-Cabrera et al. 2016). Most of the research conducted on the negative effects of acaricides on honey bees has focused mainly on fluvalinate. Notable negative effects include reduced brood survival (Dai et al. 2017, 2018), the production of smaller queens (Haarmann et al. 2002), increased susceptibility to viruses (Locke et al. 2012), and reduced learning and memory (Frost et al. 2013).
The efficacy of Bayvarol has remained relatively high, killing 73–97 % of mites (Smodiš Škerl et al. 2011, Olmstead et al. 2019), though resistance to Bayvarol has also been reported (Surlis et al. 2016, Table 3). PolyVar Yellow is flumethrin formulated as a strip. However, instead of being hung between brood frames, the strip is placed at the hive entrance and has holes through which the bees enter and leave, thereby becoming exposed to the AI. Where tested, PolyVar Yellow has proved incredibly effective, killing 99.9% of mites in one study (Blacquière et al. 2017). The negative effects to honey bees associated with flumethrin appear to be considerably less severe than those elicited by fluvalinate, with only increased adult stress being observed (Qi et al. 2020).
Abandoned Synthetic Acaricides
There are a few synthetic acaricide treatments that were used for a period of time but were abandoned due to ineffectiveness or concerns over honey bee health. For instance, cymiazole, an iminophenyl thiazolidine derivative formulated in the product Apitol, was fed to bees via sugar syrup. Apitol is a systemic acaricide, working through the honey bee hemolymph (Stanimirovic et al. 2005). However, field efficacy of Apitol has not demonstrated much success (Imdorf et al. 1996), possibly due to the fact that Varroa primarily feed on fat tissue instead of hemolymph as was once believed (Ramsey et al. 2019). Furthermore, cymiazole is water-soluble and could be easily detected in honey (Cabras et al. 1994, Wallner 1999).
Another abandoned acaricide was bromopropylate, commercialized as fumigation strips as the product Folbex-VA. Bromopropylate has been used to control twospotted spider mites (Tetranychus urticae) (Van Leeuwen et al. 2010) but was also used in the early 1980s in Europe to control Varroa (Ravoet et al. 2015). Though Folbex-VA proved moderately effective (Marchetti et al. 1984), its use in bee colonies was banned in Europe because of the consistent contamination of hive products (Lodesani et al. 1992, Wallner 1999, Bogdanov 2006, Ravoet et al. 2015).
Fenpyroximate, a pyrazole that acts as a METI (mitochondrial electron transport inhibitor) acaricide, is another example of an abandoned Varroa treatment. It was first introduced into the U.S. in 2007 as Hivastan, formulated as a patty (reviewed by Johnson et al. 2010). Fenpyroximate was used to kill two-spotted spider mites, but they became resistant (Kim et al. 2004). After issues of fenpyroximate affecting honey bee health (Johnson et al. 2013a, b), Hivastan quickly lost popularity among beekeepers.
Residue Control
Acaricides are among the most abundantly detected chemical residues in honey bee colonies (Mullin et al. 2010, Wu et al. 2011, Sanchez-Bayo and Goka 2014, Ostiguy et al. 2019). Amitraz, bromopropylate, coumaphos, flumethrin and tau-fluvalinate can be found in pollen, bee bread, and, most commonly, beeswax (vanEngelsdorp 2009, Johnson et al. 2010, Mullin et al. 2010). Given that most synthetic acaricides used to control Varroa are lipophilic and nonvolatile (reviewed by Wilmart et al. 2016), except for cymiazole (Wallner et al. 1999), they readily accumulate in wax. The chronic exposure of mites to acaricides via wax residues is thought to contribute to the development of mite resistance to these compounds (Medici et al. 2016).
Numerous studies highlight the negative effects of these residues on honey bee health and their potential interactions with other stressors (Johnson et al. 2009, Boncristiani et al. 2012, Medici et al. 2012, Wu et al. 2011, Berry et al. 2013, Johnson et al. 2013a, b, Williamson and Wright 2013). Many beekeepers attempt to eliminate pesticide residues in a colony by replacing old wax combs with new foundation, thus encouraging bees to build new comb (Johnson et al. 2010). While traces of acaricides can be found in most wax foundations around the world, (Wallner 1999, Mullin et al. 2010), rotating combs every few years appears to be a worthwhile endeavor (Berry and Delaplane 2001, Döke et al. 2015).
Resistance Management
Varroa has rapidly evolved resistance to several of the noted acaricides due to AI overuse or misuse by beekeepers. Resistance to the prevalent synthetic chemicals amitraz, coumaphos, flumethrin, and fluvalinate has been well-documented worldwide (Lodesani et al. 1995, Thompson et al. 2002, Elzen and Westervelt 2002, 2004; Pettis 2004, Goodwin et al. 2005, Sammataro et al. 2000; Gracia-Salinas et al. 2006, Maggi et al. 2009, 2010, Bak et al. 2012, Kamler et al. 2016, Table 3). Fortunately, most organic chemicals used to control Varroa have a low risk of accumulating in bee products as they are water soluble, more volatile and generally break down faster (Wallner 1999). Therefore, Varroa have a lower likelihood of developing resistance to organic chemicals after repeated exposure to the AIs (Rosenkranz et al. 2010).
Rotation of Chemical Treatments
Rotating among the different acaricides is the optimal strategy for preventing the development of Varroa resistance to any one AI (Sudo et al. 2018). An effective resistance management plan should incorporate as many different chemical classes as possible to avoid Varroa developing cross-resistance, when resistance to one acaricide confers resistance to another (FAO 2012). Acaricide rotation plans have been suggested by honey bee researchers (Elzen et al. 2001). However, each treatment should be unique to specific regions. If the steps of IPM are followed, chemical treatments will be used only when necessary, in combination with other non-chemical treatments, and will be selected according to the efficacy and appropriate timing for a given region. Therefore, it is not appropriate to prescribe specific treatment plans to every beekeeper.
The rotation of chemical treatments may only be a short-term solution for beekeepers if not adopted by the beekeeping community (Rosenkranz et al. 2010). Mites can move to neighboring colonies, hitching rides on drifting or robbing workers (Peck and Seeley 2019). If careless beekeepers increase mite resistance to a certain chemical treatment due to overuse, those mites could eventually migrate to colonies appropriately managed. Researchers have identified the molecular mechanisms of chemical resistance in Varroa populations to specific acaricides (Gonzalez-Cabrera et al. 2013, Strachecka et al. 2015, Gonzalez-Cabrera et al. 2016), though additional work is needed in this field of study. Once the mechanisms of resistance are identified, more research efforts could be invested into targeting those resistance genes and silencing them through RNAi to maintain the efficacy of chemical treatments currently available (See RNAi).
Resistance Detection
When an acaricide appears to not be as effective as expected, resistance is not always to blame. Product performance problems may include the incorrect timing of the treatment, poor application coverage, or the use of an incorrect dose (FAO 2012). However, frequent use of synthetic chemical treatments can and do lead resistance and should be monitored closely (Roth et al. 2020). Monitoring colony Varroa populations using techniques described previously (see Quantifying Varroa Populations) before and after treatment is key to early detection of chemical resistance.
Simple field assays have been used to detect Varroa resistance to synthetic acaricides formulated into strips (Pettis et al. 1998, Rinkevich 2020). Rinkevich (2020) used the following method to determine Apivar resistance in commercial apiaries. (1) Cut a small 4 × 4 cm square from the chemical strip and glue it perpendicularly to the bottom of a disposable plastic cup. (2) Collect about 300 adult bees from brood frames and place them into the container with the chemical treatment. (3) Fashion a lid from screen mesh, attach it to the container and invert the container. (4) Suspend the container a few cm over a sticky board in the shade at ambient field conditions for an amount of time determined by the researcher (usually several hours). Standardization of the exposure time is critical to compare resistance across colonies. (5) At the end of the testing period, wash the bees in the containers with warm water, dislodging the remaining “resistant” mites. (6) Add the number of remaining mites to the number of dropped mites to determine the total Varroa in the sample. (7) Finally, divide the number of dropped Varroa by the total Varroa to calculate the treatment efficacy.
Screening for New Acaricides
Chemical control of Varroa will likely remain a major part of Varroa IPM in the foreseeable future. Ensuring that there are enough effective chemical controls that can be rotated as part of a management regimen is important to the sustainability of the existing controls. As such, the discovery of new compounds active against Varroa is worthy of continual pursuit. However, it is not enough simply to find a compound toxic to Varroa. Both Varroa and honey bees belong to the phylum Arthropoda and, as such, have somewhat similar physiologies. Identifying new compounds requires extensive testing on chemical toxicity for both species before a compound can be approved for use against Varroa while demonstrating low risk to honey bees. An ideal compound will be toxic to Varroa in low dosages, while only toxic to honey bees at extremely high dosages, or not toxic to them at all. Observations of the selectivity of a compound can be made by dividing the toxicity of a compound to honey bees by the toxicity of the same compound to the mite (Lindberg et al. 2000). Such selectivity ratios (SR) provide a simple, yet efficient way to compare compounds and to make comparisons between studies.
For years, researchers worldwide have been screening compounds for toxic selectivity to the mite (Lindberg et al. 2000, Fassbinder et al. 2002, Ruffinengo et al. 2005, Damiani et al. 2009, Gashout and Guzman-Novoa 2009, Riva et al. 2019, Lin et al. 2020). In most cases, research groups are able to select some promising candidates to move forward into field level tests. However, the costs required for chemical companies to bring a product to market is so high that it prohibits most positive hits from future testing, calling into question the logic of exploring new chemistries. Nevertheless, there are many chemicals designed to target other mites, insects or arthropods that are already on the market and have not yet been tested on Varroa or honey bees. Thus, chemical screenings are still a worthwhile endeavor, though to increase the likelihood of adding new legal products to beekeeper’s arsenal of treatments, more targeted screenings are required.
Chemical Treatment vs. Non-Treatment
Many beekeepers do not want to put chemicals into their hives for fear of the negative effects these chemicals might have on honey bee health. Treating honey bee colonies with chemicals to control Varroa can lead to unintended negative side effects for the drones, queens and workers (Johnson et al. 2009, Boncristiani et al. 2012, Wu et al. 2011, Berry et al. 2013, Johnson et al. 2013a, b, Williamson and Wright 2013, Chaimanee et al. 2016). However, without any kind of beekeeper intervention, Varroa and their associated viruses will almost certainly overcome managed colonies (Frey and Rosenkranz 2014, Thompson et al. 2014, Haber et al. 2019, Grozinger and Flenniken 2019). While thoroughly vetted and labeled chemical treatments can harm honey bees, we argue that the harm caused by Varroa is worse than that of the approved acaricidal controls. Of course, it should be reiterated that chemical control should not be the sole method of control but should be used sparingly and in combination with other measures to reduce Varroa populations below the economic threshold (Flint 2012). Thus, diligent Varroa monitoring strategies should demonstrate the need for chemical intervention.
Emerging Varroa Control Technologies
With mites becoming increasingly resistant to once effective acaricides and with other IPM tactics offering only minor relief against Varroa infestations, the sustainable control of Varroa in honey bee colonies remains an expanding frontier of research. There are many avenues of Varroa control research currently in development (Dietemann et al. 2012). Here, we mention two technologies that appear to have the most promise, or at least the most resources devoted to exploring their efficacy against Varroa.
RNAi
Varroa researchers have placed an increased emphasis on new genomic approaches that can be used to target Varroa efficiently and disrupt the mite’s lifecycle since the partial sequencing of the Varroa genome (Cornman et al. 2010). Once such strategy involves RNA interference (RNAi) technology. RNAi works by reducing the RNA of specific, critical target genes, causing a reduced expression of that gene (Garbian et al. 2012, Scott et al. 2013). RNAi, in theory, is thought to have limited impacts on non-target organisms (Niu et al. 2018). The process starts by feeding honey bees with double stranded (dsRNA) corresponding to specific Varroa RNA sequences. The dsRNA presumably moves from the bee gut to its hemolymph, where it is acquired by feeding mites. This, in theory, ultimately causes gene expression changes that are lethal to or causes reduced fitness of the mites. This has been accomplished, for example, with a few bee viruses, where feeding bees viral dsRNA reduced titers of the target virus (Maori et al. 2009, Hunter et al. 2010, Desai et al. 2012, Chen et al. 2014). Despite the promise of RNAi, a great deal of research is required to ensure that the dsRNA does not contain fragments that match genes in the honey bee, as this may impact bees detrimentally (Nunes et al. 2013). Furthermore, studies are needed to determine whether chronic exposure to dsRNA will impact the honey bee immune system (Grozinger and Robinson 2015).
Recently, Huang et al. (2019) discovered target genes important to Varroa survival and reproduction via injection. They found two genes that significantly reduced Varroa survival, killing 96% and 70% of mites 72 h post-injection, and four genes that reduced Varroa reproduction, three of them by >50%. These genes, as well as many others, should be explored further as possible target sites in future research. A new method of delivery of dsRNA to Varroa has also recently opened new research avenues. Leonard et al. (2020) found that engineered symbiotic bacteria within the honey bee guts could reach Varroa with target dsRNA, thus providing a new tool to study RNAi technology for honey bee health.
Chemical Ecology
The discovery of the chemical composition of female Varroa sex pheromones (Ziegelmann et al. 2013) highlights the role chemical ecology may play in the future control of Varroa. For example, the discovery of the sex pheromones offers a new control approach for the mite, possibly via the disruption of mite mating behavior. Ziegelmann and Rosenkranz (2014) tested the ability of the sex pheromones to disrupt mating behavior in both a laboratory assay and in the field study. The laboratory assay demonstrated that male mites cannot distinguish between receptive and unreceptive females during mating attempts after exposure to the pheromones. Furthermore, the time the mites spent mating was reduced significantly. In the field, female daughters of foundress mites found in brood combs and sprayed with components of the mite sex pheromone had significantly fewer spermatozoa, suggesting reduced mating success.
Eliash et al. (2014) discovered compounds that caused Varroa present on the bodies of adult nurse bees to move towards foraging bees within the laboratory. This movement away from nurse bees to forager bees is interesting, as mites within the hive could potentially be carried away from the brood area and be more exposed to acaricidal applications. If in the future these compounds were formulated into a Varroa treatment, other treatments may become more effective, though higher incidents of drifting could be more likely to occur (Plettner et al. 2017). Regardless of the method, any future Varroa control manipulating the chemical ecology of the mite will likely be difficult to implement within honey bee colonies which, themselves, are filled with chemical signals (Nazzi and Le Conte 2016). Nevertheless, the promise of manipulating Varroa behavior to the benefit of the honey bee is exciting and should be explored further.
Holistic Control of Varroa Using IPM
Varroa control treatments may vary in efficacy due to abiotic (location, temperature, humidity, season, etc.) or biotic factors (mite resistance, honey bee colony population size, colony sensitivity to treatment, etc.). Consequently, there will never be a single Varroa control strategy that will work for every beekeeper. Beekeepers must be aware of the available and effective treatments for their own location and situation. Nevertheless, we have created a treatment decision chart to aid beekeepers in selecting the best treatments for their situation (Fig. 4). The chart recommendations are based on efficacy data reported in the literature (Table 1).
Fig. 4.
Treatment decision chart to aid beekeepers in selecting the most appropriate Varroa treatments for their colony situation and management goals. To aid in development of this chart, several assumptions about beekeeper efforts to prevent and manage Varroa are made. Hyperthermia recommendations are made based on the research referenced in Table 1, but caution is required when using this control method, as research regarding the safety of these devices is limited. Specific organic and synthetic chemical treatments are not mentioned in the figure; thus, beekeepers must determine which chemical treatments are available to them and follow the label of the given treatment. Recommendations in this figure are only intended to guide the beekeeper to available treatment options given the colony situation and management goals.
We make a broad recommendation that all beekeepers, regardless of operational size, practice Varroa prevention measures as part of their routine management strategy, use Varroa resistant stock and equip hives with screened bottom boards during warm seasons. We developed the decision tree in Fig. 4 assuming these best management recommendations are followed. From there, it is necessary to know ones Varroa infestation rate as the tree’s initial decision is predicated on whether or not colonies have infestation rates ≥3 mites/100 adult bees, the standard economic threshold. Following that decision, the beekeeper must know if colony populations are decreasing or increasing naturally, if they are being used for production, etc.
Once all questions are answered, the beekeeper arrives at a list of various IPM treatment levels/categories: cultural, mechanical, organic chemical and synthetic chemical. These are either recommended (check) or discouraged (X), based on their general efficacy specific to that condition. Biological controls are not included in the figure, as presently no effective commercial treatments are available to beekeepers. Additionally, we do not advise that beekeepers use synthetic chemical treatments when Varroa thresholds are below the 3 mites/100 bee ratio. We do not recommend specific treatment strategies within a given IPM treatment level/category. For example, we do not recommend which synthetic chemical should be used if the use of synthetic chemicals is checked in the flow chart. Beekeepers can review the efficacy of each specific treatment in Table 1 and determine which they are comfortable using. While we do not provide the financial costs for these different treatments, it is important that beekeepers determine which treatments are economically feasible for their own operations. Furthermore, not all treatment options are available in every country (Table 2). Thus, we leave that decision to the beekeeper. Ultimately, we believe that this decision tree, when followed, represents a holistic IPM strategy for controlling Varroa effectively, regardless of where the colony is located.
Conclusion
Varroa continues to be a severe problem for honey bees despite decades of research into its control. The sustainable control of Varroa likely will not be achieved using a single control approach, but rather via integrating multiple approaches to achieve maximum efficacy. However, given that our understanding of how Varroa/virus transmission affects honey bees is poor and that our current economic threshold is narrow (2 vs. >3 mites/100 bees), it is fair to consider if IPM is even a viable approach to Varroa control at all. Here, we highlight what we believe to be important gaps in collective knowledge related to Varroa control and the development of IPM protocols.
Finish annotating the Varroa destructor genome. Annotation will allow researchers to identify new RNAi target sites or develop new molecular/genetic approaches for better Varroa control.
Develop a Varroa in vitro rearing method. An in vitro rearing method will allow for high-throughput screenings of chemical treatments and will greatly increase the speed in which Varroa may be studied (Jack et al. 2020b).
-
Improve chemical control of Varroa. This might be accomplished by:
a) screening additional compounds,
b) identifying the physiological means Varroa use to acquire resistance to existing chemicals, and
c) improving the formulation and application of existing miticides.
-
Advance existing alternative Varroa control strategies. This might be accomplished by:
a) continuing breeding programs aimed at improving honey bees resistance and tolerance to Varroa,
b) utilizing chemical ecology strategies such as pheromonal disruption of mating, attractants or repellants, and
c) identifying new candidate biological control agents.
-
Advance integrated pest management strategies for Varroa. This might be accomplished by:
a) quantifying injury to a colony in terms of percent of bees with a virus per Varroa,
b) outlining specific economic injury levels regionally to determine when chemical treatment of Varroa is necessary,
c) investigating the efficacy of different combinations of treatment regimes, and
d) determining beekeeper barriers to adoption of IPM strategies.
-
Reduce the impact of Varroa-vectored viruses. This might be accomplished by:
a) understanding the mechanisms by which Varroa transmit viruses,
b) investigating the impact of mite infestations on virus prevalence in colonies, and
c) using novel technologies, such as RNAi, to reduce the impact of viruses on colonies.
Varroa has had a devastating impact on honey bee health and the sustainability of beekeeping globally. Despite this, beekeepers have managed to keep colonies alive through labor intensive, costly Varroa control management programs. We believe addressing Varroa infestations using the basic principles of IPM is possible and will benefit both honey bees and beekeepers alike.
Acknowledgments
We would like to thank Mary Bammer and Emily Noordyke for their help with the graphic design of the figures.
Author Contributions
CJ: Conceptualization; Writing - Original Draft; Writing - review & editing. JE: Conceptualization; Writing - Original Draft; Writing - review & editing.
References Cited
- Al Naggar, Y., Tan Y., Rutherford C., Connor W., Griebel P., Giesy J. P., and Robertson A. J.. . 2015. Effects of treatments with Apivar® and Thymovar® on V. destructor populations, virus infections and indoor winter survival of Canadian honey bee (Apis mellifera L.) colonies. J. Apicult. Res. 54: 548–554. [Google Scholar]
- Al Toufailia, H., Scandian L., and Ratnieks F. L. W.. . 2015. Towards integrated control of varroa: 2) comparing application methods and doses of oxalic acid on the mortality of phoretic Varroa destructor mites and their honey bee hosts. J. Apicult. Res. 54(2): 108–120. [Google Scholar]
- Al Toufailia, H., Scandian L., Shackleton K., and Ratnieks F. L. W.. . 2018. Towards integrated control of varroa: 4) Varroa mortality from treating broodless winter colonies twice with oxalic acid via sublimation. J. Apicult. Res. 57(3): 438–443. [Google Scholar]
- Ali, S., Zhang C., Wang Z., Wang X. M., Wu J. H., Cuthbertson A. G. S., and Qiu B. L.. . 2017. Toxicological and biochemical basis of synergism between the entomopathogenic fungus Lecanicillium muscarium and the insecticide Matrine against Bemisia tabaci (Gennadius). Sci. Rep. 7: 46558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aliano, N. P., and Ellis. M. D.. 2005. A strategy for using powdered sugar to reduce Varroa populations in honey bee colonies. J. Apicult. Res. 44: 54–57. [Google Scholar]
- Aliano, N. P., and Ellis. M. D.. 2008. Bee-to-bee contact drives oxalic acid distribution in honey bee colonies. Apidologie. 39(5): 481–487. [Google Scholar]
- Aliano, N. P., Ellis M. D., and Siegfried B. D.. . 2006. Acute contact toxicity of oxalic acid to Varroa destructor (Acari: Varroidae) and their Apis mellifera (Hymenoptera: Apidae) hosts in laboratory bioassays. J. Econ. Entomol. 99: 1579–1582. [DOI] [PubMed] [Google Scholar]
- Alissandrakis, E., Ilias A., and Tsagkarakou A.. . 2017. Pyrethroid target site resistance in Greek populations of the honey bee parasite Varroa destructor (Acari: Varroidae). J. Apicult. Res. 56: 625–630. [Google Scholar]
- Almecija, G., Poirot B., Cochard P., and Suppo C.. . 2020. Inventory of Varroa destructor susceptibility to amitraz and tau-fluvalinate in France. Exp. Appl. Acarol. 82: 1–16. [DOI] [PubMed] [Google Scholar]
- Alquisira-Ramírez, E. V., Paredes-Gonzalez J. R., Hernández-Velázquez V. M., Ramírez-Trujillo J. A., and Peña-Chora G.. . 2014. In vitro susceptibility of Varroa destructor and Apis mellifera to native strains of Bacillus thuringiensis. Apidologie. 45: 707–718. [Google Scholar]
- Alquisira-Ramírez, E. V., Peña-Chora G., Hernández-Velázquez V. M., Alvear-García A., Arenas-Sosa I., and Suarez-Rodríguez R.. . 2017. Effects of Bacillus thuringiensis strains virulent to Varroa destructor on larvae and adults of Apis mellifera. Ecotoxicol. Environ. Saf. 142: 69–78. [DOI] [PubMed] [Google Scholar]
- Amdam, G. V., and Omholt S. W.. . 2002. The regulatory anatomy of honeybee lifespan. J. Theor. Biol. 216: 209–228. [DOI] [PubMed] [Google Scholar]
- Anderson, D. L., and Trueman J. W.. . 2000. Varroa jacobsoni (Acari: Varroidae) is more than one species. Exp. Appl. Acarol. 24: 165–189. [DOI] [PubMed] [Google Scholar]
- Andino, G. K., and Hunt G. J.. . 2011. A scientific note on a new assay to measure honeybee mite-grooming behavior. Apidologie. 42: 481–484. [Google Scholar]
- Arechavaleta-Velasco, M. E., and Guzmán-Novoa E.. . 2001. Relative effect of four characteristics that restrain the population growth of the mite Varroa destructor in honey bee (Apis mellifera) colonies. Apidologie. 32: 157–174. [Google Scholar]
- Arechavaleta-Velasco, M. E., Alcala-Escamilla K., Robles-Rios C., Tsuruda J. M., and Hunt G. J.. . 2012. Fine-scale linkage mapping reveals a small set of candidate genes influencing honey bee grooming behavior in response to Varroa mites. Plos One. 7: e47269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asha, G. R., and Sharma S. K.. . 2009. Efficacy of screen floor and powdered sugar against Varroa destructor Anderson and Trueman in Apis mellifera L. colonies. Biopestic. Int. 5: 1–9. [Google Scholar]
- Aumeier, P. 2001. Bioassay for grooming effectiveness towards Varroa destructor mites in Africanized and Carniolan honey bees. Apidologie. 32: 81–90. [Google Scholar]
- Aumeier, P., Rosenkranz P., and Francke W.. . 2002. Cuticular volatiles, attractivity of worker larvae and invasion of brood cells by Varroa mites. A comparison of Africanized and European honey bees. Chemoecology. 12: 65–75. [Google Scholar]
- Bacandritsos, N., Papanastasiou I., Saitanis C., Nanetti A., and Roinioti E.. . 2007. Efficacy of repeated trickle applications of oxalic acid in syrup for varroosis control in Apis mellifera: influence of meteorological conditions and presence of brood. Vet. Parasitol. 148: 174–178. [DOI] [PubMed] [Google Scholar]
- Bak, B., Wilde J., and Siuda M.. . 2012. Characteristics of north-eastern population of Varroa destructor resistant to synthetic pyrethroids. Medycyna Weterynaryjna 68: 603–606. [Google Scholar]
- BeeAware . 2021. Varroa mites.https://beeaware.org.au/archive-pest/varroa-mites/#ad-image-0. Accessed 10 March 2021.
- Berry, J. A., and Delaplane K. S.. . 2001. Effects of comb age on honey bee colony growth and brood survivorship. J. Apicult. Res. 40: 3–8. [Google Scholar]
- Berry, J. A., Owens W. B., and Delaplane K. S.. . 2010. Small-cell comb foundation does not impede Varroa mite population growth in honey bee colonies. Apidologie. 41: 40–44. [Google Scholar]
- Berry, J. A., Afik O., Nolan M. P., and Delaplane K. S.. . 2012. Revisiting powdered sugar for varroa control on honey bees (Apis mellifera L.). J. Apicult. Res. 51: 367–368. [Google Scholar]
- Berry, J. A., Hood W. M., Pietravalle S., and Delaplane K. S.. . 2013. Field-level sublethal effects of approved bee hive chemicals on honey bees (Apis mellifera L). Plos One. 8: e76536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer, M., Junk J., Eickermann M., Clermont A., Kraus F., Georges C., Reichart A., and Hoffmann L.. . 2018. Winter honey bee colony losses, Varroa destructor control strategies, and the role of weather conditions: results from a survey among beekeepers. Res. Vet. Sci. 118: 52–60. [DOI] [PubMed] [Google Scholar]
- Bhatia, S., Baral S. S., Vega Melendez C., Amiri E., and Rueppell O.. . 2021. Comparing survival of Israeli acute paralysis virus infection among stocks of U.S. honey bees. Insects. 12: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blacquière, T., Altreuther G., and Krieger K. J.. . 2017. Evaluation of the efficacy and safety of flumethrin 275 mg bee-hive strips (PolyVar Yellow®) against Varroa destructor in naturally infested honey bee colonies in a controlled study. Parasitol. Res. 116: 109–122. [DOI] [PubMed] [Google Scholar]
- Boecking, O., and Genersch E.. . 2008. Varroosis—the ongoing crisis in bee keeping. J. Consum. Prot. Food Safety. 3: 221–228. [Google Scholar]
- Boecking, O., and Ritter W.. . 1993. Grooming and removal behavior of Apis mellifera-intermissa in Tunisia against Varroa jacobsoni. J. Apicult. Res. 32: 127–134. [Google Scholar]
- Boecking, O., and Spivak M.. . 1999. Behavioral defenses of honey bees against Varroa jacobsoni Oud. Apidologie. 30: 141–158. [Google Scholar]
- Boecking, O., Bienefeld K., and Drescher W.. . 2000. Heritability of the Varroa-specific hygienic behaviour in honey bees (Hymenoptera: Apidae). J. Anim. Breed. Genetics. 117: 417–424. [Google Scholar]
- Bogdanov, S. 2006. Contaminants of bee products. Apidologie. 37: 1–18. [Google Scholar]
- Boncristiani, H., Underwood R., Schwarz R., Evans J. D., Pettis J., and vanEngelsdorp D.. . 2012. Direct effect of acaricides on pathogen loads and gene expression levels in honey bees Apis mellifera. J. Insect Physiol. 58: 613–620. [DOI] [PubMed] [Google Scholar]
- Boncristiani, H., Ellis J., Bustamonte T., Kimmel C., Jack C., Graham J., Mortensen A., and Schmehl D.. . 2021. World honey bee health: the global distribution of western honey bee (Apis mellifera L.) pests and pathogens. Bee World. 98(1): 2–6. [Google Scholar]
- Boot, W. J., Calis J. N. M., and Beetsma J.. . 1992. Differential periods of Varroa mite invasion into worker and drone cell. Exp. Appl. Acarol. 16: 295–301. [Google Scholar]
- Boot, W. J., Beetsma J., and Calis. J. N. M.. 1994. Behaviour of Varroa mites invading honey bee brood cells. Exp. Appl. Acarol. 18: 371–379. [Google Scholar]
- Boot, W., Schoenmaker J., Calis J., and Beetsma J.. . 1995. Invasion of Varroa jacobsoni into drone brood cells of the honey bee, Apis mellifera. Apidologie. 26: 109–118. [Google Scholar]
- Brachman, B. 2009. The Russian honey bee breeder’s association. Bee Culture. 137: 46–47. [Google Scholar]
- Branco, M. R., Kidd N. A. C., and Pickard. R. S.. 2006. A comparative evaluation of sampling methods for Varroa destructor (Acari: Varroidae) population estimation. Apidologie. 37: 452–461. [Google Scholar]
- Broadschneider, R., Brus J., and Danihlik J.. . 2019. Comparison of apiculture and winter mortality of honey bee colonies (Apis mellifera) in Austria and Czechia. Agri. Ecosyst. Environ. 274: 24–32. [Google Scholar]
- Brown, P., Newstrom-Lloyd L. E., Foster B. J., Badger P. H., and McLean J. A.. . 2018. Winter 2016 honey bee colony losses in New Zealand. J. Apicult. Res. 57(2): 278–291. [Google Scholar]
- Bruce, W., Henegar R., and Hackett K.. . 1991. An artificial membrane for in vitro feeding of Varroa jacobsoni and Acarapis woodi, mite parasites of honey bees. Apidologie. 22(5): 503–507. [Google Scholar]
- Bruce, W. A., Chiesa F., Marchetti S., and Griffiths D. A.. . 1988. Laboratory feeding of Varroa jacobsoni Oudemans on natural and artificial diets (Acari: Varroidae). Apidologie. 19: 209–218. [Google Scholar]
- Büchler, R., Drescher W., and Tornier I.. . 1992. Grooming behavior of Apis cerana, Apis mellifera and Apis dorsata and its effect on the parasitic mites Varroa jacobsoni and Tropilaelaps clareae. Exp. Appl. Acarol. 16: 313–319. [Google Scholar]
- Büchler, R., Berg S., and Le Conte Y.. . 2010. Breeding for resistance to Varroa destructor in Europe. Apidologie. 41: 393–408. [Google Scholar]
- Büchler, R., Costa C., Hatjina F., Andonov S., Meixner M. D., Conte Y. L., Uzunov A., Berg S., Bienkowska M., Bouga M., . et al. 2014. The influence of genetic origin and its interaction with environmental effects on the survival of Apis mellifera L. colonies in Europe. J. Apicult. Res. 53: 205–214. [Google Scholar]
- Büchler, R., Uzunov A., Kovaˇci´c M., Prešern J., Pietropaoli M., Hatjina F., Pavlov B., Charistos L., Formato G., Galarza E., . et al. 2020. Summer brood interruption as integrated management strategy for effective Varroa control in Europe. J. Apicult. Res. 59(5): 764–773. [Google Scholar]
- Budavari, S. (ed.). 1989. The Merck Index—encyclopedia of chemicals, drugs and biologicals. Merck and Co., Inc., Rahway, NJ. [Google Scholar]
- Cabras, P., Martini M. G., Floris I., and Spanedda L.. . 1994. Residue of cymiazole in honey and honey bees. J. Apicult Res. 33: 83–86. [Google Scholar]
- Cabras, P., Floris I., Garau V. L., Melis M., and Prota R.. . 1997. Fluvalinate content of Apistan strips during treatment and efÞcacy in colonies containing sealed worker brood. Apidologie. 28: 91–96. [Google Scholar]
- Calderone, N. W. 1999. Evaluation of formic acid and thymol-based blend of natural products for fall control of Varroa jacobsoni (Acari: Varroidae) in colonies of Apis mellifera (Hymenoptera: Apidae). J. Econ. Entomol. 92: 253–260. [Google Scholar]
- Calderone, N. W. 2005. Evaluation of drone brood removal for management of Varroa destructor (Acari: Varroidae) in colonies of Apis mellifera (Hymenoptera: Apidae) in the northeastern United States. J. Econ. Entomol. 98: 645–650. [DOI] [PubMed] [Google Scholar]
- Calderone, N. W., and Kuenen L. P.. . 2001. Effects of western honey bee (Hymenoptera: Apidae) colony, cell type, and larval sex on host acquisition by female Varroa destructor (Acari: Varroidae). J. Econ. Entomol. 94: 1022–1030. [DOI] [PubMed] [Google Scholar]
- Calderone, N. W., and Kuenen. L. P. S.. 2003. Differential tending of worker and drone larvae of the honey bee, Apis mellifera, during the 60 hours prior to cell capping. Apidologie. 34: 543–552. [Google Scholar]
- Calderone, N. W., and Lin S.. . 2001. Behavioral responses of Varroa destructor (Acari: Varroidae) to extracts of larvae, cocoons and brood food of worker and drone honey bees, Apis mellifera (Hymenoptera: Apidae). Physiol. Entomol. 26: 341–350. [Google Scholar]
- Calderone, N. W., and Lin S.. . 2003. Rapid determination of the numbers of Varroa destructor, a parasitic mite of the honey bee, Apis mellifera, on sticky-board collection devices. Apidologie. 34:11–17. [Google Scholar]
- Calis, J. N. M., Boot, W. J., Beetsma J., van den Eijnde J., de Ruijter A., and van der Steen. J. J. M.. 1999. Effective biotechnical control of Varroa: applying knowledge on brood cell invasion to trap honey bee parasites in drone brood. J. Apicult. Res. 38(1–2): 49–61. [Google Scholar]
- Camazine, S. 1986. Differential reproduction of the mite, Varroa jacobsoni (Mesostigmata: Varroidae), on Africanized and European honey bees (Hymenoptera: Apidae). Ann. Entomol. Soc. Am. 79: 801–803. [Google Scholar]
- Carreck, N. L., Ball B. V., and Martin. S. J.. 2010. Honey bee colony collapse and changes in viral prevalence associated with Varroa destructor. J. Apicult. Res. 49: 93–94. [Google Scholar]
- Casida, J. E., and Durkin K. A.. . 2013. Neuroactive insecticides: targets, selectivity, resistance, and secondary effects. Ann. Rev. Entomol. 58: 99–117. [DOI] [PubMed] [Google Scholar]
- Castilho, R. C., de Moraes G. J., Silva E. S., Freire R. A. P., and Da Eira F. C.. . 2009. The predatory mite Stratiolaelaps scimitus as a control agent of the fungus gnat Bradysia matogrossensis in commercial production of the mushroom Agaricus bisporus. Int J. Pest Manag. 55: 181–185. [Google Scholar]
- Castillo Lopez, D., Zhu-Salzman K., Ek-Ramos M. J., and Sword G. A.. . 2014. The entomopathogenic fungal endophytes Purpureocillium lilacinum (formerly Paecilomyces lilacinus) and Beauveria bassiana negatively affect cotton aphid reproduction under both greenhouse and field conditions. Plos One. 9: e103891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaimanee, V., Evans J. D., Chen Y., Jackson C., and Pettis J. S.. . 2016. Sperm viability and gene expression in honey bee queens (Apis mellifera) following exposure to the neonicotinoid insecticide imidacloprid and the organophosphate acaricide coumaphos. J. Insect Physiol. 89: 1–8. [DOI] [PubMed] [Google Scholar]
- Chandler, D., Sunderland K., Ball B., and Davidson G.. . 2001. Prospective biological control agents of Varroa destructor n. sp., an important pest of the European honeybee, Apis mellifera. Biocontrol Sci. Technol. 11: 429–448. [Google Scholar]
- Charrière, J. D., and Imdorf A.. . 2002. Oxalic acid treatment by trickling against Varroa destructor: recommendations for use in central Europe and under temperate climate conditions. Bee World. 83: 51–60. [Google Scholar]
- Charriere, J. D., Imdorf A., Bachofen B., and Tschan A.. . 2003. The removal of capped drone brood: an effective means of reducing the infestation of varroa in honey bee colonies. Bee World. 84: 117–124. [Google Scholar]
- Chen, A. C., He H., and Davey R. B.. . 2007. Mutations in a putative octopamine receptor gene in amitraz-resistant cattle ticks. Vet. Parasitol. 148: 379–383. [DOI] [PubMed] [Google Scholar]
- Chen, W., and Hillyer J. F.. . 2013. FlyNap (Triethylamine) increases the heart rate of mosquitoes and eliminates the cardioacceleratory effect of the neuropeptide CCAP. PLoS One. 8(7): e70414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y. P., Pettis J. S., Corona M., Chen W. P., Li C. J., Spivak M., Visscher P. K., DeGrandi-Hoffman G., Boncristiani H., Zhao Y., . et al. 2014. Israeli acute paralysis virus: epidemiology, pathogenesis and implications for honey bee health. Plos Pathog. 10: e1004261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiesa, F., and Milani N.. . 1988. Some preliminary observations on the behavior of Varroa jacobsoni Oud. on its natural host under laboratory conditions, pp. 113–124. InCavalloro R., (ed.), European research on varroatosis control: Proceedings of a meeting of the EC experts’ group. Lux-CEC, Luxemborg. [Google Scholar]
- Chiesa, F., Milani N., and D’Agaro M.. . 1989. Observations of the reproductive behavior of Varroa jacobsoni Oud: Techniques and preliminary results, pp. 213–222. InCavalloro R., (ed), Present status of Varroatosis in Europe and progress of the Varroa mite control: Proceedings of a meeting of the EC experts’ group. CEC, Luxemborg. [Google Scholar]
- Coffey, M. F., and Breen J.. . 2013. Efficacy of Apilife Var® and Thymovar® against Varroa destructor as an autumn treatment in a cool climate. J. Apic. Res. 52: 210–218. [Google Scholar]
- Coffey, M. F., Breen J., Brown M. J. F., and McMullan J. B.. . 2010. Brood-cell size has no influence on the population dynamics of Varroa destructor mites in the native western honey bee, Apis mellifera mellifera. Apidologie. 41: 522–530. [Google Scholar]
- Colin, M. E., Vandame R., Jourdan P., and Di Pasquale S.. . 1997. Fluvalinate resistance of Varroa jacobsoni Oudemans (Acari: Varroidae) in Mediterranean apiaries of France. Apidologie. 28: 375–384. [Google Scholar]
- Cornman, S. R., Schatz M. C., Johnston S. J., Chen Y. P., Pettis J., Hunt G., Bourgeois L., Elsik C., Anderson D., Grozinger C. M., . et al. 2010. Genomic survey of the ectoparasitic mite Varroa destructor, a major pest of the honey bee Apis mellifera. BMC Genomics. 11: 602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crailsheim, K. 1990. The protein balance of the honey-bee worker. Apidologie. 21: 417–429. [Google Scholar]
- Crailsheim, K., Brodschneider R., Aupinel P., Behrens D., Genersch E., Vollmann J., and Reissberger-Gallé U.. . 2013. Standard methods for artificial rearing of Apis mellifera larvae. J. Apicult. Res. 52: 1–16. [Google Scholar]
- Croft, B. A., and Macrae I. V.. . 1992. Biological control of apple mites by mixed populations of Metaseiulus occidentalis (Nesbitt) and Typhlodromus pyri Scheuten (Acari: Phytoseiidae). Environ. Entomol. 21: 202–209. [Google Scholar]
- Currie, R. W., and Gatien P.. . 2006. Timing acaricide treatments to prevent Varroa destructor (Acari: Varroidae) from causing economic damage to honey bee colonies. Can. Entomol. 138: 238–252. [Google Scholar]
- Currie, R. W., and Tahmasbi G. H.. . 2008. The ability of high- and low-grooming lines of honey bees to remove the parasitic mite Varroa destructor is affected by environmental conditions. Can. J. Zool. -Rev. Can. Zool. 86: 1059–1067. [Google Scholar]
- Currie, R. W., Pernal S. F., and Guzmán-Novoa D. E.. . 2010. Honey bee colony losses in Canada. J. Apicult. Res. 49(1): 104–106. [Google Scholar]
- Dahlgren, L., Johnson R. M., Siegfried B. D., and Ellis M. D.. . 2012. Comparative toxicity of acaricides to honey bee (Hymenoptera: Apidae) workers and queens. J. Econ. Entomol. 105: 1895–1902. [DOI] [PubMed] [Google Scholar]
- Dai, P., Jack C. J., Mortensen A. N., and Ellis J. D.. . 2017. Acute toxicity of five pesticides to Apis mellifera larvae reared in vitro. Pest Manag. Sci. 73: 2282–2286. [DOI] [PubMed] [Google Scholar]
- Dai, P., Jack C. J., Mortensen A. N., Bustamante T. A., and Ellis J. D.. . 2018. Chronic toxicity of amitraz, coumaphos and fluvalinate to Apis mellifera L. larvae reared in vitro. Sci. Rep. 8: 5635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damiani, N., Gende L. B., Bailac P., Marcangeli J. A., and Eguaras M. J.. . 2009. Acaricidal and insecticidal activity of essential oils on Varroa destructor (Acari: Varroidae) and Apis mellifera (Hymenoptera: Apidae). Parasitol. Res. 106: 145–152. [DOI] [PubMed] [Google Scholar]
- Danka, R. G., Rinderer T. E., Kuznetsov V. N., and Delatte G. T.. . 1995. A USDA-ARS project to evaluate resistance to Varroa jacobsoni by honey-bees of far-eastern Russia. Am. Bee J. 135: 746–748. [Google Scholar]
- Danka, R. G., De Guzman L. I., Rinderer T. E., Sylvester H. A., Wagener C. M., Bourgeois A. L., Harris J. W., and Villa J. D.. . 2012. Functionality of Varroa-resistant honey bees (Hymenoptera: Apidae) when used in migratory beekeeping for crop pollination. J. Econ. Entomol. 105: 313–321. [DOI] [PubMed] [Google Scholar]
- Danka, R. G., Rinderer T. E., Spivak M., and Kefuss J.. . 2013. Comments on: “Varroa destructor: research avenues towards sustainable control”. J. Apicult. Res. 52(2): 69–71. [Google Scholar]
- Danka, R. G., Harris J. W., and Dodds G. E.. . 2016. Selection of VSH- derived “Pol-line” honey bees and evaluation of their Varroa-resistance characteristics. Apidologie. 47: 483–490. [Google Scholar]
- Davis, A. R. 2009. Regular dorsal dimples on Varroa destructor—damage symptoms or developmental origin? Apidologie. 40: 151–162. [Google Scholar]
- DeGrandi-Hoffman, G., Ahumada F., Probasco G., and Schantz L.. . 2012. The effects of beta acids from hops (Humulus lupulus) on mortality of Varroa destructor (Acari: Varroidae). Exp. Appl. Acarol. 58: 407–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Guzman, L. I., Rinderer T. E., Bigalk M., Tubbs H., and Bernard S. J.. . 2005. Russian honey bee (Hymenoptera: Apidae) colonies: Acarapis woodi (Acari: Tarsonemidae) infestations and overwintering survival. J. Econ. Entomol. 98: 1796–1801. [DOI] [PubMed] [Google Scholar]
- De la Mora, A., Emsen B., Borges D., Eccles L., Kelly P. G., Goodwin P. H., and Guzman-Novoa E.. . 2020. Selective breeding for low and high Varroa destructor growth in honey bee (Apis mellifera) colonies: initial results of two generations. Insects. 11: 864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jong, D., and Soares A. E. E.. . 1997. An isolated population of Italian bees that has survived Varroa jacobsoni infestation without treatment for over 12 years. Am. Bee J. 137: 742–745. [Google Scholar]
- Delaney, D. A., Keller J. J., Caren J. R., and Tarpy D. R.. . 2011. The physical, insemination, and reproductive quality of honey bee queens (Apis mellifera L.). Apidologie. 42: 1–13. [Google Scholar]
- Delaplane, K. S. 1997. Strictly for the hobbyist: Varroa- how and when to treat. Am. Bee J. 137: 571–573. [Google Scholar]
- Delaplane, K. S., and Hood W. M.. . 1997. Effects of delayed acaricide treatment in honey bee colonies parasitized by Varroa jacobsoni and a late-season treatment threshold for the southeastern USA. J. Apicult. Res. 36: 125–132. [Google Scholar]
- Delaplane, K. S., and Hood W. M.. 1999. Economic threshold for Varroa jacobsoni Oud. in the southeastern USA. Apidologie. 30: 383–395. [Google Scholar]
- Delaplane, K. S., Berry J. A., Skinner J. A., Parkman J. P., and Hood W. M.. . 2005. Integrated pest management against Varroa destructor reduces colony mite levels and delays treatment threshold. J. Apicult. Res. 44(4): 157–162. [Google Scholar]
- Delaplane, K. S., van der Steen J., and Guzman-Novoa E.. . 2013. Standard methods for estimating strength parameters of Apis mellifera colonies. J. Apicult. Res. 52(1): 1–12. [Google Scholar]
- Delaplane, K. S., Pietravalle S., Brown M. A., and Budge G. E.. . 2015. Honey bee colonies headed by hyperpolyandrous queens have improved brood rearing efficiency and lower infestation rates of parasitic Varroa mites. Plos One. 10: e0142985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delfinado-Baker, M., Rath W., and Boecking O.. . 1992. Phoretic bee mites and honeybee grooming behavior. Int. J. Acarol. 18: 315–322. [Google Scholar]
- Desai, S. D., Eu Y. J., Whyard S., and Currie R. W.. . 2012. Reduction in deformed wing virus infection in larval and adult honey bees (Apis mellifera L.) by double-stranded RNA ingestion. Insect Mol. Biol. 21: 446–455. [DOI] [PubMed] [Google Scholar]
- Dietemann, V., Pflugfelder J., Anderson D., Charrière J. D., Chejanovsky N., Dainat B., De Miranda J., Delaplane K., Dillier F-X., Fuch S., . et al. 2012. Varroa destructor: research avenues towards sustainable control. J. Apicult. Res. 51: 125–132. [Google Scholar]
- Dietemann, V., Nazzi F., Martin S. J., Anderson D. L., Locke B., Delaplane K. S., Wauquiez Q., Tannahill C., Frey E., Ziegelmann B., Rosenkranz P., . et al. 2013. Standard methods for Varroa research. J. Apicult. Res. 52: 1–54. [Google Scholar]
- Dillier, F. X., Flluri P., and Imdorf A.. . 2006. Review of the orientation behaviour in the bee parasitic mite Varroa destructor: Sensory equipment and cell invasion behaviour. Rev. Suisse Zool. 113(4): 857–877. [Google Scholar]
- Dong, K., Du Y., Rinkevich F., Nomura Y., Xu P., Wang L., Silver K., and Zhorov B. S.. . 2014. Molecular biology of insect sodium channels and pyrethroid resistance. Insect Biochem Mol Biol. 50: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Döke, M. A., Frazier M., and Grozinger C. M.. . 2015. Overwintering honey bees: biology and management. Curr. Opin. Insect Sci. 10: 185–193. [DOI] [PubMed] [Google Scholar]
- Donovan, B. J., and Paul F.. . 2005. Pseudoscorpions: the forgotten benficials inside beehives and their potential for management for control of varroa and other arthropod pests. Bee World. 86: 83–87. [Google Scholar]
- Donovan, B. J., and Paul F.. . 2006. Pseudoscorpions to the rescue? Am. Bee J. 146: 867–869. [Google Scholar]
- Donzé, G., and Guerin P. M.. . 1994. Behavioral attributes and parental care of Varroa mites parasitizing honeybee brood. Behav. Ecol. Sociobiol. 34: 305–319. [Google Scholar]
- Donzé, G., and Guerin P. M.. . 1997. Time-activity budgets and space structuring by the different life stag. J. Insect Behav. 10: 371–393. [Google Scholar]
- Donzé, G., Schnyder-Candrian S., Bogdanov S., Diehl P. A., Guerin P. M., Kilchenman V., and Monachon F.. . 1998. Aliphatic alcohols and aldehydes of the honey bee cocoon induce arrestment behavior in Varroa jacobsoni (Acari: Mesostigmata), an ectoparasite of Apis mellifera. Arch. Insect Biochem. Physiol. 37: 129–145. [Google Scholar]
- Doublet, V., Labarussias M., de Miranda J. R., Moritz R. F., and Paxton R. J.. . 2015. Bees under stress: sublethal doses of a neonicotinoid pesticide and pathogens interact to elevate honey bee mortality across the life cycle. Environ. Microbiol. 17: 969–983. [DOI] [PubMed] [Google Scholar]
- Dynes, T. L., Berry J. A., Delaplane K. S., Brosi B. J., and de Roode J. C.. . 2019. Reduced density and visually complex apiaries reduce parasite load and promote honey production and overwintering survival in honey bees. Plos One. 14: e0216286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easterbrook, M. A., Fitzgerald J. D., and Solomon M. G.. . 2001. Biological control of strawberry tarsonemid mite Phytonemus pallidus and two-spotted spider mite Tetranychus urticae on strawberry in the UK using species of Neoseiulus (Amblyseius) (Acari: Phytoseiidae). Exp. Appl. Acarol. 25: 25–36. [DOI] [PubMed] [Google Scholar]
- Egekwu, N. I., Posada F., Sonenshine D. E., and Cook S. C.. . 2018. Using an in vitro system for maintaining Varroa destructor mites on Apis mellifera pupae as hosts; studies of mite longevity and feeding behavior. Exp. Appl. Acarol. 74: 301–315. [DOI] [PubMed] [Google Scholar]
- Eguaras, M., Palacio M. A., Faverin C., Basualdo M., Del Hoyo M. L., Velis G., and Bedascarrasbure E.. . 2003. Efficacy of formic acid in gel for Varroa control in Apis mellifera L.: importance of the dispenser position inside the hive. Vet. Parasitol. 111: 241–245. [DOI] [PubMed] [Google Scholar]
- Eischen, F. A. 1998. Trials(and tribulations) with formic acid for varroa control. Am. Bee J. 138: 734–735. [Google Scholar]
- Eliash, N., Singh N. K., Kamer Y., Pinnelli G. R., Plettner E., and Soroker V.. . 2014. Can we disrupt the sensing of honey bees by the bee parasite Varroa destructor? Plos One. 9: e106889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis, J. D., and Munn P. A.. . 2005. The worldwide health status of honey bees. Bee World. 86(4): 88–101. [Google Scholar]
- Ellis, J. D., Delaplane K. S., and Hood W. M.. . 2001. Efficacy of a bottom screen device, Apistan (TM), and Apilife VAR (TM), in controlling Varroa destructor. Am. Bee J. 141: 813–816. [Google Scholar]
- Ellis, A. M., Hayes G. W., and Ellis J. D.. . 2009a. The efficacy of small cell foundation as a varroa mite (Varroa destructor) control. Exp. Appl. Acarol. 47: 311–316. [DOI] [PubMed] [Google Scholar]
- Ellis, A. M., Hayes G. W., and Ellis J. D.. . 2009b. The efficacy of dusting honey bee colonies with powdered sugar to reduce varroa mite populations. J. Apicult. Res. 48: 72–76. [Google Scholar]
- Elzen, P. J., and Westervelt D.. . 2002. Detection of coumaphos resistance in Varroa destructor in Florida. Am. Bee J. 142(4): 291–292. [Google Scholar]
- Elzen, P., and Westervelt D.. . 2004. A scientific note on reversion of fluvalinate resistance to a degree of susceptibility in Varroa destructor. Apidologie. 35: 519–520. [Google Scholar]
- Elzen, P. J., Eischen F. A., Baxter J. R., Pettis J., Elzen G. W., and Wilson W. T.. . 1998. Fluvalinate resistance in Varroa jacobsoni from several geographic locations. Am. Bee J. 138: 674–676. [Google Scholar]
- Elzen, P. J., Baxter J. R., Spivak M., and Wilson W. T.. . 1999. Amitraz resistance in Varroa: new discovery in North America. Am. Bee J. 139(5): 362. [Google Scholar]
- Elzen, P. J., Baxter J. R., Spivak M., and Wilson W. T.. . 2000. Control of Varroa jacobsoni Oud. resistant to fluvalinate and amitraz using coumaphos. Apidologie. 31: 437–441. [Google Scholar]
- Elzen, P. J., Baxter J. B., Westervelt D., Causey D., Randall C., Cutts L., and Wilson W. T.. . 2001. Acaricide rotation plan for control of varroa. Am. Bee J. 141: 412. [Google Scholar]
- Elzen, P. J., Westervelt D., and Lucas R.. . 2004. Formic acid treatment for control of Varroa destructor (Mesostigmata: Varroidae) and safety to Apis mellifera (Hymenoptera: Apidae) under southern United States conditions. J. Econ. Entomol. 97: 1509–1512. [DOI] [PubMed] [Google Scholar]
- Emsen, B., Guzman-Novoa E., and Kelly P. G.. . 2014. Honey production of honey bee (Hymenoptera: Apidae) colonies with high and low Varroa destructor (Acari: Varroidae) infestation rates in eastern Canada. Can. Entomol. 146: 236–240. [Google Scholar]
- Estroup, A., Soulignac M., and Cornuet J.. . 1994. Precise measurement of the number of patrilines and of genetics relatedness in honeybee colonies. Proc. Royal Soc. B. 258: 1–7. [Google Scholar]
- Evans, P. D., and Gee J. D.. . 1980. Action of formamidine pesticides on octopamine receptors. Nature. 287: 60–62. [DOI] [PubMed] [Google Scholar]
- Evans, J. D., and Spivak M.. . 2010. Socialized medicine: individual and communal disease barriers in honey bees. J. Invertebr. Pathol. 103(Suppl 1): S62–S72. [DOI] [PubMed] [Google Scholar]
- Fagan, L. L., Nelson W. R., Meenken E. D., Howlett B. G., Walker M. K., and Donovan B. J.. 2012. Varroa management in small bites. J. Appl. Entomol. 136: 473–475. [Google Scholar]
- Fakhimzadeh, K. 2001. The effects of powdered sugar varroa control treatments on Apis mellifera colony development. J. Apicult. Res. 40: 105–109. [Google Scholar]
- Fakhimzadeh, K., Ellis J. D., and Hayes J. W.. . 2011. Physical control of varroa mites (Varroa destructor): the effects of various dust materials on varroa mite fall from adult honey bees (Apis mellifera) in vitro. J. Apicult. Res. 50: 203–211. [Google Scholar]
- Fassbinder, C., Grodnitzky J., and Coats J.. . 2002. Monoterpenoids as possible control agents for Varroa destructor. J. Apicult. Res. 41: 83–88. [Google Scholar]
- Fisher, A., 2nd, and Rangel J.. . 2018. Exposure to pesticides during development negatively affects honey bee (Apis mellifera) drone sperm viability. Plos One. 13: e0208630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint, M. L. 2012. IPM in practice: principles and methods of integrated pest management. University of California Agriculture and Natural Resources,Oakland, CA. [Google Scholar]
- Floris, I., Satta A., Cabras P., Garau V. L., and Angioni A.. . 2004. Comparison between two thymol formulations in the control of Varroa destructor: effectiveness, persistence, and residues. J. Econ. Entomol. 97: 187–191. [DOI] [PubMed] [Google Scholar]
- Floris, I., Satta A., Garau V. L., Melis M., Cabras P., and Aloul N.. . 2001. Effectiveness, persistance, and residue of amitraz plastic strips in the apiary control of Varror destructor. Apidologie 32: 577–585. [Google Scholar]
- Flores, J. M., Gil S., and Padilla F.. . 2015. Reliability of the main field diagnostic methods of Varroa in honey bee colonies. Arch. Zootec. 64: 161–165. [Google Scholar]
- Fluri, P., Luscher M., Wille H., and Gerig L.. . 1982. Changes in weight of the pharyngeal gland and hemolymph titers of juvenile-hormone, protein and vitellogenin in worker honey bees. J. Insect Physiol. 28: 61–68. [Google Scholar]
- Food and Agriculture Organization of the United Nations . 2012. International Code of Conduct on the Distribution and Use of Pesticides – Guidelines on Prevention and Management of Pesticide Resistance, E-ISBN 978-92-5-107348-3. FAO, Rome, Italy. p. 55. [Google Scholar]
- Forlani, L., Pedrini N., Girotti J. R., Mijailovsky S. J., Cardozo R. M., Gentile A. G., Hernández-Suárez C. M., Rabinovich J. E., and Juárez M. P.. . 2015. Biological control of the chagas disease vector Triatoma infestans with the entomopathogenic fungus Beauveria bassiana combined with an aggregation cue: field, laboratory and mathematical modeling assessment. Plos Negl. Trop. Dis. 9: e0003778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier, M., Muli E., Conklin T., Schmehl D., Torto B., Frazier J., Tumlinson J., Evans J. D., and Raina S.. . 2010. A scientific note on Varroa destructor found in East Africa; threat or opportunity? Apidologie. 41: 463–465. [Google Scholar]
- Free, J. B., and Spencer-Booth Y.. . 1959. The longevity of worker honey bees (Apis mellifera). Proc. Royal Entomol. Soc. London. 34A: 141–150. [Google Scholar]
- Frey, E., and Rosenkranz P.. . 2014. Autumn invasion rates of Varroa destructor (Mesostigmata: Varroidae) into honey bee (Hymenoptera: Apidae) colonies and the resulting increase in mite populations. J. Econ. Entomol. 107: 508–515. [DOI] [PubMed] [Google Scholar]
- Frey, E., Schnell H., and Rosenkranz P.. . 2011. Invasion of Varroa destructor mites into mite-free honey bee colonies under the controlled conditions of a military training area. J. Apicult. Res. 50(20): 138–144. [Google Scholar]
- Fries, I., and Bommarco R.. . 2007. Possible host-parasite adaptations in honey bees infested by Varroa destructor mites. Apidologie. 38: 525–533. [Google Scholar]
- Fries, I., and Camazine S.. . 2001. Implications of horizontal and vertical pathogen transmission for honey bee epidemiology. Apidologie. 32: 199–214. [Google Scholar]
- Fries, I., and Rosenkranz P.. . 1996. Number of reproductive cycles of Varroa jacobsoni in honey-bee (Apis mellifera). Exp. Appl. Acarol. 20: 103–112. [Google Scholar]
- Fries, I., Aarhus A., Hansen H., and Korpela S.. . 1991. Comparison of diagnostic methods for detection of low infestation levels of Varroa jacobsoni in honeybee (Apis mellifera) colonies. Exp. Appl. Acarol. 1: 279–287. [Google Scholar]
- Fries, I., Imdorf A., and Rosenkranz P.. . 2006. Survival of mite infested (Varroa destructor) honey bee (Apis mellifera) colonies in a Nordic climate. Apidologie. 37: 564–570. [Google Scholar]
- Frisbee, R. E., and Luna J. M.. . 1989. Integrated pest management systems: protecting profits and the environment. Farm Management: The 1989 Yearbook of Agriculture, Washington, DC. p. 226–230. [Google Scholar]
- Frost, E. H., Shutler D., and Hillier N. K.. . 2013. Effects of fluvalinate on honey bee learning, memory, responsiveness to sucrose, and survival. J. Exp. Biol. 216: 2931–2938. [DOI] [PubMed] [Google Scholar]
- Fuchs, S. 1990. Preference for drone brood cells by Varroa jacobsoni Oud in colonies of Apis mellifera carnica. Apidologie. 21: 193–199. [Google Scholar]
- Fuchs, S., and Langenbach, K. 1989. Multiple infestation of Apis mellifera brood cells and reproduction in Varroa jacobsoni Oud. Apidologie. 20: 257–266. [Google Scholar]
- Fukuda, H., and Sekiguchi K.. . 1966. Seasonal change of the honeybee worker longevity in Sapporo, North Japan, with notes on some factors affecting the life span. Japan. J. Ecol. 16: 206–212. [Google Scholar]
- Garbian, Y., Maori E., Kalev H., Shafir S., and Sela I.. . 2012. Bidirectional transfer of RNAi between honey bee and Varroa destructor: Varroa gene silencing reduces Varroa population. Plos Pathog. 8: e1003035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido, C., Rosenkranz P., Paxton R. J., and Gonçalves L. S.. . 2003. Temporal changes in Varroa destructor fertility and haplotype in Brazil. Apidologie. 34: 535–541. [Google Scholar]
- Gashout, H. A., and Guzman-Novoa E.. . 2009. Acute toxicity of essential oils and other natural compounds to the parasitic mite, Varroa destructor, and to larval and adult worker honey bees (Apis mellifera L.). J. Apicult. Res. 48(4): 263–269. [Google Scholar]
- Gashout, H. A., Guzman-Novoa E., Goodwin P. H., and Correa-Benítez A.. . 2020. Impact of sublethal exposure to synthetic and natural acaricides on honey bee (Apis mellifera) memory and expression of genes related to memory. J. Insect Physiol. 121: 104014. [DOI] [PubMed] [Google Scholar]
- Giacomelli, A., Pietropaoli M., Carvelli A., Iacoponi F., and Formato G.. . 2016. Combination of thymol treatment (Apiguard®) and caging the queen technique to fight Varroa destructor. Apidologie. 47(4): 606–616. [Google Scholar]
- Gillespie, D. R. 1989. Biological control of thrips [Thysanoptera: Thripidae] on greenhouse cucumber by Amblyseius cucumeris. Entomophaga. 34: 185–192. [Google Scholar]
- Gilliam, M., Taber S. III, Lorenz B. J., and Prest D. B.. . 1988. Factors affecting development of chalkbrood disease in colonies of honey bees, Apis mellifera, fed pollen contaminated with Ascosphaera apis, J. Invertebr. Pathol. 52: 314–325. [Google Scholar]
- Giovenazzo, P., and Dubreuil P.. . 2011. Evaluation of spring organic treatments against Varroa destructor (Acari: Varroidae) in honey bee Apis mellifera (Hymenoptera: Apidae) colonies in eastern Canada. Exp. Appl. Acarol. 55: 65–76. [DOI] [PubMed] [Google Scholar]
- Giusti, M., Sabelli C., Di Donato A., Lamberti D., Paturzo C. E., Polignano V., Lazzari R., and Felicioli A.. . 2017. Efficacy and safety of varterminator, a new formic acid medicine against the Varroa mite. J. Apicult. Res. 56(2): 162–167. [Google Scholar]
- Glavan, G., and Božič J.. . 2013. The synergy of xenobiotics in honey bee Apis mellifera: Mechanisms and effects. Acta Biol. Slovenica. 56(1): 11–25. [Google Scholar]
- Goetz, B., and Koeniger N.. . 1993. The distance between larva and cell opening triggers brood cell invasion by Varroa jacobsoni. Apidologie. 24: 67–72. [Google Scholar]
- Gómez, R. G., Otero-Colina G., Villanueva-Jiménez J. A., Santillán-Galicia M. T., Peña-Valdivia C. B., and Santizo-Rincón J. A.. . 2016. Effects of neem (Azadirachta indica) on honey bee workers and queens, while applied to control Varroa destructor. J. Apicult. Res. 55(5),: 413–421. [Google Scholar]
- González-Cabrera, J., Davies T. G., Field L. M., Kennedy P. J., and Williamson M. S.. . 2013. An amino acid substitution (L925V) associated with resistance to pyrethroids in Varroa destructor. Plos One. 8: e82941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Cabrera, J., Rodríguez-Vargas S., Davies T. G. E., Field L. M., Schmehl D., Ellis J. D., Krieger K., and Williamson M. S.. . 2016. Novel mutations in the voltage-gated sodium channel of pyrethroid-resistant Varroa destructor populations from the Southeastern USA. PLoS One. 11(5): e0155332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Cabrera, J., Bumann H., Rodriguez-Vargas S., Kennedy P. J., Krieger K., . et al. 2018. A single mutation is driving resistance to pyrethroids in European populations of the parasitic mite, Varroa destructor. J. Pest Sci. 91: 1137–1144. [Google Scholar]
- Goodwin, M., and Van Eaton C.. . 2001. Control of Varroa: A guide for New Zealand Beekeepers. New Zealand Ministry of Agriculture and Forestry, Wellington, New Zealand. [Google Scholar]
- Goodwin, R. M., Taylor M. A., McBrydie H. M., and Cox H. M.. . 2005. Base levels of resistance to common control compounds by a New Zealand population of Varroa destructor. New Zeal. J. Crop Hortic. Sci. 33: 347–352. [Google Scholar]
- Goras, G., Tananaki C. H., Gounari S., Dimou M., Lazaridou E., Karazafiris E., Kanelis D., Liolios V., El Taj H. F., and Thrasyvoulou A.. . 2015. Hyperthermia—a non-chemical control strategy against varroa. J. Hellenic Vet. Med. Soc. 66(4): 249–256. [Google Scholar]
- Goswami, V., and Khan. M. S.. 2013. Management of varroa mite, Varroa destructor by essential oil and formic acid in Apis mellifera Linn. colonies. J. Nat. Prod. 6: 206–210. [Google Scholar]
- Gracia, M. J., Moreno C., Ferrer M., Sanz A., Peribáñez M. Á., and Estrada R.. . 2017. Field efficacy of acaricides against Varroa destructor. Plos One. 12: e0171633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracia-Salinas, M. J., Ferrer-Dufol M., Latorre-Castro E., Monero-Manera C., Castillo-Hernández J. A., Lucientes-Curd J., and Peribáñez-López M. A.. . 2006. Detection of fluvalinate resistance in Varroa destructor in Spanish apiaries. J. Apicult. Res. 45: 101–105. [Google Scholar]
- Gregorc, A., and Sampson B.. . 2019. Diagnosis of varroa mite (Varroa destructor) and sustainable control in honey bee (Apis mellifera) colonies—a review. Diversity. 11(12): 243. [Google Scholar]
- Gregorc, A., and Planinc I.. . 2001. Acaricidal effect of oxalic acid in honeybee (Apis mellifera) colonies. Apidologie. 32(4): 333–340. [Google Scholar]
- Gregorc, A., and Planinc I.. . 2012. Use of thymol formulations, amitraz, and oxalic acid for the control of the varroa mite in honey bee (Apis mellifera carnica) colonies. J. Apic. Sci. 56: 61–69. [Google Scholar]
- Gregorc, A., Pogacnik A., and Bowen I. D.. 2004. Cell death in honeybee (Apis mellifera) larvae treated with oxalic or formic acid. Apidologie. 35(5): 453–460. [Google Scholar]
- Gregorc, A., Adamczyk J., Kapun S., and Planinc I.. . 2016. Integrated Varroa control in honey bee (Apis mellifera carnica) colonies with or without brood. J. Apicult. Res. 55: 253–258. [Google Scholar]
- Gregorc, A., Alburaki M., Werle C., Knight P. R., and Adamczyk J.. . 2017. Brood removal or queen caging combined with oxalic acid treatment to control varroa mites (Varroa destructor) in honey bee colonies (Apis mellifera). Apidologie. 48(6): 821–832. [Google Scholar]
- Gregorc, A., Alburaki M., Sampson B., Knight P. R., and Adamczyk J.. . 2018. Toxicity of selected varroacides to honey bees (Apis mellifera) and varroa (Varroa destructor Anderson and Trueman) and their use in controlling Varroa within honey bee colonies. Insects. 9: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grozinger, C. M., and Flenniken M. L.. . 2019. Bee viruses: ecology, pathogenicity, and impacts. Annu. Rev. Entomol. 64: 205–226. [DOI] [PubMed] [Google Scholar]
- Grozinger, C. M., and Robinson G. E.. . 2015. The power and promise of applying genomics to honey bee health. Curr. Opin. Insect Sci. 10: 124–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guichard, M., Dietemann V., Neuditschko M., and Dainat B.. . 2020. Advances and perspectives in selecting resistance traits against the parasitic mite Varroa destructor in honey bees. Genet. Sel. Evol. 52: 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Guzman, L. I., Rinderer T. E., and Frake A. M.. . 2008. Comparative reproduction of Varroa destructor in different types of Russian and Italian honey bee combs. Exp. Appl. Acarol. 44: 227–238. [DOI] [PubMed] [Google Scholar]
- Guzmán-Novoa, E., Eccles L., Calvete Y., Mcgowan J., Kelly P. G., and Correa-Benítez A.. . 2010. Varroa destructor is the main culprit for the death and reduced populations of overwintered honey bee (Apis mellifera) colonies in Ontario, Canada. Apidologie. 41: 443–450. [Google Scholar]
- Guzman-Novoa, E., Emsen B., Unger P., Espinosa-Montaño L. G., and Petukhova T.. . 2012. Genotypic variability and relationships between mite infestation levels, mite damage, grooming intensity, and removal of Varroa destructor mites in selected strains of worker honey bees (Apis mellifera L.). J. Invertebr. Pathol. 110: 314–320. [DOI] [PubMed] [Google Scholar]
- Haarmann, T., Spivak M., Weaver D., Weaver B., and Glenn T.. . 2002. Effects of fluvalinate and coumaphos on queen honey bees (Hymenoptera: Apidae) in two commercial queen rearing operations. J. Econ. Entomol. 95: 28–35. [DOI] [PubMed] [Google Scholar]
- Haber, A. I., Steinhauer N. A., and vanEngelsdorp D.. . 2019. Use of chemical and nonchemical methods for the control of varroa destructor (Acari: Varroidae) and Associated Winter Colony Losses in U.S. Beekeeping Operations. J. Econ. Entomol. 112: 1509–1525. [DOI] [PubMed] [Google Scholar]
- Hamiduzzaman, M. M., Sinia A., Guzman-Novoa E., and Goodwin P. H.. . 2012. Entomopathogenic fungi as potential biocontrol agents of the ecto-parasitic mite, Varroa destructor, and their effect on the immune response of honey bees (Apis mellifera L.). J. Invertebr. Pathol. 111: 237–243. [DOI] [PubMed] [Google Scholar]
- Hamiduzzaman, M. M., Emsen B., Hunt G. J., Subramanyam S., Williams C. E., Tsuruda J. M., and Guzman-Novoa E.. . 2017. Differential Gene Expression Associated with Honey Bee Grooming Behavior in Response to Varroa Mites. Behav. Genet. 47: 335–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbo, J. R., and Harris J. W.. 1999. Selecting honey bees for resistance to Varroa jacobsoni. Apidologie. 30: 183–196. [Google Scholar]
- Harbo, J. R., and Harris J. W.. . 2001. Resistance to Varroa destructor (Mesostigmata: Varroidae) when mite-resistant queen honey bees (Hymenoptera: Apidae) were free-mated with unselected drones. J. Econ. Entomol. 94: 1319–1323. [DOI] [PubMed] [Google Scholar]
- Harbo, J. R., and Harris J. W.. 2004. Effect of screen floors on populations of honey bees and parasitic mites (Varroa destructor). J. Apicult. Res. 43: 114–117. [Google Scholar]
- Harbo, J. R., and Harris J. W.. 2005. Suppressed mite reproduction explained by the behaviour of adult bees. J. Apicult. Res. 44: 21–23. [Google Scholar]
- Harbo, J. R., and Hoopingarner R. A.. 1997. Honey bees (Hymenoptera:Apidae) in the United States that express resistance to Varroa jacobsoni (Mesostigmata:Varroidae). J. Econ. Entomol. 90: 893–898. [Google Scholar]
- Harris, J. W. 2007. Bees with Varroa sensitive hygiene preferentially remove mite infested pupae aged five days post capping. J. Apic. Res. 46: 134–139. [Google Scholar]
- Harris, J. W., and Harbo J. R.. 2000. Changes in reproduction of Varroa destructor after honey bee queens were exchanged between resistant and susceptible colonies. Apidologie. 31: 689–699. [Google Scholar]
- Hartfelder, K., Bitondi M. M. G., Brent C. S., Guidugli-Lazzarini K. R., Simões Z. L. P., Stabentheiner A., Tanaka É. D., and Wang Y.. . 2013. Standard methods for physiology and biochemistry research in Apis mellifera. J. Apicult. Res. 52: 1–48. [Google Scholar]
- Hatjina, F., and Haristos L.. . 2005. Indirect effects of oxalic acid administered by trickling method on honey bee brood. J. Apicult. Res. 44(4): 172–174. [Google Scholar]
- Higes, M., A.Meana.,Suarez M., and Llorente J.. . 1999. Negative long-term effects on bee colonies treated with oxalic acid against Varroa jacobsoni Oud. Apidologie. 30: 289–292. [Google Scholar]
- Higes, M., Martín-Hernández R., Hernández-Rodríguez C. S., and González-Cabrera J.. . 2020. Assessing the resistance to acaricides in Varroa destructor from several Spanish locations. Parasitol. Res. 119: 3595–3601. [DOI] [PubMed] [Google Scholar]
- Higley, L. G., and Peterson R. K. D.. 2009. “Economic decision rules for IPM”, 25–32. In: Radcliffe E. B., Hutchison W. D., and Cancelado R. E., (eds.), Integrated Pest Management: Concepts, tactics, strategies and case studies. Cambridge University Press, Cambridge. [Google Scholar]
- Honey Bee Health Coalition . 2018. Tools for Varroa management: a guide to effective Varroa sampling and control. Seventh Edition. The Keystone Policy Center on behalf of The Honey Bee Health Coalition, 1–29. https://honeybeehealthcoalition.org/wp-content/uploads/2018/06/HBHC-Guide_Varroa_Interactive_7thEdition_June2018.pdf. Accessed 10 March, 2021. [Google Scholar]
- Hoppe, H., and Ritter W.. . 1987. Experiments using combined heat therapy to control Varroa disease. Apidologie. 18: 383–385. [Google Scholar]
- Huang, Z. 2001. Mite Zapper – a new and effective method for Varroa mite control. Am. Bee J. 141: 730–732. [Google Scholar]
- Huang, Z. Y., and Robinson G. E.. . 1995. Seasonal changes in juvenile hormone titers and rates of biosynthesis in honey bees. J. Comp. Physiol. B. 165: 18–28. [DOI] [PubMed] [Google Scholar]
- Huang, Z. Y., Bian G., Xi Z., and Xie X.. . 2019. Genes important for survival or reproduction in Varroa destructor identified by RNAi. Insect Sci. 26: 68–75. [DOI] [PubMed] [Google Scholar]
- Hunt, G., Given J. K., Tsuruda J. M., and Andino G. K.. . 2016. Breeding mite-biting bees to control Varroa. Bee Culture. 8: 41–47. [Google Scholar]
- Hunter, W., Ellis J., Vanengelsdorp D., Hayes J., Westervelt D., Glick E., Williams M., Sela I., Maori E., Pettis J., . et al. 2010. Large-scale field application of RNAi technology reducing Israeli acute paralysis virus disease in honey bees (Apis mellifera, Hymenoptera: Apidae). Plos Pathog. 6: e1001160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim, A., and Spivak M.. . 2006. The relationship between hygienic behavior and suppression of mite reproduction as honey bee (Apis mellifera) mechanisms of resistance to Varroa destructor. Apidologie. 37: 31–40. [Google Scholar]
- Ibrahim, A., Reuter G. S., and Spivak M.. . 2007. Field trial of honey bee colonies bred for mechanisms of resistance against Varroa destructor. Apidologie. 38: 67–76. [Google Scholar]
- Ifantidis, M. D. 1983. Ontogenesis of the mite Varroa jacobsoni in worker and drone honeybee brood cells. J. Apicult. Res. 22: 200–226. [Google Scholar]
- Ifantidis, M. D. 1988. Some aspects of the process of varroa jacobsoni mite entrance into honey bee (Apis mellifera) brood cells. Apidologie. 19: 387–396. [Google Scholar]
- Imdorf, A., Bogdanov S., Kilchenmann V., and Maquelin C.. . 1995. Apilife Var- a new varroacide with thymol as the main ingredient. Bee World. 76: 77–83. [Google Scholar]
- Imdorf, A., Charrière J. D., Maquelin C., Kilchenmann V., and Bachofen B.. . 1996. Alternative Varroa control. Am. Bee J. 136: 189–193. [Google Scholar]
- Imdorf, A., Bogdanov S., Ochoa R. I., and Calderone N. W.. . 1999. Use of essential oils for the control of Varroa jacobsoni Oud. in honey bee colonies. Apidologie. 30(2–3): 209–228. [Google Scholar]
- Infantidis, M. O. 1983. Ontogenesis of the mite Varroa jacobsoni Oudemans in worker and drone brood cells of the honeybee. Apis mellifera cecropia. J. Apicult. Res. 3: 200–206. [Google Scholar]
- International Labour Organization . 2009. International Chemical Safety Card 0707 – Oxalic Acid Dihydrate. November 2009, https://www.ilo.org/dyn/icsc/showcard.display?p_version=2&p_card_id=0707. Accessed 22 November 2019.
- Iwasaki, J. M., Barratt B. I., Lord J. M., Mercer A. R., and Dickinson K. J.. . 2015. The New Zealand experience of varroa invasion highlights research opportunities for Australia. Ambio. 44: 694–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack, C. J., Sperry N., Mortensen A., and Ellis J. D.. . 2019. How to quantify Varroa destructor in honey bee (Apis mellifera L.) colonies. University of Florida IFAS Extension, ENY173, Gainesville, FL. [Google Scholar]
- Jack, C. J., van Santen E., and Ellis J. D.. . 2020a. Evaluating the efficacy of oxalic acid vaporization and brood interruption in controlling the honey bee pest Varroa destructor (Acari: Varroidae). J. Econ. Entomol. 113: 582–588. [DOI] [PubMed] [Google Scholar]
- Jack, C. J., Dai P. L., van Santen E., and Ellis J. D.. . 2020b. Comparing four methods of rearing Varroa destructor in vitro. Exp. Appl. Acarol. 80: 463–476. [DOI] [PubMed] [Google Scholar]
- Jack, C. J., van Santen E., and Ellis J. D.. . 2021. Determining the dose of oxalic acid applied via vaporization needed for the control of the honey bee (Apis mellifera) pest Varroa destructor. J. Apicult. Res. 60: 414–420. [Google Scholar]
- Johnson, R. M., Pollock H. S., and Berenbaum M. R.. . 2009. Synergistic interactions between in-hive miticides in Apis mellifera. J. Econ. Entomol. 102: 474–479. [DOI] [PubMed] [Google Scholar]
- Johnson, R. M., Ellis M. D., Mullin C. A., and Frazier M.. . 2010. Pesticides and honey bee toxicity - USA. Apidologie. 41(3): 312–331. [Google Scholar]
- Johnson, R. M., Dahlgren L., Siegfried B. D., and Ellis M. D.. . 2013a. Acaricide, fungicide and drug interactions in honey bees (Apis mellifera). PLoS One. 8(1): e54092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, R. M., Dahlgren L., Siegfried B. D., and Ellis M. D.. . 2013b. Effect of in-hive miticides on drone honey bee survival and sperm viability. J. Apicult. Res. 52: 88–95. [Google Scholar]
- Jones, G., Campbell C. A. M., Pye B. J., Maniar S. P., and Mudd A.. . 1996. Repellent and oviposition-deterring effects of hop beta-acids on the two-spotted spider mite Tetranychus urticae. Pest. Sci. 47: 165–169. [Google Scholar]
- Kamler, M., Nesvorna M., Stara J., Erban T., and Hubert J.. . 2016. Comparison of tau-fluvalinate, acrinathrin, and amitraz effects on susceptible and resistant populations of Varroa destructor in a vial test. Exp. Appl. Acarol. 69: 1–9. [DOI] [PubMed] [Google Scholar]
- Kanga, L. H. B., Jones W. A., and James R. R.. . 2003. Field trials using the fungal pathogen, Metarhizium anisopliae (Deuteromycetes: Hyphomycetes) to control the ectoparasitic mite, Varroa destructor (Acari: Varroidae) in honey bee, Apis mellifera (Hymenoptera: Apidae) colonies. J. Econ. Entomol. 96: 1091–109. [DOI] [PubMed] [Google Scholar]
- Kast, C., Kilchenmann V., and Droz B.. . 2020. Distribution of coumaphos in beeswax after treatment of honeybee colonies with CheckMite (R) against the parasitical mite Varroa destructor. Apidologie. 51: 112–122. [Google Scholar]
- Kefuss, J., Vanpoucke J., De Lahitte J. D., and Ritter W.. . 2004. Varroa tolerance in France of intermissa bees from Tunisia and their naturally mated descendants: 1993–2004. Am. Bee J. 144: 563–568. [Google Scholar]
- Kefuss, J., Vanpoucke J., Bolt M., and Kefuss C.. . 2009. Practical Varroa resistance selection for beekeepers. Abstracts 41st Apimondia congress 15–20.09, Montpellier, pp. 82. [Google Scholar]
- Khongphinitbunjong, K., de Guzman L. I., Rinderer T. E., Tarver M. R., Frake A. M., Chen Y. P., and Chantawannakul P.. . 2016. Responses of Varroa-resistant honey bees (Apis mellifera L.) to Deformed wing virus. J. Asia-Pac. Entomol. 19(4): 921–927. [Google Scholar]
- Kim, Y. J., Lee S. H., Lee S. W., and Ahn Y. J.. . 2004. Fenpyroximate resistance in Tetranychus urticae (Acari: Tetranychidae): cross-resistance and biochemical resistance mechanisms. Pest Manag. Sci. 60: 1001–1006. [DOI] [PubMed] [Google Scholar]
- Kirrane, M. J., de Guzman L. I., Whelan P. M., Frake A. M., and Rinderer T. E.. . 2018. Evaluations of the removal of Varroa destructor in russian honey bee colonies that display different levels of varroa sensitive hygienic activities. J. Insect Behav. 31: 283–297. [Google Scholar]
- Kleanthus, D., Papafotiou M., Christodoulou E., Papandreou G., and Thrasyvoulou A.. . 1999. Resistance of Varroa to fluvalinate in Cyprus. Melis Epitheor. 13(6): 260–263. [Google Scholar]
- Kralj, J., and Fuchs S.. . 2006. Parasitic Varroa destructor mites influence flight duration and homing ability of infested Apis mellifera foragers. Apidologie. 37: 577–587. [Google Scholar]
- Kretzschmar, A., Durand E., Maisonnasse A., Vallon J., and Le Conte Y.. . 2015. A new stratified sampling procedure which decreases error estimation of Varroa Mite number on sticky boards. J. Econ. Entomol. 108: 1435–1443. [DOI] [PubMed] [Google Scholar]
- Kuenen, L. P. S., and Calderone N. W.. 1997. Transfers of Varroa mites from newly emerged bees: Preferences for age. J. Insect Behav. 10: 213–228. [Google Scholar]
- Kulincevic, J. M., Rinderer T. E., and Mladjan V. J.. . 1991. Effects of fluvalinate and amitraz on bee lice (Braula coeca Nitzsch) in honey bee (Apis mellifera L.) colonies in Yugoslavia. Apidologie. 22: 43–47. [Google Scholar]
- Kulhanek, K., Steinhauer N., Rennich K., Caron D. M., Sagili R. R., Pettis J. S., Ellis J. D., Wilson M. E., Wilkes J. T., Tarpy D. R., . et al. 2017. A national survey of managed honey bee 2015–2016 annual colony losses in the USA. J. Apicult. Res. 56(4): 328–340. [Google Scholar]
- Lattorff, H. M., Buchholz J., Fries I., and Moritz R. F.. . 2015. A selective sweep in a Varroa destructor resistant honeybee (Apis mellifera) population. Infect. Genet. Evol. 31: 169–176. [DOI] [PubMed] [Google Scholar]
- Le Conte, Y., Arnold G., and Desenfant Ph.. . 1990. Influence of brood temperature and hygrometry variations on the development of the honey bee ectoparasite Varroa jacobsoni (Mesostigmata: Varroidae). Environ. Entomol. 19: 1780–5. [Google Scholar]
- Le Conte, Y., Ellis M., and Ritter W.. . 2010. Varroa mites and honey bee health: can Varroa explain part of the colony losses? Apidologie. 41: 353–363. [Google Scholar]
- Le Conte, Y., Meixner M. D., Brandt A., Carreck N. L., Costa C., Mondet F., and Büchler R.. . 2020. Geographical distribution and selection of European honey bees resistant to Varroa destructor. Insects. 11: 873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclercq, G., Francis F., Gengler N., and Blacquière T.. . 2018a. Bioassays to quantify hygienic behavior in honey bee (Apis mellifera L.) colonies: a review. J. Apicult. Res. 57(5): 663–673. [Google Scholar]
- Leclercq, G., Blacquière T., Gengler N., and Francis F.. . 2018b. Hygienic removal of freeze-killed brood does not predict Varroa-resistance traits in unselected stocks. J. Apicult. Res. 57(2): 292–299. [Google Scholar]
- Lee, K. V., Moon R. D., Burkness E. C., Hutchison W. D., and Spivak M.. . 2010. Practical sampling plans for Varroa destructor (Acari: Varroidae) in Apis mellifera (Hymenoptera: Apidae) colonies and apiaries. J. Econ. Entomol. 103: 1039–1050. [DOI] [PubMed] [Google Scholar]
- Lee, K. V., Steinhauer N., Rennich K., Wilson M. E., Tarpy D. R., Caron D. M., Rose R., Delaplane K. S., Baylis K., Lengerich E. J., . et al. 2015a. A national survey of managed honey bee 2013–2014 annual colony losses. Apidologie. 46: 292–305. [Google Scholar]
- Lee, S. J., Kim, S., Yu J. S., Kim J. C., Nai Y. S., and Kim J. S.. . 2015b. Biological control of Asian tiger mosquito, Aedes albopictus (Diptera: Culicidae) using Metarhizium anisopliae JEF-003 millet grain. J. Asia-Pac. Entomol. 18: 217–221. [Google Scholar]
- Leonard, S. P., Powell J. E., Perutka J., Geng P., Heckmann L. C., Horak R. D., Davies B. W., Ellington A. D., Barrick J. E., and Moran N. A.. . 2020. Engineered symbionts activate honey bee immunity and limit pathogens. Science. 367: 573–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, Z. G., Su X. L., Wang, S.Ji, T., Hu F. L., and Zheng H. Q.. . 2020. Fumigant toxicity of eleven Chinese herbal essential oils against an ectoparasitic mite (Varroa destructor) of the honey bee (Apis mellifera). J. Apicult. Res. 59(2): 204–210. [Google Scholar]
- Lindberg, C. M., Melathopoulos A. P., and Winston M. L.. . 2000. Laboratory evaluation of miticides to control Varroa jacobsoni (Acari: Varroidae), a honey bee (Hymenoptera: Apidae) parasite. J. Econ. Entomol. 93: 189–198. [DOI] [PubMed] [Google Scholar]
- Locke, B., and Fries I.. . 2011. Characteristics of honey bee colonies (Apis mellifera) in Sweden surviving Varroa destructor infestation. Apidologie. 42: 533–542. [Google Scholar]
- Locke, B., Forsgren E., Fries I., and de Miranda J. R.. . 2012. Acaricide treatment affects viral dynamics in Varroa destructor-infested honey bee colonies via both host physiology and mite control. Appl. Environ. Microbiol. 78: 227–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locke, B., Forsgren E., and de Miranda J. R.. . 2014. Increased tolerance and resistance to virus infections: a possible factor in the survival of Varroa destructor-resistant honey bees (Apis mellifera). Plos One. 9: e99998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodesani, M., and Costa C.. . 2008. Maximizing the efficacy of a thymol based product against the mite Varroa destructor by increasing the air space in the hive. J. Apic. Res. 47: 113–117. [Google Scholar]
- Lodesani, M., Pellacani A., Bergomi S., Carpana E., Rabitti T., and Lasagni P.. . 1992. Residue determination for some products used against Varroa infestation in bees. Apidologie. 23: 257–272. [Google Scholar]
- Lodesani, M., Colombo M., and Spreafico M.. . 1995. Ineffectiveness of Apistan(R) treatment against the mite Varroa jacobsoni Oud. in several districts of Lombardy (Italy). Apidologie. 26: 67–72. [Google Scholar]
- Lodesani, M., Vecchi M. A., Tommasini S., and Bigliardi M.. . 1996. A study on different kinds of damage to Varroa jacobsoni in Apis mellifera ligustica colonies. J. Apicult. Res. 35: 49–56. [Google Scholar]
- Lodesani, M., Costa C., Besana A., Dall’Olio D., Franceschetti S., Tesoriero D., and Vaccari G.. . 2014. Impact of control strategies for Varroa destructor on colony survival and health in northern and central regions of Italy. J. Apicult. Res. 53(1): 155–164. [Google Scholar]
- Macedo, P. A., Wu J., and Ellis M. D.. . 2002. Using inert dusts to detect and assess varroa infestations in honey bee colonies. J. Apicult. Res. 41: 3–7. [Google Scholar]
- Maggi, M. D., Ruffinengo S. R., Damiani N., Sardella N. H., and Eguaras M. J.. . 2009. First detection of Varroa destructor resistance to coumaphos in Argentina. Exp. Appl. Acarol. 47: 317–320. [DOI] [PubMed] [Google Scholar]
- Maggi, M. D., Ruffinengo S. R., Negri P., and Eguaras M. J.. . 2010. Resistance phenomena to amitraz from populations of the ectoparasitic mite Varroa destructor of Argentina. Parasitol. Res. 107: 1189–1192. [DOI] [PubMed] [Google Scholar]
- Maggi, M., Tourn E., Negri P., Szawarski N., Marconi A., Gallez L., Medici S., Ruffinengo S., Brasesco C., De Feudis L., . et al. 2016. A new formulation of oxalic acid for Varroa destructor control applied in Apis mellifera colonies in the presence of brood. Apidologie. 47: 596–605. [Google Scholar]
- Maggi, M. D., Damiani N., Ruffinengo S. R., Brasesco M. C., Szawarski N., Mitton G., Mariani F., Sammataro D., Quintana S., and Eguaras M. J.. . 2017. The susceptibility of Varroa destructor against oxalic acid: a study case. Bull. Insectol. 70(1): 39–44. [Google Scholar]
- Maggi, M. D., Ruffinengo S. R., Gende L. B., Eguaras M. J., and Sardella N. H.. . 2008. LC50 baseline levels of amitraz, coumaphos, fluvalinate and flumethrin in populations of Varroa destructor from Buenos Aires Province, Argentina. J. Apicult. Res. 47(4): 292–295. [Google Scholar]
- Manning, R. 1996. Packaged honey bees: the export market, package bee research and economics of the enterprise. A reference for Western Australian beekeepers. Western Australian Department of Agriculture 17/96, South Perth, Western Australia. pp. 59. [Google Scholar]
- Maori, E., Paldi N., Shafir S., Kalev H., Tsur E., Glick E., and Sela I.. . 2009. IAPV, a bee-affecting virus associated with Colony Collapse Disorder can be silenced by dsRNA ingestion. Insect Mol. Biol. 18: 55–60. [DOI] [PubMed] [Google Scholar]
- Marchetti, S., and Barbattini R.. . 1984. Comparative effectiveness of treatments used to control Varroa jacobsoni Oud. Apidologie. 15: 363–378. [Google Scholar]
- Marčić, D., and Međo I.. . 2014. Acaricidal activity and sublethal effects of an oxymatrine-based biopesticide on two-spotted spider mite (Acari: Tetranychidae). Exp. Appl. Acarol. 64: 375–391. [DOI] [PubMed] [Google Scholar]
- Martel, A. C., Zeggane S., Aurieres C., Drajnudel P., Faucon J. P., and Aubert M.. . 2007. Acaricide residues in honey and wax after treatment of honey bee colonies with Apivar® or Asuntol®50. Apidologie. 38: 534–544. [Google Scholar]
- Martin, S. J. 1994. Ontogeny of the mite Varroa jacobsoni Oud in worker brood of the honeybee Apis mellifera L. under natural conditions. Exp. Appl. Acarol. 18: 87–100. [Google Scholar]
- Martin, S. J. 1995. Ontogeny of the mite Varroa jacobsoni Oud in drone brood of the honeybee Apis mellifera L. under natural conditions. Exp. Appl. Acarol. 19: 199–210. [Google Scholar]
- Martin, S., and Kemp D.. . 1997. Average number of reproductive cycles performed by Varroa jacobsoni in honey bee (Apis mellifera colonies). J. Apicult. Res. 36: 113–123. [Google Scholar]
- Martin, S. J., Hawkins G. P., Brettell L. E., Reece, N., Correia-Oliveira M. E., and Allsopp M. H.. . 2019. Varroa destructor reproduction and cell re-capping in mite-resistant Apis mellifera populations. Apidologie. 51:369–381. [Google Scholar]
- Martin, S. J., Highfield A. C., Brettell L., Villalobos E. M., Budge G. E., Powell M., Nikaido S., and Schroeder D. C.. . 2012. Global honey bee viral landscape altered by a parasitic mite. Science. 336: 1304–1306. [DOI] [PubMed] [Google Scholar]
- Masterman, R., Ross R., Mesce K., and Spivak M.. . 2001. Olfactory and behavioral response thresholds to odors of diseased brood differ between hygienic and non-hygienic honey bees (Apis mellifera L.). J. Comparat. Physiol. A. 187(6): 441–452. [DOI] [PubMed] [Google Scholar]
- Mathieu, L., and Faucon J. P.. 2000. Changes in the response time for Varroa jacobsoni exposed to amitraz. J. Apicult. Res. 39: 155–158. [Google Scholar]
- Maucourt, S., Fortin F., Robert C., and Giovenazzo P.. . 2020. Genetic parameters of honey bee colonies traits in a Canadian selection program. Insects. 11: 587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maul, V., Klepsch A., and Assmannwerthmuller U.. . 1988. The trapping comb technique as part of bee management under strong infestation by Varroa jacobsoni Oud. Apidologie. 19(2): 139–154. [Google Scholar]
- Maurizio, A. 1950. The influence of pollen feeding and brood rearing on the length of life and physiological conditions of the honeybee. Bee World. 31: 9–12. [Google Scholar]
- McMenamin, A. J., and Genersch E.. . 2015. Honey bee colony losses and associated viruses. Curr. Opin. Insect Sci. 8: 121–129. [DOI] [PubMed] [Google Scholar]
- Medici, S. K., Castro A., Sarlo E. G., Marioli J. M., and Eguaras M. J.. . 2012. The concentration effect of selected acaricides present in beeswax foundation on the survival of Apis mellifera colonies. J. Apicult. Res. 51: 164–168. [Google Scholar]
- Medici, S. K., Maggi M. D., Sarlo E. G., Ruffinengo S., Marioli J. M., and Eguaras M. J.. . 2016. The presence of synthetic acaricides in beeswax and its influence on the development of resistance in Varroa destructor. J. Apicult. Res. 54(3): 267–274. [Google Scholar]
- Meikle, W. G., Sammataro D., Neumann P., and Pflugfelder J.. . 2012. Challenges for developing pathogen-based biopesticides against Varroa. Apidologie. 43: 501–514. [Google Scholar]
- Melathopoulos, A. P., and Gates J.. . 2003. Comparison of two thymol-based acaricides, Apilifevar and Apiguard, for the control of Varroa mites. Am. Bee J. 143: 489–493. [Google Scholar]
- Message, D., and Goncalves L. S.. . 1995. Effect of the size of worker brood cells of africanized honey bees on infestation and reproduction of the ectoparasitic mite Varroa jacobsoni Oud. Apidologie. 26: 381–386. [Google Scholar]
- Milne, C. P.Jr. 1983. Honey bee (Hymenoptera: Apidae) hygienic behavior and resistance to chalkbrood. Ann. Entomol. Soc. Am. 76: 384–387. [Google Scholar]
- Milone, J. P., Rinkevich F. D., McAfee A., Foster L. J., and Tarpy D. R.. . 2020. Differences in larval pesticide tolerance and esterase activity across honey bee (Apis mellifera) stocks. Ecotoxicol. Environ. Saf. 206: 111213. [DOI] [PubMed] [Google Scholar]
- Mitton, G. A., Quintana S., Giménez-Martínez P., Mendoza Y., Ramallo G., Brasesco C., Villalba A., Eguaras M., Maggi M. D., and Ruffinengo S.. . 2016. First record of resistance to flumethrin in a varroa population from Uruguay. J. Apicult. Res. 55(5): 422–427. [Google Scholar]
- Mitton, G. A., Szawarski N., Ramos F., Fuselli S., Meroi-Arcerito F. R., Eguaras M. J., Ruffinengo S. R., and Maggi M. D.. . 2018. Varroa destructor: when reversion to coumaphos resistance does not happen. J. Apicult. Res. 57(4): 536–540. [Google Scholar]
- Mohammadbeigi, A., and Port G.. . 2015. Effect of Infection by Beauveria bassiana and Metarhizium anisopliae on the feeding of Uvarovistia zebra. J. Insect Sci. 15(1): 88. [Google Scholar]
- Moon, R. D., and Wilson L. T.. . 2009. “Sampling for detection, estimation and IPM decision making”, pp. 75–89InRadcliffe E. B., Hutchison W. D., and Cancelado R. E., (ed), Integrated pest management: concepts, tactics, strategies and case studies. Cambridge University Press, Cambridge. [Google Scholar]
- Morawetz, L., Köglberger H., Griesbacher A., Derakhshifar I., Crailsheim K., Brodschneider R., and Moosbeckhofer R.. . 2019. Health status of honey bee colonies (Apis mellifera) and disease-related risk factors for colony losses in Austria. Plos One. 14: e0219293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretto, G., Goncalves L. S., and Dejong D.. . 1993. Heritability of africanized and european honey-bee defensive behavior against the mite Varroa jacobsoni. Rev. Brasil. Genet. 16: 71–77. [Google Scholar]
- Morfin, N., Given K., Evans M., Guzman-Novoa E., and Hunt G. J.. . 2020. Grooming behavior and gene expression of the Indiana “mite-biter” honey bee stock. Apidologie. 51: 267–275. [Google Scholar]
- Morse, R. A., and Calderone N. W.. . 2000. The value of honey bees as pollinators of U.S. crops in 2000. Bee Culture. 128: 1–15. [Google Scholar]
- Mortensen, A. N., and Ellis J. D.. 2018. The effects of artificial rearing environment on the behavior of adult honey bees, Apis mellifera L. Behav. Ecol. Sociobiol. 72: 92. [Google Scholar]
- Mortensen, A. N., Jack C. J., and Ellis J. D.. . 2018. The discovery of Varroa destructor on drone honey bees, Apis mellifera, at drone congregation areas. Parasitol. Res. 117: 3337–3339. [DOI] [PubMed] [Google Scholar]
- Mortensen, A. N., Bruckner S., Williams G. R., and Ellis J. D.. . 2019. Comparative morphology of adult honey bees, Apis mellifera, reared in vitro or by their parental colony. J. Apicult. Res. 58(4): 580–586. [Google Scholar]
- Mossebeckhofer, R., and Derakhshifar I.. . 1986. Comparison of the effectiveness of perizin, folbex-va and formic acid treatments for controlling Varroa jacobsoni in honey bee colony nuclei. Apidologie. 17(4): 376–379. [Google Scholar]
- Mozes-Koch, R., Slabezki Y., Efrat H., Kalev H., Kamer Y., Yakobson B. A., and Dag A.. . 2000. First detection in Israel of fluvalinate resistance in the varroa mite using bioassay and biochemical methods. Exp. Appl. Acarol. 24: 35–43. [DOI] [PubMed] [Google Scholar]
- Mullin, C. A., Frazier M., Frazier J. L., Ashcraft S., Simonds R., Vanengelsdorp D., and Pettis J. S.. . 2010. High levels of miticides and agrochemicals in North American apiaries: implications for honey bee health. Plos One. 5: e9754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanetti, A., Besana A. M., Baracani G., Romanelli R., and Galuppi R.. . 2011. Artificial brood interruption in combination with oxalic acid trickling in the control of varroa mite. In Proceedings of 42nd International Apicultural Congress, Buenos Aires, Argentina. [Google Scholar]
- Navajas, M., Migeon A., Alaux C., Martin-Magniette M., Robinson G., Evans J., Cros-Arteil S., Crauser D., and Le Conte Y.. . 2008. Differential gene expression of the honey bee Apis mellifera associated with Varroa destructor infection. BMC Genomics. 9: 301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazzi, F., and Le Conte Y.. . 2016. Ecology of Varroa destructor, the Major Ectoparasite of the Western Honey Bee, Apis mellifera. Annu. Rev. Entomol. 61: 417–432. [DOI] [PubMed] [Google Scholar]
- Nazzi, F., and Milani N.. . 1994. A technique for reproduction of Varroa jacobsoni Oud under laboratory conditions. Apidologie. 25: 579–584. [Google Scholar]
- Niu, J., Shen G., Christiaens O., Smagghe G., He L., and Wang J.. . 2018. Beyond insects: current status and achievements of RNA interference in mite pests and future perspectives. Pest Manag. Sci. 74: 2680–2687. [DOI] [PubMed] [Google Scholar]
- Noël, A., Le Conte Y., and Mondet F.. . 2020. Varroa destructor: how does it harm Apis mellifera honey bees and what can be done about it? Emerg. Top. Life Sci. 4: 45–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan, M. P., and Delaplane K. S.. . 2017. Distance between honey bee Apis mellifera colonies regulates populations of Varroa destructor at a landscape scale. Apidologie. 48: 8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes, F. M., Aleixo A. C., Barchuk A. R., Bomtorin A. D., Grozinger C. M., and Simões Z. L.. . 2013. Non-target effects of Green Fluorescent Protein (GFP)-Derived Double-Stranded RNA (dsRNA-GFP) used in honey bee RNA interference (RNAi) Assays. Insects. 4: 90–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neil, R. J., and Obrycki J. J.. . 2009. “Introduction and augmentation of biological control agents”, pp. 107–115InRadcliffe E. B., Hutchison W. D., and Cancelado R. E., (eds.), Integrated pest management: concepts, tactics, strategies and case studies. Cambridge University Press, Cambridge. [Google Scholar]
- O’Neal, S. T., Brewster C. C., Bloomquist J. R., and Anderson T. D.. . 2017. Amitraz and its metabolite modulate honey bee cardiac function and tolerance to viral infection. J. Invertebr. Pathol. 149: 119–126. [DOI] [PubMed] [Google Scholar]
- OECD . 1998. Test No. 214: honeybees, acute contact toxicity test. OECD Guidelines for the Testing of Chemicals, Section 2. 1998: 2–4. OECD Publishing, Paris, France. https://read.oecd-ilibrary.org/environment/test-no-214-honeybees-acute-contact-toxicity-test_9789264070189-en#page1 [Google Scholar]
- Oddie, M., Büchler R., Dahle B., Kovacic M., Le Conte Y., Locke B., de Miranda J. R., Mondet F., and Neumann P.. . 2018. Rapid parallel evolution overcomes global honey bee parasite. Sci. Rep. 8: 7704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oddie, M., Neumann P., and Dahle B.. . 2019. Cell size and Varroa destructor mite infestations in susceptible and naturally-surviving honeybee (Apis mellifera) colonies. Apidologie. 50: 1–10. [Google Scholar]
- Oliver, R. 2014. Amitraz: red flags or red herrings. Am. Bee J. 154: 1119–1112. [Google Scholar]
- Olmstead, S., Menzies C., McCallum R., Glasgow K., and Cutler C.. . 2019. Apivar® and Bayvarol® suppress varroa mites in honey bee colonies in Canadian Maritime Provinces. J. Acad. Entomol. Soc. 15: 46–49. [Google Scholar]
- Ontario Ministry of Agriculture, Food and Rural Affairs . 2020. Varroa mite—sampling and monitoring infestation levels. Omafra.gov.on.ca. www.omafra.gov.on.ca/english/food/inspection/bees/varroa-sampling.htm. Accessed 3 June 2020. [PMC free article] [PubMed]
- Ostermann, D. J., and Currie R. W.. . 2004. Effect of formic acid formulations on honey bee (Hymenoptera: Apidae) colonies and influence of colony and ambient conditions on formic acid concentration in the hive. J. Econ. Entomol. 97: 1500–1508. [DOI] [PubMed] [Google Scholar]
- Ostiguy, N., and Sammataro D.. . 2000. A simplified technique for counting Varroa jacobsoni Oud. on sticky boards. Apidologie. 31: 707–716. [Google Scholar]
- Ostiguy, N., Drummond F. A., Aronstein K., Eitzer B., Ellis J. D., Spivak M., and Shepherd W. S.. . 2019. Honey bee exposure to pesticides: a four-year nationwide study. Insects. 10(1): 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostlie, K. R., and Pedigo L. P.. . 1987. Incorporating pest survivorship into economic thresholds for pest management. J. Econ. Entomol. 80: 297–303. [Google Scholar]
- Oxley, P. R., Spivak M., and Oldroyd B. P.. . 2010. Six quantitative trait loci influence task thresholds for hygienic behaviour in honeybees (Apis mellifera). Mol. Ecol. 19: 1452–1461. [DOI] [PubMed] [Google Scholar]
- Palacio, M. A., Figini E. E., Ruffinengo S. R., Rodriguez E. M., del Hoyo M. L., and Bedascarasbure E. L.. . 2000. Changes in a population of Apis mellifera L. selected for hygienic behaviour and its relation to brood disease tolerance. Apidologie. 31: 471–478. [Google Scholar]
- Panziera, D., Langevelde F., and Blacquière T.. . 2017. Varroa sensitive hygiene contributes to naturally selected varroa resistance in honey bees. J. Apicult. Res. 56(5): 635–642. [Google Scholar]
- Papezikova, I., Palikova M., Kremserova S., Zachova A., Peterova H., Babak V., and Navratil S.. . 2017. Effect of oxalic acid on the mite Varroa destructor and its host the honey bee Apis mellifera. J. Apicult. Res. 56(4): 400–408. [Google Scholar]
- Peck, D. T., and Seeley T. D.. . 2019. Mite bombs or robber lures? The roles of drifting and robbing in Varroa destructor transmission from collapsing honey bee colonies to their neighbors. Plos One. 14: e0218392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck, D. T., Smith M. L., and Seeley T. D.. . 2016. Varroa destructor mites can nimbly climb from flowers onto foraging honey bees. Plos One. 11: e0167798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedigo, L. P. 1995. Closing the gap between IPM theory and practice. J. Agric. Entomol. 12(4): 171–181. [Google Scholar]
- Pedigo, L. P., and Rice M. E.. 2009. Entomology and pest management, 6th ed. Pearson Education, Inc, Upper Saddle River, NJ. [Google Scholar]
- Pedigo, L. P., Hutchins S. H., and Higley L. G.. . 1986. Economic injury levels in theory and practice. Ann. Rev. Entomol. 31: 341–368. [Google Scholar]
- Peng, Y. S., Fang Y. Z., Xu S. Y., and Ge L. S.. . 1987. The resistance mechanism of the Asian honey bee, Apis cerana Fabr., to an ectoparasitic mite, Varroa jacobsoni Oudemans. J. Inverteb. Pathol. 49: 54–60. [Google Scholar]
- Perez-Santiago, G., Otero-Colina G., Mota-Sanchez D., Ramírez Guzmán M. E., and Vandame R.. . 2000. Comparing effects of three acaricides on Varroa jacobsoni (Acari: Varroidae) and Apis mellifera (Hymenoptera: Apidae) using two application techniques. Fla. Entomol. 83(4): 468–476. [Google Scholar]
- Pernal, S., Sewalem A., and Melathopoulos A.. . 2012. Breeding for hygienic behaviour in honeybees (Apis mellifera) using free-mated nucleus colonies. Apidologie. 43: 403–424. [Google Scholar]
- Pettis, J. S. 2004. A scientific note on Varroa destructor resistance to coumaphos in the United States. Apidologie. 35: 91–92. [Google Scholar]
- Pettis, J. S., and Shimanuki H.. . 1999. A hive modification to reduce varroa populations. Am. Bee J. 139: 471–473. [Google Scholar]
- Pettis, J., Shimanuki H., and Feldlaufer M.. . 1998. An assay to detect fluvalinate resistance in Varroa mites. Am. Bee J. 138: 538–541. [Google Scholar]
- Pietropaoli, M., and Formato G.. . 2019. Acaricide efficacy and honey bee toxicity of three new formic acid-based products to control Varroa destructor. J. Apicult. Res. 58(5): 824–830. [Google Scholar]
- Pietropaoli, M., Giacomelli A., Milito M., Gobbi C., Scholl F., and Formato G.. . 2012. Integrated pest management strategies against Varroa destructor, the use of oxalic acid combined with innovative cages to obtain the absence of brood. Eur. J. Int. Med. 4(1): 93. [Google Scholar]
- Piou, V., Tabart J., Urrutia V., Hemptinne J. L., and Vétillard A.. . 2016. Impact of the phoretic phase on reproduction and damage caused by Varroa destructor (Anderson and Trueman) to Its Host, the European Honey Bee (Apis mellifera L.). Plos One. 11: e0153482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plapp, F. W.Jr, and Vinson S. B.. . 1977. Comparative toxicities of some insecticides to the tobacco budworm and its ichneumonid parasite, Campoletis sonorensis. Environ. Entomol. 6: 381–384. [Google Scholar]
- Plettner, E., Eliash N., Singh N. K., Pennelli G. R., and Soroker V.. . 2017. The chemical ecology of host-parasite interaction as a target of Varroa destructor control agents. Apidologie. 48: 78–92. [Google Scholar]
- Popov, E. T., Melnik V. N., and Matchinev V. N.. . 1989. Application of oxalic acid in varroatosis. Proceedings of XXXII International Congress Apimondia, Rio de Janeiro, Apimondia Publitioning House, Bucharest. pp. 149. [Google Scholar]
- Pritchard, D. J. 2016. Grooming by honey bees as a component of varroa resistant behavior. J. Apicult. Res. 55(1): 38–48. [Google Scholar]
- Qi, S., Niu X., Wang D. H., Wang C., Zhu L., Xue X., Zhang Z., and Wu L.. . 2020. Flumethrin at sublethal concentrations induces stresses in adult honey bees (Apis mellifera L.). Sci. Total Environ. 700: 134500. [DOI] [PubMed] [Google Scholar]
- Råberg, L., Graham A. L., and Read A. F.. . 2009. Decomposing health: tolerance and resistance to parasites in animals. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 364: 37–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademacher, E., and Harz M.. . 2006. Oxalic acid for the control of varroosis in honey bee colonies—a review. Apidologie. 37(1): 98–120. [Google Scholar]
- Rademacher, E., Harz M., and Schneider S.. . 2017. Effects of oxalic acid on Apis mellifera (Hymenoptera: Apidae). Insects. 8(3): 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey, S. D., Ochoa R., Bauchan G., Gulbronson C., Mowery J. D., Cohen A., Lim D., Joklik J., Cicero J. M., Ellis J. D., . et al. 2019. Varroa destructor feeds primarily on honey bee fat body tissue and not hemolymph. Proc. Natl. Acad. Sci. U. S. A. 116: 1792–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangel, J., and Ward L.. . 2018. Evaluation of the predatory mite Stratiolaelaps scimitus for the biological control of the honey bee ectoparasitic mite Varroa destructor. J. Apicult. Res. 39: 57, 425–432. [Google Scholar]
- Rath, W. 1999. Co-adaptation of Apis cerana Fabr. and Varroa jacobsoni Oud. Apidologie. 30: 97–110. [Google Scholar]
- Ravoet, J., Reybroeck W., and de Graaf D. C.. . 2015. Pesticides for apicultural and/or agricultural application found in Belgian honey bee wax combs. Bull. Environ. Contam. Toxicol. 94: 543–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read, S., Howlett B. G., Donovan B. J., Nelson W. R., and van Toor R. F.. . 2014. Culturing chelifers (Pseudoscorpions) that consume Varroa mites. J. Appl. Entomol. 138: 260–266. [Google Scholar]
- Rademacher, E., Harz M, and Schneider S.. . 2015. The development of HopGuard® as a winter treatment against Varroa destructor in colonies of Apis mellifera. Apidologie 46: 748–759. [Google Scholar]
- Reinbacher, L., Fernández-Ferrari María C., Angeli S., and Schausberger P.. . 2018. Effects of Metarhizium anisopliae on host choice of the bee-parasitic mite Varroa destructor. Acarologia. 58(2): 287–295. [Google Scholar]
- Rinderer, T. E., De Guzman L. I., Delatte G. T., Stelzer J. A., Lancaster V. A., Williams J. L., Beaman L. D., Kuznetsov V., Bigalk M., Bernard S. J.. et al. 2001a. Multi-state field trials of ARS—Russian honey bees—2. Honey production 1999, 2000. Am. Bee J. 141: 726–729. [Google Scholar]
- Rinderer, T. E., de Guzman L. I., Delatte G. T., Stelzer J. A., Lancaster V. A., Kuznetsov V., Beaman L., Watts R., and Harris J. W.. . 2001b. Resistance to the parasitic mite Varroa destructor in honey bees from far-eastern Russia. Apidologie. 32: 381–394. [Google Scholar]
- Rinderer, T. E., De Guzman L. I., Delatte G. T., and Harper C.. . 2003. An evaluation of ARS Russian honey bees in combination with other methods for the control of varroa mites. Am. Bee J. 143: 410–413. [Google Scholar]
- Rinderer, T. E., Harris J. W., Hunt G. J., and de Guzman L. I.. . 2010. Breeding for resistance to Varroa destructor in North America. Apidologie. 41: 409–424. [Google Scholar]
- Rinderer, T. E., De Guzman L. I., and Frake A. M.. . 2013. Associations of parameters related to the fall of Varroa destructor (Mesostigmata: Varroidae) in Russian and Italian honey bee (Hymenoptera: Apidae) colonies. J. Econ. Entomol. 106: 566–575. [DOI] [PubMed] [Google Scholar]
- Rinderer, T. E., Danka R. G., Johnson S., Bourgeois A. L., Frake A. M., Villa J. D., De Guzman L. I., and Harris J. W.. . 2014a. Functionality of Varroa-resistant honey bees (Hymenoptera: Apidae) when used for western U.S. honey production and almond pollination. J. Econ. Entomol. 107: 523–530. [DOI] [PubMed] [Google Scholar]
- Rinderer, T. E., De Guzman L. I., Frake A. M., Tarver M. R., and Khongphinitbunjong K.. . 2014b. An evaluation of the associations of parameters related to the fall of Varroa destructor (Acari: Varroidae) from commercial honey bee (Hymenoptera: Apidae) colonies as tools for selective breeding for mite resistance. J. Econ. Entomol. 107: 516–522. [DOI] [PubMed] [Google Scholar]
- Rinkevich, F. D. 2020. Detection of amitraz resistance and reduced treatment efficacy in the Varroa Mite, Varroa destructor, within commercial beekeeping operations. Plos One. 15: e0227264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riva, C., Suzanne P., Charpentier G., Dulin F., Halm-Lemeille M. P., and Sopkova-de Oliveira Santos J.. . 2019. In silico chemical library screening and experimental validation of novel compounds with potential varroacide activities. Pestic. Biochem. Physiol. 160: 11–19. [DOI] [PubMed] [Google Scholar]
- Robertson, A. J., Trost B., Scruten E., Robertson T., Mostajeran M., Connor W., Kusalik A., Griebel P., and Napper S.. . 2014. Identification of developmentally-specific kinotypes and mechanisms of Varroa mite resistance through whole-organism, kinome analysis of honeybee. Front. Genet. 5: 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson, A. J., Scruten E., Mostajeran M., Robertson T., Denomy C., Hogan D., Roesler A., Rutherford C., Kusalik A., Griebel P., . et al. 2020. Kinome analysis of honeybee (Apis mellifera L.) dark-eyed pupae identifies biomarkers and mechanisms of tolerance to varroa mite infestation. Sci. Rep. 10: 2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Dehaibes, S. R., Otero-Colina G., Pardio Sedas V., and Villanueva Jimenez J. A.. . 2005. Resistance to amitraz and flumethrin in Varroa destructor populations from Veracruz, Mexico. J. Apicult. Res. 443: 124–125. [Google Scholar]
- Rodríguez-Dehaibes, S. R., Otero-Colina G., Villanueva Jiménez J. A., and Corcuera P.. . 2011. Susceptibility of Varroa destructor (Gamasida: Varroidae) to four pesticides used in three Mexican apicultural regions under two different management systems. Int. J. Acarol. 37: 441–447. [Google Scholar]
- Rolke, D., Fuchs S., Grünewald B., Gao Z., and Blenau W.. . 2016. Large-scale monitoring of effects of clothianidin-dressed oilseed rape seeds on pollinating insects in Northern Germany: effects on honey bees (Apis mellifera). Ecotoxicology. 25: 1648–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rondeau, S., Giovenazzo P., and Fournier V.. . 2018. Risk assessment and predation potential of Stratiolaelaps scimitus (Acari: Laelapidae) to control Varroa destructor (Acari: Varroidae) in honey bees. Plos One. 13: e0208812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rondeau, S., Giovenazzo P., and Fournier V.. . 2019. The use of the predatory mite Stratiolaelaps scimitus (Mesostigmata: Laelapidae) to control Varroa destructor (Mesostigmata: Varroidae) in honey bee colonies in early and late fall. J. Econ. Entomol. 112: 534–542. [DOI] [PubMed] [Google Scholar]
- Rosenkranz, P., Fries I., Boecking O., and Stürmer M.. . 1997. Damaged Varroa mites in the debris of honey bee (Apis mellifera L) colonies with and without hatching brood. Apidologie. 28: 427–437. [Google Scholar]
- Rosenkranz, P., Aumeier P., and Ziegelmann B.. . 2010. Biology and control of Varroa destructor. J. Invertebr. Pathol. 103(Suppl 1): S96–119. [DOI] [PubMed] [Google Scholar]
- Roth, M. A., Wilson J. M., Taylor K. R., and Gross A. D.. . 2020. Biology and management of Varroa destructor (Mesostigmata: Varroidae) in Apis mellifera (Hymenoptera: Apidae) colonies. J. Integr. Pest Manag. 11(1): 1–8. [Google Scholar]
- Rothenbuhler, W. C. 1964. Behaviour genetics of nest cleaning in honey bees. I. Responses of four inbred lines to disease-killed brood. Anim. Behav. 12: 578–583 [DOI] [PubMed] [Google Scholar]
- Ruffinengo, S. R., and Maggi M. D.. . 2018. Varroa destructor: when reversion to coumaphos resistance does not happen. J. Apicult. Res. 57(4): 536–540. [Google Scholar]
- Ruffinengo, S., Eguaras M., Floris I., Faverin C., Bailac P., and Ponzi M.. . 2005. LD50 and repellent effects of essential oils from Argentinian wild plant species on Varroa destructor. J. Econ. Entomol. 98: 651–655. [DOI] [PubMed] [Google Scholar]
- Ruttner, F., and Hanel H.. . 1992. Active defense against varroa mites in a carniolan strain of honeybee (Apis mellifera carnica Pollmann). Apidologie. 23: 173–187. [Google Scholar]
- Sabahi, Q., Gashout H., Kelly P. G., and Guzman-Novoa E.. . 2017. Continuous release of oregano oil effectively and safely controls Varroa destructor infestations in honey bee colonies in a northern climate. Exp. Appl. Acarol. 72: 263–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sammataro, D., Gerson U., and Needham G.. . 2000. Parasitic mites of honey bees: life history, implications, and impact. Annu. Rev. Entomol. 45: 519–548. [DOI] [PubMed] [Google Scholar]
- Sanchez-Bayo, F., and Goka K.. . 2014. Pesticide residues and bees–a risk assessment. Plos One. 9: e94482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saucy, F. 2014. On the natural cell size of European honey bees: a “fatal error” or distortion of historical data? J. Apicult. Res. 53(3): 327–36. [Google Scholar]
- Satta, A., Floris I., Eguaras M., Cabras P., Garau V. L., and Melis M.. . 2005. Formic acid-based treatments for control of Varroa destructor in a mediterranean area. J. Econ. Entomol. 98: 267–273. [DOI] [PubMed] [Google Scholar]
- Schmehl, D. R., Tome H. V. V., Mortensen A. N., Martins G. F., and Ellis J. D.. . 2016. Protocol for the in vitro rearing of honey bee (Apis mellifera L.) workers. J. Apicult. Res. 55: 113–129. [Google Scholar]
- Schmid-Hempel, P. 1998. Parasites in social insects. Princeton University Press, Princeton, NJ. [Google Scholar]
- Schneider, S., Eisenhardt D., and Rademacher E.. . 2012. Sublethal effects of oxalic acid on Apis mellifera (Hymenoptera: Apidae): changes in behaviour and longevity. Apidologie. 43(2): 218–225. [Google Scholar]
- Scott, J. G., Michel K., Bartholomay L. C., Siegfried B. D., Hunter W. B., Smagghe G., Zhu K. Y., and Douglas A. E.. . 2013. Towards the elements of successful insect RNAi. J. Insect Physiol. 59: 1212–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley, T. D., and Griffin S. R.. . 2011. Small-cell comb does not control Varroa mites in colonies of honeybees of European origin. Apidologie. 42: 526–532. [Google Scholar]
- Seeley, T. D., and Smith M. L.. 2015. Crowding honey bee colonies in apiaries raises their vulnerability to the deadly ectoparasite Varroa destructor. Apidologie. 46: 716–727. [Google Scholar]
- Semkiw, P., Skubida P., and Pohorecka K.. . 2013. The amitraz strips efficacy in control of Varroa destructor after many years application of amitraz in apiaries. J. Apic. Sci. 57: 107–121. [Google Scholar]
- Seitz, N., Traynor K. S., Steinhauer N., Rennich K., Wilson M. E., Ellis J. D., Rose R., Tarpy D. R., Sagili R. R., Caron D. M., . et al. 2016. A national survey of managed honey bee 2014–2015 annual colony losses in the USA. J. Apicult. Res. 54: 292–304. [Google Scholar]
- Shaw, K. E., Davidson G., Clark S. J., Ball B. V., Pell J. K., Chandler D., and Sunderland K. D.. . 2002. Laboratory bioassays to assess the pathogenicity of mitosporic fungi to Varroa destructor (Acari: Mesostigmata), an ectoparasitic mite of the honeybee, Apis mellifera. Biol. Control. 24: 266–276. [Google Scholar]
- Siede, R., Meixner M. D., Almanza M. T., Schöning R., Maus C., and Büchler R.. . 2018. A long-term field study on the effects of dietary exposure of clothianidin to varroosis-weakened honey bee colonies. Ecotoxicology. 27: 772–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simone-Finstrom, M. 2017. Social immunity and the superorganism: Behavioral defenses protecting honey bee colonies from pathogens and parasites. Bee World. 94: 21–29. [Google Scholar]
- Sinia, A., and Guzman-Novoa E.. . 2018. Evaluation of the entomopathogenic fungi Beauveria bassiana GHA and Metarhizium anisopliae UAMH 9198 alone or in combination with thymol for the control of Varroa destructor in honey bee (Apis mellifera) colonies. J. Apicult. Res. 57: 308–316. [Google Scholar]
- Skinner, J. A., Parkman J. P., and Studer M. D.. . 2001. Evaluation of honey bee miticides, including temporal and thermal effects on formic acid gel vapours, in the central south-eastern USA. J. Apic. Res. 40: 81–89. [Google Scholar]
- Smart, M., Pettis J., Rice N., Browning Z., and Spivak M.. . 2016. Linking measures of colony and individual honey bee health to survival among apiaries exposed to varying agricultural land use. Plos One. 11: e0152685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, J., Cleare X. L., Given K., and Li-Byarlay H.. . 2021. Morphological changes in the mandibles accompany the defensive behavior of Indiana mite biting honey bees against Varroa destructor. Front. Ecol. Evol. 9: 638308. [Google Scholar]
- Smodiš Škerl, M. I., Nakrst M., Žvokelj L., and Gregorc A.. . 2011. The acaricidal effect of flumethrin, oxalic acid and amitraz against Varroa destructor in honey bee (Apis mellifera carnica) colonies. Acta Vet. Brno. 80(1): 51–56. [Google Scholar]
- Spivak, M., and Danka R. G.. 2021. Perspectives on hygienic behavior in Apis mellifera and other social insects. Apidologie. 52: 1–16. [Google Scholar]
- Spivak, M. 1996. Honey bee hygienic behavior and defense against Varroa jacobsoni. Apidologie. 27: 245–260. [Google Scholar]
- Spivak, M., and Downey D. L.. . 1998. Field assays for hygienic behavior in honey bees (Hymenoptera: Apidae). J. Econ. Entomol. 91: 64–70. [Google Scholar]
- Spivak, M., and Gilliam M.. . 1998. Hygienic behaviour of honey bees and its application for control of brood diseases and varroa - Part II. Studies on hygienic behaviour since the Rothenbuhler era. Bee World. 79: 169–186. [Google Scholar]
- Spivak, M., and Reuter G. S.. . 1998. Performance of hygienic honey bee colonies in a commercial apiary. Apidologie. 29: 291–302. [Google Scholar]
- Spivak, M., and Reuter G. S.. . 2001a. Resistance to American foulbrood disease by honey bee colonies Apis mellifera bred for hygienic behavior. Apidologie. 32: 555–565. [Google Scholar]
- Spivak, M., and Reuter G. S.. . 2001b. Varroa destructor infestation in untreated honey bee (Hymenoptera: Apidae) colonies selected for hygienic behavior. J. Econ. Entomol. 94: 326–331. [DOI] [PubMed] [Google Scholar]
- Spivak, M., and Reuter G. S.. 2008. New direction for the minnesota hygienic line of bees. Am. Bee J. 148: 1085–1086. [Google Scholar]
- Spreafico, M., Eordegh F. R., Bernardinelli I., and Colombo M.. . 2001. First detection of strains of Varroa destructor resistant to coumaphos. Results of laboratory tests and field trials. Apidologie. 32: 49–55. [Google Scholar]
- Stanimirović, Z., Stevanović J, Jovanovic S., and Andjelkovic M.. . 2005. Evaluation of genotoxic effects of Apitol® (cymiazole hydrochloride) in vitro by measurement of sister chromatid exchange. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 588: 152–157. [DOI] [PubMed] [Google Scholar]
- Stanimirović, Z., Stevanović J., Mirilović M., and Stojić V.. . 2008. Heritability of hygienic behaviour in grey honey bees (Apis mellifera carnica). Acta Vet. (Beograd). 58(5–6): 593–601. [Google Scholar]
- Stanimirovic, Z., Stevanovic J., Aleksic N., and Stojic V.. . 2010. Heritability of grooming behaviour in grey honey bee (Apis mellifera carnica). Acta Vet. (Beograd). 60: 313–323. [Google Scholar]
- Steinhauer, N. A., Rennich K., Wilson M. E., Caron D. M., Lengerich E. J., Pettis J. S., Rose R., Skinner J. A., Tarpy D. A., Wilkes J. T.. et al. 2014. A national survey of managed honey bee 2012–2013 annual colony losses in the USA: results from the bee informed partnership. J. Apicult. Res. 53: 1–18. [Google Scholar]
- Stern, V. M., Smith R. F., van den Bosch R., and Hagen K. S.. . 1959. The integrated control concept. Hilgardia. 29: 81–101. [Google Scholar]
- Stevanovic, J., Stanimirovic Z., Lacic N., Djelic N., and Radovic I.. . 2012. Stimulating effect of sugar dusting on honey bee grooming behavior. Entomol. Exp. Appl. 143(1): 23–30. [Google Scholar]
- Strachecka, A., Borsuk G., Olszewski K., and Paleolog J.. . 2015. A new detection method for a newly revealed mechanism of pyrethroid resistance development in Varroa destructor. Parasitol. Res. 114: 3999–4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange, J. P., and Sheppard W. S.. . 2001. Optimum timing of miticide applications for control of Varroa destructor (Acari: Varroidae) in Apis mellifera (Hymenoptera: Apidae) in Washington State, USA. J. Econ. Entomol. 94: 1324–1331. [DOI] [PubMed] [Google Scholar]
- Sudo, M., Takahashi D., Andow D. A., Suzuki Y., and Yamanaka T.. . 2018. Optimal management strategy of insecticide resistance under various insect life histories: Heterogeneous timing of selection and interpatch dispersal. Evol. Appl. 11: 271–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surlis, C., Carolan J. C., Coffey M. F., and Kavanagh K.. . 2016. Proteomic analysis of Bayvarol® resistance mechanisms in the honey bee parasite Varroa destructor. J. Apicult. Res. 54: 49–64. [Google Scholar]
- Swale, D. R., Carlier P. R., Hartsel J. A., Ma M., and Bloomquist J. R.. . 2015. Mosquitocidal carbamates with low toxicity to agricultural pests: an advantageous property for insecticide resistance management. Pest Manag. Sci. 71: 1158–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sewify, G. H., Ibrahim Y. Y., and Salah El-Deen M.. . 2015. Beauveria bassiana, a potential mycopesticide for efficient control of the honey bee ectoparasitic mite, Varroa destructor Anderson and Trueman. Egypt. J. Pest Control. 25: 333–337. [Google Scholar]
- Szabo, T. I., and Walker C. R. T.. 1995. Damages to dead Varroa jacobsoni caused by the larvae of Galleria mellonella. Am. Bee J. 135: 421–422. [Google Scholar]
- Tabor, K. L., and Ambrose J. T.. . 2001. The use of heat treatment for control of the honey bee mite, Varroa destructor. Am. Bee J. 141: 733–736. [Google Scholar]
- Tarpy, D. R., and Page R. E.. . 2002. Sex determinantion and the evolution of polyandry in honey bees (Apis mellifera). Behav. Ecol. Sociobiol. 52: 143–150. [Google Scholar]
- Tarpy, D. R., Nielsen R., and Nielsen D. I.. . 2004. A scientific note on the revised estimates of effective paternity frequency in Apis. Insect. Soc. 51: 203–204. [Google Scholar]
- Tarpy, D. R., Summers J., and Keller J. J.. . 2007. Comparison of parasitic mites in Russian-hybrid and Italian honey bee (Hymenoptera: Apidae) colonies across three different locations in North Carolina. J. Econ. Entomol. 100: 258–266. [DOI] [PubMed] [Google Scholar]
- Tarpy, D. R., Delaney D. A., and Seeley T. D.. . 2015. Mating frequencies of honey bee queens (Apis mellifera L.) in a population of feral colonies in the Northeastern United States. Plos One. 10: e0118734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor, M. A., Goodwin R. M., McBrydie H. M., and Cox H. M.. . 2008. The effect of honey bee worker brood cell size on Varroa destructor infestation and reproduction. J. Apicult. Res. 47: 239–242. [Google Scholar]
- Terpin, B., Perkins D., Richter S., Kraft-Leavey J., Snell T. W. and Pierson J. A.. . 2019. A scientific note on the effect of oxalic acid on honey bee larvae. Apidologie. 50: 363. [Google Scholar]
- Thapa, R., Wongsiri S., Lee M. L., and Choi Y. S.. . 2013. Predatory behaviour of pseudoscorpions (Ellingsenious indicus) associated with Himalayan Apis cerana. J. Apicult. Res. 52: 219–226. [Google Scholar]
- Thompson, H. M., Brown M. A., Ball R. F., and Bew M. H.. . 2002. First report of Varroa destructor resistance to pyrethroids in the UK. Apidologie. 33: 357–366. [Google Scholar]
- Thompson, C. E., Biesmeijer J. C., Allnutt T. R., Pietravalle S., and Budge G. E.. . 2014. Parasite pressures on feral honey bees (Apis mellifera sp.). Plos One. 9: e105164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tihelka, E. 2016. History of varroa heat treatment in Central Europe (1981–2013). Bee World 93: 4–6. [Google Scholar]
- Tomé, H. V. V., Schmehl D. R., Wedde A. E., Godoy R. S. M., Ravaiano S. V., Guedes R. N. C., Martins G. F., and Ellis J. D.. . 2020. Frequently encountered pesticides can cause multiple disorders in developing worker honey bees. Environ. Pollut. 256: 113420. [DOI] [PubMed] [Google Scholar]
- Toomemaa, K., Martin A. J., and Williams I. H.. 2010. The effect of different concentrations of oxalic acid in aequous and sucrose solution on Varroa mites and honey bees. Apidologie. 41: 643–653. [Google Scholar]
- van Toor, R. F., Thompson S. E., Gibson D. M., and Smith G. R.. . 2015. Ingestion of Varroa destructor by pseudoscorpions in honey bee hives confirmed by PCR analysis. J. Apicult. Res. 54: 555–562. [Google Scholar]
- Trouiller, J. 1998. Monitoring Varroa jacobsoni resistance to pyrethroids in western Europe. Apidologie. 29: 537–546. [Google Scholar]
- Tsagou, V., Lianou A., Lazarakis D., Emmanouel N., and Aggelis G.. . 2004. Newly isolated bacterial strains belonging to Bacillaceae (Bacillus sp.) and Micrococcaceae accelerate death of the honey bee mite, Varroa destructor (V. jacobsoni), in laboratory assays. Biotechnol. Lett. 26: 529–532. [DOI] [PubMed] [Google Scholar]
- Tsuruda, J. M., Harris J. W., Bourgeois L., Danka R. G., and Hunt G. J.. . 2012. High-resolution linkage analyses to identify genes that influence Varroa sensitive hygiene behavior in honey bees. Plos One. 7: e48276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu, S., Qiu X., Cao L., Han R., Zhang Y., and Liu X.. . 2010. Expression and characterization of the chitinases from Serratia marcescens GEI strain for the control of Varroa destructor, a honey bee parasite. J. Invertebr. Pathol. 104: 75–82. [DOI] [PubMed] [Google Scholar]
- Underwood, R., and Currie R.. . 2003. The effects of temperature and dose of formic acid on treatment efficacy against Varroa destructor (Acari: Varroidae), a parasite of Apis mellifera (Hymenoptera: Apidae). Exp. Appl. Acarol. 29: 303–313. [DOI] [PubMed] [Google Scholar]
- Unger, P., and Guzmán-Novoa E.. . 2010. Maternal effects on the hygienic behavior of Russian × Ontario hybrid honeybees (Apis mellifera L.). J. Hered. 101(1): 91–96. [DOI] [PubMed] [Google Scholar]
- United States Department of Agriculture, Animal and Plant Health Inspection Service . 2020. USDA APHIS Honey Bee Pests and Diseases Survey Project Plan for 2020. https://www.aphis.usda.gov/plant_health/plant_pest_info/honey_bees/downloads/SurveyProjectPlan.pdf. Accessed 5 March 2021.
- Vandervalk, L. P., Nasr M. E., and Dosdall L. M.. . 2014. New miticides for integrated pest management of Varroa destructor (Acari: Varroidae) in Honey Bee Colonies on the Canadian Prairies. J. Econ. Entomol. 107: 2030–2036. [DOI] [PubMed] [Google Scholar]
- Vanengelsdorp, D., Evans J. D., Saegerman C., Mullin C., Haubruge E., Nguyen B. K., Frazier M., Frazier J., Cox-Foster D., Chen Y., . et al. 2009. Colony collapse disorder: a descriptive study. Plos One. 4: e6481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Leeuwen, T., Vontas J., Tsagkarakou A., Dermauw W., and Tirry L.. . 2010. Acaricide resistance mechanisms in the two-spotted spider mite Tetranychus urticae and other important Acari: a review. Insect Biochem. Mol. Biol. 40: 563–572. [DOI] [PubMed] [Google Scholar]
- Vandenberg, J. D., and Shimanuki H.. . 1990. Viability of Bacillus thuringiensis and its efficacy for larvae of the greater wax moth (Lepidoptera: Pyralidae) following storage of treated combs. J. Econ. Entomol. 83: 760–765. [Google Scholar]
- Vlogiannitis, S., Mavridis K., Dermauw W., Snoeck S., Katsavou E., Morou E., Harizanis P., Swevers L., Hemingway J., Feyereisen R., . et al. 2021. Reduced proinsecticide activation by cytochrome P450 confers coumaphos resistance in the major bee parasite Varroa destructor. Proc. Natl. Acad. Sci. 118(6): e2020380118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagnitz, J. J., and Ellis M. D.. . 2010. Combining an artificial break in brood rearing with oxalic acid treatment to reduce Varroa mite levels. Sci. Bee Culture. 2(2): 6–8. [Google Scholar]
- Wagoner, K. M., Millar J. G., Schal C., and Rueppell O.. . 2020. Cuticular pheromones stimulate hygienic behavior in the honey bee (Apis mellifera). Sci. Rep. 10: 7132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallner, K. 1999. Varroacides and their residues in bee products. Apidologie. 30: 235–248. [Google Scholar]
- Wantuch, H. A., and Tarpy D. R.. . 2009. Removal of drone brood from Apis mellifera (Hymenoptera: Apidae) colonies to control Varroa destructor (Acari: Varroidae) and retain adult drones. J. Econ. Entomol. 102: 2033–2040. [DOI] [PubMed] [Google Scholar]
- Ward, K., Danka R., and Ward R.. . 2008. Comparative performance of two mite-resistant stocks of honey bees (Hymenoptera: Apidae) in Alabama beekeeping operations. J. Econ. Entomol. 101: 654–659. [DOI] [PubMed] [Google Scholar]
- Watanabe, M. E. 1994. Pollination worries rise as honey bees decline. Science. 265: 1170. [DOI] [PubMed] [Google Scholar]
- Webster, T. C., Thacker E. M., and Vorisek F. E.. . 2000. Live Varroa jacobsoni (Mesostigmata: Varroidae) fallen from honey bee (Hymenoptera: Apidae) colonies. J. Econ. Entomol. 93: 1596–1601. [DOI] [PubMed] [Google Scholar]
- Wheeler, M. W., Park R. M., and Bailer A. J.. . 2006. Comparing median lethal concentration values using confidence interval overlap or ratio tests. Environ. Toxicol. Chem. 25: 1441–1444. [DOI] [PubMed] [Google Scholar]
- Whitehead, H. 2017. Varroa mite management among small-scale beekeepers: characterizing factors that affect IPM adoption, and exploring drone brood removal as an IPM tool. (Unpublished Master’s thesis). The Ohio State University, Columbus, OH. [Google Scholar]
- Wilde, J., Fuchs S., Bratkowski J., and Siuda M.. . 2005. Distribution of Varroa destructor between swarms and colonies. J. Apicult. Res. 44(4): 190–194. [Google Scholar]
- Wilkinson, D., and Smith G. C.. . 2002. Modeling the efficiency of sampling and trapping Varroa destructor in the drone brood of honey bees (Apis mellifera). Am. Bee J. 142: 209–212. [Google Scholar]
- Williamson, S. M., and Wright G. A.. . 2013. Exposure to multiple cholinergic pesticides impairs olfactory learning and memory in honeybees. J. Exp. Biol. 216: 1799–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmart, O., Legrève A., Scippo M. L., Reybroeck W., Urbain B., de Graaf D. C., Steurbaut W., Delahaut P., Gustin P., Nguyen B. K., . et al. 2016. Residues in beeswax: a health risk for the consumer of honey and beeswax? J. Agric. Food Chem. 64: 8425–8434. [DOI] [PubMed] [Google Scholar]
- Winston, M. L. 1987. “The biology of the honey bee”. Harvard University Press, Cambridge, MA. [Google Scholar]
- Wu, J. Y., Anelli C. M., and Sheppard W. S.. . 2011. Sub-lethal effects of pesticide residues in brood comb on worker honey bee (Apis mellifera) development and longevity. Plos One. 6: e14720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanardi, O. Z., Ribeiro L. P., Ansante T. F., Santos M. S., Bordini G. P., . et al. 2015. Bioactivity of a matrine-based biopesticide against four pest species of agricultural importance. Crop Protect. 67: 160–167. [Google Scholar]
- van der Zee, R., Brodschneider R., Brusbardis V., Charrière J. D., Chlebo R., . et al. 2014. Results of international standardised beekeeper surveys of colony losses for winter 2012 – 2013: analysis of winter loss rates and mixed effects modelling of risk factors for winter loss. J. Apicult. Res. 53: 19–34. [Google Scholar]
- van der Zee, R., Gray A., Pisa L., and de Rijk T.. . 2015. An observational study of honey bee colony winter losses and their association with Varroa destructor, neonicotinoids and other risk factors. Plos One. 10: e0131611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakar, E., Javor A., and Kusza S.. . 2014. Genetic basis of tolerance to Varroa destructor in honey bees (Apis mellifera L.). Insect Soc. 613: 207–215. [Google Scholar]
- Ziegelmann, B., and Rosenkranz P.. . 2014. Mating disruption of the honeybee mite Varroa destructor under laboratory and field conditions. Chemoecology. 24: 137–144. [Google Scholar]
- Ziegelmann, B., Tolasch T., Steidle J. L. M., and Rosenkranz P.. . 2013. The mating behavior of Varroa destructor is triggered by a female sex pheromone. Part 2: Identification and dose-dependent effects of components of the Varroa sex pheromone. Apidologie. 44: 481–490. [Google Scholar]
- Ziegelmann, B., Abele E., Hannus S., Beitzinger M., Berg S., and Rosenkranz P.. . 2018. Lithium chloride effectively kills the honey bee parasite Varroa destructor by a systemic mode of action. Sci. Rep. 8: 683. [DOI] [PMC free article] [PubMed] [Google Scholar]