Abstract
Objective
The study sought to conduct a systematic review to explore the functions utilized by electronic cancer survivorship care planning interventions and assess their effects on patient and provider outcomes.
Materials and Methods
Based on PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) guidelines, studies published from January 2000 to January 2020 were identified in PubMed, CINAHL, EMBASE, PsychINFO, Scopus, Web of Science, and the ACM Digital Library . The search combined terms for cancer, survivorship, care planning, and health information technology (HIT). Eligible studies evaluated the effects of a HIT intervention on usability, knowledge, process, or health-related outcomes. A total of 578 abstracts were reviewed, resulting in 60 manuscripts describing 40 studies. Thematic analyses were used to define meta-themes of system functions, and Fisher’s exact tests were used to examine associations between functions and outcomes.
Results
Patients were the target end users for 18 interventions, while 12 targeted providers and 10 targeted both groups. Interventions used patient-reported outcomes collection (60%), automated content generation (58%), electronic sharing (40%), persistent engagement (28%), and communication features (20%). Overall, interventions decreased the time to create survivorship care plans (SCPs) and supported care planning knowledge and abilities, but results were mixed for effects on healthcare utilization, SCP sharing, and provoking anxiety. Persistent engagement features were associated with improvements in health or quality-of-life outcomes (17 studies, P = .003).
Conclusions
Features that engaged users persistently over time were associated with better health and quality-of-life outcomes. Most systems have not capitalized on the potential of HIT to share SCPs across a care team and support care coordination.
Keywords: cancer, neoplasms, survivors, patient care planning, systematic review, informatics
INTRODUCTION
Background and Significance
Advances in the detection and treatment of cancer have led to improved mortality rates and health outcomes for cancer patients.1 The number of post-treatment cancer survivors is estimated to reach 22.1 million people in 2030 in the United States, and cancer survivors are living longer lives after their diagnosis.2 Post-treatment care is complex, multifaceted, and requires substantial care coordination and communication. Quality survivorship care includes surveillance for new and recurrent cancers, management of physical and psychosocial effects of both cancer and its treatments, and general health maintenance.3 Prior research suggests that care coordination interventions improve outcomes across the cancer care continuum.4 However, many of these interventions include the use of professional navigation or case management services,4 which can be resource-intensive in an already burdened system.5
The Institute of Medicine’s foundational report on survivorship care, “Lost in Transition,” suggested that compiling a patient’s cancer-related information into a survivorship care plan (SCP) document would aid in supporting care coordination.6 An SCP contains a summary of the patient’s cancer diagnosis and the course of their treatment (a “treatment summary”), and a future care plan for cancer surveillance and the management of late and long-term effects.6 SCPs have recently gained prominence, and the provision of SCPs is a requirement for accreditation by the Commission on Cancer.7 To date, systematic reviews of SCP interventions, which have been largely paper based, have found little evidence that SCPs improve care.8 Experts have criticized current SCP interventions as being poorly implemented,8–10 as there is generally poor adherence to the creation and dissemination of SCPs8,9 and failure to measure process outcomes such as the sharing of SCPs across the care team.10–12
Recently, there has been renewed interest in leveraging health information technology (HIT), such as home-based self-management systems and electronic health records (EHRs), to improve SCP interventions.5,13,14 HIT can facilitate care coordination, clinical decision making, communication across care teams, and could provide new ways to measure care quality.15 While many EHR vendors have functionalities that support oncology care, recent research suggests that only a minority of SCPs are developed within EHRs or leverage EHR documentation.16 EHR systems also often lack adequate data entry functions and structured data elements necessary to adequately document SCPs for oncology care.17 To date, no studies have specifically examined what types of HIT systems and functionalities have been implemented or may be effective in supporting cancer survivorship care planning activities. While prior systematic reviews of SCPs have focused on the creation and use of SCP documents,8 none have explored what types of HIT can support the generation and use of electronic SCPs and related coordination activities, and how those HIT functions impact care delivery.
Objective
The objective of this systematic review was to examine the efficacy of various HIT tools to support post-treatment cancer survivors through survivorship care planning and care coordination.
MATERIALS AND METHODS
Review framework
A pragmatic systematic review with a systematic search and a rapid review process was conducted.18 The design of the review and the search strategy were guided by the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines.19
Search strategy
This review included interventions aimed at supporting cancer survivorship care planning after the end of primary treatment, and focused on the concept of the SCP due to its recent national focus. Search terms (see Supplementary Appendix A for search strings) consisted of a conjunction of terms related to cancer, survivorship, care planning, and HIT. Terms for care planning were supplemented with terms such as “follow-up care” or “needs assessment,” which are used outside of the United States. A search was conducted in PubMed, CINAHL, EMBASE, PsychINFO, Scopus, Web of Science, and the ACM Digital Library, using structured terminologies like MeSH (Medical Subject Headings) when applicable. Reference lists of review articles found through the initial search, and authors’ personal libraries were also reviewed for additional articles. The search included all full-text articles published between January 1, 2000, and January 1, 2020.
Eligibility criteria
All SCP interventions aimed at adult cancer survivors (18 years of age and older) that had a HIT component were included. Studies were not restricted based on cancer population or target end users of the technology (ie, patient or provider). Studies were included if research evaluated the intervention’s effects on user satisfaction, usability, usefulness, measurements of cancer care knowledge or management ability, health outcomes and quality of life (QOL), healthcare processes, or workload. Interventions supporting end-of-life care were excluded because these are beyond the scope of survivorship care,4 as were interventions focused on the specific needs of adolescent and young adult survivors. Interventions only addressing physical activity, diet, or weight loss in cancer survivors were also excluded, as such interventions have been reviewed extensively.20–22
Study screening and selection
Two independent reviewers (S.P.M. and A.C.G.) performed the screening and full text reviews. For each stage, both researchers iteratively examined 10% of the articles for inclusion, calculated agreement with Cohen’s kappa, and discussed discrepancies for adjudication. For both stages, a kappa of 0.8 was reached between reviewers after 3 iterations (30% of articles), after which the rest of the stage was completed by a single reviewer (S.P.M.). When studies were described in multiple manuscripts, the results from those manuscripts were grouped together by study. Article screening and review processes were tracked using Covidence (Melbourne, Australia).
Data extraction and synthesis
Two independent reviewers (S.P.M. and A.C.G.) initially extracted data for the included studies into an Excel spreadsheet. Each reviewer extracted data from half of the studies, assigned through the use of the random number function in Excel. Extracted data included the HIT system name, text describing system functions, country of origin, cancer population, target users, and study methodology. Descriptions of system functionality were extracted from appendices and referenced design studies when necessary.
Defining system functionality
A thematic synthesis process was used to identify HIT functionalities within each system described.23 One reviewer (S.P.M.) reviewed all text descriptions to inductively form themes around system functions. A second reviewer (A.C.G.) also independently reviewed half of the descriptions, and themes were harmonized through discussion. Themes were then grouped into larger meta-themes, which were used to categorize interventions in each study.
Analyzing associations between functions and outcomes
After function meta-themes were formed, study results were extracted. Both reviewers (S.P.M., A.C.G.) iteratively dual-extracted results from a randomized sample of 10% of the studies and categorized results into 4 areas: (1) usability, (2) knowledge, (3) processes, and (4) health-related outcomes. Each result was categorized as being positive if it reported a high score for a positive outcome (ie, upper half of a given measurement scale) or negative if it reported a low score. Results were subsequently categorized as being statistically significant if there was improvement or degradation in an outcome for HIT intervention users derived from pre/post or intervention/control comparisons that met the significance level defined in the study (usually P < .05). After each review iteration, result categorizations were harmonized through discussion. After 3 iterations of this process, S.P.M. completed result extraction.
Statistical analyses were performed to assess associations between function meta-themes and study results related to knowledge, processes, and health. For each type of result, sets of contingency tables were constructed comparing the numbers of studies with and without a given feature that had positive, significant positive, negative, or significant negative results, and associations were assessed with a 2-sided Fisher’s exact test using a significance level of P < .05. A similar analysis was performed to determine whether “complex interventions,” which included supplementary non-HIT features such as consultation phone calls or paper-based informational materials, performed better than interventions with only HIT components.
Quality appraisal
During results extraction, the reviewers assessed the quality of the studies with the Mixed Method Appraisal Tool (MMAT).24 Agreement in quality assessment was achieved by calculating the cumulative sum of “yes” answers to MMAT questions for each study and for each reviewer and calculating a 2-way mixed, single score intraclass correlation coefficient (ICC) through the “irr” package in R software version 3.6.2 (R Foundation for Statistical Computing, Vienna, Austria). Once the ICC for the quality metrics reached a threshold of 0.75 for an iteration, a single reviewer (S.P.M.) completed the rest of the quality appraisals.
RESULTS
Description of studies
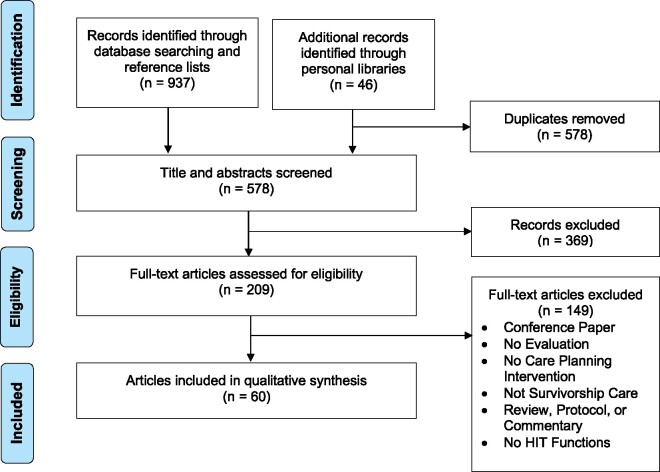
After removing duplicates, 578 abstracts were identified and 209 underwent full text review (see Figure 1). Sixty articles encompassing 40 different research studies met inclusion criteria. Supplementary Appendix B lists the reviewed studies and their characteristics. Interventions most often targeted patients or patient-caregiver dyads (n = 18, 45%), followed by providers (n = 12, 30%) and both patients and providers (n = 10, 25%). The majority of interventions focused on breast cancer (n = 16, 40%), followed by general interventions addressing multiple cancer types (n = 12, 30%), and then head and neck cancers (n = 7, 18%). Twenty-eight (70%) studies used an electronic system developed outside of an EHR, 7 (18%) studies used an EHR or existing data registry, and 6 (15%) studies used a combination of an EHR and an external system. A substantial portion of these studies (n = 17, 43%) used electronic tools as part of a complex intervention that included written educational materials, clinical visits, in-person review of SCP documents, or provider consultations.
Figure 1.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram. HIT: health information technology.
Thematic analysis identified 5 meta-themes for system functions (see Table 1). Systems collecting patient-reported outcomes (PROs) used both standardized and nonstandardized instruments to collect data. Automated content generation involved either the auto-population of a treatment summary template with existing EHR data, or assembling care plans from libraries of premade content using rules-based algorithms considering diagnosis, treatment, or symptom data. One system reported the use of electronic interfacing to send existing EHR data to an external system that then generated an SCP from premade content.25 Electronic sharing consisted of exporting the SCP in a digital format such as a PDF or using e-mail, and there were none that used interfacing tools to digitally send the SCP between EHRs. Pervasive engagement features consisted of periodic notifications through text or e-mail that requested data entry, encouraged logging into the system, provided educational content, or alerted the user when concerning PRO data were entered. In 1 case, a Fitbit was integrated to create visualizations of step count data.26 Communication tools included the use of messaging or Web-based message boards for patients; messaging features between patients, providers, and caregivers; or the ability to send referrals to other providers, which were always sent internally through an EHR.
Table 1.
Meta-themes of system functions, theme descriptions, and examples
| Theme | Description |
|---|---|
| Collection of patient-reported outcomes | Collecting information from the patient about current symptoms or health status. |
| Automated SCP content generation | Assembling portions of an SCP from existing EHR data and/or libraries of educational content. |
| Electronic sharing | Tools to facilitate the sharing of a completed SCP document through electronic means. |
| Pervasive engagement features | The use of alerts, reminders, or persistent data entry to encourage system engagement over time. |
| Communication tools | Tools to facilitate electronic messaging between the patient and their care team or other survivors. |
EHR: electronic health record; SCP: survivorship care plan.
The collection of PROs, persistent engagement tools, and communication tools were most often targeted at patients, and electronic sharing tools were most often targeted toward providers. Table 2 summarizes the number of studies utilizing each type of function by target user group.
Table 2.
Distribution of system functions across target user groups
| Users Engaged | Studies | PRO | Automated Content | Electronic Sharing | Persistent Engagement | Communication Tools |
|---|---|---|---|---|---|---|
| Patient (and caregiver) | 18 | 14 | 9 | 4 | 10 | 6 |
| Provider | 12 | 2 | 9 | 7 | 0 | 0 |
| Provider and patient | 10 | 8 | 5 | 5 | 1 | 2 |
| Total | 40 | 24 | 23 | 16 | 11 | 8 |
PRO: patient-reported outcome.
Effects on the SCP generation process
Some studies discussed the time required to create an SCP and the accuracy of information in SCPs. Creation of an SCP through manual data entry required approximately an hour.27,28 Systems leveraging rules-based algorithms required 6 to 22 minutes of data entry.25,29–34 Systems that auto-populated SCPs with existing EHR data were estimated to require between 2 and 30 minutes of additional manual data entry.35–37 One system that utilized both existing EHR data and a library of educational materials found that the SCPs took less than a minute to generate.25 Barriers noted for efficiently creating and using SCPs included the time required to review medical records from multiple organizations,27 inability to update SCPs after the initial SCP was created,33 and the lack of data sharing between the SCP and EHR.38
Studies exploring the completeness and accuracy of SCP information found that manually entered data tended to have more errors than auto-populated EHR data, but that auto-populated EHR data may omit important tumor staging information.33,35,39 The evaluation of an intervention that mixed manual data entry and auto-populated EHR data found the EHR data to be more complete than manually entered data, which included information on follow-up care and late-term effects.40 While electronic HIT interventions could decrease the amount of time required to create an SCP, some found that additional time was spent providing technical support to patients.26,41
Effects on patient and provider outcomes
Knowledge and care management abilities
Eighteen (45%) studies reported results related to changes in cancer care knowledge and the ability to manage care.26,29,31,38,41–56 Positive results (n = 17, 94%) across studies included high scores for knowledge of cancer surveillance and care coordination,26,29,31,43 cancer and its treatments,31,41,43,46,48,51,52,55–57 and symptom management29,53; increased patient activation,26,50 personal control,47 empowerment,45,46,49 and confidence in communication abilities38; and improved reflectiveness about their condition.38 Negative results (n = 2, 11%) included lack of patient empowerment52 or confidence in their knowledge for when to speak to a physician.57
Healthcare processes
Seventeen (43%) studies reported outcomes related to healthcare processes such as the creation and sharing of an SCP, engagement with clinical services, or changing care management practices.28–32,34,42,44–46,50,52–56,58–64 Fifteen (88%) studies reported positive results, including effective symptom management,44,63,64 adherence to follow-up care,29 service efficiency,29 patient-provider communication,44,46 and improved goal setting.28 Providers also noted willingness to incorporate the intervention into practice31 and increased identification of patient concerns through the use of an SCP.32
Effects on engagement with healthcare services were mixed. Some analyses found increased healthcare usage44,53 and contact with primary care providers and specialists,62 but others found that there were fewer specialist visits54 or concerns that patients may not engage with their clinicians if they have online information.49 Two studies reported increased referrals to outside resources,45,55 though one also noted that few referrals were acted upon.29 While some results indicated patient willingness to change behaviors or engage in supportive care,31,50,52 others indicated little willingness to engage with suggestions in the SCP.31,50,58
There were mixed results related to sharing an SCP among the patient’s care team. Intentions to share care plans ranged from 56%29 to 90%.34 One study found that participants regularly reporting an intent to share the SCP with their primary care provder,46 but 2 studies found that very few patients reported actually sharing an SCP.46,52 Patients in one study reported that they did not plan to share their SCP due to perceptions that their provider would not care about the SCP, or that it did not accurately reflect their current care practices.52,60,61
Health and QOL
Eighteen (45%) studies reported outcomes related to health or QOL, such as physical and psychosocial symptoms or measures of unmet needs.26,29,32,38,41,42,44–46,49,53,54,56,63,65–73 Positive results included psychosocial effects such as high levels of social functioning,41 low rates of depression,29,70 fatigue,26,70 high general QOL,32,44,46,72 and decreases in unmet needs.29,72 Some systems also saw improvement in physical symptom distress,29 symptom burden,42,63 and improved physical self-efficacy.66 There were also positive effects on health behaviors, such as improvements in physical activity,26,41,69 vegetable intake,69 and digestion and sleep.26 One system that engaged caregivers saw decreases in caregiver depression and unmet needs.29 Another study found a statistically significant negative impact on a measure of employment-related concern.42
There were mixed effects on fear and anxiety on having an SCP. Five studies found statistically significant improvements in anxiety, fear, health-related distress, and emotional state.26,63,66,67,72 Qualitative data in 2 studies indicated that SCPs did not lead to anxiety41 or worry56 for most participants. However, one trial found statistically significant negative effects related to symptom-related concerns and changes in emotional states,53,71 and a decreased belief that treatment would help.54,71 Other studies found that warning messages in SCPs could cause anxiety65 or remind people of their condition.38,56 Healthcare providers felt that interventions could make patients dwell on their disease,49 and suggested that providing information beyond the conditions directly affecting the patient could cause concern.73
Associations between features and outcomes
When assessing the associations between HIT features and outcomes (knowledge, processes, and health) among studies, only 1 significant relationship was found (see Table 3). Studies assessing interventions with persistent engagement features had a higher proportion of statistically significant positive results related to health or QOL (89% with persistent engagement features, 13% without; P = .003). There were no significant associations between HIT features and knowledge or process outcomes.
Table 3.
Proportions of positive, statistically significant positive, negative, and statistically significant negative results between interventions with and without given functional features.
| Feature Present? | Studies | Total Positive % | P | Significant Positive% | P | Total Negative % | P | Significant Negative % | P | |
|---|---|---|---|---|---|---|---|---|---|---|
| Studies with knowledge/ability results (n = 18) | ||||||||||
| Collects PROs | Yes | 13 | 1.000 | .278 | 0.308 | .326 | 0.154 | 1.000 | 0.000 | 1.000 |
| No | 5 | 0.800 | 0.600 | 0.000 | 0.000 | |||||
| Automated content generation | Yes | 10 | 1.000 | .444 | 0.600 | .066 | 0.200 | .477 | 0.000 | 1.000 |
| No | 8 | 0.875 | 0.125 | 0.000 | 0.000 | |||||
| Electronic sharing | Yes | 6 | 1.000 | 1.000 | 0.500 | .627 | 0.167 | 1.000 | 0.000 | 1.000 |
| No | 12 | 0.917 | 0.333 | 0.083 | 0.000 | |||||
| Persistent engagement features | Yes | 5 | 0.800 | .278 | 0.400 | 1.000 | 0.000 | 1.000 | 0.000 | 1.000 |
| No | 13 | 1.000 | 0.385 | 0.154 | 0.000 | |||||
| Communication | Yes | 3 | 0.667 | .167 | 0.333 | 1.000 | 0.000 | 1.000 | 0.000 | 1.000 |
| No | 15 | 1.000 | 0.400 | 0.133 | 0.000 | |||||
| Complex intervention | Yes | 10 | 0.900 | 1.000 | 0.500 | .367 | 0.000 | .183 | 0.000 | 1.000 |
| No | 8 | 1.000 | 0.250 | 0.250 | 0.000 | |||||
| Studies with process results (n = 17) | ||||||||||
| Collects PROs | Yes | 13 | 0.846 | 1.000 | 0.154 | 1.000 | 0.538 | .577 | 0.000 | .235 |
| No | 4 | 0.750 | 0.250 | 0.250 | 0.250 | |||||
| Automated content generation | Yes | 8 | 0.875 | 1.000 | 0.250 | .577 | 0.875 | .577 | 0.125 | .471 |
| No | 9 | 0.778 | 0.111 | 0.111 | 0.000 | |||||
| Electronic sharing | Yes | 6 | 0.667 | .515 | 0.000 | .515 | 0.667 | .335 | 0.000 | 1.000 |
| No | 11 | 0.909 | 0.182 | 0.364 | 0.091 | |||||
| Persistent engagement features | Yes | 4 | 0.750 | 1.000 | 0.500 | .121 | 0.250 | .577 | 0.000 | 1.000 |
| No | 13 | 0.846 | 0.077 | 0.538 | 0.077 | |||||
| Communication | Yes | 3 | 0.667 | .465 | 0.333 | .465 | 0.333 | 1.000 | 0.000 | 1.000 |
| No | 14 | 0.857 | 0.143 | 0.500 | 0.071 | |||||
| Complex intervention | Yes | 10 | 0.900 | .537 | 0.200 | 1.000 | 0.400 | .637 | 0.100 | 1.000 |
| No | 7 | 0.714 | 0.143 | 0.571 | 0.000 | |||||
| Studies with health/quality-of-life results (n = 17) | ||||||||||
| Collects PROs | Yes | 12 | 0.750 | .600 | 0.583 | .620 | 0.250 | .280 | 0.000 | .074 |
| No | 5 | 0.600 | 0.400 | 0.600 | 0.400 | |||||
| Automated content generation | Yes | 6 | 0.667 | 1.000 | 0.667 | .620 | 0.333 | 1.000 | 0.167 | 1.000 |
| No | 11 | 0.727 | 0.455 | 0.364 | 0.091 | |||||
| Electronic sharing | Yes | 4 | 0.750 | 1.000 | 0.250 | .294 | 0.250 | 1.000 | 0.000 | 1.000 |
| No | 13 | 0.692 | 0.615 | 0.385 | 0.154 | |||||
| Persistent engagement features | Yes | 9 | 0.889 | .131 | 0.889 | .003a | 0.222 | .335 | 0.111 | 1.000 |
| No | 8 | 0.500 | 0.125 | 0.500 | 0.125 | |||||
| Communication | Yes | 4 | 1.000 | .261 | 1.000 | .082 | 0.250 | 1.000 | 0.250 | .427 |
| No | 13 | 0.615 | 0.385 | 0.385 | 0.077 | |||||
| Complex intervention | Yes | 10 | 0.600 | .338 | 0.400 | .335 | 0.400 | 1.000 | 0.200 | .485 |
| No | 7 | 0.857 | 0.714 | 0.286 | 0.000 | |||||
The results are split by type of outcome considered: knowledge/ability, healthcare processes, and health/quality of life. All P values were calculated with a 2-sided Fisher’s exact test.
PRO: patient-reported outcome.
Significant at a level of P < .05.
Satisfaction, usability, and usefulness
Across studies, quantitative measures of usability, usefulness, and satisfaction were overall positive.25,26,28–31,33–35,38,40–42,44–46,48–50,52–56,58–61,65,73–82 Participants appreciated having all of their cancer care information in one place.26,28,46 Patients provided negative feedback when they felt that SCPs provided general information that was felt to be impersonal or vague.41,57,78 Providers also preferred integrating SCPs into their existing EHRs.80
Patients also found some features to be difficult to use. One system that provided patients access to limited EHR data found that the data confused 40% of participants.41 Interventions that collected PROs found that some participants had difficulties understanding41,58 or answering50,58 questions in the instruments used, and that graphs of sensor data such as step counts could be difficult to interpret.26 Providers were conflicted as to whether the collection of PROs would positively impact care by bringing new issues to light,45 or whether it would cause patients to report old or clinically irrelevant information.33
Patient feedback for 2 interventions called for the ability to communicate with their care team in real time.57,74 However, other studies reported low usefulness ratings and little use of care team-patient communication functions.44,75 Other qualitative feedback indicated that patients would rather talk to their providers in person than through online methods.75 Feedback from one study suggested that persistent provider engagement encourages greater system usage by patients.41 Similarly, system usage statistics from some studies indicated that systems with active engagement features prompted subjects to use the systems regularly,26,44 whereas systems lacking such features were visited only a few times.35,78,83
Quality appraisal
Across the 60 manuscripts reviewed, 4 (6%) used only qualitative methods, 16 (27%) used mixed quantitative and qualitative methods, and 40 (67%) used quantitative methods only. The range of MMAT metrics that were adequately addressed within the manuscripts ranged from none to all with an average score of 47%. The MMAT metrics that were least likely to be adequately addressed included accounting for confounders in nonrandomized studies (11%), groups in randomized studies being comparable at baseline (11%), and participants representing target population in nonrandomized studies (17%).
DISCUSSION
This systematic review demonstrates there are a variety of HIT tools currently used to support the creation and use of SCPs, and many studies reported positive outcomes for cancer survivors, caregivers, and care teams. Thematic analyses found 5 main types of HIT tools: electronic PROs, automated SCP content generation, electronic sharing of SCPs, pervasive engagement features, and communication tools. Collectively, these tools demonstrated improvements in user knowledge and abilities, healthcare processes, and health and QOL. However, HIT features to persistently engage users, such as reminders, were the only type of tool found to be significantly associated with improved health and QOL outcomes. This may be due to the increased engagement fostered by those systems, which suggests that SCP interventions should be dynamic and change longitudinally along with patients’ needs.14 The use of algorithms to generate SCPs based on existing data reduced time to create an SCP, and importing existing data from an EHR into an SCP treatment summary could also improve the accuracy and completeness of data in the SCP. Although the most commonly used, PROs were not associated with improvements in any outcome area. However, in many interventions, PRO measures were only collected during the creation of the SCP and not longitudinally, limiting the ability of the interventions to evaluate changes in status over time.
Implications for cancer survivorship and research
Few studies addressed the sharing of SCPs across a care team or communication between providers in a care team, demonstrating that HIT interoperability has not been adequately explored for SCPs. This may be due to the small number of studies that contained HIT tools integrated within the EHR in this review. Recent research indicates that most SCPs in the U.S. are not developed within EHRs, and that providers often rely on fax and mail to distribute SCPs across the care team.16 The reliance on manual work to share an SCP and patient perceptions that their care team would not use an SCP may have led to the mixed results related to intentions to share SCPs.
None of the interventions supported referrals or coordination activities that crossed organizational boundaries. Using technology to share information between care settings and providers is a substantial opportunity because many oncology offices are small, community-based practices84 that may not offer diverse ancillary services. A majority of cancer survivors also prefer that oncologists and primary care providers share responsibilities for their care.85 Additionally, a lack of care coordination and communication between these settings can lead to adverse patient outcomes.86 Medical records for cancer survivors can be spread across multiple specialties and settings,87 making it difficult to aggregate data for SCPs. While HIT tools could ease information sharing across settings, few interventions leveraged SCPs as a care coordination tool.10 Future research should explore the use of data exchange standards and standardized terminologies to exchange granular SCP information between digital systems. Future EHR functions and stand-alone systems could leverage the existing eCOTPS (electronic Clinical Oncology Treatment Plan and Summary) standard88 to communicate information about post-treatment plans between care settings. Also, newer standards such as the mCODE (Minimal Common Oncology Data Elements) standard,17 which leverages FHIR (Fast Healthcare Interoperability Resources), should be further developed to consider survivorship use cases.
There were mixed results regarding whether the information in an SCP could provoke anxiety over the cancer diagnosis or be comforting due to improved ability to address the condition. Mixed results related to the perceived usefulness and usability of functions such as PRO collection and communication tools also suggests that HIT to support SCP processes may require a collection of features that can be tailored to the needs of specific patients and their care teams, and that these may have the most value if used longitudinally. A tailored approach is in line with current survivorship care trends toward the provision of personalized care.13
Limitations
A potential limitation of this review is the quality of the studies assessed, which was moderate overall. Many studies did not adequately address representativeness of study populations, address differences between comparison groups, or consider potential confounding variables. Future studies should assess diverse groups of survivors to better understand how user characteristics can impact system usage. Some studies did assess differences in suitability for different groups, finding that system effectiveness could vary based on treatment types and educational attainment,70 perceived personal control,89 information coping styles,79 age,67,70 cancer types,77 and Internet usage.90 Future studies should also explore the specific needs of subpopulations and the use of system configurability to address the needs of multiple populations.
Owing to the heterogeneity of the results considered and study designs, a meta-analysis of the results could not be conducted. Instead, the results were categorized into binary positive and negative categorizations, and therefore cannot account for measured effect sizes across results. Many of the interventions were complex and included multiple features, and statistical analyses could not explore potential interactions between different features due to a lack of statistical power from the small sample sizes. Some systems, such as OncoKompas and OncoLink, were evaluated in multiple studies which could potentially bias the results due to the undue influence of these systems. Also, the paucity of negative outcome results overall may indicate publication bias in this literature. Overall, however, the breadth of results provides a wide range of guidance for future SCP system designers.
CONCLUSION
The current body of literature on SCP interventions with HIT components indicates that there is a positive though limited impact on cancer survivorship care. Interventions that had the greatest impact on the patient’s health and QOL had features that engaged the user longitudinally, emphasizing that cancer survivors need a dynamic care plan that changes along with their health status. Most systems have not capitalized on HIT to facilitate communication of information across a care team to better coordinate care. Future system designers should focus not only on engaging system users, but also on connecting users to their care teams and resources to improve survivorship care.
FUNDING
SPM is supported by a National Institutes of Health/National Cancer Institute National Research Service Award sponsored by the Lineberger Comprehensive Cancer Center at the University of North Carolina (T32 CA116339). ACG is supported by National Institutes of Health/National Library of Medicine training grant 5T15LM012500-04.
AUTHOR CONTRIBUTIONS
SPM, ACG, and AEC conceived and designed the study and methods. SPM and ACG extracted data and coding of themes, and undertook the analyses. All authors contributed to interpretation of results and approved the final version of the submitted manuscript.
SUPPLEMENTARY MATERIAL
Supplementary material is available at Journal of the American Medical Informatics Association online.
DATA AVAILABILITY STATEMENT
The data underlying this article will be shared on reasonable request to the corresponding author.
CONFLICT OF INTEREST STATEMENT
Authors have no conflicts of interest to disclose.
Supplementary Material
REFERENCES
- 1.Alfano CM, Leach CR, Smith TG, et al. Equitably improving outcomes for cancer survivors and supporting caregivers: A blueprint for care delivery, research, education, and policy. CA Cancer J Clin 2019; 69 (1): 35–49. [DOI] [PubMed] [Google Scholar]
- 2.Miller KD, Nogueira L, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin 2019; 69 (5): 363–85. [DOI] [PubMed] [Google Scholar]
- 3.Nekhlyudov L, Mollica MA, Jacobsen PB, et al. Developing a quality of cancer survivorship care framework: implications for clinical care, research, and policy. J Natl Cancer Inst 2019; 111 (11): 1120–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gorin SS, Haggstrom D, Han PKJ, et al. Cancer care coordination: a systematic review and meta-analysis of over 30 years of empirical studies. Ann Behav Med 2017; 51 (4): 532–46. [DOI] [PubMed] [Google Scholar]
- 5.Mayer DK, Alfano CM.. Personalized risk-stratified cancer follow-up care: its potential for healthier survivors, happier clinicians, and lower costs. J Natl Cancer Inst 2019; 111 (5): 442–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Institute of Medicine. From Cancer Patient to Cancer Survivor: Lost in Transition. Washington, DC: National Academies Press; 2005. [Google Scholar]
- 7.Shulman LN, Ferris L, Takanishi DM, et al. Treatment summaries and survivorship care plans: the approach by the commission on cancer to increase use. J Oncol Pract 2015; 11 (1): 40–1. [DOI] [PubMed] [Google Scholar]
- 8.Hill RE, Wakefield CE, Cohn RJ, et al. Survivorship care plans in cancer: a meta‐analysis and systematic review of care plan outcomes. Oncologist 2020; 25 (2): e351–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Birken SA, Clary AS, Bernstein S, et al. Strategies for successful survivorship care plan implementation: Results from a qualitative study. J Oncol Pract 2018; 14 (8): e462–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salz T, Baxi S.. Moving survivorship care plans forward: focus on care coordination. Cancer Med 2016; 5 (7): 1717–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jacobsen PB, DeRosa AP, Henderson TO, et al. Systematic review of the impact of cancer survivorship care plans on health outcomes and health care delivery. J Clin Oncol 2018; 36 (20): 2088–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mayer DK, Birken SA, Chen RC.. Avoiding implementation errors in cancer survivorship care plan effectiveness studies. J Clin Oncol 2015; 33 (31): 3528–30. [DOI] [PubMed] [Google Scholar]
- 13.Alfano CM, Jefford M, Maher J, et al. Building personalized cancer follow-up care pathways in the United States: lessons learned from implementation in England, Northern Ireland, and Australia. Am Soc Clin Oncol Educ Book 2019; 39: 625–39. [DOI] [PubMed] [Google Scholar]
- 14.Tevaarwerk AJ, Klemp JR, van Londen GJ, et al. Moving beyond static survivorship care plans: a systems engineering approach to population health management for cancer survivors. Cancer 2018; 124 (22): 4292–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bates DW, Bitton A.. The future of health information technology in the patient-centered medical home. Health Aff (Millwood) 2010; 29 (4): 614–21. [DOI] [PubMed] [Google Scholar]
- 16.Birken SA, Raskin S, Zhang Y, et al. Survivorship care plan implementation in US cancer programs: a national survey of cancer care providers. J Cancer Educ 2019; 34 (3): 614–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Osterman TJ, Terry M, Miller RS.. Improving cancer data interoperability: the promise of the minimal Common Oncology Data Elements (mCODE) initiative. JCO Clin Cancer Inform 2020; 4: 993–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Featherstone RM, Dryden DM, Foisy M, et al. Advancing knowledge of rapid reviews: an analysis of results, conclusions and recommendations from published review articles examining rapid reviews. Syst Rev 2015; 4: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 2009; 6 (7): e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haberlin C, O'Dwyer T, Mockler D, et al. The use of eHealth to promote physical activity in cancer survivors: a systematic review. Support Care Cancer 2018; 26 (10): 3323–36. [DOI] [PubMed] [Google Scholar]
- 21.Harvey J, Dittus K, Mench E.. eHealth and behavioral weight loss interventions for female cancer survivors: a review. Womens Health (Lond) 2017; 13 (3): 80–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roberts AL, Fisher A, Smith L, et al. Digital health behaviour change interventions targeting physical activity and diet in cancer survivors: a systematic review and meta-analysis. J Cancer Surviv 2017; 11 (6): 704–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thomas J, Harden A.. Methods for the thematic synthesis of qualitative research in systematic reviews. BMC Med Res Methodol 2008; 8: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hong Q, Pluye P, Fàbregues S, et al. Mixed Methods Appraisal Tool (MMAT), Version 2018. http://mixedmethodsappraisaltoolpublic.pbworks.com/w/file/fetch/127916259/MMAT_2018_criteria-manual_2018-08-01_ENG.pdf. Accessed March 25, 2020.
- 25.Hill-Kayser CE, Jacobs LA, Gabriel P, et al. Feasibility study of an electronic interface between internet-based survivorship care plans and electronic medical records. J Oncol Pract 2016; 12 (4): e380–7. [DOI] [PubMed] [Google Scholar]
- 26.Napoles AM, Santoyo-Olsson J, Chacon L, et al. Feasibility of a mobile phone app and telephone coaching survivorship care planning program among Spanish-speaking breast cancer survivors. JMIR Cancer 2019; 5 (2): e13543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dulko D, Pace CM, Dittus KL, et al. Barriers and facilitators to implementing cancer survivorship care plans. Oncol Nurs Forum 2013; 40 (6): 575–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosales AR, Byrne D, Burnham C, et al. Comprehensive survivorship care with cost and revenue analysis. J Oncol Pract 2014; 10 (2): e81–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sterba KR, Armeson K, Zapka J, et al. Evaluation of a survivorship needs assessment planning tool for head and neck cancer survivor-caregiver dyads. J Cancer Surviv 2019; 13 (1): 117–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hill-Kayser CE, Vachani C, Hampshire MK, et al. An internet tool for creation of cancer survivorship care plans for survivors and health care providers: design, implementation, use and user satisfaction. J Med Internet Res 2009; 11 (3): e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O’Hea E, Wu J, Dietzen L, et al. The Polaris Oncology Survivorship Transition (POST) system: a patient- and provider-driven cancer survivorship planning program. J Oncol Navig Surviv 2016; 7: 11–24. [PMC free article] [PubMed] [Google Scholar]
- 32.Rose P, Quail H, McPhelim J, et al. Experiences and outcomes of lung cancer patients using electronic assessments. Cancer Nurs Pract 2017; 16 (7): 26–30. [Google Scholar]
- 33.Salz T, Schnall RB, McCabe MS, et al. Incorporating multiple perspectives into the development of an electronic survivorship platform for head and neck cancer. JCO Clin Cancer Inform 2018; 2: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Szalda D, Schwartz L, Schapira MM, et al. Internet-based survivorship care plans for adult survivors of childhood cancer: a pilot study. J Adolesc Young Adult Oncol 2016; 5 (4): 351–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tevaarwerk AJ, Wisinski KB, Buhr KA, et al. Leveraging electronic health record systems to create and provide electronic cancer survivorship care plans: A pilot study. J Oncol Pract 2014; 10 (3): e150–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garcia SF, Kircher SM, Oden M, et al. Survivorship care planning in a comprehensive cancer center using an implementation framework. J Commun Support Oncol 2016; 14 (5): 192–9. [DOI] [PubMed] [Google Scholar]
- 37.Boehm L, Weisberg T, Linendoll N, et al. Development of phase-specific breast cancer survivorship care plans. Clin Breast Cancer 2019; 19 (6): e723–30. [DOI] [PubMed] [Google Scholar]
- 38.Clarke AL, Roscoe J, Appleton R, et al. My gut feeling is we could do more…” a qualitative study exploring staff and patient perspectives before and after the implementation of an online prostate cancer-specific holistic needs assessment. BMC Health Serv Res 2019; 19 (1): 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tevaarwerk AJ, Hocking WG, Zeal JL, et al. Accuracy and thoroughness of treatment summaries provided as part of survivorship care plans prepared by two cancer centers. J Oncol Pract 2017; 13 (5): e486–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Browning KK, Tan A, Ghosh-Berkebile R, et al. Utilization of an audit tool to evaluate accuracy of treatment summary and survivorship care plans. J Cancer Surviv Res Surviv 2019; 13 (6): 890–8. [DOI] [PubMed] [Google Scholar]
- 41.Kuijpers W, Groen WG, Oldenburg HS, et al. eHealth for breast cancer survivors: use, feasibility and impact of an interactive portal. JMIR Cancer 2016; 2 (1): e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nahm E-S, Miller K, McQuaige M, et al. Testing the impact of a cancer survivorship patient engagement toolkit on selected health outcomes. Oncol Nurs Forum 2019; 46 (5): 572–84. [DOI] [PubMed] [Google Scholar]
- 43.Beck J.A road map for creating a CPRS template for a cancer survivorship treatment summary and care plan. Fed Pract 2017; 34: 51S–56S. [PMC free article] [PubMed] [Google Scholar]
- 44.van den Brink JL, Moorman PW, de Boer MF, et al. Involving the patient: a prospective study on use, appreciation and effectiveness of an information system in head and neck cancer care. Int J Med Inform 2005; 74 (10): 839–49. [DOI] [PubMed] [Google Scholar]
- 45.Semple C, Lannon D, Qudairat E, et al. Development and evaluation of a holistic surgical head and neck cancer follow-up clinic using touchscreen technology. Psychooncology 2017; 26 (4): e12809. [DOI] [PubMed] [Google Scholar]
- 46.Smith KC, Tolbert E, Hannum SM, et al. Comparing web-based provider-initiated and patient-initiated survivorship care planning for cancer patients: a randomized controlled trial. JMIR Cancer 2016; 2 (2): e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Willems RA, Bolman CAW, Mesters I, et al. Short-term effectiveness of a web-based tailored intervention for cancer survivors on quality of life, anxiety, depression, and fatigue: randomized controlled trial. Psychooncology 2017; 26 (2): 222–30. [DOI] [PubMed] [Google Scholar]
- 48.Kuijpers W, Groen WG, Loos R, et al. An interactive portal to empower cancer survivors: a qualitative study on user expectations. Support Care Cancer 2015; 23 (9): 2535–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Duman-Lubberding S, van Uden-Kraan CF, Peek N, et al. An eHealth application in head and neck cancer survivorship care: health care professionals’ perspectives. J Med Internet Res 2015; 17 (10): e235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Melissant HC, Verdonck-de Leeuw IM, Lissenberg-Witte BI, et al. Oncokompas’, a web-based self-management application to support patient activation and optimal supportive care: a feasibility study among breast cancer survivors. Acta Oncol 2018; 57 (7): 924–34. [DOI] [PubMed] [Google Scholar]
- 51.Bulloch KJ, Irwin ML, Chagpar AB, et al. Systematic approach to providing breast cancer survivors with survivorship care plans: a feasibility study. J Oncol Pract 2015; 11 (2): e170–6. [DOI] [PubMed] [Google Scholar]
- 52.Hill-Kayser CE, Vachani CC, Hampshire MK, et al. Impact of internet-based cancer survivorship care plans on health care and lifestyle behaviors. Cancer 2013; 119 (21): 3854–60. [DOI] [PubMed] [Google Scholar]
- 53.Nicolaije KAH, Ezendam NPM, Vos MC, et al. Impact of an automatically generated cancer survivorship care plan on patient-reported outcomes in routine clinical practice: longitudinal outcomes of a pragmatic, cluster randomized trial. J Clin Oncol 2015; 33 (31): 3550–9. [DOI] [PubMed] [Google Scholar]
- 54.de Rooij BH, Ezendam NPM, Nicolaije KAH, et al. Effects of Survivorship Care Plans on patient reported outcomes in ovarian cancer during 2-year follow-up – the ROGY care trial. Gynecol Oncol 2017; 145 (2): 319–28. [DOI] [PubMed] [Google Scholar]
- 55.Clarke AL, Roscoe J, Appleton R, et al. Promoting integrated care in prostate cancer through online prostate cancer-specific holistic needs assessment: a feasibility study in primary care. Support Care Cancer 2020; 28 (4): 1817–27. 10.1007/s00520-019-04967-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Blaauwbroek R, Barf HA, Groenier KH, et al. Family doctor-driven follow-up for adult childhood cancer survivors supported by a web-based survivor care plan. J Cancer Surviv 2012; 6 (2): 163–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kuijpers W, Groen WG, Oldenburg HS, et al. Development of MijnAVL, an interactive portal to empower breast and lung cancer survivors: an iterative, multi-stakeholder approach. JMIR Res Protoc 2015; 4 (1): e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Duman-Lubberding S, van Uden-Kraan CF, Jansen F, et al. Feasibility of an eHealth application “OncoKompas” to improve personalized survivorship cancer care. Support Care Cancer 2016; 24 (5): 2163–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Frick M, Vachani C, Hampshire MK, et al. Survivorship after treatment of pancreatic cancer: insights via an internet-based survivorship care plan tool. Int J Radiat Oncol Biol Phys 2017; 99 (2): E534–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Frick MA, Vachani CC, Hampshire MK, et al. Survivorship after lower gastrointestinal cancer: patient-reported outcomes and planning for care. Cancer 2017; 123 (10): 1860–8. [DOI] [PubMed] [Google Scholar]
- 61.Frick MA, Vachani CC, Hampshire MK, et al. Patient-reported survivorship care practices and late effects after treatment of Hodgkin and non-Hodgkin lymphoma. JCO Clin Cancer Inform 2018; 2: 1–10. [DOI] [PubMed] [Google Scholar]
- 62.Jeppesen MM, Ezendam NPM, Pijnenborg JMA, et al. The impact of the survivorship care plan on health care use: 2-year follow-up results of the ROGY care trial. J Cancer Surviv 2018; 12 (1): 18–27. [DOI] [PubMed] [Google Scholar]
- 63.Irene Su H, Stark S, Kwan B, et al. Efficacy of a web-based women’s health survivorship care plan for young breast cancer survivors: a randomized controlled trial. Breast Cancer Res Treat 2019; 176 (3): 579–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wheelock AE, Bock MA, Martin EL, et al. SIS.NET: a randomized controlled trial evaluating a web-based system for symptom management after treatment of breast cancer. Cancer 2015; 121 (6): 893–9. [DOI] [PubMed] [Google Scholar]
- 65.Kapoor A, Nambisan P.. Usability and acceptance evaluation of ACESO: a web-based breast cancer survivorship tool. J Cancer Surviv 2018; 12 (3): 316–25. [DOI] [PubMed] [Google Scholar]
- 66.van den Brink JL, Moorman PW, de Boer MF, et al. Impact on quality of life of a telemedicine system supporting head and neck cancer patients: a controlled trial during the postoperative period at home. J Am Med Inform Assoc 2007; 14 (2): 198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Syrjala KL, Yi JC, Artherholt SB, et al. An online randomized controlled trial, with or without problem-solving treatment, for long-term cancer survivors after hematopoietic cell transplantation. J Cancer Surviv 2018; 12 (4): 560–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kanera IM, Bolman CAW, Willems RA, et al. Lifestyle-related effects of the web-based Kanker Nazorg Wijzer (Cancer Aftercare Guide) intervention for cancer survivors: a randomized controlled trial. J Cancer Surviv 2016; 10 (5): 883–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kanera IM, Willems RA, Bolman CAW, et al. Long-term effects of a web-based cancer aftercare intervention on moderate physical activity and vegetable consumption among early cancer survivors: a randomized controlled trial. Int J Behav Nutr Phys Act 2017; 14 (1): 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Willems RA, Mesters I, Lechner L, et al. Long-term effectiveness and moderators of a web-based tailored intervention for cancer survivors on social and emotional functioning, depression, and fatigue: randomized controlled trial. J Cancer Surviv 2017; 11 (6): 691–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.de Rooij BH, Ezendam NPM, Nicolaije KAH, et al. Survivorship care plans have a negative impact on long-term quality of life and anxiety through more threatening illness perceptions in gynecological cancer patients: the ROGY care trial. Qual Life Res 2018; 27 (6): 1533–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fang S-Y, Wang Y-L, Lu W-H, et al. Long-term effectiveness of an E-based survivorship care plan for breast cancer survivors: a quasi-experimental study. Patient Educ Couns 2020; 103 (3): 549–55. [DOI] [PubMed] [Google Scholar]
- 73.Haq R, Heus L, Baker NA, et al. Designing a multifaceted survivorship care plan to meet the information and communication needs of breast cancer patients and their family physicians: results of a qualitative pilot study. BMC Med Inform Decis Mak 2013; 13: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Badr H, Lipnick D, Diefenbach MA, et al. Development and usability testing of a web-based self-management intervention for oral cancer survivors and their family caregivers. Eur J Cancer Care (Engl) 2016; 25 (5): 806–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tarver WL, Robb BW, Haggstrom DA.. Usefulness and usability of a personal health record and survivorship care plan for colorectal cancer survivors: survey study. JMIR Cancer 2019; 5 (2): e10692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kanera IM, Willems RA, Bolman CAW, et al. Use and appreciation of a tailored self-management eHealth intervention for early cancer survivors: process evaluation of a randomized controlled trial. J Med Internet Res 2016; 18 (8): e229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Frick MA, Vachani CC, Bach C, et al. Survivorship and the chronic cancer patient: Patterns in treatment-related effects, follow-up care, and use of survivorship care plans. Cancer 2017; 123 (21): 4268–76. [DOI] [PubMed] [Google Scholar]
- 78.Pauwels E, Van Hoof E, Charlier C, et al. Design and process evaluation of an informative website tailored to breast cancer survivors’ and intimate partners’ post-treatment care needs. BMC Res Notes 2012; 5: 548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.de Rooij BH, Ezendam NPM, Vos MC, et al. Patients’ information coping styles influence the benefit of a survivorship care plan in the ROGY Care Trial: new insights for tailored delivery. Cancer 2019; 125 (5): 788–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Donohue S, Sesto ME, Hahn DL, et al. Evaluating primary care providers’ views on survivorship care plans generated by an electronic health record system. J Oncol Pract 2015; 11 (3): e329–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hill-Kayser CE, Vachani C, Hampshire MK, et al. High level use and satisfaction with internet-based breast cancer survivorship care plans. Breast J 2012; 18 (1): 97–9. [DOI] [PubMed] [Google Scholar]
- 82.Hill-Kayser CE, Vachani C, Hampshire MK, et al. The role of internet-based cancer survivorship care plans in care of the elderly. J Geriatr Oncol 2011; 2 (1): 58–63. [Google Scholar]
- 83.Syrjala KL, Crouch M-L, Leisenring WM, et al. Engagement with INSPIRE, an online program for hematopoietic cell transplantation survivors. Biol Blood Marrow Transplant 2018; 24 (8): 1692–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kirkwood MK, Hanley A, Bruinooge SS, et al. The state of oncology practice in America, 2018: results of the ASCO practice census survey. J Oncol Pract 2018; 14 (7): e412–20. [DOI] [PubMed] [Google Scholar]
- 85.Smith TG, Strollo S, Hu X, et al. Understanding long-term cancer survivors’ preferences for ongoing medical care. J Gen Intern Med 2019; 34 (10): 2091–2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Klabunde CN, Haggstrom D, Kahn KL, et al. Oncologists’ perspectives on post-cancer treatment communication and care coordination with primary care physicians. Eur J Cancer Care 2017; 26 (4): e12628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.National Cancer Policy Forum, Board on Health Care Services, Health and Medicine Division; Board of Health Care Services; National Cancer Policy Forum. Long-Term Survivorship Care after Cancer Treatment: Proceedings of a Workshop. Washington, DC: National Academies Press; 2018. [PubMed] [Google Scholar]
- 88.Warner JL, Maddux SE, Hughes KS, et al. Development, implementation, and initial evaluation of a foundational open interoperability standard for oncology treatment planning and summarization. J Am Med Inform Assoc 2015; 22 (3): 577–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Willems RA, Lechner L, Verboon P, et al. Working mechanisms of a web-based self-management intervention for cancer survivors: a randomised controlled trial. Psychol Health 2017; 32 (5): 605–25. [DOI] [PubMed] [Google Scholar]
- 90.Nicolaije KA, Ezendam NP, Pijnenborg JM, et al. Paper-based survivorship care plans may be less helpful for cancer patients who search for disease-related information on the internet: results of the Registrationsystem Oncological Gynecology (ROGY) care randomized trial. J Med Internet Res 2016; 18 (7): e162. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.