Summary
Neoagarobiose (NA2) derived from agar marine biomass is a rare reagent that acts as an anti‐melanogenesis reagent and moisturizer. Here, for the economical manufacturing of NA2, we developed the co‐secretory production system of endo‐type β‐agarases (DagA) and exo‐type β‐agarases (EXB3) in Corynebacterium glutamicum. For this purpose, we first developed a secretory system of DagA via Tat pathway. To improve the secretion efficiency, we coexpressed two Tat pathway components (TatA and TatC), and to improve the purity of secreted DagA in the culture supernatant, two endogenous protein genes (Cg2052 and Cg1514) were removed. Using the engineered strain (C. glutamicum SP002), we confirmed that DagA as high as 1.53 g l‐1 was successfully produced in the culture media with high purity (72.7% in the supernatant protein fraction). Next, we constructed the expression system (pHCP‐CgR‐DagA‐EXB3) for the simultaneous secretion of EXB3 via Sec‐pathway together with DagA, and it was clearly confirmed that DagA and EXB3 were successfully secreted as high as 54% and 24.5%, respectively. Finally, using culture medium containing DagA and EXB3, we successfully demonstrated the conversion of high‐concentration agar (40 g l‐1) into NA2 via a two‐stage hydrolysis process.
Short abstract
Agar is a potential and renewable raw macromolecule in macroalgae, and neoagarobiose (NA2) generated by hydrolysis of agar, is a rare reagent acting as a moisturizing and anti‐melanogenesis. Here, we report the development of two types of β‐agarases (endo‐ and exo‐type) secretory system in C. glutamicum for economical production of NA2.
Introduction
Agar is a major cell wall component of red macroalgae (Gracilaria, Gelidium, Pterocladia) and has recently gained considerable interest as a potential and renewable raw material of carbohydrates because of its health benefits (Rajapakse and Kim, 2011). Agar is composed of neutral agarose and charged agaropectin, and agarose is a linear polysaccharide composed of repeating disaccharide blocks of D‐galactose and 3, 6‐anhydro‐l‐galactose (L‐AHG), which are alternately linked by α‐1, 3 and β‐1, 4‐glycosidic bonds (Lahaye et al.,1989; Knutsen et al.,1994). Through the liquefaction process using chemicals or agarolytic enzymes, agar can be hydrolysed into agarooligosaccharides (AOSs) or neoagarooligosaccharides (NAOSs), which consist of a series of even‐numbered monomers including neoagarobiose (NA2), neoagarotetraose (NA4) and neoagarohexaose (NA6) (Kim et al., 2017a; Yun et al., 2017; Jiang et al., 2020). Following liquefaction, these oligosaccharides are further hydrolysed to the monomers, L‐AHG and D‐galactose by enzymatic reactions (Yun et al., 2017). Sugars and oligosaccharides from agar exhibit biological activities such as antioxidative activities and anti‐inflammation effects and have high potential for industrial applications (Hong et al., 2017; Kim et al., 2017b; Park et al., 2020). Among these sugars, NA2, an α‐1, 3 glycosidic linkage of L‐AHG and D‐galactose, is a rare reagent that acts as a moisturizing, anti‐melanogenesis reagent (Kobayashi et al., 1997). Given that the demand for NA2 is steadily increasing, there is an urgent need to explore the economical bioprocess for NA2 production from agar biomass. NA2 is usually produced by the combination of exo‐type and endo‐type β‐agarases that cleave β‐1, 4‐glycosidic bonds of agarose (Temuujin et al., 2012; Yan et al., 2020). For the industrial production of NA2, the development of an economical β‐agarase production system with high purity is necessary. Heterologous production of exo/endo‐type β‐agarases has been performed in various hosts, including Escherichia coli (Ko et al., 2012; Seo et al., 2014; Yoon et al., 2017), Bacillus subtilis (Lee et al., 2008) and Streptomyces lividans (Temuujin et al., 2011; Gullón et al., 2015); however, in most studies, the production yields were not high enough and/or enzymes needed to be purified, which makes it difficult to develop an economic bioprocess.
Corynebacterium glutamicum is a generally recognized as safe (GRAS) strain, and it has been traditionally used as an industrial workhorse for the production of amino acids including lysine, glutamate, arginine (Krämer, 1994; Hermann, 2003) and various commodities or bulk chemicals, including cadaverine, putrescine and γ‐aminobutyrate (GABA) (Woo and Park, 2014; Choi et al., 2015; Kogure and Inui, 2018). Recently, many efforts have been made to use C. glutamicum as a host for the production of recombinant proteins, owing to several beneficial characteristics (Becker and Wittmann, 2012; Wieschalka et al., 2013; Liu et al., 2016; Freudl, 2017). C. glutamicum is a Gram‐positive bacteria that does not possess an outer membrane, so the production of recombinant proteins in C. glutamicum can be achieved by the direct release of recombinant proteins into the culture medium (An et al., 2013; Lee and Kim, 2018). Secretory production into the culture medium does not require a cell lysis process during the purification process, which facilitates the downstream process and significantly reduces the production costs of a target protein (Liu et al., 2016; Yim et al., 2016a). In addition, due to the lack of detectable extracellular proteases (Suzuki et al., 2009), proteolytic degradation of the secreted proteins is not frequently observed, which leads to an increase in production yield (Li et al., 2004).
In this study, we sought to develop a bioprocess for the production of NA2 from the enzymatic hydrolysis of agar. For this purpose, we engineered C. glutamicum for the co‐secretory production of endo‐type β‐agarases (DagA) and exo‐type β‐agarases (EXB3) into the culture medium (Fig. 1). First, we developed a secretory production system for DagA via twin arginine translocation (Tat) pathway in C. glutamicum. To improve the secretion efficiency, we coexpressed two Tat pathway components (TatA and TatC) and engineered C. glutamicum to improve the purity of secreted DagA in the culture medium by eliminating two endogenous genes. Next, in order to co‐secrete DagA and EXB3, the DagA secretion system was combined with a sec‐pathway dependent EXB3 secretion system that we previously developed (Jeong et al., 2019). Using the engineered C. glutamicum, fed‐batch cultivation was performed to produce both DagA and EXB3 into culture medium, and using culture supernatant containing both enzymes, we demonstrated the conversion of high‐concentration agar (40 g l‐1) into NA2 via a two‐stage hydrolysis process.
Fig. 1.

Overall scheme of production of neoagarobiose (NA2) from agar by secretory production of exo‐type and endo‐type β‐agarases (EXB3 and DagA, respectively) in C. glutamicum. Arrows indicate the β‐1,4‐glycosidic bonds cleaved by β‐agarases.
Results
Construction of DagA secretion system via Tat pathway
For hydrolysis of agar, C. glutamicum needs to produce two enzymes including endo‐type β‐agarase and exo‐type β‐agarase in the culture medium (Fig. 1). Previously, we successfully developed the secretory production of exo‐type β‐agarase (Jeong et al., 2019); therefore, we first focussed on the development of secretory production of endo‐type β‐agarase via Tat pathway in C. glutamicum. DagA from S. coelicolor acts as an endo‐type β‐agarase and hydrolyses agarose into NA4 and NA6 (Temuujin et al., 2011). For the secretory production of DagA via Tat pathway, we employed the CgR0949 signal peptide, which has been preferably used for Tat pathway dependent secretion (Teramoto et al., 2011; Freudl, 2017). To increase the expression level of the dagA gene, the expression system was constructed in a high‐copy‐number plasmid (pHCMS) (Choi et al., 2018), yielding pHCP‐CgR‐DagA, in which dagA gene expression was regulated under the strong synthetic promoter (PH36) with CgR0949 signal peptide. After flask cultivation with C. glutamicum harbouring pHCP‐CgR‐DagA, the secretion of DagA into the culture medium was analysed by SDS‐PAGE. However, we could not detect any production of DagA in the culture medium (Fig. 2A). From the SDS‐PAGE analysis of the cytoplasmic fraction, it was confirmed that most DagA was present in the cytoplasm (data not shown), indicating that DagA was successfully produced but could not be secreted into the culture medium via Tat pathway. Previously, it was reported that the coexpression of major components (TatA, TatB and TatC) of Tat machinery could assist in the secretion of recombinant proteins via Tat pathway (De Keersmaeker et al., 2006; Kikuchi et al., 2008; Chi et al., 2011). Therefore, to improve secretion efficiency, we examined the coexpression of two combinations – (i) TatA and TatC (TatAC) and (ii) TatA, TatB and TatC (TatABC). In the flask cultivation, secretory production of DagA into the culture medium was successfully observed in both coexpression systems (Fig. 2A). Western blotting showed that the levels of DagA in both systems were very similar. However, the assay of β–agarase activity with culture supernatant samples confirmed that the coexpression of TatAC gave more positive effects on the secretory production of DagA, that is, the activity in TatAC coexpression (16.1 ± 0.74 U ml‐1) was 2.8‐fold higher than that in TatABC coexpression (5.7 ± 1.16 U ml‐1) (Fig. 2B). These results indicate that the coexpression of TatA and TatC was sufficient for the secretory production of DagA via Tat pathway.
Fig. 2.
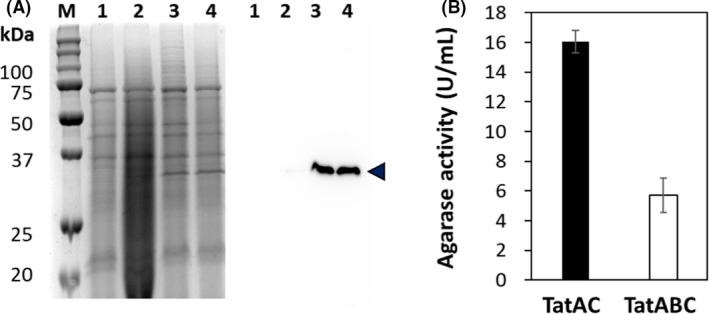
Analysis of DagA secretion level in C. glutamicum ATCC13032 and activity. A. SDS‐PAGE (left panel) and Western blotting (right panel) analysis of culture supernatant. Lane M represent protein size markers (kDa). Lane 1 to 4 represent a protein sample of cells harbouring pCES‐PLPV as a negative control, pHCP‐CgR‐DagA, pHCP‐CgR‐DagA‐TatAC and pHCP‐CgR‐DagA‐TatACB. DagA is indicated as arrowhead (◀). B. DagA activity from the extracellular medium of C. glutamicum harbouring pHCP‐CgR‐DagA‐TatAC (black bar) and C. glutamicum harbouring pHCP‐CgR‐DagA‐TatACB (white bar).
Next, to validate the feasibility of the DagA secretion system in large‐scale cultivation, we performed fed‐batch cultivation with C. glutamicum harbouring pHCP‐CgR‐DagA‐TatAC in a lab‐scale (5 l) bioreactor. At 28 h, cells grew up to 262.5 ± 7.8 of OD600 with a specific growth rate of 0.126 h‐1 (Fig. S1A). The culture supernatant samples were collected during fed‐batch cultivation and analysed by SDS‐PAGE. At 12 h, DagA was produced and its content gradually increased (Fig. 3A). Densitometric analysis confirmed that the maximum content of DagA reached 33.1% of the total secreted proteins, which corresponds to 0.48 g l‐1. Interestingly, in this SDS‐PAGE analysis, we also found another major protein band with a slightly lower molecular weight (~ 27 kDa) than that of DagA (36 kDa) (Fig. 3A). The content of this unknown protein accounted for 34.9% of the total secreted proteins. Although DagA was successfully produced in the culture medium, the purity of DagA was not high enough because of the contamination by this unknown protein.
Fig. 3.

SDS‐PAGE analysis of culture supernatant fraction in fed‐batch cultivation.
A. Fed‐batch cultivation with C. glutamicum ATCC13032 harbouring pHCP‐CgR‐DagA‐TatAC.
B. Fed‐batch cultivation with C. glutamicum SP002 harbouring pHCP‐CgR‐DagA‐TatAC. Lane M represents protein size markers (kDa), and each lane represents culture times (0–48 h). DagA (◀) and unknown protein (◁) are indicated as arrowheads.
Deletion of Cg2052 and Cg1514 for enhanced production of DagA
In secretory production, the purity and production titre of target proteins may be improved by reducing the secretion of competitive proteins, which can be achieved by deletion of genes encoding competitive proteins in the chromosome if the competitive proteins are not essential for cell viability. To identify the unknown protein in the culture medium, the protein was purified from the culture supernatant by fast protein liquid chromatography (FPLC), and the N‐terminal sequence was determined. The first five amino acids were determined as EEVSG, and this sequence corresponds to the N‐terminal sequence of Cg2052 which is a hypothetical secretory protein (Taniguchi et al., 2017). To prevent the contamination of endogenous Cg2052 in the culture medium, the gene encoding Cg2052 was deleted, and the strain was named C. glutamicum SP001. The secretory production of DagA in this engineered strain (C. glutamicum SP001 harbouring pHCP‐CgR‐DagA‐TatAC) was evaluated by fed‐batch cultivation. In this cultivation, DagA was produced well, and its content was slightly increased; however, surprisingly, we also found another major protein band with a similar molecular weight (Fig. S2). After purification and N‐terminal amino acid sequence analysis, the second unknown protein was identified as Cg1514 which is also hypothetical secretory protein (Yim et al., 2016a). To further improve the purity of DagA in the culture medium, the coding sequence of Cg1514 was also deleted from the chromosome of the C. glutamicum SP001 strain, which was named C. glutamicum SP002. The secretory production of DagA in this engineered strain (C. glutamicum SP002 harbouring pHCP‐CgR‐DagA‐TatAC) was evaluated by fed‐batch cultivation. In this cultivation, cells grew up to 251 ± 9.9 of OD600 with a specific growth rate of 0.124 h‐1 (Fig. S1B). The culture supernatant samples were collected during fed‐batch cultivation and analysed by SDS‐PAGE. DagA was clearly observed as a single major protein in the culture medium; its purity was improved by as much as 73% in the total supernatants, which was approximately 2.2‐fold higher than that of wild‐type cells (Fig. 3B). In this cultivation, the titre of DagA in the culture medium was 1.53 g l‐1.
Purification and activity analysis of DagA
From 30 ml of culture supernatant, DagA was purified by filtration, followed by ion‐exchange column chromatography (Fig. 4A), and β‐agarase activity was analysed using agarose as a substrate. It was clearly confirmed that NA4 and NA6 were produced as major products (Fig. 4B), indicating that DagA produced from C. glutamicum SP002 has endo‐type β–agarase activity.
Fig. 4.

Purification of DagA and analysis of its hydrolysis activity.
A. SDS‐PAGE analysis of DagA purification. Lane M represents protein size markers (kDa), and lane 1 represents purified DagA. DagA (◀) is indicated as arrowheads.
B. NA4 and NA6 were determined in the enzyme reaction mixture by time profiles (10–120 min). White and black bars represent NA4 and NA6, respectively.
Secretory production of exo‐ and endo‐type β‐agarases in C. glutamicum SP002
In addition to endo‐type β‐agarase (DagA), cells need to produce exo‐type β‐agarase for further degradation of agar to NA2. Previously, we isolated a novel exo‐type β‐agarase (EXB3) from Gilvimarinus chinensis, and its secretory production system via the Sec‐pathway with the Cg1514 signal peptide was successfully developed in C. glutamicum (Jeong et al., 2019). In that work, we clearly confirmed that NA2 was produced as a major product directly from agarose by reaction with EXB3 alone. For the hydrolysis of agar into NA2, both secretory systems of DagA and EXB3 were combined, yielding pHCP‐CgR‐DagA‐EXB3 in which DagA and EXB3 were secreted via Tat‐ and Sec‐pathways, respectively (Fig. 5A). During the fed‐batch fermentation with C. glutamicum SP002 harbouring pHCP‐CgR‐DagA‐EXB3, both enzymes (DagA and EXB3) were successfully produced in the culture medium: the contents of DagA and EXB3 in culture medium were 54% and 24.5%, respectively (Fig. 5B).
Fig. 5.

Coexpression of DagA and EXB3 in C. glutamicum SP002.
A. Coexpression system of pHCP‐CgR‐DagA‐EXB3. pCG1 ori and p15A ori indicate the origin of replication for C. glutamicum and E. coli, respectively.
B. SDS‐PAGE analysis of the culture supernatant by fed‐batch cultivation. Each lane represents protein size markers (M) and culture times (0–48 h). EXB3 (◀) and DagA (◁) are indicated as arrowheads.
Hydrolysis of agar into NA2 in two‐stage process
After fed‐batch fermentation, culture supernatants containing DagA and EXB3 were collected and mixed with agar to confirm the hydrolysis of agar to NA2 in the two‐stage hydrolysis process. Below 40°C, agar is solidified and cannot be hydrolysed by enzymes. However, after hydrolysis of agar, NAOSs are not solidified and can be further hydrolysed below 40°C. Therefore, hydrolysis reactions were conducted in two stages: (i) at 40°C for hydrolysis of agar (40 g l‐1) into NAOSs by endo‐type DagA and (ii) at 30°C for further hydrolysis of NAOSs to NA2 by exo‐type EXB3. In the first stage (40°C), NA4 and NA6 or higher oligosaccharides were generated from the hydrolysis of agar in the reaction, but NA2 was not detected (Fig. 6). Next, in the second stage (30°C), NA2 was produced as a major product, and a negligible amount of NA4 and NA6 was observed at 8 h (Fig. 6). During the two‐stage process, the solidification of agar was not observed, which also indicates that most of agar used for reaction were successfully hydrolysed to NA2.
Fig. 6.
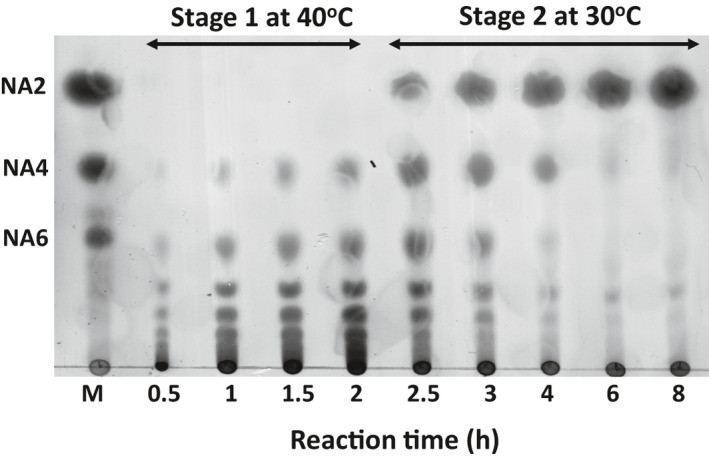
Analysis of the two‐stage hydrolysis of agar with crude fermentation samples by thin‐layer chromatography. Lane M represents NAOSs standard. NA2, Neoagarobiose; NA4, Neoagarotetraose; NA6, Neoagarohexaose. Each number of lane represents the hydrolysis reaction time (h).
Discussion
Despite the steady increase in the demand for NA2, its industrial application has been hampered mainly because of the absence of efficient processes for NA2 production from agar biomass. In the present work, to develop an efficient bioprocess, we engineered C. glutamicum which possesses enormous potential as an alternative platform organism for the secretory production of heterologous proteins, and as demonstrated, both exo‐type and endo‐type β‐agarases (DagA and EXB3) were efficiently produced in culture medium. After cultivation of the engineered strain, culture medium containing both enzymes was directly applied for two‐stage hydrolysis without any purification process, and NA2 was successfully produced by the hydrolysis of agar. Very recently, Yan et al. reported the production of NA2 from agar through two novel β‐agarases (AgaA and AgaB) and a two‐stage hydrolysis process (Yan et al., 2020). Although NA2 was successfully produced from agar (10 g l‐1), both enzymes were produced in the cytoplasm of E. coli, and the hydrolysis reaction could be conducted with purified enzymes that require a multi‐step purification process, including cell disruption and separation using magnetic beads. In general (particularly at the industrial scale), these purifications are highly expensive and laborious, and they also cause the loss of enzymes during purification (Lichty et al., 2005). In contrast, the use of C. glutamicum allowed the secretory production of both enzymes into the culture medium, so enzymes could be directly used for the hydrolysis reaction without further purification and loss of enzymes. A simple and cost‐effective production process can be developed through the elimination of expensive and laborious purification steps.
For the secretory production of DagA in C. glutamicum, we used the CgR0949 signal peptide which mediates the secretion of folded proteins via Tat pathway, and to improve the secretion efficiency, we introduced the coexpression of TatA and TatC, which are the major and essential components of the Tat secretion machinery (Pop et al., 2002; Jongbloed et al., 2004). It is known that TatA forms an active pore in the membrane, and the target protein translocates across the pore formed by TatA and TatC, which acts as the motor for translocation and proceeds protein secretion into the culture medium (Hou and Brüser, 2011; Palmer and Berks, 2012). In this regard, overproduction of TatAC could increase the secretory efficiency of DagA in C. glutamicum. In addition to TatA and TatC, it is also known that TatB helps to improve translocation efficiency although it is not an essential component for Tat function (Hou and Brüser, 2011). Kikuchi et al. reported that the overproduction of all components (TatA‐TatB‐TatC) increased the expression of pro‐PG by more than 10‐fold in C. glutamicum (Kikuchi et al., 2008). In the present study, we also found that the coexpression of all components could improve the translocation efficiency of DagA, but the efficiency was lower than that of TatAC coexpression (Fig. 2). We do not know the exact reason for the lower efficiency of TatABC coexpression, but we think that high expression levels of all genes might result in excessive metabolic loads on cells (particularly on the cytoplasmic membranes). In Gram‐positive bacteria such as Bacillus subtilis, it is known that TatA has TatA‐TatB functionalities in the absence of TatB, and bifunctional TatA interacts with TatC to form a two‐component Tat system or a minimal Tat system (Hou and Brüser, 2011). In this regard, the overexpression of two components (TatA and TatC) is sufficient for Tat‐dependent secretion. In addition, the ratio of each component in the membrane is critical for the translocation efficiency via Tat machinery (Kikuchi et al., 2008), which means that the tuning of each gene expression level can provide a more positive effect on the function of Tat machinery than the simple overexpression of all components under the single strong promoter. For this purpose, we think it is necessary to find the optimal expression levels of each component, which may be achieved using promoters of different strengths (Yim et al., 2016b).
In the enzymatic process, the purity of the enzyme in solution is also an important parameter, and the use of a solution with high enzyme content and purity makes the reaction more reliable and efficient (Santos et al., 2015; Ronghua et al., 2020). To improve the purity of enzymes in culture medium, we deleted two endogenous genes (Cg2052 and Cg1514) which were contaminated in the culture supernatant fractions, and with the engineered C. glutamicum SP002, it was clearly confirmed that the purity of DagA significantly improved as high as 73%, and the production titre was also increased up to 1.5 g l‐1 (Fig. 3). Co‐production of DagA and EXB3 in the C. glutamicum SP002 strain also showed similar contents and purity (Fig. 5), which consequently resulted in the efficient hydrolysis of high‐concentration of agar (40 g l‐1) without any further purification. In addition, we believe that our engineered C. glutamicum SP002 strain has high potential for the secretory production of various recombinant proteins. Teramoto et al. reported the secretory production of green fluorescent protein (GFP) via Tat pathway in C. glutamicum, and although GFP was successfully produced as high as 1.8 g l‐1, this result could be achieved by adding a high‐concentration of calcium ions (Ca2+) during cultivation (Teramoto et al., 2011). The addition of Ca2+ also affected the transcription of the gfp gene and cellular accumulation of the GFP protein in the cytoplasm. Using the C. glutamicum SP002 strain, we could produce DagA as high as 1.5 g l‐1 without Ca2+ addition, and cellular accumulation of DagA was not observed, which means that highly efficient secretion of recombinant protein with high purity can be achieved in the C. glutamicum SP002 strain with coexpression of TatAC.
In this study, we engineered C. glutamicum for the efficient secretory production of β‐agarases (DagA and EXB3) and successfully developed an efficient bioprocess for the production of NA2 from agar biomass. By engineering of C. glutamicum, both enzymes could be produced into culture medium with high contents, so without further purification, the culture medium was directly used for the hydrolysis process, and to the end, a cost‐effective and highly efficient process could be developed. As shown here, a high‐concentration of agar (40 g l‐1) was successfully hydrolysed into NA2 in the two‐stage process, and to the best of our knowledge, this is the highest concentration of agar in the enzymatic hydrolysis of agar (Yan et al., 2020). We also believe that the engineered C. glutamicum SP002 strain can be a potential host for the secretory production of various recombinant proteins with high purity, and our efforts in the engineering of C. glutamicum will provide new insight for the economic and industrial production of recombinant proteins as well as high‐value biochemicals.
Experimental procedures
Bacterial strains, culture media and growth conditions
The bacterial strains used in this study are listed in Table 1. E. coli XL1‐Blue was used for DNA manipulation and plasmid maintenance. C. glutamicum ATCC 13032 and its engineered strains were used to produce recombinant proteins. E. coli was cultivated in Luria‐Bertani (LB) medium (BD, Franklin Lakes, NJ, USA) at 37°C with shaking (200 rpm). To produce recombinant proteins, C. glutamicum was inoculated in brain heart infusion (BHI) medium (BD). After the overnight cultivation of C. glutamicum at 30°C, the cells were transferred into a 250 ml baffled flask containing 50 ml of fresh semi‐defined medium for 24 h with shaking (200 rpm). The semi‐defined medium contained 20 g glucose, 7 g casamino acid, 15 g yeast extract, 3 g K2HPO4, 1 g KH2PO4, 2 g urea, 10 g (NH4)2SO4, 2 g MgSO4, 200 μg biotin, 5 mg thiamine, 10 mg calcium pantothenate, 10 mg FeSO4, 1 mg MnSO4, 1 mg ZnSO4, 200 μg CuSO4 and 10 mg of CaCl2 in 1 l. In all cultivations, kanamycin (Km, 25 μg ml‐1) was added to the culture medium as the sole antibiotic.
Table 1.
Bacterial strains and plasmids used in this study.
| Relevant characteristics | Reference or Source | |
|---|---|---|
| Strains | ||
| E. coli | ||
| XL1‐blue | recA1 endA1 gyrA96 thi‐1 hsdR17 supE44 relA1 lac [F′ proABlacIq ZΔM15 Tn10 (Tetr)] | Stratagenea |
| C. glutamicum | ||
| ATCC 13032 | Biotin‐auxotrophic wild type | ATCC |
| SP001 | ATCC 13032 with in frame deletion of Cg2052 | This study |
| SP002 | SP001 with in frame deletion of Cg1514 | This study |
| Plasmids | ||
| pCES‐PLPV | pCES208 derivative; MCS and rrn terminator,Kmr | Yim et al. (2016a) |
| pXD5 | pCES208 derivative; Cg1514 signal peptide with xlnA and CgR0949 signal peptide with xynB | Yim et al. (2017) |
| pUWL201PW‐DagA | dagA expression plasmid in Streptomyces cells | Temuujin et al. (2011) |
| pHCMS | pCES‐PLPV derivative; parB nonsense mutation,Kmr | Choi et al. (2018) |
| pHCP‐CgR | pCES‐PLPV derivative; CgR0949 signal peptide from pXD5 | This study |
| pHCP‐CgR‐DagA | pHCMS derivative; CgR0949 signal peptide fused with dagA FLAG tag | This study |
| pHCP‐CgR‐DagA‐TatAC | pHCP‐CgR‐DagA derivative; tatAC | This study |
| pHCP‐CgR‐DagA‐TatACB | pHCP‐CgR‐DagA derivative; tatACB | This study |
| pCG‐S‐EXB3 | pCG‐S derivatives; EXB3 from G. chinensis, Kmr | Jeong et al. (2019) |
| pHCP‐CgR‐DagA‐EXB3 | pHCP‐CgR‐DagA‐TatAC derivative; CG‐S‐EXB3 | This study |
| pK19mobSacB | Mobilizable vector, Kmr | Schäfer et al. (1994) |
| pK19‐∆Cg2052ss | pK19mobsacB derivative; flanking region of Cg2052 signal peptide | This study |
| pK19‐∆Cg1514ss | pK19mobsacB derivative; flanking region of Cg1514 signal peptide | This study |
New England Biolabs, Beverly, MA, USA.
DNA manipulation
All restriction enzymes used for gene manipulation were purchased from Enzynomics (Daejeon, Korea). Polymerase chain reaction (PCR) was performed in a C1000TM Thermal Cycler (Bio‐Rad, Hercules, CA, USA) using Prime STAR HS polymerase (Takara Bio Inc., Shiga, Japan). All primers used for the PCR are listed in Table S1. The CgR0949 signal peptide was amplified from pXD5 as a template (Yim et al., 2017) by PCR with CgR0949‐F and CgR0949‐R primers using pXD5. The PCR product was digested with BamHI and XbaI and cloned into high‐copy number plasmid (pHCMS) (Choi et al., 2018), yielding pHCP‐CgR. To express the endo‐type β‐agarase, Streptomyces coelicolor A3(2) β‐agarase (dagA) gene was amplified from pUWL201PW‐DagA (Temuujin et al., 2011) by PCR with DagA‐F and DagA‐R primers. The PCR product was digested with SfiI and cloned into pHCP‐CgR, yielding pHCP‐CgR‐DagA. For coexpression of Tat pathway proteins, tatA and tatC genes were amplified from the chromosomal DNA of C. glutamicum ATCC13032 by PCR with two primers, TatAC‐F and TatAC‐R. The PCR product was digested with NotI and cloned into pHCP‐CgR‐DagA, yielding pHCP‐CgR‐DagA‐TatAC. In addition, the tatB gene was amplified from the chromosomal DNA of C. glutamicum by PCR using TatB‐F and TatB‐R. After digestion with BglII and SpeI, the PCR product was cloned into the same restriction enzyme site of pHCP‐CgR‐DagA‐TatAC, yielding pHCP‐CgR‐DagA‐TatACB. For the coexpression of DagA and EXB3 genes, pHCP‐CgR‐DagA‐EXB3 was constructed using Gibson assembly (Thomas et al., 2015). DNA fragments consisting of the EXB3 gene expression cassette with Cg1514 promoter (PCg1514) and Cg1514 signal peptide were obtained from pCG‐S‐EXB3 (Jeong et al., 2019) by PCR with DagB Gib‐F and DagB Gib‐R. After digestion of pHCP‐CgR‐DagA‐TatAC with BglII and SpeI, the PCR product and pHCP‐CgR‐DagA‐TatAC were assembled by Gibson assembly, yielding pHCP‐CgR‐DagA‐EXB3.
Deletion of Cg2052 and Cg1514 genes in chromosome
For gene deletion, we mainly used the double crossover method using pK19mobSacB (Schäfer et al., 1994). For the deletion of the Cg2052 gene, 500 bp DNA fragment A upstream of Cg2052 was amplified by PCR with Cg2052‐A‐F and Cg2052‐A‐R primers, and the other 500 bp DNA fragment B downstream of Cg2052 was amplified by PCR with Cg2052‐B‐F and Cg2052‐B‐R. Both the PCR products were used as templates for overlap PCR with Cg2052‐A‐F and Cg2052‐B‐R. The resulting PCR products were digested with SalI and BamHI and cloned into pK19mobsacB with the same restriction enzyme sites to yield pK19‐∆Cg2052ss. C. glutamicum ATCC13032 was transformed with pK19‐∆Cg2052ss, and the plasmid‐integrated cells were screened in BHIS (BHI with 30 g l‐1 sorbitol) plates containing kanamycin. The isolated cells were inoculated in BHI media and spread on a 10% (w/v) sucrose LB plate to pop out the integrated plasmid. With the isolated clones, the deletion of the Cg2052 gene was confirmed by colony PCR with the two primers Cg2052‐A‐F and Cg2052‐B‐R. The Cg2052 gene deleted strain was C. glutamicum SP001. The same method was used to delete the Cg1514 gene. Cg1514‐A‐F, Cg1514‐A‐R, Cg1514‐B‐F and Cg1514‐B‐R primers were used in Cg1514 gene deletion. The digested PCR product was cloned into pK19mobsacB to yield pK19‐∆Cg1514ss. C. glutamicum SP001 was transformed with pK19‐∆Cg1514ss and plasmid‐integrated cells to yield C. glutamicum SP002.
Fed‐batch cultivation
After overnight cultivation in BHI media, the cells were inoculated into four flasks containing 50 ml of semi‐defined medium in each baffled flask. After cultivation at 30°C for 24 h with shaking (200 rpm), the cells were transferred into 2 l semi‐defined medium in a 5 l jar bioreactor (BioCNS, Daejeon, Korea). The culture temperature was maintained at 30°C throughout cultivation, and the pH was controlled at pH 7.0 by adding a 20% ammonia solution. The dissolved oxygen (DO) concentration was maintained at 30% (v/v) by online monitoring and automatically increasing the agitation speed up to 1200 rpm. Cell growth was determined by measuring optical density at 600 nm (OD600) using a spectrophotometer (Mecasys, Daejeon, Korea).
Protein sample preparation and analysis
After flask cultivation, cells were precipitated by centrifugation (13 000 rpm, 10 min and 4°C), and the extracellular proteins were collected from the culture supernatant using the acetone precipitation method (Jiang et al., 2004). The culture supernatant was mixed with the same volume of cold acetone and incubated at −20°C for 2 h. After centrifugation (13 000 rpm, 10 min and 4°C), the pellet was resuspended in protein sampling buffer (50 mM Tris–HCl, pH 10, 10% glycerol, 4% SDS, 8 M urea, 2% β‐mercaptoethanol and 0.02% bromophenol blue). For the fed‐batch cultivation samples, the culture supernatant (5 μl) was mixed with protein sampling buffer without any concentration.
Protein samples were analysed using SDS‐PAGE and Western blotting (Jeong et al., 2019). After SDS‐PAGE scanning, protein densitometry was performed using the GelAnalyzer2010 software (http://www.gelanalyzer.com). For Western blotting, the polyvinyl difluoride (PVDF) membrane was incubated with a blocking solution containing horseradish peroxidase (HRP)‐conjugated monoclonal anti‐FLAG M2 antibody (Sigma Aldrich, St. Louis, MO, USA) for the detection of FLAG‐tagged proteins. After incubation, the membrane was washed with TBS‐T (four times, 10 min each), and the signal was developed using an ECL kit (GE Healthcare Bio‐Science AB, Buckinghamshire, UK).
Purification of DagA
For the purification of DagA, culture broth, after fed‐batch cultivation, was centrifuged at 13 000 rpm for 30 min at 4°C. The supernatant sample was collected and dialysed with 50 mM Tris‐HCl buffer (pH 7.0) as an equilibrium buffer for 24 h. The residual insoluble matters were removed by filtration using a 0.45 μm syringe filter (Sartorius, Goettingen, Germany). After filtration, the sample was applied to a HiTrap Q HP column (GE Healthcare Life Sciences, Uppsala, Sweden) mounted on an ÄKTA pure anion exchange chromatography (GE Healthcare Life Sciences). After washing with equilibrium buffer (50 mM Tris‐HCl, 1 M NaCl, and pH 7.0), proteins were eluted with a NaCl concentration gradient (0 to 1 M NaCl) using a UV detector.
β‐agarase activity assay
The DagA activity assay was performed using the 3, 5‐dinitrosalicylic acid (DNS) method (Park et al., 2014). After cultivation, the culture supernatant (2 ml) was collected by centrifugation at 13,000 rpm for 10 min. The supernatant (25 μl) was mixed with 975 μL of 0.2% agarose (Higel‐Agarose ClearTM, E&S, Daejeon, Korea) in 50 mM Tris‐HCl buffer (pH 7.0). The mixture reacted at 40°C for 10 min. After the reaction, 1 ml of DNS reagent was added and boiled at 100°C for 10 min. After cooling, the absorbance of the sample was measured at 540 nm by UV spectrometry (Mecasys, Korea). One unit (U) of endo‐type β‐agarase activity was defined as the amount of enzyme required to liberate 1 μmol of reducing galactose per minute, at pH 7.0 and 40°C.
Two‐stage hydrolysis of agar
For the hydrolysis of agar (Difco, Detroit, MI, USA), 1 l of semi‐defined media containing 40 g agar was autoclaved for sterilization and solubilization of agar, after which the agar medium was cooled to 40°C. In the first stage, 20 ml of filtered crude fermentation samples containing DagA and EXB3 was added, and the hydrolysis reaction was conducted at 40°C for 2 h. Next, in the second stage, the reaction mixture was cooled to 30°C and 20 ml of the fermentation sample was added. The second hydrolysis reaction was conducted at 30°C for 6 h. The reaction products in each stage were analysed by thin‐layer chromatography (TLC) method (Jeong et al., 2019).
Analysis of NAOSs
The content of NAOSs in the hydrolysis reaction with purified DagA was determined by high‐performance liquid chromatography (HPLC) (Park et al., 2014). The same method of the β‐agarase activity assay was used. The reaction sample was centrifuged at 13 000 rpm for 10 min, and the supernatant was analysed using a Waters 2960 HPLC system (Waters, Milford, MA, USA). Asahipak NH2P‐50 4E column (Shodex, Tokyo, Japan) was used, and 80% CH3CN was used as the mobile phase. The flow rate was 1.0 ml min‐1. Detection was performed using an evaporative light scattering detector (ELSD).
Protein identification
The endogenous proteins (Cg1514 and Cg2052) were identified by N‐terminal amino acid sequencing or MALDI‐TOF‐MS. After SDS‐PAGE analysis of the supernatant, a major secreted protein band was extracted and the following analytical method was performed in EMASS Co. (Seoul, Korea).
Funding Information
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Ministry of Science and ICT (MSIT) (No. NRF‐2020R1A2C2012537) and the "Cooperative Research Program for Agriculture Science and Technology Development (project number PJ015613022021)" of the Rural Development Administration, Republic of Korea.
Conflict of interests
The authors declare no competing financial interests.
Author contributions
Eun Jung Jeon and Jae Woong Choi involved in investigation, formal analysis and writing – original draft preparation. Min Soo Cho involved in investigation, validation and data curation. Ki Jun Jeong involved in methodology, conceptualization, supervision, writing – reviewing and editing, and funding acquisition.
Supporting information
Fig. S1. Time profile of fed‐batch cultivation. A. Time profile of cell growth (⬤), glucose concentration (◇) and dry cell weight (■) of C. glutamicum ATCC13032. B. Time profile of cell growth (⬤), glucose concentration (▵) and dry cell weight (■) of C. glutamicum SP002.
Fig. S2. Production of DagA in engineered C. glutamicum SP001 by fed‐batch cultivation. DagA (◀), second unknown protein (◁). Each lane represents protein size marker (M) and culture times (0–49 h).
Table S1. List of oligonucleotides used in the PCR experiment.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Ministry of Science and ICT (MSIT) (No. NRF‐2020R1A2C2012537) and the "Cooperative Research Program for Agriculture Science and Technology Development (project number PJ015613022021)" of the Rural Development Administration, Republic of Korea.
Microb. Biotechnol. (2021) 14(5), 2164–2175
References
- An, S.J., Yim, S.S., and Jeong, K.J. (2013) Development of a secretion system for the production of heterologous proteins in Corynebacterium glutamicum using the Porin B signal peptide. Protein Expr Purif 89: 251–257. [DOI] [PubMed] [Google Scholar]
- Becker, J., and Wittmann, C. (2012) Bio‐based production of chemicals, materials and fuels ‐Corynebacterium glutamicum as versatile cell factory. Curr Opin Biotechnol 23: 631–640. [DOI] [PubMed] [Google Scholar]
- Chi, W.J., Oh, E.A., Kim, J.H., and Hong, S.K. (2011) Enhancement of protein secretion by TatAC overexpression in Streptomyces griseus. Biotechnol Bioprocess Eng 16: 59–71. [Google Scholar]
- Choi, J.W., Yim, S.S., Lee, S.H., Kang, T.J., Park, S.J., and Jeong, K.J. (2015) Enhanced production of gamma‐aminobutyrate (GABA) in recombinant Corynebacterium glutamicum by expressing glutamate decarboxylase active in expanded pH range. Microb Cell Fact 14: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, J.W., Yim, S.S., and Jeong, K.J. (2018) Development of a high‐copy‐number plasmid via adaptive laboratory evolution of Corynebacterium glutamicum . Appl Microbiol Biotechnol 102: 873–883. [DOI] [PubMed] [Google Scholar]
- Freudl, R. (2017) Beyond amino acids: use of the Corynebacterium glutamicum cell factory for the secretion of heterologous proteins. J Biotechnol 258: 101–109. [DOI] [PubMed] [Google Scholar]
- Gullón, S., Vicente, R.L., Valverde, J.R., Marín, S., and Mellado, R.P. (2015) Exploring the feasibility of the Sec route to secrete proteins using the Tat route in Streptomyces lividans . Mol Biotechnol 57: 931–938. [DOI] [PubMed] [Google Scholar]
- Hermann, T. (2003) Industrial production of amino acids by coryneform bacteria. J Biotechnol 104: 155–172. [DOI] [PubMed] [Google Scholar]
- Hong, S.J., Lee, J.H., Kim, E.J., Yang, H.J., Park, J.S., and Hong, S.K. (2017) Anti‐obesity and anti‐diabetic effect of neoagarooligosaccharides on high‐fat diet‐induced obesity in mice. Mar Drugs 15: 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou, B., and Brüser, T. (2011) The Tat‐dependent protein translocation pathway. Biomol Concepts 2: 507–523. [DOI] [PubMed] [Google Scholar]
- Jeong, Y.J., Choi, J.W., Cho, M.S., and Jeong, K.J. (2019) Isolation of Novel Exo‐type β‐Agarase from Gilvimarinus chinensis and high‐level secretory production in Corynebacterium glutamicum . Biotechnol Bioprocess Eng 24: 250–257. [Google Scholar]
- Jiang, C., Liu, Z., Cheng, D., and Mao, X. (2020) Agarose degradation for utilization: enzymes, pathways, metabolic engineering methods and products. Biotechnol Adv 45: 107641. [DOI] [PubMed] [Google Scholar]
- Jiang, L., He, L., and Fountoulakis, M. (2004) Comparison of protein precipitation methods for sample preparation prior to proteomic analysis. J Chromatogr A 1023: 317–320. [DOI] [PubMed] [Google Scholar]
- Jongbloed, J.D., Grieger, U., Antelmann, H., Hecker, M., Nijland, R., Bron, S., and Van Dijl, J.M. (2004) Two minimal Tat translocases in Bacillus . Mol Microbiol 54: 1319–1325. [DOI] [PubMed] [Google Scholar]
- De Keersmaeker, S., Vrancken, K., Van Mellaert, L., Lammertyn, E., Anné, J., and Geukens, N. (2006) Evaluation of TatABC overproduction on Tat‐and Sec‐dependent protein secretion in Streptomyces lividans . Arch Microbiol 186: 507–512. [DOI] [PubMed] [Google Scholar]
- Kikuchi, Y., Itaya, H., Date, M., Matsui, K., and Wu, L.F. (2008) TatABC overexpression improves Corynebacterium glutamicum Tat‐dependent protein secretion. Appl Environ Microbiol 75: 603–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J.H., Yun, E.J., Seo, N., Yu, S., Kim, D.H., Cho, K.M., et al. (2017a) Enzymatic liquefaction of agarose above the sol‐gel transition temperature using a thermostable endo‐type beta‐agarase, Aga16B. Appl Microbiol Biotechnol 101: 1111–1120. [DOI] [PubMed] [Google Scholar]
- Kim, J.H., Yun, E.J., Yu, S., Kim, K.H., and Kang, N.J. (2017b) Different levels of skin whitening activity among 3,6‐anhydro‐L‐galactose, agarooligosaccharides, and neoagarooligosaccharides. Mar Drugs 15: 321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutsen, S.H., Myslabodski, D.E., Larsen, B., and Usov, A.I. (1994) A modified system of nomenclature for red algal galactans. Bot Mar 37: 163–169. [Google Scholar]
- Ko, H.J., Park, E., Song, J., Yang, T.H., Lee, H.J., Kim, K.H., and Choi, I.G. (2012) Functional cell surface display and controlled secretion of diverse agarolytic enzymes by Escherichia coli with a novel ligation‐independent cloning vector based on the autotransporter YfaL. Appl Environ Microbiol 78: 3051–3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi, R., Takisada, M., Suzuki, T., Kirimura, K., and Usami, S. (1997) Neoagarobiose as a novel moisturizer with whitening effect. Biosci Biotechnol Biochem 61: 162–163. [DOI] [PubMed] [Google Scholar]
- Kogure, T., and Inui, M. (2018) Recent advances in metabolic engineering of Corynebacterium glutamicum for bioproduction of value‐added aromatic chemicals and natural products. Appl Microbiol Biotechnol 102: 8685–8705. [DOI] [PubMed] [Google Scholar]
- Krämer, R. (1994) Secretion of amino‐acids by bacteria ‐ physiology and mechanism. FEMS Microbiol Rev 13: 75–93. [Google Scholar]
- Lahaye, M., Yaphe, W., Viet, M.T.P., and Rochas, C. (1989) 13C‐NMR spectroscopic investigation of methylated and charged agarose oligosaccharides and polysaccharides. Carbohydr Res 190: 249–265. [Google Scholar]
- Lee, D.G., Jang, M.K., Lee, O.H., Kim, N.Y., Ju, S.A., and Lee, S.H. (2008) Over‐production of a glycoside hydrolase family 50 beta‐agarase from Agarivorans sp. JA‐1 in Bacillus subtilis and the whitening effect of its product. Biotechnol Lett 30: 911–918. [DOI] [PubMed] [Google Scholar]
- Lee, M.J., and Kim, P. (2018) Recombinant protein expression system in Corynebacterium glutamicum and its application. Front Microbiol 9: 2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, W., Zhou, X., and Lu, P. (2004) Bottlenecks in the expression and secretion of heterologous proteins in Bacillus subtilis . Res Microbiol 155: 605–610. [DOI] [PubMed] [Google Scholar]
- Lichty, J.J., Malecki, J.L., Agnew, H.D., Michelson‐Horowitz, D.J., and Tan, S. (2005) Comparison of affinity tags for protein purification. Protein Expr Purif 41: 98–105. [DOI] [PubMed] [Google Scholar]
- Liu, X., Yang, Y., Zhang, W., Sun, Y., Peng, F., Jeffrey, L., et al. (2016) Expression of recombinant protein using Corynebacterium glutamicum: progress, challenges and applications. Crit Rev Biotechnol 36: 652–664. [DOI] [PubMed] [Google Scholar]
- Palmer, T., and Berks, B.C. (2012) The twin‐arginine translocation (Tat) protein export pathway. Nat Rev Microbiol 10: 483–496. [DOI] [PubMed] [Google Scholar]
- Park, J., Hong, S.K., and Chang, Y.K. (2014) Production of DagA, a beta‐agarase, by Streptomyces lividans in glucose medium or mixed‐sugar medium simulating microalgae hydrolysate. J Microbiol Biotechnol 24: 1622–1628. [DOI] [PubMed] [Google Scholar]
- Park, S.H., Lee, C.R., and Hong, S.K. (2020) Implications of agar and agarase in industrial applications of sustainable marine biomass. Appl Microbiol Biotechnol 104: 2815–2832. [DOI] [PubMed] [Google Scholar]
- Pop, O., Martin, U., Abel, C., and Müller, J.P. (2002) The twin‐arginine signal peptide of PhoD and the TatAd/Cd proteins of Bacillus subtilis form an autonomous Tat translocation system. J Biol Chem 277: 3268–3273. [DOI] [PubMed] [Google Scholar]
- Rajapakse, N., and Kim, S.K. (2011) Nutritional and digestive health benefits of seaweed. Adv Food Nutr Res 64: 17–28. [DOI] [PubMed] [Google Scholar]
- Ronghua, Z., Xianqing, L., Fang, L., Qing, D., Wei, C., YaPing, W., and Ben, R. (2020) High‐level Expression of an acidic and thermostable chitosanase in Pichia pastoris using multi‐copy expression strains and high‐cell‐density cultivation. Biotechnol Bioprocess Eng 25: 562–570. [Google Scholar]
- Santos, J.C.S.D., Barbosa, O., Ortiz, C., Berenguer‐Murcia, A., Rodrigues, R.C., and Fernández Lafuente, R. (2015) Importance of the support properties for immobilization or purification of enzymes. ChemCatChem 7: 2413–2432. [Google Scholar]
- Schäfer, A., Tauch, A., Jäger, W., Kalinowski, J., Thierbach, G., and Pühler, A. (1994) Small mobilizable multi‐purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: selection of defined deletions in the chromosome of Corynebacterium glutamicum . Gene 145: 69–73. [DOI] [PubMed] [Google Scholar]
- Seo, Y.B., Lu, Y., Chi, W.J., Park, H.R., Jeong, K.J., Hong, S.K., and Chang, Y.K. (2014) Heterologous expression of a newly screened beta‐agarase from Alteromonas sp.GNUM1 in Escherichia coli and its application for agarose degradation. Process Biochem 49: 430–436. [Google Scholar]
- Suzuki, N., Watanabe, K., Okibe, N., Tsuchida, Y., Inui, M., and Yukawa, H. (2009) Identification of new secreted proteins and secretion of heterologous amylase by C. glutamicum . Appl Microbiol Biotechnol 82: 491–500. [DOI] [PubMed] [Google Scholar]
- Taniguchi, H., Busche, T., Patschkowski, T., Niehaus, K., Pátek, M., Kalinowski, J., and Wendisch, V.F. (2017) Physiological roles of sigma factor SigD in Corynebacterium glutamicum . BMC Microbiol 17: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temuujin, U., Chi, W.J., Lee, S.Y., Chang, Y.K., and Hong, S.K. (2011) Overexpression and biochemical characterization of DagA from Streptomyces coelicolor A3(2): an endo‐type beta‐agarase producing neoagarotetraose and neoagarohexaose. Appl Microbiol Biotechnol 92: 749–759. [DOI] [PubMed] [Google Scholar]
- Temuujin, U., Chi, W.J., Chang, Y.K., and Hong, S.K. (2012) Identification and biochemical characterization of Sco3487 from Streptomyces coelicolor A3(2), an exo‐ and endo‐type beta‐agarase‐producing neoagarobiose. J Bacteriol 194: 142–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teramoto, H., Watanabe, K., Suzuki, N., Inui, M., and Yukawa, H. (2011) High yield secretion of heterologous proteins in Corynebacterium glutamicum using its own Tat‐type signal sequence. Appl Microbiol Biotechnol 91: 677–687. [DOI] [PubMed] [Google Scholar]
- Thomas, S., Maynard, N.D., and Gill, J. (2015) DNA library construction using Gibson Assembly®. Nat Methods 12: i–ii. [Google Scholar]
- Wieschalka, S., Blombach, B., Bott, M., and Eikmanns, B.J. (2013) Bio‐based production of organic acids with Corynebacterium glutamicum . Microb Biotechnol 6: 87–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo, H.M., and Park, J.B. (2014) Recent progress in development of synthetic biology platforms and metabolic engineering of Corynebacterium glutamicum . J Biotechnol 180: 43–51. [DOI] [PubMed] [Google Scholar]
- Yan, J., Chen, P., Zeng, Y., Yang, J., Men, Y., Zhu, Y., and Sun, Y. (2020) Production of neoagarobiose from agar through a dual‐enzyme and two‐stage hydrolysis strategy. Int J Biol Macromol 160: 288–295. [DOI] [PubMed] [Google Scholar]
- Yim, S.S., Choi, J.W., Lee, R.J., Lee, Y.J., Lee, S.H., Kim, S.Y., and Jeong, K.J. (2016a) Development of a new platform for secretory production of recombinant proteins in Corynebacterium glutamicum . Biotechnol Bioeng 113: 163–172. [DOI] [PubMed] [Google Scholar]
- Yim, S.S., Choi, J.W., Lee, S.H., and Jeong, K.J. (2016b) Modular optimization of a hemicellulose‐utilizing pathway in Corynebacterium glutamicum for consolidated bioprocessing of hemicellulosic biomass. ACS Synth Biol 5: 334–343. [DOI] [PubMed] [Google Scholar]
- Yim, S.S., Choi, J.W., Lee, S.H., Jeon, E.J., Chung, W.J., and Jeong, K.J. (2017) Engineering of Corynebacterium glutamicum for consolidated conversion of hemicellulosic biomass into Xylonic Acid. Biotechnol J 12: 1700040. [DOI] [PubMed] [Google Scholar]
- Yoon, S.Y., Lee, H.M., Kong, J.N., and Kong, K.H. (2017) Secretory expression and enzymatic characterization of recombinant Agarivorans albus beta‐agarase in Escherichia coli . Prep Biochem Biotechnol 47: 1037–1042. [DOI] [PubMed] [Google Scholar]
- Yun, E.J., Yu, S., and Kim, K.H. (2017) Current knowledge on agarolytic enzymes and the industrial potential of agar‐derived sugars. Appl Microbiol Biotechnol 101: 5581–5589. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Time profile of fed‐batch cultivation. A. Time profile of cell growth (⬤), glucose concentration (◇) and dry cell weight (■) of C. glutamicum ATCC13032. B. Time profile of cell growth (⬤), glucose concentration (▵) and dry cell weight (■) of C. glutamicum SP002.
Fig. S2. Production of DagA in engineered C. glutamicum SP001 by fed‐batch cultivation. DagA (◀), second unknown protein (◁). Each lane represents protein size marker (M) and culture times (0–49 h).
Table S1. List of oligonucleotides used in the PCR experiment.


