Summary
Water‐soluble polymers (WSPs) are a versatile group of chemicals used across industries for different purposes such as thickening, stabilizing, adhesion and gelation. Synthetic polymers have tailored characteristics and are chemically homogeneous, whereas plant‐derived biopolymers vary more widely in their specifications and are chemically heterogeneous. Between both sources, microbial polysaccharides are an advantageous compromise. They combine naturalness with defined material properties, precisely controlled by optimizing strain selection, fermentation operational parameters and downstream processes. The relevance of such bio‐based and biodegradable materials is rising due to increasing environmental awareness of consumers and a tightening regulatory framework, causing both solid and water‐soluble synthetic polymers, also termed ‘microplastics’, to have come under scrutiny. Xanthan gum is the most important microbial polysaccharide in terms of production volume and diversity of applications, and available as different grades with specific properties. In this review, we will focus on the applicability of xanthan gum in agriculture (drift control, encapsulation and soil improvement), considering its potential to replace traditionally used synthetic WSPs. As a spray adjuvant, xanthan gum prevents the formation of driftable fine droplets and shows particular resistance to mechanical shear. Xanthan gum as a component in encapsulated formulations modifies release properties or provides additional protection to encapsulated agents. In geotechnical engineering, soil amended with xanthan gum has proven to increase water retention, reduce water evaporation, percolation and soil erosion – topics of high relevance in the agriculture of the 21st century. Finally, hands‐on formulation tips are provided to facilitate exploiting the full potential of xanthan gum in diverse agricultural applications and thus providing sustainable solutions.
Synthetic water‐soluble polymers are used for different purposes in agriculture, but there are increasing concerns regarding their environmental impact. Microbial polysaccharides obtained by industrial fermentation are a sustainable, eco‐friendly alternative with precisely controlled material characteristics. This is shown on the example of xanthan gum applied for soil improvement, drift control and encapsulation.

Introduction
Efficiency in agriculture is continuously being improved by the implementation of new and high‐performance materials. These are applied to beneficially modify the properties of soil, water, fertilizers and plant protection products.
One important class of chemical compounds are WSPs, which are widely used as thickeners, stabilizers and binders. Being of synthetic origin, their properties can be tailored and precisely controlled to obtain versatile functionalities and high reliability in performance. However, despite their high molecular weight, synthetic WSPs can break down over time into potentially toxic and carcinogenic monomers like in the case of polyacrylamides (Xiong et al., 2018). Their degradation is usually slow and has been estimated to be around 10% per year (Hennecke et al., 2018), which may result in deleterious effects on the environment. Due to a lack of analytical methods for the quantification of WSPs in environmental matrices, this impact is still under debate, but has been compared to issues caused by solid microplastics nowadays (Huppertsberg et al., 2020). In the case of synthetic WSPs, the research is still on the very beginning (Arp and Knutsen, 2019). Therefore, they have come under scrutiny by environmental protection agencies and regulatory bodies. Regulatory pressure peaked when the European Chemicals Agency (ECHA) published its proposal for a restriction of intentionally added microplastics in January 2019 (ECHA European Chemicals Agency, 2019), which in its initial version comprised not only solids, but also synthetic WSPs in the definition of microplastics. Although in the latest version these are not comprised any more, researchers have further emphasized the importance of considering synthetic WSPs in the risk evaluation and regulating their release into the environment (Arp and Knutsen, 2019). With consumers becoming more and more aware of the broadened scope of microplastics, re‐formulation efforts are intensifying to avoid the use of synthetic WSPs, especially in the cosmetics and home care segment. In the agricultural sector, manufacturers face additional regulatory changes. According to the new Regulation (EU) 2019/1009, non‐nutrient polymers used in fertilizing products for the EU market will have to comply with biodegradability criteria (The European Parliament and the Council of the European Union, 2019). In practice, this means that non‐biodegradable polymers used for controlled nutrient release or improved water retention will have to be replaced by biodegradable alternatives.
In view of recent findings on the presence of solid microplastic particles in drinking water (Pivokonsky et al., 2018; Eerkes‐Medrano et al., 2019; Novotna et al., 2019) and fresh produce (Oliveri Conti et al., 2020), it is not unlikely that retailers will increasingly be confronted with end consumers calling for ‘zero residue’ products – also referring to microplastic residues. This may push for farming practices, in which only biodegradable, eco‐friendly co‐formulants, adjuvants, technological processing aids or soil amendments are used. Furthermore, the future might bring about stricter regulations on carbon emissions, thus favouring natural solutions over synthetic polymers of petrochemical origin.
These regulatory and market developments promote the search for biodegradable and environmentally friendly WSPs, which degrade into non‐contaminating compounds. One alternative to synthetic polymers are natural polysaccharides. They can be subdivided into two main categories: the starch‐based and non‐starch polysaccharides. Non‐starch polysaccharides can be obtained from plants (e.g. guar gum, locust bean gum, pectin), by microbial fermentation (e.g. xanthan gum and gellan gum), from animal origin (e.g. chitosan and gelatin) or from marine plants (e.g. alginate and carrageenan) (Nobre et al., 2015). Plant‐derived polysaccharides often struggle with fluctuating availability, yearly production and variations in their chemical characteristics. Their production is relatively cheap but season‐dependent and taking place under uncontrolled environmental conditions (Giavasis, 2013). In contrast, polysaccharides derived from microbial fermentation are bio‐synthesized under precisely controlled conditions, requiring limited space and production time. This results in stable chemical characteristics with narrow specifications and a high purity. It also ensures broad availability in the market. Therefore, microbial polysaccharides unite the aspects of naturalness and compound quality in a unique manner.
Elaborating on the potential of one of the most prominent microbial polysaccharides, xanthan gum, to replace synthetic WSPs, we will look briefly into the function of microbial polysaccharides in nature, the transfer to industrial production and how the fermentation process can be fine‐tuned to create products with distinct properties. We will discuss the use of xanthan gum in relevant technical applications and summarize further aspects influencing the performance of xanthan gum in a formulation.
Microbial polysaccharides: functionalities and their applicability in industry
In nature, microbial polysaccharides occur as essential components of biofilms. Biofilms are considered the main support of the oldest, most widely distributed and successful form of life on earth (Flemming, et al., 2016b), and the predominant form of microorganisms lifestyle (Cavalcante et al., 2017; Penesyan et al., 2019). These biofilms are aggregates of microorganisms, in which cells are embedded within a self‐produced matrix of extracellular polymeric substance (EPS) (Vert et al., 2012; Flemming, et al., 2016b). This matrix is mainly composed of polysaccharides, proteins, lipids and nucleic acids (Powell et al., 2018), but the relative composition and molecular structure of components vary depending on the producing microorganism and environmental conditions. This fact can be exploited in industrial fermentation to obtain a range of microbial polysaccharides with different properties. Apart from xanthan gum, commercially relevant microbial exopolysaccharides include gellan gum produced by Sphingomonas paucimobilis (Giavasis et al., 2000), pullulan synthesized by Aureobasidium pullulans (Cheng et al., 2011) and welan gum obtained from Alicaligenes sp. (Kaur et al., 2014).
Microorganisms produce polysaccharides for different purposes, many of which can be transferred to technological applications:
-
i.
Adhesive and structural properties: One of the most important functions of microbial polysaccharides is to increase the adhesion capacity of microorganisms to each other and to surfaces (Smith et al., 2016; Alaa, 2018). This adhesive characteristic also facilitates cell‐to‐cell interactions (Dos Santos et al., 2018). In addition, polysaccharides provide a structure with viscoelastic properties to increase resistance of the biofilm to mechanical challenges (Peterson et al., 2015). Taking advantage of this mechanism, increasing the viscosity of aqueous liquids is one of the most relevant uses of purified microbial polysaccharides in technical applications. Furthermore, adhesion of particles to each other or to a target site is a central aim in different applications.
-
ii.
Protection from environment: Biofilms help microorganisms to protect themselves in extreme environments (Yin et al., 2019), against ultraviolet radiation (UV) (de Carvalho, 2017), extreme temperatures (Smith et al., 2016; Kent et al., 2018), pH variations (Narayanan et al., 2016), salinity or drought stress (Alaa, 2018; Wang et al., 2019) and limitation of nutrients (Gingichashvili et al., 2020). In general, a biofilm can be described as a protective matrix in which both the polysaccharide‐producing microorganisms and co‐living cells are encapsulated. Looking at the industry, encapsulation is a relevant strategy in formulation of active agents to enhance their stability and ensure controlled release and improved efficacy. Additionally, the water retention capacity of polysaccharides can be utilized to support biological processes.
-
iii.
Nutrient availability: Biofilms are a source of nutrients able to be used by the biofilm community (Flemming et al., 2016a). They also facilitate the uptake and accumulation of nutrients from the environment (Kurniawan and Yamamoto, 2019). Latest studies described a channel structure in E. coli biofilms that allows for a distribution of nutrients through the biofilm (Rooney et al., 2020). This channel structure could potentially be used as new route for plant nutrient availability and could have implications for the use of microbial polysaccharides in encapsulation.
Considering these functionalities, humans can imitate nature and use microbial polysaccharides with different purposes. We will depict the potential of xanthan gum in three relevant applications in agriculture:
Soil improvement: The water holding capacity and adhesiveness of xanthan gum open a wide field of uses in soil improvement, for example to ameliorate drought stress of plants and reduce erosion.
Drift control: The capacity of xanthan gum to modify the rheology of liquids and to increase adhesiveness is important in the effectiveness of foliar applications.
Encapsulation: In this formulation approach, which is highly relevant for the growing market of biostimulants and biocontrol agents, xanthan gum is a natural surrogate to protect microorganisms, thus potentially improving performance of the formulations.
Xanthan gum: Origin and structure
Isolated for the first time in 1959 (Jeanes et al., 1961), xanthan gum is an EPS produced by different Xanthomonas spp. Even though mainly X. campestris strains are used in the production of xanthan gum, it can be also obtained from other Xanthomonas spp., like X. citri subsp. citri (Conforte et al., 2019), X. hortorum or X. axonopodis (Demirci et al., 2019).
Being a heteropolysaccharide, the primary structure consists of a cellulose‐like backbone of β‐1,4‐linked glucose units, substituted alternately with a trisaccharide side chain (Jansson et al., 1967). This side chain is composed of two mannose units separated by a glucuronic acid, where the internal mannose is mostly O‐acetylated and the terminal mannose may be substituted by a pyruvic acid residue (Fig. 1). Due to the presence of glucuronic and pyruvic acid in the side chain, xanthan gum represents a highly charged polysaccharide with a very rigid polymer backbone. It has a high molecular weight of about 2 × 106 to 2 × 107 g mol‐1 with a narrow distribution. Variations in fermentation conditions used in the production can influence the molecular weight of xanthan gum (Casas et al., 2000). Via X‐ray Powder Diffraction (XRD) measurements, the secondary structure of xanthan gum could be elucidated as a five‐fold helical conformation, where the backbone is stabilized by the side chains (Moorhouse et al., 1977).
Fig. 1.
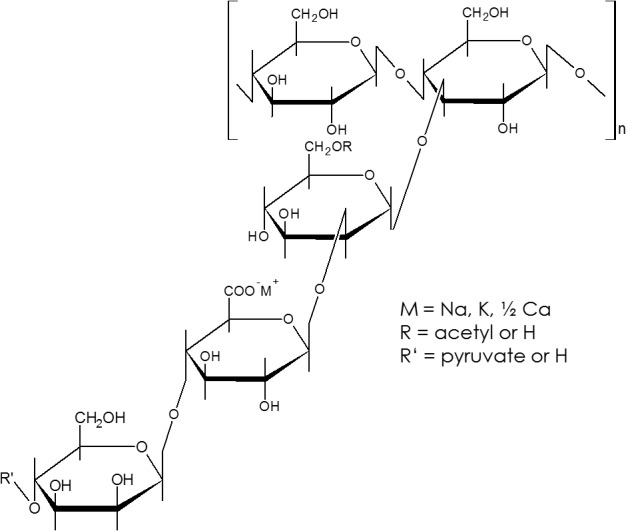
Haworth formula of xanthan gum. The backbone consists of β‐1,4‐linked glucose units with a trisaccharide side chain whose terminal mannose unit is linked fifty–fifty to a pyruvate group and the non‐terminal residue usually carries an acetyl group. Redrawn from (Sworn, 2009).
As a naturally occurring microbial polysaccharide, xanthan gum can be biodegraded into oligosaccharides, monosaccharides and ultimately water and carbon dioxide. The depolymerization of the polysaccharide is achieved by enzymatic attack, mainly xanthanase (endo‐β‐d‐glucanase) (Cadmus et al., 1982) and xanthan lyase (Sutherland, 1987) secreted by other microorganisms. Production of xanthan gum degrading enzymes has been observed, e.g. in Bacillus (Cadmus et al., 1982), Paenibacillus (Ashraf et al., 2018), Enterobacter (Chen et al., 2014) and Cellumonas (Liu et al., 2005). While it was found that for a complete depolymerization into monosaccharides, as many as five enzymes are required (Nankai et al., 1999), even a partial reduction in polymerization can be sufficient to reduce viscosity and functionality of xanthan gum (Chen et al., 2014). The speed of degradation depends on the presence of microorganisms producing relevant enzymes and environmental conditions (Hovland, 2015). For example, xanthan gum was shown to be degraded by bacteria of activated sludge within 7 days (Muchová et al., 2009). Unpublished own data confirm this fast degradation, showing a degradation by 78% within 28 days measured by a manometric respiratory test according to OECD test guideline 301F. On the other hand, some authors observed a certain resistance of xanthan gum to microbial degradation in the range of several months (Cadmus et al., 1982; Hovland, 2015). This is beneficial when a long‐term effect of xanthan gum is desired, such as in soil application.
Xanthan gum: industrial production
Xanthan gum is one of the industrially most relevant microbial polysaccharides. Compared with other microbial polysaccharides, its price is competitive and it is therefore a viable option not only regarding performance but also from an economic point of view (Chang et al., 2020). The main manufacturers of xanthan gum are Jungbunzlauer, ADM, Cargill, CP Kelco, Deosen Biochemicals, Fufeng Group, IFF (Dupont) and Meihua Group.
The production of xanthan gum can be separated into two main steps as shown in Fig. 2. The whole production starts with the fermentation step, where simple carbohydrate molecules are metabolized by X. campestris to synthesize xanthan gum. For this, X. campestris strain is cultured in a well‐aerated and well‐agitated fermenter and is expanded by growth on solid surfaces or in liquid media to obtain the inoculum (Katzbauer, 1998; Garcia‐Ochoa et al., 2000). Apart from the precise medium composition, factors such as type of bioreactor used, mode of operation (batch or continuous), medium composition, and culture conditions (temperature, pH, dissolved oxygen concentration) have an influence on the growth of the microorganism and xanthan gum production. Culture environment and operational conditions also influence the molecular structure of xanthan gum. There is extensive literature on the production conditions, medium composition, used microorganism and how these can be improved (Garcia‐Ochoa et al., 2000; Rosalam and England, 2006; Rottava et al., 2009; Palaniraj and Jayaraman, 2011; Lopes De Mônaco et al., 2015; Habibi and Khosravi‐Darani, 2017). The main parameters to adjust yield and molecular structure are related to nutrients content, temperature and production kinetics. X. campestris needs several micronutrients (e.g. sodium, potassium, iron and calcium salts) and macronutrients such as carbon (glucose or fructose) and nitrogen to produce xanthan gum. At the end of the fermentation, the broth is pasteurized to inactivate the microorganisms.
Fig. 2.
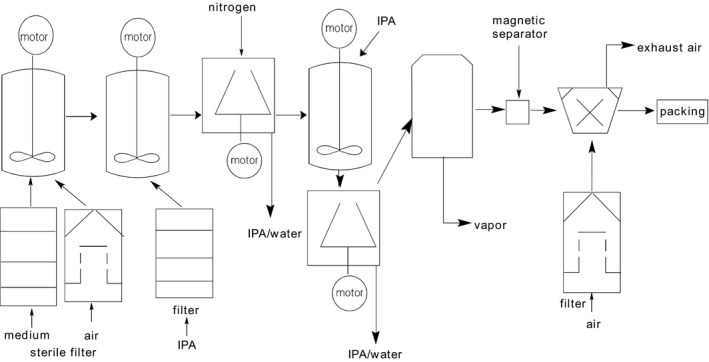
Xanthan gum production steps: fermentation, pasteurization, precipitation with IPA (isopropanol), decantation, dehydration, drying, milling/sifting, packaging.
The second step in production is a comprehensive recovery process including precipitation, drying and milling. The xanthan gum is recovered by precipitation with water‐miscible non‐solvents such as isopropyl alcohol and further purified by pH adjustments (Garcia‐Ochoa et al., 2000). These recovery steps can vary depending on the desired purity and quality of the xanthan gum product, which in turn determines the end use, e.g. in oil, technical, food, personal care or pharma applications. For example, for xanthan gum to be applied as food additive, it should be free from viable cells of X. campestris and reagents used in the recovery process. For personal care or pharma applications, even stricter purity requirements are in place, because active enzymes still present after an inadequate recovery may influence the final application. Once the polysaccharide is obtained as a wet precipitate, it is dried in batch or continuous dryers, under vacuum or with forced circulation of an inert gas. Most commercial xanthan gum products have a final moisture content of about 10–15%. After drying, the polysaccharide is milled to a predetermined mesh size and in some cases agglomerated to control dispersability and dissolution rates. Prior to packaging, xanthan gum routinely undergoes a quality control in which viscosity, microbial plate count and heavy metal residues are examined to comply with product specifications.
Further optimization of the xanthan gum production process is a subject of ongoing research. Apart from ‘traditional’ approaches such as optimizing growth conditions, the use of agro‐industrial, organic by‐products as a sustainable, alternative nutrient source is an interesting aspect (Murad et al., 2019). In this sense, a variety of waste materials has been evaluated, for example shrimp shell (De Sousa Costa et al., 2014), lignocellulosic waste such as coconut shell and cocoa husk (da Silva et al., 2018) as well as chicken feather peptone and sugar beet molasses (Ozdal and Kurbanoglu, 2019). Nevertheless, the transfer of these approaches to industrial scale is limited, since the choice of the nutrient source is tightly coupled to considerations regarding supply security and continuously high raw material quality, absence of impurities and required upstream processes.
Regarding production optimization, it has also been found that xanthan gum molecular properties such the substitution pattern with acetyl and pyruvate groups and thus rheological behaviour can be fine‐tuned on a genetic level (Gansbiller et al., 2019). Strain genetic engineering also proved feasible to increase whiteness of the xanthan gum product while using lower amounts of ethanol in the downstream processing (Dai et al., 2019). However, on an industrial scale, this approach may be prohibitive due to regulatory compliance and product labelling. Therefore, several producers of xanthan gum, including Jungbunzlauer, guarantee a GMO‐free production process.
Xanthan gum: general applications and properties
Dissolved in cold water, xanthan gum produces highly viscous solutions with a weak gel character. It has long been used as a thickener or suspending agent in food applications such as salad dressings, sauces, instant products, desserts, bakery, dairy products and fruit juices as well as in various low‐calorie foods. In cosmetics, xanthan gum serves as stabilizer and thickener, for example in toothpastes, creams, lotions, gels and shampoos. It is also relevant for pharmaceutical applications, modifying drug release from delivery systems such as tablets, films, hydrogels and nanoformulations (Cortes et al., 2020) and potential base material in tissue engineering (Petri, 2015; Kumar et al., 2018). Typical industrial applications of xanthan gum are in cleaners, paints, ceramic glazes, inks, oil drilling fluids or agricultural flowables (Katzbauer, 1998). More specifically, the latter comprise suspension concentrates (SC) and flowable concentrates for seed treatment (FS) type of formulations, in which xanthan gum is widely used.
Due to its strong polyelectrolyte nature, xanthan gum solutions are highly viscous even at very low concentrations. Under shearing, xanthan gum solutions show a strong pseudoplastic behaviour, i.e. the viscosity decreases with increasing shear rate (Milas et al., 1990). The viscosity depends on polymer concentration, temperature, salt and pH. The viscosity of xanthan gum solutions increases strongly with increasing concentration of the polymer. With increasing temperature, the viscosity declines caused by a conformational transition from a rigid helical to a flexible coil molecular structure (Norton et al., 1984). The presence of salts in solution influences the xanthan gum viscosity. At low polysaccharide concentration (approximately < 0.3 wt%), the viscosity decreases when a small amount of salt (approximately < 0.1 wt%) is added, whereas at higher xanthan gum concentration or when a larger amount of salt is added, the viscosity increases (Zatz and Knapp, 1984). Xanthan gum as an anionic polysaccharide exhibits a certain sensitivity to pH changes since its charge density is changed and therefore the molecular associations between the single molecules are influenced (Pastor et al., 1994).
Commercially, there are different xanthan gum grades available, offering different distinct properties (Table 1). They can be classified according to their purity, particle size and rheological performance. The purity of the available grades refers to microbiological plate count, residual isopropanol (IPA) and heavy metal contaminations, but does not impact flow and stabilization behaviour. The particle size or granulation of xanthan gum strongly influences the hydrating and dispersing performance. A fine granulation results in a fast hydration but with the compromise of a worse dispersibility due to a higher tendency to form lumps. Coarser granulations hydrate slower, but are easier to disperse. There is also an agglomerated xanthan gum available on the market allowing for an easy dispersion without the formation of lumps. This grade is especially suited if no or low performance mixers are used for hydration (Fig. 3).
Table 1.
Compilation of possible modifications of xanthan gum, resulting properties and their relevance for agricultural applications (own data).
| Property | Description | Modifications | Relevance for application |
|---|---|---|---|
| Reduced pseudoplasticity |
|
Fermentation conditions |
|
| Salt tolerance |
|
Fermentation conditions |
|
| Acid stable |
|
Fermentation conditions |
|
| Dust free |
|
Oil coating |
|
| Clear solution |
|
Additional clarification |
|
| Easily dispersible |
|
Agglomeration |
|
Fig. 3.
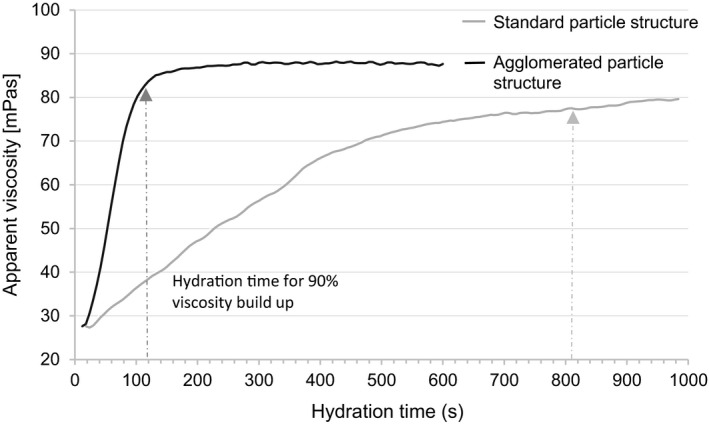
Model curves of viscosity build‐up of xanthan gum of two different particle structures, added to distilled H2O at 0.3% [w/v]. The agglomerated grade reaches 90% of the maximum viscosity after approx. 115 s. The standard grade reaches 90% of the maximum viscosity after approx. 810 s.
Different rheological properties may be important for applications containing high amounts of salts, with highly acidic conditions or where specific smooth flow properties are desired in the end product. Here, the commercial market offers different possibilities including, e.g. particularly salt‐tolerant xanthan gum or xanthan gum exhibiting a reduced pseudoplastic flow behaviour (Fig. 4). Salt‐tolerant xanthan gum has been developed for use with high concentrations of salt (up to 20% NaCl) maintaining an excellent viscosity forming potential. A typical technical application example is the formulation of fire retardants, where high concentrations of ammonium phosphates are present in combination with colouring particles of iron oxide, which need stabilization. In the food industry, salt‐tolerant xanthan gum is used in sauces and dressings with high salt content.
Fig. 4.
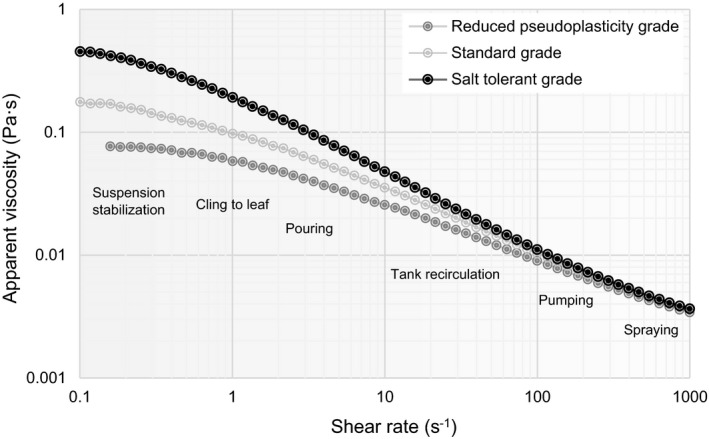
Model flow curves of solutions of 0.1% [w/v] different xanthan gum grades in standard tap water (1 g l‐1 NaCl, 0.15 g l‐1 CaCl2·2H2O). Typical application‐related shear rates are denoted. Differences in apparent viscosity are particularly pronounced in the low shear regime with implications for grade selection depending on desired behaviour.
Besides the rheological properties, the transparency of the xanthan gum solution or the dusting of the xanthan gum powder can be adjusted by different special grades. Clear solution xanthan gum, e.g. shows the same functional rheological properties as standard xanthan gum but allows the preparation of transparent solutions. Transparency is obtained by an additional treatment to remove cell residues. Dust free xanthan gum has been developed to avoid dusting during handling in industrial processes. The product is treated with 1% of an edible oil to bind the smallest particles and thus eliminate dust formation.
The availability of such different xanthan gum grades gives formulators the opportunity to select the material to best match the specific application requirements.
Soil improvement with xanthan gum
Soil improvement or soil conditioning describes a wide range of activities to modify soil characteristics according to specific needs. In general, this term has been used mostly in the context of soil physical properties in geotechnical engineering (Mirzababaei et al., 2017; Armistead et al., 2020; Chen et al., 2020), but also in agriculture (Majeed et al., 2018), forestry (Stöcker et al., 2020) and other fields. Therefore, soil improvement is not restricted to a specific industry and could be defined as the modification of any soil property by any method. In this sense, soil modifications can be of physical, chemical or biological nature (Molnár et al., 2016; Cheng et al., 2017; Oliveira et al., 2017; de Melo et al., 2019; Jiang et al., 2020).
Due to their diversity and versatility, polymers are a group of widely used materials for soil improvement purposes. Specifically, in agriculture, the use of polymers is self‐evident, since their mode of action resembles the functions of the microbial polysaccharides produced by the soil microbiota. Simulating those functions, polymers are applied to the soil mainly in order to: (i) modify water retention capacity (superabsorbents) (Vundavalli et al., 2015), (ii) improve soil structure or physical characteristics (Yang and Antonietti, 2020), (iii) as a controlled release system for fertilizers or phytochemicals (Guilherme et al., 2015; Tran et al., 2018), (iv) in soil remediation for removal of heavy metals (Guo et al., 2020) and oil contamination (Cao et al., 2016; Jung and Hu, 2017) or (v) as a biocide (Chauhan, 2019).
We will focus on the use of polymers and specifically xanthan gum to improve water retention and plant development. In face of an increasing need to use scarce water sources more efficiently, the number of research publications and the commercialization of polymers for soil improvement has grown in the last years (Milani et al., 2017), underlining the relevance of this topic.
Superabsorbent polymers (SAPs) or hydrogels are cross‐linked hydrophilic polymers able to absorb significant quantities of water of up to 1000 times their dry weight (Li et al., 2014; Lejcuś et al., 2018). By binding a large amount of water in the soil and making it available to the plants for longer periods, crop yields can be improved, especially in arid regions (Hou et al., 2018; Satriani et al., 2018; Jahan and Nassiri Mahallati, 2020).
At the moment, the most relevant SAPs for soil application are potassium polyacrylates, but also other polyacrylates and polyacrylamides as well as their cross‐linked derivatives. Non‐soluble, cross‐linked SAPs are incorporated into the soil either in a powder form by homogeneously mixing during ploughing, or already hydrated, using specific machinery allowing its injection at high pressure into the soil. The use of synthetic SAPs is common despite their slow degradation and the potential release of toxic compounds. For example, polyacrylamide can degrade into acrylamide monomers over time, which are known as toxins and potential carcinogens (Xiong et al., 2018).
The increasing awareness about these drawbacks of synthetic SAPs has triggered a demand for biopolymers to be used as readily biodegradable, environmental‐friendly SAPs. In this sense, different biopolymers such as cellulose, starch, guar gum, alginate and chitosan as well as composites have been investigated. Some examples are biodegradable and biocompatible SAP made from two cellulose derivatives, sodium carboxymethyl cellulose (CMCNa) and hydroxyethyl cellulose (HEC), using citric acid as cross‐linking agent (Montesano et al., 2015; Thombare et al., 2018; Saruchi et al., 2019).
Xanthan gum has an extraordinary water binding capacity in comparison with other microbial polysaccharides (Wallingford and Labuza, 1983; Sánchez et al., 1995) and it is thus a suitable candidate to be used as SAP. Its suitability as soil improver has been demonstrated in geotechnical engineering, improving soil mechanical properties (Dehghan et al., 2019) such as soil strengthening and stabilization (Chang et al., 2015a; Latifi et al., 2017; Dehghan et al., 2019). Results with xanthan gum are remarkable in many cases when compared to other polysaccharides (Soldo et al., 2020). In engineering, xanthan gum application rates lay normally between 0.5 and 4%. Nevertheless, positive effects can already be observed when using concentrations of xanthan gum as low as 0.1% (Chen et al., 2019).
The improved water retention can be ascribed to the formation of a crystallized top soil layer promoted by the microbial polysaccharide after drying, which maintains moisture in the inner bulk soil (Chen et al., 2019). These results were particularly pronounced in sandy soils, where the initial water content after saturation doubled when as little as 0.5% of xanthan gum was added, delaying also water drainage (Tran et al., 2018). Moreover, positive results were observed in other substrates. Xanthan gum at a concentration of 0.5% outperformed starch in terms of water retention and plant survival under drought stress, using Korean standard poor graded sand (Tran et al., 2019). Also, on silty soils and garden substrates, xanthan gum has proven a suitable amendment in order to increase water retention, seed germination and plant growth (Abobatta, 2018; Chang et al., 2020). Another beneficial effect is the reduction of soil erosion on different soils (Chang et al., 2015b).
From a practical point of view, xanthan gum has the advantage of being able to be applied as a water‐based solution. Xanthan gum is not only compatible with diverse irrigation methods like drop by drop or sprinklers, but also is an effective drag reducer, decreasing frictions in the flow of the irrigation water (Han and Choi, 2017). This implies a potential double function of xanthan gum in irrigation.
In the case of application of SAPs as a dry powder, their hydration capacity and hydration speed are affected by the depth they are delivered to, i.e. the soil load they bear. Increasing loads simulating different depths resulted in a significant reduction in the capacity of the SAPs to absorb water (Lejcuś et al., 2018). This makes xanthan gum a more efficient and thus potentially less costly solution.
One of the main problems regarding studies on xanthan gum in soil amendment is that frequently, a similar concentration range is used for agriculture‐related applications as for geotechnical engineering. This could result in some phenomena like cemented soil, in which plant growth or seed germination can be negatively affected (Tran et al., 2019). Also, the use of high concentrations of xanthan gum can cause clogging of soil pores, resulting in a slow infiltration of the xanthan gum (Chang et al., 2020). These properties are desired in soil engineering, but not in agriculture, so the approach for both applications needs to be different (Rahbari et al., 2016).
While xanthan gum has proven to confer beneficial effects by its own, latest studies have investigated the possibility to cross‐link it with other compounds, resulting in similar characteristics as synthetic SAPs. Composite materials have shown potential to be used to control the release of fertilizers (Singh and Dhaliwal, 2020), to improve the development of Camelina sativa L. under drought stress conditions (Lim et al., 2018) or to enhance water retention capacity (Feng et al., 2014).
The increasing impact of climate change, as well as the limited availability of water and good quality soils, will make soil improvement techniques more and more important in the future. Xanthan gum as an eco‐friendly and sustainable polymer is one of the most promising materials to be used in agriculture, both conventional and organic.
Drift control – potential replacement of synthetic polymers by xanthan gum
The delivery of pesticides as liquids to the plant foliage constitutes a highly relevant pathway of pesticide treatments. Across all techniques of spray delivery, unwanted drift compromises on safety and efficacy, causing losses of up to 10% (De Ruiter et al., 2003). Drift control measures include the incorporation of drift control adjuvants (DCAs) into the spray liquid. These aim to reduce the fraction of droplets with a diameter < 100–200 µm, generally referred to as driftable fines (Hilz and Vermeer, 2013). Frequently, WSPs of high molecular weight ranging around 106 g mol‐1 are used, such as polyacrylamide (PAM), polyethyleneoxide (PEO), polyvinylppyrrolidon (PVP) and polysaccharides (Spanoghe et al., 2007; Lewis et al., 2016). Even highly diluted, typically to 100–1000 ppm (Lewis et al., 2016), these polymers are effective in delaying the break‐up of the spray sheet and reducing the formation of satellite droplets (Harrison et al., 1999). Polymer rigidity strongly influences extensional viscosity in response to the strain the liquid experiences when ejected through a nozzle. Comparing 50 ppm solutions of PAM as a flexible polymer and xanthan gum as a rigid representative, Harrison et al. (1999) found a significant difference in the Trouton ratio of both materials. PAM reached a Trouton ratio (ƞe/ƞs) of more than 20, while xanthan gum reached a value of 8. This indicates that even though rigid xanthan gum does induce a delay in the break‐up of the spray sheet, flexible polyacrylamide does so more efficiently.
In practice, however, integrity of the molecular structure and thus functionality of polymers is often an issue. DCAs experience continuous shear forces of between 50 s‐1 during recirculation in the spray tank, reaching up to 105 s‐1 at the nozzle outlet (De Ruiter et al., 2003), resulting in breakage of molecules into smaller fractions (Lewis et al., 2016). For example, using a 400 ppm solution of non‐ionic PEO, the volumetric percentage of droplets < 200 µm (% V < 200 µm) grew from 5.1% before recirculation to 17.3% after recirculation. In contrast, xanthan gum only experienced a minor increase in % V < 200 µm from 7.5 to 11.0 (Zhu et al., 1997). Thus, xanthan gum shows a significantly higher resistance to mechanical stress and therefore better performance in drop size enhancement than many synthetic WSPs.
The molecular integrity and quick re‐build of viscosity of xanthan gum further benefit the deposition of droplets on the target surface, improving cling to leaf and reducing run‐off. Wang et al. (2018) demonstrated that the maximum retention [ml cm‐³] of an aqueous surfactant solution on a 45° inclined maize leaf could be doubled by adding xanthan gum in concentrations as low as 0.05%. They also showed that the addition of xanthan gum significantly decreased the dynamic spreading [mm² s‐1]. This implies the formation of more slowly evaporating ‘hot spots’ of AI (active ingredient), allowing for a higher concentration and longer contact time on the target surface (Flemmens et al., 2018).
DCAs conferring a good droplet retention and moisture maintenance are also relevant in spray application of biocontrol agents. Biocompatibility of DCAs is a crucial requirement in this application (Peng et al., 2000). Particularly, xanthan gum may take on the function of providing a protective, surrogate biofilm to live organisms. As shown for the spray application of entomopathogenic nematodes, both PVA and xanthan gum were compatible with nematode viability. In addition, xanthan gum at 0.3% had the benefit of retarding settlement (Beck et al., 2013). Another study investigated the effect of xanthan gum, PAM and other adjuvants on the performance of fungal bioherbicides. In the model system Pyricularia setariae spores against green foxtail, xanthan gum showed the best compatibility among all tested adjuvants, improving spore germination from 75% in the control to 83%. In contrast, PAM decreased sporulation to 61%, resulting in a significant efficacy loss (Byer et al., 2006).
Clearly, there are pros and cons of replacing synthetic WSPs by xanthan gum in drift control. Looking particularly at extensional viscosity, PAMs seem to be superior to xanthan gum. However, molecular stability under high mechanical stress conditions and full biocompatibility make xanthan gum a viable alternative not only for spray treatments with biologicals.
Encapsulation and controlled release formulations with xanthan gum
In an effort to improve safety and efficacy of agrochemicals, controlled release systems (CRS) are rising in popularity. In a CRS, AIs are immobilized in carriers to ensure the timely release on the target site, reduce loss into the environment and avoid exposure of the end‐user (Singh et al., 2020). CRS may also refer to enhanced efficiency fertilizers (EEF), in which plant nutrients are formulated using coating or immobilization techniques (França et al., 2019). Generally, the term CRS comprises manifold techniques and materials, which have been reviewed elsewhere (Campos et al., 2015; Sinha et al., 2019; Singh et al., 2020).
Encapsulation is one of the most relevant techniques in CRS, with a broad application potential for polymers. Polymers from synthetic origin (polyamides, polyesters, polyvinylalcohol, polyurethane, polylactic acid etc.) and biopolymers (chitosan, sodium alginate, cyclodextrin, starch and cellulose) have been used (Sinha et al., 2019; Singh et al., 2020).
Generally being considered a non‐gelling polymer by itself, xanthan gum is rather underrepresented in studies investigating bio‐based encapsulation materials for CRS in agriculture. To the best of our knowledge, there are no studies comparing the use of synthetic, film‐forming polymers with xanthan gum directly. Nevertheless, taking inspiration from other application fields such as biotechnology, food and pharma, there are several encapsulation approaches in which xanthan gum in combination with other materials has proven beneficial.
Matrix encapsulation based on xanthan gum in combination with other components is applicable for a variety of compounds such as secondary metabolites (Da Rosa et al., 2014; Ravichandran et al., 2014), enzymes (Liu et al., 2011) and microorganisms (Jiménez‐Pranteda et al., 2012). Frequently, the basic principle is to add the AI to the xanthan gum hydrogel and subject the blend to drying by lyophilization or spray drying. ‘Co‐carriers’, for example other hydrocolloids like alginate or chitosan, inorganic fillers such as clay (Liu et al., 2011) or carbohydrates such as maltodextrin (Ravichandran et al., 2014) may be incorporated. By help of these additives, properties such as capsule structure, encapsulation efficiency and release patterns can be shaped. Matrix encapsulation was also achieved by dripping blends of xanthan gum and gellan gum into a recovery bath of CaCl2. At a ratio of 1:0.75 (xanthan: gellan gum), encapsulation of Lactobacilli resulted in an increased resistance of the probiotic microorganisms to simulated bile conditions (Jiménez‐Pranteda et al., 2012).
Another possibility is the preparation of core‐shell capsules with solid or liquid core and one or several outer layers. This is possible by exploiting the electrostatic interaction between anionic xanthan gum and cationic chitosan. For example, Shu et al. (2018a,2018b) dispersed Bifidobacterium bifidum in xanthan gum hydrogel and extruded the blend into a chitosan solution to obtain capsules, which improved the survival of B. bifidum in milk. By alternating between xanthan gum solution and chitosan solution, the amount of coating layers can be modified. The beneficial effect of an additional layer was shown for Lactobacillus acidophilus in xanthan‐chitosan capsules, resulting in a better control of the release as well as improved viability during storage in a dairy beverage and exposure to simulated gastric juice (Shu et al., 2018a,2018b).
Core‐shell capsules also can be produced using alginate as shell material and xanthan gum to adjust viscosity of the liquid core and thus stabilize encapsulated agents. This approach was pursued to encapsulate Lactococcus lactis and thus increase its viability and antimicrobial activity against food‐spoilage bacteria Listeria monocytogenes (Bekhit et al., 2016).
One of the less investigated approaches is the direct cross‐linking of xanthan gum. This can be achieved by exposure to trivalent ions. For example, Pacheco‐Aguirre et al. (2016) demonstrated the encapsulation of the biocontrol agent Bacillus subtilis in xanthan gum by extrusion into a FeCl3 bath. In this form, the biocontrol of the plant‐pathogenic nematode Meleidogyne incognita tended to be more efficient than when inoculating plants with non‐encapsulated biocontrol agent.
Even though limited data are available on encapsulation of AIs for agriculture, the examples illustrate the possibilities to use xanthan gum in encapsulated formulations, giving rise to potential new approaches.
Formulating with xanthan gum: practical tips and compatibilities
Xanthan gum hydrates both in hot and cold water. Working in an aqueous system, a proper incorporation of xanthan gum into the water phase is essential to achieve optimum performance. One of the main issues that may be observed is the formation of difficult to dissolve lumps. These lumps are caused by adding xanthan gum to the hydration medium all at once, too fast or by using improper techniques, impeding the complete hydration and thus its effective use. With the right approach, the formation of these lumps can be prevented.
The first requirement is a uniform dispersion of xanthan gum particles. Under fast agitation of the hydration medium, for example by a propeller stirrer, the bulk xanthan gum is ideally added to the upper part of the vortex where mixing velocity is highest. This is especially relevant for fine‐particle‐sized xanthan gum, which is difficult to disperse with low shear equipment. If the particles are not uniformly dispersed, they may stick together and the fast hydration of the outer surface can form a gel layer, preventing the entry of water. The resulting swollen lumps are also known as ‘fish eyes’, which can only be dissolved with high shear mixing equipment and/or prolonged mixing time (Sandford et al., 1981). In many applications, this is not an option, so alternative approaches can be pursued:
-
i.
Preparation of a dry blend: Depending on additional ingredients present in the formulation, the preparation of a dry blend may be feasible. For this, the particle sizes of the individual components should be similar to avoid separation. Suitable components are for example sugars, salts, starches and clays (Sworn, 2009). In this approach, a fine particle size xanthan gum is typically used, and the focus is on ensuring a homogeneous distribution and spatial separation of the single xanthan gum particles, which facilitates hydration upon addition of water.
-
ii.
Slurry in non‐aqueous liquids: Similarly to a dry matrix, a liquid medium can serve for homogeneous distribution of xanthan gum particles. For this, it is important to choose non‐hydrating liquids. These could be (partly) water‐miscible liquids like glycerol, ethanol, propylenglycol, ethyl lactate or triethyl citrate as well as oils. For oils, a ratio of xanthan gum to liquid of 1:5 to 1:10 is recommended, whereas for water‐miscible liquids, the recommendation is 1:2 to 1:10. The slurry can be mixed with water under agitation for the hydration of xanthan gum.
-
iii.
Preparation of a stock paste: If additional components are not acceptable in the formulation, an aqueous stock solution of xanthan gum can be prepared. Stock pastes of up to 6% are feasible and can be preserved and stored long‐term until later dilution with water (Sworn, 2009). Obviously, this implies that high shear equipment has to be available during preparation of the stock solution.
-
iv.
Use of an easily dispersible xanthan gum grade: The most efficient way of ensuring fast hydration with low shear equipment and no need to modify components of the formulation is the use of a commercially available, ‘easily dispersible’ xanthan gum grade. These specifically processed grades are agglomerates with porous structure, which allows for a fast entry of water and prevention of fish‐eye formation.
Xanthan gum effectively thickens water‐based solutions even at low concentrations. To give a starting point, typical usage concentrations are between 0.2% and 1.0% in relation to the aqueous phase for the stabilization of suspensions, depending on particle characteristics. When aiming for sprayable solutions, a concentration around 0.08–0.10% is recommended, depending on nozzle type and operational parameters.
Particularly regarding the transfer of laboratory‐based research to application, it should be noted that viscosity build of xanthan gum differs in distilled water and when salts are present, such as in tap water.
In full formulations, possible interactions with xanthan gum and other components need to be considered. For example, typical anti‐freeze agents such as glycerol, ethylene glycol and propylene glycol are compatible with xanthan gum in concentrations up to 50%. Ethanol can be incorporated up to approximately 40% before precipitation of xanthan gum occurs. Xanthan gum is largely compatible with non‐ionic and anionic surfactants used in typical concentrations (approximately 15% active substance). However, due to its anionic nature, xanthan gum can interfere with cationic surfactants and amphoteric surfactants below their isoelectric point, where positive charge dominates.
The cellulose backbone of xanthan gum is well protected against enzymatic attack by its trisaccharide side chains and therefore shows better stability in presence of pectinases or amylases than other biopolymers. Nevertheless, for long‐term storage of xanthan gum solutions, a preservative system is usually required, which may be based, e.g. on isothiazolinones or less sensitizing or toxic alternatives such as benzoates and sorbates.
Considering these aspects is important to ensure successful use of xanthan gum.
Conclusion
In this review, we elaborated on the use of WSPs in different agricultural applications, focussing on eco‐friendly alternatives to synthetic materials. The process of biological synthesis by microbes, i.e. fermentation, delivers biodegradable polymers with narrow specifications and of high purity. Xanthan gum is one of the most prominent and commercially relevant examples for a microbial biopolymer. While xanthan gum is established in agriculture for formulation of suspension concentrates, there are still innovative applications to be explored. Among others, these comprise the use as spray adjuvant (drift control), co‐formulant in encapsulation and as soil improver, where classically synthetic WSPs are employed. Changes in regulatory frameworks, an increasing environmental awareness and a promising performance of xanthan gum underline the feasibility of this direction of research. Choosing the most suitable xanthan gum grade in terms of rheological behaviour and downstream modifications requires material‐specific knowledge and is crucial for successful application.
Funding Information
No funding information provided.
Conflict of interest
All authors are employees of Jungbunzlauer Ladenburg GmbH or were so in the past.
Microb. Biotechnol. (2021) 14(5), 1881–1896
References
- Abobatta, W. (2018) Impact of hydrogel polymer in agricultural sector. Adv Agric Environ Sci 1: 59–64. [Google Scholar]
- Alaa, F.M. (2018) Effectiveness of exopolysaccharides and biofilm forming plant growth promoting rhizobacteria on salinity tolerance of faba bean (Vicia faba L.). Afr J Microbiol Res 12: 399–404. [Google Scholar]
- Armistead, S.J., Rawlings, A.E., Smith, C.C., and Staniland, S.S. (2020) Biopolymer stabilization/solidification of soils: a rapid, micro‐macro, cross‐disciplinary approach. Environ Sci Technol 54: 13963–13972. [DOI] [PubMed] [Google Scholar]
- Arp, H.P.H., and Knutsen, H. (2019) Could we spare a moment of the spotlight for persistent, water‐soluble polymers? Environ Sci Technol 54: 3–5. [DOI] [PubMed] [Google Scholar]
- Ashraf, S., Soudi, M.R., Amoozegar, M.A., Nikou, M.M., and Spröer, C. (2018) Paenibacillus xanthanilyticus sp. nov., a xanthan‐degrading bacterium isolated from soil. Int J Syst Evol Microbiol 68: 76–80. [DOI] [PubMed] [Google Scholar]
- Beck, B., Brusselman, E., Nuyttens, D., Moens, M., Pollet, S., Temmerman, F., and Spanoghe, P. (2013) Improving foliar applications of entomopathogenic nematodes by selecting adjuvants and spray nozzles. Biocontrol Sci Techn 23: 507–520. [Google Scholar]
- Bekhit, M., Sánchez‐González, L., Ben Messaoud, G., and Desobry, S. (2016) Design of microcapsules containing Lactococcus lactis subsp. lactis in alginate shell and xanthan gum with nutrients core. LWT ‐ Food Sci Technol 68: 446–453. [Google Scholar]
- Byer, K.N., Peng, G., Wolf, T.M., and Caldwell, B.C. (2006) Spray retention for liquid and mycoherbicide inoculum in three weed‐biocontrol systems. Biocontrol Sci Techn 16: 815–823. [Google Scholar]
- Cadmus, M.C., Jackson, L.K., Burton, K.A., Plattner, R.D., and Slodki, M.E. (1982) Biodegradation of xanthan gum by Bacillus sp. Appl Environ Microbiol 44: 5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos, E.V.R., de Oliveira, J.L. , Fraceto, L.F., Singh, B., Ramos Campos, E.V., Luiz de Oliveira, J., et al. (2015) Polysaccharides as safer release systems for agrochemicals. Agron Sustain Dev 35: 47–66. [Google Scholar]
- Cao, S.C., Bate, B., Hu, J.W., and Jung, J. (2016) Engineering behavior and characteristics of water‐soluble polymers: Implication on soil remediation and enhanced oil recovery. Sustainability 8: 205. [Google Scholar]
- Casas, J.A., Santos, V.E., and García‐Ochoa, F. (2000) Xanthan gum production under several operational conditions: molecular structure and rheological properties. Enzyme Microb Technol 26: 282–291. [DOI] [PubMed] [Google Scholar]
- Cavalcante, J.J.V., Cardoso, A., and de Pádua, V.L.M. (2017) Concept of mono and mixed biofilms and their role in soil and in plant association. In Biofilms in Plant and Soil Health. Wiley Online Books. Ahmad, I., and Husain, F.M. (eds). Hoboken, NJ: Wiley‐Blackwell, p. 43. [Google Scholar]
- Chang, I., Im, J., Prasidhi, A.K., and Cho, G.C. (2015a) Effects of Xanthan gum biopolymer on soil strengthening. Constr Build Mater 74: 65–72. [Google Scholar]
- Chang, I., Lee, M., Tran, A.T.P., Lee, S., Kwon, Y.M., Im, J., and Cho, G.C. (2020) Review on biopolymer‐based soil treatment (BPST) technology in geotechnical engineering practices. Transp Geotech 24: 100385. [Google Scholar]
- Chang, I., Prasidhi, A.K., Im, J., Shin, H.D., and Cho, G.C. (2015) Soil treatment using microbial biopolymers for anti‐desertification purposes. Geoderma 253–254: 39–47. [Google Scholar]
- Chauhan, N.P.S. (2019) Biocidal Polymers. Berlin: Walter de Gruyter GmbH & Co KG. [Google Scholar]
- Chen, C., Peng, Z., Gu, J., Peng, Y., Huang, X., and Wu, L. (2020) Exploring environmentally friendly biopolymer material effect on soil tensile and compressive behavior. Int J Environ Res Public Health 17: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, C., Wu, L., Perdjon, M., Huang, X., and Peng, Y. (2019) The drying effect on xanthan gum biopolymer treated sandy soil shear strength. Constr Build Mater 197: 271–279. [Google Scholar]
- Chen, X., Wang, M., Yang, F., Tang, W., and Li, X. (2014) Isolation and characterization of xanthan‐degrading Enterobacter sp. nov. LB37 for reducing the viscosity of xanthan in petroleum industry. World J Microbiol Biotechnol 30: 1549–1557. [DOI] [PubMed] [Google Scholar]
- Cheng, K.‐C., Demirci, A., and Catchmark, J.M. (2011) Pullulan: biosynthesis, production, and applications. Appl Microbiol Biotechnol 92: 29–44. [DOI] [PubMed] [Google Scholar]
- Cheng, L., Shahin, M.A., and Cord‐Ruwisch, R. (2017) Surface percolation for soil improvement by biocementation utilizing in situ enriched indigenous aerobic and anaerobic ureolytic soil microorganisms. Geomicrobiol J 34: 546–556. [Google Scholar]
- Conforte, V.P., Yaryura, P.M., Bianco, M.I., Rodríguez, M.C., Daglio, Y., Prieto, E., et al. (2019) Changes in the physico‐chemical properties of the xanthan produced by Xanthomonas citri subsp. citri in grapefruit leaf extract. Glycobiology 29: 269–278. [DOI] [PubMed] [Google Scholar]
- Cortes, H., Caballero‐Florán, I.H., Mendoza‐Muñoz, N., Escutia‐Guadarrama, L., Figueroa‐González, G., Reyes‐Hernández, O.D., et al. (2020) Xanthan gum in drug release. Cell Mol Biol 66: 199–207. [PubMed] [Google Scholar]
- Da Rosa, C.G., Borges, C.D., Zambiazi, R.C., Rutz, J.K., da Luz, S.R., Krumreich, F.D., et al. (2014) Encapsulation of the phenolic compounds of the blackberry (Rubus fruticosus). LWT ‐ Food Sci Technol 58: 527–533. [Google Scholar]
- Da Silva, J.A., Cardoso, L.G., de Jesus Assis, D. , Gomes, G.V.P., Oliveira, M.B.P.P., de Souza, C.O. , and Druzian, J.I. (2018) Xanthan gum production by Xanthomonas campestris pv. campestris IBSBF 1866 and 1867 from lignocellulosic agroindustrial wastes. Appl Biochem Biotechnol 186: 750–763. [DOI] [PubMed] [Google Scholar]
- Dai, X., Gao, G., Wu, M., Wei, W., Qu, J., Li, G., and Ma, T. (2019) Construction and application of a Xanthomonas campestris CGMCC15155 strain that produces white xanthan gum. Microbiologyopen 8: e631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Carvalho, C.C.C.R. (2017) Biofilms: Microbial strategies for surviving UV exposure. In Ultraviolet Light in Human Health, Diseases and Environment. Cham: Springer, pp. 233–239. [Google Scholar]
- De Melo, T.R., Pereira, M.G., de Cesare Barbosa, G.M. , da Silva Neto, E.C. , Andrello, A.C., and Filho, J.T. (2019) Biogenic aggregation intensifies soil improvement caused by manures. Soil Tillage Res 190: 186–193. [Google Scholar]
- De Ruiter, H., Holterman, H.J., Kempenaar, C., Mol, H.G.J., de Vlieger, J.J. , and van de Zande, J.C. (2003) Influence of adjuvants and formulations on the emission of pesticides to the atmosphere. In A literature study for the Dutch Research Programme Pesticides and the Environment (DWK) theme C‐2. Wageningen: Plant Research International B.V. [Google Scholar]
- De Sousa Costa, L.A., Campos, M.I., Druzian, J.I., De Oliveira, A.M., and De Oliveira, E.N. (2014) Biosynthesis of xanthan gum from fermenting shrimp shell: yield and apparent viscosity. Int J Polymer Sci 2014: 1–8. [Google Scholar]
- Dehghan, H., Tabarsa, A., Latifi, N., and Bagheri, Y. (2019) Use of xanthan and guar gums in soil strengthening. Clean Technol Environ Policy 21: 155–165. [Google Scholar]
- Demirci, A.S., Palabiyik, I., Apaydın, D., Mirik, M., and Gumus, T. (2019) Xanthan gum biosynthesis using Xanthomonas isolates from waste bread: process optimization and fermentation kinetics. LWT 101: 40–47. [Google Scholar]
- Dos Santos, A.L.S.d., Galdino, A.C.M., Mello, T.P.d., Ramos, L.d.S., Branquinha, M.H., Bolognese, A.M., et al. (2018) What are the advantages of living in a community? A microbial biofilm perspective!. Memórias do Instituto Oswaldo Cruz 113: e180212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECHA European Chemicals Agency (2019) Annex XV Restriction Report ‐ Microplastics. URL echa.europa.eu. [Google Scholar]
- Eerkes‐Medrano, D., Leslie, H.A., and Quinn, B. (2019) Microplastics in drinking water: a review and assessment. Curr Opin Environ Sci Health 7: 69–75. [Google Scholar]
- Feng, E., Ma, G., Wu, Y., Wang, H., and Lei, Z. (2014) Preparation and properties of organic‐inorganic composite superabsorbent based on xanthan gum and loess. Carbohydr Polym 111: 463–468. [DOI] [PubMed] [Google Scholar]
- Flemmens, M.S.S., Lotz, C., Gray, V., Akins, M., and Nieto, M. (2018) Improved adjuvants for agricultural chemicals. U.S. Patent Application No. 15/760,951. [Google Scholar]
- Flemming, H.C., Neu, T.R., and Wingender, J. (2016a) The perfect slime ‐ and the “dark matter” of biofilms. In The Perfect Slime: Microbial Extracellular Polymeric Substances (EPS). Flemming, H.‐C., Neu, T.R., and Wingender, J. (eds). London: IWA Publishing, pp. 1–14. [Google Scholar]
- Flemming, H.C., Wingender, J., Szewzyk, U., Steinberg, P., Rice, S.A., and Kjelleberg, S. (2016b) Biofilms: an emergent form of bacterial life. Nat Rev Microbiol 14: 563. [DOI] [PubMed] [Google Scholar]
- França, D., Messa, L.L., Souza, C.F., and Faez, R. (2019) Nano and microencapsulated nutrients for enhanced efficiency fertilizer. In Polymers for Agri‐Food Applications. Gutiérrez, T.J. (ed). Cham: Springer International Publishing, pp. 29–44. [Google Scholar]
- Gansbiller, M., Schmid, J., and Sieber, V. (2019) In‐depth rheological characterization of genetically modified xanthan‐variants. Carbohydr Polym 213: 236–246. [DOI] [PubMed] [Google Scholar]
- Garcia‐Ochoa, F., Santos, V.E., Casas, J.A., and Gomez, E. (2000) Xanthan gum: production, recovery, and properties. Biotechnol Adv 18: 549–579. [DOI] [PubMed] [Google Scholar]
- Giavasis, I. (2013) Production of microbial polysaccharides for use in food. In Microbial Production of Food Ingredients, Enzymes and Nutraceuticals. McNeil, B., Archer, D., Giavasis, I., and Harvey, L. (eds). Sawston: Woodhead Publishing Limited, pp. 413–468. [Google Scholar]
- Giavasis, I., Harvey, L.M., and McNeil, B. (2000) Gellan gum. Crit Rev Biotechnol 20: 177–211. [DOI] [PubMed] [Google Scholar]
- Gingichashvili, S., Duanis‐Assaf, D., Shemesh, M., Featherstone, J.D.B.B., Feuerstein, O., and Steinberg, D. (2020) The adaptive morphology of Bacillus subtilis biofilms: a defense mechanism against bacterial starvation. Microorganisms 8: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilherme, M.R., Aouada, F.A., Fajardo, A.R., Martins, A.F., Paulino, A.T., Davi, M.F.T., et al. (2015) Superabsorbent hydrogels based on polysaccharides for application in agriculture as soil conditioner and nutrient carrier: a review. Eur Polym J 72: 365–385. [Google Scholar]
- Guo, J., Yang, J., Yang, J., Zheng, G., Chen, T., Huang, J., et al. (2020) Water‐soluble chitosan enhances phytoremediation efficiency of cadmium by Hylotelephium spectabile in contaminated soils. Carbohydr Polym 246: 116559. [DOI] [PubMed] [Google Scholar]
- Habibi, H., and Khosravi‐Darani, K. (2017) Effective variables on production and structure of xanthan gum and its food applications: a review. Biocatal Agric Biotechnol 10: 130–140. [Google Scholar]
- Han, W.J., and Choi, H.J. (2017) Role of bio‐based polymers on improving turbulent flow characteristics: materials and application. Polymers 9: 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison, G.M., Mun, R., Cooper, G., and Boger, D.V. (1999) A note on the effect of polymer rigidity and concentration on spray atomisation. J Nonnewton Fluid Mech 85: 93–104. [Google Scholar]
- Hennecke, D., Bauer, A., Herrchen, M., Wischerhoff, E., and Gores, F. (2018) Cationic polyacrylamide copolymers (PAMs): environmental half life determination in sludge‐treated soil. Environ Sci Eur 30: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilz, E., and Vermeer, A.W.P.P. (2013) Spray drift review: the extent to which a formulation can contribute to spray drift reduction. Crop Prot 44: 75–83. [Google Scholar]
- Hou, X., Li, R., He, W., Dai, X., Ma, K., and Liang, Y. (2018) Superabsorbent polymers influence soil physical properties and increase potato tuber yield in a dry‐farming region. J Soils Sediments 18: 816–826. [Google Scholar]
- Hovland, B. (2015) Assessment of the biodegradability of xanthan in offshore injection water. Master's Thesis, The University of Bergen. [Google Scholar]
- Huppertsberg, S., Zahn, D., Pauelsen, F., Reemtsma, T., and Knepper, T.P. (2020) Making waves: Water‐soluble polymers in the aquatic environment: an overlooked class of synthetic polymers? Water Res 181: 115931. [DOI] [PubMed] [Google Scholar]
- Jahan, M., and Nassiri Mahallati, M. (2020) Can superabsorbent polymers improve plants production in arid regions? Adv Polymer Technol 2020: 1–8. [Google Scholar]
- Jansson, P.‐E., Kenne, L., and Lindberg, B. (1967) Structure of the extracellular polysaccharide from Xanthomonas campestris . Carbohydr Res 45: 275–282. [DOI] [PubMed] [Google Scholar]
- Jeanes, A., Pittsley, J.E., and Senti, F.R. (1961) Polysaccharide B‐1459: a new hydrocolloid polyelectrolyte produced from glucose by bacterial fermentation. J Appl Polym Sci 5: 519–526. [Google Scholar]
- Jiang, N.J., Tang, C.S., Hata, T., Courcelles, B., Dawoud, O., and Singh, D.N. (2020) Bio‐mediated soil improvement: the way forward. Soil Use Manag 36: 185–188. [Google Scholar]
- Jiménez‐Pranteda, M.L., Poncelet, D., Náder‐Macías, M.E., Arcos, A., Aguilera, M., Monteoliva‐Sánchez, M., and Ramos‐Cormenzana, A. (2012) Stability of lactobacilli encapsulated in various microbial polymers. J Biosci Bioeng 113: 179–184. [DOI] [PubMed] [Google Scholar]
- Jung, J., and Hu, J.W. (2017) Characterization of polyethylene oxide and sodium alginate for oil contaminated‐sand remediation. Sustainability 9: 62. [Google Scholar]
- Katzbauer, B. (1998) Properties and applications of xanthan gum. Polym Degrad Stab 59: 81–84. [Google Scholar]
- Kaur, V., Bera, M.B., Panesar, P.S., Kumar, H., and Kennedy, J.F. (2014) Welan gum: microbial production, characterization, and applications. Int J Biol Macromol 65: 454–461. [DOI] [PubMed] [Google Scholar]
- Kent, A.G., Garcia, C.A., and Martiny, A.C. (2018) Increased biofilm formation due to high‐temperature adaptation in marine Roseobacter . Nat Microbiol 3: 989–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, A., Rao, K.M., and Han, S.S. (2018) Application of xanthan gum as polysaccharide in tissue engineering: a review. Carbohydr Polym 180: 128–144. [DOI] [PubMed] [Google Scholar]
- Kurniawan, A., and Yamamoto, T. (2019) Accumulation of NH4 + and NO3 ‐ inside biofilms of natural microbial consortia: implication on nutrients seasonal dynamic in aquatic ecosystems. Int J Microbiol 2019: 6473690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latifi, N., Horpibulsuk, S., Meehan, C.L., Abd Majid, M.Z., Tahir, M.M., and Mohamad, E.T. (2017) Improvement of problematic soils with biopolymer‐an environmentally friendly soil stabilizer. J Mater Civ Eng 29: 04016204. [Google Scholar]
- Lejcuś, K., Śpitalniak, M., and Dabrowska, J. (2018) Swelling behaviour of superabsorbent polymers for soil amendment under different loads. Polymers 10: 271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis, R.W., Evans, R.A., Malic, N., Saito, K., and Cameron, N.R. (2016) Polymeric drift control adjuvants for agricultural spraying. Macromol Chem Phys 217: 2223–2242. [Google Scholar]
- Li, X., He, J.Z., Hughes, J.M., Liu, Y.R., and Zheng, Y.M. (2014) Effects of super‐absorbent polymers on a soil‐wheat (Triticum aestivum L.) system in the field. Appl Soil Ecol 73: 58–63. [Google Scholar]
- Lim, H.G., Kim, H.S., Lee, H.S., Sin, J.H., Kim, E.S., Woo, H.S., and Ahn, S.J. (2018) Amended soil with biopolymer positively affects the growth of Camelina sativa L. under drought stress. Ecol Resil Infrastruct 5: 163–173. [Google Scholar]
- Liu, H., Huang, C., Dong, W., Du, Y., Bai, X., and Li, X. (2005) Biodegradation of xanthan by newly isolated Cellulomonas sp. LX, releasing elicitor‐active xantho‐oligosaccharides‐induced phytoalexin synthesis in soybean cotyledons. Process Biochem 40: 3701–3706. [Google Scholar]
- Liu, H., Nakagawa, K., Kato, D.I., Chaudhary, D., and Tadé, M.O. (2011) Enzyme encapsulation in freeze‐dried bionanocomposites prepared from chitosan and xanthan gum blend. Mater Chem Phys 129: 488–494. [Google Scholar]
- Lopes De Mônaco, B., Lopes Lessa, V., Moré Silva, B., Da Sila Carvalho Filho, M.A., Schnitzler, E., and Gustavo Lacerda, L., et al. (2015) Xanthan gum: properties, production conditions, quality and economic perspective. J Food Nutr Res 54: 185–194. [Google Scholar]
- Majeed, A., Muhammad, Z., and Ahmad, H. (2018) Plant growth promoting bacteria: role in soil improvement, abiotic and biotic stress management of crops. Plant Cell Rep 37: 1599–1609. [DOI] [PubMed] [Google Scholar]
- Milani, P., França, D., Balieiro, A.G., and Faez, R. (2017) Polymers and its applications in agriculture. Polimeros 27: 256–266. [Google Scholar]
- Milas, M., Rinaudo, M., Knipper, M., and Schuppiser, J.L. (1990) Flow and viscoelastic properties of xanthan gum solutions. Macromolecules 23: 2506–2511. [Google Scholar]
- Mirzababaei, M., Arulrajah, A., and Ouston, M. (2017) Polymers for stabilization of soft clay soils. Procedia Eng 189: 25–32. [Google Scholar]
- Molnár, M., Vaszita, E., Farkas, É., Ujaczki, É., Fekete‐Kertész, I., Tolner, M., et al. (2016) Acidic sandy soil improvement with biochar – A microcosm study. Sci Total Environ 563: 855–865. [DOI] [PubMed] [Google Scholar]
- Montesano, F.F., Parente, A., Santamaria, P., Sannino, A., and Serio, F. (2015) Biodegradable superabsorbent hydrogel increases water retention properties of growing media and plant growth. Agric Agric Sci Procedia 4: 451–458. [Google Scholar]
- Moorhouse, R., Walkinshaw, M.D., and Arnott, S. (1977) Xanthan gum ‐ molecular conformation and interactions. In Extracellular Microbial Polysaccharides. Sandford, P.A., and Laskin, A. (eds). Washington, DC: American Chemical Society, pp. 90–102. [Google Scholar]
- Muchová, M., Růžička, J., Julinová, M., Doležalová, M., Houser, J., Koutný, M., and Buňková, L. (2009) Xanthan and gellan degradation by bacteria of activated sludge. Water Sci Technol 60: 965–973. [DOI] [PubMed] [Google Scholar]
- Murad, H.A., Abo‐Elkhair, A., and Azzaz, H. (2019) Production of xanthan gum from nontraditional substrates with perspective of the unique properties and wide industrial applications. JSMC Microbiol 1: 6. [Google Scholar]
- Nankai, H., Hashimoto, W., Miki, H., Kawai, S., and Murata, K. (1999) Microbial system for polysaccharide depolymerization: enzymatic route for xanthan depolymerization by Bacillus sp. strain GL1. Appl Environ Microbiol 65: 2520–2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan, V., Sànchez i Nogué, V., van Niel, E.W.J., and Gorwa‐Grauslund, M.F. (2016) Adaptation to low pH and lignocellulosic inhibitors resulting in ethanolic fermentation and growth of Saccharomyces cerevisiae . AMB Express 6: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobre, C., Cerqueira, M.Â., Rodrigues, L.R., Vicente, A.A., and Teixeira, J.A. (2015) Production and extraction of polysaccharides and oligosaccharides and their use as new food additives. In Industrial Biorefineries and White Biotechnology. Pandey, A., Rainer, H., Mohammad, T., Madhavan, K.N., and Larroche, C. (eds). Amsterdam: Elsevier, pp. 653–679. [Google Scholar]
- Norton, I.T., Goodall, D.M., Frangou, S.A., Morris, E.R., and Rees, D.A. (1984) Mechanism and dynamics of conformational ordering in xanthan polysaccharide. J Mol Biol 175: 371–394. [DOI] [PubMed] [Google Scholar]
- Novotna, K., Cermakova, L., Pivokonska, L., Cajthaml, T., and Pivokonsky, M. (2019) Microplastics in drinking water treatment – Current knowledge and research needs. Sci Total Environ 667: 730–740. [DOI] [PubMed] [Google Scholar]
- Oliveira, P.J.V., Freitas, L.D., and Carmona, J.P.S.F. (2017) Effect of soil type on the enzymatic calcium carbonate precipitation process used for soil improvement. J Mater Civ Eng 29: 04016263. [Google Scholar]
- Oliveri Conti, G., Ferrante, M., Banni, M., Favara, C., Nicolosi, I., Cristaldi, A., et al. (2020) Micro‐ and nano‐plastics in edible fruit and vegetables. The first diet risks assessment for the general population. Environ Res 187: 109677. [DOI] [PubMed] [Google Scholar]
- Ozdal, M., and Kurbanoglu, E.B. (2019) Use of chicken feather peptone and sugar beet molasses as low cost substrates for xanthan production by Xanthomonas campestris MO‐03. Fermentation 5: 8–10. [Google Scholar]
- Pacheco‐Aguirre, J., Ruiz‐Sánchez, E., Reyes‐Ramírez, A., Cristóbal‐Alejo, J., Tun‐Suárez, J., and Borges‐Gómez, L. (2016) Polymer‐based encapsulation of Bacillus subtilis and its effect on Meloidogyne incognita in tomato. Phyton‐Int J Exp Bot 85: 1–6. [Google Scholar]
- Palaniraj, A., and Jayaraman, V. (2011) Production, recovery and applications of xanthan gum by Xanthomonas campestris . J Food Eng 106: 1–12. [Google Scholar]
- Pastor, M.V., Costell, E., Izquierdo, L., and Durán, L. (1994) Effects of concentration, pH and salt content on flow characteristics of xanthan gum solutions. Top Catal 8: 265–275. [Google Scholar]
- Penesyan, A., Nagy, S.S., Kjelleberg, S., Gillings, M.R., and Paulsen, I.T. (2019) Rapid microevolution of biofilm cells in response to antibiotics. NPJ Biofilms Microbiomes 5: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng, G., Wolf, T.M., Byer, K.N., and Caldwell, B.C. (2000) Spray retention and its impact on bioherbicide efficacy. Pest Technol 2008: 70–80. [Google Scholar]
- Peterson, B.W., He, Y., Ren, Y., Zerdoum, A., Libera, M.R., Sharma, P.K., et al. (2015) Viscoelasticity of biofilms and their recalcitrance to mechanical and chemical challenges. FEMS Microbiol Rev 39: 234–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petri, D.F.S.S. (2015) Xanthan gum: a versatile biopolymer for biomedical and technological applications. J Appl Polym Sci 132: 23. [Google Scholar]
- Pivokonsky, M., Cermakova, L., Novotna, K., Peer, P., Cajthaml, T., and Janda, V. (2018) Occurrence of microplastics in raw and treated drinking water. Sci Total Environ 643: 1644–1651. [DOI] [PubMed] [Google Scholar]
- Powell, L.C., Pritchard, M.F., Ferguson, E.L., Powell, K.A., Patel, S.U., Rye, P.D., et al. (2018) Targeted disruption of the extracellular polymeric network of Pseudomonas aeruginosa biofilms by alginate oligosaccharides. NPJ Biofilms Microbiomes 4: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahbari, M., Kianirad, M., Heydarian, S.M., and Mirdamadi, S.S. (2016) The effects of temperature, salt, and pH on xanthan infiltration in sandy soil. Soil Sci Soc Am J 80: 1135–1144. [Google Scholar]
- Ravichandran, K., Palaniraj, R., Saw, N.M.M.T., Gabr, A.M.M.M., Ahmed, A.R., Knorr, D., and Smetanska, I. (2014) Effects of different encapsulation agents and drying process on stability of betalains extract. J Food Sci Technol 51: 2216–2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney, L.M., Amos, W.B., Hoskisson, P.A., and McConnell, G. (2020) Intra‐colony channels in E. coli function as a nutrient uptake system. ISME J 14: 2461–2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosalam, S., and England, R. (2006) Review of xanthan gum production from unmodified starches by Xanthomonas comprestris [sic!] sp. Enzyme Microb Technol 39: 197–207. [Google Scholar]
- Rottava, I., Batesini, G., Silva, M.F., Lerin, L., de Oliveira, D., Padilha, F.F., et al. (2009) Xanthan gum production and rheological behavior using different strains of Xanthomonas sp. Carbohydr Polym 77: 65–71. [Google Scholar]
- Sánchez, V.E., Bartholomai, G.B., and Pilosof, A.M.R. (1995) Rheological properties of food gums as related to their water binding capacity and to soy protein interaction. LWT ‐ Food Sci Technol 28: 380–385. [Google Scholar]
- Sandford, P.A., Baird, J., and Cottrell, I.W. (1981) Xanthan gum with improved dispersibility. In Solution Properties of Polysaccharides. ACS Symposium Series. Brant, D.A. (ed). Washington: American Chemical Society. pp. 31–41. [Google Scholar]
- Saruchi, Kumar, V., Mittal, H. & Alhassan, S.M. (2019) Biodegradable hydrogels of tragacanth gum polysaccharide to improve water retention capacity of soil and environment‐friendly controlled release of agrochemicals. Int J Biol Macromol 132: 1252–1261. [DOI] [PubMed] [Google Scholar]
- Satriani, A., Catalano, M., and Scalcione, E. (2018) The role of superabsorbent hydrogel in bean crop cultivation under deficit irrigation conditions: a case‐study in Southern Italy. Agric Water Manag 195: 114–119. [Google Scholar]
- Shu, G., He, Y., Chen, L., Song, Y., Meng, J., and Chen, H. (2018a) Microencapsulation of Bifidobacterium bifidum BB01 by xanthan–chitosan: preparation and its stability in pure milk. Artif Cells Nanomed Biotechnol 46: 588–596. [DOI] [PubMed] [Google Scholar]
- Shu, G., He, Y., Chen, L.i., Song, Y., Cao, J., and Chen, H. (2018b) Effect of Xanthan – Chitosan Microencapsulation on the Survival of Lactobacillus acidophilus in simulated gastrointestinal fluid and dairy beverage. Polymers 10: 588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, A., Dhiman, N., Kar, A.K., Singh, D., Purohit, M.P., Ghosh, D., and Patnaik, S. (2020) Advances in controlled release pesticide formulations: prospects to safer integrated pest management and sustainable agriculture. J Hazard Mater 385: 121525. [DOI] [PubMed] [Google Scholar]
- Singh, J., and Dhaliwal, A.S. (2020) Water retention and controlled release of KCl by using microwave‐assisted green synthesis of xanthan gum‐cl‐poly (acrylic acid)/AgNPs hydrogel nanocomposite. Polym Bull 77: 4867–4893. [Google Scholar]
- Sinha, T., Bhagwatwar, P., Krishnamoorthy, C., and Chidambaram, R. (2019) Polymer based micro‐ and nanoencapsulation of agrochemicals. In Polymers for Agri‐Food Applications. Gutiérrez, T.J. (ed). Cham: Springer International Publishing, pp. 5–28. [Google Scholar]
- Smith, H.J., Schmit, A., Foster, R., Littman, S., Kuypers, M.M., and Foreman, C.M. (2016) Biofilms on glacial surfaces: hotspots for biological activity. NPJ Biofilms Microbiomes 2: 16008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldo, A., Miletić, M., and Auad, M.L. (2020) Biopolymers as a sustainable solution for the enhancement of soil mechanical properties. Sci Rep 10: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanoghe, P., De Schampheleire, M., Van der Meeren, P., and Steurbaut, W. (2007) Influence of agricultural adjuvants on droplet spectra. Pest Manag Sci 63: 4–16. [DOI] [PubMed] [Google Scholar]
- Stöcker, C.M., Bamberg, A.L., Stumpf, L., Monteiro, A.B., Cardoso, J.H., and de Lima, A.C.R. (2020) Short‐term soil physical quality improvements promoted by an agroforestry system. Agrofor Syst 94: 2053–2064. [Google Scholar]
- Sutherland, I.W. (1987) Xanthan lyases–novel enzymes found in various bacterial species. J Gen Microbiol 133: 3129–3134. [DOI] [PubMed] [Google Scholar]
- Sworn, G. (2009) Xanthan gum. In Handbook of hydrocolloids. Wiley Online Books. Phillips, G.O., and Williams, P.A. (eds). Cambridge: Woodhead Publishing Limited, pp. 186–203. [Google Scholar]
- The European Parliament and the Council of the European Union (2019) Regulation (EU) 2019/1009 laying down rules on the making available on the market of EU fertilising products and amending Regulation (EC) No 1069/2009 and (EC) No 1107/2009 and repealing Regulation (EC) No 2003/2003. Off J Eur Union L 170: 1. [Google Scholar]
- Thombare, N., Mishra, S., Siddiqui, M.Z., Jha, U., Singh, D., and Mahajan, G.R. (2018) Design and development of guar gum based novel, superabsorbent and moisture retaining hydrogels for agricultural applications. Carbohydr Polym 185: 169–178. [DOI] [PubMed] [Google Scholar]
- Tran, A.T.P., Chang, I., and Cho, G.C. (2019) Soil water retention and vegetation survivability improvement using microbial biopolymers in drylands. Geomech Eng 17: 475–483. [Google Scholar]
- Tran, A.T.P., Cho, G.C., Lee, S.J., and Chang, I. (2018) Effect of xanthan gum biopolymer on the water retention characteristics of unsaturated sand. In International Conference on Unsaturated Soil. [Google Scholar]
- Vert, M., Doi, Y., Hellwich, K.‐H., Hess, M., Hodge, P., Kubisa, P., et al. (2012) Terminology for biorelated polymers and applications (IUPAC Recommendations 2012). Pure Appl Chem 84: 377–410. [Google Scholar]
- Vundavalli, R., Vundavalli, S., Nakka, M., and Rao, D.S. (2015) Biodegradable nano‐hydrogels in agricultural farming ‐ alternative source for water resources. Procedia Mater Sci 10: 548–554. [Google Scholar]
- Wallingford, L., and Labuza, T.P. (1983) Evaluation of the water binding properties of food hydrocolloids by physical/chemical methods and in a low fat meat emulsion. J Food Sci 48: 1–5. [Google Scholar]
- Wang, D.C., Jiang, C.H., Zhang, L.N., Chen, L., Zhang, X.Y., and Guo, J.H. (2019) Biofilms positively contribute to Bacillus amyloliquefaciens 54‐induced drought tolerance in tomato plants. Int J Mol Sci 20: 6271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, S., He, X., Song, J., Wang, S., Jia, X., and Ling, Y. (2018) Effects of xanthan gum on atomization and deposition characteristics in water and Silwet 408 aqueous solution. Int J Agric Biol Eng 11: 29–34. [Google Scholar]
- Xiong, B., Loss, R.D., Shields, D., Pawlik, T., Hochreiter, R., Zydney, A.L., and Kumar, M. (2018) Polyacrylamide degradation and its implications in environmental systems. npj Clean Water 1: 1–9. [Google Scholar]
- Yang, F., and Antonietti, M. (2020) The sleeping giant: a polymer view on humic matter in synthesis and applications. Prog Polym Sci 100: 101182. [Google Scholar]
- Yin, W., Wang, Y., Liu, L., and He, J. (2019) Biofilms: the microbial “protective clothing” in extreme environments. Int J Mol Sci 20: 3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatz, J.L., and Knapp, S. (1984) Viscosity of xanthan gum solutions at low shear rates. J Pharm Sci 73: 468–471. [DOI] [PubMed] [Google Scholar]
- Zhu, H., Dexter, R.W., Fox, R.D., Reichard, D.L., Brazee, R.D., and Ozkan, H.E. (1997) Effects of polymer composition and viscosity on droplet size of recirculated spray solutions. J Agric Eng Res 67: 35–45. [Google Scholar]


