Summary
Corals are colonized by symbiotic microorganisms that profoundly influence the animal’s health. One noted symbiont is a single‐celled alga (in the dinoflagellate family Symbiodiniaceae), which provides the coral with most of its fixed carbon. Thermal stress increases the production of reactive oxygen species (ROS) by Symbiodiniaceae during photosynthesis. ROS can both damage the algal symbiont’s photosynthetic machinery and inhibit its repair, causing a positive feedback loop for the toxic accumulation of ROS. If not scavenged by the antioxidant network, excess ROS may trigger a signaling cascade ending with the coral host and algal symbiont disassociating in a process known as bleaching. We use Exaiptasia diaphana as a model for corals and constructed a consortium comprised of E. diaphana–associated bacteria capable of neutralizing ROS. We identified six strains with high free radical scavenging (FRS) ability belonging to the families Alteromonadaceae, Rhodobacteraceae, Flavobacteriaceae and Micrococcaceae. In parallel, we established a consortium of low FRS isolates consisting of genetically related strains. Bacterial whole genome sequences were used to identify key pathways that are known to influence ROS.
The field of coral microbiome engineering is in its infancy and is currently limited by a lack of definitive information about the functional roles of cnidarian microbiome members. Outlined in this manuscript is the start of a complex process to identify, evaluate, and select durable and useful candidate consortium members that may buffer the coral host against climate warming. We identified six diverse bacterial strains with high free radical scavenging (FRS) ability and six conspecific/congeneric low FRS strains with a view to including them in an inoculum to mitigate the effects of thermal stress in cnidarians.
Introduction
Coral reefs are among the most biologically and economically valuable ecosystems on Earth (Cesar et al., 2003; Alder et al., 2006; Fisher et al., 2015). While they cover less than 0.1% of the ocean floor (Spalding and Grenfell, 1997), coral reefs support economic activities relating to fisheries, tourism, pharmaceuticals and coastal development with a global value of $8.9 trillion “international $” per year (de Groot et al., 2012). Corals and other reef organisms have been dying, largely due to anthropogenic influences such as climate change (Hughes et al., 2017; Stuart‐Smith et al., 2018), which has led to an increased frequency, intensity and duration of summer heat waves that cause coral bleaching (Hughes et al., 2018; Hoegh‐Guldberg et al., 2019).
The coral holobiont, which is the sum of the coral animal and its associated microbiota, including algae, fungi, protozoans, bacteria, archaea and viruses (Rohwer et al., 2002), is an ecosystem engineer. By secreting a calcium carbonate skeleton, scleractinian corals form the literal foundation of the coral reef ecosystem. The success of corals to survive and build up reefs over thousands of years (Devlin‐Durante et al., 2016) is tightly linked to their obligate yet fragile symbiosis with endosymbiotic dinoflagellates of the family Symbiodiniaceae (Glynn, 1996).
Intracellular Symbiodiniaceae translocate photosynthetically fixed carbon to the coral host (Muscatine and Porter, 1977; Tremblay et al., 2014) in exchange for inorganic nutrients and location in a high light environment with protection from herbivory (Venn et al., 2008; Yellowlees et al., 2008). During periods of thermal stress, the relationship between the coral host and their Symbiodiniaceae can break down, resulting in a separation of the partners and significantly, a fixed carbon shortage for the host (Radecker et al., 2021). This phenomenon, ‘coral bleaching’, is devastating to the host and detrimental to the reef system. The ecosystem‐wide effects of bleaching on the coral include reduced skeletal growth and reproductive activity, a lowered capacity to shed sediments, and an inability to resist invasion of competing species and diseases. Severe and prolonged bleaching can impact coral health and ultimately cause colony death, sequentially observed as diminished reef growth, the transformation of reef‐building communities to alternate, non‐reef building community types, bioerosion and ultimately the disappearance of reef structures (Glynn, 1996).
There are several hypotheses detailing the mechanisms driving bleaching (Weis, 2008; Cunning and Baker, 2012; Wiedenmann et al., 2012; Wooldridge, 2013), with a common theme being the overproduction and toxic accumulation of reactive oxygen species (ROS). ROS production is a normal part of the physiology and functioning of organisms, including as a product of photosynthesis. The light triggered splitting of water molecules in the oxygen evolving complex (OEC), and subsequent transfer of electrons from photosystem II (PSII) to photosystem I (PSI) generates ROS in chloroplasts (Trubitsin et al., 2014). Additionally, photodamage, particularly to PSII, is a normal part of the photosynthetic light cycle, with mechanisms available to efficiently repair this damage (Aro et al., 1993). Heat affects the fluidity and integrity of the thylakoid membrane, disturbing PSI, PSII and the OEC, while simultaneously inducing the production of ROS (Farooq et al., 2016). Elevated intracellular ROS levels can both increase damage to (Mathur et al., 2014) and inhibit the repair of the D1 protein in the PSII apparatus (Warner et al., 1999; Nishiyama et al., 2006), and, in a positive feedback mechanism, excess ROS is generated. Once generated, ROS can trigger the oxidation of essential photosynthetic molecules, such as thylakoid membranes (Tchernov et al., 2004) and enzymes of the Calvin‐Benson cycle (Lesser and Farrell, 2004), thereby interfering with the supply of fixed carbon to the holobiont (Lesser, 2004, 2004).
It is hypothesized that ROS, specifically hydrogen peroxide (H2O2), can transfer from the algal symbiont to the surrounding host cell (Szabó et al., 2020). ROS can also be generated from actively growing bacteria (Zinser, 2018; Hansel and Diaz, 2021), these include bacterial coral symbionts (Zhang et al., 2016). Excess ROS may damage both the host and symbiont cellular machinery. Once damaged, Symbiodiniaceae are no longer able to maintain their role in the relationship with corals and separate from the host tissue via in situ degradation or exocytosis (Weis, 2008). Thus, any mechanism that might neutralize ROS in host or Symbiodiniaceae cells could reduce coral bleaching.
Microbiome engineering through the addition of a selection of beneficial bacteria has been proposed as a strategy to facilitate adaptation to changing environmental conditions by enhancing the coral holobiont with the metabolic capabilities of the introduced bacteria (van Oppen et al., 2015; Damjanovic et al., 2017; Peixoto et al., 2017; van Oppen et al., 2017; Damjanovic et al., 2019; Epstein et al., 2019, 2019,2019, 2019; van Oppen and Blackall, 2019; Peixoto et al., 2021). The differences in the bacterial community composition and stability of healthy and thermally stressed corals (Vega Thurber et al., 2009; Mouchka et al., 2010; Sunagawa et al., 2010; Littman et al., 2011; Epstein et al., 2019, 2019,2019, 2019; Pootakham et al., 2019) and the coral model Exaiptasia diaphana (Plovie, 2010; Ahmed et al., 2019; Hartman et al., 2020) demonstrate an adaptation of the host‐associated microbiome to changing external environments and support the potential utility of microbiome engineering in cnidarian health. In addition, a disruption to the bacterial community of Pocillopora damicornis with antibiotic treatment diminished the resilience of the holobiont during thermal stress, whereas intact microbial communities conferred resilience to thermal stress and increased the rate of holobiont recovery after bleaching events (Gilbert et al., 2012). The relative stability of coral‐associated bacterial communities has also been linked to coral heat tolerance; for instance, the bacterial community of heat‐sensitive Acropora hyacinthus corals shifted when transplanted to thermal stress conditions, whereas heat‐tolerant A. hyacinthus corals harbored a stable bacterial community (Ziegler et al., 2017).
In recent years, researchers have begun to explore microbiome engineering in corals and E. diaphana. To inhibit the progression of white pox disease, caused by pathogenic Serratia marcescens, an Alphaproteobacteria cocktail containing several Marinobacter spp. was applied to E. diaphana (Alagely et al., 2011); these introduced strains were able to inhibit both biofilm formation and swarming of S. marcescens, which halted disease progression. The Marinobacter‐based inoculum was deemed effective as anemones exposed to both the cocktail and pathogen survived after seven days, while anemones in the S. marcescens control treatment died. A bacterial consortium native to the coral Mussismilia harttii was selected to degrade water‐soluble oil fractions (dos Santos et al., 2015). This bioremediation strategy reduced the negative impacts of oil on M. harttii health and accelerated the degradation of petroleum hydrocarbons (dos Santos et al., 2015). Coral microbiomes have also been manipulated through addition of a consortium of native or seawater‐derived bacteria to the surface of P. damicornis to mitigate the effects of thermal stress (Rosado et al., 2018). The results from this study suggest the consortium was able to partially mitigate coral bleaching.
Our goal was to identify bacterial strains suitable for use in a microbiome engineering approach to mitigate the effects of thermal stress in E. diaphana. Given the potential role of ROS in the bleaching process and the prevalence of bacteria in and on hosts (Lesser et al., 2004; Work and Aeby, 2014) and intracellular Symbiodiniaceae (Ainsworth et al., 2015; Maire et al., 2021), our focus was to select diverse E. diaphana–sourced bacterial isolates with an extracellular free radical scavenging (FRS) phenotype.
Results
Diversity of culturable bacteria associated with E. diaphana
A total of 842 isolates were obtained from four genotypes of Great Barrier Reef (GBR)–sourced E. diaphana. There were no significant differences in bacterial colony forming units (CFUs) between the four genotypes, regardless of growth medium, with 5.9–10.3 × 103 CFUs per anemone on Reasoner's 2A agar (R2A) and 6.3–10.4 × 103 CFUs per anemone on marine agar (MA) (P > 0.05). Partial 16S rRNA gene sequences (˜ 1000 bp) were used to identify the closest matches from GenBank using the Basic Local Alignment Search Tool (Blastn). In total there were 109 species in 64 genera, 27 families and six phyla (Fig. 1). The most abundant genera (Table 1) were Alteromonas, Labrenzia and Ruegeria. Gram‐positive bacteria comprised 23 species, including Microbacterium (31 isolates) and Micrococcus (28 isolates). Eight genera were found to be associated with all four genotypes (Table 1); these eight genera made up 59.4% of all E. diaphana–associated bacterial isolates.
Fig. 1.
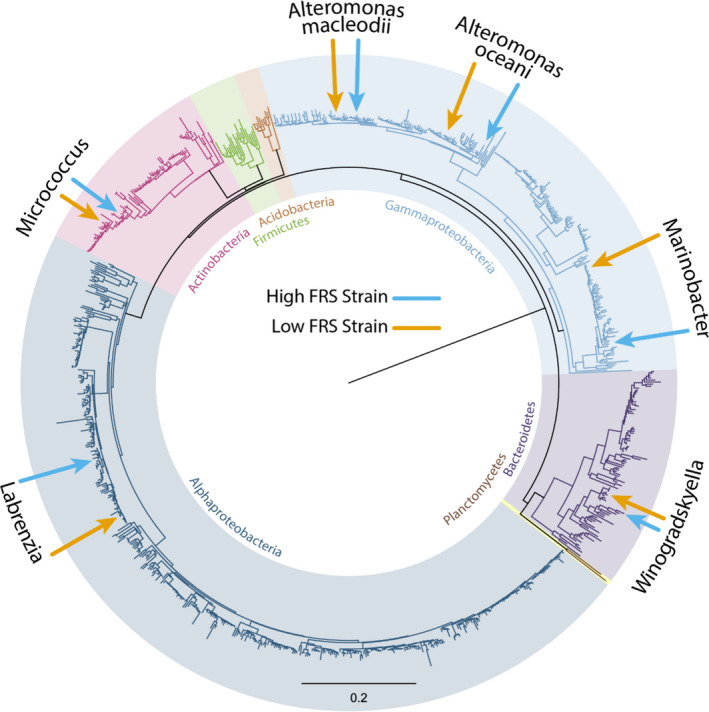
Neighbor‐Joining tree showing an overview of the phylogenetic relationship between the 842 E. diaphana–associated bacterial isolates inferred using partial 16S rRNA sequences. These isolates covered six phyla indicated by shading over the tree with Proteobacteria split into the classes Gammaproteobacteria and Alphaproteobacteria. The positions of selected strains are highlighted by arrows with blue arrows indicating the high FRS strains and orange arrows indicating low FRS strains.
Table 1.
Bacterial genera associated with all four genotypes of GBR‐sourced E. diaphana (AIMS1‐4).
| Genus | Class | No. of species | AIMS1 | AIMS2 | AIMS3 | AIMS4 | Total isolatesa |
|---|---|---|---|---|---|---|---|
| Alteromonas | Gamma‐proteobacteria | 6 | 10 | 52 | 24 | 30 | 116 |
| Labrenzia | Alpha‐proteobacteria | 4 | 11 | 10 | 26 | 38 | 85 |
| Marinobacter | Gamma‐proteobacteria | 4 | 7 | 25 | 10 | 13 | 55 |
| Muricauda | Flavobacteriia | 1 | 16 | 11 | 10 | 5 | 42 |
| Roseovarius | Alpha‐proteobacteria | 3 | 9 | 12 | 5 | 6 | 32 |
| Ruegeria | Alpha‐proteobacteria | 3 | 29 | 8 | 5 | 39 | 81 |
| Shimia | Alpha‐proteobacteria | 2 | 2 | 3 | 9 | 40 | 54 |
| Vibrio | Gamma‐proteobacteria | 3 | 3 | 7 | 31 | 15 | 56 |
521 out of the 842 isolates obtained from the four E. diaphana genotypes.
Bacterial consortium selection
A high extracellular FRS phenotype was the primary selection criteria in selecting E. diaphana–sourced bacterial isolates for inclusion in the consortium. The FRS phenotype was measured using the stable free radical 2,2‐diphenyl‐1‐picrylhydrazyl (DPPH), which is reduced in the presence of an antioxidant molecule, undergoing a color change from a violet to a colorless solution. Of the original 842 isolates, 709 were qualitatively screened for their ability to scavenge exogenous free radicals, divided into positive (144), weakly positive (121) and negative (444). Ninety‐eight strains representing eight families and 18 genera were then quantitatively assessed for FRS. There was no clear pattern of FRS capacity at the family level (Fig. 2) with strain‐specific responses evident. Consortium members were selected by choosing E. diaphana–associated bacterial isolates, where conspecific or congeneric pairs of strains displayed a high and low FRS ability (Fig. 2; Table 2). Of the 12 selected bacterial isolates, seven were catalase positive and five were catalase negative (Table 2). In each consortium set (i.e., high or low FRS strains), none of the selected isolates showed antagonistic activity against one other as evidenced by the absence of any zone of inhibition and growth from each combination of isolates on a plate. Growth curves show that after 48 h at 37°C, each selected isolate was in the stationary phase of growth (Figs S1–S12).
Fig. 2.

Quantitative FRS ability of E. diaphana–associated bacterial isolates, separated by Family. Families with high relative abundance among all cultured bacteria (Rhodobacteraceae – A, Alteromonadaceae – B, Pseudoalteromonadaceae – C, Flavobacteraceae – D, and Micrococcaceae – E) were separately analyzed to identify strains with a high FRS ability (blue font) and a corresponding conspecific or congeneric strain with a low FRS ability (orange font). In each panel, the light dashed vertical line on the left represents the mean FRS of a 0.025% (w/v) ascorbic acid standard, the middle dark dashed vertical line is the mean FRS for 0.05% (w/v) ascorbic acid standard, and the far‐right dashed line is the mean FRS of the 0.075% (w/v) ascorbic acid standard.
Table 2.
Overview of high S bacterial strains and conspecific/congeneric low FRS strains. All sequence data can be found under BioProjecta PRJNA574193. References to each selected strain at the Genus level are identified in the last three columns.
| Strain | Bacteria species (Phylum or Class) | FRSa (% ± SE) | High versus low FRSc | Strain versus growth mediumc | Catalase | E. diaphana literature | Coral literature | Probiotic literature |
|---|---|---|---|---|---|---|---|---|
| MMSF01163 (n = 3) | Alteromonas oceani (Gammaproteobacteria) | 61.7 ± 5.2 | 0.065 | 0.004 | Negative | Binsarhan (2016), Röthig et al. (2016), Herrera et al. (2017) | Chiu et al. (2012), Ceh et al. (2013), Röthig et al. ( 2017), Damjanovic et al. (2019) | Riquelme et al. (1997), Kesarcodi‐Watson et al. (2010), Hai (2015), Haryanti et al. (2017) |
| MMSF00404 (n = 4) | 35.5 ± 13.7 | 0.571 | Negative | |||||
| MMSF00958 (n = 3) | Alteromonas macleodii (Gammaproteobacteria) | 62.0 ± 4.2 | 0.175 | 0.017 | Negative | |||
| MMSF00257 (n = 3) | 30.3 ± 0.9 | 0.431 | Positive | |||||
| MMSF00132 (n = 8) | Labrenzia aggregata (Biebl et al., 2007) (Alphaproteobacteria) | 53.6 ± 8.7 | 0.016 | 0.101 | Negative | Binsarhan (2016) | Littman et al. (2011), Chiu et al. (2012) | Mancuso et al. (2015) |
| MMSF00249 (n = 3) | 14.0 ± 3.6 | 0.103 | Positive | |||||
| MMSF01190 (n = 3) | Marinobacter salsuginis (Gammaproteobacteria) | 62.0 ± 4.5 | 0.041 | < 0.0001 | Negative | Binsarhan (2016), Brown et al. (2017), Herrera et al. (2017) | Littman et al. (2009), Sharp et al. (2012), Röthig et al. (2017) | Alagely et al. (2011) |
| MMSF00964 (n = 3) | 43.7 ± 5.8 | 0.020 | Positive | |||||
| MMSF00068 (n = 6) | Micrococcus luteus (Actinobacteria) | 56.3 ± 7.3 | 0.210 | 0.002 | Positive | Binsarhan (2016) | Kellogg et al. (2013) | El‐Rhman et al. (2009), Osman et al. (2010), Hai (2015) |
| MMSF00107 (n = 3) | Micrococcus yunnanensis (Actinobacteria) | 38.0 ± 8.0 | 0.306 | Positive | ||||
| MMSF00046 (n = 3) | Winogradskyella poriferorum (Lau et al., 2005) (Bacteroidetes) | 73.3 ± 1.9 | 0.284 | 0.010 | Negative | b | dos Santos et al. (2015), Franco et al. (2018) | NA |
| MMSF00910 (n = 3) | 36.0 ± 3.5 | 0.174 | Negative |
Mean R2A FRS (% ± SE) was 27.2 ± 2.3% (n = 12), and it was catalase negative.
While there are no instances of Winogradskyella specifically identified in current E. diaphana literature, there are several Flavobacteriaceae that are not resolved to the genus level from metabarcoding data (Plovie, 2010; Röthig et al., 2016; Herrera et al., 2017; Ahmed et al., 2019).
Indicates P values for pairwise comparisons from respective one‐way analysis of variance (Tukey HSD) or Kruskal‐Wallis rank sum test (Dunn test) with bold values representing significant differences.
Comparative genomics
As part of the characterization of the 12 isolates (six high FRS and six conspecific or congeneric low FRS isolates), draft genome sequences were assembled and analyzed. A summary of the data and metrics for the draft genome sequences is presented in Table S1. The diversity of the six pairs of isolates is indicated by the %G + C range (35% to 72%) and genome size (2.4 Mb to 6.8 Mb). Each isolate pair was classified as the same species, according to 16S rRNA gene sequence identity, except the Micrococcus stains. Isolates MMSF00068 (high FRS strain) and MMSF00107 (low FRS strain) are classified as Micrococcus luteus and M. yunnanensis, respectively.
Core genome comparison provides an overview of the genetic relationship between the conspecific or congeneric isolate pairs. A wide range of genome variation between strain pairs was observed, which ranged from ˜ 190 000 single nucleotide polymorphism (SNP) differences between the Alteromonas oceani strains to fewer than five core genome SNPs between the Labrenzia aggregata isolates (Table S2). It should be noted that core genome comparison cannot be used to establish isolate identity with differences in the accessory genome content necessarily not included. In our study, this is best illustrated with the Winogradskyella poriferorum isolates MMSF00046 and MMSF00910; the core genomes differ by fewer than ten pairwise SNPs, but there are accessory genome differences.
Genes of interest
The annotated genome sequences of each selected candidate consortium member were searched for key genes relevant to extracellular ROS scavenging capabilities (Tables S3 and S4). Dimethylsulfoniopropionate (DMSP) cleavage to dimethylsulfide (DMS) was identified by presence of one or more of the DMSP lyase genes; dddP, dddD, dddL, dddW and dddQ. Only L. aggregata strains contained DMSP lyase genes (dddP and dddL) in their whole genome sequences. DMSP biosynthesis was identified by the presence of dsyB, which is the only described gene for an enzyme in the DMSP biosynthesis pathway. The cobP gene was used as an indicator for the presence of the dynamic vitamin B12 pathway, which contains 27 genes (Table S4). Again, only the L. aggregata isolates contained cobP. Catalase positive strains were identified by the presence of katG; all high and low FRS strains contained katG except the Micrococcus spp. strains, in which katA and katE were detected.
16S rRNA gene copy number
The 16S rRNA gene copy numbers of the 12 draft genomes were estimated using a read depth approach (Table S1). The copy numbers were similar within pairs of isolates, in which the pair of A. macleodii isolates (MMSF00257 and MMSF00958) contained the most copies (5.15 and 4.79, respectively), corresponding with copy numbers in a published closed genome of A. macleodii (Gonzaga et al., 2012). W. poriferorum isolates (MMSF00046 and MMSF00910) contained the fewest copies (1.03 and 0.77, respectively).
Discussion
The 842 E. diaphana bacterial isolates reported here comprise 109 species from 64 genera and six phyla. Using metabarcoding, studies of microbiomes associated with E. diaphana have revealed a similar diversity at the phylum level for E. diaphana sourced from the GBR (Hartman et al., 2020; Dungan et al., 2021), Hawaii (strain H2; Herrera et al., 2017), Pacific and Caribbean (Brown et al., 2017), Atlantic (strain CC7; Röthig et al., 2016) and Red Sea (Ahmed et al., 2019), as well as stony corals (Blackall et al., 2015). Thus, our culture collection of E. diaphana bacterial isolates captures the diversity of the E. diaphana–associated microbiome. The broader culture collection contains bacteria with a wide range of FRS capacity, and our candidate consortia comprise greater bacterial diversity than others (Alagely et al., 2011; Rosado et al., 2018).
The consistent and frequent reporting of our selected bacterial genera in E. diaphana and coral studies (Table 2) suggests these bacteria likely have key functions in cnidarian holobionts. Among these potential functions are the production and secretion of antioxidants and other reducing agents. Antioxidants of interest include DMSP and the breakdown of DMSP to other antioxidants (Sunda et al., 2002). L. aggregata have been reported to produce DMSP in the absence of any methylated sulfur compounds with dsyB identified as the first DMSP biosynthesis gene in any organism (Curson et al., 2017). dsyB was found in the genome sequences of both high and low FRS L. aggregata strains (Table S3). Many of the E. diaphana–sourced bacterial species, in particular, bacteria related to our selected isolates, have been implicated in the degradation of DMSP to DMS (Alteromonas spp., Raina et al., 2009; Labrenzia spp., Hatton et al., 2012). The dddP gene which encodes for the enzyme responsible for cleaving DMSP to DMS, was used as an indicator of a DMSP degradation genotype. Using the products identified in the Prokka annotation of each of the genomes, only the L. aggregata isolates contained genes responsible for DMSP degradation (Table S3).
Carotenoids are among the strongest antioxidants and are highly reactive against ROS and other free radicals (Fiedor et al., 2005; Asker et al., 2007; Shindo et al., 2007; Fiedor and Burda, 2014; Flórez et al., 2015). Carotenoids are lipid‐soluble pigments, and in bacteria they give an orange‐yellow hue to colonies. Two of the five selected genera produce orange/yellow colonies (Winogradskyella, Micrococcus), and there is evidence of carotenoid production by marine Flavobacteriaceae (Shindo et al., 2007; Gammone et al., 2015) and Micrococcus (Mohana et al., 2013). A marine Flavobacteriaceae (strain GF1) was found to produce the potent antioxidant carotenoid zeaxanthin that protected Symbiodiniaceae from thermal and light stress (Motone et al., 2020).
Vitamin B12 is a cofactor required for the biosynthesis of the amino acid methionine, a fundamental component of every protein, and in diverse metabolic pathways including generation of the antioxidants glutathione and DMSP (Croft et al., 2005). Vitamin B12 is synthesized by many heterotrophic bacteria (Raux et al., 2000). Genomic evidence suggests that Symbiodiniaceae have lost the capacity to synthesize vitamin B12 (Matthews et al., 2020), which is in agreement with another work showing that free‐living Symbiodiniaceae rely on bacterial symbionts for this important cofactor (Agostini et al., 2009). The genes involved in the biosynthesis of vitamin B12 have been found in coral‐associated bacteria (Robbins et al., 2019), specifically L. aggregata cultured from the Caribbean coral, Orbicella faveolata (Smith, 2018). Eighteen genes, including cobP (Raux et al., 2000), are present in each L. aggregata isolate (MMSF00132 and MMSF00249), suggesting both are capable of vitamin B12 biosynthesis. None of the other sequenced isolated had genes related to vitamin B12 biosynthesis, except in the Marinobacter salsuginis isolates, where cobO was detected, strongly indicating that none of the remaining ten isolates were capable of vitamin B12 biosynthesis (Table S4).
Bacteria have developed highly specific mechanisms to protect themselves against oxidative stress, in particular using enzymatic antioxidants such as catalase, peroxidase, and superoxide dismutase (Imlay, 2018). It has been suggested that increasing the in hospite concentration of catalase in the coral holobiont by the application of a bacterial consortium with catalase‐positive organisms could possibly minimize the impact of thermal stress by neutralizing H2O2 (Peixoto et al., 2017). Here we tested isolates for catalase production using a standard H2O2 assay (Taylor and Achanzar, 1972). Catalase participates in cellular antioxidant defense by enzymatically breaking down H2O2 to H2O and O2. The enzymes involved are hydroperoxidase I (HPI, encoded by katG), a bifunctional enzyme with both catalase and peroxidase activities expressed during aerobic growth, and hydroperoxidase II (HPII, katE), a monofunctional enzyme with catalase activity expressed during stationary phase (von Ossowski et al., 1991; Cabiscol et al., 2000). Each of the isolates had at least one katG or katE (Table S3). The lack of correlation between genotype and phenotype in relation to catalase activity may be associated with the culture conditions used, the concentration of H2O2 used in this assay [chosen to replicate previous studies (Rosado et al., 2018)], or undetected, as not all ROS scavenging activity can be observed by bubble formation.
The selected isolates were chosen from a highly diverse pool of E. diaphana–sourced isolates. While the selected isolates are phylogenetically diverse, other potentially promising beneficial bacteria in the culture collection were omitted based on our selection criteria. For example, Ruegeria spp. have been reported to breakdown DMSP and participate in denitrification (Smith, 2018). Muricauda isolates were found to have high FRS abilities, but were excluded from the consortium due to sporadic growth on the medium used in the study. Muricauda isolates have genes for denitrification (Smith, 2018), can breakdown DMSP (Hatton et al., 2012) and produce potent carotenoids (Prabhu et al., 2014) that can mitigate thermal and light stresses in Symbiodiniaceae cultures (Motone et al., 2020). Both Ruegeria and Muricauda should be included in future consortium evaluations.
Interactions among members of the microbiota associated with marine animals are undoubtedly complex. Results presented in this manuscript show that pure cultured bacteria from E. diaphana can scavenge free radicals in a strain‐specific fashion, reinforcing our approach of phenotypic selection. While there has been a surge of research and interest in microbial ROS production (Rose et al., 2008; Sutherland et al., 2019; Hansel and Diaz, 2021), there is a lack of data on ROS scavenging by specific microbial isolates. Therefore, the finding that E. diaphana–sourced bacterial isolates have a range of capacities to quench the stable N radical associated with DPPH improves our understanding of the overall extracellular FRS potential of these cultures. However, while ROS are all free radicals, there are many other types of free radicals whose activity our assay would have captured. Thus, a high FRS phenotype cannot perfectly translate to high ROS scavenging. Ultimately the role of these bacteria in coral/anemone physiology and health and the ROS dynamics within the cnidarian holobiont remains unknown.
The 12 candidate isolates making up the consortium were grown for 48 h while these cells were in stationary phase (Figs S1–S12). The life stage can play a critical role in FRS behavior with stationary phase Roseobacter isolates known to be more efficient in degrading superoxide (Hansel et al., 2019). We recognize that this may not be representative of the extracellular FRS for actively growing/respiring bacteria cells in hospite.
Inoculation with high FRS strains could be beneficial to the host under high oxidative stress conditions, such as those that potentially contribute to coral bleaching. While excessive ROS have been implicated in the onset of oxidative stress and subsequent bleaching of corals, this link has yet to be definitively shown and the role of ROS in coral health is nuanced and complex (Krueger et al., 2015; Nielsen et al., 2018) as ROS also provides beneficial benefits to the host (Hansel and Diaz, 2021). Future research should explore other beneficial bacterial functions such as alternative carbon fixation mechanisms, nitrogen fixation, and quorum quenching to disrupt cell‐to‐cell signalling of pathogens (Peixoto et al., 2021).
The field of coral microbiome engineering is in its infancy and is currently limited by a lack of definitive information about the functional roles of cnidarian microbiome members (van Oppen and Blackall, 2019; Blackall et al., 2020). Information that would aid development includes determination of bacterial phenotypes that are beneficial to the host, such as extracellular free radical scavenging. Outlined here is the start of a complex process to identify, evaluate and select durable and useful candidate consortium members that may buffer the coral host against climate warming. This consortium can be explored in the future to determine whether their addition provides a benefit to the host (i.e., are probiotics) and the underlying mechanism. Conspecific or congeneric pairs of bacteria provide an opportunity to determine the genetic basis for measured phenotypic differences between the pairs.
Experimental procedures
Isolation of bacterial isolates
Exaiptasia diaphana of GBR origin were maintained in the laboratory at 26°C (Dungan et al., 2020) and used to establish a bacterial isolate culture collection. Sixteen individuals from each of four E. diaphana genotypes (AIMS1‐4) were collected using sterile disposable pipets and gently transferred to filter‐sterilized (0.2 µm) reverse osmosis (RO) water reconstituted Red Sea Salt™ (Red Sea; RSS) at ˜ 34 parts per thousand (ppt) salinity (fRSS). After 30 min, each anemone was transferred to a sterile glass homogenizer with 1 ml of fRSS. Each homogenate was used to prepare serial dilutions from 10−1 to 10−4. From each dilution, 50 µl was spread plate–inoculated onto three replicate plates each of MA (Difco™ Marine Agar 2216, BD, Sparks, MD, USA) and R2A (R2A Agar CM0906, Oxoid, Oxoid Ltd., Basingstoke, Hampshire, England) supplemented with 40 g l−1 RSS and incubated at 26˚C. After one week, colony counts were completed. Individual colonies were sub‐cultured to purification from plates with < 100 colonies isolation medium. Single colonies (purified bacterial isolates) were resuspended individually in 40% glycerol, aliquoted into 1.2 ml cryotubes and stored at −80°C for long‐term preservation.
Identification of E. diaphana‐sourced isolates
Colony PCR with the universal bacterial primers 27f (5′‐AGA GTT TGA TCM TGG CTC AG‐3′) and 1492r (5′‐TAC GGY TAC CTT GTT ACG ACT T‐3′) (Lane, 1991) was used to generate 16S rRNA gene amplicons from each isolate. Briefly, cells from each pure culture were suspended in 20 µl Milli‐Q water and denatured at 95°C for 10 min. The suspension was then centrifuged at 2000 g at 4°C for 2 min, and the supernatant was used as the DNA template for PCR amplification. The PCR was performed with 20 µl Mango Mix™ (Bioline, London, UK), 0.25 µM of each primer and 2 µl of DNA template in a final volume of 40 µl with nuclease free water (Ambion, Thermo Fisher Scientific Inc., Austin, TX, USA). The thermal cycling protocol was as follows: 95°C for 5 min; 35 cycles of 95°C for 1 min, 50°C for 1 min and 72°C for 1 min; and a final extension of 10 min at 72°C. Amplicons were purified and sequenced on an ABI sequencing instrument by Macrogen (Seoul, South Korea) or by the Australian Genome Research Facility using the 1492r primer. Trimmed high‐quality read data from each isolate were used for presumptive identification by querying the 16S rRNA gene sequences via Blastn. For some isolates the near‐complete 16S rRNA gene sequence was determined by sequencing with additional primers [27f, 357f (5′‐CCT ACG GGA GGC AGC AG‐3′ (Muyzer et al., 1993)], 926f [5′‐CCG TCA ATT CMT TTR AGT TT‐3′ (Lane et al., 1985)], 519r [5′‐GWA TTA CCG CGG CKG CTG‐3′ (Muyzer et al., 1993)], 926r [5′‐AAA CTR AAA MGA ATT GAC GG‐3′ (Lane et al., 1985), and 1492r]. The six reads for each isolate were aligned using Geneious prime 2019.1.2 (https://www.geneious.com) via the Geneious global alignment tool using default settings and automatic determination of read direction. From this alignment, a consensus sequence for the 16S rRNA gene was constructed based on the frequency of a base and its quality (from chromatogram data) in each alignment column. The consensus sequence length for each of the six isolate pairs ranged from 1352 to 1495 nucleotides. GenBank accession numbers for sequences are shown in Table 1.
Qualitative free radical scavenging assay
DPPH is a stable free radical that is purple in its oxidized state but becomes white‐yellow if reduced by antioxidants and has been used to identify FRS marine bacteria (Takao et al., 1994; Velho‐Pereira et al., 2015). To qualitatively assess E. diaphana–associated bacterial isolates for FRS ability, a sterile Whatman #1 filter paper was gently pressed against fresh colonies from a streak plate grown for 2‐4 days. Plates (with filter paper) were then incubated overnight at 26°C. The following day, filter papers were removed with forceps and allowed to dry in a fume hood for 30 min, and 500 µl of a 0.2 mM DPPH (Cat# D9132, Sigma‐Aldrich, St. Louis, MO, USA) solution in methanol was applied with a pipette over individual colonies. As a positive control, a few drops of 0.1% (w/v) L‐ascorbic acid (Cat# A7631, Sigma‐Aldrich) were placed on a separate filter. The response of each isolate to DPPH was recorded within 3 min of DPPH application; a positive response was recorded if a white‐yellow halo appeared around individual colonies within 1 min, a weak positive response was assigned to strains that had a halo form between 1 and 3 min after DPPH application, and a negative response was listed for strains that failed to form a halo (Fig. 3). Approximately 700 isolates were screened using the qualitative DPPH assay.
Fig. 3.

DPPH is a stable free radical that is purple in its oxidized state. When reduced by an antioxidant, a white‐yellow halo will appear around individual bacteria colonies (A), this was qualitatively deemed a positive response. Isolates that did not have a halo around colonies within 3 min of DPPH application were deemed negative (B).
Quantitative free radical scavenging assay
To quantitatively assess the FRS ability, select isolates were grown in R2A broth (Table S5). Volumes of 50 ml of autoclaved medium were added to sterile 250 ml Erlenmeyer flasks, and each flask was inoculated with an isolate colony grown from a R2A plate culture. Cultures were grown with shaking (150 rpm; Ratek orbital incubator) at 37˚C for 48 h. Sterile uninoculated R2A was used as a control. A minimum of three replicate cultures were grown per isolate. After 48 h, the optical density of each culture was measured at 600 nm (OD600, CLARIOstar PLUS, BMG Labtech, Mornington, VIC, Australia), and the cultures (including negative medium controls) were centrifuged at 3000 g at 4˚C for 30 min (Allegra X‐12R) to pellet the bacterial cells. Supernatants were collected, frozen at −80˚C, freeze dried (Alpha 1‐4 LDplus, Martin Christ) and stored under inert gas in a dark, dry environment until analysis. Antioxidants were extracted from the freeze‐dried supernatant by resuspending at 50 mg ml−1 in 100% methanol, sonicating (Branson 2510) for 5 min and then centrifuging at 3000 g at 4˚C for 5 min. Quantitative DPPH assays were run by creating a 1:1 solution of 0.2 mM DPPH in methanol and CFS extract to a final volume of 1 ml, vortexing, and incubation in the dark at room temperature for 30 min. Samples were then vortexed briefly, and three 300 µl replicates for each sample were transferred into a well of a 96‐well plate. Decolorization of DPPH was determined by measuring the decrease in absorbance at 517 nm (Enspire 2300 plate reader, PerkinElmer, Waltham, MA, USA), and the FRS activity was calculated according to the formula, % DPPH scavenging activity = (Control – Sample)/Control × 100, where Control is the absorbance of the DPPH control (1:1 0.2 mM DPPH:methanol) and Sample is the absorbance of CFS extract in DPPH. All samples were measured against a 100% methanol blank. Positive controls consisting of 0.01–0.001% (w/v) L‐ascorbic acid were run on each 96‐well plate. FRS activity ranged from 0–90%.
Catalase assay
The pelleted cells from above were resuspended in 4.5 ml fRSS and 500 µl 3% (w/v) H2O2 (Rosado et al., 2018). If bubbles appeared, the organism was considered catalase positive. If there were no bubbles, the organism was classified as catalase negative.
Inhibition testing
Each paired set of high and low FRS strains were inoculated crosswise along the middle of R2A plates to test for antagonism. Plates were kept at 26°C and monitored daily for up to 7 days for antagonistic activity by documenting the presence or absence of both inoculated isolates and if there was a zone of inhibition between them.
Bacterial growth curves
Diluted cultures for each isolate were established by resuspending one isolate colony grown from a R2A plate culture in 500 µl of autoclaved R2A broth (Table S5). Volumes of 2 ml of autoclaved R2A broth were then added to each well of two sterile 24‐well plates. Three replicate randomly distributed wells were inoculated with 100 µl of the respective diluted cultures. There were six replicates of sterile uninoculated R2A blanks per plate. Bacterial growth curves were documented for each of the twelve selected isolates at 37°C over 56 h. Mixing was set to 300 rpm for the duration of the growth curve and OD600 measurements were taken every 10 min (CLARIOstar PLUS, BMG Labtech, Mornington, VIC, Australia). For each isolate, the growth curves for the triplicate cultures were averaged and blank corrected. Results were plotted in the MARS Data Analysis Software with OD600 values shown over time.
Phylogenetic analysis
All partial 16S rRNA gene sequences (842) were aligned with reference sequences (72) of closely related organisms using Geneious prime 2019.1.2 (https://www.geneious.com). This alignment was used to construct a neighbor‐joining phylogenetic tree using the Jukes‐Cantor method. Maximum‐likelihood dendrograms were generated with bootstrap values of 1000.
Whole genome sequence analysis
High FRS isolates along with conspecific or congeneric low FRS isolates were selected for genome sequencing; in total, six pairs of isolates were sequenced. Genomic DNA was isolated from a single colony using a JANUS Chemagic Workstation and Chemagic Viral DNA/RNA kit (PerkinElmer). Libraries were prepared with the Nextera XT DNA sample preparation kit (Illumina, San Diego, CA, USA). Readsets were produced using the Illumina sequencing platform (Instrument: Illumina NextSeq 500, 150 base, paired‐end) and the whole genome shotgun (WGS) method. Read depth coverage was approximately 100 times assuming a genome size of 4 M bases.
Illumina readsets for each isolate were assembled using Skesa (Souvorov et al., 2018), and the draft genome sequence was annotated using Prokka (Seemann, 2014). A genome sequence–based taxonomic classification for each isolate was determined using Kraken2 (Wood and Salzberg, 2014) with the Genome Taxonomy Database (GTDB; Parks et al., 2020) as the curated genomic data source. Classification was primarily based on the genome sequence of related isolates (within the relevant species where possible), which were obtained from GenBank. In situations where genomes of taxonomically relevant strains were available, a species‐level classification was possible. Where available, closed genome sequences from GenBank were used for comparative genomics analysis. For each of the six pairs of isolates, core genome (i.e. genes shared between the isolate pair) comparisons were performed, as implemented in Nullarbor (https://github.com/tseemann/nullarbor), with phylogenies inferred using core SNP differences. Genes for DMSP synthesis and degradation, vitamin B12 synthesis, and catalase were identified from the annotated genome sequence (GFF format) produced by Prokka; specific genes were identified by both name and Refseq accession number.
16S rRNA gene copy number estimation
The 16S rRNA gene copy number of the 12 draft genomes was predicted by the 16Stimator pipeline (Perisin et al., 2016). Briefly, all the 12 genomic assemblies were submitted to the rapid annotations using the subsystems technology (RAST) server (Brettin et al., 2015), and the positions of 16S rRNA and a set of single‐copied housekeeping genes (Table S6) were extracted from the RAST annotations. The clean read sets were mapped back to the corresponding genomic assemblies by Bowtie 2 (Langmead and Salzberg, 2012) to determine the read depth of each position. Finally, the 16S copy number of each isolate was calculated by dividing the median depth of 16S gene by the median depth of the single‐copied housekeeping genes after the read depths were calibrated by the model parameters provided by 16Stimator.
Statistical analysis
CFU counts were analyzed in R (v3.6.2, R Core Team, 2019) by first checking the assumptions of equal variance and homogeneity. An analysis of variance test was used to detect differences in the mean number of bacterial colonies from each anemone genotype by solid growth media (R2A or MA). A one‐way analysis of variance (one‐way ANOVA; Chambers and Hastie, 1992) was used to determine if there were significant differences between FRS abilities of selected high FRS, low FRS, and media controls, and pairwise comparisons were performed using Tukey’s HSD (Miller, 1981; Yandell, 2017). Each congeneric/conspecific pair and media control was tested to determine if data met the assumptions of normality and homoscedasticity. If either assumption was violated, the non‐parametic Kruskal‐Wallis rank sum test (Holland and Wolfe, 1973) was used with a Dunn test (Dunn, 1964) for multiple comparisons [P‐values adjusted with the Benjamini‐Hochberg method (Ferreira and Zwinderman, 2006)] with the R package “FSA” (Ogle, 2017).
Conflict of interest
The authors declare that they have no conflict of interest.
Author contributions
AMD, MvO and LLB conceived and designed the study. AMD performed the sampling and sample processing. AMD, DB and HL completed bioinformatic analyses. AMD wrote the first draft. All authors edited and approved the final manuscript.
Supporting information
Fig. S1. Growth curve of high FRS bacterial isolate MMSF00046 (Winogradskyella poriferorum) over 56 h at 300 rpm and 37°C.
Fig. S2. Growth curve of low FRS bacterial isolate MMSF00910 (Winogradskyella poriferorum) over 56 h at 300 rpm and 37°C.
Fig. S3. Growth curve of high FRS bacterial isolate MMSF00068 (Micococcus luteus) over 56 h at 300 rpm and 37°C.
Fig. S4. Growth curve of low FRS bacterial isolate MMSF00107 (Micococcus yunnanensis) over 56 h at 300 rpm and 37°C.
Fig. S5. Growth curve of high FRS bacterial isolate MMSF00132 (Labrenzia aggregata) over 56 h at 300 rpm and 37°C.
Fig. S6. Growth curve of low FRS bacterial isolate MMSF00249 (Labrenzia aggregata) over 56 h at 300 rpm and 37°C.
Fig. S7. Growth curve of high FRS bacterial isolate MMSF00958 (Alteromonas macleodii) over 56 h at 300 rpm and 37°C.
Fig. S8. Growth curve of low FRS bacterial isolate MMSF00257 (Alteromonas macleodii) over 56 h at 300 rpm and 37°C.
Fig. S9. Growth curve of high FRS bacterial isolate MMSF01163 (Alteromonas oceani) over 56 h at 300 rpm and 37°C.
Fig. S10. Growth curve of low FRS bacterial isolate MMSF00404 (Alteromonas oceani) over 56 h at 300 rpm and 37°C.
Fig. S11. Growth curve of high FRS bacterial isolate MMSF01190 (Marinobacter salsuginis) over 56 h at 300 rpm and 37°C.
Fig. S12. Growth curve of low FRS bacterial isolate MMSF00964 (Marinobacter salsuginis) over 56 h at 300 rpm and 37°C.
Table S1. Isolate Genome Sequence Data Summary. Strains are presented as high FRS (grey) followed by low FRS (white). 16S rRNA gene presumptive identity is derived from the NCBI classification of near‐complete 16S rRNA gene sequences. *We were unable to determine the 16S rRNA copy number of isolate MMSF00068.
Table S2. Pairwise comparison of the genome sequences between the pairs of isolates.
Table S3. Search outcomes for genes of interest.
Table S4. Summary of vitamin B12 biosynthesis pathway genes. A “+” indicates the presence of the gene in the respective isolate, whereas a “–” represents the absence of that gene. Genes in red were not found in any isolate.
Table S5. Composition of R2A broth adjusted to suit marine bacteria. Final pH = 7.2 +/‐ 0.2 at 26 °C. R2A broth was made by suspending 43.12 g of combined reagents in 1 l of MilliQ water, dissolving the medium completely, and sterilization by autoclaving at 121˚C for 15 min.
Table S6. Single‐copy housekeeping genes extracted from the RAST annotations.
Acknowledgements
This research was supported by the Australian Research Council Discovery Project grant DP160101468 (to MJHvO and LLB). MJHvO acknowledges Australian Research Council Laureate Fellowship FL180100036. We are grateful to Dr. Leon Hartman, Giulia Holland and Shona Elliot‐Kerr for their contributions in the preliminary culturing and screening of anemone‐associated bacteria. Dr. Gayle Philip contributed with bacterial whole genome sequence analysis, and Dr. Leon Hartman assisted with figure designs and reviewed the manuscript. Xavier Smith assisted with bacterial inhibition tests. Whole genome sequencing was organized by Dr. Glen Carter at the Peter Doherty Institute, Melbourne, Australia.
Microb. Biotechnol. (2021) 14(5), 2025–2040
Funding information
This research was supported by the Australian Research Council Discovery Project grant DP160101468 (to MJHvO and LLB). MJHvO acknowledges Australian Research Council Laureate Fellowship FL180100036.
Data availability statement
WGS raw reads are freely available in the Sequence Read Archive under BioProject PRJNA574193; the complete data set is listed in Table S1.
References
- Abd El‐Rhman A.M., Khattab Y,A.E., and Shalaby A.M.E. (2009) Micrococcus luteus and pseudomonas species as probiotics for promoting the growth performance and health of nile tilapia, oreochromis niloticus. Fish Shellfish Immunol 27: 175–180. 10.1016/j.fsi.2009.03.020. [DOI] [PubMed] [Google Scholar]
- Agostini, S., Suzuki, Y., Casareto, B.E., Nakano, Y., Hidaka, M., and Badrun, N. (2009) Coral symbiotic complex: Hypothesis through vitamin B12 for a new evaluation. Galaxea 11: 1–11. [Google Scholar]
- Ahmed, H.I., Herrera Sarrias, M., Liew, Y.J., and Aranda, M. (2019) Long‐term temperature stress in the coral model Aiptasia supports the “Anna Karenina principle” for bacterial microbiomes. Front Microbiol 10: 975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alagely, A., Krediet, C.J., Ritchie, K.B., and Teplitski, M. (2011) Signaling‐mediated cross‐talk modulates swarming and biofilm formation in a coral pathogen Serratia marcescens . ISME J 5: 1609–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alder, J., Arthurton, R., Ash, N., Diop, S., Heileman, S., Huber, M., et al. (2006). Brown, C., Corcoran, E., Herkenrath, P., and Thonell, J. (eds). Marine and coastal ecosystems and human well‐being: a synthesis report based on the findings of the Millennium Ecosystem Assessment. Nairobi, Kenya: United Nations Environment Programme, https://www.millenniumassessment.org/en/Articlee27e.html?id=76. [Google Scholar]
- Aro, E., Virgin, I., and Andersson, B. (1993) Photoinhibition of photosystem II. Inactivation, protein damage and turnover. Biochim Biophys Acta Bioenerg 1143: 113–134. [DOI] [PubMed] [Google Scholar]
- Asker, D., Beppu, T., and Ueda, K. (2007) Mesoflavibacter zeaxanthinifaciens gen. nov., sp. nov., a novel zeaxanthin‐producing marine bacterium of the family Flavobacteriaceae. Syst Appl Microbiol 30: 291–296. [DOI] [PubMed] [Google Scholar]
- Biebl H., Pukall R., Lünsdorf H., Schulz S., Allgaier M., Tindall B.J., and Wagner‐Döbler I. (2007) Description of labrenzia alexandrii gen. nov., sp. nov., a novel alphaproteobacterium containing bacteriochlorophyll a, and a proposal for reclassification of stappia aggregata as labrenzia aggregata comb. nov., of stappia marina as labrenzia marina comb. nov. and of stappia alba as labrenzia alba comb. nov., and emended descriptions of the genera pannonibacter, stappia and roseibium, and of the species roseibium denhamense and roseibium hamelinense. Int J Syst Evol 57: 1095–1107. 10.1099/ijs.0.64821-0. [DOI] [PubMed] [Google Scholar]
- Binsarhan, M. (2016) Comparative analysis and culturing of the microbial community of aiptasia pallida, a sea anemone model for coral biology. Doctoral dissertation.
- Blackall, L.L., Dungan, A.M., Hartman, L.M., and van Oppen, M.J.H. (2020) Probiotics for corals. Microbiol Aust 41: 100–104. [Google Scholar]
- Blackall, L.L., Wilson, B., and van Oppen, M.J.H. (2015) Coral‐the world's most diverse symbiotic ecosystem. Mol Ecol 24: 5330–5347. [DOI] [PubMed] [Google Scholar]
- Brettin, T., Davis, J.J., Disz, T., Edwards, R.A., Gerdes, S., Olsen, G.J., et al. (2015) RASTtk: a modular and extensible implementation of the RAST algorithm for building custom annotation pipelines and annotating batches of genomes. Sci Rep 5: 8365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, T., Otero, C., Grajales, A., Rodriguez, E., and Rodriguez‐Lanetty, M. (2017) Worldwide exploration of the microbiome harbored by the cnidarian model, Exaiptasia pallida (Agassiz in Verrill, 1864) indicates a lack of bacterial association specificity at a lower taxonomic rank. PeerJ 5: e3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabiscol, E., Tamarit, J., and Ros, J. (2000) Oxidative stress in bacteria and protein damage by reactive oxygen species. Int Microbiol 3: 3–8. [PubMed] [Google Scholar]
- Ceh J., van Keulen M., and Bourne D.G. (2013) Intergenerational transfer of specific bacteria in corals and possible implications for offspring fitness. Microb Ecol 65: 227–231. 10.1007/s00248-012-0105-z. [DOI] [PubMed] [Google Scholar]
- Cesar, H., Burke, L., and Pet‐Soede, L. (2003) The economics of worldwide coral reef degradation. report by Cesar Environmental Economics Consulting (CEEC).
- Chambers, J.M., and Hastie, T.J. (1992) Statistical models in S. Pacific Grove, CA: Wadsworth & Brooks/Cole Advanced Books & Software. [Google Scholar]
- Chiu J.M.Y., Li S., Li A., Po B., Zhang R., Shin P.K.S., and Qiu J.W. (2012) Bacteria associated with skeletal tissue growth anomalies in the coral platygyra carnosus. FEMS Microbiol Ecol 79: 380–391. 10.1111/j.1574-6941.2011.01225.x. [DOI] [PubMed] [Google Scholar]
- Croft, M.T., Lawrence, A.D., Raux‐Deery, E., Warren, M.J., and Smith, A.G. (2005) Algae acquire vitamin B12 through a symbiotic relationship with bacteria. Nature 438: 90. [DOI] [PubMed] [Google Scholar]
- Cunning, R., and Baker, A.C. (2012) Excess algal symbionts increase the susceptibility of reef corals to bleaching. Nat Clim Chang 3: 259–262. [Google Scholar]
- Curson, A.R.J., Liu, J.i., Bermejo Martínez, A., Green, R.T., Chan, Y., Carrión, O., et al. (2017) Dimethylsulfoniopropionate biosynthesis in marine bacteria and identification of the key gene in this process. Nat Microbiol 2: 17009. [DOI] [PubMed] [Google Scholar]
- D Ainsworth, T., Krause, L., Bridge, T., Torda, G., Raina, J.‐B., Zakrzewski, M., et al. (2015) The coral core microbiome identifies rare bacterial taxa as ubiquitous endosymbionts. ISME J 9: 2261–2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damjanovic, K., Blackall, L.L., Webster, N.S., and van Oppen, M.J.H. (2017) The contribution of microbial biotechnology to mitigating coral reef degradation. Microb Biotechnol 10: 1236–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damjanovic, K., van Oppen, M.J.H. , Menéndez, P., and Blackall, L.L. (2019) Experimental inoculation of coral recruits with marine bacteria indicates scope for microbiome manipulation in Acropora tenuis and Platygyra daedalea . Front Microbiol 10: 1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot, R., Brander, L., van der Ploeg, S., Costanza, R., Bernard, F., Braat, L., et al. (2012) Global estimates of the value of ecosystems and their services in monetary units. Ecosyst Serv 1: 50–61. [Google Scholar]
- Devlin‐Durante, M.k., Miller, M.w., Precht, W.f., Baums, I.b., Carne, L., Smith, T.B., et al. (2016) How old are you? Genet age estimates in a clonal animal. Mol Ecol 25: 5628–5646. [DOI] [PubMed] [Google Scholar]
- Dungan A.M., Hartman L.M., Tortorelli G., Belderok R., Lamb A.M., Pisan L., et al. (2020) Exaiptasia diaphana from the great barrier reef: a valuable resource for coral symbiosis research. Symbiosis 80: 195–206. 10.1007/s13199-020-00665-0. [DOI] [Google Scholar]
- Dungan, A.M., van Oppen, M.J.H. , and Blackall, L.L. (2021) Short‐term exposure to sterile seawater reduces bacterial community diversity in the sea anemone, Exaiptasia diaphana . Front Mar Sci 7: 599314. [Google Scholar]
- Dunn, O.J. (1964) Multiple comparisons using rank sums. Technometrics 6: 241–252. [Google Scholar]
- Epstein, H.E., Smith, H.A., Torda, G., and van Oppen, M.J.H. (2019) Microbiome engineering: enhancing climate resilience in corals. Front Ecol Environ 17: 100–108. [Google Scholar]
- Epstein, H.E., Torda, G., and van Oppen, M.J.H. (2019) Relative stability of the Pocillopora acuta microbiome throughout a thermal stress event. Coral Reefs 38: 373–386. [Google Scholar]
- Farooq, M., Rehman, A., Wahid, A., and Siddique, K.H.M. (2016) Photosynthesis under Heat Stress. In Handbook of Photosynthesis Pessarakli, M. (ed). CRC Press Taylor & Francis Group, pp. 697–701. [Google Scholar]
- Ferreira, J.A., and Zwinderman, A.H. (2006) On the Benjamini‐Hochberg method. Ann Statist 34: 1827–1849. [Google Scholar]
- Fiedor, J., and Burda, K. (2014) Potential role of carotenoids as antioxidants in human health and disease. Nutrients 6: 466–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedor, J., Fiedor, L., Haeßner, R., and Scheer, H. (2005) Cyclic endoperoxides of β‐carotene, potential pro‐oxidants, as products of chemical quenching of singlet oxygen. Biochim Biophys Acta Bioenerg 1709: 1–4. [DOI] [PubMed] [Google Scholar]
- Fisher, R., O’Leary, R.A., Low‐Choy, S., Mengersen, K., Knowlton, N., Brainard, R.E., and Caley, M.J. (2015) Species richness on coral reefs and the pursuit of convergent global estimates. Curr Biol 25: 500–505. [DOI] [PubMed] [Google Scholar]
- Flórez, L.V., Biedermann, P.H.W., Engl, T., and Kaltenpoth, M. (2015) Defensive symbioses of animals with prokaryotic and eukaryotic microorganisms. Nat Prod Rep 32: 904–936. [DOI] [PubMed] [Google Scholar]
- Franco A., Busse H.J., Schubert P., Wilke T., Kämpfer P., and Glaeser S.P. (2018) Winogradskyella pocilloporae sp. nov. isolated from healthy tissue of the coral pocillopora damicornis. Int J Syst Evol 68: 1689–1696. 10.1099/ijsem.0.002731. [DOI] [PubMed] [Google Scholar]
- Gammone, M., Riccioni, G., and D'Orazio, N. (2015) Marine carotenoids against oxidative stress: effects on human health. Mar Drugs 13: 6226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert, J.A., Hill, R., Doblin, M.A., and Ralph, P.J. (2012) Microbial consortia increase thermal tolerance of corals. Mar Biol 159: 1763–1771. [Google Scholar]
- Glynn, P.W. (1996) Coral reef bleaching: facts, hypotheses and implications. Glob Chang Biol 2: 495–509. [Google Scholar]
- Gonzaga, A., López‐Pérez, M., Martin‐Cuadrado, A., Ghai, R., and Rodriguez‐Valera, F. (2012) Complete Genome Sequence of the Copiotrophic Marine Bacterium Alteromonas macleodii Strain ATCC 27126. J Bacteriol 194: 6998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hai N.V. (2015) The use of probiotics in aquaculture. J App Microbiol 119: 917–935. 10.1111/jam.12886. [DOI] [PubMed] [Google Scholar]
- Hansel C.M., Diaz J.M., Plummer S. (2019) Tight regulation of extracellular superoxide points to Its vital role in the physiology of the globally relevant roseobacter clade. mBio 10: 10.1128/mbio.02668-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansel, C.M., and Diaz, J.M. (2021) Production of extracellular reactive oxygen species by marine biota. Ann Rev Mar Sci 13: 177–200. [DOI] [PubMed] [Google Scholar]
- Hartman, L.M., van Oppen, M.J.H. , and Blackall, L.L. (2020) The effect of thermal stress on the bacterial microbiome of Exaiptasia diaphana . Microorganisms 8: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatton, A.D., Shenoy, D.M., Hart, M.C., Mogg, A., and Green, D.H. (2012) Metabolism of DMSP, DMS and DMSO by the cultivable bacterial community associated with the DMSP‐producing dinoflagellate Scrippsiella trochoidea . Biogeochemistry 110: 131–146. [Google Scholar]
- Haryanti H., Sugama K., Tsumura S., and Nishijima R. (2017) Enhance production of black tiger shrimp penaeus monodon postlarvae by probiotic bacterium alteromonas sp.. Indones Fish Res J 7: 1. 10.15578/ifrj.7.1.2001.1-6. [DOI] [Google Scholar]
- Herrera, M., Ziegler, M., Voolstra, C.R., and Aranda, M. (2017) Laboratory‐cultured strains of the sea Anemone Exaiptasia reveal distinct bacterial communities. Front Mar Science 4: 115. [Google Scholar]
- Hoegh‐Guldberg, O., Jacob, D., Taylor, M., Guillén Bolaños, T., Bindi, M., Brown, S., et al. (2019) The human imperative of stabilizing global climate change at 1.5° C. Science 365: eaaw6974. [DOI] [PubMed] [Google Scholar]
- Holland, M., and Wolfe, D. (1973) Nonparametric statistical methods. New York, NY, USA: John Wiley & Sons. [Google Scholar]
- Hughes, T.P., Anderson, K.D., Connolly, S.R., Heron, S.F., Kerry, J.T., Lough, J.M., et al. (2018) Spatial and temporal patterns of mass bleaching of corals in the Anthropocene. Science 359: 80–83. [DOI] [PubMed] [Google Scholar]
- Hughes, T.P., Kerry, J.T., Álvarez‐Noriega, M., Álvarez‐Romero, J.G., Anderson, K.D., Baird, A.H., et al. (2017) Global warming and recurrent mass bleaching of corals. Nature 543: 373–377. [DOI] [PubMed] [Google Scholar]
- Imlay, J.A. (2018) Where in the world do bacteria experience oxidative stress? Environ Microbiol 21: 521–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg C.A., Piceno Y.M., Tom L.M., DeSantis T.Z., Gray M.A., Zawada D.G., and Andersen G.L. (2013) Comparing bacterial community composition between healthy and white plague‐Like disease states in orbicella annularis using PhyloChip™ G3 microarrays. PLoS ONE 8: e79801 10.1371/journal.pone.0079801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesarcodi‐Watson A., Kaspar H., Lategan M.J., and Gibson L. (2010) Alteromonas macleodii 0444 and neptunomonas sp. 0536, two novel probiotics for hatchery‐reared greenshell™ mussel larvae, perna canaliculus. Aquaculture 309: 49–55. 10.1016/j.aquaculture.2010.09.019. [DOI] [Google Scholar]
- Krueger, T., Hawkins, T.D., Becker, S., Pontasch, S., Dove, S., Hoegh‐Guldberg, O., et al. (2015) Differential coral bleaching—Contrasting the activity and response of enzymatic antioxidants in symbiotic partners under thermal stress. Comp Biochem Physiol A Mol Integr Physiol 190: 15–25. [DOI] [PubMed] [Google Scholar]
- Lane, D.J. (1991) 16S/23S rRNA sequencing. In Nucleic Acid Techniques in Bacterial Systematics. New York, NY, USA: John Wiley & Sons, pp. 115–175. [Google Scholar]
- Lane, D.J., Pace, B., Olsen, G.J., Stahl, D.A., Sogin, M.L., and Pace, N.R. (1985) Rapid determination of 16S ribosomal RNA sequences for phylogenetic analyses. Proc Natl Acad Sci USA 82: 6955–6959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead, B., and Salzberg, S.L. (2012) Fast gapped‐read alignment with Bowtie 2. Nat Methods 9: 357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau S.C.K., Tsoi M.M.Y., Li X., Plakhotnikova I., Dobretsov S., Lau K.W.K., et al. (2005) Winogradskyella poriferorum sp. nov., a novel member of the family flavobacteriaceae isolated from a sponge in the Bahamas. Int J Syst Evol 55: 1589–1592. 10.1099/ijs.0.63661-0. [DOI] [PubMed] [Google Scholar]
- Lesser, M.P. (2004) Experimental biology of coral reef ecosystems. J Exp Mar Biol Ecol 300: 217–252. [Google Scholar]
- Lesser, M.P., and Farrell, J.H. (2004) Exposure to solar radiation increases damage to both host tissues and algal symbionts of corals during thermal stress. Coral Reefs 23: 367–377. [Google Scholar]
- Lesser, M.P., Mazel, C.H., Gorbunov, M.Y., and Falowski, P.G. (2004) Discovery of symbiotic nitrogen‐fixing cyanobacteria in corals. Sci Rep 305: 997–1000. [DOI] [PubMed] [Google Scholar]
- Littman, R., Willis, B.L., and Bourne, D.G. (2011) Metagenomic analysis of the coral holobiont during a natural bleaching event on the Great Barrier Reef. Environ Microbiol Rep 3: 651–660. [DOI] [PubMed] [Google Scholar]
- Littman R.A., Willis B.L., Pfeffer C., and Bourne D.G. (2009) Diversities of coral‐associated bacteria differ with location, but not species, for three acroporid corals on the Great Barrier Reef. FEMS Microbiol Ecol 68: 152–163. 10.1111/j.1574-6941.2009.00666.x. [DOI] [PubMed] [Google Scholar]
- Maire J., Girvan S.K., Barkla S.E., Perez‐Gonzalez A., Suggett D.J., Blackall L.L., and van Oppen M.J.H. (2021) Intracellular bacteria are common and taxonomically diverse in cultured and in hospite algal endosymbionts of coral reefs. ISME J 10.1038/s41396-021-00902-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancuso, M., Rappazzo, A., Genovese, M., El Hady, M., Ghonimy, A., Ismail, M., et al. (2015) In vitro selection of bacteria and isolation of probionts from farmed sparus aurata with potential for use as probiotics. Int J Anim Biol 1: 93–98. [Google Scholar]
- Mathur, S., Agrawal, D., and Jajoo, A. (2014) Photosynthesis: response to high temperature stress. J Photochem Photobiol B 137: 116–126. [DOI] [PubMed] [Google Scholar]
- Matthews, J.L., Raina, J.‐B., Kahlke, T., Seymour, J.R., van Oppen, M.J.H. , and Suggett, D.J. (2020) Symbiodiniaceae‐bacteria interactions: rethinking metabolite exchange in reef‐building corals as multi‐partner metabolic networks. Environ Microbiol 22: 1675–1687. [DOI] [PubMed] [Google Scholar]
- Miller, R.G. (1981) Normal univariate techniques. In: Simultaneous Statistical Inference. Springer, pp. 37–108. [Google Scholar]
- Mohana, D., Thippeswamy, S., and Abhishe, R. (2013) Antioxidant, antibacterial, and ultraviolet‐protective properties of carotenoids isolated from Micrococcus spp. Radiat Protect Environ 36: 168–174. [Google Scholar]
- Motone, K., Takagi, T., Aburaya, S., Miura, N., Aoki, W., and Ueda, M. (2020) A zeaxanthin‐producing bacterium isolated from the algal phycosphere protects coral endosymbionts from environmental stress. MBio 11: e01019‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouchka, M.E., Hewson, I., and Harvell, C.D. (2010) Coral‐associated bacterial assemblages: current knowledge and the potential for climate‐driven impacts. Integr Comp Biol 50: 662–674. [DOI] [PubMed] [Google Scholar]
- Muscatine, L., and Porter, J.W. (1977) Reef corals: mutualistic symbioses adapted to nutrient‐poor environments. Bioscience 27: 454–460. [Google Scholar]
- Muyzer, G., De Waal, E.C., and Uitterlinden, A.G. (1993) Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction‐amplified genes coding for 16S rRNA. Appl Environ Microbiol 59: 695–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen, D.A., Petrou, K., and Gates, R.D. (2018) Coral bleaching from a single cell perspective. ISME J 12: 1558–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama, Y., Allakhverdiev, S.I., and Murata, N. (2006) A new paradigm for the action of reactive oxygen species in the photoinhibition of photosystem II. Biochim Biophys Acta Bioenerg 1757: 742–749. [DOI] [PubMed] [Google Scholar]
- Ogle, D.H. (2017) FSA: fisheries stock analysis. R package version 0.8 17: 636. [Google Scholar]
- van Oppen, M.J.H. , and Blackall, L.L. (2019) Coral microbiome dynamics, functions and design in a changing world. Nat Rev Microbiol 17: 557–567. [DOI] [PubMed] [Google Scholar]
- Oppen, M.J.H., Gates, R.D., Blackall, L.L., Cantin, N., Chakravarti, L.J., Chan, W.Y., et al. (2017) Shifting paradigms in restoration of the world's coral reefs. Glob Chang Biol 23: 3437–3448. [DOI] [PubMed] [Google Scholar]
- van Oppen, M.J.H. , Oliver, J.K., Putnam, H.M., and Gates, R.D. (2015) Building coral reef resilience through assisted evolution. Proc Natl Acad Sci USA 112: 2307–2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osman, H.A.M., Ibrahim, T.B., Soliman, W., and Aboud, O.(2010) Improvement growth and immune status using a potential probiotic bacteria micrococcus species among culured oreochromis niloticus. N Y Sci J 3: 5–11. [Google Scholar]
- von Ossowski, I. , Mulvey, M.R., Leco, P.A., Borys, A., and Loewen, P.C. (1991) Nucleotide sequence of Escherichia coli katE, which encodes catalase HPII. J Bacteriol 173: 514–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks, D.H., Chuvochina, M., Chaumeil, P., Rinke, C., Mussig, A.J., and Hugenholtz, P. (2020) A complete domain‐to‐species taxonomy for Bacteria and Archaea. Nat Biotechnol 38: 1079–1086. [DOI] [PubMed] [Google Scholar]
- Peixoto, R.S., Rosado, P.M., Leite, D.C., Rosado, A.S., and Bourne, D.G. (2017) Beneficial Microorganisms for Corals (BMC): proposed mechanisms for coral health and resilience. Front Microbiol 8: 341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peixoto, R.S., Sweet, M., Villela, H.D.M., Cardoso, P., Thomas, T., Voolstra, C.R., et al. (2021) Coral probiotics: premise, promise, prospects. Annu Rev Anim Biosci 9: 265–288. [DOI] [PubMed] [Google Scholar]
- Perisin, M., Vetter, M., Gilbert, J.A., and Bergelson, J. (2016) 16Stimator: statistical estimation of ribosomal gene copy numbers from draft genome assemblies. ISME J 10: 1020–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plovie, A. (2010) Comparison of bacterial communities associated with healthy and bleached Aiptasia pallida, a novel model organism for coral studies: implications and variation during bleaching. Biochemistry and Biotechnology, Liège University. [Google Scholar]
- Pootakham, W., Mhuantong, W., Yoocha, T., Putchim, L., Jomchai, N., Sonthirod, C., et al. (2019) Heat‐induced shift in coral microbiome reveals several members of the Rhodobacteraceae family as indicator species for thermal stress in Porites lutea . Microbiologyopen 8: e935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhu, S., Rekha, P.D., and Arun, A.B. (2014) Zeaxanthin biosynthesis by members of the genus Muricauda . Pol J Microbiol 63: 115–119. [PubMed] [Google Scholar]
- R Core Team (2019) A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2012. URL https://www.R‐project.org [Google Scholar]
- Radecker, N., Pogoreutz, C., Gegner, H.M., Cardenas, A., Roth, F., Bougoure, J., et al. (2021) Heat stress destabilizes symbiotic nutrient cycling in corals. Proc Natl Acad Sci USA 118: e2022653118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raina, J.B., Tapiolas, D.M., Willis, B.L., and Bourne, D.G. (2009) Coral‐associated bacteria and their role in the biogeochemical cycling of sulfur. Appl Environ Microbiol 75: 3492–3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raux, E., Schubert, H.L., and Warren, M.J. (2000) Biosynthesis of cobalamin (vitamin B12): a bacterial conundrum. Cell Mol Life Sci 57: 1880–1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riquelme C., Araya R., Vergara N., Rojas A., Guaita M., and Candia M. (1997) Potential probiotic strains in the culture of the Chilean scallop Argopecten purpuratus (Lamarck, 1819). Aquaculture 154: 17–26. 10.1016/s0044-8486(97)00043-4. [DOI] [Google Scholar]
- Robbins, S.J., Singleton, C.M., Chan, C.X., Messer, L.F., Geers, A.U., Ying, H., et al. (2019) A genomic view of the reef‐building coral Porites lutea and its microbial symbionts. Nat Microbiol 4: 2090–2100. [DOI] [PubMed] [Google Scholar]
- Rohwer, F., Seguritan, V., Azam, F., and Knowlton, N. (2002) Diversity and distribution of coral‐associated bacteria. Mar Ecol Prog Ser 243: 10. [Google Scholar]
- Rosado, P.M., Leite, D.C.A., Duarte, G.A.S., Chaloub, R.M., Jospin, G., Nunes da Rocha, U., et al. (2018) Marine probiotics: increasing coral resistance to bleaching through microbiome manipulation. ISME J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose, A.L., Webb, E.A., Waite, T.D., and Moffett, J.W. (2008) Measurement and implications of nonphotochemically generated superoxide in the equatorial Pacific Ocean. Environ Sci Technol 42: 2387–2393. [DOI] [PubMed] [Google Scholar]
- Röthig, T., Costa, R.M., Simona, F., Baumgarten, S., Torres, A.F., Radhakrishnan, A., et al. (2016) Distinct Bacterial Communities Associated with the Coral Model Aiptasia in Aposymbiotic and Symbiotic States with Symbiodinium . Front Mar Sci 3: 234. [Google Scholar]
- Röthig T., Yum L.K., Kremb S.G., Roik A., and Voolstra C.R. (2017) Microbial community composition of deep‐sea corals from the Red Sea provides insight into functional adaption to a unique environment. Sci Rep 7: 10.1038/srep44714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- dos Santos, F.A.H. , Duarte, G.A., Rachid, C.T., Chaloub, R.M., Calderon, E.N., Marangoni, L.F., et al. (2015) Impact of oil spills on coral reefs can be reduced by bioremediation using probiotic microbiota. Sci Rep 5: 18268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seemann, T. (2014) Prokka: rapid prokaryotic genome annotation. Bioinformatics 30: 2068–2069. [DOI] [PubMed] [Google Scholar]
- Sharp K.H., Distel D., and Paul V.J. (2012) Diversity and dynamics of bacterial communities in early life stages of the Caribbean coral Porites astreoides. ISME J 6: 790–801. 10.1038/ismej.2011.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shindo, K., Kikuta, K., Suzuki, A., Katsuta, A., Kasai, H., Yasumoto‐Hirose, M., et al. (2007) Rare carotenoids, (3R)‐saproxanthin and (3R,2′S)‐myxol, isolated from novel marine bacteria (Flavobacteriaceae) and their antioxidative activities. Appl Microbiol Biotechnol 74: 1350. [DOI] [PubMed] [Google Scholar]
- Smith, S.M. (2018) Complementarity in the Coral Holobiont: a genomic analysis of bacterial isolates of Orbicella faveolata and Symbiodinium spp. In Doctoral Dissertation. Penn State. [Google Scholar]
- Souvorov, A., Agarwala, R., and Lipman, D.J. (2018) SKESA: strategic k‐mer extension for scrupulous assemblies. Genome Biol 19: 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding, M.D., and Grenfell, A.M. (1997) New estimates of global and regional coral reef areas. Coral Reefs 16: 225–230. [Google Scholar]
- Stuart‐Smith, R.D., Brown, C.J., Ceccarelli, D.M., and Edgar, G.J. (2018) Ecosystem restructuring along the Great Barrier Reef following mass coral bleaching. Nature 560: 92–96. [DOI] [PubMed] [Google Scholar]
- Sunagawa, S., Woodley, C.M., and Medina, M. (2010) Threatened corals provide underexplored microbial habitats. PLoS One 5: e9554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunda, W., Kieber, D.J., Kiene, R.P., and Huntsman, S. (2002) An antioxidant function for DMSP and DMS in marine algae. Nature 418: 317–320. [DOI] [PubMed] [Google Scholar]
- Sutherland, K.M., Coe, A., Gast, R.J., Plummer, S., Suffridge, C.P., Diaz, J.M., et al. (2019) Extracellular superoxide production by key microbes in the global ocean. Limnol Oceanogr 64: 2679–2693. [Google Scholar]
- Szabó, M., Larkum, A.W.D., and Vass, I. (2020) A Review: The Role of Reactive Oxygen Species in Mass Coral Bleaching. In Photosynthesis in Algae: Biochemical and Physiological Mechanisms. Larkum, A.W.D., Grossmann, A.R., and Raven, J.A. (eds). Cham, Switzerland: Springer International Publishing, pp. 459–488. [Google Scholar]
- Takao, T., Kitatani, F., Watanabe, N., Yagi, A., and Sakata, K. (1994) A simple screening method for antioxidants and isolation of several antioxidants produced by marine bacteria from fish and shellfish. Biosci Biotechnol Biochem 58: 1780–1783. [Google Scholar]
- Taylor, W.I., and Achanzar, D. (1972) Catalase test as an aid to the identification of Enterobacteriaceae. Appl Microbiol 24: 58–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchernov, D., Gorbunov, M.Y., De Vargas, C., Yadav, S.N., Milligan, A.J., Häggblom, M., and Falkowski, P.G. (2004) Membrane lipids of symbiotic algae are diagnostic of sensitivity to thermal bleaching in corals. Proc Natl Acad Sci USA 101: 13531–13535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay, P., Grover, R., Maguer, J.‐F., Hoogenboom, M., and Ferrier‐Pagès, C. (2014) Carbon translocation from symbiont to host depends on irradiance and food availability in the tropical coral Stylophora pistillata. Coral Reefs 33: 1–13. [Google Scholar]
- Trubitsin, B.V., Mamedov, M.D., Semenov, A.Y., and Tikhonov, A.N. (2014) Interaction of ascorbate with photosystem I. Photosynth Res 122: 215–231. [DOI] [PubMed] [Google Scholar]
- Vega Thurber, R., Willner‐Hall, D., Rodriguez‐Mueller, B., Desnues, C., Edwards, R.A., Angly, F., et al. (2009) Metagenomic analysis of stressed coral holobionts. Environ Microbiol 11: 2148–2163. [DOI] [PubMed] [Google Scholar]
- Velho‐Pereira, S., Parvatkar, P., and Furtado, I.J. (2015) Evaluation of antioxidant producing potential of halophilic bacterial bionts from marine invertebrates. Indian Jf Pharmaceut Sci 77: 183–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venn, A.A., Loram, J.E., and Douglas, A.E. (2008) Photosynthetic symbioses in animals. J Exp Bot 59: 1069–1080. [DOI] [PubMed] [Google Scholar]
- Warner, M.E., Fitt, W.K., and Schmidt, G.W. (1999) Damage to photosystem II in symbiotic dinoflagellates: a determinant of coral bleaching. Proc Natl Acad Sci USA 96: 8007–8012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis, W.M. (2008) Cellular mechanisms of Cnidarian bleaching: stress causes the collapse of symbiosis. J Exp Biol 211: 3059–3066. [DOI] [PubMed] [Google Scholar]
- Wiedenmann, J., D’Angelo, C., Smith, E.G., Hunt, A.N., Legiret, F., Postle, A.D., and Achterberg, E.P. (2012) Nutrient enrichment can increase the susceptibility of reef corals to bleaching. Nat Clim Chang 3: 160. [Google Scholar]
- Wood, D.E., and Salzberg, S.L. (2014) Kraken: ultrafast metagenomic sequence classification using exact alignments. Genome Biol 15: R46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooldridge, S.A. (2013) Breakdown of the coral‐algae symbiosis: towards formalising a linkage between warm‐water bleaching thresholds and the growth rate of the intracellular zooxanthellae. Biogeosciences 10: 1647–1658. [Google Scholar]
- Work, T.M., and Aeby, G.S. (2014) Microbial aggregates within tissues infect a diversity of corals throughout the Indo‐Pacific. Mar Ecol Prog Ser 500: 1–9. [Google Scholar]
- Yandell, B.S. (2017) Practical Data Analysis for Designed Experiments. London, UK: Routledge. [Google Scholar]
- Yellowlees, D., Rees, T.A.V., and Leggat, W. (2008) Metabolic interactions between algal symbionts and invertebrate hosts. Plant Cell Environ 31: 679–694. [DOI] [PubMed] [Google Scholar]
- Zhang, T., Diaz, J.M., Brighi, C., Parsons, R.J., McNally, S., Apprill, A., and Hansel, C.M. (2016) Dark production of extracellular superoxide by the Coral Porites astreoides and representative symbionts. Front Mar Sci 3: 232. [Google Scholar]
- Ziegler, M., Seneca, F.O., Yum, L.K., Palumbi, S.R., and Voolstra, C.R. (2017) Bacterial community dynamics are linked to patterns of coral heat tolerance. Nat Commun 8: 14213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinser, E.R. (2018) Cross‐protection from hydrogen peroxide by helper microbes: the impacts on the cyanobacterium Prochlorococcus and other beneficiaries in marine communities. Environ Microbiol Rep 10: 399–411. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Growth curve of high FRS bacterial isolate MMSF00046 (Winogradskyella poriferorum) over 56 h at 300 rpm and 37°C.
Fig. S2. Growth curve of low FRS bacterial isolate MMSF00910 (Winogradskyella poriferorum) over 56 h at 300 rpm and 37°C.
Fig. S3. Growth curve of high FRS bacterial isolate MMSF00068 (Micococcus luteus) over 56 h at 300 rpm and 37°C.
Fig. S4. Growth curve of low FRS bacterial isolate MMSF00107 (Micococcus yunnanensis) over 56 h at 300 rpm and 37°C.
Fig. S5. Growth curve of high FRS bacterial isolate MMSF00132 (Labrenzia aggregata) over 56 h at 300 rpm and 37°C.
Fig. S6. Growth curve of low FRS bacterial isolate MMSF00249 (Labrenzia aggregata) over 56 h at 300 rpm and 37°C.
Fig. S7. Growth curve of high FRS bacterial isolate MMSF00958 (Alteromonas macleodii) over 56 h at 300 rpm and 37°C.
Fig. S8. Growth curve of low FRS bacterial isolate MMSF00257 (Alteromonas macleodii) over 56 h at 300 rpm and 37°C.
Fig. S9. Growth curve of high FRS bacterial isolate MMSF01163 (Alteromonas oceani) over 56 h at 300 rpm and 37°C.
Fig. S10. Growth curve of low FRS bacterial isolate MMSF00404 (Alteromonas oceani) over 56 h at 300 rpm and 37°C.
Fig. S11. Growth curve of high FRS bacterial isolate MMSF01190 (Marinobacter salsuginis) over 56 h at 300 rpm and 37°C.
Fig. S12. Growth curve of low FRS bacterial isolate MMSF00964 (Marinobacter salsuginis) over 56 h at 300 rpm and 37°C.
Table S1. Isolate Genome Sequence Data Summary. Strains are presented as high FRS (grey) followed by low FRS (white). 16S rRNA gene presumptive identity is derived from the NCBI classification of near‐complete 16S rRNA gene sequences. *We were unable to determine the 16S rRNA copy number of isolate MMSF00068.
Table S2. Pairwise comparison of the genome sequences between the pairs of isolates.
Table S3. Search outcomes for genes of interest.
Table S4. Summary of vitamin B12 biosynthesis pathway genes. A “+” indicates the presence of the gene in the respective isolate, whereas a “–” represents the absence of that gene. Genes in red were not found in any isolate.
Table S5. Composition of R2A broth adjusted to suit marine bacteria. Final pH = 7.2 +/‐ 0.2 at 26 °C. R2A broth was made by suspending 43.12 g of combined reagents in 1 l of MilliQ water, dissolving the medium completely, and sterilization by autoclaving at 121˚C for 15 min.
Table S6. Single‐copy housekeeping genes extracted from the RAST annotations.
Data Availability Statement
WGS raw reads are freely available in the Sequence Read Archive under BioProject PRJNA574193; the complete data set is listed in Table S1.


