Abstract
Background
The outbreak of COVID-19 has developed into a pandemic. Data are required that specifically address the psychological consequences in COVID-19 confirmed patients. This study mainly aimed to examine posttraumatic stress disorder (PTSD) symptoms and sleep quality among COVID-19 confirmed patients during hospitalization.
Methods
An observational study was conducted in two designated hospitals in Wuhan, China. Data were collected from 190 patients hospitalized with laboratory-confirmed COVID-19 infection between February 10, 2020 and March 13, 2020.
Results
The mean age of the 190 confirmed patients was 55.7 years (SD = 13.7), of which 96 (50.5%) were female and 88 (46.3%) had family members or acquaintances infected with COVID-19. Lymphocytopenia was presented in 62 (32.6%) patients and 25 (13.2%) patients showed oxygen desaturation. The prevalence of high PTSD symptoms was 22.6% among the 190 patients. The median time from symptom onset to first medical visit and hospitalization was 2 days (IQR, 1–5) and 16 days (IQR, 10–27), respectively. Patients’ PTSD symptoms were positively related to the time from symptom onset to first medical visit (r = 0.156, p < 0.05) and hospitalization (r = 0.181, p < 0.01). There were significant correlations between sleep quality and PTSD symptoms (r = 0.312–0.547, p < 0.01).
Conclusion
The prevalence of high PTSD symptoms was 22.6% among hospitalized COVID-19 patients. Early diagnosis and treatment of COVID-19 symptoms are beneficial to infected patients both physically and psychologically. With the recovery of physical symptoms, psychological intervention is desired to promote the trauma recovery in COVID-19 patients.
Keywords: COVID-19, patients, psychological, PTSD, sleep quality
Introduction
The coronavirus disease 2019 (COVID-19) caused by a novel coronavirus, now officially named as SARA-CoV-2,1 emerged at the end of 2019.2,3 Since then, COVID-19 has captured global attention due to its rapid spread around the world.4 Data published by the World Health Organization (WHO) on 11 March 2020 showed that the number of laboratory-confirmed COVID-19 cases had exceeded 110,000 across over 100 countries.5 The reality that COVID-19 has developed into a pandemic presented a serious threat to global public health.6 As one of the countries initially affected by COVID-19, large numbers of Chinese were negatively influenced both physically and psychologically during the early stage.7,8 To prevent and control the epidemic, China responded immediately by adopting and implementing a series of strong measures (eg, national lockdown), which has shown encouraging effects. The recovery rate had been rising steadily and over 90% COVID-19 patients were discharged from hospital by late March 2020. However, it should be noted that, in the early stage of the COVID-19 outbreak, much emphasis was placed on the biological, epidemiological, and clinical characteristics of COVID-19, whereas limited research has addressed the association between laboratory indicators and psychological consequences among COVID-19 patients.
Major infectious diseases are known to have adverse impacts on patients’ physical condition and mental health.9,10 Patients may develop negative psychological distress, such as anger, fear, anxiety, stigma, and depression due to the uncertainty about the infection.11–14 Clusters occurrence, community transmission, and subsequent isolated treatment may further aggravate these symptoms. For confirmed patients, being isolated with families and treated alone in hospital also increased their stress, anxiety, and loneliness. Without intervention, acute or chronic mental disorders were most probably developed, especially posttraumatic stress disorder (PTSD),15–17 a serious stress-related psychiatric disorder arising from a traumatic event.18 Moreover, being involved in a major infectious outbreak might be a traumatic experience.19 Accumulative evidence showed that individuals experiencing public health emergencies continuously suffered from varying degrees of stress-related disorders.20–22 Research on the psychological impact of SARS on survivors found that 10–18% of the sample reported PTSD-related symptoms, anxiety, and depression one month after they were discharged from hospital.23 Follow-up studies evaluating long-term psychiatric morbidities among SARS survivors demonstrated that over 40% respondents had experienced psychiatric disorders, and PTSD was the most common one.15,17 Apart from PTSD, long-term sleep disturbance was commonly observed among the post-SARS patients.24 Related review on the sleep problems during the COVID-19 pandemic demonstrated that sleep problems were prevalent among different populations, including the general and health-care populations, as well as COVID-19 patients.25 Previous studies also pointed out that sleep disturbances were a core characteristic of PTSD26 and highly prevalent in PTSD.27 Furthermore, a multidisciplinary observational cohort study assessing long-term sequelae in Ebola virus disease (EVD) survivors reported a positive relationship between survivors’ psychosocial distress and physical symptoms.28 Up to now, recent research has confirmed that COVID-19 patients have poorer mental health than general population.29 Therefore, it is important to provide early intervention to prevent potential adverse psychological responses in COVID-19 patients. Besides, investigating the association between laboratory indicators and psychological responses among COVID-19 patients may shed new light on the disease recovery and prognosis.
In this observational study, the laboratory indicators of concern included the minimum lymphocyte count and lowest oxygen saturation (SaO2). The severity of disease classification was investigated to divide patients into two subgroups: severe group and non-severe group. Demographic factors (gender and age), and having family, friends or acquaintances infected were taken into consideration, because they have been identified as predictors for PTSD among people exposed to major outbreak of epidemics in previous studies.30–32 Recent research showed that patients’ condition might get worse after the illness onset without treatment.33 It was assumed that the duration from symptom onset to first medical visit and hospitalization might impact patients’ psychological condition. Thus, hospital visit time after patients contracted COVID-19 was also investigated to further examine its impact on psychological responses. The principal objectives of the present study were threefold: (1) to evaluate the psychological responses in hospitalized COVID-19 patients, including PTSD symptoms and sleep quality; (2) to address the association between laboratory indicators and psychological responses in COVID-19 patients; (3) to examine associated factors for psychological responses in COVID-19 patients. Based on prior studies, we hypothesized that poorer psychological responses were related to worse laboratory indicators, and age, gender, having family, friends or acquaintances infected, as well as hospital visit time after patients contracted COVID-19 were associated with psychological responses among COVID-19 hospitalized patients.
Materials and Methods
Sample and Procedures
The study was conducted in two designated hospitals in Wuhan, Huoshenshan Hospital and Hubei Maternal and Child Health Hospital Guanggu District, between February 10, 2020 and March 13, 2020, a period when the COVID-19 pandemic was progressively under control in China. According to medical records from the two hospitals, there were 1597 patients admitted to Huoshenshan Hospital and more than 700 patients admitted to Hubei Maternal and Child Health Hospital Guanggu District by March 1, 2020. The sample size was calculated with α set as 0.05, β as 0.2, and the overall prevalence of PTSD estimated as 33%.34 A minimum of 180 patients were required in this study, among which 100 from Huoshenshan Hospital and 80 from Hubei Maternal and Child Health Hospital Guanggu District. Considering 80% response rate, 225 patients were planned to recruit. Patients met the following criteria were enrolled in this study: (1) admitted to hospital with laboratory-confirmed COVID-19 infection; (2) age≥18 years old; (3) clear state of consciousness; (4) normal ability of speech comprehension and expression. Exclusion criteria were unable to communicate effectively due to physical condition or refusing to participate in the study. The protocol for this study was reviewed and approved by the Ethics Committee of Naval Medical University. All patients provided written or verbal informed consent before participation.
Data Collection and Measures
Two clinicians and one psychiatrist were jointly responsible for data collection. Clinicians received professional training regarding related questionnaires before data collection, and the psychiatrist offered professional guidance during collection. Clinical data were extracted from electronic medical records of patients by clinicians, including the severity of disease classification, the minimum lymphocyte count and lowest SaO2. The severity of disease classification was performed based on the Clinical Classification in the Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia. Information about hospital visit of patients infected with COVID-19 was collected from patients on dates of symptom onset, first medical visit, and hospitalization. Then, a self-constructed questionnaire regarding demographic information (age and gender), presence of family members or acquaintances infected with COVID-19, and sleep quality was administered to patients. Finally, patients completed a self-report questionnaire, the PTSD Checklist for DSM-5 (PCL-5).
Items assessing sleep quality were selected from Pittsburgh sleep quality index (PSQI),35 a self-rated questionnaire developed to measure sleep quality and disturbances, including subjective sleep quality, difficulty falling asleep, frequent nocturnal or early morning awakening, and sleep duration. Patients were asked to rate their sleep quality on a scale from 0 to 3, with higher scores indicating worse sleep quality. The Chinese version of PSQI was demonstrated to be of high diagnostic sensitivity (98.3%) and specificity (90.2%).36 The Cronbach alpha for the four items in the current sample was 88.3%.
PCL-5 with 20-item was used to measure the level of PTSD symptoms.37 Each item is scored on a 5-point Likert scale ranging from 0 (not at all) to 4 (extremely), representing the degree to which an individual has been bothered by PTSD-related symptoms during the past month. The total score is the sum of 20 items with a range of 0 to 80. Meanwhile, the 20 items of PCL-5 could be classified into four symptom clusters: intrusive experience (Criterion B, item 1–5), persistent avoidance (Criterion C, item 6–7), negative alterations in cognitions and mood (Criterion D, item 8–14), and marked alterations in arousal and reactivity (Criterion E, item 15–20). Each item rated as 2 (moderately) or higher was regarded as a symptom endorsed, and symptomatic assessment criteria for a probable PTSD was at least 1 item in Criterion B, 1 item in Criterion C, 2 items in Criterion D, and 2 items in Criterion E, which was regarded as high level of PTSD symptoms in this study. The Chinese version of the PCL-5 was adapted by a two-stage process of translation (initial and back translation). It has been documented in substantial studies as a valid screening tool with reliable sensitivity and specificity.38–40 The Cronbach alpha for PCL-5 in the current sample was 93.7%.
Statistical Analysis
Patients recruited in this study were divided into severe cases group and non-severe cases group based on the severity of disease classification. Descriptive statistics were conducted with categorical variables presented as frequencies and percentage, and continuous variables presented as mean and standard deviation (SD) or median and interquartile range (IQR). The differences in the demographic and disease-related variables between the two groups were examined. If the data met normality, the t-test was used; otherwise, Mann–Whitney U-test was used. The influence of demographic factors and laboratory indicators on psychological responses was analyzed by one-way analysis of covariance (ANCOVA). When one variable was entered as an independent variable, the remaining four variables were entered as covariates. Spearman correlation analysis was carried out to determine the association between hospital visit time and psychological responses of patients. The value of p < 0.05 was taken as statistically significant. All analyses were performed with SPSS software, version 21.0.
Results
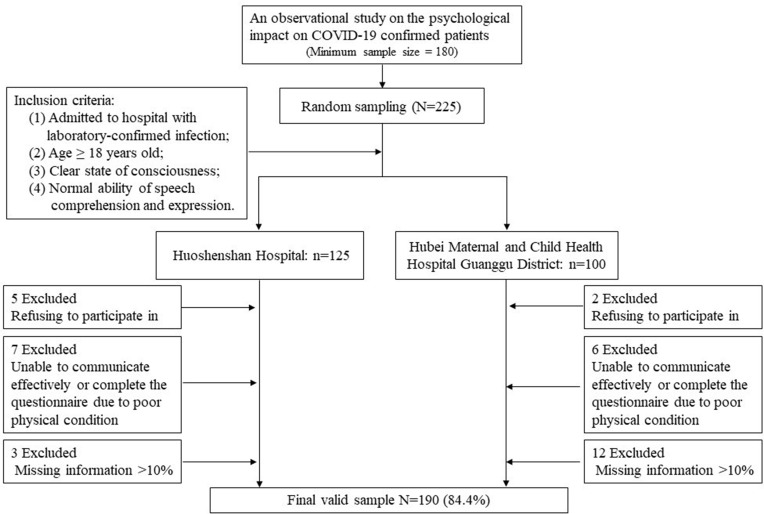
Figure 1 shows the flow chart of the enrollment process. Initially, a total of 225 COVID-19 patients were eligible for the study, among whom 7 were excluded as they refused to participate in the study and 13 were excluded due to extremely poor physical condition (eg, under mechanical ventilation). After data quality control, 15 patients were excluded because of missing information > 10%. Our final sample comprised 190 confirmed COVID-19 patients, with 32 patients in the severe group and 158 in the non-severe group.
Figure 1.
Enrollment of patients with COVID-19 from two centers.
Demographic and Disease-Related Characteristics of Patients
Table 1 summarizes the demographic and disease-related characteristics of patients. Among the 190 included patients, 96 (50.5%) were females; 33 (17.4%) aged 18–40 years, 69 (36.3%) were 41–60 years, 81 (42.6%) were more than 60 years old, and the mean age was 55.7 years (SD = 13.7); 88 (46.3%) had family members or acquaintances infected with COVID-19.
Table 1.
Demographic and Disease-Related Characteristics of Patients Confirmed with COVID-19
| Total (n=190) | Severe (n=32) | Non-Severe (n=158) | Z/x2/t | p | ||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |||
| Age (M, SD) a | 55.70 | 13.70 | 61.55 | 13.10 | 54.54 | 13.56 | 2.644 | 0.009 |
| 18–40 | 33 | 17.4 | 2 | 6.3 | 31 | 19.6 | ||
| 41–60 | 69 | 36.3 | 9 | 28.1 | 60 | 38.0 | ||
| ≥60 | 81 | 42.6 | 21 | 65.6 | 60 | 38.0 | ||
| Missing values | 7 | 3.7 | 0 | 0.000 | 7 | 4.4 | ||
| Gender b | ||||||||
| Male | 87 | 45.8 | 16 | 50.0 | 71 | 44.9 | 0.094 | 0.759 |
| Female | 96 | 50.5 | 16 | 50.0 | 80 | 50.6 | ||
| Missing values | 7 | 3.7 | 0 | 0.0 | 7 | 4.4 | ||
| Family members or acquaintances infected b | ||||||||
| No | 95 | 50.0 | 18 | 56.3 | 77 | 48.7 | 0.292 | 0.589 |
| Yes | 88 | 46.3 | 14 | 43.8 | 74 | 46.8 | ||
| Missing values | 7 | 3.7 | 0 | 0 | 7 | 4.4 | ||
| Symptom onset to, days a | Median | (IQR) | Median | (IQR) | Median | (IQR) | ||
| First Medical Visit | 2 | (1–5) | 3 | (1–11) | 2 | (0–5) | −1.625 | 0.104 |
| Hospitalization | 16 | (10–27) | 18.5 | (11.25–29.75) | 16 | (9–26) | −0.875 | 0.382 |
| Missing values | 9 | 0 | 8 | |||||
| Lymphocyte count, ×10^9/L b | 1.5 | (1.11–1.96) | 1.19 | (0.63–1.53) | 1.57 | (1.18–2.00) | −3.775 | <0.001 |
| ≥1.5 | 122 | 64.2 | 15 | 46.9 | 107 | 67.7 | ||
| <1.5 | 62 | 32.6 | 17 | 53.1 | 45 | 28.5 | ||
| Missing values | 6 | 3.2 | 0 | 0 | 6 | 3.8 | ||
| The lowest SaO2, % b | 96 | (95–98) | 93 | (90–96) | 96 | (96–98) | −5.048 | <0.001 |
| >93% | 164 | 86.3 | 13 | 40.6 | 151 | 95.6 | ||
| ≤93% | 25 | 13.2 | 19 | 59.4 | 6 | 3.8 | ||
| Missing values | 1 | 0.5 | 0 | 0 | 1 | 0.6 | ||
| Sleep quality c | Mean | SD | Mean | SD | Mean | SD | ||
| Subjective sleep quality | 1.32 | 0.88 | 1.22 | 0.94 | 1.34 | 0.87 | −0.718 | 0.474 |
| Difficulty falling asleep | 1.36 | 1.06 | 1.31 | 1.09 | 1.37 | 1.05 | −0.265 | 0.791 |
| Frequent nocturnal or early morning awakening | 1.49 | 1.07 | 1.50 | 1.16 | 1.49 | 1.05 | 0.061 | 0.951 |
| Sleep duration | 1.49 | 1.04 | 1.53 | 0.98 | 1.49 | 1.05 | 0.218 | 0.828 |
| PCL-5 c | ||||||||
| Total score | 17.69 | 13.95 | 17.00 | 12.31 | 17.83 | 14.29 | −0.306 | 0.760 |
| Criterion B | 5.15 | 4.49 | 5.22 | 4.27 | 5.14 | 4.54 | 0.091 | 0.927 |
| Criterion C | 2.25 | 2.08 | 2.19 | 2.28 | 2.26 | 2.05 | −0.178 | 0.859 |
| Criterion D | 5.22 | 4.83 | 5.09 | 4.15 | 5.24 | 4.97 | −0.156 | 0.876 |
| Criterion E | 5.07 | 4.19 | 4.50 | 3.53 | 5.19 | 4.31 | −0.849 | 0.397 |
| High PTSD symptoms b | ||||||||
| Yes | 43 | 22.6 | 5 | 15.6 | 38 | 24.1 | 1.080 | 0.300 |
| No | 147 | 77.4 | 27 | 84.4 | 120 | 75.9 | ||
Notes:aMann–Whitney U-test; bChi-square test; ct-test.
The median time from symptom onset to first medical visit and hospitalization was 2 days (IQR, 1–5) and 16 days (IQR, 10–27), respectively. The median lymphocyte count was 1.5×109/L (IQR, 1.11–1.96×109/L), and 62 (32.6%) patients showed lymphocytopenia (a lymphocyte level below 1.5×109/L); the median SaO2 was 96% (IQR, 95–98%), and 25 (13.2%) had experienced oxygen desaturation, identified as SaO2 below 93% in a resting state.
Regarding sleep quality, the mean rating scores of sleep quality, including subjective sleep quality, difficulty falling asleep, frequent nocturnal or early morning awakening and sleep duration were lower than 2, suggesting majority of patients in this study had quite good sleep quality. Of the total sample, the mean score on the PCL-5 was 17.69 (SD = 13.95).
The results revealed that age distribution was significantly different between the severe and non-severe group. The proportion of the elderly was higher in severe group than that in non-severe group (p = 0.009). Additionally, the lymphocyte count (p < 0.001) and the lowest SaO2 (p < 0.001) were generally lower in patients in severe group than that in non-severe group. Of the 32 severe patients, the median lymphocyte count was 1.19×109/L (IQR, 0.63–1.53×109/L), and the median SaO2 was 93% (IQR, 90–96%). There was no significant difference in gender, presence of family members or acquaintances infected, days from symptom onset to first medical visit and hospitalization, sleep quality, and PCL-5 scores between the two groups.
According to the symptomatic assessment criteria, the prevalence of high PTSD symptoms was 22.6% in the 190 confirmed patients, with 15.6% in the severe group and 24.1% in the non-severe group.
The Influence of Demographic Factors and Laboratory Indicators on Psychological Responses
Group differences on PCL-5 scores by demographic factors and laboratory indicators are displayed in Table 2. ANCOVA with covariates of age, presence of family members or acquaintances infected, lymphocyte count, and the lowest SaO2 showed that gender difference existed in the total score of PCL-5 (p < 0.01), the score of Criterion B (p < 0.01), Criterion C (p < 0.01), Criterion D (p < 0.05) and Criterion E (p < 0.05), indicating that females reported higher level of PTSD symptoms. However, there was no significant difference on PCL-5 scores in age, presence of family members or acquaintances infected, lymphocyte count, or the lowest SaO2.
Table 2.
Group Differences of PCL-5 Scores by Demographic Factors and Laboratory Indicators
| Total Score | F | Criterion B | F | Criterion C | F | Criterion D | F | Criterion E | F | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||||||
| Age | |||||||||||||||
| 18–40 | 15.15 | 13.73 | 0.814 | 4.48 | 4.23 | 0.411 | 1.82 | 1.78 | 0.853 | 4.64 | 4.88 | 0.472 | 4.21 | 4.13 | 2.065 |
| 41–60 | 19.29 | 14.63 | 5.33 | 4.70 | 2.43 | 2.25 | 5.65 | 5.10 | 5.87 | 4.41 | |||||
| ≥60 | 17.40 | 13.74 | 5.31 | 4.53 | 2.26 | 2.05 | 5.16 | 4.66 | 4.67 | 4.03 | |||||
| Gender | |||||||||||||||
| Male | 14.52 | 12.43 | 8.961** | 4.02 | 4.03 | 11.476** | 1.84 | 1.99 | 7.813** | 4.33 | 4.23 | 6.036* | 4.32 | 3.95 | 4.513* |
| Female | 20.59 | 14.91 | 6.21 | 4.72 | 2.61 | 2.11 | 6.08 | 5.24 | 5.69 | 4.38 | |||||
| Family members or acquaintances infected | |||||||||||||||
| No | 17.36 | 15.02 | 0.120 | 4.89 | 4.70 | 0.798 | 2.11 | 2.19 | 1.042 | 5.48 | 5.05 | 0.500 | 4.87 | 4.57 | 0.266 |
| Yes | 18.08 | 13.07 | 5.47 | 4.35 | 2.40 | 1.96 | 5.00 | 4.66 | 5.22 | 3.84 | |||||
| Lymphocyte count, ×10^9/L | |||||||||||||||
| ≥1.5 | 17.50 | 12.17 | 0.129 | 5.16 | 4.12 | 0.006 | 2.27 | 2.02 | 0.016 | 5.08 | 4.32 | 0.273 | 4.98 | 3.67 | 0.319 |
| <1.5 | 18.03 | 17.20 | 5.11 | 5.19 | 2.26 | 2.26 | 5.47 | 5.84 | 5.19 | 5.17 | |||||
| The lowest SaO2, % | |||||||||||||||
| >93% | 17.35 | 14.44 | 1.509 | 5.07 | 4.59 | 0.971 | 2.16 | 2.06 | 3.128 | 5.05 | 4.99 | 2.081 | 5.05 | 4.31 | 0.248 |
| ≤93% | 20.24 | 10.29 | 5.80 | 3.85 | 2.88 | 2.15 | 6.32 | 3.57 | 5.24 | 3.46 | |||||
Notes: *p-value < 0.05; **p-value < 0.01.
Group differences on sleep quality (subjective sleep quality, difficulty falling asleep, frequent nocturnal or early morning awakening and sleep duration) by demographic factors and laboratory indicators are displayed in Table 3. Patients who had oxygen desaturation reported shorter sleep duration (p < 0.05). There was no significant difference on any aspect of sleep quality in age, gender, having family members or acquaintances infected and lymphocyte count.
Table 3.
Group Differences of Sleep Quality by Demographic Factors and Laboratory Indicators
| Subjective Sleep Quality | F | Difficulty Falling Asleep | F | Frequent Nocturnal or Early Morning Awakening | F | Sleep Duration | F | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||||
| Age | ||||||||||||
| 18–40 | 1.24 | 0.90 | 1.327 | 1.30 | 0.88 | 2.976 | 1.36 | 1.08 | 1.385 | 1.27 | 1.01 | 1.500 |
| 41–60 | 1.45 | 0.98 | 1.61 | 1.06 | 1.67 | 1.09 | 1.65 | 1.08 | ||||
| ≥60 | 1.25 | 0.80 | 1.21 | 1.10 | 1.43 | 1.06 | 1.49 | 1.00 | ||||
| Gender | ||||||||||||
| Male | 1.29 | 0.90 | 0.549 | 1.24 | 1.09 | 2.721 | 1.52 | 1.13 | 0.045 | 1.46 | 0.99 | 1.265 |
| Female | 1.35 | 0.88 | 1.50 | 1.03 | 1.50 | 1.04 | 1.56 | 1.08 | ||||
| Family members or acquaintances infected | ||||||||||||
| No | 1.33 | 0.92 | 0.001 | 1.42 | 1.09 | 0.171 | 1.40 | 1.11 | 2.156 | 1.43 | 1.03 | 1.880 |
| Yes | 1.32 | 0.86 | 1.33 | 1.04 | 1.63 | 1.04 | 1.60 | 1.05 | ||||
| Lymphocyte count, ×10^9/L | ||||||||||||
| ≥1.5 | 1.36 | 0.88 | 0.120 | 1.42 | 1.06 | 0.201 | 1.56 | 1.07 | 0.289 | 1.52 | 1.05 | 0.004 |
| <1.5 | 1.27 | 0.91 | 1.31 | 1.06 | 1.44 | 1.08 | 1.50 | 1.04 | ||||
| The lowest SaO2, % | ||||||||||||
| >93% | 1.28 | 0.90 | 2.880 | 1.31 | 1.07 | 2.395 | 1.46 | 1.06 | 1.296 | 1.43 | 1.06 | 4.983* |
| ≤93% | 1.60 | 0.76 | 1.64 | 0.99 | 1.76 | 1.09 | 1.96 | 0.79 | ||||
Note: *p-value < 0.05.
The Association Between Hospital Visit Time and Psychological Responses of Patients
As shown by Table 4, Spearman correlation analysis involving the whole sample revealed that days from symptom onset to first medical visit were positively correlated with the total score of PCL-5 (r = 0.156, p < 0.05), Criterion C (r = 0.158, p < 0.05), and Criterion E (r = 0.175, p < 0.05), indicating that patients having longer duration from symptom onset to first medical visit reported higher level of PTSD symptoms, especially more avoidance and hyperarousal symptoms. It also demonstrated that days from symptom onset to hospitalization were positively correlated with the total score of PCL-5 (r = 0.181, p < 0.01), Criterion B (r = 0.175, p < 0.01), Criterion C (r = 0.140, p < 0.05), Criterion D (r = 0.181, p < 0.01), and Criterion E (r = 0.139, p < 0.05). No significant correlation was found between hospital visit time and sleep quality. In addition, it was worthy to be noted that there were significantly positive correlations between sleep quality and PCL-5 scores of patients (r = 0.312–0.547, p < 0.01).
Table 4.
Spearman Correlation Coefficient for Hospital Visit Time, Sleep Quality and PCL-5 Scores
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Time from symptom onset to First Medical Visit | 1.000 | ||||||||||
| 2. Time from symptom onset to Hospitalization | 0.289** | 1.000 | |||||||||
| 3. Subjective sleep quality | 0.070 | 0.104 | 1.000 | ||||||||
| 4. Difficulty falling asleep | 0.090 | 0.074 | 0.683** | 1.000 | |||||||
| 5. Frequent nocturnal or early morning awakening | 0.031 | 0.115 | 0.657** | 0.691** | 1.000 | ||||||
| 6. Sleep duration | 0.018 | 0.041 | 0.776** | 0.668** | 0.661** | 1.000 | |||||
| 7. PCL-5 Total score | 0.156* | 0.181** | 0.481** | 0.490** | 0.454** | 0.442** | 1.000 | ||||
| 8. PCL-5 Criterion B | 0.117 | 0.175** | 0.405** | 0.421** | 0.409** | 0.417** | 0.913** | 1.000 | |||
| 9. PCL-5 Criterion C | 0.158* | 0.140* | 0.364** | 0.370** | 0.312** | 0.345** | 0.807** | 0.728** | 1.000 | ||
| 10. PCL-5 Criterion D | 0.138 | 0.181** | 0.355** | 0.355** | 0.347** | 0.276** | 0.883** | 0.721** | 0.634** | 1.000 | |
| 11. PCL-5 Criterion E | 0.175* | 0.139* | 0.546** | 0.547** | 0.499** | 0.481** | 0.873** | 0.730** | 0.613** | 0.693** | 1.000 |
Notes: *p-value < 0.05; **p-value < 0.01.
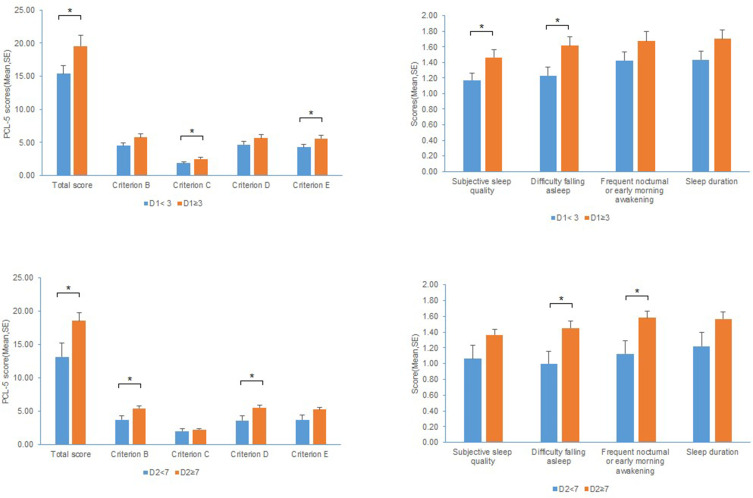
Considering that patients’ condition progressively worsened after symptom onset,33 further analysis was performed to determine the influence of hospital visit time on PTSD symptoms and sleep quality in COVID-19 patients (Figure 2). First, we divided the enrolled patients into two subgroups based on the days from symptom onset to first medical visit (more than 3 days and less than 3 days), to explore the group differences on PTSD symptoms and sleep quality. There were significant differences on the total scores of PCL-5 (p < 0.05), Criterion C (p < 0.05) and Criterion E (p < 0.05), suggesting that patients who made first medical visit more than 3 days after symptom onset experienced higher level of PTSD symptoms, specifically higher persistent avoidance and marked alterations in arousal and reactivity. Regarding sleep quality, patients who made first medical visit more than 3 days after symptom onset experienced worse subjective sleep quality (p < 0.05) and difficulty falling asleep (p < 0.05). Similarly, we divided the enrolled patients into another two subgroups based on the days from symptom onset to hospitalization (more than 7 days and less than 7 days).33,41 The results showed that patients admitted to hospital more than 7 days after symptom onset reported higher PTSD symptoms (p < 0.05), specifically higher intrusive experience (p < 0.05) and negative alterations in cognitions and mood (p < 0.05). Besides, these patients also suffered from worse difficulty falling asleep (p < 0.05) and frequent nocturnal or early morning awakening (p < 0.05).
Figure 2.
The influence of hospital visit time on PCL-5 scores and sleep quality.
Note: *p-value < 0.05.
Abbreviations: D1, days from symptom onset to first medical visit; D2, days from symptom onset to hospitalization.
Discussion
By July 20, 2021, more than 190,671,330 confirmed cases and 4,098,758 deaths of COVID-19 have been reported globally.42 The pandemic has placed a devastating threat to the physical health and mental condition of people affected by COVID-19. Our team firstly carried a preliminary investigation on acute posttraumatic stress symptoms of COVID-19 patients and found that COVID-19 patients suffered from varying degrees of posttraumatic stress symptoms and sleep disturbances.43 Based on this, the current study was further conducted to investigate the psychological status and explore the potential association between laboratory indicators and psychological responses in COVID-19 patients. It was concluded that the prevalence of high PTSD symptoms was 22.6% among the 190 patients, and most patients had quite good sleep quality. Age distribution and the level of laboratory indicators were significantly different between the severe and non-severe groups, while PTSD symptoms and sleep quality did not differ between the two groups. No significant impact was observed in the level of two indicators on patients’ PTSD symptoms and sleep quality, suggesting that the association between laboratory indicators and psychological responses in this study was not significant. Additionally, females reported higher PTSD symptoms compared with males.
In comparison with the prevalence of high PTSD symptoms among the general public and patients hospitalized with rheumatic diseases (RD) during the same stage of the COVID-19 pandemic in China, which was about 4.6%44 and 12.1%,45 respectively, the prevalence of high PTSD symptoms among COVID-19 patients in this study was quite high (22.6%), indicating that COVID-19 patients were more vulnerable to PTSD symptoms during the pandemic than the general public and patients hospitalized with other diseases. Previous study has also found that SARS patients reported the highest occurrence of PTSD compared to hospital staffs and the public exposed to SARS.46 The findings sounded an alarm to us, and more importance should be attached to the psychological health of patients, particularly PTSD-related symptoms.
In line with recent studies reporting that COVID-19 was more likely to affect older males with underlying diseases and could lead to severe and fatal diseases,47,48 we found older COVID-19 patients were more vulnerable to severe cases. However, no significant difference was observed in age on PTSD symptoms and sleep quality. In this regard, inconsistencies exist in findings within and across research on age. One study on the psychological impact of SARS outbreak suggested that older age was associated with higher prevalence and level of PTSD.31 In contrast, Norris et al indicated that the psychological disorders resulting from traumatic exposure might alleviate with age,49 while Bleich et al found that there was no difference in emotional impact of trauma exposure between the elderly and younger adults.50 The impact of age remains unclear and requires further research.
Although gender had little impact on the severity of disease and sleep quality, significant difference was found in gender on PTSD symptoms in the present study. Females reported higher level of PTSD symptoms, specifically more intrusive experience, persistent avoidance, and negative alternations in cognitions and mood. This was in line with our previous findings: female could be identified as a risk factor for PTSD symptoms in people affected by COVID-19.44,51 Previous research has also demonstrated that females are more inclined to develop PTSD than males.52,53
Two laboratory indicators (the minimum lymphocyte count and lowest SaO2) were examined in this study to identify whether patients had lymphocytopenia/oxygen desaturation or not. Our results found that the level of the two indicators had little effect on patients’ PTSD symptoms and sleep quality. However, study on COVID-19 symptoms and mental health outcomes found that physical symptoms were associated with psychological symptoms.54 It might be that in some cases laboratory results were not exactly equivalent to physical symptoms among COVID-19 patients.
No significant difference was observed on PTSD symptoms and sleep quality between the severe group and the non-severe group; that is to say, no impact of the severity of disease on PTSD symptoms and sleep quality was demonstrated in COVID-19 patients in the current study. Existing studies have reported inconsistent findings regarding the associations between disease severity and PTSD. Some studies reported that the severity of disease was not relevant to PTSD.55 Whereas, others found that the disease severity was predictive for the development of PTSD.11 Future research with a more comprehensive and rigorous design is needed to determine the relationship between the severity of the disease and the development of PTSD.
However, it was worth noting that there was a higher prevalence of PTSD symptoms in the non-severe group than the severe group (24.1% vs 15.6%), though no significant difference was observed. Such insignificance could be explained by the following two reasons: first, patients who were unable to communicate effectively due to poor physical condition were excluded during the recruitment process, causing the sample in the severe group was small (n=32), which might influence the prevalence of PTSD symptoms in this group; second, severe patients in this particular situation might focus more on physical recovery rather than psychological health, which deserves follow-up research.
With regard to the association between hospital visit time and psychological responses of patients, the results showed that the duration from symptom onset to first medical visit and hospitalization was positively related to PTSD symptoms. Particularly, patients who made first medical visit more than 3 days and admitted to hospital more than 7 days after symptom onset experienced higher level of PTSD symptoms and worse sleep quality, suggesting that the time from symptom onset to first medical visit and admission played an important role in the development of PTSD symptoms in COVID-19 patients. Therefore, early diagnosis and treatment of COVID-19 symptoms may be beneficial to the prevention of PTSD symptoms among infected patients. Besides, our study revealed a significant correlation between sleep quality and the level of PTSD symptoms among COVID-19 patients. Sleep disturbances were common among people involved in the COVID-19 pandemic44,51 and other similar major epidemics.28,56 Compared with PTSD, sleep disturbances are more likely to be recognized by clinicians. Timely identification and treatment for trauma-induced sleep problems may prevent the development of PTSD.57 The improvement of sleep quality might have clinical implications for treating psychological disorders like PTSD. Furthermore, the most evidence-based treatment might be cognitive behavior therapy (CBT), especially the Internet CBT that can prevent the spread of infection during the pandemic.58
Generalization
Since participants were recruited based on rigorous sample size calculation and the study was conducted under the cooperation of clinicians and psychiatrists, the findings of this study can be generalized to a larger population among COVID-19 patients. As mentioned above, PTSD is one of the most common chronic psychiatric sequelaes.15,17 Psychological intervention and trauma recovery should be the focus after COVID-19 patients being discharged. The present study demonstrated that the duration from symptom onset to first medical visit and hospitalization were associated with the severity of PTSD symptoms in COVID-19 patients. Early diagnosis and treatment of COVID-19 symptoms among patients may alleviate their PTSD symptoms through mitigating fear and uncertainty related to the disease.13 Moreover, clinicians and psychiatrists could consider the associated factors identified in this study (eg, female gender and poor sleep quality) when developing targeted psychological interventions for COVID-19 patients.
Limitations of This Study
The findings presented in this study should be viewed with the following limitations. First, considering the total number of confirmed cases of COVID-19 in China, the sample size was quite small in this study, though it met the minimum sample size designed by statistical calculation. Second, since clinical interview for psychological evaluation was difficult to conduct due to the infectious nature of the disease and the stay-at-home proposal, this study mainly used self-report questionnaires to measure psychiatric symptoms and did not make clinical diagnosis. However, it should be noted that the gold standard for establishing psychiatric diagnosis involved structured clinical interview and functional neuroimaging.59 Third, potential bias might exist in this study, such as selection bias in the recruitment of participants (the extremely severe patients were excluded whose psychological responses might be worse) and information bias in the participants’ psychological condition prior to admission (participants’ baseline psychological condition might influence their psychological responses during hospitalization). To reduce or avoid potential bias, clinicians and psychiatrists worked together to communicate with participants and collect information during the period of study design and conduction. Furthermore, data quality control was practiced rigidly during the whole process of study. Fourth, laboratory indicators examined in this study were limited. Future research should conduct more comprehensive assessments to identify the association between laboratory indicators and psychological disorders in COVID-19 patients, preferably based on multiple centers.
Conclusion
The prevalence of high PTSD symptoms was 22.6% in hospitalized COVID-19 patients. With the recovery from COVID-19, early psychological intervention should be conducted to relieve PTSD symptoms or prevent its onset among patients. Moreover, sleep quality of patients during hospitalization requires special attention. These findings may possess clinically referential significance on the improvement of PTSD symptoms and other psychological consequences in future epidemics.
Acknowledgments
The authors would like to acknowledge all health-care workers involved in the diagnosis and treatment of patients in Wuhan; and we thank all the patients participated in the study. The authors thank Xiaoqian Yu from the University of South Florida for language editing.
Funding Statement
This work was supported by the National Natural Science Foundation of China (grant number 32071086) and Military Medical Research Foundation (grant number CWS20J007).
Data Sharing Statement
The data are available on reasonable request from the corresponding author.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. Verbal informed consent was acceptable and the protocol for this study was approved by the Ethics Committee of Naval Medical University.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li Q; The 2019-nCoV Outbreak Joint Field Epidemiology Investigation Team. Notes from the field: an outbreak of NCIP (2019-nCoV) infection in China — Wuhan, Hubei Province, 2019–2020. China CDC Wkly. 2020;2(5):79–80. doi: 10.46234/ccdcw2020.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang C, Horby PW, Hayden FG, Gao GF. A novel coronavirus outbreak of global health concern. Lancet. 2020;395(10223):470–473. doi: 10.1016/s0140-6736(20)30185-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO. Coronavirus Disease 2019 (COVID-19): Situation Report-51, 11 March 2020. Geneva: World Health Organization; 2020. Available from:https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200311-sitrep-51-covid-19.pdf?sfvrsn=1ba62e57_4. Assessed March12, 2020. [Google Scholar]
- 6.WHO. WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19-11 March 2020. Geneva: World Health Organization; 2020. Available from:https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19—11-march-2020. Assessed March12, 2020. [Google Scholar]
- 7.Wang C, Pan R, Wan X, et al. A longitudinal study on the mental health of general population during the COVID-19 epidemic in China. Brain Behav Immun. 2020;87:40–48. doi: 10.1016/j.bbi.2020.04.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guan W-J, Ni Z-Y, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jalloh MF, Li W, Bunnell RE, et al. Impact of Ebola experiences and risk perceptions on mental health in Sierra Leone, July 2015. BMJ Glob Health. 2018;3(2):e000471. doi: 10.1136/bmjgh-2017-000471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mcalonan GM, Lee AM, Cheung V, Cheung C, Wong JGWS. Immediate and sustained psychological impact of an emerging infectious disease outbreak on health care workers. Can J Psychiatry. 2007;52(4):241–247. doi: 10.1177/070674370705200406 [DOI] [PubMed] [Google Scholar]
- 11.Wu KK, Chan SK, Ma TM. Posttraumatic stress after SARS. Emerg Infect Dis. 2005;11(8):1297–1300. doi: 10.3201/eid1108.041083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tine VB, Basnayake A, Wurie F, Jambai M, Nellums LB. Psychosocial effects of an Ebola outbreak at individual, community and international levels. Bull World Health Organ. 2016;94(3):210–214. doi: 10.2471/BLT.15.158543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Person B, Sy F, Holton K, Govert B, Liang A; Team tNSCO. Fear and stigma: the epidemic within the SARS outbreak. Emerg Infect Dis. 2004;10(2):358–363. doi: 10.3201/eid1002.030750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.da Silva ML, Rocha RSB, Buheji M, Jahrami H, Cunha KDC. A systematic review of the prevalence of anxiety symptoms during coronavirus epidemics. J Health Psychol. 2021;26(1):115–125. doi: 10.1177/1359105320951620 [DOI] [PubMed] [Google Scholar]
- 15.Lam H-BM. Mental morbidities and chronic fatigue in severe acute respiratory syndrome survivors. Arch Intern Med. 2009;169(22):2142. doi: 10.1001/archinternmed.2009.384 [DOI] [PubMed] [Google Scholar]
- 16.Kwek SK, Chew WM, Ong KC, et al. Quality of life and psychological status in survivors of severe acute respiratory syndrome at 3 months postdischarge. J Psychosom Res. 2006;60(5):513–519. doi: 10.1016/j.jpsychores.2005.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mak IWC, Chu CM, Pan PC, Yiu MG, Chan VL. Long-term psychiatric morbidities among SARS survivors. Gen Hosp Psychiatry. 2009;31(4):318–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kessler RC. Posttraumatic stress disorder: the burden to the individual and to society. J Clin Psychiatry. 2000;61 Suppl 5(5):4–12;discussion 13–4. [PubMed] [Google Scholar]
- 19.Mundy E, Baum A. Medical disorders as a cause of psychological trauma and posttraumatic stress disorder. Curr Opin Psychiatry. 2004;17(2):123–127. doi: 10.1097/00001504-200403000-00009 [DOI] [Google Scholar]
- 20.Cheng SK, Wong CW, Tsang J, Wong KC. Psychological distress and negative appraisals in survivors of severe acute respiratory syndrome (SARS). Psychol Med. 2004;34(7):1187–1195. doi: 10.1017/s0033291704002272 [DOI] [PubMed] [Google Scholar]
- 21.Maunder R, Hunter J, Vincent L, Bennett J, Mazzulli T. The immediate psychological and occupational impact of the 2003 SARS outbreak in a teaching hospital. Can Med Assoc J. 2003;168(10):1245–1251. [PMC free article] [PubMed] [Google Scholar]
- 22.Hugo M, Declerck H, Fitzpatrick G, et al. Post-traumatic stress reactions in Ebola virus disease survivors in Sierra Leone. Emerg Med. 2015;05(06):1–4. doi: 10.4172/2165-7548.1000285 [DOI] [Google Scholar]
- 23.Wu KK, Chan SK, Ma TM. Posttraumatic stress, anxiety, and depression in survivors of severe acute respiratory syndrome (SARS). J Trauma Stress. 2005;18(1):39–42. doi: 10.1002/jts.20004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moldofsky H, Patcai J. Chronic widespread musculoskeletal pain, fatigue, depression and disordered sleep in chronic post-SARS syndrome; a case-controlled study. BMC Neurol. 2011;11(1). doi: 10.1186/1471-2377-11-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jahrami H, BaHammam AS, Bragazzi NL, Saif Z, Faris M, Vitiello MV. Sleep problems during the COVID-19 pandemic by population: a systematic review and meta-analysis. J Clin Sleep Med. 2021;17(2):299–313. doi: 10.5664/jcsm.8930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spoormaker VI, Montgomery P. Disturbed sleep in post-traumatic stress disorder: secondary symptom or core feature? Sleep Med Rev. 2008;12(3):169–184. doi: 10.1016/j.smrv.2007.08.008 [DOI] [PubMed] [Google Scholar]
- 27.Janna M, Helms SM, Weymann KB, Capaldi VF, Lim MM. Sleep quality and emotion regulation interact to predict anxiety in veterans with PTSD. Behav Neurol. 2018;2018:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Etard J-F, Sow MS, Leroy S, et al. Multidisciplinary assessment of post-Ebola sequelae in Guinea (Postebogui): an observational cohort study. Lancet Infect Dis. 2017;17(5):2783–2790. doi: 10.1016/S1473-3099(16)30516-3 [DOI] [PubMed] [Google Scholar]
- 29.Hao F, Tam W, Hu X, et al. A quantitative and qualitative study on the neuropsychiatric sequelae of acutely ill COVID-19 inpatients in isolation facilities. Transl Psychiatry. 2020;10(1):355. doi: 10.1038/s41398-020-01039-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mak IWC, Chu CM, Pan PC, Yiu MG, Ho SC, Chan VL. Risk factors for chronic post-traumatic stress disorder (PTSD) in SARS survivors. Gen Hosp Psychiatry. 2010;32(6):590–598. doi: 10.1016/j.genhosppsych.2010.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee TM, Chi I, Chung LW, Chou KL. Ageing and psychological response during the post-SARS period. Aging Ment Health. 2006;10(3):303–311. doi: 10.1080/13607860600638545 [DOI] [PubMed] [Google Scholar]
- 32.Ho SMY, Kwong-Lo RSY, Mak CWY, Wong JS. Fear of Severe Acute Respiratory Syndrome (SARS) among health care workers. J Consult Clin Psychol. 2005;73(2):344–349. doi: 10.1037/0022-006x.73.2.344 [DOI] [PubMed] [Google Scholar]
- 33.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/s0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arora T, Grey I, Östlundh L, Lam KBH, Omar OM, Arnone D. The prevalence of psychological consequences of COVID-19: a systematic review and meta-analysis of observational studies. J Health Psychol. 2020. Oct 29;1359105320966639. doi: 10.1177/1359105320966639 [DOI] [PubMed] [Google Scholar]
- 35.Buysse DJ, Iii CFR, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4 [DOI] [PubMed] [Google Scholar]
- 36.Liu X, Tang M, Hu L, et al. Reliability and validity of the Pittsburgh sleep quality index. Chin J Psychiatry. 1996;29(2):103–107. [Google Scholar]
- 37.Weathers FW, Litz BT, Keane TM, Palmieri PA, Marx BP, Schnurr PP. The PTSD checklist for DSM-5 (PCL-5). 2013. Available from:http://www.ptsd.va.gov/professional/assessment/adult-sr/ptsd-checklist.asp. Accessed August25, 2021.
- 38.Fung HW, Chan C, Lee CY, Ross CA. Using the Post-traumatic Stress Disorder (PTSD) checklist for DSM-5 to screen for PTSD in the Chinese context: a Pilot Study in a psychiatric sample. J Evid Based Soc Work. 2019;16(6):643–651. doi: 10.1080/26408066.2019.1676858 [DOI] [PubMed] [Google Scholar]
- 39.Liu P, Wang L, Cao C, et al. The underlying dimensions of DSM-5 posttraumatic stress disorder symptoms in an epidemiological sample of Chinese earthquake survivors. J Anxiety Disord. 2014;28(4):345–351. doi: 10.1016/j.janxdis.2014.03.008 [DOI] [PubMed] [Google Scholar]
- 40.Zhou X, Wu X, Zhen R. Self-esteem and hope mediate the relations between social support and post-traumatic stress disorder and growth in adolescents following the Ya’an earthquake. Anxiety Stress Coping. 2018;31(1):32–45. doi: 10.1080/10615806.2017.1374376 [DOI] [PubMed] [Google Scholar]
- 41.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.WHO. Coronavirus (COVID-19), 20 July 2021. Geneva: World Health Organization; 2021. Available from:https://covid19.who.int/. Assessed July21, 2021. [Google Scholar]
- 43.Wu L, Shang Z, Zhang F, Sun L, Liu W. [A preliminary study of post-traumatic stress symptoms of two confirmed and six suspected coronavirus disease 2019 patients]. Acad J Second Mil Med Univ. 2020;41(2):130–134. in Chinese. doi: 10.16781/j.0258-879x.2020.02.0186 [DOI] [Google Scholar]
- 44.Sun L, Sun Z, Wu L, et al. Prevalence and risk factors for acute posttraumatic stress disorder during the COVID-19 outbreak. J Affect Disord. 2021;283:123–129. doi: 10.1101/2020.03.06.20032425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu X, Geng X, Shang Z, et al. Post-traumatic stress disorder in patients with rheumatic disease during the COVID-19 outbreak. BMJ Open. 2021. (In Press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang KR, Xu Y, Yang H, et al. Investigation by comparison on the posttraumatic stress response among SARS patients, hospital staffs and the public exposed to SARS. Chin J Behav Med Sci. 2006;15(04):358–360. [Google Scholar]
- 47.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/s0140-6736(20)30211-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N Engl J Med. 2020;382(13):1199–1207. doi: 10.1056/NEJMoa2001316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Norris FH, Friedman MJ, Watson PJ, Byrne CM, Diaz E, Kaniasty K. 60,000 disaster victims speak: part I. An empirical review of the empirical literature, 1981–2001. Psychiatry. 2002;65(3):207–239. doi: 10.1521/psyc.65.3.207.20173 [DOI] [PubMed] [Google Scholar]
- 50.Bleich A, Gelkopf M, Melamed Y, Solomon Z. Emotional impact of exposure to terrorism among young-old and old-old Israeli citizens. Am J Geriatr Psychiatry. 2005;13(8):705–712. doi: 10.1097/00019442-200508000-00010 [DOI] [PubMed] [Google Scholar]
- 51.Liu N, Zhang F, Wei C, et al. Prevalence and predictors of PTSS during the COVID-19 outbreak in China hardest-hit areas: gender differences matter. Psychiatry Res. 2020;287:112921. doi: 10.1016/j.psychres.2020.112.921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Christiansen DM, Elklit A. Sex differences in PTSD. In: Lazinica A, Ovuga E, editors. Posttraumatic Stress Disorder in a Global Context. Rijeka, Croatia: InTech Open Access Book; 2012:113–142. [Google Scholar]
- 53.Kessler RC. Posttraumatic stress disorder in the national comorbidity survey. Archgenpsychiatry. 1995;52(12):1048. [DOI] [PubMed] [Google Scholar]
- 54.Wang C, Chudzicka-Czupala A, Tee ML, et al. A chain mediation model on COVID-19 symptoms and mental health outcomes in Americans, Asians and Europeans. Sci Rep. 2021;11(1):6481. doi: 10.1038/s41598-021-85943-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tedstone JE, Tarrier N. Posttraumatic stress disorder following medical illness and treatment. Clin Psychol Rev. 2003;23(3):409–448. doi: 10.1016/s0272-7358(03)00031-x [DOI] [PubMed] [Google Scholar]
- 56.Clark DV, Kibuuka H, Millard M, et al. Long-term sequelae after Ebola virus disease in Bundibugyo, Uganda: a retrospective cohort study. Lancet Infect Dis. 2015;15(8):905–912. doi: 10.1016/s1473-3099(15)70152-0 [DOI] [PubMed] [Google Scholar]
- 57.Sinha SS. Trauma-induced insomnia: a novel model for trauma and sleep research. Sleep Med Rev. 2016;25:74–83. doi: 10.1016/j.smrv.2015.01.008 [DOI] [PubMed] [Google Scholar]
- 58.Soh HL, Ho RC, Ho CS, Tam WW. Efficacy of digital cognitive behavioural therapy for insomnia: a meta-analysis of randomised controlled trials. Sleep Med. 2020;75:315–325. doi: 10.1016/j.sleep.2020.08.020 [DOI] [PubMed] [Google Scholar]
- 59.Husain SF, Tang TB, Yu R, et al. Cortical haemodynamic response measured by functional near infrared spectroscopy during a verbal fluency task in patients with major depression and borderline personality disorder. EBioMedicine. 2020;51:102586. doi: 10.1016/j.ebiom.2019.11.047 [DOI] [PMC free article] [PubMed] [Google Scholar]