Abstract
In Saccharomyces cerevisiae, the protein phosphatase type 1 (PP1)-binding protein Reg1 is required to maintain complete repression of ADH2 expression during growth on glucose. Surprisingly, however, mutant forms of the yeast PP1 homologue Glc7, which are unable to repress expression of another glucose-regulated gene, SUC2, fully repressed ADH2. Constitutive ADH2 expression in reg1 mutant cells did require Snf1 protein kinase activity like constitutive SUC2 expression and was inhibited by unregulated cyclic AMP-dependent protein kinase activity like ADH2 expression in derepressed cells. To further elucidate the functional role of Reg1 in repressing ADH2 expression, deletions scanning the entire length of the protein were analyzed. Only the central region of the protein containing the putative PP1-binding sequence RHIHF was found to be indispensable for repression. Introduction of the I466M F468A substitutions into this sequence rendered Reg1 almost nonfunctional. Deletion of the central region or the double substitution prevented Reg1 from significantly interacting with Glc7 in two-hybrid analyses. Previous experimental evidence had indicated that Reg1 might target Glc7 to nuclear substrates such as the Snf1 kinase complex. Subcellular localization of a fully functional Reg1-green fluorescent protein fusion, however, indicated that Reg1 is cytoplasmic and excluded from the nucleus independently of the carbon source. When the level of Adr1 was modestly elevated, ADH2 expression was no longer fully repressed in glc7 mutant cells, providing the first direct evidence that Glc7 can repress ADH2 expression. These results suggest that the Reg1-Glc7 phosphatase is a cytoplasmic component of the machinery responsible for returning Snf1 kinase activity to its basal level and reestablishing glucose repression. This implies that the activated form of the Snf1 kinase complex must cycle between the nucleus and the cytoplasm.
Protein phosphatase type 1 (PP1) plays a key role in regulating a diverse variety of processes in eukaryotic cells (3, 48). The amino acid sequences of the mammalian and yeast homologues of the PP1 catalytic subunit (PP1c) are more than 80% identical, suggesting that their function and the regulatory mechanisms that control their activity have been conserved throughout evolution. The gene coding for the Saccharomyces cerevisiae homologue of PP1c is GLC7. Glc7 is required for the appropriate regulation of a number of cellular processes, including glycogen biosynthesis; translation; cell cycle progression; chromosome segregation, meiosis, and sporulation; and repression of many glucose-regulated genes (54). Unlike protein kinase catalytic subunits which can recognize a window of amino acid sequence surrounding the phosphorylation site (39), PP1c exhibits little inherent substrate specificity in vitro (11, 39). There is now a large body of evidence suggesting that specificity is conferred by regulatory subunits. When complexed with PP1c, they target it to specific substrates (11, 22). In S. cerevisiae, a number of Glc7-binding proteins affecting specific cellular processes have been identified (54). These potential regulatory subunits include Gac1 and Pig1, which affect glycogen accumulation (9, 55); Reg2 and Sds22, which affect growth and cell cycle progression (24, 33, 41); Gip1, which is required for completion of meiosis and sporulation (59); Scd5, which affects the vesicular secretory pathway (59); Pig2, whose function is unknown (9); and Reg1, which affects glucose repression, growth, and glycogen accumulation (23, 24, 35, 42, 44).
In S. cerevisiae, glucose repression is the major mechanism through which the expression of genes involved in the utilization of alternative or fermentable carbon sources is coordinately regulated (25). In the presence of high concentrations of glucose, the expression of glucose-regulated genes is low or repressed. When the concentration of glucose drops below 0.2%, expression of these genes is activated or derepressed. For glucose-repressible genes like SUC2, GAL1, and ADH2, this change in the level of expression can be 200-fold or greater (7, 20, 27). A number of genes have been identified as playing integral roles in glucose repression (25). Among these are REG1, GLC7, and SNF1, the yeast homologue of the catalytic subunit of AMP-activated protein kinase (30). SNF1 is required for derepression of gene expression in glucose-limited cells (4, 10, 67), while REG1 and GLC7 are required for the maintenance of the fully repressed state (23, 42, 44). A combination of genetic, two-hybrid, and coimmunoprecipitation experiments have indicated that Snf1 is complexed with Snf4 and one member of the Sip/Gal83 class of proteins (7, 65). Snf1 is thought to be anchored in the complex by its C-terminal regulatory domain to the centrally located KIS domain of the Sip/Gal83 protein (38). Snf4 is also anchored in the complex by interacting with the Sip/Gal83 protein; however, this interaction is with the C-terminal ACS domain. These interactions do not appear to be carbon source regulated. The interaction of Snf1 with Snf4, however, does appear to be carbon source regulated (37). In repressed cells, the N-terminal kinase domain of Snf1 appears to interact with its C-terminal regulatory domain, which is thought to inhibit kinase activity. Upon depletion of glucose from the growth medium, Snf4 is thought to bind to the kinase domain, displacing the regulatory domain and, thereby, freeing the Snf1 kinase domain from autoinhibition. Two-hybrid and coimmunoprecipitation experiments have also suggested that Reg1 and Glc7 act together as a complex (59). Like interactions with the Sip/Gal83 component of the Snf1 complex, the interaction between Reg1 and Glc7 does not appear to be glucose regulated. Recently, evidence has been presented indicating that Reg1 interacts with the kinase domain of Snf1, altering protein-protein interactions within the kinase complex (40). Two-hybrid experiments have suggested that Reg1 interacts weakly with the kinase domain of Snf1 in repressed cells and strongly in derepressed cells. This interaction required amino acid T210 in the activation loop, which is essential for Snf1 kinase activity and for the interaction with Snf4. Based on these observations, it was proposed that Reg1 targets Glc7 to an active Snf1 complex by binding to the kinase domain. Once bound, Glc7 could then dephosphorylate Snf1, thereby releasing Snf4 from the kinase regulatory domain and returning the complex to an autoinhibited state.
Although the Reg1-Glc7 complex has been clearly implicated in the repression of SUC2 expression, surprisingly, only Reg1 has been demonstrated to play a role in repressing ADH2 expression (20). Even though reg1 mutant cells growing under normally repressing conditions have up to 40-fold greater ADH2 expression than wild-type cells, a glc7-T152K mutant, which has a constitutively high level of SUC2 expression, is fully repressed for ADH2 expression (20). The level of this constitutive ADH2 expression is similar to that seen in cells with an ADR1c allele. ADR1c mutations fall within or near the cyclic AMP-dependent protein kinase (cAPK) phosphorylation site at serine 230 of ADR1 (18), the major activator of ADH2 transcription (17). Reg1 appears to act independently of this phosphorylation site, however, since ADR1c alleles synergistically increase ADH2 expression in reg1 mutant cells under normally repressing growth conditions (20). Like activated ADH2 expression in derepressed wild-type cells, constitutive ADH2 expression in reg1 mutant cells under normally repressing growth conditions requires ADR1. Cells with mutations in both ADR1 and REG1 have fully repressed ADH2 expression during growth on a high-glucose medium. Also, a reporter gene with promoter sequences containing UAS1, the Adr1 binding site in the ADH2 promoter (51), is constitutively expressed in reg1 mutant cells (20). The level of Adr1 in reg1 mutant cells under normally repressing growth conditions is nearly the same as that in derepressed wild-type cells. This 3-fold higher level of Adr1, however, cannot fully account for the 40-fold higher level of ADH2 expression, since ADH2 expression appears to increase linearly with the level of ADR1 expression in repressed cells (15). Also, recent data from our lab has shown that repressed cells having the same level of Adr1 as derepressed cells do not constitutively express ADH2 (53).
ADH2 expression has the same requirement for Snf1 as SUC2 expression (10), and repression of both ADH2 and SUC2 in a high-glucose medium has the same requirement for Reg1 (4, 20). This raised the question of how Reg1 could function in the apparent absence of a requirement for Glc7. In the study presented here, we provide the first evidence showing that even though repression of ADH2 expression has an apparent differential requirement for Reg1 and Glc7, Glc7 may indeed play a role in repressing ADH2 expression. A sequence similar to the mammalian PP1-binding motif (R/K)(V/I)XF (22) was identified as being essential for repression of both ADH2 and SUC2 expression and for the interaction of Reg1 with Glc7. We also show that the level of Adr1 in glc7 mutant cells is limiting for constitutive ADH2 expression under repressing growth conditions. Subcellular localization of Reg1-green fluorescent protein (GFP) suggested that Reg1 is cytoplasmic and excluded from the nucleus. These findings suggest that the Reg1-Glc7 phosphatase complex is part of the cytoplasmic machinery for resetting Snf1 kinase activity to a basal level and imply that the activated form of the Snf1 complex rapidly cycles between the nucleus and the cytoplasm.
MATERIALS AND METHODS
Yeast strains, plasmids, media, and growth conditions.
The yeast strains used in this study are listed in Table 1. Strains created for this study were constructed by using standard genetic methods (29). Strains KDY80, KDY82, KDY88, KDY90, and KDY92 have the S288C genetic background. KDY80 and KDY82 are congenic segregants derived from a REG1/reg1-1966::URA3 ADH2/ADH2::YIp24ADH2-lacZ diploid that was otherwise homozygous at other loci. The snf1::URA3 and bcy1::HIS3 alleles were introduced into these strains by using the one-step gene disruption technique described by Rothstein (47). Their presence was confirmed as described previously (21). Strains KDY18, KDY37, and KDY38 are isogenic with MC71-18Bα. Strain KDY107 was created by changing the URA3 gene marking the (lexAop)8-lacZ reporter to TRP1 by transforming strain L40 (34) with SmaI-digested pUT11 DNA as described by Cross (13). The glc7-127 allele was introduced into cells carrying four copies of the ADR1 gene by first transforming KT1640 with the ADH2-lacZ reporter plasmid pLGADH2-lacZ. One transformant was then crossed with JSY14, which had been constructed previously by integrating three copies of the ADR1 gene into the genome of strain HHY10 (53). The resulting diploid was sporulated, and segregants were screened for growth on leucine-deficient raffinose–2-deoxyglucose medium. Some of the desired segregants were auxotrophic for uracil, indicating that they did not contain the pLGADH2-lacZ plasmid, so they were then transformed with the ADH2-lacZ reporter centromeric plasmid pBGM18, creating strains VBY1, VBY2, and VBY3. Yeast cells were transformed by using a modified version of the lithium acetate procedure as described by Gietz et al. (26). The plasmids used in the present study are listed in Table 2. Their construction is described in subsequent sections.
TABLE 1.
Yeast strains used in this study
| Strain | Genotype | Reference or source |
|---|---|---|
| SB90 | MATα glc7::LEU2 his4 leu2 trp1-1 ura3-52 pNC160(TRP1)-GLC7 | 1 |
| SB214 | MATα glc7::LEU2 his4 leu2 trp1-1 ura3-52 pNC160(TRP1)-glc7-127 | 1 |
| SB241(HA) | MATα glc7::LEU2 his4 leu2 trp1-1 ura3-52 pNC160(TRP1)-glc7-131 | 1 |
| SB259 | MATα glc7::LEU2 his4 leu2 trp1-1 ura3-52 pNC160(TRP1)-glc7-133 | 1 |
| SB219 | MATα glc7::LEU2 his4 leu2 trp1-1 ura3-52 pNC160(TRP1)-glc7-134 | 1 |
| KDY80 | MATa ade2-1010 ADH2::YIp23ADH2-lacZ his3Δ200 leu2 lys2-801aura3-52 trp1Δ1 | 21 |
| KDY82 | MATa ade2-1010 ADH2::YIp23ADH2-lacZ his3Δ200 leu2 lys2-801areg1-1966::LEU2 ura3-52 trp1Δ1 | This study |
| KDY88 | MATa ade2-1010 ADH2::YIp23ADH2-lacZ his3Δ200 leu2 lys2-801asnf1::URA3 ura3-52 trp1Δ1 | This study |
| KDY89 | MATa ade2-1010 ADH2::YIp23ADH2-lacZ his3Δ200 leu2 lys2-801areg1-1966::LEU2 snf1::URA3 ura3-52 trp1Δ1 | This study |
| KDY90 | MATa ade2-1010 ADH2::YIp23ADH2-lacZ bcy1::HIS3 his3Δ200 leu2 lys2-801aura3-52 trp1Δ1 | 21 |
| KDY92 | MATa ade2-1010 ADH2::YIp23ADH2-lacZ bcy1::HIS3 his3Δ200 leu2 lys2-801areg1-1966::LEU2 ura3-52 trp1Δ1 | This study |
| MC71-18Bα | MATα adh1Δ adh3 leu2 trp1 ura3 | 20 |
| KDY18 | MATα adh1Δ adh3 leu2 reg1-1966::LEU2 trp1 ura3 | 20 |
| KDY37 | MATα adh1Δ adh3 leu2 snf1::URA3 trp1 ura3 | This study |
| KDY38 | MATα adh1Δ adh3 leu2 reg1-1966::LEU2 snf1::URA3 trp1 ura3 | This study |
| KDY107 | MATa ade2 his3Δ200 leu2-3,112 LYS2::(lexAop)4-HIS3 trp1-901 ura3::TRP1::(lexAop)8-lacZ | This study |
| BV370 | MATa glc7-133 his3 leu2 ura3 | K. Tatchell |
| KT1591 | MATα leu2 ppz1::LEU2 ppz2::URA3 ura3 | K. Tatchell |
| x209-5d | MATα glc7-133 his3 leu2 ppz1::URA3 ppz2::LEU2 ura3 | K. Tatchell |
| KT1640 | MATα glc7-127 his3 leu2 ura3 | K. Tatchell |
| JSY14 | MATa adh3 LEU2::(pRS305-ADR1)3 trp1 ura3 | This lab |
| HHY10 | MATa adh3 leu2 trp1 ura3 | This lab |
| VBY1 | Leu− Ura+ 2-DOGr segregant from KT1640(pBMG18) × JSY14 | This study |
| VBY2 | Leu+ Ura+ 2-DOGs segregant from KT1640(pBMG18) × JSY14 | This study |
| VBY3 | Leu+ Ura+ 2-DOGr segregant from KT1640(pBMG18) × JSY14 | This study |
TABLE 2.
Plasmids used in this study
| Plasmid | Vector | Description | Reference or source |
|---|---|---|---|
| pBGM18 | pRS316 | URA3-CEN ADH2-lacZ reporter plasmid (codon 109 of ADH2 fused to lacZ) | This lab |
| pLGADH2-lacZ | pLG669Z | 2μm-URA3 ADH2-lacZ reporter plasmid (codon 23 of ADH2 fused to lacZ) | This lab |
| pHDY10 | pLG669Z | 2μm-URA3 UAS1 reporter plasmid | This lab |
| YIp23ADH2-lacZ | YIp5 | Integrating TRP1 ADH2-lacZ reporter plasmid | 21 |
| pKD63 | pRS316 | URA3-CEN plasmid with REG1 | This study |
| pKD89 | pRS316 | URA3-CEN plasmid with HA-REG1 | This study |
| pKD93 | pRS316 | pKD89 with the Δ1 deletion | This study |
| pKD94 | pRS316 | pKD89 with the Δ2 deletion | This study |
| pKD95 | pRS316 | pKD89 with the Δ3 deletion | This study |
| pKD96 | pRS316 | pKD89 with the Δ4 deletion | This study |
| pKD92 | pRS316 | pKD89 with the Δ5 deletion | This study |
| pKD97 | pRS316 | pKD89 with the Δ6 deletion | This study |
| pKD98 | pRS316 | pKD89 with the Δ7 deletion | This study |
| pKD104 | pRS316 | pKD89 with the Δ8 deletion | This study |
| pKD111 | pRS316 | pKD89 with the Δ9 deletion | This study |
| pKD112 | pRS316 | pKD89 with the Δ10 deletion | This study |
| pKD114 | pRS316 | pKD89 with the I466M F468A substitutions | This study |
| pKD115 | pRS316 | pKD95 with the deleted region restored | This study |
| pKD109 | pRS316 | pKD63 with REG1 fused in frame at codon 1002 to GFP | This study |
| pKD110 | pRS316 | pKD89 with HA-REG1 fused in frame at codon 1002 to GFP | This study |
| pRSM306 | pRS306 | pRS306 with the 2μm origin from YEp24 | T. Davis |
| pKD123 | pRSM306 | URA3 2-hybrid lexA-REG1 expression plasmid | This study |
| pKD125 | pRSM306 | URA3 2-hybrid lexA-REG1 plasmid with the Δ3 deletion | This study |
| pKD126 | pRSM306 | URA3 2-hybrid lexA-REG1 plasmid with the Δ8 deletion | This study |
| pKD127 | pRSM306 | URA3 2-hybrid lexA-REG1 plasmid with the I466M F468A substitutions | This study |
| pGAD-GLC7 | pGAD | LEU2 2-hybrid GAD-GLC7 plasmid | 57 |
| pTT49 | pGAD | LEU2 2-hybrid GAD-ORC1 plasmid | R. Sternglanz |
Media and culture conditions were essentially as described by Sherman (49). Yeast cells were grown at 30°C in yeast extract-peptone (YEP) medium or, when appropriate, selective synthetic medium (SM), which lacks amino acids and uracil and contains either 0.4% Casamino Acids and tryptophan to select for URA3 plasmids or amino acid dropout solution lacking leucine to simultaneously select for URA3 and LEU2 plasmids. Unless otherwise indicated, repressed and derepressed cells were prepared essentially as described previously (21).
Construction of CEN plasmids carrying the wild-type and mutant HA-tagged REG1 genes.
To make Reg1 easily detectable by Western blotting, its amino terminus was tagged with the hemagglutinin (HA) epitope. First, an EcoRI-SalI fragment containing the REG1 promoter, open reading frame, and 3′ noncoding sequences from pUCSRN1 (61) was cloned between the EcoRI and SalI sites of pRS316 (52) to create plasmid pKD63. Next, sequence coding for a single HA tag was introduced immediately after the ATG start codon by using the recombinant PCR strategy described by Higuchi (32). Primers 62-A, 62-B, 62-C, and 62-D (Table 3) were used to simultaneously insert the HA tag and amplify the REG1 sequence from pUCSRN1 with Vent DNA polymerase (New England Biolabs, Inc., Beverly, Mass.). The sequence amplified starts just 5′ of the NheI site in the promoter and ends just past the ClaI site in the REG1 open reading frame. The resulting DNA fragment was treated with Klenow, digested with ClaI, and then ligated between the SmaI and ClaI sites of pGEM7ZF(+) (Promega Corporation, Madison, Wis.) to create plasmid pKD88. The insert was sequenced on both strands and shown to be error-free. Finally, a 0.3-kb NheI-ClaI fragment from pKD88, which has the HA-tagged 5′ end of the REG1 open reading frame and some proximal promoter sequence; a 3.3-kb ClaI-SalI fragment from pUCSRN1, which has the remainder of the open reading frame and 3′ noncoding sequence; and a 5.7-kb vector fragment from pKD63 were assembled in a three-way ligation. This created plasmid pKD89, which has the complete REG1 gene, including the HA-tagged open reading frame carried on a pRS316 backbone.
TABLE 3.
Oligonucleotide primers used in plasmid constructions
| Oligonucleotide primer | Sequence (5′→3′) |
|---|---|
| 62-A | ACCACCTCCTGAAAGAGAAC |
| 62-B | AGCGTAGTCTGGCACGTCATATGGGTACATTTTTGGATTTTTCTTATCTCGTCTTCG |
| 62-C | ATGTACCCATATGACGTGCCAGACTACGCTTCAACAAATCTAGCAAATTACTTCGCCG |
| 62-D | CAATATATTCATCAAGAAGGCCC |
| 89-1-3′ | GTGCCAGACTACGCTAGATCTTGGAGAACATGGGC |
| 89-1-5′ | GCCCATGTTCTCCAAGATCTAGCGTAGTCTGGCAC |
| 89-2-5′-N | CTTCTCCCTTGTCACCAGATCTCCATTCATGTGACAG |
| 89-2-3′-N | GCCAGAAAGACATGTTAGATCTAATTCAAATGGTGGCGG |
| 89-3-3′ | CGAACAGTAGCGTTAGATCTGAAGAACACGGCGG |
| 89-3-5′ | CCGCCGTGTTCTTCAGATCTAACGCTACTGTTCG |
| 89-4-3′ | CCATTGCTAGCCATTCAGAGATCTTCATCAGATAGCG |
| 89-4-5′ | CGCTATCTGATGAAGATCTCTGAATGGCTAGCAATGG |
| 89-5-3′ | GAAAAAAGATCTAGTGATGTTGCCATAGAGGG |
| 89-5-5′ | ATCACTAGATCTTTTTTCCTTGGATTCTACCGC |
| 89-6-3′ | CACCAGCAAACAGATCTTAGAAGAAAGAATTTTGAAGTCAAC |
| 89-6-5′-N | ATACGCTAGATCTAACCAGCTGACTGTTAGCGGGTAATGGTC |
| 89-(455-475)-3′ | CCTCAAACCCAAGTGAAAGATCTTGTATGGCACTACGATATCC |
| 89-(455-475)-5′ | GGATATCGTAGTGCCATACAAGATCTTTCACTTGGGTTTGAGG |
| 89-(349-554)-3′ | CCGTGAGATCTATCATCAGAATGTGATGAAGATGATGATTGTG |
| 89-(349-554)-5′ | AACAGTGGATCCAGTTTGAAGAGTCAACACTCTGAC |
| pRS-5′ | GCAACTGTTGGGAAGGGCGATCGGTGCGGG |
| pRS-3′ | CCATGATTACGCCAAGCTCGGAATTAACCC |
| 89-I466M, F468A-3′ | CCTACTAAAAATAGACATATGCATGCTAATGACAGGGTGG |
| 89-I466M, F468A-5′ | CCACCCTGTCATTAGCATGCATATGTCTATTTTTAGTAGG |
| XhoI-GFP-3′ | CACTATCTCGAGAATTGGAGCTCGGTACCAG |
| AatII-GFP-5′ | TCTAGGACGTCCGCAGGCGCTGGAGCCGGTG |
Mutations were introduced into the REG1 open reading frame of pKD89 by using two complementary strategies. All primers used in these constructions are listed in Table 3. Deletions Δ1, Δ3, Δ4, Δ5, Δ8, and Δ9 were introduced with primer pairs 89-1-3′ and 89-1-5′, 89-3-3′ and 89-3-5′, 89-4-3′ and 89-4-5′, 89-5-3′ and 89-5-5′, 89-(455-475)-3′ and 89-(445-475)-5′, and 89-1-5′ and 89-2-3′-N, respectively, by the Quick-Change method (Stratagene, La Jolla, Calif.) with the high fidelity thermostable DNA polymerase Pfu. These primer pairs created a BglII site at the deletion point. The remaining mutant constructs were created by using a combination of preparative PCR and traditional cloning. For deletion Δ2, sequences 5′ to the deletion point were amplified from pKD89 by Pfu polymerase with the primer combination of pRS-5′ and 89-2-5′-N. The resulting DNA fragment was then digested with EcoRI and BglII. Sequences 3′ to the deletion point were created in a similar way with the primer combination of pRS-3′ and 89-2-3′-N. The resulting DNA fragment was then digested with BglII and XhoI. Sequences 5′ to the deletion point were combined with sequences 3′ to the deletion point and cloned between the EcoRI and SalI sites of pRS316. Deletions Δ6 and Δ10 were similarly created, except that either the 5′ or 3′ side of the deletion was prepared from other constructs and not by PCR. Deletion Δ6 was created by ligating a 3.6-kb PCR fragment, which was created with primers 89-6-5′-N and pRS-5′, to the 5.3-kb EcoRI-BglII vector fragment of pKD112. For deletion Δ10, a 3-kb EcoRI-BglII fragment from pKD92 was combined with a 0.4-kb BglII-XhoI PCR fragment, which was created with primers 89-6-3′ and pRS-3′, and an EcoRI-SalI vector fragment of pRS316 in a three-way ligation. Deletion Δ7 was created by cloning a 0.98-kb EcoRI-BglII fragment from pKD93 into pKD92. The I466M F468A double substitution was also introduced by using a similar strategy. Sequences 5′ to the substitution were amplified from pKD89 with the primer combination of pRS-5′ and 89-I466M F468A-5′. The resulting DNA fragment was then digested with EcoRI and SphI. Sequences 3′ to the substitution were created with the primer combination pRS-3′ and 89-I466M F468A-3′. This DNA fragment was then digested with SphI and XhoI. The 5′ and 3′ fragments were then cloned between the EcoRI and SalI sites of pRS316 in a three-way ligation. The Δ3+ reconstruction of the REG1 gene was prepared by cloning a 0.64-kb BamHI-BglII PCR fragment, which was created with primers 89-(349-554)-5′ and 89-(349-554)-3′, into the BglII site of pKD95.
Construction of wild-type and mutant URA3-selectable LexA-REG1 expression plasmids.
To create a LexA-REG1 expression plasmid into which the REG1 mutations could be easily transferred, a 5.4-kb NarI-SalI fragment from plasmid pLexA-REG1, which had the NarI end blunted with T4 polymerase, was ligated between the SmaI and SalI sites in the polylinker of pRSM306, a 2μm derivative of pRS316, which was kindly provided by the lab of Trisha Davis. The resulting plasmid, pKD123, has a unique ClaI site in the REG1 open reading frame 0.16 kb from the fusion junction with lexA. Mutations could then be introduced by cloning a ClaI-SalI fragment from one of the mutant REG1 centromeric plasmids into pKD123. Plasmids pKD125, pKD126, and pKD127 were created in this way.
Subcellular localization of Reg1-GFP by fluorescence microscopy.
For this study, a CEN plasmid carrying the REG1 promoter and coding sequence fused in-frame at codon 1002 to GFP was constructed. A 0.9-kb fragment encoding the F64L,S65T enhanced version of GFP (12) with an AatII site introduced immediately 5′ to sequences coding for an amino terminal alanine-glycine flexible linker was created by PCR with plasmid pLI2000, which was generously supplied by Eric Muller, as the template by using primers XhoI-GFP-3′ and AatII-GFP-5′ (Table 3). The resulting PCR fragment was digested with AatII and XhoI, gel purified, and then ligated to the 8.8-kb AatII-XhoI vector fragment of pKD89 to create pKD106. Next, this plasmid was digested with AatII, treated with T4 polymerase to blunt the ends, and religated to place the REG1 coding sequence in frame with GFP to create plasmid pKD109.
In preparation for fluorescence microscopy, cells were grown at 30°C in SM broth lacking uracil with 5% glucose as the carbon source and 1.5 mM adenine to suppress the endogenous vacuolar fluorescence of these ade2 mutant cells. At a density of 107 cells/ml, an aliquot of cells was prepared for viewing. Another aliquot was washed once with cold SM lacking amino acids, adenine, uracil, or glucose. Then the cell pellet was suspended in prewarmed selective SM with 0.05% glucose as the carbon source and 1.5 mM adenine and incubated at 30°C. At 2, 4, 8, and 12 h after shifting to derepressing medium, aliquots of cells were prepared for viewing. Additional aliquots were saved for invertase and β-galactosidase assays at each time point to monitor the course of SUC2 and ADH2 derepression. To prepare cells for viewing, they were stained in culture with 4′,6-diamidino-2-phenylindole (DAPI; Sigma Chemical Co., St. Louis, Mo.), as described by Shero et al. (50). Then an 18-μl aliquot of each DAPI-stained culture was mixed with 6 μl of melted 0.6% agarose containing fresh growth medium and mounted on a microscope slide. Cells were viewed as described by Moser et al. (43) with a Zeiss Axioplan microscope fitted with the appropriate filters for discriminating between DAPI and GFP fluorescence. Images were processed by using Adobe Photoshop, version 4.0 (Adobe Systems, Inc., San Jose, Calif.), and prepared for publication by using Microsoft PowerPoint, version 4.0 (Microsoft Corp., Redmond, Wash.).
Western blot analyses.
HA-tagged Reg1 and Reg1 fusion proteins were analyzed in native whole-cell extracts. At a density of between 1 × 107 and 4 × 107 cells/ml, 40 ml of each culture was centrifuged and the resulting cell pellets were washed once with 5 ml of cold buffer A (25 mM HEPES [pH 7.5], 5 mM MgCl2, 0.01 mM EDTA, 10% glycerol) supplemented with 50 mM KCl, 1 mM β-mercaptoethanol, and 1 mM phenylmethylsulfonyl fluoride (PMSF). Then each washed cell pellet was suspended at a density of 9 × 107 cells/ml in buffer A supplemented with 200 mM KCl, 1 mM dithiothreitol, 1 mM sodium pyrophosphate, 1 mM sodium orthovanadate, 1 mM β-glycerophosphate, 1 mM EGTA, 10 mM sodium fluoride, 1 mM PMSF, and 1 μg (each) of pepstatin, aprotinin, and leupeptin per ml. Approximately 110 mg of 500-μm-diameter acid-washed glass beads was added for every 100 μl of cell suspension, and the suspensions were vortexed six times at high speed for 2 min each, with the addition of extra PMSF and 2 min of cooling on ice between each round of vortexing. To clarify the extracts, they were spun at high speed in a microcentrifuge two to four times for 15 min. The resulting clarified extracts were quick-frozen in powdered dry ice and stored at −80°C until needed. Separation of proteins by denaturing polyacrylamide gel electrophoresis and the subsequent transfer of proteins from the gel to nitrocellulose membrane were carried out by using the Mini-Protean II gel system from Bio-Rad Laboratories (Hercules, Calif.) according to the manufacturer’s instructions. Blots were probed with either anti-HA monoclonal antibody 12CA5 (Boehringer Mannheim, Indianapolis, Ind.) at a concentration of 5 μg/ml, anti-LexA monoclonal antibody 2-12 (Santa Cruz Biotechnology, Inc., Santa Cruz, Calif.) at a concentration of 2 μg/ml, or polyclonal anti-GFP antibodies, which were kindly provided by T. Davis’s lab. Adr1 was analyzed in denatured whole-cell extracts as previously described (20, 21).
Enzyme and protein assays.
β-Galactosidase activities were determined in permeabilized yeast cells as described by Guarente (28). Invertase activities were measured in whole cells essentially as described by Celenza and Carlson (5). ADH enzyme activity was analyzed in yeast extracts either by directly assaying enzyme activity for adh1 adh3 mutant cells as described by Denis et al. (17) or by nondenaturing polyacrylamide gel electrophoresis followed by chromogenic staining as described by Williamson et al. (63) for ADH wild-type cells. The protein concentration of native cell extracts was determined with the Bio-Rad protein assay reagent (Bio-Rad Laboratories). To measure the protein concentration of denatured cell extracts, a 5-μl aliquot of each extract was diluted to 125 μl with 100 mM potassium phosphate (pH 7.5), incubated at room temperature for 10 min, and centrifuged in a microcentrifuge at high speed for 10 min to precipitate excess sodium dodecyl sulfate (SDS) (66). Then the Bio-Rad reagent was used to determine the protein concentration in the supernatant.
RESULTS
Mutant alleles of GLC7 that are defective in repressing SUC2 expression fully repress ADH2 expression.
Previously, we had reported that ADH2 expression is undetectable in cells carrying the glc7-T152K allele under repressing growth conditions (20). Speculating that this lack of expression might be allele specific, four different mutants, each with a different glucose repression-defective allele of glc7, were assayed for ADH2 expression by ADH native gel analysis (data not shown). ADHII enzyme activity was undetectable in repressed cells expressing either the glc7-127, glc7-131, glc7-133, or glc7-134 allele (1). Additionally, an episomal ADH2-lacZ reporter plasmid, pLGADH2-lacZ, was introduced into cells having the glc7-131 allele, and β-galactosidase was assayed as a more sensitive measure of ADH2 expression (Table 4). Invertase activity was also assayed as a measure of SUC2 expression. β-Galactosidase activity in glc7-131 mutant cells under normally repressing growth conditions was only 2-fold higher than that in wild-type cells, while invertase activity was 18-fold higher. Therefore, unlike for SUC2 expression, ADH2 expression is not significantly affected by mutations in GLC7. Similar observations were obtained with the UAS1-lacZ reporter pHDY10 (data not shown), suggesting that, unlike mutations in REG1, mutations in GLC7 do not significantly affect ADR1-dependent expression. These results confirmed our earlier observation and caused us to question whether Reg1 is repressing ADH2 expression via the same mechanism as SUC2 expression.
TABLE 4.
Glc7 proteins defective in repressing SUC2 expression fully repress ADH2 expression
| Strain | Relevant genotype | β-Galactosi-dase activitya (Miller units)
|
Invertase activityb (nmol)
|
||
|---|---|---|---|---|---|
| R | DR | R | DR | ||
| SB90 | GLC7 | 3 | 450 | 3 | 200 |
| SB241(HA) | glc7-131 | 6 | 320 | 54 | 170 |
ADH2 expression was assayed as β-galactosidase activity expressed by the ADH2 reporter plasmid pLGADH2-lacZ. These values are the means for at least two independent transformants assayed in duplicate and had a maximum standard deviation of 30%. Repressed cells (R) had 5% glucose and derepressed cells (DR) had 2% glycerol, 2% lactate, 2% ethanol, and 0.1% glucose as the carbon sources, respectively.
SUC2 expression was assayed as invertase activity in nanomoles of sucrose hydrolyzed per min per unit of optical density at 600 nm of culture used. Each assay was performed in duplicate and had an average range of 30%. R had 5% glucose and DR had 0.1% glucose as the carbon source, respectively.
SNF1 and BCY1 are required for constitutive ADH2 expression in reg1 mutant cells.
If Reg1 represses ADH2 expression via the same mechanism that it uses to repress SUC2, then SNF1, which is required for constitutive expression of SUC2 in reg1 mutant cells under normally repressing growth conditions (44), as well as for derepression of both ADH2 and SUC2 in wild-type cells (4, 10), should also be required for constitutive expression of ADH2. To test this argument, strains with an integrated ADH2-lacZ reporter and various combinations of reg1 and snf1 deletions were prepared and β-galactosidase activity was assayed as a measure of ADH2 expression (Table 5). Deletion of REG1 increased the expression of the reporter gene by 23-fold in repressed cells, while deletion of SNF1 completely abolished derepression. When combined, the SNF1 deletion was epistatic to the REG1 deletion, completely preventing constitutive expression in repressed cells as well as derepression of the reporter. Similar results were obtained for the expression of the native ADH2 gene in a different strain background, MC71-18Bα (20), indicating that expression of the ADH2-lacZ reporter was faithfully mimicking that of the endogenous gene and that these results were not strain specific (data not shown). Therefore, constitutive ADH2 expression in reg1 mutant cells has the same requirement for SNF1 as constitutive SUC2 expression and derepressed ADH2 expression.
TABLE 5.
Deletion of snf1 or bcy1 suppresses constitutive ADH2 expression
| Strain | Relevant genotype | β-Galactosidase activitya (Miller units)
|
|
|---|---|---|---|
| R | DR | ||
| KDY80 | BCY1 REG1 SNF1 | 9.5 | 1,140 |
| KDY88 | BCY1 REG1 snf1 | 6.0 | 6.0 |
| KDY90 | bcy1 REG1 SNF1 | 4.8 | 9.0 |
| KDY82 | BCY1 reg1 SNF1 | 220 | 2,740 |
| KDY89 | BCY1 reg1 snf1 | 4.9 | 4.8 |
| KDY92 | bcy1 reg1 SNF1 | 12 | 22 |
ADH2 expression was assayed as β-galactosidase activity expressed from the integrated reporter YIp23ADH2-lacZ. The values for KDY80 and KDY82 are means of three independent assays performed in duplicate. All other values are means for three independent disruptants derived from either KDY80 or KDY82 and assayed in duplicate. Each measurement had a standard deviation of less than 20%. Repressed cells (R) had 5% glucose and derepressed cells (DR) had 3% ethanol as the carbon source, respectively.
Constitutive ADH2 expression in reg1 mutant cells is also ADR1 dependent (20). Since unregulated cAPK activity inhibits ADR1-dependent gene expression, it was of interest to determine whether deletion of BCY1, the gene coding for the regulatory subunit of cAPK in S. cerevisiae, was also epistatic to the REG1 deletion. To address this issue, BCY1 was deleted in the wild-type and reg1 mutant strains containing the ADH2-lacZ reporter and β-galactosidase activity was assayed (Table 5). Deletion of BCY1 prevented the reporter from significantly derepressing. When combined with the REG1 deletion, no constitutive activity of the reporter was observed in repressed cells and, as for the single mutant, the double mutant did not significantly derepress reporter gene expression. Thus, constitutive ADH2 expression in reg1 mutant cells under normally repressing growth conditions has the same requirement for BCY1 as ADH2 expression in derepressed wild-type cells.
Delineation of the regions of Reg1 required for function by deletion analysis.
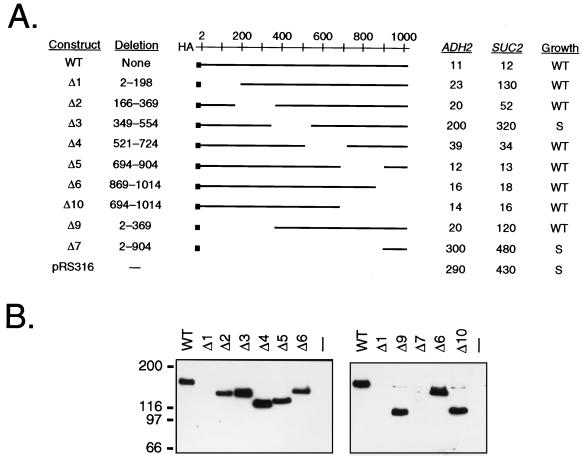
Constitutive ADH2 expression in reg1 mutant cells required at least one factor needed for SUC2 expression and appeared to be under the same controls as derepressed ADH2 expression in wild-type cells. To further elucidate the role of Reg1 in repressing ADH2 expression, we have delineated the regions of Reg1 required for function. We hoped to determine whether the same regions needed to repress ADH2 expression were also needed to repress SUC2 expression. Reg1 was tagged with a single HA epitope at its amino terminus to allow for sensitive and specific detection of the wild-type and mutant proteins in Western blot analyses. When expressed in reg1 mutant cells from either a CEN plasmid or an integrating plasmid, the HA-tagged and untagged versions of Reg1 behaved identically, fully suppressing constitutive ADH2 expression and the slow growth phenotype (data not shown), which is typical of reg1 mutant cells having an otherwise wild-type genetic background (24). A series of six deletions of approximately 200 amino acids each, scanning the entire length of the protein, were constructed (Fig. 1A). Several larger N- and C-terminal deletions were also prepared. ADH2-lacZ and SUC2 expression were assessed in reg1 mutant cells containing the wild-type or deletion constructs on CEN plasmids. The levels of ADH2-lacZ expression fell into two main groups: those with a high mutant level of β-galactosidase activity, deletion constructs Δ3 and Δ7, and those with a low wild-type level of β-galactosidase activity, most of the remaining constructs. This indicates that one region of Reg1 required for repressing ADH2 expression lies between amino acids 349 and 554. The same region was also required for repressing SUC2 expression. However, cells carrying constructs Δ1 and Δ9, each of which had wild-type low levels of β-galactosidase activity, exhibited partially constitutive SUC2 expression, having more than 25% of the constitutive level of invertase activity in reg1 mutant cells. This suggests that an additional region N terminal to amino acid 198 also plays a role in mediating repression of SUC2.
FIG. 1.
Deletion analysis of Reg1. (A) Phenotypic analyses of REG1 deletion constructs. Two independently derived clones of each deletion construct were transformed into strain KDY82. Transformants were grown under repressing conditions in SM broth lacking uracil with 5% glucose as the carbon source. Aliquots of each culture were assayed for ADH2 and SUC2 expression. Values for ADH2 expression measured as ADH2-lacZ reporter gene expression are β-galactosidase activities (in Miller units). Values for SUC2 expression are invertase activities in nanomoles of sucrose hydrolyzed per min per 107 cells. Three transformants were analyzed for each clone, and each value presented represents an average for three independent transformants of the two independent clones. Standard deviations for these values were less than 20%. Growth was assayed by streaking individual transformants to single colonies on SM agar lacking uracil with 2% glucose as the carbon source and assessing colony size after 2 days. WT, wild-type colony size; S, small colony size. (B) Western blot analysis of REG1 deletion constructs. Proteins from 100 μg of each cell extract were separated on SDS–5.5% acrylamide protein gels. After transfer from each gel to nitrocellulose, HA-Reg1 proteins were identified by chemiluminescence with anti-HA monoclonal antibody as the probe. To the left of the blots are marked the positions of standard molecular weight markers. The right panel was exposed to X-ray film for a fivefold longer period of time than the left panel.
In addition to assessing the effect of each deletion on constitutive ADH2 and SUC2 expression, the slow growth phenotype was examined. The growth rate of cells expressing each deletion was assessed as colony size on selective medium after incubation at 30°C for 3 days. In general, the deletions that were unable to suppress constitutive ADH2 expression, Reg1Δ3 and Reg1Δ7, were also unable to fully suppress the slow growth phenotype (Fig. 1A). Therefore, the same region of Reg1, from amino acids 349 to 554, was required to suppress all three mutant phenotypes, constitutive ADH2 expression, constitutive SUC2 expression, and slow growth.
Western blot analysis showed that most of the HA-Reg1 deletion proteins were expressed at approximately the same level as the wild-type protein (Fig. 1B). The Reg1Δ7 protein was not detectable under the experimental conditions used because it was only 13 kDa and migrated off the polyacrylamide protein gels used in the analysis. Only a faint band was detected for Reg1Δ1. This was very surprising, since cells expressing it were almost fully repressed for ADH2-lacZ expression. A quantitative comparison by Western blot analysis indicated that this protein was indeed being expressed, however, at a five- to eightfold lower level than the other proteins and also appeared to be less stable (data not shown). Reg1Δ9 was equally defective in repressing SUC2 expression but it was present at the same level as wild-type Reg1, lending further support for a functional role of the N terminus in repressing SUC2 expression. Therefore, the region from amino acids 349 to 554 is required for repression of both ADH2 and SUC2 and for a wild-type growth rate, while the first 198 amino acids appear to be required mainly for repression of SUC2.
The Western blot analysis also revealed that Reg1 migrates anomalously under denaturing conditions in a polyacrylamide protein gel (Fig. 1B). Its predicted molecular mass is 114 kDa, yet it migrated as a protein larger than 150 kDa. All of the deletion derivatives of Reg1 also migrated at anomalously large molecular masses. If this anomalous migration is due to posttranslational modification of the protein, then the modifications must not be restricted to one portion of the protein. This anomalous migration also was not altered in derepressed cells (data not shown). Therefore, the major posttranslational modifications responsible for the anomaly are probably not subject to the controls of glucose repression.
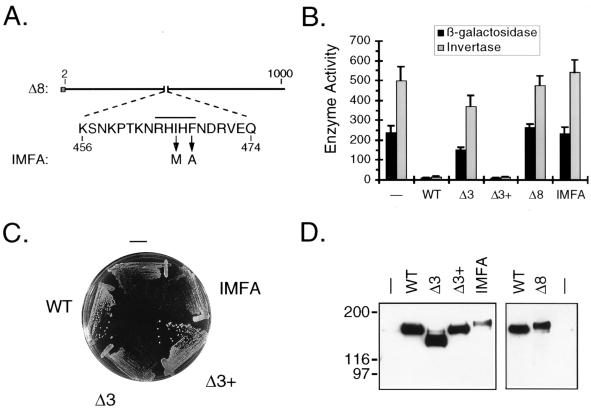
A PP1-binding motif is required both for Reg1 function and for the interaction with Glc7.
Egloff et al. (22) have pointed out that Reg1 contains the sequence RHIHF, which is homologous to the PP1-binding motif (R/K)(V/I)XF found in mammalian PP1-binding subunits. This putative PP1-binding sequence falls within the region that we have delineated as being required for most, if not all, Reg1 functions. In order to determine the importance of this sequence for function, several new derivatives of Reg1 were constructed and their effects on ADH2-lacZ expression, SUC2 expression, and growth were assessed (Fig. 2). Reg1Δ3+p had amino acids 349 to 554 restored to Reg1Δ3. It was used as a control to determine if any mistakes which could make the protein nonfunctional had been inadvertently introduced into Reg1Δ3 during its construction. Cells expressing this protein had the same level of ADH2-lacZ and SUC2 expression and the same growth rate as cells expressing the wild-type protein (Fig. 2B and C). Therefore, no crucial mistakes were present in the Reg1Δ3 sequence. Reg1Δ8 had amino acids 456 to 474, which contained the RHIHF sequence, deleted, and Reg1-IMFA contained the double amino acid substitution I466M F468A (Fig. 2A). If the RHIHF sequence functions in a manner analogous to that of the mammalian motif, then these two derivatives of Reg1 should be nonfunctional. Cells expressing either one of these proteins had the same levels of ADH2-lacZ and SUC2 expression and the same growth rate as reg1 mutant cells (Fig. 2B and C). Therefore, both the Δ8 and IMFA derivatives of Reg1 appeared to be nonfunctional. However, Western blot analysis complicated this interpretation because it showed that these two proteins were being expressed at a significantly lower level than the wild-type protein (Fig. 2D). Since Reg1Δ1 is expressed at an even lower level (data not shown) and yet is still functional, this suggests that the levels of expression of the Δ8 and IMFA derivatives should be high enough to suppress the phenotypes of reg1 mutant cells. Therefore, both derivatives must be nonfunctional, suggesting that the RHIHF sequence is critical for the function of Reg1.
FIG. 2.
Analysis of PP1-binding site mutants. (A) Diagram of the Δ8 and IMFA derivatives of Reg1. The amino acid sequence presented is that which is deleted in the Δ8 construct. The overline indicates the position of the putative PP1-binding sequence. Arrows indicate the amino acid substitutions introduced to create the IMFA construct. (B) Analysis of ADH2 and SUC2 expression. KDY82 cells transformed with either pRS316 (−), pKD89 (WT), pKD95 (Δ3), pKD115 (Δ3+), pKD104 (Δ8), or pKD114 (IMFA) were grown in synthetic selective broth containing 5% glucose. ADH2 expression was assayed as β-galactosidase activity (in Miller units) expressed from the integrated ADH2 reporter plasmid YIp23ADH2-lacZ. Each measurement represents the mean for six independent transformants, and error bars represent the standard deviation. SUC2 expression was assayed as invertase activity (in nanomoles of sucrose hydrolyzed per min per 107 cells). A single transformant having β-galactosidase activity nearest the average was assayed in triplicate. (C) Effect of PP1-binding site mutations on growth. Transformants were streaked to single colonies on SM agar lacking uracil and containing 2% glucose. The agar plate was incubated for 2 days before being photographed. (D) Western blot analysis of binding site mutants. Protein blots of KDY82 transformants were prepared and analyzed as described in the legend for Fig. 1B. The right panel was exposed to X-ray film for a fivefold longer period of time than the left panel. WT, wild type.
Western blot analysis of the new Reg1 derivatives also revealed that both Reg1Δ1 and Reg1-IMFA migrate more slowly than the wild-type protein in a denaturing polyacrylamide protein gel (Fig. 2D). This mobility shift, however, did not change when cells were derepressed (data not shown). Therefore, this altered mobility also does not appear to reflect a change in the state of glucose-regulated posttranslational modifications.
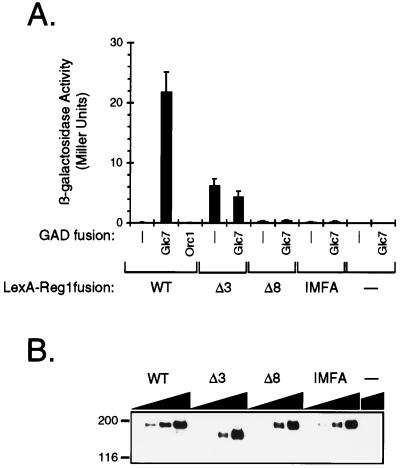
Since the RHIHF sequence was critical for function, it was of interest to determine whether the Δ3 and Δ8 deletions and the IMFA double substitution reduced or eliminated the interaction with Glc7. The two-hybrid assay with which the Reg1-Glc7 interaction had been first demonstrated was used to address this issue (58). The ability of GAD-Glc7 to specifically stimulate expression of a lexAop-lacZ reporter gene in the presence of LexA-Reg1 versions containing these changes was assayed. The LexA-Reg1 fusion proteins behaved similarly to their nonfusion counterparts in their ability to suppress the slow growth phenotype of reg1 mutant cells (data not shown). GAD-Glc7 stimulated reporter gene expression more than 100-fold in the presence of wild-type LexA-Reg1 (Fig. 3A). Neither GAD nor the nonspecific control GAD-Orc1 significantly stimulated expression. For the versions of LexA-Reg1 containing the Δ3 or Δ8 deletion as well as the IMFA double substitution, GAD-Glc7 was unable to significantly stimulate expression of the reporter gene. Western blot analysis of LexA-Reg1 expression showed that the binding-defective versions of the protein were expressed at a level no less than one-half that of the wild-type protein (Fig. 3B). Together, these results suggest that the RHIHF sequence is critical for the interaction of Reg1 with Glc7.
FIG. 3.
Effect of PP1-binding site mutations on the two-hybrid interaction between Reg1 and Glc7. (A) Two-hybrid interaction as measured by lexAop-lacZ reporter gene expression. Strain KDY107 was transformed with combinations of plasmids that allowed the simultaneous expression of wild-type (WT) or mutant versions of LexA-Reg1 and a GAD fusion protein. Transformants were grown in SM lacking leucine and uracil and containing 5% glucose. β-Galactosidase activity was assayed as a measure of lexAop-lacZ reporter gene expression. Stimulation of reporter gene expression by GAD-Glc7 reflects an interaction with LexA-Reg1. Each measurement is the average of four to nine independent transformants. Each error bar represents the standard deviation of the measurement. (B) Western blot analysis of wild-type and mutant LexA-Reg1 proteins. Cell extracts were prepared from transformants expressing GAD-Glc7 and the various LexA-Reg1 proteins. Twofold serial dilutions, as represented by the dark triangles, starting at 50 μg of protein, were loaded in reverse order onto an SDS–5.5% acrylamide gel. Proteins were transferred to nitrocellulose, and LexA-Reg1 fusion proteins were detected with monoclonal antibodies directed against LexA.
Reg1 is localized in the cytoplasm and excluded from the nucleus independently of the carbon source.
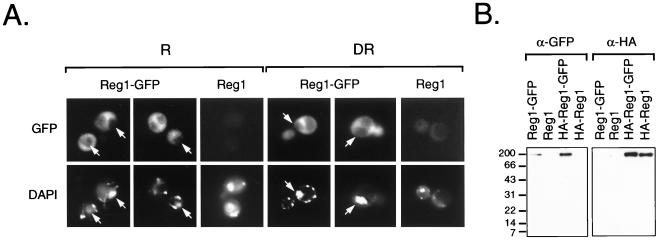
Niederacher and Entian have reported that Reg1 is likely to be nuclearly localized based on subcellular fractionation experiments with a Reg1–β-galactosidase fusion protein (46). Therefore, one possible explanation for the inability of the nonfunctional versions of Reg1 to suppress the mutant phenotypes was that they were unable to accumulate in the nucleus. To address this issue, a Reg1-GFP fusion and an HA-tagged version were constructed and their subcellular localization was determined by fluorescence microscopy. These GFP fusions were able to fully suppress constitutive ADH2-lacZ and SUC2 expression, as well as the slow growth phenotype (data not shown). Contrary to our expectation, Reg1-GFP was localized in the cytoplasm and appeared to be excluded from the nucleus (Fig. 4A). Cytoplasmic localization was observed in both repressed and derepressed cells and did not change even up to 12 h after the start of derepression (data not shown). Identical results were obtained with the HA-tagged version of the fusion protein (data not shown). Western blot analysis of the Reg1-GFP fusion proteins showed that they were not being cleaved to release the GFP portion of the fusion protein (Fig. 4B), which then would be localized in the cytoplasm (45). Since wild-type Reg1-GFP was not nuclearly localized, it seemed reasonable to assume that the nonfunctional versions would not be either. Therefore, it seems unlikely that mislocalization to the cytoplasm can explain the defect in function of the mutant Reg1 proteins.
FIG. 4.
Subcellular localization of Reg1-GFP. (A) Fluorescence micrographs of KDY82 cells expressing Reg1-GFP. Cells expressing Reg1-GFP as the sole source of functional Reg1 were created by transforming strain KDY82 with plasmid pKD109. KDY82 expressing HA-Reg1 from plasmid pKD89 served as the negative control for yeast cell autofluorescence. Repressed cells (R) were grown in medium containing 5% glucose. A portion of these cells was derepressed (DR) by shifting them to medium containing 0.05% glucose. The derepressed cells shown in these micrographs were prepared 4 h after the shift. By this time, ADH2-lacZ expression was beginning to derepress and SUC2 expression had fully derepressed (data not shown). Cells examined at 2, 4, 8, and 12 h after the shift showed the same distribution of GFP fluorescence. Arrows in the micrographs mark the position of nuclei based on the location of DAPI fluorescence. (B) Western blot analysis of Reg1-GFP. Cell extracts were prepared from repressed KDY82 transformants expressing Reg1-GFP from plasmid pKD109, Reg1 from plasmid pKD63, HA-Reg1-GFP from plasmid pKD110, and HA-Reg1 from plasmid pKD89. Proteins from 100 μg of each cell extract were separated on an SDS–12% acrylamide gel and transferred to nitrocellulose. GFP-tagged proteins were detected with anti-GFP polyclonal antibodies (α-GFP), and HA-tagged proteins were detected with anti-HA monoclonal antibodies (α-HA).
ADH2 is constitutively expressed in glc7-131 mutant cells when the level of Adr1 is elevated.
The critical role of the RHIHF sequence in Reg1 function suggests that Glc7 or another PP1-like protein phosphatase is required for full repression of ADH2 expression. S. cerevisiae has three genes coding for protein phosphatases homologous to PP1: PPZ1, PPZ2, and PPQ1 (8, 14). Strains containing several combinations of deletions of PPZ1 and PPZ2 and the glc7-133 mutation were tested by ADH native gel analysis for constitutive ADH2 expression. Strains having the glc7-133 mutation alone or having deletions of both PPZ1 and PPZ2 or having a combination of glc7-133 and both PPZ deletions showed no detectable ADHII enzyme activity when they were grown in glucose medium (data not shown). Therefore, the Ppz1 and Ppz2 phosphatases do not appear to act redundantly with Glc7 to keep ADH2 expression repressed. PPQ1 was not tested because it seemed unlikely that a phosphatase which plays a role in translational accuracy (62) would directly affect repression of ADH2 expression which occurs at the level of transcription (2, 17).
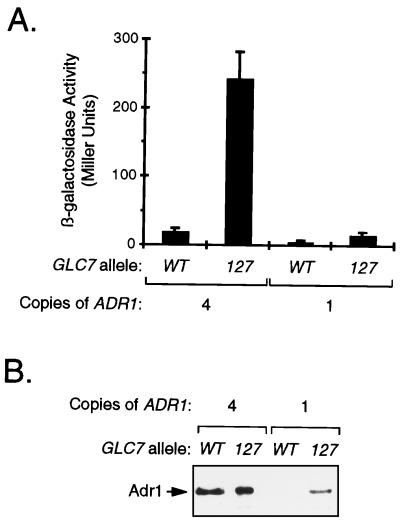
Since other GLC7 homologues either did not affect ADH2 expression or seemed unlikely to have an effect, we decided to focus on the glc7 mutant strains. Constitutive ADH2 expression in reg1 mutant cells requires ADR1 (20), and the level of Adr1 is high in some strains and barely detectable in others. Therefore, we wondered whether the level of Adr1 in repressed glc7 mutant cells was sufficient to allow constitutive ADH2 expression. To address this issue, expression of an ADH2-lacZ reporter gene under repressing growth conditions was assayed in glc7 mutant cells that express the same level of Adr1 as derepressed cells. Comparison of the Adr1 level in JSY14, which has three integrated copies of the ADR1 gene in addition to the genomic copy, with that in HHY10, the strain from which it was created, showed that JSY14 grown under repressing conditions had approximately the same amount of Adr1 as derepressed HHY10 cells (53). When the glc7-127 mutation was crossed into JSY14 containing an ADH2-lacZ reporter gene, the activity of the reporter gene in several segregants derived from two different crosses was always higher than in the GLC7 wild-type counterpart, as indicated by blue color after growth on X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) plates (data not shown). The level of reporter gene activity in one segregant of each type was quantitated by β-galactosidase assays (Fig. 5A). The glc7 mutant strain with four copies of ADR1 VBY3 had 13-fold more β-galactosidase activity than the GLC7 wild-type counterpart VBY2. The glc7 mutant strain with only one copy of ADR1 VBY1 had only threefold more β-galactosidase activity than the GLC7 wild-type counterpart HHY10. Similar results were obtained when expression of the chromosomal ADH2 gene was monitored by ADH native gel analysis (data not shown). This indicated that reporter gene expression was faithfully mimicking that of the chromosomal gene. Western blot analysis showed that the amount of Adr1 in each segregant with multiple copies of the ADR1 gene did not correlate with the level of reporter gene expression. Both VBY2 and VBY3 had equally high levels of Adr1 (Fig. 5B). HHY10 had a very low level, while VBY1 had an intermediate level. This intermediate level of Adr1 may be responsible for the slightly higher level of reporter gene expression in VBY1 than in HHY10 (Fig. 5A). These results indicate that the level of Adr1 is limiting for constitutive ADH2 expression in glc7 mutant cells and also provide the first direct evidence that the Glc7 protein phosphatase can play a role in repressing ADH2 expression.
FIG. 5.
Constitutive expression of ADH2-lacZ in repressed glc7 mutant cells having a derepressed level of Adr1 protein. (A) Analysis of ADH2-lacZ expression. Strains HHY10 (GLC7; one copy of ADR1), VBY1 (glc7-127; one copy of ADR1), VBY2 (GLC7; four copies of ADR1), and VBY3 (glc7-127; four copies of ADR1), all containing the pBGM18 ADH2-lacZ reporter plasmid, were grown initially in SM lacking uracil and containing 8% glucose and then transferred to YEP broth containing 8% glucose to more fully repress expression of the reporter. After incubation overnight, β-galactosidase activity was assayed. Each bar represents the average of four to eight measurements, and each error bar represents the standard deviation of the measurement. (B) Western blot analysis of Adr1 levels. Denatured whole-cell extracts were prepared from repressed cells, and equal amounts of protein were loaded in each lane of an SDS–5.5% acrylamide gel. After transfer to nitrocellulose, Adr1 was detected by using polyclonal antibodies prepared against amino acids 335 to 740 of Adr1.
DISCUSSION
In this paper, we provide the first evidence suggesting that the yeast PP1 homologue Glc7 plays a role in repressing ADH2 expression. It had previously been reported that the glc7-T152K allele, which is defective in glucose repression of SUC2 expression, was able to fully repress ADH2 expression (20). Here, we confirm this observation by using four other glucose repression-defective alleles of GLC7. We also provide evidence suggesting that, like SUC2 expression, constitutive ADH2 expression in reg1 mutant cells requires the SNF1 regulatory pathway. For SUC2 expression, this pathway includes the Reg1-Glc7 PP1 complex as a member. Two independent lines of evidence are presented, suggesting that Glc7 does play a role in repressing ADH2 expression. First, the region of Reg1 containing a PP1-binding motif was shown to be required for repressing ADH2 expression. Additionally, this motif was shown to be essential for the interaction of Reg1 with Glc7. Second, GLC7 was required to maintain full repression of ADH2 expression in a strain expressing the derepressed level of Adr1. This suggests that the level of Adr1 in the original glc7 mutant strains was probably too low to support constitutive ADH2 expression. Consistent with this interpretation is the almost complete repression of ADH2 expression seen for the glc7 mutant strain expressing the lower than derepressed level of Adr1. REG1 mutant cells express the fully derepressed level of Adr1 (20). Therefore, it is likely that the glc7 alleles tested were not defective enough to allow the needed increase in Adr1 level for constitutive ADH2 expression.
Our deletion analysis has identified the RHIHF sequence starting at amino acid 464 as the only nonredundant region of Reg1 that is essential for function and for binding to the yeast PP1 homologue Glc7. This is consistent with the previous observation that the N-terminal 317 amino acids are dispensable for function (46). A concurrent study in the Tatchell lab has shown that a related sequence, KNVRF, is required for the function of Gac1 as well as for binding to Glc7 (64). Cells expressing a Gac1 protein that is missing the N-terminal 130 amino acids which contain this sequence or that has the phenylalanine in the sequence replaced with alanine, F73A, are unable to accumulate normal levels of glycogen. The F73A mutant is also unable to interact with Glc7 in a two-hybrid assay. Both the RHIHF and KNVRF sequences conform to the motif (R/K)X(I/V)XF, which is present in a number of other putative Glc7 regulatory subunits (22). However, the importance of this motif for their function or for binding to Glc7 has not yet been reported.
The crystal structure of the mammalian PP1 catalytic subunit complexed with a peptide containing the binding motif from the muscle glycogen-targeting subunit shows that the peptide binds in an extended conformation to a hydrophobic channel on the surface of the protein (22). This channel is located at the junction of two β-sheets of the β-sandwich opposite the catalytic site. By analogy with the mammalian PP1 catalytic subunit, I466 and F468 of Reg1 would be expected to make extensive hydrophobic contacts with the β-strands comprising the channel. These contacts would lie primarily in β-strands or the loop between two strands. Of the known mutations in GLC7 that are defective in glucose repression, none fall within this region of the protein. We have shown that the region of Reg1 containing the RHIHF sequence is the only contiguous part of the protein absolutely required for the interaction with Glc7. Since the glc7-T152K mutation appears to weaken but not abolish the interaction with Reg1 (58), the known GLC7 mutations may be altering the position of one or more β-strands comprising the hydrophobic pocket rather than disrupting direct protein-protein contacts. Alternatively, the mutant residues may form weak secondary contacts that are not essential for maintaining the interaction.
Another region of Reg1, within the first 198 amino acids, appears to play a differential role in glucose repression. This region had a much stronger influence on SUC2 repression than on ADH2 repression. Part of this effect might be attributable to a decrease in protein stability because the level of Reg1 missing this region is much lower than that of the wild-type protein. If decreased stability does play a role, then the region of Reg1 from amino acids 166 to 369 must be responsible for destabilizing the protein. However, instability is probably not the entire picture. A version of Reg1 missing the first 369 amino acids was just as stable as full-length Reg1 and was just as defective in repressing SUC2 expression as the protein missing the first 198 amino acids. There are at least two possible explanations for this behavior. In one scenario, the N terminus of Reg1 would play a role in stimulating the activity of Glc7 in the PP1 complex. The PP1 holoenzyme containing the N-terminal deletion would still have phosphatase activity; however, the activity would be at a lower level than that with the wild-type version of Reg1. This could account for the observed differential repression if ADH2 expression required less PP1 activity to be fully repressed than SUC2 expression. In a second scenario, a protein specifically required for full repression of SUC2 expression would interact with the N terminus of Reg1. The implication of this protein not playing a major role in repressing ADH2 expression would be that the Reg1-Glc7 complex which represses SUC2 expression is different in composition from that which represses ADH2 expression.
Constitutive ADH2 expression in reg1 mutant cells under normally repressing growth conditions is likely to be controlled by the same regulatory mechanisms as derepressed ADH2 expression, because both were activated by the SNF1 regulatory pathway and both were inhibited by the cAPK pathway. The SNF1 requirement of constitutive ADH2 expression fits nicely with the proposed role of Reg1 in regulating Snf1 activity (40) and suggests that the Reg1-Glc7-Snf1 regulatory mechanism is fundamentally the same as that for SUC2 expression. In contrast to ADH2 expression, however, SUC2 expression is not inhibited by the cAPK regulatory pathway (36). This suggests that the inhibitory effect of cAPK on ADH2 expression is not at the level of the Snf1 complex but rather at a downstream step. This also agrees with previous observations indicating that Snf1 acts independently of cAPK and Adr1 in controlling ADH2 expression (16) and that cAPK acts in part by inhibiting expression of Adr1 (21). Other examples of glucose-regulated processes in yeast where the relationship between Reg1-Glc7-Snf1 and cAPK has been examined include RNA processing and glycogen accumulation (31, 35, 56, 60, 61). For RNA processing, cAPK acts as it did for constitutive ADH2 expression by suppressing the mutant reg1 phenotype (60, 61). cAPK and Reg1-Glc7-Snf1 also do not appear to affect glycogen accumulation by identical mechanisms because bcy1 and snf1 mutations have different effects on expression of GSY2, the gene coding for the predominant glycogen synthase activity in S. cerevisiae (31).
During the course of this study, we were surprised to find that the subcellular location of Reg1 was cytoplasmic and not nuclear. In fact, our data with a fully intact and functional Reg1-GFP fusion protein suggest that Reg1 may actually be excluded from the nucleus. Niederacher and Entian (46) had reported previously that a LacZ fusion protein containing the first 316 amino acids of Reg1 was nuclear and that the amino acid sequence of Reg1 contained several possible nuclear targeting signals. The Reg1-GFP fusion protein that we used in this study was not too large to enter the nucleus, and the GFP portion of the protein did not block nuclear entry because a substantially larger Adr1-GFP fusion protein was able to be accumulate in the nucleus (data not shown). This difference in results might be due to the artifactual accumulation of the Reg1-LacZ fusion in the nucleus when tetramers form through the association of the LacZ portion of the protein. GFP monomers are not known to associate into higher-ordered structures. Alternatively, a cryptic nuclear localization signal within the N-terminal segment of Reg1 may have been uncovered in the LacZ fusion protein used previously. The GFP fusion used in this study was missing only the C-terminal 12 amino acids of Reg1.
The cytoplasmic localization of Reg1 has an interesting implication for the regulation of Snf1 protein kinase activity and perhaps for glucose repression in general. One of the proposed roles for the Reg1-Glc7 protein phosphatase is its participation with the Snf1 kinase complex in a regulatory circuit that controls the subcellular localization of Mig1 (19), a glucose-regulated zinc finger protein involved in repressing expression of genes involved in the utilization of alternate carbon sources (25). In cells growing on glucose, Mig1 is nuclearly localized (19). Upon removal of glucose from the growth medium, Mig1 undergoes Snf1-dependent phosphorylation (57) and is rapidly translocated out of the nucleus (19). Two-hybrid and coimmunoprecipitation experiments have suggested that there is a direct interaction between Snf1 and Mig1 (57). Therefore, upon removal of glucose, the activated Snf1 complex is most likely located in the nucleus where it can phosphorylate Mig1. Since Reg1 is primarily cytoplasmically localized, any interaction between the Snf1 complex and the Reg1-Glc7 complex is likely to occur in the cytoplasm. This is consistent with the results of indirect immunofluorescence studies indicating that both Snf1 and Snf4 are distributed throughout the cytoplasm as well as being localized in the nucleus (6, 7). However, since the activated Snf1 complex is probably nuclear, the activated form of Snf1 most likely cycles rapidly between the nucleus and the cytoplasm, where it can be inactivated by the Reg1-Glc7 form of PP1 when glucose is added to the growth medium.
ACKNOWLEDGMENTS
We gratefully thank Kelly Tatchell for strains and helpful discussions and Xiaolin Wu from his lab for communicating unpublished results on Gac1. We also thank Marian Carlson and Rolf Sternglanz for supplying two-hybrid reagents. Thanks also go to Marian Carlson and Kelly Tatchell for critically reading this paper and providing thoughtful comments. Additionally, we thank Eric Muller and Trisha Davis for materials and advice on creating and examining the subcellular localization of GFP fusion proteins. Finally, we acknowledge the support of Jim Sloan and other members of the Young lab for materials, logistics, and useful discussions.
Support for this research was provided by Public Health Service grant GM26073 from the National Institutes of Health.
REFERENCES
- 1.Baker S H, Frederick D L, Bloecher A, Tatchell K. Alanine-scanning mutagenesis of protein phosphatase type 1 in the yeast Saccharomyces cerevisiae. Genetics. 1997;145:615–626. doi: 10.1093/genetics/145.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blumberg H. Dissertation. Seattle: University of Washington; 1987. [Google Scholar]
- 3.Bollen M, Stalmans W. The structure, role, and regulation of type 1 protein phosphatases. Crit Rev Biochem Mol Biol. 1992;27:227–281. doi: 10.3109/10409239209082564. [DOI] [PubMed] [Google Scholar]
- 4.Carlson M, Osmond B C, Botstein D. Mutants of yeast defective in sucrose utilization. Genetics. 1981;98:25–40. doi: 10.1093/genetics/98.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Celenza J L, Carlson M. Cloning and genetic mapping of SNF1, a gene required for expression of glucose-repressible genes in Saccharomyces cerevisiae. Mol Cell Biol. 1984;4:49–53. doi: 10.1128/mcb.4.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Celenza J L, Carlson M. A yeast gene that is essential for release from glucose repression encodes a protein kinase. Science. 1986;233:1175–1180. doi: 10.1126/science.3526554. [DOI] [PubMed] [Google Scholar]
- 7.Celenza J L, Eng F J, Carlson M. Molecular analysis of the SNF4 gene of Saccharomyces cerevisiae: evidence for physical association of the SNF4 protein with the SNF1 protein kinase. Mol Cell Biol. 1989;9:5045–5054. doi: 10.1128/mcb.9.11.5045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen M X, Chen Y H, Cohen P T. PPQ, a novel protein phosphatase containing a Ser + Asn-rich amino-terminal domain, is involved in the regulation of protein synthesis. Eur J Biochem. 1993;218:689–699. doi: 10.1111/j.1432-1033.1993.tb18423.x. . (Erratum, 221:1133, 1994.) [DOI] [PubMed] [Google Scholar]
- 9.Cheng C, Huang D, Roach P J. Yeast PIG genes: PIG1 encodes a putative type 1 phosphatase subunit that interacts with the yeast glycogen synthase Gsy2p. Yeast. 1997;13:1–8. doi: 10.1002/(SICI)1097-0061(199701)13:1<1::AID-YEA49>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 10.Ciriacy M. Isolation and characterization of yeast mutants defective in intermediary carbon metabolism and in carbon catabolite derepression. Mol Gen Genet. 1977;154:213–220. doi: 10.1007/BF00330840. [DOI] [PubMed] [Google Scholar]
- 11.Cohen P. The structure and regulation of protein phosphatases. Annu Rev Biochem. 1989;58:453–508. doi: 10.1146/annurev.bi.58.070189.002321. [DOI] [PubMed] [Google Scholar]
- 12.Cormack B P, Valdivia R H, Falkow S. FACS-optimized mutants of the green fluorescent protein (GFP) Gene. 1996;173:33–38. doi: 10.1016/0378-1119(95)00685-0. [DOI] [PubMed] [Google Scholar]
- 13.Cross F R. ‘Marker swap’ plasmids: convenient tools for budding yeast molecular genetics. Yeast. 1997;13:647–653. doi: 10.1002/(SICI)1097-0061(19970615)13:7<647::AID-YEA115>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 14.Da Cruz e Silva E F, Hughes V, McDonald P, Stark M J, Cohen P T. Protein phosphatase 2Bw and protein phosphatase Z are Saccharomyces cerevisiae enzymes. Biochim Biophys Acta. 1991;1089:269–272. doi: 10.1016/0167-4781(91)90023-f. [DOI] [PubMed] [Google Scholar]
- 15.Denis C L. The effects of ADR1 and CCR1 gene dosage on the regulation of the glucose-repressible alcohol dehydrogenase from Saccharomyces cerevisiae. Mol Gen Genet. 1987;208:101–106. doi: 10.1007/BF00330429. [DOI] [PubMed] [Google Scholar]
- 16.Denis C L, Audino D C. The CCR1 (SNF1) and SCH9 protein kinases act independently of cAMP-dependent protein kinase and the transcriptional activator ADR1 in controlling yeast ADH2 expression. Mol Gen Genet. 1991;229:395–399. doi: 10.1007/BF00267461. [DOI] [PubMed] [Google Scholar]
- 17.Denis C L, Ciriacy M, Young E T. A positive regulatory gene is required for accumulation of the functional messenger RNA for the glucose-repressible alcohol dehydrogenase from Saccharomyces cerevisiae. J Mol Biol. 1981;148:355–368. doi: 10.1016/0022-2836(81)90181-9. [DOI] [PubMed] [Google Scholar]
- 18.Denis C L, Fontaine S C, Chase D, Kemp B E, Bemis L T. ADR1c mutations enhance the ability of ADR1 to activate transcription by a mechanism that is independent of effects on cyclic AMP-dependent protein kinase phosphorylation of Ser-230. Mol Cell Biol. 1992;12:1507–1514. doi: 10.1128/mcb.12.4.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Vit M J, Waddle J A, Johnston M. Regulated nuclear translocation of the Mig1 glucose repressor. Mol Biol Cell. 1997;8:1603–1618. doi: 10.1091/mbc.8.8.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dombek K M, Camier S, Young E T. ADH2 expression is repressed by REG1 independently of mutations that alter the phosphorylation of the yeast transcription factor ADR1. Mol Cell Biol. 1993;13:4391–4399. doi: 10.1128/mcb.13.7.4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dombek K M, Young E T. Cyclic AMP-dependent protein kinase inhibits ADH2 expression in part by decreasing expression of the transcription factor gene ADR1. Mol Cell Biol. 1997;17:1450–1458. doi: 10.1128/mcb.17.3.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Egloff M P, Johnson D F, Moorhead G, Cohen P T, Cohen P, Barford D. Structural basis for the recognition of regulatory subunits by the catalytic subunit of protein phosphatase 1. EMBO J. 1997;16:1876–1887. doi: 10.1093/emboj/16.8.1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Entian K D, Zimmermann F K. Glycolytic enzymes and intermediates in carbon catabolite repression mutants of Saccharomyces cerevisiae. Mol Gen Genet. 1980;177:345–350. doi: 10.1007/BF00267449. [DOI] [PubMed] [Google Scholar]
- 24.Frederick D L, Tatchell K. The REG2 gene of Saccharomyces cerevisiae encodes a type 1 protein phosphatase-binding protein that functions with Reg1p and the Snf1 protein kinase to regulate growth. Mol Cell Biol. 1996;16:2922–2931. doi: 10.1128/mcb.16.6.2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gancedo J M. Yeast carbon catabolite repression. Microbiol Mol Biol Rev. 1998;62:334–361. doi: 10.1128/mmbr.62.2.334-361.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gietz R D, Schiestl R H, Willems A R, Woods R A. Studies on the transformation of intact yeast cells by the LiAc/SS-DNA/PEG procedure. Yeast. 1995;11:355–360. doi: 10.1002/yea.320110408. [DOI] [PubMed] [Google Scholar]
- 27.Griggs D W, Johnston M. Regulated expression of the GAL4 activator gene in yeast provides a sensitive genetic switch for glucose repression. Proc Natl Acad Sci USA. 1991;88:8597–8601. doi: 10.1073/pnas.88.19.8597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guarente L. Yeast promoters and lacZ fusions designed to study expression of cloned genes in yeast. Methods Enzymol. 1983;101:181–191. doi: 10.1016/0076-6879(83)01013-7. [DOI] [PubMed] [Google Scholar]
- 29.Guthrie C, Fink G R. Guide to yeast genetics and molecular biology. Vol. 194. New York, N.Y: Academic Press, Inc.; 1991. [Google Scholar]
- 30.Hardie D G, Carling D, Carlson M. The AMP-activated/SNF1 protein kinase subfamily: metabolic sensors of the eukaryotic cell? Annu Rev Biochem. 1998;67:821–855. doi: 10.1146/annurev.biochem.67.1.821. [DOI] [PubMed] [Google Scholar]
- 31.Hardy T A, Huang D, Roach P J. Interactions between cAMP-dependent and SNF1 protein kinases in the control of glycogen accumulation in Saccharomyces cerevisiae. J Biol Chem. 1994;269:27907–27913. [PubMed] [Google Scholar]
- 32.Higuchi R. Recombinant PCR. In: Innis M A, Gelfand D H, Sninsky J J, White T W, editors. PCR protocols: a guide to methods and applications. New York, N.Y: Academic Press, Inc.; 1990. pp. 177–83. [Google Scholar]
- 33.Hisamoto N, Frederick D L, Sugimoto K, Tatchell K, Matsumoto K. The EGP1 gene may be a positive regulator of protein phosphatase type 1 in the growth control of Saccharomyces cerevisiae. Mol Cell Biol. 1995;15:3767–3776. doi: 10.1128/mcb.15.7.3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hollenberg S M, Sternglanz R, Cheng P F, Weintraub H. Identification of a new family of tissue-specific basic helix-loop-helix proteins with a two-hybrid system. Mol Cell Biol. 1995;15:3813–3822. doi: 10.1128/mcb.15.7.3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang D, Chun K T, Goebl M G, Roach P J. Genetic interactions between REG1/HEX2 and GLC7, the gene encoding the protein phosphatase type 1 catalytic subunit in Saccharomyces cerevisiae. Genetics. 1996;143:119–127. doi: 10.1093/genetics/143.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hubbard E J, Yang X L, Carlson M. Relationship of the cAMP-dependent protein kinase pathway to the SNF1 protein kinase and invertase expression in Saccharomyces cerevisiae. Genetics. 1992;130:71–80. doi: 10.1093/genetics/130.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang R, Carlson M. Glucose regulates protein interactions within the yeast SNF1 protein kinase complex. Genes Dev. 1996;10:3105–3115. doi: 10.1101/gad.10.24.3105. [DOI] [PubMed] [Google Scholar]
- 38.Jiang R, Carlson M. The Snf1 protein kinase and its activating subunit, Snf4, interact with distinct domains of the Sip1/Sip2/Gal83 component in the kinase complex. Mol Cell Biol. 1997;17:2099–2106. doi: 10.1128/mcb.17.4.2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kennelly P J, Krebs E G. Consensus sequences as substrate specificity determinants for protein kinases and protein phosphatases. J Biol Chem. 1991;266:15555–15558. [PubMed] [Google Scholar]
- 40.Ludin K, Jiang R, Carlson M. Glucose-regulated interaction of a regulatory subunit of protein phosphatase 1 with the Snf1 protein kinase in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1998;95:6245–6250. doi: 10.1073/pnas.95.11.6245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.MacKelvie S H, Andrews P D, Stark M J. The Saccharomyces cerevisiae gene SDS22 encodes a potential regulator of the mitotic function of yeast type 1 protein phosphatase. Mol Cell Biol. 1995;15:3777–3785. doi: 10.1128/mcb.15.7.3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matsumoto K, Yoshimatsu T, Oshima Y. Recessive mutations conferring resistance to carbon catabolite repression of galactokinase synthesis in Saccharomyces cerevisiae. J Bacteriol. 1983;153:1405–1414. doi: 10.1128/jb.153.3.1405-1414.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moser M J, Flory M R, Davis T N. Calmodulin localizes to the spindle pole body of Schizosaccharomyces pombe and performs an essential function in chromosome segregation. J Cell Sci. 1997;110:1805–1812. doi: 10.1242/jcs.110.15.1805. [DOI] [PubMed] [Google Scholar]
- 44.Neigeborn L, Carlson M. Mutations causing constitutive invertase synthesis in yeast: genetic interactions with snf mutations. Genetics. 1987;115:247–253. doi: 10.1093/genetics/115.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Niedenthal R K, Riles L, Johnston M, Hegemann J H. Green fluorescent protein as a marker for gene expression and subcellular localization in budding yeast. Yeast. 1996;12:773–786. doi: 10.1002/(SICI)1097-0061(19960630)12:8%3C773::AID-YEA972%3E3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 46.Niederacher D, Entian K D. Characterization of Hex2 protein, a negative regulatory element necessary for glucose repression in yeast. Eur J Biochem. 1991;200:311–319. doi: 10.1111/j.1432-1033.1991.tb16187.x. [DOI] [PubMed] [Google Scholar]
- 47.Rothstein R J. One-step gene disruption in yeast. Methods Enzymol. 1983;101:202–211. doi: 10.1016/0076-6879(83)01015-0. [DOI] [PubMed] [Google Scholar]
- 48.Shenolikar S. Protein serine/threonine phosphatases—new avenues for cell regulation. Annu Rev Cell Biol. 1994;10:55–86. doi: 10.1146/annurev.cb.10.110194.000415. [DOI] [PubMed] [Google Scholar]
- 49.Sherman F. Getting started with yeast. Methods Enzymol. 1991;194:3–21. doi: 10.1016/0076-6879(91)94004-v. [DOI] [PubMed] [Google Scholar]
- 50.Shero J H, Koval M, Spencer F, Palmer R E, Hieter P, Koshland D. Analysis of chromosome segregation in Saccharomyces cerevisiae. Methods Enzymol. 1991;194:749–773. doi: 10.1016/0076-6879(91)94057-j. [DOI] [PubMed] [Google Scholar]
- 51.Shuster J, Yu J, Cox D, Chan R V, Smith M, Young E. ADR1-mediated regulation of ADH2 requires an inverted repeat sequence. Mol Cell Biol. 1986;6:1894–1902. doi: 10.1128/mcb.6.6.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sikorski R S, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sloan, J. 1998. Personal communication.
- 54.Stark M J. Yeast protein serine/threonine phosphatases: multiple roles and diverse regulation. Yeast. 1996;12:1647–1675. doi: 10.1002/(SICI)1097-0061(199612)12:16%3C1647::AID-YEA71%3E3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 55.Stuart J S, Frederick D L, Varner C M, Tatchell K. The mutant type 1 protein phosphatase encoded by glc7-1 from Saccharomyces cerevisiae fails to interact productively with the GAC1-encoded regulatory subunit. Mol Cell Biol. 1994;14:896–905. doi: 10.1128/mcb.14.2.896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thompson-Jaeger S, Francois J, Gaughran J P, Tatchell K. Deletion of SNF1 affects the nutrient response of yeast and resembles mutations which activate the adenylate cyclase pathway. Genetics. 1991;129:697–706. doi: 10.1093/genetics/129.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Treitel M A, Kuchin S, Carlson M. Snf1 protein kinase regulates phosphorylation of the Mig1 repressor in Saccharomyces cerevisiae. Mol Cell Biol. 1998;18:6273–6280. doi: 10.1128/mcb.18.11.6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tu J, Carlson M. REG1 binds to protein phosphatase type 1 and regulates glucose repression in Saccharomyces cerevisiae. EMBO J. 1995;14:5939–5946. doi: 10.1002/j.1460-2075.1995.tb00282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tu J, Song W, Carlson M. Protein phosphatase type 1 interacts with proteins required for meiosis and other cellular processes in Saccharomyces cerevisiae. Mol Cell Biol. 1996;16:4199–4206. doi: 10.1128/mcb.16.8.4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tung K S, Hopper A K. The glucose repression and RAS-cAMP signal transduction pathways of Saccharomyces cerevisiae each affect RNA processing and the synthesis of a reporter protein. Mol Gen Genet. 1995;247:48–54. doi: 10.1007/BF00425820. [DOI] [PubMed] [Google Scholar]
- 61.Tung K S, Norbeck L L, Nolan S L, Atkinson N S, Hopper A K. SRN1, a yeast gene involved in RNA processing, is identical to HEX2/REG1, a negative regulator in glucose repression. Mol Cell Biol. 1992;12:2673–2680. doi: 10.1128/mcb.12.6.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vincent A, Newnam G, Liebman S W. The yeast translational allosuppressor, SAL6: a new member of the PP1-like phosphatase family with a long serine-rich N-terminal extension. Genetics. 1994;138:597–608. doi: 10.1093/genetics/138.3.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Williamson V M, Bennetzen J, Young E T, Nasmyth K, Hall B D. Isolation of the structural gene for alcohol dehydrogenase by genetic complementation in yeast. Nature. 1980;283:214–216. doi: 10.1038/283214a0. [DOI] [PubMed] [Google Scholar]
- 64.Wu, X. 1999. Personal communication.
- 65.Yang X, Jiang R, Carlson M. A family of proteins containing a conserved domain that mediates interaction with the yeast SNF1 protein kinase complex. EMBO J. 1994;13:5878–5886. doi: 10.1002/j.1460-2075.1994.tb06933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zaman Z, Verwilghen R L. Quantitation of proteins solubilized in sodium dodecyl sulfate-mercaptoethanol-Tris electrophoresis buffer. Anal Biochem. 1979;100:64–69. doi: 10.1016/0003-2697(79)90110-6. [DOI] [PubMed] [Google Scholar]
- 67.Zimmermann F K, Kaufmann I, Rasenberger H, Haubetamann P. Genetics of carbon catabolite repression in Saccharomyces cerevisiae: genes involved in the derepression process. Mol Gen Genet. 1977;151:95–103. doi: 10.1007/BF00446918. [DOI] [PubMed] [Google Scholar]