Abstract
Background
Sars-Cov-2 is a novel corona virus associated with significant morbidity and mortality. Remdesivir and Dexamethasone are two treatments that have shown to be effective against the Sars-Cov-2 associated disease. However, a cost-effectiveness analysis of the two treatments is still lacking.
Objective
The cost-utility of Remdesivir, Dexamethasone and a simultaneous use of the two drugs with respect to standard of care for treatment Covid-19 hospitalized patients is evaluated, together with the effect of Remdesivir compared to the base model but based on alernative assumptions.
Methods
A decision tree for an hypothetical cohort of Covid-19 hospitalized patients, from an health care perspective and a one year horizon is specified. Efficacy data are retrieved from a literature review of clinical trials, whilst costs and utility are obtained from other published studies.
Results
Remdesivir, if health care costs are related to the days of hospitalization, is a cost saving strategy. Dexamethasone is cost effective with an ICER of <DOLLAR/>5208/QALY, and the concurrent use of Remdesivir and Dexamethasone is the most favorable strategy for higher level of willingness to pay thresholds. Moreover, if Remdesivir has a positive effect on mortality the utility is three times higher respect to base case. Whereas, if health care costs are not related to the length of patient hospitalization Remdesivir has an ICER respect to standard of care of <DOLLAR/>384412.8/QALY gained, which is not cost effective. We also find that Dexaamethasone is cost effective respect to standard care if we compute the cost for live saved with an ICER of <DOLLAR/>313.79 for life saved. The uncertainty of the model parameters is also tested through both a one-way deterministic sensitivity analysis and a probabilistic sensitivity analysis.
Conclusion
We find that the use of Remdesivir and/or Dexamethasone is effective from an economic standpoint.
Keywords: Sars-Cov-2, Covid-19, Remdesivir, Dexamethasone, Cost Utility, Cost Effectiveness, pharmacoeconomics, Decision tree
Background
COrona VIrus Disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was first reported in China in December 2019 [1], however phylogenetic estimates support that the Covid-19 pandemic started as far as October 2019 [2]. As of December 2020 the novel corona virus has caused significant mortality and morbidity, as there have been more than 70 million documented cases and more than one million deaths [3], both are probably globally under counted [4, 5]. Consequently there is a great interest in finding potential treatments for the respiratory disease.
Although there was a great enthusiasm for many possible candidate drugs such hydroxycloroquine or lopiravir [6, 7], only two therapeutic agents have shown benefits in robust Randomized Control Trials (RCT): Remdesivir and Dexamethasone. Remdesivir is a 1’-cyano-substituted adenosine nucleotide analogue prodrug developed for the treatment of Ebola virus disease [8], but has also showed in vitro effect against SARS-CoV-2 [9] and in a RCT sponsored by the American National Institute of Allergy and Infectious Diseases [10]. The use of Remdesivir on hospitalized patients showed a significant reduction of length of stay from 15 (95% CI: 13-18) to 10 (95% CI: 9-11) days and suggests, also, a reduction in mortality of 27% (95% CI: -3% to 48%). Although a more recent RCT, sponsored by the World Health Organization (WHO), showed little or no impact on survival [11, 12], the drug has been authorized for treatment in UK, EU and the US, with a list price of <DOLLAR/>2340 for a 5-days treatment course [13, 14]. Dexamethasone, instead, is a broadly used corticosteroid with a low price of approximately 15<DOLLAR/> for a 10-days treatment course [15]. It was authorized by the Food and Drug administration in 1958 [16], and recently was recommended for use in Covid-19 patients with severe respiratory symptoms [17], as it has been showed that it reduces mortality by 36% (95% CI: 19% to 49%) for ventilated ICU patients and 18% (95% CI: 6% to 28%) for non-ventilated patients requiring oxygen [18].
At this stage the only published economic evaluation of Remdesivir is from the Institute for Clinical and Economic Review [19]. It estimates, from the health system perspective, the cost-effectiveness price benchmark of Remdesivir versus standard of care. It finds the price benchmark for Remdesivir to be cost effective for a population similar to the ACTT-1 trial [10], at a willingness to pay threshold of <DOLLAR/>50000 per quality adjusted life year (QALY) gained of <DOLLAR/>2470. In contrast, there are no published economic evaluations on the use of Dexamethasone for Covid-19 hospitalized patients.
The objective of this paper is to evaluate the cost utility of drug therapies for Covid-19. We do this through a cost utility decision tree model, using an health care prospective and one year horizon for an hypothetical cohort of Covid-19 hospitalized patients which represents the cohort of the Remdesivir clinical trial of [10]. We also test different alternative scenarios respect the base case, one when there is a mortality benefit for Remdesivir and one where hospital costs are taken into account in a different manner. We also compute the cost for life saved for Dexamethasone, as it is the only drug which has been proved to have a positive effect on mortality. Given the pandemic situation, resources should be directed to treatment and control of Covid-19, to save as much lives as possible. However, because of the rollout of effective vaccines [20], the pandemic is withdrawing in many part of the world [3], so the focus is shifting again on treatments for those who experience vaccine breakthrough infections and for those who cannot or won’t be vaccinated [21]. Therefore, an economic evaluation of the treatments should not be dismissed. Particularly, on Remdesivir doubts persist on its effectiveness respect to the trial results and the cost-effectiveness of the drug [22, 23]. Lately, beside the US and Europe even low-income countries have begun to use Remdesivir [24], sometimes even putting a ban on export [25]. Thus, because of the unproven effect on mortality this analysis will use utilities to compare the cost-effectiveness among treatments.
The remainder of the paper is structured as follows. “Method” section explains the model structure and the data used for the economic evaluations. In “Results” section we present the results of the analysis which serve as background for a more accurate discussion in “Discussion and concluding remarks”. “Discussion and concluding remarks” section ends the paper with some concluding remarks.
Method
The study is a cost utility analysis based on a decision tree model developed with the R-package Heemod [26, 27]. Costs were considered from the perspective of the US health care services, thus consideration of societal costs is beyond the scope of this study. The use of Remdesivir, Dexamethasone or a hypothetical use of both drugs is compared to the alternative of standard care. The time horizon is one year so we take into account the utility for the survivors and the health care follow up costs.
Model structure
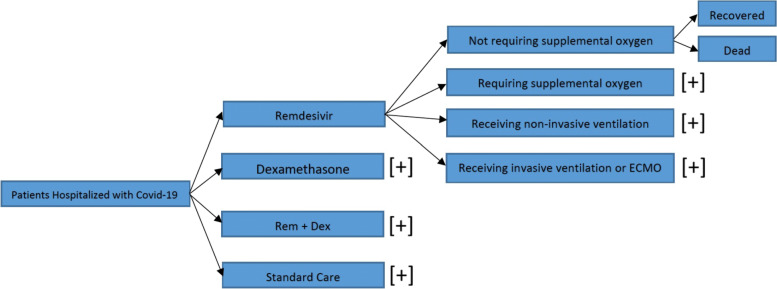
The structure of the decision tree is shown in Fig. 1. We have 4 hypothetical cohort of 1000, 60 years old, Covid-19 hospitalized patients. One cohort is treated with Remdesivir, the second with Dexamethasone and the third with both drugs, while the control group receives standard care. The specification of the decision tree derives from a representation of the Remdesivir clinical trial [10].
Fig. 1.
Decision Tree Schematic
All the patients, therefore, as enrolled in the trial, enter one of four status which represents the baseline severeness of illines indicated in the ordinal score of the RCT:
Hospitalized, not requiring supplemental oxygen;
Hospitalized, requiring supplemental oxygen;
Hospitalized, receiving noninvasive ventilation or high-flow oxygen devices;
Hospitalized, receiving invasive mechanical ventilation or ExtraCorporeal Membrane Oxygenation (ECMO).
Each patient admitted in one of the four categories described above would recover from the infection, and survive, or die, without any transition between the different degrees of hospitalization.
In the considered strategies, the patients receive the drugs as they are enrolled. The use of Remdesivir will have effect on the length of hospitalization resulting in a higher utility for the patients, and a decrease on the cost for the health care service.
We assume costs are divided equally in two parts: a fixed part that does not depend on the length of stay and a variable part correlated with the days of hospitalization. Of course, Remdesivir affects the latter.
There is no available information about whether Dexamethasone reduces the length of stay for the hospitalized patients. But, it has been demonstrated that Dexamethasone has an positive effect on the mortality for patients requiring external oxygen [18]. This positive effect affects the total utility gained as more patients survive. Moreover, there are no studies that examine the use of Dexamethasone together with Remdesivir.
In this paper, we assume that the effects of Remdesivir and Dexamethasone would add to each other: Remdesivir affects the hospital length of stay for all patients and Dexamethasone affects the mortality for patients requiring external oxygen, respectively. If a patient dies neither cost or utility is attached to the dead state, whilst the model takes into account the cost and utility of the patient while hospitalized even if she dies. In contrast, health care follow up costs are considered if she survives, as well as the utility for a year of healthy life. Since the dis-utilities of sequelae for Covid-19 hospitalization have not yet been estimated properly, they are non considered in this study. Moreover, there are not confirmed effects on the improved survival rate for Remdesivir patients. Thus, in our analysis we assume Remdesivir does not affect mortality rates. However, we run an additional base case scenario in which there is an effect as described in the meta analysis of [12].
We also run two more scenarios concerning the use of Remdesivir: one where costs of hospitalization are fixed and do not depend on the length of stay of patients, and another where hospital costs depend totally on the length of hospitalization.
Model parameters
As for the specification of model parameters, information about data collection are as follows: - Data about the probability of being hospitalized in one of the four categories, the probability of outcome, the length of stay until recovery for both the control and the analyzed strategy cohorts are retrieved from the Remdesivir RCT [10]. - Data concerning Hazard Mortality Ratio (HMR) for the use of Dexamethasone in patient requiring external oxygen are instead retrieved from [18]. - Data about the Hazard mortality ratio for the Remdesivir’s scenario in which the anti viral has a positive effect on mortality are collected from the meta analysis of the World Health Organization (WHO) solidarity trial [12].
Costs of hospitalization, and follow up costs come from [28] and [29]. These studies estimate the costs of hospitalization in US particularly for Covid-19 patients, differentiating among the degree of the treatment (hospitalized, non-ventilated, and ventilated). Unfortunately, utilities for hospitalized Covid-19 patients have not been estimated yet, so we use as their proxies the utilities derived from pneumonia and influenza hospitalization, that are found in previous literature [30–32]. As base utility we use the mean utility of a 60 years old person in US [33]. All the model inputs and their references are reported in Table 1.
Table 1.
Parameters of the model
| Data Input Parameter | Value (range) | Distribution | Ref. |
|---|---|---|---|
| % of patients not requiring supplemental oxygen | 13% | Point estimate | [10] |
| % of patients requiring supplemental oxygen | 42% | Point estimate | [10] |
| % of patients receiving noninvasive ventilation | 18.2% | Point estimate | [10] |
| % of patients receiving invasive ventilation or ECMO | 26.8% | Point estimate | [10] |
| Prob. to die if hospitalized without supplemental oxygen | 4.8% (1.6-14.3) | Beta(1.427,29.02) | [10] |
| Prob. to die if hospitalized with oxygen | 12.7% (8.8-18.3) | Beta(21.05,143.41) | [10] |
| Prob. to die if receiving noninvasive ventilation | 20.4%(13.7-29.8) | Beta(17.03,65.94) | [10] |
| Prob. to die if in ECMO | 19.3% (13.8-26.5) | Beta(24.51,102.92) | [10] |
| Remdesivir Rate Ratio for time to recovery | 1.29 (1.21-1.49) | LogN(0.255, 0.0735) | [10] |
| Remdesivir Hazard Mortality Ratio | 0.91 | Point Estimate | [12] |
| (only for the alternative scenario) | |||
| Dexamethasone HMR for hospitalized with supplemental oxygen | 0.82 (.72-.94) | LogN(-.198, 0.07) | [18] |
| Dexamethasone HMR for ventilated patients (invasive and non invasive) | 0.64 (.51-.81) | LogN(-.446, 0.12) | [18] |
| Days of hospitalization without supplemental oxygen | 6 (4-7) | Triangular | [10] |
| Days of hospitalization with supplemental oxygen | 9 (7-10) | Triangular | [10] |
| Days of hospitalization receiving noninvasive ventilation | 20 (14-26) | Triangular | [10] |
| Days of hospitalization receiving invasive ventilation or ECMO | 28 (24-30) | Triangular | [10] |
| Cost of Remdesivir | <DOLLAR/>2340 (1755-2925) | Gamma(2340, 305) | [14] |
| Cost of Dexamethasone | <DOLLAR/>15 | Point estimate | [15] |
| Total cost of hospitalization in standard care without supplemental oxygen | <DOLLAR/>9763 (7322 - 12203) | Gamma(9763, 1195) | [28] |
| Total cost of hospitalization in standard care with supplemental oxygen | <DOLLAR/>13767 (10325 - 17208) | Gamma(13767, 1711) | [28] |
| Total cost of hospitalization in standard care with non invasive ventilation | <DOLLAR/>34223 (25667 - 42778) | Gamma(34223,4113) | [28] |
| Total cost of hospitalization in standard care with invasive ventilation or ECMO | <DOLLAR/>61169 (45876 - 76461) | Gamma(61169,7403) | [28] |
| One year follow up costs for Covid-19 hospitalized survivors | <DOLLAR/>4132 (3099 - 5165) | Gamma(4132, 501) | [28] |
| Utility for hospitalized patients without supplemental oxygen | 0.581(.472 -.729) | Beta(34.2, 23.97) | [30] |
| Utility for hospitalized patients with supplemental oxygen | 0.5 (.4 -.6) | Beta(47.37, 47.37) | [30] |
| Utility for hospitalized patients with non invasive ventilation | 0.23 (.18 -.23) | Beta(61.43, 207.97) | [31] |
| Utility for hospitalized patients with invasive ventilation or ECMO | 0.05 (.02 -.08) | Beta(9.137, 171.78) | [32] |
| Base Utility | 0.851 | Point Estimate | [33] |
Utilities and costs are attached to each node of the decision tree and to compute the their value for a individual hospitalized patient we proceed as follows:
-
Utilities: for each kind of hospitalized patient we attach to the decision node a utility. This depends on the severeness of illness and consequently on the kind of hospitalization and, in addition, on the time spent in the ward. Without loss of generality, let us call the utility given to a generic hospitalized patient in standard care (e.g.: patients not requiring supplemental oxygen), DH the days spent in the ward, and QD the QALY of hospitalization. Then we have:
1 If a patient receives the Remdesivir treatment, she has its length of hospitalization shortened by the Remdesivir rate ratio (RR). Thus, for patients receiving the anti viral treatment we have:2 Paradoxically, if we compute the utility in this manner we would have more utility for the no-Remdesivir arm with respect the Remdesivir’s one. Thus, we need to suppose that patients spend 30 days in this state, and so for Remdesivir patients we have:3 whilst for standard care patients we have:4 where QB is the base QALY for a 60 year old patient. Moreover, we use 30 days because in our analysis this time span is the longest time of hospitalization for any patient (see Table 1, Days of hospitalization receiving invasive ventilation or ECMO). Basically, each patient stays in the hospitalization state for 30 days. She can spend these 30 days as hospitalized (DH) with an attached utility of (QH), or as not hospitalized (30−DH) with an attached utility of QH. After 30 days, she moves to the next state, which is labelled as “recovered” if she survives, or as “dead” if she does not. Since the use of Dexamethasone does not influence the length of hospitalization, we have that the utility for the patients treated with Dexamethasone only is equal to the utility of standard care patients:5 whilst for patients receiving both Dexamethasone and Remdesivir the utility is equal to that of the Remdesivir patients:6 These utilities vary for all the four kinds of hospitalization. Each kind of hospitalization has a different QH and length of hospitalization DH, that would result in different UH. As a results, if a patient survives a year utility of healthy life QB is attached to the recovered node.
-
Costs: for the hospitalization nodes we suppose that costs are divided equally in two parts, and from this assumption we can compute all costs for every kind of hospitalized patients in the four treatment strategies. First, without loss of generality, we take the total cost spent for a hospitalized patient in standard of care CostSC (e.g.total cost of hospitalization in standard care without supplemental oxygen), and divide it by two:
7 with8 Thus, is the fixed part9 and the varying part, from which we compute the daily cost CostDay:10 The cost associated to a hospitalized patient in standard of care is:11 To compute the cost associated to patients receiving Dexamethasone, say , we just need to add the price of the treatment (CostDex) to Eq. 11 :12 Patients receiving Remdesivir, however, have a shorter length of hospitalization. Thus, to compute the cost associated to patients receiving Remdesivir, say , we need to divide DH by the rate ratio RR, just as we did for utilities, in the varying part of the total cost, and next we have to add the price of the treatment:13 Finally, to compute the associated cost for patients that receive both treatments, we add the price of Dexamethasone to Eq. 13:14 These costs vary depending on the severeness and on the length of the hospitalization. For example, in the base case scenario and for patients hospitalized not requiring supplemental oxygen we have DH=6 and Costfix=<DOLLAR/>9763/2, and so on for all the other three kinds. Then, if the patient survives, a year for follow up costs is attached to the recovered state.
Uncertainty analysis
One-way deterministic sensitivity analysis (DSA) is performed to examine the relative importance of the parameters respect to the standard of care for each of the three analyzed strategies. Thus probabilities, costs and utilities are varied by a lower and a higher value derived from the base case. These values are usually identified with respect to their 95% confidence interval, if available. Otherwise, the base case reference value is varied by ±25% from the point estimate. The low-high possible values allows us to determine the range of the Incremental Cost Effectiveness Ratio (ICER) due to parameter uncertainty, and to produce Tornado diagrams. Finally, a Probabilistic Sensitivity Analysis (PSA) with 10,000 Monte Carlo simulations is carried out in order to assess how changes of several parameters influence ICER. Simulations are done by choosing a random value from the distribution of the parameters. As suggested by [34], probabilities and utilities follow a beta distribution, whilst costs follow a gamma distribution. The reference distribution for each parameter can be found in Table 1. The results of the PSA are presented through the cost-effectiveness acceptability curves and in the incremental cost-effectiveness plane. Hereby, the probabilistic sensitivity analysis evaluates the level of confidence, i.e. the reliability, of the results of the analysis.
Results
Base case
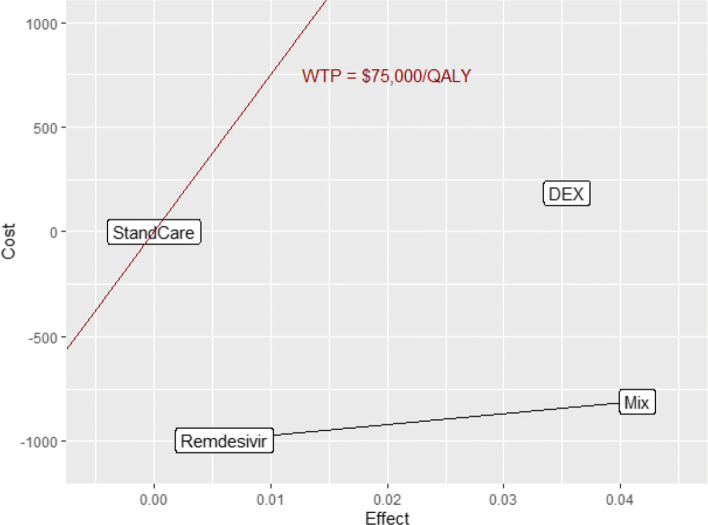
The base case analysis results are presented in Tables 2 and 3. Standard care for hospitalized Covid-19 patients has a cost of <DOLLAR/>33369.9 for 0.7673 QALY. The use of Dexamethasone for patients has an incremental cost effectiveness ratio of <DOLLAR/>5208/QALY versus standard care. The Remdesivir and the Remdesivir plus Dexamethasone (Rem + Dex) treatments both dominated the standard care treatment, as they were less costly and more effective. Table 3 shows how Dexamethasone and Rem+Dex do compare to Remdesivir alone. With respect to Remdesivir, the Dexamethasone treatment had an ICER of <DOLLAR/>40735.2/QALY, whilst the Rem + Dex treatment had an ICER of <DOLLAR/>5221.9/QALY with respect to the solo Remdesivir and it dominates the Dexamethasone treatment. Figure 2 summarizes these results in the incremental cost effectiveness plane. Hereby, we find that Remdesivir is the most cost saving strategy. This is due to the fact that Remdesivir would shorten the length of stay in hospital for patients, resulting in lower cost and a slight increase in utility. Dexamethasone, instead, is the most costly treatment, because it has no effect on the length of stay for the hospitalized patients but has a positive effect on the mortality. Moreover, since in this analysis we take into account the follow up cost for the survivors, the use of Dexamethasone leads to an increase of both cost and utility. The third treatment, which consists in the simultaneous use of Remdesivir and Dexamethasone, is the one that leads to the highest increase in utility and however is less costly than standard of care and Dexamethasone. This finding is probably due to our assumption that the effects of Remdesivir and Dexamethasone would add to each other, resulting in a shorter hospitalization thanks to Remdesivir and in a positive effect on mortality due to Dexamethasone.
Table 2.
Base Case results (ref. Standard care)
| Strategy | Cost | Δ Cost | QALY | Δ QALY | ICER |
|---|---|---|---|---|---|
| Standard Care | 33369.9 | 0.7673 | |||
| Dexamethasone | 33555.6 | 185.8 | 0.803 | 0.0357 | 5208 |
| Remedesivir | 32354.4 | -1015.5 | 0.7734 | 0.0061 | Dominant |
| Rem + Dex | 32540.2 | -829.71 | 0.809 | 0.0417 | Dominant |
Table 3.
Base Case results (ref. Remdesivir)
| Strategy | Δ Cost | Δ QALY | ICER |
|---|---|---|---|
| Remdesivir | ref. | ref. | |
| Dexamethasone | 1201.3 | 0.0295 | 40735.2 |
| Rem + Dex | 185.8 | 0.0356 | 5221.9 |
Fig. 2.
Base Case Cost-effectiveness plane
Alternative Remdesivir scenarios
We also study the effect of Remdesivir depending on other assumptions which differ respect to our base model, and compute the cost for life saved for Dexamethasone. The results of this analysis are shown in Table 4.
Table 4.
Base case results of the alternative scenarios
| Strategy | Cost | Δ Cost | QALY | Δ QALY | ICER |
|---|---|---|---|---|---|
| Standard Care | 33369.9 | 0.7673 | |||
| Remdesivir | 32409.7 | -960.2 | 0.7848 | 0.01748 | Dominant |
| (Assuming mortality benefit) | |||||
| Remdesivir | 35709.9 | 2340 | 0.7734 | 0.0061 | 384412.8 |
| (Assuming only fixed costs) | |||||
| Remdesivir | 28861.2 | -4330.7 | 0.7734 | 0.0061 | Dominant |
| (Assuming only cost per day) | |||||
| Strategy | Cost | Δ Cost | Life Saved | ICER | |
| Standard Care | 29,686,258 | ref. | |||
| Dexamethasone | 29,673,208 | 13050 | 42 | 313.79 |
In the first alternative scenario, we analyze the cost utility of Remdesivir in the case this drug has a positive effect on the patient mortality. So far the effect on mortality of Remdesivir has been disputed, therefore we run a scenario where Remdesivir has an hazard mortality ratio of 0.91 as found in the meta-analysis in [12]. Results show that the utility is almost three times greater because of the greater number of lives saved, but this would mean also higher costs due to the follow up of survived patients that cause this treatment to be not dominant respect to the standard of care. The other scenarios concern the assumption, of the base case model, that hospital costs are equally divided in two parts: a fixed part and a varying part that depends on the days of hospitalization. If we assume that hospital cost depends only on the fixed part, Remdesivir has an ICER of <DOLLAR/>384412.8/QALY, meaning that is not cost effective for any reasonable willingness to pay threshold. On the other hand, if costs depends on the days of hospitalization only, using Remdesivir would lead to a substantial saving in health care related costs. In the last possible scenario, we compute the cost for life saved for the Dexamethasone arm compared to standard care only, as Dexamethasone is the only treatment that has showed to have a positive effect on patient mortality. In this scenario, we do not consider the utility resulting from the length of staying hospitalized, neither the follow up costs for survivors. The results in Table 4 show a difference of <DOLLAR/>13050 for 1000 hospitalized patients, and 41.6 lives saved. This result leads to a cost for life saved of <DOLLAR/>315.79, well below any willingness to pay threshold.
Sensitivity analysis
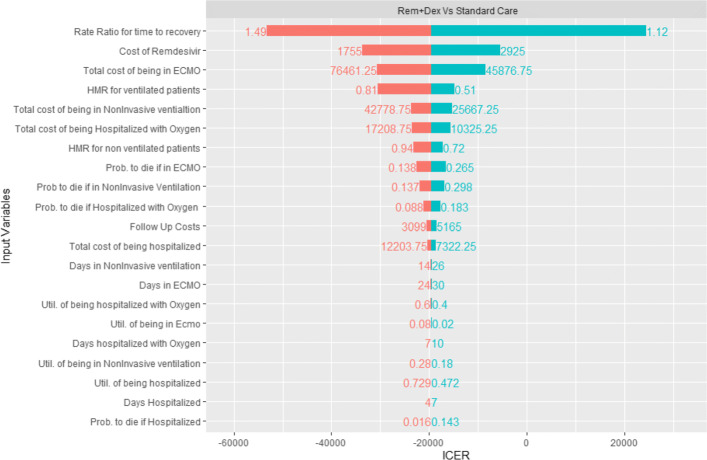
We run three one-way deterministic sensitivity analysis, one for each treatment (Dexamethasone, Remdesivir, and Remdesivir plus Dexamethasone) that is contrasted against the standard of care to check how model results are sensitive to parameter values. The results of these analysis are show in Figs. 3, 4 and 5 as tornado diagrams.
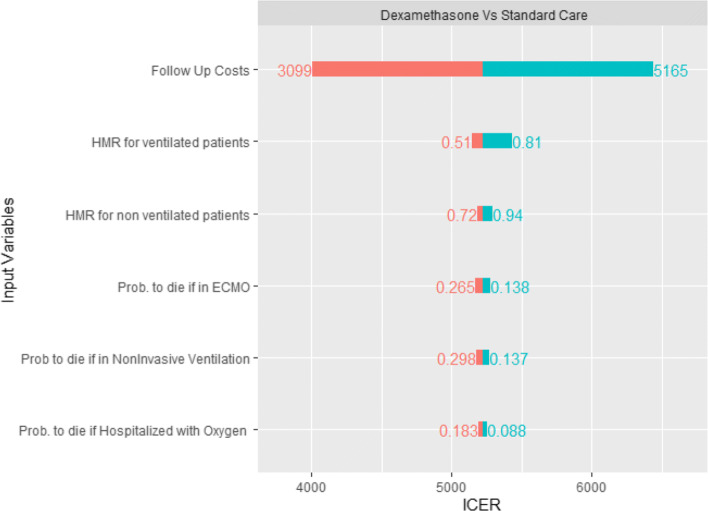
Fig. 3.
DSA Dexamethasone vs Standard Care
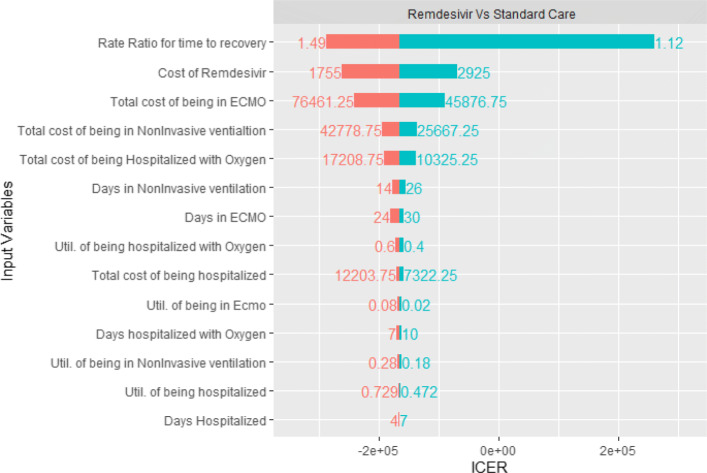
Fig. 4.
DSA Remdesivir vs Standard Care
Fig. 5.
DSA Rem + Dex vs Standard Care
Results show that, for the Dexamethasone treatment, follow up costs are the most important parameter, but regardless the range of the variables the results are robust, as the maximum ICER is <DOLLAR/>6438 per QALY gained, and the use of Dexamethasone is still cost effective. The Remdesivir and the Rem+Dex treatments are still dominant respect to standard of care for any range of parameter, except when the Remdesivir rate ratio for time to recovery is in the lower bound of the confidence interval. In this case, when the rate ratio is 1.12, we have a maximum ICER of <DOLLAR/>24438/QALY for the Rem+Dex treatment, and a maximum ICER of <DOLLAR/>260614/QALY for the solo Remdesivir treatment. The results of the 10,000 Monte Carlo simulations are shown in Table 5. They do not differ from the base case scenario.
Table 5.
PSA results (ref. Standard care)
| Strategy | Cost | Δ Cost | QALY | Δ QALY | ICER |
|---|---|---|---|---|---|
| Standard Care | 33371.7 | 0.7677 | |||
| Dexamethasone | 33555.6 | 183.9 | 0.8028 | 0.0352 | 5229.1 |
| Remedesivir | 32388.4 | -983.3 | 0.7736 | 0.0059 | Dominant |
| Rem + Dex | 32572.2 | -799.4 | 0.8087 | 0.0411 | Dominant |
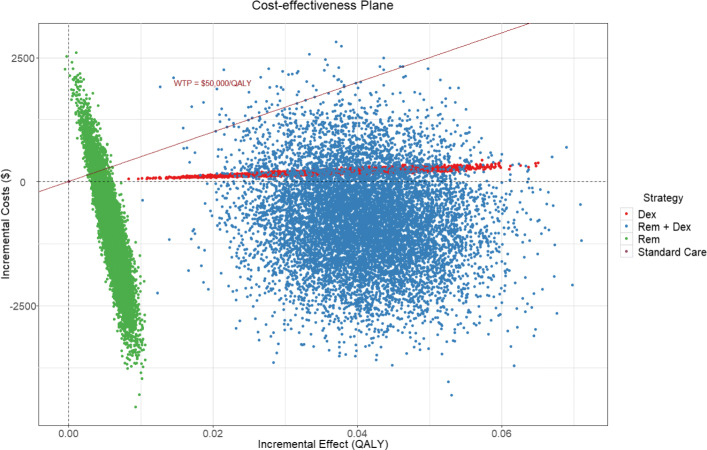
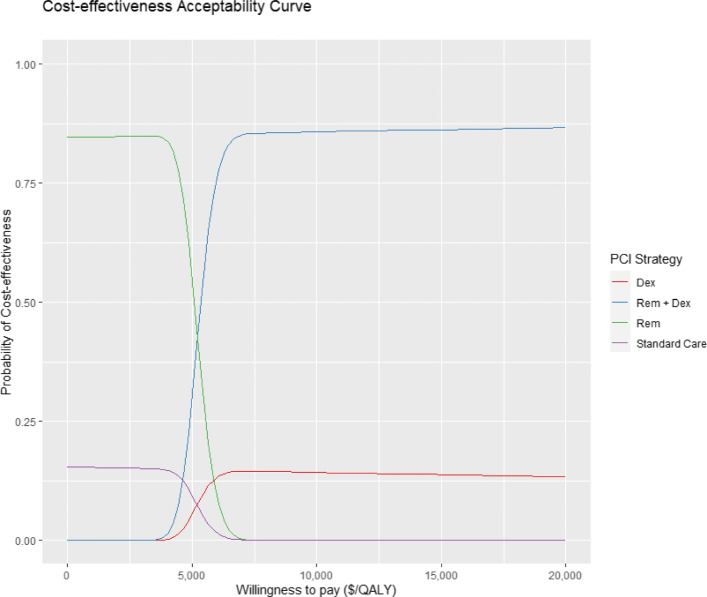
Moreover, we consider a representation of the simulation in the cost effectiveness plane and the willingness to pay threshold for <DOLLAR/>50000 in Fig. 6, and the acceptability curves for all the strategies in Fig. 7. From Fig. 6 we see that the majority of simulations for all the considered treatments lie below the <DOLLAR/>50000 WTP threshold. In particular, only the Remdesivir treatment has a significant part of the simulation outcomes located above the threshold line. In contrast, the Dexamethasone treatment is always below the threshold line. The cost effectiveness acceptability curve in Fig. 7 shows the probability of the interventions being cost effective under different WTP thresholds. For example, for a WTP below <DOLLAR/>5000 the most cost-effective treatment would be Remdesvir, but as the threshold increases the Rem+Dex treatment would be more likely to be favoured.
Fig. 6.
PSA Cost effectiveness plane
Fig. 7.
PSA Cost effectiveness acceptability curves
Discussion and concluding remarks
In the base case analysis, we found the Remdesivir is the most cost saving treatment. This could be counter intuitive given the drug high price, but if we assume that the health costs are related to the length of hospitalization (and they probably are to some extend) we can see why it is cost saving. Another point in favour of the use of Remdesvir is the fact that hospital beds in many countries are a scarce resource, and when the pandemic surges they rapidly get full [35]. Thus, the use of a drug that allows us to vacate hospital beds and human resources should be suggested, even if it does not have effect on mortality. In contrast, Dexamethasone is not cost saving respect to standard of care, although is a low cost treatment. This finding is due to the fact that, for the health care perspective considered in this study, we take into account up to a year of follow-up costs for the survivors, which are in a greater number respect to Remdesvir and standard of care treatments, and also because from the clinical trials there are no sign of reduction for the length of hospitalization. As stated in “Model structure” section, there are not yet findings from randomized control trials on the use of Remdesivir together with Dexamethasone but, if the effects of the two treatments would add each other, this analysis shows that this combined treatment would lead to a substantial saving in health care costs and in the greatest increase in utility for patients respect to standard of care, as it results as the most favorable strategy as shown by the acceptability curves represented in Fig. 7.
Although this study analyzes the cost utility of Covid-19 treatments from a United States prospective, as costs and utility are estimated from studies on this country, we might argue that results are still robust for other developed countries such as Canada, UK or the EU. However, because the main cost driver is hospitalization, for developing countries where hospitalization costs are much lower than the US or other G8 countries, the use of Remdesivir for a price of <DOLLAR/>2430 could be problematic, and depending on the WTP threshold results may lead to favour Dexamethasone.
There are several limitations to our analysis. The cost utility analysis is based on very limited literature on the effectiveness of the treatments analyzed in this model. Moreover, the use of Dexamethasone and Remdesivir taken together has not been proved to work in any randomized control trial, but we think this joint treatment should be analyzed anyway as there is a specific trial [36] currently in progress. Hence, given that Covid-19 is fairly a new disease, new information could become available and the model assumptions could change. Moreover, as we tried to mimic as best as possible the Remdesivir RCT, this analysis did not take into account potential disease progression over time, nor the difference in age, comorbidities, sex and ethnicity among the patient cohort, nor the potential adverse events in terms of nosocomial infection that could be associated with a prolonged hospital stay [37, 38]. Also, the effect of Remdesivir for reduction of days of hospitalization is still a disputed issue, as there have been mixed results in other RCTs as, for example, the WHO solidarity trial [12]. In the latter study, however, the trial has not been designed to primarily test the time to recovery [39]. Moreover, other observational studies have shown the benefits of Remdesivir even for ventilated patients [40, 41]. Next, hospital costs where assumed to be related at some extend to the patients length of stay. We do not know how much length of stay influences the total cost of hospitalization, and to account for this uncertainty we also considered the two alternative simulation scenarios. Given the burden on mortality for the pandemic, we also compute the cost for life saved for the Dexamethasone arm, which has shown to be very convenient, and below any WTP threshold.
Last but not least, data concerning utility for Covid-19 has not been estimated yet, so we had to rely on data derived from influenza and pneumonia hospitalization. Finally, we have assumed that after the discharge from hospital there would not be any impact on mortality or utility and, consequently, there are concerns on effects of Covid-19 post infection [42]. These are yet to be determined, hence they are not considered in this analysis, For these reasons, the time horizon of the model is only one year long, but we are aware that a different model time span could cause important changes in the outcomes [43]. If we had computed the cost and utility for a longer time horizon (e.g. lifetime horizon) we would have taken into account the baseline mortality risk, that would differ substantially with the cohort patient’s age. Moreover, we should have been looking at the decrease on baseline QALY [33] and the increase in the healthcare cost that survived patients would face in their remaining life.
In conclusion, any of the treatments analyzed in this study resulted to be cost effective for the willingness to pay threshold of developed countries. Caveats apply on the use of Remdesivir as its effectiveness depends strongly on the hospital costs. Moreover, its effect is still unknown when it is used concurrently with Dexamethasone, so additional information is required on this topic. Further research about economic evaluations is needed, as more treatments for Covid-19 patients are being introduced, such as monoclonal antibodies, and the treatments guidelines are continually updated [44, 45]. Anyway, this analysis supports the idea that the use of Remdesivir and/or Dexamethasone is effective from an economic standpoint as it has been shown that it allows to save costs and lives.
Acknowledgements
Not applicable.
Abbreviations
- COVID-19
COrona VIrus Disease 2019
- Sars-Cov-2
Severe acute respiratory syndrome coronavirus 2
- RCT
Randomized Control Trials
- WHO
World Health Organization
- ICU
Intensive care unit
- QALY
Quality adjusted life year
- ECMO
ExtraCorporeal Membrane Oxygenation
- HMR
Hazard Mortality Ratio
- RR
Rate Ratio
- DSA
Deterministic sensitivity analysis
- ICER
Incremental Cost Effectiveness Ratio
- PSA
Probabilistic Sensitivity Analysis
- WTP
Willingness to pay
Authors’ contributions
AC developed the theoretical formalism, performed the analytic calculations, and performed the numerical simulations. CC supervised the project. All authors contributed to the final version of the manuscript.
Funding
Financial support for this study was provided by a grant from the Department of Business and Economics, University of Cagliari, within the research project Dipartimento di Eccellenza 2018, Grant n. 1.005.14/2019. The funders had no role in the study design, data collection, analysis, interpretation, decision to publish, or preparation of the manuscript.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Guan W-j, Ni Z-y, Hu Y, Liang W-h, Ou C-q, He J-x, Liu L, Shan H, Lei C-l, Hui DS, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–20. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Dorp L, Acman M, Richard D, Shaw LP, Ford CE, Ormond L, Owen CJ, Pang J, Tan CCS, Boshier FAT, Ortiz AT, Balloux F. Emergence of genomic diversity and recurrent mutations in sars-cov-2. Infect Genet Evol. 2020;83:104351. doi: 10.1016/j.meegid.2020.104351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roser M, Ritchie H, Ortiz-Ospina E, Hasell J. Coronavirus pandemic (covid-19). Our World in Data. 2020. Available at: https://ourworldindata.org/coronavirus. Accessed 12 Dec 2020.
- 4.Pollán M, Pérez-Gómez B, Pastor-Barriuso R, Oteo J, Hernán MA, Pérez-Olmeda M, Sanmartín JL, Fernández-García A, Cruz I, de Larrea NF, et al. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet. 2020;396(10250):535–44. doi: 10.1016/S0140-6736(20)31483-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alicandro G, Remuzzi G, La Vecchia C. Italy’s first wave of the COVID-19 pandemic has ended: no excess mortality in May, 2020. Lancet. 2020;396(10253):27–8. doi: 10.1016/S0140-6736(20)31865-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Skipper CP, Pastick KA, Engen NW, Bangdiwala AS, Abassi M, Lofgren SM, Williams DA, Okafor EC, Pullen MF, Nicol MR, et al. Hydroxychloroquine in nonhospitalized adults with early covid-19: a randomized trial. Ann Intern Med. 2020;173(8):623–31. doi: 10.7326/M20-4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, Ruan L, Song B, Cai Y, Wei M, et al. A trial of lopinavir–ritonavir in adults hospitalized with severe covid-19. N Engl J Med. 2020;382:1787–99. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tchesnokov EP, Feng JY, Porter DP, Götte M. Mechanism of inhibition of Ebola virus RNA-dependent RNA polymerase by remdesivir. Viruses. 2019;11(4):326. doi: 10.3390/v11040326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pan X, Dong L, Yang N, Chen D, Peng C. Potential drugs for the treatment of the novel coronavirus pneumonia (COVID-19) in China. Virus Research. 2020;286, 198057:1–12. doi: 10.1016/j.virusres.2020.198057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, Hohmann E, Chu HY, Luetkemeyer A, Kline S, et al. Remdesivir for the treatment of Covid-19. N Engl J Med. 2020;383(19):1813–26. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dyer O. Covid-19: Remdesivir has little or no impact on survival, WHO trial shows. BMJ. 2020;371:m4057. doi: 10.1136/bmj.m4057. [DOI] [PubMed] [Google Scholar]
- 12.WHO Solidarity Trial Consortium et al. Repurposed antiviral drugs for COVID-19–interim WHO SOLIDARITY trial results. N Engl J Med. 2021;384:497–511. doi: 10.1056/NEJMoa2023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldman JD, Lye DC, Hui DS, Marks KM, Bruno R, Montejano R, Spinner CD, Galli M, Ahn M-Y, Nahass RG, et al. Remdesivir for 5 or 10 days in patients with severe covid-19. N Engl J Med. 2020;383(19):1827–37. doi: 10.1056/NEJMoa2015301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Day D. An open letter from daniel o’day, chairman & ceo, gilead sciences: Gilead Sciences.2020. Available at: https://stories.gilead.com/articles/an-open-letterfrom-daniel-oday-june-29. Accessed 12 Dec 2020.
- 15.Dexamethasone Prices, Coupons and Patient Assistance Programs. Drugs.com. 2020. Available from: https://www.drugs.com/price-guide/dexamethasone. Accessed 12 Dec 2020.
- 16.Benedek T. History of the development of corticosteroid therapy. Clin Exp Rheumatol. 2011;29(5 Suppl 68):5–12. [PubMed] [Google Scholar]
- 17.U.S. Department of Health and Human Services . Temporary policy for compounding of certain drugs for hospitalized patients by outsourcing fa-cilities during the COVID-19 public health emergency: guidance for industry. Silver Spring: Food and Drug Administration, Center for Drug Evaluation and Research; 2020. [Google Scholar]
- 18.Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, Staplin N, Brightling C, Ustianowski A, Elmahi E, et al. Dexamethasone in hospitalized patients with covid-19-preliminary report. N Engl J Med. 2020:693–704. 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed]
- 19.Whittington MD, Campbell JD. Alternative pricing models for remdesivir and other potential treatments for COVID-19. 2020. http://icer.org/wp-content/uploads/2020/11/ICER-COVID_Updated_Report_11102020.pdf. Accessed 12 Dec 2020.
- 20.Forni G, Mantovani A. Covid-19 vaccines: where we stand and challenges ahead. Cell Death Differ. 2021;28(2):626–39. doi: 10.1038/s41418-020-00720-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hacisuleyman E, Hale C, Saito Y, Blachere NE, Bergh M, Conlon EG, Schaefer-Babajew DJ, DaSilva J, Muecksch F, Gaebler C, et al. Vaccine breakthrough infections with SARS-CoV-2 variants. N Engl J Med. 2021;384(23):2212–8. doi: 10.1056/NEJMoa2105000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kelleni MT. Tocilizumab, remdesivir, favipiravir, and dexamethasone repurposed for covid-19: A comprehensive clinical and pharmacovigilant reassessment. SN Compr Clin Med. 2021;3(4):919–23. doi: 10.1007/s42399-021-00824-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dal-Ré R, Banzi R, Georgin-Lavialle S, Porcher R, Sofat R, Zeitlinger M, Rosendaal FR. Remdesivir for COVID-19 in Europe: will it provide value for money? Lancet Respir Med. 2021;9(2):127–8. doi: 10.1016/S2213-2600(20)30568-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adhikari S, Khadka S, Dahal S, Shrestha DB, Shahi J, Bajgain Y. Remdesivir in covid-19 management: availability and relevance to low-and middle-income countries. Drugs Ther Perspect. 2021;37(1):26–8. doi: 10.1007/s40267-020-00791-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.India bans exports of anti-viral drug Remdesivir as COVID-19 cases surge. Thomson Reuters. 2021. Available at: https://www.reuters.com/world/india/india-bans-exports-anti-viral-drug-remdesivir-covid-19-cases-surge-2021-04-11/. Accessed 2 May 2021.
- 26.Filipovic-Pierucci A, Zarca K, Durand-Zaleski I. Markov models for health economic evaluation modelling in r with the heemod package. Value Health. 2016;19(7):369. doi: 10.1016/j.jval.2016.09.133. [DOI] [Google Scholar]
- 27.R Core Team. R: A language and environment for statistical computing. R Found Stat Comput Vienna Austria. 2019. https://www.R-project.org/.
- 28.Rae M, Claxton G, Kurani N, McDermott D, Cox C. Potential costs of COVID-19 treatment for people with employer coverage. In: Peterson Center on Healthcare and Kaiser Family Foundation: 2020. Available at: https://www.healthsystemtracker.org/brief/potential-costs-of-coronavirus-treatment-for-people-with-employer-coverage/. Accessed 12 Dec 2020.
- 29.Bartsch SM, Ferguson MC, McKinnell JA, O’Shea KJ, Wedlock PT, Siegmund SS, Lee BY. The Potential Health Care Costs And Resource Use Associated With COVID-19 In The United States: A simulation estimate of the direct medical costs and health care resource use associated with COVID-19 infections in the United States. Health Affairs. 2020;39(6):927–35. doi: 10.1377/hlthaff.2020.00426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang J, Jit M, Zheng Y, Feng L, Liu X, Wu JT, Yu H. The impact of influenza on the health related quality of life in China: an EQ-5D survey. BMC Infect Dis. 2017;17(1):686. doi: 10.1186/s12879-017-2801-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hollmann M, Garin O, Galante M, Ferrer M, Dominguez A, Alonso J. Impact of influenza on health-related quality of life among confirmed (H1N1) 2009 patients. PLoS ONE. 2013;8(3):60477. doi: 10.1371/journal.pone.0060477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brown KL, Wray J, Wood TL, Mc Mahon AM, Burch M, Cairns J. Cost utility evaluation of extracorporeal membrane oxygenation as a bridge to transplant for children with end-stage heart failure due to dilated cardiomyopathy. J Heart Lung Transplant. 2009;28(1):32–8. doi: 10.1016/j.healun.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 33.Sullivan PW, Ghushchyan V. Preference-based EQ-5D index scores for chronic conditions in the United States. Med Dec Making. 2006;26(4):410–20. doi: 10.1177/0272989X06290495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Briggs AH, O’Brien BJ, Blackhouse G. Thinking outside the box: recent advances in the analysis and presentation of uncertainty in cost-effectiveness studies. Ann Rev Public Health. 2002;23(1):377–401. doi: 10.1146/annurev.publhealth.23.100901.140534. [DOI] [PubMed] [Google Scholar]
- 35.Giannakeas V, Bhatia D, Warkentin MT, Bogoch II, Stall NM. Estimating the maximum capacity of COVID-19 cases manageable per day given a health care system’s constrained resources. Ann Intern Med. 2020;173(5):407–10. doi: 10.7326/M20-1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adaptive covid-19 treatment trial 4 (actt-4). Updated February 25, 2021. https://clinicaltrials.gov/ct2/show/NCT04640168. Accessed 2 May 2021.
- 37.Hughes S, Troise O, Donaldson H, Mughal N, Moore LS. Bacterial and fungal coinfection among hospitalized patients with COVID-19: a retrospective cohort study in a UK secondary-care setting. Clin Microbiol Infect. 2020;26(10):1395–9. doi: 10.1016/j.cmi.2020.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kangas-Dick A, Gazivoda V, Ibrahim M, Sun A, Shaw JP, Brichkov I, Wiesel O. Clinical characteristics and outcome of pneumomediastinum in patients with covid-19 pneumonia. J Laparoendosc Adv Surg Tech. 2021;31(3):273–8. doi: 10.1089/lap.2020.0692. [DOI] [PubMed] [Google Scholar]
- 39.Aleissa MM, Silverman EA, Acosta LMP, Nutt CT, Richterman AG, Marty FM. New perspectives on antimicrobial agents: remdesivir treatment for COVID-19. Antimicrob Agents Chemother. 2020;65(1):01814–20. doi: 10.1128/AAC.01814-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Olender SA, Perez KK, Go AS, Balani B, Price-Haywood EG, Shah NS, Wang S, Walunas TL, Swaminathan S, Slim J, Chin B, De Wit S, Ali SM, Soriano Viladomiu A, Robinson P, Gottlieb RL, Tsang TYO, Lee IH, Haubrich RH, Chokkalingam AP, Lin L, Zhong L, Bekele BN, Mera-Giler R, Gallant J, Smith LE, Osinusi AO, Brainard DM, Hu H, Phulpin C, Edgar H, Diaz-Cuervo H, Bernardino JI. Remdesivir for severe covid-19 versus a cohort receiving standard of care. Clin Infect Dis. 2020:ciaa1041. 10.1093/cid/ciaa1041.
- 41.Lapadula G, Bernasconi DP, Bellani G, Soria A, Rona R, Bombino M, et al. Remdesivir use in patients requiring mechanical ventilation due to COVID-19. Open Forum Infect Dis. 2020;7(11):ofaa481. doi: 10.1093/ofid/ofaa481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mahase E. Covid-19: What do we know about “long covid”? BMJ. 2020;370:m2815. doi: 10.1136/bmj.m2815. [DOI] [PubMed] [Google Scholar]
- 43.Carta A, Conversano C. On the use of Markov models in pharmacoeconomics: Pros and cons and implications for policy makers. Front Public Health. 2020; 8. 10.3389/fpubh.2020.569500. [DOI] [PMC free article] [PubMed]
- 44.Deb P, Molla MMA, Saif-Ur-Rahman KM. An update to monoclonal antibody as therapeutic option against COVID-19. Biosaf Health. 2021;3(2):87–91. doi: 10.1016/j.bsheal.2021.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health. 2021. Available at https://www.covid19treatmentguidelines.nih.gov/. Accessed 2 May 2020. [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.