Abstract
Objective
To identify significant pathways and genes in intervertebral disc degeneration (IDD) based on bioinformatics analysis.
Design
The GEO database was used to download the GSE124272 dataset. Differentially expressed genes (DEGs) were analyzed using Limma package in R language. Then, gene ontologies (GO), Kyoto encyclopedia of genes and genomes (KEGG), and protein-protein interaction (PPI) networks were used to further identify hub genes. The mRNA expression levels of top six hub genes were verified.
Results
We found 563 DEGs, of which 214 were upregulated and 349 were downregulated. The top 5 GO terms and pathways were shown including immune response, cell cycle, and p53 pathway. Based on the PPI analysis, we verified the mRNA expression levels of 6 hub genes. The mRNA levels of CHEK1, CDCA2, SKA3, and KIF20A were upregulated in degenerative NP tissue than in healthy NP tissue. However, the mRNA level of BUB1 and SPC25 was downregulated.
Conclusions
This study may provide new biomarkers for the IDD and treatments to repair IDD related to CHEK1, CDCA2, SKA3, BUB1, KIF20A, and SPC25.
1. Introduction
Intervertebral disc degeneration (IDD) is a significant cause of intervertebral disc degeneration diseases including low back pain, stenosis, lumbar disc herniation, and ischialgia, which can cause the worldwide economic and social burden and seriously affect quality of life [1–8]. Boden et al. found that all subjects between 60 and 80 years of age showed IDD, though 35% of subjects between 20 and 39 years of age showed at least one lumbar level of IDD [9]. There is currently no effective treatment for IDD to reverse and repair IDD [10]. In recent decades, finding effective treatments and developing appropriate treatment procedures for IDD have become the focus of research [11–17].
The anatomical structure of a complete intervertebral disc consists of the surrounding annulus fibrosus (AF), the central nucleus pulposus (NP), and the cartilage endplate (CEP) [18]. During IDD, inflammation, oxidative stress, apoptosis, senescence, and other pathological factors are involved. IDD is considered a result of multifactorial contributions including trauma, inflammation, age-related changes, and local nutritional and vascular dysfunction [19]. At present, conservation and surgical treatments are often used in the treatment of IDD, but those methods can only relieve symptoms and may recur repeatedly, limiting spinal activity [20]. One key reason for the current situation is the lack of a clear understanding of its pathophysiology and molecular mechanisms [21].
Hub genes are defined as having the highest degree of connectivity, suggesting functional importance in the diseases. Thus, investigating the hub genes and key pathways associated with IDD is necessary to clarify the pathophysiology and molecular mechanisms, which provides potential effective therapeutic strategies.
Recently, microarray technology and bioinformatics analysis have become popular methods of exploration of disease pathogenesis and identification of biomarkers for disease progression [22]. The purpose of this study is to identify potential molecular targets and signaling pathways associated with IDD based on Gene Expression Omnibus datasets. To identify potential hub genes among these DEGs, we constructed protein-protein interaction (PPI) networks. These hub genes were also validated using human nucleus pulposus (NP) samples, which reveals potential molecular mechanisms associated with IDD.
2. Research Materials and Methods
2.1. Retrieving Data
The GEO database (http://www.ncbi.nlm.nih.gov/geo) is a gene expression database created by NCBI (the National Biotechnology Information Center of the United States). This GSE124272 dataset was downloaded from the GEO database, which consisted of 16 whole blood samples from 8 patients with intervertebral disc degeneration and 8 patients with healthy discs. This dataset was published by Wang Yi et al. [23]in 2019, and the patients were genotyped using the GPL21185 Agilent-072363 SurePrint G3 Human GE v3 8x60K Microarray.
2.2. Data Processing and Identification of DEGs
We used affy package (https://bioconductor.org/biocLite.R) in the R software bioconductor to read the data. The robust multiarray averaging (RMA) algorithm was used to normalize the data. Finally, we used the Limma package (Limma package R 3.4.3) to identify DEGs. P < 0.05 and |log2FC | ≥1 were used to identify DEGs from these samples, and volcano plot was constructed.
2.3. Functional Enrichment Analysis of DEGs
The DAVID (http://david.abcc.ncifcrf.gov/) database is mainly used for functional enrichment analysis of DEGs [24]. We used the DAVID database to enrich and analyze the functions and pathways including gene ontology (GO) terms and Kyoto encyclopedia of genes and genomes (KEGG) terms with a significant threshold of P < 0.05. GO terms consisted of three categories: biological process (BP), cellular component (CC), and molecular functional (MF).
2.4. PPI Analysis
PPI networks were constructed to predict protein-protein interactions of DEGs using the STRING database (http://www.stringdb.Org). Then, these data were uploaded into Cytoscape software (https://cytoscape.org/) to visualize the networks of DEGs. Finally, we used MCODE plug-in in Cytoscape to identify the hub genes based on the previously constructed PPI networks.
2.5. Verification of Hub Genes
We obtained nucleus pulposus (NP) tissues from two patients with acute lumbar disc herniation or degenerative disc disease. One patient underwent posterior lumbar interbody fusion, and another underwent percutaneous endoscopic lumbar discectomy. The relatively healthy NP tissues were grades I~II, and degenerated NP tissues were grades III~V according to Pfirrmann classification score by magnetic resonance imaging. The Pfirrmann grades of these two NP tissues, respectively, are grade II and grade V. The NP tissues were harvested under sterile conditions and immediately sent to the laboratory. Written informed consent was obtained from each patient. The study was approved by the Ethics Committee of Hubei Provincial Hospital of Traditional Chinese Medicine.
2.6. RNA Extraction and Quantitative Real-Time- (qRT-) PCR
We used TRIzol reagent (Ambion, Foster City, CA, USA) to extract total RNA from human NP tissues according to the manufacturer's instructions. We used PrimeScript RT Master Mix (Takara Bio, Shiga, Japan) to reverse transcribe total RNA according to the manufacturer's instructions. Then, qRT-PCR was performed using the One-Step SYBR PrimeScript RT-PCR Kit (Takara Bio) to quantify the mRNA expression levels of SPC25, CHEK1, CDCA2, SKA3, BUB1, and KIF20A. Endogenous housekeeping gene GAPDH was used to normalize these mRNA expression levels. The 2−ΔΔCt method was used to compute these relative expression levels. Table 1 shows the primer sequences used for qRT-PCR.
Table 1.
Primer sequences.
| Gene | Sequence | Size |
|---|---|---|
| CDCA2 | Forward: 5′-TGTGGGCAGCTCTGTAGAAA-3′ Reverse: 5′-GGGAAGTGGAAGGAAGTGGA-3′ |
185 bp |
| KIF20A | Forward: 5′-CGCAGTCACAGCATCTTCTC-3′ Reverse: 5′-GACGAAGGGCAGCAATACAG-3′ |
202 bp |
| SPC25 | Forward: 5′-TGCAGAGAGGTTGAAAAGGC-3′ Reverse: 5′-TGAGGGGCACTATCTGACAC-3′ |
198 bp |
| CHEK1 | Forward: 5′-TCAGGTGGTGTGTCAGAGTC-3′ Reverse: 5′-GACATGTGGGCTGGGAAAAG-3′ |
211 bp |
| SKA3 | Forward: 5′-AGCCCGTAATTGTAACCCCA-3′ Reverse: 5′-TCTGTATCTATGGCCTCCTCAC-3′ |
211 bp |
| BUB1 | Forward: 5′-TCCCCTCTGTACATTGCCTG-3′ | 168 bp |
| Reverse: 5′-AGCTGGCAAATGGGTTTCAG-3′ | ||
| GAPDH | Forward: 5′-TCAAGAAGGTGGTGAAGCAGG-3′ Reverse: 5′-TCAAAGGTGGAGGAGTGGGT-3′ |
115 bp |
2.7. Statistical Analysis
SPSS statistical software 24.0 was used for statistical analysis (SPSS, Inc., Chicago, IL, USA). Each experiment was carried out at least three times. The results are performed as the mean ± standard deviation (SD). These data were analyzed using Student's t-test. To evaluate the difference in gene expression level between two groups, P < 0.05 was considered significant.
3. Results
3.1. Identification of DEGs
We analyzed the DEGs between two groups in the GSE124272 based on P < 0.05 and ∣log2FC | ≥1 and found 563 DEGs, of which 214 were upregulated and 349 were downregulated. Volcano plot was shown to visualize the DEGs in Figure 1.
Figure 1.
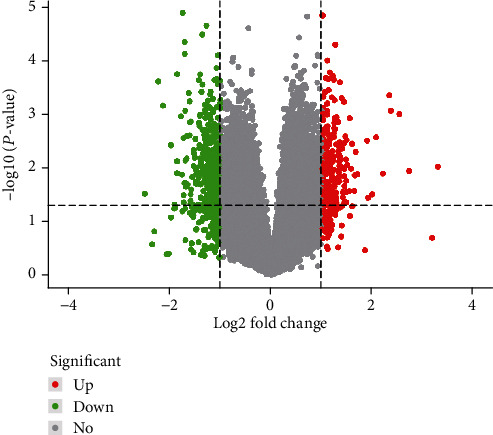
Volcano plot of DEGs. Red represents high expression, green represents low expression, and black represents no difference.
3.2. GO and KEGG Functional Enrichment Analysis of DEGs
We analyzed the functional enrichment of DEGs to explore the potential molecular mechanism and related genes. The top five enriched GO terms and the KEGG pathway of DEGs are shown in Figures 2 and 3 and Table 2.
Figure 2.
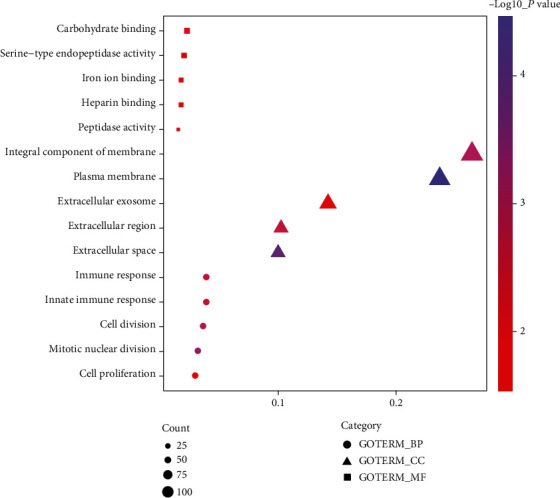
Top five enriched GO terms associated with the DEGs.
Figure 3.
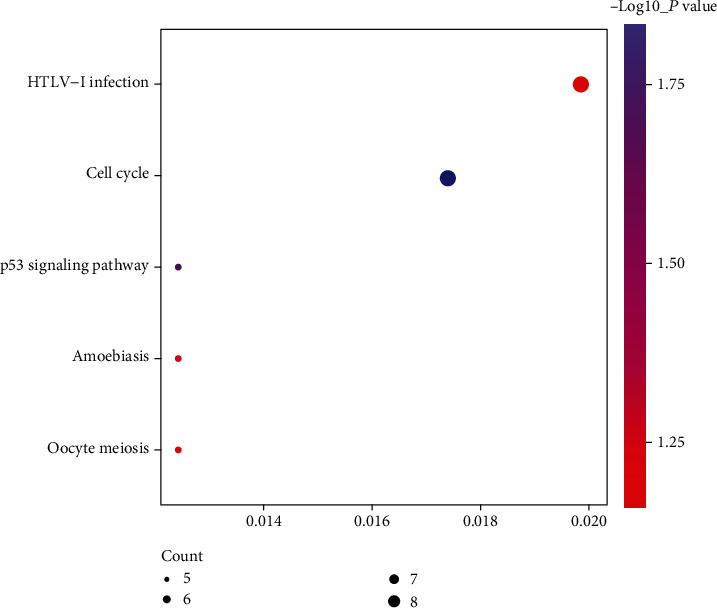
Top five enriched KEGG terms associated with the DEGs.
Table 2.
Functional and pathway enrichment analysis of DEGs.
| Category | Term | Count | Gene ratio (%) | P value |
|---|---|---|---|---|
| GOTERM_BP | Defense response to gram-negative bacterium | 7 | 1.75% | 2.00E − 04 |
| GOTERM_BP | Mitotic nuclear division | 13 | 3.24% | 5.04E − 04 |
| GOTERM_BP | Chromosome segregation | 7 | 1.75% | 6.38E − 04 |
| GOTERM_BP | Response to yeast | 4 | 1.00% | 9.36E − 04 |
| GOTERM_BP | Cell division | 15 | 3.74% | 1.16E − 03 |
| GOTERM_CC | Plasma membrane | 95 | 23.71% | 2.95E − 05 |
| GOTERM_CC | Specific granule | 5 | 1.25% | 3.70E − 05 |
| GOTERM_CC | Neuronal cell body | 17 | 4.24% | 3.75E − 05 |
| GOTERM_CC | Extracellular space | 40 | 9.98% | 1.29E − 04 |
| GOTERM_CC | Cell surface | 20 | 4.99% | 8.98E − 04 |
| GOTERM_MF | Carbohydrate binding | 9 | 2.25% | 1.09E − 02 |
| GOTERM_MF | Peptidase activity | 6 | 1.50% | 1.25E − 02 |
| GOTERM_MF | Iron ion binding | 7 | 1.75% | 3.08E − 02 |
| GOTERM_MF | Galactosyltransferase activity | 3 | 0.75% | 3.38E − 02 |
| GOTERM_MF | Cytokine binding | 3 | 0.75% | 3.38E − 02 |
| KEGG_pathway | Cell cycle | 7 | 1.75% | 1.05E − 02 |
| KEGG_PATHWAY | p53 signaling pathway | 5 | 1.25% | 1.77E − 02 |
| KEGG_PATHWAY | Amoebiasis | 5 | 1.25% | 7.41E − 02 |
| KEGG_PATHWAY | PPAR signaling pathway | 4 | 1.00% | 7.86E − 02 |
| KEGG_PATHWAY | Oocyte meiosis | 5 | 1.25% | 8.45E − 02 |
3.3. PPI Network and Hub Gene Analysis
In order to identify the potential interaction between the DEGs, we built a PPI network and used the Cytoscape software to visualize it. In the intervertebral disc degeneration group, the PPI network included 207 nodes and 668 edges (Figure 4), and the top 20 hub genes were identified by MCODE (Figure 5).
Figure 4.
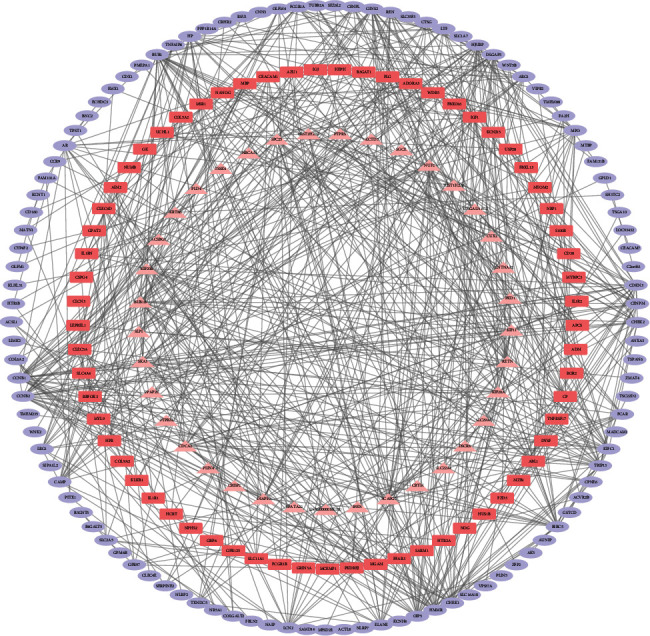
Protein-protein interaction (PPI) network of DEG.
Figure 5.
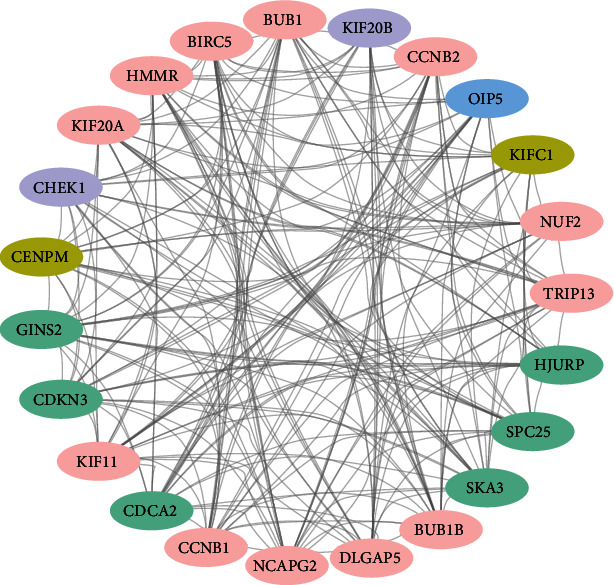
Top 20 hub genes identified by PPI.
3.4. Validation of the Hub Genes
To validate the identified hub genes, we used two human NP tissues including one from a patient with healthy disc and one from a patient with degenerated disc to identify the mRNA levels of the top six hub genes. The mRNA levels of CHEK1, CDCA2, SKA3, and KIF20A were upregulated in degenerative NP tissue than in healthy NP tissue. However, the mRNA level of BUB1 and SPC25 was downregulated in degenerative NP tissue. These results are shown in Figure 6.
Figure 6.
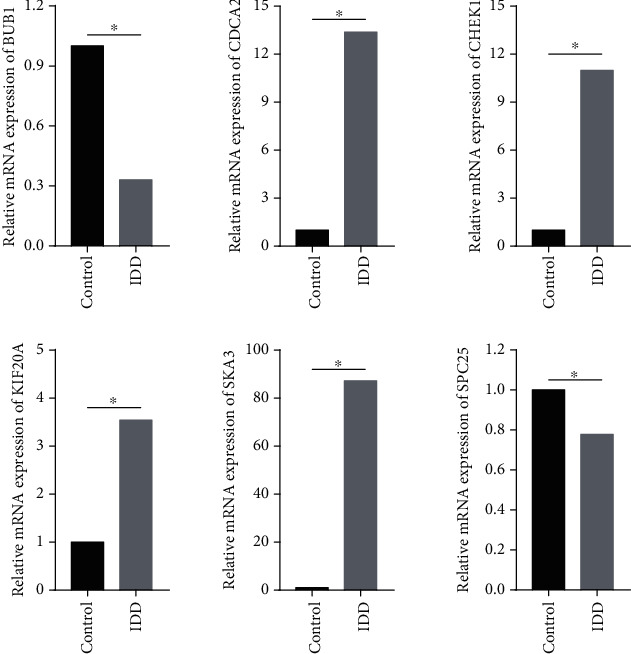
Validation of the mRNA expression levels of the top six hub genes.
4. Discussion
IDD plays an important role in spine-related diseases. It is difficult to reverse the IDD progression with current treatments. Although numerous studies have studied the mechanisms of IDD, the underlying mechanisms remain unclear. In our study, the GSE124272 was used to identify the DEGs of two groups. GO and KEGG enrichment analysis were performed to find significant biological processes and signal pathways. The important biological processes are related to immune response, innate immune response, cell division, mitotic nuclear division, and cell proliferation. It is worth mentioning that cell proliferation plays an important role in IDD. Many studies have confirmed that the proliferation level of intervertebral disc cells is diminished during IDD progression. On the contrary, the apoptosis rate of intervertebral disc cells increases relatively [25–31]. Many molecules regulate this process of IDD, including proteins, microRNAs, and long noncoding RNAs [25, 32–40]. Wang et al. [33] demonstrated that lncRNA-RMRP promoted NP cell proliferation and upregulated expression of cyclin D1 and PCNA.
KEGG pathway analysis showed several pathways associated with IDD. p53 signaling pathway have been widely studied in IDD [27, 41–44]. Many biological processes are involved in p53 pathway, including cell proliferation, apoptosis, and senescence. p53 is a transcription factor and well-known as a tumor suppressor in humans and other mammals, which participates in the regulation of biological processes. It has been reported that the p53 pathway regulates the senescence process of cartilage end plate cells and p53 phosphorylation level can been decreased through silencing of SUMO2 [27].
PPI network analysis was used to identify hub genes in IDD. We validated six hub genes including CHEK1, CDCA2, SKA3, BUB1, SPC25, and KIF20A. Cell division cycle associated 2 (CDCA2) is a cell-related protein, which is related to CDCA1, 3, and 4-8 [45]. It has been reported that upregulation of CDCA2 regulated by HIF-1α inhibited apoptosis and promoted proliferation in prostate cancer [46]. HIF-1α, a hypoxia-inducible factor, is a transcriptional factor which affects the homeostatic maintenance of NP tissue and extracellular matrix metabolism [47]. Uchida et al. [48] confirmed that silencing of CDCA2 significantly inhibited cellular proliferation and promoted apoptosis. In addition, CDCA2 performs regulation function through signal pathways including the MAPK pathway [49].
SKA3, a subunit located in the kinetochore outer layer of the SKA complex, performs biological function related to the NDC80 complex to affect proper mitotic exit during mitosis, which regulates cell proliferation and migration [50]. In CRC cells, silencing of SKA3 reduced cell growth rates and increased apoptosis, inducing G2/M arrest and decreasing migration, and anchorage-independent growth [51]. Accumulating evidence indicates that SKA3 induces the expression of matrix metalloproteinase- (MMP-) 2, MMP-7, and MMP-9 via activating the PI3K/AKT signal pathway which regulates numerous cellular functions mainly including angiogenesis, metabolism, cell growth, cell proliferation, protein synthesis, transcription activity, and cell apoptosis [52, 53].
It is found that SPC25 plays an important role in regulating cell proliferation, apoptosis, and invasion. Cui et al. demonstrated that knockdown of SPC25 suppressed cell proliferation through decreasing in the number of cells in the S phase and increasing in the number of cells in the G2/M phase [54]. Chen et al. [55] demonstrated that SPC25 was upregulated in lung cancer tissues and was involved in the regulation of tumor cell proliferation and metastasis. Additionally, SPC25 knockdown upregulated expression level of p53, indicating that the p53 signaling pathway is a potential pathway associated with SPC25 [56]. p53, a cellular stress sensor, responds to diverse stress signals including DNA damage, hypoxia by regulating cell senescence, and apoptosis in the intervertebral disc [57].
Kinesin-like family member 20A (KIF20A), a mammalian mitotic kinesin-like motor protein of the Kinesin superfamily proteins, is related to Golgi apparatus dynamics and considered a significant molecule for cell cycle regulation. It is also found that KIF20A regulates the localization of subset of central spindle components [58]. A further study indicated that KIF20A affected the process of porcine early embryo development.
Zhao et al. [59] demonstrated that KIF20A can promote tumor cell proliferation and inhibit apoptosis in vivo and in vitro. Likewise, Duan et al. [60] confirmed that cellular proliferation and invasion were promoted through upregulation of KIF20A, and cell viability and invasion capacity were inhibited via silencing of KIF20A. These findings indicate that KIF20A may be a novel target associated with NP cell degeneration.
Checkpoint kinase 1 (CHEK1), a serine/threonine-specific protein kinase, regulates the DNA damage response and cell cycle checkpoint reactions and plays a significant role in the S and G2 cell cycle checkpoints [61, 62]. In order to inhibit damaged cells from developing throughout the cell cycle, CHEK1 is activated to affect the initiation of cell cycle checkpoints, cell cycle arrest, DNA repair, and cell death, which regulates the phosphorylation level of several downstream effectors to trigger a pleiotropic cellular response [63]. Upregulation of CHEK1 is involved in various types of cancer and promotes tumor progression via affecting cell cycle and DNA damage response including breast cancer, pancreatic cancer, and oral squamous cell carcinoma [64]. It has been demonstrated that upregulation of CHEK1 can ameliorate the overall survival of non-small-cell lung cancer patients and miR-195 downregulates the expression level of CHEK1, which inhibits cell migration, growth, or invasion [65].
BUB1, a component of the spindle assembly checkpoint which is a surveillance mechanism of ensuring genome stability by delaying anaphase, is involved in cell divisions through regulating SAC function and yielding a highly aberrant mitosis. Accumulating evidence indicates that centromeres in BUB1-deficient cells separate prematurely, which reveals that BUB1 is essential for the proliferation of fibroblasts. Schliekelman et al. found that the upregulation level of BUB1 promoted tumorigenesis and had oncogenic properties [66–68]. Likewise, Zhu et al. [69] demonstrated that BUB1 overexpression promoted liver cancer cell proliferation and downexpression of BUB1 suppressed cell proliferation by activating the phosphorylation level of SMAD2. It has been reported that SMAD2 regulates NP cell proliferation and anabolic processes by activation of TGF-beta 1. Additionally, silencing of BUB1 activates a p53-dependent premature senescence response, which induces IDD through cell cycle arrest, cellular senescence, or apoptosis in the intervertebral disc [70].
In conclusion, we have used bioinformatics analysis to identify CHEK1, CDCA2, SKA3, BUB1, SPC25, and KIF20A as hub genes related to IDD, which provided a new insight into IDD pathogenesis and treatment. Further studies should be performed to verify these results.
Acknowledgments
The present study was supported by the Traditional Chinese Medicine Research Project Awarded by the Health and Family Planning Commission of Hubei Province (Grant number ZY2019M044) and the Natural Science Foundation of Hubei Province (Grant number 2019CFC901).
Data Availability
Our data can be found in the GEO database.
Conflicts of Interest
All authors declare no conflicts of interest.
Authors' Contributions
Zhiwen Zhang, Chengjian He, and Qiong Wang are responsible for the design of the study. Yang Li, Liming Zheng, and Bangzhi Li are assigned to the data curation, writing, formal analysis, and experiment. All authors have read and agreed to the published version of the manuscript. Zhiwen Zhang and Qiong Wang contributed equally to this work.
References
- 1.Kang L., Liu S., Li J., Tian Y., Xue Y., Liu X. The mitochondria-targeted anti-oxidant MitoQ protects against intervertebral disc degeneration by ameliorating mitochondrial dysfunction and redox imbalance. Cell Proliferation. 2020;53(3, article e12779) doi: 10.1111/cpr.12779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loibl M., Wuertz-Kozak K., Vadala G., Lang S., Fairbank J., Urban J. P. Controversies in regenerative medicine: should intervertebral disc degeneration be treated with mesenchymal stem cells? JOR Spine. 2019;2(1, article e1043) doi: 10.1002/jsp2.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ma K., Chen S., Li Z., et al. Mechanisms of endogenous repair failure during intervertebral disc degeneration. Osteoarthritis and Cartilage. 2019;27:41–48. doi: 10.1016/j.joca.2018.08.021. [DOI] [PubMed] [Google Scholar]
- 4.Miki T., Naoki F., Takashima H., Takebayashi T. Associations between paraspinal muscle morphology, disc degeneration, and clinical features in patients with lumbar spinal stenosis. Progress in Rehabilitation Medicine. 2020;5, article e1043 doi: 10.2490/prm.20200015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rustenburg C. M. E., Faraj S. S. A., Ket J. C. F., Emanuel K. S., Smit T. H. Prognostic factors in the progression of intervertebral disc degeneration: which patient should be targeted with regenerative therapies? JOR Spine. 2019;2, article e1063 doi: 10.1002/jsp2.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun Z., Liu Z. H., Chen Y. F., et al. Molecular immunotherapy might shed a light on the treatment strategies for disc degeneration and herniation. Medical Hypotheses. 2013;81(3):477–480. doi: 10.1016/j.mehy.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 7.Wang Y., Shen J., Chen Y., et al. PINK1 protects against oxidative stress induced senescence of human nucleus pulposus cells via regulating mitophagy. Biochemical and Biophysical Research Communications. 2018;504(2):406–414. doi: 10.1016/j.bbrc.2018.06.031. [DOI] [PubMed] [Google Scholar]
- 8.Zhang G. Z., Deng Y. J., Xie Q. Q., et al. Sirtuins and intervertebral disc degeneration: roles in inflammation, oxidative stress, and mitochondrial function. Clinica Chimica Acta. 2020;508:33–42. doi: 10.1016/j.cca.2020.04.016. [DOI] [PubMed] [Google Scholar]
- 9.Boden S. D., Davis D. O., Dina T. S., Patronas N. J., Wiesel S. W. Abnormal magnetic-resonance scans of the lumbar spine in asymptomatic subjects. A prospective investigation. The Journal of Bone and Joint Surgery American Volume. 1990;72(3):403–408. doi: 10.2106/00004623-199072030-00013. [DOI] [PubMed] [Google Scholar]
- 10.Kang L., Xiang Q., Zhan S., et al. Restoration of autophagic flux rescues oxidative damage and mitochondrial dysfunction to protect against intervertebral disc degeneration. Oxidative Medicine and Cellular Longevity. 2019;2019:27. doi: 10.1155/2019/7810320.7810320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dong W., Liu J., Lv Y., et al. miR-640 aggravates intervertebral disc degeneration via NF-κB and WNT signalling pathway. Cell Proliferation. 2019;52, article e12664 doi: 10.1111/cpr.12664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kadow T., Sowa G., Vo N., Kang J. D. Molecular basis of intervertebral disc degeneration and herniations: what are the important translational questions? Clinical Orthopaedics and Related Research. 2015;473(6):1903–1912. doi: 10.1007/s11999-014-3774-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sadowska A., Hitzl W., Karol A., et al. Differential regulation of TRP channel gene and protein expression by intervertebral disc degeneration and back pain. Scientific Reports. 2019;9(1, article 18889) doi: 10.1038/s41598-019-55212-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sampara P., Banala R. R., Vemuri S. K., Av G. R., Gpv S. Understanding the molecular biology of intervertebral disc degeneration and potential gene therapy strategies for regeneration: a review. Gene Therapy. 2018;25(2):67–82. doi: 10.1038/s41434-018-0004-0. [DOI] [PubMed] [Google Scholar]
- 15.van Uden S., Silva-Correia J., Oliveira J. M., Reis R. L. Current strategies for treatment of intervertebral disc degeneration: substitution and regeneration possibilities. Biomaterials Research. 2017;21(1):p. 22. doi: 10.1186/s40824-017-0106-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xie L., Huang W., Fang Z., et al. Circ ERCC2 ameliorated intervertebral disc degeneration by regulating mitophagy and apoptosis through miR-182-5p/SIRT1 axis. Cell Death & Disease. 2019;10(10):p. 751. doi: 10.1038/s41419-019-1978-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu J., Yu W., Wang Y., et al. lncRNAs: function and mechanism in cartilage development, degeneration, and regeneration. Stem Cell Research & Therapy. 2019;10(1):p. 344. doi: 10.1186/s13287-019-1458-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo H. Y., Guo M. K., Wan Z. Y., Song F., Wang H. Q. Emerging evidence on noncoding-RNA regulatory machinery in intervertebral disc degeneration: a narrative review. Arthritis Research & Therapy. 2020;22(1):p. 270. doi: 10.1186/s13075-020-02353-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu P. H., Kim H. S., Jang I. T. Intervertebral disc diseases PART 2: a review of the current diagnostic and treatment strategies for intervertebral disc disease. International Journal of Molecular Sciences. 2020;21 doi: 10.3390/ijms21062135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang F., Nan L. P., Zhou S. F., et al. Injectable hydrogel combined with nucleus pulposus-derived mesenchymal stem cells for the treatment of degenerative intervertebral disc in rats. Stem Cells International. 2019;2019:17. doi: 10.1155/2019/8496025.8496025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang W., Li P., Xu J., et al. Resveratrol attenuates high glucose-induced nucleus pulposus cell apoptosis and senescence through activating the ROS-mediated PI3K/Akt pathway. Bioscience Reports. 2018;38 doi: 10.1042/bsr20171454. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Hui J., Liu R., Zhang H., He S., Wei A. Screening and identification of critical biomarkers in erectile dysfunction: evidence from bioinformatic analysis. PeerJ. 2020;8, article e8653 doi: 10.7717/peerj.8653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y., Dai G., Li L., et al. Transcriptome signatures reveal candidate key genes in the whole blood of patients with lumbar disc prolapse. Experimental and Therapeutic Medicine. 2019;18(6):4591–4602. doi: 10.3892/etm.2019.8137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dennis G., Jr., Sherman B. T., Hosack D. A., et al. DAVID: database for annotation, visualization, and integrated discovery. Genome Biology. 2003;4(5):p. P3. doi: 10.1186/gb-2003-4-5-p3. [DOI] [PubMed] [Google Scholar]
- 25.Chen J., Jia Y. S., Liu G. Z., et al. Role of LncRNA TUG1 in intervertebral disc degeneration and nucleus pulposus cells via regulating Wnt/β-catenin signaling pathway. Biochemical and Biophysical Research Communications. 2017;491(3):668–674. doi: 10.1016/j.bbrc.2017.07.146. [DOI] [PubMed] [Google Scholar]
- 26.Guo Y., Tian L., Liu X., He Y., Chang S., Shen Y. ERRFI1 inhibits proliferation and inflammation of nucleus pulposus and is negatively regulated by miR-2355-5p in intervertebral disc degeneration. Spine. 2019;44(15):E873–e881. doi: 10.1097/BRS.0000000000003011. [DOI] [PubMed] [Google Scholar]
- 27.Jin L. Z., Lu J. S., Gao J. W. Silencing SUMO2 promotes protection against degradation and apoptosis of nucleus pulposus cells through p53 signaling pathway in intervertebral disc degeneration. Bioscience Reports. 2018;38(3) doi: 10.1042/BSR20171523. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Long J., Wang X., du X., et al. JAG2/Notch2 inhibits intervertebral disc degeneration by modulating cell proliferation, apoptosis, and extracellular matrix. Arthritis Research & Therapy. 2019;21(1):p. 213. doi: 10.1186/s13075-019-1990-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tan H., Zhao L., Song R., Liu Y., Wang L. microRNA-665 promotes the proliferation and matrix degradation of nucleus pulposus through targeting GDF5 in intervertebral disc degeneration. Journal of Cellular Biochemistry. 2018;119(9):7218–7225. doi: 10.1002/jcb.26888. [DOI] [PubMed] [Google Scholar]
- 30.Zhang C., Smith M. P., Zhou G. K., et al. Phlpp1 is associated with human intervertebral disc degeneration and its deficiency promotes healing after needle puncture injury in mice. Cell Death & Disease. 2019;10(10):p. 754. doi: 10.1038/s41419-019-1985-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou X., Chen L., Grad S., et al. The roles and perspectives of microRNAs as biomarkers for intervertebral disc degeneration. Journal of Tissue Engineering and Regenerative Medicine. 2017;11(12):3481–3487. doi: 10.1002/term.2261. [DOI] [PubMed] [Google Scholar]
- 32.Wang X., Lv G., Li J., Wang B., Zhang Q., Lu C. LncRNA-RP11-296A18.3/miR-138/HIF1A pathway regulates the proliferation ECM synthesis of human nucleus pulposus cells (HNPCs) Journal of Cellular Biochemistry. 2017;118(12):4862–4871. doi: 10.1002/jcb.26166. [DOI] [PubMed] [Google Scholar]
- 33.Wang X., Peng L., Gong X., Zhang X., Sun R., Du J. LncRNA-RMRP promotes nucleus pulposus cell proliferation through regulating miR-206 expression. Journal of Cellular and Molecular Medicine. 2018;22(11):5468–5476. doi: 10.1111/jcmm.13817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao K., Zhang Y., Yuan H., Zhao M., Zhao D. Long noncoding RNA LINC00958 accelerates the proliferation and matrix degradation of the nucleus pulposus by regulating miR-203/SMAD3. Aging. 2019;11(23):10814–10825. doi: 10.18632/aging.102436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang R., Wen B., Sun D. miR-573 regulates cell proliferation and apoptosis by targeting Bax in nucleus pulposus cells. Cellular & Molecular Biology Letters. 2019;24(1):p. 2. doi: 10.1186/s11658-018-0132-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu X., Li Z., Shen J., et al. MicroRNA-10b promotes nucleus pulposus cell proliferation through RhoC-Akt pathway by targeting HOXD10 in intervetebral disc degeneration. PLoS One. 2013;8(12, article e83080) doi: 10.1371/journal.pone.0083080. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 37.Zhao Z., Zheng J., Ye Y., Zhao K., Wang R., Wang R. MicroRNA-25-3p regulates human nucleus pulposus cell proliferation and apoptosis in intervertebral disc degeneration by targeting Bim. Molecular Medicine Reports. 2020;22(5):3621–3628. doi: 10.3892/mmr.2020.11483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen J., Mei Z., Huang B., et al. IL-6/YAP1/β-catenin signaling is involved in intervertebral disc degeneration. Journal of Cellular Physiology. 2019;234(5):5964–5971. doi: 10.1002/jcp.27065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Z., Wang G., Zhu X., Geng D., Yang H. Interleukin-2 is upregulated in patients with a prolapsed lumbar intervertebral disc and modulates cell proliferation, apoptosis and extracellular matrix metabolism of human nucleus pulposus cells. Experimental and Therapeutic Medicine. 2015;10(6):2437–2443. doi: 10.3892/etm.2015.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu J. W., Wang J., Yang K., et al. FBW7 inhibits nucleus pulposus cells proliferation by downregulation of cyclin E in the intervertebral disc degeneration. European Review for Medical and Pharmacological Sciences. 2020;24(2):508–516. doi: 10.26355/eurrev_202001_20026. [DOI] [PubMed] [Google Scholar]
- 41.Feng C., Liu H., Yang M., Zhang Y., Huang B., Zhou Y. Disc cell senescence in intervertebral disc degeneration: causes and molecular pathways. Cell Cycle. 2016;15(13):1674–1684. doi: 10.1080/15384101.2016.1152433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ge J., Cheng X., Yuan C., et al. Syndecan-4 is a novel therapeutic target for intervertebral disc degeneration via suppressing JNK/p53 pathway. International Journal of Biological Sciences. 2020;16(5):766–776. doi: 10.7150/ijbs.40189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li Y., Cao L., Li J., et al. Influence of microgravity-induced intervertebral disc degeneration of rats on expression levels of p53/p16 and proinflammatory factors. Experimental and Therapeutic Medicine. 2019;17(2):1367–1373. doi: 10.3892/etm.2018.7085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang Z., Lin J., Nisar M., et al. The Sirt1/P53 Axis in diabetic intervertebral disc degeneration pathogenesis and therapeutics. Oxidative Medicine and Cellular Longevity. 2019;2019:21. doi: 10.1155/2019/7959573.7959573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walker M. G. Drug target discovery by gene expression analysis: cell cycle genes. Current Cancer Drug Targets. 2001;1(1):73–83. doi: 10.2174/1568009013334241. [DOI] [PubMed] [Google Scholar]
- 46.Zhang Y., Cheng Y., Zhang Z., et al. CDCA2 inhibits apoptosis and promotes cell proliferation in prostate cancer and is directly regulated by HIF-1α pathway. Frontiers in Oncology. 2020;10:p. 725. doi: 10.3389/fonc.2020.00725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tran C. M., Fujita N., Huang B. L., et al. Hypoxia-inducible Factor (HIF)-1α and CCN2 Form a Regulatory Circuit in Hypoxic Nucleus Pulposus Cells: The Journal of Biological Chemistry. 2013;288(18):12654–12666. doi: 10.1074/jbc.M112.448860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Uchida F., Uzawa K., Kasamatsu A., et al. Overexpression of CDCA2 in human squamous cell carcinoma: correlation with prevention of G1 phase arrest and apoptosis. PLoS One. 2013;8(2, article e56381) doi: 10.1371/journal.pone.0056381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.de Castro I. J., Budzak J., di Giacinto M. L., et al. Repo-Man/PP1 regulates heterochromatin formation in interphase. Nature Communications. 2017;8(1, article 14048) doi: 10.1038/ncomms14048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hu R., Wang M. Q., Niu W. B., et al. SKA3 promotes cell proliferation and migration in cervical cancer by activating the PI3K/Akt signaling pathway. Cancer Cell International. 2018;18(1):p. 183. doi: 10.1186/s12935-018-0670-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chuang T. P., Wang J. Y., Jao S. W., et al. Over-expression of AURKA, SKA3 and DSN1 contributes to colorectal adenoma to carcinoma progression. Oncotarget. 2016;7(29):45803–45818. doi: 10.18632/oncotarget.9960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hu D. D., Chen H. L., Lou L. M., Zhang H., Yang G. L. SKA3 promotes lung adenocarcinoma metastasis through the EGFR-PI3K-Akt axis. Bioscience Reports. 2020;40(2) doi: 10.1042/BSR20194335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hemmings B. A., Restuccia D. F. PI3K-PKB/Akt pathway. Cold Spring Harbor Perspectives in Biology. 2012;4, article a011189 doi: 10.1101/cshperspect.a011189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cui F., Hu J., Fan Y., Tan J., Tang H. Knockdown of spindle pole body component 25 homolog inhibits cell proliferation and cycle progression in prostate cancer. Oncology Letters. 2018;15(4):5712–5720. doi: 10.3892/ol.2018.8003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen J., Chen H., Yang H., Dai H. SPC25 upregulation increases cancer stem cell properties in non-small cell lung adenocarcinoma cells and independently predicts poor survival. Biomedicine & Pharmacotherapy. 2018;100:233–239. doi: 10.1016/j.biopha.2018.02.015. [DOI] [PubMed] [Google Scholar]
- 56.Chen F., Zhang K., Huang Y., Luo F., Hu K., Cai Q. SPC25 may promote proliferation and metastasis of hepatocellular carcinoma via p53. FEBS Open Bio. 2020;10(7):1261–1275. doi: 10.1002/2211-5463.12872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jiang D., Attardi L. D. Engaging the p53 metabolic brake drives senescence. Cell Research. 2013;23(6):739–740. doi: 10.1038/cr.2013.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang Y., Liu J., Peng X., et al. KIF20A regulates porcine oocyte maturation and early embryo development. PLoS One. 2014;9(7, article e102898) doi: 10.1371/journal.pone.0102898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhao X., Zhou L. L., Li X., et al. Overexpression of KIF20A confers malignant phenotype of lung adenocarcinoma by promoting cell proliferation and inhibiting apoptosis. Cancer Medicine. 2018;7(9):4678–4689. doi: 10.1002/cam4.1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Duan J., Huang W., Shi H. Positive expression of KIF20A indicates poor prognosis of glioma patients. Oncotargets and Therapy. 2016;9:6741–6749. doi: 10.2147/OTT.S115974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McNeely S., Beckmann R., Bence Lin A. K. CHEK again: Revisiting the development of CHK1 inhibitors for cancer therapy. Pharmacology & Therapeutics. 2014;142(1):1–10. doi: 10.1016/j.pharmthera.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 62.Abe H., Alavattam K. G., Kato Y., et al. CHEK1 coordinates DNA damage signaling and meiotic progression in the male germline of mice. Human Molecular Genetics. 2018;27(7):1136–1149. doi: 10.1093/hmg/ddy022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang Y., Hunter T. Roles of Chk1 in cell biology and cancer therapy. International Journal of Cancer. 2014;134(5):1013–1023. doi: 10.1002/ijc.28226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xie Y., Wei R. R., Huang G. L., Zhang M. Y., Yuan Y. F., Wang H. Y. Checkpoint kinase 1 is negatively regulated by miR-497 in hepatocellular carcinoma. Medical Oncology. 2014;31(3):p. 844. doi: 10.1007/s12032-014-0844-4. [DOI] [PubMed] [Google Scholar]
- 65.Bao J., Yu Y., Chen J., et al. MiR-126 negatively regulates PLK-4 to impact the development of hepatocellular carcinoma via ATR/CHEK1 pathway. Cell Death & Disease. 2018;9(10):p. 1045. doi: 10.1038/s41419-018-1020-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Perera D., Tilston V., Hopwood J. A., Barchi M., Boot-Handford R. P., Taylor S. S. Bub1 maintains centromeric cohesion by activation of the spindle checkpoint. Developmental Cell. 2007;13(4):566–579. doi: 10.1016/j.devcel.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 67.Schliekelman M., Cowley D. O., O'Quinn R., et al. Impaired Bub1 function in vivo compromises tension-dependent checkpoint function leading to aneuploidy and tumorigenesis. Cancer Research. 2009;69(1):45–54. doi: 10.1158/0008-5472.CAN-07-6330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ricke R. M., Jeganathan K. B., van Deursen J. M. Bub1 overexpression induces aneuploidy and tumor formation through Aurora B kinase hyperactivation. The Journal of Cell Biology. 2011;193(6):1049–1064. doi: 10.1083/jcb.201012035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhu L. J., Pan Y., Chen X. Y., Hou P. F. BUB1 promotes proliferation of liver cancer cells by activating SMAD2 phosphorylation. Oncology Letters. 2020;19(5):3506–3512. doi: 10.3892/ol.2020.11445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gjoerup O. V., Wu J., Chandler-Militello D., et al. Surveillance mechanism linking Bub1 loss to the p53 pathway. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(20):8334–8339. doi: 10.1073/pnas.0703164104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Our data can be found in the GEO database.


