Abstract
Chlorpyrifos (CP) is a persistent organophosphorus pesticide (OP) used in soil ecosystem for insect control. Bioremediation process has been proven promising in degrading these toxic molecules and restoring the physio-chemical properties of soil. This work reports a laboratory microcosm study in both non-sterile & sterile conditions, conducted over a period of 56 days to examine the combined effect of additional supplements like biostimulants (BSs) such as N, P, and K in the presence of suitable carrier materials (compost, wheat straw, and corncob) along with bioaugmentation by a Ochrobactrum sp. CPD-03 on CP degradation from the contaminated soil. CP degradation was thoroughly monitored at an interval of 7 days over a period of 56 days. Results showed biostimulation and bioaugmentation along with compost as carrier material had shown higher CP degradation efficiency of 76 ± 2.8 and 74 ± 1.6% in non-sterile and sterile microcosms over a period of 56 days. Moreover, bacterial community profiling (16s rRNA and opd gene) demonstrated increased microbial counts, corroborating the efficiency of the bioremediation process. The survival of CPD-03 at the end of the assay validated its ability of colonizing modified soils. By this integrated method with compost as carrier material, bioremediation process could be enhanced for restoration CP-contaminated soils.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13205-021-02980-9.
Keywords: Chlorpyrifos, Microcosm, Biostimulation, Bioaugmentation, Carrier materials, Bioremediation
Introduction
Chlorpyrifos (CP) is a persistent organophosphorus pesticide (OP) widely used in farming and soil treatment in various crops (Tejada et al. 2011; Zhang et al. 2012). Nonetheless, because of their widespread and inappropriate use, CP is of major environmental concern because of its intensive farming with uncontrolled use resulting risk towards soil and groundwater contamination (Korade and Fulekar 2009). CP degradation leads to the formation of a highly toxic, persistent and mobile pollutant, TCP (3,5,6-trichloro-2-pyridinol) (Lu et al. 2013). In addition, TCP has antimicrobial properties that prevent the spread of CP-degrading microbes in the soil (Singh and Walker 2006). Therefore, it is important to alleviate the dangerous effects of this pollutant.
The removal of CPs from the ecosystem is critical and requires quick attention. Physical and chemical approaches for removing CPs contamination from the environment may result in secondary pollution. As a result, biodegradation is seen as a more dependable, profitable, and environmentally friendly method of decontaminating CPs (Govarthanan et al. 2020). Organic amendments make a significant contribution to soil fertility and microbial activity (Fernández et al. 2009), hence, these might contribute towards the degradation of pesticides in soil. There are numerous reports on the use of these organic materials, such as peanut shell agro-residues, coconut husk and rice husk, municipal solid waste, cow dung, compost and vermi-compost, etc. in bioremediation. Since these organic compounds are nitrogen-rich, their addition to the soil can stimulate soil CP degradation (Kadian et al. 2012; Romyen et al. 2007; Tejada et al. 2011). Recently, there has been growing concern about the use of biostimulants (BS) obtained from various organic materials (García-Martínez et al. 2010). This process of soil degradation can mitigate the environmental problems that pesticides cause. BSs can boost microbial behavior and thus encourage CP degradation in contaminated soil (Aceves-Diez et al. 2015).
The BSs are also documented to be low molecular weight proteins with high polysaccharide and humic contents and this could help to control pesticide degradation, such as molecules that would activate soil microorganisms (Tejada et al. 2014). Recent studies have shown the impact of soil organic matter and humic content could help in enhanced (Man et al. 2021; Krohn et al. 2021; Cuiying et al. 2021) biodegradation process. This aspect may be of significant environmental interest, as very few studies have been made using specific BS to degrade CP. Moreover, this bioremediation process is mostly carried out by the indigenous microbial community present in soil. The use of particular microorganisms to breakdown or convert contaminants in polluted soil and water is referred to as bioaugmentation (Govarthanan et al. 2013). Understanding the microbial community and recognizing the bioaugmentation and biostimulation impact would affect the degradation ability of bacterial strains adapting to new environmental conditions and is, therefore, of great interest to ensure that pesticide-contaminated soil is effectively bioremediated (Liu et al. 2012; Taccari et al. 2012). Additionally, this bioaugmentation process is less expensive and environmentally friendly, but it is heavily influenced by a number of factors, including bacterial survival in contaminated soil or water, the impact of abiotic factors on bacterial growth, the mechanism of degradation, the genetic expression, and the impact of pollutants on bacterial activity (Govarthanan et al. 2016).
Biostimulation can reinforce the bioaugmentation strategy, which is an effective technique involving the introduction of stimulants such as fertilizers and nutrients along with organic amendments to enhance biodegradation practices (Al-Saleh and Hassan 2016). Once organic modifications are introduced in soils with high levels of carbohydrates, nitrogen, and phosphorus, indigenous microbial metabolism regulate the soil ecosystem with availability of adequate micronutrients and macronutrients (Ren et al. 2018). Organic modifications also support the growth of these indigenous microbes which may be involved in the removal of pollutants during bioremediation process (Rubio-Bellido et al. 2015).
Microbes present in soil ecosystem contribute to enhance the soil fertility followed by organic matter degradation (Wichern et al. 2007). Hence, a major concern would be the toxic effect of CP on soil microbes (Tejada et al. 2011). Quantitative assessment of microbial degradation measurement followed by community analysis can provide information on the nature and sustainable behavior of microorganisms at genetic level as well as the impact of CP on microbial metabolic activity in the soil. This analysis can serve as an important index of the impact on soil health of pesticide dynamics of CP in soil (Tejada et al. 2011; Zhang et al. 2010).
Earlier reports have suggested that compost may enhance the biodegradation of pesticides (Cardinali et al. 2010), corncob (Huang et al. 2018) and wheat straw (Diez et al. 2015; Yang et al. 2006) help to regulate the microbial community to adapt to rich organic matter, nutrition as nitrogen and phosphorus (Fernandes et al. 2005) during the degradation process. Nonetheless, little work on the loads of organophosphorus pesticide residue in soil and the remediation of CP contamination is still scarce. Therefore, consideration of the existence of microbial community in CP remediation is meaningful (Abed et al. 2015; Hassanshahian et al. 2014). This present work aimed to inspect the impact of BSs along with additional nutrients such as NPK on CP degradation followed by the bacterial community assessment.
Materials and methods
Preparation of soil sample and chemicals
Preparation of soil sample was according to our previous work (Nayak et al. 2020). The paddy field soil sample was collected from nearby campus of KIIT University [20.3551° N, 85.8187° E], India, at a depth of 20–25 cm from the ground. Soil sample was air-dried in shade and passed through a 2-mm mesh sieve and stored in laboratory at 18–20 °C and was used to analyze its physical and chemical characteristics. Portions of (100 g) of soil were kept in a non-sterile (glass beaker) and a sterile (conical flask) and moistened with sterile distilled water to 40% water holding capacity, to reaction in soil. Analytical grade CP (99.9% purity) and TCP (99.9% purity) were purchased from Sigma–Aldrich, USA. Another commercial grade CP was purchased from locally available and was used for laboratory microcosm. All other reagents were of analytical grade used in this analysis. Three carrier materials were used, wheat straw (WS), compost (CO), and corncob (CB). These were collected from local shops in Bhubaneswar, Odisha.
Bacterial strain
CP-degrading bacterial strain Ochrobactrum sp. CPD-03 (Genbank Accession no. KT366923; Taxonomy ID: 1707152), previously isolated from an agricultural paddy rhizospheric soil upon repeated application of CP followed by enrichment culture technique, was used in this study. CPD-03 strain was grown in minimal salts (MS) media (Nayak et al. 2020) supplemented with CP (100 mg l−1) at 30 °C. To obtain 0.9–1 × 108 cells ml−1 to be used as inoculum in subsequent medium, cells are harvested and resuspended in sterile normal saline (0.9% NaCl). For bioaugmentation purpose, CPD-03 was transformed with a pCJLA-GFP vector (Lab stock) containing KanR as selectable marker using an already established protocol (Seleem et al. 2008). To monitor the growth (cells mg−1 soil) in microcosms with the bioaugmented CPD-03 strain, soil samples were taken in an interval of 7 days for over a period of 56 days, serially diluted using sterile normal saline (0.9% NaCl) and plated on Luria Bertani Agar (HiMedia, India) containing Kanamycin (50 µg ml−1) and incubated at 37 °C.
Biostimulation experiment design in sterile (conical flask) and non-sterile (open glass beakers) microcosms
Biostimulation experiments were conducted in sterile (in conical flasks) and in non-sterile (glass beaker) microcosms containing 100 g of soil to monitor the CP degradation and changes in the bacterial community structure with regard to CP degradation over a period of 56 d. An aqueous solution of the commercial CP (Hilban, 20% EC commercial grade) was added to the microcosms to provide a final concentration of 100 mg kg−1. These microcosms were kept at 27 ± 2 °C. Loss of moisture during incubation was offset by adding weekly intervals of the required amount of sterile distilled water. At periodic intervals of 7 days over a total incubation period of 56 days, residues of CP in the microcosms were analyzed using high performance liquid chromatography (HPLC). The detailed experimental treatments are mentioned below in Table.1. Each treatment was maintained in triplicates. Three types of biostimulants (BS) were used in this study, i.e., N, P, and K (N:P2O5:K2O) in a ratio of 4:2:1 along with above mentioned carrier materials. 10 g of each carrier materials are applied to each experimental setup.
Table 1.
Experimental treatments
| S.no | Abbreviation | Description |
|---|---|---|
| Microcosm | ||
| 1 | BSM-1 | Soil |
| 2 | BSM-2 | Soil + CP |
| 3 | BSM-3 | Soil + CP + NPK |
| 4 | BSM-4 | Soil + CP + CPD-03 |
| 5 | BSM-5 | Soil + CP + NPK + CPD-03 |
| 6 | BSM-6 | Soil + CP + NPK + Compost |
| 7 | BSM-7 | Soil + CP + NPK + Compost + CPD-03 |
| 8 | BSM-8 | Soil + CP+ NPK + Wheat straw |
| 9 | BSM-9 | Soil + CP + NPK + Wheat straw + CPD-03 |
| 10 | BSM-10 | Soil + CP + NPK + Comcob |
| 11 | BSM-11 | Soil + CP + NPK + Comcob + CPD-03 |
| 12 | BSM-12 | Soil + CP + Compost |
| 13 | BSM-13 | Soil + CP + Compost + CPD-03 |
| 14 | BSM-14 | Soil + CP + Wheat straw |
| 15 | BSM-15 | Soil + CP + Wheat straw + CPD-03 |
| 16 | BSM-16 | Soil + CP + Comcob |
| 17 | BSM-17 | Soil + CP + Comcob + CPD-03 |
| Sterile condition | ||
| 18 | BSM-1 | Soil |
| 19 | BSM-2 | Soil + CP |
| 20 | BSM-3 | Soil + CP + NPK |
| 21 | BSM-4 | Soil + CP + CPD-03 |
| 22 | BSM-5 | Soil + CP + NPK + CPD-03 |
| 23 | BSM-6 | Soil + CP + NPK + Compost |
| 24 | BSM-7 | Soil + CP + NPK + Compost + CPD-03 |
| 25 | BSM-8 | Soil + CP + NPK + Wheat straw |
| 27 | BSM-9 | Soil + CP + NPK + Wheat straw + CPD-03 |
| 28 | BSM-10 | Soil+CP + NPK + Comcob |
| 29 | BSM-11 | Soil + CP + NPK + Comcob + CPD-03 |
| 30 | BSM-12 | Soil + CP + Compost |
| 31 | BSM-13 | Soil + CP + Compost + CPD-03 |
| 32 | BSM-14 | Soil + CP + Wheat straw |
| 33 | BSM-15 | Soil + CP + Wheat straw + CPD-03 |
| 34 | BSM-16 | Soil + CP + Comcob |
| 35 | BSM-17 | Soil + CP + Comcob + CPD-03 |
BSM bioremediation in soil microcosm, BSS bioremediation in sterile solution
Determination of residual CP
CP extraction from soil was carried out following (Tejada et al. 2011) with a slight modification. Residual CP was extracted using acetone:methanol (1:1, v/v). The extract was concentrated to 5 ml using a rotavapor (Eyela, Japan). 25 ml of dichloromethane (DCM) was added to the extracted sample. The pooled DCM extract was passed through sodium sulfate (Na2SO4, anhydrous) column, evaporated at 42 ± 2 °C using rotavapor (Eyela, Japan), and eventually re-dissolved in methanol (HPLC Grade). Before HPLC analysis, the final sample was filtered using a 0.22 μm membrane filter.
HPLC analysis
Quantification of residual CP quantification was carried on a C18 column (Eclipse Plus, Agilent Technologies, USA) connected to a HPLC system (Agilent Technologies) equipped with a UV/VIS detector. Isocratic mode of mobile phase solvent system containing methanol:water (85:15) was used with a flow rate of 1.2 ml min−1. Injection volume was 20 µL. The CP recoveries at concentration of 1, 2, 5, 10, and 50 mg l−1, ranged from 98.2 to 100.0%.
Scanning electron microscopy
Samples of treated and untreated CPD-03 cells for Field Emission Scanning Electron Microscope (FE-SEM) were prepared according to previously reported protocol (Das et al. 2016).
DNA extraction and quantification
Total DNA from the soil was extracted as per the protocol reported previously with slight modifications (Tsai and Olson 1991). Briefly, 10 g soil samples (from both sterile and non-sterile microcosm) was collected separately and was mixed with 20 ml of washing buffer (contains 100 mM sodium phosphate buffer, pH 8.0) in a 50 ml polypropylene Oke Ridge centrifuge tube to make a slurry. It was then vigorously vortexed for 1 min and allowed to sit with occasional mixing for 15 min. The slurry was then harvested at 8500 rpm for 10 min followed by supernatant was discarded. This step was repeated twice for sufficient removal of extra cellular DNA. The pellet was suspended with 10 ml lysis solution I (150 mM Nacl; 100 mM EDTA, pH 8; 10 mg/ml lysozyme; prepared immediately before use), mixed and incubated at 370C with mixing for 60 min. Then 10 ml lysis solution II (100 mM NaCl; 500 mM Tris–HCL, pH 8; 10% SDS) was added, followed by three cycle of freezing at -800C for 20 min and thawing at 65 °C for 15 min. Then it was subjected to centrifugation at 8000 rpm for 10 min and pellet was discarded. To the supernatant, 3 ml of 5 M NaCl (final concentration of 0.7 M NaCl) and 2.1 ml of 10% cetyltrimethylammonium bromide (CTAB; final concentration of 1%) in 0.7 M NaCl was added, mixed, and incubated at 650C for 10 min. An equal volume of chloroform–isoamyl alcohol (24:1) was added and vortexed briefly to achieve emulsion. The phase separation was carried out by centrifugation at 5000 rpm for 7 min. The upper phase was treated with an equal volume of polyethylene glycol (13% prepared in 0.7 M NaCl) and incubated on ice for 15 min, harvested at 10,000 rpm for 15 min to obtain the DNA pellet. The DNA pellet was washed with 2 volumes of 70% ethanol, dried and resuspended in 750 µl TE and transferred to a 1.5 ml microfuge tube.
Bacterial community determination based on quantitative PCR (qPCR)
Quantitative analysis of 16 s rRNA and opd gene derived from microcosm DNA samples (Steinberg and Regan 2009; Stubner 2004) was performed in qPCR (Bio-Rad CFX-96, USA) using the primer set from a previous study (Kwak et al. 2012) with the Master Mix (USB®VeriQuest® SYBR® Green qPCR, Affymetrix, Inc, USA). The qPCR cycle consists of 40 cycles at 97 °C for 10 s, 56 °C for 10 s and 72 °C for 30 s along with a melting curve for the specific amplification of the target gene using 0.5 °C temperature increase in every 10 s in the range 65–95 °C. Data analysis was performed using Bio-Rad CFX Manager Software (V1.6) for three independent experiments consisting three replicates.
Statistical analysis
All experiments have been conducted in triplicates. Results obtained were analyzed statistically and reported as the mean ± one standard deviation (SD) using two-way ANOVA (GraphPad Prism, version 8).
Results and discussion
Agricultural soil analysis
The key composition of the agricultural soil drives many metabolic interactions. Hence it is important to monitor the soil characteristics. The physico-chemical properties of this soil contain clay, 6.8%; silt, 15.2%; sand, 73%; total organic carbon, 0.76%; total nitrogen, 0.08%, pH (1:1.25 H2O), 5.73, and water holding capacity of 40 ± 2%. Two weeks before the beginning of the soil microcosm setup, the soil was kept in 40C for 7 days with an intermediate wetting close to its water potential for a week. The pesticide content was 1.25 ± 0.36 mg kg−1 soil. The soil had a neutral pH with a little amount of biogenic matter.
Effects of biostimulation and bioaugmentation on CP degradation in microcosm (sterile & non-sterile)
Biostimulation with organic amendments such as N, P, and K has been shown to enrich soils with nutrients, to regulate the biodegradation processes (Agamuthu et al. 2013; Chorom et al. 2010; Yang et al. 2017). Reports have suggested the positive impact of biostimulation and bioaugmentation for driving the toxic compound degradation (Al-Kindi and Abed 2016; Das and Adhya 2014; Tejada et al. 2011, 2014; Wu et al. 2016; Yang et al. 2018). In a preliminary experiment with MSM media, CPD-03 displayed 87–89% degradation of 100 mg l−1 within 2 days. Thus, a minimum concentration was tested for microcosms studies. Therefore, biostimulation and bioaugmentation along with suitable carrier materials use were investigated in this work.
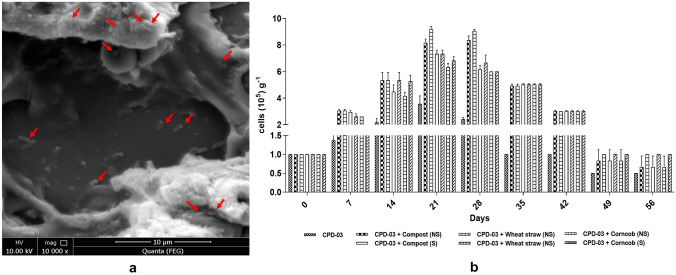
Biostimulation was carried by NPK (N:P2O5:K2O) addition in a ratio of 4:2:1. The carrier materials used in this study were preliminarily checked for their adherence and growth supporting characteristics for CPD-03 (Fig. 1). It was depicted from the analysis that, CPD-03 with compost (10 gm) as a potential carrier material, had shown the highest amount of adherence of CPD-03 along with the growth from 1 ± 0.2 × 105 to 8.6 ± 0.6 × 105 over a period of 28 days and gradually reduced up to 1.2 ± 0.2 × 105 after 56 days. This decreased growth was found due to the utilization of available nutrients.
Fig. 1.
a Scanning electron microscopy of Ochrobactrum sp. CPD-03 adherence to compost; b Growth pattern of Ochrobactrum sp. CPD-03 transformed with pCJLA. Data were recorded at various days of incubation. Data were represented in the mean ± SD of three replicates and analysis were performed using ANOVA. All tests were at p < 0.001*** significance level
CP degradation was also monitored over a period of 56 days in both sterile and non-sterile condition using BSs along with CPD-03 (initial cells count: 1.2 × 108 cells ml−1). After 56 days of incubation, CP was reduced from 100 mg kg−1 soil to 23 ± 3.2 mg kg−1 and 27 ± 1.8 mg kg−1 soil in non-sterile and sterile microcosm with a degradation efficiency of 75 ± 2.8 and 73 ± 1.6%, respectively (Fig. 2). For the first 30–40 days in both non-sterile and sterile condition, CP concentrations in the soils were decreased. However, after 56 days of incubation, there was no further reduction in CP concentrations and plateaus of degradation were obtained. It was depicted from the analysis that CP degradation was also efficiently triggered upon addition of BSs along with the potential carrier materials. Both biostimulation and bioaugmentation in BSM-7 and BSS-7 (abbreviation mentioned in Table 1) had shown the highest degradation rates along with the growth of CPD-03 (Fig. 3). After incubation, CPD-03 population reached the maximum value of 9 × 106 cells g−1 soil by 21 days and retained this amount by 28 days in both non-sterile and sterile microcosms. The BSs and compost treatment have activated the growth of CPD-03 CP degraders after during incubation, and this was maintained in both non-sterile and sterile microcosms through 28 days. Afterwards, the CPD-03 populations got declined in the seventh week and a simultaneous degradation plateau was also occurred. This indicates that nutrient application to the native microorganisms would be an effective method for enhancing pesticide degradation in soil (Svobodová and Novotný 2018; Wu et al. 2016).
Fig. 2.
a Degradation of CP (100 mg kg−1) by Ochrobactrum sp. CPD-03 resting cell (1–1.2 × 108 cells ml−1) in a non-sterile microcosm; b of CP (100 mg kg−1) by CPD-03 resting cell (1–1.2 × 108 cells ml−1) in a sterile microcosm. The values were obtained from HPLC analysis. Data were represented in the mean of three replicates and analysis were performed using ANOVA with the Prism8. All were tested at the p < 0.001*** significance level
Fig. 3.
Degradation of CP (100 mg kg−1) by Ochrobactrum sp. CPD-03 (1–1.2 × 108 cells ml−1) in a non-sterile and sterile microcosm along with the growth pattern of bioaugmented CPD-03 transformed with pCJLA. Data were represented in the mean of three replicates and analysis were performed using ANOVA with the Prism8. All were tested at the p < 0.001*** significance level
Effects of bioaugmentation and biostimulation on bacterial community in CP-contaminated soil
Pesticide degradation is influenced by the soil content and microbial metabolism (Tatar et al. 2020). The analysis of the 16s rRNA and opd gene by qPCR was confirmed using microcosm DNA samples. Enhanced CP degradation was monitored due to the indigenous microbes present in the soil along with the bioaugmented CPD-03. The 16s rRNA gene conferring the bacterial community and the opd gene encoding organophosphate hydrolase, responsible for the hydrolysis of the ester bond in OPs, was evaluated by the qPCR analysis as the target gene to assess the bacterial population responsible for CP degradation (Kwak et al. 2012).
The use of relative abundance in overall microbial community structure analysis using next generation sequencing (NGS) might lead to misinterpretation, since an increase in a single taxon results in a simultaneous reduction in compositional data for the other(s). Despite the fact that different DNA- and cell-based methods, as well as statistical approaches, have been developed to overcome the compositionality problem along with the biological relevance of absolute bacterial abundances, microbiome research has yet to adopt these methods, most likely due to feasibility concerns and cost effectivity. Quantitative PCR (qPCR) gives an accurate assessment of absolute taxon abundances when compared to NGS data, according to a recent study, and, therefore, provides an attainable solution to compositionality in high-throughput microbial community analysis (Jian et al. 2020).
The bacterial metabolism of pesticides degradation enables the development of bioremediation/biodegradation techniques for polluted ecosystem (Erguven et al. 2021). The CP-degrading microbial communities in different treatment microcosms are represented at different incubation days over a period of 56 days (Fig. 4). It summarizes the bacterial community composition based on 16 s rRNA and opd gene copy number. The bioaugmentation of CPD-03 into paddy soil microcosms under both non-sterile and sterile conditions had no significant effects on the composition of the bacterial population. Studies have reported that the addition of BSs and carrier materials significantly increased the population of soil microorganisms (Wang et al. 2017). Although there was some inconsistency with previous reports which showed that the introduction of pesticides like compounds led to a significant change in the composition of paddy soil in the bacterial community (Macur et al. 2007; Yang et al. 2017). This might be attributed to the different types of soils used in experiment purpose.
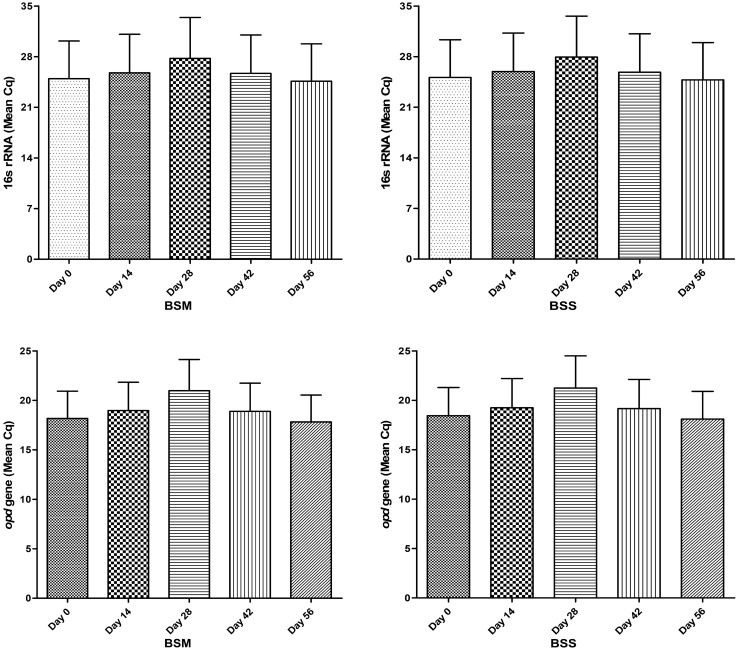
Fig. 4.
Comparative assessment of 16 s rRNA and opd gene in both non-sterile and sterile microcosm. Quantitation of 16s rRNA and opd gene was determined as Mean Cq in the soil DNA (g soil−1). Data were represented in the mean of three replicates and analysis were performed using ANOVA with the Prism8. All were tested at the p < 0.001*** significance level
The total microbial population and CP degraders were observed based on the copy number on 16s rRNA gene and opd gene by qPCR technique. During the third and fourth week (21 and 28 days) evaluation period, the amount of CP in both non-sterile and sterile conditions gradually decreased and showed an increased microbial count (copy no. of 16s rRNA and opd gene) based on qPCR analysis (Supplementary Fig. 1&2). It was assumed that the CP degradation obtained in non-control soil samples could be due to indigenous microbes that produce OPH. Therefore, the opd gene abundance was effectively evaluated using qPCR assay with target specific primers.
Conclusion
In comparison to intrusive physical and chemical treatments, bioremediation is regarded to be a cost-effective soil cleanup approach. BSs elements are suitable agents for bioremediation which can be used effectively to improve the activity of pesticide-degrading bacteria to accelerate the degradation process. CP was not previously known to experience increased degradation in a combined cycle along with carrier materials, the current study offers compelling evidence of increased degradation in sterile and non-sterile environments, perhaps due to natural augmentation of the indigenous microbial population. This work demonstrated a possible combined method including bioaugmentation and biostimulation towards bioremediation of CP-contaminated soil. The present study indicates that CP (100 mg g−1 soil) degradation was enhanced in soil microcosms by a combined approach includes biostimulation and bioaugmentation along with the application of suitable carrier material like compost. Thus, providing an appropriate number of BSs nutrients for biostimulation of indigenous microbial community and bioaugmention with CPD-03 along with compost have evidently increased the numbers of pertinent degraders in a CP-contaminated soil, leading to more active CP degradation. Additionally, the use of omics technologies (genomics/transcriptomics/proteomics/metabolomics) can be widely used to obtain insights into the ecosystem's basic genetics and microbial community at the bioremediation site. A well-coordinated use of these methods can aid in the investigation of complicated metabolic pathways and molecular alterations in the microbial population.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank Department of Biotechnology (DBT), Govt. of India, New Delhi for the research grant (BT/PR7580/BCE/8/1009/2013). All authors remain highly grateful to the Director, School of Biotechnology, KIIT University for infrastructure facility which had enabled us to work.
Declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial relationships that could be construed as a potential conflict of interest.
References
- Abed RM, Al-Kharusi S, Al-Hinai M. Effect of biostimulation, temperature and salinity on respiration activities and bacterial community composition in an oil polluted desert soil. Int Biodeterior Biodegrad. 2015;98:43–52. doi: 10.1016/j.ibiod.2014.11.018. [DOI] [Google Scholar]
- Aceves-Diez AE, Estrada-Castañeda KJ, Castañeda-Sandoval LM. Use of Bacillus thuringiensis supernatant from a fermentation process to improve bioremediation of chlorpyrifos in contaminated soils. J Environ Manage. 2015;157:213–219. doi: 10.1016/j.jenvman.2015.04.026. [DOI] [PubMed] [Google Scholar]
- Agamuthu P, Tan Y, Fauziah S. Bioremediation of hydrocarbon contaminated soil using selected organic wastes. Proc Environ Sci. 2013;18:694–702. doi: 10.1016/j.proenv.2013.04.094. [DOI] [Google Scholar]
- Al-Kindi S, Abed RM. Effect of biostimulation using sewage sludge, soybean meal, and wheat straw on oil degradation and bacterial community composition in a contaminated desert soil. Front Microbiol. 2016;7:240. doi: 10.3389/fmicb.2016.00240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Saleh E, Hassan A. Enhanced crude oil biodegradation in soil via biostimulation. Int J Phytoremed. 2016;18:822–831. doi: 10.1080/15226514.2016.1146223. [DOI] [PubMed] [Google Scholar]
- Cardinali A, Otto S, Vischetti C, Brown C, Zanin G. Effect of pesticide inoculation, duration of composting, and degradation time on the content of compost fatty acids, quantified using two methods. Appl Environ Microbiol. 2010;76:6600–6606. doi: 10.1128/AEM.00824-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chorom M, Sharifi H, Motamedi H. Bioremediation of a crude oil-polluted soil by application of fertilizers. Iran J Environ Health Sci Eng. 2010;7:319–326. [Google Scholar]
- Cuiying LI, Jianling FA, Xianghua XU, Yu WU. Enhanced anaerobic degradation of hexachlorobenzene in a Hydragric Acrisol using humic acid and urea. Pedosphere. 2021;31(1):172–179. doi: 10.1016/S1002-0160(20)60062-5. [DOI] [Google Scholar]
- Das S, Adhya TK. Effect of combine application of organic manure and inorganic fertilizer on methane and nitrous oxide emissions from a tropical flooded soil planted to rice. Geoderma. 2014;213:185–192. doi: 10.1016/j.geoderma.2013.08.011. [DOI] [Google Scholar]
- Das B, Khan MI, Jayabalan R, Behera SK, Yun SI, Tripathy SK, Mishra A. Understanding the antifungal mechanism of Ag@ ZnO core-shell nanocomposites against Candida krusei. Sci Rep. 2016;6(1):1–2. doi: 10.1038/s41598-016-0001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diez M, et al. Rhizosphere effect on pesticide degradation in biobeds under different hydraulic loads. J Soil Sci Plant Nutr. 2015;15:410–421. [Google Scholar]
- Erguven GO, Tatar Ş, Serdar O, Yildirim NC. Evaluation of the efficiency of chlorpyrifos-ethyl remediation by Methylobacterium radiotolerans and Microbacterium arthrosphaerae using response of some biochemical biomarkers. Environ Sci Pollut Res. 2021;28(3):2871–2879. doi: 10.1007/s11356-020-10672-9. [DOI] [PubMed] [Google Scholar]
- Fernandes SAP, Bettiol W, Cerri CC. Effect of sewage sludge on microbial biomass, basal respiration, metabolic quotient and soil enzymatic activity. Appl Soil Ecol. 2005;30:65–77. doi: 10.1016/j.apsoil.2004.03.008. [DOI] [Google Scholar]
- Fernández JM, Plaza C, García-Gil JC, Polo A. Biochemical properties and barley yield in a semiarid mediterranean soil amended with two kinds of sewage sludge. Appl Soil Ecol. 2009;42:18–24. doi: 10.1016/j.apsoil.2009.01.006. [DOI] [Google Scholar]
- García-Martínez AM, Tejada M, Díaz AI, Rodriguez-Morgado B, Bautista J, Parrado J. Enzymatic vegetable organic extracts as soil biochemical biostimulants and atrazine extenders. J Agric Food Chem. 2010;58:9697–9704. doi: 10.1021/jf101289n. [DOI] [PubMed] [Google Scholar]
- Govarthanan M, Lee KJ, Cho M, Kim JS, Kamala-Kannan S, Oh BT. Significance of autochthonous Bacillus sp. KK1 on biomineralization of lead in mine tailings. Chemosphere. 2013;90(8):2267–2272. doi: 10.1016/j.chemosphere.2012.10.038. [DOI] [PubMed] [Google Scholar]
- Govarthanan M, Mythili R, Selvankumar T, Kamala-Kannan S, Rajasekar A, Chang YC. Bioremediation of heavy metals using an endophytic bacterium Paenibacillus sp. RM isolated from the roots of Tridax procumbens. 3 Biotech. 2016;6(2):1–7. doi: 10.1007/s13205-016-0560-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govarthanan M, Ameen F, Kamala-Kannan S, Selvankumar T, Almansob A, Alwakeel SS, Kim W. Rapid biodegradation of chlorpyrifos by plant growth-promoting psychrophilic Shewanella sp. BT05: an eco-friendly approach to clean up pesticide-contaminated environment. Chemosphere. 2020;247:125948. doi: 10.1016/j.chemosphere.2020.125948. [DOI] [PubMed] [Google Scholar]
- Hassanshahian M, Yakimov MM, Denaro R, Genovese M, Cappello S. Using Real-Time PCR to assess changes in the crude oil degrading microbial community in contaminated seawater mesocosms. Int Biodeterior Biodegrad. 2014;93:241–248. doi: 10.1016/j.ibiod.2014.06.006. [DOI] [Google Scholar]
- Huang Y, Xiao L, Li F, Xiao M, Lin D, Long X, Wu Z. Microbial degradation of pesticide residues and an emphasis on the degradation of cypermethrin and 3-phenoxy benzoic acid: a review. Molecules. 2018;23:2313. doi: 10.3390/molecules23092313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jian C, Luukkonen P, Järvinen HY, Salonen A, Korpela K. Quantitative PCR provides a simple and accessible method for quantitative microbiota profiling. PLoS ONE. 2020;15(1):227285. doi: 10.1371/journal.pone.0227285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadian N, Malik A, Satya S, Dureja P. Effect of organic amendments on microbial activity in chlorpyrifos contaminated soil. J Environ Manag. 2012;95:S199–S202. doi: 10.1016/j.jenvman.2010.10.023. [DOI] [PubMed] [Google Scholar]
- Korade DL, Fulekar M. Rhizosphere remediation of chlorpyrifos in mycorrhizospheric soil using ryegrass. J Hazard Mater. 2009;172:1344–1350. doi: 10.1016/j.jhazmat.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Krohn C, Jin J, Wood JL, Hayden HL, Kitching M, Ryan J, Fabijański P, Franks AE, Tang C. Highly decomposed organic carbon mediates the assembly of soil communities with traits for the biodegradation of chlorinated pollutants. J Hazard Mater. 2021;15(404):124077. doi: 10.1016/j.jhazmat.2020.124077. [DOI] [PubMed] [Google Scholar]
- Kwak Y, Kim S-J, Rhee I-K, Shin J-H. Application of quantitative real-time polymerase chain reaction on the assessment of organophosphorus compound degradation in in situ soil. J Korean Soc Appl Biol Chem. 2012;55:757–763. doi: 10.1007/s13765-012-2168-4. [DOI] [Google Scholar]
- Liu P-WG, Wang S-Y, Huang S-G, Wang M-Z. Effects of soil organic matter and ageing on remediation of diesel-contaminated soil. Environ Technol. 2012;33:2661–2672. doi: 10.1080/09593330.2012.673017. [DOI] [PubMed] [Google Scholar]
- Lu P, Li Q, Liu H, Feng Z, Yan X, Hong Q, Li S. Biodegradation of chlorpyrifos and 3, 5, 6-trichloro-2-pyridinol by Cupriavidus sp. DT-1. Bioresour Technol. 2013;127:337–342. doi: 10.1016/j.biortech.2012.09.116. [DOI] [PubMed] [Google Scholar]
- Macur RE, Wheeler JT, Burr MD, Inskeep WP. Impacts of 2, 4-D application on soil microbial community structure and on populations associated with 2, 4-D degradation. Microbiol Res. 2007;162:37–45. doi: 10.1016/j.micres.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Man M, Wagner-Riddle C, Dunfield KE, Deen B, Simpson MJ. Long-term crop rotation and different tillage practices alter soil organic matter composition and degradation. Soil Tillage Res. 2021;209:104960. doi: 10.1016/j.still.2021.104960. [DOI] [Google Scholar]
- Nayak T, Panda AN, Kumari K, Adhya TK, Raina V. Comparative genomics of a paddy field bacterial isolate Ochrobactrum sp. CPD-03: analysis of Chlorpyrifos degradation potential. Indian J Microbiol. 2020;60(3):325–333. doi: 10.1007/s12088-020-00864-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X, et al. The potential impact on the biodegradation of organic pollutants from composting technology for soil remediation. Waste Manag. 2018;72:138–149. doi: 10.1016/j.wasman.2017.11.032. [DOI] [PubMed] [Google Scholar]
- Romyen S, Luepromchai E, Hawker D, Karnchanasest B. Potential of agricultural by-product in reducing chlorpyrifos leaching through soil. J Appl Sci. 2007;7:2686–2690. doi: 10.3923/jas.2007.2686.2690. [DOI] [Google Scholar]
- Rubio-Bellido M, Madrid F, Morillo E, Villaverde J. Assisted attenuation of a soil contaminated by diuron using hydroxypropyl-β-cyclodextrin and organic amendments. Sci Total Environ. 2015;502:699–705. doi: 10.1016/j.scitotenv.2014.09.052. [DOI] [PubMed] [Google Scholar]
- Seleem MN, Rajasekaran P, Ali M, Boyle SM, Sriranganathan N. Simple method for transformation of Ochrobactrum anthropi. World J Microbiol Biotechnol. 2008;24:2111–2114. doi: 10.1007/s11274-008-9716-4. [DOI] [Google Scholar]
- Singh BK, Walker A. Microbial degradation of organophosphorus compounds. FEMS Microbiol Rev. 2006;30:428–471. doi: 10.1111/j.1574-6976.2006.00018.x. [DOI] [PubMed] [Google Scholar]
- Steinberg LM, Regan JM. mcrA-targeted real-time quantitative PCR method to examine methanogen communities. Appl Environ Microbiol. 2009;75:4435–4442. doi: 10.1128/AEM.02858-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubner S. Quantification of Gram-negative sulphate-reducing bacteria in rice field soil by 16S rRNA gene-targeted real-time PCR. J Microbiol Methods. 2004;57:219–230. doi: 10.1016/j.mimet.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Svobodová K, Novotný Č. Bioreactors based on immobilized fungi: bioremediation under non-sterile conditions. Appl Microbiol Biotechnol. 2018;102:39–46. doi: 10.1007/s00253-017-8575-z. [DOI] [PubMed] [Google Scholar]
- Taccari M, Milanovic V, Comitini F, Casucci C, Ciani M. Effects of biostimulation and bioaugmentation on diesel removal and bacterial community. Int Biodeterior Biodegrad. 2012;66:39–46. doi: 10.1016/j.ibiod.2011.09.012. [DOI] [Google Scholar]
- Tatar S, Yildirim NC, Serdar O, Erguven GO. Can toxicities induced by insecticide methomyl be remediated via soil bacteria Ochrobactrum thiophenivorans and Sphingomonas melonis? Curr Microbiol. 2020;77:1301–1307. doi: 10.1007/s00284-020-02042-y. [DOI] [PubMed] [Google Scholar]
- Tejada M, Gómez I, del Toro M. Use of organic amendments as a bioremediation strategy to reduce the bioavailability of chlorpyrifos insecticide in soils. effects on soil biology. Ecotoxicol Environ Saf. 2011;74:2075–2081. doi: 10.1016/j.ecoenv.2011.07.005. [DOI] [PubMed] [Google Scholar]
- Tejada M, Rodríguez-Morgado B, Gómez I, Parrado J. Degradation of chlorpyrifos using different biostimulants/biofertilizers: effects on soil biochemical properties and microbial community. Appl Soil Ecol. 2014;84:158–165. doi: 10.1016/j.apsoil.2014.07.007. [DOI] [Google Scholar]
- Tsai Y-L, Olson BH. Rapid method for direct extraction of DNA from soil and sediments. Appl Environ Microbiol. 1991;57:1070–1074. doi: 10.1128/aem.57.4.1070-1074.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Zhou Z, Liu H, Li J, Wang Y, Xu H. Application of acclimated sewage sludge as a bio-augmentation/bio-stimulation strategy for remediating chlorpyrifos contamination in soil with/without cadmium. Sci Total Environ. 2017;579:657–666. doi: 10.1016/j.scitotenv.2016.11.044. [DOI] [PubMed] [Google Scholar]
- Wichern F, Mayer J, Joergensen RG, Müller T. Release of C and N from roots of peas and oats and their availability to soil microorganisms. Soil Biol Biochem. 2007;39:2829–2839. doi: 10.1016/j.soilbio.2007.06.006. [DOI] [Google Scholar]
- Wu M, et al. Bioaugmentation and biostimulation of hydrocarbon degradation and the microbial community in a petroleum-contaminated soil. Int Biodeterior Biodegrad. 2016;107:158–164. doi: 10.1016/j.ibiod.2015.11.019. [DOI] [Google Scholar]
- Yang Y, Sheng G, Huang M. Bioavailability of diuron in soil containing wheat-straw-derived char. Sci Total Environ. 2006;354:170–178. doi: 10.1016/j.scitotenv.2005.01.026. [DOI] [PubMed] [Google Scholar]
- Yang Z, Xu X, Dai M, Wang L, Shi X, Guo R. Rapid degradation of 2, 4-dichlorophenoxyacetic acid facilitated by acetate under methanogenic condition. Bioresour Technol. 2017;232:146–151. doi: 10.1016/j.biortech.2017.01.069. [DOI] [PubMed] [Google Scholar]
- Yang Z, Xu X, Dai M, Wang L, Shi X, Guo R. Combination of bioaugmentation and biostimulation for remediation of paddy soil contaminated with 2, 4-dichlorophenoxyacetic acid. J Hazard Mater. 2018;353:490–495. doi: 10.1016/j.jhazmat.2018.04.052. [DOI] [PubMed] [Google Scholar]
- Zhang C, Liu X, Dong F, Xu J, Zheng Y, Li J. Soil microbial communities response to herbicide 2, 4-dichlorophenoxyacetic acid butyl ester. Eur J Soil Biol. 2010;46:175–180. doi: 10.1016/j.ejsobi.2009.12.005. [DOI] [Google Scholar]
- Zhang X, Shen Y, Yu X-y, Liu X-j. Dissipation of chlorpyrifos and residue analysis in rice, soil and water under paddy field conditions. Ecotoxicol Environ Saf. 2012;78:276–280. doi: 10.1016/j.ecoenv.2011.11.036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.