Abstract
Purpose:
To conduct a first-in-human study of [18F]-BF3-Cy3-ACUPA by determining its safety, biodistribution, radiation dosimetry, and feasibility of tumor detection by preoperative positron emission tomography (PET) as well as intraoperative fluorescence imaging in patients with prostate-specific membrane antigen (PSMA) positive tumors.
Methods:
Ten patients aged 66 ± 7 years received a 6.5 ± 3.2 mCi intravenous injection of [18F]-BF3-Cy3-ACUPA and underwent PET/computed tomography (PET/CT) imaging. Radiation dosimetry of [18F]-BF3-Cy3-ACUPA, normal organ biodistribution, and tumor uptakes were examined. Two patients were pre-scheduled for radical prostatectomy (RP) with extended pelvic lymphadenectomy ~ 24 hours following [18F]-BF3-Cy3-ACUPA injection and imaging. Without reinjection, intraoperative fluorescence imaging was performed on freshly excised tissue during RP. Frozen sections of excised tissue during RP underwent confirmatory histopathology and multiphoton microscopy.
Results:
Absorbed doses by organs including the kidneys and salivary glands were similar to 68Ga-PSMA-11 imaging. [18F]-BF3-Cy3-ACUPA physiologic radiotracer accumulation and urinary/biliary excretion closely resembled the distribution of other published PSMA tracers including [18F]-JK-PSMA-7, [18F]-PSMA-1007, [18F]-DCFPyL, [18F]-DCFBC. 19F-BF3-Cy3-ACUPA was retained in PSMA+ cancer in patients for at least 24 hours, allowing for intraoperative fluorescence assessment of the prostate and of embedded prostate cancer without contrast reinjection. After 24 hours, the majority had decayed or cleared from the blood pool. Preoperative PET and fluorescence imaging findings were confirmed with final histopathology and multiphoton microscopy.
Conclusions:
Our first-in human results demonstrate that [18F]-BF3-Cy3-ACUPA is both safe and useful in humans. Larger trials with this PET tracer are expected to further define its capabilities and its clinical role in the management of PSMA+ tumors, especially in prostate cancer.
Keywords: prostate-specific membrane antigen, prostate cancer, fluorescence imaging, image-guided surgery, positron emission tomography
Microabstract
A number of radioactive tracers are currently being actively examined to image prostate-specific membrane antigen (PSMA) positive tumors, especially primary and recurrent prostate cancer. We showed a first-in-human study of [18F]-BF3-Cy3-ACUPA by determining its safety, biodistribution, radiation dosimetry, and feasibility of tumor detection by preoperative positron emission tomography (PET) as well as intraoperative fluorescence imaging in patients with PSMA positive tumors.
INTRODUCTION
Prostate cancer (PCa)-specific positron emission tomography (PET) agents are increasingly used to indicate patient candidacy for radical prostatectomy (RP), distinguish localized from disseminated disease, and monitor treatment response. In recent years, many new PET agents targeting prostate-specific membrane antigen (PSMA) have been developed and clinically translated in light of the frequent overexpression of PSMA in PCa. However, while such PET agents, notably 68Ga-PSMA-11, have become invaluable in the diagnosis, staging, and treatment planning of PCa, they are of less value for intraoperative guidance during RP, the most commonly used curative intervention in PCa. One important aspect during surgical resection of PCa is to determine lymph node status. As lymph node metastases can be only a few millimeters in diameter, they can escape detection on PET using the currently available agents due to limited spatial resolution of the modality.
Contrast agents that can demarcate small, early-stage metastases during surgery are critically needed to ensure curative RP. It has been reported that 4–48% of patients will have recurrent disease after RP, bringing along considerable health and cost implications 1-6. To address the aforementioned need, preoperative planning using PET imaging followed by intraoperative fluorescence imaging during surgery have been explored to improve the clinical success of RP, reduce biochemical recurrence, and reduce costs associated with disease recurrence 7. However, most efforts in this area have focused on using single modality agents for preoperative PET imaging and intraoperative fluorescence imaging, respectively; this requires multiple injections, and moreover, since the same receptor is targeted, this can lead to PSMA receptor saturation which may cause false negatives and result in inadequate intervention 8-14.
In contrast to a single modality agent approach, a multimodality agent approach combining PET and fluorescence into a single molecule have been alternatively proposed 14,15. In this vein, our group has developed [18F]-BF3-Cy3-ACUPA, a PSMA+ PCa-specific multimodal positron-emitting (18F) and fluorescence imaging agent 16,17. Our probe uniquely employs an alkylammonio methyl trifluoroborate (AMBF3) fluoride isotopic exchange (IEX) trap to allow rapid and direct 18F labeling to produce [18F]-BF3-Cy3-ACUPA 18-23. Since the clinical potential of the AMBF3 IEX 18F-PET prosthetic group has never been evaluated in patients, in this study, we explored the first-in-human use of [18F]-BF3-Cy3-ACUPA by determining its safety, biodistribution, radiation dosimetry, and feasibility of tumor detection by preoperative PET and intraoperative fluorescence imaging.
MATERIALS AND METHODS
Patients
This study was approved by the local Institutional Ethical Committee (Cerrahpasa University School of Medicine protocol #-29281604.01.01-139206). Written informed consent was obtained from all patients to receive [18F]-BF3-Cy3-ACUPA. All procedures were performed in accordance with the ethical standards of the institution, national research committee and with the 1964 Helsinki declaration and its later amendments. Patient studies were conducted between October 2018 and December 2019 at Istanbul University- Cerrahpasa, Cerrahpasa Medical Faculty. The inclusion criteria were suspected PCa (including a prior digital rectal examination, elevated prostate-specific antigen (PSA) serum level, and confirmed PCa (existing TNM and/or Gleason-scored biopsy) as well prior PSMA+ non-prostate cancers.
To evaluate the safety of [18F]-BF3-Cy3-ACUPA administration, vital parameters such as body temperature, blood pressure, and heart rate were measured at multiple time points up to 4 h after injection.
Radiosynthesis and Quality Control of [18F]-BF3-Cy3-ACUPA
19F-BF3-Cy3-ACUPA (Fig. 1) was synthesized and radiolabeled in 140 μg (100 nmol) aliquots as previously described 16,17. Radiolabeling, purification, and preparation of the agent was performed in a certified Good Laboratory Practice (GLP) facility (Istanbul University-Cerrahpasa, Cerrahpasa Medical Faculty). Pharmaceutical grade [18F]-fluoride ion (for bone imaging) was used for isotopic exchange. On average, IEX was conducted with 152 ± 45 mCi of [18F]-fluoride ion and resulted in 11.6 ± 7.7 mCi of [18F]-BF3-Cy3-ACUPA (decay uncorrected). The obtained specific activity was greater than 0.116 ± 0.077 mCi/nmol. Radiolabeling yield and purity was confirmed by radio-high performance liquid chromatography (HPLC), where an average HPLC purity of 94 ± 11 % was observed. Products were tested for sterility, endotoxins, pH, fluorescence, and absence of precipitates. All materials for injection were passed through sterile 0.2 μm syringe filters prior to patient injection.
Fig.1. Structure and radiosynthesis.
A) Structure of [18F]-BF3-Cy3-ACUPA, containing AMBF3, Cy3, and PSMA targeting moieties, that was radiolabeled through IEX radiochemistry. B) Radio-HPLC showing a pure [18F]-BF3-Cy3-ACUPA radiosynthesis (Patient No. 1). C) Chemical structures of other contemporary standalone PET-only [18F]-PSMA tracers including [18F]-JK-PSMA-7, [18F]-DCFPyL, [18F]-PSMA-1007, [18F]-DCFBC, and [68Ga]-PSMA-11.
[18F]-BF3-Cy3-ACUPA PET/CT and PET/MRI Acquisition
All ten patients underwent PET/computed tomography (CT) using a Discovery 710 PET/CT scanner (GE Healthcare). Additionally, pelvic PET/magnetic resonance imaging (MRI) was performed in six patients using a Signa PET/MRI scanner (GE Healthcare). Whole-body PET images were acquired from the top of the skull to the mid-thigh. Patients received an average of 6.5 ± 3.2 mCi of [18F]-BF3-Cy3-ACUPA through the cephalic vein by simple injection using a shielded syringe in 3–5 mL saline. An average time of 73 ± 27 min was allowed to pass between injection and imaging acquisition. PET data was acquired using QuantWorks (GE Healthcare).
Emission data was corrected for random scatter and decay, and reconstruction was performed using the ordered subset expectation maximization (OSEM) algorithm. Image co-registration was performed for PET and CT datasets. Attenuation correction was performed using unenhanced low-dose CT data (120 kV; 59 mAs) for PET/CT acquisitions, whereby CT images were reconstructed to a slice thickness of 3.75 mm, increment of 0.12 mm, and noise index of 15; for PET/MRI acquisitions, Zero Echo Time (ZTE) MRI sequences focusing on the bone were used for soft tissue attenuation correction of PET images.
Regions of Interest on Preoperative PET Imaging
All PET images were assessed by qualified nuclear radiologists. Regions of interest (ROIs) were drawn around PSMA+ lesions (areas of focally increased uptake). Additionally, ROIs with 20 mm diameter spherical volumes (4.19 mL) were manually drawn around other organ tissues of interest including the right/left (R/L) lacrimal, R/L parotid, R/L submandibular, urinary bladder, red bone marrow, gallbladder, adrenal, brain, breast, upper/lower large intestine wall, stomach wall, kidney, liver, lungs, muscle, pancreas, osteogenic cell, skin, spleen, testes, and thyroid.
Radiation Dosimetry and Tracer Biodistribution
Radiation dosimetry analysis including absorbed and effective dose calculations were performed using OLINDA/EXM 1.0 software (Vanderbilt University). Total counts were obtained by adding all counts from manually drawn ROIs on individual CT slices.
Tracer biodistribution to PSMA+ lesions and other organ tissues of interest was determined by calculating the mean standard uptake value (SUVmean) within the aforementioned ROIs across sequentially acquired PET images using the OLINDA/EXM 1.0 or Amide 1.0.4 software. Blood radioactivity concentration was measured in the left ventricle of the heart.
Intraoperative Fluorescence Imaging
Of the ten patients included in this study, two patients were pre-scheduled for RP with extended pelvic lymphadenectomy without reinjection ~ 24 hours following [18F]-BF3-Cy3-ACUPA injection and preoperative imaging. There is no commercially available fluorescence imaging device for wide-field fluorescence imaging of Cy3 fluorophore-containing tissue in the operating room (OR). Therefore, a first-generation custom fluorescence interference filter block device was assembled and placed on the OR back table. This device contained a Solis 525C LED illuminator (Thor Labs) with a dominant wavelength of 525 nm and a typical collimated optical power of 3.1W. The illuminating light was focused through a plano-convex lens through a fluorescence interference filter block containing a dichroic mirror with a 533–580 nm reflected band and a 595–800 nm transmission band. A 600 ± 40 nm bandpass barrier filter was used to control emitted light that was transmitted to an attached color Complementary Metal Oxide Semiconductor (CMOS) camera. An exciter filter was not used in this setup because of potential concern that important anatomical landmark data would be lost if optical filtration was too stringent. This device was used to collect fluorescence images and videos of whole, intact, fresh, resected tissue and associated margins immediately following surgical resection to qualitatively ascertain the fluorescence 19F-BF3-Cy3-ACUPA signal. Imaging was performed ex vivo and did not interfere with standard-of-care RP. TIFF files were captured using ThorCam Software v3.2.0 and converted to .AVI using Image J v.1.51j8. ThorCam Software v3.2.0 was used to transform red channel CMOS data into black and white images.
Histopathological Confirmation
Following RP, excised tissue was submitted to pathology where frozen sections were first prepared from excised 18/19F-BF3-Cy3-ACUPA-containing tissue and then stained for immunohistochemistry to confirm preoperative PET and intraoperative fluorescence colocalization within PSMA+ tissue under a confocal microscope. Histopathological evaluation was performed by dedicated uropathologists, using International Society of Urological Pathology nomenclature, blinded to preoperative PET/CT, preoperative PET/MRI (Patient 6 shown in Fig. S5), and intraoperative fluorescence imaging results. Human prostate tissue sections were stained with DAPI (blue, nuclear stain). Control, neighboring sections were stained with hematoxylin (violet, nuclear stain), eosin (light blue, cytoplasm stain), and horse-radish-peroxidase (HRP) conjugated PSMA+ antibody, and then treated with 3,3'-Diaminobenzidine (brown, oxidized polybenzimidazole product).
Confirmation by Post-Surgical Multiphoton Microscopy
Post-surgical Cy3 fluorescence imaging was also performed using multiphoton microscopy (MPM) on excised, frozen tissue sections with an Olympus FV1000 multiphoton microscope at the Microscopy CoRE and Advanced Bioimaging Center at the Icahn School of Medicine at Mount Sinai, at room temperature and at 780 nm excitation. First, intact biopsy cores were examined at low resolution for fast tissue orientation and gross PSMA+ area assessment (z-resolution = 1.1 μm/pixel, NA objective = 4×/0.4). Next, intact biopsy cores were examined at high resolution tissue to yield a tiled image (2D optical section at a focal plane approximately 20 μm below tissue’s surface) (z-resolution = 0.48, 4, and 1.2 μm/pixel; NA objective = 25×/1.0).
Statistical Analysis
All errors were reported as ± standard deviation. SigmaPlot 10.0 software was used for statistical analysis of dosimetry data including the calculation of median, mean, minimum, maximum, 25th percentile, and 75th percentile values. Dosimetry data of [18F]-BF3-Cy3-ACUPA was compared to that of contemporary [18F]-PET tracers including [18F]-JK-PSMA-7, [18F]-PSMA-1007, [18F]-DCFPyL, and [18F]-DCFBC. To determine whether differences in dosimetry data were significant between [18F]-BF3-Cy3-ACUPA and the other tracers, P-values were calculated using the following equation with a two-tailed t distribution:
where t is the statistical value used to calculate p; χ1, s1, and n1 are the published average dosimetry value, standard deviation, and sample size, respectively, for each tested tissue using [18F]-JK-PSMA-7, [18F]-PSMA-1007, [18F]-DCFPyL, or [18F]-DCFBC; and χ2, s2, and n2 are the average dosimetry value, standard deviation, and sample size (n = 10) for [18F]-BF3-Cy3-ACUPA measured in this study. P-values < 0.05 were considered statistically significant.
Tumor-to-blood ratios (TBR) were calculated by the dividing SUVmean values of lesions by the SUVmean value in the red bone marrow for individual patients.
RESULTS
Patient Characteristics
A total of ten patients were included in this study (mean age = 66 ± 7 years). Detailed patient characteristics are provided in Table 1. Nine patients (Patients 1–3, 5–10) were candidates for [18F]-BF3-Cy3-ACUPA PET imaging because of clinically suspected PCa; an additional patient (Patient 4) with PSMA+ colorectal cancer and was included in the study to achieve an adequate sample size for biodistribution measurements. Of the nine patients with clinically suspected PCa, PSA levels were measured immediately prior to [18F]-BF3-Cy3-ACUPA PET imaging, and the mean elevated serum PSA level was 31 ± 63 μg/L. Eight of these patients had undergone prostate biopsies yielding Gleason scores > 4+3 (or 3+4). The same eight patients had also undergone CT and/or MRI a minimum of 3 weeks prior to the study and received TNM classifications based on CT and/or MRI. As PET studies were not available at the time of TNM classification, TNM classifications as reported in Table 1 may not corroborate with the data obtained from [18F]-BF3-Cy3-ACUPA PET/CT and/or PET/MRI.
Table 1.
Patient Characteristics
| Patient No. |
Age | Diagnosis (post biopsy) * |
Gleason | TNM (Clinical) ┼ |
PSA (time of scan, ug/L) |
Mets Imaged |
Dose (mCi) |
Imaging Modality |
Imaging Time Point(s) (min post injection) |
HPLC (%) |
Starting Activity [18F]- fluoride ion (mCi) |
Recovered Labeled Product (mCi) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 72 | Metastatic Prostate Cancer | 4+3 | T2bN1M1 | 199 | Lung, adrenal, bone, LN | 3.25 | GE 710 PETCT | Multiple, 9-119 min | 99.1 | 120 | 3.5 |
| 2 | 60 | Prostatectomize d LN met | 5+3 | T2aN0M0 | 12 | LN | 2.79 | GE 710 PETCT+MR | Multiple, 6-121 min | 74 | 182 | 3.18 |
| 3 | 62 | No Biopsy | n.a. | n.a | 5.14 | N | 7.28 | GE 710 PETCT+MR | 46 min | 99.8 | 101 | 17 |
| 4 | 57 | Histology confirmed PSMA+ Cancer | n.a. | n.a. | n.a. | LN | 6.53 | GE 710 PETCT+MR | 60 min | 99.8 | 101 | 17 |
| 5 | 65 | Prostatectomize d LN mets | 4+3 | T3bN0M0 | 0.25 | LN | 3.20 | GE 710 PETCT | 64 min | 98.3 | 224 | 13.2 |
| 6 | 74 | Prostate Cancer | 4+4 | T3bN1M0 | 8 | LN | 9.84 | GE 710 PETCT+MR | 132 min | 72 | 160 | 22 |
| 7 | 65 | Metastatic Prostate Cancer | 4+3 | T3N1M0 | 12 | Prostate ECE | 5.00 | GE 710 PETCT+MR | 85 min | 98.3 | 224 | 13.2 |
| 8 | 73 | Prostate Cancer | 3+4 | T2aN0M0 | 10.5 | N | 5.66 | GE 710 PETCT | 78 min | 99.5 | 134 | 11 |
| 9 | 74 | Prostate Cancer | 4+4 | T3aN1M0 | 6.46 | LN | 10.99 | GE 710 PETCT | 60 min | 99.8 | 152 | 32.5 |
| 10 | 57 | Prostate Cancer | 4+3 | T3bN0M0 | 32 | LN, EPI | 10.89 | GE 710 PETCT+MR | 59 min | 99 | 128 | 29.2 |
| Average (SD) | 66 (± 7) | 31 (± 63 ug/L)◊ | 6.5 (± 3.2 mCi) | Δ Injection to First scan╪ 73 (± 27 min) | 94 (± 11%) | 152 (± 45 mCi) | 11.6 (± 7.7 mCi) |
Diagnosis made by urologists, independent of [18F]-PET imaging.
SD-variation due to high PSA value in Patient 1.
Patients receiving multiple scans were not included in the calculation.
Adverse Events
All patients tolerated the drug product well. No signs or symptoms of adverse effects from injection of [18F]-BF3-Cy3-ACUPA tracer were reported.
Biodistribution
Maximum intensity projection (MIP) images of [18F]-BF3-Cy3-ACUPA PET/CT in Patient 1 with widely disseminated PCa and Patients 6 and 8 with localized PCa are depicted in Fig. 2, 3, and 4, respectively. In Patient 1 who had multiorgan metastatic disease involving the bones, lymph nodes, and adrenals, the pattern of physiologic biodistribution was not visually affected by the presence of metastatic disease.
Fig. 2. [18F]-BF3-Cy3-ACUPA PET/CT imaging of Patient 1 with disseminated PCa.
Patient 1 had undergone RP and was receiving adjuvant luteinizing hormone-releasing hormone (LHRH) analog therapy prior to this study. Patient 1 also had a hip replacement surgery prior to this study. The patient’s serum PSA was 199 μg/L. A) Coronal and B) Sagittal whole-body PET (red)/CT (grey) MIP images show general and focal [18F]-BF3-Cy3-ACUPA distribution. Specific PCa lesions (“a–d”) are indicated by magenta arrows. C) CT only (inverted, grey) and D) [18F]-BF3-Cy3-ACUPA PET (red)/CT (inverted, grey) transverse cross-sections show specific PCa lesions (indicated by magenta markings).
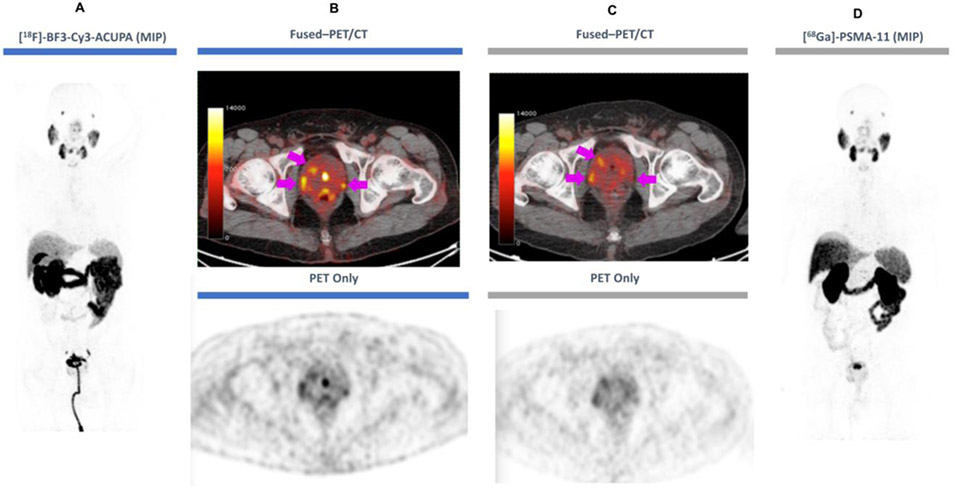
Fig 3. [18F]-BF3-Cy3-ACUPA and [68Ga]-PSMA-11 PET/CT imaging in Patient 6.
Patient 6 received [68Ga]-PSMA-11 PET/CT one month prior to [18F]-BF3-Cy3-ACUPA PET/CT. Serum PSA was measured at 8 μg/L prior to receiving [18F]-BF3-Cy3-ACUPA. A) Coronal PET (grey) MIP image (left) shows general and focal [18F]-BF3-Cy3-ACUPA distribution (patient with urinary catheter). B) Fused PET (red)/CT (grey, top) or CT only (grey, bottom) transverse cross-sections with [18F]-BF3-Cy3-ACUPA and C) fused PET (red)/CT (grey, top) or CT only (grey, bottom) transverse cross-sections with [68Ga]-PSMA-11 show the same lesions (magenta arrows). D) Coronal PET (grey) MIP image with [68Ga]-PSMA-11 shows similar general distribution and focal PCa to A).
Fig. 4. [18F]-BF3-Cy3-ACUPA PET/CT imaging of Patient 8.
Patient 8 was a candidate for RP based on prior biopsy and [68Ga]-PSMA-11 imaging. As part of this study, this patient underwent [18F]-BF3-Cy3-ACUPA injection and PET/CT imaging, followed by RP ~24 hours later. A) [68Ga]-PSMA-11 PET MIP image. B) [18F]-BF3-Cy3-ACUPA PET MIP image. C) [68Ga]-PSMA-11 PET/CT image shows localized PSMA+ disease (arrow) but no nodal or distant disease. D) Similar PET delineations (magenta arrowheads) are observed on the 90-min post-injection [18F]-BF3-Cy3-ACUPA PET/CT image. Without reinjection, the patient underwent RP 24 hours later (after 18F decayed). E) Fluorescence images of excised prostate tissue using a first-generation back-table Cy3 fluorescence imaging device. F) Post-surgical prostate co-registration shows PCa in an MRI apparent diffusion coefficient image, PET image, and fluorescence image; post-surgical fluorescence image shows a positive margin in the posterior urethra/bladder neck (indicated by a dashed magenta border).
Patients 1 and 2 were repeatedly imaged 6, 31, 61, and 121 min post injection. Fig. S6 shows decay uncorrected, relative percentile changes in organ activity in Patient 2.
Radiation Dosimetry
The total body dose was 7.07 ± 1.49 E-03 mSv/MBq. The effective dose equivalent was 1.30 ± 0.54 E-02 mSv/MBq and the effective dose was 8.87 ± 2.17 E-03 mSv/MBq. The highest organ uptakes and radiation doses were observed in the salivary glands, kidneys, and lacrimal glands Partial organ absorbed dose distribution of [18F]-BF3-Cy3-ACUPA to different organ tissues are reported in Table S1, Fig. S1, and Fig. S2.
Comparison to Contemporary 18F-PSMA Tracers
In the entire patient cohort, major physiologic radiotracer accumulation was observed in the salivary and lacrimal glands, liver, spleen and intestines in a pattern resembling the distribution of other [18F]-PSMA tracers (Table S1). Box plots illustrate the closeness of dosimetry between [18F]-BF3-Cy3-ACUPA and other published PSMA+ tracers in Fig. S1, wherein the dose of [18F]-BF3-Cy3-ACUPA was statistically similar or lower than that of other published PSMA+ tracers. Tracer biodistribution in an expanded organ panel is reported in Fig. S2.
Tumor-to-Blood Ratio of PSMA+ Lesions and Lymph Nodes
TBRs are presented in Table 2. One patient had distant metastatic PCa, six patients had PCa nodal metastasis, and one patient had PSMA+ colorectal cancer. TBRs were quantified for five lesions within the prostate gland, one bone lesion, one lesion in the adrenal gland, and four lymph nodes. PSMA+ lesions within the prostate fossa showed a TBR of 13 ± 11 (SUVmean). Lymph nodes showed a TBR of 19 ± 21 (SUVmean).
Table 2.
Tumor-to-blood ratios (TBR) of primary and metastatic lesions
| Patient No. | Age | Gleason | TNM (Clinical)┼ | PSA (time of scan, ug/L) |
TBR - Primary Prostate Lesion/Marrow* |
TBR - Metastasis/Marrow* |
|---|---|---|---|---|---|---|
| 1 | 72 | 4+3 | T2bN1M1 | 199 | 2.8 | 34.5 (Bone) 34.6 (Adrenal) |
| 2 | 60 | 5+3 | T2aN0M0 | 12 | n.a. | n.a. |
| 3 | 62 | n.a. | n.a | 5.14 | n.a. | n.a. |
| 4 | 57 | n.a. | n.a. | n.a. | n.a. | n.a. |
| 5 | 65 | 4+3 | T3bN0M0 | 0.25 | n.a. | 3.6 (LN) |
| 6 | 74 | 4+4 | T3bN1M0 | 8 | 10.7 | 3.9 (LN) |
| 7 | 65 | 4+3 | T3N1M0 | 12 | 32.5 | 50.2 (LN) |
| 8 | 73 | 3+4 | T2aN0M0 | 10.5 | 12.5 | n.a. |
| 9 | 74 | 4+4 | T3aN1M0 | 6.46 | n.a. | 31.0 (LN) |
| 10 | 57 | 4+3 | T3bN0M0 | 32 | 7.4 | 4.8 (LN) |
LN = lymph node.
Intraoperative Fluorescence Imaging of Resected PSMA+ Lesions and Lymph Nodes
Of the total ten patients, only five patients (Patients 1, 2, 5, 6, and 8) were candidates for RP based on [68Ga]-PSMA-11 PET imaging at 10 months, 3 months, 2 months, 1 month, and 1 month prior (Patient 6 shown in Fig. S4). Three patients (Patients 1, 2, and 5) had undergone RP prior to this study. The two remaining patients (Patients 6 and 8) were pre-scheduled for RP with extended pelvic lymphadenectomy ~ 24 hours following [18F]-BF3-Cy3-ACUPA injection and PET imaging.
In Patients 6 and 8, the superficial presence of PSMA+ tissue was suggestive of positive PCa margins (see Movie S1). In Patient 6, four resected lymph nodes were imaged following pelvic lymph node dissection (see Fig. S3). To best observe a visible difference in fluorescence intensity between PCa containing and non-PCa containing nodes, all four nodes were imaged together using same-intensity fluorescence imaging settings. Fluorescence imaging intensity was greater in two of the four removed nodes. All nodes were sent to histopathology for analysis. The two nodes with greater fluorescence imaging were confirmed to contain PCa foci.
Post-Surgical Confirmation by Histopathology and MPM Fluorescence Imaging of Resected PSMA+ Lesions
[18F]-BF3-Cy3-ACUPA left a permanent fluorescence record in frozen sections of excised tissue, allowing confirmation of PSMA+ status as determined by preoperative PET imaging and intraoperative fluorescence imaging. In MPM, the emitted two-photon excited signal was detected by three photomultiplier tube (PMT) channels: 1) second harmonic generation (SHG) at 390 nm for collagen specific contrast (Blue); 2) intrinsic fluorescence at 420–460 nm (granular and stromal tissue; green); and 3) Cy3 fluorescence at 500–550 nm for capturing PSMA+ cells throughout the imaged tissue (red). Fig. 5 shows a representative MPM image of a biopsy core, where the red signal indicates Cy3 fluorescence originating from glandular tissue within the prostate. Additional morphological contrast from stromal collagen enhanced the visualization of PSMA+ glands in the imaged tissue and provided additional morphological tissue information, not seen by confocal imaging (histopathology) alone.
Fig 5. Corroborative 18F-BF3-Cy3-ACUPA fluorescence imaging, diaminobenzidine histopathology, and multiphoton microscopy.
Patient 6 underwent [18F]-BF3-Cy3-ACUPA injection and PET/CT imaging, followed by RP ~24 hours later. A prostate specimen containing BF3-Cy3-ACUPA was excised during RP and sent to histopathology. A) At histopathology, after staining with DAPI (purple, nuclear stain). The specimen containing BF3-Cy3-ACUPA appears pink and is localized to glandular tissue under a confocal microscope. B) A neighboring frozen section was created from the prostate specimen and stained with hematoxylin (violet, nuclear stain), eosin (light blue, cytoplasm stain), horse-radish-peroxidase (HRP) conjugated PSMA+ antibody and then treated with 3,3'-Diaminobenzidine (DAB brown, oxidized polybenzimidazole product). DAB produced by the PSMA+ antibody is brown and is also localized to glandular tissue. Note the colocalization of ACUPA-Cy3-BF3 (pink signal) with PSMA+ immunohistochemistry (right, brown) in glandular prostate tissue. C–F) MPM imaging of unprocessed frozen sections containing ACUPA-Cy3-BF3 capturing endogenous two-photon excited PSMA+ fluorescence from Cy3 (red), cellular and elastin intrinsic fluorescence (green), and second harmonic from stromal collagen (Blue). Examples of C–E) PSMA+ fluorescence glandular tissue and a F) PSMA− specimen. Note that MPM enhances the visualization of collagen fibers surrounding the prostate glands. Scale bars: A–B) 100 μm, C) 1000 μm, D–F) 100 μm.
DISCUSSION
This was a first-in-human study to investigate the safety, biodistribution, radiation dosimetry, and tumor-detecting ability of multimodal PET and fluorescence [18F]-BF3-Cy3-ACUPA in patients with PSMA+ malignancy. Altogether, our findings demonstrate that [18F]-BF3-Cy3-ACUPA is safe and useful in the clinical setting.
In our study, we determined that [18F]-BF3-Cy3-ACUPA dosimetry to normal, healthy tissue was generally lower than that of contemporary [18F]-PET tracers including [18F]-JK-PSMA-7, [18F]-PSMA-1007, [18F]-DCFPyL, and [18F]-DCFBC. Specifically, its distribution to the kidney, lung, liver, and splenic tissue was 1.4 to 6-fold lower than that of the published tracers (Table S1). An exception was its distribution to salivary and lacrimal tissue, which was similar (0.5–1.6×) to that of the published tracers; this may be due to the specific targeting of natural levels of PSMA expression in lacrimal and salivary gland tissue. [18F]-BF3-Cy3-ACUPA distribution to the bone and marrow was minimal, indicating that it does not defluoridate to produce significant concentrations of [18F]-fluoride ions in humans. Dosimetry findings, taken together, suggest that [18F]-BF3-Cy3-ACUPA may have superior in-patient PSMA+ affinity compared with contemporary [18F]-PSMA tracers. A larger patient sample is needed to confirm this hypothesis.
In this study of ten patients, nine had clinically suspected PCa. We also showed that [18F]-BF3-Cy3-ACUPA can be used to accurately delineate PCa in a diverse group of nine patients including those with metastatic disease, those undergoing RP, and those with suspected PCa, with varying PSA serum levels, Gleason scores, and TNM staging. PSMA+ PCa was visible in the prostate gland (n = 5) and lymph nodes (n = 6) on [18F]-BF3-Cy3-ACUPA PET imaging. Like other [18F]-PSMA tracers, [18F]-BF3-Cy3-ACUPA PET was useful in distinguishing between disseminated and localized PSMA+ cancer. The TBR of [18F]-BF3-Cy3-ACUPA for small lymph node metastases ranged from 3.6 to 50.2, suggesting that [18F]-BF3-Cy3-ACUPA may be exceptionally sensitive for identifying tiny PSMA+ tumor deposits in the body.
19F-BF3-Cy3-ACUPA is also useful for fluorescence imaging within ~24 h of injection to confirm PSMA+ cancer in the intraoperative (back-table) setting. In light of this, the [18F]-BF3-Cy3-ACUPA tracer stands apart from other contemporary [18F]-PSMA tracers which lack fluorescence utility and are therefore not useful in the intraoperative setting. The ~24 h temporal delay between injection and preoperative PET imaging and intraoperative fluorescence imaging is advantageous for three reasons: 1) The delay gives urologists more time to assess the PET image in determining the extent of lymph node dissection. 2) The delay removes ionizing radiation (from 18F decay) from the OR procedure (18F has a half-life of 108 min). 3) The delay allows for quantitative clearance of unbound 19F-BF3-Cy3-ACUPA from bloodstream and the urine. With the temporal delay, a patient can also travel and undergo delayed fluorescence-guided RP with the urologist of his or her choice without reinjection.
This first-in-human study demonstrated first time, to our knowledge, using [18F]-PET/FL multimodality imaging probe. There is currently no FDA-approved PSMA+ PET probe or [18F]-PET/FL multimodality imaging probe. Combined PET/FL contrast preclinical development is current and popular 14,15. It is possible to compete with a multimodality probe by employing a mixture of standalone PET and FL probes. However, a multimodal probe would obviate the well-recognized complications associated with co-injected mixtures of standalone PET or FL contrast, including differences in blood clearance and non-specific tissue accumulation. In cases of limited ligand expression, using standalone probes would lead to PSMA+ receptors binding sites’ being blocked by a competing PET or FL probe, reducing the absolute PET or FL signal.
We used a unique IEX AMBF3 radiochemistry to yield 19F-BF3-Cy3-ACUPA. IEX AMBF3 radiochemistry is less technically challenging compared to many current strategies for [18F]-PET radio labeling. No immediate or long-term adverse effects were reported or observed in patients receiving [18F]-BF3-Cy3-ACUPA, suggesting that the AMBF3 prosthetic group can be safely translated to other agents to yield contrast that is safe for human use.
Intraoperative fluorescence imaging of ex vivo prostate or lymph node tissue in the OR did not interfere with standard-of-care prostate removal. However, our Cy3 fluorescence imaging system has a few limitations. Firstly, given the lack of a standard device for Cy3 fluorescence wavelength (554 nm excitation, 565 nm emission) imaging in the OR 24, standardized thresholds or approved parameters for molecularly targeted fluorescence PCa margin imaging do not exist and it was necessary to build a custom fluorescence imaging camera for real-time Cy3 wavelength imaging and perform qualitative evaluation. Secondly, our Cy3 fluorescence imaging system would benefit from future equipment modifications. From this study, our current wish list is: stereotaxic visualization, photomultiplier incorporation, lens autofocusing, and interchangeable exciter filters. Thirdly, it would be ideal if Cy3 fluorescence imaging could be used to image margins within the sterile field and not on a back-table. Fourthly, clear mathematical parameters to define certain signal-to-noise ratios as the positive margin are needed. These fluorescence parameters and improvements can only be developed for use in the OR with input from experienced urological surgeons.
CONCLUSION
In summary, this study demonstrates that [18F]-BF3-Cy3-ACUPA is both safe and useful in humans. Developing a multimodal, single molecule PET and FL contrast agent will be cheaper and faster than developing separate standalone PET and FL contrast agents in parallel. Larger trials with this PET tracer are expected to further define its capabilities and its clinical role in the management of PSMA+ malignancy, especially in PCa.
Supplementary Material
CLINICAL PRACTICE POINTS.
Prostate-specific membrane antigen (PSMA), which is highly expressed in both localized and metastatic prostate cancer (PCa) as well as non-prostate origin cancer, is an ideal target for imaging and therapy of PCa.
We showed that [18F]-BF3-Cy3-ACUPA is both safe and useful in humans. 18F]-BF3-Cy3-ACUPA demonstrated high PSMA-targeting efficiency and a favorable pharmacokinetic profile, allowing for sensitive and high-contrast detection of PSMA positive lesions by PET and optical imaging methods (intraoperative imaging/in vivo and ex vivo fluorescence microscopy)
Our findings also suggested that [18F]-BF3-Cy3-ACUPA is versatile hybrid imaging agents.
ACKNOWLEDGMENTS
We thank the patients whose contributions made this work possible. We also thank Erdogan and Behzat Senkaya for their support and donation to the Department of Nuclear Medicine at the Istanbul University-Cerrahpasa, Cerrahpasa Medical Faculty, and the personnel at the Department of Nuclear Medicine for their hard work and enthusiasm.
Funding: Omer Aras and Oguz Akin were partially funded by NIH/NCI Cancer Center Support Grant P30 CA008748.
Footnotes
Conflict of Interest: Richard Ting and Omer Aras are minor stakeholders in Trace Imaging Technologies. Richard Ting is also a minor stakeholder in alpha-9 therapeutics. The other authors declare that they have no conflict of interest.
Ethical Approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: All patients were informed about the in-house production of the novel radiopharmaceutical, according to the standards of the institution and/or national research committee and gave written informed consent.
Statement of human rights: We retrospectively report observations from clinical practice. For this type of study no formal clinical trial registration is required. All studies were conducted following IRB approval from Istanbul University Cerrahpasa Medical Faculty, Ethical Committee (approval#-29281604.01.01-139206, Cerrahpasa University School of Medicine, Department of Nuclear Medicine). All procedures performed involving human participants were performed in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Tringale KR, Pang J, Nguyen QT: Image-guided surgery in cancer: A strategy to reduce incidence of positive surgical margins. Wiley Interdiscip Rev Syst Biol Med 10:e1412, 2018 [DOI] [PubMed] [Google Scholar]
- 2.Cookson MS, Chang SS: Margin control in open radical prostatectomy: what are the real outcomes? Urol Oncol 28:205–9, 2010 [DOI] [PubMed] [Google Scholar]
- 3.Silberstein JL, Eastham JA: Significance and management of positive surgical margins at the time of radical prostatectomy. Indian J Urol 30:423–8, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shah SK, Fleet TM, Williams V, et al. : SEER coding standards result in underestimation of positive surgical margin incidence at radical prostatectomy: results of a systematic audit. J Urol 186:855–9, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Stephenson AJ, Wood DP, Kattan MW, et al. : Location, extent and number of positive surgical margins do not improve accuracy of predicting prostate cancer recurrence after radical prostatectomy. J Urol 182:1357–63, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Halpern JA, Sedrakyan A, Hsu WC, et al. : Use, complications, and costs of stereotactic body radiotherapy for localized prostate cancer. Cancer 122:2496–504, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kularatne SA, Thomas M, Myers CH, et al. : Evaluation of Novel Prostate-Specific Membrane Antigen-Targeted Near-Infrared Imaging Agent for Fluorescence-Guided Surgery of Prostate Cancer. Clin Cancer Res 25:177–187, 2019 [DOI] [PubMed] [Google Scholar]
- 8.Pourkavoos N: Unique Risks, Benefits, and Challenges of Developing Drug-Drug Combination Products in a Pharmaceutical Industrial Setting. Combination Products in Therapy 2:2, 2012 [Google Scholar]
- 9.Hensbergen AW, van Willigen DM, van Beurden F, et al. : Image-Guided Surgery: Are We Getting the Most Out of Small-Molecule Prostate-Specific-Membrane-Antigen-Targeted Tracers? Bioconjug Chem 31:375–395, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sengupta S, Asha Krishnan M, Chattopadhyay S, et al. : Comparison of prostate-specific membrane antigen ligands in clinical translation research for diagnosis of prostate cancer. Cancer Reports 2:e1169, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olson MT, Ly QP, Mohs AM: Fluorescence Guidance in Surgical Oncology: Challenges, Opportunities, and Translation. Mol Imaging Biol 21:200–218, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Derks YHW, Lowik D, Sedelaar JPM, et al. : PSMA-targeting agents for radio- and fluorescence-guided prostate cancer surgery. Theranostics 9:6824–6839, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao J, Chen J, Ma S, et al. : Recent developments in multimodality fluorescence imaging probes. Acta Pharm Sin B 8:320–338, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li D, Zhang J, Chi C, et al. : First-in-human study of PET and optical dual-modality image-guided surgery in glioblastoma using 68Ga-IRDye800CW-BBN. Theranostics 8:2508–2520, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Society of Nuclear Medicine: Preclinical evaluation of dual-labeled PSMA-inhibitors for the diagnosis and therapy of prostate cancer, in Socieity of Nuclear Medicine and Molecular Imaging tAM, June10-14, 2017, Denver, Colo; (ed), 2017 [Google Scholar]
- 16.Kommidi H, Guo H, Nurili F, et al. : (18)F-Positron Emitting/Trimethine Cyanine-Fluorescent Contrast for Image-Guided Prostate Cancer Management. J Med Chem 61:4256–4262, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo H, Kommidi H, Vedvyas Y, et al. : A Fluorescent, [(18)F]-Positron-Emitting Agent for Imaging Prostate-Specific Membrane Antigen Allows Genetic Reporting in Adoptively Transferred, Genetically Modified Cells. ACS Chem Biol 14:1449–1459, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kostikov AP, Chin J, Orchowski K, et al. : Synthesis of [(18)F]SiFB: a prosthetic group for direct protein radiolabeling for application in positron emission tomography. Nat Protoc 7:1956–63, 2012 [DOI] [PubMed] [Google Scholar]
- 19.Wangler B, Quandt G, Iovkova L, et al. : Kit-like 18F-labeling of proteins: synthesis of 4-(di-tert-butyl[18F]fluorosilyl)benzenethiol (Si[18F]FA-SH) labeled rat serum albumin for blood pool imaging with PET. Bioconjug Chem 20:317–21, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Richarz R, Krapf P, Zarrad F, et al. : Neither azeotropic drying, nor base nor other additives: a minimalist approach to (18)F-labeling. Org Biomol Chem 12:8094–9, 2014 [DOI] [PubMed] [Google Scholar]
- 21.Perrin DM: [(18)F]-Organotrifluoroborates as Radioprosthetic Groups for PET Imaging: From Design Principles to Preclinical Applications. Acc Chem Res 49:1333–43, 2016 [DOI] [PubMed] [Google Scholar]
- 22.Liu Z, Li Y, Lozada J, et al. : Stoichiometric leverage: rapid 18F-aryltrifluoroborate radiosynthesis at high specific activity for click conjugation. Angew Chem Int Ed Engl 52:2303–7, 2013 [DOI] [PubMed] [Google Scholar]
- 23.Bernard-Gauthier V, Bailey JJ, Liu Z, et al. : From Unorthodox to Established: The Current Status of (18)F-Trifluoroborate- and (18)F-SiFA-Based Radiopharmaceuticals in PET Nuclear Imaging. Bioconjug Chem 27:267–79, 2016 [DOI] [PubMed] [Google Scholar]
- 24.Sajedi S, Sabet H, Choi Hak S: Intraoperative biophotonic imaging systems for image-guided interventions, Nanophotonics, 2018, pp 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.