Abstract
OBJECTIVES:
Brain-derived neurotrophic factor (BDNF) has been implicated in central neurological processes. We hypothesize that greater pain catastrophizing is associated with higher urinary BDNF levels in women with bladder pain syndrome.
METHODS:
Secondary analysis of a database of women with urinary urgency. We identified women who met AUA criteria of bladder pain syndrome. Urinary symptoms, pain catastrophizing, and neuropathic pain were measured using the Female Genitourinary Pain Index (F-GUPI), Pain Catastrophizing Scale (PCS) and painDETECT questionnaires respectively. Relationship of catastrophizing score with urinary BDNF (primary outcome) and other urinary biomarkers including nerve growth factor (NGF), vascular endothelial growth factor (VEGF) and osteopontin was evaluated using univariable and multivariable analyses.
RESULTS:
In 62 women with bladder pain syndrome, 15 (24%) reported pain catastrophizing symptoms (PCS score >30). Higher catastrophizing scores were associated with worse urinary symptoms, greater pelvic pain, greater neuropathic pain, and worse quality of life scores (all p<0.01). On multivariable analysis, after controlling for age, BMI and urinary symptoms, higher pain catastrophizing score was associated with lower BDNF (p=0.04) and lower VEGF levels (p=0.03). Urinary urgency was associated with higher NGF level (p=0.04) while bladder pain was associated with higher levels of NGF (p=0.03) and VEGF (p=0.01).
CONCLUSIONS:
Neuroinflammatory mechanisms contribute to the central processing of pain in women with bladder pain syndrome. Worse urinary symptoms are associated with higher NGF and VEGF levels, but worse pain catastrophizing is associated with lower BDNF and VEGF levels. Urinary BDNF levels may be useful in phenotyping women who have central augmentation of pain processing.
Keywords: bladder pain syndrome, BDNF, NGF, pain catastrophizing, interstitial cystitis
INTRODUCTION
Bladder pain syndrome (BPS), characterized by bladder pain, urinary urgency, and frequency in the absence of known underlying pathology, is a chronic debilitating pain condition that affects 2–6% of adult women in the United States.1,2 In BPS, urinary symptoms are commonly associated with central symptoms such as depression, anxiety, and insomnia, however, the underlying mechanism of co-existing urinary and central symptoms are poorly understood.3
Animal studies have revealed that BPS is characterized by inflammation in both the peripheral and central nervous systems.4 Peripheral neuroinflammation is indicated by chronic activation of C-afferent pain fibers, while activation of the somatosensory cortex and limbic system in the central nervous system in animal models of BPS resulted in bladder sensitization.5,6 In women with BPS, neuroimaging studies have identified changes in gray matter density in the somatosensory cortex and regions of the brain involved with processing emotional response, specifically the amygdala and hippocampus within the limbic system.7 In addition, Kilpatrick et al reported increased functional connectivity of the somatosensory and viscerosensory cortices to the frontal cortex, a finding that correlated with evoked pain during bladder filling.8 These findings suggest that the sensorimotor component of pain in women with BPS is closely associated with cognitive function including emotional processing, and central augmentation of pain likely occurs in women with BPS.
Catastrophizing is a maladaptive, self-reported coping mechanism that occurs in response to the central processing of afferent pain stimuli and shapes an individual’s experience of pain.9 Catastrophizing can be measured in three domains including rumination, magnification, and helplessness using the validated Pain Catastrophizing Scale.10 In BPS, hypersensitivity to the visceral stimulus of bladder filling has been shown to be associated with a catastrophizing response and worsening chronic pain.11 Patients with high scores on the Pain Catastrophizing Scale report higher pain severity, greater physical disability, and lower pain thresholds.12 When associated with overwhelming helplessness and rumination, catastrophizing in patients with BPS has been implicated in higher rates of depression, anxiety and suicidal ideation.13 A peripheral urinary biomarker of pain catastrophizing would both help to elucidate underlying mechanism of this complex neurobehavioral symptom and potentially allow early identification of these patients.
Brain-derived neurotrophic factor (BDNF) is a biomarker of neurogenic dysfunction and progressive decline of neuronal activity.14 BDNF production is primarily concentrated within the limbic system, specifically, the hippocampus.15 In an animal model, systemic change in serum BDNF reflected changes in BDNF expression within the central nervous system.15 Furthermore, in a BDNF knockout mouse model with chronic neuropathic inflammation, BDNF depletion signaled hypersensitivity to pain stimuli and the development of chronic pain syndromes.16 Given that prior studies have reported elevated BDNF levels in adults with lower urinary tract symptoms, a common mechanism may explain the co-existence of urinary and central nervous symptoms in women with BPS.17 Several other urinary neuropeptides known to be associated with lower urinary tract inflammation including nerve growth factor (NGF), vascular endothelial growth factor (VEGF) and osteopontin have also been implicated in central nervous system conditions including cognitive dysfunction, anxiety, and major depression.18–20 Investigating the relationship between the level of pain catastrophizing and urinary neuropeptides could help elucidate the mechanisms of central augmentation of pain processing in women with BPS.
Our primary hypothesis was that in women with chronic bladder pain, pain catastrophizing, a central neurobehavioral symptom, was associated with elevated levels of urinary BDNF. We also explored the relationship of pain catastrophizing with other urinary neuropeptides including NGF, VEGF, and osteopontin.
METHODS
Our study was approved by the Institutional Review Board at the University of Pennsylvania. We conducted a secondary analysis of a database of adult women with urinary urgency without incontinence. Subjects in this database were enrolled from the Urogynecology Division at the Hospital of the University of Pennsylvania and the Pelvic and Sexual Health Institute from March to July 2013. Inclusion criteria for the original database were: women age 18 years or older and who had moderate or severe urinary urgency on the Indevus Urgency Severity Scale (score ≥2).21 The Indevus Urgency Severity Scale is a four point (0 to 4) validated questionnaire used to assess urinary urgency.21 For the secondary analysis performed in the present study, we identified women who met the American Urologic Association diagnostic criteria of BPS (pain in the bladder associated with other urinary tract symptoms for more than 6 weeks duration in the absence of urinary tract infection). Bladder pain was defined as pain of severity ≥3 on a visual analog scare (VAS) from 0 to 10.22 Exclusion criteria were neurogenic bladder, kidney stones, recent or acute urinary tract infection and recent pregnancy (<3 months postpartum).
Demographic data including history of anxiety and depression were extracted from the database.
Data on urinary biomarkers NGF, BDNF, VEGF and osteopontin levels normalized to urinary creatinine (Cr) concentration (NGF/Cr, BDNF/Cr, VEGF/Cr and osteopontin/Cr levels) were extracted from database. Collection technique of urine specimens and laboratory analysis have been previously described.26
Data on urinary symptoms, pain catastrophizing, and neuropathic pain measured using the following validated questionnaires were also extracted.
The Pain Catastrophizing Scale (PCS) is a 13-item validated questionnaire that measures severity of catastrophizing thoughts in patients with chronic pain. Each item in the PCS measures severity of thoughts on a scale of 0 to 4 (total score range 0 to 52). PCS contains three subscales: rumination (0 to 16), magnification (0 to 12) and helplessness (0 to 24).10 Rumination subscale measures the attention to pain symptoms and perseverating painful thoughts. Magnification measures catastrophizing thoughts that the pain will worsen or transform into a more serious problem. Helplessness measures concerns that the pain will never improve.10 Total pain catastrophizing scores > 30 correspond to the 75th percentile of chronic pain respondents and subjects with score > 30 are classified as demonstrating pain catastrophizing.
The Female Genitourinary Pain Index (F-GUPI) is a 9-item validated questionnaire that measures bladder pain and lower urinary tract symptoms with a total score ranging from 0 to 45. The F-GUPI contains three domains: pain (0 to 23), urinary symptoms (0 to 10) and quality of life (0 to 12). The pain domain contains a 10-point VAS which was used as study inclusion criteria. Higher F-GUPI scores indicate worse lower urinary tract symptoms and pain.23
The Interstitial Cystitis Symptom and Problem Index (ICSI and ICPI) are validated indices to quantify severity of lower urinary tract symptoms consistent with interstitial cystitis/BPS. ICSI and ICPI measure urinary urgency, frequency, nocturia and bladder pain. Total score for each instrument ranges from 0 to 20 with higher scores indicating worse symptoms and greater impact on quality of life.24
The PainDETECT questionnaire is a 9-item validated instrument to measure the severity of neuropathic pain (score range from −1 to 38). PainDETECT measures the following seven sensory neurologic symptoms: paresthesia, burning, pain associated with light touch and pressure, thermal hyperalgesia, numbness and electric shocks. Two additional items assess the course and radiation of pain. Neuropathic pain is diagnosed based on a score ≥19.25
Based on the PCS total score, women with BPS were divided into two groups: high pain catastrophizing score (score > 30) and low pain catastrophizing score (score ≤30). We compared baseline demographic data, clinical questionnaire results and urinary biomarker levels between subjects with high and low catastrophizing scores using parametric and non-parametric t-tests. Categorical variables were compared using Pearson Chi-square and Fisher Exact tests. We determined the relationship between pain catastrophizing and urinary BDNF levels (primary outcome) and other neuropeptides (NGF, VEGF, osteopontin) using univariable and multivariable linear regression analysis. The a priori multivariable regression model based on prior analysis included age, BMI, bladder pain severity, neuropathic pain and urinary urgency.26 Statistical analyses were conducted using STATA 15.1 (Stata Corp, College Station, TX).
RESULTS
A total of 62 women met inclusion criteria of BPS out of the total of 103 women in the database. Of the 62 women with completed questionnaires, 51 (82.3%) provided urinary samples. Eleven women did not return proper urine specimens.
Mean (SD) age, BMI, and median (range) parity for the 62 women in the BPS cohort was 44.1 (+/−2.3), 24.4 (+/−4.9), 0 (0–5) respectively. The mean (SD) pain catastrophizing score for the cohort was 18.8 (+/−13.0). The mean (SD) for the PCS subscales were as follows: rumination 6.4 (+/−5.0), magnification 3.6 (+/−3.0) and helplessness 8.9 (+/−6.4).
Fifteen subjects (24.2%) were categorized in the higher pain catastrophizing score group (PCS score >30) and 47 (75.8%) were in the lower pain catastrophizing group (PCS score ≤30). There were no significant differences in age, race, BMI, parity, menopausal status or current treatments for BPS, antidepressants, and anxiolytics between the higher and lower pain catastrophizing score groups (Table 1). Women in the higher pain catastrophizing score group were more likely to have a concomitant diagnosis of vulvodynia and less likely to have undergone hysterectomy. There were no significant differences in other co-morbidities including anxiety and depression (Table 1).
Table 1.
Demographics of patients with bladder pain syndrome (N=62).
| Lower Pain Catastrophizing score (PCS≤30) N = 47 | Higher Pain Catastrophizing score (PCS>30) N = 15 | P-value | |
|---|---|---|---|
| Age (years) | 44.7 (+/−2.2) | 42.1 (+/−3.7) | 0.57a |
| Race (N, %) | |||
| Caucasian | 41 (87.2) | 14 (93.3) | 0.68b |
| African American | 4 (8.5) | 1 (6.7) | |
| Other | 2 (4.3) | 0 (0.0) | |
| BMI (kg/m2) | 25.0 (+/−5.2) | 22.6 (+/−3.5) | 0.10a |
| Parity (median, range) | 0 (0–5) | 0 (0–2) | 0.93c |
| Postmenopausal (N, %) | 19 (40.4) | 6 (40.0) | 0.99b |
| Current Treatment(s) (N, %) | |||
| SSRIs/SNRIs | 14 (30.0) | 4 (26.7) | 0.55d |
| Tricyclic Anti-depressants | 10 (21.2) | 3 (20.0) | 0.62d |
| Anxiolytics | 13 (27.7) | 8 (53.3) | 0.07b |
| Antipsychotics | 2 (4.3) | 0 (0.0) | 0.57d |
| Lamotrigine | 2 (4.3) | 0 (0.0) | 0.57d |
| Gabapentin | 4 (8.5) | 2 (13.3) | 0.24d |
| Antihistamines | 14 (29.8) | 5 (33.3) | 0.79b |
| Anticholinergics | 2 (4.3) | 0 (0.0) | 0.53d |
| Analgesics | 20 (42.6) | 6 (40.0) | 0.86b |
| Pentosan polysulfate | 7 (14.9) | 3 (20.0) | 0.46d |
| Bladder Instillations | 3 (6.4) | 4 (46.7) | 0.05d |
| Botox | 1 (2.1) | 0 (0.0) | 0.76d |
| Interstim | 0 (0.0) | 0 (0.0) | |
| Past Surgical History (N, %) | |||
| Hysterectomy | 11 (23.4) | 0 (0.0) | 0.03 d |
| Prolapse | 1 (2.1) | 0 (0.0) | 0.76d |
| Incontinence Procedure | 1 (2.1) | 1 (6.7) | 0.43d |
| Oophorectomy | 6 (12.8) | 0 (0.0) | 0.18d |
| Appendectomy | 10 (21.3) | 1 (6.7) | 0.19d |
| Past Medical History (N, %) | |||
| Depression | 11 (23.4) | 5 (33.3) | 0.34b |
| Anxiety | 14 (29.8) | 6 (40.0) | 0.46b |
| Vulvodynia | 2 (4.3) | 4 (26.7) | 0.03 d |
| Pelvic Floor Dysfunction | 3 (6.4) | 4 (26.7) | 0.05d |
| Fibromyalgia | 3 (6.4) | 4 (26.7) | 0.05d |
| Chronic Fatigue Syndrome | 4 (8.5) | 1 (6.7) | 0.65d |
| Migraine | 8 (17.0) | 6 (40.0) | 0.06b |
| Irritable Bowel Syndrome | 12 (25.5) | 6 (40.0) | 0.28b |
| Diabetes | 3 (6.4) | 0 (0.0) | 0.43d |
| Hypertension | 4 (8.5) | 1 (6.7) | 0.65d |
Independent t-test
Chi2 test
Equality of medians
Fisher exact test
Statistical significance for all tests, p<0.05
PCS=pain catastrophizing scale
SSRIs/SNRIs=selective serotonin reuptake inhibitors/serotonin and norepinephrine reuptake inhibitors
Urinary symptom score as measured by the ICSI and ICPI were not significantly different between the higher and lower pain catastrophizing groups (Table 2). Similarly, there were no significant differences in the urinary subscale scores of the F-GUPI and the Indevus urgency scale scores between groups. The higher pain catastrophizing score group had significantly higher (worse) scores for the pain and quality life domains of the F-GUPI and higher (worse) bladder pain/burning on the ICSI than the lower pain catastrophizing score group. The mean neuropathic pain score as measured by painDETECT was greater in the higher pain catastrophizing group but did not reach significant levels.
Table 2.
Urinary symptoms and urinary biomarker levels of patients with bladder pain syndrome (N=62).
| Lower Pain Catastrophizing score (PCS≤30) N = 47 | Higher Pain Catastrophizing score (PCS>30) N = 15 | P-value | |
|---|---|---|---|
| Clinical Symptoms | |||
| Indevus Urgency Severity Scale | |||
| Moderate | 19 (40.4) | 6 (40.0) | 0.96a |
| Interstitial Cystitis Symptom Index (ICSI) Total Score | 9.4 (+/−4.6) | 11.5 (+/−5.5) | 0.15b |
| Urgency (ICSI, Question 1) | 1.8 (+/−1.5) | 1.9 (+/−1.5) | 0.89b |
| Frequency (ICSI, Question 2) | 3.1 (+/−1.6) | 3.1 (+/−1.5) | 0.97b |
| Nocturia (ICSI, Question 3) | 2.8 (+/−1.9) | 3.5 (+/−2.1) | 0.23b |
| Pain/burning in bladder (ICSI, Question 4) | 1.7 (+/−1.7) | 2.9 (+/−2.1) | 0.03 b |
| Interstitial Cystitis Problem Index (ICPI) Total Score | 8.7 (+/−3.9) | 9.0 (+/−6.2) | 0.80b |
| Female Genitourinary Pain Index (F-GUPI) Total Score | 26.4 (+/−6.2) | 33.3 (+/−6.4) | <0.01 b |
| Pain Subscale | 13.3 (+/−3.3) | 17.3 (+/−2.8) | <0.01 b |
| Urinary Subscale | 5.0 (+/−2.9) | 6.2 (+/−3.2) | 0.18b |
| Quality of Life Subscale | 8.1 (+/−2.3) | 10.0 (+/−2.3) | <0.01 b |
| PainDETECT Total Score | 12.6 (+/−8.0) | 16.2 (+/−9.5) | 0.15b |
| Neuropathic Painc | 10 (21.3) | 6 (40.0) | 0.15a |
| Urinary Biomarkers | N= 36 | N= 15 | |
| BDNF | 3.13 (+/−3.46) | 3.28 (+/−4.79) | 0.90b |
| NGF | 0.23 (+/−0.61) | 0.47 (+/−0.74) | 0.23b |
| VEGF | 0.35 (+/−0.45) | 0.40 (+/−0.54) | 0.72b |
| Osteopontin | 3.89 (+/−3.98) | 3.34 (+/−5.32) | 0.69b |
Chi2 test
Independent t-test
Statistical significance for all tests, p<0.05
Neuropathic pain defined as painDETECT score ≥19
PCS=pain catastrophizing scale
NGF= nerve growth factor
BDNF= brain-derived neurotrophic factor
VEGF= vascular endothelial growth factor
We noted significant association between total catastrophizing score and neuropathic painDETECT score (Table 3). Higher rumination and helplessness subscale scores of the PCS were associated with higher painDETECT scores, however, no significant relationship was noted with the Magnification subscale.
Table 3.
Relationship between neuropathic pain and pain catastrophizing scale in patients with bladder pain syndrome (N=62).
| Neuropathic Paina | ||
|---|---|---|
| Coefficientb (95% CI) | P value | |
| Pain Catastrophizing Scale (PCS) Total Score | 0.22 (0.06–0.38) | 0.01 |
| Rumination Subscale | 0.48 (0.05–0.09) | 0.03 |
| Magnification Subscale | 0.54 (−0.17–1.24) | 0.13 |
| Helplessness Subscale | 0.51 (0.19–0.83) | <0.01 |
Neuropathic pain is defined as painDETECT Total Score ≥19.
Univariable linear regression analysis, statistical significance p<0.05
Mean neuropeptide levels between the higher and lower pain catastrophizing score groups were not significantly different (Table 2). The relationship between urinary neuropeptides and selected clinical variables are presented in Table 4. On univariable analysis, we observed a significant association of pain scores (bladder pain and neuropathic pain) with NGF and VEGF levels (Table 4). Urinary urgency was also associated with NGF levels.
Table 4.
Relationship of clinical variables with urinary neuropeptide levels: Univariable and Multivariable Analyses
| Coefficienta (95% CI) | P value | Coefficientb (95% CI) | P value | |
|---|---|---|---|---|
| Pain Catastrophizing Scale | ||||
| BDNF | −0.07 (−0.15–0.01) | 0.09 | −0.10 (−0.19– −0.003) | 0.04 |
| NGF | 0.001 (−0.01–0.01) | 0.99 | −0.01 (−0.03–0.01) | 0.16 |
| VEGF | −0.01 (−0.02–0.003) | 0.17 | −0.01 (−0.03– −0.001) | 0.03 |
| Osteopontin | 0.05 (−0.14–0.04) | 0.29 | −0.11 (−0.23–0.02) | 0.09 |
| Urinary Urgency c | ||||
| BDNF | 2.13 (−0.10–4.35) | 0.06 | 0.77 (−1.34–2.88) | 0.47 |
| NGF | 0.38 (0.006–0.76) | 0.04 | 0.22 (−0.18–0.63) | 0.27 |
| VEGF | 0.09 (−0.19–0.37) | 0.54 | −0.01 (−0.30–0.29) | 0.96 |
| Osteopontin | 0.89 (−1.70–3.48) | 0.49 | 0.28 (−2.64–3.19) | 0.85 |
| Bladder Pain d | ||||
| BDNF | 0.27 (−0.38–0.92) | 0.41 | 0.42 (−0.28–1.11) | 0.23 |
| NGF | 0.11 (0.01–0.22) | 0.03 | 0.11 (−0.02–0.24) | 0.10 |
| VEGF | 0.08 (0.003–0.16) | 0.04 | 0.13 (0.04–0.22) | 0.01 |
| Osteopontin | 0.51 (−0.21–1.23) | 0.16 | 0.85 (−0.08–1.79) | 0.07 |
| Neuropathic Pain e | ||||
| BDNF | 0.48 (−2.04–3.00) | 0.70 | 0.57 (−1.74–2.88) | 0.62 |
| NGF | 0.44 (0.04–0.84) | 0.03 | 0.39 (−0.04–0.81) | 0.08 |
| VEGF | 0.06 (−0.24–0.36) | 0.67 | −0.01 (−0.32–0.30) | 0.95 |
| Osteopontin | 0.96 (−1.81–3.73) | 0.49 | 0.40 (−2.69–3.19) | 0.80 |
Univariable linear regression analysis, statistical significance p<0.05
A priori multivariable linear regression controlled for age, BMI, urgency, bladder pain and neuropathic pain, statistical significance p<0.05
As ascertained from the Indevus Urgency Severity Scale
Interstitial Cystitis Symptom Index (ICSI, Question 4)
Neuropathic pain defined as painDETECT Total Score ≥19
NGF= nerve growth factor
BDNF= brain-derived neurotrophic factor
VEGF= vascular endothelial growth factor
Catastrophizing total and subscale scores were not significantly associated with urinary neuropeptide levels on univariable analysis. On multivariable analysis, after controlling for age, BMI, urinary urgency, and bladder pain, pain catastrophizing score was negatively associated with BDNF (p=0.04) and VEGF levels (p=0.03) (Figure 1).
Figure 1.
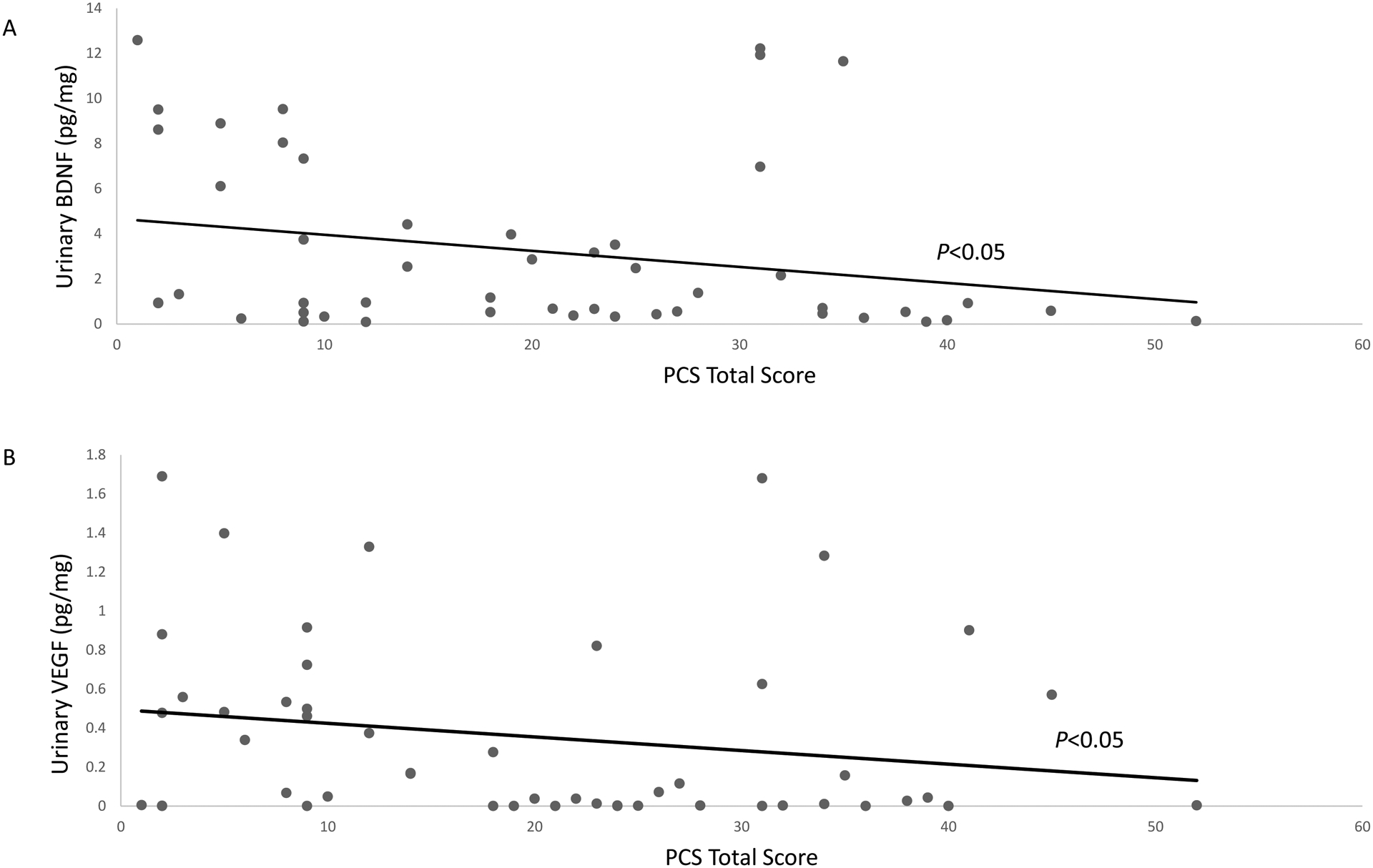
Relationship of pain catastrophizing scale (PCS) total scores with urinary BDNF (A) and VEGF (B) levels. P values adjusted for age, BMI, urinary urgency, bladder pain, and neuropathic pain.
DISCUSSION
We rejected our hypothesis that pain catastrophizing is associated with higher urinary BDNF levels in women with BPS. This was based on our finding that pain catastrophizing, a central neurobehavioral representation of an individual’s experience of pain, was significantly associated with higher bladder pain and neuropathic pain scores, but lower urinary BDNF level. Specifically, two domains of pain catastrophizing, rumination and helplessness, were associated with higher neuropathic pain scores, a clinical biomarker of neuroinflammation. Additionally, higher total pain catastrophizing scores were associated with lower urinary BDNF level after controlling for confounding effects of age and BMI. Taken together, these findings suggest that neuroinflammatory mechanisms may be involved in the central processing of chronic bladder pain.
Pain catastrophizing is conceptualized as a negative cognitive–affective response to pain.27 Clinically, catastrophizing should not be viewed simply as a maladaptive response. Prior studies suggest that helplessness and rumination domains of catastrophizing are significantly associated with major depression and suicidal ideation.13 In our study, we noted significant association of pain catastrophizing with the severity of bladder pain and neuropathic pain scores. These findings have useful clinical implications. First, neuropathic pain was associated with the rumination and helplessness but not the magnification domain of pain catastrophizing. These findings should reassure clinicians that women with BPS are not exaggerating their bladder pain experience. Next, given the close relationship of helplessness and rumination with depression and suicidal ideation, our findings suggest that patients who report severe bladder pain and/or neuropathic pain should be evaluated for pain catastrophizing using the PCS questionnaire that takes less than 5 minutes to complete.13 Finally, our findings could help to guide treatment for subjects who report pain catastrophizing. According to the appraisal theory, rumination (persistent thinking about pain) reflects a primary appraisal and evaluation of a painful stimulus as extremely threatening, whereas helplessness reflects secondary appraisal of inability to cope.28 Accordingly, catastrophizing may represent reduced self-efficacy to control a stimulus perceived as extremely threatening.12 These findings suggest that even relatively simple measures such as urinary analgesics that improve self-efficacy for controlling pain in the short-term could help reduce central symptoms in women with BPS. In the long-term, subjects with pain catastrophizing would likely benefit from interventions that improve their ability to cope with pain such as cognitive behavioral therapy.
We noted a significant negative association between higher pain catastrophizing scores and urinary BDNF levels after adjusting for the confounding effect of age and BMI. Though several studies have investigated a variety of psychosocial factors in pain catastrophizing, relatively few have examined whether pain catastrophizing is associated with alteration in endogenous pain modulation.29,30 The limbic system, specifically the hippocampus, contains the primary concentration of BDNF within the cerebral cortex.15 In animal models, chronic emotional stress resulted in neuronal dysfunction and apoptosis within the hippocampus, in addition to a decrease in serum BDNF levels.31 In BPS patients, fMRI studies demonstrate altered gray matter and abnormal functional connectivity of the hippocampus.32 Our findings that catastrophizing is associated with urinary BDNF levels suggest that central neurogenic inflammatory processes contribute to central symptoms in women with BPS. Furthermore, given that higher catastrophizing scores were associated with lower urinary BDNF levels, presence of BDNF in the urine of women with BPS could serve as a clinical predictor of less severe central augmentation. Larger studies in which urinary and affective symptoms are correlated with BDNF levels are required to confirm our findings.
We also explored the relationship between pain catastrophizing and other urinary biomarkers such as NGF, VEGF, and osteopontin. Consistent with prior studies, we noted significant associations of urgency with NGF levels, and between bladder pain and NGF and VEGF levels.33,34 Similar to BDNF, pain catastrophizing was negatively associated with lower urinary VEGF levels. In adults with major depression, a decrease in gray matter density within the hippocampus has been shown to be associated with decreased systemic VEGF levels.35 Therefore, similar underlying central neuroinflammatory mechanisms may explain lower BDNF and VEGF levels in the urine of women with BPS and who exhibit prominent pain catastrophizing.
Our study provides evidence that BPS patients with higher pain catastrophizing also have higher rates of vulvodynia. The rate of pelvic floor dysfunction, irritable bowel syndrome and fibromyalgia was also higher in women with higher PCS scores, and although not statistically significant, these findings contribute to a theory of pelvic organ crosstalk. Cross-sensitization between urinary and reproductive organs may explain our findings. The convergence of pain stimuli from urinary and reproductive organs are mediated by peripheral and central mechanisms.36 Supraspinal mechanisms of cross-sensitization are processed within the thalamus and limbic system which results in hyperexcitability and hyperalgesia of pelvic organs.37 In addition, NGF has been implicated in pelvic organ crosstalk. Elevated levels of bladder NGF was associated with enhanced sensitivity of other pelvic organs outside the bladder.38 Cross-sensitization between the bladder and vulva may explain our findings of higher pain catastrophizing due to hyperalgesia and central processing of pain. However, the role of urinary biomarkers and supraspinal pain processing among pelvic organs requires further investigation.
Strengths of our study include a clearly defined cohort of women with BPS. We purposely excluded patients with minimal to no bladder pain in order to capture pain catastrophizing symptoms in women with recent pain episodes. We utilized validated questionnaires to measure clinical variables and urinary neuropeptides that were previously studied in women with lower urinary tract symptoms. Our study is limited by the research design of a secondary analysis, limited sample size, and absence of a control group. Due to the nature of a retrospective secondary analysis, we were only able to extract urine biomarker levels and serum biomarker values were not available in the database. Therefore, we used serum creatinine to normalize urinary biomarker values. Future directions may include investigation of serum BDNF and VEGF in patients with BPS and catastrophizing symptoms.
In conclusion, our study offers evidence that neuroinflammatory mechanisms in women with BPS contribute to the central processing of bladder pain. Our study also demonstrates that urinary BDNF and VEGF could potentially be developed as biomarkers for phenotyping subjects with consequential central symptoms such as catastrophizing.
FUNDING:
The study was supported by a grant from International Urogynecological Association (IUGA), and by R01 DK121506 (to A.P.M)
REFERENCES:
- 1.Berry SA, Elliott MN, Suttorp M, Bogart LM, Stolo MA, Eggers P, Nyberg L, Clemens Q. Prevalence of symptoms of bladder pain syndrome/interstitial cystitis among adult females in the United States. J Urol 2011; 186 (8): 540–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McLennan MT. Interstitial cystitis: epidemiology, pathophysiology, and clinical presentation. Obstet Gynecol Clin North Am 2014; 41 (3): 385–395. [DOI] [PubMed] [Google Scholar]
- 3.Chuang YC, Weng SF, Hsu YW, et al. Increased risks of healthcare-seeking behaviors of anxiety, depression and insomnia among patients with bladder pain syndrome/interstitial cystitis: a nationwide population-based study. Int Urol Nephrol 2015; 47:275–281. [DOI] [PubMed] [Google Scholar]
- 4.Wang Z, Chang HH, Gao Y, Zhang R, Guo Y, Holschneider DP, et al. Effects of water avoidance stress on peripheral and central responses during bladder filling in the rat: a multidisciplinary approach to the study of urologic chronic pelvic pain syndrome (MAPP) research network study. PLoS One 2017;12:e0182976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruggemann J, Shi T, Apkarian AV. Viscero-somatic neurons in the primary somatosensory cortex (SI) of the squirrel monkey. Brain research 1997; 756(1): 297–300. [DOI] [PubMed] [Google Scholar]
- 6.Hur EM, Youssef S, Haws EM, et al. Osteopontin-induced relapse and progression of autoimmune brain disease through enhanced survival of activated T cells. Nature Immunology 2007; 8:74–83. [DOI] [PubMed] [Google Scholar]
- 7.Asiri MD, Banjar R., Al-Qahtani W, Goodarzynejad, H, Hassouna M. Central Nervous System Changes in Pelvic Inflammation/Pain Patients. Current Bladder Dysfunction Reports 2019;14(4): 223–230. [Google Scholar]
- 8.Kilpatrick LA, Kutch JJ, Tillisch K, et al. Alterations in resting state oscillations and connectivity in sensory and motor networks in women with interstitial cystitis/painful bladder syndrome. Journal of Urology 2014; 192(3): 947–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Theunissen M, Peters ML, Bruce J, Gramke HF, Marcus MA. Preoperative anxiety and catastrophizing. The Clinical Journal of Pain 2012; 28(9): 819–841. [DOI] [PubMed] [Google Scholar]
- 10.Sullivan MJ, Bishop SR. The pain catastrophizing scale: development and validation. Psychological Assessment 1995; 7(4):524–532. [Google Scholar]
- 11.Lowenstein L, Kenton K, Mueller ER, Brubaker L, Heneghan M, Senka J, Fitzgerald MP. Patients with painful bladder syndrome have altered response to thermal stimuli and catastrophic reaction to painful experiences. Neuro Uro 2009; 28:400–404. [DOI] [PubMed] [Google Scholar]
- 12.Sulivan MJ, Thorn B, Haythornthwaite JA, et al. Theoretical perspective on the relation between castrophizing and pain. Clin J Pain 2001; 17: 52–64. [DOI] [PubMed] [Google Scholar]
- 13.Tripp DA, Nickel JC, Krsmanovic A, et al. Depression and catastrophizing predict suicidal ideation in tertiary care patients with interstitial cystitis/bladder pain syndrome. Can Urol Assoc J 2016; (11–12): 383–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mariga A, Mitre M, Chao MV. Consequences of brain-derived neurotrophic factor withdrawal in CNS neurons and implications in disease. Neurobiology of Disease 2017: 97, 73–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hofer M, Pagliusi SR, Hohn A, Leibrock J, Barde YA. Regional distribution of brain-derived neurotrophic factor mRNA in the adult mouse brain. EMBO J 1990; 9: 2459–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sikandar S, Minett MS, Millet Q, Santana-Varela S, Lau J, Wood JN, Zhao J. Brain-derived neurotrophic factor derived from sensory neurons plays a critical role in chronic pain. Brain 2018; 141(4): 1028–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang LW, Han XM., Chen CH, et al. Urinary brain-derived neurotrophic factor: a potential biomarker for objective diagnosis of overactive bladder. Int Urol Nephrol. 2014;46:341–347. [DOI] [PubMed] [Google Scholar]
- 18.Wiener CD, Ferreira SM, Moreira FP, et al. Serum levels of nerve growth factor (NGF) in patients with major depression disorder and suicide risk. J Affect Disord 2015; 184: 245–248. [DOI] [PubMed] [Google Scholar]
- 19.Xie T, Stathopoulou M, de Andrés F et al. VEGF-related polymorphisms identified by GWAS and risk for major depression. Transl Psychiatry. 2017;7: e1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hur EM, Youssef S, Haws EM, et al. Osteopontin-induced relapse and progression of autoimmune brain disease through enhanced survival of activated T cells. Nature Immunology 2007; 8: 74–83. [DOI] [PubMed] [Google Scholar]
- 21.Nixon A, Colman S, Sabounjian L, et al. A validated patient reported measure of urinary urgency severity in overactive bladder for use in clinical trials. J Urol 2005; 174: 604–607. [DOI] [PubMed] [Google Scholar]
- 22.Hanno PM, Erickson D, Moldwin R, Faraday M. Diagnosis and treatment of interstitial cystitis/bladder pain syndrome: AUA guideline amendment. J Urol 2015; 193(5): 1545–1553. [DOI] [PubMed] [Google Scholar]
- 23.Clemens JQ, Calhoun EA, Litwin MS, et al. Validation of a modified National Institutes of Health chronic prostatitis symptom index to assess genitourinary pain in both men and women. Urology. 2009; 74 (5): 983–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Leary MP, Sant GR, Fowler FJ, et al. The interstitial cystitis symptom index and problem index. Urology. 1997;49:58. [DOI] [PubMed] [Google Scholar]
- 25.Arya LA, Harvie HS, Andy UU, et al. Construct validity of an instrument to measure neuropathic pain in women with bladder pain syndrome. Neurourol Urodyn 2013; 32:424. [DOI] [PubMed] [Google Scholar]
- 26.Soriano A, Andy U, Hassani D, Whitmore K, Harvie H, Malykhina AP, Arya L. Relationship of bladder pain with clinical and urinary markers of neuroinflammation in women with urinary urgency. FPMRS 2020; doi: 10.1097/SPV.0000000000000951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quartana PJ, Campbell CM, Edwards RR. Pain catastrophizing: a critical review. Expert Rev Neurother 2009;9(5): 745–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Severeijns R, Vlaylen JWS, van den Hout MA. Do we need a communal coping model of pain catastrophizing? An alternative explanation. Pain. 2004;111: 226–229. [DOI] [PubMed] [Google Scholar]
- 29.Meints SM, Edwards RR. Evaluating psychosocial contributions to chronic pain outcomes. Progress in Neuro-Psychopharmacology and Biological Psychiatry 2018;87: 168–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Edwards RR, Dworkin RH, Sullivan MD, Turk DC, Wasan AD. The Role of Psychosocial Processes in the Development and Maintenance of Chronic Pain. The Journal of Pain 2016; 17(9): T70–T92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim BK, Ko IG, Kim SE, et al. Impact of several types of stresses on short term memory and apoptosis in the hippocampus of rats. Int Neurourol J 2013;17(3): 114–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martucci KT, Shirer WR, Bagarinao E, et al. The posterior medical cortex in urologic chornic pelvic pain syndrome: detachment from default mode network. A resting-state study from the MAPP research network. Pain 2006; 156(9): 1755–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saban R, Saban MR, Maier J, et al. Urothelial expression of neuropilins and VEGF receptors in control and interstitial cystitis patients. Am J Physiol Renal Physiol 2008; 295(6): F1613–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim SW IM YJ, Choi HC, et al. Urinary nerve growth factor correlates with the severity of urgency and pain. Int Urogynecol J 2014; 25(11): 1561–1567. [DOI] [PubMed] [Google Scholar]
- 35.Xie T, Stathopoulou M, de Andrés F. et al. VEGF-related polymorphisms identified by GWAS and risk for major depression. Transl Psychiatry 2017;7: e1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Winnard KP, Dmitrieva N, Berkley KJ Cross-organ interactions between reproductive gastrointestinal and urinary tracts: modulation by estrous stage and involvement of the hypogastric nerve. Am J Physiol Regul Integr Comp Physiol. 2006;291(6):R1592–R1601. [DOI] [PubMed] [Google Scholar]
- 37.Qin C, Greenwood-Van Meerveld B, Formean RD. Spinal neuronal responses to urinary bladder stimulation in rats with corticosterone or aldosterone onto the amygdala. J Neurophysiol. 2003;90:2180–2189. [DOI] [PubMed] [Google Scholar]
- 38.Bielefeldt K, Lamb K, Gebhart GF Convergence of sensory pathways in the development of somatic and visceral hypersensitivity. Am J Physiol Gastrointest Liver Physiol 2006; 291: G658–G665. [DOI] [PubMed] [Google Scholar]


