Abstract
Within the brain, traumatic brain injury (TBI) alters synaptic plasticity and increases neuroinflammation and neuronal death. Yet, there lacks effective TBI treatments providing pleiotropic beneficial effects on these diverse cellular processes necessary for functional recovery. Here, we show the diabetes drug, metformin, significantly improves cognitive functions after controlled cortical impact (CCI) injury in mice, showing improved spatial learning and nest building. Furthermore, injured animals treated with metformin exhibit increased ramification of microglia processes, indicating reduced neuroinflammation. Finally, metformin treatment in vitro increased neuronal activation of partitioning defective 1 (Par1), a family of Ser/Thr kinases playing a key role in synaptic plasticity. These results suggest metformin is a promising therapeutic agent for targeting multiple cellular processes necessary for functional TBI recovery.
Keywords: metformin, TBI, neuroinflammation, microglia, Par1/MARK
Introduction
Each year traumatic brain injury (TBI) affects an estimated 10 million individuals worldwide. TBI is a leading cause of death and disability, especially among children and the elderly (Langlois et al., 2006; Roozenbeek et al., 2013). TBI leads to long-term consequences, such as seizures, sensorimotor deficits, emotional instability, and impaired learning and memory (Wood and Worthington, 2017). In addition, TBI is an important risk factor for neurodegenerative diseases, including Alzheimer’s disease (Li et al., 2017), Parkinson’s disease, and chronic traumatic encephalopathy (Blennow et al., 2016; Gardner et al., 2018). Even though TBI is a significant public health concern, the clinical setting currently lacks effective treatment options.
The primary injury of TBI results from external force to the brain followed by a prolonged secondary injury occurring within hours to days after the initial traumatic event. Since the primary injury is difficult to reverse, therapeutic strategies generally aim to prevent or alleviate secondary injuries. Secondary injuries include excitotoxicity, mitochondria dysfunction, oxidative stress, and overzealous neuroinflammation, all of which further exacerbate the physical neuronal damage (Blennow et al., 2016). Even mild TBI causes chronic impairments in hippocampal synaptic plasticity (Aungst et al., 2014; Logue et al., 2016; White et al., 2017), which is thought to underlie the learning and memory deficits in affected patients. Thus, functional recovery from TBI needs to involve not only neuroprotection and reduced neuroinflammation but also enhanced synaptic plasticity to alleviate cognitive deficits. However, no current TBI medications provide pleiotropic beneficial effects encompassing these diverse cellular processes necessary for recovery.
Metformin is currently emerging as a pharmacotherapeutic agent for CNS injuries. For decades, it has been used in the clinical setting as a diabetes medication. However, beyond glucose regulation, metformin also stimulates adult hippocampal dentate gyrus neurogenesis (Wang et al., 2012) and provides potent anti-inflammatory properties (Afshari et al., 2018; Cameron et al., 2016; Kothari et al., 2016; Muri et al., 2019). This makes it an exciting candidate for treating CNS injuries. Indeed, several recent studies demonstrate the neuroprotective effects of metformin in different CNS injury models (Hill et al., 2016; Tao et al., 2018; Wang et al.,2020). Nevertheless, improvements of behavioral outcomes of TBI due to metformin treatment remain unclear, and the molecular mechanisms of metformin are not well understood.
Here, we demonstrate that metformin treatment following TBI significantly improves cognitive function by increasing spatial learning in the Morris Water Maze. In addition, we observe increased nest building in metformin-treated animals, indicating improved emotional stability and well-being. Furthermore, microglia in metformin-treated injured animals exhibit increased ramified and decreased amoeboid morphology compared with vehicle controls, suggesting metformin treatment reduces neuroinflammation. Finally, applying metformin to primary cortical neurons activates partitioning defective 1 (Par1 or microtubule affinity regulating kinase, MARK), a family of Ser/Thr kinases we recently established as important for synaptic plasticity (Bernard and Zhang, 2015; Wu et al., 2012; Wu et al., 2017) and a key regulator of microglia activation (DiBona et al., 2019). Taken together, our studies suggest metformin is a highly promising therapeutic agent to improve TBI recovery through a pleiotropic mechanism by reducing neuroinflammation and stimulating synaptic plasticity, ultimately contributing to functional recovery.
Material and Methods
Animals and Genotyping of Mouse Models
All animal procedures were carried out following approval from the Rutgers University Institutional Animal Care and Use Committee. 32-to 38-day old male CD-1 mice were purchased from Charles River. All animals were housed in a clean, temperature regulated facility maintained a strict 12-hour light/dark cycle with free access to water and food. Animals were randomly assigned with a letter and number name to blind all behavior testing and tissue processing throughout each experiment until after quantifications.
Traumatic Brain Injury
Adult mice were subjected to a controlled cortical impact (CCI) injury. CCI was performed as described previously with minor modifications (DiBona et al., 2019; Pleasant et al., 2011). Briefly, mice were anesthetized with isoflurane and administered perisurgical analgesics (0.025% bupivacaine and buprenorphine (0.1mg/kg)). A craniectomy was performed midway between bregma and lambda and halfway between the sagittal suture and temporal crest, centered over the hippocampus. To expose the dura mater, we used a 2.7 mm hand-held trephine ensuring no damage to the dura mater. The CCI device was placed over the exposed dura mater and lowered until contact was made. A computer-controlled cortical impact (Hatteras Instruments Model PCI1300) was performed using an impactor probe with a flat tip, 2.5 mm in diameter. Sample parameters include impact speed of 3 m/sec, dwell time of 100 msec, and cortical deformation of 1 mm. For sham-operated controls, only a craniectomy was performed. Following injury, the skin was re-secured over the skull with Vetbond and animals were returned to their home cages on heating pads for recovery.
Metformin Treatments
CCI-TBI, sham-operated control, and naïve CD-1 mice were treated with either a water vehicle or 200 mg/kg of metformin by intraperitoneal (i.p.) injection 1 hour immediately following the completion of surgery. 200mg/kg was selected as our treatment dosage as it is the most widely-used and supported in rodent studies (Duca et al., 2016; He et al., 2009; Shaw et al., 2005; Wang et al., 2012). Additional vehicle or metformin injections were administered once per day thereafter (Supplemental Figure 1). Nomenclature for surgery groups and treatments include: Naive (N), Sham-Operated (Sham or S), CCI-TBI (TBI or T), Vehicle (Veh or V) and Metformin (Met or M).
Behavior Testing: Morris Water Maze (MWM)
Two days prior to surgery, animals were acclimated, and baseline tested using a visible platform in a circular pool filled with water made opaque with black paint. Four quadrants with wall - mounted cues divided the pool and the platform was visible as a control for motor and visual deficits. Learning and memory testing was started 1-day post-injury (1dpi), using a hidden platform with visible cues placed on the walls. Animals were placed in each quadrant once for a total of 4 trials per mouse per day. Latency to reach the platform from each quadrant was measured each trial for 6 days. For each trial maximum swim time was capped at 60 seconds. At 7dpi the platform was removed, and the time spent in each quadrant, the number of crossings in each quadrant, and average swim velocity were recorded and quantified. Mice averaging 50 seconds or more latency to platform at day 6 were considered non-performers. Three (3) mice total were excluded from the MWM and all other experiments as non-performers. Exclusions included: two (2) Naive-Veh and one (1) TBI-Met as non-performers (Vorhees and Williams, 2006).
Behavior Testing: Nesting
Each animal was placed into a new cage with a new nestlet after surgery. The degree of nesting made from each animal tearing up the nestlet was rated on a scale of 1 to 5, with 1 representing no nest and 5 representing a full nest, starting at 1dpi (Deacon, 2006) (Supplemental Figure 4). The next morning, all nests and bedding were removed from the cage and a new nestlet introduced. Scoring and replacement of nestlets was completed each morning until 7dpi.
Behavior Testing: Pole Test
A vertical pole with rough surface 1 cm in diameter and 30 cm tall was used within a large rat cage. Mice were placed with their head upward at the top of pole. Two times were recorded: 1. The time it took for the mouse to right itself so its body and all paws were facing downward, and 2. The latency to reach the ground. Completion of both objectives were capped at 60 seconds. After completion of the trial, the mouse was placed in its home cage and the pole was cleaned with ethanol. This procedure was repeated for three non-consecutive trials at 1, 4, and 7dpi (Matsuura et al., 1997).
Cardiac Perfusion and Tissue Processing
Animals were anesthetized with ketamine (100 mg/kg) / xylazine (10 mg/kg) and sacrificed via cardiac perfusion with a perfusion pump. Brains were cleared with phosphate-buffered saline (PBS) and then fixed with 4% paraformaldehyde (PFA). Extracted brains were submerged in 4% PFA for 1-2 hours to post-fix. Each brain was washed with PBS and transferred through sucrose gradients (10%, 20%, and 30%) for 24 hours each at 4°C. 70-μm frozen, free-floating coronal tissue sections were made using a frozen sliding microtome. Sections were stored at −20°C in cryoprotectant solutions (25% glycerol, 25% ethylene glycol, 30% sucrose in 0.1M PBS) until immunohistochemistry was performed.
Cortical Disruption Image Quantification
A section from both vehicle and metformin injured tissue within the center of the impact region (midway between bregma and lambda and halfway between the sagittal suture and temporal crest, centered over the hippocampus) was placed on a charged slide.Each section was imaged with a standard light microscope using an 4x objective. ImageJ was used to measure 1. the entire ipsi-lateral cortical area, and 2. a freehand trace of the injured cavity where tissue was disrupted. The percentage of cortical tissue that was disrupted was Quantified.
Immunohistochemistry (IHC)
Tissue was washed 4 times with 1xPBS to remove cryoprotectant residue. Following thorough washing, tissue was permeabilized in blocking solution (0.3% Triton-X 100, 5% Donkey Serum, 1x PBS) for 30 minutes at room temperature. Sham-operated control and CCI-TBI tissue were then incubated with donkey-anti-mouse Fab fragment IgG (Jackson ImmunoResearch, West Grove, PA, 1:60 dilution) for at least 1 hour at room temperature. After removing the blocking solution, tissue was incubated in primary antibody overnight at room temperature (Iba1, Wako Chemicals USA, Inc, Richmond, VA, 1:1000; GFAP, Millipore-Sigma, St. Louis, MO, 1:1000). The following day, tissue was washed, and DyLight 594 Donkey anti-rabbit (Jackson ImmunoResearch, West Grove, PA; 1:500 dilution), DyLight 488 Donkey anti-mouse secondary antibody (Jackson ImmunoResearch, West Grove, PA; 1:500 dilution) and DAPI were applied and incubated at room temperature for 2 hours. Tissue was washed and mounted using Vectashield Hardset (Vector Laboratories, Burlingame, CA).
IHC Microscopy and Image Quantification
All tissue sections were imaged on an Olympus FV1000MPE microscope using either an Olympus UPlan SApo 20x objective (NA 0.75) or an Olympus LUMPLanFLN 60x objective (NA 1.0).
For microglia density:
Single images (20x and 60x) from the V1 region of the cortex ipsilaterally to injury were captured. Microglia density was measured manually by counting individual Iba1+ cells and measuring total tissue area using ImageJ.
For astrocyte fluorescent intensity:
Single images (20x) from the V1 region of the cortex ipsilaterally to injury were captured. Astrocyte fluorescent intensity was measured in ImageJ by acquiring the mean fluorescent intensity of a freehand tracing of the entire tissue area subtracted from a background area.
For morphology analysis:
To ensure imaging a complete cell, z-stack images were obtained by focusing on microglia somas in the middle of the tissue (20x Objective digitally zoomed to 60x magnification, 1 μm step size). All microglia cells fully encompassed within the imaged z-stack were traced and reconstructed using Neurolucida software (Microbrightfield Inc., Colchester, VT) to obtain analysis of segmentation, cell bodies, Sholl, and more.
Primary Cortical Neuron Cultures
Cortical neuron cultures were prepared from embryonic rats as previously described (Sun et al., 2013). Briefly, cortices were dissected from embryonic day 18 Sprague-Dawley rats, trypsinized, and triturated through a glass Pasteur pipette. Dissociated neurons were plated at desired density in a 12-well plate. Cultures were grown in Neurobasal medium (Invitrogen) supplemented with B-27 (Invitrogen) and 2 mM GlutaMAX (Invitrogen). At 10 days in vitro (DIV), cells were treated with 1mM metformin or water vehicle control and incubated for 2 hours.
Western Blotting
Primary cortical neuron cultures were lysed in RIPA buffer (20mM Tris-HCl, 150mM NaCl, 0.5% NP40, 1.0% Trition-X, 2.0mM EDTA, 2.0mM EGTA, and 0.25% DOC) supplemented with protease inhibitor cocktail (Sigma Aldrich, Saint Louis, MO), phosphatase inhibitor cocktail (Sigma Aldrich, Saint Louis, MO), 10 mM β-glycerophosphate, 10 mM NaF, 10 mM Dithiothreitol (DTT), and 10 mM Phenylmethylsulfonyl fluoride (PMSF). Lysed cells were incubated on ice for 10 minutes and centrifuged at 13,000xg at 4°C for 10 min to obtain cleared lysate. 30μg of total lysate was run on 10% SDS-PAGE gels and transferred to PVDF membrane. Blots were probed with primary antibodies Par1c/MARK1 (ProteinTech, Rosemont, IL: 1:1000) and p-Par1 (Cell Signaling, Danvers, MA: 1:1000). Horseradish peroxidase conjugated goat anti-rabbit and goat anti-mouse antibody (Jackson ImmunoResearch, West Grove, PA) was used at 1:5,000. Imaging of blots by enhanced chemiluminescence was obtained using Syngene G:Box/iChemi-XR and the GeneSnap software (Version 7.09.a) (Syngene, Frederick, MD).
Statistical Analysis
StatPlus:mac Pro (version 6.1.60), GraphPad Prism (version 6.0) and/or IBM SPSS (v25) were used for statistical analysis. Data are expressed as mean ± standard error mean (SEM). One-way or two-way analysis of variance (ANOVA) was utilized for behavioral and cell culture experiment as appropriate for all groups combined. Tukey post-hoc testing was performed. Significance was considered as a p value of < 0.05. For cellular morphology and fluorescence, SPSS was used to perform a linear mixed model with a type III test of fixed effects to determine significance.
Results
Metformin improves cognitive functions after TBI
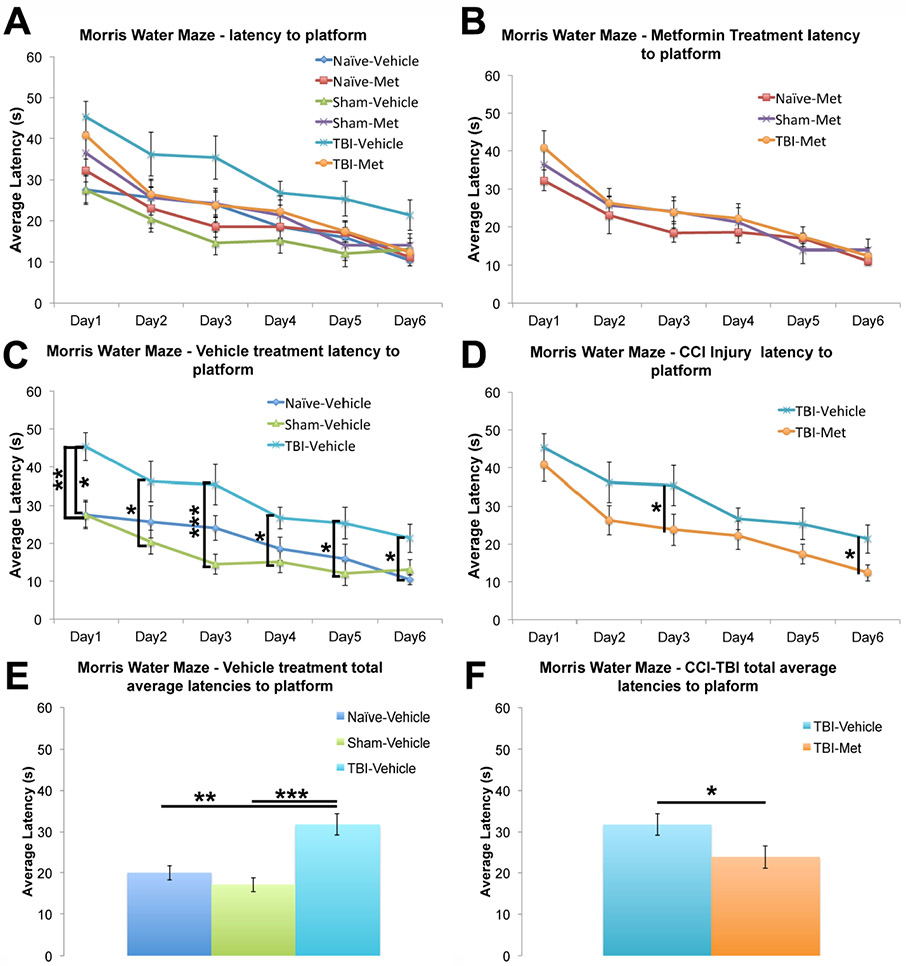
Metformin is emerging as a promising therapeutic agent for CNS injury through its neuroprotective mechanisms. However, its effects on hippocampal-dependent learning processes following TBI remains elusive even though many TBI patients exhibit hippocampal-dependent cognitive impairments (An et al., 2016; Sandry et al., 2015; Whiting et al., 2006). Thus, we sought to determine whether metformin can rescue hippocampal-dependent cognitive defects following a controlled cortical impact (CCI) injury in mice using the Morris water maze (MWM). The injury was performed midway between bregma and lambda and halfway between the sagittal suture and temporal crest, centered over the hippocampus. Adult male CD-1 mice were tested two-days pre-surgery with a visible platform as a baseline measurement. Following injury mice were tested with a hidden platform from 1 day post injury (1dpi) through 7dpi (Supplemental Figure 1). The average latency to reach the platform was analyzed for each experimental group with a 2 (injury type) by 2 (treatment type) ANOVA performed (Figure 1A; Supplemental Table 1).
Figure 1. Metformin significantly improved spatial learning following CCI-TBI.
Adult CD-1 male mice were tested using the Morris Water Maze for six days starting one day post-surgery. The latency for each mouse to find the submerged platform was measured daily (data represent the average of 4 trials per day). A Two-way ANOVA was performed assessing all groups and treatments. A. The average latencies for each experimental group were plotted together. The following plots are extracted from graph A for illustrative purposes. B. Injured mice given metformin were plotted against their sham and naïve controls. No significant difference was observed between different groups. C. Injured mice given a vehicle were plotted against their sham and naïve controls. The injured mice consistently performed significantly worse when compared to their sham and naïve controls. D. Average latencies for the injured mice treated with either vehicle or metformin. Metformin treated mice performed significantly better than vehicle treated mice as shown by the shorter latency to reach the hidden platform. E. The total average latency across all six days for injured mice given a vehicle was plotted against their sham and naïve controls. F. The total average latency across all six days for the injured mice treated with either vehicle or metformin. Data represent the mean ± SEM. A 2-way ANOVA was performed with Tukey’s post-hoc test, *p<0.05, **p<0.01, ***p<0.001. N (animals) = Vehicle: Naive=8, Sham=12, TBI=10; Metformin: Naive=10, Sham=12, TBI=11.
As expected, CCI-TBI (T) mice treated with the vehicle control performed significantly worse than both naïve (N) and sham-operated (S) vehicle treated controls by having significantly increased latencies in finding the platform each day (Figure 1C; Supplemental Table 1) and increased latency to the platform when all days were combined (Figure 1E; Supplemental Table 1), indicating impaired spatial learning following injury. Remarkably, CCI-TBI mice treated with metformin performed significantly better when compared to CCI-TBI mice treated with the vehicle control, having significantly decreased latencies in finding the platform in multiple days (Figure 1D; Supplemental Table 1)and decreased latency to the platform when all days were combined (Figure 1F; Supplemental Table 1). Metformin treatment nearly fully recovered the injury-induced learning impairment, as no significant difference was found between CCI-TBI mice treated with the metformin when compared to both naïve and sham-operated controls treated with metformin (Figure 1B; Supplemental Table 1). At 7dpi during the probe test, the platform was removed from the target quadrant to test the spatial memory of the platform location. No significant differences were found in memory retention measured by time in target quadrant, quadrant crossings, and mean swim speed (Supplemental Figure 2) between any of the experimental groups. To further rule out possible motor deficits in the injured mice, we performed a pole test. We found no significant differences between any of the experimental groups in time to right (data not shown) or latency to descend once righted, indicating no motor dysfunction in the injured mice (Supplemental Figure 3, Supplemental Table 2). Taken together, these data demonstrate metformin treatment rescues the defects in hippocampal-dependent spatial learning following TBI.
Metformin treatment improves nest building after injury
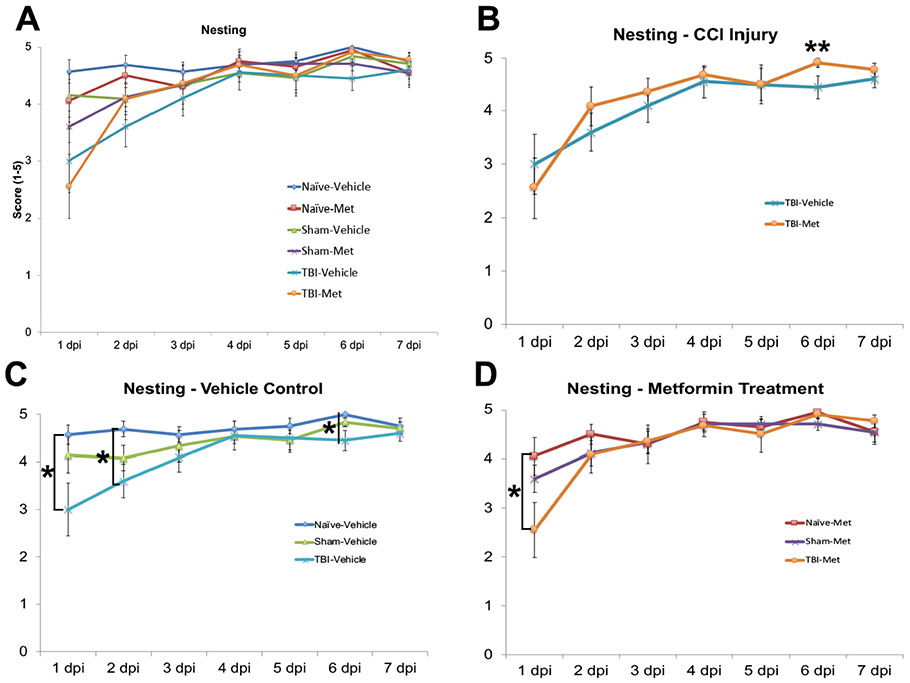
Nest building is an effective method for analyzing rodent behavioral changes (Aubert, 1999; Deacon, 2006; Gaskill et al., 2013; Jirkof, 2014). Rodents (both male and female) are highly motivated to build a nest when provided with nesting material, such as a nestlet or crinkled paper. Poor or no nest building is a measure used to identify changes in an animal’s well-being, and can indicate stress, illness, or pain. Quantitative measurements of nest building in laboratory animals have now been established (Gaskill et al., 2013). Thus, we scored the nesting of metformin or vehicle treated CCI-TBI (T), sham-operated (S), and naïve (N) control mice from 1dpi through 7dpi (Supplemental Figure 1). Each day the degree of nesting was ranked on a scale from 1 to 5, with 1 representing no nest and 5 representing a full nest (Supplemental Figure 4). The degree of nesting was average for each group and a 2 (injury type) by 2 (treatment type) ANOVA was performed (Figure 2A; Supplemental Table 3).
Figure 2. Metformin significantly improved nesting behavior following CCI-TBI.
Adult CD-1 male mice had their home cage nests ranked each morning starting 1-day post-surgery to assess for stress, anxiety, and other emotional disturbances. A Two-way ANOVA was performed assessing all groups and treatments. A. The nesting score for the naïve, sham and injured mice treated with either vehicle or metformin were plotted together. The following plots are extracted from graph A for illustrative purpose. B. Injured mice given metformin were plotted against their sham and naïve controls. No significant differences were found between different groups. C. Injured mice given a vehicle were plotted against their sham and naïve controls. Injured mice display significant impairments in nest building as compared to vehicle treated naïve and sham controls. D. Injured mice treated with metformin show significantly improved nesting behavior starting the first day post-injury when compared to vehicle treated injured mice. Data represent the mean ± SEM. A 2-way ANOVA was performed with Tukey’s post-hoc test, *p<0.05, **p<0.01, ***p<0.001.N (animals) = Vehicle: Naive=8, Sham=12, TBI=10; Metformin: Naive=10, Sham=12, TBI=11.
Both metformin and vehicle treated injured animals showed poor nest building 1dpi when compared to sham and naïve controls (Figure 2C and D). Injured mice treated with metformin showed significant improvement in their nesting by 2dpi, displaying nests similar to those of the sham and naïve controls (Figure 2D). This improvement in nesting behavior continued through 7dpi. By the end of the week, injured animals treated with vehicle control built worse nests than injured animals treated with metformin (Figure 2B; Supplemental Table 3). These results show metformin treatment improves nest-building behavior following TBI, indicating improved overall well-being in mice.
Metformin treatment reduces microglial, but not astrocyte, activation after TBI
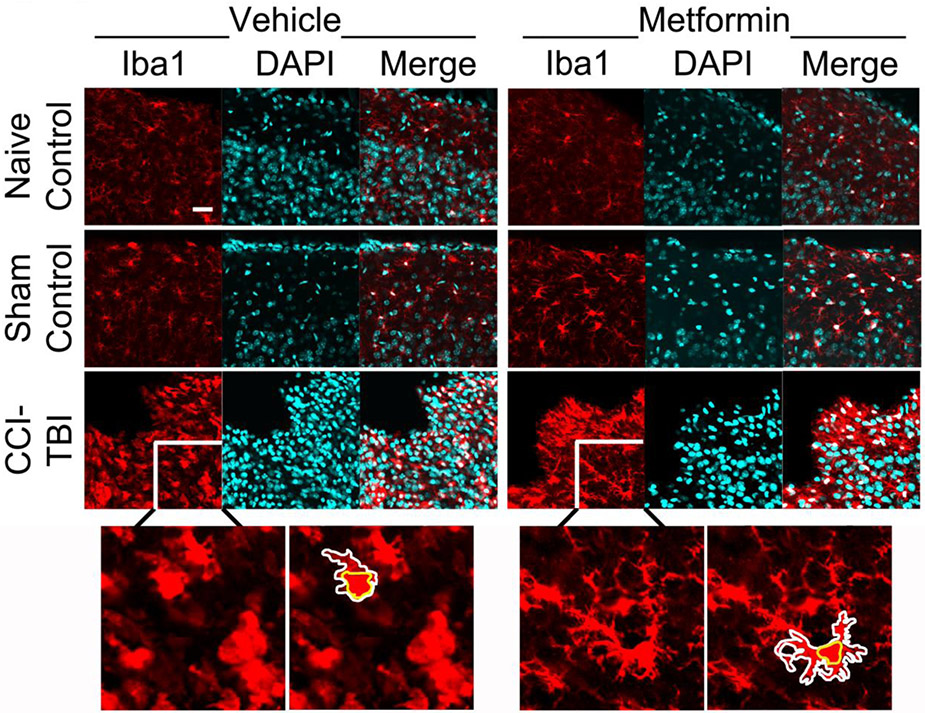
One of the most striking responses following TBI is the inflammatory response, which consists of massive recruitment of activated microglial and astrocytes cells to the injury site. Chronic activation of microglia is believed to largely contribute to the secondary injuries after TBI. Because metformin has been shown to provide anti-inflammatory properties during health and following injury and illness (Afshari et al., 2018; Cameron et al., 2016; Kothari et al., 2016; Muri et al., 2019), we sought to determine whether metformin can reduce neuroinflammation after TBI. To do this, we examined both microglial morphology and astrocyte activation in control and injured animals treated with vehicle or metformin. Mice were sacrificed at 7dpi (Supplemental Figure 1). First, we measured the extent of cortical disruption in injured mice and found no significant difference between the treatment groups, indicating that the injuries were done uniformly (Supplementary Figure 5). Next, tissue sections were immunostained with a microglia marker (Iba1) or astrocyte marker (GFAP) and DAPI. Tissue sections were imaged at the peripheral site of injury (Figure 3; Supplemental Figure 6).
Figure 3. Microglia response following treatment with metformin post-surgery.
Naïve, sham-operated control or CCI-TBI mice were treated with either vehicle or metformin beginning at 1-hour post-surgery and daily thereafter. At 7 days post-injury, mice were sacrificed, their brains sectioned and immunostained for microglia marker Iba1 (red) and nuclear marker DAPI (cyan). Confocal images were acquired of the ipsi-lateral cortex for all mouse tissue at 60x. The periphery of a microglia cell is outlined in white, and somas are outlined in yellow. Scale bar = 10μm.
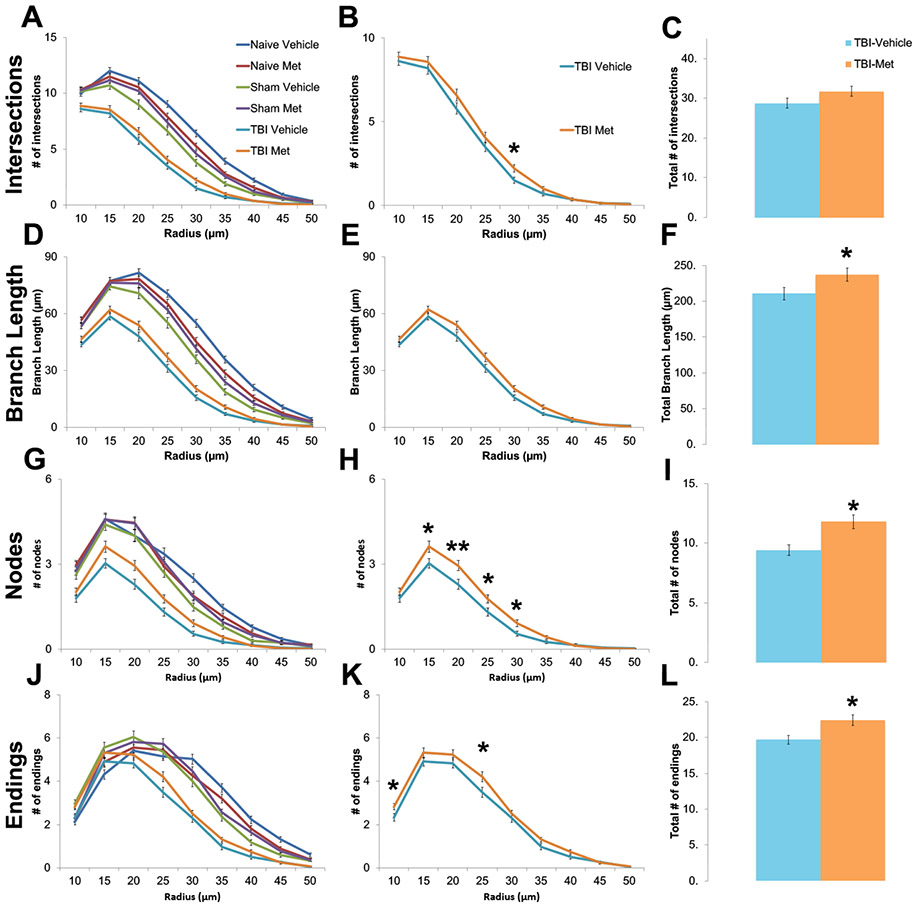
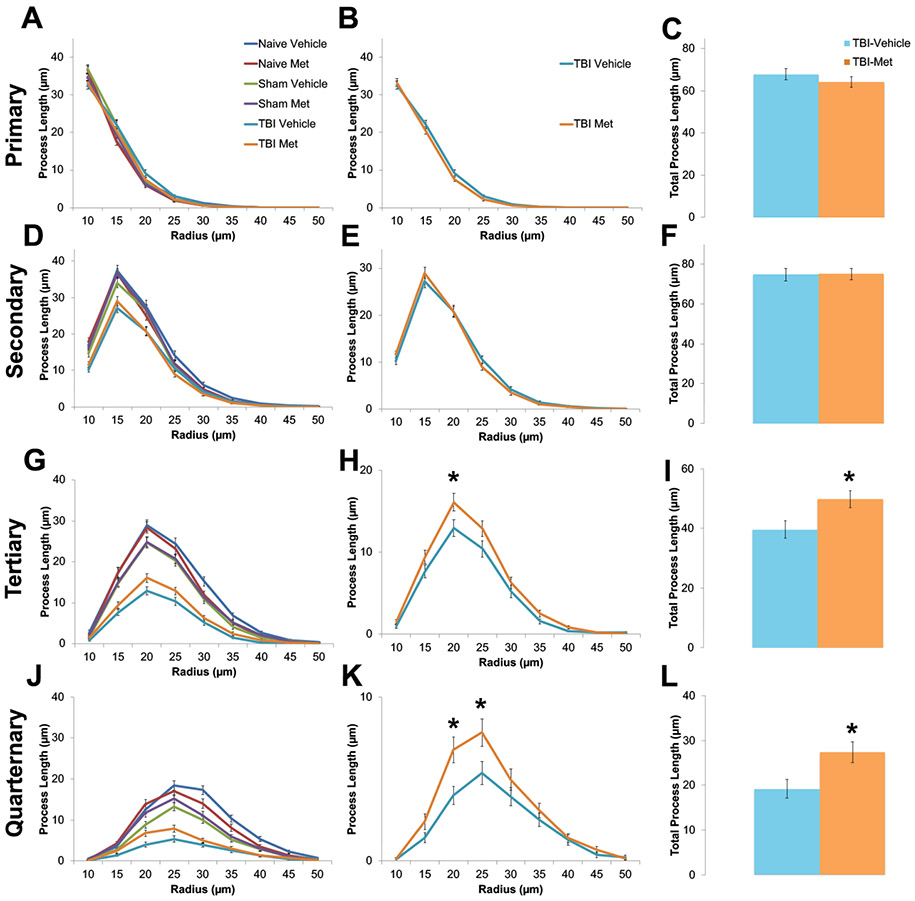
Under physiological conditions, microglia display highly ramified morphology including increased higher order branching and processes. Upon injury, microglia processes retract transitioning to an amoeboid morphology with significantly reduced branching and process lengths. To quantify changes in microglial morphology, z-stack images were obtained from the ipsi-lateral region of the cortex for each animal. Then, Neurolucida was used to create 3D reconstructions of individual microglia (Supplemental Figure 7). From the 3D reconstructions, Sholl analysis and various morphological aspects were measured, including number of intersections; average branch length, nodes, and endings (Figure 4); and total lengths of primary, secondary, tertiary, and quaternary processes (Figure 5). A 2 (injury type) by 2 (treatment type) ANOVA was performed (Supplemental Table 5).
Figure 4. Metformin treatment after injury increased microglia branching complexity.
Z-stack images were acquired from the cortex of naïve, sham and CCI-TBI animal tissue treated with either vehicle or metformin. Neurolucida software was used to create 3D reconstructions of individually isolated microglia. Various morphological aspects were measured following Sholl Analysis with a somal radius of 10 μm, including number of intersections (A,B), average branch length (D,E), nodes (G, H), and endings (J,K). Injured mice treated with metformin were found to have significantly more intersections (C), average branch length (F), nodes (I) and endings (L) than injured mice treated with vehicle. Data represent the mean ± SEM. N (animals/microglia) = Vehicle-TBI=10/120; Metformin-TBI=12/144, *p<0.05, **p<0.01 by type III test of fixed effects using a linear mixed model analysis.
Figure 5. Metformin treatment after injury increased high order branching of microglia.
Z-stack images were acquired from the cortex of naïve, sham and CCI-TBI animal tissue treated with either vehicle or metformin. Neurolucida software was used to create 3D reconstructions of individually isolated microglia. Orders of branch complexity was measured following Sholl Analysis with a somal radius of 10 μm, including primary (A,B), secondary (D,E), tertiary (G,H) and quaternary (J,K) orders. Injured mice treated with metformin were found to have higher complexity at the tertiary (I) and quaternary (L) orders than injured mice treated with vehicle. Data represent the mean ± SEM. N (animals/microglia) = Vehicle-TBI=10/120; Metformin- TBI=12/144, *p<0.05, **p<0.01 by type III test of fixed effects using a linear mixed model analysis.
Interestingly, injured animals treated with metformin show significantly more ramified microglia morphology than injured animals treated with the vehicle control. As seen in Figures 4 and 5, the metformin treated group exhibit longer average branch lengths; more intersections, nodes, and endings, and increased higher order tertiary and quaternary branches (Supplemental Table 5). These increases in higher order branching and processes suggest decreased immune activation. Since the overall density of microglia remained unaltered (Supplemental Figure 8, Supplemental Table 4), metformin treatment does not alter microglia recruitment or proliferation at the injury site. These results demonstrate that while microglia are still recruited to and proliferate at the injury site, injured animals treated with metformin exhibit more ramified microglia morphology, indicating reduced neuroinflammation.
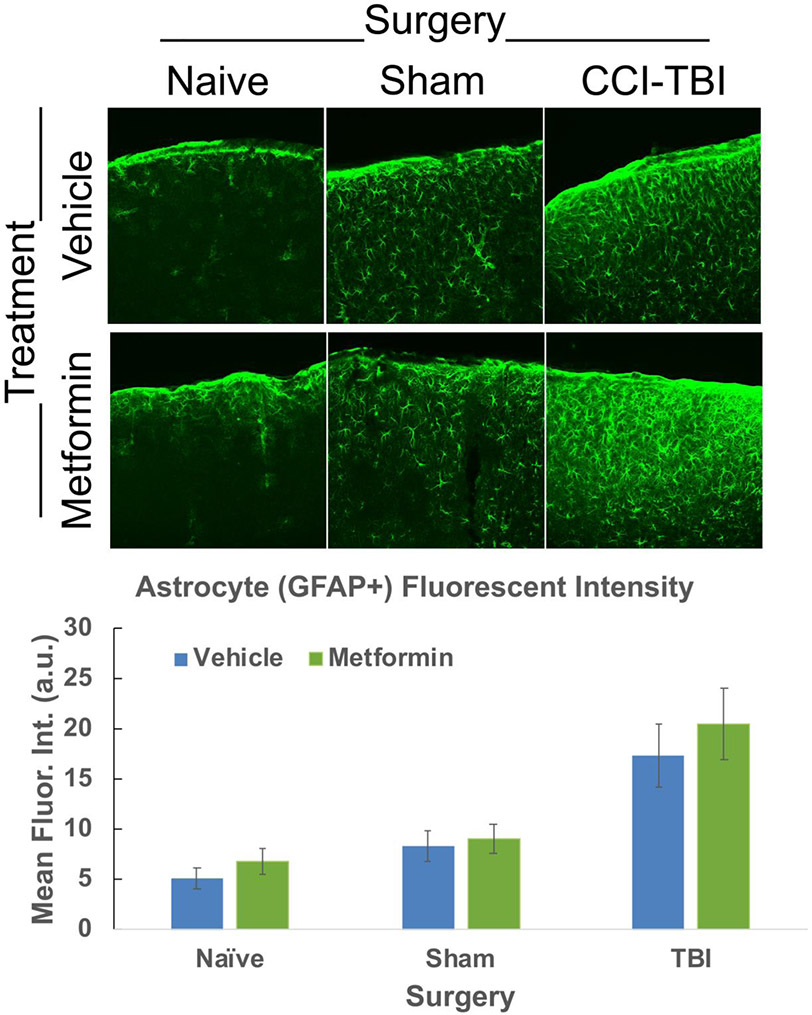
Under normal conditions, astrocytes are found largely throughout the brain performing many vital functions, such as regulation of Blood Brain Barrier (BBB) and maintenance of neuronal homeostasis (Matejuk and Ransohoff, 2020; Sofroniew, 2020). However, injury causes astrocyte activation and proliferation near the injury site (Guttenplan et al., 2020; Liddelow et al., 2017). To assess changes in astrocyte activation, alterations in overall fluorescent intensity of GFAP, a known marker of astrocyte activation, at the injury site were quantified. No significant intensity changes were found between any of the vehicle or metformin treated groups (Fig. 6, Supplemental Table 6), suggesting metformin does not alter astrocyte activation.
Figure 6. Astrocyte activation is not significantly altered following Metformin treatment.
Confocal images were acquired of the ipsi-lateral cortex of CCI-TBI, sham-operated and naïve control mouse tissue and the fluorescent intensity was manually quantified of GFAP+ cells using ImageJ. No significant difference in fluorescent intensity of GFAP+ labeled astrocytes was found between vehicle or metformin treated Naïve, sham-operated control or CCI-TBI mice. Data represent the mean ± SEM. A 2-way ANOVA was performed. N (animals/images) = Vehicle: Naive=8/24, Sham=12/32, TBI=10/31; Metformin: Naive=10/27, Sham=12/37,TBI=11/36. Data were analyzed by type III test of fixed effects using a linear mixed model analysis. No significant differences were observed between the experimental groups.
Metformin significantly activates Par1 in primary neurons
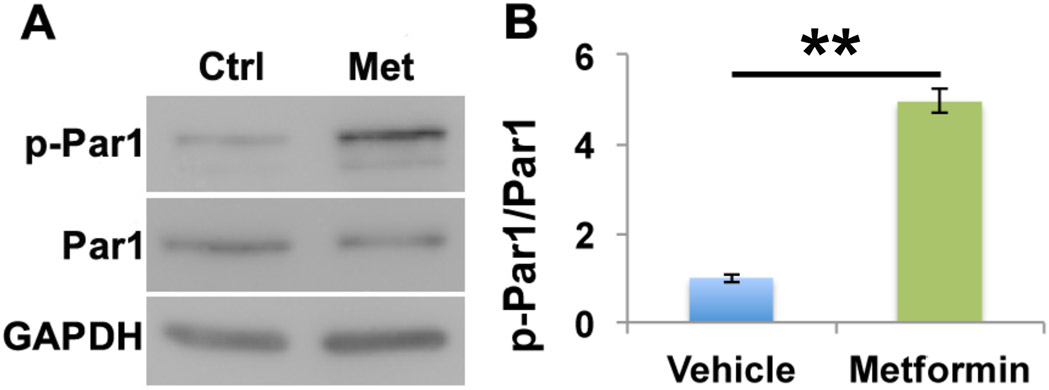
Given the promising functional benefits of metformin in treating brain injury, we wanted to explore the downstream molecular targets of metformin in the brain. Previous studies propose the Ser/Thr kinase known as liver kinase B1 (LKB1) as a major effector of metformin (Shaw et al., 2005). LKB1 is best known for activating AMP-activated protein kinase (AMPK) by phosphorylation, making the LKB1-AMPK pathway a major metabolic cellular checkpoint (Shackelford and Shaw, 2009). Another downstream target of LKB1 is the Ser/Thr kinase partitioning defective 1 (Par1), also known as microtubule affinity regulating kinase (MARK) (Lizcano et al., 2004). Interestingly, we recently found that Par1 plays an important role in synaptic plasticity, the key cellular basis for learning and memory (Bernard and Zhang, 2015;Wu et al., 2012; Wu et al., 2017), and in regulating microglia activation (DiBona et al., 2019). To investigate whether metformin affects Par1 activity, we treated primary cortical neurons with metformin or vehicle control. Lysed neurons analyzed via Western blot revealed metformin treatment significantly increased Par1 activation, as shown by increased phosphorylation of the Par1 using an antibody against the activation loop threonine of the Par1/MARK family (Fig. 7, Supplemental Table 7). This suggests Par1/MARK family kinases are activated downstream of metformin in neurons.
Figure 7. Metformin significantly activates Par1 in primary cortical neurons.
Primary neuronal cultures were treated with 1mM of vehicle or metformin for 2 hours. Protein lysates were probed for Par1, phospho-Par1 (p-Par1) and GAPDH as a loading control. Par1 was significantly activated following metformin treatment as determined by a significant increase in the p-Par1/Par1 ratio. Data represent the mean ±SEM. A 1-way ANOVA was performed with Tukey’s post-hoc test, **p<0.01.
Discussion
TBI causes multifaceted changes within the brain, including oxidative stress, neuroinflammation, and impaired synaptic plasticity, leading to chronic deficits, such as emotional instability and cognitive dysfunction in patients (Blennow et al., 2016). Thus, for full functional recovery treatment strategies need to improve diverse aspects of cellular and cognitive brain functions. Yet, current TBI medications generally only alleviate certain symptoms and do not fundamentally address the injury mechanisms needed for functional recovery.
In this study, we show that metformin significantly enhances cognitive recovery after TBI demonstrated by improved spatial learning in the Morris Water Maze (MWM). Remarkably, injured animals treated with metformin behave indistinguishably from the naïve and sham groups in the MWM test, which indicates a full functional recovery in spatial learning. While TBI caused a significant impairment in spatial learning, it did not affect memory retention once the learning trials were completed, as evidenced by the lack of significant differences in the probe trial observed between any of the experimental groups. Interestingly, a recent study did not show improvements in spatial learning after metformin treatment of injured animals (Hill et al., 2016). One likely reason for this discrepancy is their shortened treatment protocol. While their mice were treated with only 3 injections of metformin after injury, mice in our study were treated with a total of 7 daily injections of metformin. Nonetheless, beneficial effects were observed including reduced neuronal loss after just three doses of metformin (Gardner et al., 2018). In addition, nest building was significantly improved, which suggests improved overall well-being in the metformin-treated injury group. Together, these results highlight metformin as a highly promising therapeutic agent for improving cognitive functions and emotional wellbeing after TBI.
Metformin also offers anti-inflammatory properties (Afshari et al., 2018; Cameron et al., 2016; Kothari et al., 2016; Muri et al., 2019). Here, we show that following injury metformin treatment results in increased ramified microglial morphology compared with vehicle controls. Microglia from injured mice treated with metformin have longer total branch lengths; increased intersections, nodes, and endings; and increased branch complexity with more tertiary and quaternary processes. Together, this alteration in microglia morphology indicates reduced neuroinflammation following metformin treatment after an injury. Indeed, a recent study demonstrated reduced cytokine production in response to metformin in a rat model of brain injury (Tao et al., 2018). Surprisingly, we found microglial density was not reduced in the metformin-treated group. This suggests metformin does not target the injury-induced microglial proliferation process since microglia are still recruited to and proliferate at the injury site to ideally aid in repair. Similarly, we did not observe a significant difference in the level of astrocyte activation at the injury sites. Since reactive astrocytes play an essential neuroprotective role in the acute brain injury response (Myer et al., 2006; Zhou et al., 2020), the lack of a dampening effect of metformin on astrocyte and microglia proliferation may be beneficial in preserving the acute neuroprotective role of the inflammatory response. Taken together, our results indicate metformin may promote a measured reduction in immune response following injury to facilitate functional recovery.
Our study consists of young adult male mice. Infant, adolescent, and young adult males not only have higher incidence rates of TBI than females but have a three times higher death rate (Caplan et al., 2017; Ciurea et al., 2011; Faul M, 2010). By age 65, the male:female incidence ratio is almost 1:1 (Mushkudiani et al., 2007; Vagnerova et al., 2008), with TBI in older females resulting in worse clinical outcomes, such as increased severity of injury and mortality (Munivenkatappa et al., 2016). Only recently has research begun to explore if, and how, sex differences affect TBI outcomes. In newborn female mice metformin activates neural stem cells and improves cognitive recovery in a puzzle box testing paradigm (Ruddy et al., 2019). This stem cell expansion is hormone dependent for both male and female mice. Estradiol increases while testosterone decreases the stem cell pool. For these reasons, future studies should examine the effects of metformin in a female cohort and in aged mice.
What are the molecular mechanisms underlying the effects of metformin? Although more studies are needed to clearly understand this question, a recent study found metformin promotes neurogenesis by activating the atypical Protein Kinase C- (aPKC) mediated phosphorylation of CREB-binding protein (CBP) pathway (Wang et al., 2012). Another study found prolonged treatment with metformin in older mice to decrease microglia activation associated with aging and diabetes, resulting in enhanced hippocampal neurogenesis. They revealed long-term metformin treatment results in the activation of insulin receptor substrate 1 (IRS1), a mediator of selective serine phosphorylation of the insulin/insulin-like growth factor 1-R (IGF1-R) signaling pathway, which is associated with adenosine monophosphate (AMP)-activated protein kinase (AMPK)- atypical protein kinase C ζ (aPKC ζ) pathway (Tanokashira et al., 2018). Multiple groups have found metformin to activate LKB1 in several different cellular contexts, including within the liver to lower blood glucose levels, within C. elegans to extend lifespan and enhance healthspan by slowing lipofuscin accumulation, and to also activate the central homeostasis regulator AMPK (Onken and Driscoll, 2010; Shaw et al., 2005; Tanokashira et al., 2018; Xie et al., 2008). Here, we show metformin activates the Ser/Thr kinase Par1/MARK, which is a downstream target of LKB1 (Lizcano et al., 2004). Interestingly, our recent studies demonstrate loss of Par1 enhances microglia sensitivity to injury (DiBona et al., 2019). This provides an additional mechanism by which metformin can reduce microglial activation after injury. Moreover, our recent studies implicate Par1 in synaptic plasticity downstream of NMDA receptors in dendritic spines (Bernard and Zhang, 2015; Wu et al., 2012; Wu et al., 2017). Additionally, the anti-inflammatory properties of metformin is thought to be mediated through nuclear factor kappa-light-chain-enhancer of activated B cells (NFkB). NFkB is known to induce the expression of pro-inflammatory genes that regulates the survival, activation and differentiation of both the innate and adaptive immune response (Hirsch et al., 2013; Isoda et al., 2006; Liu et al., 2017). Thus, our studies provide an additional molecular pathway through which metformin restores cognitive functions by stimulating synaptic plasticity and moderating an overzealous immune response.
In summary, our studies demonstrate that metformin treatment restores cognitive and emotional deficits in mice subjected to TBI. Metformin treatment shifts microglia towards a more ramified and less ameboid morphology, indicating reduced neuroinflammation. Furthermore, we found metformin stimulates the Ser/Thr kinase Par1/MARK activity, which we have recently established to play an important role in synaptic plasticity. Taken together, our studies suggest metformin is a highly promising pharmacotherapeutic agent to potentially restore cognitive and emotional deficits in TBI patients through a pleiotropic mechanism by reducing neuroinflammation and enhancing synaptic plasticity.
Supplementary Material
Highlights.
Metformin improves cognitive functions of spatial learning and nest building post injury in mice.
Injured animals treated with metformin had increased ramification of microglia processes.
Metformin treatment in vitro increased neuronal activation of partitioning defective 1 (Par1).
Metformin is a promising therapeutic for targeting multiple cellular processes in TBI recovery.
Acknowledgements:
We would like to thank Dr. Long-Jun Wu for expert advice and helpful discussions, Drs. George Wagner and Alexander Kusnecov for support with animal behavior studies, and Dr. Qian Wu, Dr. Miao Sun and Laura P. Bernard for technical assistance. This work was supported by NIH grants NS065183 and NS089578 to HZ., and New Jersey Commission for Brain Injury Research predoctoral fellowship and NIH Institutional Research and Career Development Award (IRACDA) 1K12GM093854 to VLD. This work was also supported by the Assistant Secretary of Defense for Health Affairs endorsed by the Department of Defense, through the Epilepsy Research Program under Award No. W81XQH-18-1-0338 to HZ. Opinions, interpretations, conclusions, and recommendations are those of the author and are not necessarily endorsed by the Department of Defense. The U.S. Army Medical Research Acquisition Activity, 820 Chandler Street, Fort Detrick MD 21702-5014 is the awarding and administering acquisition office. MKS, KJK, and WZ received support from the Aresty Center for Undergraduate Research at Rutgers University. DMS was supported by the Summer Undergraduate Research Program in Molecular and Developmental Neurobiology (NIH grant R25MH095722).
Footnotes
Competing interests
The authors declare they have no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Afshari K, Dehdashtian A, Haddadi NS, Haj-Mirzaian A, Iranmehr A, Ebrahimi MA, Tavangar SM, Faghir-Ghanesefat H, Mohammadi F, Rahimi N, Javidan AN, and Dehpour AR. 2018. Anti-inflammatory effects of Metformin improve the neuropathic pain and locomotor activity in spinal cord injured rats: introduction of an alternative therapy. Spinal Cord. 56:1032–1041. [DOI] [PubMed] [Google Scholar]
- An C, Jiang X, Pu H, Hong D, Zhang W, Hu X, and Gao Y. 2016. Severity-Dependent Long-Term Spatial Learning-Memory Impairment in a Mouse Model of Traumatic Brain Injury. Transl Stroke Res. 7:512–520. [DOI] [PubMed] [Google Scholar]
- Aubert A 1999. Sickness and behaviour in animals: a motivational perspective. Neurosci Biobehav Rev. 23:1029–1036. [DOI] [PubMed] [Google Scholar]
- Aungst SL, Kabadi SV, Thompson SM, Stoica BA, and Faden AI. 2014. Repeated mild traumatic brain injury causes chronic neuroinflammation, changes in hippocampal synaptic plasticity, and associated cognitive deficits. J Cereb Blood Flow Metab. 34:1223–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard LP, and Zhang H. 2015. MARK/Par1 Kinase Is Activated Downstream of NMDA Receptors through a PKA-Dependent Mechanism. PloS one. 10:e0124816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blennow K, Brody DL, Kochanek PM, Levin H, McKee A, Ribbers GM, Yaffe K, and Zetterberg H. 2016. Traumatic brain injuries. Nat Rev Dis Primers. 2:16084. [DOI] [PubMed] [Google Scholar]
- Cameron AR, Morrison VL, Levin D, Mohan M, Forteath C, Beall C, McNeilly AD, Balfour DJ, Savinko T, Wong AK, Viollet B, Sakamoto K, Fagerholm SC, Foretz M, Lang CC, and Rena G. 2016. Anti-Inflammatory Effects of Metformin Irrespective of Diabetes Status. Circ Res. 119:652–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan HW, Cox CS, and Bedi SS. 2017. Do microglia play a role in sex differences in TBI? J Neurosci Res. 95:509–517. [DOI] [PubMed] [Google Scholar]
- Ciurea AV, Gorgan MR, Tascu A, Sandu AM, and Rizea RE. 2011. Traumatic brain injury in infants and toddlers, 0-3 years old. Journal of medicine and life. 4:234–243. [PMC free article] [PubMed] [Google Scholar]
- Deacon RM 2006. Assessing nest building in mice. Nat Protoc. 1:1117–1119. [DOI] [PubMed] [Google Scholar]
- DiBona VL, Zhu W, Shah MK, Rafalia A, Ben Cheikh H, Crockett DP, and Zhang H. 2019. Loss of Par1b/MARK2 primes microglia during brain development and enhances their sensitivity to injury. Journal of neuroinflammation. 16:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duca FA, Cote CD, Rasmussen BA, Zadeh-Tahmasebi M, Rutter GA, Filippi BM, and Lam TK. 2016. Corrigendum: Metformin activates a duodenal Ampk-dependent pathway to lower hepatic glucose production in rats. Nat Med. 22:217. [DOI] [PubMed] [Google Scholar]
- Faul M, X.L., Wald MM, Coronado VG. 2010. Traumatic Brain Injury in the United States:Emergency Department Visits, Hospitalizations and Deaths 2002–2006. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control, Atlanta (GA). [Google Scholar]
- Gardner RC, Byers AL, Barnes DE, Li Y, Boscardin J, and Yaffe K. 2018. Mild TBI and risk of Parkinson disease: A Chronic Effects of Neurotrauma Consortium Study. Neurology. 90:e1771–e1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskill BN, Karas AZ, Garner JP, and Pritchett-Corning KR. 2013. Nest building as an indicator of health and welfare in laboratory mice. Journal of visualized experiments : JoVE. 51012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttenplan KA, Stafford BK, El-Danaf RN, Adler DI, Munch AE, Weigel MK, Huberman AD, and Liddelow SA. 2020. Neurotoxic Reactive Astrocytes Drive Neuronal Death after Retinal Injury. Cell Rep. 31:107776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, Sabet A, Djedjos S, Miller R, Sun X, Hussain MA, Radovick S, and Wondisford FE. 2009. Metformin and insulin suppress hepatic gluconeogenesis through phosphorylation of CREB binding protein. Cell. 137:635–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JL, Kobori N, Zhao J, Rozas NS, Hylin MJ, Moore AN, and Dash PK. 2016. Traumatic brain injury decreases AMP-activated protein kinase activity and pharmacological enhancement of its activity improves cognitive outcome. Journal of neurochemistry. 139:106–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch HA, Iliopoulos D, and Struhl K. 2013. Metformin inhibits the inflammatory response associated with cellular transformation and cancer stem cell growth. Proc Natl Acad Sci U S A. 110:972–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isoda K, Young JL, Zirlik A, MacFarlane LA, Tsuboi N, Gerdes N, Schonbeck U, and Libby P. 2006. Metformin inhibits proinflammatory responses and nuclear factor-kappaB in human vascular wall cells. Arterioscler Thromb Vasc Biol. 26:611–617. [DOI] [PubMed] [Google Scholar]
- Jirkof P 2014. Burrowing and nest building behavior as indicators of well-being in mice. J Neurosci Methods. 234:139–146. [DOI] [PubMed] [Google Scholar]
- Kothari V, Galdo JA, and Mathews ST. 2016. Hypoglycemic agents and potential anti-inflammatory activity. J Inflamm Res. 9:27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlois JA, Rutland-Brown W, and Wald MM. 2006. The epidemiology and impact of traumatic brain injury: a brief overview. The Journal of head trauma rehabilitation. 21:375–378. [DOI] [PubMed] [Google Scholar]
- Li Y, Li Y, Li X, Zhang S, Zhao J, Zhu X, and Tian G. 2017. Head Injury as a Risk Factor for Dementia and Alzheimer's Disease: A Systematic Review and Meta-Analysis of 32 Observational Studies. PloS one. 12:e0169650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, Bennett ML, Munch AE, Chung WS, Peterson TC, Wilton DK, Frouin A, Napier BA, Panicker N, Kumar M, Buckwalter MS, Rowitch DH, Dawson VL, Dawson TM, Stevens B, and Barres BA. 2017. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 541:481–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Zhang L, Joo D, and Sun SC. 2017. NF-kappaB signaling in inflammation. Signal Transduct Target Ther. 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizcano JM, Goransson O, Toth R, Deak M, Morrice NA, Boudeau J, Hawley SA, Udd L, Makela TP, Hardie DG, and Alessi DR. 2004. LKB1 is a master kinase that activates 13 kinases of the AMPK subfamily, including MARK/PAR-1. The EMBO journal. 23:833–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logue OC, Cramer NP, Xu X, Perl DP, and Galdzicki Z. 2016. Alterations of functional properties of hippocampal networks following repetitive closed-head injury. Exp Neurol. 277:227–243. [DOI] [PubMed] [Google Scholar]
- Matejuk A, and Ransohoff RM. 2020. Crosstalk Between Astrocytes and Microglia: An Overview. Front Immunol. 11:1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura K, Kabuto H, Makino H, and Ogawa N. 1997. Pole test is a useful method for evaluating the mouse movement disorder caused by striatal dopamine depletion. J Neurosci Methods. 73:45–48. [DOI] [PubMed] [Google Scholar]
- Munivenkatappa A, Agrawal A, Shukla DP, Kumaraswamy D, and Devi BI. 2016. Traumatic brain injury: Does gender influence outcomes? Int J Crit Illn Inj Sci. 6:70–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muri L, Le ND, Zemp J, Grandgirard D, and Leib SL. 2019. Metformin mediates neuroprotection and attenuates hearing loss in experimental pneumococcal meningitis. Journal of neuroinflammation. 16:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mushkudiani NA, Engel DC, Steyerberg EW, Butcher I, Lu J, Marmarou A, Slieker F,McHugh GS, Murray GD, and Maas AI. 2007. Prognostic value of demographic characteristics in traumatic brain injury: results from the IMPACT study. J Neurotrauma. 24:259–269. [DOI] [PubMed] [Google Scholar]
- Myer DJ, Gurkoff GG, Lee SM, Hovda DA, and Sofroniew MV. 2006. Essential protective roles of reactive astrocytes in traumatic brain injury. Brain : a journal of neurology. 129:2761–2772. [DOI] [PubMed] [Google Scholar]
- Onken B, and Driscoll M. 2010. Metformin induces a dietary restriction-like state and the oxidative stress response to extend C. elegans Healthspan via AMPK, LKB1, and SKN-1. PLoS One. 5:e8758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleasant JM, Carlson SW, Mao H, Scheff SW, Yang KH, and Saatman KE. 2011. Rate of neurodegeneration in the mouse controlled cortical impact model is influenced by impactor tip shape: implications for mechanistic and therapeutic studies. J Neurotrauma. 28:2245–2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozenbeek B, Maas AI, and Menon DK. 2013. Changing patterns in the epidemiology of traumatic brain injury. Nat Rev Neurol. 9:231–236. [DOI] [PubMed] [Google Scholar]
- Ruddy RM, Adams KV, and Morshead CM. 2019. Age- and sex-dependent effects of metformin on neural precursor cells and cognitive recovery in a model of neonatal stroke. Sci Adv. 5:eaax1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandry J, DeLuca J, and Chiaravalloti N. 2015. Working memory capacity links cognitive reserve with long-term memory in moderate to severe TBI: a translational approach. J Neurol. 262:59–64. [DOI] [PubMed] [Google Scholar]
- Shackelford DB, and Shaw RJ. 2009. The LKB1-AMPK pathway: metabolism and growth control in tumour suppression. Nat Rev Cancer. 9:563–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw RJ, Lamia KA, Vasquez D, Koo SH, Bardeesy N, Depinho RA, Montminy M, and Cantley LC. 2005. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science. 310:1642–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew MV 2020. Astrocyte Reactivity: Subtypes, States, and Functions in CNS Innate Immunity. Trends Immunol. 41:758–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M, Bernard LP, Dibona VL, Wu Q, and Zhang H. 2013. Calcium phosphate transfection of primary hippocampal neurons. J Vis Exp:e50808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanokashira D, Kurata E, Fukuokaya W, Kawabe K, Kashiwada M, Takeuchi H, Nakazato M, and Taguchi A. 2018. Metformin treatment ameliorates diabetes-associated decline in hippocampal neurogenesis and memory via phosphorylation of insulin receptor substrate 1. FEBS Open Bio. 8:1104–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao L, Li D, Liu H, Jiang F, Xu Y, Cao Y, Gao R, and Chen G. 2018. Neuroprotective effects of metformin on traumatic brain injury in rats associated with NF-kappaB and MAPK signaling pathway. Brain Res Bull. 140:154–161. [DOI] [PubMed] [Google Scholar]
- Vagnerova K, Koerner IP, and Hurn PD. 2008. Gender and the injured brain. Anesth Analg. 107:201–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees CV, and Williams MT. 2006. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 1:848–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Zheng Z, Han W, Yuan Y, Li Y, Zhou K, Wang Q, Xie L, Xu K, Zhang H, Xu H, Wu Y, and Xiao J. 2020. Metformin Promotes Axon Regeneration after Spinal Cord Injury through Inhibiting Oxidative Stress and Stabilizing Microtubule. Oxid Med Cell Longev. 2020:9741369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Gallagher D, DeVito LM, Cancino GI, Tsui D, He L, Keller GM, Frankland PW, Kaplan DR, and Miller FD. 2012. Metformin activates an atypical PKC-CBP pathway to promote neurogenesis and enhance spatial memory formation. Cell Stem Cell. 11:23–35. [DOI] [PubMed] [Google Scholar]
- White ER, Pinar C, Bostrom CA, Meconi A, and Christie BR. 2017. Mild Traumatic Brain Injury Produces Long-Lasting Deficits in Synaptic Plasticity in the Female Juvenile Hippocampus. J Neurotrauma. 34:1111–1123. [DOI] [PubMed] [Google Scholar]
- Whiting MD, Baranova AI, and Hamm RJ. 2006. Cognitive Impairment following Traumatic Brain Injury. InAnimal Models of Cognitive Impairment. Levin ED and Buccafusco JJ, editors, Boca Raton (FL). [PubMed] [Google Scholar]
- Wood RL, and Worthington A. 2017. Neurobehavioral Abnormalities Associated with Executive Dysfunction after Traumatic Brain Injury. Front Behav Neurosci. 11:195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, DiBona VL, Bernard LP, and Zhang H. 2012. The polarity protein partitioning-defective 1 (PAR-1) regulates dendritic spine morphogenesis through phosphorylating postsynaptic density protein 95 (PSD-95). J Biol Chem. 287:30781–30788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Sun M, Bernard LP, and Zhang H. 2017. Postsynaptic density 95 (PSD-95) serine 561 phosphorylation regulates a conformational switch and bidirectional dendritic spine structural plasticity. J Biol Chem. 292:16150–16160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Dong Y, Scholz R, Neumann D, and Zou MH. 2008. Phosphorylation of LKB1 at serine 428 by protein kinase C-zeta is required for metformin-enhanced activation of the AMP-activated protein kinase in endothelial cells. Circulation. 117:952–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Shao A, Yao Y, Tu S, Deng Y, and Zhang J. 2020. Dual roles of astrocytes in plasticity and reconstruction after traumatic brain injury. Cell Commun Signal. 18:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.