Abstract
Zaire ebolavirus (EBOV), Sudan ebolavirus (SUDV), and Marburg marburgvirus (MARV) are the most prevalent and pathogenic species of filovirus. Previously, we showed that glycoprotein antigens from each virus could be lyophilized to create thermostable monovalent subunit vaccines. However, cross-protection is not expected from the monovalent vaccines and therefore developing a trivalent filovirus vaccine would be desirable. Subunit protein vaccines often require the addition of an adjuvant to sufficiently boost the immunogenicity. Typically, liquid suspensions or emulsions of adjuvants and lyophilized antigens are stored in separate vials to avoid destabilizing interactions and are only mixed immediately before administration. Herein, we describe the development and characterization of monovalent and trivalent filovirus vaccines that are co-lyophilized with a squalane-in-water emulsion adjuvant. We found that the single-vial presentation retained adjuvant particle diameter and zeta potential after lyophilization and reconstitution. Furthermore, the trivalent vaccines elicited high antibody levels against all three antigens in mice and non-human primates. These results advance the prospect of developing a single-vial trivalent filovirus vaccine, which would enable easier distribution and administration of the vaccine to resource-poor areas.
Keywords: co-lyophilization, emulsion adjuvant, glycoprotein subunit vaccine, formulation development
1. Introduction
Many vaccine antigens experience instabilities in liquid formulations due to water-mediated degradation pathways that are often accelerated by elevated temperatures [1]. Thus, cold-chain systems are used to maintain vaccines at frozen or refrigerated temperatures throughout manufacturing, transportation, and storage [2]. Vaccine stability can be enhanced by removing bulk water through drying processes such as lyophilization, which can greatly reduce water-mediated degradation [3,4]. Lyophilization of vaccines may prolong their shelf life and lessen dependence on the cold chain by increasing thermostability [5]. Subunit protein vaccines are seen as safer alternatives to attenuated virus vaccines, but they often require the addition of an adjuvant to enhance the immunogenicity of these antigens [6]. Typically, dry formulations of vaccine antigens and diluents comprising liquid suspensions or emulsions of adjuvants are bedside-mixed just before administration to avoid instabilities arising from interactions between antigens and adjuvants, as is the case with SHINGRIX [7]. This requires complex multi-vial presentations where the antigen and adjuvant are stored separately to maintain stability and efficacy. In fact, no lyophilized adjuvanted vaccine that is currently marketed is provided in a single-vial presentation [8]. Single-vial presentations would be easier to transport, distribute, and administer, especially in the context of resource-poor areas, and particularly in low- and middle-income countries.
Lyophilization of adjuvants presents additional challenges. Aluminum salts are the most commonly used adjuvants, but because they are freeze-sensitive [9], their use in formulations marketed to date is limited to liquid suspensions [10,11], although we have previously demonstrated thermostable, lyophilized alum-adjuvanted vaccines [12–16]. Other adjuvants such as oil-in-water emulsions are prone to phase separation, coalescence, and aggregation during lyophilization [17,18]. The adjuvant used in this study was CoVaccine HT™, a squalane-in-water emulsion from Protherics Medicines Development Ltd [19]. CoVaccine HT is comprised of a 1:4:1 ratio of an immunostimulant sucrose fatty acid sulfate ester (SFASE), squalane, and the surfactant polysorbate 80 emulsified in phosphate-buffered saline (PBS) [20].
Filoviruses such as Zaire ebolavirus (EBOV), Sudan ebolavirus (SUDV) and Marburg marburgvirus (MARV) are some of the most lethal viruses known. Previously, we showed that we could thermostabilize monovalent EBOV, SUDV, and MARV glycoprotein vaccines through lyophilization [21]. After incubation for up to 12 weeks at 40°C, glycoprotein quaternary structure and immunogenicity were maintained in lyophilized formulations. A vaccine against EBOV infection has been approved [22], but there are no marketed vaccines for either SUDV or MARV. Moreover, the EBOV vaccine ERVEBO® requires storage at temperatures not to exceed −60°C [23], effectively limiting its use to ring vaccination strategies. In recent years, an increase in epidemic activity [24] has demonstrated the need for a vaccine against multiple species of filovirus [25], and thus developing a trivalent filovirus vaccine is imperative. In our previous study, each of the lyophilized monovalent filovirus vaccines was reconstituted with water, combined with CoVaccine HT and immediately injected into mice [21]. Herein, we describe our effort to reduce the number of vials to a presentation wherein all three glycoprotein antigens and the emulsion adjuvant are co-lyophilized in a single vial. A thermostable single-vial trivalent filovirus vaccine could have a significant impact on incidence of disease by enabling easier, faster, and more widespread vaccine distribution. Throughout this study, we focus on optimizing the adjuvant and antigen concentrations, characterizing adjuvant stability after lyophilization, and assessing the immunogenicity of monovalent and trivalent co-lyophilized formulations (antigens and adjuvants lyophilized together) in both mice and non-human primates.
2. Materials and methods
2.1. Materials
Recombinant filovirus glycoproteins EBOV-GP, MARV-GP, and SUDV-GP were produced at the University of Hawaiʻi at Mānoa as previously described [26]. Three mL Type I borosilicate glass vials, 13 mm two-leg butyl rubber stoppers, and 13 mm aluminum seals were purchased from DWK Life Sciences, LLC (Millville, NJ). Ammonium acetate was purchased from J.T. Baker (Avantor, Radnor, PA) and trehalose was purchased from Pfanstiehl, Inc. (Waukegan, IL). Coomassie Brilliant Blue G-250 was purchased from Amresco (Solon, OH). Precision Plus Protein™ All Blue Standards were purchased from Bio-Rad Laboratories (Hercules, CA).
2.2. Vaccine formulation
Glycoprotein stock solutions were stored at −80°C in 10 mM PBS at pH 7.4. After thawing to room temperature, stock solutions were dialyzed overnight with three buffer exchanges into 10 mM ammonium acetate at pH 7 using Slide-A-Lyzer™ 10,000 MWCO Dialysis Cassettes (Thermo Fisher, Waltham, MA). Protein stocks were then diluted with additions of trehalose, CoVaccine HT, and ammonium acetate to reach the final concentrations as described in Table 1. The formulations were filter sterilized (0.22-micron polyethersulfone membrane) and 1.0 mL aliquots were dispensed into autoclaved 3 mL Type I borosilicate glass vials and stoppered halfway with autoclaved rubber stoppers.
Table 1.
Summarized antigen and adjuvant concentrations for co-lyophilized formulations. In addition to antigen and CoVaccine HT (CoV) at the specified concentrations, each formulation also contained 9.5% (w/v) trehalose and 10 mM ammonium acetate at pH 7. CoVaccine HT concentration refers to the concentration of the immunostimulant SFASE.
| Study | Group ID | EBOV-GP (ug/mL) |
SUDV-GP (ug/mL) |
MARV-GP (ug/mL) |
CoVaccine HT (mg/mL) |
|---|---|---|---|---|---|
|
Study A: Adjuvant Dose Response Study in Mice
(Dose Volume = 0.1 mL) |
E1 | 10 | --- | --- | 0 |
| E10 | 100 | --- | --- | 0 | |
| E1+CoV0.3 | 10 | --- | --- | 3 | |
| E10+CoV0.3 | 100 | --- | --- | 3 | |
| E1+CoV0.5 | 10 | --- | --- | 5 | |
| E10+CoV0.5 | 100 | --- | --- | 5 | |
| E1+CoV0.7 | 10 | --- | --- | 7 | |
| E10+CoV0.7 | 100 | --- | --- | 7 | |
| E1+CoV1 | 10 | --- | --- | 10 | |
| E10+CoV1 | 100 | --- | --- | 10 | |
| CoV1 | --- | --- | --- | 10 | |
|
Study B: Antigen Dose Response Study in Mice
(Dose Volume = 0.1 mL) |
E0.1+CoV0.3 | 1 | --- | --- | 3 |
| E1+CoV0.3 | 10 | --- | --- | 3 | |
| S0.1+CoV0.3 | --- | 1 | --- | 3 | |
| S1+CoV0.3 | --- | 10 | --- | 3 | |
| M0.1+CoV0.3 | --- | --- | 1 | 3 | |
| M1+CoV0.3 | --- | --- | 10 | 3 | |
| E0.1+S0.1+M0.1+CoV0.3 | 1 | 1 | 1 | 3 | |
| E1+S1+M1+CoV0.3 | 10 | 10 | 10 | 3 | |
|
Study C: NHP Immunogenicity Study
(Dose Volume = 0.5 mL) |
E25+CoV5 | 50 | --- | --- | 10 |
| E25+S25+M10+CoV5 | 50 | 50 | 20 | 10 | |
| E25+M10+CoV5 | 50 | --- | 20 | 10 | |
| E25+S25+M5+CoV5 | 50 | 50 | 10 | 10 | |
| CoV5 | --- | --- | --- | 10 |
Liquid CoVaccine HT control sample consisted of CoVaccine HT at 10 mg/mL with 9.5% (w/v) trehalose in 10 mM ammonium acetate at pH 7.
2.3. Lyophilization
Samples of monovalent, bivalent, and trivalent vaccine formulations were lyophilized using an FTS Systems LyoStar lyophilizer (Warminster, PA) using a protocol as previously described [21]. Briefly, the lyophilizer shelves were pre-cooled to −10°C. Half-stoppered sample vials were surrounded with unstoppered dummy vials containing 1 mL of water. During freezing, the shelf temperature was decreased to −40°C at a rate of 0.5°C/min then held at temperature for 1 h. The primary drying phase (at 60 mTorr) started with a temperature ramp of 1°C/min to −20°C followed by a hold for 20 h. Then, during secondary drying, the shelf temperature was increased to 0°C at 0.2 °C/min, then to 30°C at 0.5°C/min, and finally was held at 30°C for 5 h. After the lyophilization cycle was complete, the lyophilizer was back-filled with filtered nitrogen and the vials were fully stoppered automatically by moving the lyophilizer shelves. The vials were removed from the lyophilizer shelves and the stoppers immediately secured with aluminum caps. All samples were kept at 4°C until analysis. Prior to use for analytical characterization or administration to mice or primates, lyophilized vaccine formulations were reconstituted with sterile, deionized (MilliQ®) water or HyClone HyPure water for injection (GE Healthcare Life Sciences, Chicago, IL) to 1.0 mL total volume.
2.4. SDS-PAGE
Antigen samples were characterized using SDS-PAGE and Coomassie staining. In order to reduce oil-in-water adjuvant interference, a sample preparation protocol was followed from Vaccine Adjuvants: Methods and Protocols [27]. Briefly, 4x Laemmli sample buffer (Bio-Rad Laboratories, Hercules, CA) was diluted with 2 parts of 20% sodium dodecyl sulfate (Sigma Aldrich, St. Louis, MO) and 1 part vaccine sample. Prepared samples were heated to 90°C for 15 minutes, after which they were analyzed using a Mini-Protean® TGX™ Gel (Bio-Rad) with a Tris-glycine running buffer per the manufacturer’s instructions. Staining was completed as developed by Studier [28].
2.5. Adjuvant analysis
Particle sizes and zeta potentials were measured using an Anton Paar Litesizer 500 (Graz, Austria). Before analysis, samples were diluted 1:100 with 20 nm-filtered water. Dynamic light scattering analysis was conducted in disposable polystyrene cuvettes using back scattering at 175°. Electrophoretic mobilities were measured in Anton Paar Omega Cuvettes (Graz, Austria). Zeta potentials were calculated from the electrophoretic mobility using the equations of Henry and Smoluchowski integrated into the analyzer software.
2.6. Immunogenicity testing in Swiss-Webster mice
Murine immunogenicity testing was completed for Study A and Study B. Vaccination groups consisted of an equal number of male and female 7–8 week old Swiss-Webster mice (n = 6 per group for Study A and n = 8 per group for Study B) that were purchased from Taconic (Rensselaer, NY) or bred at the University of Hawaiʻi from stocks purchased from Taconic (Rensselaer, NY). Mice were immunized intramuscularly at two sites with a total 100 μL of the prepared vaccine three times, three weeks apart (Days 0, 21, and 42). Serum samples were collected two weeks after each dose, and mice were euthanized on Day 56 and bled by cardiac puncture.
All mouse immunogenicity studies were approved by the University of Hawaiʻi Institutional Animal Care and Use Committee (IACUC), and conducted in strict accordance with local, state, federal, and institutional policies established by the National Institutes of Health and the University of Hawaiʻi IACUC. The University of Hawaiʻi John A. Burns School of Medicine (JABSOM) Laboratory Animal Facility is accredited by the American Association for Accreditation of Laboratory Animal Care (AAALAC). Animal experiments were conducted in consultation with veterinary and animal care staff at the University of Hawaiʻi.
2.7. Immunogenicity testing in non-human primates
Filovirus-naive, adult cynomolgus macaques (Macaca fascicularis) were housed at BIOQUAL, Inc. (Rockville, MD) during the vaccination period. Animals were vaccinated three times intramuscularly into both deltoids with 0.5 mL of vaccine or with formulations containing adjuvant only at weeks 0, 3 and 8. Serum or plasma was collected periodically until week 10 after the first vaccination.
Non-human primate (NHP) experiments were approved by the BIOQUAL, Inc. Institutional Animal Care and Use Committee (IACUC) and conducted in strict accordance with local, state, federal and institutional policies established by the United States National Institutes of Health. The BIOQUAL, Inc. Animal Facilities are accredited by the American Association for Accreditation of Laboratory Animal Care (AAALAC). Animal experiments were conducted in consultation with veterinary and animal care staff at BIOQUAL, Inc.
2.8. Antigen-specific IgG immunoassays
Concentrations of filovirus GP-reactive IgG in mouse sera were determined as described previously [21] using a Luminex®-based (Austin, TX) microsphere immunoassay. The same method and modifications were also used to analyze NHP serum samples.
2.9. Statistical analysis
Significant differences in IgG antibody concentrations between groups of animals were determined using an ANOVA analysis with multiple comparisons using OriginPro, Version 2020 (Northampton, MA). Significant differences between two sets of biophysical data were determined using unpaired t tests. P-values < 0.05 were considered significant.
3. Results
3.1. Antigen and adjuvant biophysical characterization
Glycoprotein and CoVaccine HT emulsion stability were quantified with a variety of biophysical characterization methods. CoVaccine HT suspensions in water are optically turbid and thus not amenable to analysis by most spectroscopic techniques that require sample transparency [27]. Because of this, a number of typical protein analyses were not possible. In addition, the high concentration of polysorbate in CoVaccine HT interferes with size exclusion chromatography, which has previously been shown to be a potent stability-indicating assay for the three glycoproteins [21]. Figure 1 shows the SDS-PAGE gel of EBOV-GP in vaccines from Study A: Adjuvant Dose Response Study. To reduce the adjuvant interference with protein bands, test mixtures were prepared with 1 part vaccine sample, 2 parts 20% SDS, and 1 part 4x concentrated Laemmli sample buffer and were incubated at 90°C for 15 minutes according to a published protocol [27]. The main EBOV-GP band appeared at 100 kDa, and all samples had similar band intensities regardless of the concentration of CoVaccine HT, indicating that substantial antigen degradation did not occur when EBOV-GP was co-lyophilized with CoVaccine HT.
Figure 1.
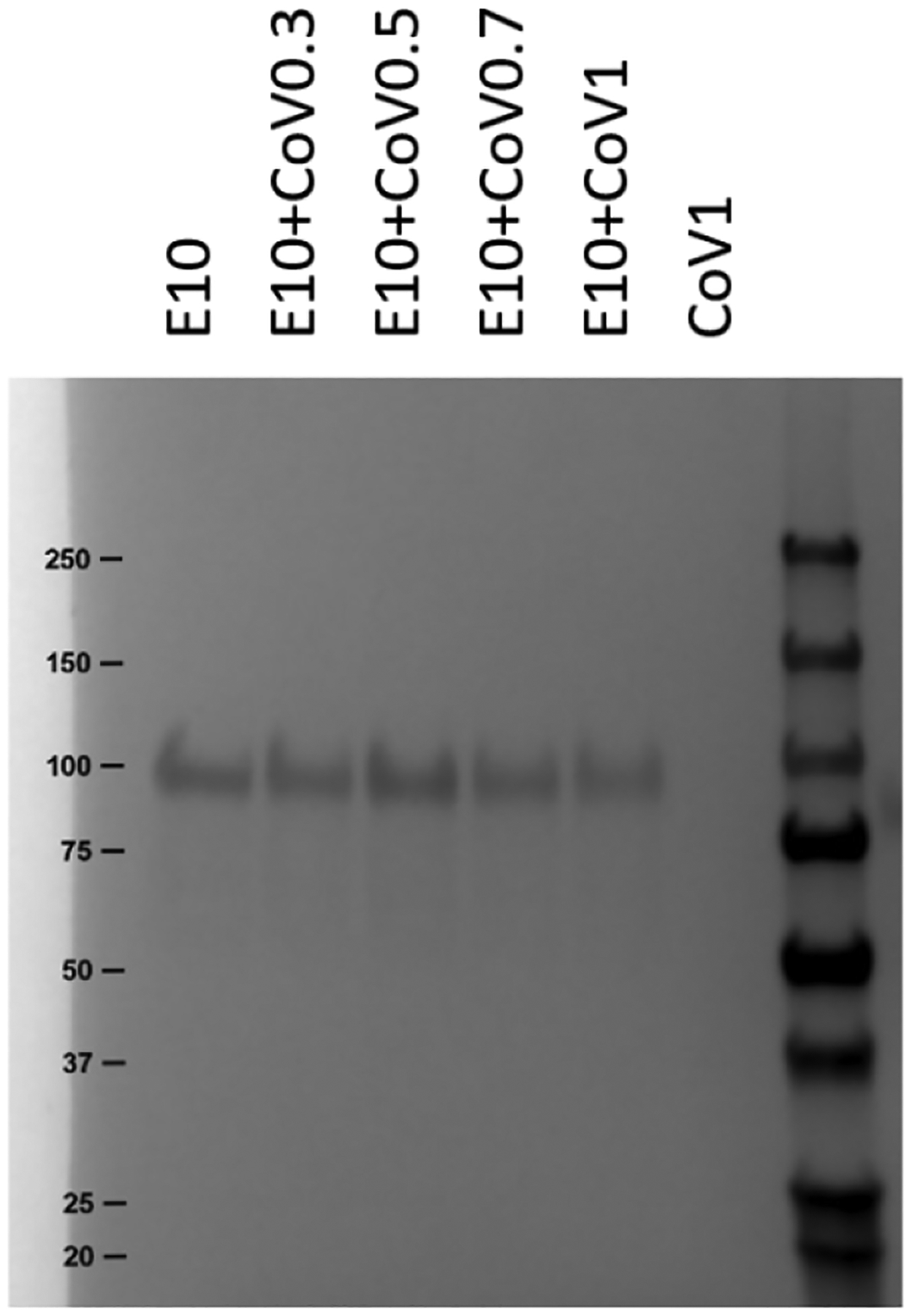
Analysis of Study A: Adjuvant Dose Response Study using SDS-PAGE with Coomassie staining. Lane labels refer to groups defined in Table 1. The CoVaccine HT (CoV) concentration ranged from 3–10 mg/mL SFASE. All formulations were lyophilized and reconstituted.
Zeta potential is a key indicator of the stability of colloidal dispersions such as those formed by CoVaccine HT emulsions in water. Zeta potentials reflect the surface charge density on particles and thus the electrostatic repulsion between charged particles, with larger magnitudes of the zeta potential representing more electrostatic repulsion and therefore less propensity for agglomeration and precipitation [29]. The zeta potential of CoVaccine HT emulsions was approximately −53 mV, which indicates good colloidal stability [30]. In samples that were lyophilized and reconstituted, this value remained unchanged (Figure 2). Formulating the CoVaccine HT with mixtures of the glycoproteins in various concentrations did not affect the zeta potential. Thus, the CoVaccine HT emulsion was stable after formulation and lyophilization.
Figure 2.
Zeta potentials of CoVaccine HT emulsion droplets in co-lyophilized adjuvanted formulations compared to zeta potentials for CoVaccine HT (CoV) emulsion droplets alone. All formulations were lyophilized and reconstituted unless otherwise noted as liquid (LIQ). Error bars are standard deviation from the average of three replicates. Instrument error was between 0.5 and 2.1 mV. Labels refer to groups defined in Table 1.
CoVaccine HT emulsions were manufactured as nanoemulsions (about 110 nm in diameter). Emulsion droplet size is important for maximizing the effectiveness of the adjuvant and to activate the correct immune response for optimal vaccine efficacy [31,32]. Maintenance of the droplet size after lyophilization and reconstitution ensures that maximal adjuvanticity is achieved. CoVaccine HT emulsions control samples diluted to 10 mg/mL had droplet hydrodynamic diameters of approximately 113 nm before and after lyophilization and reconstitution (Figure 3). The hydrodynamic diameters of emulsion droplets in the various co-lyophilized formulations after reconstitution were between 110–120 nm, with no dependence on the concentration of CoVaccine HT or antigens.
Figure 3.
Hydrodynamic diameter of CoVaccine HT emulsion droplets determined from DLS experiments. All formulations were lyophilized and reconstituted unless otherwise noted as liquid (LIQ). Error bars are standard deviation from the average of three replicates. Labels refer to groups defined in Table 1.
3.2. Immunogenicity of co-lyophilized vaccine formulations
The immunogenicities of various vaccine formulations were tested in mouse and primate models. Study A probed the effect of various concentrations of CoVaccine HT (from 0.3–1.0 mg/dose) with two EBOV-GP dose levels (either 1 or 10 μg) in Swiss-Webster mice. After dose 2, potent IgG responses were seen in groups immunized with co-lyophilized vaccines (Figure 4a). The control samples, E1+CoV1 LIQ and E10+CoV1 LIQ, consisted of liquid EBOV-GP and liquid CoVaccine HT that were formulated fresh from stocks immediately before administration. The corresponding samples co-lyophilized in single vials elicited similar responses. A wider range of antibody responses among animals within a group with lower geometric mean titers (GMTs) were observed at the lower EBOV-GP dose level (1 μg) than at the higher dose level (10 μg). After dose 3 (Figure 4b), the immune response range among animals within a group was considerably tightened, and there were no statistically significant differences between IgG concentrations observed in relation to EBOV-GP dose levels for groups receiving the same adjuvant dose. In addition, the study showed that after three doses, lower concentrations of CoVaccine HT were equally as effective at inducing antigen-specific IgG as the highest concentration of 10 mg/mL.
Figure 4.
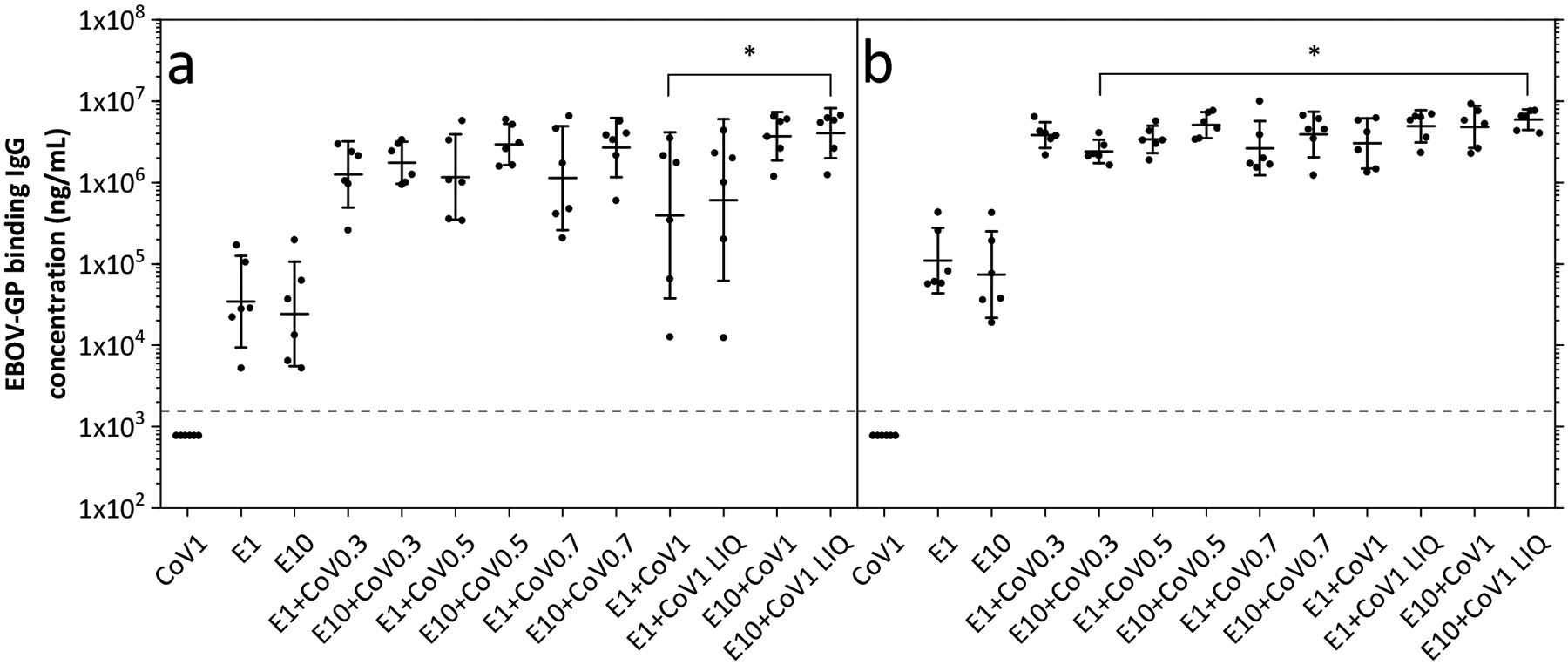
IgG responses in Study A: Adjuvant Dose Response Study. IgG concentrations specific to EBOV-GP from (a) post-dose 2 and (b) post-dose 3 mouse serum samples. Data are shown as geometric mean titers (GMT) ± 95% confidence interval. Each dot represents the IgG concentration measured from serum of one individual mouse. The dotted line represents the limit of quantification (LOQ); any values measured below this limit are shown here as half the LOQ. The numbers in the group labels are defined in Table 1 and refer to the dose of EBOV-GP (E; in μg) and adjuvant (CoV; in mg). Statistically significant differences are only shown determined for groups receiving both antigen and adjuvant, where * = p < 0.05.
Because lower levels of CoVaccine HT were shown to elicit equal immunogenicity in co-lyophilized formulations, the antigen dose response study (Study B) was completed with CoVaccine HT doses at 0.3 mg. Both monovalent and trivalent formulations at low (0.1 μg) and high (1 μg) antigen dose levels were tested in Swiss-Webster mice, with IgG antibody responses after dose 2 and 3 against all three immunogens shown in Figure 5. As expected, antibody concentrations were generally higher after vaccine dose 3. Trivalent co-lyophilized vaccines were seen to be somewhat more immunogenic than their monovalent vaccine counterparts at the same antigen dose, which was especially evident in the IgG responses against SUDV-GP and MARV-GP. This result may suggest that the antibody titers were bolstered when additional antigens were added into formulations, possibly due to a degree of cross-reactivity.
Figure 5.
Mouse immunogenicity results from Study B: Antigen Dose Response Study. Responses to immunizations are shown as IgG concentrations against (a,b) EBOV-GP, (c,d) SUDV-GP, and (e,f) MARV-GP. Each dot represents the antibody concentration measured from serum of one individual mouse. The dotted line represents the limit of quantification (LOQ); any values measured below this limit are shown here as half the LOQ. Data are shown at GMTs ± 95% confidence interval (where all values within a group are above the LOQ) from serum collected post dose two (PD2, left panels) and three (PD3, right panels). The numbers in the group labels are defined in Table 1 and refer to the dose of antigen (in μg) and adjuvant (in mg).
In addition to evaluating immunogenicity in mice, single-vial vaccines were also tested for immunogenicity in non-human primates (NHPs). The main focus of Study C was to confirm that co-lyophilized vaccines were as immunogenic in primates as they were previously shown to be in mice. Previous data (not shown) suggested that MARV-GP might show immunodominance over EBOV-GP or SUDV-GP responses. To further explore this possible effect, monovalent EBOV-GP formulations were tested against bivalent and trivalent formulations using MARV-GP at two different dose levels (5 or 10 μg). As an additional strategy, an EBOV-GP prime followed by two trivalent boosters was attempted to minimize interference from MARV-GP on the EBOV-specific response. Besides the CoVaccine HT-only control group, all primates received an EBOV-GP dose of 25 μg whether in monovalent or multivalent preparations. Starting at week 5 (two weeks after the second immunization), there was very little difference in the IgG responses against EBOV-GP across all test groups (Figure 6a). Antibody responses against EBOV-GP or SUDV-GP for groups receiving 25 μg SUDV-GP for all three doses were nearly equivalent, indicating that increasing the MARV-GP dose from 5 μg to 10 μg did not produce the suspected dominating effect (Figure 6b). Antibody responses against MARV-GP were similar regardless of whether MARV-GP was formulated in bivalent or trivalent vaccines or if MARV-GP was given as a 5 or 10 μg dose (Figure 6c). The prime/boost test group (blue triangles) that received a monovalent EBOV-GP for the first dose and a trivalent mixture for the second and third doses showed interesting results. Since the primates received the same amount of EBOV-GP for all three doses, there was little difference between these groups and the EBOV-GP monovalent group or any of the trivalent groups for EBOV-specific IgG. The response against MARV-GP was absent after dose one and showed a lower IgG concentration after dose 2, but by two weeks after dose 3, the prime/boost group had similar antibody levels to groups that received MARV-GP for all three doses. This effect was not seen for SUDV-GP, where the prime/boost group had similar antibody concentrations as groups not receiving doses of SUDV-GP throughout the study, highlighting the importance of proper timing and combination of immunizations.
Figure 6.
NHP immunogenicity from Study C with IgG responses against (a) EBOV-GP, (b) SUDV-GP, and (c) MARV-GP. Data are shown as GMTs from n = 3 animals per group, except for the CoV10 group with n = 1. The dotted line represents the limit of quantification (LOQ). If any primate in a group had a response less than the LOQ, the GMT is shown as half the LOQ. The numbers in the group labels are defined in Table 1 and refer to the dose of antigen (in μg) and adjuvant (in mg).
4. Discussion
No currently licensed lyophilized vaccine that contains adjuvant is presented in a single-vial format. While there are numerous studies demonstrating the possibility of co-lyophilizing antigens with aluminum salt adjuvants [12–16] and liposomal formulations [33–35], fewer reports of successfully co-lyophilizing antigens with oil-in-water emulsions are available to the public domain. One example, however, is a tuberculosis vaccine developed by Orr et al. [36] and Kramer et al. [37] that was stabilized by co-lyophilizing a M. tuberculosis fusion protein with a squalene-in-water nanoemulsion.
In this study, co-lyophilization of the glycoprotein antigens and CoVaccine HT oil-in-water emulsion adjuvant did not significantly impact the physical characteristics of the emulsion. After reconstitution, adjuvant parameters such as particle diameter and zeta potential were not substantially different from those in the liquid starting emulsions. Zeta potential measurement is a technique that can be used for determining the surface charge of adjuvants in colloidal suspensions. The magnitude of the zeta potential is representative of the colloidal stability of the suspension. CoVaccine HT emulsion droplets were measured to be around −53 mV, which indicates good stability. This stability was unchanged after lyophilization and reconstitution, with the zeta potential of co-lyophilized vaccines determined to be in the range of −50 to −55 mV. A large magnitude in zeta potential indicates strong repulsion between emulsion droplets, which helps inhibit agglomeration. If agglomeration did occur, this would be reflected in increases in the droplet hydrodynamic diameters. We did not observe any large increase in droplet size; using dynamic light scattering, the droplet diameter was consistently measured to be between 110–120 nm for all the co-lyophilized and reconstituted test vaccines and controls. Additionally, formulating the co-lyophilized vaccines as trivalent preparations also did not significantly impact the emulsion physical characteristics.
Co-lyophilizing filovirus glycoproteins and CoVaccine HT did not impair their immunological activity. In Study A, single-vial presentations of EBOV-GP and CoVaccine HT were as immunogenic as the liquid two-vial preparation. Furthermore, excellent emulsion stability at all points was seen and formulations with lower levels of CoVaccine HT were as immunogenic as those formulated at the highest concentration tested. However, this was only seen post-dose three. After dose two, formulations with lower levels of CoVaccine HT yielded reduced titers, especially in the groups receiving the lower EBOV-GP doses. The highest CoVaccine HT and EBOV-GP dose (10 μg dose of EBOV-GP and 1 mg dose of CoVaccine HT), had high GMTs after dose two that only increased slightly after an additional dose. For the lower CoVaccine HT groups, larger increases in the GMTs after dose 3 were seen so that they were at equivalent antibody concentrations regardless of CoVaccine HT dose. This might indicate that the number of doses could be decreased if higher doses of both CoVaccine HT and antigen are used.
In formulating trivalent vaccines, we sought to uncover the effect of immunodominance, as there was a concern that MARV-GP specific responses could dominate those of the ebolavirus species. In monovalent formulations from Study B, MARV-GP was shown to elicit higher anti-MARV-GP specific antibody concentrations than respective anti-SUDV-GP antibody concentrations elicited from monovalent SUDV-GP formulations. When mice were immunized with all three antigens, antibody concentrations were higher than those resulting from administration of monovalent formulations of the same antigen dose. Due to the high degree of primary structure conservation between EBOV-GP and SUDV-GP, there might be antibodies formed that are cross-reactive to the two glycoproteins. However, it is unclear whether cross-reactive antibodies also provide cross-neutralization at a level necessary for immune protection [38,39]. Overall, the addition of more antigens did not seem to negatively affect the respective humoral responses against a specific glycoprotein. This was also observed in the NHP immunogenicity Study C. In terms of anti-EBOV-GP antibody response, the trivalent mixtures in Study C elicited similar concentrations of antibodies in NHPs as compared to the monovalent EBOV-GP vaccine as all formulations contained the same dose of EBOV-GP. In Study C, we observed that immunization with MARV-GP elicited higher antibody concentrations than the other two glycoproteins even though the MARV-GP doses were much lower, confirming observations seen in mice. Further work is needed to effectively balance antigens to achieve an optimized immune response against all three glycoproteins.
A combination of our formulation and process conditions likely helped to stabilize the antigens and CoVaccine HT adjuvant during lyophilization. Glass-forming excipients such as trehalose are often added to lyophilized protein formulations to stabilize antigens both directly through the replacement of hydrogen bonds formed previously with water and indirectly by reducing molecular mobility through vitrification of antigens in a glassy state [40,41]. Similarly, glassy trehalose matrices can also decrease the interaction between hydrocarbon chains in oil-in-water emulsions by maintaining the spatial organization and distance between the droplets [42]. Trehalose especially has been shown to be effective for lyophilizing emulsions as compared to other saccharides [37,43]. It has also been shown that higher concentrations of cryoprotectants in conjunction with faster freezing rates helps to maximize the stabilization effect [44]. Here, we used a formulation with 9.5% (w/v) trehalose and a freezing rate of 0.5°C/min. During freezing, trehalose concentrates as more water is converted to ice, eventually reaching a maximum concentration of about 80% (w/v) [45]. Therefore, the maximum amount that the rest of the solutes (e.g. the glycoproteins, ammonium acetate salt, and CoVaccine HT droplets) can cryoconcentrate is 8-fold. The fast freezing rate also further limits the time the solutes spend in a freeze-concentrated, but still liquid state. Ammonium acetate is a volatile salt, and partially sublimates during lyophilization. This can reduce the potentially damaging effects of concentrated salts during reconstitution that could cause aggregation of both antigens and emulsion adjuvants through pH shifts and colloidal instabilities [46,47].
Overall, we found that formulating filovirus glycoproteins with adjuvant as a single-vial presentation through co-lyophilization did not have a significant impact on the biophysical characteristics of the adjuvant or the immunogenicity of the vaccine formulation. Moreover, trivalent vaccine formulations produced high antibody concentrations against all three glycoproteins showing full retention of antigen and adjuvant integrity. A single-vial trivalent vaccine eases storage and administration, which is beneficial for distribution to resource-poor areas. Previously, we have shown that monovalent lyophilized glycoprotein vaccines could withstand temperatures up to 40°C for 12 weeks. Future studies will focus on evaluating the thermal stability of co-lyophilized trivalent formulations with the objective of significantly reducing cold chain requirements for transport and storage.
5. Acknowledgements
Funding for this project was provided by the National Institute of Allergy and Infectious Diseases under grants R01AI119185 and R01AI132323 (PI: Dr. Axel Lehrer) and sponsored by Soligenix, Inc. We would like to thank Protherics Medicines Development (London, UK) for the gift of CoVaccine HT for this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Interests
The University of Colorado has licensed intellectual property related to thermostabilization of lyophilized vaccines to VitriVax, Inc., a company in which TWR holds equity. OD is an employee of Soligenix, Inc. ATL and OD are inventors on patents related to vaccine technology, including technology specific to filovirus vaccines.
Declaration of interests
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
The University of Colorado has licensed intellectual property related to thermostabilization of lyophilized vaccines to VitriVax, Inc., a company in which TWR holds equity. OD is an employee of Soligenix, Inc. ATL and OD are inventors on patents related to vaccine technology, including technology specific to filovirus vaccines.
References
- [1].Kanojia G, Willems GJ, Frijlink HW, Kersten GFA, Soema PC, Amorij JP. A Design of Experiment approach to predict product and process parameters for a spray dried influenza vaccine. Int J Pharm 2016;511:1098–111. 10.1016/j.ijpharm.2016.08.022. [DOI] [PubMed] [Google Scholar]
- [2].Kumru OS, Joshi SB, Smith DE, Middaugh CR, Prusik T, Volkin DB. Vaccine instability in the cold chain: Mechanisms, analysis and formulation strategies. Biologicals 2014;42:237–59. 10.1016/j.biologicals.2014.05.007. [DOI] [PubMed] [Google Scholar]
- [3].Burke CJ, Hsu TA, Volkin DB. Formulation, stability, and delivery of live attenuated vaccines for human use. Crit Rev Ther Drug Carrier Syst 1999;16:1–83. 10.1615/critrevtherdrugcarriersyst.v16.i1.10. [DOI] [PubMed] [Google Scholar]
- [4].Rexroad J, Wiethoff CM, Jones LS, Middaugh CR. Lyophilization and the thermostability of vaccines. Cell Preserv Technol 2002;1:91–104. 10.1089/153834402320882593. [DOI] [Google Scholar]
- [5].Chen D, Kristensen D. Opportunities and challenges of developing thermostable vaccines. Expert Rev Vaccines 2014;8:547–57. 10.1586/erv.09.20. [DOI] [PubMed] [Google Scholar]
- [6].Dumpa N, Goel K, Guo Y, McFall H, Pillai AR, Shukla A, et al. Stability of Vaccines. AAPS PharmSciTech 2019;20:1–11. 10.1208/s12249-018-1254-2. [DOI] [PubMed] [Google Scholar]
- [7].Pasteur Sanofi. Package Insert - SHINGRIX. US Food Drug Adm; 2019. https://www.fda.gov/media/108597/download. [Google Scholar]
- [8].PATH. Summary of Stability Data for Licensed Vaccines. Seattle, WA: 2012. [Google Scholar]
- [9].Clapp T, Munks MW, Trivedi R, Kompella UB, Braun LTJ. Freeze-thaw stress of Alhydrogel® alone is sufficient to reduce the immunogenicity of a recombinant hepatitis B vaccine containing native antigen. Vaccine 2014;32:3765–71. 10.1016/j.vaccine.2014.05.037. [DOI] [PubMed] [Google Scholar]
- [10].Clapp T, Siebert P, Chen D, Braun LTJ. Vaccines with aluminum-containing adjuvants: Optimizing vaccine efficacy and thermal stability. J Pharm Sci 2010;100:388. 10.1002/jps.22284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kurzątkowski W, Kartoğlu Ü, Górska P, Główka M, Woźnica K, Zasada AA, et al. Physical and chemical changes in Alhydrogel™ damaged by freezing. Vaccine 2018;36:6902–10. 10.1016/j.vaccine.2018.10.023. [DOI] [PubMed] [Google Scholar]
- [12].Hassett KJ, Cousins MC, Rabia LA, Chadwick CM, O’Hara JM, Nandi P, et al. Stabilization of a recombinant ricin toxin A subunit vaccine through lyophilization. Eur J Pharm Biopharm 2013;85:279–86. 10.1016/j.ejpb.2013.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Westfall J, Yates JL, Van Slyke G, Ehrbar D, Measey T, Straube R, et al. Thermal stability and epitope integrity of a lyophilized ricin toxin subunit vaccine. Vaccine 2018;36:5967–76. 10.1016/j.vaccine.2018.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hassett KJ, Vance DJ, Jain NK, Sahni N, Rabia LA, Cousins MC, et al. Glassy-state stabilization of a dominant negative inhibitor anthrax vaccine containing aluminum hydroxide and glycopyranoside lipid a adjuvants. J Pharm Sci 2015;104:627–39. 10.1002/jps.24295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hassett KJ, Meinerz NM, Semmelmann F, Cousins MC, Garcea RL, Randolph TW. Development of a highly thermostable, adjuvanted human papillomavirus vaccine. Eur J Pharm Biopharm 2015;94:220–8. 10.1016/j.ejpb.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Chisholm CF, Kang TJ, Dong M, Lewis K, Namekar M, Lehrer AT, et al. Thermostable Ebola virus vaccine formulations lyophilized in the presence of aluminum hydroxide. Eur J Pharm Biopharm 2019;136:213–20. 10.1016/j.ejpb.2019.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Iyer V, Cayatte C, Marshall JD, Sun J, Schneider-Ohrum K, Maynard SK, et al. Feasibility of Freeze-Drying Oil-in-Water Emulsion Adjuvants and Subunit Proteins to Enable Single-Vial Vaccine Drug Products. J Pharm Sci 2017;106:1490–8. 10.1016/j.xphs.2017.02.024. [DOI] [PubMed] [Google Scholar]
- [18].Morais ARDV, Alencar ÉDN, Xavier Júnior FH, De Oliveira CM, Marcelino HR, Barratt G, et al. Freeze-drying of emulsified systems: A review. Int J Pharm 2016;503:102–14. 10.1016/j.ijpharm.2016.02.047. [DOI] [PubMed] [Google Scholar]
- [19].Hilgers LAT, Blom AG. Sucrose fatty acid sulphate esters as novel vaccine adjuvant. Vaccine 2006;24:S81–2. 10.1016/j.vaccine.2005.01.133. [DOI] [PubMed] [Google Scholar]
- [20].Blom AG, Hilgers LAT. Sucrose fatty acid sulphate esters as novel vaccine adjuvants: effect of the chemical composition. Vaccine 2004;23:743–54. 10.1016/j.vaccine.2004.07.021. [DOI] [PubMed] [Google Scholar]
- [21].Preston KB, Monticello CR, Wong TAS, To A, Donini O, Lehrer AT, et al. Preservation of Quaternary Structure in Thermostable, Lyophilized Filovirus Glycoprotein Vaccines: A Search for Stability-Indicating Assays. J Pharm Sci 2020;109:3716–27. 10.1016/j.xphs.2020.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].U.S. Food and Drug Administration. First FDA-approved vaccine for the prevention of Ebola virus disease, marking a critical milestone in public health preparedness and response n.d. https://www.fda.gov/news-events/press-announcements/first-fda-approved-vaccine-prevention-ebola-virus-disease-marking-critical-milestone-public-health (accessed January 16, 2020).
- [23].Arnemo M, Viksmoen Watle SS, Schoultz KM, Vainio K, Norheim G, Moorthy V, et al. Stability of a Vesicular Stomatitis Virus-Vectored Ebola Vaccine. J Infect Dis 2016;213:930–3. 10.1093/infdis/jiv532. [DOI] [PubMed] [Google Scholar]
- [24].Rugarabamu S, Mboera L, Rweyemamu M, Mwanyika G, Lutwama J, Paweska J, et al. Forty-two years of responding to Ebola virus outbreaks in Sub-Saharan Africa: A review. BMJ Glob Heal 2020;5:1–10. 10.1136/bmjgh-2019-001955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Jacob ST, Crozier I, Fischer WA, Hewlett A, Kraft CS, de La Vega MA, et al. Ebola virus disease. vol. 6. Springer; US; 2020. 10.1038/s41572-020-0147-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lehrer AT, Wong TAS, Lieberman MM, Humphreys T, Clements DE, Bakken RR, et al. Recombinant proteins of Zaire ebolavirus induce potent humoral and cellular immune responses and protect against live virus infection in mice. Vaccine 2018;36:3090–100. 10.1016/j.vaccine.2017.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Fox CB. Vaccine Adjuvants: Methods and Protocols. 2017.
- [28].Studier FW. Protein production by auto-induction in high density shaking cultures. Protein Expr Purif 2005;41:207–34. 10.1016/j.pep.2005.01.016. [DOI] [PubMed] [Google Scholar]
- [29].Bhattacharjee S DLS and zeta potential - What they are and what they are not? J Control Release 2016;235:337–51. 10.1016/j.jconrel.2016.06.017. [DOI] [PubMed] [Google Scholar]
- [30].Kumar A, Dixit CK. Methods for characterization of nanoparticles. Adv Nanomedicine Deliv Ther Nucleic Acids 2017:44–58. 10.1016/B978-0-08-100557-6.00003-1. [DOI] [Google Scholar]
- [31].Shah RR, O’hagan DT, Amiji MM, Brito LA. The impact of size on particulate vaccine adjuvants. Nanomedicine 2014;9:2671–81. 10.2217/nnm.14.193. [DOI] [PubMed] [Google Scholar]
- [32].Iyer V, Cayatte C, Guzman B, Schneider-Ohrum K, Matuszak R, Snell A, et al. Impact of formulation and particle size on stability and immunogenicity of oil-in-water emulsion adjuvants. Hum Vaccin 2015;11:1853–64. 10.1080/21645515.2015.1046660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Wui SR, Kim KS, Ryu JI, Ko A, Do HTT, Lee YJ, et al. Efficient induction of cell-mediated immunity to varicella-zoster virus glycoprotein E co-lyophilized with a cationic liposome-based adjuvant in mice. Vaccine 2019;37:2131–41. 10.1016/j.vaccine.2019.02.048. [DOI] [PubMed] [Google Scholar]
- [34].Mabrouk MT, Huang W, Deng B, Li-purcell N, Seffouh A, Ortega J, et al. Lyophilized, antigen-bound liposomes with reduced MPLA and enhanced thermostability 2020;589. 10.1016/j.ijpharm.2020.119843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Fortpied J, Collignon S, Moniotte N, Renaud F, Bayat B, Lemoine D. The thermostability of the RTS,S/AS01 malaria vaccine can be increased by co-lyophilizing RTS,S and AS01. Malar J 2020;19. 10.1186/s12936-020-03253-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Orr MT, Kramer RM, Barnes LV., Dowling QM, Desbien AL, Beebe EA, et al. Elimination of the cold-chain dependence of a nanoemulsion adjuvanted vaccine against tuberculosis by lyophilization. J Control Release 2014;177:20–6. 10.1016/j.jconrel.2013.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Kramer RM, Archer MC, Orr MT, Dubois Cauwelaert N, Beebe EA, Huang PWD, et al. Development of a thermostable nanoemulsion adjuvanted vaccine against tuberculosis using a design-of-experiments approach. Int J Nanomedicine 2018;13:3689–711. 10.2147/IJN.S159839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Natesan M, Jensen SM, Keasey SL, Kamata T, Kuehne AI, Stonier SW, et al. Human survivors of disease outbreaks caused by Ebola or Marburg virus exhibit cross-reactive and long-lived antibody responses. Clin Vaccine Immunol 2016;23:717–24. 10.1128/CVI.00107-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Wang Y, Howell KA, Brannan J, Agans KN, Turner HL, Wirchnianski AS, et al. Prominent Neutralizing Antibody Response Targeting the Ebolavirus Glycoprotein Subunit Interface Elicited by Immunization. J Virol 2021;95:1–24. 10.1128/jvi.01907-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Ohtake S, Wang YJ. Trehalose: Current use and future applications. J Pharm Sci 2011;100:2020–53. 10.1002/jps.22458. [DOI] [PubMed] [Google Scholar]
- [41].Crowe LM, Reid DS, Crowe JH. Is trehalose special for preserving dry biomaterials? Biophys J 1996;71:2087–93. 10.1016/S0006-3495(96)79407-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Abdelwahed W, Degobert G, Stainmesse S, Fessi H. Freeze-drying of nanoparticles: Formulation, process and storage considerations. Adv Drug Deliv Rev 2006;58:1688–713. 10.1016/j.addr.2006.09.017. [DOI] [PubMed] [Google Scholar]
- [43].Li F, Wang T, He HB, Tang X. The properties of bufadienolides-loaded nano-emulsion and submicro-emulsion during lyophilization. Int J Pharm 2008;349:291–9. 10.1016/j.ijpharm.2007.08.011. [DOI] [PubMed] [Google Scholar]
- [44].Lee MK, Kim MY, Kim S, Lee J. Cryoprotectants for freeze drying of drug nano-suspensions: Effect of freezing rate. J Pharm Sci 2009;98:4808–17. 10.1002/jps.21786. [DOI] [PubMed] [Google Scholar]
- [45].Chen T, Fowler A, Toner M. Literature review: Supplemented phase diagram of the trehalose-water binary mixture. Cryobiology 2000;40:277–82. 10.1006/cryo.2000.2244. [DOI] [PubMed] [Google Scholar]
- [46].Nail SL, Akers MJ. Development and Manufacture of Protein Pharmaceuticals. 2002.
- [47].Costantino HR, Langer R, Klibanov AM. Aggregation of a Lyophilized Pharmaceutical Protein, Recombinant Human Albumin: Effect of Moisture and Stabilization by Excipients. Nat Biotechnol 1995;13:493–6. 10.1038/nbt0595-493. [DOI] [PubMed] [Google Scholar]


