Abstract
Background:
Patients with residual muscle-invasive urinary tract cancer after neoadjuvant chemotherapy (NAC) have a high risk of recurrence.
Objective:
To retrospectively evaluate whether additional adjuvant chemotherapy (AC) improves outcomes compared with surveillance in patients with significant residual disease despite NAC.
Design, setting, and participants:
We identified 474 patients who received NAC from the Retrospective International Study of Cancers of the Urothelium database, of whom 129 had adverse residual disease (≥ypT3 and/or ypN+).
Outcome measurements and statistical analysis:
Time to relapse (TTR) was the primary endpoint assessed starting from 2 mo after surgery to minimize immortal time bias. Secondary endpoints included overall survival (OS), incidence of AC use, and chemotherapy patterns. Kaplan-Meier and Cox regression models estimated TTR, OS, and associations with AC, adjusting for the type of NAC, age, and pathological stage in multivariable analyses.
Results and limitations:
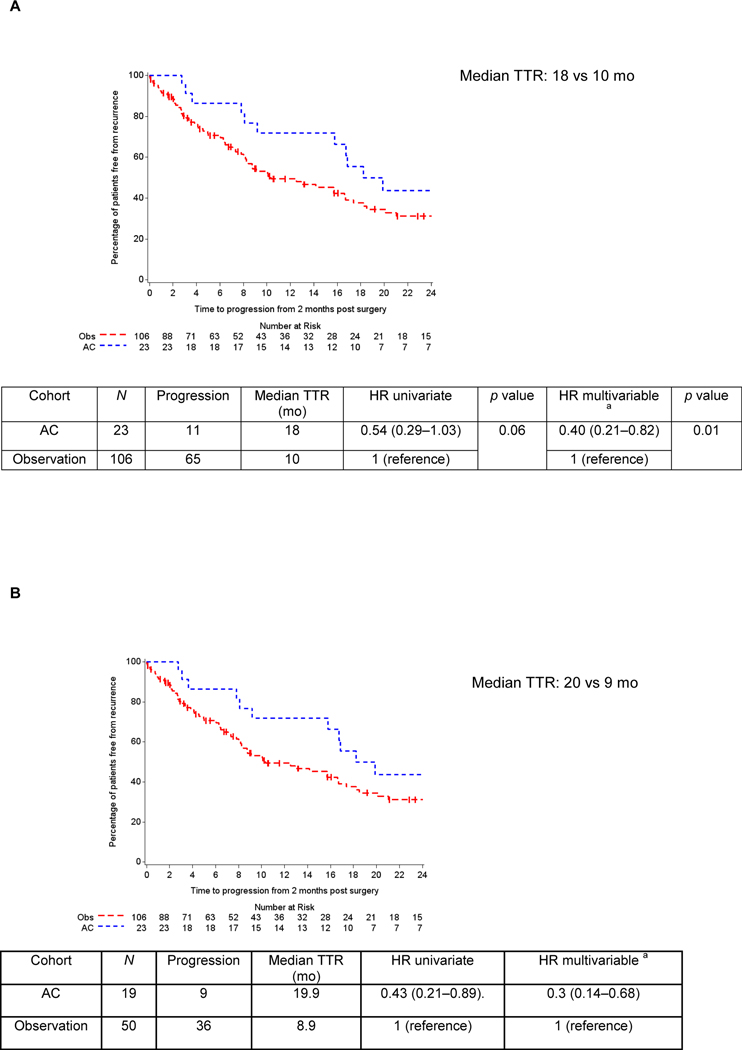
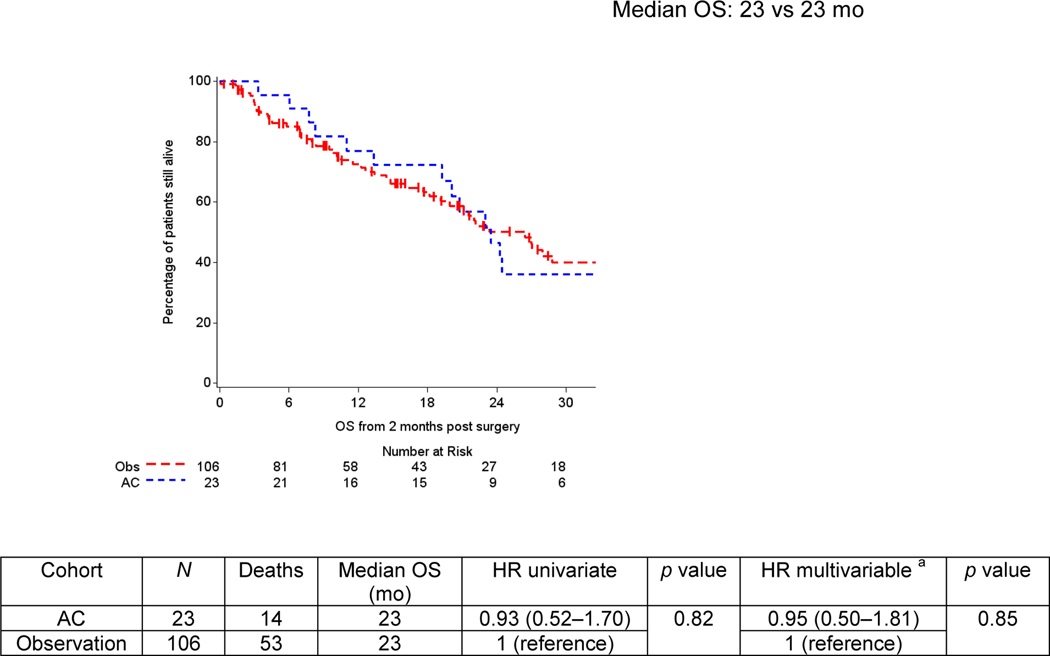
A total of 106 patients underwent surveillance, while 23 received AC. Gemcitabine-cisplatin was the most frequent regimen employed in both settings (30.4%), and the majority (82.6%) of the patients switched to a different regimen. Median follow-up was 30 mo. Over 50% of patients developed a recurrence. Median TTR was 16 mo (range: <1–108 mo). Longer median TTR was observed with AC compared with surveillance (18 vs 10 mo, p = 0.06). Risk of relapse significantly decreased with AC when adjusted in multivariable analyses (p = 0.01). The subgroup analyses of ypT4b/ypN+ patients (AC: 19; surveillance: 50) who received AC had significantly greater median TTR (20 vs 9 mo; hazard ratio 0.43; 95% confidence interval: 0.21–0.89). No difference in OS was found. Limitations include the retrospective design.
Conclusions:
The utilization of AC after NAC in patients with high-risk residual disease is not frequent in clinical practice but might reduce the risk of recurrence. Further investigation is needed in this high-risk population to identify optimal therapy and to improve clinical outcomes such as the ongoing adjuvant immunotherapy trials.
Patient summary:
We found that administering additional chemotherapy in patients who had significant residual disease despite preoperative chemotherapy is not frequent in clinical practice. While it might reduce the risk of recurrence, it did not clearly increase overall survival. We encourage participation in the ongoing immunotherapy trials to see whether we can improve outcomes using a different type of therapy that stimulates the immune system.
Keywords: Adjuvant chemotherapy, Muscle-invasive bladder cancer, Muscle-invasive urinary tract cancer, Neoadjuvant chemotherapy, Residual disease, Risk of relapse, Time to recurrence
1. Introduction
Treatment of localized muscle-invasive urinary tract cancers aims to achieve cure by eliminating the primary tumor with local therapies and eradicating micrometastases with systemic chemotherapy. Currently, the gold standard is cisplatin-based neoadjuvant chemotherapy (NAC) in eligible patients followed by radical cystectomy with bilateral pelvic lymph node dissection. Cisplatin-based NAC increases survival without affecting the feasibility and safety of the surgery [1–3]. The most commonly used cisplatin-based regimens in the perioperative setting are gemcitabine/cisplatin (GC) and dose-dense methotrexate/vinblastine/adriamycin/cisplatin (ddMVAC) [4–9]. Pathological complete response (pCR) or downstaging to non–muscle-invasive disease is an indicator of biological sensitivity to cytotoxic agents and is associated with improved survival [10,11].
In the absence of upfront NAC, adjuvant chemotherapy (AC) is generally reserved for cisplatin-fit patients with high-risk pathological features for recurrence at cystectomy such as extravesicular extension (pT3–4), lymph node positive disease, or lymphovascular invasion. Meta-analyses collating a heterogeneous mix of phase 3 studies that failed to accrue successfully suggest that cisplatin-based AC significantly decreases the risk of recurrence and prolongs survival in patients without prior NAC [12–14]. The most recent phase 3 study by the European Organisation for Research and Treatment of Cancer evaluated immediate adjuvant versus delayed therapy at relapse. While it closed early due to poor accrual, there was a clear benefit of improved progression-free survival (PFS) with earlier AC: median PFS of 2.9 versus 0.9 yr (p > 0.0001) and 5-yr PFS of 46.8% versus 29.5% (p > 0.0001) [15]. No overall survival (OS) benefit was observed. In addition, for patients with upper tract disease, recent data from the randomized POUT study demonstrated that adjuvant platinum-based chemotherapy significantly improved disease-free survival (hazard ratio [HR] 0.47; 95% confidence interval [CI]: 0.29–0.74) [16]. Survival data are maturing, but a retrospective analysis leveraging the National Cancer Database found a significant improvement in OS with adjuvant therapy in upper tract disease [17].
An unresolved clinical question is whether patients who do not achieve a pCR or significant downstaging should receive additional AC, given their high risk of relapse. Limited evidence exists to guide the management of this subgroup of patients, and the question has never been studied prospectively. Using an international database collaboration (Retrospective International Study of Cancers of the Urothelium [RISC]), we evaluated the clinical outcomes of patients with residual disease after NAC who received additional AC compared with a control cohort of patients who underwent surveillance. Additionally, we strove to assess the incidence and practice patterns of AC administration after NAC.
2. Patients and methods
Regulatory approval was obtained from 23 sites participating in the RISC database. Time of therapy administration was restricted from 1991 to 2013 to ensure modern chemotherapy practices and adequate follow-up of a minimum of 2 yr. Patients who received NAC followed by surgical resection of the primary tumor (upper or lower urinary tract) and had adverse residual disease, defined as pathological stage T3 or greater (ypT3 or ypT4) and/or involved pathological lymph node (ypN+), were included. To capture a real-world experience, we included patients with radiologically evident lymph node disease (cN+), urothelial carcinoma (UC), and variant histologies, and patients who also received non–cisplatin combination therapies such as carboplatin-based regimens or cisplatin monotherapy. Patients who had <2 mo of follow-up data or who were lost to follow-up without adequate recurrence or survival data were excluded. AC was defined as any chemotherapy administered at ≤3 mo from surgery in the absence of metastatic disease. Surveillance plans were as per institutional preference or trial requirement, and generally consisted of clinical monitoring and radiological imaging every 3–6 mo during the first 2 yr and then every 6–12 mo.
Clinicodemographic variables, including age, gender, race, smoking history, performance status, comorbidities, date of diagnosis, tumor primary site, clinical stage, and measurable disease status, were collected, as were pathological features (histology, number of nodes removed and pathological involvement, presence of lymphovascular invasion, and margin positivity), treatment data (chemotherapy regimens administered, including number of cycles, duration of treatment, and use of adjuvant radiation), response data (pathological response and objective radiological response in cN+), and time to event outcomes (dates of recurrence, initiation of new systemic treatment for recurrence, death, and last known alive).
The primary endpoint was to assess time to relapse (TTR) in patients who received NAC and surgery followed by AC (study arm) compared with those under surveillance (control arm) for residual disease. TTR was defined as time from primary surgery to development of metastatic disease or censored at the last known date without metastases. To minimize immortal time bias, landmark analysis of TTR was performed starting at 2 mo after surgery. The time of 2 mo was chosen because the median time from surgery to AC initiation was 1.8 mo. Secondary endpoints were OS, incidence of AC use after NAC, and patterns of chemotherapy administration among the RISC centers, including NAC/AC regimen type used and the number of cycles received.
We employed descriptive statistics to characterize the study cohort overall and by chemotherapy regimen in terms of baseline clinicodemographic and surgical data. Pathological response rates were calculated with the denominator of all treated patients, including those who did not complete all NAC. The association between pathological stage and use of AC was assessed using Fisher’s exact test. TTR and OS were estimated by Kaplan-Meier method. OS was defined as the time from 2 mo after surgery to death or censored at the date last known to be alive. Associations between AC and TTR and OS were evaluated using a Cox regression model with univariate and multivariable analyses adjusted for pathological stage, age, and type of NAC. Types of NAC were grouped according to the receipt of cisplatin-based, carboplatin-based, or other regimens. Pathological stages was grouped according to the American Joint Committee on Cancer (AJCC) seventh edition into stage III (ypT3N0) and stage IV (ypN+/ypT4b) [18]. HRs for AC administration within stages were estimated by including an AC use-by-stage interaction in the model and using contrasts. A two-sided p value of ≤0.05 was considered statistically significant.
3. Results
Of the 474 patients who received NAC, 27.2% (N = 129) had adverse residual disease (ypT3/T4 and/or ypN+). Clinicodemographic baseline characteristics were balanced between both groups; only age was higher in the observation group (p = 0.03) and corrected for in the multivariate analyses (Table 1). Most patients had primary bladder tumors (96%). Upper urinary tract tumors were rare (renal pelvis 0.8%, ureter 0.8%) and unknown (2.3%). Most cancers were pure UC or mixed histologies with a UC component (87%). A minority of patients (16%) had cN+ and the majority of them (93%) had lymphadenectomy. The median numbers of lymph nodes resected were 19 for AC and 14 for no AC. Of the 129 patients with adverse residual disease, 17.8% (N = 23) received AC while 82.2% (N = 106) started surveillance. The specific AC regimen was not included in the multivariable analysis, given the small number of patients within each regimen.
Table 1 –
Clinicodemographic data
| Clinicodemographic | AC (N = 23) | Observation (N = 106) | p value | ||
|---|---|---|---|---|---|
|
|
|||||
| N | %, median (Q1, Q3) | N | %, median (Q1, Q3) | ||
| Age at diagnosis | 23 | 61 (51, 68) | 105 | 66 (59, 71) | 0.03 |
| Gender (male/female) | 17/6 | 74%/26% | 75/31 | 71%/29% | 0.20 |
| Smoking history | 0.95 | ||||
| Current | 4 | 17% | 18 | 17% | |
| Former | 9 | 39% | 45 | 42% | |
| Never | 10 | 43% | 40 | 38% | |
| Missing | 0 | 0% | 3 | 3% | |
| Performance status a | 0.84 | ||||
| 0–1 | 15 | 65% | 89 | 84% | |
| 2–3 | 0 | 0% | 2 | 2% | |
| Missing | 8 | 35% | 15 | 14% | |
| Charlson score a | 0.33 | ||||
| 0 | 9 | 39% | 51 | 48% | |
| 1 –2 | 8 | 35% | 26 | 25% | |
| 3–4 | 1 | 4% | 12 | 11% | |
| ≥5 | 5 | 22% | 14 | 14% | |
| Missing | 0 | 0% | 3 | 3% | |
| cT stage | 0.31 | ||||
| cT1 b | 0 | 0% | 4 | 4% | |
| cT2 | 16 | 70% | 60 | 57% | |
| cT3 | 3 | 13% | 33 | 31% | |
| cT4 | 2 | 9% | 8 | 8% | |
| Missing | 2 | 9% | 1 | 1% | |
| cN+ stage | 0.75 | ||||
| cN0 | 12 | 52% | 57 | 54% | |
| cN1 | 3 | 13% | 7 | 7% | |
| cN2 | 1 | 4% | 8 | 8% | |
| cN3 | 0 | 0% | 1 | 1% | |
| cNx | 7 | 30% | 33 | 31% | |
| Histology | 0.77 | ||||
| Urothelial c | 18 | 78% | 83 | 78% | |
| Squamous | 2 | 9% | 5 | 5% | |
| Small cell | 0 | 0 | 3 | 3% | |
| Adenocarcinoma | 0 | 0 | 2 | 2% | |
| Sarcomatoid | 0 | 0 | 1 | 1% | |
| Others/unknown | 3 | 13% | 12 | 11% | |
AC = adjuvant chemotherapy; cT = clinical tumoral stage; cN+ = clinical lymph nodes stage; Q = quartile.
Percentages may not add up to 100% due to rounding.
At the time of neoadjuvant chemotherapy initiation.
T1 disease was included only in case of concurrent lymph node positive disease.
Urothelial includes pure and mixed histologies with predominant urothelial component.
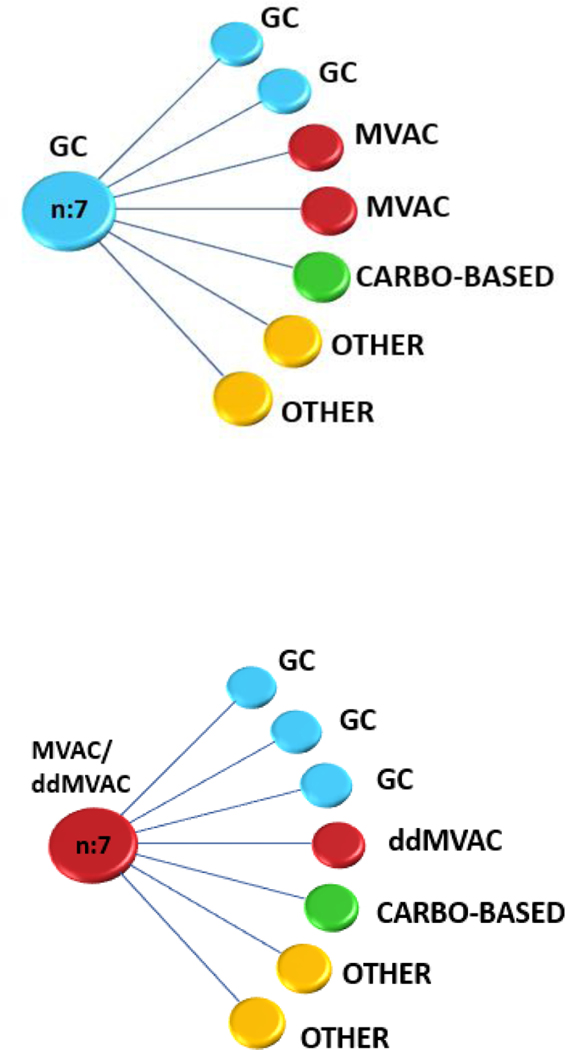
The AC group had a significantly higher proportion of stage IV patients (ypN+/ypT4b; AJCC seventh edition) compared with the observation group (83% vs 47%, p = 0.002; Table 2). There were similar rates of positive surgical margins (17% vs 16%) but a numerically higher rate of adjuvant radiation in the AC group compared with the observation cohort (17%, n = 4 vs 1%, n = 1). The most frequently used chemotherapy regimens in the neoadjuvant setting were GC (57.4%) and ddMVAC (21.2%). Carboplatin-based regimens (16%) and cisplatin monotherapy (2%) were administered less frequently. Of the 23 patients treated with NAC and AC, GC was the most frequent regimen employed in both settings (30.4%). Dose-dense MVAC was the second most frequently used NAC regimen (26.1%), but it was rarely employed in the adjuvant setting (4.3%). Carboplatin was generally a part of a triplet with gemcitabine and taxanes with a similar rate of usage in NAC and AC settings (21.7%). Most patients switched to a different AC regimen (82.6%, Fig. 1). Only four patients (17.3%) received the same agents for NAC and AC, all of whom had node positive disease at surgery. The median numbers of cycles administered were 3 for NAC and 4 for AC. The median time between NAC initiation and surgery was 3.4 mo (interquartile range [IQR] 2.8–4.4 mo) and 1.8 mo (IQR 1.6–2.5 mo) between tumor resection and AC initiation. No significant differences existed in the type of chemotherapy regimens (cisplatin based, non–cisplatin based, and other) administered from 1991 to 2003 compared with those administered from 2003 to 2015.
Table 2 –
Treatment characteristic and pathological stage after neoadjuvant chemotherapy
| Treatment data | AC (N = 23) | Observation (N = 106) | p value | ||
|---|---|---|---|---|---|
|
|
|||||
| N | %, median (range) | N | %, median (range) | ||
| NAC regimen | 0.45 | ||||
| GC | 7 | 30% | 50 | 47% | |
| ddMVAC | 6 | 26% | 15 | 14% | |
| MVAC | 1 | 4% | 5 | 5% | |
| CMV | 0 | 0% | 2 | 2% | |
| Cisplatin | 1 | 4% | 2 | 2% | |
| GCa | 0 | 0% | 10 | 9% | |
| GCaP | 4 | 17% | 6 | 6% | |
| MCaVi | 1 | 4% | 0 | 0% | |
| Other/unknown | 3 | 13% | 16 | 15% | |
| No. of NAC cycles | 22 | 3 (3–4) | 102 | 3 (3–4) | 0.87 |
| Pathological stage a | 0.002 | ||||
| III (ypT3a-b, ypT4a) | 4 | 17% | 56 | 53% | |
| IV(ypT4b, ypN+) | 19 | 83% | 50 | 47% | |
| Positive surgical margin | 4 | 17% | 17 | 16% | 0.75 |
| Adjuvant RT | 4 | 17% | 1 | 1% | 0.003 |
| AC regimen | |||||
| GC | 7 | 30% | NA | NA | NA |
| ddMVAC | 1 | 4% | |||
| MVAC | 3 | 13% | |||
| GCaP | 2 | 9% | |||
| CaP | 3 | 13% | |||
| Other/unknown | 7 | 30% | |||
| No. of AC cycles | 20 | 4 (3–4) | NA | NA | NA |
AC = adjuvant chemotherapy; AJCC = American Joint Committee on Cancer; CaP = carboplatin-paclitaxel; CMV = cisplatin-methotrexate-vinblastine; ddMVAC = dose-dense methotrexate-vinblastine-adriamycin-cisplatin; GC = gemcitabine-cisplatin; GCa = gemcitabine-carboplatin; GCaP = gemcitabine-carboplatin-paclitaxel; MCaVi = methotrexate-carboplatin-vinblastine; MVAC = methotrexate-vinblastine-adriamycin-cisplatin; NA = not applicable; NAC = neoadjuvant chemotherapy; RT = radiotherapy.
Percentages may not add up to 100% due to rounding.
Pathological stage classified following the AJCC seventh edition.
Fig. 1 –
Most frequently administered chemotherapeutic regimens in the neoadjuvant setting with the corresponding adjuvant therapy. Each line represents one patient’s pathway. Carbo = carboplatin; ddMVAC = dose-dense methotrexate-vinblastine-adriamycin-cisplatin; GC = gemcitabine-cisplatin; MVAC = methotrexate-vinblastine-adriamycin-cisplatin.
Median follow-up in the overall cohort from 2 mo after surgery was 30 mo (<1–108 mo), at which time >50% (76/129) of patients had relapsed. The median TTR was 16 mo. On univariate analysis, median TTR was numerically but not statistically significantly longer with the use of AC (18 vs 10 mo in the observation cohort; p = 0.06, HR 0.54; 95% CI: 0.29–1.03; Fig. 2A). Adjusting for pathological stage, type of NAC, and age, patients who received AC had a reduced risk of disease relapse (p = 0.01, HR 0.40; 95% CI: 0.21–0.82). In the subgroup analyses of patients with ypT4b and/or ypN+ disease, a significantly greater median TTR was observed in the AC group (20 vs 9 mo, HR 0.43; 95% CI: 0.21–0.89; Fig. 2B). This finding was confirmed on multivariable analysis (HR 0.30; 95% CI: 0.14–0.68) with the use of AC correlating with a 70% reduction in the risk of experiencing recurrence in this highest-risk cohort. Year of surgery was not significantly associated with TTR in the univariate analysis (p = 0.61). Median OS was 23 mo (<1–108 mo) with 67 deaths, of which 75% were related to metastatic UC. No difference in OS was observed between cohorts, with both surviving a median of 23 mo (HR 0.93; 95% CI: 0.52–1.70; p = 0.82; Fig. 3). Forty-nine patients (46%) received salvage chemotherapy in the observation group. The lack of survival difference persisted on multivariable analyses (HR 0.95; 95% CI: 0.50–1.81; p = 0.85), which incorporated NAC regimen type, pathological stage, and age. When excluding the pure variant histologies and assessing the urothelial cohort (AC: 18; observation: 83), median TTR was not reached with AC compared with TTR of 8.8 mo with observation. Median OS was 24.3 versus 27 mo. No differences in TTR or OS were found among the small subset of patients who received adjuvant radiotherapy (n = 4) versus no adjuvant radiation (n = 19), the same NAC and AC regimens (n = 4) versus different regimens (n = 19), or the AC regimen type classified as cisplatin based (n = 11) versus non–cisplatin based (n = 12).
Fig. 2 –
Time to relapse (TTR) in patients with adverse residual disease (ypT3-4 and/or ypN+) despite neoadjuvant chemotherapy based on adjuvant chemotherapy use. (A) Patients with ypT3-4 and/or ypN+ residual disease. (B) Patients with ypT4b and/or ypN+ residual disease. Kaplan-Meier curves represent crude survival estimates. AC = adjuvant chemotherapy; NAC = neoadjuvant chemotherapy; Obs = observation. a Multivariable analyses were adjusted for pathological stage (stage III and stage IV disease), type of NAC (cisplatin based, carboplatin based, and other regimens), and age.
Fig. 3 –
Overall survival (OS) by use of adjuvant chemotherapy. Kaplan-Meier curves represent crude survival estimates. AC = adjuvant chemotherapy; NAC = neoadjuvant chemotherapy; Obs = observation. a Multivariable analyses were adjusted for pathological stage (stage III and stage IV disease), type of NAC (cisplatin-based and carboplatin-based and other regimens), and age.
4. Discussion
There is level one evidence for cisplatin-based NAC in eligible patients with localized urothelial cancers [1], and achieving a major pathological response (<ypT2 and ypN0) to NAC portends improved survival [10,11]. A higher risk of recurrence is described among patients with residual disease despite NAC [2,3]. Little is known whether administering AC in these patients who do not achieve an ideal pathological response will improve outcomes.
We strove to retrospectively investigate the unresolved clinical question of whether administering AC in patients with adverse pathological features despite NAC improves clinical outcomes and assessed the field’s current patterns of care. Currently, there is no standard of care. No prospective randomized clinical trial has evaluated this question, and there have been conflicting reports from retrospective series that cumulatively encompass >700 patients [19–23]. Only one study showed a significant survival benefit with AC. Among 788 patients with adverse residual disease after NAC, 184 received additional AC. Median OS was significantly longer for the AC subgroup compared with the observation group (29.9 vs 24.2 mo, p = 0.046), and OS benefit decreased significantly with age (p = 0.02) [16]. However, detailed chemotherapy data on the type and number of cycles was not reported in the three largest studies [21–23]. Further, as there is no evidence to support that cisplatin monotherapy or noncisplatin regimens improve outcomes in the perioperative setting [24,25], incorporation of chemotherapy information is key when analyzing differences in TTR and OS, as imbalances may sway the results. Outside of our report, the type of chemotherapy administered has been described only in two small studies. No benefit in relapse-free or cancer-specific survival was observed in a retrospective analysis comparing 29 patients who received additional AC with 51 patients who received NAC only [20]. This study highlights the importance of understanding the potential impact of chemotherapy as 55% of the population received carboplatin-based AC, which may have influenced the results. The second study focused on the ypN+ population exclusively. Here, only 11 of the 37 patients received AC after NAC, mostly with cisplatin-based treatment (73%), and a significant benefit in relapse-free survival (13 vs 4.7 mo, p = 0.001) was described [19].
In the current study, we performed a subset analysis of the highest-risk patients (AJCC seventh edition) and observed an increase in median TTR with AC, suggesting that additional chemotherapy may delay or reduce the risk of recurrence in patients with adverse residual disease after NAC. However, we did not detect a significant difference of OS between the surveillance and AC groups. This lack of survival difference could have been confounded by the small number of patients who received AC, potential detrimental effects of AC, the possibility that salvage chemotherapy at the time of recurrence is just as effective, the low number of OS events, adjustment for multiple variables, inclusion of non-UC variants, and incomplete data on any differences in subsequent treatments (which may have differed between these groups and were prior to routine use of checkpoint blockade).
Our work is limited by its retrospective nature. The receipt of perioperative chemotherapy was not standardized, and reasons for treatment choice versus observation such as performance status, renal function, comorbidities, or physician belief in utility were not captured. AC patients were likely to be healthier patients who could tolerate further chemotherapy (healthy user bias). Additional biases may have been inflicted by inclusion of non–standard-of-care chemotherapy regimens (cisplatin monotherapy, carboplatin), non-UC histology, and upper urinary tumors. Acknowledging that these subgroups may have a different natural history that could have influenced the observed outcomes, they represented a minority of cases and were well balanced between cohorts. The AC group was composed of a greater number of patients with ypT4b/N+ disease, which likely drove the decision to administer AC and radiation. However, this higher percentage of high-risk disease should have biased the AC group to worse outcomes. Finally, although we reviewed the clinical outcomes of patients spanning 22 yr, there were no significant differences in outcomes in terms of chemotherapy regimen (cisplatin based, carboplatin based, or other) administered or year of surgery.
Acknowledging these limitations, this study represents the largest multicenter collaboration to incorporate specific information about chemotherapy regimens in terms of the type and duration of treatment. This international series underscores that the use of AC for adverse residual disease after NAC is not frequent in current clinical practice, and when AC is administered, generally a different regimen is preferred. Cisplatin-based chemotherapy, specifically GC, was most frequently used in the NAC and AC settings.
Given the proven efficacy of checkpoint immunotherapy in the metastatic setting [26–29], the question of whether to add AC may become moot. Currently, several phase 3 trials are testing adjuvant nivolumab (NCT02632409), atezolizumab (NCT02450331), and pembrolizumab (NCT03244384). These trials are specifically targeting patients at a high risk of recurrence, defined as pT3–4 and/or pN+ or adjuvant cisplatin ineligible if no prior NAC, or patients with significant residual disease after prior NAC (ypT2–4 and/or ypN+).
Identification of predictive biomarkers that move beyond pathological stage to personalize treatment is needed. Intrinsic molecular subtypes of urothelial cancer and genomic alterations have been identified as potential predictive biomarkers but require prospective validation [30]. A prospective study (SWOG 1314) is underway in the neoadjuvant setting to evaluate the ability of a gene-expression profiling algorithm (COXEN) to predict pathological responses to GC and ddMVAC (NCT02177695).
5. Conclusions
Interrogation of an international database of nonmetastatic urinary tumors treated with NAC suggests that AC administration after surgical resection might delay or reduce the risk of recurrence in patients with adverse amounts of residual disease, especially ypT4 and/or ypN+ disease. Given the hypothesis-generating nature of this work and lack of definite OS benefit, the consensus from the RISC group is to encourage participation in the ongoing adjuvant immunotherapy studies in patients with significant residual disease after platinum-based NAC and to develop other prospective studies that might definitely test this question.
The utilization of adjuvant chemotherapy after neoadjuvant chemotherapy in patients with high-risk residual disease is not frequent but might reduce the risk of recurrence. Participation in the ongoing adjuvant immunotherapy trials and development of other prospective studies are warranted to resolve this question.
Acknowledgments
Funding/Support and role of the sponsor: The work presented was supported in part by National Cancer Institute Cancer Center Support grant P30 CA008748.
Financial disclosures: Lauren C. Harshman certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: S.K. Pal: consulting—Genentech, Aveo, Eisai, Roche, Pfizer, Novartis, Exelixis, Ipsen, BMS, and Astellas; honoraria—Genentech. E.Y. Yu: consulting—Amgen, AstraZeneca, Bayer, EMD Serono, Incyte, Janssen, Merck, and Tolmar; research funding to institution—Bayer, Dendreon, Merck, and Seattle Genetics. S.J. Crabb: consulting/advisory—Roche, Clovis Oncology, Bayer, Janssen Cilag, and Merck; research support—AstraZeneca, Astex Pharmaceuticals, Plexxikon, and Clovis Oncology. J.E. Rosenberg: research funding—Roche/Genetech; consulting— Roche/Genentech, Merck, BMS, and AstraZeneca; speaker fee—Chugai. S.A. Hussain: advisory board—Roche, Merck, Janssen, Astellas, Astra Zeneca, Pierre-Febre, Bayer, Ipsen, BMS, Sotio, Pfizer, and Pierre-Fabre; research funding to institution—Janssen, Bayer, Boehringer Ingelheim, Pierre-Fabre, and Eli Lilly. M.I. Milowsky: consulting—BioClin Therapeutics. N. Agarwal: consultancy: Pfizer, Novartis, Merck, Genentech, Eisai, Exelixis, Clovis, EMD Serono, BMS, Astra Zeneca, and Astellas. C.N. Sternberg: honoraria—Lilly; consultancy—BMS, Merck, Clovis, and Janssen. J. Bellmunt: advisory—Bayer, Genentech, Pfizer, Genentech, Merck, and Exelixis; research to the institution—Bayer, Merck, Takeda, and Pfizer. M.D. Galsky: advisory—BMS, Pfizer, Genentech, and Merck. L.C. Harshman: advisory—Bayer, Genentech, Dendreon, Pfizer, Medivation/Astellas, Kew Group, Theragene, Corvus, Merck, Exelixis, and Novartis; research to the institution—Bayer, Sotio, Bristol-Myers Squib, Merck, Takeda, Dendreon/Valient, Janssen, Medivation/Astellas, Genentech, and Pfizer.
Footnotes
Study concept and design: Harshman, Galsky.
Acquisition of data: All investigators.
Analysis and interpretation of data: All investigators.
Drafting of the manuscript: Chanza, Harshman.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Werner.
Obtaining funding: None.
Administrative, technical, or material support: Harshman, Galsky.
Supervision: Harshman.
Other: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Flaig TW, Spiess PE, Agarwal N, et al. NCCN guidelines insights: bladder cancer version 5. J Natl Compr Canc Netw 2018;16:1041–53. [DOI] [PubMed] [Google Scholar]
- [2].Advanced Bladder Cancer (ABC) Meta-analysis Collaboration. Neoadjuvant chemotherapy in invasive bladder cancer: Update of a systematic review and meta-analysis of individual patient data advanced bladder cancer (ABC) meta-analysis collaboration. Eur Urol 2005;48:202–5; discussion 205–6. [DOI] [PubMed] [Google Scholar]
- [3].International Collaboration of Trialists, Medical Research Council Advanced Bladder Cancer Working Party (now the National Cancer Research Institute Bladder Cancer Clinical Studies Group), European Organization for Research and Treatment of Cancer Genito-Urinary Tract Cancer Group, et al. International phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: long-term results of the BA06 30894 trial. J Clin Oncol 2011;29:2171–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Choueiri TK, Jacobus S, Bellmunt J, et al. Neoadjuvant dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin with pegfilgrastim support in muscle-invasive urothelial cancer: pathologic, radiologic, and biomarker correlates. J Clin Oncol 2014;32:1889–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Plimack ER, Hoffman-Censits JH, Viterbo R, et al. Accelerated methotrexate, vinblastine, doxorubicin, and cisplatin is safe, effective, and efficient neoadjuvant treatment for muscle-invasive bladder cancer: Results of a multicenter phase II study with molecular correlates of response and toxicity. J Clin Oncol 2014;32:1895–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Galsky MD, Pal SK, Chowdhury S, et al. Comparative effectiveness of gemcitabine plus cisplatin versus methotrexate, vinblastine, doxorubicin, plus cisplatin as neoadjuvant therapy for muscle-invasive bladder cancer. Cancer 2015;121:2586–93. [DOI] [PubMed] [Google Scholar]
- [7].Pal SK, Ruel NH, Wilson TG, Yuh BE. Retrospective analysis of clinical outcomes with neoadjuvant cisplatin-based regimens for muscle-invasive bladder cancer. Clin Genitourin Cancer 2012;10:246–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lee FC, Harris W, Cheng HH, et al. Pathologic response rates of gemcitabine/cisplatin versus methotrexate/vinblastine/adriamycin/cisplatin neoadjuvant chemotherapy for muscle invasive urothelial bladder cancer. Adv Urol 2013;2013:317190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Zargar H, Shah JB, Van Rhijn BW, et al. Neoadjuvant dose dense MVAC versus GC in patients with cT3–4aN0M0 bladder cancer treated with radical cystectomy. J Urol 2018;199:1452–8. [DOI] [PubMed] [Google Scholar]
- [10].Zargar H, Zargar-Shoshtari K, Lotan Y, et al. Final pathological stage after neoadjuvant chemotherapy and radical cystectomy for bladder cancer—does pT0 predict better survival than pTa/Tis/T1? J Urol 2016;195(4 Pt 1):886–93. [DOI] [PubMed] [Google Scholar]
- [11].Sonpavde G, Goldman BH, Speights VO, et al. Quality of pathologic response and surgery correlate with survival for patients with completely resected bladder cancer after neoadjuvant chemotherapy. Cancer 2009;115:4104–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Leow JJ, Martin-Doyle W, Rajagopal PS, et al. Adjuvant chemotherapy for invasive bladder cancer: a 2013 updated systematic review and meta-analysis of randomized trials. Eur Urol 2014;66:42–54. [DOI] [PubMed] [Google Scholar]
- [13].Ruggeri EM, Giannarelli D, Bria E, et al. Adjuvant chemotherapy in muscle-invasive bladder carcinoma: a pooled analysis from phase III studies. Cancer 2006;106:783–8. [DOI] [PubMed] [Google Scholar]
- [14].Galsky MD, Stensland KD, Moshier E, et al. Effectiveness of adjuvant chemotherapy for locally advanced bladder cancer. J Clin Oncol 2016;34:825–32. [DOI] [PubMed] [Google Scholar]
- [15].Sternberg CN, Skoneczna I, Kerst JM, et al. Immediate versus deferred chemotherapy after radical cystectomy in patients with pT3-pT4 or N+ M0 urothelial carcinoma of the bladder (EORTC 30994): an intergroup, open-label, randomized phase 3 trial. Lancet Oncol 2015;16:76–86. [DOI] [PubMed] [Google Scholar]
- [16].Birtle AJ, Chester JD, Jones RJ, et al. Results of POUT: a phase III randomized trial of perioperative chemotherapy versus surveillance in upper tract urothelial cancer (UTUC). J Clin Oncol 2018;36(6_suppl):407. [Google Scholar]
- [17].Seisen T, Krasnow RE, Bellmunt J, et al. Effectiveness of adjuvant chemotherapy after radical nephroureterectomy for locally advanced and/or positive regional lymph node upper tract urothelial carcinoma. J Clin Oncol 2017;35:852–60. [DOI] [PubMed] [Google Scholar]
- [18].American Joint Committee on Cancer. AJCC cancer staging manual. Urinary bladder. ed. 7. New York, NY: Springer; 2010. p. 497–502. [Google Scholar]
- [19].Kassouf W, Agarwal PK, Grossman HB, et al. Outcome of patients with bladder cancer with pN+ disease after preoperative chemotherapy and radical cystectomy. Urology 2009;73:147–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Zargar-Shoshtari K, Kongnyuy M, Sharma P, et al. Clinical role of additional adjuvant chemotherapy in patients with locally advanced urothelial carcinoma following neoadjuvant chemotherapy and cystectomy. World J Urol 2016;34:1567–73. [DOI] [PubMed] [Google Scholar]
- [21].Parker WP, Habermann EB, Day CN, et al. Adverse pathology after neoadjuvant chemotherapy and radical cystectomy: the role of adjuvant chemotherapy. Clin Genitourin Cancer. In press. 10.1016/j.clgc.2017.07.010 [DOI] [PubMed] [Google Scholar]
- [22].Seisen T, Jamzadeh A, Leow JJ, et al. Adjuvant chemotherapy vs observation for patients with adverse pathologic features at radical cystectomy previously treated with neoadjuvant chemotherapy. JAMA Oncol 2018;4:225–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Sui W, Lim EA, Joel Decastro G, Mckiernan JM, Anderson CB. Use of adjuvant chemotherapy in patients with advanced bladder cancer after neoadjuvant chemotherapy. Bladder Cancer 2017;3:181–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Mertens LS, Meijer RP, Kerst JM, et al. Carboplatin based induction chemotherapy for non-organ confined bladder cancer—a reasonable alternative for cisplatin unfit patients? J Urol 2012;188:1108–13. [DOI] [PubMed] [Google Scholar]
- [25].Murasawa H, Koie T, Ohyama C, et al. The utility of neoadjuvant gemcitabine plus carboplatin followed by immediate radical cystectomy in patients with muscle-invasive bladder cancer who are ineligible for cisplatin-based chemotherapy. Int J Clin Oncol 2017;22:159–65. [DOI] [PubMed] [Google Scholar]
- [26].Apolo AB, Infante JR, Balmanoukian A, et al. Avelumab, an anti- programmed death ligand 1 antibody, in patients with refractory metastatic urothelial carcinoma: results from a multicenter, phase Ib study. J Clin Oncol 2017;35:2117–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Bellmunt J, de Wit R, Vaughn DJ, et al. Pembrolizumab as second- line therapy for advanced urothelial carcinoma. N Engl J Med 2017;376:1015–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Sharma P, Callahan MK, Bono P, et al. Nivolumab monotherapy in recurrent metastatic urothelial carcinoma (CheckMate 032): a multi-center, open-label, two-stage, multi-arm, phase1/2 trial. Lancet Oncol 2016;17:1590–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Rosenberg JE, Hoffman-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicenter, phase 2 trial. Lancet 2016;387:1909–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Seiler R, Ashab HAD, Erho N, et al. Impact of molecular subtypes in muscle-invasive bladder cancer on predicting response and survival after neoadjuvant chemotherapy. Eur Urol 2017;72:544–54. [DOI] [PubMed] [Google Scholar]