Abstract
Background
There is significant interest in determining risk factors in individuals at risk of rheumatoid arthritis (RA). A core set of risk factors for clinical arthritis development has not been defined.
Methods
A literature search and systematic literature review (SLR) was conducted to identify risk factors in individuals at risk of RA using Medline, Embase, PubMed and Central databases.
Results
3854 articles were identified by the literature search. After screening of titles, 138 abstracts were reviewed and 96 articles finally included. Fifty-three articles included data on risk factors including autoantibodies, subclinical inflammation on imaging, clinical features, serum and cellular biomarkers and genetic markers. Risk factors were dependent on the at-risk population. There was good evidence for serum anticitrullinated protein antibodies (ACPA) levels, as risk factors for arthritis in all at-risk populations (n=13 articles). Subclinical inflammation on ultrasound (n=12) and MRI (n=6) was reported as a risk factor in multiple studies in at-risk individuals with musculoskeletal (MSK) symptoms and undifferentiated arthritis (UA). Clinical features were reported as a risk factor in at-risk individuals with MSK symptoms and UA (n=13). Other risk factors, including serum and cellular markers were less frequently reported.
Conclusions
Risk factors for arthritis development in RA are specific to the at-risk population. Serum ACPA confers risk in all populations; subclinical inflammation on imaging and clinical features confer risk in at-risk individuals with MSK symptoms. This SLR informed the EULAR taskforce for points to consider on conducting clinical trials and studies in individuals at risk of RA.
Keywords: arthritis, rheumatoid, autoimmune diseases, autoimmunity
Key messages.
Risk factors for arthritis development in rheumatoid arthritis (RA) are specific to the at-risk population.
Serum anticitrullinated protein antibodies (ACPA) confer risk of RA in both asymptomatic and symptomatic at-risk populations.
Serum ACPA, clinical features and subclinical inflammation on imaging should be considered as ‘core risk factors’ in individuals at risk of RA who have symptoms without clinical arthritis.
Introduction
Furthering our understanding of the preclinical phase of rheumatoid arthritis (RA) is likely to hold the key to disease prevention. The identification, follow-up and scrutiny of individuals at risk of RA is a central part of this approach. At-risk populations have been identified based on the presence of a few well-recognised risk factors for the development of RA. These include a family history of RA, the presence of anticitrullinated protein antibodies (ACPA) and certain musculoskeletal (MSK) symptoms. Within the different at-risk populations, data on several other risk factors have also been reported. These include different RA-related autoantibodies, imaging biomarkers, various clinical features and serological markers. Typically the risk factors collected vary in different observational studies and clinical trials. Consequently, the relative importance of these risk factors in at-risk populations can be difficult to interpret. There is also variation in which risk factors are used to select at-risk individuals for clinical trials.
The EULAR task force for conducting clinical trials and studies in individuals at risk of RA was convened to help align future work in this area through the provision of data-driven guidance and consensus. The task force agreed that population-specific core sets of risk factors should be stipulated for inclusion in future observational studies an clinical trials. The task force also felt that the frequency at which risk factor assessment should be repeated was an important question to be addressed. When defining the points to consider, participants of the EULAR task force were guided by the findings of this systematic literature review.
Methods
An international multidisciplinary EULAR task force was convened to define points to consider for conducting clinical trials and studies in individuals at risk of RA (co-convened by KM and PE). At the first meeting (October 2019 in Amsterdam, The Netherlands), the task force agreed on four key questions to be addressed by systematic literature reviews (SLR). A key question agreed and prioritised by the task force was: ‘In individuals at risk of rheumatoid arthritis (RA), is there core set of risk factors and how frequently should they be measured?’ The results of the corresponding SLR are presented in the current manuscript. Three other questions were also proposed and are addressed in the EULAR points to consider. However, the SLR on risk factors was deemed the most novel and contained the most data. The task force agreed that this SLR should be submitted for independent publication.
Study protocol
A literature search was carried out by two of the authors (KM and HS) and the expert health librarian (JK), following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.1 For the literature search, relevant keywords determined by the librarian were used in Medline, Embase, PubMed and Central databases (searched from 1944 to October 2019); see online supplemental materials for full search strategy.
rmdopen-2021-001768supp001.pdf (122.9KB, pdf)
Inclusion criteria
We included studies, which demonstrated risk factors for arthritis development in individuals at risk of RA. Studies identifying risk factors in the following at-risk populations were eligible for inclusion: first-degree relatives (FDRs) of RA probands, indigenous North American populations, ACPA-positive individuals with/without MSK symptoms, seropositive arthralgia, clinically suspect arthralgia, undifferentiated arthritis (UA), palindromic rheumatism (PR). Abstracts published from January 2018 onwards were included. This was to ensure important recent data, yet to be published in full articles, were also included. Articles not published in English were excluded. Duplicates were excluded. Meta-analyses were included but all other reviews and study protocols were excluded. Manually searched articles either from the references of selected manuscripts or identified by task force members could also be included.
Study selection
Studies retrieved from the searches were recorded on a central database. After removing conference abstracts (pre-2018), two investigators (KM and HS) independently screened all titles. Abstracts of titles identified as potentially eligible for inclusion were then independently assessed against the inclusion and exclusion criteria by the two investigators. Disagreements were settled by discussion between the two investigators and through discussion with a third investigator (ADM) where required. Discussions were held with the expert EULAR task force members to ensure additional relevant articles could also be identified (‘hand searched’).
Quality assessment
Quality assessment of studies was performed using the Newcastle-Ottowa Scale (NOS) for assessing the quality of non-randomised studies in meta-analysis.2 This was conducted by two of the investigators independently (ADM and DA-R). Any disagreement between the two investigators was resolved by a third independent investigator (KM). The NOS scores studies according to three items: selection, comparability and outcome. The final score (range 0–9) is a sum of the item scoring. The higher the score, the better the methodological quality and the lower risk of bias (RoB); studies with ≥6 stars were considered low RoB, those with 4 or 5 stars intermediate RoB and those with <4 stars at high RoB.
Results
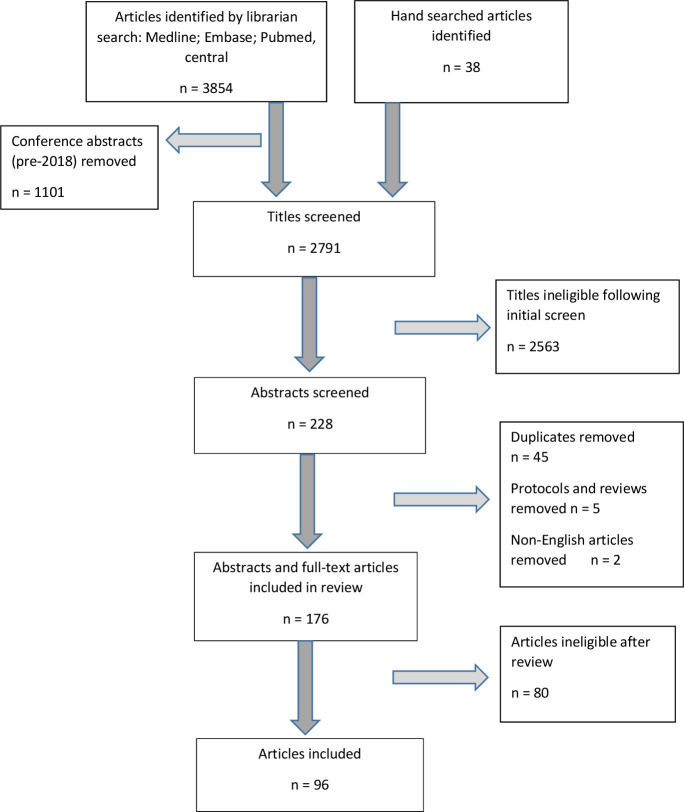
A total of 3854 articles were identified by the librarian search. A further 38 articles were identified by hand searches. No specific pattern was identified by the health librarian to explain why these articles were missed by the searches. After screening of titles, 176 abstracts and/or articles were reviewed and 96 articles were evaluated in detail (figure 1).
Figure 1.
Flow chart for article selection according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.
Articles identifying risk factors for the development of RA fell into one of two categories: (1) risk factors identified in prospective cohorts of individuals at risk of developing RA, or (2) risk factors for development of RA in the general population using retrospective (predominantly case-control) studies. As the research question specifies risk factors in at-risk individuals, rather than the background population, articles which were in the first category were reviewed in detail.
Of the 96 articles evaluated in detail, 53 studies identifying risk factors in at-risk individuals were grouped according to the specific risk factor(s) identified in the study (table 1). All but two of the articles assessed were of high quality, that is, low RoB according to the NOS score (see online supplemental tables 1–4). Seven articles were abstracts or letters and two were meta-analyses. These studies could not be assessed for RoB.
Table 1.
Research articles identified by the literature search according to the different at-risk populations
| Risk factor | At-risk population | Total | ||
| Asymptomatic* | MSK symptoms without clinical arthritis | UA/PR | ||
| Subclinical inflammation on ultrasound | 0 | 8 | 4 | 12 |
| Subclinical inflammation on MRI | 0 | 4 | 2 | 6 |
| BMD | 0 | 1 | 1 | 2 |
| ACPA/RF/Other autoantibodies | 6 | 4 | 3 | 13 |
| Clinical features | 0 | 4 | 9 | 13 |
| Demographic features | 0 | 1 | 0 | 1 |
| Serum markers | 1 | 2 | 1 | 4 |
| Cellular markers | 0 | 2 | 0 | 2 |
| Genetic markers | 0 | 1 | 1 | 2 |
| Total | 7 | 27 | 21 | 53 |
Fifty-three articles were identified: one article reported on both clinical and genetic risk factors; one article reported on both autoantibodies and serum markers.
*Including relatives of patients with RA.
ACPA, anticitrullinated protein antibodies; BMD, bone mineral density; MSK, musculoskeletal; PR, palindromic rheumatism; RA, rheumatoid arthritis; RF, rheumatoid factor; UA, undifferentiated arthritis.
In these studies, risk factors are usually considered in a population-specific manner; for example, risk factors such as clinical features and subclinical inflammation on imaging are relevant in at-risk individuals with MSK symptoms but not in asymptomatic populations such as FDRs of RA probands.
ACPA and other autoantibodies
Thirteen articles specifically addressed ACPA and other autoantibodies as risk factors for arthritis development; 10/13 had a low RoB and 2/13 had intermediate RoB. One article was a systematic review and meta-analysis.
Presence of RA-related autoantibodies, especially ACPAs, is the best characterised risk factor for arthritis development across the various at-risk populations, from those without symptoms through to those with early synovitis (table 2). Indeed, serum ACPAs were identified prior to the onset of arthritis in multiple seminal studies in this field.3–5 Considering asymptomatic populations (ie, without MSK symptoms), ACPA-positive individuals may be identified by screening in the general population or testing individuals with a heightened genetic risk of RA, that is, relatives of RA probands or Indigenous North American (INA) populations. There is good evidence that the latter groups are at higher risk of RA development, whereas there are relatively few published longitudinal data on arthritis development in ACPA-positive individuals screened from the general population. In a large Mexican cohort study, 819 healthy relatives (79% FDRs) of RA probands were followed prospectively for 5 years to investigate for RA development.6 RA developed in 17 (2.1%) of the relatives, with a positive predictive value (PPV) of 64% when both anti-CCP and rheumatoid factor (RF) were present, and 58% when only anti-CCP was positive.6 In a recent longitudinal study of healthy relatives of INAs with RA, in which autoantibody levels were serially measured, 4.8% developed inflammatory arthritis (IA) during follow-up. All were seropositive at IA onset. Thirty per cent of anti-CCP/RF double positive individuals developed IA. Interestingly, there was a 71% likelihood of an ACPA-positive state reverting to seronegative after 5 years.7 Furthermore, glycosylation of the IgG ACPA variable domain is present in FDRs of INAs with RA and is strongly associated with the future development of RA (HR 6.1, 95% CI 1.5 to 25.2, p=0.01).8 Prior to the availability of ACPA testing, a large longitudinal study of >2000 INAs monitored bi-annually for 19 years revealed a highly significant association between RF level and RA development (p<0.01, controlling for age and sex).9 In a recent study of anti-CCP3-positive individuals identified by population screening at health fairs in the USA, 35 of 250 individuals who tested anti-CCP3 positive were recruited and followed longitudinally for mean of 2.56 years.10 Forty per cent of these subjects developed IA.10
Table 2.
Articles reporting on RA-related autoantibodies as isolated risk factors in individuals at risk of RA
| Study | Population studied | Cohort size | Risk factor | Frequency assessed | Main outcome |
| Asymptomatic | |||||
| del Puente et al9 | Healthy NAN | 2712 | RF | Multiple: bi-annually |
RA development Incidence of RA (cases per 1000 person-years) increased according to RF titre (p<0.001): cases per 1000 person-years=2.4 (RF titre <1:2); 6.7 (titre 1:2–1:16); 11.0 (titre 1:32–1:256); 48.3 (titre >1:256) |
| Ramos-Remus et al6 | FDRs and relatives of RA probands | 819 relatives of 252 RA probands | Relatives of RA probands | Once (BL) | Development of RA All relatives Anti-CCP2+/RF+ versus seronegative: HR 52.5 (95% CI 19.1 to 144.1), p<0.01 Offspring versus other relatives HR 3.1 (95% CI 1.2 to 8.1) |
| Gan et al52 | Anti-CCP3-positive individuals screened from the general population | 35 | Anti-CCP3 | Once (BL) | Development of IA Anti-CCP3: 14/35 (40%) of anti-CCP3+ individuals developed IA at 2.56 years follow-up |
| Verheul et al60 | Pre-RA individuals, FDRs | 379 (pre-RA) 246 (FDR) |
Anti-CCP RF Anti-CarP |
Meta-analysis | Development of RA Number of different autoantibodies present: one antibody: OR 12 (95% CI 6 to 21) two antibodies: OR 30 (95% CI 11 to 83) three antibodies: OR 112 (95% CI 6 to 2122) |
| Tanner et al7 | Unaffected relatives of NAN RA-probands in Canada and Alaska | 374 | ACPA RF |
Multiple: BL and annually |
Development of IA Cases of IA per 1000 person-years: ACPA+/RF+ 97.1 ACPA−/RF+ 7.2 ACPA+/RF− 36.4 |
| Hafkenscheid et al8 | ACPA-positive FDRs of patients with RA, in NANs | 126 | IgG ACPA V‐domain glycosylation | Development of RA IgG ACPA variable‐domain glycosylation: HR=6.07 (95% CI 1.46 to 25.2, p=0.013) |
|
| MSK symptoms without arthritis | |||||
| Bos et al11 | Seropositive arthralgia (ACPA and/or RF) | 147 (50 ACPA+, 52 RF+, 45 ACPA+/RF+) |
Anti-CCP2 IgM RF |
Once (BL) | Development of IA Compared with RF: anti-CCP: HR 6.0 (95% CI 1.8 to 19.8); p=0.004 In anti-CCP+ only: anti-CCP+/IgM RF+: HR 3.0 (95% CI 1.4 to 6.9), p=0.01. High anti-CCP level: HR 1.7; (95% CI 1.1 to 2.5), p=0.008 |
| van de Stadt et al12 | Seropositive arthralgia (ACPA and/or RF) | 244 | Reactivity to five citrullinated peptides: cFib1 cFib2 cFib3 enolase vimentin |
Once (BL) | Development of IA Recognition of 2–5 additional citrullinated peptides: HR 1.7 (95% CI 0.93 to 3.16), p=0.08 |
| Shi et al16 | Seropositive arthralgia (anti-CCP2 and/or RF) | 340 | Anti-CarP Ab | Once (BL) | Development of IA Anti-CarP antibodies: HR=1.56 (95% CI 1.06 to 2.29), p=0.003. In anti-CCP+ subgroup only: anti-CarP antibodies: HR=2.23 (95% CI 1.31 to 3.79), p=0.003 |
| Ten Brinck et al13 | CSA | 241 | ACPA, RF and anti-CarP Ab | Once (BL) | Development of IA Anti-CCP2: HR=5.1 (95% CI 2.0 to 13.2) RF: 2.0 (95% CI 0.81 to 4.9) Anti-CarP: 1.04 (95% CI 0.46 to 2.4) |
| Early clinical arthritis | |||||
| Kudo-Tanaka et al17 | Recent-onset (<2 years) UA (arthritis in ≥2 joints not meeting RA or other classification criteria) | 146 | Anti-CCP IgM RF MMP3 CARF |
Once (BL) | RA development (PPV, NPV and diagnostic accuracy) anti-CCP2: 65.2%, 97.2%, 91.7%, respectively; IgM RF: 34.1%, 95.6%, 76.3%, respectively; CARF: 30.2%, 97.5%, 70.5%, respectively; MMP3: 25.0%, 91.5%, 69.0%, respectively |
| van der Linden et al19 | Early (<2 years) UA | 625 | Anti-CCP2 Anti-CCP3 Anti-MCV |
Once (BL) | RA development (PPV) Anti-CCP2: 67.1% Anti-CCP3: 64% Anti-MCV: 56.3% |
| Mjaavatten et al18 | UA (≥1 clinically swollen joint of ≤16 weeks duration) | 376 | Anti-CCP2 IgM RF Anti-CCP2+/IgM RF+ |
Once (BL) | Development of persistent IA Anti-CCP: OR 3.567 (95% CI 1.572 to 8.094) IgM RF: OR 2.716 (95% CI 1.189 to 6.202) Anti-CCP+/RF+: OR 7.948 (95% CI 2.659 to 23.752) |
HR/OR and 95% CIs have been reported where available.
+, positive; Ab, antibodies; ACPA, anticyclic citrullinated peptide antibodies; BL, baseline; CARF, anti-agalactosyl IgG antibodies; CarP, carbamilated protein; CCP, cyclic citrullinated peptide; CCP2, second-generation anti-CCP Ab; CCP3, third-generation anti-CCP Ab; CSA, clinically suspect arthralgia; FDRs, first-degree relatives; IA, inflammatory arthritis; MCV, mutated citrullinated vimentin; MMP-3, matrix metalloproteinase 3; NAN, native American nations; NPV, negative predictive value; PPV, positive predictive value; RA, rheumatoid arthritis; RF, rheumatoid factor; UA, undifferentiated arthritis.
In at-risk individuals with MSK symptoms (seropositive arthralgia, clinically suspect arthralgia, ACPA positive with MSK symptoms), ACPA levels are also strongly associated with progression to IA. In a Dutch seropositive (anti-CCP+ and/or RF+) arthralgia cohort, positive anti-CCP antibodies were associated with progression to IA (HR 6.0, 95% CI 1.8 to 19.8, p<0.01). Within anti-CCP-positive subjects, a high level of anti-CCP antibodies and/or the additional presence of IgM RF were further associated with progression to IA (HR 1.7, 95% CI 1.1 to 2.5, p<0.01 and HR 3.0, 95% CI 1.4 to 6.9, p=0.01, respectively).11 In a subsequent study from the same group, higher anti-CCP levels were associated with a broader ACPA repertoire; patients recognising ≥2 of 5 candidate ACPAs were at higher risk of IA development (p=0.04).12 In the Dutch clinically suspect arthralgia (CSA) cohort, where subjects are recruited based on their symptoms alone rather than serological status, anti-CCP positivity, but not RF or anti-carbamylated protein antibodies (anti-CarP), was associated with development of IA (HR 5.1) in the multivariable analysis. However, in addition to anti-CCP positivity, lone RF positivity was also associated with IA development compared with seronegative individuals (HR 2.6).13 High anti-CCP level was also the strongest risk factor in two clinical prediction rules in Dutch seropositive patients with arthralgia14 and the Leeds cohort of ACPA-positive individuals with non-specific MSK symptoms.15 One study has demonstrated a positive association between serum anti-CarP antibodies and IA development in a cohort of anti-CCP/RF-positive patients with arthralgia (HR 1.6, p=0.02).16 This association remained positive in the anti-CCP-positive subgroup (OR 2.2, p<0.01).
In patients with early UA, anti-CCP antibodies are also a strong risk factor for disease progression to RA.17–19 Anti-CCP2 antibodies were the strongest predictive biomarker for progression to RA in a Japanese cohort of patients with UA, with other diagnostic biomarkers (including IgM RF and C reactive protein) adding minimal additional value.17 Anti-CCP2 antibodies were compared with anti-CCP3, RF and antimutated citrullinated vimentin (MCV) antibodies in a prospective study of 625 patients with UA.19 Overall, anti-CCP2 antibodies had the highest PPV for RA development (67.1%), followed by anti-CCP3 (64%), RF (61.7%) and anti-MCV (56.3%).
In 376 Norwegian early arthritis clinic patients, the association between anti-CCP and RF levels and arthritis persistence was tested.18 Almost 50% of these patients had persistent arthritis at 1 year. Sensitivity/Specificity for persistent arthritis was 28%/95% for RF alone, 30%/95% for anti-CCP alone and 37%/92% for both autoantibodies. Likelihood of persistent arthritis increased with increasing levels of both RF and anti-CCP.18
One important caveat when interpreting these studies is that patients were classified as UA based on failure to meet now outdated RA classification criteria. It is therefore likely that a significant proportion of these patients with UA would actually classify as RA based on contemporary criteria.20
Clinical features
Thirteen articles addressed clinical features as risk factors for arthritis development; 11/13 had a low RoB. Of the two articles without low RoB, one article was an abstract and one was a systematic review and meta-analysis.
Clinical features have not been well characterised in at-risk populations who do not have MSK symptoms (ie, FDRs and INA populations). A range of clinical features related to the MSK system have been characterised in ACPA-positive individuals with MSK symptoms, patients with seropositive arthralgia, patients with CSA and patients with early arthritis (ie, UA and PR) (table 3). Whether non-MSK symptoms, for example, fatigue, are relevant to disease progression will be an important area for future research. Composite risk prediction tools incorporating multiple clinical features have been reported for seropositive arthralgia14 and ACPA-positive individuals with MSK symptoms.15 The Dutch risk prediction tool was based on an analysis of 374 patients with seropositive arthralgia, 254 (68%) of whom were anti-CCP positive. One hundred thirty-one (35%) patients developed arthritis after a median of 12 months. In the multivariate Cox regression analysis, six clinical MSK features were associated with arthritis development: duration of symptoms <12 months, intermittent symptoms, location of symptoms in the upper and lower extremities, visual analogue scale (VAS) pain ≥50, early morning stiffness (EMS) ≥1 hour, swollen joints reported by the patient. Of these, VAS pain ≥50 had the strongest association with progression (HR 2.3) and was given two points in the risk score while all other clinical features were given one point.14 A subsequent UK risk prediction tool (Leeds CCP cohort) was based on a cohort of 100 anti-CCP-positive individuals with non-specific MSK symptoms, of whom 50 developed arthritis after a median of 7.9 months.15 In this study, only two clinical features were associated with arthritis development, tenderness of small joints and EMS (≥30 min and ≥60 min). Pain VAS ≥50, intermittent symptoms and symptoms in the upper and lower extremities were not predictive.15 In both risk prediction tools, autoantibody status had a far stronger association with arthritis development than any clinical feature.14 15 The EULAR definition of arthralgia suspicious for progression to RA (ie, the accepted case definition of CSA) is a set of six clinical features (and one genetic factor) deemed by rheumatologists to represent an increased risk for arthritis development.21 These include symptoms duration <1 year, metacarpophalangeal (MCP) joint symptoms, EMS ≥60 min, most severe symptoms in morning, difficulty making a fist, positive MCPJ squeeze test. This definition was subsequently validated in two independent cohorts of patients with CSA from Leiden (The Netherlands) and Umea (Sweden).22 Recruitment into these cohorts preceded the EULAR definition. Individuals meeting criteria for a positive definition of CSA (≥3 of the above listed factors present) had an increased risk of developing arthritis compared with definition-negative patients (HR 2.1, 95% CI 0.9 to 4.7), although statistical significance was not reported. The sensitivity was 84% and the PPV was 30%.22
Table 3.
Articles reporting clinical features alone, or as part of a risk prediction tool, as risk factors in individuals at risk of RA
| Study | Population | Cohort size | Risk factor | Frequency | Part of composite risk prediction tool? | Outcome |
| MSK symptoms without arthritis | ||||||
| van de Stadt et al14 | Seropositive arthralgia (ACPA and/or RF) | 374 | Symptoms duration <12M Intermittent symptoms Symptoms in upper and lower extremities VAS pain ≥50 EMS >60 min Swollen joints reported |
BL | Y |
Development of IA Symptoms duration <12M: HR=1.72 (95% CI 1.39 to 2.15) Intermittent symptoms: HR=1.75 (95% CI 1.41 to 2.16) Symptoms upper and lower extremities: HR=1.37 (95% CI 1.12 to 1.68) VAS pain ≥50: HR=2.29 (95% CI 1.85 to 2.85) EMS >60 min: HR=1.67 (95% CI 1.29 to 2.15) Swollen joints reported: HR=1.78 (95% CI 1.46 to 2.20) |
| Rakieh et al15 | Anti-CCP+ individuals with non-specific MSK symptoms | 100 | EMS ≥30 min | BL | Y |
Development of IA HR=1.85 (95% CI 1.02 to 3.35), p=0.043 |
| Burgers et al22 | CSA | 354 (2 cohorts) | Positive definition of CSA (EULAR definition for suspicious arthralgia) ≥3 parameters present | BL | Y |
Development of IA HR=2.1 (95% CI 0.9 to 4.7) |
| Nakajima et al61 | ACPA+ individuals without clinical synovitis | 18 | Tenderness of DAS-28 subject joints at the first visit | BL | N |
Development of IA Progressors versus non-progressors: tenderness present in 10/10 patients vs 2/8 patients (p=0.0044) |
| Early clinical arthritis | ||||||
| Gonzalez-Lopez et al31 | PR—Gonzalez Lopez criteria | 127 | Frequency of PR attacks | BL | Y | Development of a chronic connective tissue disease HR 1.03 (95% CI 1.01 to 1.05), p=0.03 |
| El Miedany et al24 | UA—synovitis >2 joints for ≤6 months | 173 | EMS duration | BL | Y | Development of persistent arthritis OR 1.15 (95% CI 1.094 to 1.222), p<0.001 |
| Kuriya et al29 | UA—symptoms ≤12 months | 105 | TJC SJC HAQ score |
BL | Y | Development of RA Progressors versus non-progressors: TJC (median 22 vs 4), p<0.001 SJC (median 12 vs 1), p<0.001 HAQ score (mean 0.94 vs 0.52), p=0.006 |
| Salaffi et al62 | UA—symptoms ≤16 weeks | 149 | Symptoms ≥6 weeks EMS >30 min |
BL | Y | Development of RA Symptoms ≥6 weeks: OR=4.97 (95% CI 1.39 to 17.88), p=0.014 EMS >30 min: OR=3.16 (95% CI 1.06 to 9.46), p=0.039 |
| Tamai et al32 | PR—Gonzalez Lopez criteria | 28 | PIP joint involvement | BL | N | Development of RA HR 27.16 (95% CI 1.55 to 474.22), p=0.024 |
| Ha et al27 | UA—(not meeting 1987 RA criteria) | 164 | EMS >30 min SJC ≥4 |
BL | Y | Development of RA EMS >30 min: OR=11.9 (95% CI 2.0 to 71.7), p=0.007 SJC ≥4: OR=13.8 (95% CI 1.7 to 112.4), p=0.014 |
| Bizzaro et al26 | UA—symptoms ≤12 weeks | 192 | Hand joint arthritis | BL | Y | Development of RA HR=2.140 (95% CI 1.128 to 4.059) p=0.02 |
| McNally et al25 | UA—up to 12M symptoms, synovitis in ≥1 joint | 1084 | Score of ≥8 on the Leiden Clinical Prediction Rule | Meta-analysis | Y | Development of RA at 1 year Specificity 95% (95% CI 92 to 97) and sensitivity 49% (95% CI 43 to 55) |
| Yiannopoulos et al30 | UA | 192 | Disease duration | BL | N | Development of RA Progressors versus non-progressors Disease duration 28.5 months vs 45.3 months, p=0.018 |
HR/OR and CIs have been reported where available.
+, positive; A, abstract only; ACPA, anticyclic citrullinated peptide; BL, baseline; CCP, cyclic citrullinated peptide; CPR, clinical prediction rule; CRP, C reactive protein; CSA, clinically suspect arthralgia; DAS-28, Disease Activity Score-28 joints; EMS, early morning stiffness; ESR, erythrocyte sedimentation rate; FDRs, first-degree relatives; HAQ, Health Assessment Questionnaire; IA, inflammatory arthritis; M, months; MSK, musculoskeletal; PD, power Doppler; PIPs, proximal interphalangeal joints; PPV, positive predictive value; PR, palindromic rheumatism; RA, rheumatoid arthritis; RADAI, rheumatoid arthritis disease activity index; RF, rheumatoid factor; STJ, swollen joints count; TJC, tender joints count; UA, undifferentiated arthritis; US, ultrasound; VAS, visual analogue scale.
The majority of published data on clinical features as risk factors in at-risk individuals are reported in patients with early clinical arthritis, classifying as UA based on criteria that precede 2010 RA classification criteria.23–30 The clinical parameters used in these studies are summarised in table 3.
In patients with PR, clinical features have also been demonstrated to be risk factors for RA development. In a retrospective review of 127 Spanish patients with PR, 36 (28%) had subsequently developed RA and early involvement of the wrist and proximal interphalangeal (PIP) joints was associated with this progression.31 However, the presence of RF was more strongly associated with development of RA compared with any clinical factors and this study predated the routine use of ACPA assays. In a subsequent Japanese cohort study, PIP joint involvement was again associated with arthritis development (OR 8.2). However, anti-CCP antibodies were much more predictive than clinical factors (OR 46.7).32
Imaging markers
Eighteen articles specifically addressed imaging markers as risk factors for arthritis development; 13/18 had a low RoB. The five articles without low RoB were abstracts, therefore RoB was not applicable.
In at-risk individuals with MSK symptoms, including anti-CCP+ individuals with MSK symptoms and patients with seropositive arthralgia, joint abnormalities on high-resolution ultrasound (US) are associated with arthritis development (table 3). US abnormalities are also predictive of disease progression in patients with UA (table 4). In a UK cohort of 136 anti-CCP-positive individuals with MSK symptoms, US features were predictive of arthritis development at both joint and patient level.33 US erosions and grey-scale (GS) synovitis were both predictive of arthritis at patient level, although intra-articular power Doppler (PD) signal had the highest predictive value (HR 3.7, 95% CI 2.0 to 6.9, p<0.01). There was an even stronger association with progression at joint level; PD grade ≥2 had an HR 31.3, 95% CI 15.6 to 62.9, p<0.01. In the same cohort, US scans acquired at multiple time points have been analysed to identify predictors of disease progression. In 44 subjects who had 3 serial US scans, those patients who developed arthritis had an increase in US inflammation (ie, total PD score) between scans 2 and 3 (preprogression and progression to arthritis) compared with the corresponding time points in non-progressors, suggesting US inflammation is a late feature which heralds the imminent onset of clinical arthritis.34 In a subsequent analysis of 307 subjects, the frequency of US PD at patient level (ie, number of joints affected) increased in the 12 months prior to arthritis development.35 In a Dutch seropositive arthralgia cohort, US changes (ie, PD signal, GS synovitis, erosions) were associated with arthritis development at joint level but not patient level, although the US protocol contained fewer joints compared with the UK cohort studies.36 In a subsequent study, GS synovitis (grade ≥2) was associated with the development of arthritis and its timing at patient level (metatarsophalangeal (MTP) joints excluded); HR 3.4. Interestingly, there was no positive association with PD in this study and the predictive capacity of US was higher in individuals at higher risk of arthritis, based on a clinical prediction rule based on this cohort.14 37 These data further emphasise the predictive utility of US in higher risk individuals with MSK symptoms. In a Swedish cohort of ACPA-positive individuals with MSK symptoms but no clinical or US synovitis, US tenosynovitis was associated with arthritis development (RR 3.0, 95% CI 1.8 to 4.8, p<0.01).38 39
Table 4.
Articles reporting imaging findings alone as risk factors in individuals at risk of RA
| Study | Population | Cohort size | Risk factor | Frequency | Outcome |
| Ultrasound alone | |||||
| MSK symptoms without arthritis | |||||
| van de Stadt et al36 | Seropositive arthralgia (ACPA and/or RF) | 192 | US PD signal (joint level) | BL | Development of IA OR=2.9 (95% CI 4.65 to 360) |
| Nam et al33 | Anti-CCP+ with non-specific MSK symptoms | 136 | US PD signal (patient level) US BE (patient level) USGS ≥2 (patient level) |
BL | Development of IA US PD signal: HR 3.7 (95% CI 2.0 to 6.9), p<0.001 US BE: HR 2.9 (95% CI 1.7 to 5.1), p<0.001 US GS ≥2: HR 2.3 (95% CI 1.0 to 4.9), p=0.038 |
| Zufferey et al40 | Anti-CCP-negative patients with arthralgia and no synovitis | 80 | US synovitis (SONAR score) | BL | Progression to RA OR=7.4 (95% CI 1.19 to 42.8), p=0.02 |
| van Beers-Tas et al37 | Seropositive arthralgia (ACPA and/or RF) | 163 | US GS ≥2 (excluding MTP joints) (patient level) |
BL | Development of IA HR=3.4 (95% CI 1.6 to 6.8), p<0.01 |
| Pentony et al34 | Anti-CCP+ with non-specific MSK symptoms | 44 | US total PD score in the wrists, MCPJs (1–5) and PIP joints (1–5) | BL Preprogression Progression |
Development of IA 54.5% progressors had increased PD signal score longitudinally vs 9.1% non-progressors |
| Kisten et al38 | Anti-CCP+ with non-specific MSK symptoms but no clinical/US inflammation | 66 | US TSV (patient level) | BL or FU visits | Development of IA RR=3.0 (95% CI 1.8 to 4.8), p=0.001 |
| Hensvold et al39 | Anti-CCP+ with non-specific MSK symptoms but no clinical/US inflammation | 66 | Combined US-TSV on US (patient level) and positive HLA-SE | BL | Development of IA HR=4.9 (95% CI 1.5 to 16), p=0.01 |
| Duquenne et al35 | Anti-CCP+ with non-specific MSK symptoms | 307 | ≥1 joint with PD signal | >12M before progression 3–12M before progression <3M before progression |
Development of IA in the next 3 months OR=7.52 |
| Early clinical arthritis | |||||
| Freeston et al41 | UA ≤12W of inflammatory arthralgia ±synovitis | 50 | US GS score ≥3, PD signal ≥1, ≥1 BE | BL | Development of persistent arthritis Probability increased from 34% to 94% |
| Filer et al42 | UA—early synovitis of ≥1 joint and symptom duration ≤3M | 58 | PD 10 index: summed PD grades of MCP joints 2–3, wrists and MTP joints 2–3 | BL | Development of RA PD 10 index combined with Leiden prediction score: AUC 0.962, compared with AUC 0.905 (Leiden score alone), p<0.05 |
| Sahbudin et al43 | UA—early synovitis of ≥1 joint and symptom duration ≤3M | 107 | US digital flexor TSV Positive PD signal in MCP3 |
BL | Development of RA OR=4.066 (95% CI 1.444 to 11.444), p=0.008 OR=3.078 (95% CI 1.047 to 9.046), p=0.041 |
| MRI±US | |||||
| MSK symptoms without arthritis | |||||
| Kleyer et al44 | ACPA-positive at-risk individuals | 20 | MRI TSV at ≥2 sites | BL | Development of RA 5/5 (100%) of individuals who developed RA had MRI TSV at ≥2 sites |
| Van Steenbergen et al46 | CSA | 150 | Subclinical MRI inflammation | BL | Development of IA HR=5.07 (95% CI 1.77 to 14.50), p=0.002 |
| Boer et al63 | Patients with CSA and patients with UA (arthritis <2 years) | 225 CSA+201 UA | MRI inflammation ‘corrected’ for MRI abnormalities in healthy individuals |
BL | ‘Corrected’ versus ‘uncorrected’ MRI inflammation Development of IA at 12 months Accuracy 60% (95% CI 54 to 67) vs 32% (95% CI 26–38) AUC 0.71 vs 0.55 Fulfilment of 1987 RA criteria at 12 months Accuracy 44% (95% CI 38 to 51) vs 22% (95% CI 17 to 29) AUC 0.65 vs 0.52 |
| Hunt et al45 | Anti-CCP+ individuals with non-specific MSK symptoms | 98 | MRI TSV US PD+ ≥2 US GS ≥2 |
BL | Development of IA HR=4.02 (95% CI 1.91 to 8.44), p=0.002 HR=5.09 (95% CI 1.93 to 13.44), p=0.006 HR=2.69 (95% CI 1.14 to 6.34), p=0.059 |
| Early clinical arthritis | |||||
| Navalho et al49 | Untreated recent onset (<1 year) polyarthritis | 32 | MRI ECU TSV MRI FT2 TSV MRI synovitis of the radioulnar joint |
BL | Development of RA OR=3.21 (95% CI 1.09 to 9.40), p=0.03 OR=9.6 (95% CI 1.17 to 78.93), p=0.03 OR=8.79 (95% CI 1.02 to 75.63), p=0.04 |
| Navalho et al47 | Untreated recent onset (<1 year) undifferentiated polyarthritis | 4 | MRI carpal joint synovitis MRI flexor tendon TSV MRI global joint and tendon count Global MRI and US scores |
BL | Development of RA OR=3.64 (95% CI 1.19 to 11.84), p=0.032 OR=5.09 (95% CI 1.62 to 16.05), p=0.005 OR=2.77 (95% CI 1.249 to 6.139), p=0.012 AUC=0.959 and=0.853, respectively, p<0.05 |
| Dakkak et al48 | UA (arthritis in ≥1 joint, symptom duration <2 years) | 123 | MRI TSV in feet adjusted for BME and synovitis of the foot MRI TSV in feet adjusted for CRP and swollen joint count MRI TSV in hands independent of BME and synovitis |
BL | Development of RA OR=3.29 (95% CI 1.03 to 10.53) OR=2.14 (95% CI 0.77 to 5.95) OR=3.99 (95% CI 1.64 to 9.69) |
HR/OR and CIs have been reported where available.
+, positive; A, abstract only; ACPA, anticyclic citrullinated peptide antibodies; AUC, area under the curve; BE, bone erosions; BL, baseline; BME, bone marrow oedema; CCP, cyclic citrullinated peptide; CRP, C reactive protein; CSA, clinically suspect arthralgia; ECU, extensor carpi ulnaris; FDRs, first-degree relatives; FT, flexor tendons; FU, follow-up; GS, grey scale; IA, inflammatory arthritis; M, months; MCPs, metacarpophalangeal joints; MSK, musculoskeletal; MSUS, musculoskeletal ultrasound; MTPs, metatarsophalangeal joints; PD, power Doppler; PPV, positive predictive value; RA, rheumatoid arthritis; RF, rheumatoid factor; RR, relative risk; TSV, tenosynovitis; UA, unclassified/undifferentiated arthritis; US, ultrasound; W, weeks.
In seronegative at-risk individuals with arthralgia, US synovitis (Swiss SONAR criteria positive) is also associated with arthritis development.40 Furthermore, in seronegative patients with early UA, US synovitis and erosions were associated with development of persistent arthritis.41 GS synovitis in the wrists and MCP joints and PD signal in the MTP joints were all associated with development of RA in a UK cohort of patients with very early UA.42 In a subsequent analysis of 107 patients with UA by the same group, US tenosynovitis was frequently identified and US finger flexor tenosynovitis was predictive of RA development (OR 3.1, 95% CI 1.05 to 9.05, p=0.04).43
In both ACPA-positive individuals with MSK symptoms and patients with CSA, subclinical inflammation on MRI has been associated with arthritis development44–46 (table 4). In both populations, MRI tenosynovitis was the most prevalent MRI abnormality and also the most strongly associated with arthritis development. In a study of 150 Dutch patients with CSA, 31% of patients with baseline subclinical MRI inflammation (either synovitis, osteitis or tenosynovitis) had developed clinical arthritis at 1 year (71% of ACPA-positive patients with CSA with MRI inflammation had developed arthritis). MRI tenosynovitis was the only MRI feature independently associated with arthritis development in the multivariable analysis (HR 8.4, 95% CI 3.4 to 20.8, p<0.01).46 Similarly, at patient level, MRI tenosynovitis was the only MRI feature associated with arthritis development in a UK cohort of 98 ACPA-positive individuals with MSK symptoms (HR 4.02, 95% CI 1.9 to 8.4, p<0.01).45
MRI findings in patients with UA are also associated with disease progression and specifically RA development.47 48 In a small study of patients with recent-onset polyarthritis, MRI tenosynovitis of the finger flexor tendons and extensor carpi ulnaris, and synovitis of the radioulnar joint were associated with progression to RA.49 In this study, MRI was more sensitive than US for joint and tendon inflammation. In a subsequent study comparing MRI and US in the same cohort, MRI was more accurate for predicting RA development.47 In patients with UA, MRI tenosynovitis in the foot was also associated with development of RA, independent of bone marrow oedema (BME) and synovitis (OR 3.3, 95% CI 1.0 to 10.5).48 However, in this study adding MRI of the foot did not improve the predictive accuracy compared with MRI of the hands alone.
Serum markers
Four articles specifically addressed serum markers as risk factors for arthritis development. Three of these articles had low RoB. The one article which did not have a low RoB was a research letter, therefore RoB could not be formally assessed.
The vast majority of published work on serum biomarkers as risk factors in at-risk individuals relates to autoantibodies. There are relatively few data on other serum biomarkers (table 5). In a Dutch seropositive arthralgia cohort, the serum inflammatory protein 14-3-3eta was detectable up to 5 years before the onset of clinical arthritis and was present more frequently and at higher levels in those who developed arthritis compared with those who did not.50 However, it was not clear from this study whether 14-3-3eta levels could predict arthritis onset independently of autoantibodies. Serum dyslipidaemia was also investigated in the same cohort; of several lipid markers measured, lower baseline levels of apolipoprotein A1 were associated with an increased risk of arthritis development, even after adjustment for anti-CCP status (HR 0.5, 95% CI 0.3 to 0.9).51
Table 5.
Other risk factors which have been reported in individuals at risk of developing RA
| Study | Population | Cohort size | Risk factor | Frequency | Outcome |
| BMI | |||||
| Deane et al59 | ACPA+ subjects without arthritis (some FDRs, some clinic patients and some health fares) | 86 | Those with incident RA had higher BMI (p=0.03) | BL | Development of IA Higher BMI in progressors versus non-progressors (32 vs 27), p=0.03 |
| Serum/Cellular/Genetic | |||||
| Asymptomatic | |||||
| Gan et al52 | Anti-CCP3+ individuals without IA (from health fairs) | 35 | Increased docosapentaenoic acid (n-3 FA) | BL and 6M assessments until IA development | Development of IA HR=0.52 (95% CI 0.27 to 0.98) |
| MSK symptoms without arthritis | |||||
| van Beers-Tas et al50 | Seropositive arthralgia (ACPA and/or RF) | 144 | 14-3-3eta | BL | Development of IA RR=2.5 (95% CI 1.2 to 5.6), p=0.02 |
| van De Stadt et al51 | Seropositive arthralgia (ACPA and/or RF) | 348 | Lower ApoA1 level | BL | Development of IA HR 0.52 (95% CI 0.29 to 0.92) |
| Rakieh et al15 | Anti-CCP+ individuals with non-specific MSK symptoms | 100 | HLA DR shared epitope | BL | Development of IA HR=1.84 (95% CI 1.02 to 3.32) |
| Hunt et al53 | Anti-CCP+ individuals with non-specific MSK symptoms | 103 | Combined clinical and T-cell subset parameters | BL and repeated at 1 year | Development of IA AUC 0.79. PPV 60% and NPV 95% |
| Early clinical arthritis | |||||
| Jacobsen et al64 | Early (<2 years) polyarthritis | 68 | Homozygous for MBL variant alleles | BL | Development of RA OR 4.7 (95% CI 1.1 to 19), p=0.02 |
| Yeo et al55 | UA—early synovitis ≥1 joints with symptoms ≤3 months | 48 | Synovial mRNA and protein expression of CXCL4 and CXCL7 | BL assessment | Development of RA at 18 months Progressors versus non-progressors Increased expression (trend) in progressors |
| BMD | |||||
| Mangnus et al56 | CSA | 108 | BMD loss | BL and one subsequent visit | Development of IA HR 6.1 (95% CI 1.7 to 21.4), p=0.005 |
| de Rooy et al57 | Early UA | 125 | Highly elevated BMD loss (>2.5 mg/cm2/month) | BL and 6 months | Development of RA OR 6.1 (95% CI 1.24 to 29.24) |
HR/OR and CIs have been reported where available.
+, positive;A, abstract only; ACPA, anticyclic citrullinated peptide antibodies; ApoA1, apolipoprotein A1; AUC, area under the curve; BCR, B cell receptor; BL, baseline; BMD, bone mineral density; BMI, body mass index; CCP, cyclic citrullinated peptide; CRP, C-reactive protein; CXCL, C-X-C motif ligand; FA, fatty acid; FDRs, first-degree relatives; FU, follow-up; HLA-SE, human leucocyte antigen-shared epitope; hs-CRP, high-sensitive C reactive protein; M, months; MBL, mannose binding-lectine; MCP‐1, monocyte chemotactic protein 1; MSK, musculoskeletal; NAN, native American nations; NPV, negative predictive value; PPV, positive predictive value; RA, rheumatoid arthritis; RF, rheumatoid factor; ROC, receiving operating characteristics; RR, relative risk.
In a small prospective cohort (n=35) of at-risk individuals identified by screening for anti-CCP3 antibodies at health fairs, increased levels of docosapentaenoic acid (a n-3 fatty acid) in red blood cells appeared to be protective for the development of IA (HR 0.5, 95% CI 0.3 to 1.0).52
Cellular markers
Two articles specifically addressed cellular markers as risk factors for arthritis development. Both had low RoB.
Circulating T-cell and B-cell biomarkers appear to be risk factors for arthritis development in at-risk individuals, although relatively few data have been published. In a UK cohort of anti-CCP positive individuals with MSK symptoms, frequencies of circulating T-naïve and T-reg cells were reduced, while inflammation-related cells were increased compared with controls.53 The presence of two or more T cell abnormalities had high specificity for arthritis development and a prediction model combining clinical factors and T-cell subsets performed better than clinical factors alone (AUC 0.8, 95% CI 0.7 to 0.9).53 In patients with seropositive arthralgia, the expansion of B-cell receptor clones (ie, presence of dominant clones) was associated with arthritis development. In both a test and validation cohort, the presence of ≥5 dominant B-cell clones was independently associated with arthritis development (Relative Risk 6.3, 95% CI 2.7 to 15, p<0.01).54 Interestingly, when at-risk individuals developed arthritis, the dominant B-cell receptor clones appeared in synovial tissue but disappeared from peripheral blood, suggesting migration into target tissue in the at-risk phase.
Genotype, gene expression and other markers
Two articles specifically addressed genotype and gene expression as risk factors for arthritis development. Two articles addressed bone mineral density and one addressed demographic features. All of these articles were deemed to have a low RoB.
There is very limited published work on genetic markers (genotype or gene expression) as independent risk factors for arthritis in individuals at risk of RA. In the Leeds cohort of anti-CCP-positive individuals with MSK symptoms, one or more copies of HLA DR shared epitope (SE) alleles was associated with development of clinical arthritis (HR 2.5, 95% CI 1.0 to 5.9). Consequently, HLA DR SE was included in the Leeds risk prediction tool.15 In a UK study of patients with very early UA, synovial mRNA expression of 117 cytokines was measured in synovitic joints. Higher mRNA levels of the inflammatory chemokines CXCL4 and CXCL7 were identified in individuals with very early RA (ie, in the early phase of persistent arthritis) compared with those with resolving arthritis.55
Two Dutch studies have demonstrated that bone mineral density (BMD) loss measured by X-ray is associated with disease progression, both in patients with CSA and patients with early UA.56,57 In 108 patients with CSA, BMD loss was associated with total MRI inflammation scores, and both factors were independently associated with arthritis development.56 In a separate cohort of 101 patients with UA, highly elevated BMD loss (≥2.5 mg/cm2/month) was associated with RA development (OR 6.1, 95% CI 1.2 to 29.2). Of the various demographic factors measured in at-risk cohorts, elevated body mass index (BMI) has been demonstrated to be associated with progression to arthritis in cohorts of patients with seropositive arthralgia and ACPA-positive individuals (from health fairs, clinic patients and some FDRs).58 59 In 83 ACPA-positive individuals recruited from health fairs, rheumatology clinics and FDRs, BMI was higher in the 10 individuals who progressed to arthritis compared with those that did not (32 vs 27, p=0.03).59 In an early analysis of the Dutch seropositive arthralgia cohort, elevated BMI and smoking history were both independently associated with the development of arthritis in the 15/55 (27%) of individuals who progressed. Of the two, smoking had the stronger association with arthritis (HR 9.6, 95% CI 1.3 to 73, p=0.03 vs HR 5.6, 95% CI 1.3 to 25, p=0.02).58
Repeat assessment of risk factors
The large majority of the prospective studies described have evaluated risk factors for arthritis development at only the baseline time point (ie, the first assessment). There is, therefore, insufficient published data to indicate the optimum frequency at which risk factors should be measured in at-risk individuals, and whether and how specific risk factors may fluctuate over time. This is an important area for future research. The limited studies that have assessed risk factor(s) at multiple time points highlight the unique insights which may be derived from this approach; sequential US assessments in ACPA-positive individuals with MSK symptoms suggest the development of US inflammation is a relatively late event, which occurs when clinical arthritis is imminent.34 35 Furthermore, serial autoantibody assessments in FDRs of INAs suggest that in many individuals, ACPA/RF resolve over time and individuals become seronegative.7 The stability and timing of other risk factors in relation to the development of arthritis in at-risk individuals has not been reported.
Discussion
This SLR was performed to address a key question raised by the EULAR task force for conducting clinical trials and studies in individuals at risk of RA. Where relevant, SLRs addressing other questions raised by the taskforce will be published separately. There is significant interest in the study of risk factors in at-risk individuals. Multiple risk factors have now been reported, across multiple biomarker modalities and in different at-risk populations. A key ambition of the task force was to provide evidence-based guidance on a ‘core set’ of risk factors for each at-risk population, so that investigators could include these in future observational studies and clinical trials. Of note, certain risk factors for RA have been identified in large case-control studies undertaken in the wider background population, but have not been identified in populations of at-risk individuals. A detailed discussion of all such risk factors was outside the scope of this review.
Across all at-risk populations, the most well-described risk factor for arthritis development is the presence of serum RA-related autoantibodies, in particular ACPA. The level of ACPA, and its combination with RF, has been consistently demonstrated to predict arthritis development in at-risk individuals across the continuum, from FDRs through to patients with UA. Imaging abnormalities (mainly on MRI and US) appear to be significant risk factors for arthritis in symptomatic at-risk populations (ie, seropositive arthralgia, ACPA-positive individuals with MSK symptoms, CSA and UA). This includes recent MRI studies, which highlight tenosynovitis as a risk factor for disease progression. Of note, imaging abnormalities have not been well studied in asymptomatic at-risk populations (ie, FDRs, genetically predisposed individuals and ACPA+ subjects screened from the general population) and further investigation is required. A range of clinical features also confer increased risk of arthritis in symptomatic at-risk populations; the majority of published data have been in UA cohorts and many describe clinical features as part of composite risk prediction tools, which also include autoantibodies. In at-risk individuals with MSK symptoms but without clinical arthritis, clinical features which indicate inflammatory type symptoms (eg, prolonged EMS duration) have been reported as risk factors for arthritis in several populations, and form important components of risk prediction tools (table 6). The significance of clinical symptoms in at-risk individuals without MSK symptoms has not been well studied.
Table 6.
Core risk factors for development of arthritis in individuals at risk of RA according to population
| At-risk population | Subpopulations | Core risk factors for arthritis |
| Asymptomatic at-risk individuals | Relatives of RA probands | Serum ACPA level±RF |
| Indigenous at-risk populations | ||
| ACPA+ individuals identified by population screening | ||
| MSK symptoms without arthritis | ACPA+ with MSK symptoms | Serum ACPA level±RF MSK symptoms Subclinical joint inflammation on US Subclinical joint and tendon inflammation on MRI |
| ACPA+/RF+ with arthralgia | Serum ACPA level±RF MSK symptoms Subclinical joint inflammation on US |
|
| Clinically suspect arthralgia | Serum ACPA level±RF MSK symptoms Subclinical joint and tendon inflammation on MRI |
|
| Early clinical arthritis | Palindromic rheumatism | Serum ACPA level±RF MSK symptoms |
| Undifferentiated arthritis | Serum ACPA level±RF MSK symptoms Subclinical joint inflammation on US Subclinical joint and tendon inflammation on MRI |
ACPA, anticitrullinated protein antibodies; MSK, musculoskeletal; RA, rheumatoid arthritis; RF, rheumatoid factor; US, ultrasound.
While there are data suggesting other serum and cellular biomarkers may be associated with arthritis development in at-risk populations, these are far fewer and largely demonstrated in single studies without validation in other cohorts. Without further evidence, these would not yet be appropriate to consider as ‘core’ risk factors. The EULAR task force agreed a research agenda, which included several open questions related to risk factors in at-risk populations which should be addressed by future research (see ‘Research agenda’ section).
The strengths of this SLR include an expert librarian-led search and the review of all titles, relevant abstracts and papers by two investigators. We also benefitted from the expert knowledge of the EULAR task force; some additional relevant manuscripts, which were not identified in the literature search, have been included in the SLR. The risk of important articles being missed is therefore low. One limitation is that the SLR is restricted to narrative review as there was significant heterogeneity in the data and populations were not comparable between studies.
The identification of specific risk factors in at-risk populations is critical both on a pragmatic level, to improve the precision of risk prediction, and also on a scientific level, to improve our understanding of the pathobiology of RA. This SLR has served to bring together this information and has informed the guidance provided in the EULAR points to consider in this key area.
Research agenda
Do the risk factors that drive RA autoimmunity and disease progression vary according to the ethnicity or geography of the population?
Which biomarkers/risk factors change as individuals progress to IA?
In individuals at risk of RA what is the sequence and timescale of the changes in biomarkers/risk factors?
How frequently should we re-assess an individual’s risk and is this subpopulation-dependent?
Should interventions be personalised to an individual’s risk factors? For example, smoking cessation, treatment of periodontitis, weight loss?
In those at high risk, should multimodal intervention be considered according to risk factors? For example, immunomodulation combined with periodontal therapy/smoking cessation/weight loss as appropriate.
Does reduction in one or more risk factors reduce the likelihood of progression?
Can the quantification of an individual’s risk be improved, and risk scores validated?
Should individuals with mucosal inflammation/dysbiosis (periodontal, lung or gut) with or without genetic predisposition or serum autoantibodies be considered as an at-risk group?
Patient and public involvement
EULAR PARE members Marios Kouloumas and Codruta Zabalan were members of the EULAR task force (CLI 115) and were involved in determining the topic of focus for this SLR.
rmdopen-2021-001768supp002.pdf (54.4KB, pdf)
Acknowledgments
The authors would like to thank Leeds Teaching Hospitals library services for providing informatics support.
Footnotes
Contributors: KM, HS and JK conducted the literature search. KM and HS reviewed and selected the articles. ADM and DA-R conducted the quality assessment. The manuscript was drafted by KM and PE. All authors commented on and revised the manuscript.
Funding: EULAR grant CLI 115.
Competing interests: KM: honoraria from AbbVie, Lilly, UCB; grants from Lilly, Gilead. HJS: none declared. AK: speakers bureau, consultancy: AbbVie, Bristol-Myers Squibb, Celgene, Eli-Lilly, Gilead, Merck Sharp and Dohme, Novartis and Pfizer. DA-R: scientific advisor for GSK. ADM: none declared. JK: none declared. DA: none declared. PE: expert advice to Pfizer, AbbVie, Amgen, MSD, Roche, Sanofi, BMS, Novartis, Lilly, Gilead, Samsung, Celltrion; grants from AbbVie, Lilly, BMS, Samsung. The review was not registered. Data collection forms and other materials used in the review available from authors on request.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not required.
References
- 1.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wells GSB, O'Connell D, Peterson J, et al. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses, 2013. Available: http://wwwohrica/programs/clinical_epidemiology/oxfordasp
- 3.Nielen MMJ, van Schaardenburg D, Reesink HW, et al. Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurements in blood donors. Arthritis Rheum 2004;50:380–6. 10.1002/art.20018 [DOI] [PubMed] [Google Scholar]
- 4.Rantapää-Dahlqvist S, de Jong BAW, Berglin E, et al. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum 2003;48:2741–9. 10.1002/art.11223 [DOI] [PubMed] [Google Scholar]
- 5.Hensvold AH, Magnusson PKE, Joshua V, et al. Environmental and genetic factors in the development of anticitrullinated protein antibodies (ACPAs) and ACPA-positive rheumatoid arthritis: an epidemiological investigation in twins. Ann Rheum Dis 2015;74:375–80. 10.1136/annrheumdis-2013-203947 [DOI] [PubMed] [Google Scholar]
- 6.Ramos-Remus C, Castillo-Ortiz JD, Aguilar-Lozano L, et al. Autoantibodies in prediction of the development of rheumatoid arthritis among healthy relatives of patients with the disease. Arthritis Rheumatol 2015;67:2837–44. 10.1002/art.39297 [DOI] [PubMed] [Google Scholar]
- 7.Tanner S, Dufault B, Smolik I, et al. A prospective study of the development of inflammatory arthritis in the family members of Indigenous North American people with rheumatoid arthritis. Arthritis Rheumatol 2019;71:1494–503. 10.1002/art.40880 [DOI] [PubMed] [Google Scholar]
- 8.Hafkenscheid L, de Moel E, Smolik I, et al. N-Linked glycans in the variable domain of IgG Anti-Citrullinated protein antibodies predict the development of rheumatoid arthritis. Arthritis Rheumatol 2019;71:1626–33. 10.1002/art.40920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.del Puente A, Knowler WC, Pettitt DJ, et al. The incidence of rheumatoid arthritis is predicted by rheumatoid factor titer in a longitudinal population study. Arthritis Rheum 1988;31:1239–44. 10.1002/art.1780311004 [DOI] [PubMed] [Google Scholar]
- 10.Gan RW, Young KA, Zerbe GO, et al. Lower omega-3 fatty acids are associated with the presence of anti-cyclic citrullinated peptide autoantibodies in a population at risk for future rheumatoid arthritis: a nested case-control study. Rheumatology 2016;55:367–76. 10.1093/rheumatology/kev266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bos WH, Wolbink GJ, Boers M, et al. Arthritis development in patients with arthralgia is strongly associated with anti-citrullinated protein antibody status: a prospective cohort study. Ann Rheum Dis 2010;69:490–4. 10.1136/ard.2008.105759 [DOI] [PubMed] [Google Scholar]
- 12.van de Stadt LA, van der Horst AR, de Koning MHMT, et al. The extent of the anti-citrullinated protein antibody repertoire is associated with arthritis development in patients with seropositive arthralgia. Ann Rheum Dis 2011;70:128–33. 10.1136/ard.2010.132662 [DOI] [PubMed] [Google Scholar]
- 13.Ten Brinck RM, van Steenbergen HW, van Delft MAM, et al. The risk of individual autoantibodies, autoantibody combinations and levels for arthritis development in clinically suspect arthralgia. Rheumatology 2017;56:2145–53. 10.1093/rheumatology/kex340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van de Stadt LA, Witte BI, Bos WH, et al. A prediction rule for the development of arthritis in seropositive arthralgia patients. Ann Rheum Dis 2013;72:1920–6. 10.1136/annrheumdis-2012-202127 [DOI] [PubMed] [Google Scholar]
- 15.Rakieh C, Nam JL, Hunt L, et al. Predicting the development of clinical arthritis in anti-CCP positive individuals with non-specific musculoskeletal symptoms: a prospective observational cohort study. Ann Rheum Dis 2015;74:1659–66. 10.1136/annrheumdis-2014-205227 [DOI] [PubMed] [Google Scholar]
- 16.Shi J, van de Stadt LA, Levarht EWN, et al. Anti-carbamylated protein antibodies are present in arthralgia patients and predict the development of rheumatoid arthritis. Arthritis Rheum 2013;65:911–5. 10.1002/art.37830 [DOI] [PubMed] [Google Scholar]
- 17.Kudo-Tanaka E, Ohshima S, Ishii M, et al. Autoantibodies to cyclic citrullinated peptide 2 (CCP2) are superior to other potential diagnostic biomarkers for predicting rheumatoid arthritis in early undifferentiated arthritis. Clin Rheumatol 2007;26:1627–33. 10.1007/s10067-007-0558-5 [DOI] [PubMed] [Google Scholar]
- 18.Mjaavatten MD, van der Heijde D, Uhlig T, et al. The likelihood of persistent arthritis increases with the level of anti-citrullinated peptide antibody and immunoglobulin M rheumatoid factor: a longitudinal study of 376 patients with very early undifferentiated arthritis. Arthritis Res Ther 2010;12:R76. 10.1186/ar2995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van der Linden MPM, van der Woude D, Ioan-Facsinay A, et al. Value of anti-modified citrullinated vimentin and third-generation anti-cyclic citrullinated peptide compared with second-generation anti-cyclic citrullinated peptide and rheumatoid factor in predicting disease outcome in undifferentiated arthritis and rheumatoid arthritis. Arthritis Rheum 2009;60:2232–41. 10.1002/art.24716 [DOI] [PubMed] [Google Scholar]
- 20.Aletaha D, Neogi T, Silman AJ, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League against rheumatism collaborative initiative. Arthritis Rheum 2010;62:2569–81. 10.1002/art.27584 [DOI] [PubMed] [Google Scholar]
- 21.van Steenbergen HW, Aletaha D, Beaart-van de Voorde LJJ. EULAR definition of arthralgia suspicious for progression to rheumatoid arthritis. Ann Rheum Dis 2016. [DOI] [PubMed] [Google Scholar]
- 22.Burgers LE, Siljehult F, Ten Brinck RM, et al. Validation of the EULAR definition of arthralgia suspicious for progression to rheumatoid arthritis. Rheumatology 2017;56:2123–8. 10.1093/rheumatology/kex324 [DOI] [PubMed] [Google Scholar]
- 23.Salaffi F, Filippucci E, Carotti M, et al. Inter-observer agreement of standard joint counts in early rheumatoid arthritis: a comparison with grey scale ultrasonography-a preliminary study. Rheumatology 2008;47:54–8. 10.1093/rheumatology/kem286 [DOI] [PubMed] [Google Scholar]
- 24.El Miedany Y, Youssef S, Mehanna AN, et al. Development of a scoring system for assessment of outcome of early undifferentiated inflammatory synovitis. Joint Bone Spine 2008;75:155–62. 10.1016/j.jbspin.2007.04.021 [DOI] [PubMed] [Google Scholar]
- 25.McNally E, Keogh C, Galvin R, et al. Diagnostic accuracy of a clinical prediction rule (CPR) for identifying patients with recent-onset undifferentiated arthritis who are at a high risk of developing rheumatoid arthritis: a systematic review and meta-analysis. Semin Arthritis Rheum 2014;43:498–507. 10.1016/j.semarthrit.2013.08.005 [DOI] [PubMed] [Google Scholar]
- 26.Bizzaro N, Bartoloni E, Morozzi G, et al. Anti-cyclic citrullinated peptide antibody titer predicts time to rheumatoid arthritis onset in patients with undifferentiated arthritis: results from a 2-year prospective study. Arthritis Res Ther 2013;15:R16. 10.1186/ar4148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ha YJ, Park Y-B, Son M-K, et al. Predictive factors related to progression toward rheumatoid arthritis in Korean patients with undifferentiated arthritis. Rheumatol Int 2012;32:1555–61. 10.1007/s00296-011-1806-1 [DOI] [PubMed] [Google Scholar]
- 28.Thabet MM, Huizinga TWJ, van der Heijde DM, et al. The prognostic value of baseline erosions in undifferentiated arthritis. Arthritis Res Ther 2009;11:R155. 10.1186/ar2832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuriya B, Cheng CK, Chen HM, et al. Validation of a prediction rule for development of rheumatoid arthritis in patients with early undifferentiated arthritis. Ann Rheum Dis 2009;68:1482–5. 10.1136/ard.2008.092676 [DOI] [PubMed] [Google Scholar]
- 30.Yiannopoulos G, Daoussis D, Melissaropoulos K, et al. Evolution of undifferentiated arthritis: a ten-year experience from the early arthritis clinic of a tertiary care hospital. Clin Exp Rheumatol 2015;33:341–6. [PubMed] [Google Scholar]
- 31.Gonzalez-Lopez L, Gamez-Nava JI, Jhangri GS, et al. Prognostic factors for the development of rheumatoid arthritis and other connective tissue diseases in patients with palindromic rheumatism. J Rheumatol 1999;26:540–5. [PubMed] [Google Scholar]
- 32.Tamai M, Kawakami A, Iwamoto N, et al. Contribution of anti-CCP antibodies, proximal interphalangeal joint involvement, HLA-DRB1 shared epitope, and PADI4 as risk factors for the development of rheumatoid arthritis in palindromic rheumatism. Scand J Rheumatol 2010;39:287–91. 10.3109/03009741003604534 [DOI] [PubMed] [Google Scholar]
- 33.Nam JL, Hensor EMA, Hunt L, et al. Ultrasound findings predict progression to inflammatory arthritis in anti-CCP antibody-positive patients without clinical synovitis. Ann Rheum Dis 2016;75:2060–7. 10.1136/annrheumdis-2015-208235 [DOI] [PubMed] [Google Scholar]
- 34.Pentony P, Mankia K, Hensor EM, et al. SAT0107 Sequential ultrasound shows a late increase in inflammatory burden in anti-ccp positive patients with non-specific musculoskeletal symptoms just before progression to inflammatory arthritis. Ann Rheum Dis 2018;77:916. [Google Scholar]
- 35.Duquenne L, Mankia K, Nam J, et al. Thu0072 ultrasound predicts imminent progression to arthritis in anti-CCP positive at-risk individuals 2019;78:304–5. [Google Scholar]
- 36.van de Stadt LA, Bos WH, Meursinge Reynders M, et al. The value of ultrasonography in predicting arthritis in auto-antibody positive arthralgia patients: a prospective cohort study. Arthritis Res Ther 2010;12:R98. 10.1186/ar3028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Beers-Tas MH, Blanken AB, Nielen MMJ, et al. The value of joint ultrasonography in predicting arthritis in seropositive patients with arthralgia: a prospective cohort study. Arthritis Res Ther 2018;20:279. 10.1186/s13075-018-1767-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kisten YRH, af Klint E, Fei G, et al. Catrina AI Tenosynovitis Detected By Ultrasound Predicts Arthritis Onset in Individuals at Risk of Developing Rheumatoid Arthritis [abstract]. Arthritis & rheumatology 2018;70. [Google Scholar]
- 39.Hensvold A, Kisten Y, Circiumaru A. Thu0080 development of ultrasound detectable arthritis among AcpA positive subjects with musculoskeletal symptoms: the risk RA prospective study. Ann Rheum Dis 2019;78:310. [Google Scholar]
- 40.Zufferey P, Rebell C, Benaim C, et al. Ultrasound can be useful to predict an evolution towards rheumatoid arthritis in patients with inflammatory polyarthralgia without anticitrullinated antibodies. Joint Bone Spine 2017;84:299–303. 10.1016/j.jbspin.2016.05.011 [DOI] [PubMed] [Google Scholar]
- 41.Freeston JE, Wakefield RJ, Conaghan PG, et al. A diagnostic algorithm for persistence of very early inflammatory arthritis: the utility of power Doppler ultrasound when added to conventional assessment tools. Ann Rheum Dis 2010;69:417–9. 10.1136/ard.2008.106658 [DOI] [PubMed] [Google Scholar]
- 42.Filer A, de Pablo P, Allen G, et al. Utility of ultrasound joint counts in the prediction of rheumatoid arthritis in patients with very early synovitis. Ann Rheum Dis 2011;70:500–7. 10.1136/ard.2010.131573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sahbudin I, Pickup L, Nightingale P, et al. The role of ultrasound-defined tenosynovitis and synovitis in the prediction of rheumatoid arthritis development. Rheumatology 2018;57:1243–52. 10.1093/rheumatology/key025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kleyer A, Krieter M, Oliveira I, et al. High prevalence of tenosynovial inflammation before onset of rheumatoid arthritis and its link to progression to RA-A combined MRI/CT study. Semin Arthritis Rheum 2016;46:143–50. 10.1016/j.semarthrit.2016.05.002 [DOI] [PubMed] [Google Scholar]
- 45.Hunt L, Nam J, Hensor EM. OP0042 In acpa positive at-risk individuals, which mri and us findings best predict development of clinical synovitis? Ann Rheum Dis 2018;77:72–3. [Google Scholar]
- 46.van Steenbergen HW, Mangnus L, Reijnierse M, et al. Clinical factors, anticitrullinated peptide antibodies and MRI-detected subclinical inflammation in relation to progression from clinically suspect arthralgia to arthritis. Ann Rheum Dis 2016;75:1824–30. 10.1136/annrheumdis-2015-208138 [DOI] [PubMed] [Google Scholar]
- 47.Navalho M, Resende C, Rodrigues AM, et al. Bilateral evaluation of the hand and wrist in untreated early inflammatory arthritis: a comparative study of ultrasonography and magnetic resonance imaging. J Rheumatol 2013;40:1282–92. 10.3899/jrheum.120713 [DOI] [PubMed] [Google Scholar]
- 48.Dakkak YJ, Boeters DM, Boer AC, et al. What is the additional value of MRI of the foot to the hand in undifferentiated arthritis to predict rheumatoid arthritis development? Arthritis Res Ther 2019;21:56. 10.1186/s13075-019-1845-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Navalho M, Resende C, Rodrigues AM, et al. Bilateral MR imaging of the hand and wrist in early and very early inflammatory arthritis: tenosynovitis is associated with progression to rheumatoid arthritis. Radiology 2012;264:823–33. 10.1148/radiol.12112513 [DOI] [PubMed] [Google Scholar]
- 50.van Beers-Tas MH, Marotta A, Boers M, et al. A prospective cohort study of 14-3-3η in AcpA and/or RF-positive patients with arthralgia. Arthritis Res Ther 2016;18:76. 10.1186/s13075-016-0975-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van de Stadt LA, van Sijl AM, van Schaardenburg D, et al. Dyslipidaemia in patients with seropositive arthralgia predicts the development of arthritis. Ann Rheum Dis 2012;71:1915–6. 10.1136/annrheumdis-2012-201709 [DOI] [PubMed] [Google Scholar]
- 52.Gan RW, Bemis EA, Demoruelle MK, et al. The association between omega-3 fatty acid biomarkers and inflammatory arthritis in an anti-citrullinated protein antibody positive population. Rheumatology 2017;56:2229–36. 10.1093/rheumatology/kex360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hunt L, Hensor EM, Nam J, et al. T cell subsets: an immunological biomarker to predict progression to clinical arthritis in ACPA-positive individuals. Ann Rheum Dis 2016;75:1884–9. 10.1136/annrheumdis-2015-207991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tak PP, Doorenspleet ME, de Hair MJH, et al. Dominant B cell receptor clones in peripheral blood predict onset of arthritis in individuals at risk for rheumatoid arthritis. Ann Rheum Dis 2017;76:1924–30. 10.1136/annrheumdis-2017-211351 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 55.Yeo L, Adlard N, Biehl M, et al. Expression of chemokines CXCL4 and CXCL7 by synovial macrophages defines an early stage of rheumatoid arthritis. Ann Rheum Dis 2016;75:763–71. 10.1136/annrheumdis-2014-206921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mangnus L, van Steenbergen HW, Reijnierse M, et al. Bone mineral density loss in clinically suspect arthralgia is associated with subclinical inflammation and progression to clinical arthritis. Scand J Rheumatol 2017;46:364–8. 10.1080/03009742.2017.1299217 [DOI] [PubMed] [Google Scholar]
- 57.de Rooy DPC, Kälvesten J, Huizinga TWJ, et al. Loss of metacarpal bone density predicts RA development in recent-onset arthritis. Rheumatology 2012;51:1037–41. 10.1093/rheumatology/ker435 [DOI] [PubMed] [Google Scholar]
- 58.de Hair MJH, Landewé RBM, van de Sande MGH, et al. Smoking and overweight determine the likelihood of developing rheumatoid arthritis. Ann Rheum Dis 2013;72:1654–8. 10.1136/annrheumdis-2012-202254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Deane KD, Nagpal S, Rao N. Sat0082 associations of baseline clinical and biomarker factors with symptoms and future development of clinically-apparent rheumatoid arthritis in an AcpA positive cohort. Ann Rheum Dis 2019;78:1105. [Google Scholar]
- 60.Verheul MK, Böhringer S, van Delft MAM, et al. Triple positivity for Anti-Citrullinated protein autoantibodies, rheumatoid factor, and Anti-Carbamylated protein antibodies conferring high specificity for rheumatoid arthritis: implications for very early identification of at-risk individuals. Arthritis Rheumatol 2018;70:1721–31. 10.1002/art.40562 [DOI] [PubMed] [Google Scholar]
- 61.Nakajima T, Nobuhara Y, Nakazawa T. AB0273 the analysis of the clinical courses of the AcpA positive patients without synovitis: whether to follow up them. Ann Rheum Dis 2019;78:1594. [Google Scholar]
- 62.Salaffi F, Ciapetti A, Gasparini S, et al. A clinical prediction rule combining routine assessment and power Doppler ultrasonography for predicting progression to rheumatoid arthritis from early-onset undifferentiated arthritis. Clin Exp Rheumatol 2010;28:686–94. [PubMed] [Google Scholar]
- 63.Boer AC, Burgers LE, Mangnus L, et al. Using a reference when defining an abnormal MRI reduces false-positive MRI results-a longitudinal study in two cohorts at risk for rheumatoid arthritis. Rheumatology 2017;56:1700–6. 10.1093/rheumatology/kex235 [DOI] [PubMed] [Google Scholar]
- 64.Jacobsen S, Madsen HO, Klarlund M, et al. The influence of mannose binding lectin polymorphisms on disease outcome in early polyarthritis. TIRA group. J Rheumatol 2001;28:935–42. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2021-001768supp001.pdf (122.9KB, pdf)
rmdopen-2021-001768supp002.pdf (54.4KB, pdf)