Abstract
Objectives
To delineate characteristics of non-radiographic axial spondyloarthritis (nr-axSpA) in Asia versus non-Asian regions, and compare radiographic axSpA (r-axSpA) with nr-axSpA within Asia.
Methods
Data were collected from the Assessment of SpondyloArthritis international Society-COMOrbidities in SPondyloArthritis database. Categorising patients by region, we compared clinical characteristics between nr-axSpA from Asia vs elsewhere (Europe, the Americas and Africa). Within Asians, we additionally compared patient characteristics of those with nr-axSpA versus r-axSpA.
Results
Among 3984 SpA cases, 1094 were from Asian countries. Of 780 axSpA patients in Asia, 112 (14.4%) had nr-axSpA, less than in non-Asian countries (486/1997, 24.3%). Nr-axSpA patients in Asia were predominantly male (75.9% vs 47.1%), younger at onset (22.8 vs 27.8 years) and diagnosis (27.2 vs 34.5 years), and experienced less diagnostic delay (1.9 vs 2.9 years) compared with nr-axSpA in non-Asian countries. Nr-axSpA in Asia exhibited higher human leucocyte antigens-B27 prevalence (90.6% vs 61.9%), fewer peripheral SpA features (53.6% vs 66.3%) and similar extra-articular and comorbid disease rates compared with those with nr-axSpA in non-Asian countries. Disease activity, functional impairment and MRI sacroiliitis were less in nr-axSpA in Asia, with higher rates of non-steroidal anti-inflammatory drug response and less methotrexate and biological disease-modifying antirheumatic drugs use. Within Asia, r-axSpA showed higher disease activity and structural damage compared with nr-axSpA, with no differences in other features.
Conclusion
Among axSpA, lower frequency of nr-axSpA was observed in Asia. Our results offer an opportunity to better understand clinical characteristics and optimise diagnostic strategies, such as ensuring access and availability of MRI resources for accurate diagnosis of nr-axSpA in Asia.
Keywords: spondylitis, ankylosing, epidemiology, arthritis, psoriatic
Key messages.
What is already known about this subject?
Among axial spondyloarthritis (SpA) patients, proportion of non-radiographic (nr)-axSpA was observed in one third to one fourth from reported cohort studies.
The patients with nr-axSpA compared with those in AS were younger but older disease onset, more female patients and similar proportion of human leucocyte antigens (HLA)-B27 positivity.
The patients with nr-axSpA have lower levels of acute phase reactants, whereas no differences were seen in disease burden such as patient-reported disease activity, function or HRQoL.
Key messages.
What does this study add?
Among axial SpA patients, substantially lower frequency of nr-axSpA was observed in Asia compared with other regions.
Nr-axSpA patients in Asia were predominantly male, and had younger disease onset with higher HLA-B27 positivity rate and less peripheral signs.
Diagnosis strategies in Asian countries should be reviewed according to the particularities of each region, specifically MRI availability, to better identify nr-axSpA patients.
How might this impact on clinical practice or further developments?
Nr-axSpA patients demonstrated unique clinical features compared with the patients in other countries.
These results offer an opportunity to improve both early diagnosis and treatment of nr-axSpA patients in Asian countries.
Introduction
Spondyloarthritis (SpA) comprises a group of inflammatory conditions that primarily affect the axial skeleton, with radiographic axial SpA (r-axSpA) or ankylosing spondylitis (AS) being the prototypical disease.1 To capture the full spectrum of the disease in research, including patients who do not have r-axSpA, the Assessment of SpondyloArthritis international Society (ASAS) developed new classification criteria for axSpA to include patients with clinical features of SpA who may not have substantial radiographic sacroiliitis.2 Patients who fulfil axSpA criteria but do not have radiographic sacroiliitis sufficiently advanced to meet modified New York Criteria are categorised as non-radiographic axSpA (nr-axSpA).
Approximately 10% of patients with nr-axSpA progress to r-axSpA within 2 years; 26%–60% develop r-axSpA within 10–15 years.1 3 Although significant radiographic damage is not seen in nr-axSpA, studies have nonetheless shown a substantial disease burden similar to that of r-axSpA, with both groups experiencing inadequate disease control.3 4 Recent studies report both tumour necrosis factor inhibitors and interleukin 17 inhibitors as effectively reducing signs and symptoms of nr-axSpA.5–7 These findings suggest the continued need for early detection and treatment of nr-axSpA to slow disease progression and improve patient outcomes.
Clinical characteristics of nr-axSpA are highly variable across patients, and may potentially vary across patient populations, particularly due to differing distributions of human leucocyte antigens (HLA) and other genetic factors. However, the majority of nr-axSpA studies have been conducted in Europe and the USA, with only small studies reported from Asia.3 4 8–12
In this study, we aim to characterise the distinguishing clinical features of nr-axSpA in patients from Asian countries, potentially allowing clinicians to improve both underdiagnosis and misdiagnosis of this increasingly treatable disease entity.
Methods
Study population and design
We used data from the ASAS-COMOrbidities in SPondyloArthritis (ASAS-COMOSPA) study, an international, multicentre, cross-sectional, observational study involving 22 countries including Africa, America, Asia and Europe.13 The initial aim of ASAS-COMOSPA was to describe the prevalence of various comorbidities among SpA patients, details of which have been previously described. To ensure appropriate international patient representation, the ASAS-COMOSPA Scientific Committee selected national principal investigators from collaborating countries, each of whom invited rheumatologists in their country to participate in the study.
Patients aged ≥18 years, with a diagnosis of SpA and fulfilling ASAS criteria for either axial or peripheral disease,2 14 and able to give written consent and complete the study questionnaire, were enrolled. Per study protocol, patients were expected to be enrolled consecutively from all ASAS-COMOSPA participating centres, thus minimising bias.
Patient characteristic assessment
Patient information was collected by a study investigator or research nurse during a face-to-face patient interview at each study site. Medical records were also examined for further information. Collected information was classified into four domains: demographics and disease characteristics, comorbidities, risk factors for comorbidities and adherence with recommendations for monitoring of comorbidities.
Demographic data included age, gender, body mass index (BMI) and smoking status. Disease characteristics included axial involvement; peripheral SpA features such as peripheral arthritis, enthesitis (heel or total peripheral enthesitis) and dactylitis; extra-articular manifestations (eg, inflammatory bowel disease (IBD), uveitis, psoriasis); and comorbidities (ie, obesity, hypertension, diabetes mellitus, dyslipidaemia, ischaemic heart disease or stroke, vertebral or fragility fractures). SpA disease activity was assessed via Bath Ankylosing Spondylitis Disease Activity Index (BASDAI)15 and Ankylosing Spondylitis Disease Activity Score calculated with C reactive protein (mg/L) (CRP) (ASDAS).16 17 SpA disease severity was assessed by history of SpA-related surgeries, presence of bamboo spine and Bath Ankylosing Spondylitis Functional Index (BASFI).18 Past and current medications (non-steroidal anti-inflammatory drugs (NSAIDs), glucocorticoids, conventional synthetic (cs) and biological (b) disease-modifying antirheumatic drugs (DMARDs)) were also examined.
Patient group definition
As our primary outcome of interest was differences in the distribution of clinical characteristics of nr-axSpA patients across countries, we grouped patients based on their region as follows: Asian countries (China, Japan, Singapore, South Korea and Taiwan), and non-Asian countries (Europe, the Americas and Africa). We classified patients into the ASAS axSpA subgroup if they met either of the ASAS axSpA criteria2: (1) imaging arm, that is, sacroiliitis on imaging (MRI or X-ray) plus ≥1 clinical characteristics of SpA or (2) clinical arm, that is, HLA-B27 positivity plus ≥2 clinical characteristics of SpA. Among those with axSpA, patients were further classified as having r-axSpA or nr-axSpA according to the presence of radiographic sacroiliitis per modified New York criteria.19 Classification of psoriatic arthritis (PsA) was based on Classification Criteria for Psoriatic Arthritis (CASPAR).20
We made regional comparisons for patients with nr-axSpA. We additionally compared the clinical characteristics of Asian patients with nr-axSpA vs those with r-axSpA.
Statistical analyses
Statistical information regarding patient demographics, disease characteristics, diagnosis and treatment were quantified and compared between Asian and non-Asian countries. Differences between nr-axSpA and r-axSpA among patients from Asian countries was also analysed using univariate analysis. In descriptive statistics, categorical variables were presented as number (with percentage) and quantitative variables as median (with IQR) considering non-normal distribution. χ2 or Fisher’s exact test was used to compare categorical variables as appropriate. To analyse differences in continuous variables, Wilcoxon rank-sum test was used for non-normally distributed variables.
For all analyses, a p<0.05 was considered statistically significant. All analyses were performed using R (V.4.0.2) using the tableone package for tables21 and Easy R (EZR) (V.1.52), a graphical user interface for R.22
Results
Frequency of nr-axSpA among axSpA by regions
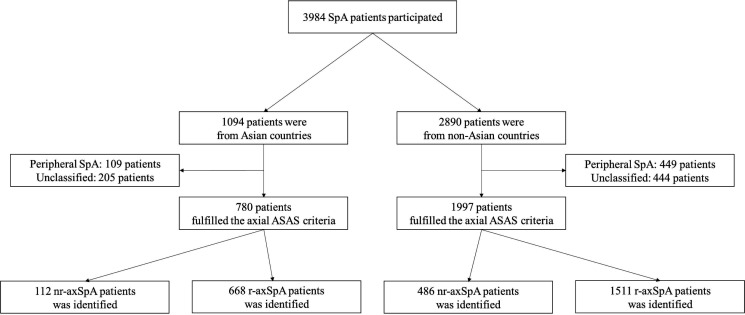
Among 3984 patients included in the study, 1094 were from centres in Asian countries; 2890 patients were from North or South America, Europe or Africa. Of these, 780 (73.1%) in Asia (China, n=210; Japan, n=77; Singapore, n=144; South Korea, n=178; Taiwan, n=171) and 1997 (69.1%) in other countries fulfilled the ASAS criteria for axSpA. Among patients with axSpA in Asian countries, 112 (14.4%, range 7.1%–25.0%, online supplemental table 1) were identified as having nr-axSpA, a substantially lower proportion than identified in non-Asian countries (486/1997, 24.3%) (figure 1).
Figure 1.
A flow diagram of SpA patients in Asian countries versus non-Asian countries. ASAS, Assessment of SpondyloArthritis international Society; nr-axSpA, non-radiographic axial spondyloarthritis; SpA, spondyloarthritis.
rmdopen-2021-001752supp001.pdf (93.6KB, pdf)
Demographic characteristics of patients with nr-axSpA by regions
The overall patient characteristics of nr-axSpA in Asia versus non-Asian countries are summarised in table 1. Nr-axSpA patients in Asian countries were younger at the time of disease onset and diagnosis compared with patients in other countries. The average time between disease onset to diagnosis was 1.9 years in Asian countries, whereas it was 2.9 years in other regions. The proportion of male patients was markedly higher in Asian countries at 75.9% vs 47.1% in other countries. There was no difference in smoking status between regions and BMI was lower in nr-axSpA patients in Asia (23.5 vs 25.2).
Table 1.
Characteristics of non-radiographic axial SpA in Asia versus non-Asian regions
| Variables | Asia | Non-Asian regions | P value |
| n | 112 | 486 | |
| Age at disease diagnosis, years | 27.2 (21.1, 39.6) | 34.5 (27.7, 41.7) | <0.001 |
| Age at disease onset, years | 22.8 (18.5, 31.0) | 27.8 (20. 5, 34.8) | 0.007 |
| Age at enrollment, years | 33.9 (24.9, 44.0) | 39.4 (31.5, 47.9) | <0.001 |
| Diagnostic delay*, years | 1.9 (0.27, 5.56) | 2.9 (0.59, 9.58) | 0.01 |
| Male (%) | 85 (75.9) | 229 (47.1) | <0.001 |
| Body mass index, kg/m2 | 23.5 (20.7, 26.6) | 25.2 (22.8, 28.2) | <0.001 |
| Smoking status | |||
| Never (%) | 67 (59.8) | 270 (55.6) | 0.48 |
| Past (%) | 17 (15.2) | 100 (20.6) | 0.24 |
| Current (%) | 28 (25.0) | 116 (23.9) | 0.90 |
| Family history of SpA (%) | 36 (32.1) | 199 (40.9) | 0.11 |
| Meeting ASAS axial SpA criteria | |||
| Imaging arm only (%) | 19 (17.0) | 219 (45.1) | <0.001 |
| Clinical arm only (%) | 63 (56.2) | 145 (29.8) | <0.001 |
| Both (%) | 30 (26.8) | 122 (25.1) | 0.80 |
| MRI tested (%) | 73 (65.2) | 415 (85.4) | <0.001 |
| Sacroiliitis among MRI tested (%) | 49 (67.1) | 341 (82.2) | 0.005 |
| HLA B27 measured (%) | 106 (94.6) | 441 (90.7) | 0.26 |
| HLA B27 positivity among measured (%) | 96 (90.6) | 273 (61.9) | <0.001 |
| Inflammatory back pain (%) | 107 (95.5) | 478 (98.4) | 0.08 |
| Arthritis, enthesitis, or dactylitis (%) | 60 (53.6) | 322 (66.3) | 0.02 |
| Peripheral arthritis (%) | 53 (47.3) | 257 (52.9) | 0.34 |
| Heel enthesitis (%) | 33 (29.5) | 160 (32.9) | 0.55 |
| Peripheral enthesitis (%) | 38 (33.9) | 202 (41.6) | 0.17 |
| Dactylitis (%) | 2 (1.8) | 63 (13.0) | 0.001 |
| Psoriasis (%) | 12 (10.7) | 82 (16.9) | 0.14 |
| CASPAR criteria (%) | 6 (5.4) | 27 (5.6) | 1 |
| Uveitis (%) | 20 (17.9) | 81 (16.7) | 0.87 |
| Inflammatory bowel disease (%) | 5 (4.5) | 27 (5.6) | 0.82 |
| Erythrocyte sedimentation rate, mm/hour | 10.0 (5.0, 19.0) | 11.0 (5.0, 23.0) | 0.11 |
| C reactive protein (CRP) level, mg/dL | 0.50 (0.30, 1.33) | 0.50 (0.10, 1.70) | 0.55 |
| Elevated CRP (%) | 37 (33.0) | 213 (43.8) | 0.05 |
| Physician global assessment (0–10) | 2.0 (1.0, 5.0) | 2.0 (1.0, 4.0) | 0.74 |
| Patient global assessment (0–10) | 3.0 (1.0, 6.0) | 4.0 (2.0, 6.0) | 0.01 |
| ASDAS-CRP | 1.40 (0.95, 2.08) | 1.97 (1.21, 2.78) | <0.001 |
| BASDAI | 2.8 (1.5, 4.2) | 4.1 (2.0, 6.3) | <0.001 |
| BASFI | 0.8(0.1, 2.7) | 2.9(0.8, 5.6) | <0.001 |
| Presence of bamboo spine (%) | 0 (0.0) | 2 (0.4) | 1 |
| History of SpA-related surgeries (%) | 0 (0.0) | 18 (3.7) | 0.03 |
| Treatment | |||
| NSAIDs use since onset (%) | 103 (92.0) | 430 (88.5) | 0.37 |
| COX-2 selective NSAIDs among user (ever) (%) | 32 (31.1) | 127 (29.5) | 0.853 |
| % days with intake ≧50% among user (ever) (%) | 68 (66.0) | 329 (76.5) | 0.039 |
| Good response to NSAIDs (%) | 80 (71.4) | 272 (56.0) | 0.004 |
| Current glucocorticoid use (%) | 10 (8.9) | 59 (12.1) | 0.43 |
| Conventional synthetic DMARDs use (%) | 60 (53.6) | 266 (54.7) | 0.91 |
| Methotrexate use (%) | 18 (16.1) | 134 (27.6) | 0.02 |
| Sulfasalazine use (%) | 51 (45.5) | 205 (42.2) | 0.59 |
| Biological DMARDs (TNF antagonists) use (%) | 27 (24.1) | 191 (39.3) | 0.004 |
| Comorbidities | |||
| Obesity (%) | 24 (21.4) | 91 (19.0) | 0.65 |
| Hypertension (%) | 15 (13.4) | 61 (12.6) | 0.93 |
| Diabetes (%) (%) | 2 (1.8) | 20 (4.1) | 0.40 |
| Dyslipidaemia (%) | 11 (9.8) | 50 (10.3) | 1 |
| Ischaemic heart disease or stroke (%) | 1 (0.9) | 8 (1.6) | 1 |
| Vertebral or fragility fractures (%) | 2 (1.8) | 7 (1.4) | 0.68 |
Values are expressed as n (%) or median (IQR) unless otherwise indicated.
*Diagnostic delay: the difference between the date of the diagnosis and the date of the first symptom.
ASAS, Assessment of SpondyloArthritis International Society; ASDAS, Ankylosing Spondylitis Disease Activity Score; BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; BASFI, Bath Ankylosing Spondylitis Functional Index; CASPAR, ClASsification for Psoriatic ARthritis; DMARD, disease-modifying antirheumatic drug; HLA, human leucocyte antigens; NSAID, non-steroidal anti-inflammatory drug; SpA, spondyloarthritis; TNF, Tumor Necrosis Factor.
Clinical characteristics of patients with nr-axSpA by regions
Among nr-axSpA patients in Asia, 65.2% underwent MRI evaluation with 67.1% presenting with positive sacroiliitis on MRI; the corresponding proportion was 82.2% in other countries (85.4% tested). HLA-B27 test results were available for more than 90% of patients in both regions, and positivity was higher in Asian countries (90.6%, range 79.6%–97.6%, online supplemental table 1) vs in other countries (61.9%). Given this difference, we examined how patients were identified as having nr-axSpA, finding different patterns between regions. In Asian countries, 56.2% of nr-axSpA patients met only the HLA-B27-based clinical criteria, a proportion substantially lower in other countries (29.8%). The proportion of patients with inflammatory back pain were similar across regions. Peripheral signs of SpA such as peripheral arthritis, enthesitis, and dactylitis were identified less frequently among nr-axSpA patients in Asia (53.6% vs 66.3%). There were no differences in extra-articular manifestations including psoriasis, uveitis and IBD.
Erythrocyte sedimentation rate (ESR) levels and physician global assessment measurements were similar in nr-axSpA patients in Asia compared with patients in other regions, while elevated CRP levels, patient global assessment, ASDAS-CRP, BASDAI and BASFI measurements were lower among patients in Asia (table 1). Although the rate of NSAID use was similar between regions, good response to NSAIDs was comparatively higher in the Asian countries (71.4% vs 56.0%). Glucocorticoid use was identified in approximately 10% of patients regardless of region. Use of csDMARDs, including methotrexate, leflunomide, hydroxychloroquine, gold salts, azathioprine and sulfasalazine, was similar between patients in Asia (53.6%) and patients in other regions (54.7%), but methotrexate use was lower in Asia (16.1% vs 27.6%), which may be explained by the axial-dominant composition of those patients. The use of bDMARDs were lower in nr-axSpA patients in Asian countries (24.1%) compared with patients in other countries (39.3%). The comorbidity profile of nr-axSpA patients in Asia was similar to those in other countries.
Difference in clinical characteristics of patients within Asia: r-axSpA vs nr-axSpA
In the axSpA subset in Asia, including both r-axSpA and nr-axSpA patients, the age of onset and age at diagnosis were similar, and we identified no statistical difference in diagnostic delay as well (table 2). The proportion of male patients and smoking status was also similar between groups.
Table 2.
Difference in characteristics of non-radiographic axial SpA vs radiographic axial SpA within Asia
| Variables | nr-axSpA | r-axSpA | P value |
| n | 112 | 668 | |
| Age at disease diagnosis, years | 27.2 (21.1, 39.6) | 26.9 (21.7, 35.6) | 0.79 |
| Age at disease onset, years | 22.8 (18.5, 31.0) | 22.7 (18.3, 29.3) | 0.41 |
| Age at enrolment, years | 33.9 (24.9, 44.0) | 34.9 (26.8, 44.0) | 0.21 |
| Diagnostic delay*, years | 1.9 (0.27, 5.56) | 2.8 (0.50, 7.34) | 0.14 |
| Male (%) | 85 (75.9) | 533 (79.8) | 0.42 |
| Body mass index, kg/m2 | 23.5 (20.7, 26.6) | 22.9 (20.4, 25.7) | 0.15 |
| Smoking status | |||
| Never (%) | 67 (59.8) | 357 (53.4) | 0.25 |
| Past (%) | 17 (15.2) | 124 (18.6) | 0.47 |
| Current (%) | 28 (25.0) | 187 (28.0) | 0.59 |
| Family history of SpA (%) | 36 (32.1) | 228 (34.1) | 0.76 |
| Meeting ASAS axial SpA criteria | |||
| Imaging arm only (%) | 19 (17.0) | 147 (22.0) | 0.28 |
| Clinical arm only (%) | 63 (56.2) | 0 (0.0) | <0.001 |
| Both (%) | 30 (26.8) | 521 (78.0) | <0.001 |
| MRI tested (%) | 73 (65.2) | 249 (37.3) | <0.001 |
| Sacroiliitis among MRI tested (%) | 49 (67.1) | 193 (77.5) | 0.10 |
| HLA B27 measured (%) | 106 (94.6) | 596 (89.2) | 0.11 |
| HLA B27 positivity among measured (%) | 96 (90.6) | 551 (92.4) | 0.64 |
| Inflammatory back pain (%) | 107 (95.5) | 650 (97.3) | 0.36 |
| Arthritis, enthesitis, or dactylitis (%) | 60 (53.6) | 336 (50.3) | 0.59 |
| Peripheral arthritis (%) | 53 (47.3) | 277 (41.5) | 0.29 |
| Heel enthesitis (%) | 33 (29.5) | 159 (23.8) | 0.24 |
| Peripheral enthesitis (%) | 38 (33.9) | 182 (27.2) | 0.18 |
| Dactylitis (%) | 2 (1.8) | 35 (5.2) | 0.18 |
| Psoriasis (%) | 12 (10.7) | 34 (5.1) | 0.03 |
| CASPAR criteria (%) | 6 (5.4) | 12 (1.8) | 0.03 |
| Uveitis (%) | 20 (17.9) | 149 (22.3) | 0.35 |
| Inflammatory bowel disease (%) | 5 (4.5) | 12 (1.8) | 0.08 |
| Erythrocyte sedimentation rate, mm/hour | 10.0 (5.0, 19.0) | 13.0 (6.0, 28.5) | 0.004 |
| C reactive protein (CRP) level, mg/dL | 0.50 (0.30, 1.33) | 0.70 (0.30, 3.00) | 0.02 |
| Elevated CRP (%) | 37 (33.0) | 393 (58.8) | <0.001 |
| Physician global assessment (0–10) | 2.0 (1.0, 5.0) | 3.0 (2.0, 5.0) | 0.03 |
| Patient global assessment (0–10) | 3.0 (1.0, 6.0) | 4.0 (2.0, 6.0) | 0.048 |
| ASDAS-CRP | 1.40 (0.95, 2.08) | 1.79 (1.04, 2.66) | 0.006 |
| BASDAI | 2.8 (1.5, 4.2) | 3.0 (1.7, 4.8) | 0.22 |
| BASFI | 0.8 (0.1, 2.7) | 1.2 (0.2, 3.1) | 0.09 |
| Presence of bamboo spine (%) | 0 (0.0) | 99 (14.8) | <0.001 |
| History of SpA-related surgeries (%) | 0 (0.0) | 36 (5.4) | 0.02 |
| Treatment | |||
| NSAIDs use since onset (%) | 103 (92.0) | 586 (87.7) | 0.26 |
| Good response to NSAIDs (%) | 80 (71.4) | 430 (64.4) | 0.18 |
| Current glucocorticoid use (%) | 10 (8.9) | 65 (9.7) | 0.93 |
| Conventional synthetic DMARDs use (%) | 60 (53.6) | 328 (49.1) | 0.44 |
| Methotrexate use (%) | 18 (16.1) | 90 (13.5) | 0.56 |
| Sulfasalazine use (%) | 51 (45.5) | 296 (44.3) | 0.89 |
| Biological DMARDs (TNF antagonists) use (%) | 27 (24.1) | 197 (29.5) | 0.29 |
| Comorbidities | |||
| Obesity (%) | 24 (21.4) | 107 (16.1) | 0.21 |
| Hypertension (%) | 15 (13.4) | 96 (14.4) | 0.90 |
| Diabetes mellitus (%) | 2 (1.8) | 22 (3.3) | 0.56 |
| Dyslipidaemia (%) | 11 (9.8) | 66 (9.9) | 1 |
| Ischaemic heart disease or stroke (%) | 1 (0.9) | 8 (1.2) | 1 |
| Vertebral or fragility fractures (%) | 2 (1.8) | 22 (3.3) | 0.56 |
Values are expressed as n (%) or median (IQR) unless otherwise indicated.
*Diagnostic delay: the difference between the date of the diagnosis and the date of the first symptom.
ASAS, Assessment of SpondyloArthritis international Society; ASDAS, Ankylosing Spondylitis Disease Activity Score; BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; BASFI, Bath Ankylosing Spondylitis Functional Index; CASPAR, ClASsification for Psoriatic ARthritis; DMARD, disease-modifying antirheumatic drug; HLA, human leucocyte antigens; nr-axSpA, non-radiographic axial spondyloarthritis; NSAID, non-steroidal anti-inflammatory drug; r-axSpA, radiographic axial spondyloarthritis; SpA, spondyloarthritis; TNF, Tumor Necrosis Factor.
Regarding criteria by which patients in Asia were classified as having r-axSpA or nr-axSpA, more r-axSpA patients fulfilled both imaging and HLA-B27-based clinical arms of the ASAS criteria compared with patients with nr-axSpA (78% vs 26.8%). The proportion of patients with positive HLA-B27, inflammatory back pain, peripheral signs was similar between groups. There were no differences in extra-articular manifestations, except psoriasis, which was more frequent among nr-axSpA patients.
Concerning the burden of the disease within Asia, patient with r-axSpA compared with those with nr-axSpA showed higher disease activity and structural damage evaluated by ASDAS-CRP (1.79 vs 1.40), ESR (13.0 vs 10.0 mm/hour) and CRP level (0.70 vs 0.50 mg/dL), as well as presence of bamboo spine (14.8 vs 0%) and history of SpA-related surgery (5.4% vs 0%). Other variables, such as patient and physician global assessment, were significantly different between r-axSpA and nr-axSpA within Asia; BASFI was also numerically higher in r-axSpA patients (table 2). We found differences in neither BASDAI or comorbidities, nor in treatment modalities, including use of NSAIDs, csDMARDs and bDMARDs, between r-axSpA and nr-axSpA in the Asian countries.
Discussion
In this study, we examined the clinical characteristics of nr-axSpA patients in Asian countries in comparison to patients in non-Asian regions in order to clarify differences in composition, clinical features and practice patterns. We found striking differences in proportion of nr-axSpA among axSpA in Asia, which appears to be much less prevalent (14.4%) compared with other regions (24.3%). Limited access to MRI, precluding meeting MRI criteria, may be a contributing factor to underdiagnosis in Asia. However, higher HLA-B27 positivity, younger age at disease onset, and less diagnostic delay in nr-axSpA patients from Asian countries, contradict that underdiagnosis alone is sufficient to explain the lower disease proportion in Asia. Previous studies have reported that male sex confers high risk for disease progression; the higher proportion of men in nr-axSpA patients in Asian countries may contribute to less structural disease represented by nr-axSpA.23 While our cross-sectional study includes consecutively enrolled patients seen at ASAS-selected rheumatology practices, specific enrolment protocols may have varied slightly from practice to practice and by country. This may have influenced the frequency of patient and disease characteristics to some extent. However, it is unlikely that this explains all the differences seen, as our data reflect a broad swath of patients at ASAS expert rheumatology practices in a wide variety of regions.
We also found regional differences in demographic characteristics of nr-axSpA patients. A recent pooled analysis from a meta-analysis and systematic literature review has reported a significantly lower prevalence of men in nr-axSpA compared with r-axSpA (53.6% vs 69.6%).8 In contrast, we found a markedly higher proportion of men among nr-axSpA patients in Asian countries (75.9%) compared with other countries (47.1%). This result corroborates previous studies from Asia, which have reported that men comprise 64%–70% of nr-axSpA groups.4 9 10 Moreover, several studies have shown a male prevalence in HLA-B27 positive r-axSpA groups.24 25 In fact, with regards to HLA-B27 status, the number of male patients with HLA-B27 was higher than male patients without HLA-B27 in those studies. The substantially higher proportion of men among nr-axSpA patients from Asian countries appears to be an ethnic characteristic of the Asian population, though overdiagnosis in HLA-B27 men cannot be entirely excluded.
We also found markedly higher proportion of HLA-B27 among nr-axSpA patients in Asia in our study. A previous study reports an HLA-B27 positivity rate of <0.3% in the general Japanese population, compared with rates of about 5%–10% in other Asian and non-Asian countries.26 27 However, we found a higher HLA-B27 positivity rate in nr-axSpA patients throughout Asian countries, ranging from 79.6% in Japan to 97.6% in Taiwan (online supplemental table 1). Similarly, high rates of HLA-B27 from other studies of Asian patients support that this is a genetic characteristic of ethnically Asian populations.9 10 Although MRI evaluation is considered integral to the diagnosis of nr-axSpA, it is not readily accessible in some Asian countries, being performed in only 65.2% of nr-axSpA patients from Asia compared with 85.4% in other countries. As such, slightly more than half of patients in Asia were classified as nr-axSpA based solely on the HLA-B27-driven clinical arm of the ASAS criteria. It is also interesting to note that a sex-based comparison of r-axSpA patients from South Korea demonstrated a greater HLA-B27 prevalence among men.28 Previous studies have shown that HLA-B27 positive patients have a younger age of onset and shorter average delay to diagnosis compared with HLA-B27 negative patients with r-axSpA,29 30 and our study suggests that nr-axSpA patients in Asian countries may have similar trend. While previous studies have reported a high overall prevalence of HLA-B27 among nr-axSpA patients at 70%–80%,4 8 11 this may not be uniformly true according to our examination of the non-Asian ASAS-COMOSPA data. For example, HLA-B27 prevalence among nr-axSpA patients from Italy and Egypt was remarkably low at only 9.8% and 37.5%, respectively, and suggests marked regional variation in HLA-B27 prevalence.
In addition, comparing findings in all patients who received MRI, we still found a lower prevalence of MRI sacroiliitis in Asian patients. The proportion of positive MRI may reflect pretest probability of axSpA and local strategies of referral. In most Asian countries, it has been our experience that rheumatologists receive more patients with mechanical back pain—that is, with a low probability of having positive MRI—compared with specialists in Western countries, who may receive more targeted referrals. This is likely due to differences in primary care and other gatekeeping systems. In addition to the substantially lower rate of MRI performed in all Asian patients, an important limitation in itself, female subjects with inflammatory back pain who were HLA-B27 negative may not have been able to fulfil the ASAS criteria without an MRI showing sacroiliitis.31 32
For many patients, nr-axSpA classification is based on clinical arm findings and is not confirmed by imaging. One might justifiably question whether given the fact that this is the population with the highest possibility of a diagnostic mistake, it would be important to see whether a significant difference existed between Asian and non-Asian nr-axSpA patients fulfilling the clinical arm of the ASAS axSpA classification criteria. We conducted a subanalysis looking at differences in nr-axSpA patients fulfilling only clinical arm of the ASAS axSpA classification criteria (online supplemental table 2). We also found that nr-axSpA patients in Asia were predominantly male (p=0.06), had less diagnostic delay (p=0.03) and peripheral signs, as well as had milder disease (numerically lower ASDAS-CRP and significantly lower rate of abnormal CRP and BAFI). A sensitivity analysis of nr-axSpA patients fulfilling only clinical arm ASAS criteria corroborated results found in the main analysis.
A previous meta-analysis showed that, except for greater impairment of mobility in r-axSpA compared with nr-axSpA, both groups showed a comparable burden of disease, range of treatment modalities and treatment effects.8 In our study, the rate of elevated CRP, patient global assessment, ASDAS-CRP, BASDAI and BASFI were lower in nr-axSpA patients in Asian countries compared with those in other countries. Despite lower overall proportion of nr-axSpA, we may hypothesise that ASAS centres in Asian countries are identifying these patients appropriately early, possibly due to lower systems-based barriers to accessing expert rheumatology care. Regarding treatment effects, although rates of NSAID use was similar between regions, we found that a higher number of nr-axSpA patients in Asian countries responded adequately to NSAIDs. This may play a contributing role to lower disease burdens in nr-axSpA patients in Asia, and suggests a reason for the lower number of patients in Asia treated with bDMARDs. Alternatively, nr-axSpA in Asia may fundamentally represent milder disease than seen elsewhere, driven by ethnic and genetic variation, obviating the need for advanced therapies.
Regarding subanalysis of SpA patients within Asia, our comparison of characteristics between r-axSpA and nr-axSpA patients showed several differences and similarities compared with previous findings. Several previous studies from outside Asia have reported that, compared with those with r-axSpA, patients with nr-axSpA were overall younger in age, but older at time of disease onset, predominantly women, and demonstrated lower levels of acute phase reactants. These studies reported no differences between r-axSpA and nr-axSpA patients with regards to HLA-B27 prevalence, patient-reported disease activity, function or health-related quality of life (HRQoL).3 4 8 11 33 Another study also demonstrated several differences between these groups, notably for classic risk factors for structural damage including longer disease duration, male gender, smoking and increased CRP, all of which were more frequently observed in patients with r-axSpA, as well as for peripheral involvement, more frequently seen in nr-axSpA patients.8 Interestingly, in our subanalysis of the data within Asia, no significant differences were found between r-axSpA and nr-axSpA with regards to sex, age of disease onset, disease duration, smoking status or presence of peripheral signs. Mean and median age of disease onset of nr-axSpA was recently reported to range from 27.7 to 33.2 years in cohorts from Germany (German spondyloarthritis inception cohort (GESPIC)), Switzerland (Swiss Clinical Quality Management in Rheumatic Diseases (SCQM)), as well as in meta-analysis,8 34 35 all of which, except the GESPIC cohort were older than r-axSpA comparators. In contrast, age of disease onset of r-axSpA and nr-axSpA were similar at 22.7 years and 22.8 years, respectively, in our study, corroborating results from a South Asian population in India.10 We found that the proportion of HLA-B27 was also similar between r-axSpA and non-radiographic groups in our study, results in line with a previous study.36 An interesting explanation is provided by these authors, who considered that HLA-B27 might be artificially over-represented in nr-axSpA, given that HLA-B27 positivity is mandatory to fulfil the clinical ASAS criteria for nr-axSpA. As expected, our data showed a higher disease activity as well as greater structural damage in r-axSpA patients compared with those with nr-axSpA. These results support the hypothesis that both diagnoses belong to the same disease spectrum. Concerning treatment modalities between r-axSpA and nr-axSpA groups, while no conclusions could be drawn due to the cross-sectional study design, the data did not suggest any differences in treatment choices. Regarding extra-articular manifestations, several studies have shown that a more common history of uveitis in r-axSpA patients,8 32 a difference not found in our study, and suggesting the ongoing need to carefully assess for uveitis in nr-axSpA patients in order to prevent missed diagnoses.
Our study has some important limitations that warrant discussion. First, though selection bias is possible, the study was conducted in ASAS-associated centres and we expect any bias towards earlier detection to be non-differential and largely independent of country-specific socioeconomic factors. Second, the sample size of nr-axSpA in Asia are small, however, a previous single-centre retrospective cohort study in Japan, among 114 SpA patients, showed a similarly low prevalence of nr-axSpA patients (9.7% r-axSpA vs 1.8% nr-axSpA).37 Similarly, while better selection of patients for treatment is also possible in ASAS centres, patients were included in the study consecutively, and the disease characteristics of the population studied is reflective of a typical SpA population. Furthermore, the cross-sectional nature of ASAS-COMOSPA precludes the study of causal links and only allows for examination of associations.38 Finally, a gender bias is possible in the lower rate of nr-axSpA in Asia. A recent study published from Germany analysing a health insurance database showed that female sex was associated with nearly doubling of risk for diagnostic delay in axSpA patients.39 Our study also showed similar trend (online supplemental table 3); therefore, underdiagnosis of nr-axSpA in women in Asia cannot be excluded. Important strengths of the study include the large patient numbers and the uniqueness of ASAS-COMOSPA as one of the largest multinational SpA datasets to date to assess the clinical features of SpA patients involving 22 countries.
In conclusion, among axSpA patients, a substantially lower frequency of nr-axSpA was observed in Asian countries compared with other regions of the world. Nr-axSpA patients in Asian countries were predominantly male, and had younger disease onset with more frequent HLA-B27 positivity and less peripheral signs, as well as better response to NSAIDs. These results offer an opportunity to better understand the clinical characteristics of this patient population and optimise diagnostic strategies for accurate diagnosis of nr-axSpA patients in Asia, such as ensuring access and availability of MRI resources.
Acknowledgments
Part of this study was presented in the EULAR2021 (#POS0975). This study was conducted under the umbrella of the International Society for Spondyloarthritis Assessment (ASAS) and COMOSPA study was supported by unrestricted grants from Pfizer, AbbVie and UCB.
Footnotes
Funding: This study was conducted under the umbrella of the International Society for Spondyloarthritis Assessment (ASAS) and COMOSPA study was supported by unrestricted grants from Pfizer, AbbVie and UCB.
Competing interests: MK: Consulting fees and/or honoraria from AbbVie, Amgen-Astellas BioPharma, Asahi-Kasei Pharma, Astellas, Ayumi Pharma, BMS, Chugai, Daiichi-Sankyo, Eisai, Eli Lilly, Gilead, Janssen, Kyowa Kirin, Novartis, Ono Pharma, Pfizer, Tanabe-Mitsubishi, Teijin Pharma and UCB Pharma. KY: Consulting fees from OM1. Funding from Corrona. KY was additionally supported by the Rheumatology Research Foundation K Bridge Award, Brigham and Women's Hospital Department of Medicine Fellowship Award, and the US National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) Career Development Award (K23AR076453). YK: Grants or speaking fees from from AbbVie, Astellas, Ayumi, Bristol-Myers Squibb, Chugai, Eisai, Eli Lilly, Hisamitsu, Jansen, Kissei, Pfizer, Sanofi, Takeda, Tanabe-Mitsubishi, and UCB. NT: Speaker fees and/or consulting fees from AbbVie, Astellas, Bristol-Myers Squibb, Eisai, Eli Lilly, Janssen, Kyowa Kirin, Mitsubishi-Tanabe and Novartis. SK: Personal fees from Kyowa Kirin, Novartis Pharma K.K., Eli Lilly Japan K.K., Chugai Pharma, Asahi Kasei Pharma, Gilead Sciences and Janssen Pharma K.K. outside the submitted work. CL-M: Speaking fees from Janssen, Abbvie and UCB. AM has received research grants from Abbvie, Bristol-Myers Squibb, Gilead, MSD, Pfizer, Jansen, UCB. DvdH: Consulting fees AbbVie, Amgen, Astellas, AstraZeneca, Bayer, BMS, Boehringer Ingelheim, Celgene, Cyxone, Daiichi, Eisai, Eli-Lilly, Galapagos, Gilead, Glaxo-Smith-Kline, Janssen, Merck, Novartis, Pfizer, Regeneron, Roche, Sanofi, Takeda, UCB Pharma. Director of Imaging Rheumatology bv. TT: Consultancy and speaker fees from AbbVie, Astellas, Bristol-Myers Squibb, Eisai, Eli Lilly and Company, Janssen, Kyowa Kirin, Mitsubishi-Tanabe, Novartis and Pfizer. All other authors have declared no conflicts of interest.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available on reasonable request.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
Hôpital Cochin, APHP institutional review board approved the ASAS-COMOSPA study protocol (#SC 3004); approval was also obtained from each study site (22 countries). The study was conducted according to guidelines for good clinical practice in all countries.
References
- 1.Wang R, Gabriel SE, Ward MM. Progression of Nonradiographic axial spondyloarthritis to ankylosing spondylitis: a population-based cohort study. Arthritis Rheumatol 2016;68:1415–21. 10.1002/art.39542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rudwaleit M, van der Heijde D, Landewé R, et al. The development of assessment of spondyloarthritis International Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis 2009;68:777–83. 10.1136/ard.2009.108233 [DOI] [PubMed] [Google Scholar]
- 3.Boonen A, Sieper J, van der Heijde D, et al. The burden of non-radiographic axial spondyloarthritis. Semin Arthritis Rheum 2015;44:556–62. 10.1016/j.semarthrit.2014.10.009 [DOI] [PubMed] [Google Scholar]
- 4.Burgos-Varga R, Wei JC-C, Rahman MU, et al. The prevalence and clinical characteristics of nonradiographic axial spondyloarthritis among patients with inflammatory back pain in rheumatology practices: a multinational, multicenter study. Arthritis Res Ther 2016;18:132. 10.1186/s13075-016-1027-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braun J, Baraliakos X, Hermann K-G, et al. Effect of certolizumab pegol over 96 weeks of treatment on inflammation of the spine and sacroiliac joints, as measured by MRI, and the association between clinical and MRI outcomes in patients with axial spondyloarthritis. RMD Open 2017;3:e000430. 10.1136/rmdopen-2017-000430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deodhar A, Blanco R, Dokoupilová E, et al. Improvement of signs and symptoms of Nonradiographic axial spondyloarthritis in patients treated with Secukinumab: primary results of a randomized, placebo-controlled phase III study. Arthritis Rheumatol 2021;73:110–20. 10.1002/art.41477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deodhar A, van der Heijde D, Gensler LS, et al. Ixekizumab for patients with non-radiographic axial spondyloarthritis (COAST-X): a randomised, placebo-controlled trial. Lancet 2020;395:53–64. 10.1016/S0140-6736(19)32971-X [DOI] [PubMed] [Google Scholar]
- 8.López-Medina C, Ramiro S, van der Heijde D, et al. Characteristics and burden of disease in patients with radiographic and non-radiographic axial spondyloarthritis: a comparison by systematic literature review and meta-analysis. RMD Open 2019;5:e001108. 10.1136/rmdopen-2019-001108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Min HK, Lee J, Ju JH, et al. Predictors of assessment of spondyloarthritis International Society (ASAS) health index in axial spondyloarthritis and comparison of ASAS health index between ankylosing spondylitis and Nonradiographic axial spondyloarthritis: data from the Catholic axial spondyloarthritis cohort (CASCO). J Clin Med 2019;8:467. 10.3390/jcm8040467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malaviya AN, Kalyani A, Rawat R, et al. Comparison of patients with ankylosing spondylitis (AS) and non-radiographic axial spondyloarthritis (nr-axSpA) from a single rheumatology clinic in New Delhi. Int J Rheum Dis 2015;18:736–41. 10.1111/1756-185X.12579 [DOI] [PubMed] [Google Scholar]
- 11.Zhao SS, Ermann J, Xu C, et al. Comparison of comorbidities and treatment between ankylosing spondylitis and non-radiographic axial spondyloarthritis in the United States. Rheumatology 2019;58:2025–30. 10.1093/rheumatology/kez171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kishimoto M, Yoshida K, Ichikawa N, et al. Clinical characteristics of patients with spondyloarthritis in Japan in comparison with other regions of the world. J Rheumatol 2019;46:896–903. 10.3899/jrheum.180412 [DOI] [PubMed] [Google Scholar]
- 13.Moltó A, Etcheto A, van der Heijde D, et al. Prevalence of comorbidities and evaluation of their screening in spondyloarthritis: results of the international cross-sectional ASAS-COMOSPA study. Ann Rheum Dis 2016;75:1016–23. 10.1136/annrheumdis-2015-208174 [DOI] [PubMed] [Google Scholar]
- 14.Rudwaleit M, van der Heijde D, Landewé R, et al. The assessment of spondyloarthritis International Society classification criteria for peripheral spondyloarthritis and for spondyloarthritis in general. Ann Rheum Dis 2011;70:25–31. 10.1136/ard.2010.133645 [DOI] [PubMed] [Google Scholar]
- 15.Garrett S, Jenkinson T, Kennedy LG, et al. A new approach to defining disease status in ankylosing spondylitis: the Bath ankylosing spondylitis disease activity index. J Rheumatol 1994;21:2286–91. [PubMed] [Google Scholar]
- 16.Lukas C, Landewé R, Sieper J, et al. Development of an ASAS-endorsed disease activity score (ASDAS) in patients with ankylosing spondylitis. Ann Rheum Dis 2009;68:18–24. 10.1136/ard.2008.094870 [DOI] [PubMed] [Google Scholar]
- 17.van der Heijde D, Lie E, Kvien TK, et al. ASDAS, a highly discriminatory ASAS-endorsed disease activity score in patients with ankylosing spondylitis. Ann Rheum Dis 2009;68:1811–8. 10.1136/ard.2008.100826 [DOI] [PubMed] [Google Scholar]
- 18.Calin A, Garrett S, Whitelock H, et al. A new approach to defining functional ability in ankylosing spondylitis: the development of the Bath ankylosing spondylitis functional index. J Rheumatol 1994;21:2281–5. [PubMed] [Google Scholar]
- 19.van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the new York criteria. Arthritis Rheum 1984;27:361–8. 10.1002/art.1780270401 [DOI] [PubMed] [Google Scholar]
- 20.Taylor W, Gladman D, Helliwell P, et al. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum 2006;54:2665–73. 10.1002/art.21972 [DOI] [PubMed] [Google Scholar]
- 21.Yoshida K, Bohn J. Package “tableone” [online]. Available: https://CRAN.R-project.org/package=tableone/ [Accessed 20 Dec 2018].
- 22.Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 2013;48:452–8. 10.1038/bmt.2012.244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sari I, Lee S, Tomlinson G, et al. Factors predictive of radiographic progression in ankylosing spondylitis. Arthritis Care Res 2021;73:275–81. 10.1002/acr.24104 [DOI] [PubMed] [Google Scholar]
- 24.Yang M, Xu M, Pan X, et al. Epidemiological comparison of clinical manifestations according to HLA-B*27 carrier status of Chinese ankylosing spondylitis patients. Tissue Antigens 2013;82:338–43. 10.1111/tan.12186 [DOI] [PubMed] [Google Scholar]
- 25.Akassou A, Yacoubi H, Jamil A, et al. Prevalence of HLA-B27 in Moroccan healthy subjects and patients with ankylosing spondylitis and mapping construction of several factors influencing AS diagnosis by using multiple correspondence analysis. Rheumatol Int 2015;35:1889–94. 10.1007/s00296-015-3342-x [DOI] [PubMed] [Google Scholar]
- 26.Ikeda N, Kojima H, Nishikawa M, et al. Determination of HLA-A, -C, -B, -DRB1 allele and haplotype frequency in Japanese population based on family study. Tissue Antigens 2015;85:252–9. 10.1111/tan.12536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reveille JD, Hirsch R, Dillon CF, et al. The prevalence of HLA-B27 in the US: data from the US National Health and Nutrition Examination Survey, 2009. Arthritis Rheum 2012;64:1407–11. 10.1002/art.33503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jung Y-O, Kim I, Kim S, et al. Clinical and radiographic features of adult-onset ankylosing spondylitis in Korean patients: comparisons between males and females. J Korean Med Sci 2010;25:532–5. 10.3346/jkms.2010.25.4.532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feldtkeller E, Khan MA, van der Heijde D, et al. Age at disease onset and diagnosis delay in HLA-B27 negative vs. positive patients with ankylosing spondylitis. Rheumatol Int 2003;23:61–6. 10.1007/s00296-002-0237-4 [DOI] [PubMed] [Google Scholar]
- 30.Wu Z, Lin Z, Wei Q, et al. Clinical features of ankylosing spondylitis may correlate with HLA-B27 polymorphism. Rheumatol Int 2009;29:389–92. 10.1007/s00296-008-0743-0 [DOI] [PubMed] [Google Scholar]
- 31.Bubová K, Forejtová Šárka, Zegzulková K, et al. Cross-sectional study of patients with axial spondyloarthritis fulfilling imaging arm of ASAS classification criteria: baseline clinical characteristics and subset differences in a single-centre cohort. BMJ Open 2019;9:e024713. 10.1136/bmjopen-2018-024713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hong C, Kwan YH, Leung Y-Y, et al. Comparison of ankylosing spondylitis and non-radiographic axial spondyloarthritis in a multi-ethnic Asian population of Singapore. Int J Rheum Dis 2019;22:1506–11. 10.1111/1756-185X.13603 [DOI] [PubMed] [Google Scholar]
- 33.Wallman JK, Kapetanovic MC, Petersson IF, et al. Comparison of non-radiographic axial spondyloarthritis and ankylosing spondylitis patients--baseline characteristics, treatment adherence, and development of clinical variables during three years of anti-TNF therapy in clinical practice. Arthritis Res Ther 2015;17:378. 10.1186/s13075-015-0897-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rudwaleit M, Haibel H, Baraliakos X, et al. The early disease stage in axial spondylarthritis: results from the German spondyloarthritis inception cohort. Arthritis Rheum 2009;60:717–27. 10.1002/art.24483 [DOI] [PubMed] [Google Scholar]
- 35.Ciurea A, Scherer A, Exer P, et al. Tumor necrosis factor α inhibition in radiographic and nonradiographic axial spondyloarthritis: results from a large observational cohort. Arthritis Rheum 2013;65:3096–106. 10.1002/art.38140 [DOI] [PubMed] [Google Scholar]
- 36.de Winter JJ, van Mens LJ, van der Heijde D, et al. Prevalence of peripheral and extra-articular disease in ankylosing spondylitis versus non-radiographic axial spondyloarthritis: a meta-analysis. Arthritis Res Ther 2016;18:196. 10.1186/s13075-016-1093-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Endo Y, Fujikawa K, Koga T, et al. Characteristics of late-onset spondyloarthritis in Japan: a retrospective cohort study. Medicine 2019;98:e14431. 10.1097/MD.0000000000014431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nikiphorou E, van der Heijde D, Norton S, et al. Inequity in biological DMARD prescription for spondyloarthritis across the globe: results from the ASAS-COMOSPA study. Ann Rheum Dis 2018;77:405–11. 10.1136/annrheumdis-2017-212457 [DOI] [PubMed] [Google Scholar]
- 39.Redeker I, Callhoff J, Hoffmann F, et al. Determinants of diagnostic delay in axial spondyloarthritis: an analysis based on linked claims and patient-reported survey data. Rheumatology 2019;58:1634–8. 10.1093/rheumatology/kez090 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2021-001752supp001.pdf (93.6KB, pdf)
Data Availability Statement
Data are available on reasonable request.