Abstract
Background
Anti-programmed death 1 (PD1)/programmed cell death ligand 1 (PD-L1) therapies have shown modest activity as monotherapy in recurrent ovarian cancer. Platinum chemotherapies induce T-cell proliferation and enhance tumor recognition. We assessed activity and safety of pembrolizumab with carboplatin in recurrent platinum-resistant ovarian cancer.
Patients and methods
This phase I/II, single-arm clinical trial studied concurrent carboplatin and pembrolizumab in recurrent platinum-resistant ovarian, fallopian tube, and primary peritoneal cancer. Primary platinum refractory patients were excluded. Patients were treated after progression on subsequent non-platinum systemic therapy after becoming platinum resistant or refractory. Pembrolizumab 200 mg was given on day 1 and carboplatin area under the curve 2 on days 8 and 15 of a 3-week cycle until progression. Imaging was assessed by blinded independent review. PD-L1 expression was assessed by immunohistochemistry. Flow cytometry on peripheral blood mononuclear cells was performed for CD3, CD4, CD8, PD1, CTLA4 and Ki67.
Results
The most common treatment-related adverse events were lymphopenia (18%) and anemia (9%) with most being grade 1 or 2 (93%). Of 29 patients treated, 23 patients were evaluable for best objective response: 10.3% (95% CI 2.2 to 27.4) had partial response (PR), 51.7% (95% CI 32.5 to 70.6) had stable disease (SD). 56.5% of patients had decreases in target lesions from baseline. All PD-L1-positive patients achieved PR (3/7, 42.8%) or SD (4/7, 57.2%). Median progression-free survival was 4.63 months (95% CI 4.3 to 4.96). Median OS was 11.3 months (95% CI 6.094 to 16.506). Peripheral CD8+PD1+Ki67+ T cells expanded after 3 (p=0.0015) and 5 (p=0.0023) cycles. CTLA4+PD1+CD8+ T cells decreased through the course of treatment up to the 12th cycle (p=0.004). When stratified by ratio of peripheral CD8+PD1+Ki67+ T cells to tumor burden at baseline, patients with a ratio ≥0.0375 who had a significantly longer median OS of 18.37 months compared with those with a ratio <0.0375 who had a median OS of 8.72 months (p=0.0099). No survival advantage was seen with stratification by tumor burden alone (p=0.24) or by CD8+PD1+Ki67+ T cells alone (p=0.53).
Conclusions
Pembrolizumab with carboplatin was well-tolerated and active in recurrent platinum-resistant ovarian cancer. A ratio of peripheral T-cell exhaustion to radiographic tumor burden may identify patients more likely to benefit from this chemoimmunotherapy.
Trial registration number
Keywords: genital neoplasms, female, CD8-positive t-lymphocytes, immunotherapy, drug therapy, combination
Introduction
Antibodies targeting the anti-programmed death 1 (PD1)/programmed cell death ligand 1 (PD-L1) pathway have thus far shown only modest activity as monotherapy to treat recurrent advanced ovarian cancer. Pembrolizumab has shown activity in recurrent advanced ovarian cancer with an 8% response rate and a median progression-free survival (PFS) of 2.1 months reported in KEYNOTE-100.1 Similar response rates and PFS duration are seen with treatment with nivolumab and avelumab in recurrent advanced ovarian cancer.2 3 As a result, there is interest in exploring combinations with anti-PD1/PD-L1 agents to improve efficacy for recurrent ovarian cancer.4–7
Cytotoxic chemotherapies have been shown to stimulate the immune system in several ways.8 Platinum chemotherapies possess unique immune properties and induce T cell proliferation and cytokine release.9 10 Cisplatin and carboplatin promote cytotoxic T cell activity in vitro at concentrations used in vivo.11 Modulation of PD-L1 and PD-L2 has been shown to be mediated through STAT V.6 and these immune effects have also been demonstrated in mouse models of ovarian cancer.12 13 Carboplatin, in particular, induces T cell proliferation in vitro to significantly higher levels compared with other cytotoxic chemotherapies.13 We hypothesized these effects could be exploited to synergize with anti-PD1 therapy. We assessed the safety and activity of pembrolizumab with carboplatin in recurrent platinum-resistant ovarian cancer.
Response rates to anti-PD1 therapies in recurrent ovarian cancer have been low, so we also explored whether immune analysis from archival tumor samples or contemporaneous measures of peripheral immune response and tumor burden could identify patients who would benefit from this approach. Higher PD-L1 expression in tumors has correlated with improved response rates to pembrolizumab in ovarian cancer, but without significant improvement in PFS.1 14 Tumor-infiltrating lymphocytes at time of diagnosis have been associated with improved survival in ovarian cancer, but their prognostic value may be abrogated after cytotoxic chemotherapy.15–17 Peripheral lymphocytes have been associated with survival in ovarian cancer, independent of tumor-infiltrating lymphocytes.18 Peripheral markers measuring invigoration of exhausted T cells in a ratio with overall tumor burden are associated with prolonged survival with pembrolizumab therapy in other malignancies.19
Patients and methods
Patient population
After informed consent, patients with recurrent ovarian, fallopian tube, or peritoneal carcinoma were enrolled from May 2017 to October 2018. Key eligibility criteria for this phase I/II single arm trial were platinum-resistant advanced ovarian, fallopian, or peritoneal carcinoma with progression on subsequent non-platinum systemic therapy after becoming platinum resistant or refractory. Primary platinum refractory patients were excluded. Enrolled patients received intravenous infusion of pembrolizumab 200 mg on day 1 followed by intravenous infusion of carboplatin area under the curve 2 on days 8 and 15 of a 3-week cycle for 2 years or until progression or unacceptable toxicity. Tumor imaging was performed prior to cycles 4 and 8, and then every 3 months.
Study design
This phase I/II, single-arm clinical trial was designed to examine the clinical response rate of concurrent platinum and pembrolizumab in patients with recurrent platinum-resistant ovarian, fallopian tube, and primary peritoneal cancer. The primary objectives were to (1) determine the clinical response rate of platinum chemotherapy and pembrolizumab and (2) evaluate whether platinum chemotherapy and pembrolizumab in platinum-resistant ovarian, fallopian tube, and primary peritoneal cancer improves PFS. To determine the clinical response of concurrent pembrolizumab and platinum chemotherapy, serial imaging studies evaluated target lesions and were assessed by both RECIST V.1.1 and irRECIST V.1.1 by blinded, independent review. Imaging was performed at baseline and prior to cycles 4 and 8 and then prior to every fourth cycle. Target lesion responses were described as (1) complete response, (2) partial response (PR), (3) progressive disease (PD) and (4) stable disease (SD). All adverse events (AEs) were reported according to NCI Common Terminology for Adverse Events V.4.0.
Evaluation of PD-L1 expression on tumor tissue
Archival tumor was obtained for immunohistochemical staining. Tissue slides were shipped to QualTek Molecular Laboratories (Newtown, Pennsylvania, USA) for PD-L1 analysis where they were stained using the PD-L1 IHC 22C3 antibody and expression of PD-L1 was scored by a board-certified pathologist. Each slide was given a modified proportion score (MPS), the overall per cent of positive cells expressing PD-L1. MPS is a variant of a typical proportion score, where mononuclear inflammatory cells that express PD-L1 are counted in conjunction with the tumor cells. Only tumor nests were scored for PD-L1 positivity, so the surrounding stroma PD-L1 staining is excluded from MPS. For this study, MPS ≥5% was defined as PD-L1 positive.
Evaluation of immune signatures by flow cytometry
Cryopreserved peripheral blood mononuclear cell (PBMC) samples from baseline and post-treatment (cycles 1–12) were thawed and rested overnight at 37°C. Then cells were stained with a viability dye (eBioscience Fixable Viability Dye eFluor 450) and a master mix of antibodies for surface stains including CD4-BV605 (BioLegend Cat# 317438, RRID:AB_11218995), CD3-PE-Cyanine5.5 (Invitrogen Cat# 35-0036-42, RRID: AB_11220085), CD8a-PE-Cyanine7 (Invitrogen Cat# 25-0088-42, RRID: AB_1659702), PD1-APC (BioLegend Cat# 329908, RRID: AB_940475), CD152-PE-Cy5 (BD Biosciences Cat# 555854, RRID:AB_396177). Cells were next fixed and permeabilized with the eBioscience Foxp3/Transcription Factor Staining Buffer Set (ThermoFisher) and subsequently stained intracellularly with Alexa Fluor 700 antihuman Ki67 antibody (BioLegend Cat# 350530, RRID: AB_2564040). Stained cells were acquired on a BD Canto RUO and analyzed with FlowJo software (FlowJo, RRID:SCR_008520).
Calculation of immune cell:tumor burden ratios
Ki67+PD1+CD8+ T cell:tumor burden ratios were calculated from flow cytometry performed on PBMC and total RECIST tumor burden measured at enrollment prior to therapy as previously reported. The ratio was calculated as %Ki67+PD1+CD8+ cells over the RECIST V.1.1 total at baseline.19
Statistical evaluation
A sample size of 27 patients was calculated to have 80% power to declare statistical significance at level 0.05 when the true response rate of the combinatorial therapy is 50%. Clinical characteristics of the study cohort at the time of initial diagnosis were tabulated. Response rate was compared with historical control rates by examining whether 95% CI cover the historical control rate. Time-to-event variables were analyzed using the Kaplan-Meier method. Kaplan-Meier estimates of the survival function with 95% CIs at specific time points (using Greenwood’s formula for the SE) were computed. Comparisons for other efficacy end points with the historical control PFS and overall survival (OS) were conducted by examining whether the 95% CI covers the historical control proportions. Statistical analyses were performed using GraphPad Prism V.8.0.2 (GraphPad Prism, RRID:SCR_002798). Correlations were determined by Pearson’s r coefficient. Repeated measures comparisons were analyzed using the mixed-effects model without the Geisser-Greenhouse correction, with Tukey’s multiple comparisons test.
Results
Patient population
A total of 29 patients were enrolled (table 1). The median age of patients was 65 years (range: 41–80). Patients were heavily pretreated with a median of 4 (range: 2–9) prior lines of systemic therapy. Prior bevacizumab and PARP inhibitor therapy was received by 72.4% and 31% of patients, respectively. Most patients had high-grade serous histology and were initially diagnosed at stage 3 or 4.
Table 1.
Patient demographics
| Characteristic | Median (range) | No. | % |
| Number of patients treated | – | 29 | – |
| Age, years | 65 (41–80) | – | – |
| Disease stage | |||
| IIC | – | 1 | 3.4 |
| III | – | 21 | 72.4 |
| IV | – | 7 | 24.1 |
| Histology | |||
| Serous | 26 | 90 | |
| Non-serous | 3 | 10 | |
| Number of previous chemotherapy regimens | 4 (2–9) | – | – |
| Prior bevacizumab therapy | – | 21 | 72.4 |
| Prior PARP inhibitor therapy | – | 9 | 31.0 |
| RECIST V.1.1 tumor burden | |||
| Number of target lesions | 3 (1–5) | 29 | – |
| Size of longest diameter in largest target lesion (mm) | 32.8 (14.8–124.4) | 29 | – |
| RECIST V.1.1 total | 65.3 (15–198.2) | 29 | – |
| BRCA status | |||
| BRCA1 | – | 4 | 13.8 |
| BRCA2 | – | 1 | 3.4 |
| Negative | – | 23 | 79.3 |
| Unknown | – | 1 | 3.4 |
Safety
Treatment with pembrolizumab and carboplatin was well tolerated overall (table 2). The most common treatment-related (TR) AEs were lymphopenia and anemia. The majority of TR AEs were grade 1 or 2 (94%). Six per cent of AEs were grade 3 with lymphopenia the most common. The two grade 4 AEs were neutropenia and lymphopenia.
Table 2.
Adverse events (AEs)
| Most common | Possibly, probably, or definitely related | All AEs | ||
| No. | % of related AEs | No. | % of all AEs | |
| Lymphopenia | 97 | 21.5 | 100 | 12.4 |
| Anemia | 51 | 11.3 | 82 | 10.2 |
| Hypoalbuminemia | 24 | 5.3 | 42 | 5.2 |
| Thrombocytopenia | 33 | 7.3 | 37 | 4.6 |
| White blood cell decreased | 33 | 7.3 | 35 | 4.3 |
| Hypocalcemia | 7 | 1.6 | 31 | 3.8 |
| Hypokalemia | 4 | 0.9 | 27 | 3.3 |
| Nausea | 21 | 4.7 | 26 | 3.2 |
| Neutropenia | 25 | 5.5 | 25 | 3.1 |
| Hypomagnesemia | 18 | 4.0 | 24 | 3.0 |
| AE gradings | ||||
| 1 | 316 | 70.0 | 597 | 74.0 |
| 2 | 107 | 24.0 | 169 | 21.0 |
| 3 | 26 | 6.0 | 40 | 5.0 |
| 4 | 2 | 0.0 | 2 | 0.0 |
| 5 | 0 | 0.0 | 0 | 0.0 |
Antitumor activity
Of 29 patients treated, 10.3% (95% CI 2.2 to 27.4) had PR, 51.7% (95% CI 32.5 to 70.6) had SD and 17.2% (95% CI 5.8 to 35.8) had PD (figure 1A). One patient achieved disease stability for 45 weeks. There was no difference in best overall rates of response between RECIST and irRECIST methodologies; 56.5% of patients had decreases in RECIST target lesions from baseline (figure 1B). Median PFS was 4.63 months (95% CI 4.3 to 4.96) (figure 2A). Median OS was 11.3 months (95% CI 6.094 to 16.506) (figure 2B). Seven of the 23 evaluable patients (30.4%) had archival tumor with modified per cent scoring ≥5 for PD-L1 and all achieved PR (3/7, 42.8%) or SD (4/7, 57.2%) as best objective response. However, there was no significant improvement in PFS (PD-L1+ median 4.63 (95% CI 4.55 to 4.73), PD-L1− median 4.70 (95% CI 0.00 to 9.41), p=0.87) or OS (PD-L1+ median 14.37 (95% CI 10.43 to 18.30), PD-L1− median 10.90 (95% CI 7.87 to 13.93), p=0.96) in the PD-L1-positive patients compared with PD-L1-negative patients.
Figure 1.
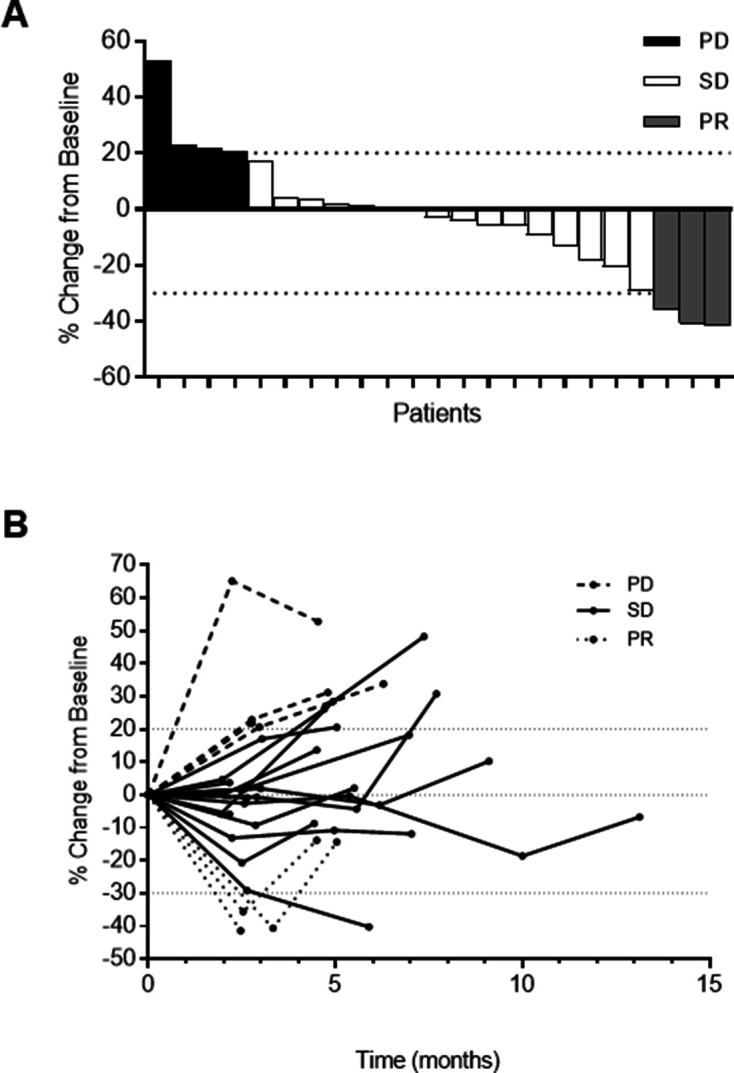
(A) Waterfall plot. Best percentage change in tumor size from baseline by RECIST V.1.1. (B) Spider plot. Percentage change in tumor size over time from baseline by RECIST V.1.1.
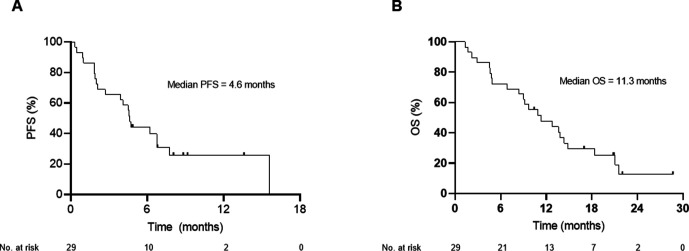
Figure 2.
Progression-free survival (PFS) and overall survival (OS). Symbols at censored patients. (A) PFS median (95% CI)=4.633 (4.301 to 4.966), 6-month PFS rate=44.14%. (B) OS median (95% CI)=11.3 (6.094 to 16.506) months.
Exploratory studies of peripheral immune markers for T cell exhaustion and radiographic tumor burden
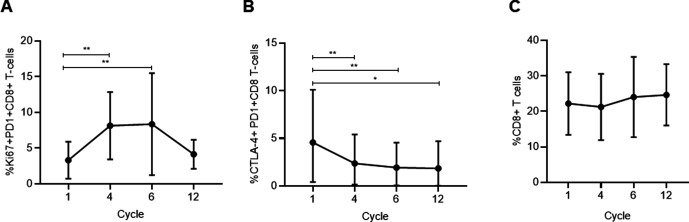
Because immunologically relevant circulating T cell populations have been shown to be a potential predictor to PD1 blockade in other malignancies and combinatorial therapy targeting markers in these subpopulations (ie, nivolumab and ipilimumab) have shown activity in ovarian cancer, we performed exploratory flow cytometry on T cells obtained during this study to assess modulation of these markers with pembrolizumab and carboplatin.7 19 Peripheral CD8+PD1+Ki67+ T cells increased significantly from baseline after 3 (p=0.0015) and 5 (p=0.0023) cycles (figure 3A). We also see significant decreases in the percentages of CTLA4+PD1+CD8+ T cells through the course of treatment up to the 12th cycle (figure 3B). There was no significant difference in the total peripheral CD8+ population after 3 (p=0.81) and 5 cycles (p=0.21) (figure 3C).
Figure 3.
(A) Change in Ki67+PD1+CD8+ T cells throughout treatment, p=0.0004. Cycle 1 vs cycle 4 p=0.0015, cycle 1 vs cycle 6 p=0.0023. Dots at mean, error bars SD. (B) Change in CTLA-4+PD1+CD8+ T cells throughout treatment, p=0.0004. Dots at mean, error bars SD. Tukey’s multiple comparisons test showed the following significant comparisons: cycle 1 vs cycle 4 p=0.0034; cycle 1 vs cycle 6 p=0.0010; cycle 1 vs cycle 12 p=0.0336. (C) Change in CD8+CD4+ T cells throughout treatment. No significant difference was observed in the total peripheral CD8+ population after 3 cycles (p=0.81) and 5 cycles (p=0.21). Dots at mean, error bars SD.
T-cell exhaustion evidenced by these subpopulations when placed in a ratio with radiographic tumor burden have predicted clinical outcome with anti-PD1 monotherapy in melanoma.19 We used RECIST V.1.1 criteria for baseline tumor burden as this is the most widely accepted methodology for radiographic tumor quantitation. While we acknowledge this limits the number of baseline target lesions, data warehouse analysis when the RECIST V.1.1 version was implemented showed no loss of information with the move to a reduced lesion number.20 21 Stratification by median baseline RECIST tumor burden alone did not yield a significant survival advantage for patients with lower volume disease (p=0.24). When patients are stratified by the median ratio of percentage of CD8+PD1+Ki67+ T cells at baseline to total RECIST tumor burden at baseline, patients with a ratio ≥0.0375 had a significantly longer median OS of 18.37 months compared with those with a ratio <0.0375 who had a median OS of 8.72 months, a 9.65-month OS advantage (p=0.0099) (figure 4). No advantage was seen in PFS (p=0.54). We tried other cutpoints above and below the median and confirmed that median yields the best contrast between the two groups. Stratification by median CD8+PD1+Ki67+ T cells at baseline alone also does not yield any significant survival advantage (p=0.53).
Figure 4.
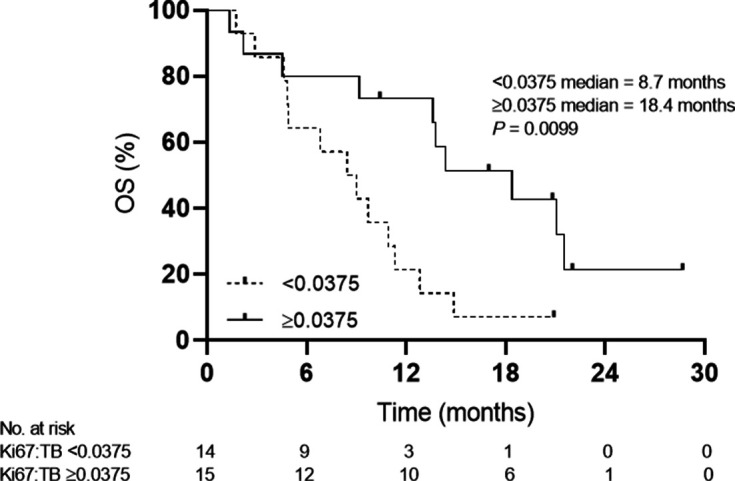
Kaplan-Meier plot of baseline Ki67/tumor burden (TB) ratio stratified by less than and greater than the median ratio (0.0375); <0.0375 median=8.72 months, ≥0.0375 median=18.37 months, p=0.0099. Symbols at censored patients. OS, overall survival.
Discussion
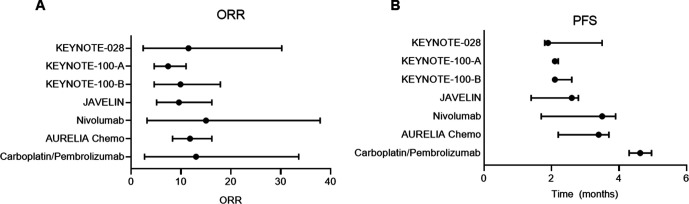
Although the response rate with pembrolizumab and low-dose carboplatin did not differ from monotherapy with anti-PD1/PD-L1 (7.4%–15%) or second-line cytotoxic chemotherapies (11.8%) (figure 5A), median PFS in our trial exceeded what has been reported for single agent cytotoxic chemotherapies without overlap in 95% CIs meeting our defined second primary end point (figure 5B).1–3 14 22 Combinations with anti-PD1/PD-L1 agents to improve efficacy for recurrent ovarian cancer have been explored in other phase II studies, but most are non-randomized and not all have been restricted to platinum-resistant patients.4–7
Figure 5.
(A) Overall response rate (ORR) comparison. (B) Progression-free survival (PFS) comparison with other monotherapies for platinum-resistant ovarian cancer: KEYNOTE (pembrolizumab),1 13 JAVELIN (avelumab),2 nivolumab,3 and AURELIA Chemo (paclitaxel, pegylated liposomal doxorubicin, topotecan).20
Low-dose carboplatin with pembrolizumab has favorable tolerability compared with other anti-PD1 combinations in recurrent ovarian cancer.4 7 Lower doses of carboplatin used in combination chemotherapy for ovarian cancer have demonstrated improved side-effect profiles, which is of particular importance for regimens contemplated for patients with recurrent ovarian cancer, where PFS must be balanced against toxicities.23 24 Both lower doses and metronomic doses of cytotoxic chemotherapies and optimization of the interval between chemotherapy and immunotherapy may also permit modulation of lymphocytes and the immune tumor microenvironment.25
The study has some limitations. It consisted of patients in a single arm and may be subject to selection bias. Patients were more heavily pretreated than similar trials in platinum-resistant ovarian cancer, and although this may represent a group that may be more or less chemoresistant, it could also represent patients with better prognosis. PD-L1-positive patients showed an improved response rate compared with KEYNOTE-028, which selected this population for treatment with pembrolizumab monotherapy, but the sample size of this study was not powered to detect a difference either in response or survival for this subgroup.14 Like the AURELIA study, patients refractory to frontline platinum were excluded in this study.22 However, unlike AURELIA, the majority of patients in this study had previously been treated with bevacizumab. While some monotherapy anti-PD1/PD-L1 studies could have included patients refractory to frontline platinum, the contribution appears to be small. In KEYNOTE 100, only 4 patients out of 291 (1.4%) with reported platinum response status were refractory to any line of platinum therapy.26 Responses to platinum chemotherapy after clinical diagnosis of platinum-resistant ovarian cancer are uncommon, but have been reported in retrospective studies. These illustrate that the definition of platinum resistance is imperfect.27–29 This study was not designed to establish whether low-dose carboplatin is augmenting response to pembrolizumab or vice versa, but instead sought to identify an additive benefit of combining an immunotherapy with low-dose carboplatin to improve response rates over monotherapy. Given the imperfect definition of platinum resistance, some cytotoxic contribution from platinum may have occurred in this study. However, the low dose used and high percentage of disease stability favors an immunotherapy effect. Finally, although the markers identified in the exploratory analyses could possess predictive or prognostic value, the design of the study permits us to suggest these as hypothesis generating only.
The OS observed for this study does not differ significantly from what would be expected in a heavily pretreated platinum-resistant ovarian cancer population with standard therapies. The absence of an OS advantage in studies that have shown improved PFS is not uncommon for ovarian cancer and has led to questions regarding the appropriate end points for all ovarian cancer clinical trials.22 30 This is particularly relevant for immunotherapies in recurrent ovarian cancer, where there may be improved efficacy with subsequent lines of chemotherapy.31
BRCA1 and BRCA2 mutated ovarian cancers have been noted to have a higher neoantigen load, greater numbers of tumor-infiltrating lymphocytes and expression of PD1/PD-L1, which may lead them to be more sensitive to PD1/PD-L1 inhibitors.32 Only five patients in our study had known BRCA1 or BRCA2 mutations so the ability to meaningfully correlate this with response in a study of this size would be limited, but this would be an important line for future inquiry. However, low response rates for immunotherapies in ovarian cancer continue to drive the need for markers to identify patients who would most benefit from these approaches.
Our exploratory analysis of peripheral markers of T cell exhaustion and tumor burden was undertaken after studies identified the significance of these measures in patients with melanoma treated with pembrolizumab monotherapy.19 The prognostic significance of residual tumor volume in ovarian cancer has long been known.33 34 Tumor-infiltrating lymphocytes have been shown to have prognostic significance, but platinum-based chemotherapy can alter the tumor microenvironment along with the prognostic significance of tumor-infiltrating lymphocytes, so greater tumor burdens may exert immune effects detectable in peripheral T cells from patients with ovarian cancer.15–18 35 This analysis generates a hypothesis that such a ratio could represent an accessible marker to select recurrent ovarian cancers for chemoimmunotherapy, since biopsies are not routinely obtained in the recurrent setting, but radiographic imaging is commonly used to confirm recurrences detected by serological tumor markers, such as CA-125.
We see evidence of T cell reinvigoration with expansion of CD8+PD1+Ki67+ T cell populations with carboplatin pembrolizumab therapy without concurrent increase in the total CD8+ population. In recurrent ovarian cancer, early detection of low volume disease is possible with CA-125 surveillance.36 We are studying the potential synergy of carboplatin with pembrolizumab with a modified regimen in patients with ovarian cancer with low volume disease, with biochemical recurrences, to optimize the tumor burden side of this ratio (NCT# 04387227). Overall, these findings suggest that strategies such as using platinum as an immune sensitizer in select patients may improve efficacy of anti-PD1/PD-L1-based therapies, even in cancers considered less immunogenic.
jitc-2021-003122supp001.pdf (157.5KB, pdf)
Footnotes
Contributors: Study concept: JL. Study design: JL, JD. Analysis and interpretation of data: JL, KH-B, WG, JD. Writing and review of manuscript: all authors.
Funding: This work was supported by the Merck Investigators Studies Program (#52440). JL is supported by the Department of Defense Ovarian Cancer Academy (W81XWH-14-1-0161).
Competing interests: JL received research funding through his institution from Merck, Forty-Seven, Sanofi-Aventis US, Harpoon Therapeutics, Sumitomo Dainippon Pharma Oncology, and Precigen. AC has a consulting or advisory role with Halozyme, Seattle Genetics, Merrimack, and AbbVie. AC received research funding from XBiotech, Newlink Genetics, Taiho Pharmaceutical, Immunomedics, Onconova Therapeutics, Lilly, Gilead Sciences, Genentech, Seattle Genetics, AbGenomics International, Halozyme, Novocure, MedImmune, and Amgen. KMcG has a consulting or advisory role with Ambry Genetics. KMcG has stock ownership of AbbVie. MLD received research funding from Pfizer, Bavarian Nordisk, Precigen, and EMD Serono. MLD has stock ownership of Epithany. BG has an immediate family member employed by Lilly.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request. All data relevant to the study are included in the article or uploaded as supplementary information. Data will be available on ClinicalTrials.gov.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
Institutional review board approval was granted by the Fred Hutchinson Cancer Research Center/University of Washington Cancer Consortium.
References
- 1.Matulonis UA, Shapira-Frommer R, Santin AD, et al. Antitumor activity and safety of pembrolizumab in patients with advanced recurrent ovarian cancer: results from the phase II KEYNOTE-100 study. Ann Oncol 2019;30:1080–7. 10.1093/annonc/mdz135 [DOI] [PubMed] [Google Scholar]
- 2.Disis ML, Taylor MH, Kelly K, et al. Efficacy and safety of Avelumab for patients with recurrent or refractory ovarian cancer: phase 1B results from the javelin solid tumor trial. JAMA Oncol 2019;5:393-401. 10.1001/jamaoncol.2018.6258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hamanishi J, Mandai M, Ikeda T, et al. Safety and antitumor activity of anti-PD-1 antibody, nivolumab, in patients with platinum-resistant ovarian cancer. J Clin Oncol 2015;33:4015–22. 10.1200/JCO.2015.62.3397 [DOI] [PubMed] [Google Scholar]
- 4.Konstantinopoulos PA, Waggoner S, Vidal GA, et al. Single-Arm phases 1 and 2 trial of Niraparib in combination with pembrolizumab in patients with recurrent platinum-resistant ovarian carcinoma. JAMA Oncol 2019;5:1141. 10.1001/jamaoncol.2019.1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zsiros E, Lynam S, Attwood KM, et al. Efficacy and safety of pembrolizumab in combination with bevacizumab and oral metronomic cyclophosphamide in the treatment of recurrent ovarian cancer: a phase 2 nonrandomized clinical trial. JAMA Oncol 2021;7:78. 10.1001/jamaoncol.2020.5945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee EK, Xiong N, Cheng S-C, et al. Combined pembrolizumab and pegylated liposomal doxorubicin in platinum resistant ovarian cancer: a phase 2 clinical trial. Gynecol Oncol 2020;159:72–8. 10.1016/j.ygyno.2020.07.028 [DOI] [PubMed] [Google Scholar]
- 7.Zamarin D, Burger RA, Sill MW, et al. Randomized phase II trial of nivolumab versus nivolumab and ipilimumab for recurrent or persistent ovarian cancer: an NRG oncology study. J Clin Oncol 2020;38:1814–23. 10.1200/JCO.19.02059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bracci L, Schiavoni G, Sistigu A, et al. Immune-Based mechanisms of cytotoxic chemotherapy: implications for the design of novel and rationale-based combined treatments against cancer. Cell Death Differ 2014;21:15–25. 10.1038/cdd.2013.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hato SV, Khong A, de Vries IJM, et al. Molecular pathways: the immunogenic effects of platinum-based chemotherapeutics. Clin Cancer Res 2014;20:2831–7. 10.1158/1078-0432.CCR-13-3141 [DOI] [PubMed] [Google Scholar]
- 10.Di Gioacchino M, Di Giampaolo L, Verna N, et al. In vitro effects of platinum compounds on lymphocyte proliferation and cytokine release. Ann Clin Lab Sci 2004;34:195–202. [PubMed] [Google Scholar]
- 11.Markasz L, Skribek H, Uhlin M, et al. Effect of frequently used chemotherapeutic drugs on cytotoxic activity of human cytotoxic T-lymphocytes. J Immunother 2008;31:283–93. 10.1097/CJI.0b013e3181628b76 [DOI] [PubMed] [Google Scholar]
- 12.Grabosch S, Bulatovic M, Zeng F, et al. Cisplatin-Induced immune modulation in ovarian cancer mouse models with distinct inflammation profiles. Oncogene 2019;38:2380–93. 10.1038/s41388-018-0581-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lesterhuis WJ, Punt CJA, Hato SV, et al. Platinum-Based drugs disrupt STAT6-mediated suppression of immune responses against cancer in humans and mice. J Clin Invest 2011;121:3100–8. 10.1172/JCI43656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Varga A, Piha-Paul S, Ott PA, et al. Pembrolizumab in patients with programmed death ligand 1-positive advanced ovarian cancer: analysis of KEYNOTE-028. Gynecol Oncol 2019;152:243–50. 10.1016/j.ygyno.2018.11.017 [DOI] [PubMed] [Google Scholar]
- 15.Lo CS, Sanii S, Kroeger DR, et al. Neoadjuvant chemotherapy of ovarian cancer results in three patterns of tumor-infiltrating lymphocyte response with distinct implications for immunotherapy. Clin Cancer Res 2017;23:925–34. 10.1158/1078-0432.CCR-16-1433 [DOI] [PubMed] [Google Scholar]
- 16.Zhang L, Conejo-Garcia JR, Katsaros D, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med 2003;348:203–13. 10.1056/NEJMoa020177 [DOI] [PubMed] [Google Scholar]
- 17.Sato E, Olson SH, Ahn J, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A 2005;102:18538–43. 10.1073/pnas.0509182102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Milne K, Alexander C, Webb JR, et al. Absolute lymphocyte count is associated with survival in ovarian cancer independent of tumor-infiltrating lymphocytes. J Transl Med 2012;10:33. 10.1186/1479-5876-10-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang AC, Postow MA, Orlowski RJ, et al. T-Cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature 2017;545:60–5. 10.1038/nature22079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bogaerts J, Ford R, Sargent D, et al. Individual patient data analysis to assess modifications to the RECIST criteria. Eur J Cancer 2009;45:248–60. 10.1016/j.ejca.2008.10.027 [DOI] [PubMed] [Google Scholar]
- 21.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 22.Pujade-Lauraine E, Hilpert F, Weber B, et al. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: the aurelia open-label randomized phase III trial. J Clin Oncol 2014;32:1302–8. 10.1200/JCO.2013.51.4489 [DOI] [PubMed] [Google Scholar]
- 23.Pignata S, Scambia G, Katsaros D, et al. Carboplatin plus paclitaxel once a week versus every 3 weeks in patients with advanced ovarian cancer (MITO-7): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol 2014;15:396–405. 10.1016/S1470-2045(14)70049-X [DOI] [PubMed] [Google Scholar]
- 24.Havrilesky LJ, Alvarez Secord A, Ehrisman JA, et al. Patient preferences in advanced or recurrent ovarian cancer. Cancer 2014;120:3651–9. 10.1002/cncr.28940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu J, Waxman DJ. Immunogenic chemotherapy: dose and schedule dependence and combination with immunotherapy. Cancer Lett 2018;419:210–21. 10.1016/j.canlet.2018.01.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matulonis UA, Shapira-Frommer R, Santin A, et al. Antitumor activity and safety of pembrolizumab in patients with advanced recurrent ovarian cancer: interim results from the phase 2 KEYNOTE-100 study. Journal of Clinical Oncology 2018;36:5511. 10.1200/JCO.2018.36.15_suppl.5511 [DOI] [PubMed] [Google Scholar]
- 27.Leitao MM, Hummer A, Dizon DS, et al. Platinum retreatment of platinum-resistant ovarian cancer after nonplatinum therapy. Gynecol Oncol 2003;91:123–9. 10.1016/S0090-8258(03)00464-5 [DOI] [PubMed] [Google Scholar]
- 28.Lindemann K, Gao B, Mapagu C, et al. Response rates to second-line platinum-based therapy in ovarian cancer patients challenge the clinical definition of platinum resistance. Gynecol Oncol 2018;150:239–46. 10.1016/j.ygyno.2018.05.020 [DOI] [PubMed] [Google Scholar]
- 29.Alvarez RD, Matulonis UA, Herzog TJ, et al. Moving beyond the platinum sensitive/resistant paradigm for patients with recurrent ovarian cancer. Gynecol Oncol 2016;141:405–9. 10.1016/j.ygyno.2016.03.005 [DOI] [PubMed] [Google Scholar]
- 30.Herzog TJ, Ison G, Alvarez RD, et al. Fda ovarian cancer clinical trial endpoints workshop: a Society of gynecologic oncology white paper. Gynecol Oncol 2017;147:3–10. 10.1016/j.ygyno.2017.08.012 [DOI] [PubMed] [Google Scholar]
- 31.Liu YL, Zhou Q, Iasonos A, et al. Subsequent therapies and survival after immunotherapy in recurrent ovarian cancer. Gynecol Oncol 2019;155:51–7. 10.1016/j.ygyno.2019.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Strickland KC, Howitt BE, Shukla SA, et al. Association and prognostic significance of BRCA1/2-mutation status with neoantigen load, number of tumor-infiltrating lymphocytes and expression of PD-1/PD-L1 in high grade serous ovarian cancer. Oncotarget 2016;7:13587–98. 10.18632/oncotarget.7277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Griffiths CT, Fuller AF. Intensive surgical and chemotherapeutic management of advanced ovarian cancer. Surg Clin North Am 1978;58:131–42. 10.1016/S0039-6109(16)41440-4 [DOI] [PubMed] [Google Scholar]
- 34.Winter WE, Maxwell GL, Tian C, et al. Tumor residual after surgical cytoreduction in prediction of clinical outcome in stage IV epithelial ovarian cancer: a gynecologic Oncology Group study. J Clin Oncol 2008;26:83–9. 10.1200/JCO.2007.13.1953 [DOI] [PubMed] [Google Scholar]
- 35.Böhm S, Montfort A, Pearce OMT, et al. Neoadjuvant chemotherapy modulates the immune microenvironment in metastases of tubo-ovarian high-grade serous carcinoma. Clin Cancer Res 2016;22:3025–36. 10.1158/1078-0432.CCR-15-2657 [DOI] [PubMed] [Google Scholar]
- 36.Giuliani M, Gui B, Valentini AL, et al. Early detection of recurrence or progression disease in patients with ovarian cancer after primary debulking surgery. Correlation between CT findings and Ca 125 levels. Minerva Ginecol 2017;69:538–47. 10.23736/S0026-4784.17.04062-X [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2021-003122supp001.pdf (157.5KB, pdf)
Data Availability Statement
Data are available on reasonable request. All data relevant to the study are included in the article or uploaded as supplementary information. Data will be available on ClinicalTrials.gov.