Abstract
COVID-19 pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has spread across the globe, posing an enormous threat to public health and safety. Traditional Chinese medicine (TCM), in combination with Western medicine (WM), has made important and lasting contributions in the battle against COVID-19. In this review, updated clinical effects and potential mechanisms of TCM, presented in newly recognized three distinct phases of the disease, are summarized and discussed. By integrating the available clinical and preclinical evidence, the efficacies and underlying mechanisms of TCM on COVID-19, including the highly recommended three Chinese patent medicines and three Chinese medicine formulas, are described in a panorama. We hope that this comprehensive review not only provides a reference for health care professionals and the public to recognize the significant contributions of TCM for COVID-19, but also serves as an evidence-based in-depth summary and analysis to facilitate understanding the true scientific value of TCM.
KEY WORDS: COVID-19, SARS-CoV-2, Traditional Chinese medicine, Clinical evidence, Potential mechanism, Viral infection, Cytokine storm, Multiple organ dysfunction
Graphical abstract
The potential mechanisms of TCM remedy in three phases of distinct disease stages for COVID-19 are systematically described within a panorama by integrating available clinical and preclinical evidence.
1. Introduction
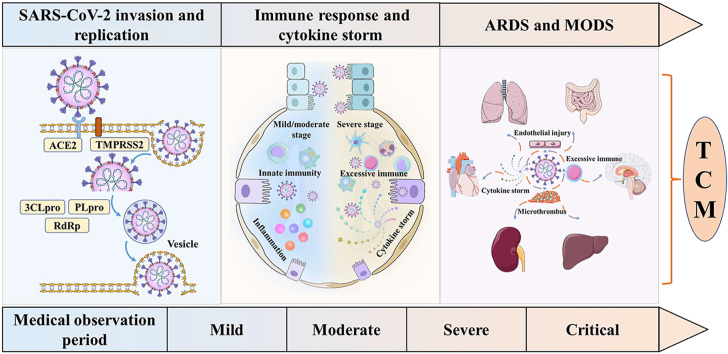
The outbreak and spread of coronavirus disease-19 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has inflicted immense losses on human lives and properties all over the world. Globally, as of August 7, 2021, there have been more than two hundred million confirmed COVID-19 cases, including more than four million of deaths (WHO, https://covid19.who.int/). SARS-CoV-2 is an enveloped, single-stranded, positive-sense, β-coronavirus RNA virus that belongs to the subfamily Coronavirinae, family Coronavirdiae, order Nidovirales. It shares about 79.6% identity of genome sequence with SARS-CoV and 96% similarity with bat coronavirus at the whole-genome level1,2. SARS-CoV-2 is transmitted from person to person via respiratory droplets, high concentration of aerosols, and occasionally feces or urine. Currently, no approved specific anti-viral drug is recommended to defeat COVID-19, which may lead to acute respiratory distress syndrome (ARDS), multiple organ dysfunction syndrome (MODS), and even death.
It is well documented that traditional Chinese medicine (TCM) has accumulated abundant clinical experience and effective prescriptions to control and treat infectious diseases in about 500 epidemics occurred in China over more than 3000 years in the past3. The combined therapy of TCM and Western medicine (WM) had significantly reduced mortality, shortened duration of fever, decreased chest radiograph abnormalities, and relieved secondary fungal infections among patients receiving glucocorticoids in combating severe acute respiratory syndrome (SARS)4. Owning to the positive role of TCM in treating previous coronavirus pneumonias such as SARS, middle east respiratory syndrome (MERS), and other epidemic diseases4, 5, 6, 7, 8, 9, the National Health Commission of China recommended to use TCM as one of the strategies for COVID-19 remedy. This epidemic was deemed as the category of “pestilence” with the pathological characteristics of “dampness, heat, toxin, deficiency, and stasis” under TCM theory10, 11, 12. Over the past year, TCM achieved remarkable efficacy in treating patients at all stages infected with SARS-CoV-2 in China. Typical clinical characteristics contain clinical manifestations, laboratory findings, and chest imaging features, as well as the pathogenesis of SARS-CoV-2 infection and therapeutic targets including SARS-CoV-2 invasion and replication, immune response, and cytokine storm, ARDS and MODS were outlined in published papers. In this review, the therapeutic efficacities and pharmacological mechanisms of TCM for this epidemic disease were systematically documented and discussed, aiming at displaying an in-depth understanding of TCM against COVID-19.
2. TCM in the treatment of COVID-19
2.1. Understanding COVID-19 in TCM theory
In the theory of TCM, COVID-19 is deemed as the category of “dampness‒toxin pestilence”10. The distinct disease stages of TCM treatment can be divided into mild, moderate, severe, and critical. The main patterns in mild stage are cold‒damp constraint and damp‒heat accumulation in the lung, where dispersing lung and removing pathogenic factors, and resolve turbidity with aroma are needed; The main patterns in moderate stage are damp‒toxin constraint in the lung and cold‒damp obstructing the lung, where eliminating heat and dampness, detoxification, and invigorate spleen are needed; The main patterns in severe stage are epidemic toxin blocking the lung, blazing of both qi and yin, where tonifying qi and yin, ventilating lung qi, co-treatment of lung and intestines are needed. The main patterns in critical stage are internal blockage and external desertion, where tonifying qi and preventing exhaustion, cool blood and nourishing yin, and restore consciousness are needed13, 14, 15. Syndrome differentiation is one of the most important principles for TCM to treat COVID-19.
2.2. The recommended TCMs for distinct stages of COVID-19 treatment
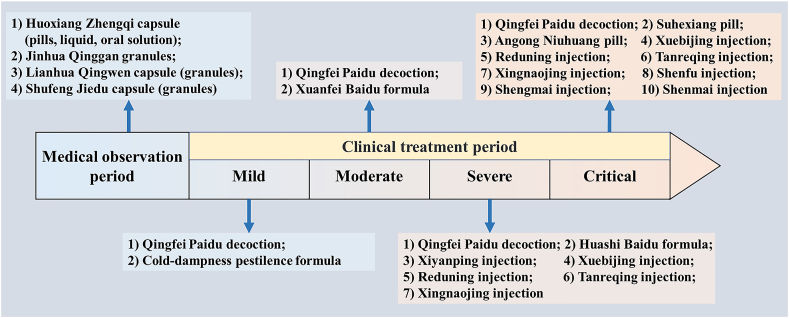
According to the officially issued 7th and 8th trial version of Diagnosis and Treatment Protocol for COVID-19 in China and other references14,16, 17, 18, 19, 20, 21, 22, 23, there are more than 18 recommended TCMs to prevent and treat COVID-19, covering from medical observation period (suspected cases) to clinical treatment period (confirmed cases) including distinct disease stages of mild, moderate, severe, and critical, as shown in Fig. 1. Among them, the highly recommended three Chinese patent medicines (CPMs) are Jinhua Qinggan granules, Lianhua Qingwen capsule (granules), and Xuebijing injection, and three Chinese medicine formulas are Qingfei Paidu decoction, Huashi Baidu formula, and Xuanfei Baidu formula, with proven efficacies in treating COVID-1924,25. Jinhua Qinggan granules clear heat and detoxifying, and diffuse the lung. It is composed of 12 herbal medicines originating from Maxingshigan‒Yinqiaosan formula, which could shorten time to fever resolution in patients with H1N1 influenza virus infection occurred in 200926. Lianhua Qingwen capsule (granules), containing 13 herbal medicines and with a clinical indication for clearing heat, diffusing the lung, and detoxifying, was an innovative CPM for the treatment of SARS in 200327,28. Xuebijing injection, a five-herbal injection medicine and with a clinical indication for dissolving stasis and detoxifying, was derived from a modified Xuefu Zhuyu decoction and was developed and marketed during SARS. The Chinese medicine formula Qingfei Paidu decoction consists of 21 herbal medicines from five classic formulas of Treatise on Febrile Diseases. It clears the lung and calm panting, and is the first recommended universal treatment formula for all stages from mild to critical of COVID-1925,29. Huashi Baidu formula is composed of 14 medicinal herbs. It serves to clearing heat and detoxifying, removing dampness, mainly suitable for the treatment of mild, moderate, and severe COVID-19 patients30,31. Xuanfei Baidu formula is derived from classic formulas including Maxing Shigan decoction and Maxing Yigan decoction, and is composed of 13 medicinal herbs. It detoxifies and removes blood stasis, diffuses the lung, removes dampness, clears heat, and is mainly applicable to treat mild and moderate COVID-19 patients32. Beyond the above mentioned medicines and formulas, Chinese herbal injections, including Xiyanping injection, Reduning injection, Tanreqing injection, Shenfu injection, Shengmai injection, and Shenmai injection, were more suitable as supplemental treatments for severe or critical COVID-19 cases with their advantages of fast absorption, high bioavailability, and clearer ingredients in contrast to orally administrated TCMs33, 34, 35.
Figure 1.
The recommended Chinese patent medicines or Chinese medicine formulas for distinct stages of COVID-19 treatment.
2.3. Clinical evidence of TCM for COVID-19
A total of 40 representative clinical trials, including 11 randomized controlled trials (RCTs), 16 retrospective cohort studies (RCSs), 5 multi-center clinical observations, and 8 others were completed and summarized27,28,30, 31, 32,36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70. According to the available clinical data, integrated TCM and WM exhibited several clinical advantages in COVID-19 treatment, including the outcomes of 1) clinical manifestations, 2) lung features, and 3) laboratory findings as shown in Table 127,28,30, 31, 32,36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70. Furthermore, based on Table 1, the clinical evidence of TCM for typical characteristics of COVID-19 were analyzed and summarized in Table 227,28,30, 31, 32,36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70.
Table 1.
Clinical efficacies of integrated TCM and WM for COVID-19 treatment.
| No. | Intervention | Method | Object (T/C) | Disease stage | Clinical manifestation | Laboratory finding | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | Jinhua Qinggan granules + WM vs. WM | Retrospective cohort study (RCS) |
44/36 | Moderate or severe | 1) Shorten the duration of nucleic acid turn negative 2) Promote the absorption of pneumonia inflammatory exudate |
Increase WBC and lymphocyte count | 36 |
| 2 | Jinhua Qinggan capsule + WM vs. WM | Randomized controlled trial (RCT) | 82/41 | Mild | Reduce the symptoms of fever, cough, fatigue, and sputum cough, and relieve the psychological anxiety | Unreported | 37 |
| 3 | Lianhua Qingwen capsule + WM vs. WM | RCT | 142/142 | Mild or moderate | 1) Shorten median time to symptom recovery 2) Shorten time to recovery of fever, fatigue, and cough 3) Improve the rate of chest CT manifestations and clinical cure |
Unreported | 27 |
| 4 | Lianhua Qingwen capsule + WM vs. WM | RCS | 63/38 | All | Relieve symptoms of fever, cough, weakness, and short of breath | Unreported | 38 |
| 5 | Lianhua Qingwen capsule + arbidol vs. arbidol | RCT | 147/148 | Mild or moderate | Relieve symptoms of fever, fatigue, cough, dry throat, sore throat, and chest tightness | Lower the levels of CRP and procalcitonin, elevate WBC and lymphocyte count | 39 |
| 6 | Lianhua Qingwen capsule + WM | Before and after comparison | 54/0 | Moderate | Relieve the symptoms and reduce the duration of fever, fatigue, and cough. | Unreported | 40 |
| 7 | Lianhua Qingwen capsule + Huoxiang Zhengqi dropping pills + WM vs. WM | RCT | 189/94 | All | 1) Improve the symptoms of fever and diarrhea, especially fatigue, nausea and vomiting, chest tightness, shortness of breath and limb soreness 2) Reduce the utilization rate of anti-infective drugs and improve the prognosis of patients 3) Block disease aggravation |
Unreported | 28 |
| 8 | Lianhua Qingwen capsule + arbidol vs. arbidol | RCS | 68/40 | Mild or moderate | 1) Shorten the median time from admission to the first negative result of nucleic acid detection 2) Reduce lung inflammation |
1) Increase lymphocytes count 2) Lower the levels of serum amyloid A and CRP |
41 |
| 9 | Xuebijing injection + WM | Case analysis | 11/0 | Severe or critical | May ameliorate lung injury | Reduce the levels of TNF-α, IP-10, MIP-1β, and RANTES | 42 |
| 10 | Xuebijing injection + WM vs. WM | RCT | 40/20 | Severe | Improve the conditions of patients, lower APACHE II score | 1) Improve the oxygenation index of PaO2/FiO2 2) Increase WBC and lymphocyte count, decrease the levels of CRP and ESR |
43 |
| 11 | Xuebijing injection + antiviral treatment vs. antiviral treatment | RCS | 22/22 | Moderate | Increase the effective rate of lung lesions absorption and the overall effective rate of treatment | Tend to improve WBC count, lymphocyte count, and the levels of CRP and ferritin | 44 |
| 12 | Qingfei Paidu decoction + WM vs. WM | RCS | 37/26 | Severe | 1) Relieve the symptoms and improve inflammation resolution in the lung 2) Tend to mitigate the extent of multi-organ impairment |
1) Improve the levels of CRP, CK, creatine kinase-myocardial band, LDH, and blood urea nitrogen 2) Increase lymphocyte count |
45 |
| 13 | Qingfei Paidu decoction + WM | Before and after comparison | 98/0 | All | 1) Nearly all adverse symptoms including fever, cough, asthma, and fatigue were relieved 2) Improve lung CT imaging |
Restore the levels of AST, ALT, D-dimer, CRP, ESR, and the percentage of lymphocyte | 46 |
| 14 | Qingfei Paidu decoction + antiviral treatment vs. antiviral treatment | RCS | 30/30 | All | 1) Shorten inpatient days and reduce the time of fever and cough 2) Promote lung CT improvement |
Unreported | 47 |
| 15 | Qingfei Paidu decoction + WM | RCS | 46/43 | All | 1) Reduce inflammation, enhance cellular immunity, improve renal function, lower hypercoagulability 2) Shorten the length of hospitalization and nucleic acid negative time |
Reduce the level of IL-6 and increase the level of CD3 | 48 |
| 16 | Qingfei Paidu decoction + WM vs. WM | Multi-center clinical observation | 199/96 | Mild or moderate | 1) Reduce mean length of hospital stay, nucleic acid negative time and improve symptom of sputum 2) Improve lung CT imaging |
Unreported | 49 |
| 17 | Qingfei Paidu decoction + WM | Multi-center clinical observation | 782/0 | All | Shorten the time of recovery, viral shedding, and the duration of hospital stay | Unreported | 50 |
| 18 | Xuanfei Baidu decoction + WM vs. WM | RCT | 22/20 | Mild | Increase the disappearance rate of symptoms of fever, cough, fatigue, and loss of appetite | 1) Elevate WBC and lymphocyte count 2) Reduce the levels of CRP and ESR |
32 |
| 19 | Huashi Baidu granule vs. WM | RCS | 23/32 | Severe | Improve chest CT imaging and lung lesion opacity | Decrease the levels of CRP, ESR, serum ferritin, and myoglobin | 30 |
| 20 | Huashi Baidu formula + TCM injection vs. Huashi Baidu formula + lopinavir‒ritonavir vs. lopinavir‒ritonavir | RCS | 20/20/20 | Mild or moderate | Shorten the clinical remission time | No significant differences in biochemical indicators such as D-dimer, CRP, and IL-6 | 31 |
| 21 | Shufeng Jiedu capsule + WM vs. WM | RCS | 34/34 | Moderate | 1) Improve the symptoms of cough, sputum, fatigue, chest tightness, and shortness of breath 2) Lower the rate of transferring to severe disease 3) Promote the absorption of lung inflammation and improve lung CT imaging |
1) Increase lymphocyte count 2) Decrease the levels of CRP, procalcitonin, and D-dimer |
51 |
| 22 | Shufeng Jiedu capsule + arbidol vs. arbidol | RCS | 100/100 | Mild | 1) Alleviate the symptoms of fever, cough, chest distress, and shortness of breath 2) Increase the absorption lung infected lesions |
Increase lymphocyte count and lymphocyte percentage | 52 |
| 23 | Shufeng Jiedu capsule + arbidol vs. arbidol | RCS | 40/30 | Mild or moderate | 1) Shorten the antipyretic time and the disappearance time of dry cough, nasal congestion, runny nose, pharyngeal pain, fatigue, and diarrhea 2) Reduce novel coronavirus negative conversion time |
Unreported | 53 |
| 24 | Shufeng Jiedu capsule + arbidol vs. arbidol | RCS | 100/100 | Moderate | 1) Shorten defervescence time 2) Improve resolution of pneumonia on chest CT |
1) Increase WBC and lymphocyte count 2) Reduce the levels of CRP and IL-6 |
54 |
| 25 | Hanshiyi formula + WM vs. WM | RCS | 430/291 | Mild or moderate | Reduce the progression to severe disease | Unreported | 55 |
| 26 | Lianhua Qingke granules + WM vs. WM | RCT | 25/32 | Mild or moderate | Ameliorate the symptoms of cough, sputum, fever, fatigue, dry throat, and sore throat, and shorten the duration of cough and sputum, reduce lung diseases, improve respiratory function | Unreported | 56 |
| 27 | Toujie Quwen granules + moxifloxacin + ambroxol vs. moxifloxacin + ambroxol | RCS | 32/33 | Mild or moderate | 1) Improve the symptoms of fever, cough, fatigue, expectoration, dry throat, and sore throat 2) Improve lung CT imaging |
1) Up-regulate lymphocyte count and neutrophil ratio 2) Down-regulate the levels of CRP, D-dimer, and procalcitonin |
57 |
| 28 | Reyanning mixture + WM vs. WM | Multi-center clinical observation | 26/23 | Moderate | 1) Improve the symptoms of dry throat, cough, fatigue, chest tightness, and headache, and shorten the duration of fever 2) Promote the improvement of lung CT 3) Improve nucleic acid negative conversion rate |
No significant differences in neutrophil count, lymphocyte count and CRP level | 58 |
| 29 | Maxing Shigan decoction + WM | Before and after comparison | 40/0 | Moderate | Improve the symptoms of fever, cough, fatigue, hemoptysis, nausea, vomiting, diarrhea, and chest pain | Decrease CRP level, increase CD4+T and CD8+T count | 59 |
| 30 | Honeysuckle oral liquid + WM vs. WM | Multi-center clinical observation | 200/100 | Moderate | 1) Shorten the length of hospitalization and the time of nucleic acid negative conversion 2) Lower right lung CT score |
No significant difference in the levels of ALT, AST, creatinine, and uric acid | 60 |
| 31 | Chansu injection + WM vs. WM | RCT | 25/25 | Severe or critical | Improve the respiratory function and shorten the respiratory support step-down time | Improve the respiratory function indicators of PaO2/FiO2 and ROX index | 61 |
| 32 | Yidu–toxicity blocking lung decoction + WM vs. WM | RCT | 15/24 | Severe | All patients are cured and discharged | Reduce the levels of IL-6 and TNF-α | 62 |
| 33 | Qingfei Dayuan granules + WM | Multi-center clinical observation | 451/0 | All | 1) Reduce the incidence of fever, cough, and fatigue 2) Improve the symptoms of aversion to cold, nasal obstruction, runny nose, sneezing, pharyngeal itch, sore throat, dyspnea, chest tightness, muscle ache or joint pain, dizziness, headache, tolerance, nausea and vomiting, abdominal distension, and loose stool 3) Thin white greasy moss, thick greasy moss, and yellow greasy moss, and improve tongue color 4) Decrease and thin lung lesion area |
1) Increase lymphocyte count 2) Reduce the levels of CRP and procalcitonin |
63 |
| 34 | “Fei Yan No. 1”+WM vs. WM | RCS | 49/35 | All | 1) Improve the rate of recovering from symptoms and shorten the time 2) Increase the proportion of testing negative for nucleic acid 3) Promote focal lung absorption and inflammation |
Reduce leukocyte count and CRP level | 64 |
| 35 | Xuanfei Huazhuo decoction + WM | Case analysis | 40/0 | All | 1) Improve the symptoms of cough, fever, sputum, diarrhea, loss of appetite, and fatigue 2) Promote the absorption of pulmonary inflammation |
1) Improve WBC count, lymphocyte, and neutrophil percentage 2) Reduce the levels of CRP, ESR, total bilirubin, LDH, and the ratio of AST/ALT |
65 |
| 36 | Keguan-1+WM vs. WM | RCT | 24/24 | All | 1) Reduce ARDS development 2) Shorten the time to fever resolution 3) Tend to improve lung injury recovery |
No significant difference in biochemical indicators such as ALT, AST, and D-dimer | 66 |
| 37 | Qingfei Touxie Fuzheng recipe + WM vs. WM | RCT | 51/49 | All | 1) Alleviate the symptoms of fever, cough, expectoration, chest tightness, and shortness of breath 2) Promote the absorption of pulmonary lesions and improve oxygenation |
Decrease the levels of ESR, CRP, and IL-6, tend to increase IFN-γ level | 67 |
| 38 | Ganlu Xiaodu decoction + Chinese medicine and WM | Case analysis | 131/0 | All | Increase the effective rate of lung lesions absorption | Increase WBC and lymphocyte count | 68 |
| 39 | Matrine injection + WM | Case analysis | 40/0 | All | 1) Improve the symptoms of cough, fatigue, appetite, and digestive tract 2) Promote absorption of lung lesions, especially for grid-like and fibrotic lesions 3) Shorten nucleic acid clearance time |
Alleviate absolute value and ratio of lymphocyte and CRP | 69 |
| 40 | Diammonium glycyrrhizinate + arbidol | Case analysis | 46/0 | All | Improve the symptoms of low-grade fever, cough, and fatigue | 1) Increase lymphocyte count and decrease ESR level 2) Decrease the levels of CRP, IL-6, and procalcitonin |
70 |
T/C, treatment/control.
Table 2.
Clinical evidence of TCM for typical characteristics of COVID-19.
| Clinical evidence | TCM |
|---|---|
| Clinical symptom | |
| Fever | Jinhua Qinggan granules37, Lianhua Qingwen capsule27,28,38, 39, 40, Qingfei Paidu decoction46,47, Xuanfei Baidu decoction32, Shufeng Jiedu capsule52,53, Lianhua Qingke granules56, Toujie Quwen granules57, Reyanning mixture58, Maxing Shigan decoction59, Qingfei Dayuan granules63, Xuanfei Huazhuo decoction65, Qingfei Touxie Fuzheng recipe67, Diammonium glycyrrhizinate70 |
| Cough | Jinhua Qinggan granules37, Lianhua Qingwen capsule27,38, 39, 40, Qingfei Paidu decoction46,47, Xuanfei Baidu decoction32, Shufeng Jiedu capsule51, 52, 53, Lianhua Qingke granules56, Toujie Quwen granules57, Reyanning mixture58, Maxing Shigan decoction59, Qingfei Dayuan granules63, Xuanfei Huazhuo decoction65, Qingfei Touxie Fuzheng recipe67, Matrine injection69, Diammonium glycyrrhizinate70 |
| Fatigue | Jinhua Qinggan granules37, Lianhua Qingwen capsule27,28,39,40, Qingfei Paidu decoction46, Xuanfei Baidu decoction32, Shufeng Jiedu capsule51,53, Lianhua Qingke granules56, Toujie Quwen granules57, Reyanning mixture58, Maxing Shigan decoction59, Qingfei Dayuan granules63, Xuanfei Huazhuo decoction65, Matrine injection69, Diammonium glycyrrhizinate70 |
| Dry throat | Lianhua Qingwen capsule39, Shufeng Jiedu capsule53, Lianhua Qingke granules56, Toujie Quwen granules57, Reyanning mixture58 |
| Sore throat | Lianhua Qingwen capsule39, Lianhua Qingke granules56, Toujie Quwen granules57, Qingfei Dayuan granules63 |
| Sputum production | Jinhua Qinggan granules37, Qingfei Paidu decoction49, Lianhua Qingke granules56, Xuanfei Huazhuo decoction65, Qingfei Touxie Fuzheng recipe67 |
| Shortness of breath | Lianhua Qingwen capsule28,38, Qingfei Paidu decoction46, Shufeng Jiedu capsule51,52, Qingfei Dayuan granules63, Qingfei Touxie Fuzheng recipe67 |
| Myalgia | Lianhua Qingwen capsule28, Shufeng Jiedu capsule53, Qingfei Dayuan granules63 |
| Diarrhea | Lianhua Qingwen capsule28, Shufeng Jiedu capsule53, Maxing Shigan decoction59, Xuanfei Huazhuo decoction65 |
| Duration of nucleic acid turn negative | Jinhua Qinggan granules36, Lianhua Qingwen capsule41, Qingfei Paidu decoction48, Shufeng Jiedu capsule53, Reyanning mixture58, Honeysuckle oral liquid60, “Fei Yan No. 1”64, Matrine injection69 |
| Time to symptom recovery | Lianhua Qingwen capsule27, Xuebijing injection44, Qingfei Paidu decoction47, 48, 49, 50, Huashi Baidu Decoction31, Honeysuckle oral liquid60, Yidu-toxicity blocking lung decoction62, “Fei Yan No. 1”64, Keguan-166 |
| The progression to severe disease | Shufeng Jiedu capsule51, Hanshiyi formula55 |
| Multiorgan injury | Xuebijing injection44, Qingfei Paidu decoction48 |
| Lung feature | |
| Lung inflammatory absorption | Jinhua Qinggan granules36, Lianhua Qingwen capsule41, Xuebijing injection44, Qingfei Paidu decoction45, Shufeng Jiedu capsule52, “Fei Yan No. 1”64, Xuanfei Huazhuo decoction65, Ganlu Xiaodu decoction68, Matrine injection69 |
| CT imaging | Lianhua Qingwen capsule27, Qingfei Paidu decoction46,47, Huashi Baidu formula30, Shufeng Jiedu capsule51, Toujie Quwen granules57, Reyanning mixture58, Honeysuckle oral liquid60 |
| Lung injury | Xuebijing injection42, Lianhua Qingke granules56, Qingfei Dayuan granules63, Keguan-166 |
| Lung function | Chansu injection61 |
| Laboratory finding | |
| WBC count | Jinhua Qinggan granules36, Lianhua Qingwen capsule39, Xuebijing injection43,44, Xuanfei Baidu decoction32, Shufeng Jiedu capsule54, “Fei Yan No. 1”64, Xuanfei Huazhuo decoction65, Ganlu Xiaodu decoction68 |
| Lymphocyte count | Jinhua Qinggan granules36, Lianhua Qingwen capsule39,41, Xuebijing injection43,44, Qingfei Paidu decoction45,46, Xuanfei Baidu decoction32, Shufeng Jiedu capsule51, Toujie Quwen granules57, Qingfei Dayuan granules63, Xuanfei Huazhuo decoction65, Ganlu Xiaodu decoction68, Matrine injection69, Diammonium glycyrrhizinate70 |
| Oxygenation index | Xuebijing injection43, Chansu injection61 |
| CRP | Lianhua Qingwen capsule39, Xuebijing injection43,44, Qingfei Paidu decoction45, Xuanfei Baidu Decoction32, Huashi Baidu formula30, Shufeng Jiedu capsule51,54, Toujie Quwen granules57, Maxing Shigan decoction59, Qingfei Dayuan granules63, “Fei Yan No. 1”64, Xuanfei Huazhuo decoction65, Qingfei Touxie Fuzheng recipe67, Matrine injection69, Diammonium glycyrrhizinate70 |
| IL-6 | Xuebijing injection42, Qingfei Paidu decoction48, Shufeng Jiedu capsule54, Yidu-toxicity blocking lung decoction62, Qingfei Touxie Fuzheng recipe67, Diammonium glycyrrhizinate70 |
| TNF-α | Xuebijing injection42, Yidu-toxicity blocking lung decoction62 |
| ESR | Xuebijing injection44, Qingfei Paidu decoction46, Xuanfei Baidu Decoction32, Huashi Baidu formula30, Xuanfei Huazhuo decoction65, Qingfei Touxie Fuzheng recipe67, Diammonium glycyrrhizinate70 |
| CK | Qingfei Paidu decoction45 |
| LDH | Qingfei Paidu decoction45, Xuanfei Huazhuo decoction65 |
| ALT | Qingfei Paidu decoction46, Xuanfei Huazhuo decoction65 |
| AST | Qingfei Paidu decoction46, Xuanfei Huazhuo decoction65 |
| Procalcitonin | Lianhua Qingwen capsule39, Shufeng Jiedu capsule51, Toujie Quwen granules57, Qingfei Dayuan granules63, Diammonium glycyrrhizinate70 |
| D-dimer | Qingfei Paidu decoction46, Shufeng Jiedu capsule51, Toujie Quwen granules57 |
| CD4+ T cell | Maxing Shigan decoction59 |
| CD8+ T cell | Maxing Shigan decoction59 |
For mild or moderate stages: 1) the most typical clinical symptoms of fever, cough, and fatigue were relieved by Jinhua Qinggan granules37, Lianhua Qingwen granules39, Shufeng Jiedu capsule51,52, Toujie Quwen granules57, Lianhua Qingke granules56, Xuanfei Baidu decoction32, and Maxing Shigan decoction59; Lianhua Qingwen granules38 and Shufeng Jiedu capsule51 improved the symptoms of short of breath and chest tightness; Jinhua Qinggan granules relieved the symptom of psychological anxiety37, and Shufeng Jiedu capsule53 improved the symptom of diarrhea. 2) Jinhua Qinggan granules36, Shufeng Jiedu capsule51, and Toujie Quwen granules57 promoted pneumonia inflammatory absorption or improve lung CT imaging. 3) Jinhua Qinggan granules36, Lianhua Qingwen granules39, Shufeng Jiedu capsule52, Xuanfei Baidu decoction32, and Toujie Quwen granules57 increased white blood cell (WBC) or lymphocyte count; Lianhua Qingwen granules39, Shufeng Jiedu capsule51, Toujie Quwen granules57, Xuanfei Baidu decoction32, and Maxing Shigan decoction59 reduced the level of C-reactive protein (CRP). Shufeng Jiedu capsule decrease the level of interleukin-6 (IL-6)54.
For severe or critical stages: 1) Xuebijing injection43 and Qingfei Paidu decoction45 improved the conditions of patients and reduced multiple organ dysfunction. 2) Xuebijing injection43, Qingfei Paidu decoction45, and Huashi Baidu formula30 improved chest CT imaging or promoted lung lesions absorption; Chansu injection61 ameliorated the respiratory function and shorten the respiratory support step-down time. 3) Both Xuebijing injection43 and Chansu injection61 improved the oxygenation index of PaO2/FiO2; Xuebijing injection43 and Qingfei Paidu decoction45 decreased the level of CRP, and increased WBC or lymphocyte count; In addition, Xuebijing injection reduced the level of inflammatory mediators of TNF-α, IP-10, MIP-1β, and RANTES42; Qingfei Paidu decoction decreased biochemical parameters of CK and LDH, and the level of blood urea nitrogen45; Maxing Shigan decoction increased CD4+ T and CD8+ T count59; Huashi Baidu formula30 decreased CRP, erythrocyte sedimentation rate (ESR), serum ferritin, and myoglobin level; Yidu-toxicity blocking lung decoction reduced the levels of IL-6 and TNF-α62.
For all stages: 1) Qingfei Paidu decoction46 and Qingfei Dayuan granules63 ameliorated extensive adverse symptoms such as fever, cough, fatigue, chest tightness, and headache; Xuanfei Huazhuo decoction relieved the symptoms of cough, fever, sputum, diarrhea, fatigue, and loss of appetite65. 2) Qingfei Paidu decoction45, Qingfei Dayuan granules63, Xuanfei Huazhuo decoction65, Keguan-166, Qingfei Touxie Fuzheng recipe67, Ganlu Xiaodu decoction68, and Matrine injection69 improved lung inflammation or lesions absorption. 3) Qingfei Paidu decoction46, Qingfei Dayuan granules63, Xuanfei Huazhuo decoction65, Ganlu Xiaodu decoction68, “Fei Yan No. 1”64, Matrine and sodium chloride injection69, and Diammonium glycyrrhizinate70 increased WBC or lymphocyte count; Qingfei Touxie Fuzheng recipe67 and Diammonium glycyrrhizinate70 decreased the level of CRP, IL-6, and ESR; Qingfei Paidu decoction46,48 and Xuanfei Huazhuo decoction65 reduced the level of CRP and ESR, and the biochemical parameters of AST and ALT. What's more, Qingfei Paidu decoction decreased the level of a thrombotic marker D-dimer46.
A plentiful of clinical studies and analyses proved that integrated Chinese and Western medicine therapy are much better than pure use of WM for COVID-1971, 72, 73, 74, 75, 76, 77, 78, 79, 80. A recent systematic review and meta-analysis of RCTs involving 2275 patients revealed that integration of TCM and WM group was more effective than WM treatment alone in the indicators of clinical cure rate, conversion rate from mild to critical, length of hospital stay, total score of clinical symptoms, symptoms of fever, cough and fatigue, TCM syndrome, negative conversion rate of viral nucleic acid, inflammatory biomarkers of CRP and lung CT without significant difference in adverse effects81,82. Another similar meta-analysis of RCTs including 1259 COVID-19 patients showed consistent results that TCM with WM treatment could improve the amounts of severe and critical conversion, length of hospital stay, time of antipyretic, and resolution rate of fever, fatigue, and tachypnea83.
In summary of clinical evidence, TCM is beneficial for treating COVID-19 in 1) relieving the typical symptoms of fever, cough, fatigue, dry throat, sore throat, sputum production, shortness of breath, myalgia, and diarrhea; shortening the duration of positive viral nucleic acid, reducing the time to symptom recovery and the progression to severe disease, and protecting against multi-organ injury; 2) improving the lung features including lung inflammatory absorption, CT imaging, lung injury, lung function, and oxygenation index; 3) regulating laboratory index including inflammatory and immune response related the count of WBC, lymphocyte, CD4+ T and CD8+ T, and the level of CRP, IL-6, TNF-α, and ESR, single or multi-organ injury related the level of procalcitonin, CK, LDH, ALT, and AST, and thrombosis related D-dimer level. Taking full advantage of integration of TCM and WM is one of the important reasons for the rapid containment of this epidemic in China. Additional high-quality RCTs are needed to demonstrate the effectiveness and adverse events of TCM in the treatment of COVID-19.
3. Potential mechanisms of TCM for COVID-19
The intervention of TCM for COVID-19 is greatly inspired by the successful experience of treating SARS in 2002–20034, 5, 6, 7, 8, 9. SARS-CoV-2 is genetically more similar with SARS-CoV (about 80%) than MERS-CoV (about 50%)1,2,84. According to sequence alignment and homology modeling, the critical targets of spike, 3C-like protease (3CLpro), papain-like protease (PLpro), and RNA-dependent RNA polymerase (RdRp) protease share 76%, 96%, 83%, 96% sequence similarity between SARS-CoV and SARS-CoV-2, respectively85, 86, 87. We collected and summarized TCMs and their ingredients to reveal the specific mechanisms of TCM for the three phases of distinct disease stages of COVID-1942,88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115, 116, 117, 118, 119, 120, 121, 122, 123, 124, 125, 126, 127, 128, 129, 130, 131, 132, 133, 134, 135, 136, 137, 138, 139, 140, 141, 142, 143, 144, 145, 146, 147, 148, 149, 150, 151, 152, 153, 154, 155, 156, 157, 158, 159, 160, 161, 162, 163, 164, seen in Table 342,88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115, 116, 117, 118, 119, 120 and Table 4 109,115, 116, 117,121, 122, 123, 124, 125, 126, 127, 128, 129, 130, 131, 132, 133, 134, 135, 136, 137, 138, 139, 140, 141, 142, 143, 144, 145, 146, 147, 148, 149, 150, 151, 152, 153, 154, 155, 156, 157, 158, 159, 160, 161, 162, 163, 164.
Table 3.
Potential mechanisms of TCM for COVID-19.
| No. | TCM | Coronavirus | Model/method | IC50 (EC50) or dosage | Potential mechanism | Ref. |
|---|---|---|---|---|---|---|
| 1 | Jinhua Qinggan | SARS-CoV-2 | Network pharmacology (NP), molecular docking | Not applicable (NA) | 1) Regulate TNF, PI3K/Akt, and HIF-1 signaling pathways via binding angiotensin converting enzyme 2 (ACE2) and acting on targets such as PTGS2, HSP90AB1, HSP90AA1, PTGS1, and NCOA2 2) Formononetin, stigmasterol, β-sitosterol, and anhydroicaritin have a high affinity with 3CLpro and ACE2 |
88 |
| 2 | Lianhua Qingwen capsule | SARS-CoV-2 | Infected Vero E6 cells and Huh-7 cells, cytopathic effect (CPE), plaque reduction assay | 411.2 μg/mL | 1) Inhibit virus replication and decrease the number of virus particles 2) Reduce pro-inflammatory cytokines of TNF-α, IL-6, MCP-1, and IP-10 production |
89 |
| 3 | Lianhua Qingwen formula | SARS-CoV-2 | NP | NA | 1) Exert antiviral effect and repair lung injury 2) Modulate inflammatory process and relieve cytokine storm 3) Improve ACE2 expression disorder caused symptoms |
90 |
| 4 | Xuebijing injection | SARS-CoV-2 | Infected Vero E6 cells and Huh-7 cells, CPE, plaque reduction assay | 11.75 mg/mL | 1) Exert antiviral effect and reduce plaque formation 2) Inhibit the expression and release of TNF-α, IL-6, MIP-1β, RANTES, and IP-10 |
42 |
| 5 | Xuebijing injection | SARS-CoV-2 | NP, molecular docking | NA | 1) Quercetin, luteolin, apigenin, and other compounds may target TNF, MAPK1, and IL6 2) Anhydrosafflor yellow B, salvianolic acid B, and rutin play the role of anti-inflammatory, antiviral, and immune response |
91 |
| 6 | Xuebijing injection | SARS-CoV-2 | NP | NA | Exert anti-inflammatory and immunoregulatory effects through RAS, NF-κB, PI3K, Akt, MAPK, VEGF, TLR, TNF, and TRP signaling pathways | 92 |
| 7 | Qingfei Paidu decoction | SARS-CoV-2 | NP, molecular docking | NA | 1) Exert antiviral and anti-inflammatory activities, regulate metabolic programming, and repair lung injury 2) Glycyrrhizin in one of the main ingredients inhibits TLR agonists induced IL-6 production in macrophage |
93, 94, 95 |
| 8 | Qingfei Paidu decoction | SARS-CoV-2 | NP, molecular docking, molecular verification | NA | 1) Exhibit the effects of immune regulation, anti-infection, anti-inflammation, and multi-organ protection 2) Four compounds of baicalin, glycyrrhizin, hesperidin, and hyperoside act on the targets including AKT1, TNF-α, IL-6, PTGS2, HMOX1, IL10, and TP53 3) Inhibit IL-6, CCL2, TNF-α, NF-κB, PTGS1/2, CYP1A1, and CYP3A4 activity, and increase IL-10 expression 4) Reduce platelet aggregation. |
96 |
| 9 | Huashi Baidu formula | SARS-CoV-2 | NP, molecular docking | NA | 1) Regulate TNF, PI3K-Akt, NOD-like, MAPK, and HIF-1 signaling pathways 2) Baicalein and quercetin are the top two compounds with a high affinity to ACE2 |
97 |
| 10 | Xuanfei Baidu | SARS-CoV-2 | NP | NA | Regulate viral, parasites and bacterial infections, and modulate energy metabolism, immunity, and inflammation | 98 |
| 11 | Shufeng Jiedu capsule | SARS-CoV-2 | NP | NA | Regulate the key targets of RELA, MAPK1, MAPK14, CASP3, CASP8, and IL-6 | 99 |
| 12 | Shufeng Jiedu capsule | SARS-CoV-2 | NP, molecular docking | NA | Regulate immunomodulatory and anti-inflammatory related targets on multiple pathways | 100 |
| 13 | Maxing Shigan decoction | SARS-CoV-2 | NP | NA | 1) Reduce inflammation and suppress cytokine storm 2) Protect pulmonary alveolar-capillary barrier and alleviate pulmonary edema 3) Regulate immune response and decrease fever |
101 |
| 14 | Maxing Shigan decoction | SARS-CoV-2 | NP, molecular docking, molecular verification | NA | 1) Inhibit IL-6 mediated JAK-STAT signal pathway 2) Amygdalin is predicted to bind ACE2, 3CLpro, and RdRp |
102 |
| 15 | Cold-damp plague formula | SARS-CoV-2 | NP, molecular docking | NA | 1) Regulate free radical production and blood circulation 2) Exert antiviral, immune-regulatory, and anti-inflammatory by targeting ACE2 and IL-6 |
103 |
| 16 | Dayuanyin | SARS-CoV-2 | NP, molecular docking | NA | 1) Play an anti-inflammatory and immunoregulatory role via acting on IL-6, IL-1β, and CCL2 2) Decrease the level of IL-6 in mild, moderate, and severe clinical cases 3) The ingredients of kaempferol, quercetin, 7-methoxy-2-methyl, isoflavone, naringenin, and formononetin target IL-6, IL-1β, and CCL2 with high affinity |
104,105 |
| 17 | Reduning injection | SARS-CoV-2 | Infected Vero E6 cells, CPE, NP | 103.420 μg/mL | 1) Exert antiviral effect 2) Regulate ACE2, 3CLpro, and PLpro activity 3) Modulate inflammation-related expressions of MAPKs, PKC, and NF-κB |
106 |
| 18 | Liu Shen capsule | SARS-CoV-2 | Infected Vero E6 cells and Huh-7 cells, CPE, plaque reduction assay | 0.6 μg/mL | 1) Inhibit virus replication and reduce plaque formation 2) Reduce pro-inflammatory cytokines of TNF-α, IL-6, IL-1β, IL-8, MCP-1, and IP-10 production, and inhibit p-NF-κB p65, p-IκBα, and p-p38 MAPK expression |
107 |
| 19 | Pudilan Xiaoyan oral liquid | SARS-CoV-2 | Infected Vero E6 cells, CPE | 1.08 mg/mL | Inhibit viral replication in vitro and in vivo | 108 |
| 20 | Shuanghuanglian preparation | SARS-CoV-2 | 1) Infected Vero cells 2) Enzyme inhibition assay |
1) 0.93–1.2 μL/mL 2) 0.06–0.09 μL/mL |
1) Inhibit viral replication 2) Inhibit 3CLpro activity |
109 |
| 21 | Yinqiao powder | SARS-CoV-2 | NP, molecular docking, surface plasmon resonance (SPR) analysis | NA | Regulate TNF, T-cell receptor, Toll-like receptor, and MAPK signaling pathways | 110 |
| 22 | Pudilan prescription | SARS-CoV-2 | NP, GSEA enrichment, molecular docking | NA | 1) Prevent SARS-CoV-2 entrance by blocking ACE2 2) Inhibit cytokine storm of CRP, IFN-γ, IL-6, IL-10, TNF, EGFR, CCL5, and TGF-β1 |
111 |
| 23 | Matrine injection | SARS-CoV-2 | NP, molecular docking | NA | 1) Inhibit viral replication, host cell apoptosis and inflammation by targeting the TNF-α, IL-6, and CASP3 in TNF signaling pathway 2) Reduce lung tissue damage and lung index 3) Decrease the production of IL-6, IL-10, TNF-α, IFN-γ, as well as the viral load in lung tissue 4) Increase the percentage of CD4+ T cells, CD8+ T cells and B cells in peripheral blood |
112,113 |
| 24 | Shenfu decoction | SARS-CoV-2 | NP, molecular docking | NA | Play antiviral role through multi-component, multi-target, and multi-pathway approach, and exert anti-inflammation, immune regulation, and multi-organ protection effects | 114 |
| 25 | Andrographis paniculate (Chuanxinlian) | SARS-CoV-2 | Infected Calu-3 cells, CPE | 0.036 μg/mL | Exert antiviral effect | 115 |
| 26 | Scutellaria baicalensis (Huangqin) | SARS-CoV-2 | 1) Enzyme inhibition assay 2) Infected Vero cells, CPE |
1) 8.52 mg/mL 2) 0.74 mg/mL |
1) Inhibit 3CLpro activity 2) Exert antiviral effect |
116 |
| 27 | Rheum officinale (Yaoyong Dahuang) | SARS-CoV | Infected Vero E6 cells, CPE, biotinylated ELISA | 1–10 μg/mL | Block spike–ACE2 interaction | 117 |
| 28 | Polygonum multiflorum (Heshouwu) | SARS-CoV | Infected Vero E6 cells, CPE, Biotinylated ELISA | 1–10 μg/mL | Block spike–ACE2 interaction | 117 |
| 29 | Houttuynia cordata (Yuxingcao) | SARS-CoV | Flow cytometry, ELISA, enzyme inhibition assay, etc. | 0–800 μg/mL | 1) Stimulate the proliferation of mouse splenic lymphocytes 2) Increase the proportion of CD4+ and CD8+ T cells 3) Increase in the secretion of IL-2 and IL-10 in mouse splenic lymphocytes 4) Inhibit 3CLpro and RdRp activity |
118 |
| 30 | Rheum palmatum (Zhangye Dahuang) | SARS-CoV | Enzyme inhibition assay | 13.76 μg/mL | Inhibit 3CLpro activity | 119 |
| 31 | Cibotium barometz (Gouji) | SARS-CoV | 1) Infected Vero E6 cells, CPE 2) Enzyme inhibition assay |
1) 8.42 μg/mL 2) 39 μg/mL |
1) Inhibit viral replication 2) Inhibit 3CLpro activity |
120 |
| 32 | Dioscorea batatas (Shanyao) | SARS-CoV | 1) Infected Vero E6 cells, CPE 2) Enzyme inhibition assay |
1) 8.06 μg/mL 2) 44 μg/mL |
1) Inhibit viral replication 2) Inhibit 3CLpro activity |
120 |
Table 4.
Potential mechanisms of TCM ingredients for COVID-19.
| No. | TCM ingredient | Source | Coronavirus | Model/method | IC50 (EC50) or dosage | Potential mechanism | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | Rhein | Rheum palmatum (Yaoyong Dahuang) | SARS-CoV-2 | Enzyme inhibition assay, molecular docking, and surface plasmon resonance (SPR) analysis | 18.33 μmol/L | Inhibit ACE2 activity | 121 |
| 2 | Forsythoside A | Forsythiae fructus (Lianqiao) fruit | SARS-CoV-2 | Enzyme inhibition assay, molecular docking, SPR analysis | Unclear | Inhibit ACE2 activity | 121 |
| 3 | Neochlorogenic acid | Lonicera japonica (Jingyinhua) | SARS-CoV-2 | Enzyme inhibition assay, molecular docking, SPR analysis | ∼40 μmol/L | Inhibit ACE2 activity | 121 |
| 4 | Quercetin | Ginkgo biloba (Yingxing) | SARS-CoV-2 | Enzyme inhibition assay | 4.48 μmol/L | Inhibit ACE2 activity | 122 |
| 5 | Ephedrine | Ephedrae Herba (Mahuang) | SARS-CoV-2 | Molecular docking, SPR analysis | Unclear | Inhibit ACE2 activity | 123 |
| 6 | Hesperidin | Citrus aurantium (Suancheng) | SARS-CoV-2 | Target-based virtual ligand screening | Unclear | Block spike–ACE2 interaction. | 124,125 |
| 7 | Geniposide | Gardenia jasminoides (Zhizi) | SARS-CoV-2 | Molecular docking | Unclear | Inhibit TMPRSS2 activity | 126 |
| 8 | Baicalin | Scutellaria baicalensis (Huangqin) | SARS-CoV-2 | 1) Infected Vero E6 cells, CPE 2) Enzyme inhibition assay |
1) 27.87 μmol/L 2) 6.41 μmol/L |
1) Inhibit viral replication 2) Inhibit 3CLpro activity |
109 |
| 9 | Baicalein | Scutellaria baicalensis (Huangqin) | SARS-CoV-2 | 1) Enzyme inhibition assay 2) Infected Vero cells |
1) 0.39 μmol/L 2) 2.9 μmol/L |
1) Inhibit 3CLpro activity 2) Exert antiviral infection effect |
116 |
| 10 | Shikonin | Lithospermum erythrorhizon (Zicao) | SARS-CoV-2 | Enzyme inhibition assay | 15.75 μmol/L | Inhibit 3CLpro activity | 127 |
| 11 | EGCG | Green tea | SARS-CoV-2 | Enzyme inhibition assay | 0.017 μmol/L | Inhibit 3CLpro activity | 128 |
| 12 | Theaflavin | Black tea | SARS-CoV-2 | Enzyme inhibition assay | 0.015 μmol/L | Inhibit 3CLpro activity | 128 |
| 13 | Scutellarein | Scutellaria baicalensis (Huangqin) | SARS-CoV-2 | Enzyme inhibition assay | 5.8 μmol/L | Inhibit 3CLpro activity | 116 |
| 14 | Myricetin | Myrica rubra (Yangmei) | SARS-CoV-2 | Enzyme inhibition assay | 2.86 μmol/L | Inhibit 3CLpro activity | 116 |
| 15 | Cannabidiol | Cannabis sativa (Dama) | SARS-CoV-2 | 1) Molecular docking 2) Infected Vero cells |
7.91 μmol/L | 1) Bind to PLpro 2) Exert antiviral effect |
129 |
| 16 | Theaflavin | Black tea | SARS-CoV-2 | Molecular docking | Unclear | Inhibit RdRp activity | 130,131 |
| 17 | Digitoxin | Digitalis purpurea (Yangdihuang) | SARS-CoV-2 | Infected Vero cells, CPE | 0.23 μmol/L | Exert antiviral effect | 132 |
| 18 | Tetrandrine | Stephania tetrandra (Fengfangji) | SARS-CoV-2 | Infected Vero cells, CPE | 3 μmol/L | Exert antiviral effect | 132 |
| 19 | Glycyrrhizin | Glycyrrhiza uralensis (Gancao) | SARS-CoV-2 | Infected Vero E6 cells, CPE | 0.53 μmol/L | Exert antiviral effect | 133 |
| 20 | Resveratrol | Polygonum cuspidatum (Huzhang) | SARS-CoV-2 | Infected Vero E6, Calu-3 and primary human bronchial epithelium cells, CPE | 66 μmol/L | Exert antiviral effect | 134 |
| 21 | Pterostilbene | Pterocarpus santalinus (Zitan) | SARS-CoV-2 | Infected Vero E6, Calu-3 and primary human bronchial epithelium cells, CPE | 19 μmol/L | Exert antiviral effect | 134 |
| 22 | Phillyrin | Forsythiae fructus (Lianqiao) | SARS-CoV-2 | Infected Vero-E6 cells and Huh-7 cells, CPE | 1) 63.9 μg/mL 2) and 3) 62.5–250 μg/mL |
1) Inhibit viral replication 2) Reduce the production of proinflammatory cytokines of TNF-α, IL-6, IL-1β, MCP-1, and IP-10 3) Suppress NF-κB signaling pathway |
135 |
| 23 | Catechin | Green tea | SARS-CoV-2 | Molecular docking | Unclear | Bind to 3CLpro, cathepsin L, RBD of S protein, NSP6, and nucleocapsid protein | 131,136 |
| 24 | Artemisinin | Artemisia annua (Qinghao) | SARS-CoV-2 | Infected Vero E6 cells, CPE | 64.45 μmol/L | Inhibit viral replication | 137 |
| 25 | Artesunate | Artemisinin derivative | SARS-CoV-2 | Infected Vero E6 cells, CPE | 12.98 μmol/L | Inhibit viral replication | 137 |
| 26 | Cepharanthine | Stephania japonica (Qianjinteng) | SARS-CoV-2 | Infected Vero E6 cells, CPE | 0.98 μmol/L | Inhibit viral entry and viral replication | 138 |
| 27 | Bufalin | Toad venom (Chansu) | SARS-CoV-2 | Infected Vero E6 cells, CPE | 18 nmol/L | Exert antiviral effect by targeting Na+/K+-ATPase | 139 |
| 28 | Bruceine A | Brucea javanica (Yadanzi) | SARS-CoV-2 | Infected Vero E6 cells, CPE | 11 nmol/L | Exert antiviral effect | 139 |
| 29 | Naringenin | Gardenia jasminoides (Zhishi) | SARS-CoV-2 | Infected Vevo E6 cells, CPE | 31.3–250 μmol/L | Target two-pore channel 2 | 140 |
| 30 | Andrographolide | Andrographis paniculate (Chuanxinlian) | SARS-CoV-2 | Infected Calu-3 cells, CPE | 0.034 μmol/L | Exert antiviral effect | 115 |
| 31 | Glycyrrhizin + vitamin C | Glycyrrhiza uralensis (Gancao) | SARS-CoV-2 | NP | Unclear | Elevate immunity and suppress inflammatory stress | 141 |
| 32 | Chlorogenic acid | Lonicera japonica (Jinyinhua) | SARS-CoV-2 | NP | Unclear | Exert antiviral effect by targeting NFE2L2, PPARG, ESR1, ACE, IL-6, and HMOX1 | 142 |
| 33 | Emodin | Rheum palmatum (Yaoyong Dahuang) | SARS-CoV | Infected Vero E6 cells, CPE, biotinylated ELISA | 200 μmol/L | Block spike–ACE2 interaction | 117 |
| 34 | Celastrol | Celastrus orbiculatus (Nansheteng) | SARS-CoV | Enzyme inhibition assay | 10.3 μmol/L | Inhibit 3CLpro activity | 143,144 |
| 35 | Tingenone | Euonymus alatus (Weimao) | SARS-CoV | Enzyme inhibition assay | 9.9 μmol/L | Inhibit 3CLpro activity | 143 |
| 36 | Curcurmin | Curcuma longa (Jianghuang) | SARS-CoV | 1) Enzyme inhibition assay; 2) Infected Vero E6 cells, CPE |
1) 23.5 μmol/L 2) 40 μmol/L |
1) Inhibit 3CLpro activity 2) Inhibit viral replication |
145,146 |
| 37 | Quercetin | Ginkgo biloba (Yingxing) | SARS-CoV | Enzyme inhibition assay | 73 μmol/L | Inhibit 3CLpro activity | 147,148 |
| 38 | Tanshinone IIA | Salvia miltiorrhiza (Danshen) | SARS-CoV | Enzyme inhibition assay | 89.1 μmol/L | Inhibit 3CLpro activity | 149 |
| 39 | Dihydrotanshinone I | Salvia miltiorrhiza (Danshen) | SARS-CoV | Enzyme inhibition assay | 14.4 μmol/L | Inhibit 3CLpro activity | 149 |
| 40 | Xanthoangelol E | Angelica keiskei (Mingriye) | SARS-CoV | Enzyme inhibition assay | 11.4 μmol/L | Inhibit 3CLpro activity | 150 |
| 41 | Sinigrin | Isatis indigotica root (Banlangen) | SARS-CoV | Enzyme inhibition assay | 217 μmol/L | Inhibit 3CLpro activity | 151 |
| 42 | Hesperetin | Isatis indigotica root (Banlangen) | SARS-CoV | Enzyme inhibition assay | 8.3 μmol/L | Inhibit 3CLpro activity | 151 |
| 43 | Pectolinarin | Cirsium japonicum (Daji) | SARS-CoV | Enzyme inhibition assay | 37.78 μmol/L | Inhibit 3CLpro activity | 152 |
| 44 | Luteolin | (Jinyinhua) | SARS-CoV | 1) Infected Vero E6 cells, CPE; 2) Enzyme inhibition assay |
1) 9.02 μmol/L 2) 20.2 μmol/L |
1) Exert antiviral effect 2) Inhibit 3CLpro activity |
153,154 |
| 45 | Hirsutenone | Alnus japonica (Chiyang) | SARS-CoV | Enzyme inhibition assay | 4.1 μmol/L | Inhibit PLpro activity | 155 |
| 46 | Tanshinone IIB | Salvia miltiorrhiza (Danshen) | SARS-CoV | Enzyme inhibition assay | 10.7 μmol/L | Inhibit PLpro activity | 149 |
| 47 | Crytotanshinone | Salvia miltiorrhiza (Danshen) | SARS-CoV | Enzyme inhibition assay | 0.8 μmol/L | Inhibit PLpro activity | 149 |
| 48 | Dihydrotanshinone I | Salvia miltiorrhiza (Danshen) | SARS-CoV | Enzyme inhibition assay | 4.9 μmol/L | Inhibit PLpro activity | 149 |
| 49 | Xanthoangelol E | Angelica keiskei (Mingriye) | SARS-CoV | Enzyme inhibition assay | 1.2 μmol/L | Inhibit PLpro activity | 150 |
| 50 | Terrestrimine | Tribulus terrestris (Cijili) fruits | SARS-CoV | Enzyme inhibition assay | 15.8 μmol/L | Inhibit PLpro activity | 156 |
| 51 | Isobavachalcone | Psoralea corylifolia (Buguzhi) seeds | SARS-CoV | Enzyme inhibition assay | 7.3 μmol/L | Inhibit PLpro activity | 157 |
| 52 | Psoralidin | Psoralea corylifolia (Buguzhi) seeds | SARS-CoV | Enzyme inhibition assay | 4.2 μmol/L | Inhibit PLpro activity | 157 |
| 53 | Tomentin A-E | Paulownia tomentosa fruits (Maopaotong) | SARS-CoV | Enzyme inhibition assay | 5.0–12.5 μmol/L | Inhibit PLpro activity | 158 |
| 54 | Glycyrrhizin | Glycyrrhiza uralensis (Gancao) | SARS-CoV | Infected Vero cells, CPE | 0.3 mg/mL | Inhibit virus replication | 159, 160, 161 |
| 55 | Cepharanthine | Stephania japonica (Qianjinteng) | SARS-CoV | Infected Vero E6 cells, CPE | 6.0–9.5 μg/mL | Exert antiviral effect | 162 |
| 56 | Ginsenoside Rb1 | Panax ginseng (Renshen) | SARS-CoV | Infected Vero E6 cells, CPE | 100 μmol/L | Exert antiviral effect | 163 |
| 57 | Aescin | Aesculus chinensis (Qiyeshu) | SARS-CoV | Infected Vero E6 cells, CPE | 6.0 μmol/L | Inhibit viral replication | 163 |
| 58 | Reserpine | Ophiorrhiza japonica (Shegencao) | SARS-CoV | Infected Vero E6 cells, CPE | 3.4 μmol/L | Inhibit viral replication | 163 |
| 59 | Lycorine | Lycoris radiata (Shisuan) | SARS-CoV | Infected Vero E6 cells, CPE | 15.7 nmol/L | Exert antiviral effect | 164 |
3.1. Potential mechanisms of TCM for SARS-CoV-2 invasion and replication
Although the direct evidence is still lacking, increasing reports suggested that TCM resource holds great promises for agents against SARS-CoV-2 invasion and replication. Numerous efforts had been made to identify the antiviral effects of CPMs and herbals, as shown in Table 3. Lianhua Qingwen capsule with a half maximal inhibitory concentration (IC50) of 411.2 μg/mL89, Liu Shen capsule107 with an IC50 of 0.6 μg/mL, and Shuanghuanglian preparation109 with an IC50 of 0.93–1.2 μL/mL were confirmed to inhibit SARS-CoV-2 replication in Vero E6 cells. In addition, Pudilan Xiaoyan oral liquid not only inhibited SARS-CoV-2-stimulated Vero E6 cells in vitro, but also showed the potential efficacy on SARS-CoV-2-infected human angiotensin converting enzyme-2 (hACE2) transgenic mice in vivo108. Six herbal extracts of Cibotium barometz (Gouji), Gentiana scabra (Longdan), Dioscorea batatas (Shanyao), Cassia tora (Juemingzi), and Taxillus chinensis (Sangjisheng) were evaluated for the anti-SARS-CoV activities by screening out from more than 200 extracts of Chinese medicinal herbs using a Vero E6 cell-based assay120. Among them, Gouji and Shanyao could significantly inhibit 3CLpro protease activity of SARS-CoV with IC50 values of 39 and 44 μg/mL120. Another screen of 312 Chinese medicinal herb extracts discovered three widely used Chinese medicinal herbs of the family Polygonaceae involving Rheum officinale (Yaoyong Dahuang), Polygonum multiflorum (Heshouwu), and Caulis polygoni multiflori (Shouwuteng) blocking the interaction of SARS-CoV Spike protein and angiotensin converting enzyme 2 (ACE2) which may protect the host from virus invasion with the IC50 values ranged from 1 to 10 g/mL117. It was not difficult to find that although several TCMs like Liu Shen capsule and Dahuang showed a good performance in suppressing viral replication or activity, more studies are still necessary to be implemented to reveal more receivable anti-viral CPMs and herbal extracts especially the recommended CPMs in vitro and in vivo.
Noticeably, a considerable number of ingredients derived from TCMs were found to have anti-viral invasion and anti-viral replication activities by targeting diverse molecules, as seen in Table 4. The interaction between spike protein and ACE2, primed by serine protease transmembrane protease serine 2 (TMPRSS2), is the key step for SARS-CoV-2 host invasion. Emodin from Yaoyong Dahuang was able to inhibit S protein and ACE2 interaction with an IC50 of 200 μmol/L117, while hesperidin from Citrus aurantium (Suancheng) was predicted to target the binding between spike RBD and ACE2 with high affinity124. Besides, geniposide from Gardenia jasminoides (Zhizi) was found through virtual screening of 2140 compounds with pharmacophoric features, which could target the active site residues of TMPRSS2 with a binding energy score of −14.69, and is even greater than that of the standard inhibitor of camostat mesylate126. Seven isolated tanshinones derived from Salvia miltiorrhiza (Danshen) including tanshinone IIA, tanshinone IIB, methyl tanshinonate, crytotanshinone, tanshinone I, dihydrotanshinone I, and rosmariquinone showed marked inhibitory activities to both proteases of 3CLpro and PLpro149. Particularly, dihydrotanshinone I exerted powerful effects with IC50 values of 14.4 μmol/L regarding 3CLpro and 4.9 μmol/L regarding PLpro149. Furthermore, crytotanshinone exhibited the most potent nanomolar level inhibitory activity toward PLpro with an IC50 of 0.8 μmol/L149. Baicalin and baicalein, the major bioactive ingredients of Shuanghuanglian preparation, were characterized as the first noncovalent and nonpeptidomimetic inhibitors of SARS-CoV-2 3CLpro, also possessed good anti-SARS-CoV-2 activity in Vero E6 cell-based system109. What's more, celastrol143,144, tingenone143, xanthoangelol E150, and hesperetin151 targeting 3CLpro, while hirsutenone155, methyl tanshinoate, tanshinone I149, xanthoangelol E150, isobavachalcone, 4′-O-methylbavachalcone, psoralidin157, and tomentin A-E158 targeting PLpro, may have relatively strong anti-viral replication efficacy with IC50 below or near 10 μmol/L. Notably, the well-known anti-malarial165, anti-tumor166, and immune modulation167 compound artemisinin from Artemisia apiacea (Qinghao), and its derivatives including arteannuin B, artesunate, dihydroartemisinin, arteether, and lumefantrine presented favorable anti-SARS-CoV-2 effects. Among these artemisinin derivatives, arteannuin B showed the highest anti-viral potential with an IC50 of 10.28 μmol/L, while lumefantrine exerted therapeutic promise owing to its high plasma and lung concentrations after multiple dosing. The deeper pharmacological mechanism analysis revealed that these two compounds acted at the post-entry step of SARS-CoV-2 infection137. Significantly, lycorine from Lycoris radiata (Shisuan) had a powerful inhibitory effect on virus activity with an IC50 of 15.7 nmol/L and may serve as a candidate for the development of new anti-SARS-CoV-2 drug in the treatment of COVID-19164. In addition, a Vero E6 cell-based large-scale anti-SARS-CoV-2 activity of 1058 natural compounds were screened, and 17 newly discovered compounds showed strong anti-virus propagation effects with the IC50 values ranging from 0.011 to 11.03 μmol/L. Among them, bufalin from toad venom (Chansu) exerted the antiviral effect with an IC50 of 18 nmol/L by targeting the ion transport function of Na+/K+-ATPase139. Theaflavin was predicted to exert anti-viral replication by inhibiting RdRp activity130. The binding affinities with the critical proteins of a portion of ingredients presented above were also predicted by in silico screening and molecular docking124,168. Whether these TCM ingredients could be used to combat COVID-19 need further in vitro and in vivo validation. Pharmacokinetic profiles including absorption, distribution, metabolism, and excretion (ADME) on the promising leads should be further studied.
3.2. Potential mechanisms of TCM for immune and inflammatory regulation
Antiviral monotherapy for patients hospitalized with COVID-19 is quite not enough, especially for severely and critically ill patients169. Except for the broad-spectrum antiviral activity, TCM process advantages in regulating immune response, suppressing cytokine storm through multiple avenues170, 171, 172. Beyond inhibiting virus replication, Lianhua Qingwen capsule89 and Liu Shen capsule107 reduced pro-inflammatory cytokines production such as TNF-α, IL-6, MCP-1, and IP-10 in SARS-CoV-2 infected Huh-7 cells. In addition, Lianhua Qingwen capsule was analyzed to repair lung injury by modulating inflammatory process and cytokine storm90. Maxing Shigan decoction is the basic prescription of “three medicines and three formulas” apart from Xuebijing injection, was revealed to regulate immunity and reduce cytokine storm, as well as protect alveolar–capillary barrier of lung and relieve pulmonary edema by utilizing integrated network pharmacological approaches101. As same as Maxing Shigan decoction, Qingfei Paidu decoction showed multiple immune regulation, anti-inflammation, and lung injury–repair activities with its main ingredients of baicalin, glycyrrhizin, hesperidin, and hyperoside by targeting proteins including TNF-α, IL-6, IL-10, and CCL293, 94, 95, 96. Furthermore, several ingredients such as baicalin and glycyrrhizin of Qingfei Paidu decoction could inhibit platelet aggregation96. Dayuanyin is the basic formula of Qingfei Dayuan granules that might process an anti-inflammatory and immunoregulatory effects via acting on IL-6, IL-1β, and MCP-1, with its ingredients containing kaempferol, isoflavone, and formononetin63,104. Glycyrrhizin is an anti-viral agent and clinically used anti-inflammatory ingredient from Glycyrrhiza uralensis (Gancao) was determined to elevate immunity and suppress inflammatory stress through T cell receptor and VEGF signaling pathways141,159,173. Matrine was not only predicted to suppress host cell apoptosis and inflammation by targeting the TNF-α, IL-6, and CASP3 in the TNF signaling, but also validated to reduce lung tissue damage and lung index by decreasing the production of IL-6, IL-10, TNF-α, and IFN-γ, increasing the percentage of CD4+ T cells, CD8+ T cells, and B cells in peripheral blood, and lessening viral load in lung tissue in a mouse model combining human coronavirus pneumonia with cold‒dampness pestilence attacking the lung112,113. Although systems pharmacology is a convenient and effective tool to propose the mechanism of action of TCM at a holistic level, all the results above need to be further validated. IL-6 was considered as one of the most important molecules in cytokine storm174, 175, 176, 177, 178, 179, 180, 181, 182. Administration with Dayuanyin reduced the level of IL-6 in mild, moderate, and even severe clinical stages of COVID-19104. Besides, Shufeng Jiedu capsule54, Yidu-toxicity blocking lung decoction62, Qingfei Touxie Fuzheng recipe67, and diammonium glycyrrhizinate70 were confirmed to decrease the level of IL-6 in COVID-19 patients, as seen in Table 1. Interestingly, except for the strong anti-SARS-CoV-2 activity137, artemisinin and its derivatives regulated multiple immune cells including macrophage, monocyte, dendritic cell, and T cell to inhibit pro-inflammatory cytokine release and cytokine storm outbreak to protect tissues from injury183 (Table 3).
3.3. Potential mechanisms of TCM for ARDS and MODS treatment
In contrast with WM therapy, TCM is adept at treating complications of COVID-19 such as ARDS and MODS which are likely caused by the concurrence of viral toxicity, endothelial damage, cytokine storm, excessive immune, and microthrombus holistically (Table 3). Xuebijing injection was certified to treat severe pneumonia, sepsis, coagulopathy, SIRS, and MODS, owing to its various effects on cytokine reduction, immunoregulation, microcirculation improvement, anti-coagulation, pro-angiogenesis, and neutralization of released bacterial cytotoxins42,184, 185, 186, 187, 188, 189. Xuebijing injection was able to improve the oxygenation index of PaO2/FiO2 and reduce the level of pro-inflammatory cytokines of TNF-α, IP-10, MIP-1β, and RANTES in the treatment of COVID-1942,43. It was also reported that Xuebijing injection could downregulate the expression of IL-6, IL-1, TLR4, MAPK, and NF-κB, maintain the balance of Tregs and Th17 cells in acute lung injury190, 191, 192, 193. Besides, Xuebijing injection processed the potential to alleviate liver damage, acute lung injury-induced left ventricular ischemia/reperfusion, sepsis-induced acute kidney injury, and sepsis-induced myocardial injury via inhibiting inflammation, apoptosis, and endothelial injury194, 195, 196, 197, 198, 199. Systems pharmacological analysis revealed that Qingfei Paidu decoction could protect multi-organ including nervous system, sensory system, digestive system, and circulatory system by regulating key enzymes, G protein-coupled receptors, ion channels, and transporters96.
In the background of great demands for acute lung injury and ARDS therapy of COVID-19, more than one hundred of natural products from TCM with their potential benefits and underlying mechanisms of anti-inflammation, antioxidant stress, anti-apoptosis, and anti-pulmonary fibrosis were summarized and categorized. According to their chemical structures, these were divided into flavonoids (e.g., luteolin, baicalein), alkaloids (e.g., berberine, matrine), terpenoids (e.g., pogostone, andrographolide), polyphenols (e.g., honokiol, curcumin), quinonoids (e.g., emodin, shikonin), and other compounds (e.g., osthole, imperatorin)200. In addition, a systematic review and meta-analysis of 19 eligible RCTs including Tanreqing injection, Shengmai injection, Shenfu injection, Danshen injection, Reduning injection, and Xuebijing injection demonstrated that Chinese medicine injections were adjuvant therapy with great potential benefits for the treatment of ALI/ARDS33. For example, based on the effects of inhibiting inflammatory cytokines of IL-6, IL-8, IL-1β, and TNF-α, regulating immune, and elevating the oxygenation index of PaO2, Tanreqing injection was proved to improve lung injury, pulmonary infection, airway inflammation, and airway mucus hypersecretion201, 202, 203, 204. Reduning injection was demonstrated to prevent pulmonary neutrophil infiltration, lung injury and severe pneumonia which may attribute to downregulating IL-1β, IL-18, TNF-α, NF-κB, and pyrin domain containing 3 levels, lowering myeloperoxidase activities, and reducing reactive oxygen species production205, 206, 207. Xiyanping injection, a famous Chinese medicinal preparation of andrographolide sulfonate, was reputed as one of the most effective alternatives to antibiotics, which has been widely used to ameliorate lung damage, bronchitis and community acquired pneumonia probably through inhibiting NF-κB and MAPK-mediated inflammatory responses208,209. Besides, Xiyanping injection and Reduning injection were used to treat diarrhea in children. Xiyanping injection could ameliorate colitis by inhibiting Th1/Th17 response in mice210.
Cardiovascular disease is a high frequent comorbidity and complication of COVID-19. Three Chinese injection medicines including Shenfu injection, Shengmai injection, and Shenmai injection, have both pulmonary and cardiac protective effects. For instance, Shenfu injection is effective in the treatment of heart failure, myocardial hypertrophy, cardiac arrest, myocardial ischemia-reperfusion injury, myocardial fibrosis, and acute viral myocarditis, partly through suppressing apoptosis and inflammation, improving microcirculation, reducing mitochondrial damage and coagulation-fibrinolysis disorders211, 212, 213, 214, 215, 216, 217, 218, 219, 220, 221. Moreover, Shenfu injection has a protective effect on gastrointestinal tract and intestinal mucosa222,223. Xingnaojing injection and Angong Niuhuang pill are different preparations share similar ingredients for stroke treatment in clinic. Both of them ameliorate cerebral ischemia/reperfusion injury, cerebral infarction, cerebral edema, blood‒brain barrier disruption, and acute cerebral hemorrhage because of their benefits in brain microvascular endothelial cells, hippocampal and cortical neurons protection, and their anti-inflammation and anti-apoptosis effects224, 225, 226, 227, 228, 229, 230, 231.
3.4. Potential mechanisms of the representative and commonly used herbs in the treatment of COVID-19
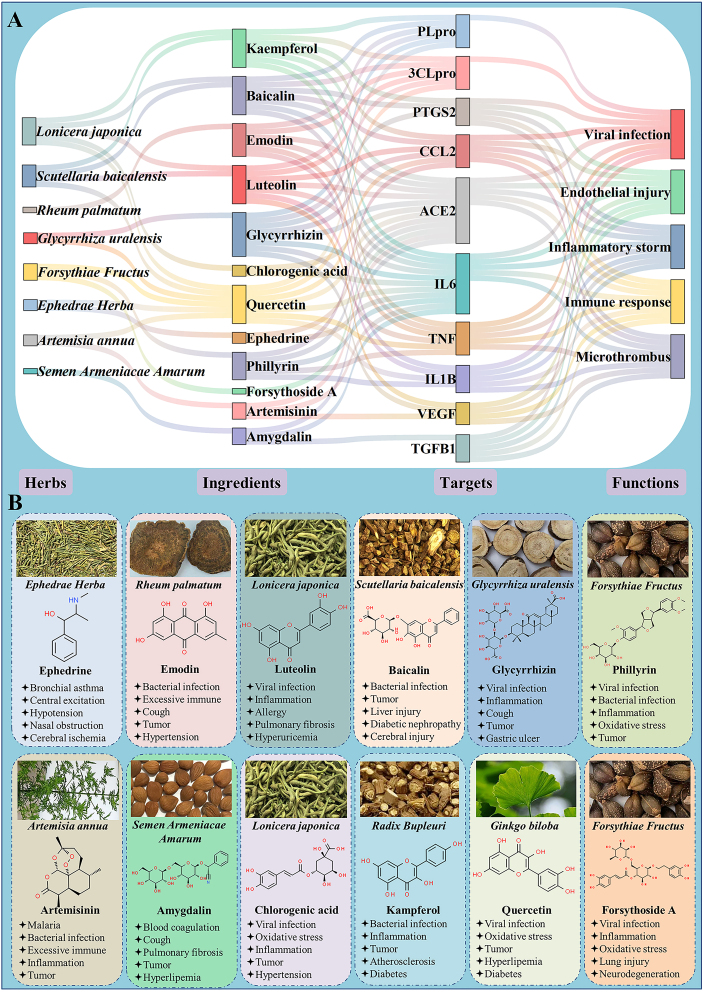
Analyses of the main compositions of the “three medicines and three formulas” and other related literatures identified G. uralensis (Gancao), Ephedrae Herba (Mahuang), Semen Armeniacae Amarum (Kuxingren), Scutellaria baicalensis (Huangqin), Forsythiae Fructus (Lianqiao), Lonicera japonica (Jingyinhua), Rheum palmatum (Dahuang), and Artemisia annua (Qinghao) as the representative and commonly used herbs for COVID-193,81,232. Herb–ingredient–target–function action network is established to elucidate the potential mechanisms of the frequently used herbs for COVID-19. In this relationship network, 8 commonly used herbs, 12 main ingredients, 10 key targets and 5 pivotal functions are involved, as shown in Fig. 2A. The portraits of commonly used herbs, chemical structures of ingredients, and main functions are illustrated in Fig. 2B.
Figure 2.
Representative herbs and their main active ingredients and functions for COVID-19. (A) The herb-ingredient-target-function network of frequently used herbs and their main ingredients, as well as their key targets and functions for COVID-19. (B) The chemical structures of main active ingredients and their main functions of commonly used herbs for COVID-19.
Gut microbiome is involved in disease severity and host inflammatory and immune responses in COVID-19 patients233. It is worth noting that the anti-COVID-19 effects and mechanisms of TCM may be exerted via the gut–lung axis and mediated by gut microbiota234, 235, 236. For example, short-term intervention of Qingfei Paidu decoction dose-dependently regulates the host metabolism and gut microbiome in rats, indicating that altering gut microbiota composition may be part of the anti-COVID-19 mechanisms of Qingfei Paidu decoction237. It is of particularly significance to consider that the solubility and bioavailability of certain TCM ingredients, such as resveratrol, quercetin, baicalin, curcurmin, emodin, and tanshinone IIA, are limited, leading to poor absorption into the bloodstream after oral administration. These ingredients may exert their therapeutic effects though interplaying with gut microbiota238. For instance, resveratrol could also alleviate intestinal inflammation and oxidative damage by modulating the composition of gut microbiota in addition to the direct antiviral effect239. What's more, to improve the bioavailability, a nano-micellar form of curcumin was used to decrease IL-6 and IL-1β expression and secretion in patients with COVID-19240.
In summary of preclinical evidence, the anti-COVID-19 effects and mechanisms of TCM include but not limited to 1) inhibiting SARS-CoV-2 invasion and replication by targeting the key proteins of spike, ACE2, TMPRSS2, 3CLpro, PLpro, RdRp, and spike–ACE2 interaction; 2) regulating immune and inflammatory response by targeting inflammatory cytokines such as IL-1, TNF-α, and IL-8, and chemokines like CCL5, CCL2, and IP-10, which are secreted by monocytes, macrophages, dendritic cells, CD4+ T cells, and CD8+ T cells; 3) protecting against ARDS and MODS by suppressing the crosstalk of viral toxicity, endothelial damage, cytokine storm, excessive immune, and microthrombus by targeting IL-6, CRP, D-dimer, and procalcitonin.
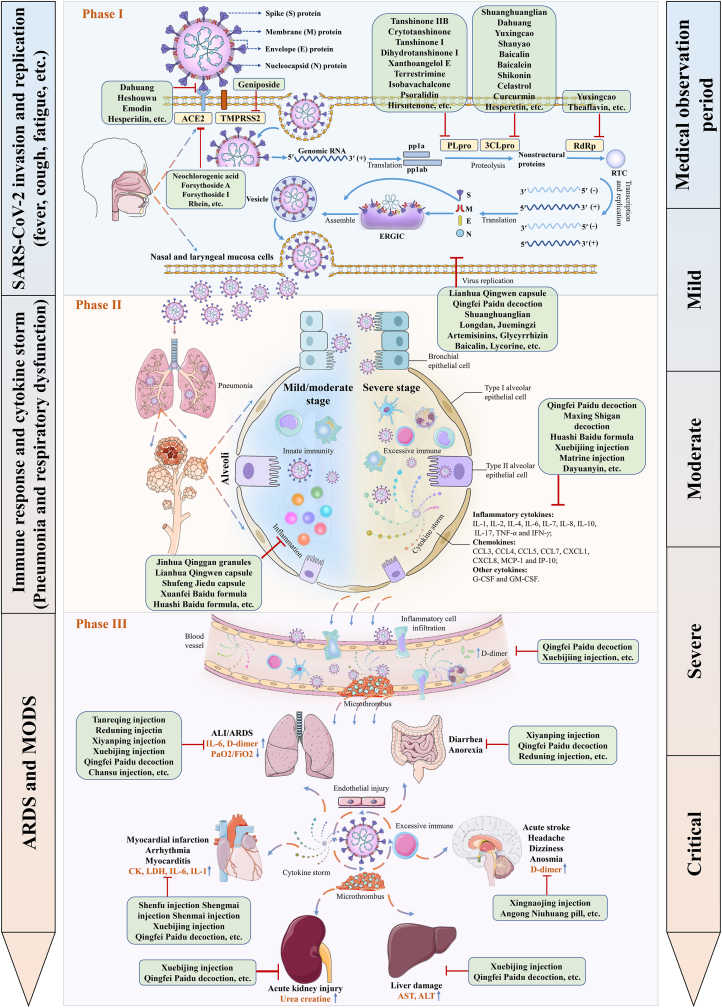
Finally, by integrating the clinical evidence and potential mechanisms of TCM for COVID-19, a panorama is drawn in Fig. 3, hoping that the effect and mechanism of TCM for COVID-19 could be viewed and understood within a single framework.
Figure 3.
An overview of pathogenesis of COVID-19 and the potential mechanisms of TCM remedy in distinct disease stages.
4. Conclusions and perspectives
Although a great quantity of review articles have been published on the topic of TCM in COVID-1913,14,19,23,25,35,71,87,168,171,241, 242, 243, 244, 245, 246, 247, 248, 249, 250, 251, 252, 253, 254, 255, 256, 257, 258, 259, 260, 261, 262, 263, 264, 265, 266, 267, 268, 269, 270, 271, 272, 273, 274, 275, 276, 277, our work offers something unique. 1) To our knowledge, this is the first review of TCM on COVID-19 that integrates evidence-based scientific findings from bedside to bench with the most comprehensive and updated literatures. 2) The pathogenesis and potential mechanisms of TCM remedy in three phases corresponding to distinct stages for COVID-19 are first systematically described and presented within a single panorama by integrating available clinical and fundamental evidence.
A valuable lesson learned from China's COVID-19 battle is that perseverance in combination of TCM and WM is the right and sensible choice71,249. Looking ahead, several critical issues need to be addressed as we prepare to face similar or even more serious global health threats in the future. Firstly, as the pandemic continues to evolve, the pathogenesis of COVID-19 is not fully elucidated. It is reasonable to postulate that the crosstalk of viral toxicity, endothelial damage, cytokine storm, excessive immune, and microthrombus are essential contributors for severely or critically ill patients with COVID-19, which need to be validated further. Secondly, due to a lack of in-depth understanding, there are still some skepticisms on the validity of treating COVID-19 with TCM278, 279, 280. More RCTs with high accuracy, clinical safety, rigorous design, and large sample, as well as in-depth mechanistic explorations with compatibility principal should be conducted to provide more reliable evidence for TCM in COVID-19 intervention, especially for the highly recommended three CPMs and three Chinese medicine formulas. Thirdly, the rehabilitative effects of TCM ought to be continuous concerned and long-term medical observed for the COVID-19 patients in recovery phase, especially for the aged. A recent paper published in The Lancet on 6-month consequences of 1733 COVID-19 patients revealed that those with severe disease discharged from hospital showed common syndromes of fatigue or muscle weakness, sleep difficulties, and anxiety or depression281,282. Meanwhile, a comparison of 425 non-treatment with 143 TCM-treated COVID-19 patients post discharge showed that TCM was beneficial for decreasing IL-6 and procalcitonin, and increasing red blood cell, hemoglobin, and platelet count283.
Overall, the purpose of this review is to scientifically and systematically evaluate the roles of TCM in combating COVID-19. The efficacies and potential mechanisms of TCM remedy in three phases of distinct stages of COVID-19 are discussed and presented comprehensively within a single panorama by integrating available clinical and preclinical evidence. Finally, although the availability of anti-COVID-19 vaccines and a global vaccination program have brought great hope for the ultimate control of the disease, threat of viral variants and new epidemics still exist. Therefore, it is of scientific value to historically and objectively summarize the contribution of TCM during the pandemic, which could be deployed in the future to combat against COVID-19 and other infectious diseases around the world.
Acknowledgments
This study was supported by grants from National Science and Technology Emergency Project (Integrated Traditional Chinese and Western Medicine to Control COVID-19, 2020yfc0841600, China), National Major Science and Technology Projects of China (2018YFC1704502, 2020YFA0708004), National Science Foundation of China (NSFC 82104431), Open project of State Key Laboratory of Component-based Chinese Medicine (CBCM2020201, China), and China Postdoctoral Science Foundation Grant (2019M650989).
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Contributor Information
Yuanlu Cui, Email: cuiyl@tju.edu.cn.
Junhua Zhang, Email: zjhtcm@foxmail.com.
Yan Zhu, Email: yanzhu.harvard@icloud.com.
Boli Zhang, Email: zhangbolipr@163.com.
Author contributions
Boli Zhang, Yan Zhu, Junhua Zhang, Yuanlu Cui, and Jigang Wang conceived, designed, and revised the manuscript; Ming Lyu, Guanwei Fan, and Guangxu Xiao wrote and revised the manuscript; Taiyi Wang, Dong Xu, Jie Gao, Shaoqin Ge, Qinglin Li, Yuling Ma, and Han Zhang revised the manuscript and discussed interpretation.
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luo H., Tang Q.L., Shang Y.X., Liang S.B., Yang M., Robinson N., et al. Can Chinese medicine be used for prevention of corona virus disease 2019 (COVID-19)? A review of historical classics, research evidence and current prevention programs. Chin J Integr Med. 2020;26:243–250. doi: 10.1007/s11655-020-3192-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu J., Manheimer E., Shi Y., Gluud C. Chinese herbal medicine for severe acute respiratory syndrome: a systematic review and meta-analysis. J Alternative Compl Med. 2004;10:1041–1051. doi: 10.1089/acm.2004.10.1041. [DOI] [PubMed] [Google Scholar]
- 5.Leung P.C. The efficacy of Chinese medicine for SARS: a review of Chinese publications after the crisis. Am J Chin Med. 2007;35:575–581. doi: 10.1142/S0192415X07005077. [DOI] [PubMed] [Google Scholar]
- 6.International expert meeting on the treatment of SARS by traditional Chinese medicine, and the integration of traditional Chinese medicine with Western medicine. SARS: clinical trials on treatment using a combination of traditional Chinese medicine and Western medicine: report of the WHO international expert meeting to review and analyse clinical reports on combination treatment for SARS, 8‒10 October 2003, Beijing, People’s Republic of China. World Health Organization. Available from: https://apps.who.int/iris/handle/10665/43029.
- 7.Lau T.F., Leung P.C., Wong E.L., Fong C., Cheng K.F., Zhang S.C., et al. Using herbal medicine as a means of prevention experience during the SARS crisis. Am J Chin Med. 2005;33:345–356. doi: 10.1142/S0192415X05002965. [DOI] [PubMed] [Google Scholar]
- 8.Hsu C.H., Hwang K.C., Chao C.L., Chang S.G., Ker C.C., Chien L.C., et al. The lesson of supplementary treatment with Chinese medicine on severe laboratory-confirmed SARS patients. Am J Chin Med. 2006;34:927–935. doi: 10.1142/S0192415X06004405. [DOI] [PubMed] [Google Scholar]
- 9.Chen Z., Nakamura T. Statistical evidence for the usefulness of Chinese medicine in the treatment of SARS. Phytother Res. 2004;18:592–594. doi: 10.1002/ptr.1485. [DOI] [PubMed] [Google Scholar]
- 10.Zhao Y.S., Hou X.Y., Gao Z.H., Wang T. Research on medication for severe type of COVID-19 based on Huashi Baidu prescription. Chin Arch Trad Chin Med. 2020;38:14–17. [Google Scholar]
- 11.Zhao Z.H., Zhou Y., Li W.H., Huang Q.S., Tang Z.H., Li H. Analysis of traditional Chinese medicine diagnosis and treatment strategies for COVID-19 based on “the diagnosis and treatment program for coronavirus disease-2019” from Chinese authority. Am J Chin Med. 2020;48:1035–1049. doi: 10.1142/S0192415X20500500. [DOI] [PubMed] [Google Scholar]
- 12.Tong X.L., Li X.Y., Zhao L.H., Li Q.W., Yang Y.Y., Lin Y.Q., et al. Discussion on traditional Chinese medicine prevention and treatment strategies of coronavirus disease 2019 (COVID-19) from the perspective of “cold‒dampness pestilence”. J Tradit Chin Med. 2020;61:465–470. [Google Scholar]
- 13.Leung E.L.H., Pan H.D., Huang Y.F., Fan X.X., Wang W.Y., He F., et al. The scientific foundation of Chinese herbal medicine against COVID-19. Engineering (Beijing) 2020;6:1099–1107. doi: 10.1016/j.eng.2020.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luo H., Gao Y., Zou J., Zhang S., Chen H., Liu Q., et al. Reflections on treatment of COVID-19 with traditional Chinese medicine. Chin Med. 2020;15:94. doi: 10.1186/s13020-020-00375-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zheng W.K., Zhang J.H., Yang F.W., Huang M., Miao Q., Qi W.S., et al. Treatment of coronavirus disease 2019 (COVID-19) from perspective of dampness‒toxicity plagues. J Tradit Chin Med. 2020;61:1024–1028. [Google Scholar]
- 16.Chinese Association of Integrated Traditional and Western Medicine Expert consensus on prevention and treatment of COVID-19 by integrating traditional Chinese and Western medicine. Chin J Integr Tradit West Med. 2020;40:1413–1423. [Google Scholar]
- 17.Li Y.X., Li J., Zhang Y., Tian Y.P., Zhang Y.G., Jin R.J., et al. Clinical practice guidelines and experts' consensuses for treatment of coronavirus disease 2019 (COVID-19) patients with Chinese herbal medicine: a systematic review. Chin J Integr Med. 2020;26:786–793. doi: 10.1007/s11655-020-3431-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee B.J., Lee J.A., Kim K.I., Choi J.Y., Jung H.J. A consensus guideline of herbal medicine for coronavirus disease 2019. Integr Med Res. 2020;9:100470. doi: 10.1016/j.imr.2020.100470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan K.W., Wong V.T., Tang S.C.W. COVID-19: an update on the epidemiological, clinical, preventive and therapeutic evidence and guidelines of integrative Chinese–Western medicine for the management of 2019 novel coronavirus disease. Am J Chin Med. 2020;48:737–762. doi: 10.1142/S0192415X20500378. [DOI] [PubMed] [Google Scholar]
- 20.Liang N., Li H., Wang J., Jiao L., Ma Y., Wang X., et al. Development of rapid advice guidelines for the treatment of coronavirus disease 2019 with traditional Chinese medicine. Am J Chin Med. 2020;48:1511–1521. doi: 10.1142/S0192415X20500743. [DOI] [PubMed] [Google Scholar]
- 21.Ho L.T.F., Chan K.K.H., Chung V.C.H., Leung T.H. Highlights of traditional Chinese medicine frontline expert advice in the China national guideline for COVID-19. Eur J Integr Med. 2020;36:101116. doi: 10.1016/j.eujim.2020.101116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liang N., Ma Y., Wang J., Li H., Wang X., Jiao L., et al. Traditional Chinese medicine guidelines for coronavirus disease 2019. J Tradit Chin Med. 2020;40:891–896. doi: 10.19852/j.cnki.jtcm.20200902.001. [DOI] [PubMed] [Google Scholar]
- 23.Qiu Q., Huang Y., Liu X., Huang F., Li X., Cui L., et al. Potential therapeutic effect of traditional Chinese medicine on coronavirus disease 2019: a review. Front Pharmacol. 2020;11:570893. doi: 10.3389/fphar.2020.570893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang J., Qi F. Traditional Chinese medicine to treat COVID-19: the importance of evidence-based research. Drug Discov Ther. 2020;14:149–150. doi: 10.5582/ddt.2020.03054. [DOI] [PubMed] [Google Scholar]
- 25.Li Q., Wang H., Li X., Zheng Y., Wei Y., Zhang P., et al. The role played by traditional Chinese medicine in preventing and treating COVID-19 in China. Front Med. 2020;14:681–688. doi: 10.1007/s11684-020-0801-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang C., Cao B., Liu Q.Q., Zou Z.Q., Liang Z.A., Gu L., et al. Oseltamivir compared with the Chinese traditional therapy maxingshigan-yinqiaosan in the treatment of H1N1 influenza: a randomized trial. Ann Intern Med. 2011;155:217–225. doi: 10.7326/0003-4819-155-4-201108160-00005. [DOI] [PubMed] [Google Scholar]
- 27.Hu K., Guan W.J., Bi Y., Zhang W., Li L., Zhang B., et al. Efficacy and safety of Lianhuaqingwen capsules, a repurposed Chinese herb, in patients with coronavirus disease 2019: a multicenter, prospective, randomized controlled trial. Phytomedicine. 2020;16:153242. doi: 10.1016/j.phymed.2020.153242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xiao M., Tian J., Zhou Y., Xu X., Min X., Lv Y., et al. Efficacy of Huoxiang Zhengqi dropping pills and Lianhua Qingwen granules in treatment of COVID-19: a randomized controlled trial. Pharmacol Res. 2020;161:105126. doi: 10.1016/j.phrs.2020.105126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ren W., Ma Y., Wang R., Liang P., Sun Q., Pu Q., et al. Research advance on Qingfei Paidu decoction in prescription principle, mechanism analysis and clinical application. Front Pharmacol. 2020;11:589714. doi: 10.3389/fphar.2020.589714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang L. Efficacy and safety assessment of severe COVID-19 patients with Chinese medicine: a retrospective case series study at early stage of the COVID-19 epidemic in Wuhan, China. J Ethnopharmacol. 2021;30:113888. doi: 10.1016/j.jep.2021.113888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shi N., Guo L., Liu B., Bian Y., Chen R., Chen S., et al. Efficacy and safety of Chinese herbal medicine versus lopinavir‒ritonavir in adult patients with coronavirus disease 2019: a non-randomized controlled trial. Phytomedicine. 2020;81:153367. doi: 10.1016/j.phymed.2020.153367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xiong W.Z., Wang G., Du J., Ai W. Efficacy of herbal medicine (Xuanfei Baidu decoction) combined with conventional drug in treating COVID-19: a pilot randomized clinical trial. Integr Med Res. 2020;9:100489. doi: 10.1016/j.imr.2020.100489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen Y.B., Liu Q., Xie H., Yin S.M., Wu L., Yu X.H., et al. Is Chinese medicine injection applicable for treating acute lung injury and acute respiratory distress syndrome? A systematic review and meta-analysis of randomized controlled trials. Chin J Integr Med. 2019;6:857–866. doi: 10.1007/s11655-019-3078-7. [DOI] [PubMed] [Google Scholar]
- 34.Song P., Zhao L., Li X., Su J., Jiang Z., Song B., et al. Interpretation of the traditional Chinese medicine portion of the diagnosis and treatment protocol for corona virus disease 2019 (Trial Version 7) J Tradit Chin Med. 2020;40:497–508. doi: 10.19852/j.cnki.jtcm.2020.03.019. [DOI] [PubMed] [Google Scholar]
- 35.Zhang D., Zhang B., Lv J.T., Sa R.N., Zhang X.M., Lin Z.J. The clinical benefits of Chinese patent medicines against COVID-19 based on current evidence. Pharmacol Res. 2020;157:104882. doi: 10.1016/j.phrs.2020.104882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu Z., Li X., Gou C., Li L., Luo X., Zhang C., et al. Effect of Jinhua Qinggan granules on novel coronavirus pneumonia in patients. J Tradit Chin Med. 2020;40:467–472. doi: 10.19852/j.cnki.jtcm.2020.03.016. [DOI] [PubMed] [Google Scholar]
- 37.Duan C., Xia W.G., Zhen C.J., Sun G.B., Li Z.L., Li Q.L., et al. Clinical observation of Jinhua Qinggan granules in treating pneumonia infected by COVID-19. J Tradit Chin Med. 2020;61:1473–1477. [Google Scholar]
- 38.Lu R.B., Wang W.J., Li X. Clinical observation on Lianhua Qingwen granules combined with Western medicine conventional therapy in the treatment of 63 suspected cases of COVID-19. J Tradit Chin Med. 2020;61:655–659. [Google Scholar]
- 39.Yu P., Li Y.Z., Wan S.B., Wang Y. Efficacy of Lianhua Qingwen granules combined with arbidol in the treatment of mild novel coronavirus pneumonia. China J Chin Mater Med. 2020;55:1042–1045. [Google Scholar]
- 40.Cheng Z.D., Li Y. Clinical effectiveness and case analysis in 54 NCP patients treated with Lanhua Qingwen Granules. World Chin Med. 2020;15:150–154. [Google Scholar]
- 41.Liu L., Shi F., Tu P., Chen C., Zhang M., Li X., et al. Arbidol combined with the Chinese medicine Lianhuaqingwen capsule versus arbidol alone in the treatment of COVID-19. Medicine (Baltimore) 2021;100 doi: 10.1097/MD.0000000000024475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ma Q., Qiu M., Zhou H., Chen J., Yang X., Deng Z., et al. The study on the treatment of Xuebijing injection (XBJ) in adults with severe or critical Corona Virus Disease 2019 and the inhibitory effect of XBJ against SARS-CoV-2. Pharmacol Res. 2020;160:105073. doi: 10.1016/j.phrs.2020.105073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wen L., Zhou Z., Jiang D., Huang K. Effect of Xuebijing injection on inflammatory markers and disease outcome of coronavirus disease 2019. Chin Crit Care Med. 2020;32:426–429. doi: 10.3760/cma.j.cn121430-20200406-00386. [DOI] [PubMed] [Google Scholar]
- 44.Zhang C.Y., Zhang S., Wang W., Jiang X.Q., Zhang C.Y., Zhang S., et al. Clinical observation of Xuebijing in the treatment of COVID-19. Chin J Hosp Pharm. 2020;40:964–967. [Google Scholar]
- 45.Xin S., Cheng X., Zhu B., Liao X., Yang F., Song L., et al. Clinical retrospective study on the efficacy of Qingfei Paidu decoction combined with Western medicine for COVID-19 treatment. Biomed Pharmacother. 2020;129:110500. doi: 10.1016/j.biopha.2020.110500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang R.Q., Yang S.J., Xie C.G., Shen Q.L., Li M.Q., Lei X., et al. Clinical observation of Qingfei Paidu decoction in the treatment of COVID-19. Pharmacol Clin Chin Mater Med. 2020;36:13–18. [Google Scholar]
- 47.Li K.Y., An W., Xia F., Chen M., Yang P., Liao Y.L., et al. Observation on clinical effect of modified Qingfei Paidu Decoction in treatment of COVID-19. Chin Herb Med. 2020;51:2046–2049. [Google Scholar]
- 48.Yu X.Y., Zhang S., Yan F.F., Su D.Z. Comparison of clinical efficacy of Qingfei Paidu decoction combined with western medicine in 43 cases and single western medicine in 46 cases in the treatment of COVID-19. J Shandong Univ (Health Sci) 2020;58:47–53. [Google Scholar]
- 49.Sun Y.N., Lv W.L., Li H., Xiao Y., Yang W., Yang H.J., et al. A multicenter clinical study on the treatment of 295 COVID-19 cases with Qingfei Paidu decoction. J Tradit Chin Med. 2020;62:599–603. [Google Scholar]
- 50.Shi N., Liu B., Liang N., Ma Y., Ge Y., Yi H., et al. Association between early treatment with Qingfei Paidu decoction and favorable clinical outcomes in patients with COVID-19: a retrospective multicenter cohort study. Pharmacol Res. 2020;161:105290. doi: 10.1016/j.phrs.2020.105290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen L., Liu F., Wu J.H., Song H.Y., Xia J.S., Sheng B., et al. Clinical efficacy of Shufeng Jiedu capsule combined with Western medicine in treatment of common COVID-19 patients by retrospective analysis. Chin J Exp Tradit Med Form. 2020;26:14–20. [Google Scholar]
- 52.Xiao Q., Jiang Y.J., Wu S.S., Wang Y., An J., Xu W.P., et al. Analysis of the value of Shufeng Jiedu capsule combined with arbidol in the treatment of mild new coronavirus pneumonia. J Emerg Tradit Chin Med. 2020;29:756–758. [Google Scholar]
- 53.Qu X.K., Hao S.L., Ma J.H., Wei G.Y., Song K.Y., Tang C., et al. Observation on clinical effect of Shufeng Jiedu capsule combined with arbidol hydrochloride capsule in treatment of COVID-19. Chin Herb Med. 2020;51:1167–1170. [Google Scholar]
- 54.Chen J., Lin S., Niu C., Xiao Q. Clinical evaluation of Shufeng Jiedu Capsules combined with umifenovir (arbidol) in the treatment of common-type COVID-19: a retrospective study. Expet Rev Respir Med. 2020;15:257–265. doi: 10.1080/17476348.2020.1822741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tian J., Yan S., Wang H., Zhang Y., Zheng Y., Wu H., et al. Hanshiyi Formula, a medicine for SARS-CoV-2 infection in China, reduced the proportion of mild and moderate COVID-19 patients turning to severe status: a cohort study. Pharmacol Res. 2020;161:105127. doi: 10.1016/j.phrs.2020.105127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sun H.M., Xu F., Zhang L., Wei C., Chen J.Y., Wang Q.X., et al. Study on the clinical efficacy of Lianhua Qingke granules in the treatment of mild and common COVID-19. Chin J Exp Tradit Med Form. 2020;26:29–34. [Google Scholar]
- 57.Fu X.X., Lin L.P., Tan X.H. Clinical observation on effect of Toujie Quwen granules in treatment of COVID-19. Chin J Exp Tradit Med Form. 2020;26:44–48. [Google Scholar]
- 58.Yang M.B., Dang S.S., Huang S., Li Y.J., Guo Y.L. Multi-center clinical observation of Reyanning mixture in treatment of COVID-19. Chin J Exp Tradit Med Form. 2020;26:7–12. [Google Scholar]
- 59.Qu Y.F., Fang W., Jin Y.Z., Qin C., Niu X.C., Zhang N., et al. Forty cases of common COVID-19 treated with modified ephedra and apricot kernel and gypsum and licorice decoction combined with Western medicine routine treatment. Henan Tradit Chin Med. 2020;40:666–669. [Google Scholar]
- 60.Hu F., Guo A.H., Huang L., Yu W.X., Liu G.F., Gao X.S., et al. Multi-center clinical observation of honeysuckle oral liquid combined with Western medicine in the treatment of common COVID-19. J Tradit Chin Med. 2020;62:510–515. [Google Scholar]
- 61.Hu F., Chen J., Chen H., Zhu J., Wang C., Ni H., et al. Chansu injection improves the respiratory function of severe COVID-19 patients. medRxiv. 2020 Available from: https://doi.org/10.1101/2020.05.20.20107607. [Google Scholar]
- 62.Zhao J., Yang X., Wang C., Song S., Cao K., Wei T., et al. Yidu-toxicity blocking lung decoction ameliorates inflammation in severe pneumonia of SARS-COV-2 patients with Yidu-toxicity blocking lung syndrome by eliminating IL-6 and TNF-α. Biomed Pharmacother. 2020;129:110436. doi: 10.1016/j.biopha.2020.110436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ba Y.M., Wang L.Q., Li W.N., Li M., Tao R., Zuo X.H., et al. Multi center clinical study on 451 cases of COVID-19 treated with ‘Pneumonia No. 1 Formula’. World Chin Med. 2020;15:1962–1966. [Google Scholar]
- 64.Ai Z., Zhou S., Li W., Wang M., Wang L., Hu G., et al. “Fei Yan No. 1” as a combined treatment for COVID-19: an efficacy and potential mechanistic study. Front Pharmacol. 2020;11:581277. doi: 10.3389/fphar.2020.581277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shi T.F., Zhou G.C., Zhang L.Y., Niu F., Ke Y.C., Zhou T., et al. Clinical efficacy of Xuanfei Huazhuo decoction on 40 cases of COVID-19. Chin J Exp Tradit Med Form. 2020;26:26–31. [Google Scholar]
- 66.Wang J.B., Wang Z.X., Jing J., Zhao P., Dong J.H., Zhou Y.F., et al. Exploring an integrative therapy for treating COVID-19: a randomized controlled trial. Chin J Integr Med. 2020;26:648–655. doi: 10.1007/s11655-020-3426-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ding X.J., Zhang Y., He D.C., Zhang M.Y., Tan Y.J., Yu A.R., et al. Clinical effect and mechanism of Qingfei Touxie Fuzheng recipe in the treatment of COVID-19. Herald Med. 2020;39:640–644. [Google Scholar]
- 68.Chen L., Cheng Z.Q., Liu F., Xia Y., Chen Y.G. Analysis of 131 cases of COVID-19 treated with Ganlu Xiaodu decoction. China J Chin Mater Med. 2020;45:2232–2238. doi: 10.19540/j.cnki.cjcmm.20200322.505. [DOI] [PubMed] [Google Scholar]
- 69.Yang M.W., Chen F., Zhu D.J., Li J.Z., Zhu J.L., Zeng W., et al. Clinical efficacy of matrine and sodium chloride injection in treatment of 40 cases of COVID-19. China J Chin Mater Med. 2020;45:2221–2231. doi: 10.19540/j.cnki.cjcmm.20200323.501. [DOI] [PubMed] [Google Scholar]
- 70.Xi J.X., Xiang S.L., Zhang H.J., Lan B.Q., Chen X.Y. Clinical observation of Arbidol combined with diammonium lycyrrhizinate in the treatment of COVID-19. Chin J Hosp Pharm. 2020;40:1287–1290. [Google Scholar]
- 71.Ni L., Chen L., Huang X., Han C., Xu J., Zhang H., et al. Combating COVID-19 with integrated traditional Chinese and Western medicine in China. Acta Pharm Sin B. 2020;10:1149–1162. doi: 10.1016/j.apsb.2020.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xia W.G., An C.Q., Zhen C.J., Zhang J.X., Huang M., Wang Y., et al. Clinical observation on 34 patients with novel coronavirus pneumonia (COVID-19) treated with integrated traditional Chinese and Western medicine. J Tradit Chin Med. 2020;61:375–382. [Google Scholar]
- 73.Yang Q., Sun Q.G., Jiang B., Xu H.J., Luo M., Xie P., et al. Retrospective clinical study on treatment of COVID-19 patients with integrated traditional Chinese and western medicine. Chin Herb Med. 2020;51:2050–2054. [Google Scholar]
- 74.Shu Z., Zhou Y., Chang K., Liu J., Min X., Zhang Q., et al. Clinical features and the traditional Chinese medicine therapeutic characteristics of 293 COVID-19 inpatient cases. Front Med. 2020;14:760–775. doi: 10.1007/s11684-020-0803-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang H.T., Huang M.X., Liu X., Zheng X.C., Li X.H., Chen G.Q., et al. Evaluation of the adjuvant efficacy of natural herbal medicine on COVID-19: a retrospective matched case-control study. Am J Chin Med. 2020;48:779–792. doi: 10.1142/S0192415X20500391. [DOI] [PubMed] [Google Scholar]
- 76.Liu M., Gao Y., Yuan Y., Yang K., Shi S., Zhang J., et al. Efficacy and safety of integrated traditional Chinese and Western medicine for corona virus disease 2019 (COVID-19): a systematic review and meta-analysis. Pharmacol Res. 2020;158:104896. doi: 10.1016/j.phrs.2020.104896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ye Y.A. Guideline-based Chinese herbal medicine treatment plus standard care for severe coronavirus disease 2019 (G-CHAMPS): evidence from China. Front Med (Lausanne) 2020;7:256. doi: 10.3389/fmed.2020.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang K., Tian M., Zeng Y., Wang L., Luo S., Xia W., et al. The combined therapy of a traditional Chinese medicine formula and Western medicine for a critically ill case infected with COVID-19. Compl Ther Med. 2020;52:102473. doi: 10.1016/j.ctim.2020.102473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen G., Su W., Yang J., Luo D., Xia P., Jia W., et al. Chinese herbal medicine reduces mortality in patients with severe and critical coronavirus disease 2019: a retrospective cohort study. Front Med. 2020;14:752–759. doi: 10.1007/s11684-020-0813-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ang L., Song E., Lee H.W., Lee M.S. Herbal medicine for the treatment of coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis of randomized controlled trials. J Clin Med. 2020;9:1583. doi: 10.3390/jcm9051583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xiong X., Wang P., Su K., Cho W.C., Xing Y. Chinese herbal medicine for coronavirus disease 2019: a systematic review and meta-analysis. Pharmacol Res. 2020;160:105056. doi: 10.1016/j.phrs.2020.105056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yu M., Zhang R., Ni P., Duan G. Chinese herbal medicine supplementation therapy on COVID-19. Pharmacol Res. 2020;160:105181. doi: 10.1016/j.phrs.2020.105181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pang W., Liu Z., Li N., Li Y., Yang F., Pang B., et al. Chinese medical drugs for coronavirus disease 2019: a systematic review and meta-analysis. Integr Med Res. 2020;9:100477. doi: 10.1016/j.imr.2020.100477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chan J.F., Kok K.H., Zhu Z., Chu H., To K.K., Yuan S., et al. Genomic characterization of the 2019 novel human‒pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg Microb Infect. 2020;9:221–236. doi: 10.1080/22221751.2020.1719902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Morse J.S., Lalonde T., Xu S., Liu W.R. Learning from the past: possible urgent prevention and treatment options for severe acute respiratory infections caused by 2019-nCoV. Chembiochem. 2020;21:730–738. doi: 10.1002/cbic.202000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu C., Zhou Q., Li Y., Garner L.V., Watkins S.P., Carter L.J., et al. Research and development on therapeutic agents and vaccines for COVID-19 and related human coronavirus diseases. ACS Cent Sci. 2020;6:315–331. doi: 10.1021/acscentsci.0c00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li H., Yang L., Liu F.F., Ma X.N., He P.L., Tang W., et al. Overview of therapeutic drug research for COVID-19 in China. Acta Pharmacol Sin. 2020;41:1133–1140. doi: 10.1038/s41401-020-0438-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gong P.Y., Guo Y.J., Li X.P., Wang N., Gu J. Exploring active compounds of Jinhua Qinggan Granules for prevention of COVID-19 based on network pharmacology and molecular docking. Chin Tradit Herb Drugs. 2020;51:1685–1693. [Google Scholar]
- 89.Li R.F., Hou Y.L., Huang J.C., Pan W.Q., Ma Q.H., Shi Y.X., et al. Lianhuaqingwen exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-CoV-2) Pharmacol Res. 2020;156:104761. doi: 10.1016/j.phrs.2020.104761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zheng S., Baak J.P., Li S., Xiao W., Ren H., Yang H., et al. Network pharmacology analysis of the therapeutic mechanisms of the traditional Chinese herbal formula Lian Hua Qing Wen in Corona virus disease 2019 (COVID-19), gives fundamental support to the clinical use of LHQW. Phytomedicine. 2020;79:153336. doi: 10.1016/j.phymed.2020.153336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xing Y., Hua Y.R., Shang J., Ge W.H., Liao J. Traditional Chinese medicine network pharmacology study on exploring the mechanism of Xuebijing Injection in the treatment of coronavirus disease 2019. Chin J Nat Med. 2020;18:941–951. doi: 10.1016/S1875-5364(20)60038-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zheng W.J., Yan Q., Ni Y.S., Zhan S.F., Yang L.L., Zhuang H.F., et al. Examining the effector mechanisms of Xuebijing injection on COVID-19 based on network pharmacology. BioData Min. 2020;13:17. doi: 10.1186/s13040-020-00227-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chen J., Wang Y.K., Gao Y., Hu L.S., Yang J.W., Wang J.R., et al. Protection against COVID-19 injury by Qingfei Paidu decoction via anti-viral, anti-inflammatory activity and metabolic programming. Biomed Pharmacother. 2020;129:110281. doi: 10.1016/j.biopha.2020.110281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yang R., Liu H., Bai C., Wang Y., Zhang X., Guo R., et al. Chemical composition and pharmacological mechanism of Qingfei Paidu Decoction and Ma Xing Shi Gan Decoction against coronavirus disease 2019 (COVID-19): in silico and experimental study. Pharmacol Res. 2020;157:104820. doi: 10.1016/j.phrs.2020.104820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang D.H., Zhang X., Peng B., Deng S.Q., Wang Y.F., Yang L., et al. Network pharmacology suggests biochemical rationale for treating COVID-19 symptoms with a traditional Chinese medicine. Commun Biol. 2020;3:466. doi: 10.1038/s42003-020-01190-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhao J., Tian S., Lu D., Yang J., Zeng H., Zhang F., et al. Systems pharmacological study illustrates the immune regulation, anti-infection, anti-inflammation, and multi-organ protection mechanism of Qing-Fei-Pai-Du decoction in the treatment of COVID-19. Phytomedicine. 2020;85:153315. doi: 10.1016/j.phymed.2020.153315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tao Q., Du J., Li X., Zeng J., Tan B., Xu J., et al. Network pharmacology and molecular docking analysis on molecular targets and mechanisms of Huashi Baidu formula in the treatment of COVID-19. Drug Dev Ind Pharm. 2020;46:1345–1353. doi: 10.1080/03639045.2020.1788070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang Y., Li X., Zhang J.H., Xue R., Qian J.Y., Zhang X.H., et al. Study on the mechanism of Xuanfei Baidu decoction for COVID-19 based on network pharmacology. China J Chin Mater Med. 2020;45:2249–2256. doi: 10.19540/j.cnki.cjcmm.20200325.401. [DOI] [PubMed] [Google Scholar]
- 99.Chen X., Yin Y.H., Zhang M.Y., Liu J.Y., Li R., Qu Y.Q. Investigating the mechanism of ShuFeng JieDu capsule for the treatment of novel coronavirus pneumonia (COVID-19) based on network pharmacology. Int J Med Sci. 2020;17:2511–2530. doi: 10.7150/ijms.46378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tao Z., Zhang L., Friedemann T., Yang G., Li J., Wen Y., et al. Systematic analyses on the potential immune and anti-inflammatory mechanisms of Shufeng Jiedu capsule against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-caused pneumonia. J Funct Foods. 2020;75:104243. doi: 10.1016/j.jff.2020.104243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang Y.X., Ma J.R., Wang S.Q., Zeng Y.Q., Zhou C.Y., Ru Y.H., et al. Utilizing integrating network pharmacological approaches to investigate the potential mechanism of Ma Xing Shi Gan Decoction in treating COVID-19. Eur Rev Med Pharmacol Sci. 2020;24:3360–3384. doi: 10.26355/eurrev_202003_20704. [DOI] [PubMed] [Google Scholar]
- 102.Li Y., Chu F., Li P., Johnson N., Li T., Wang Y., et al. Potential effect of Maxing Shigan decoction against coronavirus disease 2019 (COVID-19) revealed by network pharmacology and experimental verification. J Ethnopharmacol. 2021;271:113854. doi: 10.1016/j.jep.2021.113854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Han L., Wei X.X., Zheng Y.J., Zhang L.L., Wang X.M., Yang H.Y., et al. Potential mechanism prediction of Cold‒Damp Plague Formula against COVID-19 via network pharmacology analysis and molecular docking. Chin Med. 2020;15:78. doi: 10.1186/s13020-020-00360-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ruan X., Du P., Zhao K., Huang J., Xia H., Dai D., et al. Mechanism of Dayuanyin in the treatment of coronavirus disease 2019 based on network pharmacology and molecular docking. Chin Med. 2020;15:62. doi: 10.1186/s13020-020-00346-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhang X.R., Li T.N., Ren Y.Y., Zeng Y.J., Lv H.Y., Wang J., et al. The important role of volatile components from a traditional Chinese medicine Dayuan-Yin against the COVID-19 pandemic. Front Pharmacol. 2020;11:583651. doi: 10.3389/fphar.2020.583651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jia S., Luo H., Liu X., Fan X., Huang Z., Lu S., et al. Dissecting the novel mechanism of Reduning injection in treating Coronavirus Disease 2019 (COVID-19) based on network pharmacology and experimental verification. J Ethnopharmacol. 2021;273:113871. doi: 10.1016/j.jep.2021.113871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ma Q., Pan W., Li R., Liu B., Li C., Xie Y., et al. Liu Shen capsule shows antiviral and anti-inflammatory abilities against novel coronavirus SARS-CoV-2 via suppression of NF-kappaB signaling pathway. Pharmacol Res. 2020;158:104850. doi: 10.1016/j.phrs.2020.104850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Deng W., Xu Y., Kong Q., Xue J., Yu P., Liu J., et al. Therapeutic efficacy of pudilan xiaoyan oral liquid (PDL) for COVID-19 in vitro and in vivo. Signal Transduct Target Ther. 2020;5:66. doi: 10.1038/s41392-020-0176-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Su H.X., Yao S., Zhao W.F., Li M.J., Liu J., Shang W.J., et al. Anti-SARS-CoV-2 activities in vitro of Shuanghuanglian preparations and bioactive ingredients. Acta Pharmacol Sin. 2020;41:1167–1177. doi: 10.1038/s41401-020-0483-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lin H., Wang X., Liu M., Huang M., Shen Z., Feng J., et al. Exploring the treatment of COVID-19 with Yinqiao powder based on network pharmacology. Phytother Res. 2021;35:2651–2664. doi: 10.1002/ptr.7012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kong Q., Wu Y., Gu Y., Lv Q., Qi F., Gong S., et al. Analysis of the molecular mechanism of Pudilan (PDL) treatment for COVID-19 by network pharmacology tools. Biomed Pharmacother. 2020;128:110316. doi: 10.1016/j.biopha.2020.110316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Peng W., Xu Y., Han D., Feng F., Wang Z., Gu C., et al. Potential mechanism underlying the effect of matrine on COVID-19 patients revealed through network pharmacological approaches and molecular docking analysis. Arch Physiol Biochem. 2020 doi: 10.1080/13813455.2020.1817944. https://www.tandfonline.com/doi/full/10.1080/13813455.2020.1817944 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sun J., Zhao R.H., Guo S.S., Shi Y.J., Bao L., Geng Z.H., et al. Effect of matrine sodium chloride injection on a mouse model combining disease with syndrome of human coronavirus pneumonia with cold-dampness pestilence attacking the lung. Acta Pharm Sin. 2020;55:366–373. [Google Scholar]
- 114.Li X., Lin H., Wang Q., Cui L., Luo H., Luo L. Chemical composition and pharmacological mechanism of shenfu decoction in the treatment of novel coronavirus pneumonia (COVID-19) Drug Dev Ind Pharm. 2020;46:1947–1959. doi: 10.1080/03639045.2020.1826510. [DOI] [PubMed] [Google Scholar]
- 115.Sa-ngiamsuntorn K., Suksatu A., Pewkliang Y., Thongsri P., Kanjanasirirat P., Manopwisedjaroen S., et al. Anti-SARS-CoV-2 activity of Andrographis paniculata extract and its major component andrographolide in human lung epithelial cells and cytotoxicity evaluation in major organ cell representatives. J Nat Prod. 2021;84:1261–1270. doi: 10.1021/acs.jnatprod.0c01324. [DOI] [PubMed] [Google Scholar]
- 116.Liu H., Ye F., Sun Q., Liang H., Li C., Li S., et al. Scutellaria baicalensis extract and baicalein inhibit replication of SARS-CoV-2 and its 3C-like protease in vitro. J Enzym Inhib Med Chem. 2021;36:497–503. doi: 10.1080/14756366.2021.1873977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ho T.Y., Wu S.L., Chen J.C., Li C.C., Hsiang C.Y. Emodin blocks the SARS coronavirus spike protein and angiotensin-converting enzyme 2 interaction. Antivir Res. 2007;74:92–101. doi: 10.1016/j.antiviral.2006.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lau K.M., Lee K.M., Koon C.M., Cheung C.S., Lau C.P., Ho H.M., et al. Immunomodulatory and anti-SARS activities of Houttuynia cordata. J Ethnopharmacol. 2008;118:79–85. doi: 10.1016/j.jep.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Luo W., Su X., Gong S., Qin Y., Liu W., Li J., et al. Anti-SARS coronavirus 3C-like protease effects of Rheum palmatum L. extracts. Biosci Trends. 2009;3:124–126. [PubMed] [Google Scholar]
- 120.Wen C.C., Shyur L.F., Jan J.T., Liang P.H., Kuo C.J., Arulselvan P., et al. Traditional Chinese medicine herbal extracts of Cibotium barometz, Gentiana scabra Dioscorea batatas, Cassia tora, and Taxillus chinensis inhibit SARS-CoV replication. J Tradit Complement Med. 2011;1:41–50. doi: 10.1016/S2225-4110(16)30055-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chen X., Wu Y., Chen C., Gu Y., Zhu C., Wang S., et al. Identifying potential anti-COVID-19 pharmacological components of traditional Chinese medicine Lianhuaqingwen capsule based on human exposure and ACE2 biochromatography screening. Acta Pharm Sin B. 2021;11:222–236. doi: 10.1016/j.apsb.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Liu X., Raghuvanshi R., Ceylan F.D., Bolling B.W. Quercetin and its metabolites inhibit recombinant human angiotensin-converting enzyme 2 (ACE2) activity. J Agric Food Chem. 2020;68:13982–13989. doi: 10.1021/acs.jafc.0c05064. [DOI] [PubMed] [Google Scholar]
- 123.Lv Y., Wang S., Liang P., Wang Y., Zhang X., Jia Q., et al. Screening and evaluation of anti-SARS-CoV-2 components from Ephedra sinica by ACE2/CMC‒HPLC‒IT‒TOF‒MS approach. Anal Bioanal Chem. 2021;413:2995–3004. doi: 10.1007/s00216-021-03233-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wu C., Liu Y., Yang Y., Zhang P., Zhong W., Wang Y., et al. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm Sin B. 2020;10:766–788. doi: 10.1016/j.apsb.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Haggag Y.A., El-Ashmawy N.E., Okasha K.M. Is hesperidin essential for prophylaxis and treatment of COVID-19 infection? Med Hypotheses. 2020;144:109957. doi: 10.1016/j.mehy.2020.109957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rahman N., Basharat Z., Yousuf M., Castaldo G., Rastrelli L., Khan H. Virtual screening of natural products against type II transmembrane serine protease (TMPRSS2), the priming agent of coronavirus 2 (SARS-CoV-2) Molecules. 2020;25:2271. doi: 10.3390/molecules25102271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jin Z., Du X., Xu Y., Deng Y., Liu M., Zhao Y., et al. Structure of M(pro) from SARS-CoV-2 and discovery of its inhibitors. Nature. 2020;582:289–293. doi: 10.1038/s41586-020-2223-y. [DOI] [PubMed] [Google Scholar]
- 128.Jang M., Park Y.I., Cha Y.E., Park R., Namkoong S., Lee J.I., et al. Tea polyphenols EGCG and theaflavin inhibit the activity of SARS-CoV-2 3CL-protease in vitro. Evid Based Complement Alternat Med. 2020;2020:5630838. doi: 10.1155/2020/5630838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Raj V., Park J.G., Cho K.H., Choi P., Kim T., Ham J., et al. Assessment of antiviral potencies of cannabinoids against SARS-CoV-2 using computational and in vitro approaches. Int J Biol Macromol. 2021;168:474–485. doi: 10.1016/j.ijbiomac.2020.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lung J., Lin Y.S., Yang Y.H., Chou Y.L., Shu L.H., Cheng Y.C., et al. The potential chemical structure of anti-SARS-CoV-2 RNA-dependent RNA polymerase. J Med Virol. 2020;92:693–697. doi: 10.1002/jmv.25761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mhatre S., Srivastava T., Naik S., Patravale V. Antiviral activity of green tea and black tea polyphenols in prophylaxis and treatment of COVID-19: a review. Phytomedicine. 2020;85:153286. doi: 10.1016/j.phymed.2020.153286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jeon S., Ko M., Lee J., Choi I., Byun S.Y., Park S., et al. Identification of antiviral drug candidates against SARS-CoV-2 from FDA-approved drugs. Antimicrob Agents Chemother. 2020;64:e00819–e00820. doi: 10.1128/AAC.00819-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sand L.V.D., Bormann M., Alt M., Schipper L., Heilingloh C.S., Todt D., et al. Glycyrrhizin effectively neutralizes SARS-CoV-2 in vitro by inhibiting the viral main protease. Viruses. 2021;13:609. doi: 10.3390/v13040609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ellen ter B.M., Dinesh N., Bouma E.M., Troost B., Pol van de D.P.I., Ende Van der-Metselaar H.H., et al. Resveratrol and pterostilbene potently inhibit SARS-CoV-2 infection in vitro. Viruses. 2021;13:1335. doi: 10.3390/v13071335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ma Q., Li R., Pan W., Huang W., Liu B., Xie Y., et al. Phillyrin (KD-1) exerts anti-viral and anti-inflammatory activities against novel coronavirus (SARS-CoV-2) and human coronavirus 229E (HCoV-229E) by suppressing the nuclear factor kappa B (NF-kappaB) signaling pathway. Phytomedicine. 2020;78:153296. doi: 10.1016/j.phymed.2020.153296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mishra C.B., Pandey P., Sharma R.D., Malik M.Z., Mongre R.K., Lynn A.M., et al. Identifying the natural polyphenol catechin as a multi-targeted agent against SARS-CoV-2 for the plausible therapy of COVID-19: an integrated computational approach. Briefings Bioinf. 2021;22:1346–1360. doi: 10.1093/bib/bbaa378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cao R., Hu H., Li Y., Wang X., Xu M., Liu J., et al. Anti-SARS-CoV-2 potential of artemisinins in vitro. ACS Infect Dis. 2020;6:2524–2531. doi: 10.1021/acsinfecdis.0c00522. [DOI] [PubMed] [Google Scholar]
- 138.Fan H.H., Wang L.Q., Liu W.L., An X.P., Liu Z.D., He X.Q., et al. Repurposing of clinically approved drugs for treatment of coronavirus disease 2019 in a 2019-novel coronavirus-related coronavirus model. Chin Med J (Engl) 2020;133:1051–1056. doi: 10.1097/CM9.0000000000000797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhang Z.R., Zhang Y.N., Li X.D., Zhang H.Q., Xiao S.Q., Deng F., et al. A cell-based large-scale screening of natural compounds for inhibitors of SARS-CoV-2. Signal Transduct Target Ther. 2020;5:218. doi: 10.1038/s41392-020-00343-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Clementi N., Scagnolari C., D'Amore A., Palombi F., Criscuolo E., Frasca F., et al. Naringenin is a powerful inhibitor of SARS-CoV-2 infection in vitro. Pharmacol Res. 2021;163:105255. doi: 10.1016/j.phrs.2020.105255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Li R., Wu K., Li Y., Liang X., Lai K.P., Chen J. Integrative pharmacological mechanism of vitamin C combined with glycyrrhizic acid against COVID-19: findings of bioinformatics analyses. Briefings Bioinf. 2021;22:1161–1174. doi: 10.1093/bib/bbaa141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wang W.X., Zhang Y.R., Luo S.Y., Zhang Y.S., Zhang Y., Tang C. Chlorogenic acid, a natural product as potential inhibitor of COVID-19: virtual screening experiment based on network pharmacology and molecular docking. Nat Prod Res. 2021 doi: 10.1080/14786419.2021.1904923. https://www.tandfonline.com/doi/full/10.1080/14786419.2021.1904923 Available from: [DOI] [PubMed] [Google Scholar]
- 143.Ryu Y.B., Park S.J., Kim Y.M., Lee J.Y., Seo W.D., Chang J.S., et al. SARS-CoV 3CLpro inhibitory effects of quinone-methide triterpenes from Tripterygium regelii. Bioorg Med Chem Lett. 2010;20:1873–1876. doi: 10.1016/j.bmcl.2010.01.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Habtemariam S., Nabavi S.F., Berindan-Neagoe I., Cismaru C.A., Izadi M., Sureda A., et al. Should we try the antiinflammatory natural product, celastrol, for COVID-19? Phytother Res. 2020;34:1189–1190. doi: 10.1002/ptr.6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wen C.C., Kuo Y.H., Jan J.T., Liang P.H., Wang S.Y., Liu H.G., et al. Specific plant terpenoids and lignoids possess potent antiviral activities against severe acute respiratory syndrome coronavirus. J Med Chem. 2007;50:4087–4095. doi: 10.1021/jm070295s. [DOI] [PubMed] [Google Scholar]
- 146.Zahedipour F., Hosseini S.A., Sathyapalan T., Majeed M., Jamialahmadi T., Al-Rasadi K., et al. Potential effects of curcumin in the treatment of COVID-19 infection. Phytother Res. 2020;34:2911–2920. doi: 10.1002/ptr.6738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Nguyen T.T., Woo H.J., Kang H.K., Nguyen V.D., Kim Y.M., Kim D.W., et al. Flavonoid-mediated inhibition of SARS coronavirus 3C-like protease expressed in Pichia pastoris. Biotechnol Lett. 2012;34:831–838. doi: 10.1007/s10529-011-0845-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Abian O., Ortega-Alarcon D., Jimenez-Alesanco A., Ceballos-Laita L., Vega S., Reyburn H.T., et al. Structural stability of SARS-CoV-2 3CLpro and identification of quercetin as an inhibitor by experimental screening. Int J Biol Macromol. 2020;164:1693–1703. doi: 10.1016/j.ijbiomac.2020.07.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Park J.Y., Kim J.H., Kim Y.M., Jeong H.J., Kim D.W., Park K.H., et al. Tanshinones as selective and slow-binding inhibitors for SARS-CoV cysteine proteases. Bioorg Med Chem. 2012;20:5928–5935. doi: 10.1016/j.bmc.2012.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Park J.Y., Ko J.A., Kim D.W., Kim Y.M., Kwon H.J., Jeong H.J., et al. Chalcones isolated from Angelica keiskei inhibit cysteine proteases of SARS-CoV. J Enzym Inhib Med Chem. 2016;31:23–30. doi: 10.3109/14756366.2014.1003215. [DOI] [PubMed] [Google Scholar]
- 151.Lin C.W., Tsai F.J., Tsai C.H., Lai C.C., Wan L., Ho T.Y., et al. Anti-SARS coronavirus 3C-like protease effects of Isatis indigotica root and plant-derived phenolic compounds. Antivir Res. 2005;68:36–42. doi: 10.1016/j.antiviral.2005.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Jo S., Kim S., Shin D.H., Kim M.S. Inhibition of SARS-CoV 3CL protease by flavonoids. J Enzym Inhib Med Chem. 2020;35:145–151. doi: 10.1080/14756366.2019.1690480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Yi L., Li Z., Yuan K., Qu X., Chen J., Wang G., et al. Small molecules blocking the entry of severe acute respiratory syndrome coronavirus into host cells. J Virol. 2004;78:11334–11339. doi: 10.1128/JVI.78.20.11334-11339.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ryu Y.B., Jeong H.J., Kim J.H., Kim Y.M., Park J.Y., Kim D., et al. Biflavonoids from Torreya nucifera displaying SARS-CoV 3CL(pro) inhibition. Bioorg Med Chem. 2010;18:7940–7947. doi: 10.1016/j.bmc.2010.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Park J.Y., Jeong H.J., Kim J.H., Kim Y.M., Park S.J., Kim D., et al. Diarylheptanoids from Alnus japonica inhibit papain-like protease of severe acute respiratory syndrome coronavirus. Biol Pharm Bull. 2012;35:2036–2042. doi: 10.1248/bpb.b12-00623. [DOI] [PubMed] [Google Scholar]
- 156.Song Y.H., Kim D.W., Curtis-Long M.J., Yuk H.J., WangY, Zhuang N., et al. Papain-like protease (PLpro) inhibitory effects of cinnamic amides from Tribulus terrestris fruits. Biol Pharm Bull. 2014;37:1021–1028. doi: 10.1248/bpb.b14-00026. [DOI] [PubMed] [Google Scholar]
- 157.Kim D.W., Seo K.H., Curtis-Long M.J., Oh K.Y., Oh J.W., Cho J.K., et al. Phenolic phytochemical displaying SARS-CoV papain-like protease inhibition from the seeds of Psoralea corylifolia. J Enzym Inhib Med Chem. 2014;29:59–63. doi: 10.3109/14756366.2012.753591. [DOI] [PubMed] [Google Scholar]
- 158.Cho J.K., Curtis-Long M.J., Lee K.H., Kim D.W., Ryu H.W., Yuk H.J., et al. Geranylated flavonoids displaying SARS-CoV papain-like protease inhibition from the fruits of Paulownia tomentosa. Bioorg Med Chem. 2013;21:3051–3057. doi: 10.1016/j.bmc.2013.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Cinatl J., Morgenstern B., Bauer G., Chandra P., Rabenau H., Doerr H.W. Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus. Lancet. 2003;361:2045–2046. doi: 10.1016/S0140-6736(03)13615-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hoever G., Baltina L., Michaelis M., Kondratenko R., Baltina L., Tolstikov G.A., et al. Antiviral activity of glycyrrhizic acid derivatives against SARS-coronavirus. J Med Chem. 2005;48:1256–1259. doi: 10.1021/jm0493008. [DOI] [PubMed] [Google Scholar]
- 161.Bailly C., Vergoten G. Glycyrrhizin: an alternative drug for the treatment of COVID-19 infection and the associated respiratory syndrome? Pharmacol Ther. 2020;214:107618. doi: 10.1016/j.pharmthera.2020.107618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zhang C.H., Wang Y.F., Liu X.J., Lu J.H., Qian C.W., Wan Z.Y., et al. Antiviral activity of cepharanthine against severe acute respiratory syndrome coronavirus in vitro. Chin Med J. 2005;118:493–496. [PubMed] [Google Scholar]
- 163.Wu C.Y., Jan J.T., Ma S.H., Kuo C.J., Juan H.F., Cheng Y.S., et al. Small molecules targeting severe acute respiratory syndrome human coronavirus. Proc Natl Acad Sci U S A. 2004;101:10012–10017. doi: 10.1073/pnas.0403596101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Li S.Y., Chen C., Zhang H.Q., Guo H.Y., Wang H., Wang L., et al. Identification of natural compounds with antiviral activities against SARS-associated coronavirus. Antivir Res. 2005;67:18–23. doi: 10.1016/j.antiviral.2005.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bridgford J.L., Xie S.C., Cobbold S.A., Pasaje C.F.A., Herrmann S., Yang T., et al. Artemisinin kills malaria parasites by damaging proteins and inhibiting the proteasome. Nat Commun. 2018;9:3801. doi: 10.1038/s41467-018-06221-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wang J., Zhang J., Shi Y., Xu C., Zhang C., Wong Y.K., et al. Mechanistic investigation of the specific anticancer property of artemisinin and its combination with aminolevulinic acid for enhanced anticolorectal cancer activity. ACS Cent Sci. 2017;3:743–750. doi: 10.1021/acscentsci.7b00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.An J., Minie M., Sasaki T., Woodward J.J., Elkon K.B. Antimalarial drugs as immune modulators: new mechanisms for old drugs. Annu Rev Med. 2017;68:317–330. doi: 10.1146/annurev-med-043015-123453. [DOI] [PubMed] [Google Scholar]
- 168.Huang F., Li Y., Leung E.L., Liu X., Liu K., Wang Q., et al. A review of therapeutic agents and Chinese herbal medicines against SARS-CoV-2 (COVID-19) Pharmacol Res. 2020;158:104929. doi: 10.1016/j.phrs.2020.104929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Cao B., Hayden F.G. Antiviral monotherapy for hospitalised patients with COVID-19 is not enough. Lancet. 2020;396:1310–1311. doi: 10.1016/S0140-6736(20)32078-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Huang C.F., Lin S.S., Liao P.H., Young S.C., Yang C.C. The immunopharmaceutical effects and mechanisms of herb medicine. Cell Mol Immunol. 2008;5:23–31. doi: 10.1038/cmi.2008.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Huang Y.F., Bai C., He F., Xie Y., Zhou H. Review on the potential action mechanisms of Chinese medicines in treating Coronavirus Disease 2019 (COVID-19) Pharmacol Res. 2020;158:104939. doi: 10.1016/j.phrs.2020.104939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ma H.D., Deng Y.R., Tian Z., Lian Z.X. Traditional Chinese medicine and immune regulation. Clin Rev Allergy Immunol. 2013;44:229–241. doi: 10.1007/s12016-012-8332-0. [DOI] [PubMed] [Google Scholar]
- 173.Cao Z.Y., Liu Y.Z., Li J.M., Ruan Y.M., Yan W.J., Zhong S.Y., et al. Glycyrrhizic acid as an adjunctive treatment for depression through anti-inflammation: a randomized placebo-controlled clinical trial. J Affect Disord. 2020;265:247–254. doi: 10.1016/j.jad.2020.01.048. [DOI] [PubMed] [Google Scholar]
- 174.Zhang C., Wu Z., Li J.W., Zhao H., Wang G.Q. Cytokine release syndrome in severe COVID-19: interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int J Antimicrob Agents. 2020;55:105954. doi: 10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Crisafulli S., Isgro V., La Corte L., Atzeni F., Trifiro G. Potential role of anti-interleukin (IL)-6 drugs in the treatment of COVID-19: rationale, clinical evidence and risks. BioDrugs. 2020;34:415–422. doi: 10.1007/s40259-020-00430-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Chakraborty C., Sharma A.R., Bhattacharya M., Sharma G., Lee S.S., Agoramoorthy G. COVID-19: consider IL-6 receptor antagonist for the therapy of cytokine storm syndrome in SARS-CoV-2 infected patients. J Med Virol. 2020;92:2260–2262. doi: 10.1002/jmv.26078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J., et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Moore J.B., June C.H. Cytokine release syndrome in severe COVID-19. Science. 2020;368:473–474. doi: 10.1126/science.abb8925. [DOI] [PubMed] [Google Scholar]
- 179.Xu X., Han M., Li T., Sun W., Wang D., Fu B., et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci U S A. 2020;117:10970–10975. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Masia M., Fernandez-Gonzalez M., Padilla S., Ortega P., Garcia J.A., Agullo V., et al. Impact of interleukin-6 blockade with tocilizumab on SARS-CoV-2 viral kinetics and antibody responses in patients with COVID-19: a prospective cohort study. EBioMedicine. 2020;60:102999. doi: 10.1016/j.ebiom.2020.102999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Dzobo K., Chiririwa H., Dandara C., Dzobo W. Coronavirus disease-2019 treatment strategies targeting interleukin-6 signaling and herbal medicine. OMICS. 2020;25:13–22. doi: 10.1089/omi.2020.0122. [DOI] [PubMed] [Google Scholar]
- 182.Nasonov E., Samsonov M. The role of Interleukin 6 inhibitors in therapy of severe COVID-19. Biomed Pharmacother. 2020;131:110698. doi: 10.1016/j.biopha.2020.110698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Tang Y., Liu J., Zhang D., Xu Z., Ji J., Wen C. Cytokine storm in COVID-19: the current evidence and treatment strategies. Front Immunol. 2020;11:1708. doi: 10.3389/fimmu.2020.01708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Song Y., Yao C., Shang H., Yao X., Bai C. Intravenous infusion of Chinese medicine Xuebijing for patients with severe pneumonia: a multicenter, randomised, double-blind controlled trial. Lancet. 2017;390:S34. [Google Scholar]
- 185.Lyu M., Zhou Z., Wang X., Lv H., Wang M., Pan G., et al. Network pharmacology-guided development of a novel integrative regimen to prevent acute graft-vs.-host disease. Front Pharmacol. 2018;9:1440. doi: 10.3389/fphar.2018.01440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Wang L., Liu Z., Dong Z., Pan J., Ma X. Effects of Xuebijing injection on microcirculation in septic shock. J Surg Res. 2016;202:147–154. doi: 10.1016/j.jss.2015.12.041. [DOI] [PubMed] [Google Scholar]
- 187.Yin Q., Li C. Treatment effects of xuebijing injection in severe septic patients with disseminated intravascular coagulation. Evid Based Complement Alternat Med. 2014;2014:949254. doi: 10.1155/2014/949254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Song Y., Yao C., Yao Y., Han H., Zhao X., Yu K., et al. XueBiJing injection versus placebo for critically ill patients with severe community-acquired pneumonia: a randomized controlled trial. Crit Care Med. 2019;47:e735–e743. doi: 10.1097/CCM.0000000000003842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Hou S.Y., Feng X.H., Lin C.L., Tan Y.F. Efficacy of Xuebijing for coagulopathy in patients with sepsis. Saudi Med J. 2015;36:164–169. doi: 10.15537/smj.2015.2.9895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Liu M.W., Su M.X., Zhang W., Wang Y.Q., Chen M., Wang L., et al. Protective effect of Xuebijing injection on paraquat-induced pulmonary injury via down-regulating the expression of p38 MAPK in rats. BMC Complement Altern Med. 2014;14:498. doi: 10.1186/1472-6882-14-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.He F., Wang J., Liu Y., Wang X., Cai N., Wu C., et al. Xuebijing injection induces anti-inflammatory-like effects and downregulates the expression of TLR4 and NF-kappaB in lung injury caused by dichlorvos poisoning. Biomed Pharmacother. 2018;106:1404–1411. doi: 10.1016/j.biopha.2018.07.111. [DOI] [PubMed] [Google Scholar]
- 192.Chen X., Feng Y., Shen X., Pan G., Fan G., Gao X., et al. Anti-sepsis protection of Xuebijing injection is mediated by differential regulation of pro- and anti-inflammatory Th17 and T regulatory cells in a murine model of polymicrobial sepsis. J Ethnopharmacol. 2018;211:358–365. doi: 10.1016/j.jep.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 193.Liu M.W., Wang Y.H., Qian C.Y., Li H. Xuebijing exerts protective effects on lung permeability leakage and lung injury by upregulating Toll-interacting protein expression in rats with sepsis. Int J Mol Med. 2014;34:1492–1504. doi: 10.3892/ijmm.2014.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Zhang H., Wei L., Zhao G., Liu S., Zhang Z., Zhang J., et al. Protective effect of Xuebijing injection on myocardial injury in patients with sepsis: a randomized clinical trial. J Tradit Chin Med. 2016;36:706–710. doi: 10.1016/S0254-6272(17)30003-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Yuxi Q., Zhang H., Baili Y., Shi S. Effects of Xuebijing injection for patients with sepsis-induced acute kidney injury after Wenchuan earthquake. Altern Ther Health Med. 2017;23:36–42. [PubMed] [Google Scholar]
- 196.Ji M., Wang Y., Wang L., Chen L., Li J. Protective effect of Xuebijing injection against acute lung injury induced by left ventricular ischemia/reperfusion in rabbits. Exp Ther Med. 2016;12:51–58. doi: 10.3892/etm.2016.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Xu Q., Liu J., Guo X., Tang Y., Zhou G., Liu Y., et al. Xuebijing injection reduces organ injuries and improves survival by attenuating inflammatory responses and endothelial injury in heatstroke mice. BMC Complement Altern Med. 2015;15:4. doi: 10.1186/s12906-015-0519-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Liu X., Hu Z., Zhou B., Li X., Tao R. Chinese herbal preparation Xuebijing potently inhibits inflammasome activation in hepatocytes and ameliorates mouse liver ischemia‒reperfusion injury. PLoS One. 2015;10 doi: 10.1371/journal.pone.0131436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Chen Y., Tong H., Zhang X., Tang L., Pan Z., Liu Z., et al. Xuebijing injection alleviates liver injury by inhibiting secretory function of Kupffer cells in heat stroke rats. J Tradit Chin Med. 2013;33:243–249. doi: 10.1016/s0254-6272(13)60133-8. [DOI] [PubMed] [Google Scholar]
- 200.He Y.Q., Zhou C.C., Yu L.Y., Wang L., Deng J.L., Tao Y.L., et al. Natural product derived phytochemicals in managing acute lung injury by multiple mechanisms. Pharmacol Res. 2020;163:105224. doi: 10.1016/j.phrs.2020.105224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Dong S., Zhong Y., Yang K., Xiong X., Mao B. Intervention effect and dose-dependent response of tanreqing injection on airway inflammation in lipopolysaccharide-induced rats. J Tradit Chin Med. 2013;33:505–512. doi: 10.1016/s0254-6272(13)60156-9. [DOI] [PubMed] [Google Scholar]
- 202.Zhong Y., Mao B., Wang G., Fan T., Liu X., Diao X., et al. Tanreqing injection combined with conventional Western medicine for acute exacerbations of chronic obstructive pulmonary disease: a systematic review. J Altern Complement Med. 2010;16:1309–1319. doi: 10.1089/acm.2009.0686. [DOI] [PubMed] [Google Scholar]
- 203.Liu W., Jiang H.L., Cai L.L., Yan M., Dong S.J., Mao B. Tanreqing injection attenuates lipopolysaccharide-induced airway inflammation through MAPK/NF-kappaB signaling pathways in rats model. Evid Based Complement Alternat Med. 2016;2016:5292346. doi: 10.1155/2016/5292346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Liu W., Zhang X., Mao B., Jiang H. Systems pharmacology-based study of Tanreqing injection in airway mucus hypersecretion. J Ethnopharmacol. 2020;249:112425. doi: 10.1016/j.jep.2019.112425. [DOI] [PubMed] [Google Scholar]
- 205.Jiang C., Zhong R., Zhang J., Wang X., Ding G., Xiao W., et al. Reduning injection ameliorates paraquat-induced acute lung injury by regulating AMPK/MAPK/NF-kappaB signaling. J Cell Biochem. 2019;120:12713–12723. doi: 10.1002/jcb.28540. [DOI] [PubMed] [Google Scholar]
- 206.Chen W., Ma Y., Zhang H., Guo Y., Guan M., Wang Y. Reduning plus ribavirin display synergistic activity against severe pneumonia induced by H1N1 influenza A virus in mice. J Tradit Chin Med. 2020;40:803–811. doi: 10.19852/j.cnki.jtcm.2020.05.010. [DOI] [PubMed] [Google Scholar]
- 207.Tang L.P., Xiao W., Li Y.F., Li H.B., Wang Z.Z., Yao X.S., et al. Anti-inflammatory effects of Reduning Injection on lipopolysaccharide-induced acute lung injury of rats. Chin J Integr Med. 2014;20:591–599. doi: 10.1007/s11655-014-1758-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Peng S., Hang N., Liu W., Guo W., Jiang C., Yang X., et al. Andrographolide sulfonate ameliorates lipopolysaccharide-induced acute lung injury in mice by down-regulating MAPK and NF-kappaB pathways. Acta Pharm Sin B. 2016;6:205–211. doi: 10.1016/j.apsb.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Shi H., Guo W., Zhu H., Li M., Ung C.O.L., Hu H., et al. Cost-effectiveness analysis of Xiyanping injection (andrographolide sulfonate) for treatment of adult community acquired pneumonia: a retrospective, propensity score-matched cohort study. Evid Based Complement Alternat Med. 2019;2019:4510591. doi: 10.1155/2019/4510591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Liu W., Guo W., Guo L., Gu Y., Cai P., Xie N., et al. Andrographolide sulfonate ameliorates experimental colitis in mice by inhibiting Th1/Th17 response. Int Immunopharm. 2014;20:337–345. doi: 10.1016/j.intimp.2014.03.015. [DOI] [PubMed] [Google Scholar]
- 211.Yin Q., Liu B., Wu C., Yang J., Hang C., Li C. Effects of Shen-Fu injection on coagulation-fibrinolysis disorders in a porcine model of cardiac arrest. Am J Emerg Med. 2016;34:469–476. doi: 10.1016/j.ajem.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 212.Yan X., Wu H., Ren J., Liu Y., Wang S., Yang J., et al. Shenfu Formula reduces cardiomyocyte apoptosis in heart failure rats by regulating microRNAs. J Ethnopharmacol. 2018;227:105–112. doi: 10.1016/j.jep.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 213.Mao Z.J., Zhang Q.L., Shang J., Gao T., Yuan W.J., Qin L.P. Shenfu Injection attenuates rat myocardial hypertrophy by up-regulating miR-19a-3p expression. Sci Rep. 2018;8:4660. doi: 10.1038/s41598-018-23137-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Zhang Q., Li C., Shao F., Zhao L., Wang M., Fang Y. Efficacy and safety of combination therapy of Shenfu injection and postresuscitation bundle in patients with return of spontaneous circulation after in-hospital cardiac arrest: a randomized, assessor-blinded, controlled trial. Crit Care Med. 2017;45:1587–1595. doi: 10.1097/CCM.0000000000002570. [DOI] [PubMed] [Google Scholar]
- 215.Jin Y.Y., Gao H., Zhang X.Y., Ai H., Zhu X.L., Wang J. Shenfu Injection inhibits inflammation in patients with acute myocardial infarction complicated by cardiac shock. Chin J Integr Med. 2017;23:170–175. doi: 10.1007/s11655-016-2749-x. [DOI] [PubMed] [Google Scholar]
- 216.Wang Y.Y., Li Y.Y., Li L., Yang D.L., Zhou K., Li Y.H. Protective effects of Shenfu injection against myocardial ischemia‒reperfusion injury via activation of eNOS in rats. Biol Pharm Bull. 2018;41:1406–1413. doi: 10.1248/bpb.b18-00212. [DOI] [PubMed] [Google Scholar]
- 217.Shao F., Li H., Li D., Li C. Effects of Shenfu injection on survival and neurological outcome after out-of-hospital cardiac arrest: a randomised controlled trial. Resuscitation. 2020;150:139–144. doi: 10.1016/j.resuscitation.2019.11.010. [DOI] [PubMed] [Google Scholar]
- 218.Wu J., Li C., Yuan W. Effects of Shenfu injection on macrocirculation and microcirculation during cardiopulmonary resuscitation. J Ethnopharmacol. 2016;180:97–103. doi: 10.1016/j.jep.2016.01.027. [DOI] [PubMed] [Google Scholar]
- 219.Ni J., Shi Y., Li L., Chen J., Li L., Li M., et al. Cardioprotection against heart failure by Shenfu Injection via TGF-beta/Smads signaling pathway. Evid Based Complement Alternat Med. 2017;2017:7083016. doi: 10.1155/2017/7083016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Tan G., Zhou Q., Liu K., Dong X., Li L., Liao W., et al. Cross-platform metabolic profiling deciphering the potential targets of Shenfu injection against acute viral myocarditis in mice. J Pharm Biomed Anal. 2018;160:1–11. doi: 10.1016/j.jpba.2018.07.042. [DOI] [PubMed] [Google Scholar]
- 221.Xu P., Zhang W.Q., Xie J., Wen Y.S., Zhang G.X., Lu S.Q. Shenfu injection prevents sepsis-induced myocardial injury by inhibiting mitochondrial apoptosis. J Ethnopharmacol. 2020;261:113068. doi: 10.1016/j.jep.2020.113068. [DOI] [PubMed] [Google Scholar]
- 222.Zhang X.J., Song L., Zhou Z.G., Wang X.M. Effect of shenfu injection on gastrointestinal microcirculation in rabbits after myocardial ischemia‒reperfusion injury. World J Gastroenterol. 2006;12:4389–4391. doi: 10.3748/wjg.v12.i27.4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Jin S., Jiang R., Lei S., Jin L., Zhu C., Feng W., et al. Shenfu injection prolongs survival and protects the intestinal mucosa in rats with sepsis by modulating immune response. Turk J Gastroenterol. 2019;30:364–371. doi: 10.5152/tjg.2019.18418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Ma X., Yang Y.X., Chen N., Xie Q., Wang T., He X., et al. Meta-analysis for clinical evaluation of Xingnaojing injection for the treatment of cerebral infarction. Front Pharmacol. 2017;8:485. doi: 10.3389/fphar.2017.00485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Zhang Y.M., Qu X.Y., Tao L.N., Zhai J.H., Gao H., Song Y.Q., et al. XingNaoJing injection ameliorates cerebral ischaemia/reperfusion injury via SIRT1-mediated inflammatory response inhibition. Pharm Biol. 2020;58:16–24. doi: 10.1080/13880209.2019.1698619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Xu M., Su W., Xu Q.P., Huang W.D. Effect of Xingnaojing injection on cerebral edema and blood‒brain barrier in rats following traumatic brain injury. Chin J Traumatol. 2010;13:158–162. [PubMed] [Google Scholar]
- 227.Qu X.Y., Zhang Y.M., Tao L.N., Gao H., Zhai J.H., Sun J.M., et al. XingNaoJing injections protect against cerebral ischemia/reperfusion injury and alleviate blood‒brain barrier disruption in rats, through an underlying mechanism of NLRP3 inflammasomes suppression. Chin J Nat Med. 2019;17:498–505. doi: 10.1016/S1875-5364(19)30071-8. [DOI] [PubMed] [Google Scholar]
- 228.Ma X., Wang T., Wen J., Wang J., Zeng N., Zou W., et al. Role of Xingnaojing injection in treating acute cerebral hemorrhage: a systematic review and meta-analysis. Medicine (Baltimore) 2020;99 doi: 10.1097/MD.0000000000019648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Liu H., Yan Y., Pang P., Mao J., Hu X., Li D., et al. Angong Niuhuang Pill as adjuvant therapy for treating acute cerebral infarction and intracerebral hemorrhage: a meta-analysis of randomized controlled trials. J Ethnopharmacol. 2019;237:307–313. doi: 10.1016/j.jep.2019.03.043. [DOI] [PubMed] [Google Scholar]
- 230.Zhang D.S., Liu Y.L., Zhu D.Q., Huang X.J., Luo C.H. Point application with Angong Niuhuang sticker protects hippocampal and cortical neurons in rats with cerebral ischemia. Neural Regen Res. 2015;10:286–291. doi: 10.4103/1673-5374.152384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Zhang D., Fu M., Song C., Wang C., Lin X., Liu Y. Expressions of apoptosis-related proteins in rats with focal cerebral ischemia after Angong Niuhuang sticker point application. Neural Regen Res. 2012;7:2347–2353. doi: 10.3969/j.issn.1673-5374.2012.30.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Huang K., Zhang P., Zhang Z., Youn J.Y., Zhang H., Cai H.L. Traditional Chinese medicine (TCM) in the treatment of viral infections: efficacies and mechanisms. Pharmacol Ther. 2021;225:107843. doi: 10.1016/j.pharmthera.2021.107843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Yeoh Y.K., Zuo T., Lui G.C., Zhang F., Liu Q., Li A.Y., et al. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut. 2021;70:698–706. doi: 10.1136/gutjnl-2020-323020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Augusti P.R., Conterato G.M.M., Denardin C.C., Prazeres I.D., Serra A.T., Bronze M.R., et al. Bioactivity, bioavailability, and gut microbiota transformations of dietary phenolic compounds: implications for COVID-19. J Nutr Biochem. 2021;97:108787. doi: 10.1016/j.jnutbio.2021.108787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Li X., Wu D., Niu J., Sun Y., Wang Q., Yang B., et al. Intestinal flora: a pivotal role in investigation of traditional Chinese medicine. Am J Chin Med. 2021;49:237–268. doi: 10.1142/S0192415X21500130. [DOI] [PubMed] [Google Scholar]
- 236.Zhang Q., Yue S., Wang W., Chen Y., Zhao C., Song Y., et al. Potential role of gut microbiota in traditional Chinese medicine against COVID-19. Am J Chin Med. 2021;49:785–803. doi: 10.1142/S0192415X21500373. [DOI] [PubMed] [Google Scholar]
- 237.Wu G.S., Zhong J., Zheng N.N., Wang C.R., Jin H.L., Ge G.B., et al. Investigation of modulating effect of Qingfei Paidu Decoction on host metabolism and gut microbiome in rats. China J Chin Mater Med. 2020;45:3726–3739. doi: 10.19540/j.cnki.cjcmm.20200609.201. [DOI] [PubMed] [Google Scholar]
- 238.Lyu M., Wang Y.F., Fan G.W., Wang X.Y., Xu S.Y., Zhu Y. Balancing herbal medicine and functional food for prevention and treatment of cardiometabolic diseases through modulating gut microbiota. Front Microbiol. 2017;8:2146. doi: 10.3389/fmicb.2017.02146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Qiu Y., Yang J., Wang L., Yang X., Gao K., Zhu C., et al. Dietary resveratrol attenuation of intestinal inflammation and oxidative damage is linked to the alteration of gut microbiota and butyrate in piglets challenged with deoxynivalenol. J Anim Sci Biotechnol. 2021;12:71. doi: 10.1186/s40104-021-00596-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Valizadeh H., Abdolmohammadi-Vahid S., Danshina S., Ziya G.M., Ammari A., Sadeghi A., et al. Nano-curcumin therapy, a promising method in modulating inflammatory cytokines in COVID-19 patients. Int Immunopharm. 2020;89:107088. doi: 10.1016/j.intimp.2020.107088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Dai Y.J., Wan S.Y., Gong S.S., Liu J.C., Li F., Kou J.P. Recent advances of traditional Chinese medicine on the prevention and treatment of COVID-19. Chin J Nat Med. 2020;18:881–889. doi: 10.1016/S1875-5364(20)60031-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Wang Z., Yang L. Chinese herbal medicine: fighting SARS-CoV-2 infection on all fronts. J Ethnopharmacol. 2021;270:113869. doi: 10.1016/j.jep.2021.113869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Lee D.Y.W., Li Q.Y., Liu J., Efferth T. Traditional Chinese herbal medicine at the forefront battle against COVID-19: clinical experience and scientific basis. Phytomedicine. 2020;80:153337. doi: 10.1016/j.phymed.2020.153337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.An X., Zhang Y., Duan L., Jin, Zhao S., Zhou R., et al. The direct evidence and mechanism of traditional Chinese medicine treatment of COVID-19. Biomed Pharmacother. 2021;137:111267. doi: 10.1016/j.biopha.2021.111267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Huang J., Tao G., Liu J., Cai J., Huang Z., Chen J.X. Current prevention of COVID-19: natural products and herbal medicine. Front Pharmacol. 2020;11:588508. doi: 10.3389/fphar.2020.588508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Jalali A., Dabaghian F., Akbrialiabad H., Foroughinia F., Zarshenas M.M. A pharmacology-based comprehensive review on medicinal plants and phytoactive constituents possibly effective in the management of COVID-19. Phytother Res. 2020;35:1925–1938. doi: 10.1002/ptr.6936. [DOI] [PubMed] [Google Scholar]
- 247.Nugraha R.V., Ridwansyah H., Ghozali M., Khairani A.F., Atik N. Traditional herbal medicine candidates as complementary treatments for COVID-19: a review of their mechanisms, pros and cons. Evid Based Complement Alternat Med. 2020;2020:2560645. doi: 10.1155/2020/2560645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Li Q., Ran Q., Sun L., Yin J., Luo T., Liu L., et al. Lian Hua Qing Wen capsules, a potent epithelial protector in acute lung injury model, block proapoptotic communication between macrophages, and alveolar epithelial cells. Front Pharmacol. 2020;11:522729. doi: 10.3389/fphar.2020.522729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Chen K., Chen H. Traditional Chinese medicine for combating COVID-19. Front Med. 2020;14:529–532. doi: 10.1007/s11684-020-0802-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Huang S.T., Lai H.C., Lin Y.C., Huang W.T., Hung H.H., Ou S.C., et al. Principles and treatment strategies for the use of Chinese herbal medicine in patients at different stages of coronavirus infection. Am J Cancer Res. 2020;10:2010–2031. [PMC free article] [PubMed] [Google Scholar]
- 251.Yang Y., Islam M.S., Wang J., Li Y., Chen X. Traditional Chinese medicine in the treatment of patients infected with 2019-new coronavirus (SARS-CoV-2): a review and perspective. Int J Biol Sci. 2020;16:1708–1717. doi: 10.7150/ijbs.45538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Khare P., Sahu U., Pandey S.C., Samant M. Current approaches for target-specific drug discovery using natural compounds against SARS-CoV-2 infection. Virus Res. 2020;290:198169. doi: 10.1016/j.virusres.2020.198169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Wiersinga W.J., Rhodes A., Cheng A.C., Peacock S.J., Prescott H.C. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. J Am Med Assoc. 2020;324:782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 254.Dhama K., Khan S., Tiwari R., Sircar S., Bhat S., Malik Y.S., et al. Coronavirus disease 2019-COVID-19. Clin Microbiol Rev. 2020;33 doi: 10.1128/CMR.00028-20. e00028-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Xian Y., Zhang J., Bian Z., Zhou H., Zhang Z., Lin Z., et al. Bioactive natural compounds against human coronaviruses: a review and perspective. Acta Pharm Sin B. 2020;10:1163–1174. doi: 10.1016/j.apsb.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Li S., Liu C., Guo F., Taleb S.J., Tong M., Shang D. Traditional Chinese medicine as potential therapy for COVID-19. Am J Chin Med. 2020;48:1263–1277. doi: 10.1142/S0192415X20500627. [DOI] [PubMed] [Google Scholar]
- 257.Shi J., Lu Y., Zhang Y., Xia L., Ye C., Lu Y., et al. Traditional Chinese medicine formulation therapy in the treatment of coronavirus disease 2019 (COVID-19) Am J Chin Med. 2020;48:1523–1538. doi: 10.1142/S0192415X20500755. [DOI] [PubMed] [Google Scholar]
- 258.Zhang L., Yu J., Zhou Y., Shen M., Sun L. Becoming a faithful defender: traditional Chinese medicine against coronavirus disease 2019 (COVID-19) Am J Chin Med. 2020;48:763–777. doi: 10.1142/S0192415X2050038X. [DOI] [PubMed] [Google Scholar]
- 259.Sun C.Y., Sun Y.L., Li X.M. The role of Chinese medicine in COVID-19 pneumonia: a systematic review and meta-analysis. Am J Emerg Med. 2020;38:2153–2159. doi: 10.1016/j.ajem.2020.06.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Zhang J., Xie B., Hashimoto K. Current status of potential therapeutic candidates for the COVID-19 crisis. Brain Behav Immun. 2020;87:59–73. doi: 10.1016/j.bbi.2020.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Liu L. Traditional Chinese medicine contributes to the treatment of COVID-19 patients. Chin Herb Med. 2020;12:95–96. doi: 10.1016/j.chmed.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Li J.G., Xu H. Chinese medicine in fighting against Covid-19: role and inspiration. Chin J Integr Med. 2021;27:3–6. doi: 10.1007/s11655-020-2860-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Tong T., Wu Y.Q., Ni W.J., Shen A.Z., Liu S. The potential insights of traditional Chinese medicine on treatment of COVID-19. Chin Med. 2020;15:51. doi: 10.1186/s13020-020-00326-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Xu J., Zhang Y. Traditional Chinese medicine treatment of COVID-19. Compl Ther Clin Pract. 2020;39:101165. doi: 10.1016/j.ctcp.2020.101165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Zhuang W., Fan Z., Chu Y., Wang H., Yang Y., Wu L., et al. Chinese patent medicines in the treatment of coronavirus disease 2019 (COVID-19) in China. Front Pharmacol. 2020;11:1066. doi: 10.3389/fphar.2020.01066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Lopez-Alcalde J., Yan Y., Witt C.M., Barth J. Current state of research about Chinese herbal medicines (CHM) for the treatment of coronavirus disease 2019 (COVID-19): a scoping review. J Altern Complement Med. 2020;26:557–570. doi: 10.1089/acm.2020.0189. [DOI] [PubMed] [Google Scholar]
- 267.Zhou L.P., Wang J., Xie R.H., Pakhale S., Krewski D., Cameron D.W., et al. The effects of traditional Chinese medicine as an auxiliary treatment for COVID-19: a systematic review and meta-analysis. J Altern Complement Med. 2021;27:225–237. doi: 10.1089/acm.2020.0310. [DOI] [PubMed] [Google Scholar]
- 268.Ling C.Q. Traditional Chinese medicine is a resource for drug discovery against 2019 novel coronavirus (SARS-CoV-2) J Integr Med. 2020;18:87–88. doi: 10.1016/j.joim.2020.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Al-Romaima A., Liao Y., Feng J., Qin X., Qin G. Advances in the treatment of novel coronavirus disease (COVID-19) with Western medicine and traditional Chinese medicine: a narrative review. J Thorac Dis. 2020;12:6054–6069. doi: 10.21037/jtd-20-1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.McKee D.L., Sternberg A., Stange U., Laufer S., Naujokat C. Candidate drugs against SARS-CoV-2 and COVID-19. Pharmacol Res. 2020;157:104859. doi: 10.1016/j.phrs.2020.104859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Ren J.L., Zhang A.H., Wang X.J. Traditional Chinese medicine for COVID-19 treatment. Pharmacol Res. 2020;155:104743. doi: 10.1016/j.phrs.2020.104743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Wang W.Y., Xie Y., Zhou H., Liu L. Contribution of traditional Chinese medicine to the treatment of COVID-19. Phytomedicine. 2021;85:153279. doi: 10.1016/j.phymed.2020.153279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Zhang J.L., Li W.X., Li Y., Wong M.S., Wang Y.J., Zhang Y. Therapeutic options of TCM for organ injuries associated with COVID-19 and the underlying mechanism. Phytomedicine. 2021;85:153297. doi: 10.1016/j.phymed.2020.153297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Zhao Z., Li Y., Zhou L., Zhou X., Xie B., Zhang W., et al. Prevention and treatment of COVID-19 using traditional Chinese medicine: a review. Phytomedicine. 2021;85:153308. doi: 10.1016/j.phymed.2020.153308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.He T., Qu R., Qin C., Wang Z., Zhang Y., Shao X., et al. Potential mechanisms of Chinese herbal medicine that implicated in the treatment of COVID-19 related renal injury. Saudi Pharm J. 2020;28:1138–1148. doi: 10.1016/j.jsps.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Cui H.T., Li Y.T., Guo L.Y., Liu X.G., Wang L.S., Jia J.W., et al. Traditional Chinese medicine for treatment of coronavirus disease 2019: a review. Tradit Med Res. 2020;5:65–73. [Google Scholar]
- 277.Mani J.S., Johnson J.B., Steel J.C., Broszczak D.A., Neilsen P.M., Walsh K.B., et al. Natural product-derived phytochemicals as potential agents against coronaviruses: a review. Virus Res. 2020;284:197989. doi: 10.1016/j.virusres.2020.197989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Gray P.E., Belessis Y. The use of Traditional Chinese medicines to treat SARS-CoV-2 may cause more harm than good. Pharmacol Res. 2020;156:104776. doi: 10.1016/j.phrs.2020.104776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Yang Y. Use of herbal drugs to treat COVID-19 should be with caution. Lancet. 2020;395:1689–1690. doi: 10.1016/S0140-6736(20)31143-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Zhang A.H., Ren J.L., Wang X.J. Reply to "The use of traditional Chinese medicines to treat SARS-CoV-2 may cause more harm than good". Pharmacol Res. 2020;157:104775. doi: 10.1016/j.phrs.2020.104775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Huang C., Huang L., Wang Y., Li X., Ren L., Gu X., et al. 6-Month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Yelin D., Wirtheim E., Vetter P., Kalil A.C., Bruchfeld J., Runold M., et al. Long-term consequences of COVID-19: research needs. Lancet Infect Dis. 2020;20:1115–1117. doi: 10.1016/S1473-3099(20)30701-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.An Y.W., Yuan B., Wang J.C., Wang C., Liu T.T., Song S., et al. Clinical characteristics and impacts of traditional Chinese medicine treatment on the convalescents of COVID-19. Int J Med Sci. 2021;18:646–651. doi: 10.7150/ijms.52664. [DOI] [PMC free article] [PubMed] [Google Scholar]